-
全氟辛烷磺酸(perfluorooctane sulfonate ,PFOS)是一种典型的全氟化合物,具有持久性、生物累积性和高毒性,2009年5月被增列入《关于持久性有机污染物的斯德哥尔摩公约》的名单中[1]。PFOS的大量生产和超稳定结构导致其在环境中持续累积,一些受污染场地的地下水中PFOS浓度可达到mg·L−1的水平[2-3]。研究发现接触PFOS可能对人体发育和生殖产生负面影响,甚至在低浓度情况下(10—15 ng·L−1)就可能存在人类健康风险[4]。由于PFOS在水中有较高的溶解度和稳定的化学结构,传统的生物处理工艺无法有效去除水中的PFOS[5-7]。吸附则是一种经济有效去除水中污染物的方法,PFOS在离子交换聚合物[8]、活性炭[9-10]、壳聚糖分子印迹聚合物[11]和树脂[12]等吸附剂上的吸附研究均有报道。
碳纳米管(carbon nanotubes,CNTs)是一种具有高稳定性和较强吸附性的纳米材料,由于其从废水中去除各种有机和无机污染物的突出能力而引起广泛关注[13-17]。近年来,许多研究集中在有机污染物在碳纳米管上的吸附[18-20]。Zhou等[21]研究发现,碳纳米管可以吸附高浓度的PFOS;Li等[22- 23]发现,由于受到了碳纳米管中氧含量的影响,电化学辅助增强了碳纳米管对PFOS的吸附。碳纳米管主要分为单壁碳纳米管(SWCNTs)和多壁碳纳米管(MWCNTs),SWCNTs是由单层石墨烯卷成柱状无缝管体而成,MWCNTs可看作由多层石墨体同轴套构而成。而双壁碳纳米管是最简单的多壁碳纳米管,将SWCNTs的形态和柔韧性与MWCNTs的物理化学阻力结合在一起,因此被认为是“碳纳米管”最好的模式[24-25]。Chen等[26]研究了3种碳纳米管对PFOS的吸附情况,其吸附能力依次为:DWCNTs>SWCNTs>MWCNTs。DWCNTs一般以纳米颗粒悬浮在水中,吸附后如何将其有效地从水环境中分离出来是值得研究的问题,而填充磁性纳米颗粒的吸附剂因具有磁性便于分离和收集,可以很好地解决这一问题[27]。
本研究采用化学沉淀法制备了磁性铁氧化物修饰的双壁碳纳米管复合材料,研究了磁性双壁碳纳米管(magnetic double-walled carbon nanotubes, m-DWCNTs )在不同pH和吸附剂投加量的情况下对低浓度PFOS的吸附影响,同时对m-DWCNTs进行表征以及吸附动力学和等温线模型的拟合,并初步探讨其吸附机理,为后续研究纳米材料处理水中的有机污染物提供了借鉴和参考。
-
主要仪器:液相色谱-串联四极杆质谱联用仪,Waters_TQS(沃特世科技(上海)有限公司); 气浴恒温振荡器,THZ-92B(青岛明博公司);漩涡振荡器,Vortex3(IKA公司);低速离心机,LXJ-IIB(上海安亭科学仪器厂);180 mm透明玻璃干燥器(广州锂阁公司);超声波清洗器,2150T(上海安谱科技有限公司)。
主要试剂:全氟辛烷磺酸(PFOS,纯度≥98%,Sigma-Aldrich公司);甲醇(HPLC,上海安谱实验科技股份有限公司);双壁碳纳米管(DWCNTs,纯度≥60%,直径:2—4 nm,长度:—50 μm, Macklin公司);氯化铁(FeCl3,纯度:98%,Alfa Aesar公司);氯化亚铁(FeCl2,纯度:99.5%,Energy-Chemical公司)。
-
PFOS采用液相色谱-串联四极杆质谱联用仪测定。色谱柱为Waters HSS C18 (2.1 mm×100 mm,0.17 μm);柱温:38 ℃;进样体积:2.0 μL;总流速:0.3 mL·min−1;流动相分为A、B两相,A:0.1%甲酸水溶液,B:甲醇溶液,梯度洗脱条件:0—1 min,30% B;1.1 min,100% B;1.1—5 min,100% B;5.1 min,30% B;5.1—7 min,30% B。质谱电离方式:电喷雾离子源(ESI);负离子模式;源温度120℃;毛细管电压3.0 kV;干燥N2温度为250 ℃;雾化器流速为800 L·h−1;干燥气流速6 L·min−1;实验采用多反应监测模式(MRM);实验定量离子对PFOS,m/z=499/80。
-
(1)纯化
将1 g原DWCNTs和100 mL混酸[
V(浓HNO3) :V(浓H2SO4) =1:3]置于500 mL锥形瓶中,超声处理2 h,使DWCNTs分散在酸溶液中。将混合物置于恒温磁力搅拌器中,55 ℃下搅拌6 h后,自然冷却至室温。用0.45 μm滤膜过滤冷却后的溶液,用去离子水和乙醇反复洗涤,直至滤液pH值接近7。收集滤膜上的黑色物质,65 ℃烘箱中干燥24 h后研磨过100目筛,即得纯化双壁碳纳米管(p-DWCNTs),放入干燥器备用。(2)磁化
称取0.6 g纯化后的DWCNTs、0.8 g FeCl3和1.2 g FeCl2,并将其置于500 mL平底烧瓶中,加入200 mL去离子水。将混合物超声处理1 h,然后缓慢加入浓度为25 %的氨水溶液,将混合溶液pH值控制在10左右,使铁氧化物沉积在p-DWCNTs上。置于磁力搅拌器中,在55 ℃下搅拌2 h后进行冷却至室温。用0.45 μm滤膜过滤冷却的溶液,去离子水和乙醇反复洗涤,清洗至滤液的pH值接近7,收集滤膜上的黑色物质,65 ℃下烘干24 h后研磨过100目筛,即得磁性双壁碳纳米管(m-DWCNTs),放入干燥器备用。
-
采用扫描电镜(SEM,ZEISS Gemini 300)观察样品的表面形貌;采用X射线衍射(XRD,D8A)检测吸附剂的晶型;采用红外光谱(FTIR,Thermo Scientific Nicolet iS5)分析吸附剂的波峰;采用振动样品磁强计(VSM,MPMS XL-7)测定吸附剂的磁性能。
-
将40 mL不同初始浓度的PFOS溶液加入到含有一定量 m-DWCNTs的50 mL聚丙烯离心管中,滴加0.1 mol·L−1的HCl和NaOH调节溶液的pH,将离心管置于25 ℃和200 r·min−1条件下的恒温振荡器中,震荡反应一定时间后,静置1 h后用0.22 μm聚丙烯微孔滤膜过滤,过滤后取1 mL滤液测定其中PFOS的含量,计算m-DWCNTs对PFOS的吸附量qe。分别研究吸附时间、初始浓度、吸附剂投加量和pH对m-DWCNTs吸收PFOS的影响。实验条件分别为:(1)设定吸附时间为1、2、4、8、12、24、36、48、60、72、96 h,其他实验条件同上述;(2)设定初始浓度为1、5、10、20、50、100、200、400 μg·L−1,其他实验条件同上述;(3)设定吸附剂投加量为1.5、5、10、15、20、40 mg,其他实验条件同上述;(4)设定pH值为2.0、3.0、4.0、5.0、6.0、7.0、8.0、9.0,其他条件同上。所有实验重复3次,取其平均值进行分析.
-
(1)PFOS的去除率和吸附量
根据公式(1)计算其去除率η(%),公式(2)计算其吸附量
qe( mg·g−1) ,计算公式如下:式中,
C0 -吸附质的初始浓度,mg·L−1;Ce -平衡时吸附质的浓度,mg·L−1;m-吸附剂的质量;V-吸附质溶液的体积,L。(2)吸附动力学和等温线模型
吸附动力学研究吸附过程中吸附量随时间变化的趋势,是吸附行为研究的重要组成部分。分别用准一级动力学模型、准二级吸附动力学模型、颗粒内扩散模型和Elovich模型就m-DWCNTs对PFOS的吸附进行拟合。计算方程如公式3—6所示:
式中,K1-反应速率常数,h−1;t-时间,h;qe-吸附平衡时吸附剂对吸附质的吸附量,mg·g−1;qt-t时刻吸附剂对吸附质的吸附量,mg·g−1;K2-反应速率常数,g·mg−1·h−1;Kd-粒子内扩散速率常数,mg·g−1·h1/2;C-与边界层厚度有关的常数,mg·g−1;a-表示初始吸附速率,g·mg−1·h−1;b-解析常数,g·mg−1。
吸附等温线是在一定温度条件下,用来描述达到吸附平衡时的浓度和吸附量之间函数关系的曲线,目前常用的吸附等温线模型有Langmuir 模型和Freundlich模型(公式7、8):
式中,KL—Langmuir 常数,L·mg−1;Ce-吸附平衡时吸附质的浓度,mg·L−1;qe-吸附平衡时吸附剂的吸附量,mg·g−1;qm-吸附剂的最大吸附量,mg·g−1; 1/n-不均匀性因数;KF-Freundlich 常数,
(mg⋅g−1)⋅(L⋅mg−1)1/n .(3)数据分析
实验数据经Office 2019处理后,使用Origin 9.0、Prism 8.0 进行模型拟合和绘图。
-
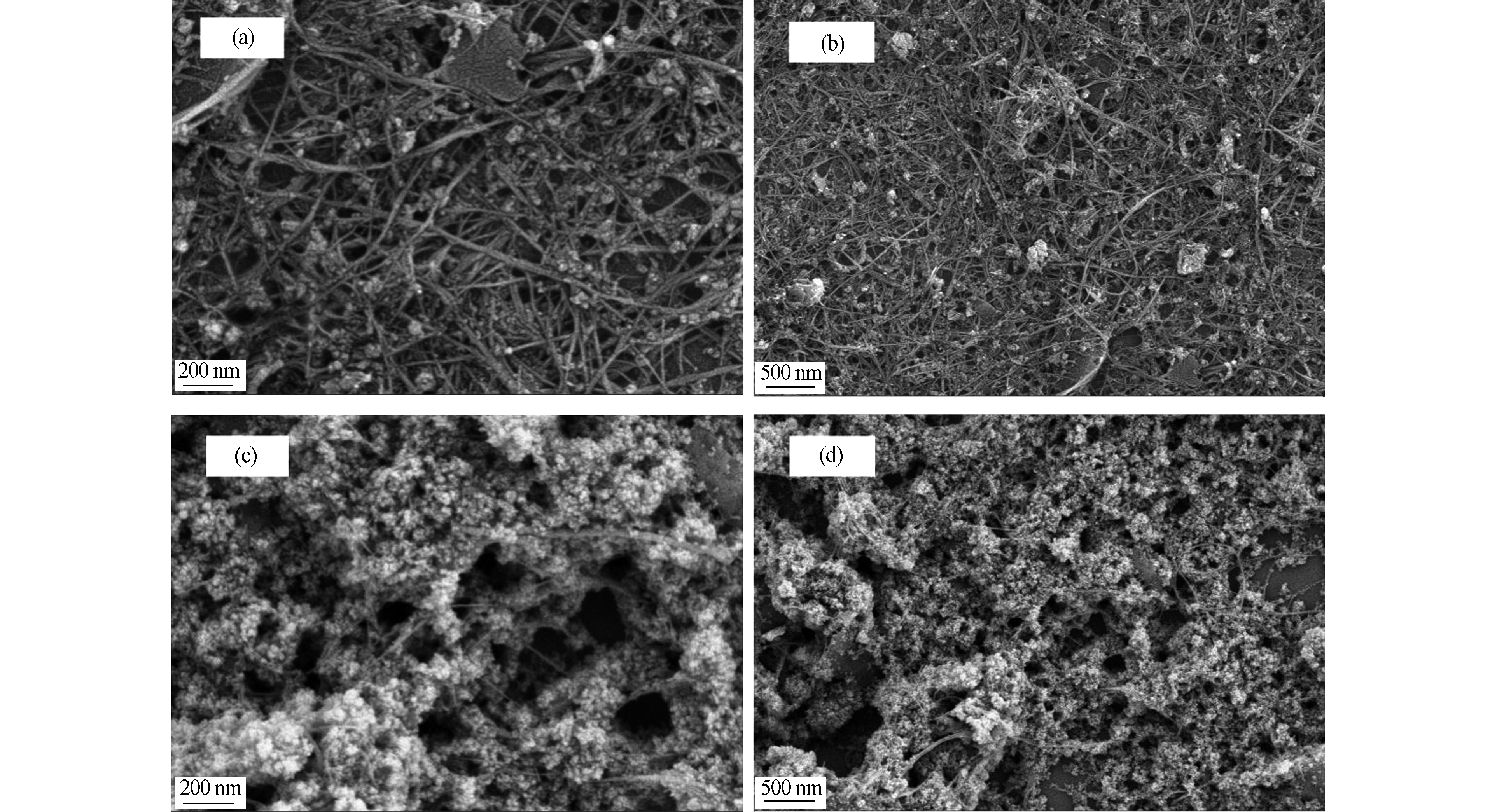
图1为纯化后DWCNTs(p-DWCNTs)和磁化后DWCNTs(m-DWCNTs)的SEM扫描电镜图。由图可知,p-DWCNTs呈相互缠绕的管状结构,表面相对比较光滑,无明显附着物。而经铁修饰后的m-DWCNTs管状结构没有发生变化,但管状结构上附着大量粒径很小的颗粒,表面明显粗糙,初步可判断磁性粒子已经成功负载在p-DWCNTs的外壁上。
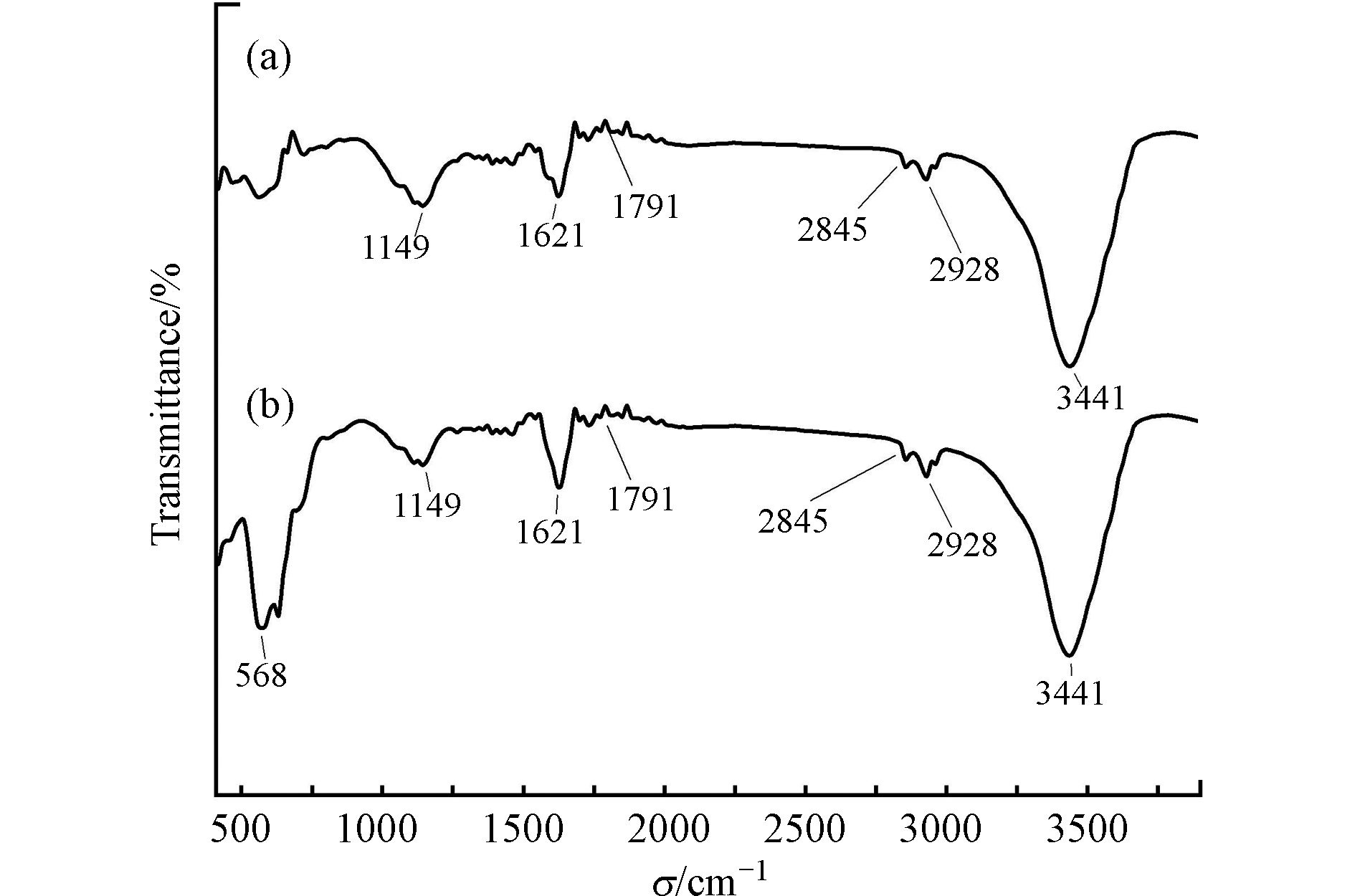
图2是p-DWCNTs(a)和m-DWCNTs(b)的傅里叶红外光谱图。由图2可知,p-DWCNTs在3441、2928、2845、1621、1149 cm−1都有特征峰。1621 cm−1处的振动峰是芳香族C=C的伸缩振动所引起的,表明了碳纳米管石墨结构的存在[28]。2845 cm−1和2928 cm−1处为饱和烃类C—H的伸缩振动特征峰,1149 cm−1为C—H的弯曲振动峰。同时可以看出在1791 cm−1的吸收峰,对应的是C=O的伸缩振动峰,说明在p-DWCNTs和m-DWCNTs表面上都存在羧基基团。与p-DWCNTs相比,磁化后的DWCNTs在568 cm−1附近增加了Fe—O键的伸缩吸收峰,表明有可能是铁氧化物包覆在碳纳米管的表面上。Zhao等[29]指出,与原碳纳米管相比,由于存在磁性氧化材料,磁化后的碳纳米管的表面可能部分被磁性氧化物覆盖,磁性氧化物显著影响碳纳米管的表面性质。
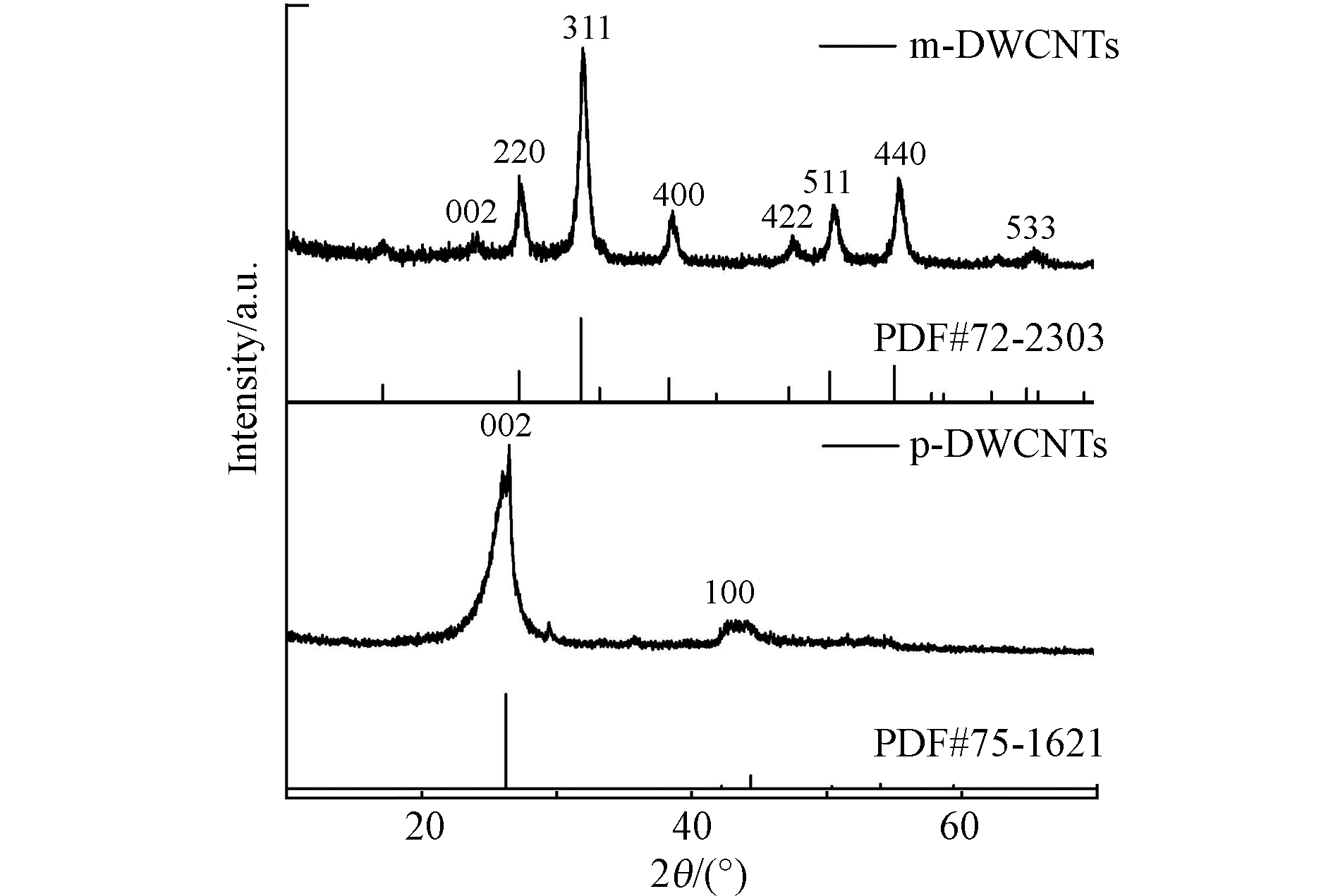
图3是p-DWCNTs和m-DWCNTs的X射线衍射(XRD)结果。p-DWCNTs的特征衍射峰位于2θ=26.46°和42.44°,对应了石墨的(002)和(100)晶面,表明碳纳米管中存在C-C结构,而p-DWCNTs经过磁化后,石墨(100)晶面对应的衍射峰消失或者强度变得非常弱,(002)晶面对应的特征峰相对于p-DWCNTs也降低了很多。m-DWCNTs对应曲线的特征衍射峰和和Fe3O4的衍射峰一致,2θ=30.065°、35.412°、43.037°、53.389°、56.912°、62.495°和73.928°分别对应于Fe3O4的晶面(220)、(311)、(400)、(422)、(511)、(440)和(533),说明磁性铁氧化物已经成功负载在DWCNTs上。
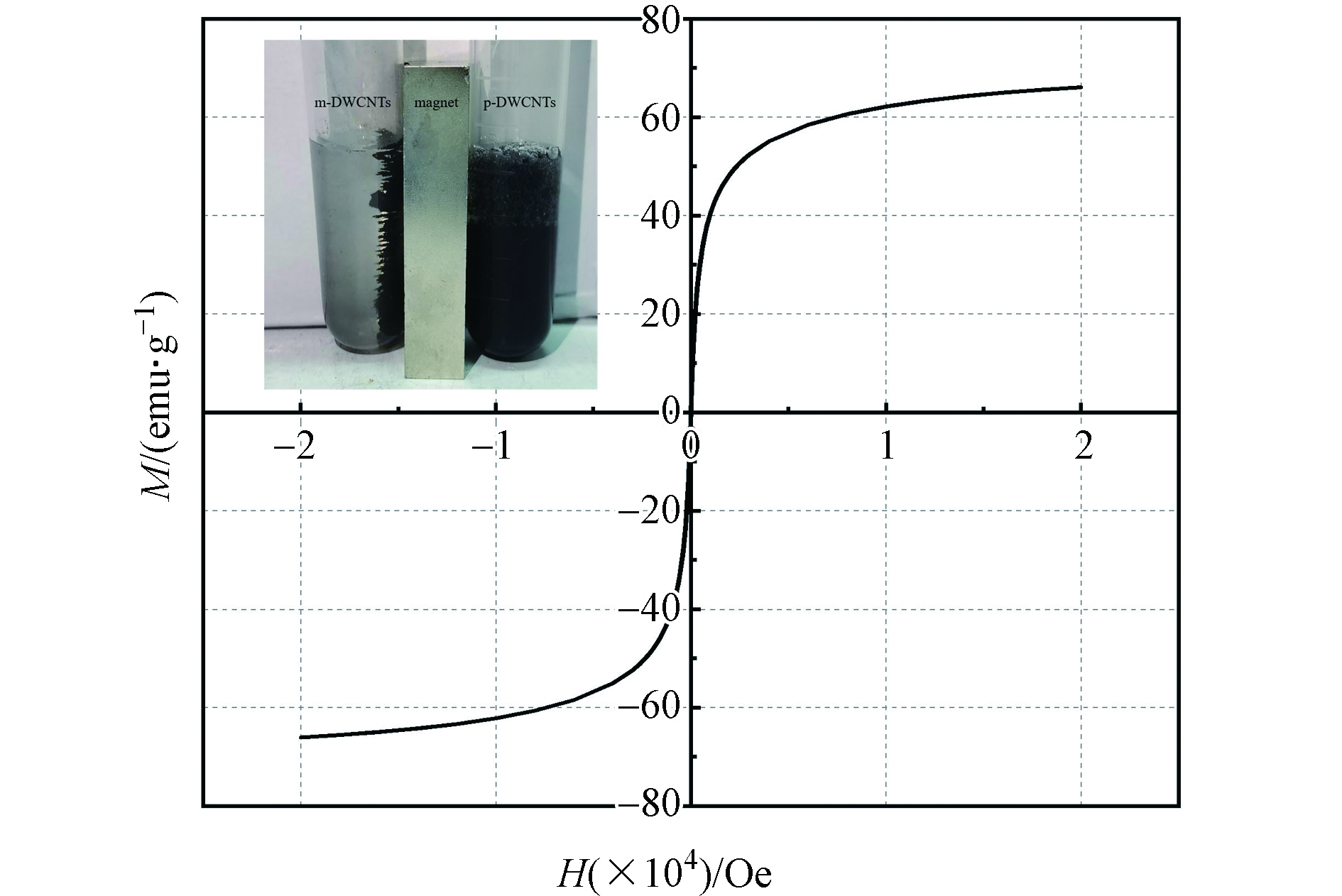
m-DWCNTs的磁滞回线如图4所示,可以看出m-DWCNTs的矫顽力和剩磁力均为0,说明m-DWCNTs具有超顺磁性,意味着m-DWCNTs复合材料吸附后能够有效分离以及再利用。对m-DWCNTs的表征结果显示磁性纳米颗粒(Fe3O4)已经成功地包覆在碳纳米管的表面。另外,由图4中的实物可以看出,左边m-DWCNTs能够很好地被磁铁吸引,而右边的p-DWCNTs则悬浮分散在水环境中,这表明经过Fe3O4修饰后的m-DWCNTs在外加磁场作用下具有良好的磁分离效果,可以快速实现固液分离。Zhao等[29]通过用MnCl2和FeCl3制成的磁性碳纳米管的饱和磁化强度仅为10.8 emu·g−1;Xue等[30]用Cu2+、Mn2+、 Ni2+和Zn2+分别与Fe3+混和制备的磁性碳纳米管饱和磁化强度最高的仅为0.65 emu·g−1;而本实验中,室温下m-DWCNTs的饱和磁化强度为66.07 emu·g−1(图4),比Zhao和Xue等[29-30]所制得的磁性碳纳米管的磁性较强,更易实现固液快速分离。
-
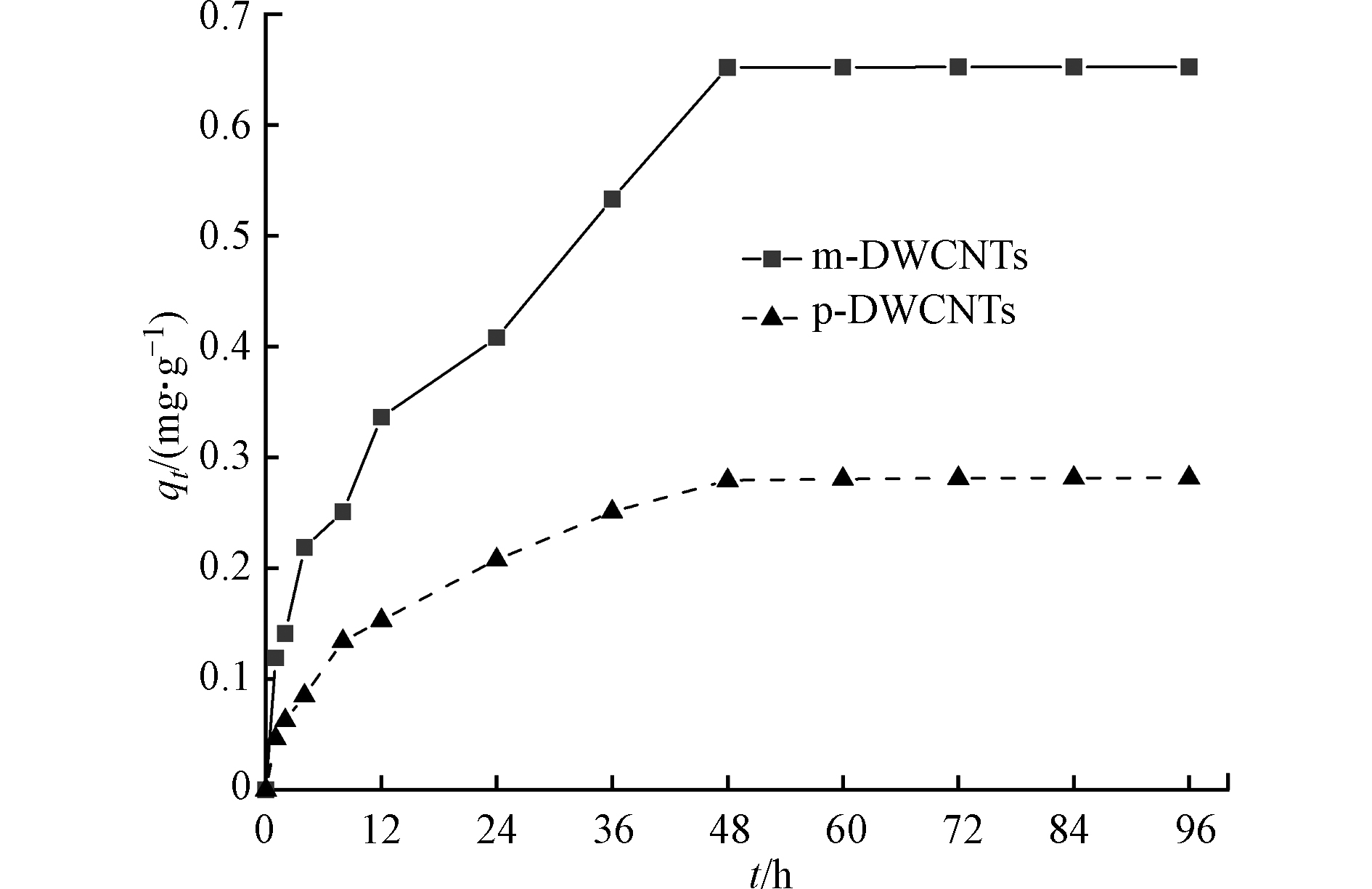
图5为磁化前后DWCNTs对PFOS吸附量随时间的变化情况,磁化后m-DWCNTs对PFOS的吸附量比未磁化的p-DWCNTs吸附量大,吸附性能显著提升,吸附量从0.28 mg·g−1增长至0.65 mg·g−1,去除率从40.36%增长至85.14%。
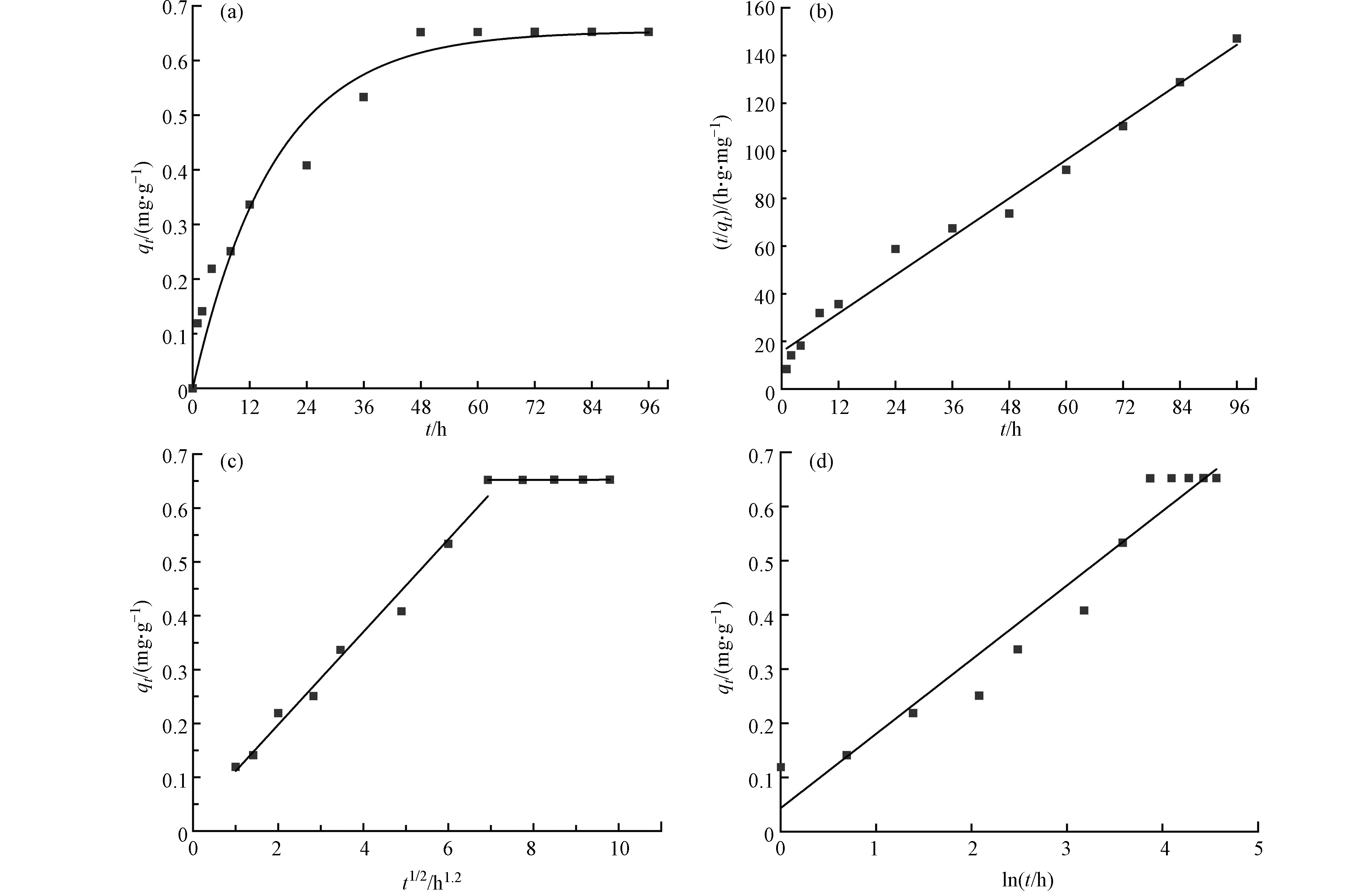
图6显示了接触时间对m-DWCNTs吸附PFOS的影响。PFOS在m-DWCNTs的吸附过程可分为2个阶段,0—48 h内PFOS吸附量随着吸附时间的延长而快速上升,吸附率最快;随后在48—96 h基本维持在平衡状态,PFOS浓度没有明显变化,吸附量基本维持在0.65 mg·g−1。这是因为在前48小时内,m-DWCNTs表面上拥有大量空余的吸附位点,可以迅速地与溶液中PFOS分子相结合;在48 h之后可供PFOS吸附的位点数大大减少,导致后期吸附速率降低。
为了进一步探讨PFOS在m-DWCNT上的吸附机理,对实验数据进行拟合,拟合参数见表1。结果表明,准一级动力学和准二级动力学与实验数据都吻合得比较好,但准二级动力学模型具有更高的相关系数R2>0.98,并且拟二级动力学得到的qe值更加接近于实验结果。准二级动力学吸附模型是基于化学吸附的假设,所以可以推断出该吸附过程可能存在化学吸附[31-32]。
由表1的扩散模型拟合可知,第一阶段R2>0.95,大于第二阶段的R2。表明在初始扩散阶段模型能够较好地描述m-DWCNTs对PFOS的吸附过程,在第一阶段,PFOS通过颗粒外部扩散过程传输到吸附剂的外表面,之后是渐进的吸附过程,PFOS扩散到吸附剂的表面孔内[33-34];随着吸附过程进入第二阶段,由于PFOS含量变低,颗粒内扩散开始减缓。因此,颗粒外部扩散和粒子内扩散同时影响m-DWCNTs对PFOS的吸附。而Elovich模型的R2<0.95与其他模型相比较小,说明Elovich模型相比之下无法很好地描述m-DWCNTs吸附PFOS的动力学行为[35]。
-
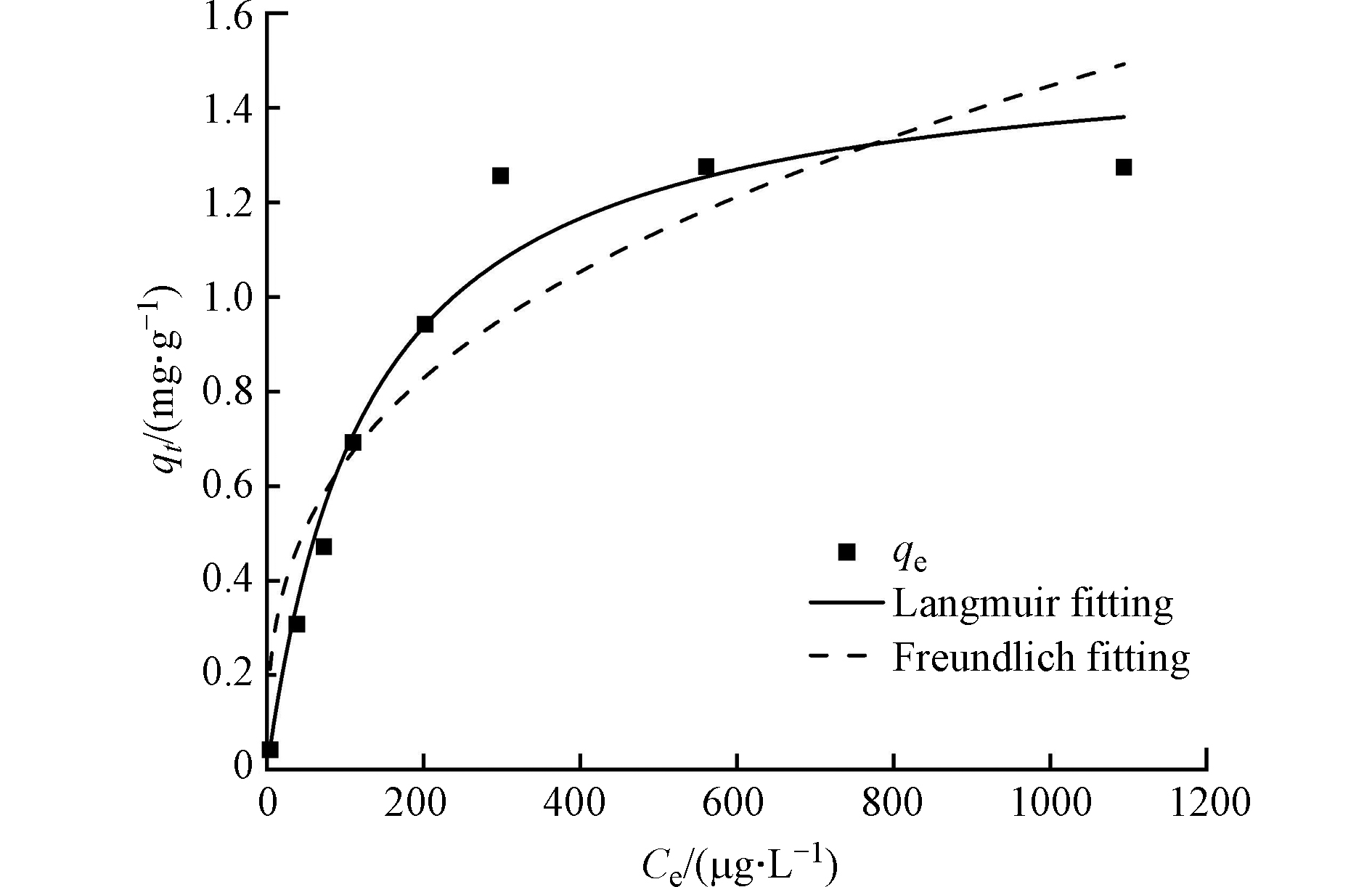
m-DWCNTs对PFOS的吸附等温线如图7所示。可以看出,随着平衡浓度的增加,吸附量qe先呈上升趋势然后逐渐趋于平缓。当平衡浓度为3.92—298.32 μg·L−1时,吸附量从0.04 mg·g−1增长到1.26 mg·g−1;当平衡浓度为560.94—1494.54 μg·L−1时,吸附量达到平衡,无显著变化,随着PFOS的浓度增大,m-DWCNTs对PFOS的吸附量趋于平衡。
为进一步研究m-DWCNTs对PFOS的吸附机理,分别采用Langmuir和Freundlich等温吸附模型对等温吸附试验数据进行拟合。Langmuir模型适用于吸附材料对吸附质的单分子层吸附,而Freundlich模型适用于吸附材料对吸附质的多分子层吸附。拟合参数如表2所示,因为Langmuir模型的线性拟合参数R2大于Freundlich模型的线性拟合参数,R2>0.9,表明m-DWCNTs对PFOS的吸附更符合Langmuir模型。由Freundlich模型拟合参数n=2.8879>1,KF和n值都为正值,表明对PFOS的吸附过程为优惠吸附,吸附性能较好[36]。再由Langmuir拟合模型可知m-DWCNTs对PFOS有可能以单分子层吸附为主,吸附发生在吸附剂表层内的特定均匀位置。最大吸附量高达1.54 mg·g−1。
-
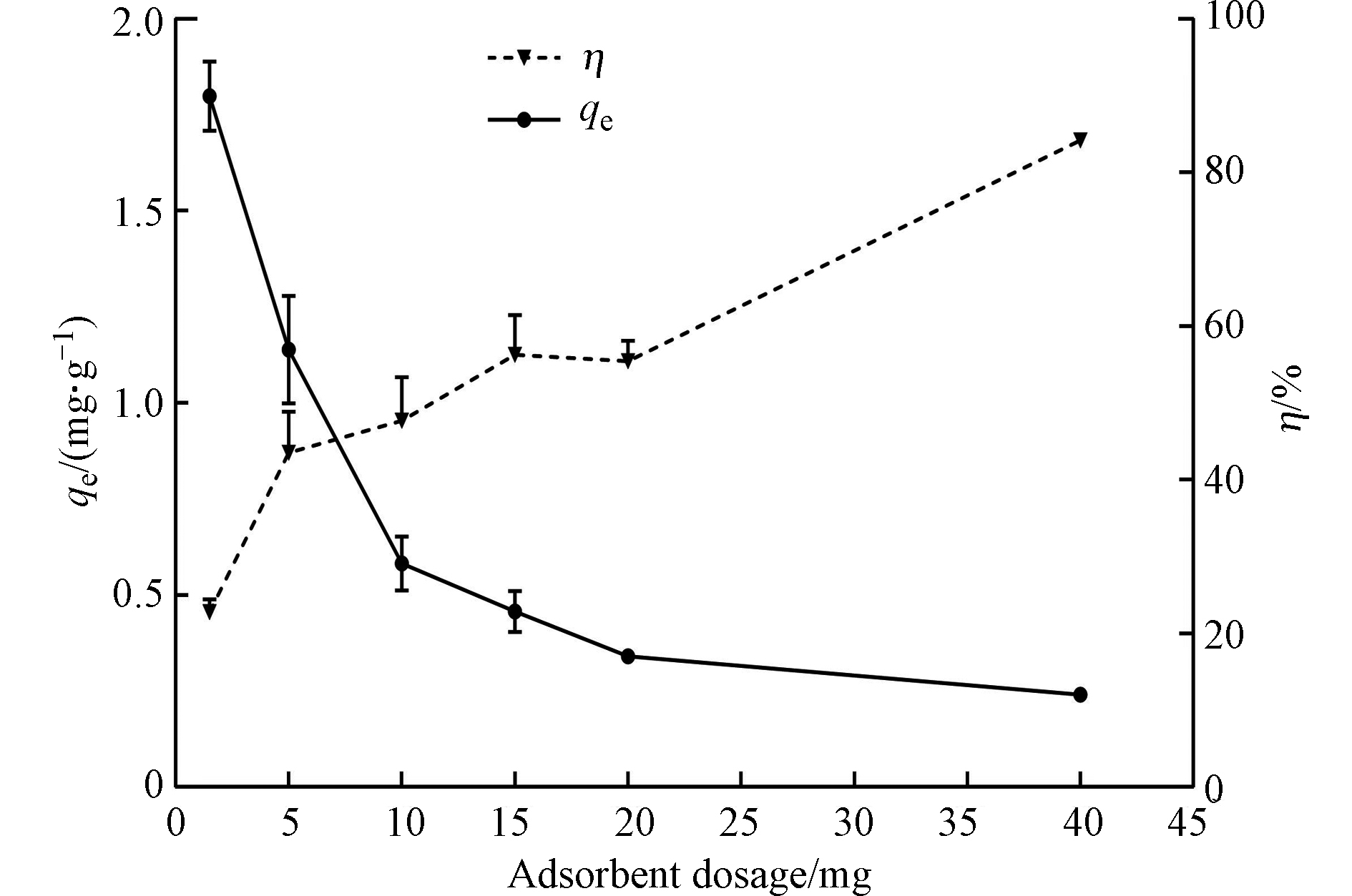
吸附剂的用量对m-DWCNTS吸附PFOS也有一定的影响,并非吸附剂用量越多越好,吸附剂用量越多可能会造成吸附剂表面吸附位点剩余,而吸附剂用量较少时可能导致吸附不完全。吸附剂用量对m-DWCNTS吸附PFOS的影响如图8所示。从图8可知,当m-DWCNTs的用量从1.5 mg增加到40 mg时,去除率从22.67%增长至84.36%,吸附量从1.80 mg·g−1降低至0.24 mg·g−1。吸附量
qe 随吸附剂用量的升高而降低可能是因为随着投加量的增加,吸附位点增加,吸附位点数量多于PFOS的含量,吸附位点未得到充分利用,致使吸附剂投加量越高得到的单位吸附量越小[37]。而根据公式(1)可知去除率与吸附在吸附剂上PFOS的浓度成正比,随着吸附剂用量的增加,表面有效吸附位点也在增加,从而导致了PFOS去除率的增加。 -
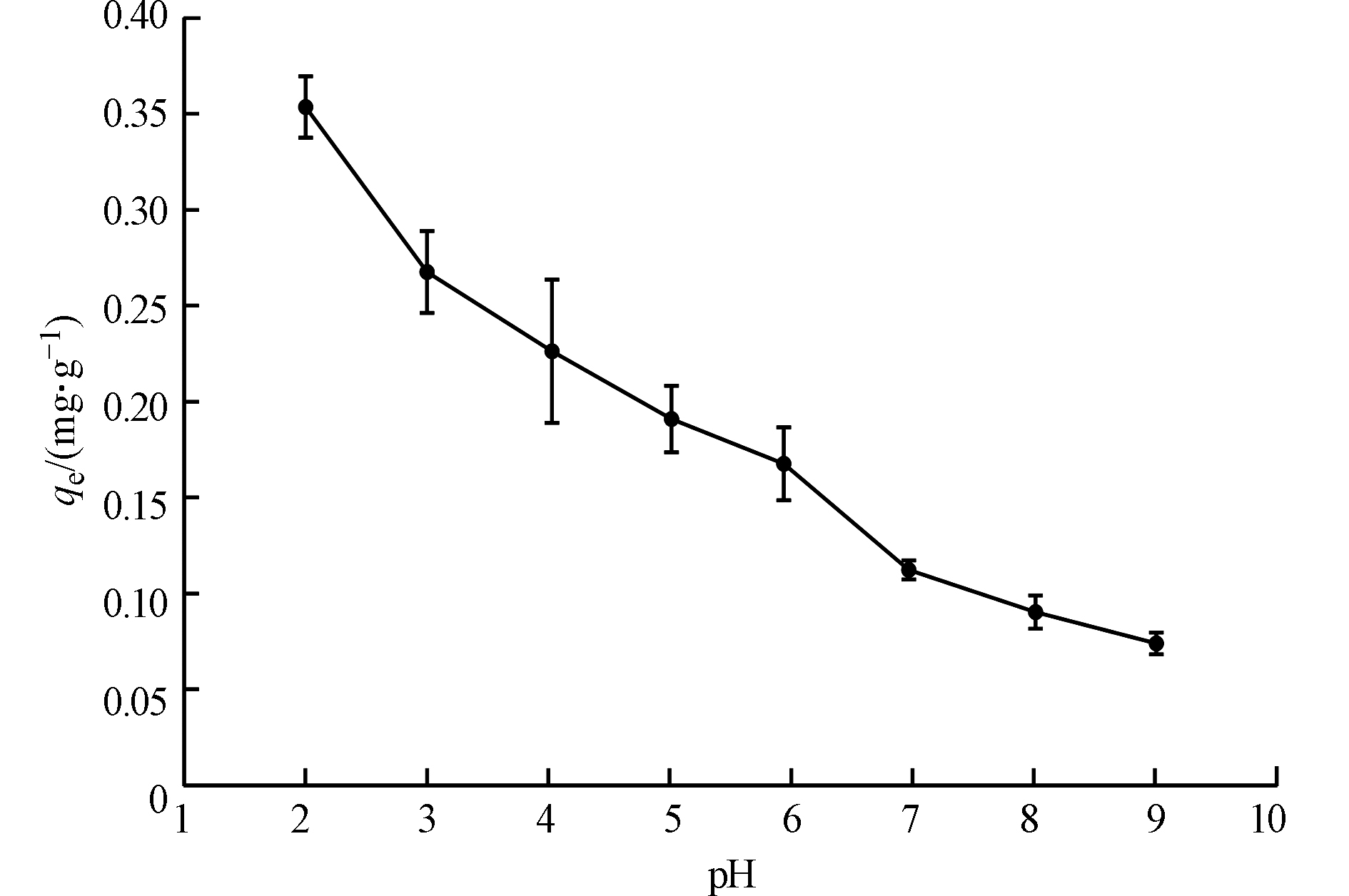
pH是控制m-DWCNTs吸附PFOS过程的重要参数之一。图9表明,pH值从2.0增至9.0,吸附量qe逐渐降低,从0.35 mg·g−1降至0.07 mg·g−1。表明静电相互作用有可能参与了PFOS在m-DWCNTs上的吸附。由于m-DWCNTs在所研究的pH范围内带负电,又因为PFOS的pKa较低(-3.27),PFOS在溶液中也以阴离子的形式存在,因此m-DWCNTs和PFOS的阴离子产生静电排斥作用。在低pH值下,m-DWCNTs表面由于质子化而带正电荷,与带着负电荷的PFOS产生了静电吸引[38]。随着pH值的升高,质子化作用减弱,m-DWCNTs表面上的负电荷也逐渐增多,静电斥力阻止了PFOS接近m-DWCNTs表面,导致了吸附量的逐渐降低。研究发现,氧化铝[39]、活性炭[40]、壳聚糖[41-42]等材料对PFOS的吸附都涉及静电作用;钟伟民等[43]发现,磁性分子印迹材料对PFOS的吸附主要为静电作用;郭盼盼等[44]也认为磁性壳聚糖对PFOS的吸附存在静电作用;Stebel等[45]发现可膨胀有机改性二氧化硅对PFOS的吸附主要为静电作用和离子交换作用;Cao等[46]发现基于碳纳米管的分子印迹聚合物对PFOS的吸附作用主要为静电作用和氢键作用。今后可对m-DWCNTs对PFOS的吸附机理开展进一步研究,例如对吸附前后的m-DWCNTs进行表征,对其所含元素、官能团及化学键等的变化进行分析等。
-
磁性DWCNTs对PFOS具有良好的吸附性能,从对m-DWCNTs的表征和不同实验条件下磁性DWCNTs吸附PFOS的结果可以得出以下结论:
(1) 纯化后的DWCNTs经过磁化后,改善了DWCNTs的表面性质。m-DWCNTs的表征观察证明了Fe3O4成功负载在DWCNTs上,并且具有超顺磁性,饱和磁化强度为66.07 emu·g−1,便于从水环境中快速分离。
(2) 用准二级动力学方程和Langmuir模型能较好地拟合PFOS在m-DWCNTs上的吸附过程,说明m-DWCNTs对PFOS的吸附可能是以化学吸附和单分子层吸附为主。
(3) 磁性DWCNTs吸附PFOS的去除率随着吸附剂用量的增加而增加,但吸附量随着吸附剂用量的增加而减少,当吸附剂投加量增加到40 mg,去除率可达84.36%。
(4) 磁性DWCNTs吸附PFOS的吸附机理与静电作用相关,在低pH值下,静电吸引作用有利于m-DWCNTs对PFOS的吸附,在高pH值下,静电排斥不利于PFOS在m-DWCNTs上的吸附。
磁性双壁碳纳米管(m-DWCNTs)对水中全氟辛烷磺酸(PFOS)的吸附
Adsorption of perfluorooctane sulfonate (PFOS) in water by magnetic double-walled carbon nanotubes (m-DWCNTs)
-
摘要: 本文以双壁碳纳米管(double-walled carbon nanotubes ,DWCNTs)为基础材料,通过化学沉淀法制备了磁性双壁碳纳米管(magnetic double-walled carbon nanotubes ,m-DWCNTs),研究了pH和吸附剂投加量对全氟辛烷磺酸(perfluorooctane sulfonate ,PFOS)的吸附影响。采用扫描电镜(SEM)、傅里叶红外光谱(FTIR)、X射线衍射(XRD)和VSM对吸附前后的m-DWCNTs进行了观察,并进行了吸附动力学模型和等温线模型拟合。结果表明,磁化后的双壁碳纳米管包覆了铁的氧化物,出现了Fe3O4的特征衍射峰,具有超顺磁性,饱和磁化强度为66.07 emu·g-1。m-DWCNTs对PFOS的吸附更符合准二级动力学方程(R2>0.95)和Langmuir等温吸附模型(R2>0.95)。随着m-DWCNTs投加量的增加,吸附量逐渐降低,去除率逐渐升高。吸附量随着pH值的增加而逐渐降低,其主要原因有可能是m-DWCNTs对PFOS的吸附产生了静电作用。本研究结果为后续纳米材料处理水中的PFOS提供了借鉴和参考。Abstract: Magnetic double-walled carbon nanotubes (m-DWCNTs) are prepared by chemical precipitation method. The adsorption effects of perfluorooctane sulfonate (PFOS) under different pH and adsorbent dosages were studied, and m-DWCNTs were characterized by SEM, FTIR, XRD and VSM. The adsorption kinetic model and isotherm model were used for fitting. The results showed that the magnetized double-walled carbon nanotubes were coated with iron oxides, and had the characteristic diffraction peaks of Fe3O4. They were superparamagnetic and had a saturation magnetization of 66.07 emu·g-1. The adsorption of PFOS by m-DWCNTs was more in line with the pseudo-second-order kinetic equation (R2>0.95) and the Langmuir isotherm adsorption model (R2>0.95). With the increase of the dosage of m-DWCNTs, the adsorption capacity of PFOS gradually decreased, and the adsorption rate gradually increased. The adsorption capacity gradually decreases with the increase of pH value. The main reason may be the electrostatic effect of m-DWCNTs on the adsorption of PFOS. The results of this study provide a reference for the subsequent treatment of PFOS in water bodies with nanomaterial.
-
Key words:
- perfluorooctane sulfonate(PFOS) /
- double-walled carbon nanotubes /
- magnetic /
- adsorption
-
全氟辛烷磺酸(perfluorooctane sulfonate ,PFOS)是一种典型的全氟化合物,具有持久性、生物累积性和高毒性,2009年5月被增列入《关于持久性有机污染物的斯德哥尔摩公约》的名单中[1]。PFOS的大量生产和超稳定结构导致其在环境中持续累积,一些受污染场地的地下水中PFOS浓度可达到mg·L−1的水平[2-3]。研究发现接触PFOS可能对人体发育和生殖产生负面影响,甚至在低浓度情况下(10—15 ng·L−1)就可能存在人类健康风险[4]。由于PFOS在水中有较高的溶解度和稳定的化学结构,传统的生物处理工艺无法有效去除水中的PFOS[5-7]。吸附则是一种经济有效去除水中污染物的方法,PFOS在离子交换聚合物[8]、活性炭[9-10]、壳聚糖分子印迹聚合物[11]和树脂[12]等吸附剂上的吸附研究均有报道。
碳纳米管(carbon nanotubes,CNTs)是一种具有高稳定性和较强吸附性的纳米材料,由于其从废水中去除各种有机和无机污染物的突出能力而引起广泛关注[13-17]。近年来,许多研究集中在有机污染物在碳纳米管上的吸附[18-20]。Zhou等[21]研究发现,碳纳米管可以吸附高浓度的PFOS;Li等[22- 23]发现,由于受到了碳纳米管中氧含量的影响,电化学辅助增强了碳纳米管对PFOS的吸附。碳纳米管主要分为单壁碳纳米管(SWCNTs)和多壁碳纳米管(MWCNTs),SWCNTs是由单层石墨烯卷成柱状无缝管体而成,MWCNTs可看作由多层石墨体同轴套构而成。而双壁碳纳米管是最简单的多壁碳纳米管,将SWCNTs的形态和柔韧性与MWCNTs的物理化学阻力结合在一起,因此被认为是“碳纳米管”最好的模式[24-25]。Chen等[26]研究了3种碳纳米管对PFOS的吸附情况,其吸附能力依次为:DWCNTs>SWCNTs>MWCNTs。DWCNTs一般以纳米颗粒悬浮在水中,吸附后如何将其有效地从水环境中分离出来是值得研究的问题,而填充磁性纳米颗粒的吸附剂因具有磁性便于分离和收集,可以很好地解决这一问题[27]。
本研究采用化学沉淀法制备了磁性铁氧化物修饰的双壁碳纳米管复合材料,研究了磁性双壁碳纳米管(magnetic double-walled carbon nanotubes, m-DWCNTs )在不同pH和吸附剂投加量的情况下对低浓度PFOS的吸附影响,同时对m-DWCNTs进行表征以及吸附动力学和等温线模型的拟合,并初步探讨其吸附机理,为后续研究纳米材料处理水中的有机污染物提供了借鉴和参考。
1. 材料和方法(Materials and methods)
1.1 主要仪器与试剂
主要仪器:液相色谱-串联四极杆质谱联用仪,Waters_TQS(沃特世科技(上海)有限公司); 气浴恒温振荡器,THZ-92B(青岛明博公司);漩涡振荡器,Vortex3(IKA公司);低速离心机,LXJ-IIB(上海安亭科学仪器厂);180 mm透明玻璃干燥器(广州锂阁公司);超声波清洗器,2150T(上海安谱科技有限公司)。
主要试剂:全氟辛烷磺酸(PFOS,纯度≥98%,Sigma-Aldrich公司);甲醇(HPLC,上海安谱实验科技股份有限公司);双壁碳纳米管(DWCNTs,纯度≥60%,直径:2—4 nm,长度:—50 μm, Macklin公司);氯化铁(FeCl3,纯度:98%,Alfa Aesar公司);氯化亚铁(FeCl2,纯度:99.5%,Energy-Chemical公司)。
1.2 仪器工作条件
PFOS采用液相色谱-串联四极杆质谱联用仪测定。色谱柱为Waters HSS C18 (2.1 mm×100 mm,0.17 μm);柱温:38 ℃;进样体积:2.0 μL;总流速:0.3 mL·min−1;流动相分为A、B两相,A:0.1%甲酸水溶液,B:甲醇溶液,梯度洗脱条件:0—1 min,30% B;1.1 min,100% B;1.1—5 min,100% B;5.1 min,30% B;5.1—7 min,30% B。质谱电离方式:电喷雾离子源(ESI);负离子模式;源温度120℃;毛细管电压3.0 kV;干燥N2温度为250 ℃;雾化器流速为800 L·h−1;干燥气流速6 L·min−1;实验采用多反应监测模式(MRM);实验定量离子对PFOS,m/z=499/80。
1.3 磁性双壁碳纳米管的制备及表征
1.3.1 磁性双壁碳纳米管的制备
(1)纯化
将1 g原DWCNTs和100 mL混酸[
V(浓HNO3) V(浓H2SO4) (2)磁化
称取0.6 g纯化后的DWCNTs、0.8 g FeCl3和1.2 g FeCl2,并将其置于500 mL平底烧瓶中,加入200 mL去离子水。将混合物超声处理1 h,然后缓慢加入浓度为25 %的氨水溶液,将混合溶液pH值控制在10左右,使铁氧化物沉积在p-DWCNTs上。置于磁力搅拌器中,在55 ℃下搅拌2 h后进行冷却至室温。用0.45 μm滤膜过滤冷却的溶液,去离子水和乙醇反复洗涤,清洗至滤液的pH值接近7,收集滤膜上的黑色物质,65 ℃下烘干24 h后研磨过100目筛,即得磁性双壁碳纳米管(m-DWCNTs),放入干燥器备用。
1.3.2 吸附材料表征
采用扫描电镜(SEM,ZEISS Gemini 300)观察样品的表面形貌;采用X射线衍射(XRD,D8A)检测吸附剂的晶型;采用红外光谱(FTIR,Thermo Scientific Nicolet iS5)分析吸附剂的波峰;采用振动样品磁强计(VSM,MPMS XL-7)测定吸附剂的磁性能。
1.4 实验方法
将40 mL不同初始浓度的PFOS溶液加入到含有一定量 m-DWCNTs的50 mL聚丙烯离心管中,滴加0.1 mol·L−1的HCl和NaOH调节溶液的pH,将离心管置于25 ℃和200 r·min−1条件下的恒温振荡器中,震荡反应一定时间后,静置1 h后用0.22 μm聚丙烯微孔滤膜过滤,过滤后取1 mL滤液测定其中PFOS的含量,计算m-DWCNTs对PFOS的吸附量qe。分别研究吸附时间、初始浓度、吸附剂投加量和pH对m-DWCNTs吸收PFOS的影响。实验条件分别为:(1)设定吸附时间为1、2、4、8、12、24、36、48、60、72、96 h,其他实验条件同上述;(2)设定初始浓度为1、5、10、20、50、100、200、400 μg·L−1,其他实验条件同上述;(3)设定吸附剂投加量为1.5、5、10、15、20、40 mg,其他实验条件同上述;(4)设定pH值为2.0、3.0、4.0、5.0、6.0、7.0、8.0、9.0,其他条件同上。所有实验重复3次,取其平均值进行分析.
1.5 数据处理
(1)PFOS的去除率和吸附量
根据公式(1)计算其去除率η(%),公式(2)计算其吸附量
qe( ) 去除率:η=(C0−Ce)C0×100\% (1) 吸附量:qe=(C0−Ce)×Vm (2) 式中,
C0 Ce (2)吸附动力学和等温线模型
吸附动力学研究吸附过程中吸附量随时间变化的趋势,是吸附行为研究的重要组成部分。分别用准一级动力学模型、准二级吸附动力学模型、颗粒内扩散模型和Elovich模型就m-DWCNTs对PFOS的吸附进行拟合。计算方程如公式3—6所示:
准一级动力学模型:qt=qe(1−e−k1t) (3) 准二级动力学模型:tqt=1(K2q2e)+tqe (4) 扩散模型:qt=Kdt1/2+C (5) Elovich模型:qt=1blnab+1blnt (6) 式中,K1-反应速率常数,h−1;t-时间,h;qe-吸附平衡时吸附剂对吸附质的吸附量,mg·g−1;qt-t时刻吸附剂对吸附质的吸附量,mg·g−1;K2-反应速率常数,g·mg−1·h−1;Kd-粒子内扩散速率常数,mg·g−1·h1/2;C-与边界层厚度有关的常数,mg·g−1;a-表示初始吸附速率,g·mg−1·h−1;b-解析常数,g·mg−1。
吸附等温线是在一定温度条件下,用来描述达到吸附平衡时的浓度和吸附量之间函数关系的曲线,目前常用的吸附等温线模型有Langmuir 模型和Freundlich模型(公式7、8):
Langmuir模型:qe=KLqmCe1+KLCe (7) Freundlich模型:qe=KFC1/ne (8) 式中,KL—Langmuir 常数,L·mg−1;Ce-吸附平衡时吸附质的浓度,mg·L−1;qe-吸附平衡时吸附剂的吸附量,mg·g−1;qm-吸附剂的最大吸附量,mg·g−1; 1/n-不均匀性因数;KF-Freundlich 常数,
(mg⋅g−1)⋅(L⋅mg−1)1/n (3)数据分析
实验数据经Office 2019处理后,使用Origin 9.0、Prism 8.0 进行模型拟合和绘图。
2. 结果和讨论(Results and discussion)
2.1 吸附剂的表征分析
图1为纯化后DWCNTs(p-DWCNTs)和磁化后DWCNTs(m-DWCNTs)的SEM扫描电镜图。由图可知,p-DWCNTs呈相互缠绕的管状结构,表面相对比较光滑,无明显附着物。而经铁修饰后的m-DWCNTs管状结构没有发生变化,但管状结构上附着大量粒径很小的颗粒,表面明显粗糙,初步可判断磁性粒子已经成功负载在p-DWCNTs的外壁上。
图2是p-DWCNTs(a)和m-DWCNTs(b)的傅里叶红外光谱图。由图2可知,p-DWCNTs在3441、2928、2845、1621、1149 cm−1都有特征峰。1621 cm−1处的振动峰是芳香族C=C的伸缩振动所引起的,表明了碳纳米管石墨结构的存在[28]。2845 cm−1和2928 cm−1处为饱和烃类C—H的伸缩振动特征峰,1149 cm−1为C—H的弯曲振动峰。同时可以看出在1791 cm−1的吸收峰,对应的是C=O的伸缩振动峰,说明在p-DWCNTs和m-DWCNTs表面上都存在羧基基团。与p-DWCNTs相比,磁化后的DWCNTs在568 cm−1附近增加了Fe—O键的伸缩吸收峰,表明有可能是铁氧化物包覆在碳纳米管的表面上。Zhao等[29]指出,与原碳纳米管相比,由于存在磁性氧化材料,磁化后的碳纳米管的表面可能部分被磁性氧化物覆盖,磁性氧化物显著影响碳纳米管的表面性质。
图3是p-DWCNTs和m-DWCNTs的X射线衍射(XRD)结果。p-DWCNTs的特征衍射峰位于2θ=26.46°和42.44°,对应了石墨的(002)和(100)晶面,表明碳纳米管中存在C-C结构,而p-DWCNTs经过磁化后,石墨(100)晶面对应的衍射峰消失或者强度变得非常弱,(002)晶面对应的特征峰相对于p-DWCNTs也降低了很多。m-DWCNTs对应曲线的特征衍射峰和和Fe3O4的衍射峰一致,2θ=30.065°、35.412°、43.037°、53.389°、56.912°、62.495°和73.928°分别对应于Fe3O4的晶面(220)、(311)、(400)、(422)、(511)、(440)和(533),说明磁性铁氧化物已经成功负载在DWCNTs上。
m-DWCNTs的磁滞回线如图4所示,可以看出m-DWCNTs的矫顽力和剩磁力均为0,说明m-DWCNTs具有超顺磁性,意味着m-DWCNTs复合材料吸附后能够有效分离以及再利用。对m-DWCNTs的表征结果显示磁性纳米颗粒(Fe3O4)已经成功地包覆在碳纳米管的表面。另外,由图4中的实物可以看出,左边m-DWCNTs能够很好地被磁铁吸引,而右边的p-DWCNTs则悬浮分散在水环境中,这表明经过Fe3O4修饰后的m-DWCNTs在外加磁场作用下具有良好的磁分离效果,可以快速实现固液分离。Zhao等[29]通过用MnCl2和FeCl3制成的磁性碳纳米管的饱和磁化强度仅为10.8 emu·g−1;Xue等[30]用Cu2+、Mn2+、 Ni2+和Zn2+分别与Fe3+混和制备的磁性碳纳米管饱和磁化强度最高的仅为0.65 emu·g−1;而本实验中,室温下m-DWCNTs的饱和磁化强度为66.07 emu·g−1(图4),比Zhao和Xue等[29-30]所制得的磁性碳纳米管的磁性较强,更易实现固液快速分离。
2.2 吸附实验结果
2.2.1 吸附时间的影响
图5为磁化前后DWCNTs对PFOS吸附量随时间的变化情况,磁化后m-DWCNTs对PFOS的吸附量比未磁化的p-DWCNTs吸附量大,吸附性能显著提升,吸附量从0.28 mg·g−1增长至0.65 mg·g−1,去除率从40.36%增长至85.14%。
图6显示了接触时间对m-DWCNTs吸附PFOS的影响。PFOS在m-DWCNTs的吸附过程可分为2个阶段,0—48 h内PFOS吸附量随着吸附时间的延长而快速上升,吸附率最快;随后在48—96 h基本维持在平衡状态,PFOS浓度没有明显变化,吸附量基本维持在0.65 mg·g−1。这是因为在前48小时内,m-DWCNTs表面上拥有大量空余的吸附位点,可以迅速地与溶液中PFOS分子相结合;在48 h之后可供PFOS吸附的位点数大大减少,导致后期吸附速率降低。
为了进一步探讨PFOS在m-DWCNT上的吸附机理,对实验数据进行拟合,拟合参数见表1。结果表明,准一级动力学和准二级动力学与实验数据都吻合得比较好,但准二级动力学模型具有更高的相关系数R2>0.98,并且拟二级动力学得到的qe值更加接近于实验结果。准二级动力学吸附模型是基于化学吸附的假设,所以可以推断出该吸附过程可能存在化学吸附[31-32]。
表 1 吸附动力学模型拟合参数Table 1. Fitting parameters of adsorption kinetic model模型Model 拟合参数Fitting parameters 数值Value 准一级Pseudo-first-order kinetic K1 0.0586 qe/(mg·g−1) 0.6538 R2 0.9547 准二级Pseudo-second-order kinetic K2/(g·mg−1·h) 0.1149 qe/(mg·g−1) 0.6521 R2 0.9814 扩散拟合 Diffusion fiiting 第一阶段The first stage Kd/(g·g−1·h−1/2) 0.0861 C/(mg·g−1) 0.0254 R2 0.9834 第二阶段The second stage Kd/(g·g−1·h−1/2) 1.6056e−4 C/(mg·g−1) 0.6510 R2 0.7851 Elovich 拟合Elovich fitting a/(g·mg−1·h−1) 1.3458 b/(g·mg−1) 3.6496 R2 0.9456 由表1的扩散模型拟合可知,第一阶段R2>0.95,大于第二阶段的R2。表明在初始扩散阶段模型能够较好地描述m-DWCNTs对PFOS的吸附过程,在第一阶段,PFOS通过颗粒外部扩散过程传输到吸附剂的外表面,之后是渐进的吸附过程,PFOS扩散到吸附剂的表面孔内[33-34];随着吸附过程进入第二阶段,由于PFOS含量变低,颗粒内扩散开始减缓。因此,颗粒外部扩散和粒子内扩散同时影响m-DWCNTs对PFOS的吸附。而Elovich模型的R2<0.95与其他模型相比较小,说明Elovich模型相比之下无法很好地描述m-DWCNTs吸附PFOS的动力学行为[35]。
2.2.2 初始浓度的影响
m-DWCNTs对PFOS的吸附等温线如图7所示。可以看出,随着平衡浓度的增加,吸附量qe先呈上升趋势然后逐渐趋于平缓。当平衡浓度为3.92—298.32 μg·L−1时,吸附量从0.04 mg·g−1增长到1.26 mg·g−1;当平衡浓度为560.94—1494.54 μg·L−1时,吸附量达到平衡,无显著变化,随着PFOS的浓度增大,m-DWCNTs对PFOS的吸附量趋于平衡。
为进一步研究m-DWCNTs对PFOS的吸附机理,分别采用Langmuir和Freundlich等温吸附模型对等温吸附试验数据进行拟合。Langmuir模型适用于吸附材料对吸附质的单分子层吸附,而Freundlich模型适用于吸附材料对吸附质的多分子层吸附。拟合参数如表2所示,因为Langmuir模型的线性拟合参数R2大于Freundlich模型的线性拟合参数,R2>0.9,表明m-DWCNTs对PFOS的吸附更符合Langmuir模型。由Freundlich模型拟合参数n=2.8879>1,KF和n值都为正值,表明对PFOS的吸附过程为优惠吸附,吸附性能较好[36]。再由Langmuir拟合模型可知m-DWCNTs对PFOS有可能以单分子层吸附为主,吸附发生在吸附剂表层内的特定均匀位置。最大吸附量高达1.54 mg·g−1。
表 2 Langmuir和Freundlich模型拟合参数Table 2. Parameters of Langmuir and Freundlich models模型Model 拟合参数Fitting parameters 数值Value Langmuir模型 Langmuir fitting KL/(L·mg−1) 0.0077 qm/(mg·g-1) 1.5442 R2 0.9669 Freundlich模型Freundlich fitting KF/(L·mg−1) 0.1322 n 2.8879 R2 0.8346 2.2.3 吸附剂投加量的影响
吸附剂的用量对m-DWCNTS吸附PFOS也有一定的影响,并非吸附剂用量越多越好,吸附剂用量越多可能会造成吸附剂表面吸附位点剩余,而吸附剂用量较少时可能导致吸附不完全。吸附剂用量对m-DWCNTS吸附PFOS的影响如图8所示。从图8可知,当m-DWCNTs的用量从1.5 mg增加到40 mg时,去除率从22.67%增长至84.36%,吸附量从1.80 mg·g−1降低至0.24 mg·g−1。吸附量
qe 2.2.4 初始pH的影响
pH是控制m-DWCNTs吸附PFOS过程的重要参数之一。图9表明,pH值从2.0增至9.0,吸附量qe逐渐降低,从0.35 mg·g−1降至0.07 mg·g−1。表明静电相互作用有可能参与了PFOS在m-DWCNTs上的吸附。由于m-DWCNTs在所研究的pH范围内带负电,又因为PFOS的pKa较低(-3.27),PFOS在溶液中也以阴离子的形式存在,因此m-DWCNTs和PFOS的阴离子产生静电排斥作用。在低pH值下,m-DWCNTs表面由于质子化而带正电荷,与带着负电荷的PFOS产生了静电吸引[38]。随着pH值的升高,质子化作用减弱,m-DWCNTs表面上的负电荷也逐渐增多,静电斥力阻止了PFOS接近m-DWCNTs表面,导致了吸附量的逐渐降低。研究发现,氧化铝[39]、活性炭[40]、壳聚糖[41-42]等材料对PFOS的吸附都涉及静电作用;钟伟民等[43]发现,磁性分子印迹材料对PFOS的吸附主要为静电作用;郭盼盼等[44]也认为磁性壳聚糖对PFOS的吸附存在静电作用;Stebel等[45]发现可膨胀有机改性二氧化硅对PFOS的吸附主要为静电作用和离子交换作用;Cao等[46]发现基于碳纳米管的分子印迹聚合物对PFOS的吸附作用主要为静电作用和氢键作用。今后可对m-DWCNTs对PFOS的吸附机理开展进一步研究,例如对吸附前后的m-DWCNTs进行表征,对其所含元素、官能团及化学键等的变化进行分析等。
3. 结论(Conclusion)
磁性DWCNTs对PFOS具有良好的吸附性能,从对m-DWCNTs的表征和不同实验条件下磁性DWCNTs吸附PFOS的结果可以得出以下结论:
(1) 纯化后的DWCNTs经过磁化后,改善了DWCNTs的表面性质。m-DWCNTs的表征观察证明了Fe3O4成功负载在DWCNTs上,并且具有超顺磁性,饱和磁化强度为66.07 emu·g−1,便于从水环境中快速分离。
(2) 用准二级动力学方程和Langmuir模型能较好地拟合PFOS在m-DWCNTs上的吸附过程,说明m-DWCNTs对PFOS的吸附可能是以化学吸附和单分子层吸附为主。
(3) 磁性DWCNTs吸附PFOS的去除率随着吸附剂用量的增加而增加,但吸附量随着吸附剂用量的增加而减少,当吸附剂投加量增加到40 mg,去除率可达84.36%。
(4) 磁性DWCNTs吸附PFOS的吸附机理与静电作用相关,在低pH值下,静电吸引作用有利于m-DWCNTs对PFOS的吸附,在高pH值下,静电排斥不利于PFOS在m-DWCNTs上的吸附。
-
表 1 吸附动力学模型拟合参数
Table 1. Fitting parameters of adsorption kinetic model
模型Model 拟合参数Fitting parameters 数值Value 准一级Pseudo-first-order kinetic K1 0.0586 qe/(mg·g−1) 0.6538 R2 0.9547 准二级Pseudo-second-order kinetic K2/(g·mg−1·h) 0.1149 qe/(mg·g−1) 0.6521 R2 0.9814 扩散拟合 Diffusion fiiting 第一阶段The first stage Kd/(g·g−1·h−1/2) 0.0861 C/(mg·g−1) 0.0254 R2 0.9834 第二阶段The second stage Kd/(g·g−1·h−1/2) 1.6056e−4 C/(mg·g−1) 0.6510 R2 0.7851 Elovich 拟合Elovich fitting a/(g·mg−1·h−1) 1.3458 b/(g·mg−1) 3.6496 R2 0.9456 表 2 Langmuir和Freundlich模型拟合参数
Table 2. Parameters of Langmuir and Freundlich models
模型Model 拟合参数Fitting parameters 数值Value Langmuir模型 Langmuir fitting KL/(L·mg−1) 0.0077 qm/(mg·g-1) 1.5442 R2 0.9669 Freundlich模型Freundlich fitting KF/(L·mg−1) 0.1322 n 2.8879 R2 0.8346 -
[1] 王亚韡, 蔡亚岐, 江桂斌. 斯德哥尔摩公约新增持久性有机污染物的一些研究进展 [J]. 中国科学:化学, 2010, 40(2): 99-123. doi: 10.1360/zb2010-40-2-99 WANG Y W, CAI Y Q, JIANG G B. Research processes of persistent organic pollutants (POPs) newly listed and candidate POPs in Stockholm Convention [J]. Scientia Sinica (Chimica), 2010, 40(2): 99-123(in Chinese). doi: 10.1360/zb2010-40-2-99
[2] CORDNER A, de la ROSA V Y, SCHAIDER L A, et al. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors [J]. Journal of Exposure Science & Environmental Epidemiology, 2019, 29(2): 157-171. [3] SHARMA B M, BHARAT G K, TAYAL S, et al. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure [J]. Environmental Pollution, 2016, 208: 704-713. doi: 10.1016/j.envpol.2015.10.050 [4] MCNAMARA J D, FRANCO R, MIMNA R, et al. Comparison of activated carbons for removal of perfluorinated compounds from drinking water [J]. Journal - American Water Works Association, 2018, 110(1): E2-E14. doi: 10.5942/jawwa.2018.110.0003 [5] 洪雷, 丁倩云, 亓祥坤, 等. 吸附法去除水中全氟化合物的研究进展 [J]. 环境化学, 2021, 40(7): 2193-2203. doi: 10.7524/j.issn.0254-6108.2020031303 HONG L, DING Q Y, QI X K, et al. The research progress of removing perfluoroalkyl substances by adsorption in water [J]. Environmental Chemistry, 2021, 40(7): 2193-2203(in Chinese). doi: 10.7524/j.issn.0254-6108.2020031303
[6] KUCHARZYK K H, DARLINGTON R, BENOTTI M, et al. Novel treatment technologies for PFAS compounds: A critical review [J]. Journal of Environmental Management, 2017, 204: 757-764. doi: 10.1016/j.jenvman.2017.08.016 [7] WANG Q, SONG H J, WANG Q M. Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation[J]. Chinese Chemical Letters, 2022, 33(2): 626-642. [8] SCHURICHT F, BOROVINSKAYA E S, RESCHETILOWSKI W. Removal of perfluorinated surfactants from wastewater by adsorption and ion exchange—Influence of material properties, sorption mechanism and modeling [J]. Journal of Environmental Sciences, 2017, 54: 160-170. doi: 10.1016/j.jes.2016.06.011 [9] ZENG C, ATKINSON A, SHARMA N, et al. Removing per- and polyfluoroalkyl substances from groundwaters using activated carbon and ion exchange resin packed columns [J]. AWWA Water Science, 2020, 2(1): 1172. doi: 10.1002/aws2.1172 [10] WANG W, MI X, SHI H L, et al. Adsorption behaviour and mechanism of the PFOS substitute OBS (sodium p -perfluorous nonenoxybenzene sulfonate) on activated carbon [J]. Royal Society Open Science, 2019, 6(9): 191069. doi: 10.1098/rsos.191069 [11] ZHONG W M, WANG Z P, ZHOU Y, et al. Study on the magnetic molecularly imprinted materials for removing the perfluorooctane sulfonate (PFOS) in water[J]. Advanced Materials Research, 2013, 864/865/866/867: 706-709. [12] GAO Y X, DENG S B, DU Z W, et al. Adsorptive removal of emerging polyfluoroalky substances F-53B and PFOS by anion-exchange resin: A comparative study [J]. Journal of Hazardous Materials, 2017, 323: 550-557. doi: 10.1016/j.jhazmat.2016.04.069 [13] DIEL J C, FRANCO D S P, IGANSI A V, et al. Green synthesis of carbon nanotubes impregnated with metallic nanoparticles: Characterization and application in glyphosate adsorption [J]. Chemosphere, 2021, 283: 131193. doi: 10.1016/j.chemosphere.2021.131193 [14] REN X M, CHEN C L, NAGATSU M, et al. Carbon nanotubes as adsorbents in environmental pollution management: A review [J]. Chemical Engineering Journal, 2011, 170(2/3): 395-410. [15] YANG S B, HU J, CHEN C L, et al. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions [J]. Environmental Science & Technology, 2011, 45(8): 3621-3627. [16] CHUDOBA D, ŁUDZIK K, JAŻDŻEWSKA M, et al. Kinetic and equilibrium studies of doxorubicin adsorption onto carbon nanotubes [J]. International Journal of Molecular Sciences, 2020, 21(21): 8230. doi: 10.3390/ijms21218230 [17] ZHANG J L, ZHAI J R, ZHENG H, et al. Adsorption, desorption and coadsorption behaviors of sulfamerazine, Pb(II) and benzoic acid on carbon nanotubes and nano-silica [J]. Science of the Total Environment, 2020, 738: 139685. doi: 10.1016/j.scitotenv.2020.139685 [18] PAN J M, ZOU X H, WANG X, et al. Adsorptive removal of 2, 4-didichlorophenol and 2, 6-didichlorophenol from aqueous solution by β-cyclodextrin/attapulgite composites: Equilibrium, kinetics and thermodynamics [J]. Chemical Engineering Journal, 2011, 166(1): 40-48. doi: 10.1016/j.cej.2010.09.067 [19] GUPTA V K, AGARWAL S, SALEH T A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes [J]. Water Research, 2011, 45(6): 2207-2212. doi: 10.1016/j.watres.2011.01.012 [20] SALEH T A, GUPTA V K. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide [J]. Journal of Colloid and Interface Science, 2012, 371(1): 101-106. doi: 10.1016/j.jcis.2011.12.038 [21] ZHOU Y P, WEN B, PEI Z G, et al. Coadsorption of copper and perfluorooctane sulfonate onto multi-walled carbon nanotubes [J]. Chemical Engineering Journal, 2012, 203: 148-157. doi: 10.1016/j.cej.2012.06.156 [22] LI X N, CHEN S, QUAN X, et al. Enhanced adsorption of PFOA and PFOS on multiwalled carbon nanotubes under electrochemical assistance [J]. Environmental Science & Technology, 2011, 45(19): 8498-8505. [23] LI X N, ZHAO H M, QUAN X, et al. Adsorption of ionizable organic contaminants on multi-walled carbon nanotubes with different oxygen contents [J]. Journal of Hazardous Materials, 2011, 186(1): 407-415. doi: 10.1016/j.jhazmat.2010.11.012 [24] 田红灯. 单壁与双壁碳纳米管的物理特性研究[D]. 无锡: 江南大学, 2009. TIAN H D. Research on physical properties of single-walled carbon nanotubes and double-walled carbon nanotubes[D]. Wuxi, China: Jiangnan University, 2009(in Chinese).
[25] FEDOSEEVA Y V, BULUSHEVA L G, KOROTEEV V O, et al. Preferred attachment of fluorine near oxygen-containing groups on the surface of double-walled carbon nanotubes [J]. Applied Surface Science, 2020, 504: 144357. doi: 10.1016/j.apsusc.2019.144357 [26] CHEN X, XIA X H, WANG X L, et al. A comparative study on sorption of perfluorooctane sulfonate (PFOS) by chars, ash and carbon nanotubes [J]. Chemosphere, 2011, 83(10): 1313-1319. doi: 10.1016/j.chemosphere.2011.04.018 [27] 熊振湖, 王璐, 周建国, 等. 磁性多壁碳纳米管吸附水中双氯芬酸的热力学与动力学 [J]. 物理化学学报, 2010, 26(11): 2890-2898. doi: 10.3866/PKU.WHXB20101130 XIONG Z H, WANG L, ZHOU J G, et al. Thermodynamics and kinetics of adsorption of diclofenac on magnetic multiwalled carbon nanotubes in an aqueous solution [J]. Acta Physico-Chimica Sinica, 2010, 26(11): 2890-2898(in Chinese). doi: 10.3866/PKU.WHXB20101130
[28] AMEN M T, BARAKAT N A M, JAMAL M A H M, et al. Anolyte in situ functionalized carbon nanotubes electrons transport network as novel strategy for enhanced performance microbial fuel cells [J]. Applied Energy, 2018, 228: 167-175. doi: 10.1016/j.apenergy.2018.06.035 [29] ZHAO W G, TIAN Y M, CHU X X, et al. Preparation and characteristics of a magnetic carbon nanotube adsorbent: Its efficient adsorption and recoverable performances [J]. Separation and Purification Technology, 2021, 257: 117917. doi: 10.1016/j.seppur.2020.117917 [30] ZHANG X, CUI C Y, WANG Y, et al. An efficient method for removal of pentachlorophenol using adsorption and microwave regeneration with different magnetic carbon nanotubes [J]. Water Science and Technology, 2020, 81(3): 585-595. doi: 10.2166/wst.2020.146 [31] 卢丽娟, 唐敏康, 陈瑛, 等. Fe2+负载颗粒活性炭去除水中全氟辛酸研究 [J]. 应用化工, 2017, 46(4): 614-619. LU L J, TANG M K, CHEN Y, et al. Removal of PFOA from aqueous solutions using Fe2+ salts supported on activated carbon [J]. Applied Chemical Industry, 2017, 46(4): 614-619(in Chinese).
[32] MALIMA N M, OWONUBI S J, LUGWISHA E H, et al. Thermodynamic, isothermal and kinetic studies of heavy metals adsorption by chemically modified Tanzanian Malangali Kaolin clay [J]. International Journal of Environmental Science and Technology, 2021, 18(10): 3153-3168. doi: 10.1007/s13762-020-03078-0 [33] CHEN G C, SHAN X Q, ZHOU Y Q, et al. Adsorption kinetics, isotherms and thermodynamics of atrazine on surface oxidized multiwalled carbon nanotubes [J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 912-918. [34] SHAHTALEBI A, SARRAFZADEH M H, MCKAY G. An adsorption diffusion model for removal of copper (Ⅱ) from aqueous solution by pyrolytic tyre char [J]. Desalination and Water Treatment, 2013, 51(28/29/30): 5664-5673. [35] ZHANG J H, ZHANG M, BAI B Q, et al. Studies on adsorption kinetics and thermodynamics of macroporous resin for rosmarinic acid [J]. Journal of Oleo Science, 2021, 70(3): 439-451. doi: 10.5650/jos.ess20305 [36] 杨海君, 邓蓉蓉, 易勇, 等. 加拿大一枝黄花茎秆生物炭的制备及其对吡啶的吸附 [J]. 环境化学, 2021, 40(6): 1922-1932. doi: 10.7524/j.issn.0254-6108.2020012701 YANG H J, DENG R R, YI Y, et al. Preparation of biochar from Solidago canadensis L. stalk and its pyridine adsorption performance [J]. Environmental Chemistry, 2021, 40(6): 1922-1932(in Chinese). doi: 10.7524/j.issn.0254-6108.2020012701
[37] FAGBAYIGBO B O, OPEOLU B O, FATOKI O S, et al. Removal of PFOA and PFOS from aqueous solutions using activated carbon produced from Vitis vinifera leaf litter [J]. Environmental Science and Pollution Research, 2017, 24(14): 13107-13120. doi: 10.1007/s11356-017-8912-x [38] BEI Y, DENG S B, DU Z W, et al. Adsorption of perfluorooctane sulfonate on carbon nanotubes: Influence of pH and competitive ions [J]. Water Science and Technology, 2014, 69(7): 1489-1495. doi: 10.2166/wst.2014.049 [39] WANG F, SHIH K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations [J]. Water Research, 2011, 45(9): 2925-2930. doi: 10.1016/j.watres.2011.03.007 [40] YU Q, ZHANG R Q, DENG S B, et al. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study [J]. Water Research, 2009, 43(4): 1150-1158. doi: 10.1016/j.watres.2008.12.001 [41] GUO P P, WANG Z P, ZHOU Y, et al. Study on the magnetic chitosan microspheres for removing the perfluorooctane sulfonate (PFOS) in water [J]. Advanced Materials Research, 2014, 1030/1031/1032: 326-329. [42] ZHANG Q Y, DENG S B, YU G, et al. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism [J]. Bioresource Technology, 2011, 102(3): 2265-2271. doi: 10.1016/j.biortech.2010.10.040 [43] 钟伟民. 磁性分子印迹材料对水中全氟辛烷磺酸的吸附研究[D]. 南京: 南京理工大学, 2014. ZHONG W M. Study on the magnetic molecularly imprinted materials for removing the perfluorooctane sulfonate (PFOS) in water[D]. Nanjing: Nanjing University of Science and Technology, 2014(in Chinese).
[44] 郭盼盼. 磁性壳聚糖的制备及其对水中全氟辛烷磺酸的吸附研究[D]. 南京: 南京理工大学, 2015. GUO P P. Preparation of magnetic chitosan and study on the adsorption of perfluorooctane sulfonate in water[D]. Nanjing: Nanjing University of Science and Technology, 2015(in Chinese).
[45] STEBEL E K, PIKE K A, NGUYEN H, et al. Absorption of short-chain to long-chain perfluoroalkyl substances using swellable organically modified silica [J]. Environmental Science:Water Research & Technology, 2019, 5(11): 1854-1866. [46] CAO F M, WANG L, YAO Y M, et al. Synthesis and application of a highly selective molecularly imprinted adsorbent based on multi-walled carbon nanotubes for selective removal of perfluorooctanoic acid [J]. Environmental Science:Water Research & Technology, 2018, 4(5): 689-700. 期刊类型引用(3)
1. 张新庄,马天奇,王丹丹. 碳纳米管磁改性及其强化含油污泥吸附降解技术进展. 炭素技术. 2025(01): 7-12 .  百度学术
百度学术
2. 石旻飞,黄珺,张瑞斌. 固定化菌藻填料强化人工湿地去除营养盐和PFOS效果研究. 安全与环境学报. 2024(01): 361-369 .  百度学术
百度学术
3. 周淑红,张博文,卫嘉华,王雪,彭其安,蔡亚君. 铁改性生物炭对几种染料的吸附机理研究. 环境化学. 2023(11): 3928-3939 .  本站查看
本站查看
其他类型引用(3)
-













 下载:
下载: