-
在饮用水处理过程中,氯因其持久氧化性及经济性是目前最为常用的氧化剂和消毒剂。然而,氯与有机物反应会生成多种具有致畸性、致癌性消毒副产物(disinfection by-products,DBPs)。我国《生活饮用水卫生标准》(GB 5749-2022)对三卤甲烷(trihalomethanes,THMs)和卤乙酸(haloacetic acid,HAAs)进行了明确管控。除了已知的包括THMs、HAAs、卤代苯酚、亚硝铵等多种DBPs之外,饮用水中还存在着大量具有较高潜在毒性风险的未知DBPs。
活性炭(activated carbon,AC)作为一种高效、经济的吸附剂广泛应用于饮用水厂和家用净水过滤系统中[1]。在预处理阶段,粉末活性炭常用于解决突发性微量污染物问题[2]。在净水过滤器中,AC可以作为其吸附剂的主要组分[3]。因此,在预处理阶段或者家用净水器端,AC不可避免的会与氯接触。之前的研究发现[4],AC本身也可与氯反应生成毒性更强的DBPs。且由于AC的催化作用,其可催化次氯酸产生氯自由基(Cl·),导致不同的氯化产物。BULMAN等[5]发现,氯光解过程中形成的多种活性氧化剂会诱导形成新兴的氯化DBPs。VOUDRIAS等[6]也发现AC会促进游离氯氧化酚类物质形成新的副产物。此外,AC作为优良的吸附剂既可以吸附溶解性天然有机物(dissolved organic matter,DOM),也可以吸附生成的DBPs,导致其对DOM氯化过程中DBPs的生成具有复杂的影响效应。因此,深入探究AC对DOM氯化过程中产生DBPs释放风险的影响具有重要意义。
傅立叶变换离子回旋共振质谱(fourier transform ion cyclotron resonance mass spectrometry,FTICR-MS)是一种高分辨率质谱仪器。为了分析的精确性,其采用较长的采集时间和上百次的谱图叠加[7],用于检测DOM中的分子结构,也可鉴定高分子质量的有机化合物[8-9]。FTICR-MS可通过分子式的元素比率和芳香度信息来分析DOM的组分特征,从而研究DOM与生物、自然介质之间的关系[10]。ZHANG等[11]通过FTICR-MS对不同分子质量DOM馏分的光学和分子特征进行了研究,发现高度不饱和的芳香族物质富含电子,其与次氯酸表现出高反应性。AC氯化后会生成分子质量为1 000~10 000 Da的副产物,但具体的种类及AC对DOM氯化的影响机制还尚未明确。
因此,本研究通过以是否在氯化过程中投加AC为变量,达到以下目的:1)研究AC对DOM氯化过程中产生已知DBPs的影响,并评价其出水产物毒性;2)通过FTICR-MS技术识别并明确AC对DOM氯化过程中产生的氯化产物种类的影响;3)通过FTICR-MS技术阐明AC对氯化过程中DOM特性转化的影响。
-
本研究中使用的DBPs标准品为色谱纯,购自Accu Standard公司(美国);甲基叔丁基醚(methyl tert-butyl ether,MTBE)为色谱纯,购自北京百灵威科技有限公司;无水硫酸钠(Na2SO4)、碳酸氢钠(NaHCO3)、浓硫酸(H2SO4)、硫代硫酸钠(NaS2O3)和次氯酸钠(NaClO)均为分析纯,购自国药集团化学试剂有限公司;AC购自宁夏光华活性炭有限公司,选取椰壳炭的物理性质包括碘值1 030 mg·g−1,比表面积1 114 m2·g−1,平均孔径3.61 nm,总孔隙体积0.78 m2·g−1,微孔和介孔体积分别为0.3 m2·g−1和0.46 m2·g−1;其表面官能团结构包括碱性、酸性、酚醛、羧基和内酯基团的含量为0.58、0.50、0.11、0.38和0.02 mmoL·g−1。AC均用去离子水洗涤至滤液pH呈中性,在115 ℃下干燥12 h后,将其制备成1 g·L−1的悬浊液。原水(raw water,RW)取自中国北京京密引水渠,本研究所用的实验水样参数:pH=8.27,浊度为1.28 NTU,以CaCO3计的碱度和硬度分别为83.38 mg·L−1和111.00 mg·L−1,UV254为0.023 cm−1,溶解性有机碳(dissolved organic carbon,DOC)为2.21 mg·L−1。
-
将AC悬浊液超声后加入到1 L 0.1 mmol·L−1 NaClO的超纯水和RW中,AC质量浓度为10 mg·L−1,使用10 mmol·L−1磷酸盐缓冲液将溶液的pH调整为7.5,同时设计另一组实验,先使用AC对RW中的DOM进行吸附,再加氯进行反应。磁力搅拌24 h,检测反应0.5、1、2、24 h后水样中THMs和HAAs的浓度,同时对反应24 h的样品进行FTICR-MS分析,使用Na2S2O3淬灭余氯并利用0.45 μm的膜过滤去除AC,滤后水中加入5 g无水Na2SO4,使用MTBE作为萃取剂提取水样,HAAs还需甲醇酸化处理,使其衍化为卤乙酸甲酯,测定DBPs以及其他指标。
-
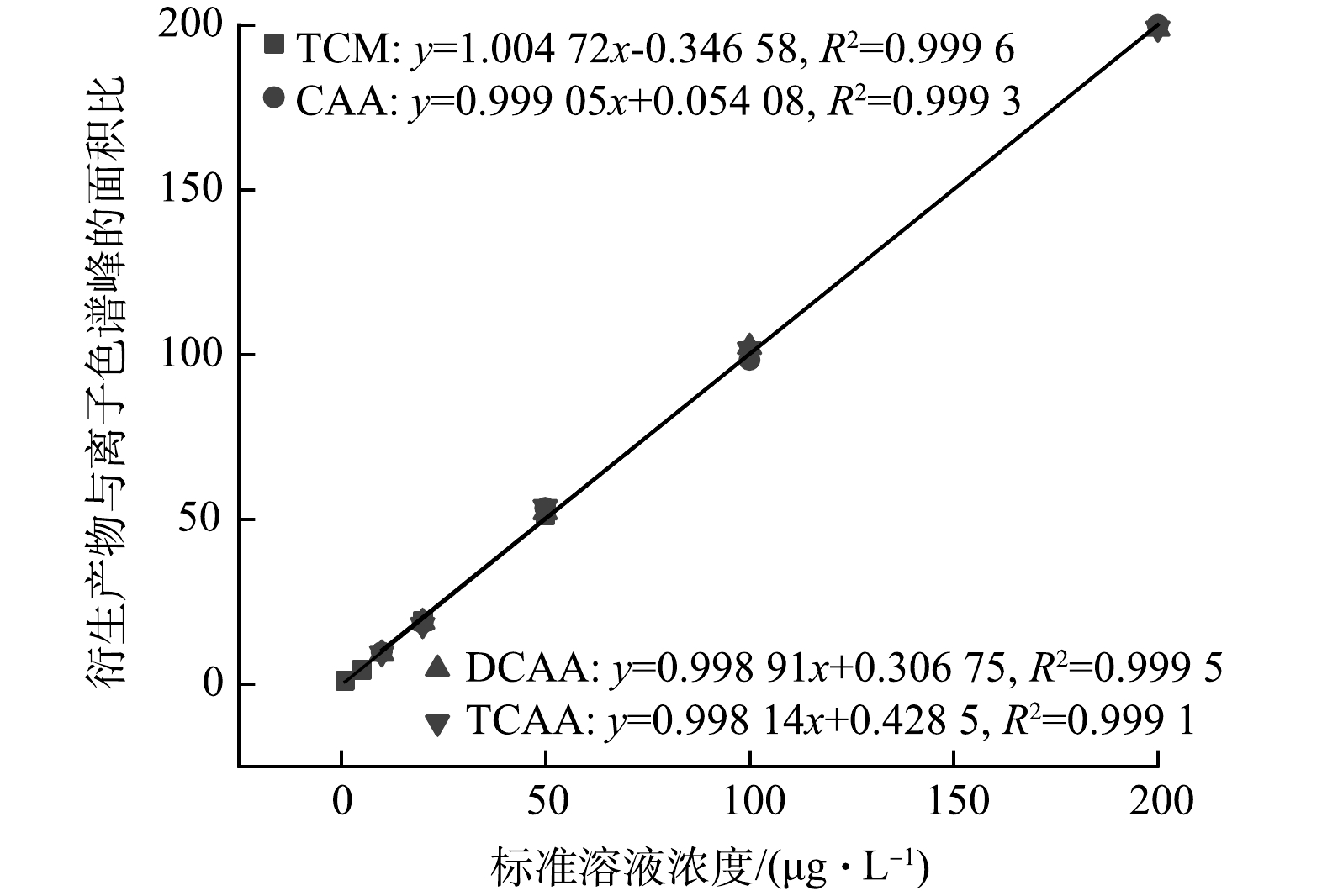
THMs和HAAs的测定参考美国环境保护署标准方法(USEPA Standard Methods 551.1和552.3),THMs和HAAs的回归曲线如图1所示。测定的4种DBPs(TCM、CAA、DCAA、TCAA)采用配备电子捕获检测器(Agilent Technologies,Santa Clara,CA,USA)的气相色谱仪(Agilent 7 890,Santa Clara,USA)进行分析[12]。气相色谱柱为HP-5型的熔融石英毛细管柱(30 mm×0.25 mm内径,薄膜厚度为0.25 mm)。氯化反应开始前的溶液使用pH计(HACHHQ 40 d,Loveland Colorado,USA)校准成中性。余氯使用N,N-二乙基对苯二胺(DPD)方法进行测定,结果以mg·L−1的Cl2表示(HACH Pocket ColorimeterII,Loveland Colorado,USA)。总有机碳分析仪(total organic carbon,TOC,Elementar公司,德国)测定AC滤后水中DOC的浓度。溶液中的有机物含量使用紫外分光光度计(UV-6 100型,中国上海)进行测定。在5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为捕获剂的条件下,采用电子自旋共振波谱仪(electron spin resonance,ESR,A300-10/12型Bruker公司,德国)检测自由基。
-
仪器参数与操作步骤使用配备有15.0 T超导磁体和电喷雾电离源的FTICR-MS(Bruker Solari X型)对样品的分子组成进行分析。样品在负离子模式下进行测试,进样方式为连续进样,进样速度为150 μL·h−1,毛细管入口电压为4 kV,离子累积时间为0.08 s,相对分子质量采集范围为100~1 000 Da,采样点数为4 ppm,时域信号叠加300次以提高信噪比.上机测试前用10 mmol·L−1甲酸钠对仪器进行校正,样品检测完成后用可溶性有机质(已知分子式)进行内标校正。经过校正后,检测的质量误差均小于1 ppm。样品检测时取原水样品200 μL,过0.22 μm滤膜以去除颗粒物等杂质,然后用甲酸酸化水样,逐滴加入甲酸直至水样pH调节至2。然后对水样中的DOM进行SPE固相萃取(萃取柱型号为Agilent Bond Elut PPL(1.0 g,6 mL)。H/Cw、O/Cw和碳归一化双键当量(DBE/Cw)等分子式参数根据每个样品中指定分子式的相对强度加权平均值计算得出[13]。数据采用DOM中已知的CHO类化合物进行内标校准,如对应多个分子式,采用同系物规则和最小杂原子个数规则进行正确分子式筛选。
-
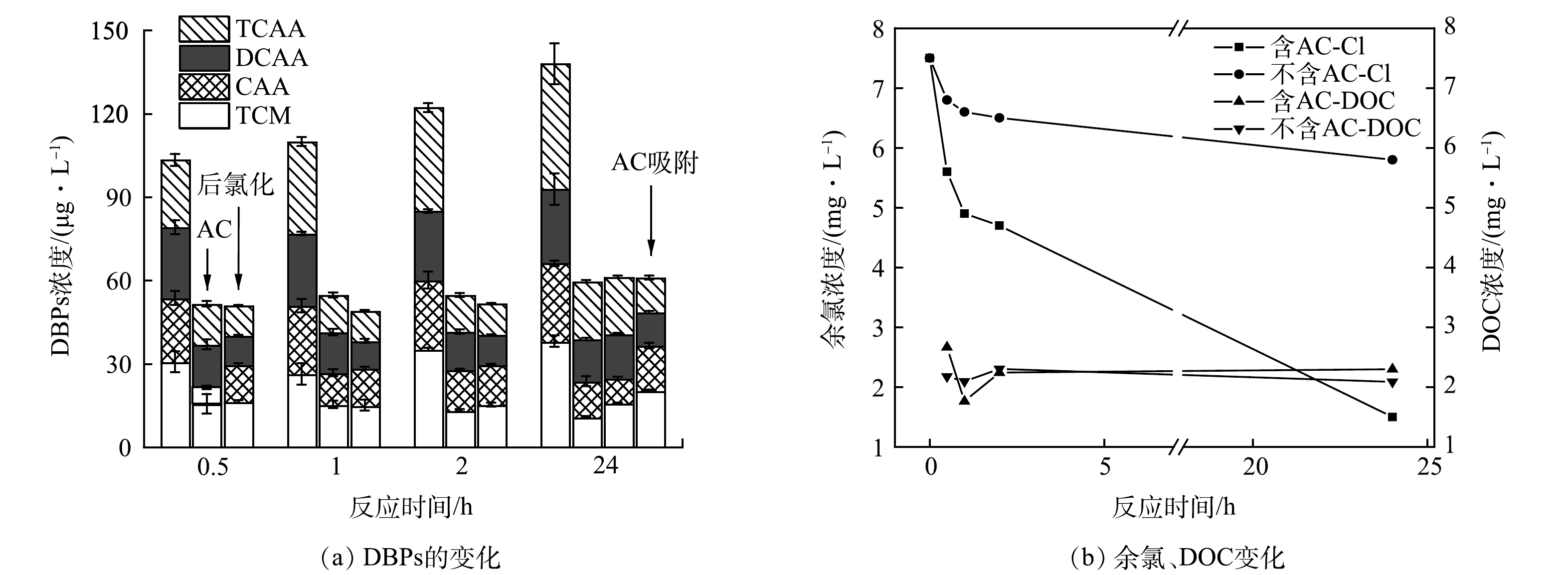
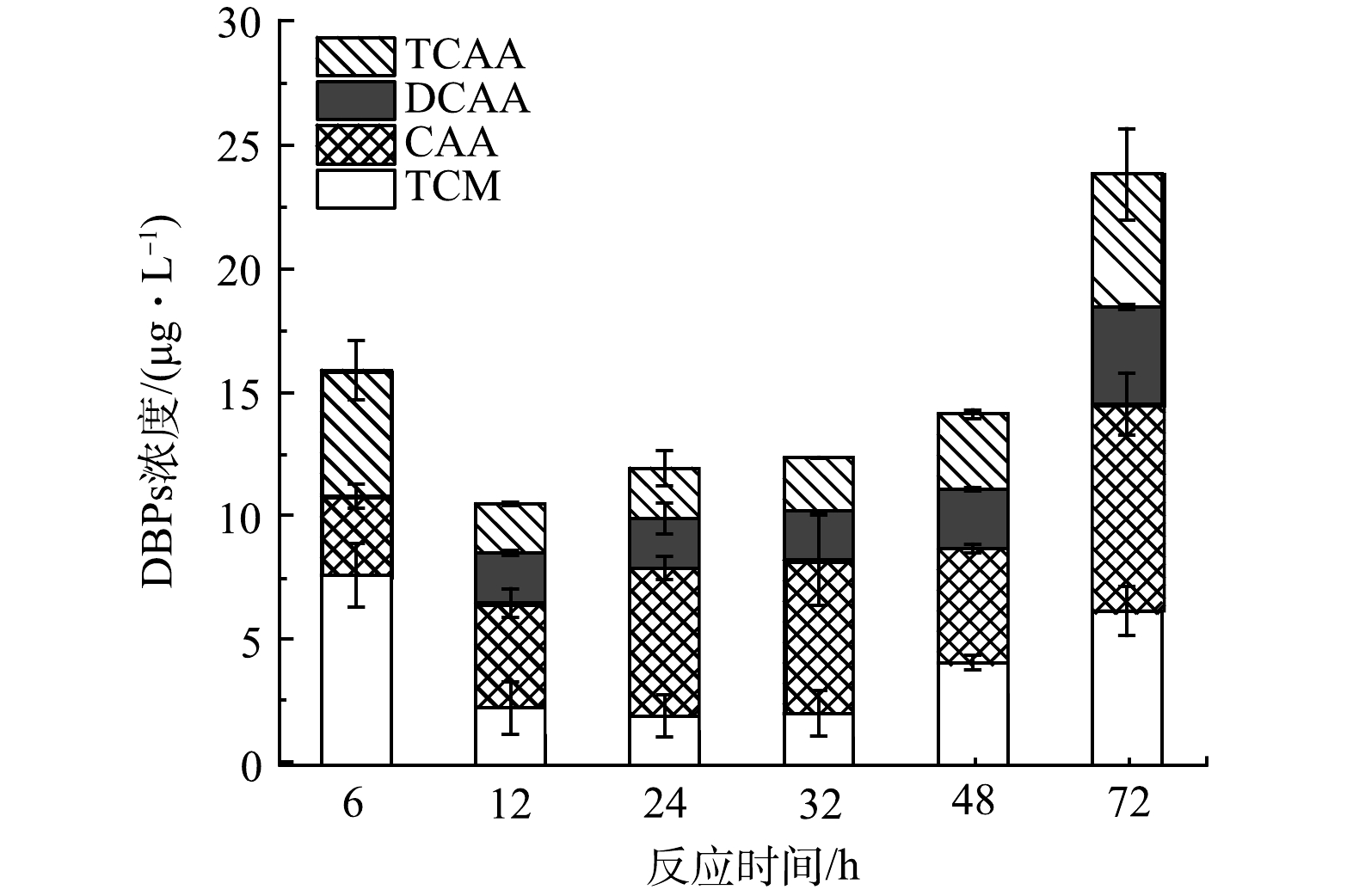
如图2所示,比较了AC是否存在和不同氯化方式对RW氯化过程中DBPs的释放情况。图2(a)所示为测定的DBPs浓度随时间变化规律,可以看到无论是否在RW中加入AC,DBPs浓度均随时间的延长升高,DBPs的总浓度在反应初始时可忽略不计。AC存在与不存在时DBPs的浓度分别从0.5 h的51.29 μg·L−1和103.19 μg·L−1上升至24 h的59.34 μg·L−1和137.87 μg·L−1,并且在2 h时达到较高水平,说明AC与0.1 mmol·L−1 NaClO在开始的2 h内剧烈反应生成大量DBPs。但加入AC的水样随着反应时间的增加,DBPs的变化并不明显,可能是由于部分DBPs及其前体物被AC快速吸附以及自由氯被大量消耗后导致反应速率下降。此外,进一步对比了在RW氯化过程中不同活性炭加入方式对DBPs生成释放的影响,结果如图2(a)所示。发现过滤掉AC后氯化方式产生的DBPs与AC一直存在的结果基本一致,说明AC在此过程中虽然可以吸附THMs、HAAs及其前体物,并且可以催化氯产生自由基,但对释放到水中THMs及HAAs影响较小。如图2(b)所示,在AC存在时,氯的衰减率明显增加,但释放到水体的目标DBPs浓度较未加入AC时更低。AC存在时余氯衰减快,测得DBPs较少。一方面是由于生成的DBPs被AC吸附,另一方面具有较强还原性的AC本身也会快速消耗自由氯。如图2(a)所示,单独在24 h时测定AC吸附的DBPs,发现即使将吸附反应后的AC经有机溶剂丙酮浸泡,并超声处理释放DBPs,测定的4种TCM、CAA、DCAA、TCAA的质量浓度分别为19.85、16.29、12.08、12.50 μg·L−1,可以发现加入AC组的DBPs总质量浓度(120.05 μg·L−1)仍低于不加入AC组(147.87 μg·L−1)。此外,如图3所示,还测定了在纯水中AC与过量氯反应产生的DBPs。发现THMs及HAAs的浓度先下降后上升。这是由于AC前期吸附性较强,后期吸附能力下降,生成的DBPs逐渐释放到水中。同时对水中的DOC进行测定,前2 h的DOC浓度均为先下降后上升,但随着反应时间的继续增加,加入AC组的DOC浓度继续上升,而未加入AC组却呈现下降趋势。这一现象说明AC影响了DOM的氯化过程,导致其结构被破坏且生成了其他副产物。VOUDRIAS等[14]发现AC会导致一系列自由基连锁反应的发生。HUANG等[4]研究了THMs和HAAs在AC存在下的含量变化,但没有研究其单独氯化DOM的情况,而且AC存在时溶液的细胞毒性也有所增强。因此,还需进一步探究AC存在时的氯化副产物的变化。
-
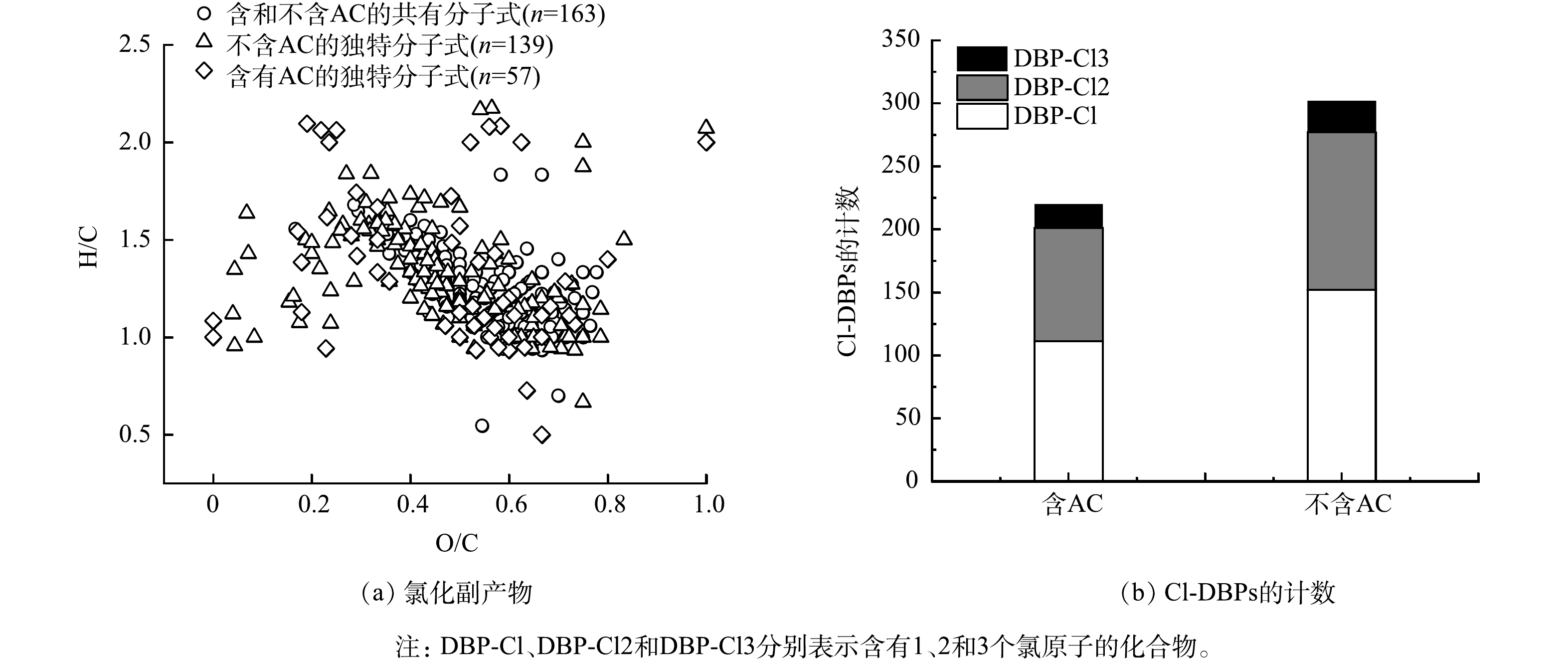
如图4所示,通过FTICR-MS探究了AC对氯化副产物的影响。由图4(a)可以看到,在RW氯化过程中,其产物匹配了302个氯化分子式,而AC存在时的氯化产物中,可对应220个氯化分子式。进一步分析302种氯化产物,其中163种分子式与AC存在时相同,因此AC存在时的氯化会导致部分氯化产物减少,但也生成了新的氯化产物包括57种在内的独特分子式,其中CHOCl、CHONCl、CHOSCl、CHNSCl、CHNOSCl分子式各生成了42、5、5、2、3种。如图4(b)所示,在有AC存在的氯化过程中,氯化副产物产生的含有2个和3个氯原子的DBPs相对较少。在AC存在的氯化水样中,生成含有2个和3个Cl原子的分子式分别为90个和19个;而在RW氯化过程中,生成含有2个和3个Cl原子的分子式为125个和25个。此外,如表1所示,经对比发现,AC存在时,CHOCl、CHONCl、CHONSCl分子式的数量减少,而CHOSCl的分子式增加,并且CHOCl、CHONCl以及CHOSCl和CHONSCl分子式的H/Cw值均低于RW的氯化过程,相反的是O/Cw均高于RW氯化。有研究[15]表明,与传统的暗氯化生成副产物的生成机制不同,活性氯物种(reactive chlorine species,RCS)与有机物的主要反应机理是氯加成、单电子转移和氢抽取反应。BEN等[16]和SUN等[17]发现氯可以通过自由基链式反应发生降解,从而减少自身与其他物质的接触时间。RCS和DOM结合也会影响靶向DBPs的生成,诱导形成新型的DBPs[5],这可能是AC存在时有Cl·的生成,从而发生的后续自由基反应导致H/Cw值较低、O/Cw较高。
-
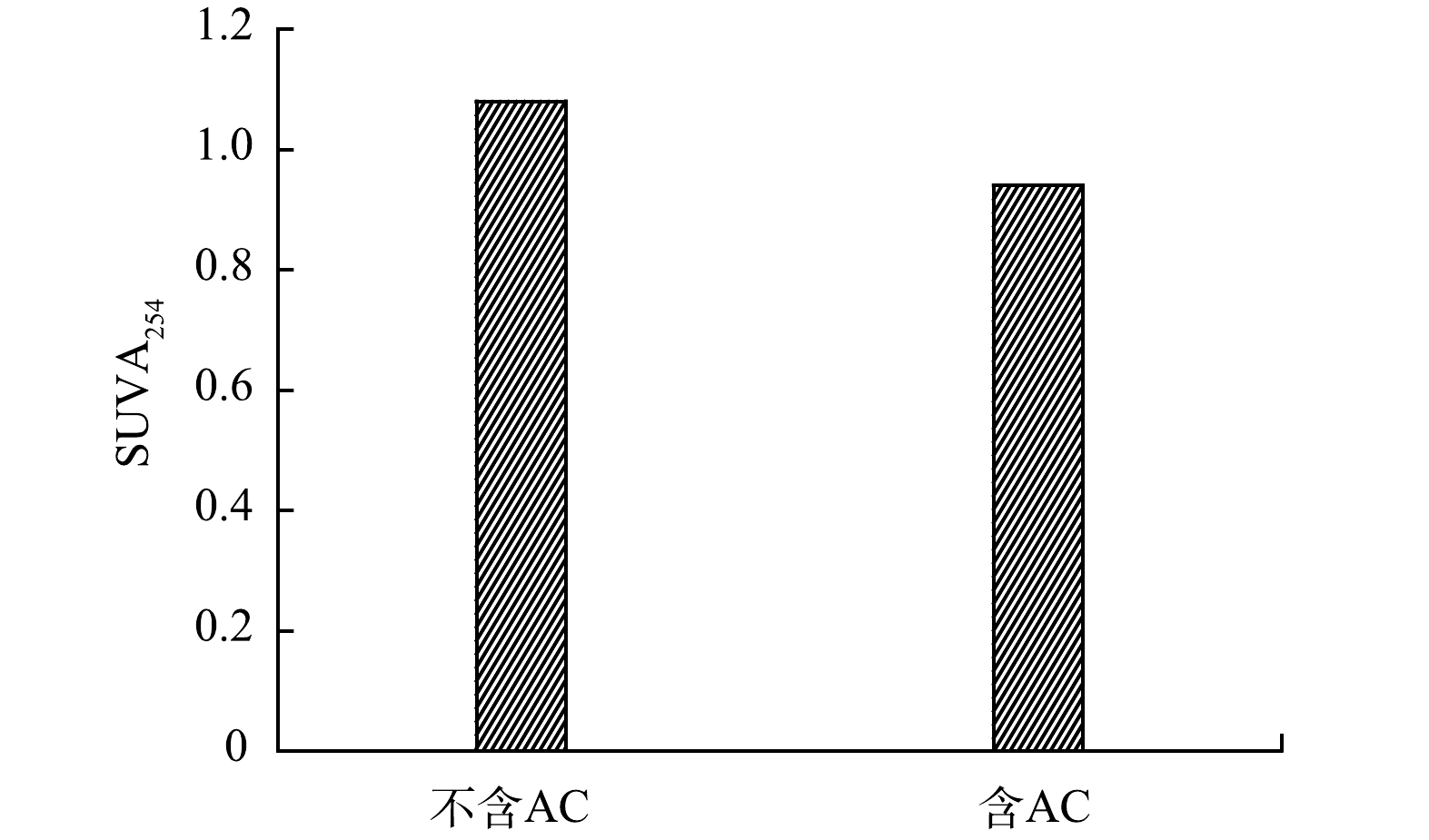
DOM的成分也会影响AC对氯的反应特性。因此,探究了AC存在时RW氯化过程中DOM的转化情况。基于修正后的芳香指数和H/C将溶解性有机质分为5类[18]:稠环多环芳烃(AImod>0.66)、多酚类物质(0.5<AImod≤0.66)、高度不饱和酚类物质(AImod≤0.5且H/C≤1.5)、脂肪类物质(AImod≤0.5和1.5<H/C≤2)和饱和类物质(H/C>2)[19]。如图5(a)和图5(b)所示,绝大部分有机物属于脂肪类物质、高度不饱和酚类物质和多酚类物质。此外,SUVA254(即UV254/DOC)可用来比较不同样品中的芳香族化合物的含量(即芳香度)[20]。芳香度与反应性有关,有机物的反应性反映了通过凝聚去除该有机物的难易程度,以及有机物与氯反应产生DBPs的可能性。如图6所示,对比了氯化后RW中是否存在AC时SUVA254的变化,THMs和HAAs的浓度随SUVA254的增加而增加[21]。图5显示AC存在时的SUVA254低于不含AC的水样,与上述结果保持一致。含AC和不含AC的RW中DOC在氯化前后仅有轻微变化,这表明DOM未发生矿化作用。SUVA254还可以表征有机物中不饱和键数量(芳香特征),氯化后的RW中,加入AC组后的SUVA254较低,因此其芳香性低,DOM转化的较多。在两种氯化过程后,SUVA254均有所下降,尤其是AC存在时,BULMAN等[5]的研究也得到了类似的结果,这表明SUVA254的大幅下降可能是由于含有芳香族DOM分子,富含芳香族结构的化合物可以提供更强的疏水作用、离子相互作用和键合作用。
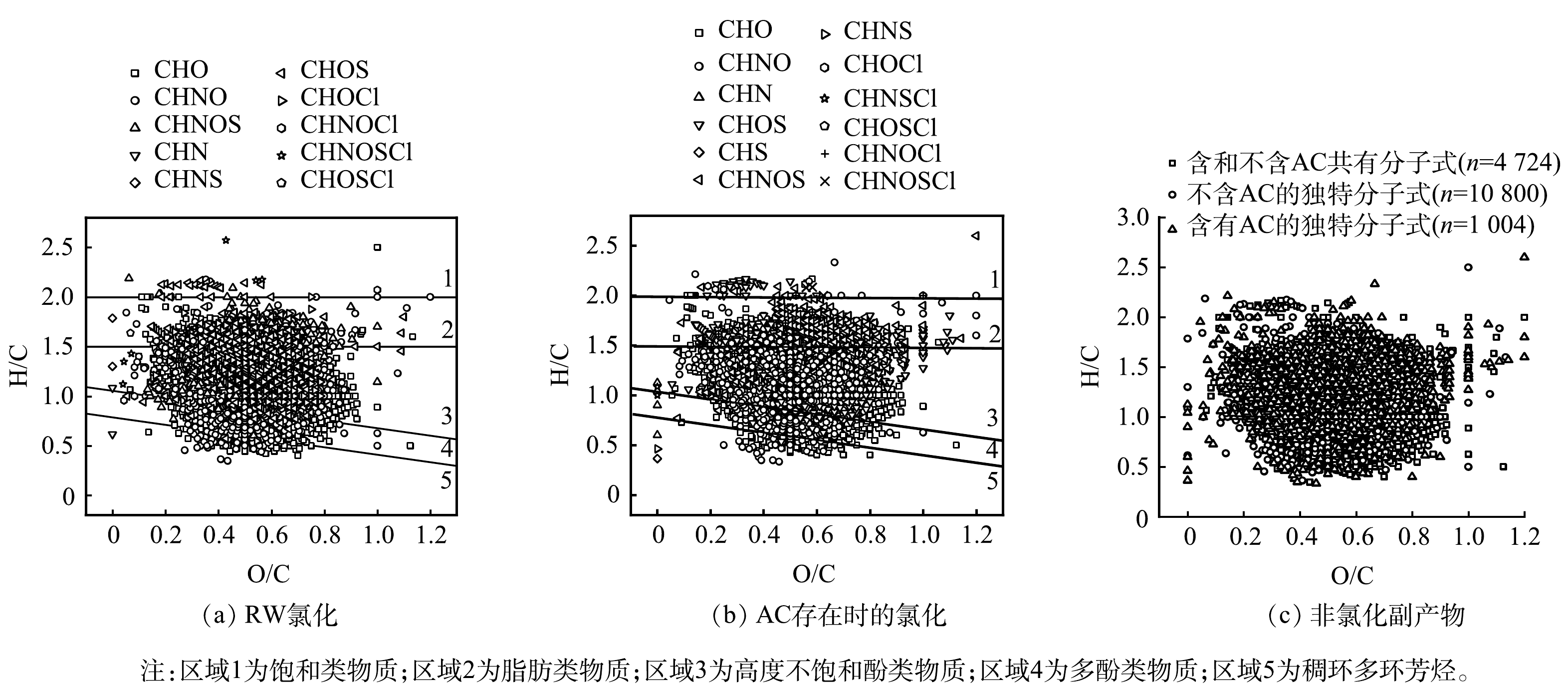
利用FTICR-MS对有无AC存在的2种情况下的无氯分子式进行比较。如图5(c)所示,2种条件下,相同分子式的比例(约70%)显著高于氯化分子式的比例。如表2所示,CHO、CHON、CHOS和CHONS分子式的H/Cw和O/Cw相似。不含AC氯化条件下CHO、CHON、CHOS和CHONS分子式的DBEw均大于AC存在时氯化条件下的DBEw。较低的DBEw表明产生的DOM平均脂肪族含量更高,与SUVA254结果相一致。有研究[15, 22]表明,AC可与氧气反应生成过氧自由基,过氧自由基经过双分子衰变或单分子衰变生成醇或醛。因此,较低的DBEw可能是由于过氧自由基在AC和氧的活化下产生了部分醇。
-
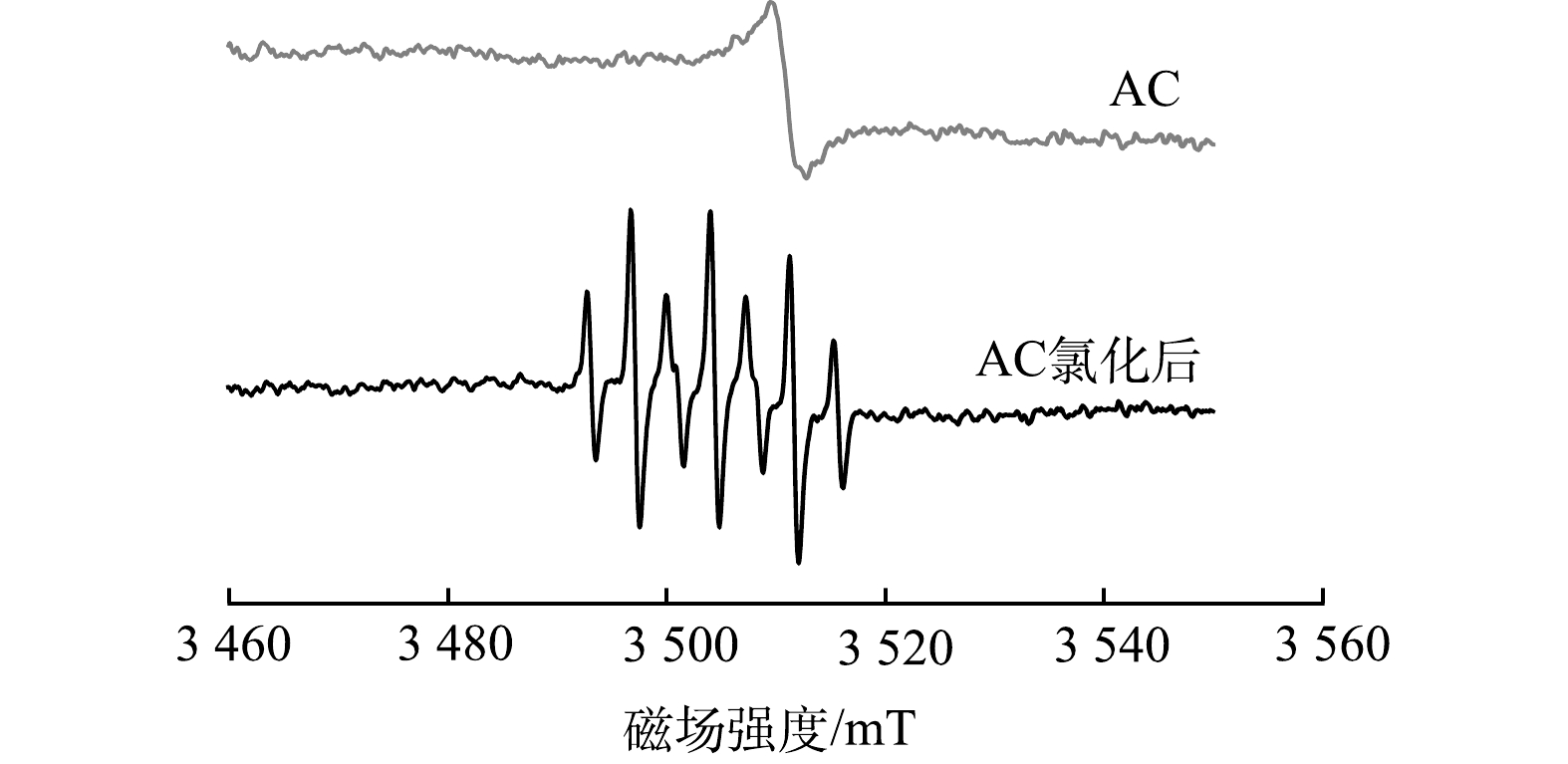
采用ESR技术对AC氯化前后产生的自由基进行检测分析。如图7所示,在只含AC时,可检测到活性炭表面的持久性自由基。在AC氯化后发现了多重峰的存在,DMPO-H2O体系中的七重峰对应·Cl/DMPO加合物,表明在此过程中产生了Cl·[23],Cl·是一种对有机化合物具有较强选择性的自由基,易发生取代反应。由于Cl·具有很强的活性,因此,能够促进DBPs的生成,诱导某些有毒副产物的形成。ESR的结果表明,AC表面持久性自由基可催化次氯酸产生Cl·,在自由基的作用下,DOM与RCS之间会产生氯化副产物,尤其是亲核反应在其中发挥着很大作用,并且DOM的芳香性变强也有助于总有机氯的形成[24]。因此,氯化过程中AC会促进自由基的产生进而诱导其他类型DBPs的生成。
-
AC作为一种优良吸附剂被广泛应用于水处理工艺和终端净水过滤;同时,氯也是一种常见的预氧化剂和消毒剂。本文阐述了AC对RW氯化过程DBPs生成及DOM转化的影响,主要结果如下。
1)虽然AC存在时余氯下降较为迅速,但生成的THMs及HAAs较少,这是由于AC优良的吸附性能以及还原性AC快速消耗氯生成其他DBPs,DOC的变化也可说明了这一变化趋势。
2) FTICR-MS检测结果表明,AC存在时含氯物质的数量减少,Cl-DBPs的种类由302种减少到220种,其中有57种特异性氯代产物,CHOSCl化合物生成较多,其他CHOCl、CHONCl、CHONSCl化合物的数量减少。
3) FTICR-MS的结果显示,AC存在时可鉴定的化合物数量呈下降趋势,其是否存在的两种情况,生成的化合物有较大区别,但大部分化合物均属于脂肪类物质、高度不饱和类及酚类物质和多酚类物质。AC存在时,SUVA254的大幅降低表明含有芳香性的DOM被转化,而未加入AC组没有发生矿化反应。
4) AC表面持久性自由基催化次氯酸产生Cl·,Cl·引发的自由基反应是造成氯化产物及有机物形态改变的主要原因。
活性炭对天然有机物氯化过程消毒副产物生成的影响及作用机制
Effect and mechanism of activated carbon on the generation of disinfection by-products during chlorination process of dissolved organic matter
-
摘要: 活性炭(AC)与氯均为水处理过程中广泛使用的药剂,在实际使用过程中二者的接触不可避免,因此,深入研究AC对于氯化过程中消毒副产物(DBPs)生成的影响对于饮用水安全有重要意义。本研究对比了AC对于溶解性天然有机物(DOM)氯化过程中已知DBPs(包括三卤甲烷(THMs)和卤乙酸(HAAs))生成释放的影响,并采用傅立叶变换离子回旋共振质谱(FTICR-MS)技术检测分析其滤后水的未知氯化副产物及有机物变化规律。结果表明,在DOM氯化过程中,AC存在时释放的THMs和HAAs浓度较低,但氯的衰减速率更快,这是由于AC本身的强还原性及其他氯代副产物生成导致的。进一步通过FTICR-MS分析未知氯代产物及DOM的变化发现,在2种条件下有163种相同的氯化产物,与不存在AC时对比,AC存在时生成了不同的氯化产物中有57种。此外,AC存在时CHOCl、CHONCl和CHONSCl分子式的数量减少,而CHOSCl分子式的数量增加,并且具有芳香结构的DOM更容易被转化。通过电子自旋共振谱仪(ESR)分析发现AC表面的持久性自由基激发次氯酸钠反应生成的氯自由基(Cl·)是导致氯化产物变化的主要原因。本研究揭示了AC对氯化过程DBPs的生成影响,对于饮用水DBPs控制具有重要意义。Abstract: Activated carbon (AC) and chlorine are both widely used agents in the water treatment process, and their contact is inevitable during the actual use. Therefore, it is important to study the effects of AC on the generation of disinfection by-products (DBPs) during the chlorination process for the safety of drinking water. Therefore, in this study, the effects of AC on the concentration of known DBPs including trihalomethanes (THMs) and haloacetic acids (HAAs) formed during the chlorination of dissolved organic matter (DOM) were compared, and the unknown chlorination byproducts and the transformation of organics in effluent were analyzed by using the Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) technique detection. The results showed that during DOM chlorination, lower concentrations of THMs and HAAs were released in the presence of AC, but the rate of chlorine decay was faster, which was due to the strong reducing property of AC itself and the generation of other chlorinated by-products. Further analysis of the unknown chlorinated products and changes in DOM by FTICR-MS revealed that there were 163 identical chlorination products under the two conditions, and 57 of the different chlorination products were generated in the presence of AC compared to the absence of AC. In addition, the numbers of CHOCl, CHONCl, and CHONSCl molecular formulas decreased, while the number of CHOSCl molecular formulas increased, and DOM with an aromatic structure was more easily converted in the presence of AC. The main reason for the change in chlorination products is the generation of chlorine radicals (Cl·) from the reaction of sodium hypochlorite activated by the persistent radicals on the surface of AC through electron spin resonance spectroscopy (ESR) analysis. This study reveals the effects of AC on the generation of DBPs during chlorination, which is important for the control of DBPs in drinking water.
-
Key words:
- disinfection by-products /
- activated carbon /
- chlorination /
- FTICR-MS /
- chlorine radical
-
在饮用水处理过程中,氯因其持久氧化性及经济性是目前最为常用的氧化剂和消毒剂。然而,氯与有机物反应会生成多种具有致畸性、致癌性消毒副产物(disinfection by-products,DBPs)。我国《生活饮用水卫生标准》(GB 5749-2022)对三卤甲烷(trihalomethanes,THMs)和卤乙酸(haloacetic acid,HAAs)进行了明确管控。除了已知的包括THMs、HAAs、卤代苯酚、亚硝铵等多种DBPs之外,饮用水中还存在着大量具有较高潜在毒性风险的未知DBPs。
活性炭(activated carbon,AC)作为一种高效、经济的吸附剂广泛应用于饮用水厂和家用净水过滤系统中[1]。在预处理阶段,粉末活性炭常用于解决突发性微量污染物问题[2]。在净水过滤器中,AC可以作为其吸附剂的主要组分[3]。因此,在预处理阶段或者家用净水器端,AC不可避免的会与氯接触。之前的研究发现[4],AC本身也可与氯反应生成毒性更强的DBPs。且由于AC的催化作用,其可催化次氯酸产生氯自由基(Cl·),导致不同的氯化产物。BULMAN等[5]发现,氯光解过程中形成的多种活性氧化剂会诱导形成新兴的氯化DBPs。VOUDRIAS等[6]也发现AC会促进游离氯氧化酚类物质形成新的副产物。此外,AC作为优良的吸附剂既可以吸附溶解性天然有机物(dissolved organic matter,DOM),也可以吸附生成的DBPs,导致其对DOM氯化过程中DBPs的生成具有复杂的影响效应。因此,深入探究AC对DOM氯化过程中产生DBPs释放风险的影响具有重要意义。
傅立叶变换离子回旋共振质谱(fourier transform ion cyclotron resonance mass spectrometry,FTICR-MS)是一种高分辨率质谱仪器。为了分析的精确性,其采用较长的采集时间和上百次的谱图叠加[7],用于检测DOM中的分子结构,也可鉴定高分子质量的有机化合物[8-9]。FTICR-MS可通过分子式的元素比率和芳香度信息来分析DOM的组分特征,从而研究DOM与生物、自然介质之间的关系[10]。ZHANG等[11]通过FTICR-MS对不同分子质量DOM馏分的光学和分子特征进行了研究,发现高度不饱和的芳香族物质富含电子,其与次氯酸表现出高反应性。AC氯化后会生成分子质量为1 000~10 000 Da的副产物,但具体的种类及AC对DOM氯化的影响机制还尚未明确。
因此,本研究通过以是否在氯化过程中投加AC为变量,达到以下目的:1)研究AC对DOM氯化过程中产生已知DBPs的影响,并评价其出水产物毒性;2)通过FTICR-MS技术识别并明确AC对DOM氯化过程中产生的氯化产物种类的影响;3)通过FTICR-MS技术阐明AC对氯化过程中DOM特性转化的影响。
1. 材料与方法
1.1 试剂与材料
本研究中使用的DBPs标准品为色谱纯,购自Accu Standard公司(美国);甲基叔丁基醚(methyl tert-butyl ether,MTBE)为色谱纯,购自北京百灵威科技有限公司;无水硫酸钠(Na2SO4)、碳酸氢钠(NaHCO3)、浓硫酸(H2SO4)、硫代硫酸钠(NaS2O3)和次氯酸钠(NaClO)均为分析纯,购自国药集团化学试剂有限公司;AC购自宁夏光华活性炭有限公司,选取椰壳炭的物理性质包括碘值1 030 mg·g−1,比表面积1 114 m2·g−1,平均孔径3.61 nm,总孔隙体积0.78 m2·g−1,微孔和介孔体积分别为0.3 m2·g−1和0.46 m2·g−1;其表面官能团结构包括碱性、酸性、酚醛、羧基和内酯基团的含量为0.58、0.50、0.11、0.38和0.02 mmoL·g−1。AC均用去离子水洗涤至滤液pH呈中性,在115 ℃下干燥12 h后,将其制备成1 g·L−1的悬浊液。原水(raw water,RW)取自中国北京京密引水渠,本研究所用的实验水样参数:pH=8.27,浊度为1.28 NTU,以CaCO3计的碱度和硬度分别为83.38 mg·L−1和111.00 mg·L−1,UV254为0.023 cm−1,溶解性有机碳(dissolved organic carbon,DOC)为2.21 mg·L−1。
1.2 实验方法
将AC悬浊液超声后加入到1 L 0.1 mmol·L−1 NaClO的超纯水和RW中,AC质量浓度为10 mg·L−1,使用10 mmol·L−1磷酸盐缓冲液将溶液的pH调整为7.5,同时设计另一组实验,先使用AC对RW中的DOM进行吸附,再加氯进行反应。磁力搅拌24 h,检测反应0.5、1、2、24 h后水样中THMs和HAAs的浓度,同时对反应24 h的样品进行FTICR-MS分析,使用Na2S2O3淬灭余氯并利用0.45 μm的膜过滤去除AC,滤后水中加入5 g无水Na2SO4,使用MTBE作为萃取剂提取水样,HAAs还需甲醇酸化处理,使其衍化为卤乙酸甲酯,测定DBPs以及其他指标。
1.3 分析方法
THMs和HAAs的测定参考美国环境保护署标准方法(USEPA Standard Methods 551.1和552.3),THMs和HAAs的回归曲线如图1所示。测定的4种DBPs(TCM、CAA、DCAA、TCAA)采用配备电子捕获检测器(Agilent Technologies,Santa Clara,CA,USA)的气相色谱仪(Agilent 7 890,Santa Clara,USA)进行分析[12]。气相色谱柱为HP-5型的熔融石英毛细管柱(30 mm×0.25 mm内径,薄膜厚度为0.25 mm)。氯化反应开始前的溶液使用pH计(HACHHQ 40 d,Loveland Colorado,USA)校准成中性。余氯使用N,N-二乙基对苯二胺(DPD)方法进行测定,结果以mg·L−1的Cl2表示(HACH Pocket ColorimeterII,Loveland Colorado,USA)。总有机碳分析仪(total organic carbon,TOC,Elementar公司,德国)测定AC滤后水中DOC的浓度。溶液中的有机物含量使用紫外分光光度计(UV-6 100型,中国上海)进行测定。在5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为捕获剂的条件下,采用电子自旋共振波谱仪(electron spin resonance,ESR,A300-10/12型Bruker公司,德国)检测自由基。
1.4 FTICR-MS分析
仪器参数与操作步骤使用配备有15.0 T超导磁体和电喷雾电离源的FTICR-MS(Bruker Solari X型)对样品的分子组成进行分析。样品在负离子模式下进行测试,进样方式为连续进样,进样速度为150 μL·h−1,毛细管入口电压为4 kV,离子累积时间为0.08 s,相对分子质量采集范围为100~1 000 Da,采样点数为4 ppm,时域信号叠加300次以提高信噪比.上机测试前用10 mmol·L−1甲酸钠对仪器进行校正,样品检测完成后用可溶性有机质(已知分子式)进行内标校正。经过校正后,检测的质量误差均小于1 ppm。样品检测时取原水样品200 μL,过0.22 μm滤膜以去除颗粒物等杂质,然后用甲酸酸化水样,逐滴加入甲酸直至水样pH调节至2。然后对水样中的DOM进行SPE固相萃取(萃取柱型号为Agilent Bond Elut PPL(1.0 g,6 mL)。H/Cw、O/Cw和碳归一化双键当量(DBE/Cw)等分子式参数根据每个样品中指定分子式的相对强度加权平均值计算得出[13]。数据采用DOM中已知的CHO类化合物进行内标校准,如对应多个分子式,采用同系物规则和最小杂原子个数规则进行正确分子式筛选。
2. 结果与讨论
2.1 AC对氯与有机物反应生成已知DBPs的影响
如图2所示,比较了AC是否存在和不同氯化方式对RW氯化过程中DBPs的释放情况。图2(a)所示为测定的DBPs浓度随时间变化规律,可以看到无论是否在RW中加入AC,DBPs浓度均随时间的延长升高,DBPs的总浓度在反应初始时可忽略不计。AC存在与不存在时DBPs的浓度分别从0.5 h的51.29 μg·L−1和103.19 μg·L−1上升至24 h的59.34 μg·L−1和137.87 μg·L−1,并且在2 h时达到较高水平,说明AC与0.1 mmol·L−1 NaClO在开始的2 h内剧烈反应生成大量DBPs。但加入AC的水样随着反应时间的增加,DBPs的变化并不明显,可能是由于部分DBPs及其前体物被AC快速吸附以及自由氯被大量消耗后导致反应速率下降。此外,进一步对比了在RW氯化过程中不同活性炭加入方式对DBPs生成释放的影响,结果如图2(a)所示。发现过滤掉AC后氯化方式产生的DBPs与AC一直存在的结果基本一致,说明AC在此过程中虽然可以吸附THMs、HAAs及其前体物,并且可以催化氯产生自由基,但对释放到水中THMs及HAAs影响较小。如图2(b)所示,在AC存在时,氯的衰减率明显增加,但释放到水体的目标DBPs浓度较未加入AC时更低。AC存在时余氯衰减快,测得DBPs较少。一方面是由于生成的DBPs被AC吸附,另一方面具有较强还原性的AC本身也会快速消耗自由氯。如图2(a)所示,单独在24 h时测定AC吸附的DBPs,发现即使将吸附反应后的AC经有机溶剂丙酮浸泡,并超声处理释放DBPs,测定的4种TCM、CAA、DCAA、TCAA的质量浓度分别为19.85、16.29、12.08、12.50 μg·L−1,可以发现加入AC组的DBPs总质量浓度(120.05 μg·L−1)仍低于不加入AC组(147.87 μg·L−1)。此外,如图3所示,还测定了在纯水中AC与过量氯反应产生的DBPs。发现THMs及HAAs的浓度先下降后上升。这是由于AC前期吸附性较强,后期吸附能力下降,生成的DBPs逐渐释放到水中。同时对水中的DOC进行测定,前2 h的DOC浓度均为先下降后上升,但随着反应时间的继续增加,加入AC组的DOC浓度继续上升,而未加入AC组却呈现下降趋势。这一现象说明AC影响了DOM的氯化过程,导致其结构被破坏且生成了其他副产物。VOUDRIAS等[14]发现AC会导致一系列自由基连锁反应的发生。HUANG等[4]研究了THMs和HAAs在AC存在下的含量变化,但没有研究其单独氯化DOM的情况,而且AC存在时溶液的细胞毒性也有所增强。因此,还需进一步探究AC存在时的氯化副产物的变化。
2.2 FTICR-MS分析氯化副产物
如图4所示,通过FTICR-MS探究了AC对氯化副产物的影响。由图4(a)可以看到,在RW氯化过程中,其产物匹配了302个氯化分子式,而AC存在时的氯化产物中,可对应220个氯化分子式。进一步分析302种氯化产物,其中163种分子式与AC存在时相同,因此AC存在时的氯化会导致部分氯化产物减少,但也生成了新的氯化产物包括57种在内的独特分子式,其中CHOCl、CHONCl、CHOSCl、CHNSCl、CHNOSCl分子式各生成了42、5、5、2、3种。如图4(b)所示,在有AC存在的氯化过程中,氯化副产物产生的含有2个和3个氯原子的DBPs相对较少。在AC存在的氯化水样中,生成含有2个和3个Cl原子的分子式分别为90个和19个;而在RW氯化过程中,生成含有2个和3个Cl原子的分子式为125个和25个。此外,如表1所示,经对比发现,AC存在时,CHOCl、CHONCl、CHONSCl分子式的数量减少,而CHOSCl的分子式增加,并且CHOCl、CHONCl以及CHOSCl和CHONSCl分子式的H/Cw值均低于RW的氯化过程,相反的是O/Cw均高于RW氯化。有研究[15]表明,与传统的暗氯化生成副产物的生成机制不同,活性氯物种(reactive chlorine species,RCS)与有机物的主要反应机理是氯加成、单电子转移和氢抽取反应。BEN等[16]和SUN等[17]发现氯可以通过自由基链式反应发生降解,从而减少自身与其他物质的接触时间。RCS和DOM结合也会影响靶向DBPs的生成,诱导形成新型的DBPs[5],这可能是AC存在时有Cl·的生成,从而发生的后续自由基反应导致H/Cw值较低、O/Cw较高。
表 1 RW氯化过程中的氯化产物分子式分子指数的强度加权平均值Table 1. Intensity-weighted average of molecular indices of molecular formulae for chlorination products during the RW chlorination process分子式 水样 H/CW O/CW DBEW AImod,w 总强度 相对丰度/% CHOCl 不含AC 1.29 0.50 6.98 0.22 4.60×109 93.77 含AC 1.23 0.54 7.24 0.25 2.8×109 94.38 CHONCl 不含AC 1.42 0.28 8.84 0.17 1.48×108 3.41 含AC 1.33 0.33 9.22 0.24 7.52×107 2.54 CHOSCl 不含AC 1.68 0.21 7.28 0.03 6.8×107 1.37 含AC 1.67 0.51 3.69 -0.31 6.15×107 2.07 CHONSCl 不含AC 1.6 0.30 7.02 -0.14 7.2×107 1.45 含AC 0.49 0.13 0.65 -0.12 4.02×107 1.01 2.3 溶解有机物的转化
DOM的成分也会影响AC对氯的反应特性。因此,探究了AC存在时RW氯化过程中DOM的转化情况。基于修正后的芳香指数和H/C将溶解性有机质分为5类[18]:稠环多环芳烃(AImod>0.66)、多酚类物质(0.5<AImod≤0.66)、高度不饱和酚类物质(AImod≤0.5且H/C≤1.5)、脂肪类物质(AImod≤0.5和1.5<H/C≤2)和饱和类物质(H/C>2)[19]。如图5(a)和图5(b)所示,绝大部分有机物属于脂肪类物质、高度不饱和酚类物质和多酚类物质。此外,SUVA254(即UV254/DOC)可用来比较不同样品中的芳香族化合物的含量(即芳香度)[20]。芳香度与反应性有关,有机物的反应性反映了通过凝聚去除该有机物的难易程度,以及有机物与氯反应产生DBPs的可能性。如图6所示,对比了氯化后RW中是否存在AC时SUVA254的变化,THMs和HAAs的浓度随SUVA254的增加而增加[21]。图5显示AC存在时的SUVA254低于不含AC的水样,与上述结果保持一致。含AC和不含AC的RW中DOC在氯化前后仅有轻微变化,这表明DOM未发生矿化作用。SUVA254还可以表征有机物中不饱和键数量(芳香特征),氯化后的RW中,加入AC组后的SUVA254较低,因此其芳香性低,DOM转化的较多。在两种氯化过程后,SUVA254均有所下降,尤其是AC存在时,BULMAN等[5]的研究也得到了类似的结果,这表明SUVA254的大幅下降可能是由于含有芳香族DOM分子,富含芳香族结构的化合物可以提供更强的疏水作用、离子相互作用和键合作用。
利用FTICR-MS对有无AC存在的2种情况下的无氯分子式进行比较。如图5(c)所示,2种条件下,相同分子式的比例(约70%)显著高于氯化分子式的比例。如表2所示,CHO、CHON、CHOS和CHONS分子式的H/Cw和O/Cw相似。不含AC氯化条件下CHO、CHON、CHOS和CHONS分子式的DBEw均大于AC存在时氯化条件下的DBEw。较低的DBEw表明产生的DOM平均脂肪族含量更高,与SUVA254结果相一致。有研究[15, 22]表明,AC可与氧气反应生成过氧自由基,过氧自由基经过双分子衰变或单分子衰变生成醇或醛。因此,较低的DBEw可能是由于过氧自由基在AC和氧的活化下产生了部分醇。
表 2 RW氯化过程后的非氯化产物分子式分子指数的强度加权平均值Table 2. Intensity-weighted average of molecular indices of molecular formulae for non-chlorinated products after the RW chlorination process分子式 水样 H/CW O/CW DBEW AImod,w 总强度 相对丰度/% CHO 不含AC 1.23 0.52 9.54 0.24 1.79×1011 80.56 含AC 1.23 0.52 9.35 0.23 1.79×1011 78.06 CHON 不含AC 1.20 0.52 9.99 0.23 3.21×1010 14.45 含AC 1.20 0.52 9.88 0.23 3.11×1010 13.56 CHOS 不含AC 1.40 0.49 6.45 0.07 8.5×109 3.83 含AC 1.42 0.53 6.40 0.03 1.54×1010 6.71 CHONS 不含AC 1.51 0.55 7.81 -0.14 2.58×109 1.16 含AC 1.55 0.60 7.12 -0.22 3.82×109 1.67 2.4 自由基的表征
采用ESR技术对AC氯化前后产生的自由基进行检测分析。如图7所示,在只含AC时,可检测到活性炭表面的持久性自由基。在AC氯化后发现了多重峰的存在,DMPO-H2O体系中的七重峰对应·Cl/DMPO加合物,表明在此过程中产生了Cl·[23],Cl·是一种对有机化合物具有较强选择性的自由基,易发生取代反应。由于Cl·具有很强的活性,因此,能够促进DBPs的生成,诱导某些有毒副产物的形成。ESR的结果表明,AC表面持久性自由基可催化次氯酸产生Cl·,在自由基的作用下,DOM与RCS之间会产生氯化副产物,尤其是亲核反应在其中发挥着很大作用,并且DOM的芳香性变强也有助于总有机氯的形成[24]。因此,氯化过程中AC会促进自由基的产生进而诱导其他类型DBPs的生成。
3. 结论
AC作为一种优良吸附剂被广泛应用于水处理工艺和终端净水过滤;同时,氯也是一种常见的预氧化剂和消毒剂。本文阐述了AC对RW氯化过程DBPs生成及DOM转化的影响,主要结果如下。
1)虽然AC存在时余氯下降较为迅速,但生成的THMs及HAAs较少,这是由于AC优良的吸附性能以及还原性AC快速消耗氯生成其他DBPs,DOC的变化也可说明了这一变化趋势。
2) FTICR-MS检测结果表明,AC存在时含氯物质的数量减少,Cl-DBPs的种类由302种减少到220种,其中有57种特异性氯代产物,CHOSCl化合物生成较多,其他CHOCl、CHONCl、CHONSCl化合物的数量减少。
3) FTICR-MS的结果显示,AC存在时可鉴定的化合物数量呈下降趋势,其是否存在的两种情况,生成的化合物有较大区别,但大部分化合物均属于脂肪类物质、高度不饱和类及酚类物质和多酚类物质。AC存在时,SUVA254的大幅降低表明含有芳香性的DOM被转化,而未加入AC组没有发生矿化反应。
4) AC表面持久性自由基催化次氯酸产生Cl·,Cl·引发的自由基反应是造成氯化产物及有机物形态改变的主要原因。
-
表 1 RW氯化过程中的氯化产物分子式分子指数的强度加权平均值
Table 1. Intensity-weighted average of molecular indices of molecular formulae for chlorination products during the RW chlorination process
分子式 水样 H/CW O/CW DBEW AImod,w 总强度 相对丰度/% CHOCl 不含AC 1.29 0.50 6.98 0.22 4.60×109 93.77 含AC 1.23 0.54 7.24 0.25 2.8×109 94.38 CHONCl 不含AC 1.42 0.28 8.84 0.17 1.48×108 3.41 含AC 1.33 0.33 9.22 0.24 7.52×107 2.54 CHOSCl 不含AC 1.68 0.21 7.28 0.03 6.8×107 1.37 含AC 1.67 0.51 3.69 -0.31 6.15×107 2.07 CHONSCl 不含AC 1.6 0.30 7.02 -0.14 7.2×107 1.45 含AC 0.49 0.13 0.65 -0.12 4.02×107 1.01 表 2 RW氯化过程后的非氯化产物分子式分子指数的强度加权平均值
Table 2. Intensity-weighted average of molecular indices of molecular formulae for non-chlorinated products after the RW chlorination process
分子式 水样 H/CW O/CW DBEW AImod,w 总强度 相对丰度/% CHO 不含AC 1.23 0.52 9.54 0.24 1.79×1011 80.56 含AC 1.23 0.52 9.35 0.23 1.79×1011 78.06 CHON 不含AC 1.20 0.52 9.99 0.23 3.21×1010 14.45 含AC 1.20 0.52 9.88 0.23 3.11×1010 13.56 CHOS 不含AC 1.40 0.49 6.45 0.07 8.5×109 3.83 含AC 1.42 0.53 6.40 0.03 1.54×1010 6.71 CHONS 不含AC 1.51 0.55 7.81 -0.14 2.58×109 1.16 含AC 1.55 0.60 7.12 -0.22 3.82×109 1.67 -
[1] MENYA E, JJAGWE J, KALIBBALA H M, et al. Progress in deployment of biomass-based activated carbon in point-of-use filters for removal of emerging contaminants from water: A review[J]. Chemical Engineering Research & Design, 2023, 192: 412-40. [2] LUO Y, GUO W, NGO H H, et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment[J]. Science of the Total Environment, 2014, 473: 619-641. [3] 滕恺, 武道吉, 任会学, 等. 家用净水器净水材料标准与卫生安全性探讨[J]. 净水技术, 2019, 38(9): 68-74+99. [4] HUANG X, YU Y, CHEN H, et al. Disinfection by-product formation and toxicity evaluation for chlorination with powered activated carbon[J]. Water Research, 2021, 205: 117660. doi: 10.1016/j.watres.2021.117660 [5] BULMAN D M, REMUCAL C K. Role of reactive halogen species in disinfection byproduct formation during chlorine photolysis[J]. Environmental Science & Technology, 2020, 54(15): 9629-9639. [6] VOUDRIAS E A, LARSON R A, SNOEYINK V L. Effects of activated carbon on the reactions of free chlorine with phenols[J]. Environmental Science & Technology, 1985, 19(5): 441-449. [7] 马超, 倪洪星, 戚羽霖. 超高效液相色谱-傅里叶变换离子回旋共振质谱法解析溶解性有机质的化学多样性[J]. 色谱, 2023, 41(8): 662-672. [8] LAVONEN E E, GONSIOR M, TRANVIK L J, et al. Selective chlorination of natural organic matter: identification of previously unknown disinfection byproducts[J]. Environmental Science & Technology, 2013, 47(5): 2264-2271. [9] ZHANG H, ZHANG Y, SHI Q, et al. Study on transformation of natural organic matter in source water during chlorination and its chlorinated products using ultrahigh resolution mass spectrometry[J]. Environmental Science & Technology, 2012, 46(8): 4396-4402. [10] HERTKORN N, RUECKER C, MERINGER M, et al. High-precision frequency measurements: indispensable tools at the core of the molecular-level analysis of complex systems[J]. Analytical and Bioanalytical Chemistry, 2007, 389(5): 1311-1327. doi: 10.1007/s00216-007-1577-4 [11] ZHANG X, KANG J, CHU W, et al. Spectral and mass spectrometric characteristics of different molecular weight fractions of dissolved organic matter[J]. Separation and Purification Technology, 2020, 253: 117390. doi: 10.1016/j.seppur.2020.117390 [12] SINHA R, GUPTA A K, GHOSAL P S. A review on trihalomethanes and haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106511. doi: 10.1016/j.jece.2021.106511 [13] WANG Y, XIANG Y, DOS SANTOS M M, et al. UV/chlorine and chlorination of effluent organic matter fractions: Tracing nitrogenous DBPs using FT-ICR mass spectrometry[J]. Water Research, 2023, 231: 119646. doi: 10.1016/j.watres.2023.119646 [14] VOUDRIAS E A, LARSON R A, SNOEYINK V L. Importance of surface free-radicals in the reactivity of antigranulocytes activated carbon under water-treatment conditions[J]. Carbon, 1987, 25(4): 503-515. doi: 10.1016/0008-6223(87)90191-6 [15] LEI Y, LEI X, WESTERHOFF P, et al. Reactivity of chlorine radicals (Cl• and Cl2•-) with dissolved organic matter and the formation of chlorinated byproducts[J]. Environmental Science & Technology, 2021, 55(1): 689-699. [16] BEN W, SUN P, HUANG C-H. Effects of combined UV and chlorine treatment on chloroform formation from triclosan[J]. Chemosphere, 2016, 150: 715-722. doi: 10.1016/j.chemosphere.2015.12.071 [17] SUN P, LEE W N, ZHANG R, et al. Degradation of deet and caffeine under UV/chlorine and simulated sunlight/chlorine conditions[J]. Environmental Science & Technology, 2016, 50(24): 13265-13273. [18] HAO Z, SHI F, CAO D, et al. Freezing-induced bromate reduction by dissolved organic matter and the formation of organobromine compounds[J]. Environmental Science & Technology, 2020, 54(3): 1668-1676. [19] 王雪凝, 张炳亮, 潘丙才. 市政污水二级出水中溶解性有机质在紫外/氯处理过程中的转化特性[J]. 环境科学, 2021, 42(8): 3847-3857. [20] LEENHEER J A, CROUé J P. Characterizing aquatic dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(1): 18A-26A. [21] ERSAN M S, LIU C, AMY G, et al. The interplay between natural organic matter and bromide on bromine substitution[J]. Science of the Total Environment, 2019, 646: 1172-1181. doi: 10.1016/j.scitotenv.2018.07.384 [22] ZHANG W, ZHOU S, WU Y, et al. Computerized pathway generator for the UV/free chlorine process: prediction of byproducts and reactions[J]. Environmental Science & Technology, 2021, 55(4): 2608-2617. [23] ZHANG Y, LI J, BAI J, et al. Total organic carbon and total nitrogen removal and simultaneous electricity generation for nitrogen-containing wastewater based on the catalytic reactions of hydroxyl and chlorine radicals[J]. Applied Catalysis B-Environmental, 2018, 238: 168-176. doi: 10.1016/j.apcatb.2018.07.036 [24] LEI Y, CHENG S, LUO N, et al. Rate constants and mechanisms of the reactions of Cl• and Cl2•- with trace organic contaminants[J]. Environmental Science & Technology, 2019, 53(19): 11170-11182. 期刊类型引用(1)
1. 何应钦. 基于活性炭吸附的自来水中THMs去除技术研究. 山西化工. 2024(10): 264-266 .  百度学术
百度学术
其他类型引用(0)
-






 下载:
下载: