-
近年来,我国秋冬季节频繁发生以PM2.5为主的细颗粒物污染. 根据《2020年中国环境状况公报》显示,在我国337个城市中,以PM2.5为首要污染物的超标天数占总超标天数的51%,PM2.5依然是我国最重要的大气污染物[1]. 我国的细颗粒物污染存在区域性污染的特点,常发生在工业化程度高的区域,如京津冀及周边地区、长三角和汾渭平原. 其次,在复杂的大气环境中,多种污染物之间的协同作用加强了大气氧化能力,使得颗粒物表面的非均相反应频繁发生,导致二次颗粒物的大量爆发,进一步加重了细颗粒污染的程度,形成了典型的复合污染特征[2-3]. 细颗粒物污染给人类健康和地球环境带来了诸多负面影响,如呼吸道疾病、心血管疾病、农业减产和影响地球循环等[4]. 因此,对细颗粒物进行成因分析对于控制和治理颗粒物污染至关重要.
细颗粒物的形成机制受其组分种类和混合态的影响,目前根据各类质谱和色谱技术检测出的细颗粒物组分主要包括以下9种:矿物颗粒、海盐、金属、飞灰、地壳元素、无机盐、氯化物、烟灰(soot)和有机物[5-7]. 由于来源的复杂性,单个颗粒物的组分常常以内部混合的形式存在[8],彼此之间发生化学反应,使得各组分的原始特性(尺寸、形状和理化性质)发生改变,从而导致颗粒物整体光学和吸湿性质的差异性[9-10]. 早期研究主要使用各类的全分析技术来分析颗粒物,如气相色谱仪、质谱仪和离子色谱仪等[11]. 全分析技术可以同时分析数百或数千个气溶胶颗粒,得到大气颗粒物的化学组成、浓度和体积等平均信息. 这些结果对于研究其来源成因及其对人体健康和气候变化的影响都十分重要,但无法在微观层面给出单个细颗粒的组成和混合状态信息.
单颗粒分析技术可弥补全分析技术的不足,提供颗粒物微观形貌及组成成分等信息. 现有的单颗粒技术主要分为电子显微镜/X射线光谱法、振动光谱法和质谱法(表1),已有研究者们对各类单颗粒分析技术进行了总结[10, 12-13]. 其中,常用的技术手段包括扫描电镜法(SEM)、透射电镜法(TEM)和拉曼(Raman)光谱法等. 前两种技术在颗粒物形貌的观测上得到了广泛的应用[14-15],但是SEM和TEM的真空操作过程会导致水或半挥发性组分的损失. 而拉曼光谱作为一种无损的灵敏指纹识别光谱技术,规避了以上缺点,逐渐发展成为一种高效可靠的细颗粒物检测手段. 本文介绍了传统拉曼技术的原理,归纳总结了拉曼技术在细颗粒物分析领域的研究进展,并介绍了相关的技术改进,最后对未来的发展方向提出展望,希望能为相关领域的研究提供参考.
-
1928年,印度科学家拉曼首先发现了拉曼散射的存在[16]. 拉曼是一种散射光谱技术,可以提供分子的振动-转动信息. 当被激发光照射时,处于基态或激发态的分子会跃迁到受激虚态,随后释放能量形成散射光. 如果散射光的频率与入射光相同,则发生了瑞利散射;反之则属于拉曼散射,有以下两种情况:散射光频率小于入射光,成为斯托克斯(stokes)线;大于入射光,则成为反斯托克斯(anti-stokes)线(图1).
与其他单颗粒分析技术相比,拉曼技术主要具有以下优点:检测所需样品量少、环境压力条件下操作的无损分析和精准的指纹识别. 使用拉曼光谱可以研究分子的对称性以及分子动力学等问题,其与红外光谱技术互为补充,综合二者可以得到分子结构的完整信息. 目前,拉曼光谱已广泛应用于无机化合物、有机化合物及高分子化合物的定性分析;生物大分子的构象变化及相互作用研究;各种材料(包括纳米材料、生物材料、金刚石)和膜(包括半导体薄膜、生物膜)的分析;矿物组成分析;宝石、文物和公安试样的无损鉴定等方面. 步入21世纪以来,拉曼技术也逐渐在细颗粒物分析领域崭露头角.
-
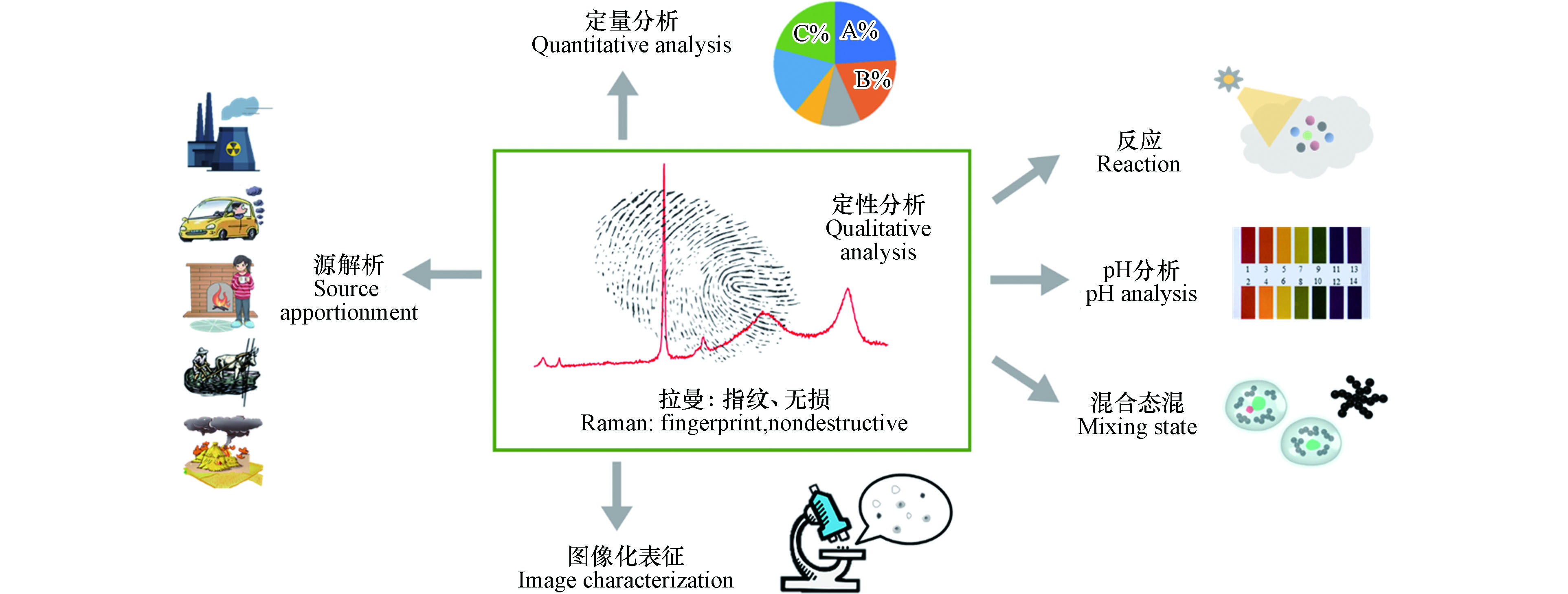
作为一种无损的指纹识别技术,拉曼光谱已经在细颗粒物定性分析、定量分析、图像化表征、混合态分析、源解析、pH分析和非均相反应等领域得到了诸多应用(图2). 在这7项应用中,定性分析是基础,为后续的识别工作提供了光谱参考.
-
当被激光照射时,物质产生的拉曼散射谱线的位移(拉曼峰位)与入射光的波长无关, 只取决于物质的振动转动能级,这就是独一无二的拉曼指纹. 因此,可以通过拉曼峰位分析出分子所含结构,避开其他干扰,实现对样品组成的精准指纹识别与指认.
-
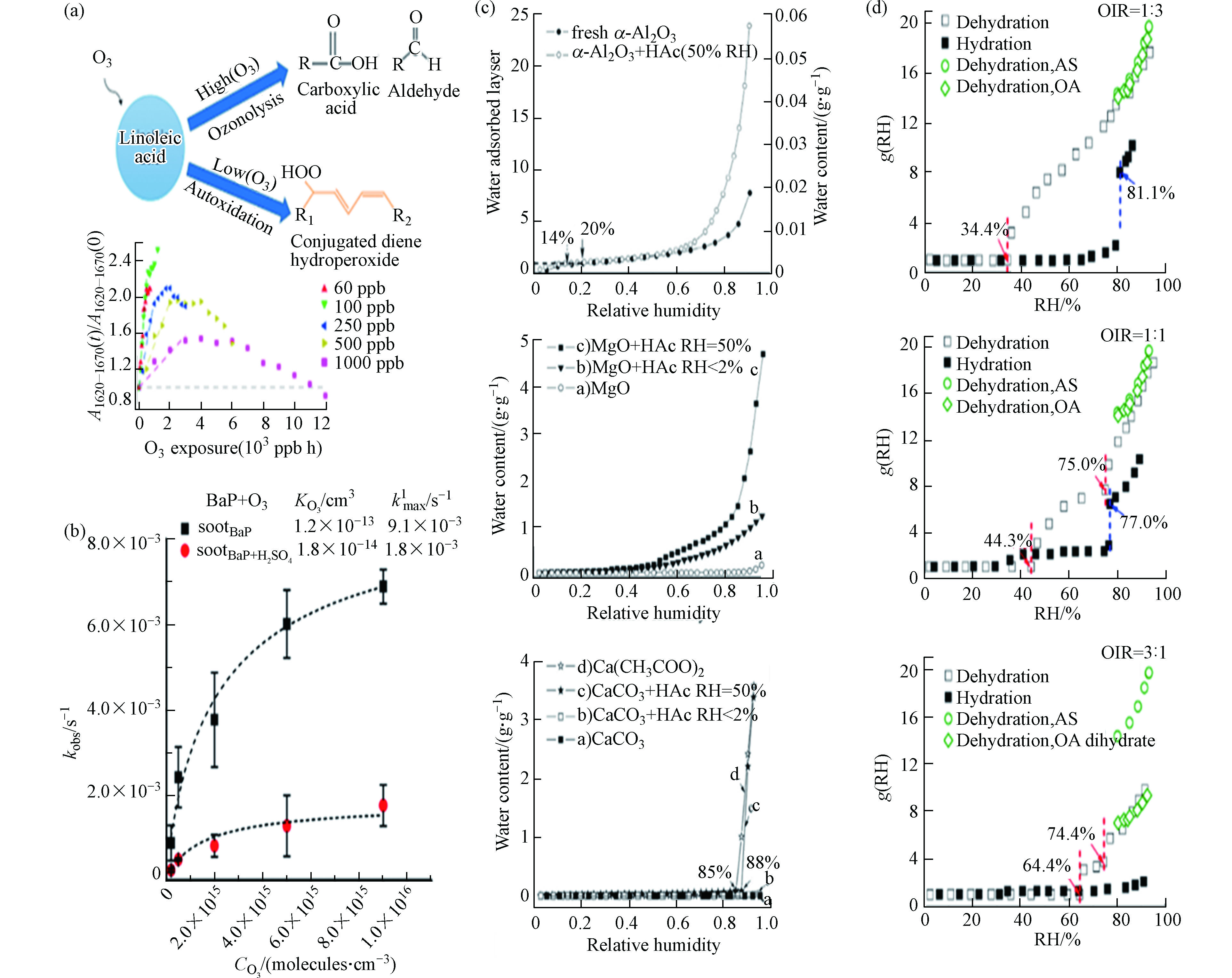
无机盐是细颗粒中的重要组分类型,由其前体物质SO2、NOx和NH3等在大气中二次转化生成,主要包括硫酸盐、硝酸盐和铵盐[17],还有部分的碳酸盐和氯化物[18-20]. 由于亲水特性,二次无机盐密切影响着颗粒的凝结成核及吸湿成长,对细颗粒物的形成具有重要作用.
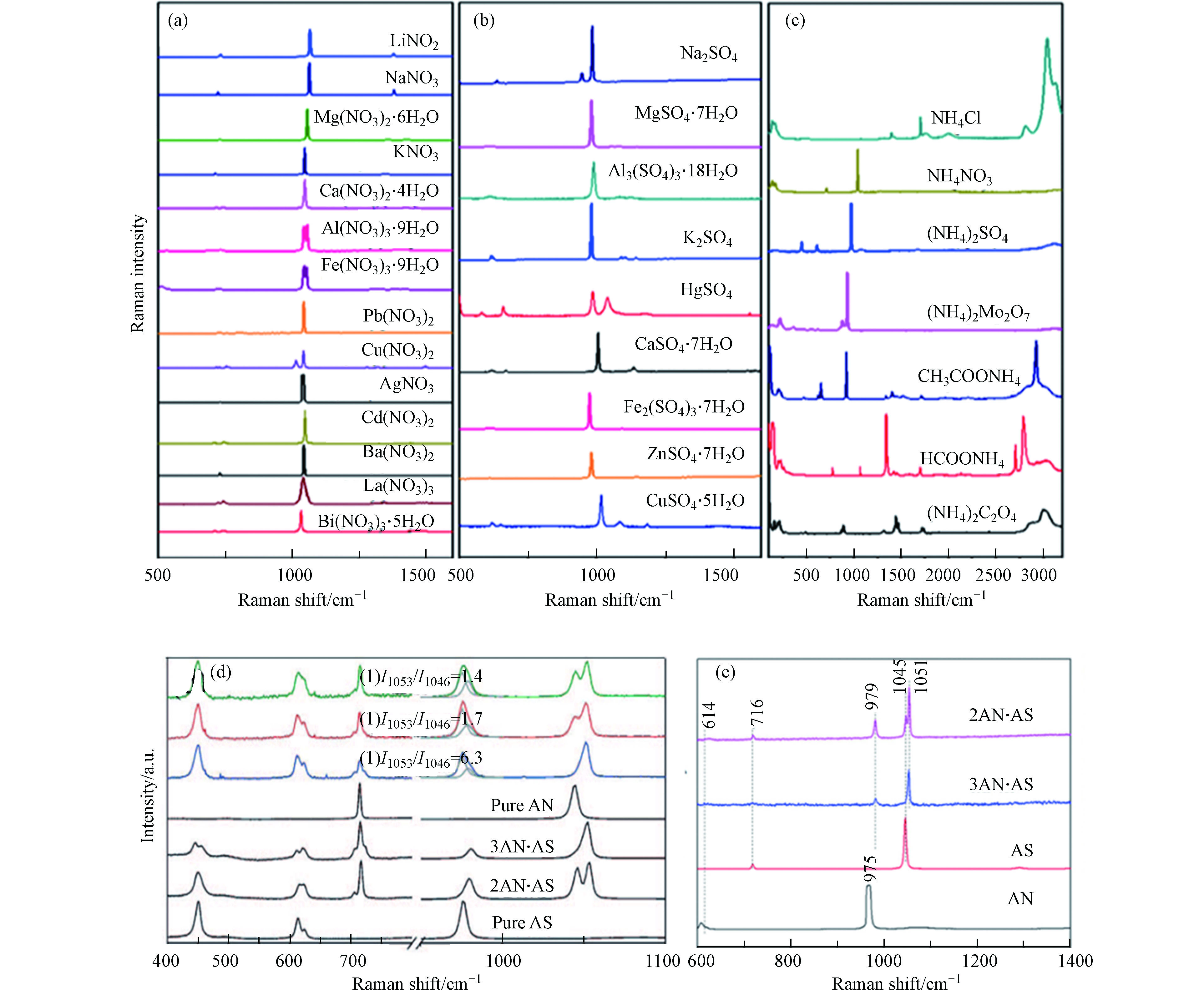
目前已有研究者们对数十种常见无机盐的标准品开展了谱线识别工作[21-23],并对其峰位进行了振动模式的分配(部分常见无机盐的拉曼谱线如图3a-c所示),例如硫酸盐最强峰位于~975 cm−1(归因于S—O的对称伸缩);硝酸盐的最强峰位于~1050 cm−1(归因于N—O的对称伸缩). 而大气颗粒物中无机盐常常是多种类共存的形式,除了简单的组分识别,拉曼技术还可以对无机盐的复合形式进行分析. Ling和Chan[24]开展了(硝酸盐/硫酸盐)过饱和溶液中的单颗粒结晶实验,观察到复盐3(NH4NO3)·(NH4)2SO4(3AN·AS)和2(NH4NO3)·(NH4)2SO4(2AN·AS)的形成(图3d),这是复盐拉曼光谱的首次报道. Feng等[25]对复盐的形成过程开展了进一步的研究.
该团队对硫酸铵和硝酸铵的等摩尔混合物进行了风化过程分析,发现当相对湿度(RH)超过50%时,混合物首先形成3AN·AS,并且在整个风化过程中观察不到2AN·AS的形成. 在实验研究的基础上,Sun等[26]收集了真实雾霾事件的细颗粒物样品,证明了雾霾天里复盐的形成(图3e). 此外,除了常见的铵根离子,无机酸根也常常与各类金属元素组成形成各类混合盐. Jentzsch等[27]根据单一溶液液滴的汽化实验,结合拉曼谱线推测了大气中K2Ca(SO4)2·H2O、(NH4)2Ca(SO4)2·H2O的形成过程,并对其进行了拉曼谱带的分配.
-
作为一类主要的细颗粒物组分,soot来源于生物质、生物燃料和化石燃料的不完全燃烧,其主要包含C和O两种元素,以及因不同来源带来的其他元素,例如S、K、Ca和Si[15]. 由于其光吸收特性,soot在全球变暖和大气光辐射的变化中起着重要作用[28]. 新鲜的soot颗粒由聚集的链状碳球组成,且在高倍率TEM图像中呈现出弯曲的洋葱状石墨层状结构. 随着雾霾污染程度的增加,soot会提供吸附界面并作为将SO2和NOX转化为SNA所需的反应部位,从而使细颗粒形成复杂的内部混合状态[29]. 不同的混合状态、形状和位置(即soot在其主体颗粒中的位置)会进一步影响soot颗粒的理化性质.
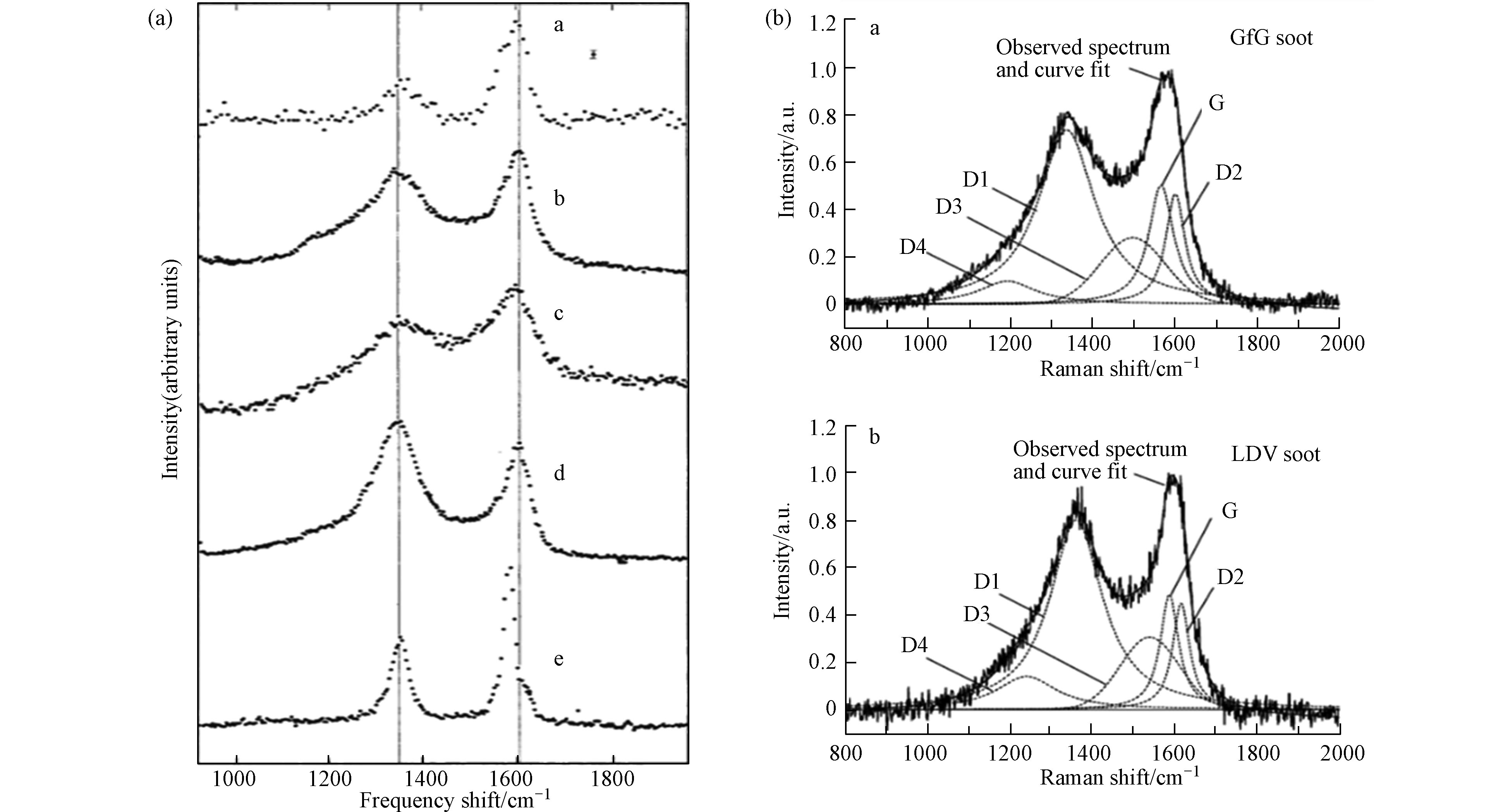
1977年,拉曼首次应用于气溶胶分析领域. Rosen和Novakov[30]使用拉曼技术分析了大气样品、机动车排放尾气、活性炭和多晶石墨的样品,发现这些样品存在两个共同的特征谱带(图4a):~1600 cm−1处的峰位表示有序石墨晶格结构的存在(G峰),~1350 cm−1表示含碳样品的无序缺陷(D峰). 两处峰位强度比值的不同表示了石墨的不同晶型组成,根据这一特点,可以使用拉曼对soot进行结构和特性的分析. 此外,研究者们还使用拉曼技术研究了不同的生长方式和反应条件,如添加的燃料种类[31]、无机盐[32-33]、有机物含量和温度[34]等,对生成或老化的soot的结构、光辐射特性、氧化反应性和相变等变化的影响[35-37].
除了直接根据观察两处特征峰的峰强对soot进行分析,研究者们还结合了数学拟合模型(主要包括Gaussian拟合、Lorentzian拟合和Voigt拟合),对D峰和G峰进行了二段或五段拟合(D1、D2、D3、D4和G)[38],得到了关于结构-峰位更详细的信息:D1频段分配给涉及石墨烯层边缘的振动模式(A1g对称,位于~1360 cm−1);D2波段是G波段旁边的肩峰,归属为表面石墨层中无序碳的E2g伸缩振动模式(位于~1620 cm−1);D3谱带归因于soot的无定形碳sp2、sp3和sp杂化碳原子的混合振动模式(位于~1530 cm−1),而弱的D4谱带则归因于无序石墨晶格(~1180 cm−1)[39].
-
有机物是细颗粒中的常见成分,根据来源的不同可以分为一次有机气溶胶和二次有机气溶胶(SOA). 最近的研究表明,在雾霾天气期间,有机物占细颗粒物含量的50%以上,尤其是在冬季供暖期和东北部寒冷地区[40]. 有机物很容易在颗粒物内部与其他类型的组分混合,从而改变了细颗粒的物理和化学性质. 另外,由于有机物对环境和人类健康的毒性,其受到了研究人员的广泛关注.
已有诸多研究者们对常见有机物的拉曼峰进行了识别[41],并对其特征峰位进行了振动模式的分类. 例如,An等[42]对一次有机物异戊二烯、萜烯、蒎烯及其混合物进行了拉曼/红外光谱的收集和振动识别,致力于建立一个用于有机物识别的拉曼/红外指纹图谱数据库. Ebben等[43]合成了异戊二烯衍生的几类二次有机气溶胶颗粒,并对其进行了表征和颗粒表面分子的拉曼光谱鉴定.
-
金属化合物主要来自于重工业和燃烧活动的排放,其含有丰富的组成,主要包含Zn、Fe和Pb等元素. 金属种类,尤其是重金属,对人类健康有着极大的负面影响,当人体中金属含量超标时,会导致智力发育迟缓、肾脏受损、癌症甚至死亡[44]. 由于种类的丰富性和生物毒性,金属化合物成为了大气颗粒物中重要的组分之一,已有许多研究者们对不同金属种类进行了拉曼识别,并对其理化特征进行了分析.
Avzianova和Brooks[45]通过将可溶的和不可溶的Ni衍生化为[Ni(dmgH)2]络合物,将Ni从颗粒物中分离出来并进行拉曼光谱识别及定量分析,这为单独研究颗粒物中某类组分提供了思路. 另一方面,也可以通过拉曼光谱得到重金属的存在形式信息. 例如,Morillas等[46]使用拉曼和扫描电镜/能谱仪(SEM/EDS),确认了真实环境颗粒物中的重金属元素大多数以氧化物形式存在,但也以不同的聚集形式嵌入颗粒物中,如铝硅酸盐、磷酸盐、卤化物和硫酸盐等. Goienaga等[47]鉴定了大气颗粒中金属的两种存在形式:分子形式(最常见的是硫化物、硫酸盐、碳酸盐或氧化物)和可吸入颗粒物形式(主要是Zn、Pb和Cu等有毒金属),并对其进行了健康风险分析.
-
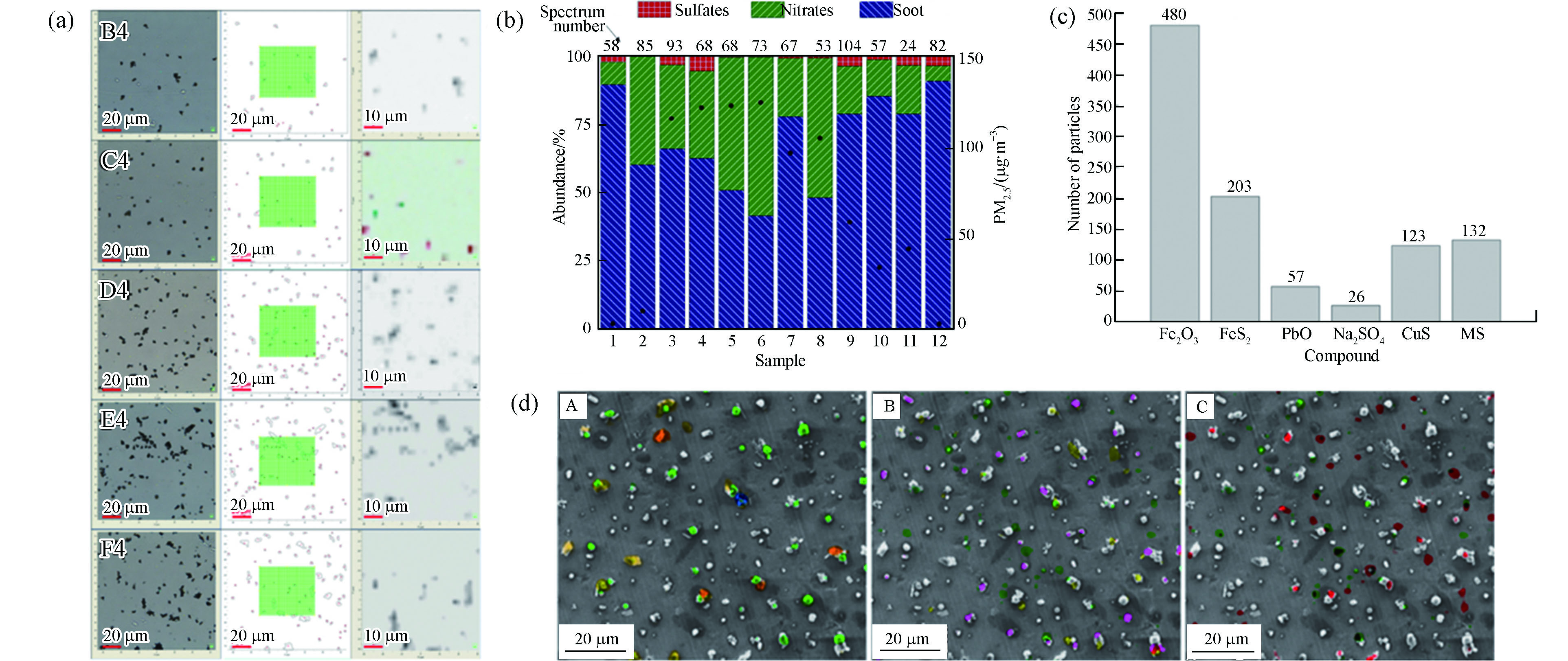
由于污染来源的复杂性,真实大气颗粒物样品通常包含成百上千种不同的组分,得益于指纹识别的特点,除了可以识别单一组分的颗粒物,还能够从复杂组分混合物中鉴别出每一类组分. Ivleva等[48]使用电动低压冲击器收集了空气颗粒物的大小可分辨样品,并鉴定了其中的soot、类腐殖质和无机化合物. Harpale等[49]收集了制糖厂周围的环境颗粒物,直接使用拉曼光谱仪测出了样品中的25处特征峰,并根据标准光谱指认出对应的物质种类.
除了对真实颗粒物进行组分识别,研究者们还通过实验室模拟合成气溶胶,以研究多组分气溶胶的行为特征以及参数对气溶胶生成和拉曼分析的影响. Sinanis等[50]合成了五类不同组成的气相/分散相气溶胶,研究不同气溶胶相态对拉曼检测效果的影响. Jentzsch等[51]设计了NaNO3-KNO3系统,在水的参与下生成了两种化合物的盐混合物,并研究在单个溶液液滴内汽化过程盐混合物的相变和拉曼光谱变化,这也是混合盐Na3(NO3)SO4·H2O首次拉曼光谱的报道.
因此,无论是对于单一种类组分,还是复杂组成的细颗粒物,拉曼技术都表现出了强大的定性分析能力. 并且,在许多研究者的共同努力下,逐渐构建出用于细颗粒物组分分析的拉曼光谱数据库,这为后续的颗粒物识别工作提供了参考和理论支持.
-
获取颗粒物化学组分的定量信息是了解颗粒物理化性质和进行PM2.5源解析的重要前提. 在定性分析的基础上,通过与各类采样手段和数学方法结合,可以实现拉曼对颗粒物的定量分析. Steer等[52]使用商业ZnO样品代表大气气溶胶,结合光谱处理软件对ZnO的3条特征峰进行了拉曼扫描,得到了ZnO在载玻片上的分布(图5a),但该工作只研究了单个组分. 对此,Chen等[53]收集了一次雾霾事件样品,根据光谱指认出了硫酸盐、硝酸盐和soot三类组分,并结合直接经典最小二乘法得到了雾霾过程中的组分占比信息(图5b),这为拉曼多组分的定量分析提供了思路.
在光谱分析的基础上,与化学定量学的联用正成为拉曼定量分析的必要趋势. Siepka等[54]使用层次聚类分析等数学方法对气溶胶样品的拉曼光谱进行分析,得到了各组分分类及占比情况(图5c). 而许多化学定量分析都是建立在图像化表征的基础上的. Ofner等[55]使用基于主成分分析-层次聚类分析的组合方法,通过融合的拉曼和扫描电镜/能量色散X射线(SEM/EDX)高光谱数据立方体,实现了更完整颗粒物组分的成像,随后使用化学计量法提取和确定了7类组分种类,并通过K值聚类找到和验证了其中6类组分及其浓度分布(图5d).
综上所述,虽然拉曼技术在定性分析领域得到了广泛的应用,但其定量分析尚处在起步阶段,未来需要对这方面开展进一步的研究.
-
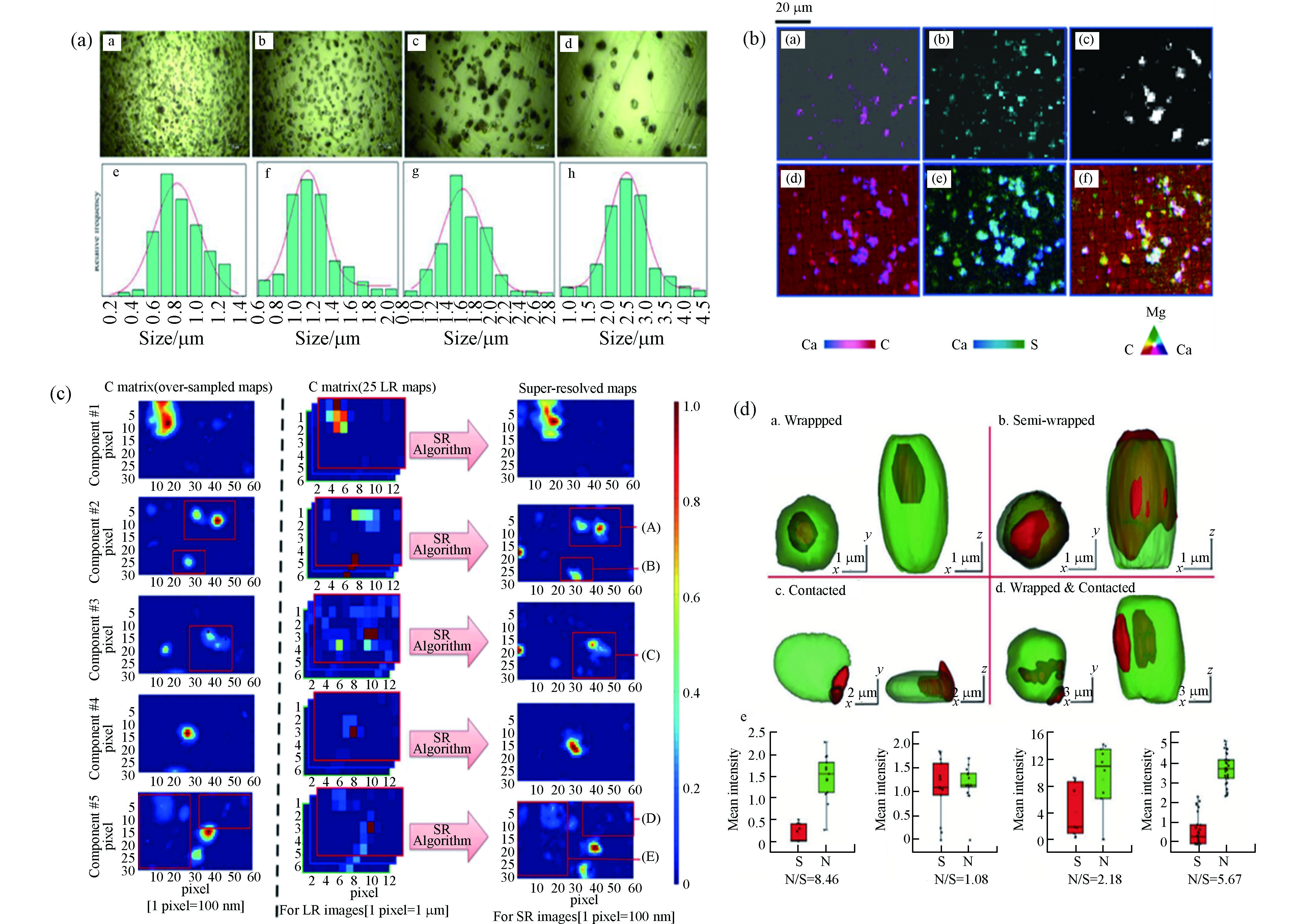
显微拉曼成像仪器的出现实现了对样品的图像化表征,通过拉曼光学图像可以直观地获得颗粒物的表面形貌信息. Sun等[23]收集了0.6—2.5 µm范围内4个尺寸区间的样品,其光学图像清楚显示了颗粒的尺寸和形貌,并据此统计出了颗粒粒径的正态分布情况(图6a).
在此基础上,借助相应的拉曼处理仪器,对样品进行一定范围内的激光扫描,可以得到该区域包含特征峰位信息的拉曼面扫描(mapping)图像. 然而,仪器的空间分辨率无法解决由于组分异质性带来的光谱重叠问题,这个难题可以通过多元曲线分辨率变换最小二乘法(MCR-ALS)方法解决. Batonneau等[56]收集了冶炼厂产生的极细、细和粗气溶胶颗粒,通过2D mapping图像的MCR来确定气溶胶颗粒水平上的异相化学. 2D mapping图像与X射线扫描获得的元素图像的高度吻合(图6b),证明了拉曼用于图像表征的有效性. 由于仪器的空间分辨率取决于衍射极限,为了进一步克服仪器分辨率的限制,Offroy等[57]在共焦拉曼成像中使用超分辨率概念和MCR-ALS算法结合,将空间分辨率提高了65%,达到了200 nm. 与低分辨率图像和超样品图像相比,高分辨率mapping图像能够清晰地观察到颗粒的边缘与形状(图6c),这使得利用常规共聚焦拉曼光谱仪表征单个气溶胶颗粒成为可能.
除了2D成像外,研究者们也逐渐对细颗粒物开展了3D mapping研究. Ao等[58]以无损方式展示了单个气溶胶颗粒物的3D化学成像的首次应用. 通过使用逐步增加Z轴深度的方达还原了颗粒物的空间3D结构,硝酸盐和硫酸盐的空间分辨分布揭示了大气颗粒的精细结构和不同的混合状态(图6d). 目前,图像化表征已经成为了拉曼作用于细颗粒物分析中不可或缺的一部分.
-
大多数颗粒物内部都存在两种及两种以上的组分,不同的来源会导致每个颗粒原始组分类型的多样性,而不同的大气化学反应过程又会进一步改变颗粒物的混合和生长老化方式. 对颗粒物混合态和结构进行分析,可以回溯出颗粒形成过程的物理化学行为,从而帮助进一步推断和了解颗粒的形成机制.
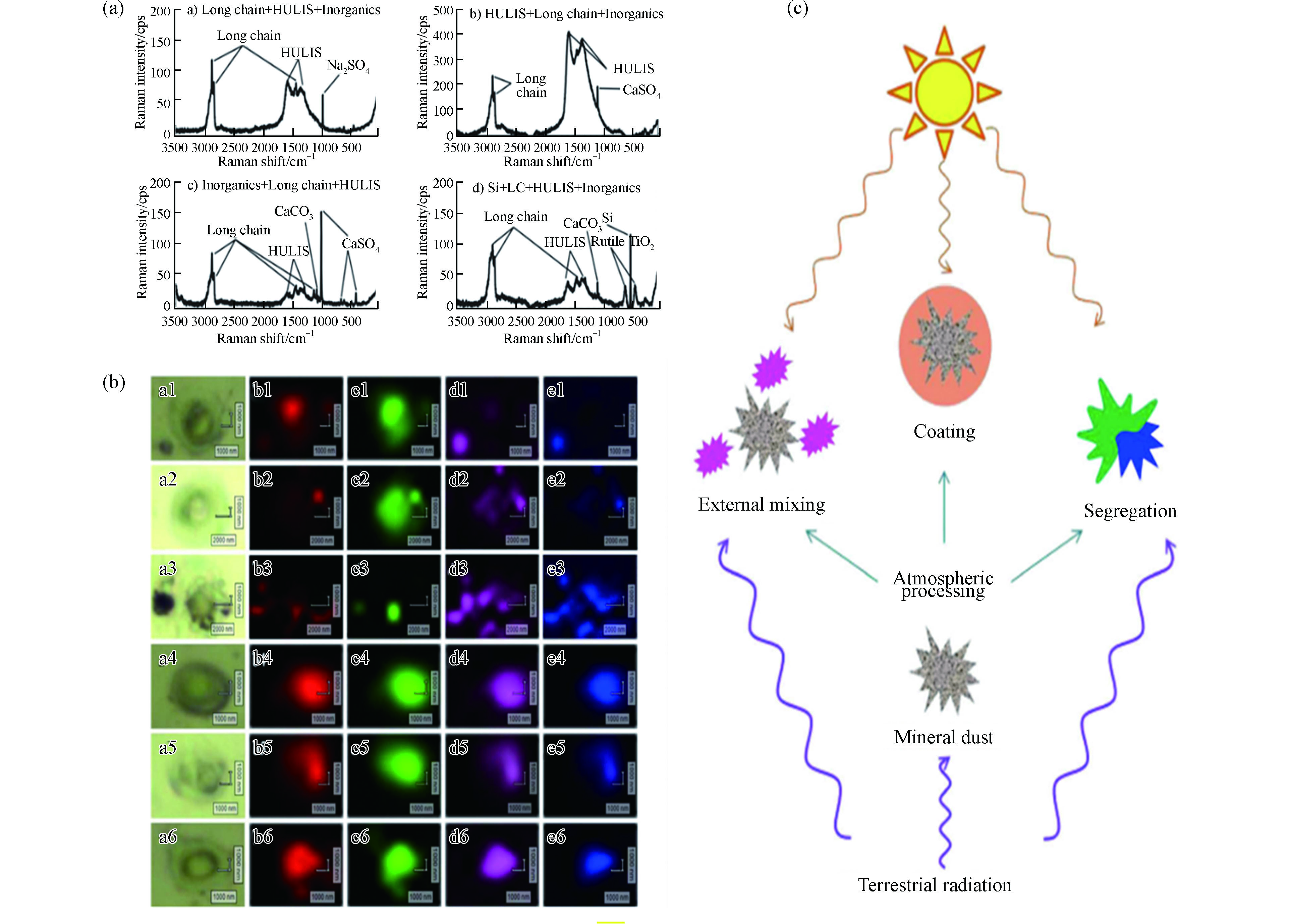
目前,已有少量文献开展了颗粒物混合态的研究. Deng等[59]研究了太平洋上空海洋气溶胶的拉曼光谱,发现海洋气溶胶包含多种组分,其中以高有机物含量主导. 长链有机物普遍存在,且会与其他有机和无机物内部混合(图7a). Chen等[53]对北京市雾霾事件的颗粒物进行了拉曼mapping分析,研究发现在雾霾形成过程中,soot充当内核,为硫酸盐和硝酸盐的生长提供吸附表面,形成SNA包裹soot的单颗粒核壳结构(图7b),该研究结果为细颗粒物核壳结构的形成提供了理论参考.
为了得到关于混合态更丰富的信息,研究者们也常将拉曼与其他表征分析技术结合. Deboudt等[60]结合了拉曼与电镜技术研究了大西洋沿岸的气溶胶颗粒,发现矿物、碳质颗粒和海洋化合物三者同时存在时属于外部混合,但两两组合属于内部混合,且碳质颗粒和海洋化合物经常在颗粒物表面形成涂层. Laskina等[61]结合拉曼、傅立叶红外消光光谱和SEM,分析了矿物粉尘和有机物经雾化后的混合物,发现有机物和矿物存在3种混合作用形式(图7c):(1)与颗粒内产物分离;(2)某些有机酸在矿物表面形成均匀的涂层;(3)外部混合. 细颗粒物混合态的多样性表明了大气环境的复杂性,对混合态展开具体分析有利于更好地研究当地的雾霾形成机制.
-
目前常规的源解析主要依靠各类模型对来源贡献进行分类和模拟[62-63],但是受到参数设置和真实环境复杂性的影响,模型源解析的结果可能会与实际的大气情况存在差异[20]. 而使用拉曼进行源解析是直接对目标颗粒物进行振动模式的分析,以进行种类和结构的判别,因此能够获得颗粒物最真实的信息. 其中,最常用的手段就是根据soot两个特征峰强度比值ID/IG进行来源的判别. 不同来源的soot颗粒的ID/IG存在差异[38, 64],根据对未知样品参数的测量,可以反推出样品的来源类型以及各来源类别的占比.
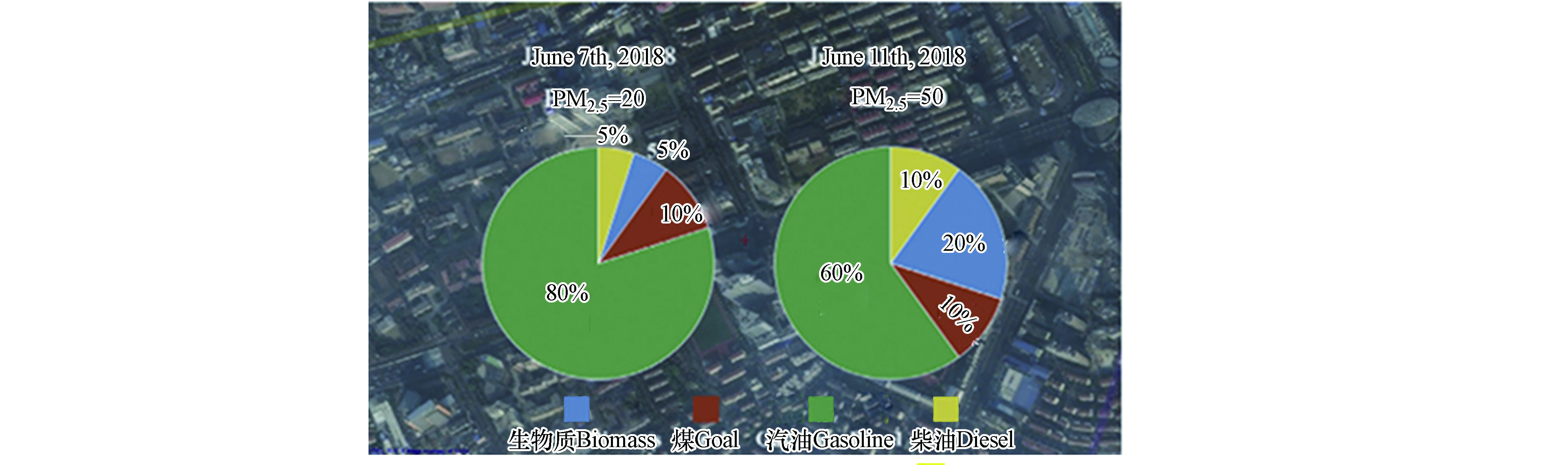
Catelani等[65]分析了soot和与碳质材料有关的矿物材料的拉曼光谱,使用ID/IG等光谱信息对其进行了鉴定,并据此对来自城市和农村的颗粒物进行了来源分配. Feng等[66]结合参数与逐步判别模型对上海市街头的soot颗粒来源进行了贡献分析,结果表明车辆排放是上海市soot颗粒的主要来源,其次是生物质燃烧和煤炭燃烧(图8). 未来的研究可以结合更多的soot颗粒样本,进行更广时空尺度范围的来源贡献分析.
同混合态分析一样,除了单独使用拉曼技术,研究者们还常常将拉曼与其他表征技术结合,在得到更丰富的颗粒物组分和形态信息的基础上,推断更准确的源解析结果. Popovicheva等[67]使用拉曼、SEM和能量色散X射线(EDX)对船舶废气进行了观察分析,发现其中含碳、硫酸盐和过渡元素的基团的存在与重质燃料油的燃烧具有很强的相关性. 由于大气区域传输的特点,细颗粒物的形成往往不仅限于当地污染贡献. Wagner等[68]使用拉曼、SEM/EDS、X射线荧光(XRF)和风蚀尘模型对高PM2.5天的污染源进行识别,确定了PM2.5的三类来源:(1)本地,来自非典型方向的风蚀尘颗粒,与模型预测的高尘埃颗粒浓度的结果一致;(2)冬季,区域性,吸湿性,富含氮和硫的盐,与硝酸铵和硫酸铵一致,以及(3)采样或记录错误.
-
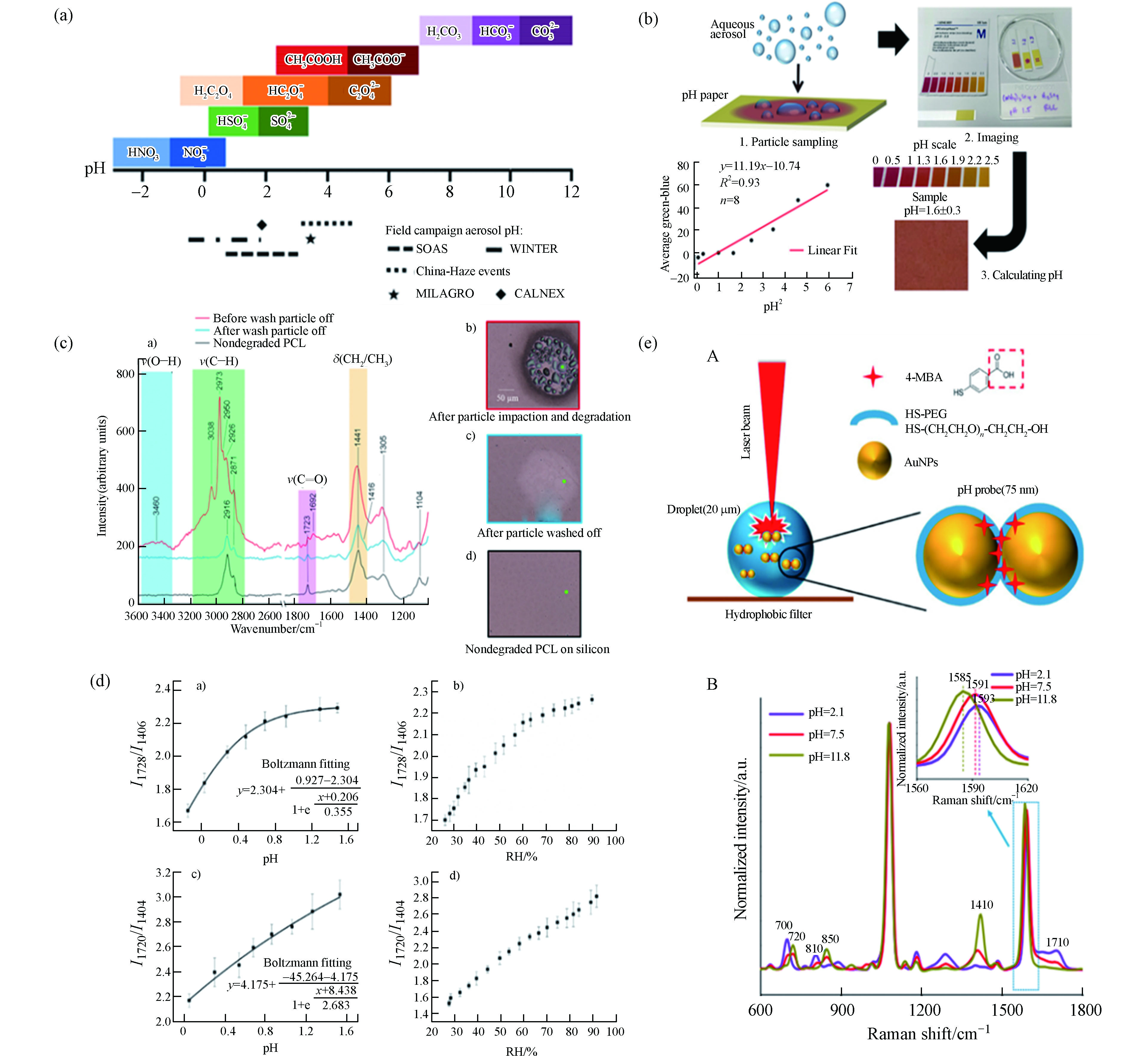
已有的研究表明,颗粒物的pH值多为酸性[19, 69-70],颗粒酸度会影响很多气溶胶的化学反应过程和特性,包括非均相反应[71]、相分离[72]和金属离子溶解度[73]等等. 当前尚无可靠的直接测量颗粒物pH值的方法,一些间接法如离子平衡、摩尔比、热力学平衡模型和相分配,常用于估算pH值大小,但其存在一定的不确定性. 因此,开发一种直接全面的检测方法对于准确测量pH值至关重要. 目前研究者们已经就使用拉曼进行颗粒物pH值测量做出了众多尝试.
Andrew P. Ault团队设计了三类pH值测量方法:(1)酸-共轭碱法[74-75](图9a),并实现了对实验室合成气溶胶颗粒pH值的优良表征;(2)基于pH指示剂(例如百里香酚蓝)和手机成像的可在现场部署的比色方法[76](图9b),将其应用于密歇根大学气溶胶的初步测试,证明了该方法在真实颗粒物中的应用潜力;(3)使用原子力显微镜、拉曼观测和表征酸催化聚合物的降解来确定pH值[77],研究发现颗粒物尺寸越小,聚合物降解程度越高,颗粒酸度更强(图9c).
同样是借助酸-共轭碱的原理,Boyer等[80]使用气溶胶光学镊子实现了对单个悬浮液滴的原位测量. 通过酸碱的峰面积比获得相对浓度,再加上分析受激腔体增强拉曼得到总溶质浓度,从而直接测量每个离子浓度以获得质子浓度,最后计算[H+]和pH值. 然而,对于纯羧酸溶液,由于其共轭碱浓度很小而无法观察到拉曼峰,此时酸-共轭碱法是不适用的. 对此,Chang等[78]开发了另一种羧酸气溶胶颗粒pH值的直接测量法(图9d). 研究者们配制了不同浓度的丙二酸和柠檬酸的标液,利用拉曼峰相对强度和pH值的关系建立标准曲线,从而可以根据研究溶液的拉曼峰强反推其pH值.
除了酸碱特性,研究者们还从其他角度入手研究了拉曼测量气溶胶的有效方法. Wei等[79]开发了一种pH值纳米探针测量法. 该团队合成了含有4-巯基苯甲酸(4-MBA)官能化的Au NPs的气溶胶颗粒,4-MBA是pH值敏感的信号分子,其在酸碱环境下有不同的官能团结合方式,从而表现出不同SERS特征峰位和强度(图9e). 该研究还发现颗粒物中心pH值更高,并且表现出稳定的pH值梯度.
综上所述,研究者们已经设计出了丰富多样的细颗粒物pH值测量方法,但大多数的研究对象为实验室合成的各类气溶胶,对真实环境颗粒物的pH分析方法还需进一步发展和验证.
-
颗粒物具有较小的粒径、较大的比表面积和特殊的表面结构,使得痕量气体很容易在其表面发生包括吸附、计量反应和催化反应在内的非均相反应[81]. 下面就反应机理和反应条件两个方面分别进行介绍.
-
根据产物的不同,非均相反应可以分为二次无机气溶胶(SIA)和SOA生成两大类型. 参与SIA生成的气相物种大多是NOX和SO2,产物为各类硫酸盐和硝酸盐.
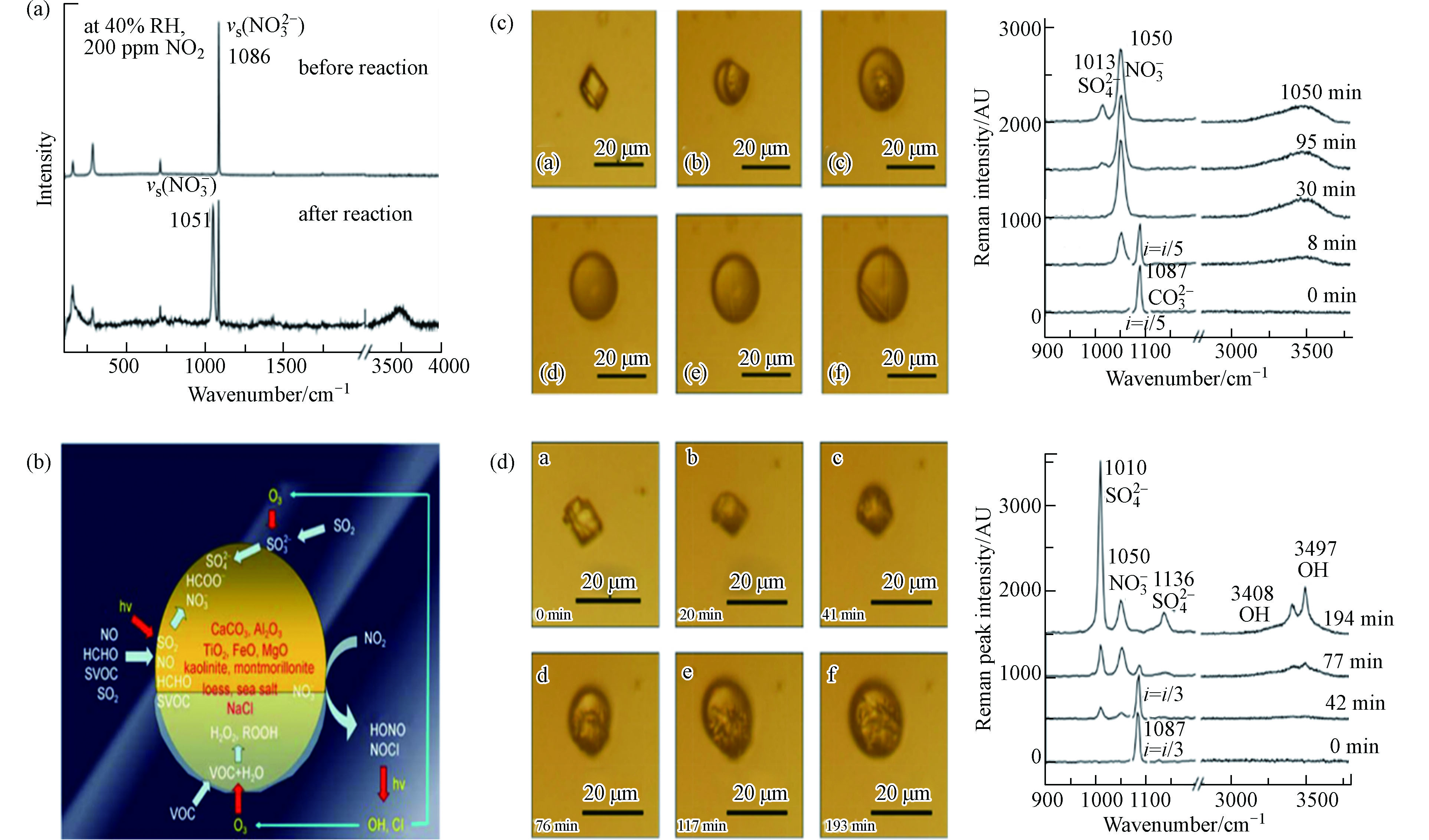
Zhao等[82]使用拉曼和自制流动反应系统研究了沉积的单个CaCO3颗粒与NO2的二元非均相反应(图10a),还可以根据拉曼峰强度比I(H2O)/I(
NO−3 )来定量Ca(NO3)2的生成. 在二元系统的基础上,Zhu等[83]利用实验室开发的综合实验方法,研究了NO2、SO2、O3和HCHO与各种颗粒的三元协同反应(图10b). 在污染过程中,颗粒表面形成了高度吸湿的硫酸盐和硝酸盐层,这种增强的颗粒吸湿性可以加速非均相反应并增加颗粒的消光系数,并据此建立了非均相反应动力学模型.SO2和NOX常作为两个体系分别研究,但其在大气中经常存在协同作用. Zhao等[84]使用显微拉曼光谱技术研究了SO2与NO2对N2中单个CaCO3颗粒的多相反应(图10c):NO2首先与CaCO3潮解形成Ca(NO3)2液滴,然后NO2氧化Ca(NO3)2中的SO2形成CaSO4. 对于该结论,Yu等[85]又开展了进一步的研究. 该团队在上述系统中引入了O2,其非均相反应过程说明O2是主要的氧化剂而不是NO2,NO2的主要角色是充当了自由基形成的引发剂(图10d).
SOA也是非均相反应重要的产物,相比于SIA,SOA产物的组分更加复杂. Lee等[86]根据拉曼光谱发现,油酸颗粒与O3的氧化产物主要由过氧化物(过氧化物和/或臭氧化物的O—O基团)、羰基和羟基组成,且保留在颗粒相中的氧化产物比其疏水性母体分子吸湿性更强. Liu等[87]研究了O3与soot的非均相反应对其结构和吸湿性的影响. 在氧化soot的拉曼光谱中观察到了表面含氧物质的生成,包括酮、内酯和酸酐. 氧化soot的结构更加紧密,其粒径的减小和表面含氧物质的形成又导致soot吸湿性的增强.
-
反应机理的研究已经让人们对大气中SIA和SOA的形成成因有了基础的了解,但是各类反应条件对非均相反应的过程和结果同样有非常重要的影响. Chu等[88]使用气溶胶流动池反应器结合原位拉曼光谱研究发现,低O3浓度和高RH有利于亚油酸颗粒氧化产物的积累(图11a). 由于大气组分的复杂性,单个颗粒表面常出现有机和无机组分共存的情况. Ray等[89]通过分子动力学和拉曼光谱的变化,观察到并解释了H2SO4涂层的存在对soot颗粒物表面苯并[a]芘臭氧速率降低的现象(图11b).
除了参与反应物种的影响,颗粒吸湿性与非均相反应也有着密切的关系[92]. 研究发现,非均相反应通常在一定的RH下发生,非均相反应改变了颗粒物的理化性质,从而影响其吸湿性. 拉曼作为一项强大的表征技术,能够直观地观察到吸湿过程颗粒物的形貌变化. Ma等[90]分析了乙酸(CH3COOH)与α-Al2O3、MgO和CaCO3颗粒的非均相反应,发现非均相反应对颗粒物的吸湿性皆有不同程度的促进(图11c). Wang等[91]使用拉曼对草酸与硫酸铵颗粒不同占比的混合物进行表征,研究发现占比的不同导致了吸湿性的差异(图11d),影响了潮解点和风化点,进而表现出不同的反应行为和产物.
另一方面,颗粒吸湿过程中的潮解形成的液相环境,又进一步影响着非均相反应的发生[93]. Ma和He[94]研究了草酸、Ca(NO3)2和CaCO3颗粒的反应过程,在吸湿过程中,中酸(草酸)会取代强酸(硝酸),而Ca(NO3)2的存在进一步促进了CaCO3和草酸之间的反应. 之后,该团队[95]又对硫酸盐和钙盐之间的反应进行分析,证明了强吸湿组分会促进非均相反应的进行. 此外,RH还会通过改变颗粒物的相态进而影响非均相反应. Wu等[96]合成了硫酸镁和戊二酸的均匀混合液滴,使用拉曼分析了脱水过程中液滴不同位置的谱线,从而确定液滴中的物质分布. 拉曼光谱表明,随着RH的降低,液滴经历了从均匀混合状态到液-液相分离,再到结晶/凝胶化的转变,该研究结果为RH对无机盐和二元酸之间非均相反应的影响提供了重要的理论支持.
由此可见,颗粒物表面的非均相反应是多元且复杂的,拉曼的无损特点能够完整表征反应过程,并有助于我们更好地了解反应过程及机理.
-
如前所述,拉曼已经在细颗粒物分析领域得到了较为全面的发展,但是拉曼散射本身是个非常弱的过程,存在信号不高的问题,导致难以识别颗粒物中的痕量组分. 为了克服传统拉曼技术的不足,研究者们在此基础上开发出了多种优化的拉曼技术,如表面增强拉曼散射(SERS)、尖端增强拉曼散射(TERS)和受激拉曼散射(SRS),下面将对其技术原理和在细颗粒物分析领域的发展情况进行介绍.
-
1974年,英国南安普顿大学的M. Fleischmann发现在粗糙的银电极表面,吡啶分子的拉曼信号得到了106倍数量级的增强[97]. 后来的研究者们通过实验重复和理论计算,将这种发生在Ag、Au和Cu等贵金属表面的信号增强现象称之为SERS效应. 当被测分子与贵金属的SERS基底接触时,会在接触表面形成近场的物理和化学增强,从而实现信号的放大.
通过观察分子在基底材料表面的SERS信号,可以确定待测物的组成和结构. 该技术在化学和材料等领域的应用十分广泛,但在大气颗粒物方面应用较少. 目前SERS分析雾霾气溶胶的方法大体上分为两种思路:底部膜式SERS增强技术与顶部接触式SERS增强技术. Ayora等[98]最早报道了SERS技术用于气溶胶的分析,研究发现在滤纸上原位还原或者沉积Ag溶胶作为底部膜式SERS材料,可以增强合成气溶胶样品中的吖啶、4-硝基苯酚和2,4-二硝基酚的拉曼特征峰强度. Craig等[99]随后在真实大气气溶胶分析中,通过SERS技术首次在单颗粒尺度上识别SOA. 以Ag纳米颗粒作为的膜式SERS材料对ν(
NO−3 )、ν(O−H)、ν(C−H)和δ(C−C)的拉曼振动均有信号增强作用(图12a). Zhang等进一步对膜式SERS材料进行优化,发展了更适合颗粒物分析的倒金字塔形状(图12b)及具有球形空隙(图12c)的有序结构基底,并提高了颗粒物SERS分析的增强因子[100-101]. Sun等建立了适用于雾霾单颗粒采集与分析的SERS方法,采用银膜同时作为颗粒物采样膜和底部膜式SERS增强基底(图12d),识别出北京市雾霾事件颗粒物中的硫酸盐、硝酸盐、soot和一些有机物的拉曼特征峰[23, 26]. 同一时期Gen等以另一种顶部接触式增强思路发展气溶胶SERS基底,利用电喷雾技术将Ag纳米颗粒覆盖在气溶胶表面(图12e),成功识别出气溶胶中的v(SO2−4 )、v(C–H)和v(O–H)振动[102-103].以上两种方法都属于2D SERS基底,已有少数研究者开始对3D基底提出了新型构想. Phan-Quang等[104]使用气雾剂将SERS胶体引入空气中,形成了被SERS基底全方位包裹的悬浮气溶胶颗粒(图12f),实现了对远距离空气(200 cm)的3D SERS检测. 随后,该团队[105]设计了一类金属-有机框架(MOF)集成平台(图12g),该平台能够对多环芳烃混合物进行3D吸附和覆盖,实现了对气溶胶的更远距离(2—10 m)检测,并将检测限优化低至十亿分之几.
-
TERS是将SERS与近场光学显微镜结合的新型光谱技术,使用金属尖端作为SERS探针,与其相接触的被测分子会产生近场信号的增强[106]. TERS已被证明是用于表征纳米材料和纳米结构的有前途的光谱和微形貌方法,该技术具有纳米级的空间分辨率,能够提供常规分析方法无法获得的系统结构和化学信息,目前已经成为薄晶体材料、碳纳米管、RNA/DNA单链、氧化还原反应、绘制单分子图像、半导体纳米结构和微腔等研究领域的有力技术[107],但其在细颗粒领域的研究尚在开发之中.
Ofner等[108]在烟雾箱内模拟盐湖上方11种大气粒子的形成,使用TERS近场光学技术研究了粒子间的光谱. 硫酸在老化成核模式颗粒物中具有重要占比,说明其是颗粒成核的重要驱动因素,而有机涂层的存在使得在较大模式颗粒中几乎检测不到硫酸盐,且不同粒子间有机物占比的不同表明了SOA生成方式的多样化. 该工作加深了研究者们对于颗粒物成核和SOA形成机制的理解.
-
SRS是一种非线性技术,当非线性介质内部的光强度达到一定(阈值)水平时,会诱发辐射跃迁过程中的受激散射,并通过迅速自催化增加形成SRS. SRS可达到的光谱范围从紫外到中红外,这取决于所使用的泵浦激光器和拉曼材料. 由于SRS是一个相干过程,其与常规拉曼技术相比,不仅能够提供更高的分辨率和对振动模式进行时间分辨分析,还可以克服研究对象自身的强荧光背景干扰从而实现高速表征[107]. 近年来,SRS显微镜由于其高特异性和高速化学成像特性,在生物、医学和材料学领域都得到了应用,并有研究者开始将其应用于细颗粒物的混合态分析与化学定量.
Ao等[58]以无损方式展示了SRS显微镜在单个气溶胶3D化学成像上的首次应用,成功揭示了颗粒物中不同化学组分的混合状态. 此外,通过从大量的甲烷中测量NO3−和SO42-的平均质量比(以N/S表示),将SRS大面积成像结果与离子色谱相关联,证明了SRS定量分析的能力,并将其应用于实际气溶胶样品的分析中.
-
综上所述,近年来拉曼已经在细颗粒物定性分析、定量分析、图像化表征、混合态分析、源解析、pH分析和非均相反应等方面展现出了重要的应用价值. 但是,随着国家对细颗粒污染治理的日益重视,需要研究者们深入对细颗粒物的分析研究,这就对现有拉曼技术提出了更高的要求,今后的拉曼技术有望在以下几个方面加强研究:
首先,细颗粒物组分,尤其是复杂有机物的组分仍在持续的识别和发展中. 未来的研究应着眼于更多的有机物种类,进一步完善细颗粒物组分的拉曼光谱库,使得拉曼成为更可靠的细颗粒物定性识别技术.
其次,大气的复杂性和区域间传输使得我国的细颗粒污染研究变得更加棘手. 拉曼的定量分析刚刚起步,与更多成熟有效的化学计量学结合有利于我们获得更加靠谱的组分占比和混合态信息,从而更好地研究细颗粒物的形成机制以及开展源解析.
最后,单一技术能提供的信息总是有限的,与其他技术联用能够帮助我们获得更加详细和全面的细颗粒物信息. 同时,设计同步、实时检测的拉曼仪器能够实现对细颗粒物的在线监控和检测,避免在样品运输和保存过程中颗粒物性质的变化,拓展拉曼技术在细颗粒物分析领域的进一步应用.
拉曼光谱在细颗粒物分析领域的应用
Applications of Raman spectroscopy in the field of fine-particle analysis
-
摘要: 近年来,作为一项无损的指纹识别技术,拉曼光谱在细颗粒物分析领域表现出巨大的应用潜力. 本文对拉曼光谱在细颗粒物定性分析、定量分析、图像化表征、混合态分析、源解析、pH分析和非均相反应七个方面的研究进展进行了综述. 对于传统拉曼光谱存在信号强度不高的问题,通过表面增强拉曼散射(SERS)、尖端增强拉曼散射(TERS)和受激拉曼散射(SRS)技术得到了改进. 最后对今后拉曼光谱在细颗粒物分析领域可能的研究方向进行了展望.Abstract: In recent years, as a nondestructive fingerprint recognition technology, Raman spectroscopy has shown great application potential in the field of fine-particle analysis. This article reviewed research process in seven aspects: qualitative analysis, quantitative analysis, image characterization, mixing-state analysis, source apportionment, pH analysis, and heterogeneous reaction. The problem of low signal strength of traditional Raman has been improved through technical improvements, such as surface-enhanced Raman scattering (SERS), tip-enhanced Raman scattering (TERS) and stimulated Raman scattering (SRS). Finally, this article prospected the possible research directions of Raman spectroscopy in the field of fine-particle analysis in the future.
-
Key words:
- Raman /
- fine-particle /
- technical improvement
-
工矿、印刷等行业的快速发展,大量含铅污染物被排放到水环境中,水质铅污染物检测及治理正成环境与材料领域的研究热点。传统的重金属检测技术主要包含质谱分析法[1]、原子吸收光谱测试法[2]、荧光分析法[3]和色谱分析法[4]等,但这些方法存在设备大、价格昂贵、操作复杂以及便携性差等局限性,限制了其在水环境中的实时监测应用。基于电化学方法的碳基传感器技术因具有便于携带、操作简单、灵敏度高、价格低廉等优势,在水质实时测定分析与治理方面具有极大优势[5]。作为一种新型介观碳材料,有序介孔碳因具有大的比表面积、有序的介孔结构、良好的导电性能,可望成为潜在的新型电极传感器材料。
最近,越来越多的研究[6-7]证实,通过氮磷等杂原子掺杂能在生物炭结构上形成更多的富电子边缘和空位缺陷等活性位和更高的极化度,进而增强材料对极性有机污染物和重金属的吸附固定;同时,改性掺杂的氮磷原子具有更高的电负性和更强的给电子能力,掺杂改性置入多孔碳材料的碳晶格中后,不仅能增强多孔碳材料的电子传递能力,并可以诱导与氮磷相邻的C原子或氧原子产生较大的自旋和电荷密度,作为氧化还原反应的活性位点,增强碳材料的氧化还原性能,从而提高其对检测物的循环伏安响应能力[8-9],如高杨等[10]研究表明,氮掺杂能明显增加石墨烯表面的活性点位,提升石墨烯的自由载流子密度和导电性能,从而增强其对硝酸根的检测性能。但也有研究表明,N/P共掺杂后多孔碳材料的氧化还原活性不仅没有提高,甚至降低[11-12],这可能是由于掺杂改性新生成的氮磷基团破坏或阻塞了多孔碳材料的微孔与介孔孔道,降低了氧化还原反应的有效活性位点和被检测物质在孔道中传递性能所致[13]。为此,本研究探索在氮磷双掺杂的同时,控制多孔碳材料的微中孔结构,规则的孔径结构与发达的孔隙率,使得氮磷等官能活性位点可以分散在不同长度的孔中并且增大了界面运输面积、提高电子转移速率,从而提升其电化学性能,保证制备的氮磷双掺杂多孔碳材料对水中痕量重金属铅的响应度与测定精确性。
在本研究中,以间苯二酚、甲醛和F127为碳源和模板剂制备出富含微孔的介孔碳,并使用生活中常见的磷氮双成份磷酸二氢铵为改性剂,在氮气气氛下,通过一步碳化实现碳材料的双重掺杂。在碳化改性过程中,磷酸二氢铵的分解可引起磷酸化反应,可有效减少碳材料中原有碳氧官能团的分解;同时,磷酸二氢铵中的氮和磷元素,在高温碳化的过程中与碳材料原有碳氧官能团结合,在碳骨架上生成大量活性氮磷结构,这将大大改善碳基材料在水性电解质中的润湿性;而且,磷酸二氢铵在高温下分解释放的氨气对多孔碳进行再次活化,可在制备碳材料上生成丰富的微孔结构。氮磷双掺杂、中孔结构的保持和微孔结构的增加,将增加氮磷掺杂介孔碳的有效活性点位,从而提升其对水中铅离子的识别敏感程度,实现对水中痕量铅离子的电化学检测。
1. 材料与方法
1.1 试剂和仪器
间苯二酚、磷酸、无水乙醇、模板剂F127、盐酸、甲醛、磷酸二氢铵均为分析纯,购于南京化学试剂有限公司。主要仪器包括比表面及微孔径分析仪(3H-2000PM2,贝士德仪器科技有限公司)、管式炉(OTF-1200X,合肥科晶材料技术有限公司)、傅里叶变化红外光谱分析仪(Nicolet 6700,美国)、元素分析仪(Elementar Vario MICRO,德国)、透射电子显微镜 (JEM-2100F,日本)等。
1.2 氮磷双改性有序介孔碳的制备
将1.729 g磷酸(磷源)和3.3 g间苯二酚溶解在30 g质量分数50%的乙醇溶液中,加入3.0 g F127,搅拌至透明后,加入0.60 g盐酸,继续搅拌1 h,滴加3 g甲醛,继续搅拌1.5 h,将得到的混合物在室温下静置96 h,去除上层清液,用玻璃棒搅拌下层沉淀物,直至形成乳白色的粘稠物,将此粘稠物在室温静置48 h,先在80 ℃下固化48 h,而后在氮气保护下于500 ℃下恒温碳化3 h,冷却至室温,获得介孔碳OMC。将磷酸二氢铵与制得的OMC材料按2.3:1的质量比溶于水,在室温下浸渍12 h,将所得到的混和材料在500 ℃恒温碳化1 h后自然降温,将得到的改性碳材料命名为OMC-MAP。
1.3 OMC-MAP改性玻碳电极的制备
在进行玻碳电极预处理时,将直径3 mm的裸玻碳电极依次用粒径为1.0、0.3、0.05 μm的Al2O3粉在麂皮上抛光至洁净,每次抛光后先冲洗掉表面污物,再移入超声水浴中精洗2~3 min,重复3次,然后再依次用质量浓度为1:1的乙醇、HNO3和蒸馏水超声清洗;最后将清洗干净的电极在0.1 mol·L−1 H2SO4溶液中,通过循环伏安法在0~0.6 V扫描10次进行活化备用。
在进行玻碳电极改性时,将50 mg过200目筛的改性介孔碳溶入5 mL 1%的Nafion溶液中,超声20 min获得均一的分散液;以微量注射器移取2.5 μL分散液,均匀地滴涂在预处理合格的裸玻碳电极表面,自然晾干后在45 ℃下固化15 min,得到OMC-MAP/GCE电极。
1.4 性质表征
用贝士德微孔吸附仪测定碳样品在77.3 K下的氮吸附量,并通过Brunauer-Emmett-Teller (BET)、Horvath-Kawazoe(HK)和Barrett-Joyner-Halenda(BJH)理论计算材料比表面积、微孔孔容和中孔孔容,并通过Density functional theory(DFT)与BJH表征材料的全孔和介孔孔径分布特征;利用傅里叶变化红外光谱分析仪和X射线光电子能谱仪分析碳材料的表面化学官能团性质。
1.5 电化学检测
在(25±1) ℃下,在铂电极、饱和甘汞电极和氮磷介孔碳改性玻碳工作电极组成的三电极体系中,使用循环伏安法(CV)对水中痕量铅离子进行电化学检测,扫描区间为-1~0.2 V、采样间隔为0.001 V、静止时间为2 s、扫速为0.1 V·s−1,铅离子浓度为1 mg·L−1、底液浓度为0.2 mol·L−1。在优化实验中,探讨底液 (磷酸氢二钠盐-磷酸二氢钠、醋酸-醋酸钠、氯化铵-氨水)、pH(3.3~4.2)、富集电位(-1.4~-1.0 V)、富集时间(180~300 s) 对溶出电流的影响,进行优化时,除变量外,底液、pH、富集电位、富集时间设为醋酸-醋酸钠、3.8、240 s、-1.2 V和240 s。实际水样检测和抗干实验均在优化条件下测定。
2. 结果与讨论
2.1 碳材料的表征
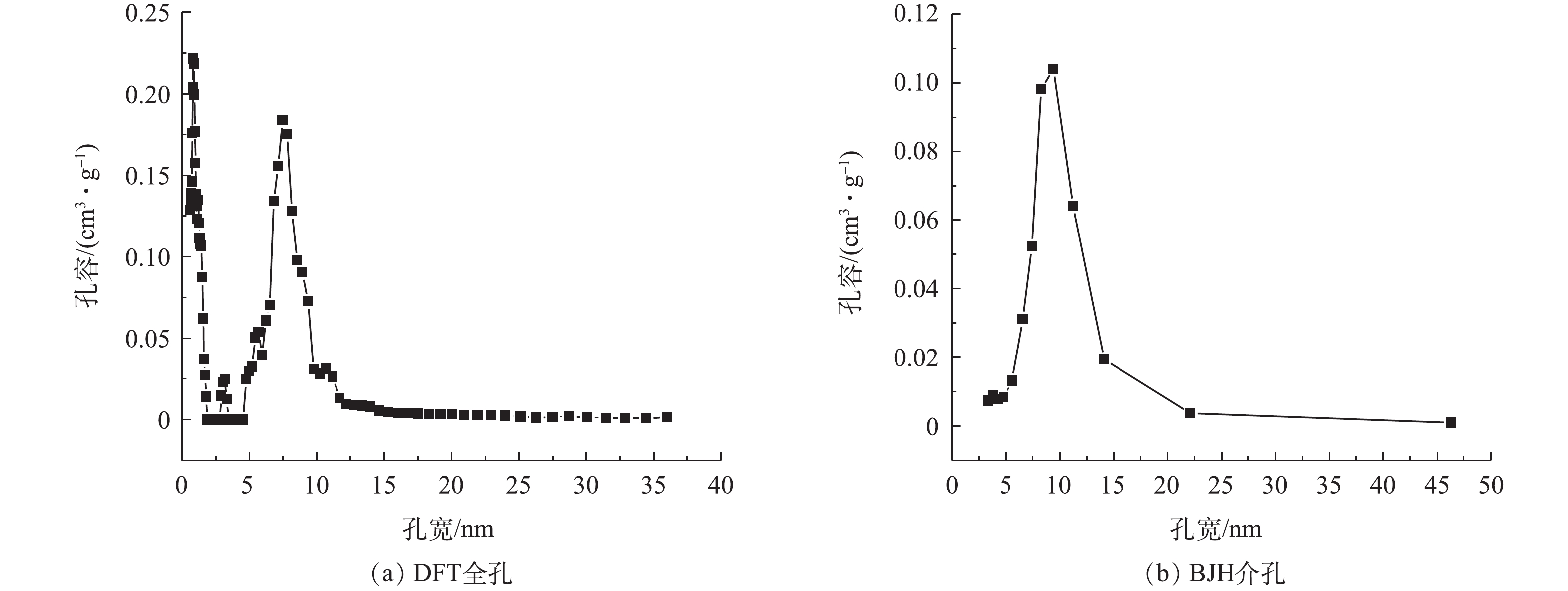
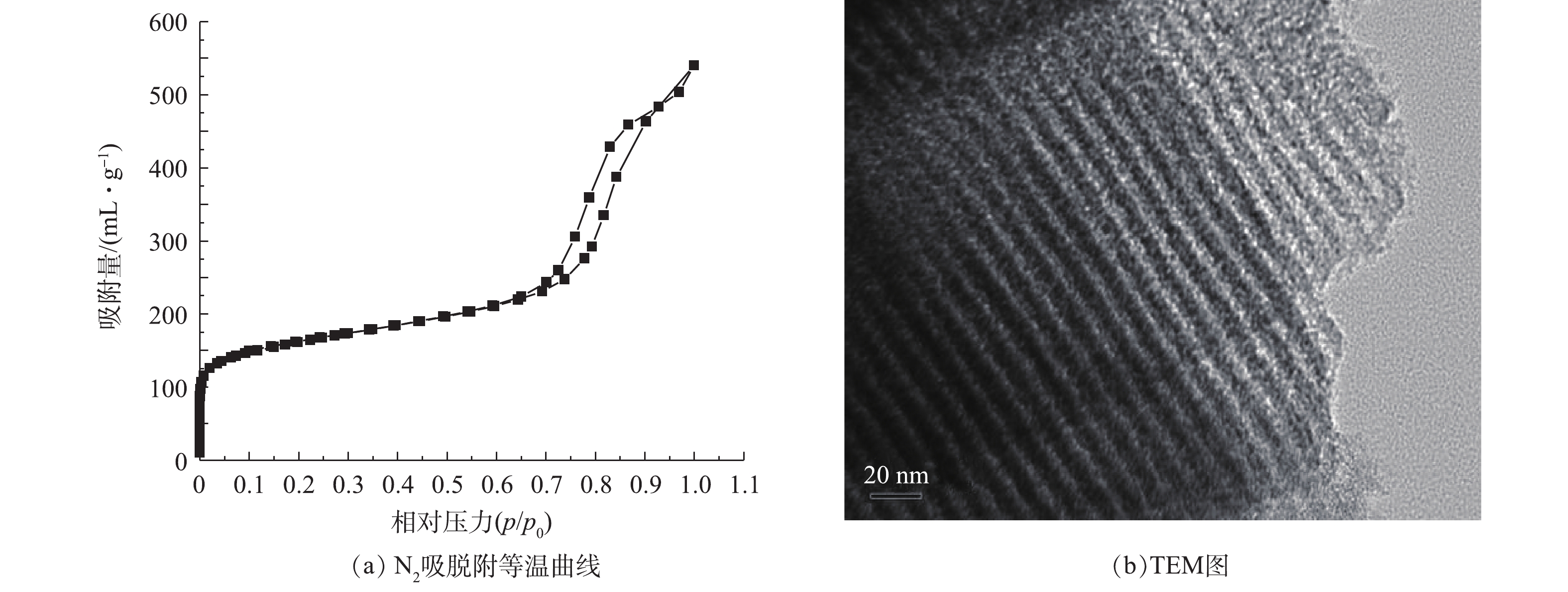
图1(a)为氮磷改性碳OMC-MAP的N2吸脱附等温曲线。在P/P0<0.01的低压区,介孔碳OMC-MAP的氮吸附量随着相对压力的增加而迅速增加,而后出现一缓慢吸附平台,这是由于微孔对氮气单分子层吸附达到饱和后,又出现了多层吸附引起的现象,这表明材料存在丰富的微孔结构,并含有一定量孔径接近于微孔的微中孔结构[14];当0.7<P/P0<0.98时,氮吸附等温线陡峭式的上升,发生毛细管凝聚,脱附线出现明显的滞后回线,这一吸附段的氮吸附量约为350 cm3·g−1,占总吸附量的60%,表明OMC-MAP材料存在大量孔径尺寸集中介孔结构。由图1(b)可见,在OMC-MAP的结构中,明显具有二维六方有序介孔结构的有序介孔碳。
图2为介孔碳OMC-MAP的DFT全孔与BJH介孔孔径分布。由DFT全孔分布(图2(a))可以看出,碳材料OMC-MAP的孔主要包括微孔和介孔,峰值孔径主要分布在0.813、1.178和7.45 nm处,且介孔分布集中于5~15 nm;由BJH介孔孔径分布(图2(b))也可以看出,OMC-MAP的介孔孔径主要分布在5~15 nm。根据氮等温吸附线数据,通过BET、HK和BJH可以计算出OMC-MAP的比表面积为579 m2·g−1,介孔孔容、微孔孔容和总孔孔容分别为0.681、0.232和0.835 cm3·g−1,介孔占总孔容的81.5%。结果表明:OMC-MAP材料是属于富含微孔和二维六方有序介孔结构的有序介孔碳,该孔结构特征有利吸附捕获溶液铅离子以及氧化还原反应产生的电子传递,从而增强该电极材料对水中痕量重金属离子的识别与测定。
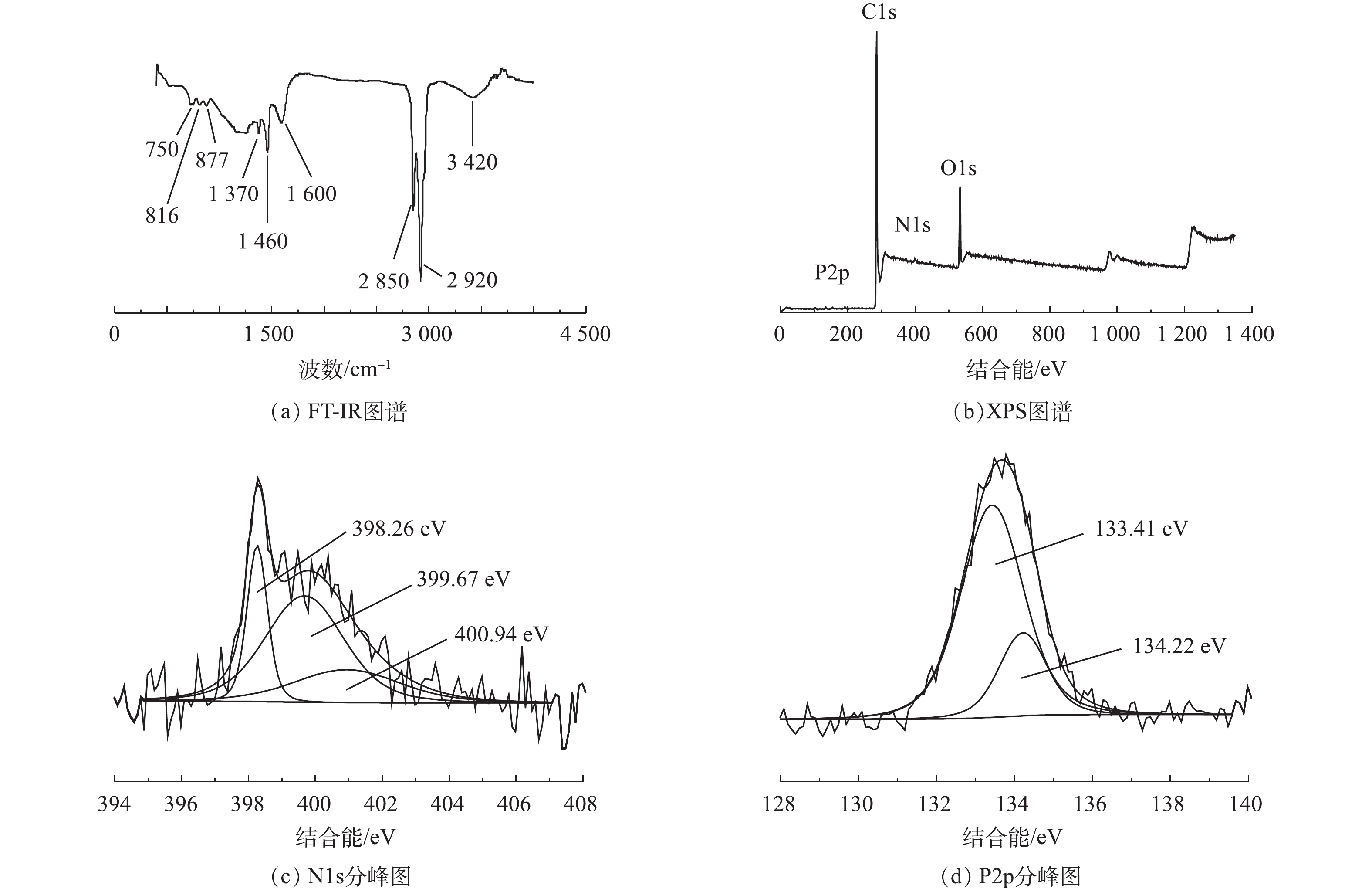
2.2 碳材料FT-IR与XPS分析
图3为碳材料FT-IR与XPS图谱。OMC-MAP的FT-IR红外光谱如图3(a)所示,可以看出OMC-MAP在750、816、877、1 370、1 460、1 600、2 850、2 920、3 420 cm−1处有吸收峰,1 460、2 850和2 920 cm−1处的吸收峰尖锐,750、816、870、1 370、1 600和3 420 cm−1处的峰谱带较宽。750 cm−1和816 cm−1处的吸收峰属于含磷官能团,其归因于P—C的伸缩振动[15]和P—O—C的对称伸缩振动[16];877、1 370和3 420 cm−1的吸附峰是由=C—H面外弯曲振动、—COOH拉伸振动和—OH伸缩振动所引起的[17-19];而1 600 cm−1吸附峰在双键区(2 000~1 500 cm−1),可能是由芳环与氧的其他不饱和体系发生共轭带而分裂出来的吸收带引起的[20]。以上结果表明:材料表面含氧官能团可能包含羟基、羧基、羰基及醌基等多种含氧酸基团;1 460 cm−1和2 850 cm−1是由氨基NH2的拉伸振动[21]和伯胺N—H键不对称伸缩[22]引起的吸收峰,这表示在介孔碳的表面含有氨基等含氮官能团。上述结果表明,介孔碳OMC-MAP的表面除含有羟、羧基等含氧酸基团外,还含有P—C、P—O—C、N—H、—NH2等含氮磷官能团。
XPS分析用于鉴定OMC-MAP的表面元素组成和化学结构。图3(b)为OMC-MAP的XPS光谱。可以看出,图谱中显示出O1s(532 eV)、C1s(284 eV)、P2p(135 eV)和N1s(399 eV)峰,其所对应的原子含量分别为85.7%、11.0%、1.75%和0.79%,这进一步说明改性样品成功掺入了N、P元素。为了进一步验证N、P元素的状态,N1s图谱可以解卷积成3个不同的分量,由图3(c)可见,其峰值分别在398.26、399.67、400.94 eV,这可归因于吡啶氮[23],吡咯氮[24]和氨基(—NH2)[25];P2p峰的精细结构表明样品存在2个主要的含磷基团,如图3(d)所示,峰值为134.22 eV表明其含有P—O—C基团[26],而峰值在133.41 eV附近可归因于P—C键[27]。这与FT-IR的分析结果保持一致。
2.3 改性电极铅检测条件优化
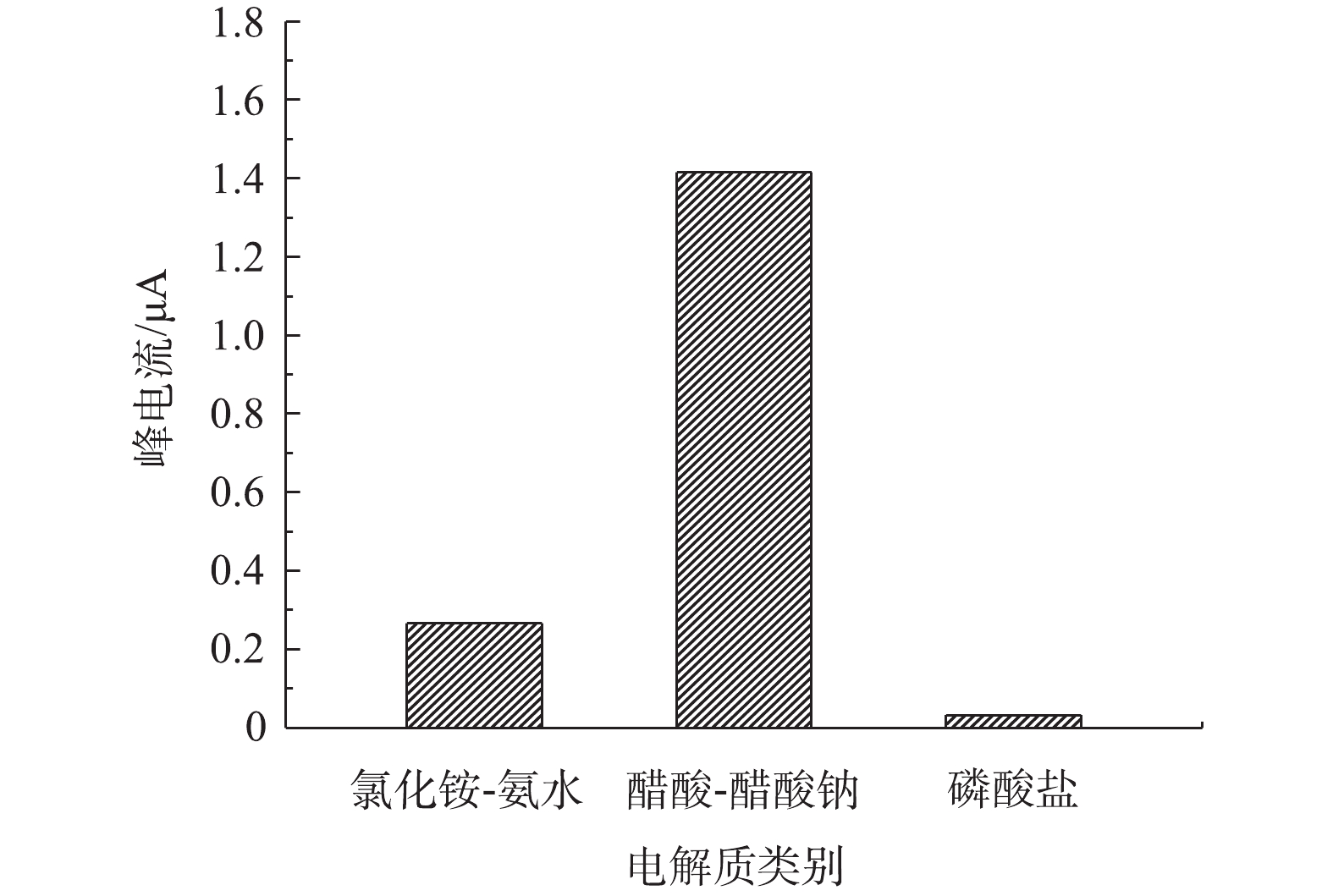
1)不同支持电解质底液对改性电极检测痕量铅的影响。由图4可知,在以磷酸氢二钠盐-磷酸二氢钠、醋酸-醋酸钠、氯化铵-氨水3种缓冲液作为支持电解质底液时,支持电解质底液类别对改性电极检测铅离子的性能具有较大影响。此外,当醋酸-醋酸钠作为支持电解质底液时,改性电极对水中铅离子的溶出电流峰值最大。这主要是由于醋酸-醋酸钠和介孔碳改性电极对目标铅离子的还原均可能产生较强的催化作用,而且醋酸也可与铅离子发生配位作用,从而成为铅离子的扩散载体,这2个方面的原因均会导致扩散速度和峰电流增大。李静等[28]比较了Na2SO4、KCl、Na2HPO4-NaH2PO4和醋酸-醋酸钠等作为支持电解质溶液对铋改性碳电极测定水中痕量铅离子的影响,也得到了相似的结论,即以醋酸-醋酸钠缓冲液作为支持电解质底液时,改性电极对铅检测响应敏感度最优。为此,在后续实验中选用醋酸-醋酸钠缓冲液作为OMC-MAP改性电极铅检测的支持电解质底液。
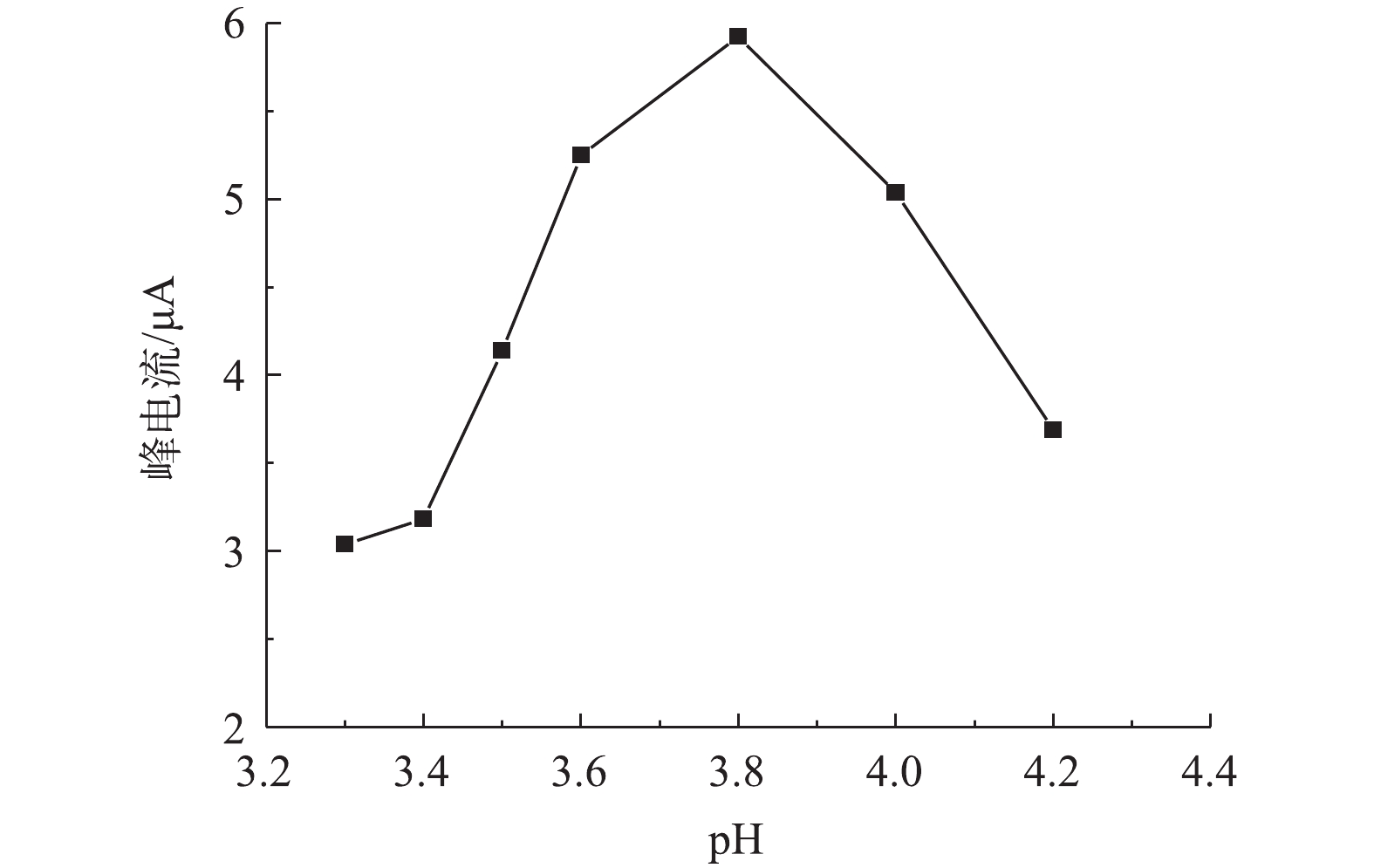
2)溶液pH对改性电极测定痕量铅的影响。由图5可知,当溶液pH由3.3增加4.2时,改性电极的铅溶出电流先增大后减小;在溶液pH=3.8时,改性电极对铅检测的峰电流值最大,说明溶液pH对改性电极检测铅有较大影响,这主要是由于改性电极表面修饰的介孔碳材料表面富含氮磷官能团,在不同pH下,氮磷官能团质子化程度发生变化,如吡咯或吡啶上的氮孤对电子在不同酸性条件下质子化程度不同,因此,其所表现出来的电性不同,导致与溶液中铅离子的静电或化学作用也有所不同,进而影响铅离子在改性电极表面结合能力和结合量,最终导致循环伏安电流变化。戴兴欣[29]在研究氨基改性对介孔硅电极测定铅的影响时,也发现溶液pH对氨基改性电极测定铅有较大影响。在对于本研究中OMC-MAP改性的玻碳电极测定溶液中铅,在pH为3.8时峰电流最强,在后续研究中选择其为最优pH。
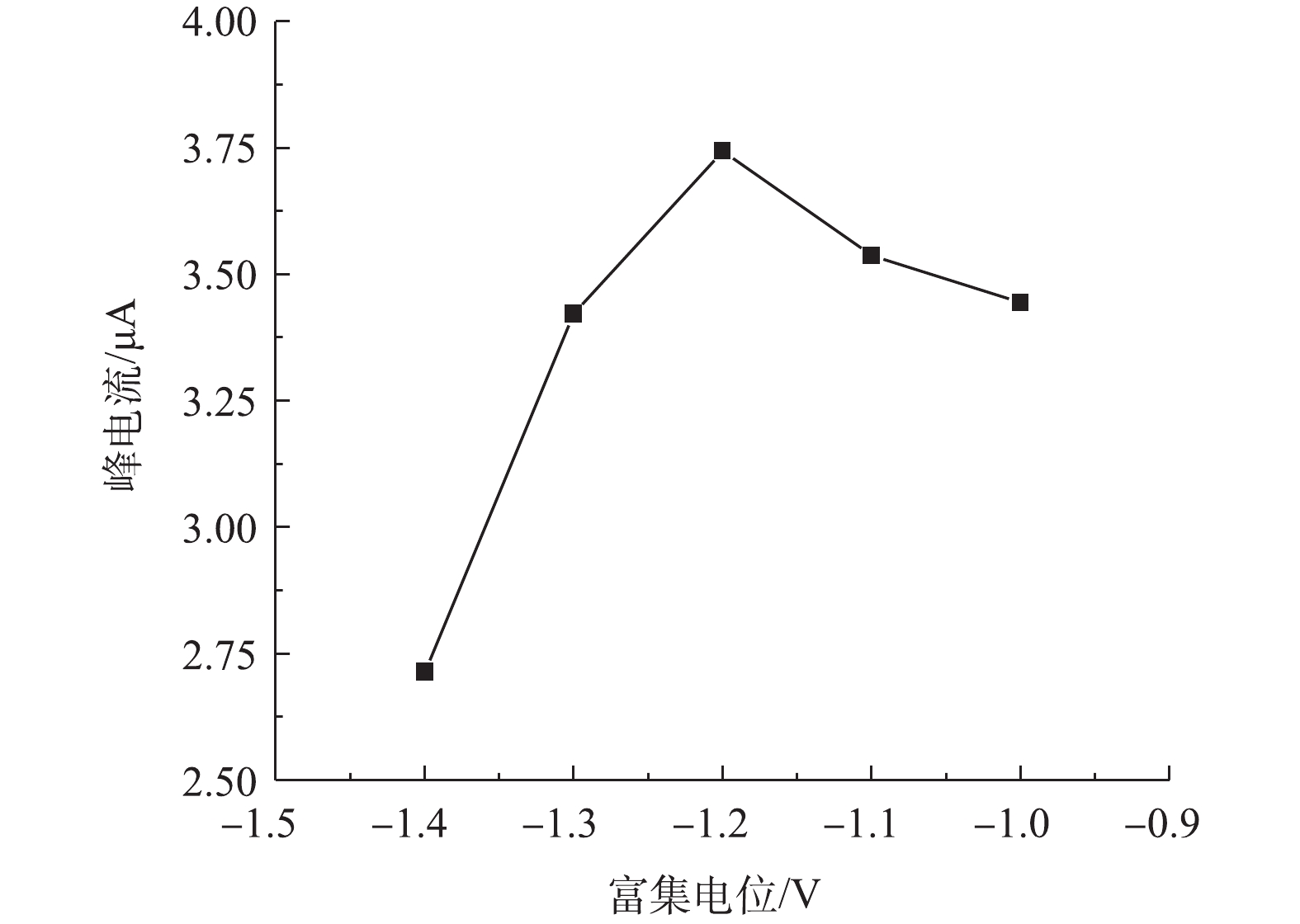
3)富集电位对改性电极检测痕量铅的影响。由图6可见,改性电极对铅的响应峰电流随着富集电位的升高先升高而后降低,当富集电位为−1.2 V时,峰电流达到最大值。当富集电位由−1.0 V降到−1.2 V时,随着富集电位绝对值的增大,峰电流增大,这可能是高电位下水中铅离子在改性电极上的还原更加充分所致;当富集电位由−1.2 V降到−1.4 V时,响应峰电流降低,这可能是高电位下溶液发生了析氢,电位越高,析氢增大,而氢离子的析出阻碍了铅离子在电极表面的沉积[30],进而导致电化学还原阶段峰电流的降低。
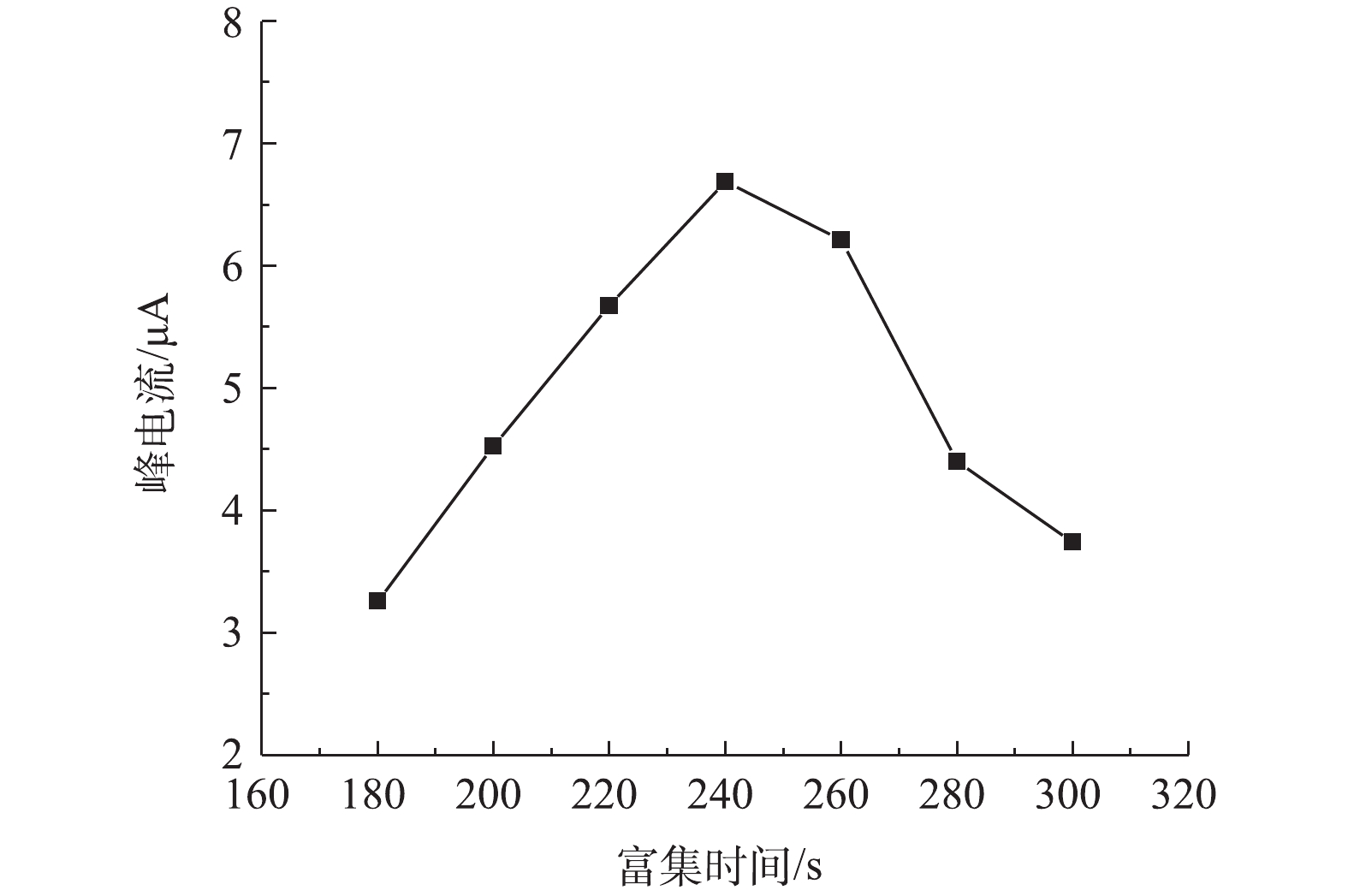
4)富集时间对改性电极测定铅的影响。由图7可见,当富集时间为240 s时,改性电极对铅的响应溶出电流达最高。当富集时间小于240 s时,铅离子在改性电极上的富集不完全,未达到最大值,因而响应溶出电流值较低。当富集时间大于240 s时,溶出峰电流随着富集时间的增加非但没有进一步增加,并且还有所降低,这可能归因于改性电极对铅离子的还原富集在240 s时已达最高值,进一步增加富集时间,改性电极对铅离子的还原富集量因活性位点饱和不再增加,反而导致已还原的铅与改性介孔碳表面氮磷官能团等活性位结合成了更稳定的化学物,阻碍电子传输,使得在随后的氧化溶出时变得困难,从而导致检测响应溶出峰电流降低。尽管不少研究表明,改性电极对铅检测的响应峰电流随富集时间的增加一直增加,达到一峰值后峰响应电流不变,但也有研究[31-32]得出与本研究相类似结果,这说明在高活性官能团改性电极测定金属离子时,优化控制富集时间对提高电极敏感性有重要的意义。
2.4 改性玻碳电极应用分析
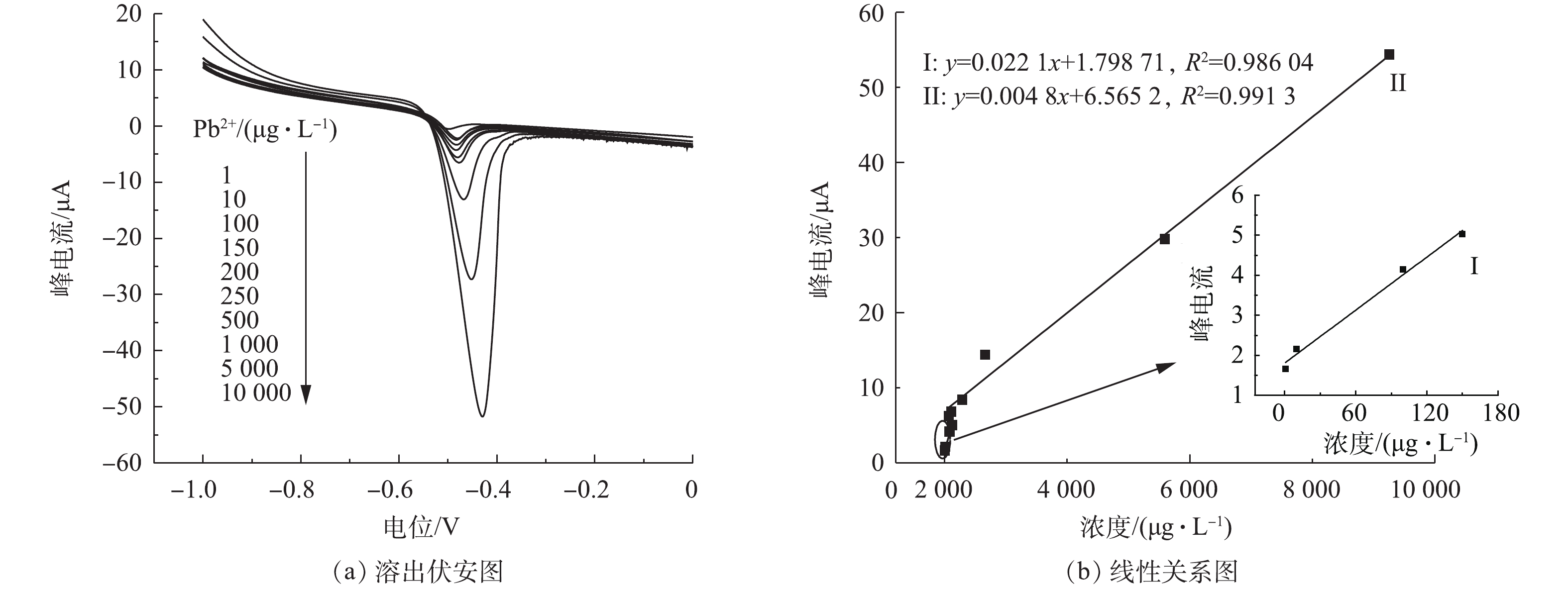
不同浓度铅离子在改性电极上的溶出伏安图及峰电流与铅离子浓度之间的线性关系见图8。图8(a)为OMC-MAP改性玻碳电极在醋酸-醋酸钠作为支持电解质底液、pH=3.8、富集电位−1.2 V、富集时间280 s的优化条件下对水中1~10 000 µg·L−1间10个不同浓度的铅离子的溶出响应伏安电流图。可以看出,响应电流峰值随着铅离子浓度的增大而增大。对响应电流值与铅离子浓度作线性相关分析可知,溶出响应电流与铅离子的浓度间呈两段线性相关(图8(b)),在铅离子为1~150 µg·L−1和150~10 000 µg·L−1时,对应的拟合方程为y=0.022 1x+1.798 71和y=0.004 8x+4.676 79,R2均大于0.98。每次测定并加相反电压后,在空白底液中经过循环伏安扫描除去电极表面的铅,以恢复电极活性,对2.18 µg·L−1的铅溶液平行测定5次的相对标准偏差为2.16%,这表明电极有良好的重现性。上述结果表明,经氮磷改性介孔碳OMC-MAP修饰的改性电极,对水中的痕量铅有良好的响应度和稳定性。
本实验的检测方法同其他文献报道出来的检测方法相比,如表1所示,具有更宽的线性范围及较低的检测限,说明氮磷改性介孔碳作为玻碳电极的修饰材料,无论在线性范围以及检测限方面都有非常大的优势,对水中的痕量铅有良好的响应度与灵敏度。
表 1 不同电极检测铅离子的比较Table 1. Comparison of detection of lead ions by different electrodes2.5 实际水样测定与抗干扰实验
在最优实验条件下,使用OMC-MAP改性玻碳电极5次重复测定长江实际取水水样中的Pb2+浓度,测定结果分别为2.08、2.12、2.04、2.13和2.11 μg·L−1,平均值为2.096 µg·L−1。使用ICP-AES法检测实际长江水样的Pb2+离子浓度为2.13 μg·L−1。OMC-MAP改性玻碳电极与ICP-AES 2种方法检测的Pb2+离子浓度的相对偏差为3.85%,表明本方法具有可靠性,其可用于实际环境水样中Pb2+浓度的检测。在长江水样中加入100倍的Ca2+、Na2+、Mg2+干扰物质后,在优化的实验条件下,对OMC-MAP改性玻碳电极检测长江水中Pb2+的浓度并无影响,这说明OMC-MAP改性玻碳电极具有良好的选择性。
3. 结论
1)以磷酸二氢铵为磷氮改性剂、间苯二酚和F127为碳源和模板剂成功制备了具有丰富微中孔结构的磷氮双改性介孔碳OMC-MAP,材料的介孔、微孔孔容分别为0.681 cm3·g−1和0.232 cm3·g−1。
2) FT-IR与XPS表征结果表明,OMC-MAP介孔孔道镶嵌有—NH2、N—H、P—C、P—O—C等氮磷官能团以及C=O、C—O、COOH等含氧官能团。
3)在以醋酸-醋酸钠支持电解质底液、pH=3.8、富集电位为−1.2 V和富集时间为240 s的条件下,当线性范围为1~10 000 µg·L−1时,循环伏安溶出响应电流与对应的铅离子呈良好的两段线性相关关系,线性相关度R2>0.98,对应的检测限为0.2 µg·L−1,这表明电极对水中的痕量铅有良好的响应度和灵敏度。
-
图 3 (a-c)常见无机盐的拉曼光谱[23];(d)四类单颗粒结晶与I1046/I1053大于1时结晶的拉曼光谱[24];(e)单盐及复盐的拉曼光谱[26]
Figure 3. (a-c) Raman spectra of common inorganic salts [23]; (d) Raman spectra of four types of single-particle crystals and crystals with I1046/I1053 greater than 1, modified from ref[24]; (e) Raman spectra of single and double salts, modified from ref[26]
图 5 (a)气溶胶样品的光学图像、拉曼光学图像和根据特征峰位得到的ZnO的拉曼数据处理图像[52];(b)雾霾事件中硫酸盐、硝酸盐和soot含量占比的时间变化,修改自参考文献[53];(c)化学定量法得到的颗粒物组分贡献[54];(d)由Raman和SEM/EDX数据获得的各组分浓度分布图像[55]
Figure 5. (a) Optical image and Raman optical image of aerosol sample, and Raman data processing image of ZnO[52]; (b) temporal variations of the proportion of sulfate, nitrate and soot during a haze event, modified from ref [53]; (c) contribution of particle compositions obtained by using chemical quantitative method[54]; (d) concentration distribution image of each component obtained from Raman and SEM/EDX data[55]
图 6 (a)四个尺寸范围的颗粒物拉曼光学图像及尺寸分布[23];(b)颗粒物的2D拉曼面扫描图像和X射线图像[56];(c)气溶胶颗粒的超样品图、低分辨率图和高分辨率图[57];(d)单个气溶胶颗粒的3D成像[58]
Figure 6. (a) Raman optical images and size distributions of particles in four size ranges[23]; (b) 2D Raman mapping image and X-ray images of particles[56]; (c) over-sample images, low-resolution images, and high-resolution images of aerosol particles[57]; (d) 3D imaging of a single aerosol particle[58]
图 7 (a)太平洋上空多组分气溶胶颗粒物的拉曼光谱[59];(b)清洁天和污染天里细颗粒物混合状态的面扫描分析[53];(c)矿物粉尘与有机物的三种混合形式[61]
Figure 7. (a) Raman spectra of multi-component aerosol particles over the Pacific[59]; (b) mapping analysis of the mixing state of fine particles in clear and polluted days[53]; (c) three mixing forms of mineral dust and organics[61]
图 9 (a)酸-共轭碱法示意图[75];(b)基于pH指示剂比色法示意图[76];(c)聚合物降解过程的拉曼光谱及图像[77];(d)标准曲线法,修改自参考文献[78];(e)pH值纳米探针作用示意图[79]
Figure 9. (a) Schematic diagram of acid-conjugate base method[75]; (b) schematic diagram of colorimetric method based on pH indicator[76]; (c) Raman spectra and image of polymer degradation process[77]; (d) standard curve line method, modified from ref[78]; (e) schematic diagram of the action of the pH value nanoprobe[79]
图 10 (a)与NO2反应前后的CaCO3颗粒拉曼光谱[82];(b)三元非均相反应协同作用示意图[83];(c)CaCO3颗粒与SO2、NO2反应过程的拉曼光学图像及光谱变化,修改自参考文献[84];(d)CaCO3颗粒与O3、SO2、NO2反应过程的拉曼光学图像及光谱变化,修改自参考文献[85]
Figure 10. (a) Raman spectra of CaCO3 particles before and after the reaction with NO2[82]; (b) schematic diagram of ternary heterogeneous reaction[83]; (c) Raman optical images and spectral changes of CaCO3 particles during the reaction process with SO2 and NO2, modified from ref[84]; (d) Raman optical image and spectral changes CaCO3 particles during the reaction process of with O3, SO2, and NO2, modified from ref[85]
图 11 (a)O3浓度和RH对亚油酸颗粒被O3异质氧化的影响[88];(b)soot表面双分子异质动力学曲线[89];(c)乙酸与α-Al2O3、MgO和CaCO3颗粒的吸附等温线,修改自参考文献[90];(d)不同占比的草酸与硫酸铵颗粒混合物的吸湿性曲线[91]
Figure 11. (a) The influence of O3 concentration and RH on the heterogeneous oxidation of linoleic acid particles by O3[88]; (b) bimolecular heterogeneous kinetic curve on the surface of soot[89]; (c) adsorption isotherm of Acetic acid and α-Al2O3, MgO and CaCO3 particles, modified from ref[90]; (d) hygroscopicity curves of mixtures of oxalic acid and ammonium sulfate particles with different proportions[91]
方法Method 尺寸范围Size range 用途Using 优势与不足Advantages and disadvantages 电子显微镜/X射线光谱 SEM和场发射SEM 20 nm分辨率,但颗粒粒径最好大于80 nm 常与能量色散X射线光谱仪(EDX)结合,能够提供表面形貌、组成和电导率等信息 空间分辨率较低;无法提供颗粒物内部信息;真空操作造成水、硫酸铵和部分有机物损失 TEM和场发射TEM 0.1 nm分辨率,但颗粒粒径最好大于10 nm且小于2 mm 常与EDX联用;可以观察单个颗粒的混合状态和形貌 空间分辨率高;能够观察到粒子内部;高能电子束和真空操作造成硝酸盐的损失 扫描透射X射线显微镜-近边X射线吸收精细结构光谱 适合粒径大于200 nm的颗粒 对轻元素(C和N)和金属元素具有优异的测量能力,能够提供混合态信息 在环境压力a下工作;可以研究碳质气溶胶中特定的键类型;需要同步辐射;空间分辨率低 原子力显微镜 2 nm分辨率,颗粒物粒径最好大于10 nm且小于2 mm 能够检测颗粒表面形貌、相态和吸湿性,进而了解颗粒体积和面积 在环境压力下工作;提供颗粒物表面信息;结果解释不明确;无化学组成信息 振动光谱 拉曼光谱 颗粒粒径大于800 nm 提供颗粒物组成和分布信息 在环境压力下工作;无损的指纹识别;丰富的光谱数据;存在荧光信号干扰和检测深度限制 表面增强拉曼散射和尖端增强拉曼散射 颗粒粒径大于10 nm 提供颗粒物组成和分布信息 在环境压力下工作;样品无损识别;与拉曼相比实现了信号数量级的增强 荧光 0.5—50 μm 主要用于检测生物气溶胶 在环境压力下工作;检测的气溶胶种类单一 单颗粒soot光度计 70—500 nm soot的质量分布 检测的种类单一 质谱 气溶胶飞行时间质谱仪 100 nm分辨率,颗粒粒径最好大于100 nm 提供化学成分、非均相反应、二次粒子的形成以及气溶胶来源等信息 提供实时数据;多成分同时检测;无法提供形态信息;真空操作造成样品损失;复杂的数据处理和操作周期 纳米级二次离子质谱仪 分辨率小于50 nm,颗粒粒径最好大于50 nm 能够检测有机物和硫酸盐/硝酸盐;提供混合态信息 可以研究C、N和O元素在气溶胶中的分布;操作费时;真空操作造成样品损失 飞行时间二次离子质谱仪 50 nm分辨率,颗粒粒径最好大于100 nm 提供表面元素和分子类型信息,可视化单个物质的分布;主要用于检测有机物 可以研究碳质气溶胶中特定的键类型;空间分辨率低;真空操作造成样品损失 -
[1] 中华人民共和国生态环境部. 2020中国生态环境状况公报[R]. 2021. [2] 贺克斌, 杨复沫, 段凤魁. 大气颗粒物与区域复合污染[M]. 北京: 科学出版社, 2011. HE K B, YANG F M, DUAN F K. Atmospheric particles and regional compound pollution[M]. Beijing: Science Press, 2011(in Chinese).
[3] 楚碧武, 马庆鑫, 段凤魁, 等. 大气“霾化学”: 概念提出和研究展望 [J]. 化学进展, 2020, 32(1): 1-4. CHU B W, MA Q X, DUAN F K, et al. Atmospheric “haze chemistry”: Concept and research prospects [J]. Progress in Chemistry, 2020, 32(1): 1-4(in Chinese).
[4] GAO J H, WOODWARD A, VARDOULAKIS S, et al. Haze, public health and mitigation measures in China: A review of the current evidence for further policy response [J]. Science of the Total Environment, 2017, 578: 148-157. doi: 10.1016/j.scitotenv.2016.10.231 [5] WANG Y C, WANG Q Y, YE J H, et al. A review of aerosol chemical composition and sources in representative regions of China during wintertime [J]. Atmosphere, 2019, 10(5): 277. doi: 10.3390/atmos10050277 [6] GU J X, BAI Z P, LI W F, et al. Chemical composition of PM2.5 during winter in Tianjin, China [J]. Particuology, 2011, 9(3): 215-221. doi: 10.1016/j.partic.2011.03.001 [7] LIANG C S, DUAN F K, HE K B, et al. Review on recent progress in observations, source identifications and countermeasures of PM2.5 [J]. Environment International, 2016, 86: 150-170. doi: 10.1016/j.envint.2015.10.016 [8] LI W J, SUN J X, XU L, et al. A conceptual framework for mixing structures in individual aerosol particles [J]. Journal of Geophysical Research:Atmospheres, 2016, 121(22): 13784-13798. doi: 10.1002/2016JD025252 [9] CHINA S, SCARNATO B, OWEN R C, et al. Morphology and mixing state of aged soot particles at a remote marine free troposphere site: Implications for optical properties [J]. Geophysical Research Letters, 2015, 42(4): 1243-1250. doi: 10.1002/2014GL062404 [10] AULT A P, AXSON J L. Atmospheric aerosol chemistry: Spectroscopic and microscopic advances [J]. Analytical Chemistry, 2017, 89(1): 430-452. doi: 10.1021/acs.analchem.6b04670 [11] WANG F, YU H F, WANG Z Y, et al. Review of online source apportionment research based on observation for ambient particulate matter [J]. Science of the Total Environment, 2021, 762: 144095. doi: 10.1016/j.scitotenv.2020.144095 [12] LI W J, SHAO L Y, ZHANG D Z, et al. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions [J]. Journal of Cleaner Production, 2016, 112: 1330-1349. doi: 10.1016/j.jclepro.2015.04.050 [13] RIEMER N, AULT A P, WEST M, et al. Aerosol mixing state: Measurements, modeling, and impacts [J]. Reviews of Geophysics, 2019, 57(2): 187-249. doi: 10.1029/2018RG000615 [14] NIU H Y, SHAO L Y, ZHANG D Z. Soot particles at an elevated site in Eastern China during the passage of a strong cyclone [J]. Science of the Total Environment, 2012, 430: 217-222. doi: 10.1016/j.scitotenv.2012.04.050 [15] FU H, ZHANG M, LI W, et al. Morphology, composition and mixing state of individual carbonaceous aerosol in urban Shanghai [J]. Atmospheric Chemistry and Physics, 2012, 12(2): 693-707. doi: 10.5194/acp-12-693-2012 [16] RAMAN C V, KRISHNAN K S. A new type of secondary radiation [J]. Nature, 1928, 121(3048): 501-502. [17] 李英红, 谭吉华, 饶志国, 等. 兰州市大气细颗粒物中水溶性离子的污染特征 [J]. 环境化学, 2016, 35(9): 1799-1807. doi: 10.7524/j.issn.0254-6108.2016.09.2015101102 LI Y H, TAN J H, RAO Z G, et al. Pollution characteristics of water soluble ions in atmospheric fine particles in Lanzhou [J]. Environmental Chemistry, 2016, 35(9): 1799-1807(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.09.2015101102
[18] LI W J, ZHOU S Z, WANG X F, et al. Integrated evaluation of aerosols from regional brown hazes over Northern China in winter: Concentrations, sources, transformation, and mixing states [J]. Journal of Geophysical Research:Atmospheres, 2011, 116(D9): D09301. [19] CHENG Y F, ZHENG G J, WEI C, et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China [J]. Science Advances, 2016, 2(12): e1601530. doi: 10.1126/sciadv.1601530 [20] 唐孝炎, 张远航, 邵敏. 大气环境化学-第2版[M]. 北京: 高等教育出版社, 2006. TANG X Y, ZHANG Y H, SHAO M. Atmospheric environmental Chemistry-2nd Edition[M]. Beijing: Higher Education Press, 2006 (in Chinese).
[21] VARGAS JENTZSCH P, KAMPE B, CIOBOTĂ V, et al. Inorganic salts in atmospheric particulate matter: Raman spectroscopy as an analytical tool [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2013, 115: 697-708. doi: 10.1016/j.saa.2013.06.085 [22] ZAPATA F, GARCÍA-RUIZ C. The discrimination of 72 nitrate, chlorate and perchlorate salts using IR and Raman spectroscopy [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2018, 189: 535-542. doi: 10.1016/j.saa.2017.08.058 [23] SUN Z L, DUAN F K, HE K B, et al. Physicochemical analysis of individual atmospheric fine particles based on effective surface-enhanced Raman spectroscopy [J]. Journal of Environmental Sciences, 2019, 75: 388-395. doi: 10.1016/j.jes.2018.06.006 [24] LING T Y, CHAN C K. Formation and transformation of metastable double salts from the crystallization of mixed ammonium nitrate and ammonium sulfate particles [J]. Environmental Science & Technology, 2007, 41(23): 8077-8083. [25] WANG F, ZHENG Y X, ZHANG Y H. Temporally and spatially resolved investigation on the efflorescence process of a mixed droplet of ammonium sulfate and ammonium nitrate [J]. Chinese Science Bulletin, 2011, 56(24): 2600-2603. doi: 10.1007/s11434-011-4617-6 [26] SUN Z L, DUAN F K, HE K B, et al. Sulfate-nitrate-ammonium as double salts in PM2.5: Direct observations and implications for haze events [J]. Science of the Total Environment, 2019, 647: 204-209. doi: 10.1016/j.scitotenv.2018.07.107 [27] JENTZSCH P V, BOLANZ R M, CIOBOTĂ V, et al. Raman spectroscopic study of calcium mixed salts of atmospheric importance [J]. Vibrational Spectroscopy, 2012, 61: 206-213. doi: 10.1016/j.vibspec.2012.03.007 [28] SHARMA N, CHINA S, BHANDARI J, et al. Physical properties of aerosol internally mixed with soot particles in a biogenically dominated environment in California [J]. Geophysical Research Letters, 2018, 45(20): 11473-11482. doi: 10.1029/2018gl079404 [29] HE G Z, MA J Z, HE H. Role of carbonaceous aerosols in catalyzing sulfate formation [J]. ACS Catalysis, 2018, 8(5): 3825-3832. doi: 10.1021/acscatal.7b04195 [30] ROSEN H, NOVAKOV T. Raman scattering and the characterisation of atmospheric aerosol particles [J]. Nature, 1977, 266(5604): 708-710. doi: 10.1038/266708a0 [31] TRIVANOVIC U, SIPKENS T A, KAZEMIMANESH M, et al. Morphology and size of soot from gas flares as a function of fuel and water addition [J]. Fuel, 2020, 279: 118478. doi: 10.1016/j.fuel.2020.118478 [32] BLADT H, IVLEVA N P, NIESSNER R. Internally mixed multicomponent soot: Impact of different salts on soot structure and thermo-chemical properties [J]. Journal of Aerosol Science, 2014, 70: 26-35. doi: 10.1016/j.jaerosci.2013.11.007 [33] CASTOLDI L, MATARRESE R, BRAMBILLA L, et al. Effect of potassium on a model soot combustion: Raman and HRTEM evidences [J]. Aerosol Science and Technology, 2016, 50(4): 405-415. doi: 10.1080/02786826.2016.1158398 [34] ESS M N, FERRY D, KIREEVA E D, et al. In situ Raman microspectroscopic analysis of soot samples with different organic carbon content: Structural changes during heating [J]. Carbon, 2016, 105: 572-585. doi: 10.1016/j.carbon.2016.04.056 [35] SETO T, INOUE A, HIGASHI H, et al. Phase transition and restructuring of carbon nanoparticles induced by aerosol laser irradiation [J]. Carbon, 2014, 70: 224-232. doi: 10.1016/j.carbon.2013.12.111 [36] ESANGBEDO C, BOEHMAN A L, PEREZ J M. Characteristics of diesel engine soot that lead to excessive oil thickening [J]. Tribology International, 2012, 47: 194-203. doi: 10.1016/j.triboint.2011.11.003 [37] NORDMANN S, BIRMILI W, WEINHOLD K, et al. Measurements of the mass absorption cross section of atmospheric soot particles using Raman spectroscopy [J]. Journal of Geophysical Research:Atmospheres, 2013, 118(21): 12075-12085. doi: 10.1002/2013JD020021 [38] GE H W, YE Z P, HE R. Raman spectroscopy of diesel and gasoline engine-out soot using different laser power [J]. Journal of Environmental Sciences, 2019, 79: 74-80. doi: 10.1016/j.jes.2018.11.001 [39] HAN C, LIU Y C, MA J Z, et al. Effect of soot microstructure on its ozonization reactivity [J]. The Journal of Chemical Physics, 2012, 137(8): 084507. doi: 10.1063/1.4747190 [40] XU L, LIU L, ZHANG J, et al. Morphology, composition, and mixing state of individual aerosol particles in northeast China during wintertime [J]. Atmosphere, 2017, 8(12): 47. doi: 10.3390/atmos8030047 [41] DU J J, XU J W, SUN Z L, et al. Au nanoparticles grafted on Fe3O4 as effective SERS substrates for label-free detection of the 16 EPA priority polycyclic aromatic hydrocarbons [J]. Analytica Chimica Acta, 2016, 915: 81-89. doi: 10.1016/j.aca.2016.02.009 [42] AN P, YUAN C Q, LIU X H, et al. Vibrational spectroscopic identification of isoprene, pinenes and their mixture [J]. Chinese Chemical Letters, 2016, 27(4): 527-534. doi: 10.1016/j.cclet.2016.01.036 [43] EBBEN C J, STRICK B F, UPSHUR M A, et al. Towards the identification of molecular constituents associated with the surfaces of isoprene-derived secondary organic aerosol (SOA) particles [J]. Atmospheric Chemistry and Physics, 2014, 14(5): 2303-2314. doi: 10.5194/acp-14-2303-2014 [44] CHEN P F, BI X H, ZHANG J Q, et al. Assessment of heavy metal pollution characteristics and human health risk of exposure to ambient PM2.5 in Tianjin, China [J]. Particuology, 2015, 20: 104-109. doi: 10.1016/j.partic.2014.04.020 [45] AVZIANOVA E, BROOKS S D. Analysis of nickel (II) in particulate matter by Raman microspectroscopy [J]. Journal of Aerosol Science, 2014, 67: 207-214. doi: 10.1016/j.jaerosci.2013.10.003 [46] MORILLAS H, MARCAIDA I, MAGUREGUI M, et al. Identification of metals and metalloids as hazardous elements in PM2.5 and PM10 collected in a coastal environment affected by diffuse contamination [J]. Journal of Cleaner Production, 2019, 226: 369-378. doi: 10.1016/j.jclepro.2019.04.063 [47] GOIENAGA N, SARMIENTO A, OLIVARES M, et al. Emerging application of a structural and chemical analyzer for the complete characterization of metal-rich particulate matter [J]. Analytical Chemistry, 2013, 85(15): 7173-7181. doi: 10.1021/ac400878y [48] IVLEVA N P, MCKEON U, NIESSNER R, et al. Raman microspectroscopic analysis of size-resolved atmospheric aerosol particle samples collected with an ELPI: Soot, humic-like substances, and inorganic compounds [J]. Aerosol Science and Technology, 2007, 41(7): 655-671. doi: 10.1080/02786820701376391 [49] HARPALE V M, GOSAVI R S, Raman spectroscopic investigation of multicomponent aerosols from the environment of sugar factory [C]. 3rd Biannual International Conference on Advanced Atmospheric Aerosol, 2010. [50] SINANIS S, ALEKSANDROVA M, SCHABER K. Characterization of multicomponent aerosols by Raman spectroscopy [J]. Aerosol Science and Technology, 2011, 45(6): 751-757. doi: 10.1080/02786826.2011.559494 [51] JENTZSCH P V, CIOBOTĂ V, KAMPE B, et al. Origin of salt mixtures and mixed salts in atmospheric particulate matter [J]. Journal of Raman Spectroscopy, 2012, 43(4): 514-519. doi: 10.1002/jrs.3064 [52] STEER B, GORBUNOV B, PRICE M C, et al. Raman spectroscopic identification of size-selected airborne particles for quantitative exposure assessment [J]. Measurement Science and Technology, 2016, 27(4): 045801. doi: 10.1088/0957-0233/27/4/045801 [53] CHEN H, DUAN F K, DU J J, et al. Surface-enhanced Raman scattering for mixing state characterization of individual fine particles during a haze episode in Beijing, China [J]. Journal of Environmental Sciences, 2021, 104: 216-224. doi: 10.1016/j.jes.2020.12.008 [54] SIEPKA D, UZU G, STEFANIAK E A, et al. Combining Raman microspectrometry and chemometrics for determining quantitative molecular composition and mixing state of atmospheric aerosol particles [J]. Microchemical Journal, 2018, 137: 119-130. doi: 10.1016/j.microc.2017.10.005 [55] OFNER J, KAMILLI K A, EITENBERGER E, et al. Chemometric analysis of multisensor hyperspectral images of precipitated atmospheric particulate matter [J]. Analytical Chemistry, 2015, 87(18): 9413-9420. doi: 10.1021/acs.analchem.5b02272 [56] BATONNEAU Y, SOBANSKA S, LAUREYNS J, et al. Confocal microprobe Raman imaging of urban tropospheric aerosol particles [J]. Environmental Science & Technology, 2006, 40(4): 1300-1306. [57] OFFROY M, MOREAU M, SOBANSKA S, et al. Pushing back the limits of Raman imaging by coupling super-resolution and chemometrics for aerosols characterization [J]. Scientific Reports, 2015, 5: 12303. doi: 10.1038/srep12303 [58] AO J P, FENG Y Q, WU S M, et al. Rapid, 3D chemical profiling of individual atmospheric aerosols with stimulated Raman scattering microscopy [J]. Small Methods, 2020, 4(2): 1900600. doi: 10.1002/smtd.201900600 [59] DENG C H, BROOKS S D, VIDAURRE G, et al. Using Raman microspectroscopy to determine chemical composition and mixing state of airborne marine aerosols over the Pacific Ocean [J]. Aerosol Science and Technology, 2014, 48(2): 193-206. doi: 10.1080/02786826.2013.867297 [60] DEBOUDT K, FLAMENT P, CHOËL M, et al. Mixing state of aerosols and direct observation of carbonaceous and marine coatings on African dust by individual particle analysis[J]. Journal of Geophysical Research: Atmospheres, 2010, 115: D24. [61] LASKINA O, YOUNG M A, KLEIBER P D, et al. Infrared extinction spectroscopy and micro-Raman spectroscopy of select components of mineral dust mixed with organic compounds [J]. Journal of Geophysical Research:Atmospheres, 2013, 118(12): 6593-6606. doi: 10.1002/jgrd.50494 [62] LIU Y, ZHENG M, YU M Y, et al. High-time-resolution source apportionment of PM2.5 in Beijing with multiple models [J]. Atmospheric Chemistry and Physics, 2019, 19(9): 6595-6609. doi: 10.5194/acp-19-6595-2019 [63] ZHU Y H, HUANG L, LI J Y, et al. Sources of particulate matter in China: Insights from source apportionment studies published in 1987-2017 [J]. Environment International, 2018, 115: 343-357. doi: 10.1016/j.envint.2018.03.037 [64] FERRUGIARI A, TOMMASINI M, ZERBI G. Raman spectroscopy of carbonaceous particles of environmental interest [J]. Journal of Raman Spectroscopy, 2015, 46(12): 1215-1224. doi: 10.1002/jrs.4753 [65] CATELANI T, PRATESI G, ZOPPI M. Raman characterization of ambient airborne soot and associated mineral phases [J]. Aerosol Science and Technology, 2014, 48(1): 13-21. doi: 10.1080/02786826.2013.847270 [66] FENG Y Q, LIU L, YANG Y, et al. The application of Raman spectroscopy combined with multivariable analysis on source apportionment of atmospheric black carbon aerosols [J]. Science of the Total Environment, 2019, 685: 189-196. doi: 10.1016/j.scitotenv.2019.05.367 [67] POPOVICHEVA O, KIREEVA E, PERSIANTSEVA N, et al. Microscopic characterization of individual particles from multicomponent ship exhaust [J]. Journal of Environmental Monitoring, 2012, 14(12): 3101-3110. doi: 10.1039/c2em30338h [68] WAGNER J, WANG Z M, GHOSAL S, et al. Source identification on high PM2.5 days using SEM/EDS, XRF, Raman, and windblown dust modeling [J]. Aerosol and Air Quality Research, 2019, 19(11): 2518-2530. doi: 10.4209/aaqr.2019.05.0276 [69] LIU M X, SONG Y, ZHOU T, et al. Fine particle pH during severe haze episodes in Northern China [J]. Geophysical Research Letters, 2017, 44(10): 5213-5221. doi: 10.1002/2017GL073210 [70] WEBER R J, GUO H Y, RUSSELL A G, et al. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years [J]. Nature Geoscience, 2016, 9(4): 282-285. doi: 10.1038/ngeo2665 [71] LIN Y H, ZHANG Z F, DOCHERTY K S, et al. Isoprene epoxydiols as precursors to secondary organic aerosol formation: Acid-catalyzed reactive uptake studies with authentic compounds [J]. Environmental Science & Technology, 2012, 46(1): 250-258. [72] LOSEY D J, PARKER R G, FREEDMAN M A. pH dependence of liquid-liquid phase separation in organic aerosol [J]. The Journal of Physical Chemistry Letters, 2016, 7(19): 3861-3865. doi: 10.1021/acs.jpclett.6b01621 [73] FANG T, GUO H Y, ZENG L H, et al. Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity [J]. Environmental Science & Technology, 2017, 51(5): 2611-2620. [74] RINDELAUB J D, CRAIG R L, NANDY L, et al. Direct measurement of pH in individual particles via Raman microspectroscopy and variation in acidity with relative humidity [J]. The Journal of Physical Chemistry A, 2016, 120(6): 911-917. doi: 10.1021/acs.jpca.5b12699 [75] CRAIG R L, NANDY L, AXSON J L, et al. Spectroscopic determination of aerosol pH from acid-base equilibria in inorganic, organic, and mixed systems [J]. The Journal of Physical Chemistry A, 2017, 121(30): 5690-5699. doi: 10.1021/acs.jpca.7b05261 [76] CRAIG R L, PETERSON P K, NANDY L, et al. Direct determination of aerosol pH: Size-resolved measurements of submicrometer and supermicrometer aqueous particles [J]. Analytical Chemistry, 2018, 90(19): 11232-11239. doi: 10.1021/acs.analchem.8b00586 [77] LEI Z Y, BLIESNER S E, MATTSON C N, et al. Aerosol acidity sensing via polymer degradation [J]. Analytical Chemistry, 2020, 92(9): 6502-6511. doi: 10.1021/acs.analchem.9b05766 [78] CHANG P P, CHEN Z, ZHANG Y H, et al. Direct measurement of aerosol pH in individual malonic acid and citric acid droplets under different relative humidity conditions via Raman spectroscopy [J]. Chemosphere, 2020, 241: 124960. doi: 10.1016/j.chemosphere.2019.124960 [79] WEI H R, VEJERANO E P, LENG W N, et al. Aerosol microdroplets exhibit a stable pH gradient [J]. PNAS, 2018, 115(28): 7272-7277. doi: 10.1073/pnas.1720488115 [80] BOYER H C, GORKOWSKI K, SULLIVAN R C. In situ pH measurements of individual levitated microdroplets using aerosol optical tweezers [J]. Analytical Chemistry, 2020, 92(1): 1089-1096. doi: 10.1021/acs.analchem.9b04152 [81] 马金珠, 刘永春, 马庆鑫, 等. 大气非均相反应及其环境效应 [J]. 环境化学, 2011, 30(1): 97-119. doi: 10.1002/etc.379 MA J Z, LIU Y C, MA Q X, et al. Atmospheric heterogeneous reactions and their environmental effects [J]. Environmental Chemistry, 2011, 30(1): 97-119(in Chinese). doi: 10.1002/etc.379
[82] ZHAO D F, ZHU T, CHEN Q, et al. Raman micro-spectrometry as a technique for investigating heterogeneous reactions on individual atmospheric particles [J]. Science China Chemistry, 2011, 54(1): 154-160. doi: 10.1007/s11426-010-4182-x [83] ZHU T, SHANG J, ZHAO D F. The roles of heterogeneous chemical processes in the formation of an air pollution complex and gray haze [J]. Science China Chemistry, 2011, 54(1): 145-153. doi: 10.1007/s11426-010-4181-y [84] ZHAO D F, SONG X J, ZHU T, et al. Multiphase oxidation of SO2 by NO2 on CaCO3 particles [J]. Atmospheric Chemistry and Physics, 2018, 18(4): 2481-2493. doi: 10.5194/acp-18-2481-2018 [85] YU T, ZHAO D F, SONG X J, et al. NO2-initiated multiphase oxidation of SO2 by O2 on CaCO3 particles [J]. Atmospheric Chemistry and Physics, 2018, 18(9): 6679-6689. doi: 10.5194/acp-18-6679-2018 [86] LEE A K Y, CHAN C K. Single particle Raman spectroscopy for investigating atmospheric heterogeneous reactions of organic aerosols [J]. Atmospheric Environment, 2007, 41(22): 4611-4621. doi: 10.1016/j.atmosenv.2007.03.040 [87] LIU Y C, LIU C, MA J Z, et al. Structural and hygroscopic changes of soot during heterogeneous reaction with O3 [J]. Physical Chemistry Chemical Physics, 2010, 12(36): 10896-10903. doi: 10.1039/c0cp00402b [88] CHU Y X, CHENG T F, GEN M S, et al. Effect of ozone concentration and relative humidity on the heterogeneous oxidation of linoleic acid particles by ozone: An insight into the interchangeability of ozone concentration and time [J]. ACS Earth and Space Chemistry, 2019, 3(5): 779-788. doi: 10.1021/acsearthspacechem.9b00002 [89] RAY D, BHATTACHARYA T S, CHATTERJEE A, et al. Hygroscopic coating of sulfuric acid shields oxidant attack on the atmospheric pollutant benzo(a)Pyrene bound to model soot particles [J]. Scientific Reports, 2018, 8: 129. doi: 10.1038/s41598-017-18292-z [90] MA Q X, LIU Y C, LIU C, et al. Heterogeneous reaction of acetic acid on MgO, α-Al2O3, and CaCO3 and the effect on the hygroscopic behaviour of these particles [J]. Physical Chemistry Chemical Physics, 2012, 14(23): 8403. doi: 10.1039/c2cp40510e [91] WANG X W, JING B, TAN F, et al. Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate [J]. Atmospheric Chemistry and Physics, 2017, 17(20): 12797-12812. doi: 10.5194/acp-17-12797-2017 [92] 常翩翩, 张韫宏. 气溶胶单颗粒的拉曼测量方法 [J]. 光散射学报, 2020, 32(4): 295-300. doi: 10.13883/j.issn1004-5929.202004001 CHANG P P, ZHANG Y H. Raman spectroscopy measurement for aerosol single particle [J]. The Journal of Light Scattering, 2020, 32(4): 295-300(in Chinese). doi: 10.13883/j.issn1004-5929.202004001
[93] 马庆鑫, 马金珠, 楚碧武, 等. 矿质和黑碳颗粒物表面大气非均相反应研究进展 [J]. 科学通报, 2015, 60(2): 122-136. doi: 10.1360/N972014-01190 MA Q X, MA J Z, CHU B W, et al. Current progress towards the heterogeneous reactions on mineral dust and soot [J]. Chinese Science Bulletin, 2015, 60(2): 122-136(in Chinese). doi: 10.1360/N972014-01190
[94] MA Q X, HE H. Synergistic effect in the humidifying process of atmospheric relevant calcium nitrate, calcite and oxalic acid mixtures [J]. Atmospheric Environment, 2012, 50: 97-102. doi: 10.1016/j.atmosenv.2011.12.057 [95] MA Q X, HE H, LIU Y C, et al. Heterogeneous and multiphase formation pathways of gypsum in the atmosphere [J]. Physical Chemistry Chemical Physics, 2013, 15(44): 19196-19204. doi: 10.1039/c3cp53424c [96] WU F M, WANG X W, JING B, et al. Liquid-liquid phase separation in internally mixed magnesium sulfate/glutaric acid particles [J]. Atmospheric Environment, 2018, 178: 286-292. doi: 10.1016/j.atmosenv.2018.02.012 [97] FLEISCHMANN M, HENDRA P J, MCQUILLAN A J. Raman spectra of pyridine adsorbed at a silver electrode [J]. Chemical Physics Letters, 1974, 26(2): 163-166. doi: 10.1016/0009-2614(74)85388-1 [98] AYORA M J, BALLESTEROS L, PÉREZ R, et al. Detection of atmospheric contaminants in aerosols by surface-enhanced Raman spectrometry [J]. Analytica Chimica Acta, 1997, 355(1): 15-21. doi: 10.1016/S0003-2670(97)81607-8 [99] CRAIG R L, BONDY A L, AULT A P. Surface enhanced Raman spectroscopy enables observations of previously undetectable secondary organic aerosol components at the individual particle level [J]. Analytical Chemistry, 2015, 87(15): 7510-7514. doi: 10.1021/acs.analchem.5b01507 [100] FU Y, KUPPE C, VALEV V K, et al. Surface-enhanced Raman spectroscopy: A facile and rapid method for the chemical component study of individual atmospheric aerosol [J]. Environmental Science & Technology, 2017, 51(11): 6260-6267. [101] DONG X, OHNOUTEK L, YANG Y, et al. Cu/Ag Sphere Segment Void Array as Efficient Surface Enhanced Raman Spectroscopy Substrate for Detecting Individual Atmospheric Aerosol [J]. Analytical Chemistry, 2019, 91(21): 13647-13657. doi: 10.1021/acs.analchem.9b02840 [102] GEN M S, CHAN C K. Electrospray surface-enhanced Raman spectroscopy (ES-SERS) for probing surface chemical compositions of atmospherically relevant particles [J]. Atmospheric Chemistry and Physics, 2017, 17(22): 14025-14037. doi: 10.5194/acp-17-14025-2017 [103] GEN M S, KUNIHISA R, MATSUKI A, et al. Electrospray surface-enhanced Raman spectroscopy (ES-SERS) for studying organic coatings of atmospheric aerosol particles [J]. Aerosol Science and Technology, 2019, 53(7): 760-770. doi: 10.1080/02786826.2019.1597964 [104] PHAN-QUANG G C, LEE H K, TENG H W, et al. Plasmonic hotspots in air: An omnidirectional three-dimensional platform for stand-off in-air SERS sensing of airborne species [J]. Angwandte Chemie (International Ed. in English), 2018, 57(20): 5792-5796. doi: 10.1002/anie.201802214 [105] PHAN-QUANG G C, YANG N C, LEE H K, et al. Tracking airborne molecules from afar: Three-dimensional metal-organic framework-surface-enhanced Raman scattering platform for stand-off and real-time atmospheric monitoring [J]. ACS Nano, 2019, 13(10): 12090-12099. doi: 10.1021/acsnano.9b06486 [106] JONES R R, HOOPER D C, ZHANG L W, et al. Raman techniques: Fundamentals and frontiers [J]. Nanoscale Research Letters, 2019, 14(1): 231. doi: 10.1186/s11671-019-3039-2 [107] LIU R, LIU J F, ZHOU X X, et al. Applications of Raman-based techniques to on-site and in-vivo analysis [J]. Trends in Analytical Chemistry, 2011, 30 (9):1462-1476 . DOI: 10.1016/j.trac.2011.06.011 [108] OFNER J, DECKERT-GAUDIG T, KAMILLI K A, et al. Tip-enhanced Raman spectroscopy of atmospherically relevant aerosol nanoparticles [J]. Analytical Chemistry, 2016, 88(19): 9766-9772. doi: 10.1021/acs.analchem.6b02760 -




 下载:
下载: