-
重金属污染已成为全球性的环境问题[1],重金属能通过到空气、水和土壤危害人体健康。据报道,长期接触镉(Cd)会引起肺腺癌、肺癌、肾功能损伤和骨折[2];长期高剂量暴露在锌(Zn)会影响胆固醇的平衡和生育能力[3];铅(Pb)中毒可能导致各种类型的疾病,包括神经系统疾病,阿尔茨海默氏病[4];慢性砷(As)可能导致角化过度,皮肤病变以及肺癌,膀胱癌和肾病[5];长期接触汞(Hg),会积聚在脂肪组织中,并损害人体中枢神经系统[6];铜(Cu)、铬(Cr)和镍(Ni)也会对人体健康产生不利影响[7]。基于重金属对生物体的高致癌、高毒害作用,2011年3月,《重金属污染综合防治“十二五”规划》获得国务院正式批复,成为我国第一个“十二五”国家级别的专项规划。由此可以看出,重金属污染防治是当前和今后一个时期环境保护工作的重中之重。
固化/稳定化是治理重金属污染的有效方法之一[8],波特兰水泥(OPC)是重金属废物固化/稳定化的常用基质,但研究表明,重金属的存在对OPC的抗压强度(降低30.4%)不利[9],但对磷酸镁水泥(MPC)的影响相对要小得多。固化重金属或重金属污染土后的磷酸镁水泥进行二次使用或者掩埋,都需要高的抗压强度和低的渗透率[10],结合磷酸镁水泥早期强度高、稳定性好和孔隙率低的特点,并且毒性特征浸出程序(TCLP)结果表明,浸出毒性浸出浓度远低于国家标准(GB5085.3-2007) [9],因此磷酸镁水泥在固化/稳定化重金属方面有着良好的前景。
基于此,本文综述MPC固化/稳定化重金属和重金属污染土的研究现状,讨论重金属种类和掺量等常见因素对MPC力学性能、凝结时间、酸碱度、水化热及水化机理等方面的影响。MPC常处理重金属主要包括Pb、Ni、Cu、Zn、Cr、Mn、Hg等物质,而其固化/稳定化重金属效果受重金属含量、重金属种类、氧化镁和磷酸盐质量比(M/P)和固化时间等众多因素影响;固化作用机理主要包括3个方面:(1)重金属离子与磷酸根离子结合形成低溶度积的化合物,如Pb5(PO4)3OH、Cd3(PO4)2等;(2)MPC水化产物对重金属离子产生较强的吸附作用;(3)MPC具有较强的物理力学性能和致密的结构,对重金属离子具有物理包裹作用。通过化学键合、吸附、物理包裹三重作用,从而实现重金属离子的高效率和大容量固化。本文以期为后续MPC固化/稳定化废弃物中重金属和重金属污染土研究提供前期的基础数据及理论依据。
-
MPC固化/稳定化重金属的处理效果与很多因素有关,主要包括重金属种类、重金属处理量、M/P和固化时间,其固化效果及影响因素见表1。重金属的固化效果主要以抗压强度、凝结时间和处理样毒性浸出特性为指标。(1)重金属种类,MPC常用来处理Pb、Cu、Cd和Ni等重金属以及放射性Sr、Cs等污染物。不同种类的重金属,固化效果不同,相同条件下,Zn的存在会降低MPC的抗压强度,Cu、Cd和Ni等会提高MPC抗压强度,但均会延长凝结时间;同时固化Pb和Cd,MPC对Pb的固化效果大于Cd;此外,不同种类重金属间也存在相互影响,例如Pb的存在会削弱Zn的固定作用。(2)重金属处理量,在相同的M/P下,重金属经MPC固化,固化过程中金属离子会与磷酸根反应形成相应的磷酸盐,当MPC处理重金属的量增加时,重金属的加入会阻碍鸟粪石的形成,减缓材料凝结速率,延长凝结时间,而固化体抗压强度则会降低、毒性浸出浓度增大。(3)M/P比,当M/P比过高时,体系水化反应的磷酸盐相对较小,这类似于重金属对MPC抗压强度的影响。在MPC的水化硬化过程中,虽然重金属离子可与磷酸根离子反应生成磷酸盐水合相,且一定程度改善材料的致密性,但其对材料强度的负作用更为突出。研究表明,过高的M/P比对MPC的抗压强度有降低作用,但对MPC的凝结时间影响不是很明显。在毒性浸出浓度方面,随着M/P比的增加,浸出浓度也增加。(4)固化时间,随着固化时间的增加,MPC主水化产物(鸟粪石)不断长大结晶,并逐渐填充体系孔隙,阻滞孔隙的贯通性,从而不断降低渗透系数、提高材料抗压强度和降低毒性浸出浓度。
综上所述,不同重金属的加入均会延长MPC的凝结时间,但其对不同重金属的作用效果不同。总体而言,MPC对Pb的作用效果较为显著;当重金属处理量恒定时,材料抗压强度和毒性浸出浓度与M/P和固化时间密切相关,延长固化时间和一定的M/P可有效抑制重金属溶出和提高材料抗压强度。
-
磷酸镁水泥(MPC)的形成是基于氧化镁(MgO)与磷酸/磷酸盐(H3PO4或NH4H2PO4、(NH4)2HPO4、KH2PO4)之间的酸碱反应,该反应的主要产物是鸟粪石(MgNH4PO4·6H2O/MgKPO4·6H2O),体系酸碱度能影响MPC水化反应速率以及水化产物的生成与结晶[23],进而影响材料宏观性能如凝结时间和力学性能。同样,体系酸碱度也是稳定处理危险废物的一个非常重要的参数,中和能力的强弱决定了重金属在MPC的化学稳定性[24]。在MPC固化重金属过程中,表1中的影响因素不同,则会影响固化体系的pH值。
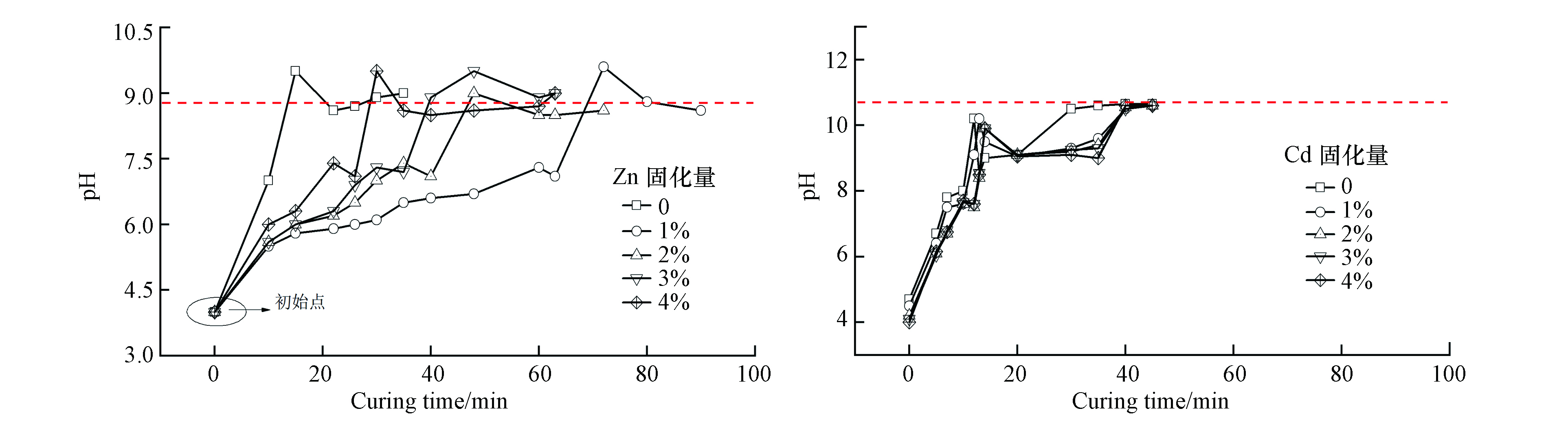
如He等[15-16]、Lai等[17]和Tao等[18]分别研究了在一定硼砂作用下,Zn2+、Cu2+、Ni2+和Cd2+掺量对MPC早期pH的影响,用PHS-3C酸度计测试不同重金属含量下MPC体系的pH变化趋势,本文重点讨论Zn2+和Ni2+对MPC的pH的影响,结果见图1。从图1可以看出,所有样品的pH都显示出随时间的推移从酸性到碱性的变化,最终pH趋近于碱性。对不添加Zn2+的样品,酸碱度存在极值;而含Zn2+物质的加入对体系的初始和最终酸碱度整体没有显著影响,仅是pH的变化速率变缓慢,其中含1%Zn2+样品的变化速率最慢,其归因于当Zn2+含量较低时,其加速H2PO4–的电离,增加H+的相对量。Cd2+的加入同样不改变MPC水化反应过程pH演变趋势,仅减缓pH变化速率。分析MPC体系pH的变化过程:早期pH的迅速增加与硼砂相关,硼砂与水混合后,会释放B4O72+和Na+,与KH2PO4电离的H+结合形成硼酸,导致溶液中H+的量减少,pH增加;随着Cd2+、Zn2+含量的增加,pH整体变化速度减慢,这表明重金属Cd2+、Zn2+的加入延缓了MPC的水化速率。由此可知,重金属的加入对MPC初始和最终pH影响不大,仅影响体系pH的变化速率。
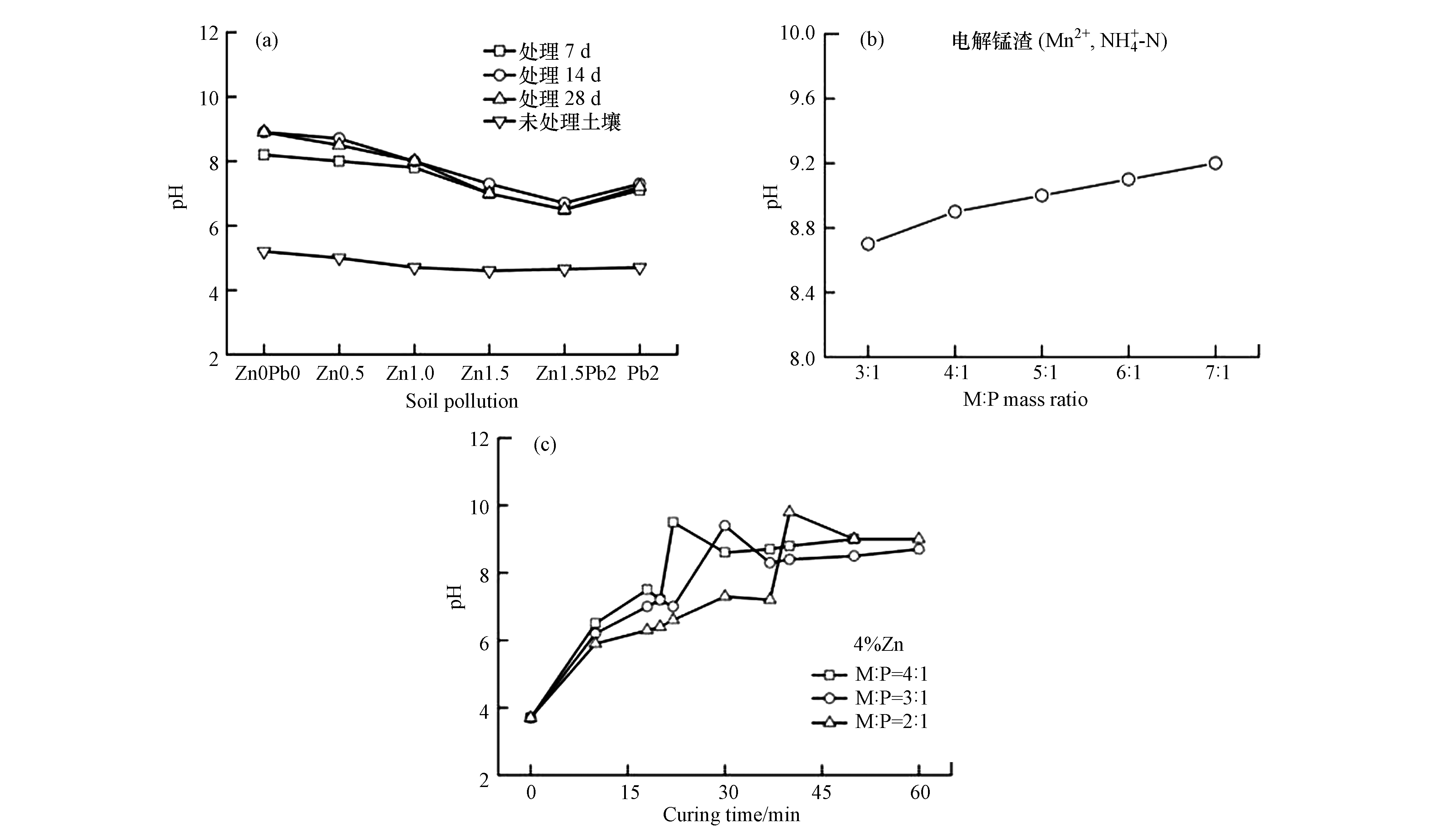
另外,有学者从不同角度研究了MPC固化重金属体系pH的变化趋势。如Du等[25]提出了一种由草酸活化磷矿、磷酸二氢钾和活性氧化镁组成的MPC,用来固化土壤中的Pb和Zn,同时探究两者之间的相互影响作用,结果如图2(a)所示。从图2(a)中可以看出,MPC固化后的渗滤液酸碱度比未经处理的污染土壤酸碱度高1.9—3.5个单位,含复合Zn2+和Pb2+污染土壤的渗滤液酸碱度比单一Zn2+或Pb2+的土壤酸碱度低0.5—0.8个单位。说明,一定浓度的重金属可提高污染土的酸中和能力。Shu等[26]和Lai等[17]探究了不同M/P和时间下固化体系pH变化规律。从图2(b, c)可以观察到,对于不同M/P的固化体系,样品的pH变化趋势相似。在重金属掺量和MPC添加量恒定的条件下,固化体系pH随着M/P比和时间的增加而增大,这是由于体系中磷酸盐的相对含量减少所致。
-
在MPC的早期水化阶段,其水化迅速,大量集中放热[27],导致体系水化温度升高,体系可从20 ℃升至90 ℃,在绝热条件下甚至超过100 ℃[28]。MPC水化释放的热影响体系温度,即反应速率,进而影响材料的凝结时间和强度。在MPC中加入重金属化合物,重金属会延缓体系的水化反应速率,降低体系早期水化温度峰值以及到峰值所需时间,使其早期水化程度降低。总结文献发现,体系早期水化热的释放情况主要是通过测定体系放热速率、累积水化热或水化温度。
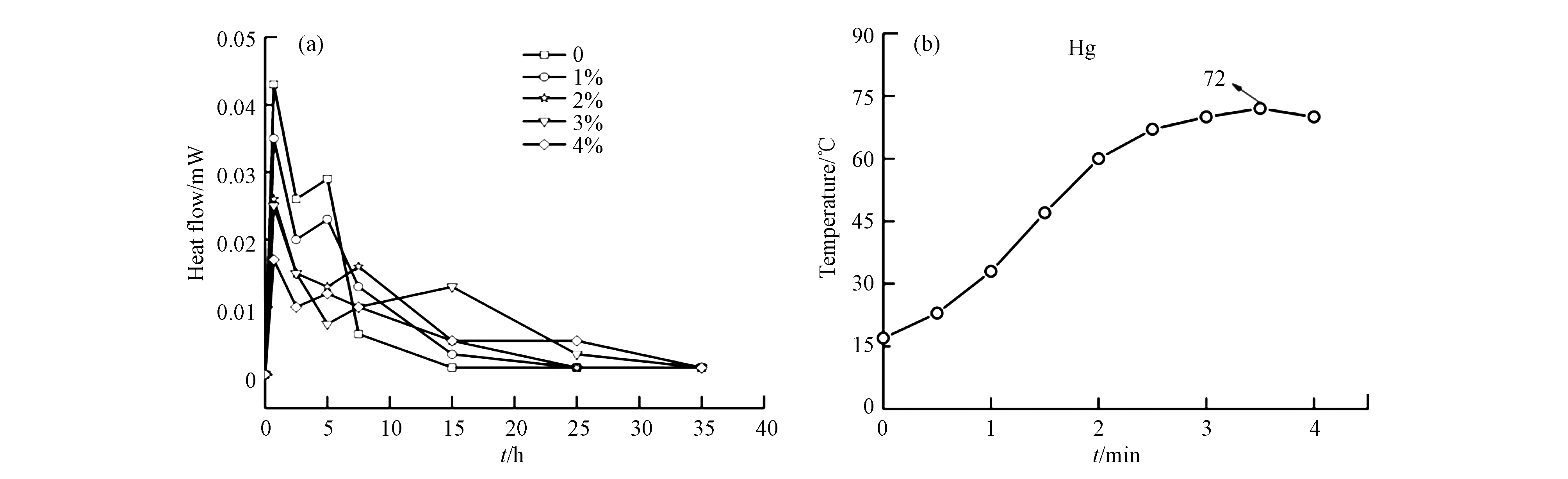
He等[15-17]和石军兵等[29]从水化热量角度分析了Cu,Cd和Zn化合物对MPC体系水化热的影响规律,3种金属对水化热量的影响规律大致相同,规律见图3(a)。从图3(a)可知,重金属含量的增加不会改变体系的放热水化趋势,均存在2个放热峰,且第1个放热峰的峰值显著高于第2个放热峰,说明体系在短时间内释放大量热量。作者认为,MPC体系的第1个放热峰是体系MgO的溶解所致:MgO+H2O→MgOH++OH–;MgOH++2H2O→Mg(OH)2+H3O+;Mg(OH)2→Mg2++2OH–。而第2个放热峰主要是由于水化反应产物鸟粪石的形成以及磷酸盐化合物能附着在MgO表面在一定程度上阻碍MPC的水化反应的双重作用结果。在MPC固化重金属体系,第1个峰值是MgO和重金属化合物如硝酸铜、硝酸铬和硝酸锌溶解的叠加效果,二者叠加使峰值降低,且这种趋势随着重金属掺量的增加而增大。重金属化合物硝酸铜、硝酸铬和硝酸锌溶解释放的重金属离子的在MPC水化过程可参与反应,形成重金属磷酸盐化合物,该化合物形成消耗一定量的酸根离子,从而导致可用于形成鸟粪石的量相对减少,减缓鸟粪石形成速率和生成量,从而延缓和降低第2个峰的峰值和达到峰值所需时间(图3a,b,c)。另外,有学者研究了MPC固化重金属体系的水化温度变化趋,Liu等[21]测定了MPC固化0.25%Hg2+体系水化温度的变化,结果如图3(b)所示。从图3(b)可以看出,体系在1 min内缓慢上升,从第2 min开始温度急剧增大。原因在于MPC中通常掺入缓凝剂硼酸,其与体系中的磷酸盐发生反应,生成了菱镁矿(Mg3(PO4) B2O3·8H2O)薄层附着在MgO表面,这阻止了MgO与磷酸盐的反应,待薄层溶解后,MgO与磷酸盐发生水合反应,放出热量。反应一段时间后,体系上升趋势平缓达温度极值(72°C),并出现下降趋势,理论上经一段时间体系将至室温。
-
MPC固化重金属后的稳定性是评估其能否安全处置的最重要的指标,其评估方式主要是通过考察外界环境对重金属MPC体系稳定性的影响。总结现有文献发现,常考察酸雨、核辐射、温度、冻融循环等对其的影响。
如王哲等[30]模拟了酸雨淋滤对MPC固化Zn2+的影响。研究发现,随着酸雨酸度的增加,Zn2+的浸出浓度和有效扩散系数不断增大,稳定性变差。其原因为:Zn2+在体系中的主要存在形式为Zn3(PO4)2·4H2O、Zn(OH)2等难溶盐,但随着酸度的增加,会发生逆转反应,体系倾向于生成更加稳定的Mg3(PO4)2·22H2O、Mg3(PO4)2·8H2O,导致体系中的Zn2+溶解。
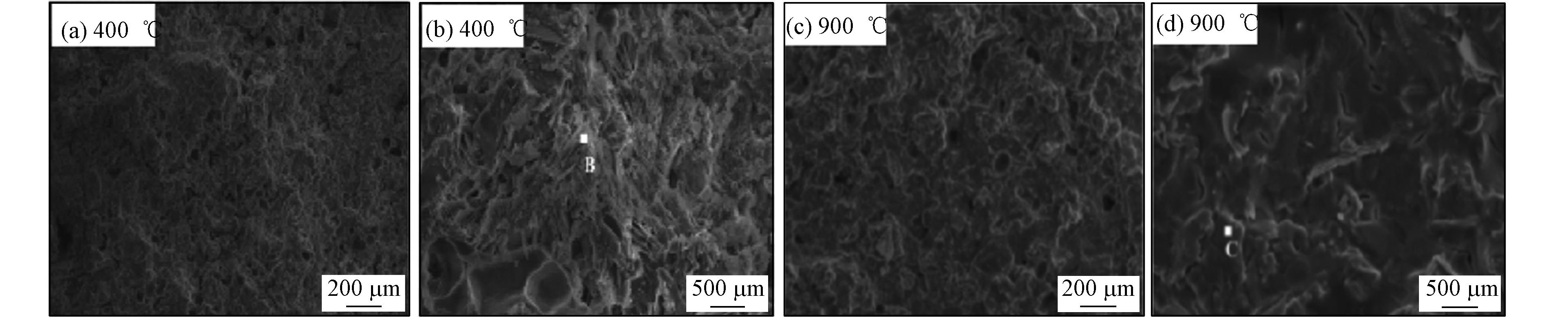
MPC在长期贮存固化放射性废物期间,体系中的水会因为放射分解,可能会产生氧气和爆炸性气体氢气,对MPC长期稳定性产生影响。Bykov等[31]探究了γ射线对MPC的影响,结果表明,在放射剂量为25 MGy时,约0.18%的水分解,这表明该材料具有很高的抗辐射性。而且体系没有生成O2,这消除了爆炸性气体混合物的形成,H2的形成不会导致MPC膨胀或破坏。即使在高吸收剂量下,也未检测到材料的机械强度有明显变化,而且体系的微观结构也没有发生明显变化。所以,此研究结果可以为MPC固化放射性废物提供有力证明,而高放射性核素的放热率大于2 kw·m-3时,可能会对重金属MPC体系的稳定性产生影响,此推测出的结论与傅明娇等[32]一致。在傅明娇等[32]考察高温对MPC固化α-高放射性核废液稳定性的影响研究中发现,在400 ℃下,重金属MPC体系浸出率高于未烧结的,900℃下则低于未烧结的,通过观察其微观形貌发现在900 ℃下具有致密的陶瓷结构,比400 ℃下固体性质更加稳定(如图4所示)。分析其原因为:400 ℃下,体系内形成多孔结构,导致浸出率增大、浸出浓度增加;900 ℃下,体系内的MgKPO4、MgCsPO4等晶体由于高温烧结熔融在一起,形成致密的陶瓷体结构,则表现出更低的浸出率。
此外,侯世伟等[33]探究了在冻融循环下MPC固化铜污染土的长期稳定性,铜污染土在MPC稳定固化后,Cu2+的浸出浓度随冻融循环次数(0、 6、 12)的增加而上升;在反复冻融条件下,体系内的水反复冻融产生膨胀力对体系造成破坏,同时体系内的胶结物也被破坏,导致Cu2+浸出浓度增加。
-
MPC为未反应完的氧化镁颗粒和主水化产物(鸟粪石)形成的致密结构体,在废物管理领域,尤其是在有毒重金属以及低水平核废料的固化/稳定化方面具有广阔的应用前景。基于MPC结构体中含有大量未反应的MgO颗粒和HPO42-、H2PO4-、PO43-、OH-等阴离子,重金属离子可与上述离子发生沉淀反应生成磷酸盐、氢氧化物等难溶性化合物,吸附于主水化产物(鸟粪石)表面,或者其包裹在体系内[30]。通过化学键合、吸附作用和物理包裹作用实现有害物质的固化/稳定固封。
-
化学键合作用就是通过向原始介质中引入化学试剂来固化或将可移动的污染物转化为低溶解度和低迁移率的沉淀物,从而将污染物固定的一种作用方式[34]。在制备MPC时需要加入磷酸或者磷酸盐,引入大量阴离子,易与重金属离子反应生成难溶盐。Shu等[26,35]、Cho等[36]和Buj等[37]探究了MPC对Mn2+等重金属的固化机理;Du等[25]、Sanderson等[38]、Feng等[39] Wang等[40]探究了MPC对土壤中Pb等重金属的固化机理。上述作者认为MPC固化/稳定化重金属的机理为:首先可溶性AH2PO4(KH2PO4/NH4H2PO4)溶解形成酸性溶液(见式(1)、式(2)和式(3)),其式中A代表NH4或K;之后,在酸性溶液中,氧化镁受H+攻击逐渐溶解释放Mg2+见式(4);Mg2+与NH4+和HPO42-、H2PO4-、PO43-等离子反应形成非晶或晶态磷酸盐水化产物(MgNH4PO4·6H2O/MgKPO4·6H2O),具体反应方程式如式(5)、式(6)和式(7)所示。在MPC水化过程中,重金属Mn、Pb、Zn、Cr、Cu等离子参与化学键合反应,生成难溶性磷酸盐如如Mn3(PO4)2·nH2O、Pb3(PO4)2·nH2O、Cd3(PO4)2·nH2O、Cu3(PO4)2·nH2O、MnHPO4·nH2O、Mn(H2PO4)2·nH2O、Zn(OH)2、Mn(OH)2等,将化学式中重金属离子记作M,具体反应方程式如式(5)—(14)所示。
-
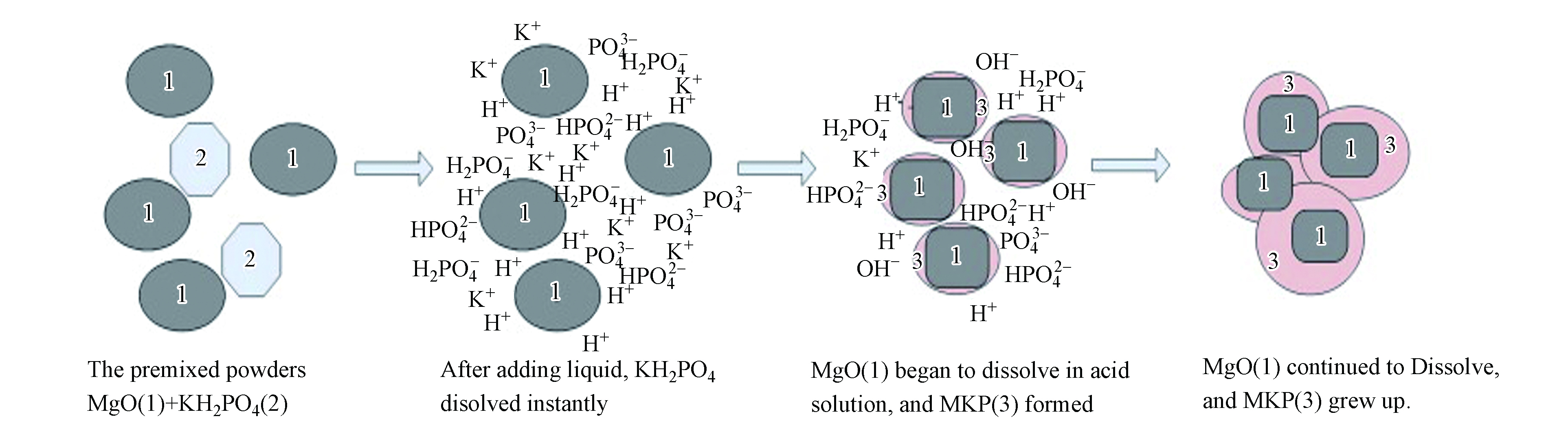
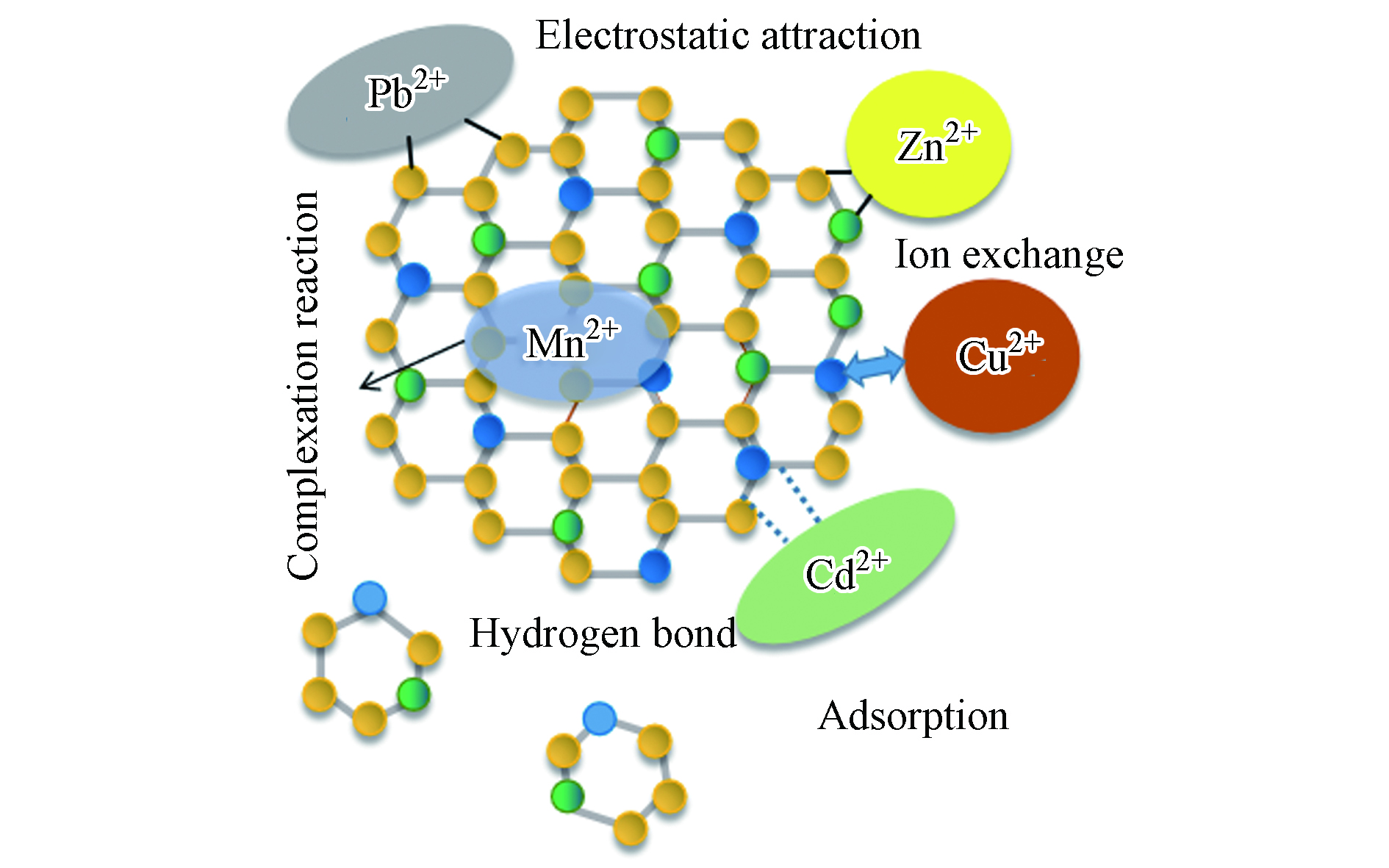
吸附根据它们的相互作用力分为物理吸附和化学吸附。静电吸附是一种众所周知的物理吸附机制,它与高性能混凝土的表面电荷状态和重金属离子的类型密切相关[41-42],主要作用力是范德华力。尽管存在静电吸附作用,但磷酸盐与重金属离子之间的化学相互作用也被认为是解释重金属离子吸附的机制[43],化学作用包括:表面的离子交换[44];通过化学吸附回收的产物形成含金属的磷酸盐沉淀[45];在重结晶过程中,其它金属取代作用[46];以及官能团和重金属在其表面上的结合[47]。然而,很难确切的说每种机制对吸附金属的贡献,可能4种机制都可以一起工作[48]。Soudée 等[49]认为MPC在水化后,Mg(H2O)62+、PO43-和NH4+可在表面由于氢键形成鸟粪石网络,具有良好的吸附性。Wang等[50]认为MgO-KH2PO4-H2O三元体系由KH2PO4的解离开始释放K+、H+ 、PO43-、HPO42-和H2PO4-等离子,体系的酸性环境诱导MgO的解离,随后生成产物MgKPO4·6H2O,然后形成一种鸟粪石网络(如图5所示)。Du等[51]探究KMPC固化土壤中的Zn2+和Pb2+机理,根据XRD图分析认为,重金属阳离子是通过交换吸附在KMPC表面,从而实现对重金属的固化。图6为吸附作用方式机理图。
-
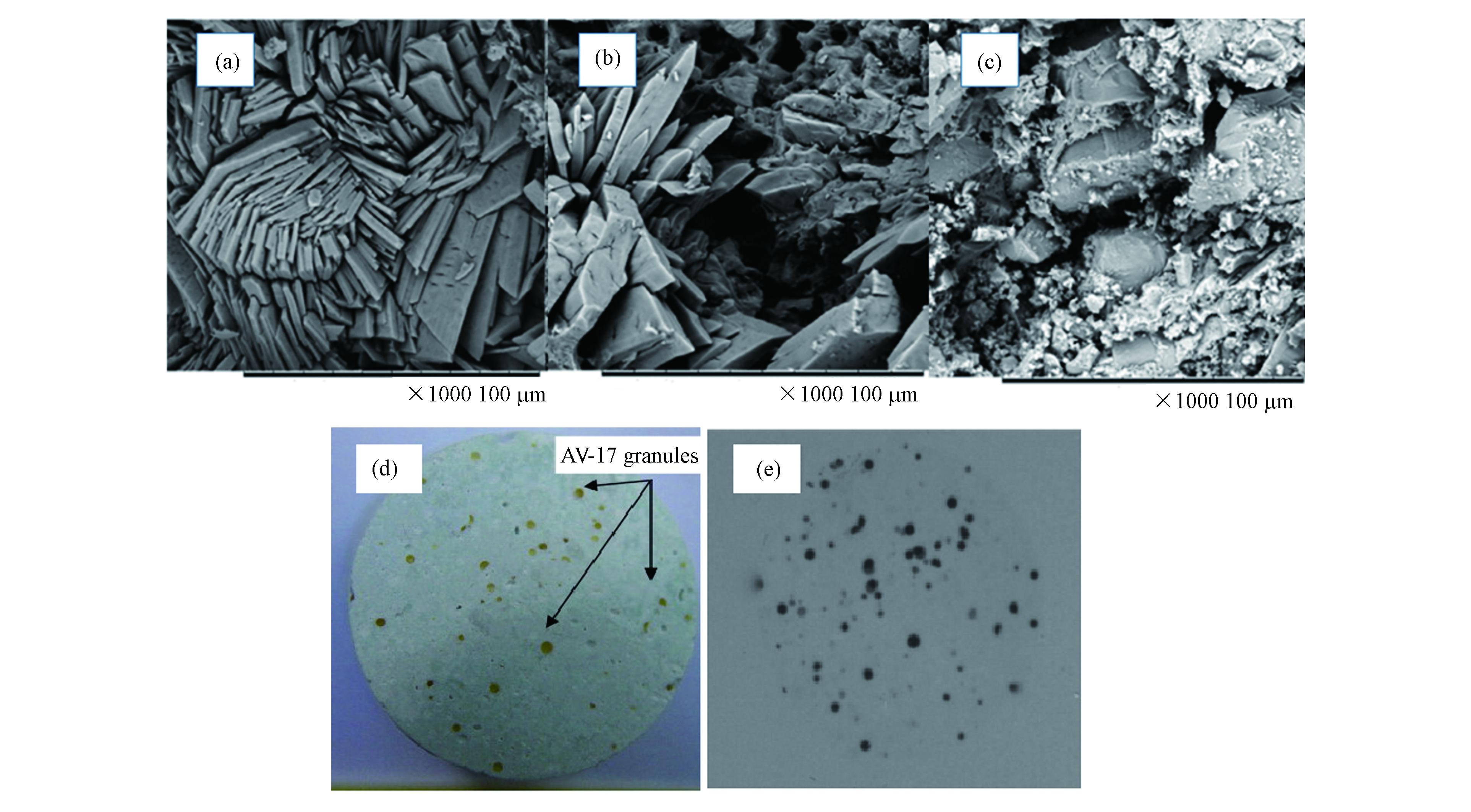
物理包裹也称为“封装”,是将重金属包含在一个结构紧密的物理屏障系统中的技术[34]。 MPC的主要水化产物为鸟粪石,根据SEM图7(a),鸟粪石晶体呈棱柱状和板状,表面相对平坦,彼此连接并形成致密结构[17],与大量未反应的MgO结合在一起,有良好的包覆性能。Haque等[52]和Randall 等[53]探究了MPC对Ni2+和Hg2+的固化机理,通过对SEM图7(b)、(c)分析得出:重金属被包裹在MPC之中,成团状或者微胶囊状,由于MPC致密的结构,因此在浸出研究中发现浸出率极低。Wagh 等[54]和Vinokurov 等[55]探究了MPC对铯等放射性元素的固化机理,固化后的重金属MPC体系如图7(d, e)所示,可以看出铯和锶均匀分布在MPC中,主要以包裹体形式存在。这为磷酸镁水泥有效固化核应急事件中放射性物质提供了有力证据。
-
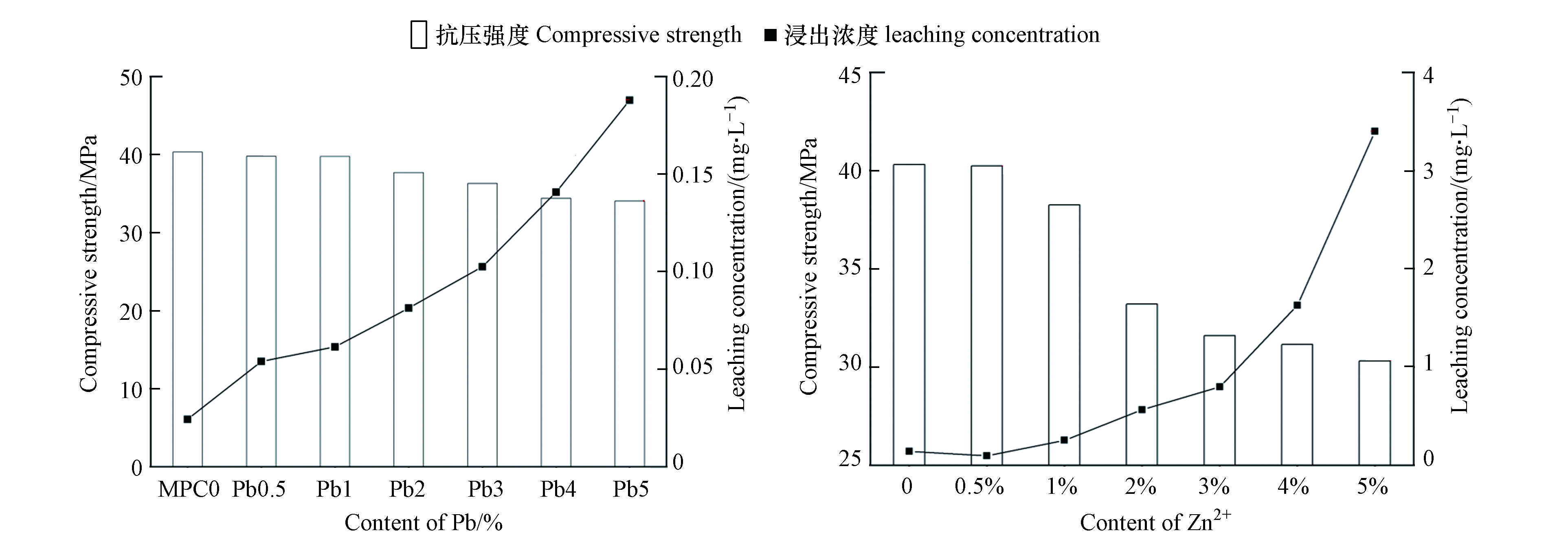
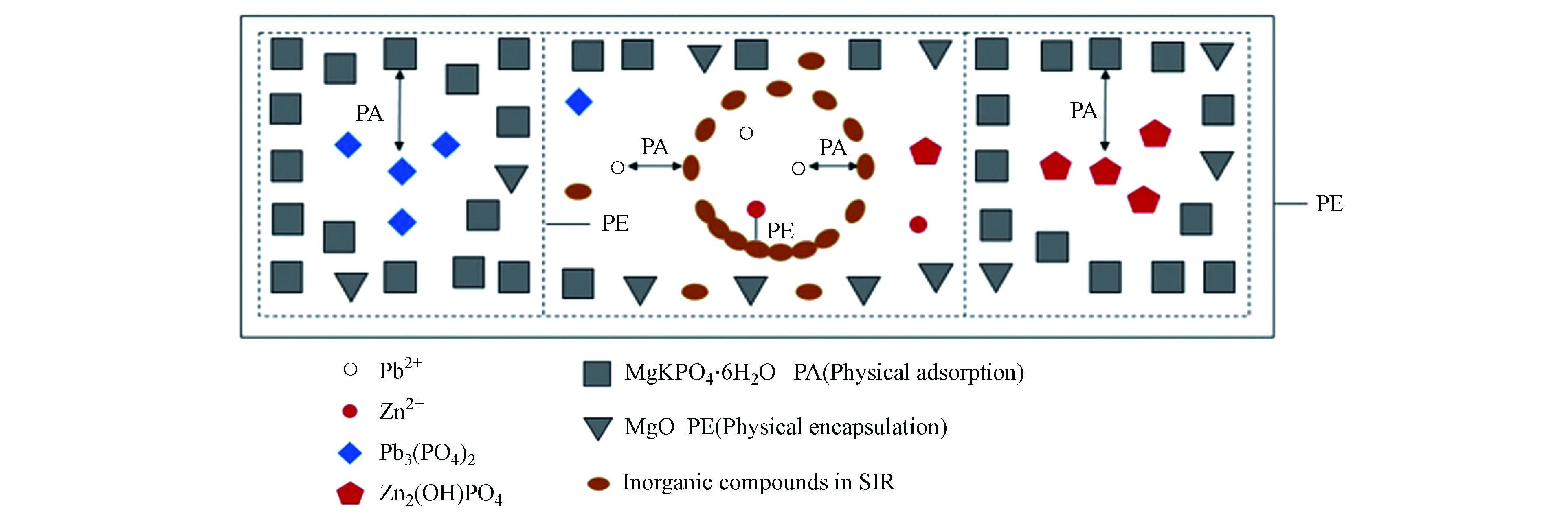
上述MPC固化/稳定化重金属的机理表明其固化机制为化学键合、吸附和物理包裹。但在具体MPC处理重金属过程中可能是三种方式中的两种或三种的协同作用如Su等[13]、Cao等[14]和Singh等[56]。学者Cao等[14]基于重金属MKPC体系抗压强度、浸出浓度与Pb2+、Zn2+掺量之间变化发现(如图8所示),体系的浸出浓度与抗压强度之间成负相关关系,这一点清楚地表明了物理作用参与了重金属的固化;同时,含Pb2+、Zn2+样品之间的浸出浓度差异远大于相应的抗压强度差异,这表明Pb2+、Zn2+在MKPC中的固化不仅有物理作用,而且还可能包括化学作用。通过结合物质Ksp,Pb2+在体系中主要是以Pb3(PO4)2(Ksp= 8×10-43)形式存在;Zn2+先以Zn3(PO4)2(Ksp= 9×10-33)形式存在,在pH>7后,转化为Zn2(OH)PO4,它们的溶解度都小于MgKPO4·6H2O(Ksp=2.4×10-11)。总结整个固化过程为:游离Pb2+和Zn2+首先发生反应并形成低溶解度Pb3(PO4)2和Zn2(OH)PO4,然后通过MgKPO4·6H2O和过量的MgO通过物理吸附和封装将Pb2+、Zn2+很好地固定在MKPC中,其固化机理如图9所示。
-
MPC具有多种性能,包括高早期强度、快速凝固和硬化、高水化热和良好的体积相容性,常应用于生物材料、路面裂缝修补、危险废物管理、纤维增强砂浆、刨花板和临床生物陶瓷等方面,尤其是在固化/稳定化重金属方面。因此,本文总结了重金属种类和掺量等常见因素对MPC力学性能、酸碱度、凝结时间和水化热等方面的影响,以及固化/稳定化有毒重金属的机理这两大方面。总结得到如下结论:
(1)不同种类的重金属MPC体系的抗压强度和浸出浓度不同;随着固化量增多,在早期会使MPC抗压强度增大,随着固化时间延长,抗压强度降低,浸出浓度增加。
(2)重金属的加入会减缓MPC的水化过程,其表现为酸碱度变化速度变慢,凝结时间增长,水化热降低,主要是重金属消耗了磷酸根导致的。
(3) MPC固化/稳定化重金属的机理主要包括化学键合作用、吸附和物理包裹作用,也有的学者认为是它们的协同作用。
MPC有着良好的发展前景,然而,它的一些特性也限制了它的发展,包括高的水化热、凝固时间短等缺陷。固化重金属后会减缓其水化速率,但是高的固化量会降低其抗压强度和增大它的浸出率,这就要求我们探究每种重金属的最佳固化量。在探究固化机理时,因为重金属掺量少,通过化学键合作用形成的化合物含量低于XRD检出限,应该适当增加重金属掺量或者采用其他检测方法。同时寻求合适的富含氧化镁工业废渣以降低制备磷酸镁水泥的成本,在固化重金属的同时,也对保护环境有着重要的意义。
磷酸镁水泥固化/稳定化重金属性能及作用机理研究进展
Research progress on solidification/stabilization mechanism and properties of heavy metals using magnesium phosphate cement
-
摘要: 磷酸镁水泥(MPC)作为一种新型无机胶凝材料,是由过烧氧化镁和磷酸盐通过酸碱中和反应制备得来,具有早期强度高、结构紧密和体积形变小等优点,在固化/稳定化重金属方面受到广泛关注,但存在放热量大、凝结速度快等缺点。本文归纳了国内外学者对磷酸镁水泥固化/稳定化重金属和重金属污染土的研究,重点讨论重金属离子对磷酸镁水泥抗压强度、凝结时间、酸碱度和水化热的影响,环境对重金属MPC体系稳定性的影响,以及其固化/稳定化重金属离子的作用机理,得出主要固化机理为化学键合、吸附作用和物理包裹,最后指出磷酸镁水泥固化重金属研究的不足和对未来研究的展望,为磷酸镁水泥固化/稳定化重金属的研究提出了新思路。Abstract: Magnesium phosphate cement (MPC) is a new type of inorganic cementing material, which is prepared by over-burned magnesium oxide and phosphate through acid-base neutralization reaction. It has the advantages of high early strength, compact structure and small volume deformation. It has received extensive attention in terms of solidification/stabilization of heavy metals, but it has the disadvantages of large heat release and fast condensation speed. This article summarizes the research of domestic and foreign research scholars on the solidification/stabilization of heavy metal and heavy metal contaminated soil by magnesium phosphate cement, focusing on the impact of heavy metal ions on the compressive strength, setting time, pH and hydration heat of magnesium phosphate cement, and the environment on heavy metal MPC The influence of system stability and the mechanism of its solidification/stabilization of heavy metal ions. The main curing mechanisms are chemical bonding, adsorption and physical encapsulation. Finally, the lack of research on magnesium phosphate cement curing heavy metals and the prospects for future research are pointed out. A new idea is proposed for the research of magnesium phosphate cement solidification/stabilization heavy metals.
-
重金属污染已成为全球性的环境问题[1],重金属能通过到空气、水和土壤危害人体健康。据报道,长期接触镉(Cd)会引起肺腺癌、肺癌、肾功能损伤和骨折[2];长期高剂量暴露在锌(Zn)会影响胆固醇的平衡和生育能力[3];铅(Pb)中毒可能导致各种类型的疾病,包括神经系统疾病,阿尔茨海默氏病[4];慢性砷(As)可能导致角化过度,皮肤病变以及肺癌,膀胱癌和肾病[5];长期接触汞(Hg),会积聚在脂肪组织中,并损害人体中枢神经系统[6];铜(Cu)、铬(Cr)和镍(Ni)也会对人体健康产生不利影响[7]。基于重金属对生物体的高致癌、高毒害作用,2011年3月,《重金属污染综合防治“十二五”规划》获得国务院正式批复,成为我国第一个“十二五”国家级别的专项规划。由此可以看出,重金属污染防治是当前和今后一个时期环境保护工作的重中之重。
固化/稳定化是治理重金属污染的有效方法之一[8],波特兰水泥(OPC)是重金属废物固化/稳定化的常用基质,但研究表明,重金属的存在对OPC的抗压强度(降低30.4%)不利[9],但对磷酸镁水泥(MPC)的影响相对要小得多。固化重金属或重金属污染土后的磷酸镁水泥进行二次使用或者掩埋,都需要高的抗压强度和低的渗透率[10],结合磷酸镁水泥早期强度高、稳定性好和孔隙率低的特点,并且毒性特征浸出程序(TCLP)结果表明,浸出毒性浸出浓度远低于国家标准(GB5085.3-2007) [9],因此磷酸镁水泥在固化/稳定化重金属方面有着良好的前景。
基于此,本文综述MPC固化/稳定化重金属和重金属污染土的研究现状,讨论重金属种类和掺量等常见因素对MPC力学性能、凝结时间、酸碱度、水化热及水化机理等方面的影响。MPC常处理重金属主要包括Pb、Ni、Cu、Zn、Cr、Mn、Hg等物质,而其固化/稳定化重金属效果受重金属含量、重金属种类、氧化镁和磷酸盐质量比(M/P)和固化时间等众多因素影响;固化作用机理主要包括3个方面:(1)重金属离子与磷酸根离子结合形成低溶度积的化合物,如Pb5(PO4)3OH、Cd3(PO4)2等;(2)MPC水化产物对重金属离子产生较强的吸附作用;(3)MPC具有较强的物理力学性能和致密的结构,对重金属离子具有物理包裹作用。通过化学键合、吸附、物理包裹三重作用,从而实现重金属离子的高效率和大容量固化。本文以期为后续MPC固化/稳定化废弃物中重金属和重金属污染土研究提供前期的基础数据及理论依据。
1. MPC固化重金属性能分析(Performance analysis of MPC solidification heavy metals)
1.1 固化效果分析
MPC固化/稳定化重金属的处理效果与很多因素有关,主要包括重金属种类、重金属处理量、M/P和固化时间,其固化效果及影响因素见表1。重金属的固化效果主要以抗压强度、凝结时间和处理样毒性浸出特性为指标。(1)重金属种类,MPC常用来处理Pb、Cu、Cd和Ni等重金属以及放射性Sr、Cs等污染物。不同种类的重金属,固化效果不同,相同条件下,Zn的存在会降低MPC的抗压强度,Cu、Cd和Ni等会提高MPC抗压强度,但均会延长凝结时间;同时固化Pb和Cd,MPC对Pb的固化效果大于Cd;此外,不同种类重金属间也存在相互影响,例如Pb的存在会削弱Zn的固定作用。(2)重金属处理量,在相同的M/P下,重金属经MPC固化,固化过程中金属离子会与磷酸根反应形成相应的磷酸盐,当MPC处理重金属的量增加时,重金属的加入会阻碍鸟粪石的形成,减缓材料凝结速率,延长凝结时间,而固化体抗压强度则会降低、毒性浸出浓度增大。(3)M/P比,当M/P比过高时,体系水化反应的磷酸盐相对较小,这类似于重金属对MPC抗压强度的影响。在MPC的水化硬化过程中,虽然重金属离子可与磷酸根离子反应生成磷酸盐水合相,且一定程度改善材料的致密性,但其对材料强度的负作用更为突出。研究表明,过高的M/P比对MPC的抗压强度有降低作用,但对MPC的凝结时间影响不是很明显。在毒性浸出浓度方面,随着M/P比的增加,浸出浓度也增加。(4)固化时间,随着固化时间的增加,MPC主水化产物(鸟粪石)不断长大结晶,并逐渐填充体系孔隙,阻滞孔隙的贯通性,从而不断降低渗透系数、提高材料抗压强度和降低毒性浸出浓度。
表 1 MPC固化重金属效果及影响因素Table 1. MPC solidification effect of heavy metals and influencing factors重金属种类Types of heavy metals 重金属含量Heavy metal content 镁/磷M/P 固化时间/dsolidification time 抗压强度/MPaCompressive strength 凝结时间/minSetting time 浸出浓度/(mg·L-1)Leaching concentration 参考文献References Pb2+ 5000 mg·kg-1 1:1 7 d 0.36 1.75 [11] Pb2+, Cd2+ 0—5% 4:1 28 d 0.15,0.37 [12] Pb2+, Cd2+ 1.2%、 0.3% 3:1 90 d 44、47 0.92,0.43 [13] Pb2+, Zn2+ 0.5%—5% 4:1 28 d 34—40、30—40 0.05—0.2, 0.2—3.4 [14] Pb2+ 1% 3:1 28 d 55 16 0.05 [9] Cu2+ 1%、2%、3%、4% 2:1 28 d 40、50、55、56 11.8、12、17.5、23.1 1.1 [15] Cu2+ 1%、2%、3%、4% 3:1 28 d 55、54、53、48 12.3、12.7、17.3、23.6 1.32 [15] Cu2+ 1%、2%、3%、4% 4:1 28 d 45、43、36、25 12.8、13.3、17.2、25 1.7 [15] Cd2+ 1%,2%,3%,4% 2:1 7 d 37、38、44、45 12.8、13.3、13.8、14.6 0.37 [16] Cd2+ 1%、2%、3%、4% 3:1 7 d 48、45、42、43 12.5、13.3、13.9、14.4 0.42 [16] Cd2+ 1%、2%、3%、4% 4:1 7 d 49、48、47、35 12.1、12.3、12.8、13.9 0.46 [16] Zn2+ 1%、2%、3%、4% 2;1 28 d 42、41、40、39 13.5、17、22、30.7 0.0155 [17] Zn2+ 1%、2%、3%、4% 3:1 28 d 46、45、38、37 13.5、15、17.5、25 0.016 [17] Zn2+ 1%、2%、3%、4%% 4:1 28 d 43、39、36、24 10、12.5、18.5、27.5 0.02 [17] Ni2+ 1%、2%、3%、4% 2:1 28 d 52、55、57、62 12.3、12.6、13.2、15.3 2.75 [18] Ni2+ 1%、2%、3%、4% 3:1 28 d 65、63、61、58 11.8、12.2、13.3、16.3 2.55 [18] Ni2+ 1%、2%、3%、4% 4:1 28 d 49、48、40、35 11.3、11.5、13.8、18.2 3.45 [18] Ni2+ 1%、2%、3%、4% 5:1 28 d 37、32、27、25 10.7、11、15.2、20 3.68 [18] Cr2+ 25 g·dm-3 1:1 21 d 20 [19] Hg2+ 380 mg·kg-1 1:1 28 d 10—36 2.48 [20] Hg2+ 0.25% 3.5:1 21 d 9.5—12.5 0.697 [21] Sr2+、 Cs+ 50% 1:1 28 d 4.2、13.2 10-7, 10-4 (g·m-2·d-1) [22] 综上所述,不同重金属的加入均会延长MPC的凝结时间,但其对不同重金属的作用效果不同。总体而言,MPC对Pb的作用效果较为显著;当重金属处理量恒定时,材料抗压强度和毒性浸出浓度与M/P和固化时间密切相关,延长固化时间和一定的M/P可有效抑制重金属溶出和提高材料抗压强度。
1.2 体系pH变化分析
磷酸镁水泥(MPC)的形成是基于氧化镁(MgO)与磷酸/磷酸盐(H3PO4或NH4H2PO4、(NH4)2HPO4、KH2PO4)之间的酸碱反应,该反应的主要产物是鸟粪石(MgNH4PO4·6H2O/MgKPO4·6H2O),体系酸碱度能影响MPC水化反应速率以及水化产物的生成与结晶[23],进而影响材料宏观性能如凝结时间和力学性能。同样,体系酸碱度也是稳定处理危险废物的一个非常重要的参数,中和能力的强弱决定了重金属在MPC的化学稳定性[24]。在MPC固化重金属过程中,表1中的影响因素不同,则会影响固化体系的pH值。
如He等[15-16]、Lai等[17]和Tao等[18]分别研究了在一定硼砂作用下,Zn2+、Cu2+、Ni2+和Cd2+掺量对MPC早期pH的影响,用PHS-3C酸度计测试不同重金属含量下MPC体系的pH变化趋势,本文重点讨论Zn2+和Ni2+对MPC的pH的影响,结果见图1。从图1可以看出,所有样品的pH都显示出随时间的推移从酸性到碱性的变化,最终pH趋近于碱性。对不添加Zn2+的样品,酸碱度存在极值;而含Zn2+物质的加入对体系的初始和最终酸碱度整体没有显著影响,仅是pH的变化速率变缓慢,其中含1%Zn2+样品的变化速率最慢,其归因于当Zn2+含量较低时,其加速H2PO4–的电离,增加H+的相对量。Cd2+的加入同样不改变MPC水化反应过程pH演变趋势,仅减缓pH变化速率。分析MPC体系pH的变化过程:早期pH的迅速增加与硼砂相关,硼砂与水混合后,会释放B4O72+和Na+,与KH2PO4电离的H+结合形成硼酸,导致溶液中H+的量减少,pH增加;随着Cd2+、Zn2+含量的增加,pH整体变化速度减慢,这表明重金属Cd2+、Zn2+的加入延缓了MPC的水化速率。由此可知,重金属的加入对MPC初始和最终pH影响不大,仅影响体系pH的变化速率。
另外,有学者从不同角度研究了MPC固化重金属体系pH的变化趋势。如Du等[25]提出了一种由草酸活化磷矿、磷酸二氢钾和活性氧化镁组成的MPC,用来固化土壤中的Pb和Zn,同时探究两者之间的相互影响作用,结果如图2(a)所示。从图2(a)中可以看出,MPC固化后的渗滤液酸碱度比未经处理的污染土壤酸碱度高1.9—3.5个单位,含复合Zn2+和Pb2+污染土壤的渗滤液酸碱度比单一Zn2+或Pb2+的土壤酸碱度低0.5—0.8个单位。说明,一定浓度的重金属可提高污染土的酸中和能力。Shu等[26]和Lai等[17]探究了不同M/P和时间下固化体系pH变化规律。从图2(b, c)可以观察到,对于不同M/P的固化体系,样品的pH变化趋势相似。在重金属掺量和MPC添加量恒定的条件下,固化体系pH随着M/P比和时间的增加而增大,这是由于体系中磷酸盐的相对含量减少所致。
1.3 水化热分析
在MPC的早期水化阶段,其水化迅速,大量集中放热[27],导致体系水化温度升高,体系可从20 ℃升至90 ℃,在绝热条件下甚至超过100 ℃[28]。MPC水化释放的热影响体系温度,即反应速率,进而影响材料的凝结时间和强度。在MPC中加入重金属化合物,重金属会延缓体系的水化反应速率,降低体系早期水化温度峰值以及到峰值所需时间,使其早期水化程度降低。总结文献发现,体系早期水化热的释放情况主要是通过测定体系放热速率、累积水化热或水化温度。
He等[15-17]和石军兵等[29]从水化热量角度分析了Cu,Cd和Zn化合物对MPC体系水化热的影响规律,3种金属对水化热量的影响规律大致相同,规律见图3(a)。从图3(a)可知,重金属含量的增加不会改变体系的放热水化趋势,均存在2个放热峰,且第1个放热峰的峰值显著高于第2个放热峰,说明体系在短时间内释放大量热量。作者认为,MPC体系的第1个放热峰是体系MgO的溶解所致:MgO+H2O→MgOH++OH–;MgOH++2H2O→Mg(OH)2+H3O+;Mg(OH)2→Mg2++2OH–。而第2个放热峰主要是由于水化反应产物鸟粪石的形成以及磷酸盐化合物能附着在MgO表面在一定程度上阻碍MPC的水化反应的双重作用结果。在MPC固化重金属体系,第1个峰值是MgO和重金属化合物如硝酸铜、硝酸铬和硝酸锌溶解的叠加效果,二者叠加使峰值降低,且这种趋势随着重金属掺量的增加而增大。重金属化合物硝酸铜、硝酸铬和硝酸锌溶解释放的重金属离子的在MPC水化过程可参与反应,形成重金属磷酸盐化合物,该化合物形成消耗一定量的酸根离子,从而导致可用于形成鸟粪石的量相对减少,减缓鸟粪石形成速率和生成量,从而延缓和降低第2个峰的峰值和达到峰值所需时间(图3a,b,c)。另外,有学者研究了MPC固化重金属体系的水化温度变化趋,Liu等[21]测定了MPC固化0.25%Hg2+体系水化温度的变化,结果如图3(b)所示。从图3(b)可以看出,体系在1 min内缓慢上升,从第2 min开始温度急剧增大。原因在于MPC中通常掺入缓凝剂硼酸,其与体系中的磷酸盐发生反应,生成了菱镁矿(Mg3(PO4) B2O3·8H2O)薄层附着在MgO表面,这阻止了MgO与磷酸盐的反应,待薄层溶解后,MgO与磷酸盐发生水合反应,放出热量。反应一段时间后,体系上升趋势平缓达温度极值(72°C),并出现下降趋势,理论上经一段时间体系将至室温。
1.4 稳定性分析
MPC固化重金属后的稳定性是评估其能否安全处置的最重要的指标,其评估方式主要是通过考察外界环境对重金属MPC体系稳定性的影响。总结现有文献发现,常考察酸雨、核辐射、温度、冻融循环等对其的影响。
如王哲等[30]模拟了酸雨淋滤对MPC固化Zn2+的影响。研究发现,随着酸雨酸度的增加,Zn2+的浸出浓度和有效扩散系数不断增大,稳定性变差。其原因为:Zn2+在体系中的主要存在形式为Zn3(PO4)2·4H2O、Zn(OH)2等难溶盐,但随着酸度的增加,会发生逆转反应,体系倾向于生成更加稳定的Mg3(PO4)2·22H2O、Mg3(PO4)2·8H2O,导致体系中的Zn2+溶解。
MPC在长期贮存固化放射性废物期间,体系中的水会因为放射分解,可能会产生氧气和爆炸性气体氢气,对MPC长期稳定性产生影响。Bykov等[31]探究了γ射线对MPC的影响,结果表明,在放射剂量为25 MGy时,约0.18%的水分解,这表明该材料具有很高的抗辐射性。而且体系没有生成O2,这消除了爆炸性气体混合物的形成,H2的形成不会导致MPC膨胀或破坏。即使在高吸收剂量下,也未检测到材料的机械强度有明显变化,而且体系的微观结构也没有发生明显变化。所以,此研究结果可以为MPC固化放射性废物提供有力证明,而高放射性核素的放热率大于2 kw·m-3时,可能会对重金属MPC体系的稳定性产生影响,此推测出的结论与傅明娇等[32]一致。在傅明娇等[32]考察高温对MPC固化α-高放射性核废液稳定性的影响研究中发现,在400 ℃下,重金属MPC体系浸出率高于未烧结的,900℃下则低于未烧结的,通过观察其微观形貌发现在900 ℃下具有致密的陶瓷结构,比400 ℃下固体性质更加稳定(如图4所示)。分析其原因为:400 ℃下,体系内形成多孔结构,导致浸出率增大、浸出浓度增加;900 ℃下,体系内的MgKPO4、MgCsPO4等晶体由于高温烧结熔融在一起,形成致密的陶瓷体结构,则表现出更低的浸出率。
此外,侯世伟等[33]探究了在冻融循环下MPC固化铜污染土的长期稳定性,铜污染土在MPC稳定固化后,Cu2+的浸出浓度随冻融循环次数(0、 6、 12)的增加而上升;在反复冻融条件下,体系内的水反复冻融产生膨胀力对体系造成破坏,同时体系内的胶结物也被破坏,导致Cu2+浸出浓度增加。
2. MPC固化/稳定化机理( MPC solidification/stabilization mechanism)
MPC为未反应完的氧化镁颗粒和主水化产物(鸟粪石)形成的致密结构体,在废物管理领域,尤其是在有毒重金属以及低水平核废料的固化/稳定化方面具有广阔的应用前景。基于MPC结构体中含有大量未反应的MgO颗粒和HPO42-、H2PO4-、PO43-、OH-等阴离子,重金属离子可与上述离子发生沉淀反应生成磷酸盐、氢氧化物等难溶性化合物,吸附于主水化产物(鸟粪石)表面,或者其包裹在体系内[30]。通过化学键合、吸附作用和物理包裹作用实现有害物质的固化/稳定固封。
2.1 化学键合作用方式
化学键合作用就是通过向原始介质中引入化学试剂来固化或将可移动的污染物转化为低溶解度和低迁移率的沉淀物,从而将污染物固定的一种作用方式[34]。在制备MPC时需要加入磷酸或者磷酸盐,引入大量阴离子,易与重金属离子反应生成难溶盐。Shu等[26,35]、Cho等[36]和Buj等[37]探究了MPC对Mn2+等重金属的固化机理;Du等[25]、Sanderson等[38]、Feng等[39] Wang等[40]探究了MPC对土壤中Pb等重金属的固化机理。上述作者认为MPC固化/稳定化重金属的机理为:首先可溶性AH2PO4(KH2PO4/NH4H2PO4)溶解形成酸性溶液(见式(1)、式(2)和式(3)),其式中A代表NH4或K;之后,在酸性溶液中,氧化镁受H+攻击逐渐溶解释放Mg2+见式(4);Mg2+与NH4+和HPO42-、H2PO4-、PO43-等离子反应形成非晶或晶态磷酸盐水化产物(MgNH4PO4·6H2O/MgKPO4·6H2O),具体反应方程式如式(5)、式(6)和式(7)所示。在MPC水化过程中,重金属Mn、Pb、Zn、Cr、Cu等离子参与化学键合反应,生成难溶性磷酸盐如如Mn3(PO4)2·nH2O、Pb3(PO4)2·nH2O、Cd3(PO4)2·nH2O、Cu3(PO4)2·nH2O、MnHPO4·nH2O、Mn(H2PO4)2·nH2O、Zn(OH)2、Mn(OH)2等,将化学式中重金属离子记作M,具体反应方程式如式(5)—(14)所示。
AH2PO4→A + + H2PO - 4 (1) H2PO - 4→H + + HPO2 - 4 (2) HPO2 - 4→H + + PO3 - 4 (3) MgO + H+→Mg2++OH− (4) Mg2++H2PO4 - +6H2O +A+→MgNH4PO4⋅6H2O↓+2H + (5) Mg2++HPO42 - +6H2O +A + →MgNH4PO4⋅6H2O↓+H + (6) Mg2++PO43 - +6H2O +A+→MgNH4PO4⋅6H2O↓ (7) 3M2++2PO43 - +nH2O →M3(PO4)2⋅nH2O↓ (8) M2++PO3 - 4+NH4 + +nH2O→NH4MPO4⋅H2O↓ (9) M2++HPO42−+nH2O →MHPO4⋅nH2O↓ (10) M2++HPO42−+NH4 + +nH2O →NH4MPO4⋅nH2O↓ + H + (11) M2++2H2PO4−+nH2O →M(H2PO4)2⋅nH2O↓ (12) M3++PO43−+nH2O →MPO4⋅nH2O↓ (13) M2++2OH - →M(OH)2↓ (14) 2.2 吸附作用方式
吸附根据它们的相互作用力分为物理吸附和化学吸附。静电吸附是一种众所周知的物理吸附机制,它与高性能混凝土的表面电荷状态和重金属离子的类型密切相关[41-42],主要作用力是范德华力。尽管存在静电吸附作用,但磷酸盐与重金属离子之间的化学相互作用也被认为是解释重金属离子吸附的机制[43],化学作用包括:表面的离子交换[44];通过化学吸附回收的产物形成含金属的磷酸盐沉淀[45];在重结晶过程中,其它金属取代作用[46];以及官能团和重金属在其表面上的结合[47]。然而,很难确切的说每种机制对吸附金属的贡献,可能4种机制都可以一起工作[48]。Soudée 等[49]认为MPC在水化后,Mg(H2O)62+、PO43-和NH4+可在表面由于氢键形成鸟粪石网络,具有良好的吸附性。Wang等[50]认为MgO-KH2PO4-H2O三元体系由KH2PO4的解离开始释放K+、H+ 、PO43-、HPO42-和H2PO4-等离子,体系的酸性环境诱导MgO的解离,随后生成产物MgKPO4·6H2O,然后形成一种鸟粪石网络(如图5所示)。Du等[51]探究KMPC固化土壤中的Zn2+和Pb2+机理,根据XRD图分析认为,重金属阳离子是通过交换吸附在KMPC表面,从而实现对重金属的固化。图6为吸附作用方式机理图。
2.3 物理包裹作用方式
物理包裹也称为“封装”,是将重金属包含在一个结构紧密的物理屏障系统中的技术[34]。 MPC的主要水化产物为鸟粪石,根据SEM图7(a),鸟粪石晶体呈棱柱状和板状,表面相对平坦,彼此连接并形成致密结构[17],与大量未反应的MgO结合在一起,有良好的包覆性能。Haque等[52]和Randall 等[53]探究了MPC对Ni2+和Hg2+的固化机理,通过对SEM图7(b)、(c)分析得出:重金属被包裹在MPC之中,成团状或者微胶囊状,由于MPC致密的结构,因此在浸出研究中发现浸出率极低。Wagh 等[54]和Vinokurov 等[55]探究了MPC对铯等放射性元素的固化机理,固化后的重金属MPC体系如图7(d, e)所示,可以看出铯和锶均匀分布在MPC中,主要以包裹体形式存在。这为磷酸镁水泥有效固化核应急事件中放射性物质提供了有力证据。
2.4 协同作用方式
上述MPC固化/稳定化重金属的机理表明其固化机制为化学键合、吸附和物理包裹。但在具体MPC处理重金属过程中可能是三种方式中的两种或三种的协同作用如Su等[13]、Cao等[14]和Singh等[56]。学者Cao等[14]基于重金属MKPC体系抗压强度、浸出浓度与Pb2+、Zn2+掺量之间变化发现(如图8所示),体系的浸出浓度与抗压强度之间成负相关关系,这一点清楚地表明了物理作用参与了重金属的固化;同时,含Pb2+、Zn2+样品之间的浸出浓度差异远大于相应的抗压强度差异,这表明Pb2+、Zn2+在MKPC中的固化不仅有物理作用,而且还可能包括化学作用。通过结合物质Ksp,Pb2+在体系中主要是以Pb3(PO4)2(Ksp= 8×10-43)形式存在;Zn2+先以Zn3(PO4)2(Ksp= 9×10-33)形式存在,在pH>7后,转化为Zn2(OH)PO4,它们的溶解度都小于MgKPO4·6H2O(Ksp=2.4×10-11)。总结整个固化过程为:游离Pb2+和Zn2+首先发生反应并形成低溶解度Pb3(PO4)2和Zn2(OH)PO4,然后通过MgKPO4·6H2O和过量的MgO通过物理吸附和封装将Pb2+、Zn2+很好地固定在MKPC中,其固化机理如图9所示。
3. 结语(Conclusion)
MPC具有多种性能,包括高早期强度、快速凝固和硬化、高水化热和良好的体积相容性,常应用于生物材料、路面裂缝修补、危险废物管理、纤维增强砂浆、刨花板和临床生物陶瓷等方面,尤其是在固化/稳定化重金属方面。因此,本文总结了重金属种类和掺量等常见因素对MPC力学性能、酸碱度、凝结时间和水化热等方面的影响,以及固化/稳定化有毒重金属的机理这两大方面。总结得到如下结论:
(1)不同种类的重金属MPC体系的抗压强度和浸出浓度不同;随着固化量增多,在早期会使MPC抗压强度增大,随着固化时间延长,抗压强度降低,浸出浓度增加。
(2)重金属的加入会减缓MPC的水化过程,其表现为酸碱度变化速度变慢,凝结时间增长,水化热降低,主要是重金属消耗了磷酸根导致的。
(3) MPC固化/稳定化重金属的机理主要包括化学键合作用、吸附和物理包裹作用,也有的学者认为是它们的协同作用。
MPC有着良好的发展前景,然而,它的一些特性也限制了它的发展,包括高的水化热、凝固时间短等缺陷。固化重金属后会减缓其水化速率,但是高的固化量会降低其抗压强度和增大它的浸出率,这就要求我们探究每种重金属的最佳固化量。在探究固化机理时,因为重金属掺量少,通过化学键合作用形成的化合物含量低于XRD检出限,应该适当增加重金属掺量或者采用其他检测方法。同时寻求合适的富含氧化镁工业废渣以降低制备磷酸镁水泥的成本,在固化重金属的同时,也对保护环境有着重要的意义。
-
表 1 MPC固化重金属效果及影响因素
Table 1. MPC solidification effect of heavy metals and influencing factors
重金属种类Types of heavy metals 重金属含量Heavy metal content 镁/磷M/P 固化时间/dsolidification time 抗压强度/MPaCompressive strength 凝结时间/minSetting time 浸出浓度/(mg·L-1)Leaching concentration 参考文献References Pb2+ 5000 mg·kg-1 1:1 7 d 0.36 1.75 [11] Pb2+, Cd2+ 0—5% 4:1 28 d 0.15,0.37 [12] Pb2+, Cd2+ 1.2%、 0.3% 3:1 90 d 44、47 0.92,0.43 [13] Pb2+, Zn2+ 0.5%—5% 4:1 28 d 34—40、30—40 0.05—0.2, 0.2—3.4 [14] Pb2+ 1% 3:1 28 d 55 16 0.05 [9] Cu2+ 1%、2%、3%、4% 2:1 28 d 40、50、55、56 11.8、12、17.5、23.1 1.1 [15] Cu2+ 1%、2%、3%、4% 3:1 28 d 55、54、53、48 12.3、12.7、17.3、23.6 1.32 [15] Cu2+ 1%、2%、3%、4% 4:1 28 d 45、43、36、25 12.8、13.3、17.2、25 1.7 [15] Cd2+ 1%,2%,3%,4% 2:1 7 d 37、38、44、45 12.8、13.3、13.8、14.6 0.37 [16] Cd2+ 1%、2%、3%、4% 3:1 7 d 48、45、42、43 12.5、13.3、13.9、14.4 0.42 [16] Cd2+ 1%、2%、3%、4% 4:1 7 d 49、48、47、35 12.1、12.3、12.8、13.9 0.46 [16] Zn2+ 1%、2%、3%、4% 2;1 28 d 42、41、40、39 13.5、17、22、30.7 0.0155 [17] Zn2+ 1%、2%、3%、4% 3:1 28 d 46、45、38、37 13.5、15、17.5、25 0.016 [17] Zn2+ 1%、2%、3%、4%% 4:1 28 d 43、39、36、24 10、12.5、18.5、27.5 0.02 [17] Ni2+ 1%、2%、3%、4% 2:1 28 d 52、55、57、62 12.3、12.6、13.2、15.3 2.75 [18] Ni2+ 1%、2%、3%、4% 3:1 28 d 65、63、61、58 11.8、12.2、13.3、16.3 2.55 [18] Ni2+ 1%、2%、3%、4% 4:1 28 d 49、48、40、35 11.3、11.5、13.8、18.2 3.45 [18] Ni2+ 1%、2%、3%、4% 5:1 28 d 37、32、27、25 10.7、11、15.2、20 3.68 [18] Cr2+ 25 g·dm-3 1:1 21 d 20 [19] Hg2+ 380 mg·kg-1 1:1 28 d 10—36 2.48 [20] Hg2+ 0.25% 3.5:1 21 d 9.5—12.5 0.697 [21] Sr2+、 Cs+ 50% 1:1 28 d 4.2、13.2 10-7, 10-4 (g·m-2·d-1) [22] -
[1] GARCÍA-CARMONA M, ROMERO-FREIRE A, SIERRA ARAGÓN M, et al. Evaluation of remediation techniques in soils affected by residual contamination with heavy metals and arsenic [J]. Journal of Environmental Management, 2017, 191: 228-236. [2] FENG L, YAN H, DAI C, et al. The systematic exploration of cadmium-accumulation characteristics of maize kernel in acidic soil with different pollution levels in China [J]. Science of The Total Environment, 2020, 729: 138972. doi: 10.1016/j.scitotenv.2020.138972 [3] WANG Y, WANG R, FAN L, et al. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, Southwest China [J]. Chemosphere, 2017, 174: 613-627. doi: 10.1016/j.chemosphere.2017.01.055 [4] DEHGHANIFIROOZABADI M, NOFERESTI P, AMIRABADIZADEH A, et al. Blood lead levels and multiple sclerosis: A case-control study [J]. Multiple Sclerosis and Related Disorders, 2019, 27: 151-155. doi: 10.1016/j.msard.2018.10.010 [5] GONG Y, QU Y, YANG S, et al. Status of arsenic accumulation in agricultural soils across China (1985–2016) [J]. Environmental Research, 2020, 186: 109525. doi: 10.1016/j.envres.2020.109525 [6] KUMARI S, AMIT, JAMWAL R, et al. Recent developments in environmental mercury bioremediation and its toxicity: A review [J]. Environmental Nanotechnology, Monitoring & Management, 2020, 13: 100283. [7] HUANG Y, CHEN Q, DENG M, et al. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China [J]. Journal of Environmental Management, 2018, 207: 159-168. [8] 张亭亭, 李江山, 王平, 等. 磷酸镁水泥固化铅污染土的应力—应变特性研究 [J]. 岩土力学, 2016, 37(S1): 215-225. ZHANG T T, LI J S, WANG P, et al. Study on stress-strain characteristics of lead-contaminated soil cured by magnesium phosphate cement [J]. Rock and Soil Mechanics, 2016, 37(S1): 215-225(in Chinese).
[9] WANG Y S, DAI J G, WANG L, et al. Influence of lead on stabilization/solidification by ordinary portland cement and magnesium phosphate cement [J]. Chemosphere, 2018, 190: 90-96. doi: 10.1016/j.chemosphere.2017.09.114 [10] 张亭亭, 李江山, 王平, 等. 磷酸镁水泥固化铅污染土的力学特性试验研究及微观机制 [J]. 岩土力学, 2016, 37(S2): 279-286. ZHANG T T, LI J S, WANG P, et al. Experimental study and micro-mechanism of mechanical properties of lead-contaminated soil cured by magnesium phosphate cement [J]. Rock and Soil Mechanics, 2016, 37(S2): 279-286(in Chinese).
[11] 张亭亭, 王平, 李江山, 等. 养护龄期和铅含量对磷酸镁水泥固化/稳定化铅污染土的固稳性能影响规律及微观机制 [J]. 岩土力学, 2018, 39(6): 2115-2123. ZHANG T T, WANG P, LI J S, et al. The effect of curing age and lead content on the stability of magnesium phosphate cement solidified/stabilized lead-contaminated soil and its micro-mechanism [J]. Rock and Soil Mechanics, 2018, 39(6): 2115-2123(in Chinese).
[12] 李晔, 方嘉淇. 磷酸镁水泥对挥发型重金属Pb2+、Cd2+的固化效果及机理的研究 [J]. 硅酸盐通报, 2019, 38(3): 901-904,917. LI Y, FANG J Q. Study on the curing effect and mechanism of magnesium phosphate cement on volatile heavy metals Pb2+ and Cd2+ [J]. Bulletin of the Chinese Ceramic Society, 2019, 38(3): 901-904,917(in Chinese).
[13] SU Y, YANG J, LIU D, et al. Effects of municipal solid waste incineration fly ash on solidification/stabilization of Cd and Pb by magnesium potassium phosphate cement [J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 259-265. doi: 10.1016/j.jece.2015.11.025 [14] CAO X, WANG W, MA R, et al. Solidification/stabilization of Pb2+ and Zn2+ in the sludge incineration residue-based magnesium potassium phosphate cement: Physical and chemical mechanisms and competition between coexisting ions [J]. Environmental Pollution, 2019, 253: 171-180. doi: 10.1016/j.envpol.2019.07.017 [15] HE X, LAI Z, YAN T, et al. Hydration characteristics and microstructure of magnesium phosphate cement in presence of Cu2+ [J]. Construction and Building Materials, 2019, 225: 234-242. doi: 10.1016/j.conbuildmat.2019.07.184 [16] HE Y, LAI Z, YAN T, et al. Effect of Cd2+ on early hydration process of magnesium phosphate cement and its leaching toxicity properties [J]. Construction and Building Materials, 2019, 209: 32-40. doi: 10.1016/j.conbuildmat.2019.03.075 [17] LAI Z, LAI X, SHI J, et al. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement [J]. Construction and Building Materials, 2016, 129: 70-78. doi: 10.1016/j.conbuildmat.2016.11.002 [18] TAO Y, ZHENYU L, ZHICHAO H, et al. Mechanical and microstructure of magnesium potassium phosphate cement with a high concentration of Ni(II) and its leaching toxicity [J]. Construction and Building Materials, 2020, 245: 118425. doi: 10.1016/j.conbuildmat.2020.118425 [19] BUJ I, TORRAS J, CASELLAS D, et al. Effect of heavy metals and water content on the strength of magnesium phosphate cements [J]. Journal of Hazardous Materials, 2009, 170(1): 345-350. doi: 10.1016/j.jhazmat.2009.04.091 [20] KIM H T, LEE T G. A simultaneous stabilization and solidification of the top five most toxic heavy metals (Hg, Pb, As, Cr, and Cd) [J]. Chemosphere, 2017, 178: 479-485. doi: 10.1016/j.chemosphere.2017.03.092 [21] LIU Z, QIAN G, ZHOU J, et al. Improvement of ground granulated blast furnace slag on stabilization/solidification of simulated mercury-doped wastes in chemically bonded phosphate ceramics [J]. Journal of Hazardous Materials, 2008, 157(1): 146-153. doi: 10.1016/j.jhazmat.2007.12.110 [22] ZHENYU L, HONGTAO W, YANG H, et al. Rapid solidification of highly loaded high‐level liquid wastes with magnesium phosphate cement [J]. Ceramics International, 2019, 45(4): 5050-5057. doi: 10.1016/j.ceramint.2018.11.206 [23] LE ROUZIC M, CHAUSSADENT T, PLATRET G, et al. Mechanisms of k-struvite formation in magnesium phosphate cements [J]. Cement and Concrete Research, 2017, 91: 117-122. doi: 10.1016/j.cemconres.2016.11.008 [24] CÔTÉ P L, BRIDLE T R. Long-term leaching scenarios for cement-based waste forms [J]. Waste Management & Research, 1987, 5(1): 55-66. [25] DU Y J, WEI M L, REDDY K R, et al. New phosphate-based binder for stabilization of soils contaminated with heavy metals: Leaching, strength and microstructure characterization [J]. Journal of Environmental Management, 2014, 146: 179-188. doi: 10.1016/j.jenvman.2014.07.035 [26] SHU J, WU H, LIU R, et al. Simultaneous stabilization/solidification of Mn2+ and NH4+-N from electrolytic manganese residue using MgO and different phosphate resource [J]. Ecotoxicology and Environmental Safety, 2018, 148: 220-227. doi: 10.1016/j.ecoenv.2017.10.027 [27] FANG Y, CHEN B, ODERJI S Y. Experimental research on magnesium phosphate cement mortar reinforced by glass fiber [J]. Construction and Building Materials, 2018, 188: 729-736. doi: 10.1016/j.conbuildmat.2018.08.153 [28] YOU C, QIAN J, QIN J, et al. Effect of early hydration temperature on hydration product and strength development of magnesium phosphate cement (MPC) [J]. Cement and Concrete Research, 2015, 78: 179-189. doi: 10.1016/j.cemconres.2015.07.005 [29] 石军兵, 赖振宇, 卢忠远, 等. 铅离子对复合磷酸盐磷酸镁水泥水化硬化特性的影响 [J]. 功能材料, 2015, 46(2): 2060-2065. doi: 10.3969/j.issn.1001-9731.2015.02.013 SHI J B, LAI Z Y, LU Z Y, et al. Effect of lead ion on the hydration and hardening characteristics of composite phosphate magnesium phosphate cement [J]. Functional Materials, 2015, 46(2): 2060-2065(in Chinese). doi: 10.3969/j.issn.1001-9731.2015.02.013
[30] 王哲, 丁耀堃, 许四法, 等. 酸雨环境下磷酸镁水泥固化锌污染土溶出特性研究 [J]. 岩土工程学报, 2017, 39(4): 697-704. doi: 10.11779/CJGE201704015 WANG Z, DING Y K, XU S F, et al. Study on the dissolution characteristics of magnesium phosphate cement solidified zinc contaminated soil under acid rain environment [J]. Chinese Journal of Geotechnical Engineering, 2017, 39(4): 697-704(in Chinese). doi: 10.11779/CJGE201704015
[31] BYKOV G L, ERSHOV V A, ERSHOV B G. Radiolysis of the magnesium phosphate cement on γ-irradiation [J]. Construction and Building Materials, 2020, 252: 119156. doi: 10.1016/j.conbuildmat.2020.119156 [32] 傅明娇, 杨海林, 吴传明, 等. 温度对含模拟α-高放核废液的磷酸镁水泥固化体性能的影响 [J]. 材料导报, 2017, 31(24): 86-90. doi: 10.11896/j.issn.1005-023X.2017.024.017 FU M J, YANG H L, WU C M, et al. The effect of temperature on the properties of solidified magnesium phosphate cement containing simulated α-high-level nuclear waste liquid [J]. Materials Review, 2017, 31(24): 86-90(in Chinese). doi: 10.11896/j.issn.1005-023X.2017.024.017
[33] 侯世伟, 张瑀哲, 李宏男, 等. 冻融循环下磷酸镁水泥固化铜污染土的淋滤特性研究 [J]. 科学技术与工程, 2020, 20(5): 1993-1999. doi: 10.3969/j.issn.1671-1815.2020.05.043 HOU S W, ZHANG Y Z, LI H N, et al. Leaching characteristics of copper-contaminated soil solidified by magnesium phosphate cement under freeze-thaw cycles [J]. Science Technology and Engineering, 2020, 20(5): 1993-1999(in Chinese). doi: 10.3969/j.issn.1671-1815.2020.05.043
[34] LIU L, LI W, SONG W, et al. Remediation techniques for heavy metal-contaminated soils: Principles and applicability [J]. Science of The Total Environment, 2018, 633: 206-219. doi: 10.1016/j.scitotenv.2018.03.161 [35] SHU J, LIU R, LIU Z, et al. Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO [J]. Journal of Hazardous Materials, 2016, 317: 267-274. doi: 10.1016/j.jhazmat.2016.05.076 [36] CHO J H, EOM Y, LEE T G. Stabilization/solidification of mercury-contaminated waste ash using calcium sodium phosphate (CNP) and magnesium potassium phosphate (MKP) processes [J]. Journal of Hazardous Materials, 2014, 278: 474-482. doi: 10.1016/j.jhazmat.2014.06.026 [37] BUJ I, TORRAS J, ROVIRA M, et al. Leaching behaviour of magnesium phosphate cements containing high quantities of heavy metals [J]. Journal of Hazardous Materials, 2010, 175(1): 789-794. [38] SANDERSON P, NAIDU R, BOLAN N, et al. Chemical stabilisation of lead in shooting range soils with phosphate and magnesium oxide: Synchrotron investigation [J]. Journal of Hazardous Materials, 2015, 299: 395-403. doi: 10.1016/j.jhazmat.2015.06.056 [39] FENG Y S, DU Y J, REDDY K R, et al. Performance of two novel binders to stabilize field soil with zinc and chloride: Mechanical properties, leachability and mechanisms assessment [J]. Construction and Building Materials, 2018, 189: 1191-1199. doi: 10.1016/j.conbuildmat.2018.09.072 [40] WANG L, CHEN L, GUO B, et al. Red mud-enhanced magnesium phosphate cement for remediation of Pb and As contaminated soil [J]. Journal of Hazardous Materials, 2020, 400: 123317. doi: 10.1016/j.jhazmat.2020.123317 [41] JI M, SU X, ZHAO Y, et al. Effective adsorption of Cr(VI) on mesoporous Fe-functionalized Akadama clay: Optimization, selectivity, and mechanism [J]. Applied Surface Science, 2015, 344: 128-136. doi: 10.1016/j.apsusc.2015.03.006 [42] NGUYEN T C, LOGANATHAN P, NGUYEN T V, et al. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies [J]. Chemical Engineering Journal, 2015, 270: 393-404. doi: 10.1016/j.cej.2015.02.047 [43] DIMA J B, SEQUEIROS C, ZARITZKY N E. Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes [J]. Chemosphere, 2015, 141: 100-111. doi: 10.1016/j.chemosphere.2015.06.030 [44] MOGHIMI F, JAFARI A H, YOOZBASHIZADEH H, et al. Adsorption behavior of Sb(Ⅲ) in single and binary Sb(Ⅲ)—Fe(Ⅱ) systems on cationic ion exchange resin: Adsorption equilibrium, kinetic and thermodynamic aspects [J]. Transactions of Nonferrous Metals Society of China, 2020, 30(1): 236-248. doi: 10.1016/S1003-6326(19)65195-2 [45] ANGKAWIJAYA A E, SANTOSO S P, BUNDJAJA V, et al. Studies on the performance of bentonite and its composite as phosphate adsorbent and phosphate supplementation for plant [J]. Journal of Hazardous Materials, 2020, 399: 123130. doi: 10.1016/j.jhazmat.2020.123130 [46] GORSKI C A, FANTLE M S. Stable mineral recrystallization in low temperature aqueous systems: A critical review [J]. Geochimica et Cosmochimica Acta, 2017, 198: 439-465. doi: 10.1016/j.gca.2016.11.013 [47] WANG H, WANG X, MA J, et al. Removal of cadmium (Ⅱ) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste–struvite/attapulgite obtained from nutrient-rich wastewater [J]. Journal of Hazardous Materials, 2017, 329: 66-76. doi: 10.1016/j.jhazmat.2017.01.025 [48] CAO X, MA L Q, RHUE D R, et al. Mechanisms of lead, copper, and zinc retention by phosphate rock [J]. Environmental Pollution, 2004, 131(3): 435-444. doi: 10.1016/j.envpol.2004.03.003 [49] SOUDÉE E, PÉRA J. Mechanism of setting reaction in magnesia-phosphate cements [J]. Cement and Concrete Research, 2000, 30(2): 315-321. doi: 10.1016/S0008-8846(99)00254-9 [50] WANG A J, ZHANG J, LI J M, et al. Effect of liquid-to-solid ratios on the properties of magnesium phosphate chemically bonded ceramics [J]. Materials Science and Engineering, 2013, 33(5): 2508-2512. doi: 10.1016/j.msec.2013.02.014 [51] DU Y J, WEI M L, REDDY K R, et al. Effect of carbonation on leachability, strength and microstructural characteristics of KMP binder stabilized Zn and Pb contaminated soils [J]. Chemosphere, 2016, 144: 1033-1042. doi: 10.1016/j.chemosphere.2015.09.082 [52] HAQUE M A. Assessment of nickel leaching phenomena from landfill waste mixed paving block for eco-friendly field application [J]. Journal of Cleaner Production, 2016, 139: 99-112. doi: 10.1016/j.jclepro.2016.08.028 [53] RANDALL P, CHATTOPADHYAY S. Advances in encapsulation technologies for the management of mercury-contaminated hazardous wastes [J]. Journal of Hazardous Materials, 2004, 114(1): 211-223. [54] WAGH A S, SAYENKO S Y, SHKUROPATENKO V A, et al. Experimental study on cesium immobilization in struvite structures [J]. Journal of Hazardous Materials, 2016, 302: 241-249. doi: 10.1016/j.jhazmat.2015.09.049 [55] VINOKUROV S E, KULYAKO Y M, SLYUNTCHEV O M, et al. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices [J]. Journal of Nuclear Materials, 2009, 385(1): 189-192. doi: 10.1016/j.jnucmat.2008.09.053 [56] SINGH D, MANDALIKA V R, PARULEKAR S J, et al. Magnesium potassium phosphate ceramic for 99Tc immobilization [J]. Journal of Nuclear Materials, 2006, 348(3): 272-282. doi: 10.1016/j.jnucmat.2005.09.026 -






 DownLoad:
DownLoad: