-
化石燃料燃烧、汽车尾气、燃煤电厂以及工业锅炉排放的NOx是大气的主要污染物之一,会造成酸雨、雾霾以及光化学烟雾等一系列环境问题,对生态环境和人类健康均造成了严重危害[1-2]。目前,NH3选择性催化还原NOx技术(NH3-SCR)被广泛研究,并在工业上应用于控制NO、NO2和N2O的排放,已经被证明是经济且高效的NOx脱除方法[3]。其中,最广泛使用的催化剂是V2O5-WO3/TiO2。然而,传统的V2O5-WO3/TiO2催化剂虽然具有较高的催化活性和耐硫性能,但存在反应温度高、操作温度窗口窄、V2O5对环境有害等缺点[4]。因此,有必要开发环保高效的低温脱硝催化剂。
多孔材料在材料学领域有着悠久的研究历史,是NH3-SCR催化剂优异的载体候选材料[5]。Song等[6]采用水热法制备了具有微孔结构的的TiO2,与传统TiO2相比,前者所具有的小孔结构使反应中间体NH3-NO3能够稳定存在,不易分解为N2O,从而使得以具有小孔结构的TiO2为载体所制备的催化剂具有更高的N2选择性和NOx转化率。Zha等[7]采用溶剂热法制备了介孔TiO2球,并通过浸渍法制备了MnOx-CeO2/m-TiO2催化剂,研究发现,由于载体的多孔结构使得活性组分可均匀吸附在其表面而不团聚,介孔TiO2的孔结构抑制了活性组分的迁移,从而使得SCR反应充分进行。
硅藻土由于价格低廉,且具有独特的介孔和微孔结构等诸多优良性能,被应用于许多重要领域,如作为有毒气体或有机染料的吸附材料等[8]。以硅藻土为原料,将其制备成具有一定形状与机械强度的多孔陶瓷,可以进一步扩大其应用领域。多孔材料的制备方法多种多样,如造孔剂法[9]、直接发泡发[10]、冷冻干燥法[11]、模板法[12]、泡沫凝胶铸造法[13]等,这些方法可直接用于或改性制备硅藻土基多孔陶瓷[8]。其中,造孔剂法制备多孔陶瓷,因为工艺简单,成本低廉而被广泛应用。目前,多孔陶瓷造孔剂大致可分为两类,有机造孔剂和无机造孔剂。有机造孔剂通常为天然有机物和高分子聚合物,而植物类的天然有机物因成本低廉且来源广泛备受青睐,无机造孔剂则可分为高温分解和氧化燃烧两种类型。本文分别选取价格低廉的淀粉、高温下分解的碳酸钙和氧化燃烧的石墨作为造孔剂进行研究,并以埃洛石黏土为添加剂增强样品的机械强度进行研究。
制备方法影响催化剂的催化活性[2],本课题组前期所开发的原位生长法无需煅烧,便可将活性组分原位沉积在载体表面,该方法与载体结构的协同效应能够促进SCR反应的进行[14]。本文采用造孔剂法制备了孔隙度较高且具有一定机械强度的硅藻土基多孔陶瓷,并以所制备的多孔陶瓷为载体,通过原位生长法将MnOx原位沉积在多孔陶瓷表面。采用XRD、SEM、XPS等方法对所制备样品进行表征,重点研究了造孔剂种类与含量对多孔陶瓷结构与性能的影响,以及对负载活性组分之后的催化剂催化活性的影响。拓宽了硅藻土基多孔陶瓷的研究领域,为多孔陶瓷类载体在SCR领域的研究提供了一定的思路。
-
埃洛石(HAL,郑州金阳光陶瓷有限公司),主要化学组成(质量分数)为: 50.31% SiO2,38.27% Al2O3,7.59% SO3,1.22% K2O,1.12% Fe2O3;硅藻土(DE,国药集团化学试剂有限公司),主要化学成分(质量分数)为:88.80% SiO2,;2.98% Al2O3, 2.06% Fe2O3,2.03% CO2,1.21% Na2O;乙酸锰 Mn(CH3COO)2;高锰酸钾KMnO4;聚乙烯醇(PVA,(CH2CHOH)n);淀粉(Starch,(C6H10O5)n);石墨(Graphite,C);碳酸钙 CaCO3;均由国药集团化学试剂有限公司提供,纯度为AR,模拟烟气由南京上元工业气体厂提供。
-
(1)多孔陶瓷的制备
首先称取总量为10 g的硅藻土与埃洛石粉末,质量比6:4,再称取适量造孔剂,采用湿法球磨(球:原料:酒精 = 2:1:0.6)将上述粉末混合均匀,110 ℃过夜干燥,过200目筛,滴加4% wt. PVA,研磨,经红外压片机4 Mpa压片成型,再经管式炉1000 ℃煅烧,得到硅藻土基多孔陶瓷。将所得多孔陶瓷破碎后过20目筛,用以作为催化剂载体,并标记为 x %wt. y-DE-HAL (x为造孔剂含量,y为造孔剂种类;x=0、2、4、6、8;y=S 淀粉,G 石墨,Ca 碳酸钙,下同)。
(2)原位生长法制备Mn/DE-HAL催化剂
称取5 g制备的硅藻土基多孔陶瓷,称取化学计量的乙酸锰溶解于一定量的去离子水中,用以作为锰的前驱体。将多孔陶瓷等体积浸渍于乙酸锰溶液中,静置24 h后加入KMnO4溶液,搅拌后静置24 h,多次抽滤洗涤至滤液澄清无色,K+通过多次去离子水反复冲洗抽滤除去,于鼓风干燥箱中110 ℃过夜干燥,即得所需催化剂(催化剂中Mn元素质量分数为8%),并标记为x %wt. y-Mn/DE-HAL。
-
催化剂的脱硝活性评价测试装置为长80 cm,内径15 mm的竖式石英玻璃常压固定床反应器,活性评价装置主要由模拟烟气 (1000 mg·L−1 NO、1000 mg·L−1 NH3、3% O2、N2为平衡气混合而成,总流量为350 mL·min−1) 、固定床反应器和分析检测装置的3部分组成。反应空速分别为21000 h−1、52000 h−1、84000 h−1,反应温度为100—300 ℃。采用德国MRU OPTIMA 7烟气分析仪对反应器进出口的烟气浓度进行在线测量。
催化剂的活性计算公式为:
式中,[NO]in、[NO]out分别为NO进口、出口浓度。
-
采用美国康塔NOVA 2200e型比表面积及孔径分析仪分析样品的比表面积和孔径孔容。采用荷兰帕纳科PANalytical X-Pert PRO MPD型X射线衍射仪对多孔陶瓷和催化剂的物相与晶型进行表征,Cu Kα射线,操作电压为40 kV,操作电流为40 mA,扫描速度为4 o·min−1,扫描区间范围2θ=5°—80° 。采用美国Thermo ESCALAB250Xi型X射线光电子能谱仪对催化剂进行XPS测试,Al Kα射线,管电压为1486.6 eV。采用德国卡尔蔡司Gemini 500型热场发射扫描电子显微镜观察多孔陶瓷与催化剂的表面微观结构。将样品制成直径为13 mm,厚度为1.4 mm的圆柱状陶瓷,采用YHKC-3A型自动颗粒强度测定仪测量样品轴向机械强度,强度数据见表1.
-
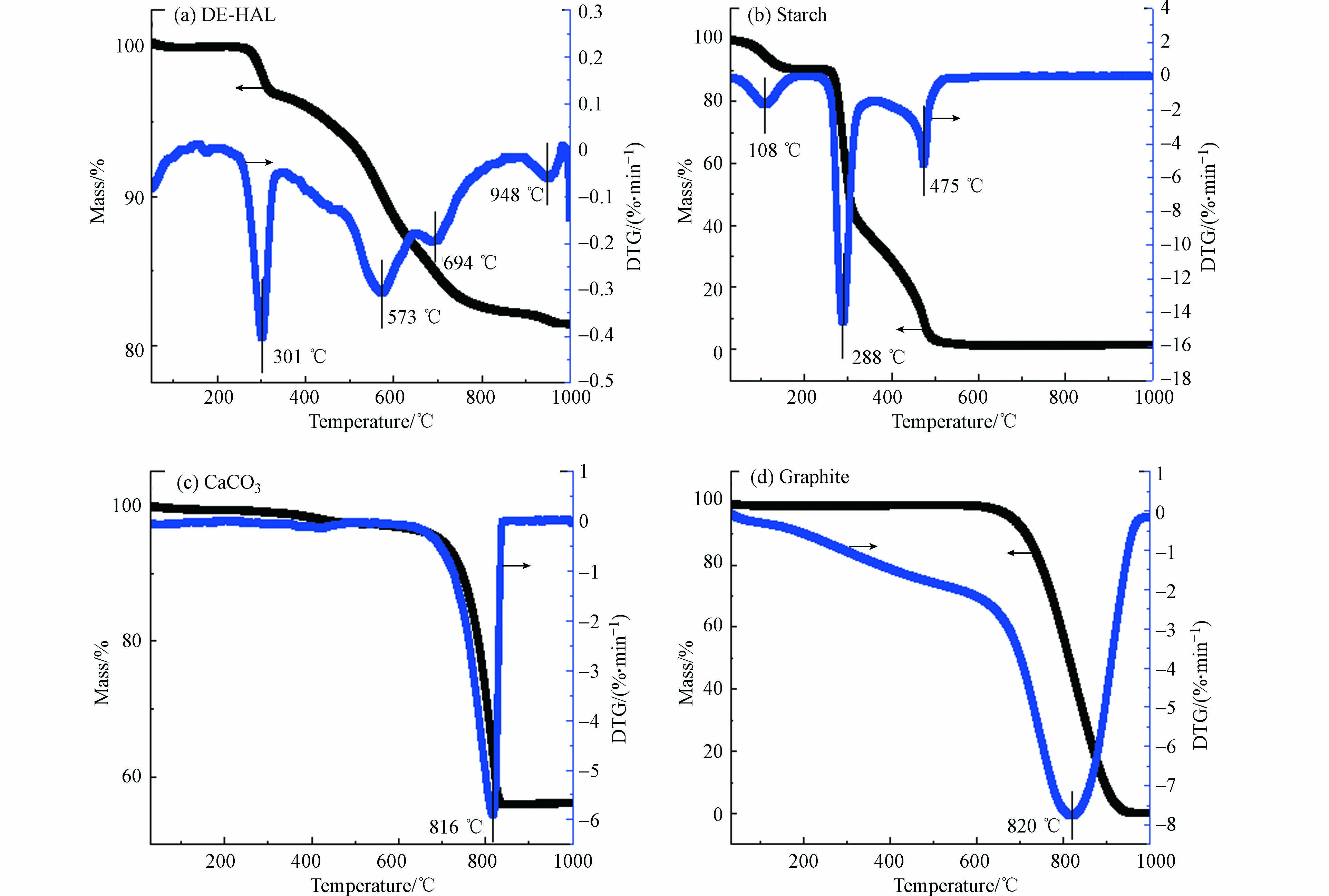
图1为陶瓷原料与3种造孔剂的热分析图。图1a为陶瓷原料DE-HAL的TG-DTG曲线,样品在250—340 ℃之间质量损失约为3%,归因于原料中硅藻土结构水的脱除[15],400—700 ℃之间的吸热谷推测是由于原料中埃洛石的脱羟基作用,948 ℃处的吸热谷归因于原料中的石英相向方石英相的转变[15]。图1b为淀粉的TG-DTG曲线,淀粉在30—1000 ℃的失重曲线分为3个阶段,50—160 ℃之间质量损失约为8%,为水分子的脱除,288 ℃处的吸热谷是由于淀粉的分解,而475 ℃处的吸热谷,因于淀粉的进一步分解,500 ℃时淀粉基本分解完全。淀粉在较低温度下脱除的水分子以及在氧气充足条件下高温分解所产生的CO2扩散到陶瓷外部后均会在陶瓷基体中留下孔隙。图1 c为CaCO3的TG-DTG曲线,CaCO3仅在650—850 ℃之间有一个吸热谷,质量损失约为45%,该部分是由于CaCO3的氧化分解,850 ℃之后曲线趋于平稳,CaCO3不再分解。CaCO3氧化分解生成CaO和CO2,其中CaO留在了陶瓷基体中,CO2扩散到了外部,并留下了孔隙。图1 d为石墨的TG-DTG曲线,石墨在30—1000 ℃之间仅在820 ℃处有1个吸热谷,在650—940 ℃之间样品的质量损失为100%,即石墨在该温度区间内燃烧完全。石墨在高温下燃烧释放出大量CO2,这些CO2在陶瓷基体中留下了较多的孔隙。对比3种造孔剂的TG-DTG曲线可知,淀粉的分解温度较低,而造孔剂在较低温度留下的孔隙,在更高温度下会烧结收缩,即采用较低温度下分解的造孔剂所制备的多孔陶瓷孔隙率较低,且容易形成闭孔。此外,淀粉与石墨在30—1000 ℃均完全分解,未在陶瓷基体中残留,而CaCO3则在陶瓷基体中留下CaO。
-
表2为多孔陶瓷的比表面积、孔道结构以及密度数据,表3为负载了MnOx的催化剂的比表面积与孔道结构数据。由表2可以看出,造孔剂种类相同的情况下,随着造孔剂含量的增加,多孔陶瓷比表面积、孔隙率逐渐增加,密度逐渐减小,孔径大小几乎没有变化。造孔剂含量相同时,采用不同种类造孔剂制备的多孔陶瓷孔径分布相似,孔容与孔隙率区别较为显著。4% wt. G-Mn/DE-HAL较之其他两个样品具有更大的孔容和更高的孔隙率。由表3可以看出,催化剂比表面积变化规律与未负载活性组分的多孔陶瓷呈相同趋势,负载MnOx之后,样品比表面积与孔容明显增加,这与活性组分在多孔陶瓷表面的分散有关,而孔径减小,可能是MnOx堵塞了多孔陶瓷的部分孔隙造成的。
-
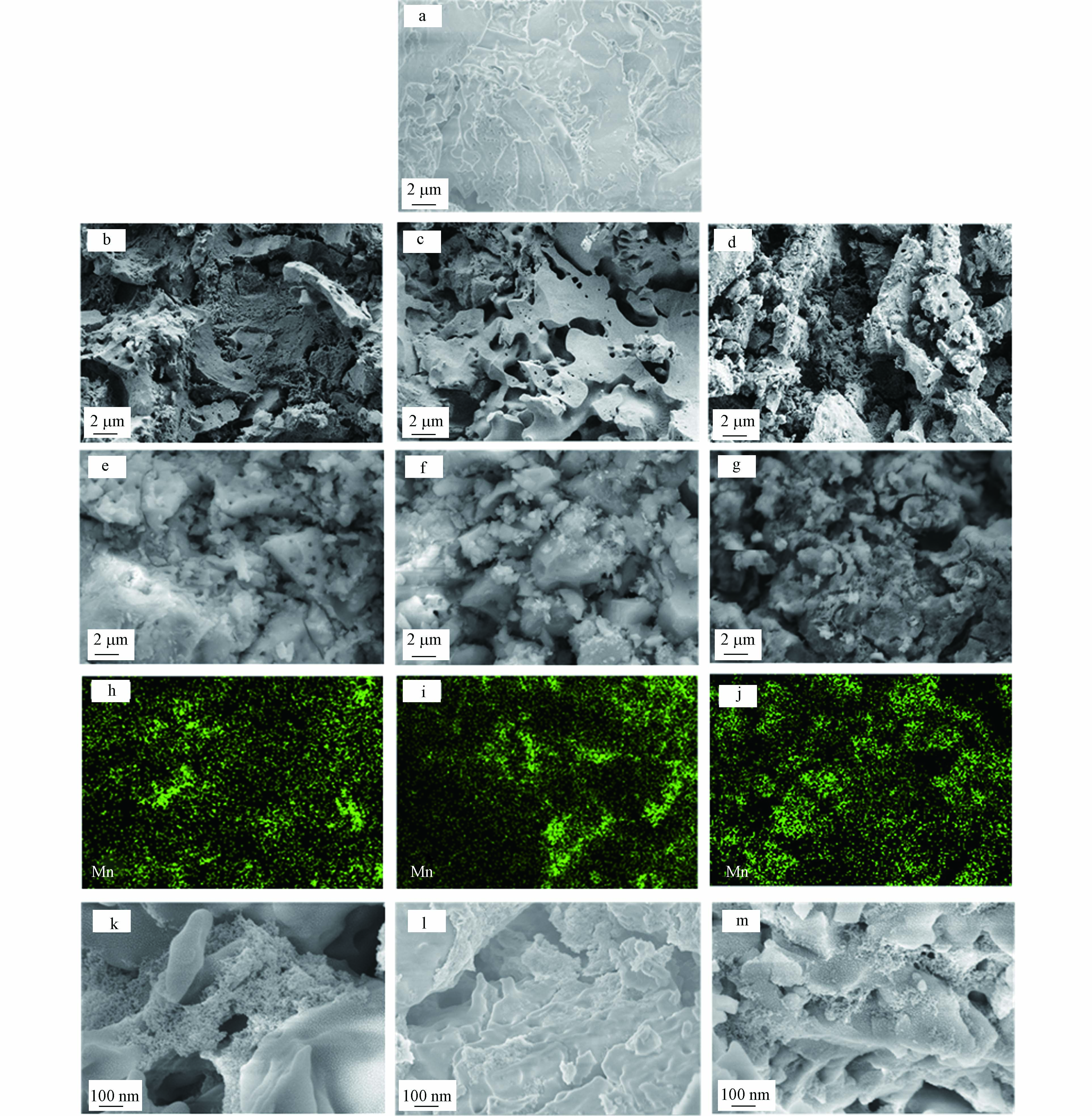
为了更直观地研究造孔剂种类对多孔陶瓷以及催化剂结构和性能的影响,对样品进行SEM和Mapping表征。图2(a)为未添加造孔剂的陶瓷载体,图2(b—d)分别为添加了3种造孔剂所制备的多孔陶瓷,如图所示,与未添加造孔剂的陶瓷相比,添加造孔剂所制备的多孔陶瓷表面均呈现出较多的大小不一的孔隙,结合氮气吸脱附以及阿基米德排水法计算的孔隙率结果可知,造孔剂成功地在陶瓷基体中留下了孔隙。此外,添加3种不同种类的造孔剂所制得的多孔陶瓷形貌差异显著,以淀粉和石墨为造孔剂所制备的多孔陶瓷表面粗糙,而以碳酸钙为造孔剂所制备的多孔陶瓷表面较为光滑。如图2(e—g)所示,负载MnOx之后,活性组分分布在多孔陶瓷的表面以及孔隙中。图2(h—j)为4 %wt. 的S-Mn/DE-HAL,4%wt. Ca-Mn/DE-HAL,4% wt. G-Mn/DE-HAL的Mapping图,可以观察到MnOx在3个样品表面的分散性差异较为明显,4% wt. Ca-Mn/DE-HAL表面MnOx的分散性最差,其次是4% wt. 的S-Mn/DE-HAL,活性组分在4% wt. G-Mn/DE-HAL表面分散的较为均匀。图2(k—m)中,4% wt. 的S-Mn/DE-HAL表面MnOx团聚较为严重,4% wt. Ca-Mn/DE-HAL表面粗糙度不高,负载的MnOx较少,而4% wt. G-Mn/DE-HAL表面凹凸不平,较多的活性组分分散在孔隙之间。可见,粗糙的表面更有利于活性组分的附着。
-
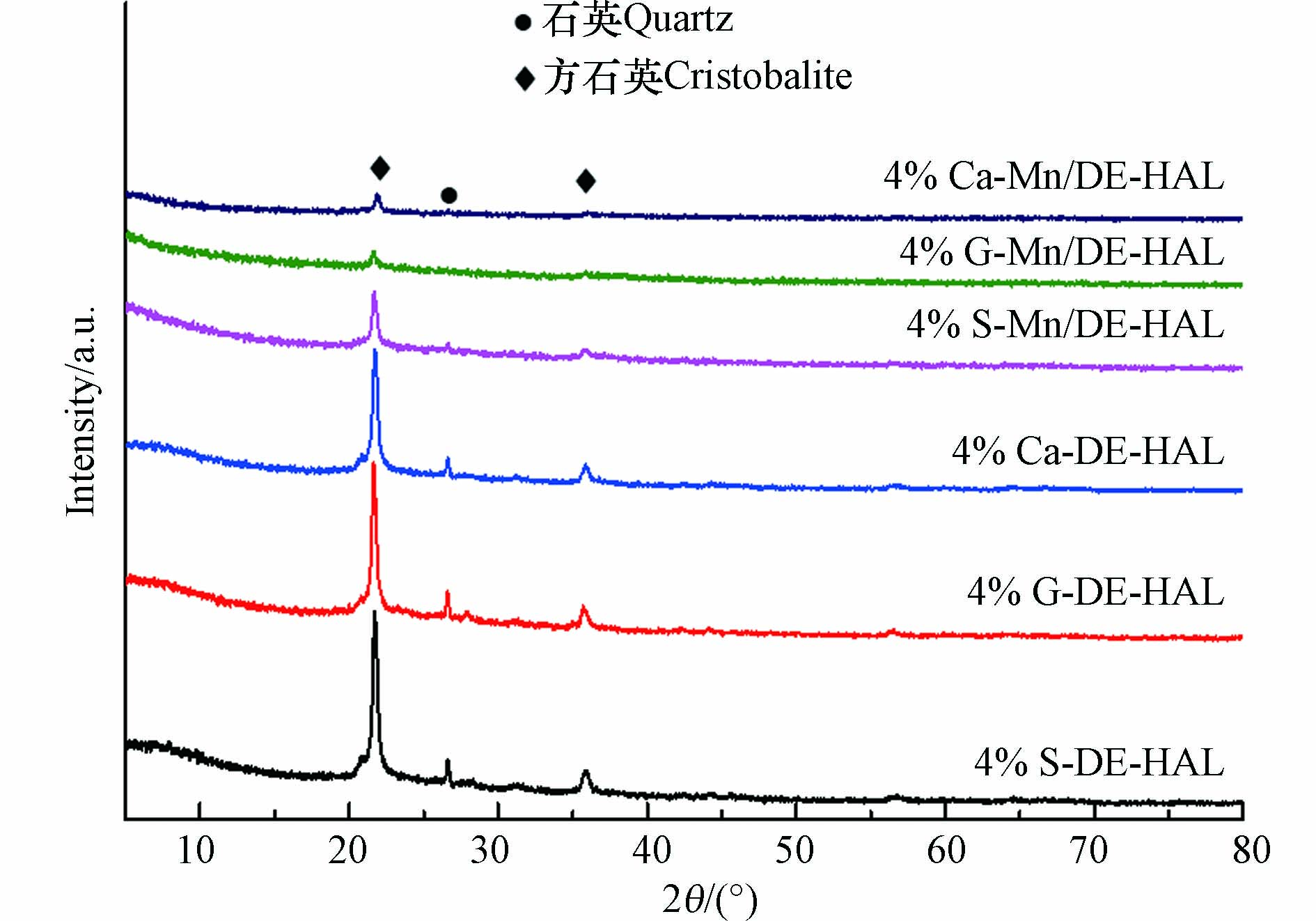
图3为采用3种造孔剂所制备的多孔陶瓷以及多孔陶瓷负载MnOx之后的XRD图。图3中并未观察到MnOx的峰,这可能是因为活性组分呈无定形态分布在载体表面[2]。21.66°和35.94°处的峰为方石英的特征衍射峰(PDF#39-1425),26.49°处的峰为石英的特征衍射峰(PDF#46-1045)。由XRD图谱可以观察到,未负载MnOx的多孔陶瓷在21.66°处的方石英峰十分尖锐,此外,未负载活性组分的3个样品衍射峰差异并不明显。多孔陶瓷负载MnOx之后,石英与方石英的衍射峰均明显减弱,这并非是因为Mn的引入破坏了多孔陶瓷的结构。一方面活性组分包裹在载体表面会削弱载体原有的衍射峰[14],另一方面由于样品的特殊性,筛分得到的样品多孔陶瓷含量较少,最终导致归属于载体的衍射峰并不明显。
-
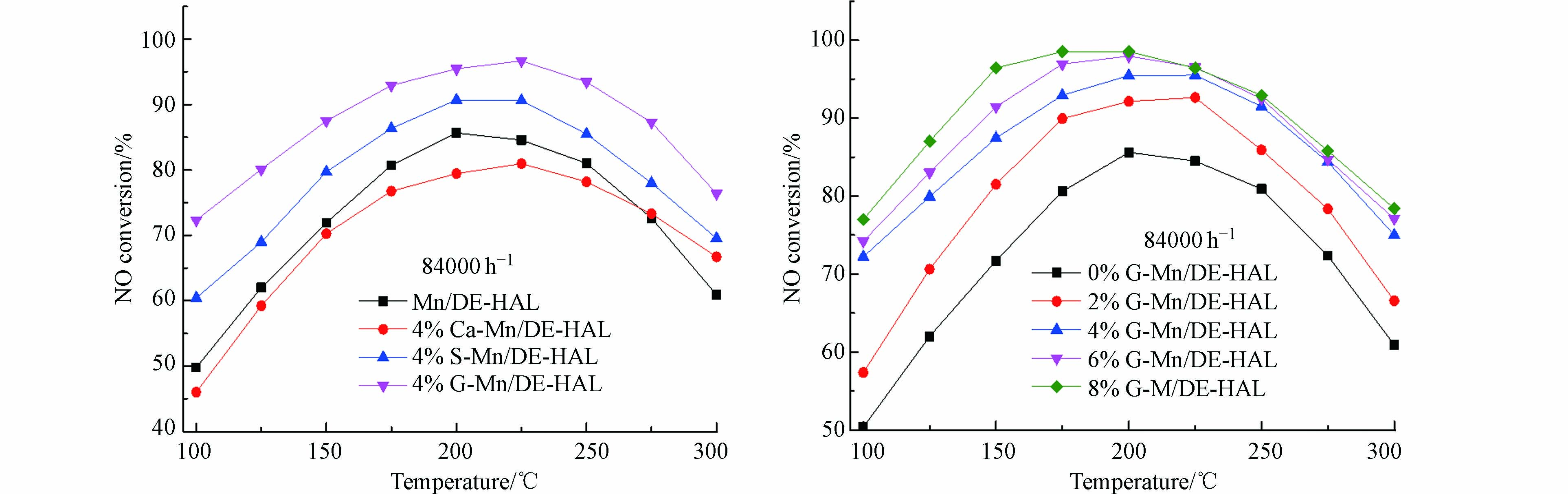
图4为造孔剂种类对催化剂NO转化率的影响。造孔剂种类的变化,直接影响多孔陶瓷的孔隙率与比表面积等理化性质,从而影响催化剂的脱硝活性。图4为Mn/DE-HAL、4% wt. S-Mn/DE-HAL、4% wt. G-Mn/DE-HAL、4% wt. Ca-Mn/DE-HAL等 4个样品在100—300 ℃范围内NO的转化率,测试过程中,每个温度点稳定40 min,分别在保温时间剩余12、6、1 min时对出口NO浓度进行测量,取3次测试的平均值作为最终数据,测试结果误差在1%以内。由图4可以看出,所有样品在100—300 ℃之间的脱硝率均呈先升高后降低的趋势。未添加造孔剂的样品Mn/DE-HAL在100 ℃时的NO转化率约为50%,200 ℃时NO转化率最高,略高于80%。4% wt. S-Mn/DE-HAL在100 ℃时NO转化率约为60%,200 ℃时NO转化率最高,约为90%。4% wt. Ca-Mn/DE-HAL在100 ℃时NO转化率低于50%,225 ℃时活性最好,接近80%,300 ℃时NO转化率约为65%。4%wt. G-Mn/DE-HAL在100 ℃时NO转化率高于70%,在175—250 ℃范围内NO转化率高于90%,300 ℃时NO转化率仍然接近75%。综合比较,4个催化剂脱硝性能强弱顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL。4% wt. Ca-Mn/DE-HAL脱硝性能最差一方面因为其表面粗糙度低,不利于MnOx负载,另一方面是因为CaCO3高温下分解为CaO和CO2,其中CaO作为碱土金属,降低了催化剂的NO转化率[16]。4% wt. G-Mn/DE-HAL与4% wt. S-Mn/DE-HAL孔隙率较高且表面更粗糙,可见,更高的孔隙率与更粗糙的表面有利于SCR反应的进行。
-
图4为造孔剂含量对于催化剂NO转化率的影响。造孔剂含量的变化会影响多孔陶瓷的孔隙率与比表面积等理化性质,从而影响催化剂的脱硝性能。图4分别为0% wt. G-Mn/DE-HAL,2% wt. G-Mn/DE-HAL,4% wt. G-Mn/DE-HAL,6% wt. G-Mn/DE-HAL,8% wt. G-Mn/DE-HAL 5个样品在100—300 ℃范围内的NO转化率。随着造孔剂含量的增加,催化剂脱硝性能逐渐增强,0% wt. G-Mn/DE-HAL在200 ℃之后NO转化率开始大幅度下降,300 ℃时NO转化率约为60%。2% wt. G-Mn/DE-HAL在150—250 ℃之间NO转化率高于80%。4% wt. G-Mn/DE-HAL与6% wt. G-Mn/DE-HAL两个样品在150—275 ℃之间NO转化率高于80%。8% wt. G-Mn/DE-HAL在150—250 ℃之间NO转化率不低于90%,300 ℃时NO转化率仍然接近80%。随着载体孔隙率的提高,催化剂脱硝性能逐渐增强,可见,适当提高孔隙率有利于增强催化剂的催化活性。
-
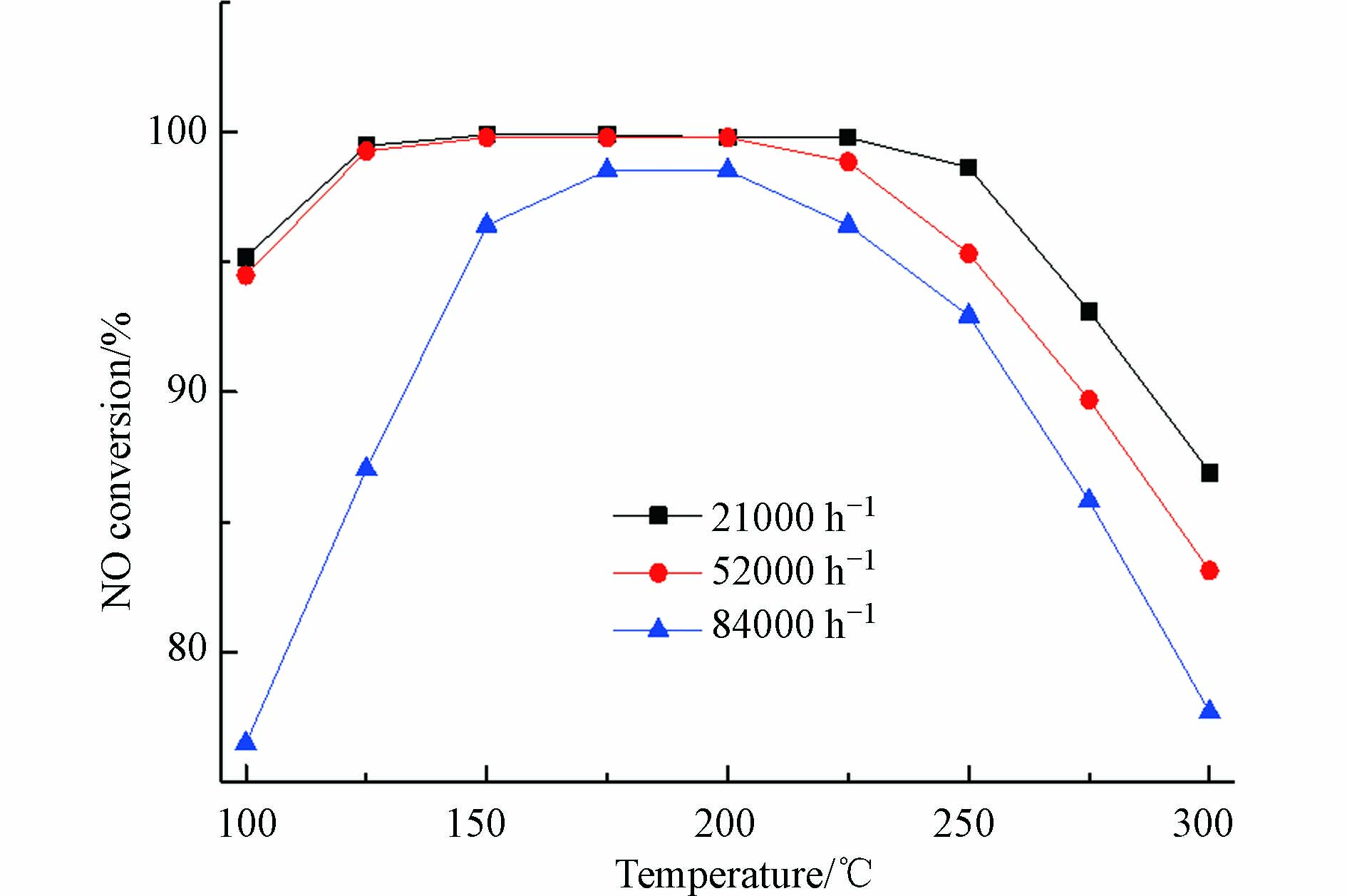
空速是工业催化剂应用的一个重要工况。图5是8% wt. G-Mn/DE-HAL催化剂分别在21000 h−1、52000 h−1、84000 h−1的3个空速下的NO转化率。由图5可以看出,随着催化反应空速的提高,催化剂NO转化率逐渐降低,这是因为反应空速越高,气体在催化剂表面的停留时间越短,而停留时间的减少会使得氨气逃逸量增加,从而影响催化剂的NO转化率。8% wt. G-Mn/DE-HAL在21000 h−1、52000 h−1下,100—250 ℃之间,NO转化率均能维持在90 %以上。300 ℃时,催化剂在21000 h−1和52000 h−1下,NO转化率高于80%,在84000 h−1下,NO转化率约为75%。
-
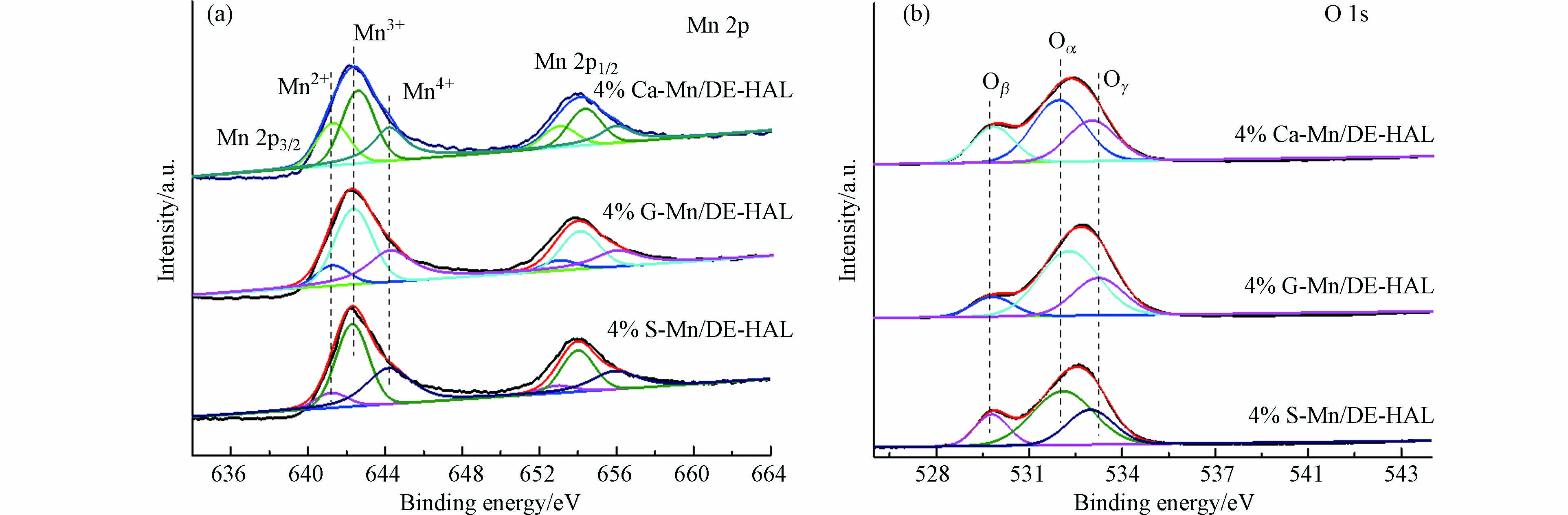
图6为催化剂的XPS图,表4为对XPS进行分峰拟合计算后所得催化剂Mn与O的表面原子比例。催化剂Mn 2p光谱如图6 a所示,图中可以观察到归属于Mn 2p3/2和Mn 2p1/2的两个主峰。Mn 2p3/2可以拟合成3个子峰,其中位于640.9—641.3 eV、641.9—642.3 eV、643.6—644.3 eV的峰分别归属于 Mn2+、Mn3+ 和 Mn4+[17-18]。研究认为,高价态离子占较大比例有利于NO氧化转化为NO2,促进“快速SCR”反应的进行,是影响脱硝催化剂低温催化性能的重要因素[19]。由表4可知,3个催化剂表面Mn4+相对百分比含量顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL,表明4% wt. G-Mn/DE-HAL将NO氧化成NO2的能力最强,这可能是因为以石墨为造孔剂所制备的载体具有更高的孔隙率和更粗糙的表面,更有利于MnOx的负载和分散。催化剂O 1s光谱如图6 (b)所示,O 1s可以拟合成3个子峰,图中化学吸附氧(Oα)、晶格氧(Oβ)、吸附的水物种(Oγ) 的峰分别位于531.7—532.4 eV、530.1—530.2 eV、533.5—534.4 eV[20-21]。研究表明,由于Oα具有更好的流动性和更高的活性,所以催化剂Oα/Oβ比例越高越有利于SCR反应的进行[20]。如表4所示,样品中Oα相对百分比含量由高到低顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL,出现这一结果可能是由于陶瓷中残留的CaO和少量淀粉降低了Mn4+的含量,抑制了Oα的形成。可见,造孔剂种类影响催化剂表面原子价态分布。
-
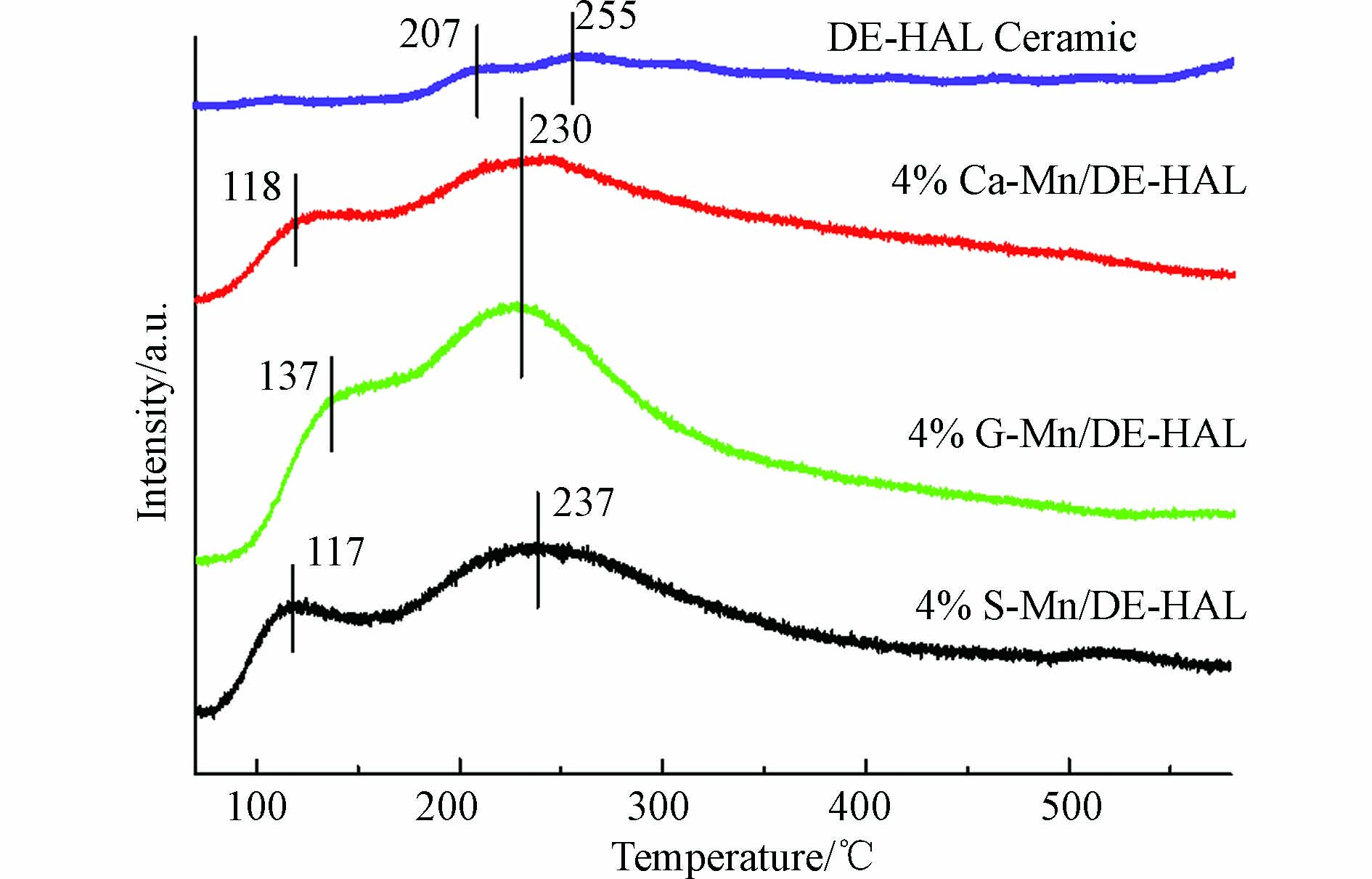
图7为多孔陶瓷载体以及以3种造孔剂所制备的多孔陶瓷负载活性组分之后的NH3-TPD图谱。低于350 ℃的脱附峰为中弱酸性位点,归属于结合在Brønsted酸位上的NH4+;高于350 ℃的脱附峰为强酸性位点,归属于Lewis酸位上的配位NH3[22]。研究表明,酸性位点的数量与分布情况是催化剂脱硝性能的重要影响因素,是保证NH3吸附和活化的前提[23-26],其中,位于100—350 ℃的酸性位点数量影响催化剂低温下的脱硝性能[18]。由图7可观察到,未负载活性组分的陶瓷在207 ℃和255 ℃处出现了中弱酸性位点,而3个催化剂酸性位点的分布以及NH3-TPD的谱图较为相似,但峰面积相差较大,NH3-TPD的峰面积与酸性位点的含量呈正比[22]。对样品的峰面积进行拟合计算,结果列于表5,由计算结果知,3个催化剂的峰面积大小顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL。以石墨为造孔剂所制备的载体可以负载更多的MnOx,从而使得催化剂具有更强的吸附和活化NH3的能力,而4% wt. Ca-DE-HAL表面较为平滑不利于MnOx的负载,且CaO作为碱土金属会占据部分酸性位点,降低了催化剂对NH3的吸附和活化能力[27]。可见,造孔剂的种类影响所制备样品的酸性位点含量,最终影响催化剂的催化活性。
-
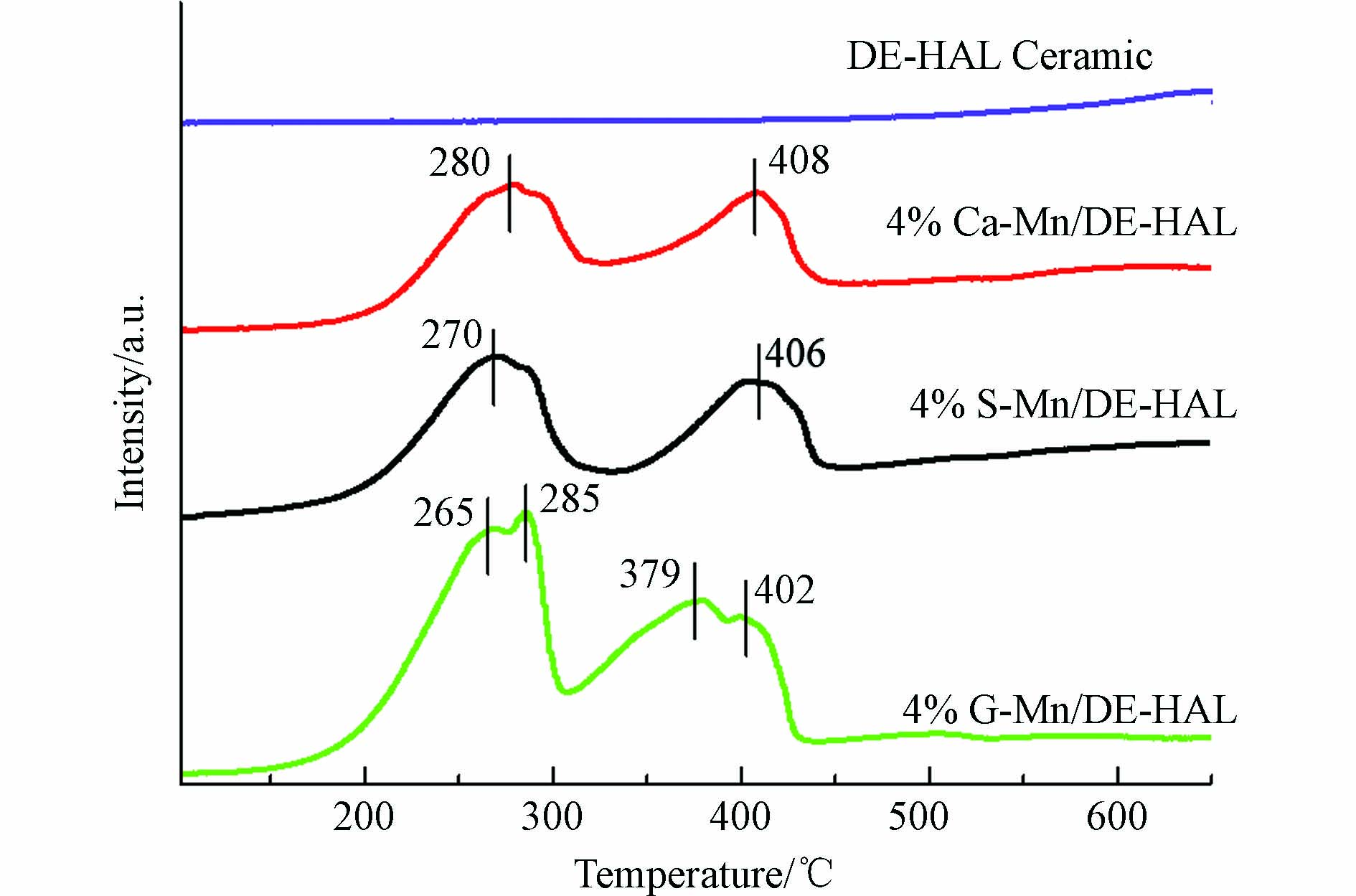
通过H2-TPR对造孔剂不同的催化剂氧化还原能力进行测试,结果如图8所示,未负载活性组分的陶瓷在100—600 ℃之间没有明显的还原峰,而采用3种造孔剂所制备的催化剂在200—300 ℃处有明显的还原峰和肩峰,这是由于MnO2到Mn2O3的还原;3个样品在300—450 ℃之间的还原峰归属于Mn2O3到MnO的还原[17]。4% wt. G-Mn/DE-HAL,4 %wt. S-Mn/DE-HAL,4% wt. Ca-Mn/DE-HAL的3个催化剂的还原峰位置依次向右偏移,而还原峰温度越低,催化剂越容易被氧化还原,即3个样品的氧化还原能力依次降低。造孔剂通过改变多孔陶瓷的孔隙率和表面粗糙程度从而改变了MnOx的负载与分散状况,最终影响了催化剂的氧化还原能力。这一结果表明,选取适当的造孔剂可以使所制备的催化剂具有更强的氧化还原能力。
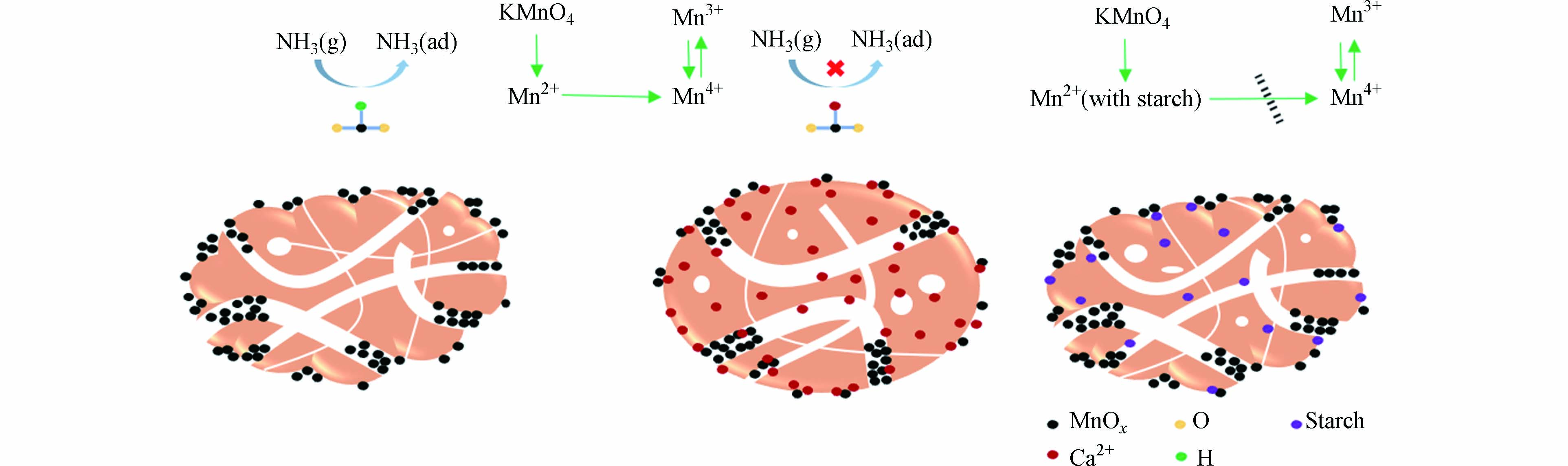
图9为造孔剂不同的催化剂反应机理图。以石墨为造孔剂所制备的催化剂具有粗糙的表面与较多的孔隙,为MnOx提供了更多的附着位点。以CaCO3为造孔剂所制备的样品中残留的CaO占据了部分酸性位点,降低了催化剂的NH3吸附量,且该样品表面较为平滑,可供MnOx附着的空间较少。以淀粉为造孔剂所制备的样品,虽然同样具有较为粗糙的表面,且基体中也不含会占据酸性位点的碱土金属,然而由于淀粉具有一定的黏性,在压片时样品会变得更加致密,煅烧时在样品内部的淀粉无法与氧气充分接触从而导致部分淀粉无法除去,负载活性组分前会将陶瓷破碎,原本在内部的淀粉则会暴露出来,淀粉具有还原性,在KMnO4氧化Mn(Ac)2的过程中会消耗部分KMnO4,从而导致更多的Mn2+无法被氧化成Mn4+,降低了催化剂的氧化还原能力。以上因素导致采用不同造孔剂所制备的样品催化活性表现出较大差异。
-
(1) 采用造孔剂法制备了硅藻土基多孔陶瓷,以该多孔陶瓷为载体,采用原位生长法制备了Mn/DE-HAL催化剂。造孔剂种类与含量影响着催化剂NO转化率,相较于CaCO3和淀粉,以石墨为造孔剂所制备的催化剂脱硝性能较为优异。此外,采用石墨为造孔剂制备的催化剂脱硝性能随石墨含量增加而增强。
(2) 造孔剂种类与含量的改变影响多孔陶瓷的结构与形貌,进而影响MnOx在载体表面的分散。载体具有更粗糙的表面更有利于MnOx的良好分散,此外,较高的孔隙率为MnOx提供了更多的负载空间,适当提高载体的孔隙率有利于MnOx的负载,二者为催化剂具有优良低温SCR脱硝性能的重要原因。
(3) 造孔剂的种类影响催化剂的理化性质,选取合适的造孔剂,可以提升催化剂Mn4+和Oα百分比含量,增加酸性位点含量并增强其氧化还原能力,从而提升催化剂的脱硝性能。
造孔剂对低温锰基多孔陶瓷NH3-SCR催化剂性能的影响
Effect of pore-forming agent on properties of NH3-SCR catalyst for low temperature manganese-based porous ceramics
-
摘要: 本文采用造孔剂法制备了以埃洛石 (HAL) 为添加剂的硅藻土 (DE) 基多孔陶瓷,并以该多孔陶瓷为载体,通过原位生长法制备了锰基硅藻土多孔陶瓷催化剂。探究了造孔剂的种类和含量对多孔陶瓷结构与形貌以及对催化剂脱硝性能的影响。采用XRD、SEM、XPS等方法对多孔陶瓷以及催化剂的理化性质进行了表征,结果表明,造孔剂的种类与含量影响多孔陶瓷的结构与形貌,更粗糙的表面和更高的孔隙率为MnOx提供了更多的附着位点。此外,选取适当的造孔剂可以促进所制备样品表面原子价态分布,提升表面酸性位点含量,并增强其氧化还原能力,增强催化剂的脱硝性能。Abstract: Diatomite (DE) based porous ceramics with halloysite (HAL) as additive were prepared by pore-forming agent method, and the porous ceramic was used as the carrier to prepare manganese based diatomite porous ceramic catalyst by in-situ deposition method. The influence of the type and content of pore-forming agent on the structure and morphology of porous ceramics and on the deNOx performance of catalyst were investigated. The physical and chemical properties of porous ceramics and catalysts were characterized by XRD, SEM, XPS and other methods. The results show that the type and content of pore-forming agent affect the structure and morphology of porous ceramics, rougher surfaces and higher porosity provide more attachment sites for MnOx. In addition, the selection of appropriate pore-forming agent can promote the surface atomic valence distribution of the sample, improve the content of acid sites on the surface, and enhance the redox ability of the catalyst, improve the deNOx performance.
-
Key words:
- porous ceramics /
- pore-forming agent /
- MnOx /
- NH3-SCR.
-
化石燃料燃烧、汽车尾气、燃煤电厂以及工业锅炉排放的NOx是大气的主要污染物之一,会造成酸雨、雾霾以及光化学烟雾等一系列环境问题,对生态环境和人类健康均造成了严重危害[1-2]。目前,NH3选择性催化还原NOx技术(NH3-SCR)被广泛研究,并在工业上应用于控制NO、NO2和N2O的排放,已经被证明是经济且高效的NOx脱除方法[3]。其中,最广泛使用的催化剂是V2O5-WO3/TiO2。然而,传统的V2O5-WO3/TiO2催化剂虽然具有较高的催化活性和耐硫性能,但存在反应温度高、操作温度窗口窄、V2O5对环境有害等缺点[4]。因此,有必要开发环保高效的低温脱硝催化剂。
多孔材料在材料学领域有着悠久的研究历史,是NH3-SCR催化剂优异的载体候选材料[5]。Song等[6]采用水热法制备了具有微孔结构的的TiO2,与传统TiO2相比,前者所具有的小孔结构使反应中间体NH3-NO3能够稳定存在,不易分解为N2O,从而使得以具有小孔结构的TiO2为载体所制备的催化剂具有更高的N2选择性和NOx转化率。Zha等[7]采用溶剂热法制备了介孔TiO2球,并通过浸渍法制备了MnOx-CeO2/m-TiO2催化剂,研究发现,由于载体的多孔结构使得活性组分可均匀吸附在其表面而不团聚,介孔TiO2的孔结构抑制了活性组分的迁移,从而使得SCR反应充分进行。
硅藻土由于价格低廉,且具有独特的介孔和微孔结构等诸多优良性能,被应用于许多重要领域,如作为有毒气体或有机染料的吸附材料等[8]。以硅藻土为原料,将其制备成具有一定形状与机械强度的多孔陶瓷,可以进一步扩大其应用领域。多孔材料的制备方法多种多样,如造孔剂法[9]、直接发泡发[10]、冷冻干燥法[11]、模板法[12]、泡沫凝胶铸造法[13]等,这些方法可直接用于或改性制备硅藻土基多孔陶瓷[8]。其中,造孔剂法制备多孔陶瓷,因为工艺简单,成本低廉而被广泛应用。目前,多孔陶瓷造孔剂大致可分为两类,有机造孔剂和无机造孔剂。有机造孔剂通常为天然有机物和高分子聚合物,而植物类的天然有机物因成本低廉且来源广泛备受青睐,无机造孔剂则可分为高温分解和氧化燃烧两种类型。本文分别选取价格低廉的淀粉、高温下分解的碳酸钙和氧化燃烧的石墨作为造孔剂进行研究,并以埃洛石黏土为添加剂增强样品的机械强度进行研究。
制备方法影响催化剂的催化活性[2],本课题组前期所开发的原位生长法无需煅烧,便可将活性组分原位沉积在载体表面,该方法与载体结构的协同效应能够促进SCR反应的进行[14]。本文采用造孔剂法制备了孔隙度较高且具有一定机械强度的硅藻土基多孔陶瓷,并以所制备的多孔陶瓷为载体,通过原位生长法将MnOx原位沉积在多孔陶瓷表面。采用XRD、SEM、XPS等方法对所制备样品进行表征,重点研究了造孔剂种类与含量对多孔陶瓷结构与性能的影响,以及对负载活性组分之后的催化剂催化活性的影响。拓宽了硅藻土基多孔陶瓷的研究领域,为多孔陶瓷类载体在SCR领域的研究提供了一定的思路。
1. 实验部分(Experimental section)
1.1 材料与试剂
埃洛石(HAL,郑州金阳光陶瓷有限公司),主要化学组成(质量分数)为: 50.31% SiO2,38.27% Al2O3,7.59% SO3,1.22% K2O,1.12% Fe2O3;硅藻土(DE,国药集团化学试剂有限公司),主要化学成分(质量分数)为:88.80% SiO2,;2.98% Al2O3, 2.06% Fe2O3,2.03% CO2,1.21% Na2O;乙酸锰 Mn(CH3COO)2;高锰酸钾KMnO4;聚乙烯醇(PVA,(CH2CHOH)n);淀粉(Starch,(C6H10O5)n);石墨(Graphite,C);碳酸钙 CaCO3;均由国药集团化学试剂有限公司提供,纯度为AR,模拟烟气由南京上元工业气体厂提供。
1.2 催化剂的制备
(1)多孔陶瓷的制备
首先称取总量为10 g的硅藻土与埃洛石粉末,质量比6:4,再称取适量造孔剂,采用湿法球磨(球:原料:酒精 = 2:1:0.6)将上述粉末混合均匀,110 ℃过夜干燥,过200目筛,滴加4% wt. PVA,研磨,经红外压片机4 Mpa压片成型,再经管式炉1000 ℃煅烧,得到硅藻土基多孔陶瓷。将所得多孔陶瓷破碎后过20目筛,用以作为催化剂载体,并标记为 x %wt. y-DE-HAL (x为造孔剂含量,y为造孔剂种类;x=0、2、4、6、8;y=S 淀粉,G 石墨,Ca 碳酸钙,下同)。
(2)原位生长法制备Mn/DE-HAL催化剂
称取5 g制备的硅藻土基多孔陶瓷,称取化学计量的乙酸锰溶解于一定量的去离子水中,用以作为锰的前驱体。将多孔陶瓷等体积浸渍于乙酸锰溶液中,静置24 h后加入KMnO4溶液,搅拌后静置24 h,多次抽滤洗涤至滤液澄清无色,K+通过多次去离子水反复冲洗抽滤除去,于鼓风干燥箱中110 ℃过夜干燥,即得所需催化剂(催化剂中Mn元素质量分数为8%),并标记为x %wt. y-Mn/DE-HAL。
1.3 催化剂活性评价
催化剂的脱硝活性评价测试装置为长80 cm,内径15 mm的竖式石英玻璃常压固定床反应器,活性评价装置主要由模拟烟气 (1000 mg·L−1 NO、1000 mg·L−1 NH3、3% O2、N2为平衡气混合而成,总流量为350 mL·min−1) 、固定床反应器和分析检测装置的3部分组成。反应空速分别为21000 h−1、52000 h−1、84000 h−1,反应温度为100—300 ℃。采用德国MRU OPTIMA 7烟气分析仪对反应器进出口的烟气浓度进行在线测量。
催化剂的活性计算公式为:
XNO=[NO]in−[NO]out[NO]in×100% 式中,[NO]in、[NO]out分别为NO进口、出口浓度。
1.4 催化剂表征
采用美国康塔NOVA 2200e型比表面积及孔径分析仪分析样品的比表面积和孔径孔容。采用荷兰帕纳科PANalytical X-Pert PRO MPD型X射线衍射仪对多孔陶瓷和催化剂的物相与晶型进行表征,Cu Kα射线,操作电压为40 kV,操作电流为40 mA,扫描速度为4 o·min−1,扫描区间范围2θ=5°—80° 。采用美国Thermo ESCALAB250Xi型X射线光电子能谱仪对催化剂进行XPS测试,Al Kα射线,管电压为1486.6 eV。采用德国卡尔蔡司Gemini 500型热场发射扫描电子显微镜观察多孔陶瓷与催化剂的表面微观结构。将样品制成直径为13 mm,厚度为1.4 mm的圆柱状陶瓷,采用YHKC-3A型自动颗粒强度测定仪测量样品轴向机械强度,强度数据见表1.
表 1 样品机械强度数据Table 1. Sample mechanical strength data样品 Samples 强度/MPa Mechanical strength DE-HAL 5.11 4 %wt. G-DE-HAL 4.95 4% wt. S-DE-HAL 5.02 4 %wt. Ca-DE-HAL 6.15 2. 结果与讨论(Results and discussion)
2.1 TG-DTG分析
图1为陶瓷原料与3种造孔剂的热分析图。图1a为陶瓷原料DE-HAL的TG-DTG曲线,样品在250—340 ℃之间质量损失约为3%,归因于原料中硅藻土结构水的脱除[15],400—700 ℃之间的吸热谷推测是由于原料中埃洛石的脱羟基作用,948 ℃处的吸热谷归因于原料中的石英相向方石英相的转变[15]。图1b为淀粉的TG-DTG曲线,淀粉在30—1000 ℃的失重曲线分为3个阶段,50—160 ℃之间质量损失约为8%,为水分子的脱除,288 ℃处的吸热谷是由于淀粉的分解,而475 ℃处的吸热谷,因于淀粉的进一步分解,500 ℃时淀粉基本分解完全。淀粉在较低温度下脱除的水分子以及在氧气充足条件下高温分解所产生的CO2扩散到陶瓷外部后均会在陶瓷基体中留下孔隙。图1 c为CaCO3的TG-DTG曲线,CaCO3仅在650—850 ℃之间有一个吸热谷,质量损失约为45%,该部分是由于CaCO3的氧化分解,850 ℃之后曲线趋于平稳,CaCO3不再分解。CaCO3氧化分解生成CaO和CO2,其中CaO留在了陶瓷基体中,CO2扩散到了外部,并留下了孔隙。图1 d为石墨的TG-DTG曲线,石墨在30—1000 ℃之间仅在820 ℃处有1个吸热谷,在650—940 ℃之间样品的质量损失为100%,即石墨在该温度区间内燃烧完全。石墨在高温下燃烧释放出大量CO2,这些CO2在陶瓷基体中留下了较多的孔隙。对比3种造孔剂的TG-DTG曲线可知,淀粉的分解温度较低,而造孔剂在较低温度留下的孔隙,在更高温度下会烧结收缩,即采用较低温度下分解的造孔剂所制备的多孔陶瓷孔隙率较低,且容易形成闭孔。此外,淀粉与石墨在30—1000 ℃均完全分解,未在陶瓷基体中残留,而CaCO3则在陶瓷基体中留下CaO。
2.2 BET及孔结构分析
表2为多孔陶瓷的比表面积、孔道结构以及密度数据,表3为负载了MnOx的催化剂的比表面积与孔道结构数据。由表2可以看出,造孔剂种类相同的情况下,随着造孔剂含量的增加,多孔陶瓷比表面积、孔隙率逐渐增加,密度逐渐减小,孔径大小几乎没有变化。造孔剂含量相同时,采用不同种类造孔剂制备的多孔陶瓷孔径分布相似,孔容与孔隙率区别较为显著。4% wt. G-Mn/DE-HAL较之其他两个样品具有更大的孔容和更高的孔隙率。由表3可以看出,催化剂比表面积变化规律与未负载活性组分的多孔陶瓷呈相同趋势,负载MnOx之后,样品比表面积与孔容明显增加,这与活性组分在多孔陶瓷表面的分散有关,而孔径减小,可能是MnOx堵塞了多孔陶瓷的部分孔隙造成的。
表 2 多孔陶瓷的比表面积、孔道结构及密度Table 2. Specific surface area, pore structure and density of porous ceramics样品Samples 比表面积/(m2·g−1)Specific surface area 孔容/(mL·g−1)Pore volume 孔径/nmPore diameter 孔隙率/%Porosity 密度/(g·cm−3)Density 0 %wt. G-DE-HAL 2.13 0.011 178.98 28.12 1.81 2 % wt.G-DE-HAL 2.45 0.011 158.66 32.73 1.53 4 %wt. G-DE-HAL 3.94 0.017 180.21 34.03 1.52 6% wt. G-DE-HAL 4.04 0.019 177.39 35.57 1.46 8 %wt. G-DE-HAL 4.17 0.020 179.47 36.32 1.43 4% wt. S-DE-HAL 2.38 0.013 184.12 33.62 1.63 4% wt.Ca-DE-HAL 2.55 0.015 164.76 32.56 1.51 表 3 催化剂的比表面积及孔道结构Table 3. Specific surface area and pore structure of catalysts样品 比表面积/(m2·g−1)Specific surface area 孔容/(mL·g−1)Pore volume 孔径/nmPore diameter 0 %wt. G-Mn/DE-HAL 23.21 0.031 48.29 2% wt. G-Mn/DE-HAL 24.90 0.052 56.33 4 %wt. G-Mn/DE-HAL 27.31 0.054 48.75 6 %wt. G-Mn/DE-HAL 31.92 0.055 49.56 8% wt. G-Mn/DE-HAL 32.26 0.057 48.75 4 %wt. S-Mn/DE-HAL 19.23 0.025 38.45 4% wt. Ca-Mn/DE-HAL 29.72 0.034 38.42 2.3 SEM分析
为了更直观地研究造孔剂种类对多孔陶瓷以及催化剂结构和性能的影响,对样品进行SEM和Mapping表征。图2(a)为未添加造孔剂的陶瓷载体,图2(b—d)分别为添加了3种造孔剂所制备的多孔陶瓷,如图所示,与未添加造孔剂的陶瓷相比,添加造孔剂所制备的多孔陶瓷表面均呈现出较多的大小不一的孔隙,结合氮气吸脱附以及阿基米德排水法计算的孔隙率结果可知,造孔剂成功地在陶瓷基体中留下了孔隙。此外,添加3种不同种类的造孔剂所制得的多孔陶瓷形貌差异显著,以淀粉和石墨为造孔剂所制备的多孔陶瓷表面粗糙,而以碳酸钙为造孔剂所制备的多孔陶瓷表面较为光滑。如图2(e—g)所示,负载MnOx之后,活性组分分布在多孔陶瓷的表面以及孔隙中。图2(h—j)为4 %wt. 的S-Mn/DE-HAL,4%wt. Ca-Mn/DE-HAL,4% wt. G-Mn/DE-HAL的Mapping图,可以观察到MnOx在3个样品表面的分散性差异较为明显,4% wt. Ca-Mn/DE-HAL表面MnOx的分散性最差,其次是4% wt. 的S-Mn/DE-HAL,活性组分在4% wt. G-Mn/DE-HAL表面分散的较为均匀。图2(k—m)中,4% wt. 的S-Mn/DE-HAL表面MnOx团聚较为严重,4% wt. Ca-Mn/DE-HAL表面粗糙度不高,负载的MnOx较少,而4% wt. G-Mn/DE-HAL表面凹凸不平,较多的活性组分分散在孔隙之间。可见,粗糙的表面更有利于活性组分的附着。
2.4 XRD分析
图3为采用3种造孔剂所制备的多孔陶瓷以及多孔陶瓷负载MnOx之后的XRD图。图3中并未观察到MnOx的峰,这可能是因为活性组分呈无定形态分布在载体表面[2]。21.66°和35.94°处的峰为方石英的特征衍射峰(PDF#39-1425),26.49°处的峰为石英的特征衍射峰(PDF#46-1045)。由XRD图谱可以观察到,未负载MnOx的多孔陶瓷在21.66°处的方石英峰十分尖锐,此外,未负载活性组分的3个样品衍射峰差异并不明显。多孔陶瓷负载MnOx之后,石英与方石英的衍射峰均明显减弱,这并非是因为Mn的引入破坏了多孔陶瓷的结构。一方面活性组分包裹在载体表面会削弱载体原有的衍射峰[14],另一方面由于样品的特殊性,筛分得到的样品多孔陶瓷含量较少,最终导致归属于载体的衍射峰并不明显。
2.5 催化剂活性测试
2.5.1 造孔剂种类对4% wt. y-Mn/DE-HAL催化剂脱硝活性影响
图4为造孔剂种类对催化剂NO转化率的影响。造孔剂种类的变化,直接影响多孔陶瓷的孔隙率与比表面积等理化性质,从而影响催化剂的脱硝活性。图4为Mn/DE-HAL、4% wt. S-Mn/DE-HAL、4% wt. G-Mn/DE-HAL、4% wt. Ca-Mn/DE-HAL等 4个样品在100—300 ℃范围内NO的转化率,测试过程中,每个温度点稳定40 min,分别在保温时间剩余12、6、1 min时对出口NO浓度进行测量,取3次测试的平均值作为最终数据,测试结果误差在1%以内。由图4可以看出,所有样品在100—300 ℃之间的脱硝率均呈先升高后降低的趋势。未添加造孔剂的样品Mn/DE-HAL在100 ℃时的NO转化率约为50%,200 ℃时NO转化率最高,略高于80%。4% wt. S-Mn/DE-HAL在100 ℃时NO转化率约为60%,200 ℃时NO转化率最高,约为90%。4% wt. Ca-Mn/DE-HAL在100 ℃时NO转化率低于50%,225 ℃时活性最好,接近80%,300 ℃时NO转化率约为65%。4%wt. G-Mn/DE-HAL在100 ℃时NO转化率高于70%,在175—250 ℃范围内NO转化率高于90%,300 ℃时NO转化率仍然接近75%。综合比较,4个催化剂脱硝性能强弱顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL。4% wt. Ca-Mn/DE-HAL脱硝性能最差一方面因为其表面粗糙度低,不利于MnOx负载,另一方面是因为CaCO3高温下分解为CaO和CO2,其中CaO作为碱土金属,降低了催化剂的NO转化率[16]。4% wt. G-Mn/DE-HAL与4% wt. S-Mn/DE-HAL孔隙率较高且表面更粗糙,可见,更高的孔隙率与更粗糙的表面有利于SCR反应的进行。
2.5.2 造孔剂含量对x% wt. G-Mn/DE-HAL催化剂脱硝活性的影响
图4为造孔剂含量对于催化剂NO转化率的影响。造孔剂含量的变化会影响多孔陶瓷的孔隙率与比表面积等理化性质,从而影响催化剂的脱硝性能。图4分别为0% wt. G-Mn/DE-HAL,2% wt. G-Mn/DE-HAL,4% wt. G-Mn/DE-HAL,6% wt. G-Mn/DE-HAL,8% wt. G-Mn/DE-HAL 5个样品在100—300 ℃范围内的NO转化率。随着造孔剂含量的增加,催化剂脱硝性能逐渐增强,0% wt. G-Mn/DE-HAL在200 ℃之后NO转化率开始大幅度下降,300 ℃时NO转化率约为60%。2% wt. G-Mn/DE-HAL在150—250 ℃之间NO转化率高于80%。4% wt. G-Mn/DE-HAL与6% wt. G-Mn/DE-HAL两个样品在150—275 ℃之间NO转化率高于80%。8% wt. G-Mn/DE-HAL在150—250 ℃之间NO转化率不低于90%,300 ℃时NO转化率仍然接近80%。随着载体孔隙率的提高,催化剂脱硝性能逐渐增强,可见,适当提高孔隙率有利于增强催化剂的催化活性。
2.5.3 空速对8% wt. G-Mn/DE-HAL催化剂脱硝活性的影响
空速是工业催化剂应用的一个重要工况。图5是8% wt. G-Mn/DE-HAL催化剂分别在21000 h−1、52000 h−1、84000 h−1的3个空速下的NO转化率。由图5可以看出,随着催化反应空速的提高,催化剂NO转化率逐渐降低,这是因为反应空速越高,气体在催化剂表面的停留时间越短,而停留时间的减少会使得氨气逃逸量增加,从而影响催化剂的NO转化率。8% wt. G-Mn/DE-HAL在21000 h−1、52000 h−1下,100—250 ℃之间,NO转化率均能维持在90 %以上。300 ℃时,催化剂在21000 h−1和52000 h−1下,NO转化率高于80%,在84000 h−1下,NO转化率约为75%。
2.6 XPS分析
图6为催化剂的XPS图,表4为对XPS进行分峰拟合计算后所得催化剂Mn与O的表面原子比例。催化剂Mn 2p光谱如图6 a所示,图中可以观察到归属于Mn 2p3/2和Mn 2p1/2的两个主峰。Mn 2p3/2可以拟合成3个子峰,其中位于640.9—641.3 eV、641.9—642.3 eV、643.6—644.3 eV的峰分别归属于 Mn2+、Mn3+ 和 Mn4+[17-18]。研究认为,高价态离子占较大比例有利于NO氧化转化为NO2,促进“快速SCR”反应的进行,是影响脱硝催化剂低温催化性能的重要因素[19]。由表4可知,3个催化剂表面Mn4+相对百分比含量顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL,表明4% wt. G-Mn/DE-HAL将NO氧化成NO2的能力最强,这可能是因为以石墨为造孔剂所制备的载体具有更高的孔隙率和更粗糙的表面,更有利于MnOx的负载和分散。催化剂O 1s光谱如图6 (b)所示,O 1s可以拟合成3个子峰,图中化学吸附氧(Oα)、晶格氧(Oβ)、吸附的水物种(Oγ) 的峰分别位于531.7—532.4 eV、530.1—530.2 eV、533.5—534.4 eV[20-21]。研究表明,由于Oα具有更好的流动性和更高的活性,所以催化剂Oα/Oβ比例越高越有利于SCR反应的进行[20]。如表4所示,样品中Oα相对百分比含量由高到低顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL,出现这一结果可能是由于陶瓷中残留的CaO和少量淀粉降低了Mn4+的含量,抑制了Oα的形成。可见,造孔剂种类影响催化剂表面原子价态分布。
表 4 造孔剂种类不同的催化剂表面原子比值Table 4. Surface atomic ratios of catalysts with different pore-forming agents样品Samples 表面原子比值/% Surface atom ratio Mn4+/Mn3+ Oα/Oβ 4%wt.Ca-Mn/DE-HAL 67.66 315.01 4%wt.G-Mn/DE-HAL 91.51 473.51 4% wt.S-Mn/DE-HAL 71.34 218.13 2.7 NH3-TPD分析
图7为多孔陶瓷载体以及以3种造孔剂所制备的多孔陶瓷负载活性组分之后的NH3-TPD图谱。低于350 ℃的脱附峰为中弱酸性位点,归属于结合在Brønsted酸位上的NH4+;高于350 ℃的脱附峰为强酸性位点,归属于Lewis酸位上的配位NH3[22]。研究表明,酸性位点的数量与分布情况是催化剂脱硝性能的重要影响因素,是保证NH3吸附和活化的前提[23-26],其中,位于100—350 ℃的酸性位点数量影响催化剂低温下的脱硝性能[18]。由图7可观察到,未负载活性组分的陶瓷在207 ℃和255 ℃处出现了中弱酸性位点,而3个催化剂酸性位点的分布以及NH3-TPD的谱图较为相似,但峰面积相差较大,NH3-TPD的峰面积与酸性位点的含量呈正比[22]。对样品的峰面积进行拟合计算,结果列于表5,由计算结果知,3个催化剂的峰面积大小顺序为4% wt. G-Mn/DE-HAL>4% wt. S-Mn/DE-HAL>4% wt. Ca-Mn/DE-HAL。以石墨为造孔剂所制备的载体可以负载更多的MnOx,从而使得催化剂具有更强的吸附和活化NH3的能力,而4% wt. Ca-DE-HAL表面较为平滑不利于MnOx的负载,且CaO作为碱土金属会占据部分酸性位点,降低了催化剂对NH3的吸附和活化能力[27]。可见,造孔剂的种类影响所制备样品的酸性位点含量,最终影响催化剂的催化活性。
表 5 造孔剂种类不同的催化剂NH3-TPD积分Table 5. NH3-TPD integration of catalysts with different pore-forming agents样品 Samples 4% wt. S-Mn/DE-HAL 4% wt. G-Mn/DE-HAL 4 %wt. Ca-Mn/DE-HAL 4% wt. G- DE-HAL NH3-TPD /(eV·s−1) 1.54×10−7 2.12×10−7 1.45×10−7 1.46×10−7 2.8 H2-TPR分析
通过H2-TPR对造孔剂不同的催化剂氧化还原能力进行测试,结果如图8所示,未负载活性组分的陶瓷在100—600 ℃之间没有明显的还原峰,而采用3种造孔剂所制备的催化剂在200—300 ℃处有明显的还原峰和肩峰,这是由于MnO2到Mn2O3的还原;3个样品在300—450 ℃之间的还原峰归属于Mn2O3到MnO的还原[17]。4% wt. G-Mn/DE-HAL,4 %wt. S-Mn/DE-HAL,4% wt. Ca-Mn/DE-HAL的3个催化剂的还原峰位置依次向右偏移,而还原峰温度越低,催化剂越容易被氧化还原,即3个样品的氧化还原能力依次降低。造孔剂通过改变多孔陶瓷的孔隙率和表面粗糙程度从而改变了MnOx的负载与分散状况,最终影响了催化剂的氧化还原能力。这一结果表明,选取适当的造孔剂可以使所制备的催化剂具有更强的氧化还原能力。
图9为造孔剂不同的催化剂反应机理图。以石墨为造孔剂所制备的催化剂具有粗糙的表面与较多的孔隙,为MnOx提供了更多的附着位点。以CaCO3为造孔剂所制备的样品中残留的CaO占据了部分酸性位点,降低了催化剂的NH3吸附量,且该样品表面较为平滑,可供MnOx附着的空间较少。以淀粉为造孔剂所制备的样品,虽然同样具有较为粗糙的表面,且基体中也不含会占据酸性位点的碱土金属,然而由于淀粉具有一定的黏性,在压片时样品会变得更加致密,煅烧时在样品内部的淀粉无法与氧气充分接触从而导致部分淀粉无法除去,负载活性组分前会将陶瓷破碎,原本在内部的淀粉则会暴露出来,淀粉具有还原性,在KMnO4氧化Mn(Ac)2的过程中会消耗部分KMnO4,从而导致更多的Mn2+无法被氧化成Mn4+,降低了催化剂的氧化还原能力。以上因素导致采用不同造孔剂所制备的样品催化活性表现出较大差异。
3. 结论(Conclusion)
(1) 采用造孔剂法制备了硅藻土基多孔陶瓷,以该多孔陶瓷为载体,采用原位生长法制备了Mn/DE-HAL催化剂。造孔剂种类与含量影响着催化剂NO转化率,相较于CaCO3和淀粉,以石墨为造孔剂所制备的催化剂脱硝性能较为优异。此外,采用石墨为造孔剂制备的催化剂脱硝性能随石墨含量增加而增强。
(2) 造孔剂种类与含量的改变影响多孔陶瓷的结构与形貌,进而影响MnOx在载体表面的分散。载体具有更粗糙的表面更有利于MnOx的良好分散,此外,较高的孔隙率为MnOx提供了更多的负载空间,适当提高载体的孔隙率有利于MnOx的负载,二者为催化剂具有优良低温SCR脱硝性能的重要原因。
(3) 造孔剂的种类影响催化剂的理化性质,选取合适的造孔剂,可以提升催化剂Mn4+和Oα百分比含量,增加酸性位点含量并增强其氧化还原能力,从而提升催化剂的脱硝性能。
-
表 1 样品机械强度数据
Table 1. Sample mechanical strength data
样品 Samples 强度/MPa Mechanical strength DE-HAL 5.11 4 %wt. G-DE-HAL 4.95 4% wt. S-DE-HAL 5.02 4 %wt. Ca-DE-HAL 6.15 表 2 多孔陶瓷的比表面积、孔道结构及密度
Table 2. Specific surface area, pore structure and density of porous ceramics
样品Samples 比表面积/(m2·g−1)Specific surface area 孔容/(mL·g−1)Pore volume 孔径/nmPore diameter 孔隙率/%Porosity 密度/(g·cm−3)Density 0 %wt. G-DE-HAL 2.13 0.011 178.98 28.12 1.81 2 % wt.G-DE-HAL 2.45 0.011 158.66 32.73 1.53 4 %wt. G-DE-HAL 3.94 0.017 180.21 34.03 1.52 6% wt. G-DE-HAL 4.04 0.019 177.39 35.57 1.46 8 %wt. G-DE-HAL 4.17 0.020 179.47 36.32 1.43 4% wt. S-DE-HAL 2.38 0.013 184.12 33.62 1.63 4% wt.Ca-DE-HAL 2.55 0.015 164.76 32.56 1.51 表 3 催化剂的比表面积及孔道结构
Table 3. Specific surface area and pore structure of catalysts
样品 比表面积/(m2·g−1)Specific surface area 孔容/(mL·g−1)Pore volume 孔径/nmPore diameter 0 %wt. G-Mn/DE-HAL 23.21 0.031 48.29 2% wt. G-Mn/DE-HAL 24.90 0.052 56.33 4 %wt. G-Mn/DE-HAL 27.31 0.054 48.75 6 %wt. G-Mn/DE-HAL 31.92 0.055 49.56 8% wt. G-Mn/DE-HAL 32.26 0.057 48.75 4 %wt. S-Mn/DE-HAL 19.23 0.025 38.45 4% wt. Ca-Mn/DE-HAL 29.72 0.034 38.42 表 4 造孔剂种类不同的催化剂表面原子比值
Table 4. Surface atomic ratios of catalysts with different pore-forming agents
样品Samples 表面原子比值/% Surface atom ratio Mn4+/Mn3+ Oα/Oβ 4%wt.Ca-Mn/DE-HAL 67.66 315.01 4%wt.G-Mn/DE-HAL 91.51 473.51 4% wt.S-Mn/DE-HAL 71.34 218.13 表 5 造孔剂种类不同的催化剂NH3-TPD积分
Table 5. NH3-TPD integration of catalysts with different pore-forming agents
样品 Samples 4% wt. S-Mn/DE-HAL 4% wt. G-Mn/DE-HAL 4 %wt. Ca-Mn/DE-HAL 4% wt. G- DE-HAL NH3-TPD /(eV·s−1) 1.54×10−7 2.12×10−7 1.45×10−7 1.46×10−7 -
[1] SHEN Q, ZHANG L Y, SUN N N, et al. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation [J]. Chemical Engineering Journal, 2017, 322: 46-55. doi: 10.1016/j.cej.2017.02.148 [2] ZHANG X L, WU Q, DIAO Q C, et al. Performance study for NH3-SCR at low temperature based on different methods of Mnx/SEP catalyst [J]. Chemical Engineering Journal, 2019, 370: 364-371. doi: 10.1016/j.cej.2019.03.065 [3] GONÇALVES A A S, CIESIELCZYK F, SAMOJEDEN B, et al. Toward development of single-atom ceramic catalysts for selective catalytic reduction of NO with NH3 [J]. Journal of Hazardous Materials, 2021, 401: 123413. doi: 10.1016/j.jhazmat.2020.123413 [4] SHI X K, GUO J X, SHEN T, et al. Enhancement of Ce doped La-Mn oxides for the selective catalytic reduction of NOx with NH3 and SO2 and/or H2O resistance [J]. Chemical Engineering Journal, 2021, 421: 129995. doi: 10.1016/j.cej.2021.129995 [5] LI M H, GUO Y Y, YANG J P. Spatially nanoconfined architectures: A promising design for selective catalytic reduction of NO X [J]. ChemCatChem, 2020, 12(22): 5599-5610. doi: 10.1002/cctc.202001024 [6] SONG I, LEE H, JEON S W, et al. Controlling catalytic selectivity mediated by stabilization of reactive intermediates in small-pore environments: A study of Mn/TiO2 in the NH3-SCR reaction [J]. ACS Catalysis, 2020, 10(20): 12017-12030. doi: 10.1021/acscatal.0c03154 [7] ZHA K W, CAI S X, HU H, et al. In situ DRIFTs investigation of promotional effects of tungsten on MnOx-CeO2/meso-TiO2 catalysts for NOx reduction [J]. The Journal of Physical Chemistry C, 2017, 121(45): 25243-25254. doi: 10.1021/acs.jpcc.7b08600 [8] HAN L, LI F L, ZHANG H J, et al. Low-temperature preparation of porous diatomite ceramics via direct-gelcasting using melamine and boric acid as cross-linker and sintering agent [J]. Ceramics International, 2019, 45(18): 24469-24473. doi: 10.1016/j.ceramint.2019.08.172 [9] YAN W, LIN X L, CHEN J F, et al. Effect of TiO2 addition on microstructure and strength of porous spinel (MgAl2O4) ceramics prepared from magnesite and Al(OH)3 [J]. Journal of Alloys and Compounds, 2015, 618: 287-291. doi: 10.1016/j.jallcom.2014.08.169 [10] GREGOROVÁ E, PABST W, UHLÍŘOVÁ T, et al. Processing, microstructure and elastic properties of mullite-based ceramic foams prepared by direct foaming with wheat flour [J]. Journal of the European Ceramic Society, 2016, 36(1): 109-120. doi: 10.1016/j.jeurceramsoc.2015.09.028 [11] WU Z, SUN L C, PAN J J, et al. Highly porous Y2 SiO5 ceramic with extremely low thermal conductivity prepared by foam-gelcasting-freeze drying method [J]. Journal of the American Ceramic Society, 2018, 101(3): 1042-1047. doi: 10.1111/jace.15330 [12] SERGEEVA A, FEOKTISTOVA N, PROKOPOVIC V, et al. Design of porous alginate hydrogels by sacrificial CaCO3Templates: Pore formation mechanism [J]. Advanced Materials Interfaces, 2015, 2(18): 1500386. doi: 10.1002/admi.201500386 [13] HAN L, DENG X G, LI F L, et al. Preparation of high strength porous mullite ceramics via combined foam-gelcasting and microwave heating [J]. Ceramics International, 2018, 44(12): 14728-14733. doi: 10.1016/j.ceramint.2018.05.101 [14] ZHANG X L, WANG P M, WU X, et al. Application of MnOx/HNTs catalysts in low-temperature NO reduction with NH3 [J]. Catalysis Communications, 2016, 83: 18-21. doi: 10.1016/j.catcom.2016.05.003 [15] 董学成. 硅藻土基孔梯度多孔陶瓷的制备与性能研究[D]. 武汉: 武汉理工大学, 2017. DONG X C. Preparation and properties of porous gradient ceramics from diatomite[D]. Wuhan: Wuhan University of Technology, 2017(in Chinese).
[16] LI C X, CHENG J, YE Q, et al. Poisoning effects of alkali and alkaline earth metal doping on selective catalytic reduction of NO with NH3 over the Nb-Ce/Zr-PILC catalysts [J]. Catalysts, 2021, 11(3): 329. doi: 10.3390/catal11030329 [17] FAN Z Y, SHI J W, GAO C, et al. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance [J]. Chemical Engineering Journal, 2018, 348: 820-830. doi: 10.1016/j.cej.2018.05.038 [18] FRANCE L J, YANG Q, LI W, et al. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx [J]. Applied Catalysis B:Environmental, 2017, 206: 203-215. doi: 10.1016/j.apcatb.2017.01.019 [19] SHI Y R, YI H H, GAO F Y, et al. Evolution mechanism of transition metal in NH3-SCR reaction over Mn-based bimetallic oxide catalysts: Structure-activity relationships [J]. Journal of Hazardous Materials, 2021, 413: 125361. doi: 10.1016/j.jhazmat.2021.125361 [20] GAO L, LI C T, LI S H, et al. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal [J]. Chemical Engineering Journal, 2019, 371: 781-795. doi: 10.1016/j.cej.2019.04.104 [21] FAN J, NING P, WANG Y C, et al. Significant promoting effect of Ce or La on the hydrothermal stability of Cu-SAPO-34 catalyst for NH3-SCR reaction [J]. Chemical Engineering Journal, 2019, 369: 908-919. doi: 10.1016/j.cej.2019.03.049 [22] ZHANG G D, HAN W L, ZHAO H J, et al. Solvothermal synthesis of well-designed ceria-tin-titanium catalysts with enhanced catalytic performance for wide temperature NH3-SCR reaction [J]. Applied Catalysis B:Environmental, 2018, 226: 117-126. doi: 10.1016/j.apcatb.2017.12.030 [23] HUANG X S, DONG F, ZHANG G D, et al. A strategy for constructing highly efficient yolk-shell Ce@Mn@TiOx catalyst with dual active sites for low-temperature selective catalytic reduction of NO with NH3 [J]. Chemical Engineering Journal, 2021, 419: 129572. doi: 10.1016/j.cej.2021.129572 [24] DAMMA D, PAPPAS D K, BONINGARI T, et al. Study of Ce, Sb, and Y exchanged titania nanotubes and superior catalytic performance for the selective catalytic reduction of NOx [J]. Applied Catalysis B:Environmental, 2021, 287: 119939. doi: 10.1016/j.apcatb.2021.119939 [25] CHEN L, YAO X J, CAO J, et al. Effect of Ti4+ and Sn4+ co-incorporation on the catalytic performance of CeO2-MnOx catalyst for low temperature NH3-SCR [J]. Applied Surface Science, 2019, 476: 283-292. doi: 10.1016/j.apsusc.2019.01.095 [26] XU J Q, TANG T, ZHANG Q, et al. Remarkable low temperature catalytic activity for SCR of NO with propylene under oxygen-rich conditions over Mn0.2La0.07Ce0.05Ox/ZSM-5 catalyst [J]. Vacuum, 2021, 188: 110174. doi: 10.1016/j.vacuum.2021.110174 [27] ZHU N, SHAN W P, SHAN Y L, et al. Effects of alkali and alkaline earth metals on Cu-SSZ-39 catalyst for the selective catalytic reduction of NOx with NH3 [J]. Chemical Engineering Journal, 2020, 388: 124250. doi: 10.1016/j.cej.2020.124250 期刊类型引用(0)
其他类型引用(1)
-






 DownLoad:
DownLoad: