-
漆酶是一类含铜多酚氧化酶,广泛存在于真菌、细菌和部分植物中,其特征在于铜氧还原蛋白结构中含有4个铜离子[1]。依据电磁学特征,T1和T2型2个铜均为单电子受体,呈现顺磁性;而T3型铜含有2个铜离子,为双电子受体,呈现反磁性。T2和T3铜位点构成三核铜簇中心,与T1铜相互连通,参与漆酶的催化氧化过程。漆酶可以利用分子氧为唯一的电子受体,氧化各种酚类、芳香族化合物及其胺类化合物[2-3]。因此,漆酶是一种优良的绿色生物催化剂,广泛应用于生物传感、医药加工、有机合成、及废水处理等工程应用领域[4]。然而,游离漆酶对环境条件表现出极高的敏感性,这意味着漆酶在自然条件下(pH、温度等)的稳定性相对较差[5-6]。漆酶的低稳定性和高生产成本导致漆酶在实际工业应用中受到限制。而且,游离漆酶在复杂环境中极易变性,回收十分困难。提高漆酶的稳定性和可重复使用性是漆酶相关研究的一个重要方向[7],因此,酶的固定化是备受关注的一项关键技术。
漆酶的固定化是将游离漆酶嫁接到不溶性载体基质上[8-10]。吸附法是指漆酶通过分子间作用力(离子键、氢键和范德华力等)吸附到载体的表面和孔隙内部[11]。吸附法制备条件相对简单,但吸附力相对较弱,易于漆酶脱落渗漏,从而降低固定化酶活性。吸附载体多样易得,如生物炭[6]、金属有机骨架材料[12]或蒙脱石[13]等。包埋法是指将漆酶固定在半透性聚合物膜或多面体结构中。常见的包埋材料包括天然凝胶(如明胶、壳聚糖珠等)以及合成凝胶(如聚丙烯酰胺和海藻酸盐珠等),其中海藻酸盐珠由于其无毒温和的凝胶特性[14]最为常见。包埋法的特殊固定方式是漆酶被包裹在高聚物基质中,因此可以有效地防止漆酶的流失和机械损伤,使得包埋后的酶不易漏出,也不会引起酶结构的变化。提高了固定化酶的稳定性。包埋法形成的固定化酶颗粒较小,底物和产物容易扩散到颗粒内部,有利于酶促反应的进行。但可能会造成漆酶与底物的接触效率低和孔扩散受到限制。
碳基材料因其物理强度高和热稳定性优良等特点,被视为一种有前途的固定化载体。生物炭是通过限氧裂解生物质废弃物而来的一类富碳颗粒,被视为廉价的、且环境友好的炭质材料[15]。由于生物质的可再生性,大量低廉的生物质废弃物可为生物炭的规模化生产提供原料。LIU 等[16]针对生物炭的经济性进行了分析,指出水稻秸秆炭和甘蔗渣炭的价格分别为0.79美元·kg−1和0.93 美元·kg−1。据国际生物炭协会(International Biochar Initiative, IBI)调查,全球生物炭价格在0.08~13.48美元·kg−1[17]。碳纳米管(CNT)是同轴圆管状的石墨烯材料,具有超轻重量、高机械强度以及化学稳定性等独特的性质。它因能应用在高性能材料和电化学传感器等领域而备受关注[19]。只是CNT生产成本高,其价格从5美元·g−1到75美元·g−1不等[18],这限制了CNT的技术应用。生物炭和CNT均具有较强的机械强度、可观的比表面积、以及易于功能化等优点,可作为固定化漆酶的碳基载体[20-22]。ZHANG 等[21]利用CNT固定化漆酶去除刚果红,研究发现漆酶-碳纳米管可在前30 min内快速吸附和降解刚果红。在漆酶-碳纳米管体系下,75%刚果红被去除时所需时间仅仅是CNT体系下的50%。
雌二醇(E2)属于典型的环境激素、广泛分布在水体环境中。它能通过干扰或破坏生物体内原生激素的合成、转运、分解等过程,使生物体无法正常代谢。据报道,E2在0.1ng·L−1 的质量浓度水平内就能表现出生物学效应[23]。E2在食物链中的积累可能会导致对人和动物的生长问题、生殖健康问题、低出生率及高肿瘤率等不良影响[23-24]。因此,利用固定化漆酶高效处理水体中E2的研究具有重要实际应用意义。本研究旨在比较性研究生物炭和CNT固定化漆酶的性能及去除水体E2的效果及机制,本研究选用木炭(WB)、竹炭(BB)和CNT作为碳基载体,通过吸附法和包埋法固定游离漆酶,对比游离漆酶和固定化漆酶的活性、稳定性、和可重复使用性;研究WB、BB和CNT固定化漆酶去除E2的效果,以及循环利用率;同时,探究金属离子浓度效应对于吸附法和包埋法固定化漆酶去除水体E2的影响。
-
实验选取来源于杂色栓菌(Trametes versicolor)的真菌漆酶(Laccase, 500 U·g−1)、E2(17β-estradiol, >98%)、一水柠檬酸、二水柠檬酸钠、2,2'-叠氮基-双(3-乙基苯并噻唑啉-6-磺酸)(ABTS)、WB(粒径<300目)、BB(粒径<300目)、CNT等购置于阿拉丁试剂(上海)有限公司。
-
通过吸附法固定漆酶,以柠檬酸-柠檬酸钠为缓冲对作为背景液,配制50 mg·L−1漆酶溶液,分别将100 mL漆酶溶液与100 mg WB、BB、CNT进行充分混合,25 °C下于摇床中振荡12 h。待吸附固定后,进行固液分离,通过0.22 μm滤膜得到的三种固定化漆酶材料,放置于4 °C冰箱中待用。利用吸附法固载漆酶于WB上,简写为AWB-L(Adsorption Wood Biochar-Laccase)。其他依次简写为ABB-L和ACNT-L。
通过包埋法固定漆酶,将100 mL的3%氯化钙溶液与100 mg的WB、BB、CNT进行充分混合,超声30 min后,在25 °C保存24 h,使3种炭质材料与Ca2+充分进行接触。待炭质材料表面充分吸附Ca2+后,进行固液分离,通过0.22 μm滤膜得到3种炭质材料,在60 °C下烘干。其次将100 mL漆酶溶液(50 mg·L−1)与100 mg富Ca2+离子的3种炭质材料充分混合。在摇床中振荡8 h后,取20 mL质量分数0.5%的海藻酸钠溶液缓慢滴入漆酶-炭质材料的混合体系,置于摇床中继续振荡4 h。最后,当转化的海藻酸钙包埋住游离漆酶固载于3种炭质材料上,进行固液分离,通过0.22 μm滤膜获得3种包埋法固定化漆酶材料。放置于4 °C冰箱中待用。利用包埋法固载漆酶于WB简写为EWB-L(Embedding Wood Biochar-Laccase),其他依次简写为EBB-L和ECNT-L。
-
使用扫描电子显微镜(SEM, S-4800, Hitachi, Japan)表征固定化前后WB、BB、CNT的微观尺度形貌结构。通过傅里叶红外光谱(FTIR, Varian 640-IR, USA)在400~4000 cm−1内分析固定化前后WB、BB、CNT的官能团。
-
ABTS向ABTS+自由基的转化用于测定漆酶的活性。一个游离酶活性单位定义为每分钟催化 1 μmol ABTS 所需的游离酶量。具体而言,取0.3 mL 1 mmol·L−1 ABTS与2.7 mL漆酶溶液充分混合,使用紫外-可见分光光度计(Ultraviolet spectrophotometer, UV-Vis)于420 nm 处测量其吸光度的增加量,2 min内的吸光度变化,每隔15 s记录1次。根据测定的吸光度,计算漆酶的酶活,计算公式如式(1)所示。
其中:K代表酶活,U·g−1;∆A为ABTS+在一段时间内的吸光度变化值;Vt为反应体系的总体积,L;ε为表ABTS+的摩尔消光系数,36 000 L·(mol·cm)−1;∆T 为反应时间,min;m 为酶的质量,g。
-
在25 °C下,将50 mg·L−1游离漆酶和40 mg固定化漆酶分别放入40 mL柠檬酸-柠檬酸钠溶液(pH=5)中,并分别在24、48、72、96、120 h时,测定其剩余活性。研究了固定化漆酶在25 °C下长达25 d的储存稳定性,并以120 h为间隔测定剩余活性。通过ABTS作为底物,研究了固定化漆酶的重复使用性,进行了5个重复循环。
-
在pH=5条件下,将40 mg WB、BB、CNT以及固定化材料加入至40 mL的E2(50 mg·L−1)溶液中,利用0.22 μm过滤器分别在20、40、60、90、120、240、480 min从反应体系中取出0.5 mL液体置于2 mL玻璃小瓶,并加入0.5 mL甲醇溶液淬灭漆酶反应活性。所有样品均一式三份,利用高效液相色谱仪(HPLC, 安捷伦1260),定量液相剩余E2的质量浓度。反应480 min后,进行固液分离,重新加入40 mL E2(50 mg·L−1)溶液。该步骤循环重复5次,每次所取出的液体经过0.22 μm的过滤器制样、保存。所有样品均一式三份,利用HPLC定定液相剩余E2的质量浓度。
-
以柠檬酸-柠檬酸钠缓冲背景液(pH=5),分别配制0.5、1.0、5.0 mmol·L−1的硝酸钾(KNO3)和一水硝酸锰(MnNO3()2·H2O)。在K+和Mn2+离子存在条件下,分别利用40 mg吸附和包埋法固定化漆酶降解 40 mL质量浓度为 50 mg·L−1 E2溶液。分别在20、40、60、90、120、240、480 min进行取样。所有样品均一式三份,利用HPLC测定液相剩余E2的质量浓度。
-
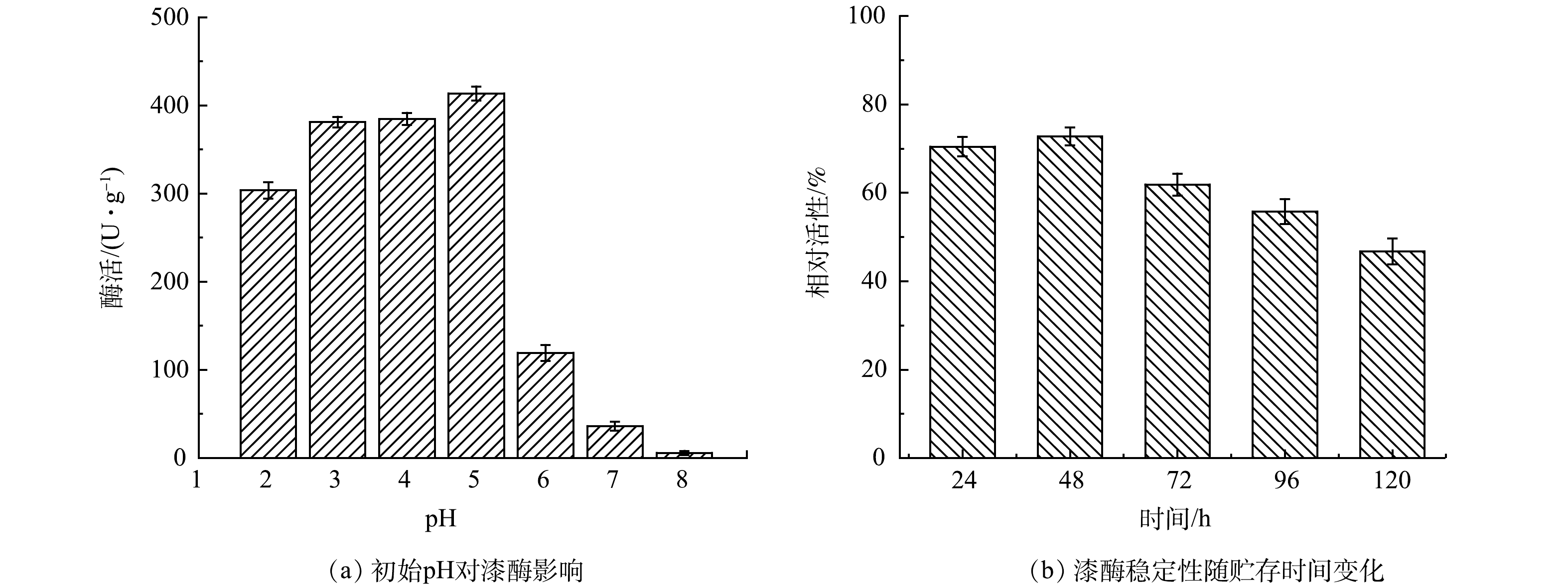
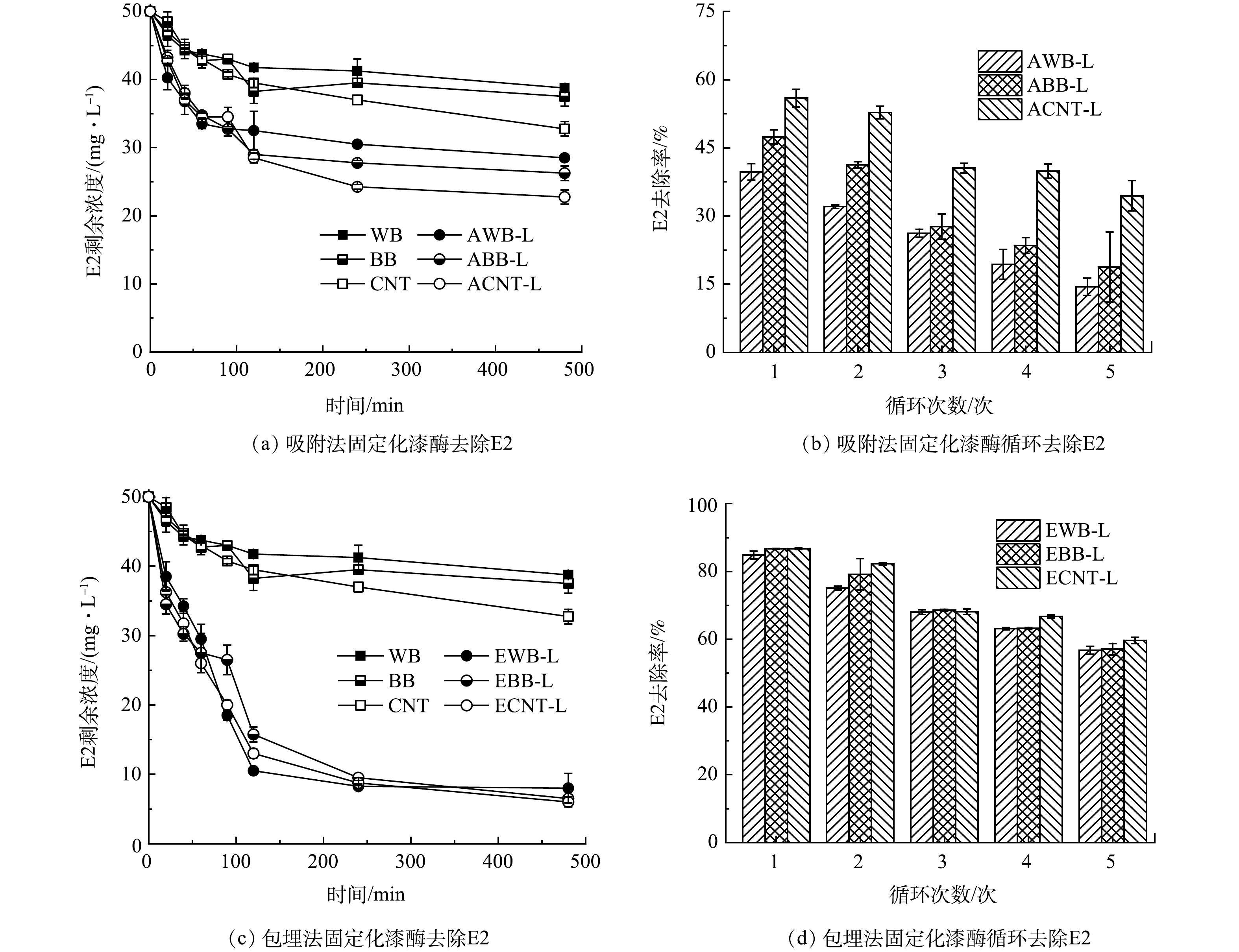
由于游离漆酶的活性受pH影响极大,因此测定了不同pH条件下的漆酶活性。如图1(a)所示,在pH=2~8的条件下,游离漆酶的活性分别为303.75、381.05、384.65、413.58、119.31、36.24、5.80 U·g−1。pH的变化可以决定漆酶中氨基酸的电离状态,从而影响其结构和活性[4]。游离漆酶的活性随着pH的提高,逐渐增加。在pH=5时游离漆酶活性最佳。随着pH继续升高,漆酶的活性出现急剧下降现象。当pH=7时,游离漆酶的活性降低至36.24 U·g−1,仅为最高酶活的8.76%。在碱性水体环境中漆酶完全丧失了其活性。这可能是由于氢氧根阴离子与漆酶活性位点的 T2/T3 铜结合而引起的抑制,从而中断了从 T1 到 T2/T3 铜位点的内部电子转移[25-26]。漆酶的 T1/T2 和T3活性位点协同作用,通过传递电子来促进漆酶催化氧化反应的进行。这些活性位点在固定化漆酶结构中保持了其原有的生物活性,使得固定化的漆酶可以有效地催化氧化反应。此外,在pH =5时游离漆酶储存的稳定性。结果表明:24、48、72、96、120 h时,游离漆酶剩余的相对活性分别为70.40%、72.78%、61.92%、55.85%、46.78%(图1(b))。可以发现,随着时间的增加,游离漆酶的活性逐渐下降。这可能是因为在长时间静置的游离漆酶中,酶活性位点的构象变化导致酶活性降低[27-28]。游离漆酶作为一种水溶性蛋白,难以在有机废水处理过程中进行回收,造成浪费。而且,漆酶的稳定性弱和成本高限制了其在环境修复领域的应用。因此,研究适当的固定化方法将游离漆酶固载,提高漆酶的循环利用率以及固定化漆酶的功能效应非常必要。
-
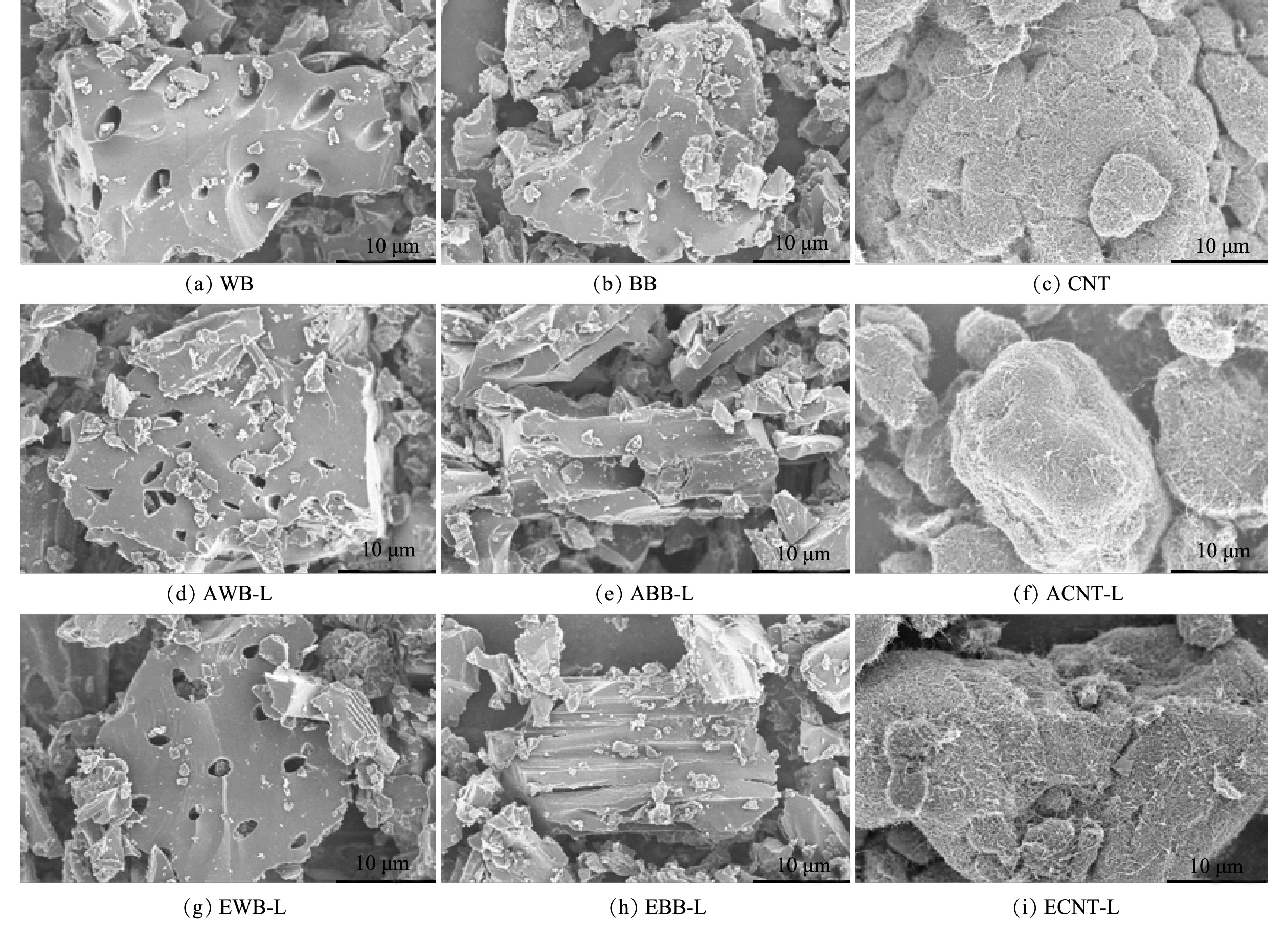
通过吸附和包埋方式将漆酶固定在WB、BB和CNT上,利用扫描电镜观察固定前后的微观形态结构。如图2(a)~(c)所示,WB、BB结构表面存有排列有序的孔状结构,这是由于木质纤维素在炭化过程中炭相的转化所致。而CNT有着细长的管状结构并容易团聚成不规则的球体。图2(d)~(i)是固化漆酶后的扫描电镜图,在图中没有观察到表面形貌的显着变化。这可能是由于漆酶蛋白(60~90 kDa)的小尺寸相当于小于 5 nm 的粒径。这个尺寸很难被放大 10 μm 的显微照片捕捉到[21,29]。 然而,在酶固定化后,AWB-L、EWB-L等表面似乎更光滑,这可能是由于WB、BB、和CNT表面有酶涂层所致[22]。有研究表明,利用磁性生物炭固定漆酶后,生物炭的表面因有酶涂层变得更为光滑[30]。
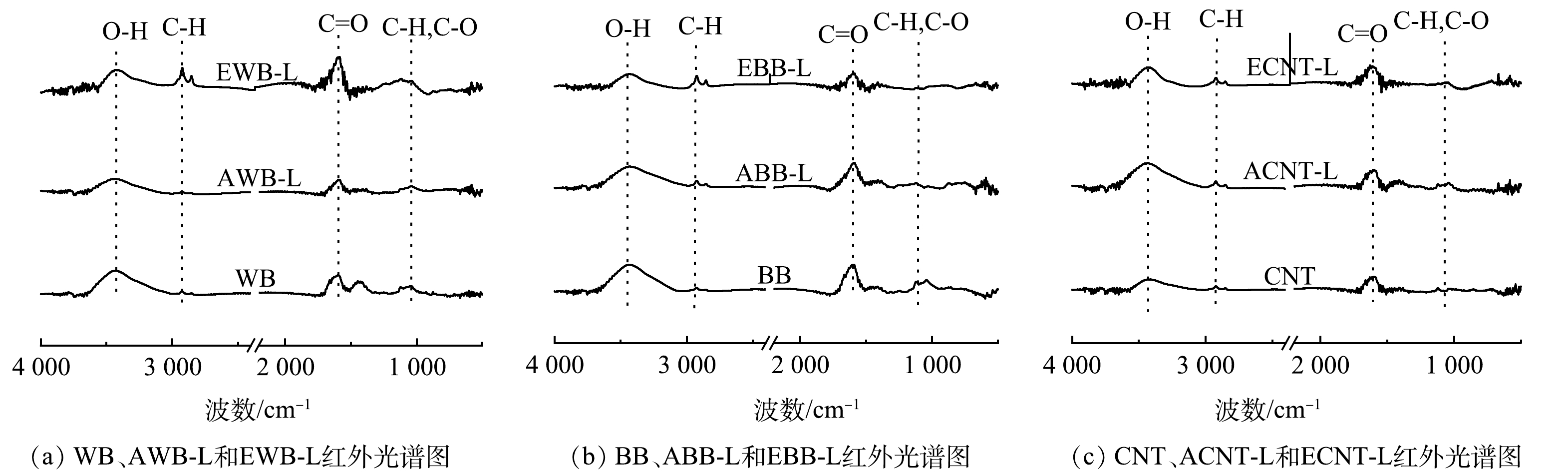
本研究利用FTIR分析了漆酶固定化前后WB、BB和CNT的结构变化。如图3所示,在3 420~3 450 cm−1处观察到的峰为—OH 伸缩振动[29]。2 924 cm−1处的峰归因于烷基引起的不对称 C—H 和对称 C—H 振动[31]。1 626~1 641 cm−1处的吸收峰可能与生物炭中羧基的C=O键和漆酶蛋白中存在的酰胺键的伸缩振动有关[32]。1 148 cm−1处的峰对应于二级酰胺结构的C—O—C伸缩振动[33]。1 050 cm−1和1 071 cm−1处的峰代表生物炭中芳环的 C—H 键[34]。而 1 026 cm−1处则代表着 C—O 基团的伸缩振动[22,34]。与WB和BB相比,CNT固载漆酶后的以上官能团的振动峰均有所增强,这是由于漆酶负载后增加了官能团的在CNT上的量。例如, WB和BB负载漆酶后,—OH峰强有所降低;而CNT负载漆酶后—OH峰则增强,CNT固载漆酶的峰强增加。包埋法负载在WB上的C=O和C—H官能团有明显增加。另外,780 cm−1附近的峰变多,进一步证明漆酶成功固定在WB和BB和CNT上[35]。
-
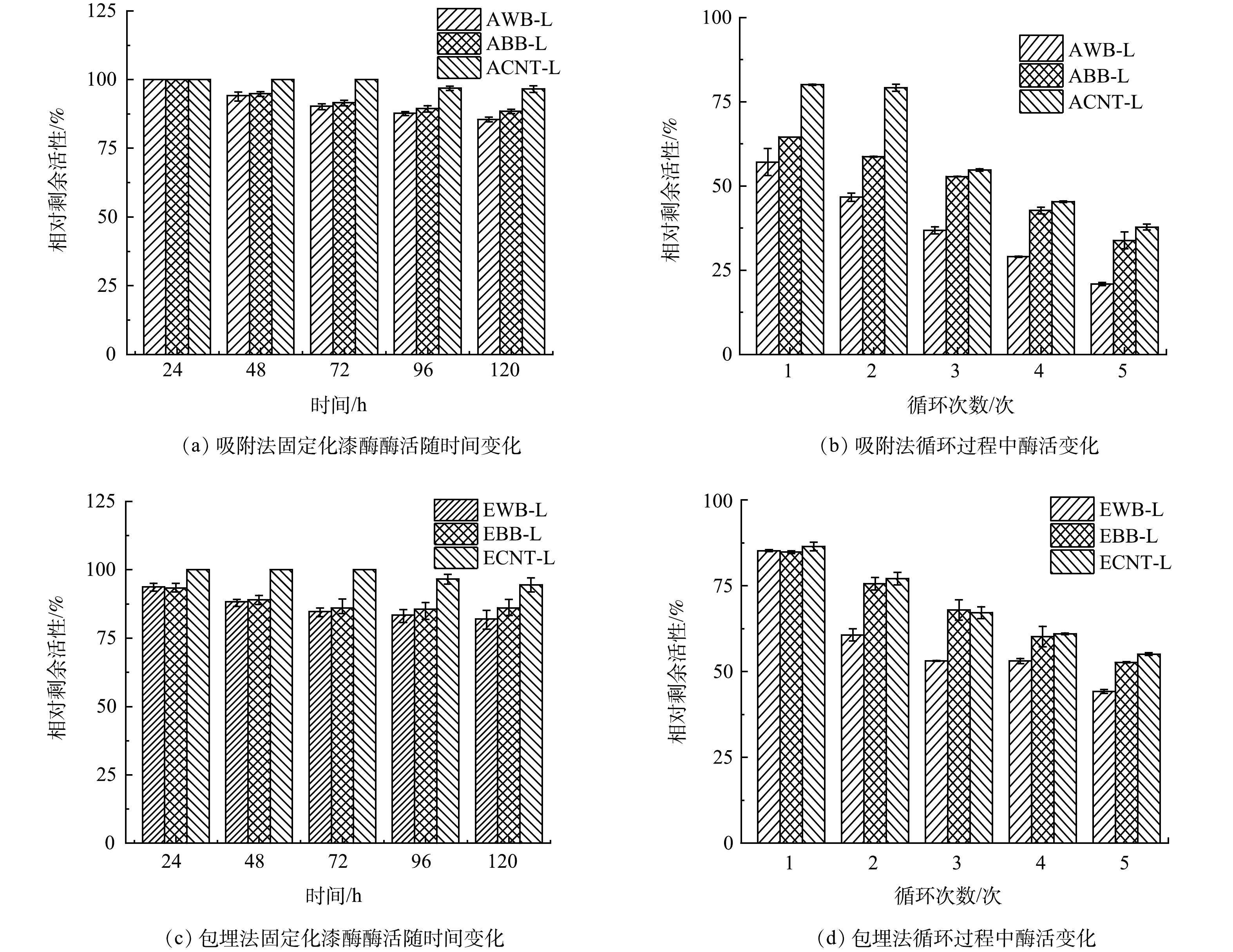
漆酶的稳定性是决定生物催化剂稳健性的重要因素,可通过探究固漆酶固定化后酶活性的时间变化,探究不同固定方式的漆酶的稳定性。本研究在25 °C下储存120 h来评估固定化漆酶的稳定性,每24 h测定其相对剩余活性。吸附法固定化漆酶的稳定性如图4(a)所示。可见,120 h后AWB-L和ABB-L的活性仍分别保留了85.82%和88.51%剩余活性,而ACNT-L更加表现出其优越的固载性能,可保留96.60%固载的漆酶。此前研究报道固定在磁性纳米颗粒上的漆酶能保存71.2%的活性[36],而固定在氨基功能化纳米颗粒上的漆酶仅表现出64.7%的活性[37]。经过包埋法固定化后(图4(c)),EWB-L、EBB-L和ECNT-L的相对剩余活性在120 h后分别为82.02%、85.07%和94.54%。这表现出在WB、BB和CNT上包埋法极大提高了游离漆酶的稳定性,防止了漆酶在水相中流失。碳基材料固定化漆酶的优越性可能关乎其刚性多孔结构,能起到高效贮存漆酶作用。
另一方面,漆酶固定后,它的重复使用性也是决定生物催化系统在工业规模上的潜在应用的重要参数。本研究以ABTS作为底物,在5次循环过程中检查固定化漆酶的可重复使用性。研究结果表明,吸附法固定化漆酶经历 5次循环后(图4(b)),AWB-L和ABB-L的剩余酶活 仅剩初始酶活的27.81%和26.62%;而ACNT-L也仅存37.99%的剩余活性。这可能是由于吸附负载过程漆酶与载体活性位点之间的结合力不强。经过多次反应和水洗后,大量漆酶从固定载体表面脱落,导致相对酶活性下降[38]。相比于吸附法,包埋法的损失率要小于吸附法的损失率。因为包埋法固定化漆酶主要利用了聚合物和载体共同包裹住漆酶,而不是利用吸附的弱作用力固定化漆酶[39],此结果与其他研究研究结果类似。WANG 等[6]利用十六烷基三甲基溴化铵-氢氧化钾(CTAB-KOH)改性生物炭,并通过吸附法固定漆酶,他们发现固定化漆酶在6次循环后失去了约 55% 的活性。而另一项研究中,将漆酶吸附固定在稻草生物炭上,其固定化率达到66%,而且固定化酶在经过6次循环的稳定性操作,能够保留40%的初始活性[40]。ZHANG 等[22]通过吸附、沉淀和交联将漆酶稳定地固定在纳米磁性生物炭(L-MBC)上,固定量达到2.25 U·mg−1,经过30 d储存,L-MBC活性还保持在64.40%。由此可知,吸附法虽然操作方法易行,但其固定化漆酶的稳定性和可重复使用性相对较弱。包埋法固定化漆酶不仅更有利于酶的稳定性,而且提高了固定化漆酶的重复利用率,从而有效降低漆酶在污水处理中的应用成本。
-
通过吸附法和包埋法使漆酶固定在WB、BB和CNT上,从而研究固定化漆酶对E2的去除。经过8 h反应,3种碳基材料对E2的吸附量遵循CNT>BB>WB,其吸附量分别达到17.23、13.01和11.32 mg·L−1。这可能是由于CNT的比表面积大所致[41]。如图5(a)所示,AWB-L的E2去除量增加到21.46 mg·L−1;ABB-L的E2去除量增加到23.83 mg·L−1。ACNT-L对E2去除最高,在8 h内对于E2去除量为27.38 mg·L−1。另外,包埋法固定化漆酶对于E2的去除效果如图5c。在8 h内,EWB-L对E2去除量增加到42.04 mg·L−1;EBB-L对E2去除量增加到43.60 mg·L−1;ECNT-L对于E2去除量达到43.93 mg·L−1。固定化漆酶对水中E2的去除效果更好,是因为固定化漆酶不仅可以吸附水体E2,而且可以通过漆酶的催化氧化降解E2[42]。另外,有研究表明,包埋法固定化漆酶去除E2的效果显著高于吸附法,可能由于漆酶吸附于载体的作用力较弱,循环利用过程中漆酶也易于再次脱落,漆酶的负载率会不断降低。对于包埋法而言,由于酶蛋白分子相对较大,漆酶易于被限制在包埋空间内,且漆酶的分子结构也不会发生改变[43-44]。而分子质量较小的污染物则可以畅通进入漆酶空间内, 当污染物分子接近该空间时, 被固定化的漆酶将参与污染物的催化氧化过程[14]。SHAO等[45]通过空心介孔碳纳米球(HMCs)固定了游离的漆酶, 发现漆酶的固定化效果极为稳定, HMCs材料上漆酶的最大载量达到835 mg·g−1。固定化漆酶对盐酸四环素和盐酸环丙沙星的去除率分别为99.40%和96.90%。
对比5个连续批次(每批次8 h)循环对E2(50 mg·L−1)的去除量的变化。如图5(b)所示, 经过5次循环后,吸附法制备的3种固定化漆酶对E2的去除率逐渐降低。AWB-L对E2的去除率由39.66%降低至15.38%;对于ABB-L,5次循环后E2的去除率也由47.44%降至19.76%;5次循环后ACNT-L对E2的去除率由54.50%降低至34.84%。这是因为固定在载体上的漆酶发生脱落,或漆酶结构发生变化导致酶活性下降,进而影响了E2的去除效率[46]。包埋法固定化漆酶去除E2的循环实验结果如图5(d)所示,对于EWB-L,5次循环后E2的去除率由88.84%降低至56.06%;EBB-L对E2的去除率由86.66%降低至57.08%;对于ECNT-L,5次循环后E2的去除率由86.76%降低至59.66%。经过多次循环后,利用包埋法固定化漆酶的去除能力也会随着循环次数的增加而逐渐减弱,因为经过多次使用,导致包埋结构遭到破坏,从而导漆酶脱落所致[47]。在5次循环后,利用包埋法固定化漆酶去除E2效果高于吸附法,可能归因于藻酸盐基质提供的保护性微环境[48]。相对于吸附法,包埋法固定漆酶对E2的效果更好,循环使用的去除率也较高。其中,吸附法WB和BB固定漆酶对E2的去除率要低于CNT固定漆酶的去除率。然而,包埋法CNT与生物炭固载漆酶对E2的去除率和循环效率都很相似,因此,包埋法生物炭固载漆酶可以代替高成本的CNT固载漆酶,也能达到相同的去除E2的效果。
-
漆酶的活性通常会受到废水或者其他环境中存在的金属离子的影响,随金属离子和酶的不同而变化。例如,Mn2+可刺激肉孢子丝菌中的漆酶但抑制来自Leptographium qinlingensis的漆酶[49,50]。所以为了进一步了解固定化漆酶的实际应用效果,考察2种金属离子(K+和Mn2+)对固定化漆酶去除E2的影响。
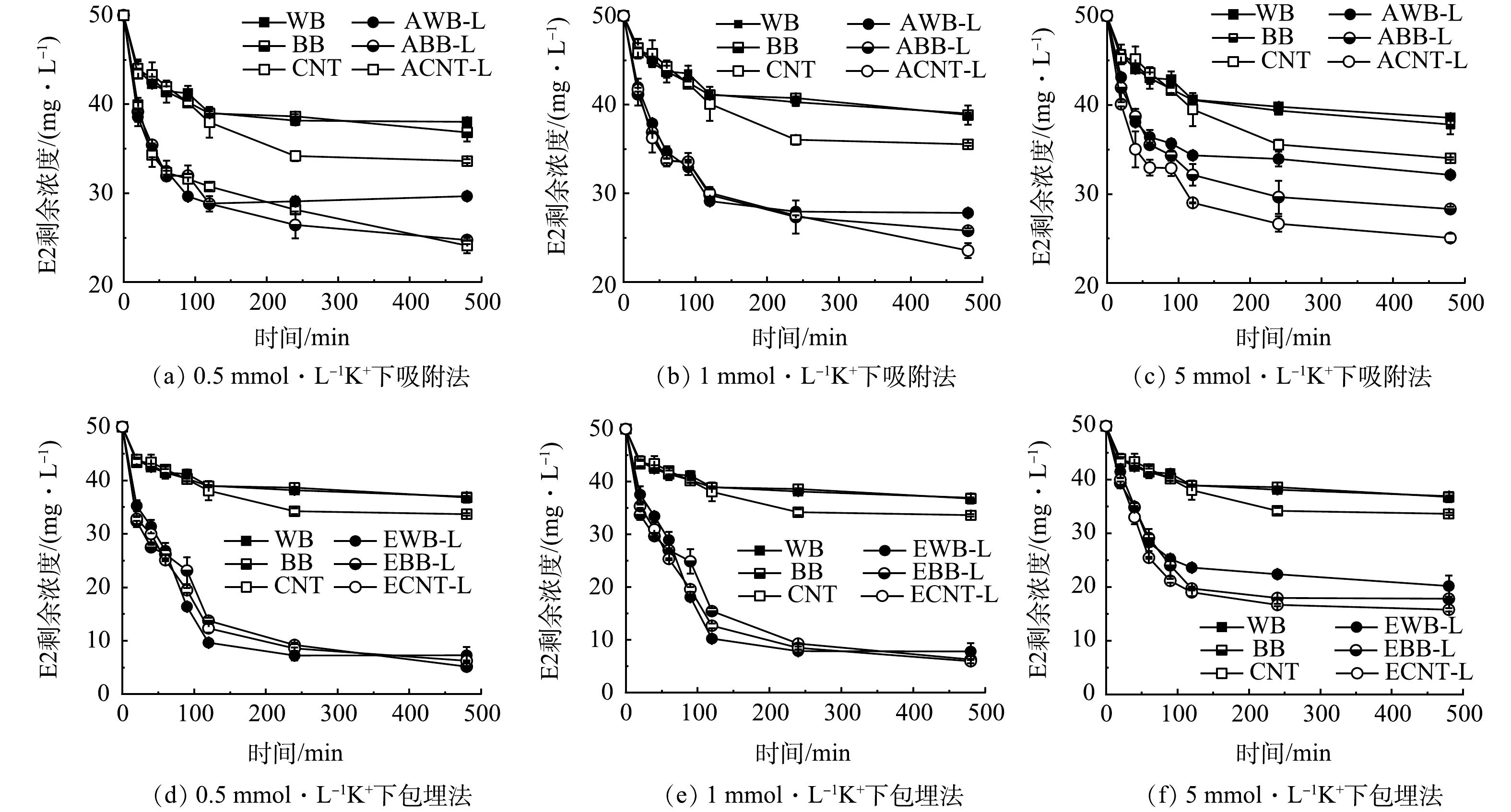
1) K+离子的影响。图6描述在0.5、1和5 mmol·L−1 K+浓度下,漆酶固定化前后炭质材料去除E2随时间变化的关系曲线。研究发现在前100 min内E2的质量浓度迅速下降,随后保持一定平衡。随着K+浓度由0.5 mmol·L−1增加到1 mmol·L−1时,固定化漆酶对于E2的去除无太大的变化现象。但是当K+浓度达到5 mmol·L−1时,E2的去除率明显降低。金属离子的抑制作用主要是通过干扰漆酶催化反应过程中电子传递以及可能影响漆酶的活性,从而导致固定化漆酶对E2的去除量降低。同样冯义平等[51]发现Na+、K+、Cd2+、Hg2+、Fe2+、Fe3+ 在10.0 mmol·L−1浓度水平下显著抑制漆酶活性。这些金属离子能够与漆酶结构中游离的半胱氨酸结合,形成失活的蛋白加合物,并因此导致漆酶失活。从而影响漆酶催化去除溴代阻燃剂四溴双酚 A。另外发现在K+离子影响下,包埋法固定化漆酶去除E2受到抑制的效果低于吸附法。一方面可能由于漆酶对载体的吸附亲和力差,导致容易脱落[52];另一方面可能由于藻酸盐包埋保护漆酶一定程度上免受溶液中金属离子的影响[53]。
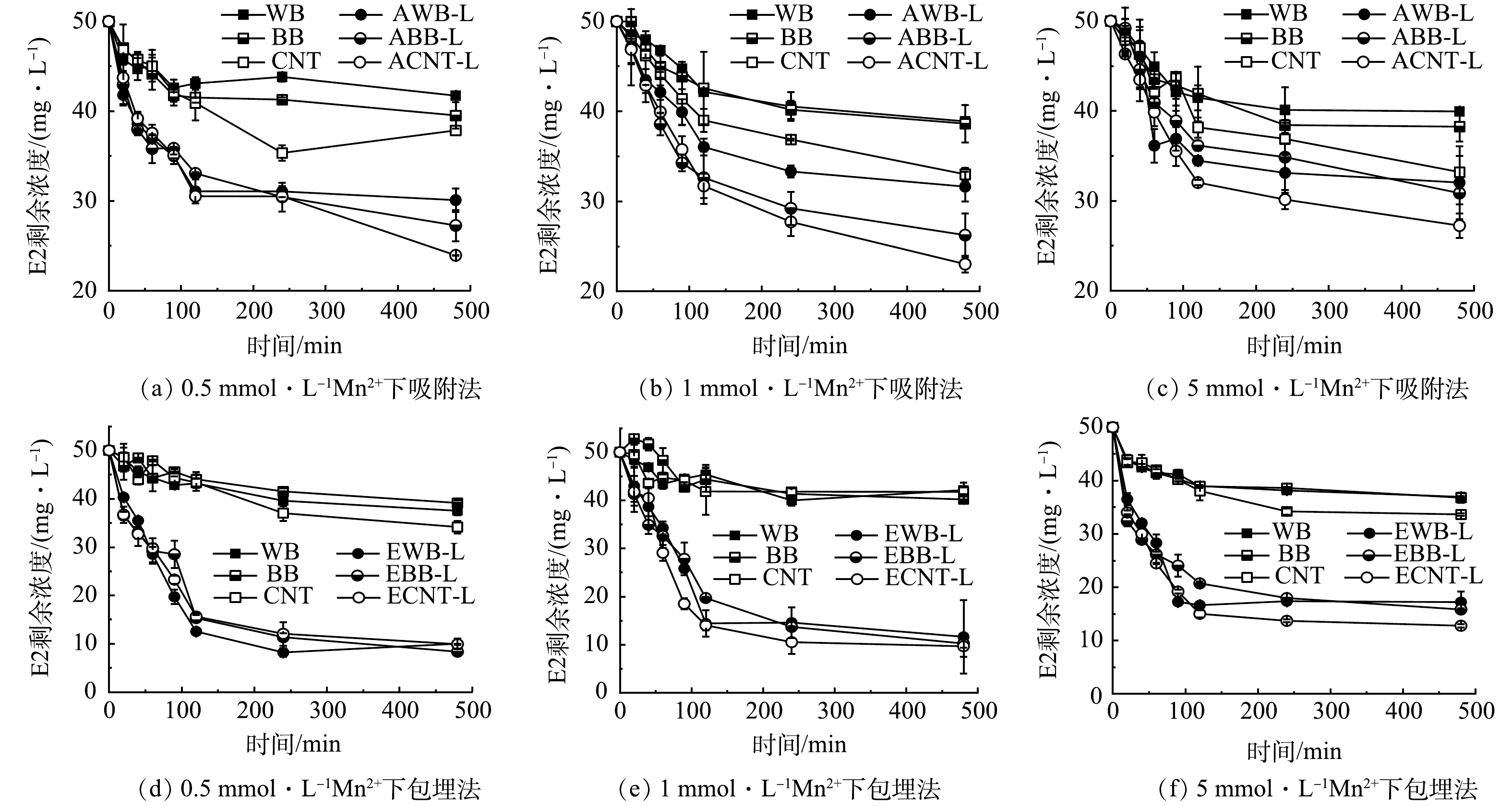
2) Mn2+的影响。将Mn2+与固定化后的漆酶在E2溶液中共同反应8 h后(图7)。当Mn2+浓度由0.5 mmol·L−1增加到1 mmol·L−1时,对于E2的去除几乎没有影响。使用吸附法和包埋法固定化漆酶后,E2的剩余质量浓度分别为28 mg·L−1和10 mg·L−1。然而当Mn2+浓度达到5 mmol·L−1时,对E2的去除出现抑制作用。这可能是由于水溶液中的漆酶带负电荷,与金属阳离子之间由于静电引力而吸附在一起,而且金属阳离子价态越高,相互之间静电力作用越强,从而导致漆酶的活性降低[54]。WAGNER 等[55]研究在pH=5.0、Mn2+浓度高达1.0 mmol·L−1的情况下,对苯酚的去除有明显抑制效果。有研究[56]表明,包括Mn(II)在内的某些金属离子与含氧配体相互作用,例如存在于酶上的羧基。这些相互作用可能会改变酶对底物的亲和力。
-
通过吸附法和包埋法制备WB、BB和CNT的固定化漆酶,系统地探究了固定化漆酶的活性和稳定性,固定化漆酶去除E2效果以及金属离子对去除效果的影响,为固定化漆酶技术在绿色化学应用研究提供了新的视角。得出如下结论。
1)对比WB、BB、CNT 3种炭质载体吸附法固定漆酶,发现CNT固定漆酶的稳定性、可重复使用性以及对E2的去除效果都高于WB和BB,这可能是因为CNT具有丰富的纳米孔隙结构所致。
2)包埋法固定的漆酶的稳定性、可重复使用性和E2的去除效果均高于吸附法固定化漆酶。WB、BB、CNT 3种炭质载体包埋法固定漆酶的稳定性、可重复使用性、及对E2去除效率差别不大。因此,生物炭可以作为替代CNT的炭质载体通过包埋法固定游离漆酶,减少固定化成本。
3)固定化漆酶的活性和去除E2的效率受金属离子种类的影响,K+和Mn2+离子的抑制固定化漆酶的活性和E2的去除效率。
生物炭和碳纳米管固定化漆酶去除水体雌二醇
Removal of β-estradiol in wastewater using laccase immobilized by biochar and carbon nano tube
-
摘要: 漆酶是一类具有高效的催化氧化能力的含铜多酚的氧化酶,而固定化漆酶技术在有机废水处理领域受到了越来越多的关注。通过比较吸附法和包埋法利用木炭(WB)、竹炭(BB)和碳纳米管(CNT)固定游离漆酶去除水体雌二醇(E2)。在25 °C 、pH=5条件下,固定化漆酶经120 h 贮存仍保留80%的活性。经过5次循环研究,固载的漆酶仍保留了27%~58%的剩余活性。结合扫描电子显微镜和傅里叶变换红外光谱,证实了漆酶成功地负载在碳基材料上。仅在 8 h内,50 mg·L−1 E2通过吸附法制备的WB、BB、CNT固定化漆酶去除了42.92%、47.66%、43.92%,而包埋法制备的WB、BB、CNT固定化漆酶分别去除84.08%、87.20%、87.86%。结果表明,包埋法固定化漆酶对E2的去除效率更优,这是由于包埋固载在生物炭和CNT的漆酶更为稳定。此外,K+和Mn2+离子可能通过干扰漆酶的电子传递过程影响漆酶的活性,从而抑制E2的去除。本研究旨在为固定化漆酶在有机废水处理的实际应用提供指导。Abstract: Laccase is a class of Cu-containing polyphenol oxidase with high catalytic oxidation ability. The immobilized laccase technique has attracted an increasing attention in organic wastewater treatment. In this study, wood- and bamboo-derived biochars (WB and BB) or carbon nano tube (CNT) were used to immobilize laccase by the adsorption and embedding methods for the removal of β-estradiol (E2) in aqueous environment. At 25 °C and pH=5, 80% activity of the immobilized laccase on WB, BB, and CNT was preserved after 120 h storage. After 5 cycles, the residual activity of the immobilized laccase was 27%~58%. Combined with scanning electron microscopy and Fourier transform infrared spectroscopy, it was confirmed that laccase was successfully loaded on carbon-based materials. Within only 8 h, 42.92%, 47.66%, and 43.92% of 50 mg·L−1 E2 were removed by WB-, BB-, and CNT-immobilized laccases using the adsorption method, while 84.08%, 87.2%, and 87.86% of 50 mg·L−1 E2 were removed by WB-, BB-, and CNT-immobilized laccases using the embedding method. The results showed that the immobilized laccase using the embedding method had a better removal efficiency for E2 due to its stronger stability onto biochars and CNT. In addition, K+ and Mn2+ ions could interfere with electron transfer of laccases, then affect the activity of laccases, diminish E2 removal. The aim of this work can provide a guidance for designing immobilized laccase for organic wastewater treatment.
-
Key words:
- biochars /
- CNT /
- immobilized laccase /
- β-estradiol /
- metal ions
-
微量元素分为潜在有毒化学致癌物和生物学意义的必需非化学致癌物[1],在生理功能、内稳态和酶活性中起着重要的作用。过量的必需微量元素或低剂量的有毒微量元素均会引起毒性作用,导致肾脏毒性、肝脏毒性、神经毒性甚至诱导肿瘤发生[2-4],严重危害人体健康。孕妇及胎儿等敏感人群更容易受到微量元素的影响。Mayumi等[5]发现镉(Cd)可能会导致孕妇早产,Mistry等[6]发现孕妇体内缺乏时硒(Se)和锰(Mn)可能会导致流产。已有研究表明砷(As)、Cd和铅(Pb)等有毒微量元素可通过胎盘屏障进入胚胎或胎儿体内[7] ,从而引起胚胎毒性和致畸作用。Boucher等[8]的研究表明孕期Pb暴露会对婴儿产生神经毒性,Cabrera-Rodríguez等[9]的研究发现孕期锑(Sb)和镍(Ni)暴露与新生儿出生体重呈负相关关系。必需微量元素的缺乏或过量也会对胎儿的生长发育产生不良影响[10]。Vigeh等[11]的研究发现Mn的缺乏与胎儿宫内发育迟缓有关,Ozel等[12]的研究发现孕妇孕期Mn水平较高与新生儿神经管畸形有关。
微量元素具有持久性、生物富集和放大、不易生物降解及不可逆等特性,其可长期存在于水、土壤、灰尘和空气等环境中[13-15]。灰尘是一种重要的室内污染物赋存介质[16],是有机和无机化合物的复杂混合物[17]。人们日益关注家庭灰尘中的微量元素对人类健康的影响[18]。室内环境是人体暴露有毒有害微量元素的重要场所[19-21];尤其是孕妇和婴儿,其大部分的时间在室内度过,更容易受到微量元素影响[22]。室内灰尘进入人体的主要途径是呼吸摄入和皮肤接触,其中的微量元素存在潜在的健康风险[23],Sharma等[24]发现室内灰尘蓄积的As和Pb是人体暴露有毒微量元素的主要来源;Doyi等[19]发现室内灰尘中的铬(Cr)和Pb对儿童健康存在潜在健康风险。因此,对于人类特别是孕妇的健康,室内灰尘中微量元素的研究具有重要意义。生物监测可作为人类评估微量元素暴露水平的指标,可为公共卫生提供有效数据[25-26]。尿液是大多数化学物质的主要排泄方式,也是评估环境暴露水平的重要途径[27] ,有60%的微量元素经尿液排出体内,其余微量元素在8 h 内被自然免疫系统还原为离子态[28]。中国多个地区已经开展人体尿液中微量元素的生物监测,系统了解当地居民尿液中微量元素含量[29-30],并检测出孕妇尿液中微量元素对胎儿生长发育的影响[31-32]。室内灰尘是室内污染的长期储存“库”,可以反映孕期微量元素经灰尘这一途径的环境暴露状况,而孕妇尿液则可作为生物监测材料,反映孕期内暴露状况。相对于普通人群,孕妇和胎儿由于其特殊的生理特点,对化学物质暴露更加敏感,因此有必要监测家庭环境和母体内微量元素的暴露情况及其潜在健康风险。
广州是具有1800万人口的沿海特大城市,是中国经济和城市化发展最快的地区。作为中国最重要的工业中心之一,主要的产业有电子通讯设备、汽车制造、石油化工等。环境中微量元素的来源主要是汽车尾气、焚化炉、工业废物和大气沉降等[33]。近年来,微量元素Cd和Pb在广州污染严重,特别是土壤中的Pb的浓度[34],邹梦遥等[35]研究发现,广东省室内灰尘中Cd和Pb的污染较为严重。环境中微量元素的增加对孕妇健康可能存在危害,因此探究广州孕妇微量元素的暴露特征。
本研究招募孕妇志愿者,采集孕妇孕晚期尿液及其生活环境的室内灰尘,分析其必需微量元素钒(V)、Mn、钴(Co)、Se、钼(Mo),以及有毒微量元素Cr、Ni、As、Cd、Sb、Pb的浓度水平及特征,探讨微量元素与室内环境和生活习惯对孕妇微量元素之间的关联暴露的影响,评估其对孕妇的健康风险,为人群健康防护提供基础数据。
1. 材料与方法(Materials and methods)
1.1 研究人群
2020年1月至2021年2月,在广东省广州市采集22份孕妇孕晚期尿液及其居住家庭灰尘样本,并收集了产妇年龄、产妇孕前身体质量指数(body mass index, BMI)、孕妇及其配偶教育程度、吸烟习惯及饮食信息。尿液使用聚丙烯尿液杯收集于棕色玻璃瓶中,−20℃储存;每户家庭室内地面、桌面等灰尘聚积的地方用软毛刷进行扫取收集,灰尘样本过100目不锈钢筛(150 μm孔径)后用塑料袋包裹,−20℃储存。所有参与者均签署知情同意书,本研究经中山大学附属第六医院伦理委员会批准。
1.2 尿液微量元素(样本)分析
冷冻尿液样本置于室温下解冻,振荡混匀,用1%硝酸以1:10比例稀释尿液,振荡混匀后静置过夜,之后于45 ℃温度下超声1 h,12000 r·min−1离心取上清液,使用电感耦合等离子体质谱仪进行分析。为了质量保证和质量控制,每批尿液样本都进行平行样本、程序空白和加标回收实验,用尿比重校正浓度。各元素的回收率在78%—107%。微量元素V、Mn、Co、Se、Mo、Cr、Ni、As、Cd、Sb、Pb检测限(limit of detection,LOD)分别为0.003、0.024、0.001、0.409、0.005、0.021、0.043、0.014、0.002、0.002、0.026 ng·g−1,标准曲线的相关系数均大于0.999。
1.3 灰尘微量元素(样本)分析
称取0.1 g左右灰尘样品,加入6 mL王水(3:1 盐酸:硝酸),在微波消解仪中进行消解。微波消解仪升温程序为:120 ℃保持2 min;150 ℃保持5 min;185 ℃保持40 min。消解液用纯水定容至50 mL,使用电感耦合等离子体质谱仪进行分析。为了质量保证和质量控制,每批灰尘样本都准备了有证标准物质(certified reference material,CRM)和程序空白。CRM中微量元素的回收率在70%—125%。微量元素V、Mn、Co、Se、Mo、Cr、Ni、As、Cd、Sb、Pb的LOD分别为0.005、0.030、0.004、0.607、0.004、0.002、0.058、0.025、0.002、0.027、0.022 ng·g−1,标准曲线的相关系数均大于0.999。
1.4 统计分析
使用 IBM SPSS Statistics(26.0)软件进行描述性统计分析、相关性分析和多元线性回归分析。计算家庭灰尘和孕妇尿液微量元素浓度在LOD以上的中值、最小值和最大值。使用频率和百分比来描述分类变量。孕妇尿液和室内灰尘浓度经 Kolmogorov-Smirnov 正态检验后呈偏态分布,故对其进行对数转换。Pearson相关分析评估孕妇尿液和室内灰尘微量元素之间的关系。采用多元线性回归模型评估孕妇尿液微量元素水平的潜在影响因素,其中人口学特征和室内灰尘对数转换浓度作为自变量,孕妇尿液微量元素的对数转换浓度作为因变量,用后退法进行分析。统计学显著性设定为P<0.05。
1.5 健康风险评估
1.5.1 家庭灰尘健康风险评估
对于检出率大于50%的微量元素,参照美国环境保护署(US Environmental Protection Agency,US-EPA)方法进行建模评估。由于模型估计结果与每种微量元素的真实水平具有不确定性,故以95%置信区间(confidence interval,CI)作为健康风险计算的代表值。人体健康风险系数见式(1)—(3)[19]:
HQ摄取=CDIRfD (1) HQ吸入=CDIRfC (2) 总风险:
HI=ΣHQ摄取;HQ吸入 (3) 式中,HQ(hazard quotient)为非致癌性风险熵,HI(hazard index )为总风险危害指数;RfD(reference dose)为摄取途径下每日最高允许摄入的参考剂量(μg·kg−1·d−1);RfC(reference concentration)为吸入途径下每日最高允许摄入得参考剂量(μg·kg−1·d−1);CDI(chronic daily intake)为每日摄入量(mg·kg−1·d−1)。
当HI值大于1.0时为超出可承受风险,应考虑采取干预措施。环境样品的六价铬(Cr(Ⅵ))较为复杂,因为在样品收集、储存和分析期间Cr会在三价态和六价态之间过渡,本研究中的数据为总Cr浓度。六价铬有毒且RfC的适宜因子仅适用于六价铬,为避免总铬健康风险表达过度,采用物种评价的保守比率(Cr(Ⅵ)=0.25:1)估算RfC值[36]。由于其他暴露介质、外部环境因素和样品微量元素生物利用度尚未得到评估,本文使用的建模方法对家庭灰尘中微量元素的健康风险提供了保守估计。如果健康风险超过可容忍限度,应对这些因素进行进一步调查。
1.5.2 孕妇尿液健康风险评估
本研究同时对孕妇尿液内暴露进行了健康风险评估,其计算公式见式(4)—(5)[37]:
DI微量元素=UE×UVFue×MW (4) HQ=DIRfD (5) 式中: DI(daily intake)为每个微量元素的日摄入量(μg·kgBW−1·d−1);UE(urinary excretion)为尿液中微量元素的浓度(μmol·L−1);UV(volume of daily urinary excretion)为24 h尿液排出量( L·d−1);MW(molecular weight)为母体微量元素的原子量;Fue(urinary rexcretion fraction for a given metabolite)表示尿液中微量元素的尿排泄分数,V、Mn、Co、Se、Mo、Cr、Ni、As、Cd、Sb、Pb的Fue分别为0.66、0.049、0.726、0.176、0.600、0.163、0.400、0.109、0.020、0.800、0.0363[38-42];RfD为参考剂量(μg·kg−1·d−1),参照同灰尘。
1.6 孕妇基本信息
本次研究的22名孕妇的基本信息见表1。孕妇的平均年龄为(31±4 )岁(周岁)。81.8%的孕妇孕前BMI在正常范围(18.5—24.5)内,81.8%的孕妇及其配偶接受过大专及以上教育,54.5%的孕妇为第一次生产。所有孕妇均不吸烟,14.3%的孕妇在孕期存在被动的烟草烟雾暴露。在妊娠期间,30.0%的孕妇饮用桶装水,所有孕妇均食用了淡水鱼及海产品,55.0%的孕妇在怀孕前补充了叶酸。
表 1 孕妇基本信息(n=22)Table 1. Basic information for pregnant women(n=22)年龄(周岁) 孕前BMI 产次 教育程度 配偶教育程度 ≤30 >30 平均 <18.5 ≥18.5 平均 初产妇 经产妇 大专以下 大专及以上 大专以下 大专及以上 28±2 34±3 31±4 16.7±0.384 21.6±1.95 20.7±2.60 54.5% 45.5% 18.2% 81.8% 18.2% 81.8% 被动吸烟 饮用桶装水 食用淡水鱼 食用海产品 补充叶酸 是 否 是 否 每周1—3次 每周4—6次 每周1—3次 每周4—6次 怀孕前 怀孕后 14.3% 85.7% 30.0% 70.0% 95.0% 5.0% 20.0% 80.0% 55.0% 45.0% 2. 结果与讨论(Results and discussion)
2.1 孕妇尿液和家庭灰尘中微量元素水平
2.1.1 家庭灰尘中微量元素水平及组成特征
11种微量元素在室内灰尘的检出率均为100%。灰尘中微量元素含量(平均值、中位值、范围浓度)由高到低排序依次是:Mn(456 μg·g−1、416 μg·g−1、131—1109 μg·g−1)> Pb(159 μg·g−1、96.3 μg·g−1、2.27—592 μg·g−1)> Ni(125 μg·g−1、99.9 μg·g−1、23.0—451 μg·g−1)> Cr(107 μg·g−1、113 μg·g−1、44.7—147 μg·g−1)> V(23.9 μg·g−1、23.0 μg·g−1、8.88—41.6 μg·g−1)> As(15.6 μg·g−1、11.1 μg·g−1、3.73—21.9 μg·g−1)> Co(9.66 μg·g−1、8.24 μg·g−1、2.78—23.3 μg·g−1)> Sb(8.78 μg·g−1、8.80 μg·g−1、2.90—19.7 μg·g−1)> Mo(5.11 μg·g−1、3.56 μg·g−1、1.75—19.7 μg·g−1)> Cd(2.21 μg·g−1、1.62 μg·g−1、0.62—7.86 μg·g−1)> Se(1.95 μg·g−1、1.70 μg·g−1、0.796—3.18 μg·g−1)。室内灰尘中微量元素含量目前还未有公认的参考值,本研究与国内外研究进行了比较,见表2。
表 2 国内外不同城市家庭灰尘微量元素浓度比较(μg·g−1)Table 2. Comparison of trace element concentration in household dust of different cities at home and abroad (μg·g−1)省/市Province/city 采样年份Sampling date 样本量Sample number 钒V 锰Mn 钴Co 硒Se 钼Mo 铬Cr 镍Ni 砷As 镉Cd 锑Sb 铅Pb 文献Reference 广州 2020—2021 22 23.9 457 9.66 1.95 5.11 107 125 15.6 2.21 8.78 159 本研究 广东省清远市 2013—2014 78 — — — — — 41.6 — — 2.45 — 214 [46] 广东省清远农村 2013—2014 78 — — — — — 29.4 — — 4.18 — 392 [46] 山西省太原市 2019 72 48.8 444 8.19 — — 134 40.5 17.4 0.560 — 50.3 [43] 四川省成都市 2014—2015 90 — — — — — 82.7 52.6 — 2.37 — 123 [23] 安徽省合肥市 2018 41 18.0 177 — — — 29.5 26.1 — 4.39 2.00 95.4 [45] 安徽省农村 2010 125 52.6 — 10.3 — — 114 38.9 4.46 — — 349 [44] 加拿大 2016 125 15.0 250 5.40 1.60 8.30 92.0 60.0 13.0 11.0 36.0 450 [15] 伊拉克 2020 50 — — — — — 289.5 106 — 14.8 — 75.6 [48] 沙特阿拉伯 2016 20 — — — — — 46.7 32.2 — 0.540 — — [47] 伊朗 2016 19 — — — — — 143 57.1 — 5.31 — 209 [49] 注:—表示无数据。表格中数据为国内外不同城市家庭灰尘微量元素平均值. — Indicates no data. The data in the table are the average values of trace elements in household dust in different cities at home and abroad. 对于必需微量元素,本研究的家庭灰尘中V浓度(23.9 μg·g−1)与国内城市[43-44](45.0—55.0 μg·g−1)相比较低,与国外城市[15](10.0—20.0 μg·g−1)浓度接近;Mn浓度(456 μg·g−1)与国内[45]和国外[15]城市(150—250 μg·g−1)相比较高,但与华东城市[43](400—500 μg·g−1)浓度一致;Co浓度(9.66 μg·g−1)高于国外城市[15](5.00—6.00 μg·g−1),与国内城市[43-44](8.00—11.0 μg·g−1)浓度相当;Se浓度(1.95 μg·g−1)与国外城市[15](1.00—2.00 μg·g−1)浓度接近;家庭灰尘中Mo浓度(5.11 μg·g−1)与国外城市[15](8.00—9.00 μg·g−1)相比较低。对于有毒微量元素,家庭灰尘中Cr浓度(107 μg·g−1)比国内南方城市[23,45-46]和沙特阿拉伯[47](25.0—85.0 μg·g−1)高,比国内北方城市[43]和亚洲国家[48-49](130—300 μg·g−1)低;Ni浓度(125 μg·g−1)与国内外城市[15,23,43-45,47,49](20.0—60.0 μg·g−1)相比较高,与伊拉克[48](100—130 μg·g−1)浓度相当;As浓度(15.6 μg·g−1)比国内南方城市[44](4.00—5.00 μg·g−1)高,与国内北方城市[43]和国外城市[15](10.0—20.0 μg·g−1)相当;Cd浓度(2.21 μg·g−1)比国外城市[15,48](10.0—15.0 μg·g−1)低,与国内南方城市[23,45-46](2.00—5.00 μg·g−1)相当;Sb浓度(8.78 μg·g−1)比国内城市[45](1.00—3.00 μg·g−1)高,比国外城市[15](30.0—40.0 μg·g−1)低;Pb浓度(159 μg·g−1)比国内农村[44,46]和加拿大[15](300—500 μg·g−1)低,与国内南方城市[23,45-46] (100—250 μg·g−1)相当。总的来说,本研究的孕妇家庭灰尘中Cr、Ni和As浓度水平较高,需要注意其对孕妇健康的危害。
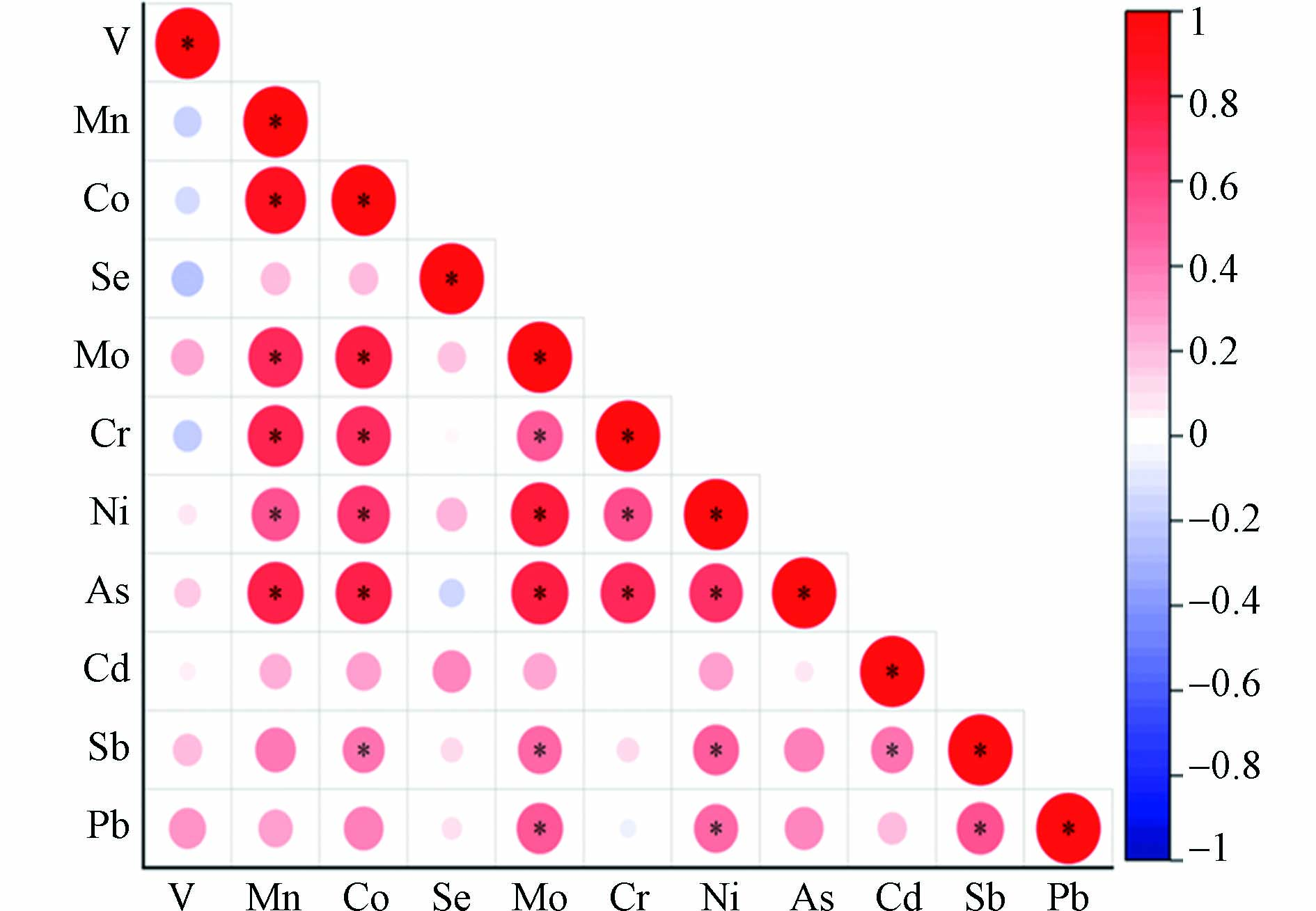
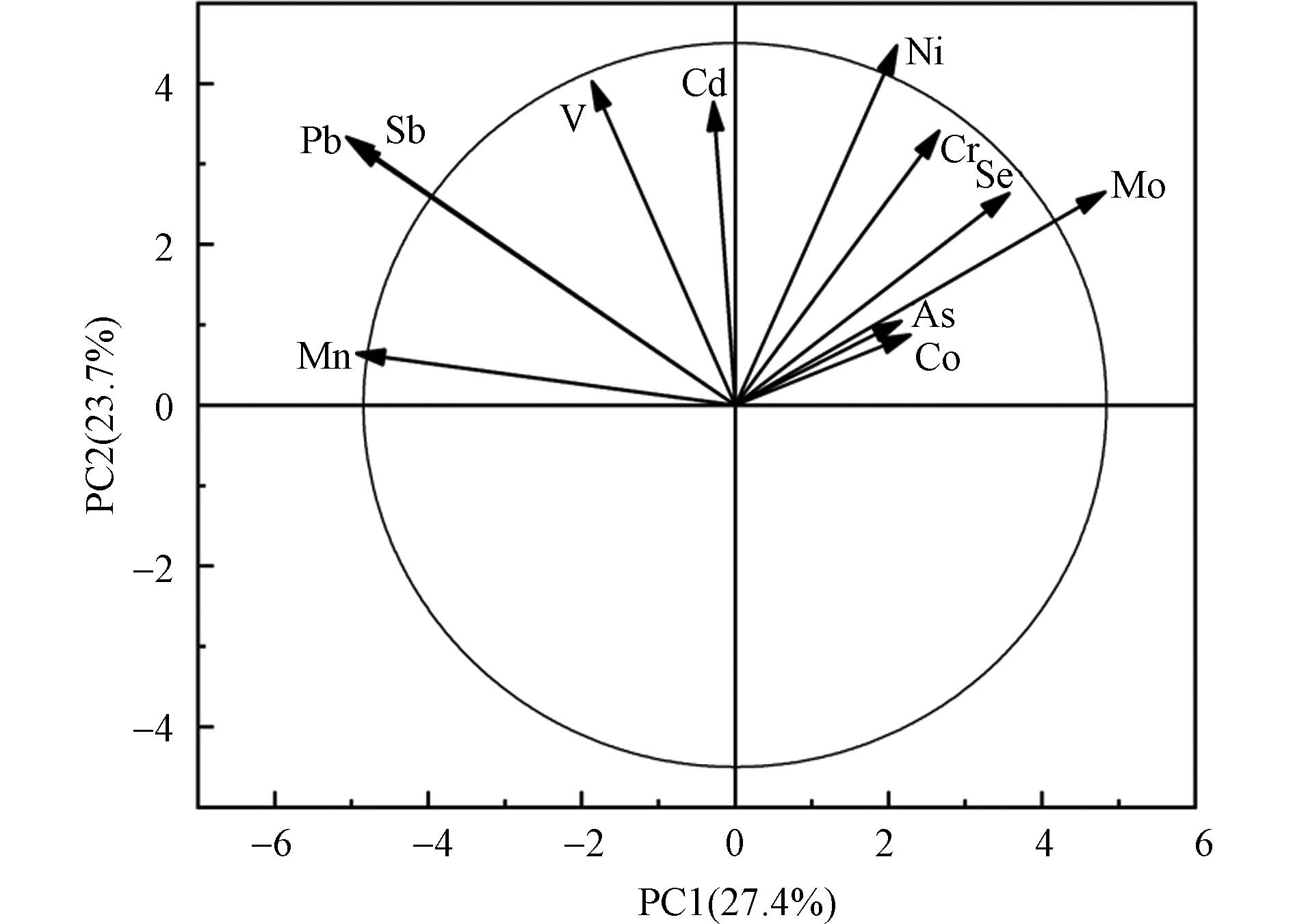
室内灰尘中微量元素之间存在相关性(图1),灰尘中Mn和Co(r=0.898)、Mo(r=0.722)、Cr(r=0.750)、Ni(r =0.559)、As(r=0.761)呈正相关关系(P<0.05)。Co和Mo(r=0.784)、Cr(r=0.714)、Ni(r=0.675)、As(r=0.768)、Sb(r=0.431)呈正相关关系(P<0.05)。Mo和Cr(r=0.521)、Ni(r=0.814)、As(r=0.791)、Sb(r=0.464)、Pb(r=0.525)呈正相关关系(P<0.05)。Cr和Ni(r=0.580)、As(r=0.736),Ni和As(r=0.696)、Sb(r=0.512)、Pb(r=0.475),Sb和Cd(r=0.436)、Pb(r=0.543)呈正相关关系(P<0.05)。主成分分析提取了4个因子,占总方差的84.3%(F1为45.5%;F2为15.9%;F3为13.9%;F4为8.98%),其中Mn、Co、Mo和As是主要变量。从图2的主成分分析载量图可以看出,家庭灰尘中Mn、Co和Mo聚集在一起,Se和Cd聚集在一起,与相关性分析的结果一致。Wang等[50]的研究表明空气中Mn和Co的强相关性可能来源于机动车自然粉尘,Gurbanov等[51]研究表明Mo存在于土壤中,所以家庭灰尘中Mn、Co和Mo可能具有共同的来源。有研究表明,Se易在土壤中富集[52],Cd在道路灰尘中是主要富集元素之一[53],家庭灰尘中Se和Cd可能共同来源于室外道路粉尘或土壤环境。
2.1.2 孕妇尿液中微量元素水平及组成特征
孕妇尿液中,除Sb的检出率为54.6%外,其余微量元素检出率均在75%以上。尿液微量元素(平均值、中位值、浓度范围)按照平均值排列为:Mo(37.2 μg·L−1、21.1 μg·L−1、1.19—123 μg·L−1)>As(26.6 μg·L−1、11.7 μg·L−1、2.29—214 μg·L−1)>Se(16.1 μg·L−1、10.0 μg·L−1、nd. —92.6 μg·L−1) >Pb (5.81 μg·L−1、4.32 μg·L−1、1.41—325 μg·L−1)>Cr(5.63 μg·L−1、4.78 μg·L−1、nd. —13.1 μg·L−1)>Mn(2.68 μg·L−1、2.56 μg·L−1、nd. —8.10 μg·L−1)>Ni(2.64 μg·L−1、2.91 μg·L−1、nd. —8.09 μg·L−1)>Cd(0.538 μg·L−1、11.7 μg·L−1、2.29—214 μg·L−1)>Co(0.495 μg·L−1、0.310 μg·L−1、nd. —1.79 μg·L−1)>V(0.339 μg·L−1、0.218 μg·L−1、nd. —1.81 μg·L−1)>Sb(0.244 μg·L−1、0.530 μg·L−1、0.0300—1.10 μg·L−1)。本研究与国内外研究孕妇和成人的尿液微量元素浓度进行了比较(表3)。
表 3 国内外不同城市居民尿液微量元素浓度比较(μg·L−1)Table 3. Comparison of urinary trace elements concentrations of residents in different cities at home and abroad (μg·L−1)省/市Province/city 人口类别Population category 采样年份Sampling date 样本量Sample number 钒V 锰Mn 钴Co 硒Se 钼Mo 铬Cr 镍Ni 砷As 镉Cd 锑Sb 铅Pb 文献Reference 广州 孕妇 2020—2021 22 0.339 2.68 0.495 16.1 37.2 5.63 2.64 26.6 0.538 0.244 5.24 本研究 中国武汉 孕妇 2014—2016 675 0.960 — — — — 1.12 — 20.0 0.630 — 2.54 [54] 中国武汉 成人 2014—2016 226 — 0.530 — 34.0 — 0.240 — 40.9 1.09 — 0.920 [30] 广东深圳 成人 2016—2017 334 — 5.80 — 30.1 — 4.46 — 48.8 1.47 — 4.69 [56] 西班牙 孕妇 2004—2008 1346 — — 0.520 17.1 38.8 — 1.27 34.4 0.230 0.350 1.14 [57] 加拿大 孕妇 2016 29 0.170 0.630 0.630 58.2 — 0.130 0.960 7.40 0.120 — 0.160 [55] 马来西亚 成人 2017—2018 817 — — — — — — 5.73 82.3 0.470 — 1.53 [60] 美国 成人 2003—2014 9537 — — 0.340 — 38.6 — — 8.85 0.240 0.06 0.50 [58] 伊朗 成人 2014 33 — — — 102 — — — — — — 16.7 [59] 注:—表示无数据。表格中数据为国内外不同城市居民尿液微量元素平均值. — Indicates no data. The data in the table are the average values of urine trace elements of residents in different cities at home and abroad. 对于必需微量元素,本研究的孕妇尿液中V浓度(0.339 μg·g−1)比国内孕妇[54](0.900—1.00 μg·g−1)低,比国外孕妇[55](0.100—0.200 μg·g−1)高;孕妇尿液中Mn浓度(2.68 μg·g−1)比武汉成人[30]和国外孕妇[55](0.500—0.700 μg·g−1)高,与广东深圳成人[56]尿液中的浓度(2.00—10.0 μg·g−1)相当;孕妇尿液中Co浓度(0.495 μg·g−1)与国外[57-58](0.300—0.800 μg·g−1)一致;孕妇尿液中Se浓度(16.1 μg·g−1)低于国内外孕妇和成人[30,55-56,59]浓度(30.0—110 μg·g−1),与西班牙孕妇[57]尿液浓度(10.0—20.0 μg·g−1)相当;Mo浓度(37.2 μg·g−1)与国外孕妇和成人[57-58]尿液浓度(30—40 μg·g−1)相当。整体来看,本研究的孕妇尿液中Se水平较低,早产与Se水平低有很强的关系[61],需要注意在妊娠期间Se的补充。
对于有毒微量元素,本研究的孕妇尿液中Cr浓度(5.63 μg·g−1)比武汉孕妇和成人[54-55](0.200—1.50 μg·g−1)高,但与广东深圳成人[56](4.00—6.00 μg·g−1)相当;孕妇尿液中Ni浓度(2.64 μg·g−1)略高于孕妇[55,57](0.500—1.50 μg·g−1)尿液浓度,低于成人[60](5.00—6.00 μg·g−1);孕妇尿液中As浓度(26.6 μg·g−1)略高于国内外孕妇和成人[54-55,58](5.00—20.0 μg·g−1);孕妇尿液中Cd浓度(0.538 μg·g−1)与国内外浓度[30,54-58,60](0.100—1.50 μg·g−1)一致;孕妇尿液中Sb浓度(0.244 μg·g−1)比美国成人[58](0—0.100 μg·g−1)高,与西班牙孕妇[57](0.100—0.500 μg·g−1)相当;孕妇尿液中Pb浓度(5.24 μg·g−1)与广东深圳浓度[56](3.00—6.00 μg·g−1)一致。本研究的孕妇尿液As和Ni浓度较高,且其家庭灰尘中As和Ni浓度水平较高,提示在妊娠期间需要注意家庭灰尘中有毒微量元素As和Ni对孕妇和胎儿的危害。
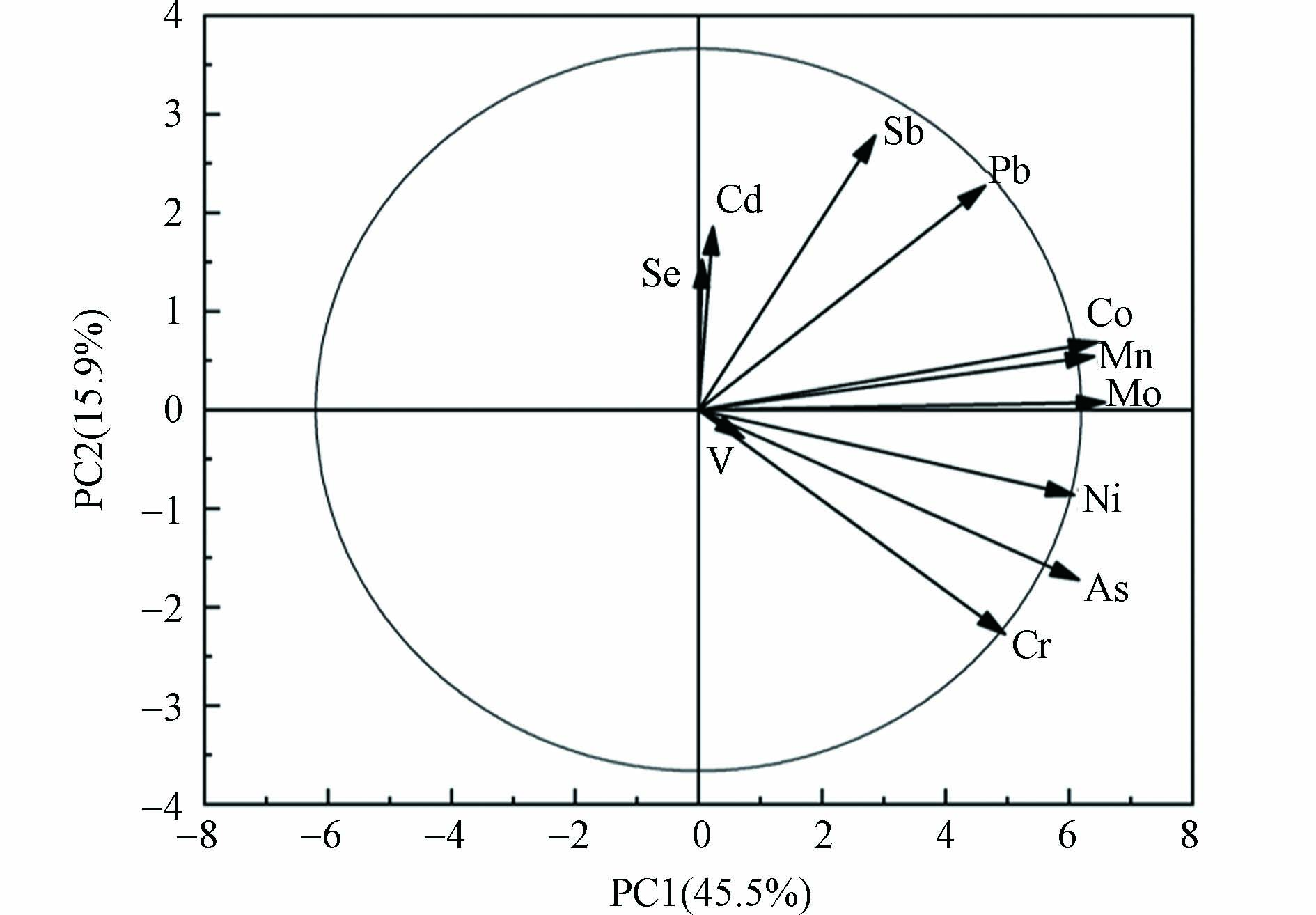
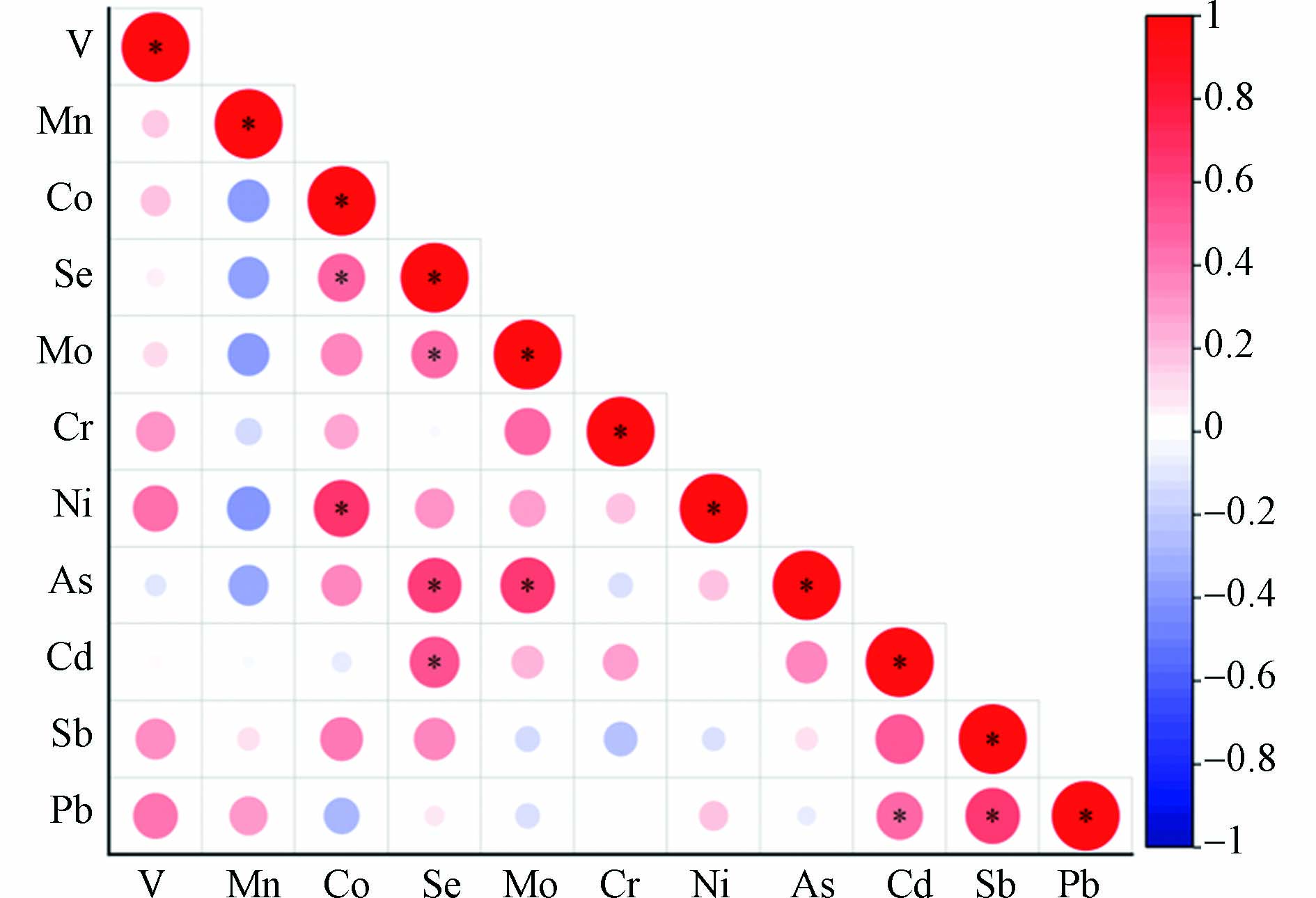
孕妇尿液中微量元素之间存在相关性(图3),孕妇尿液中Se和Co(r=0.481)、Mo(r=0.472)、As(r=0.631)、Cd(r=0.546)呈正相关关系(P<0.05);Co和Ni(r=0.676)呈正相关关系(P<0.05)。这与Maxime等[62]研究中Co和Ni的相关性一致,Zhao 等[63]也发现尿液中Co和Ni、Cd和Se之间存在显著相关性。孕妇尿液中Mo和As(r=0.646,P<0.05)呈正相关关系。Pb和Cd(r=0.475)、Sb(r=0.647)呈正相关关系(P<0.05),有研究表明Pb和Cd之间具有免疫抑制的协同效应[64]。主成分分析提取了4个因子,占总方差的76.5%(F1为27.4%;F2为23.7%;F3为15.6%;F4为9.73%),其中Pb、Sb、Mo和Ni是主要变量。从图4孕妇尿液主成分分析载量图可以看出,孕妇尿液中Pb和Sb聚集在一起,Mo、Se、As和Co聚集在一起,与相关分析结果一致。近年来,Pb和Sb广泛应用和大规模开采,导致其在水和土壤中的污染日益严重[65-66],Pb和Sb可能经口进入体内。有研究表明Mo、Se、As和Co主要来源与饮食有关[57,67-69]。所以孕妇尿液中Pb和Sb,Mo、Se、As和Co可能具有相同的来源或代谢过程。该结果与家庭灰尘的结果不尽相同,表明孕妇尿液中微量元素的影响因素不只是家庭灰尘,还需要进一步分析其他影响因素,如饮水和食物等途径。
2.2 人口学特征的多元回归分析
根据环境因素和饮食习惯的调查问卷,使用多元线性回归研究其对孕妇尿液微量元素浓度的潜在影响。每种微量元素的最终回归模型的结果如表4。结果显示,显著影响孕妇尿液微量元素浓度的因素是有限的;室内灰尘中Cd的浓度是孕妇尿液Cd浓度(β=−0.870,P<0.01)的重要预测因素。孕妇尿液Mn(β=0.848,P=0.001)、Cd(β=1.24,P=0.001)和Pb(β=1.51,P<0.01)浓度增加,Co(β=−0.594,P<0.01)和Mo(β=1.94,P=0.004)浓度减少,均与被动吸烟有关。Isley等[36]的研究发现尿液中较高浓度的Mn和Pb可能与室内吸烟有关;De 等[70]发现吸烟可使尿液中Cd含量增加;Manuel等[57]研究发现Co含量降低与怀孕期间被动吸烟有关。有毒微量元素会对孕妇和胎儿造成不良影响。Eguchi等[71]发现孕妇体内Mn的浓度水平与新生儿的出生头围呈负相关。Cd是Ⅰ类人类致癌物,妊娠期暴露Cd会影响胎儿的生长和器官发育[72]。高浓度的Pb会引起神经管缺陷[73],Daniali等[74]发现孕妇体内Pb浓度水平与新生儿出生体重呈负相关。因此在妊娠期应警惕生活环境如烟尘带来的危害。
表 4 尿液微量元素浓度影响因素的多元线性回归模型结果Table 4. Multivariate linear regression model results of factors influencing urinary trace element concentration微量元素Trace elements 影响因素Influence factor 标准化系数Standardized Coefficients 95%置信区间95%Confidence interval R2 调整R2Adjust R2 Beta Sig 下界Lower bound 上界Upper bound Mn 常量 — 0.050 −3.64 0.004 0.566 0.499 被动吸烟(是) 0.848 0.001 0.912 2.93 — — Co 常量 — 0.302 −1.17 3.44 0.757 0.669 教育程度(大专以下) −0.962 0.003 −4.06 −1.05 — — 被动吸烟(是) −0.594 0.005 3.05 −0.672 — — 食用海产品(4—6次/周) 0.698 0.020 0.425 3.95 — — Se 补充叶酸(怀孕前) 0.909 0.000 1.08 2.69 — — 常量 — 0.023 −2.47 −0.212 0.500 0.428 补充叶酸(怀孕前) 0.552 0.012 0.245 1.68 — — Mo 常量 — 0.183 −1.22 5.78 0.558 0.422 产次(经产妇) 0.574 0.012 0.307 2.04 — — 配偶教育程度(大专及以上) 1.94 0.004 1.84 7.66 — — 被动吸烟(是) −1.54 0.004 −6.79 −1.63 — — 食用海产品(4—6次/周) −0.786 0.024 −4.69 −0.396 — — Ni 常量 — 0.002 2.02 6.42 0.842 0.771 饮用桶装水(是) −0.373 0.032 −1.55 −0.086 — — Cd 常量 — 0.638 −2.19 1.40 0.785 0.695 Cd(灰尘) −0.870 0.000 −1.33 −0.600 — — 被动吸烟(是) 1.24 0.001 1.72 5.04 — — Pb 常量 — 0.214 −2.28 0.554 0.744 0.709 被动吸烟(是) 1.51 0.000 2.72 5.48 — — 注:—表示无数据。— Indicates no data. 同时,孕妇尿液中Co(β=0.698,P=0.020)的浓度增加,尿Mo(β=−0.786,P=0.024)的浓度减少,与频繁食用海产品有关;经产妇的尿Mo浓度比初产妇高(β=0.574,P=0.012)。Baraquoni等[75]发现新生儿Mo浓度高与发育迟缓有关,Chaoqun等[76]发现妊娠期间暴露Mo与胎儿神经发育有关。孕妇体内Se(β=0.552,P=0.012)和Co(β=0.909,P<0.01)的浓度增加与早期补充叶酸有关;孕妇尿液中Co与教育程度呈正相关(β=−0.962,P=0.003)。Co在体内是必需微量元素之一,Zhi等[77]研究表明孕妇体内Co水平较低可能会引发早产。Se在妊娠期对机体起到保护作用[78],Hawkes等[79]发现孕妇Se的水平过低会导致妊娠糖尿病,Se的浓度过低会增加胎儿过小的风险[80];Wang 等[69]的研究显示尿Mn浓度与孕期补充叶酸显著相关,本研究未发现此结果,但是尿Se浓度增加这一结果与本研究一致。本研究结果提示可以通过食用海产品补充孕妇体内Co的含量,尽早补充叶酸增加孕妇体内Se的含量。
2.3 健康风险评估
表5列出了孕妇微量元素暴露的健康风险评估;家庭灰尘Mn、Mo、Cr、As和Pb的总风险分别为(4.07±1.94)、(1.59×10−3±1.25×10−3)、(5.61×10−3±4.80×10−3)、(4.77×10−1±4.73×10−1)和(4.00×10−2±3.83×10−2). 本研究家庭灰尘Mn的总风险较高,其中主要为吸入暴露贡献。然而,以往研究普遍发现吸入微量元素对人体的健康风险较低[23,81-82]。建议孕妇妊娠期需要注意及时打扫室内卫生,防止灰尘堆积。由于本研究样本量较少,且室内灰尘健康风险评价的准确性受到其他因素的影响,如灰尘粒径大小、采样时的环境、居住地周围环境等未收集分析,所以对于室内灰尘的健康风险评估还需要进一步开展深入研究。
表 5 健康风险评估Table 5. Health risk assessment微量元素Trace element RfD/(μg·kg−1·d−1) RfC/(μg·kg−1·d−1) 家庭灰尘 均值±标准差(范围)Household dust Mean ± Standard deviation(range) 孕妇尿液 均值±标准差(范围)Pregnant women urine Mean ± Standard deviation(range) HQ摄取HQ ingestion HQ吸入HQ inhalation HI DI/(μg·kg-1BW·d−1) HQ V 5.04 0.1 (2.16×10−3±9.39×10−4)(7.55×10−4—4.01×10−3) (1.09×10−1±4.73×10−2)(3.81×10−2—2.02×10−1) (1.11×10−1±4.83×10−2) 0.0470±0.0563(nd.—0.252) 0.00933±0.0112(nd.—0.0500) Mn 140 0.05 (1.45×10−3±6.92×10−4)(5.63×10−4—3.55×10−3) 4.07±1.94(1.58—9.94) 4.07±1.94 5.40±4.73(nd.—16.3) 0.0386±0.0338(nd.—0.117) Co 0.3 0.006 (1.42×10−2±6.42×10−3)(5.56×10−3—3.47×10−2) (7.11×10−1±3.21×10−1)(2.78×10−1—1.74) (7.26×10−1±3.28×10−1) 0.0695±0.0726(nd.—0.262) 0.232±0.242(nd.—0.872) Se 5 20 (1.78×10−4±6.83×10−5)(7.35×10−5—3.24×10−4) (4.42×10−5±1.71×10−5 )(1.71×10−5—8.10×10−5) (2.21×10−4±8.54×10−5) 1.01±0.854(nd.—28.0) 0.261±0.317(nd.—15.0) Mo 5 2 (4.53×10−4±3.57×10−4)(1.62×10−4—1.77×10−3) (1.13×10−3±8.93×10−4)(4.04×10−4—4.42×10−3) (1.59×10−3±1.25×10−3) 10.7±9.51(0.343—35.4) 2.14±1.90(0.0685—7.07) Cr 3 0.1 (1.81×10−4±1.55×10−4)(5.74×10−5—5.84×10−4) (5.43×10−3±4.65×10−3)(1.72×10−3—1.75×10−2) (5.61×10−3±4.80×10−3) 3.23±2.64(nd.—7.50) 1.08±0.881(nd.—2.50) Ni 20 0.09 (2.75×10−3±1.96×10−3 )(6.89×10−4—9.09×10−3) (6.11×10−1±4.35×10−1)(1.53×10−1—2.02) (6.14×10−1±4.37×10−1) 0.698±0.502(nd.—2.14) 0.0349±0.021(nd.—0.107) As 0.3 0.015 (2.27×10−2±2.25×10−2)(7.46×10−3—1.10×10−1) (4.55×10−1±4.51×10−1)(1.49×10−1—2.20) (4.77×10−1±4.73×10−1) 18.4±14.3(2.83—52.6) 44.5±27.1(9.43—102) Cd 0.1 0.01 (9.63×10−3±7.48×10−3)(3.56×10−3—3.32×10−2) (9.63×10−2±7.48×10−2)(3.56×10−2—3.32×10−1) (1.06×10−1±8.22×10−2) 5.44±3.31(0.303—11.1) 0.544±0.331(0.0303—1.11) Sb 0.4 0.3 (9.77×10−3±4.63×10−3)(2.86×10−3—2.08×10−2) (1.30×10−2±6.18×10−3)(3.82×10−3—2.78×10−2) (2.28×10−2±1.08×10−2) 0.0668±0.225(nd.—1.09) 0.167±0.561(nd.—2.72) Pb 3.5 3.52 (2.01×10−2±1.92×10−2)(2.67×10−4—7.15×10−2) (2.00×10−2±1.91×10−2)(2.65×10−4—7.10×10−2) (4.00×10−2±3.83×10−2) 46.0±18.3(14.5—83.5) 13.2±7.50(4.14—23.9) 注:nd.,未检出。nd.,not detected. 针对孕妇尿液的健康风险评估中显示Mo、Cr、As、Pb处于潜在风险(HQ>1),Cd和Sb分别有3人和1人HQ>1,Zhong等[83]研究也表明广州居民尿液中As具有潜在风险。孕妇尿液种Cr和Pb水平相对较高,且家庭灰尘Cr和Pb水平也较高,说明家庭灰尘可能会影响孕妇尿液Cr和Pb的浓度水平。在家庭灰尘中Mn的HQ>1,而孕妇尿液中Mn的HQ<1,说明家庭灰尘可能不是影响孕妇体内Mn含量的主要因素。Mo、Cr、As、Pb在家庭灰尘中HQ<1,而在孕妇尿液中HQ>1,这说明灰尘摄入可能并非Mo、Cr、As和Pb进入孕妇体内的主要途径。
3. 结论(Conclusion)
本研究检测了孕妇尿液及家庭灰尘中必需微量元素(V、Mn、Co、Se和Mo)和有毒微量元素(Cr、Ni、As、Cd、Sb和Pb)的浓度水平,家庭灰尘中的Cr、Ni和As含量较高;孕妇尿液中As和Ni的含量较高,Se的含量偏低。在吸烟环境会使孕妇尿液中Mn、Cd和Pb的浓度增加,建议在妊娠期避免在室内抽烟。本研究健康风险评估家庭灰尘中Mn的HQ>1,孕妇尿液中Mo、Cr、As和Pb的HQ>1,虽然本研究没有观察到早产、自然流产和畸形等严重的临床影响,但是根据本研究的分析,这些微量元素对孕妇及胎儿的健康影响可能存在危害,还要考虑采取干预措施。由于本研究的样本量较少,还需要进一步进行调查研究,以便更好地阐明微量元素对孕妇和胎儿的影响。
-
-
[1] LIU J, SUN K, ZHU R, et al. Biotransformation of bisphenol A in vivo and in vitro by laccase-producing Trametes hirsuta La-7: Kinetics, products, and mechanisms[J]. Environmental Pollution, 2023, 321: 121155-121166. doi: 10.1016/j.envpol.2023.121155 [2] GUPTA V, BALDA S, GUPTA N, et al. Functional substitution of domain 3 (T1 copper center) of a novel laccase with Cu ions[J]. International journal of biological macromolecules, 2019, 123: 1052-1061. doi: 10.1016/j.ijbiomac.2018.11.174 [3] DATTA S, VEENA R, SAMUEL M S, et al. Immobilization of laccases and applications for the detection and remediation of pollutants: a review[J]. Environmental Chemistry Letters, 2021, 19(1): 521-538. doi: 10.1007/s10311-020-01081-y [4] WANG Z, REN D, YU H, et al. Preparation optimization and stability comparison study of alkali-modified biochar immobilized laccase under multi-immobilization methods[J]. Biochemical Engineering Journal, 2022, 181: 108401-108412. doi: 10.1016/j.bej.2022.108401 [5] BRUGNARI T, PEREIRA M G, BUBNA G A, et al. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A[J]. Science of the Total Environment, 2018, 634: 1346-1351. doi: 10.1016/j.scitotenv.2018.04.051 [6] WANG Z, REN D, JIANG S, et al. The study of laccase immobilization optimization and stability improvement on CTAB-KOH modified biochar[J]. BMC Biotechnology, 2021, 21: 1-13. doi: 10.1186/s12896-020-00660-9 [7] PRIMOŽIČ M, KRAVANJA G, KNEZ Ž, et al. Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio) degradation[J]. Journal of Cleaner Production, 2020, 275: 124121-124137. doi: 10.1016/j.jclepro.2020.124121 [8] REN D, WANG Z, JIANG S, et al. Recent environmental applications of and development prospects for immobilized laccase: A review[J]. Biotechnology and Genetic Engineering Reviews, 2020, 36(2): 81-131. doi: 10.1080/02648725.2020.1864187 [9] KOŁODZIEJCZAK-RADZIMSKA A, ZEMBRZUSKA J, SIWIŃSKA-CIESIELCZYK K, et al. Catalytic and physicochemical evaluation of a TiO2/ZnO/laccase biocatalytic system: Application in the decolorization of Azo and Anthraquinone dyes[J]. Materials, 2021, 14(20): 6030-6047. doi: 10.3390/ma14206030 [10] KOŁODZIEJCZAK-RADZIMSKA A, BUDNA A, CIESIELCZYK F, et al. Laccase from Trametes versicolor supported onto mesoporous Al2O3: Stability tests and evaluations of catalytic activity[J]. Process Biochemistry, 2020, 95: 71-80. doi: 10.1016/j.procbio.2020.05.008 [11] NEEDHIDASAN S, RAMALINGAM C. Stratagems employed for 2, 4-dichlorophenoxyacetic acid removal from polluted water sources[J]. Clean Technologies and Environmental Policy, 2017, 19: 1607-1620. doi: 10.1007/s10098-017-1371-8 [12] HAN Z, FAN X, YU S, et al. Metal-organic frameworks (MOFs): A novel platform for laccase immobilization and application[J]. Journal of Environmental Chemical Engineering, 2022: 108795-108821. [13] LI G, NANDGAONKAR A G, LU K, et al. Laccase immobilized on PAN/O-MMT composite nanofibers support for substrate bioremediation: a de novo adsorption and biocatalytic synergy[J]. RSC advances, 2016, 6(47): 41420-41427. doi: 10.1039/C6RA00220J [14] LE T T, MURUGESAN K, LEE C-S, et al. Degradation of synthetic pollutants in real wastewater using laccase encapsulated in core–shell magnetic copper alginate beads[J]. Bioresource Technology, 2016, 216: 203-210. doi: 10.1016/j.biortech.2016.05.077 [15] CHU G, ZHAO J, HUANG Y, et al. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores[J]. Environmental Pollution, 2018, 240: 1-9. doi: 10.1016/j.envpol.2018.04.003 [16] LIU Y, YANG X, ZHANG J, et al. Process Simulation of Preparing Biochar by Biomass Pyrolysis Via Aspen Plus and Its Economic Evaluation[J]. Waste and Biomass Valorization, 2022, 13(5): 2609-2622. doi: 10.1007/s12649-021-01671-z [17] CAMPBELL R M, ANDERSON N M, DAUGAARD D E, et al. Financial viability of biofuel and biochar production from forest biomass in the face of market price volatility and uncertainty[J]. Applied Energy, 2018, 230: 330-343. doi: 10.1016/j.apenergy.2018.08.085 [18] MRINAL B. Polymer nanocomposites: A comparison between carbon nanotubes, graphene, and clay as nanofillers[J]. Materials, 2016, 9(4): 262. doi: 10.3390/ma9040262 [19] VITHANAGE M, HERATH I, ALMAROAI Y A, et al. Effects of carbon nanotube and biochar on bioavailability of Pb, Cu and Sb in multi-metal contaminated soil[J]. Environmental geochemistry and health, 2017, 39: 1409-1420. doi: 10.1007/s10653-017-9941-6 [20] XU R, TANG R, ZHOU Q, et al. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes[J]. Chemical Engineering Journal, 2015, 262: 88-95. doi: 10.1016/j.cej.2014.09.072 [21] ZHANG W, YANG Q, LUO Q, et al. Laccase-carbon nanotube nanocomposites for enhancing dyes removal[J]. Journal of Cleaner Production, 2020, 242: 118425-118436. doi: 10.1016/j.jclepro.2019.118425 [22] ZHANG Y, PIAO M, HE L, et al. Immobilization of laccase on magnetically separable biochar for highly efficient removal of bisphenol A in water[J]. RSC advances, 2020, 10(8): 4795-4804. doi: 10.1039/C9RA08800H [23] COLEMAN H M, EGGINS B R, BYRNE J A, et al. Photocatalytic degradation of 17-β-oestradiol on immobilized TiO 2[J]. Applied Catalysis B Environmental, 2000, 24(1): 1-5. doi: 10.1016/S0926-3373(99)00091-0 [24] IMRAN, ALI, ZEID, et al. Supra molecular mechanism of the removal of 17-β-oestradiol endocrine disturbing pollutant from water on functionalized iron nano particles[J]. Journal of Molecular Liquids, 2017: 123-129. [25] XU F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases[J]. Journal of biological chemistry, 1997, 272(2): 924-928. doi: 10.1074/jbc.272.2.924 [26] HUAN W W, YANG Y X, WU B, et al. Degradation of 2, 4‐DCP by the immobilized laccase on the carrier of Fe3O4@ SiO2‐NH2[J]. Chinese Journal of Chemistry, 2012, 30(12): 2849-2860. doi: 10.1002/cjoc.201200718 [27] HAN Z, WANG H, ZHENG J, et al. Ultrafast synthesis of laccase-copper phosphate hybrid nanoflowers for efficient degradation of tetracycline antibiotics[J]. Environmental Research, 2023, 216: 114690-114701. doi: 10.1016/j.envres.2022.114690 [28] MAURYA S S, NADAR S S, RATHOD V K. Dual activity of laccase-lysine hybrid organic–inorganic nanoflowers for dye decolourization[J]. Environmental Technology & Innovation, 2020, 19: 100798-100811. [29] PANDEY D, DAVEREY A, DUTTA K, et al. Bioremoval of toxic malachite green from water through simultaneous decolorization and degradation using laccase immobilized biochar[J]. Chemosphere, 2022, 297: 134126-134134. doi: 10.1016/j.chemosphere.2022.134126 [30] HE L, YANG Y, KIM J, et al. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water[J]. Chemical Engineering Journal, 2020, 384: 123276-123286. doi: 10.1016/j.cej.2019.123276 [31] LIU L, LI Y, FAN S. Preparation of KOH and H3PO4 modified biochar and its application in methylene blue removal from aqueous solution[J]. Processes, 2019, 7(12): 891-911. doi: 10.3390/pr7120891 [32] LEPORE M, PORTACCIO M. Optical detection of different phenolic compounds by means of a novel biosensor based on sol–gel immobilized laccase[J]. Biotechnology and Applied Biochemistry, 2017, 64(6): 782-792. doi: 10.1002/bab.1551 [33] BOKOV D O, MAHMOUD M Z, WIDJAJA G, et al. Transfer hydrogenation of nitroarenes using cellulose filter paper-supported Pd/C by filtration as well as sealed methods[J]. RSC Advances, 2022, 12(18): 10933-10949. doi: 10.1039/D2RA01151D [34] ZHU Y, YI B, YUAN Q, et al. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar[J]. RSC advances, 2018, 8(36): 19917-19929. doi: 10.1039/C8RA03018A [35] ZHENG Z, LIU W, ZHOU Q, et al. Effects of co-modified biochar immobilized laccase on remediation and bacterial community of PAHs-contaminated soil[J]. Journal of Hazardous Materials, 2023, 443: 130372-130383. doi: 10.1016/j.jhazmat.2022.130372 [36] PATEL S K, GUPTA R K, KIM S Y, et al. Rhus vernicifera Laccase Immobilization on Magnetic Nanoparticles to Improve Stability and Its Potential Application in Bisphenol A Degradation[J]. Indian Journal of Microbiology, 2020, 61(1): 45-54. [37] PATEL S K, ANWAR M Z, KUMAR A, et al. Fe2O3 yolk-shell particle-based laccase biosensor for efficient detection of 2, 6-dimethoxyphenol[J]. Biochemical Engineering Journal, 2018, 132: 1-8. doi: 10.1016/j.bej.2017.12.013 [38] WANG Z, REN D, WU J, et al. Study on adsorption-degradation of 2, 4-dichlorophenol by modified biochar immobilized laccase[J]. International Journal of Environmental Science and Technology, 2022, 19: 1393-1406. doi: 10.1007/s13762-021-03151-2 [39] YAOHUA G, PING X, FENG J, et al. Co-immobilization of laccase and ABTS onto novel dual-functionalized cellulose beads for highly improved biodegradation of indole[J]. Journal of hazardous materials, 2018, 365: 118-124. [40] IMAM A, SUMAN S K, SINGH R, et al. Application of laccase immobilized rice straw biochar for anthracene degradation[J]. Environmental Pollution, 2021, 268: 115827-115841. doi: 10.1016/j.envpol.2020.115827 [41] REN X, CHEN C, NAGATSU M, et al. Carbon nanotubes as adsorbents in environmental pollution management: A review[J]. Chemical Engineering Journal, 2011, 170(2-3): 395-410. doi: 10.1016/j.cej.2010.08.045 [42] 任海燕, 纪树兰, 崔成武, 等. 17α-乙炔基雌二醇的降解及其共基质代谢特性[J]. 环境科学研究, 2006, 19(4): 61-64. doi: 10.3321/j.issn:1001-6929.2006.04.012 [43] DARONCH N A, KELBERT M, PEREIRA C S, et al. Elucidating the choice for a precise matrix for laccase immobilization: A review[J]. Chemical Engineering Journal, 2020, 397: 125506-125521. doi: 10.1016/j.cej.2020.125506 [44] ASGHER M, NOREEN S, BILAL M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers[J]. International journal of biological macromolecules, 2017, 95: 54-62. doi: 10.1016/j.ijbiomac.2016.11.012 [45] SHAO B, LIU Z, ZENG G, et al. Immobilization of laccase on hollow mesoporous carbon nanospheres: noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal[J]. Journal of hazardous materials, 2019, 362: 318-326. doi: 10.1016/j.jhazmat.2018.08.069 [46] LONAPPAN L, LIU Y, ROUISSI T, et al. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac[J]. Chemical Engineering Journal, 2018, 351: 985-994. doi: 10.1016/j.cej.2018.06.157 [47] LI G, NANDGAONKAR A G, WANG Q, et al. Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo-and bio-catalytic dye degradation[J]. Journal of membrane science, 2017, 525: 89-98. doi: 10.1016/j.memsci.2016.10.033 [48] A CHAUDHARI S, R KAR J, S SINGHAL R. Immobilization of proteins in alginate: Functional properties and applications[J]. Current Organic Chemistry, 2015, 19(17): 1732-1754. doi: 10.2174/1385272819666150429232110 [49] OLAJUYIGBE F M, FATOKUN C O. Biochemical characterization of an extremely stable pH-versatile laccase from Sporothrix carnis CPF-05[J]. International Journal of Biological Macromolecules, 2017, 94: 535-543. doi: 10.1016/j.ijbiomac.2016.10.037 [50] JAISWAL N, PANDEY V P, DWIVEDI U N. Purification of a thermostable alkaline laccase from papaya (Carica papaya) using affinity chromatography[J]. International Journal of Biological Macromolecules, 2015, 72: 326-332. doi: 10.1016/j.ijbiomac.2014.08.032 [51] 冯义平, 沈梦瑶, 张伊健, 等. 典型金属离子对漆酶催化降解溴代阻燃剂四溴双酚A的影响研究[J]. 环境科学学报, 2020, 40(6): 2082-2089. [52] YANG B, TANG K, WEI S, et al. Preparation of functionalized mesoporous silica as a novel carrier and immobilization of laccase[J]. Applied Biochemistry and Biotechnology, 2021, 193: 2547-2566. doi: 10.1007/s12010-021-03556-2 [53] YAMAK O, KALKAN N A, AKSOY S, et al. Semi-interpenetrating polymer networks (semi-IPNs) for entrapment of laccase and their use in Acid Orange 52 decolorization[J]. Process Biochemistry, 2009, 44(4): 440-445. doi: 10.1016/j.procbio.2008.12.008 [54] LU J, SHI Y, JI Y, et al. Transformation of triclosan by laccase catalyzed oxidation: The influence of humic acid-metal binding process[J]. Environmental Pollution, 2017, 220: 1418-1423. doi: 10.1016/j.envpol.2016.10.092 [55] WAGNER M, NICELL J A. Impact of dissolved wastewater constituents on peroxidase‐catalyzed treatment of phenol[J]. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 2002, 77(4): 419-428. [56] BRZYSKA M, CIESZCZYK M, ŁOBARZEWSKI J. The effect of the simultaneous operation of two metal ions on soluble and immobilized peroxidase[J]. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental and Clean Technology, 1997, 68(1): 101-109. -




 DownLoad:
DownLoad: