-
21世纪以来,随着农业、医疗、工业的飞速发展,产生大量难降解的污染物。因环保意识和管理制度的欠缺,我国每年有80%以上的工业废水和生活污水未经完善的处理就直接排入河流[1]。随着我国对污染物处理标准的不断完善,污水处理厂的规模和标准也在不断扩大和提高,但传统的污水处理技术难以降解工业废水和特殊行业废水中的微量污染物。因此,研发高效的新型水处理技术具有重要意义。
电催化臭氧技术(electro-peroxone)耦合了O3和电化学技术,克服了传统臭氧氧化和电化学氧化对污染物降解的局限性,并有效利用了产O3过程中逸散的O2,通过两电子还原反应在阴极原位电产H2O2(式(1)),与产生的O3发生臭氧化反应生成HO•(式(2)),并利用体系中的强氧化性物质(HO•、O3)实现有机污染物的快速降解和矿化[2]。有研究[3]表明,在含氯和含溴废水中,电催化臭氧技术可以在有效提高污染物去除的同时,阴极产生的H2O2还可有效淬灭阳极地表水中氯离子和溴离子氧化产生的次氯酸盐和溴酸盐,从而抑制含氯和含溴副产物的生成。与传统臭氧氧化和电化学氧化相比,电催化臭氧工艺可显著提升对二次出水中TOC和COD的去除效果[4],消毒效果也高于臭氧和电化学的总和。外加添加MnO2和H2O2或电化学产生的H2O2可以不同程度地促进O3向HO•的转化[5]。
碳纳米管(carbon nanotubes,CNTs)具有特殊的中空管状结构,与其他多孔材料相比,具有更高的比表面积和电导率,可以有效降低电子传递的内阻[6]。蒽醌类物质常用于产H2O2,其自身的氧化还原能力可以促进分子氧的催化还原,但由于蒽醌的电导率较低,极大地限制了其应用[7]。有研究[8]表明,蒽醌类与碳材料掺杂可以提高H2O2的产量。由于碳纳米管电极吸附能力较强,长时间电解后表面会附着污染物,从而导致电极电催化效果降低。采用聚四氟乙烯作为电极粘合剂,可以提高电极的疏水性,克服电极长时间电解易被污染的问题[9]。
西玛津是一种三嗪类含氮芳香族除草剂,具有一定的毒性,可干扰生物体的内分泌系统和免疫神经系统,被广泛用于防除农林业杂草,在亚洲、欧洲、美洲和澳大利亚的自然环境中常被检测到[10]。传统的生物法或物化法对西玛津去除效率较低,且需要投加药剂。因此,本研究探究了电催化臭氧技术对西玛津的降解效果,以聚四氟乙烯为粘结剂制备碳纳米管/蒽醌修饰(CNT/TBAQ)浸没曝气电极,探究了电极的形貌特征和降解西玛津的性能,在此基础上,考察了电催氧化臭氧技术用于实际有机废水处理的可行性,以期为微污染物的高效减排提供一种有应用前景的技术。
-
主要试剂与材料:西玛津、聚四氟乙烯乳液、2-叔丁基蒽醌、草酸钛钾、无水硫酸钠购自上海麦克林生化科技公司,均为分析纯。多壁碳纳米管和炭黑购自苏州碳丰科技,镍网和铂片购自腾丰金属材料。
主要仪器:直流稳压稳流电源(DH1719A-5)、臭氧发生器(3S-A5)、臭氧检测器(UV-300B)、管式炉(OTF-1200X)。
-
CNT/TBAQ电极是以镍网为支撑层,炭黑(CB)为扩散层,碳纳米管(CNT)为催化层,制备方法如下:1)将镍网剪成2 cm×5 cm,分别在丙酮、乙醇和超纯水中超声10 min后烘干;2)称取0.18 g碳黑于50 mL的烧杯中,加入10 mL 无水乙醇,铝箔纸封口超声10 min,加入1 mL 60%(质量百分比)聚四氟乙烯乳液后封口超声20 min;将混合乳液置于电炉上80 ℃加热,玻璃棒不断搅拌成膏状,压成0.5 mm 厚的薄片贴在镍网一侧,为扩散层;3)称取0.20 g碳纳米管和4.0 mg 叔丁基蒽醌于50 mL的烧杯中,加入10 mL无水乙醇,铝箔纸封口超声10 min,加入113 μL 60%聚四氟乙烯乳液封口超声20 min;将混合乳液置于电炉上80 ℃加热,玻璃棒不断搅拌成膏状,压成0.5 mm厚的薄片贴在镍网的另一侧,为催化剂层;4)使用压片机,20 MPa下保持1 min将电极压成0.55 mm的薄片,放入管式炉内350 ℃下煅烧 60 min,自然降温得到CNT/TBAQ电极。
-
电催化臭氧技术降解西玛津实验采用通臭氧的圆形玻璃反应器完成,装置组成如图1所示,主要包括:玻璃反应器(内径7 cm,高 8.5 cm,反应液体积为200 mL)、铂片阳极、CNT/TBAQ阴极、磁力搅拌器、臭氧发生器、直流稳压稳流电源、臭氧检测器等。通过接通或断开直流稳压稳流电源和臭氧发生器即可实现单独臭氧技术、单独电化学技术、电催化臭氧技术的研究。
-
反应器进口臭氧质量浓度使用臭氧检测仪进行实时检测,西玛津质量浓度采用液相色谱仪检测,总有机碳(TOC)质量浓度采用岛津TOC分析仪检测,H2O2质量浓度采用草酸钛钾分光光度法测定,HO•采用对二苯甲酸(TA)捕获间接检测。西玛津降解中间体采用 Ultimate 3 000 UHPLC-Q Exactive 液质联用仪(LC-MS,美国 Thermo Scientific 公司)鉴定。
电催化氧化、臭氧氧化和电催化臭氧技术矿化污染物过程中的能量消耗(E)如式(3)~(5)所示,污染物降解过程中臭氧的消耗量根据式(6)计算。
式中:E为处理每千克目标污染物所消耗的能量,kWh·kg−1;V为反应液体积,0.2 L;I为反应电流,A;U为电压,V;t为反应时间,min;r为每千克O3生成所消耗的能量,15 kWh·kg−1 [8];C1为消耗的O3量,g;C0为溶液初始污染物质量浓度,mg·L−1;Ct是t时刻溶液的污染物质量浓度,mg·L−1。Qg为气体流量,L·min−1;C1与C2分别为O3进出口的质量浓度,mg·L−1。
-
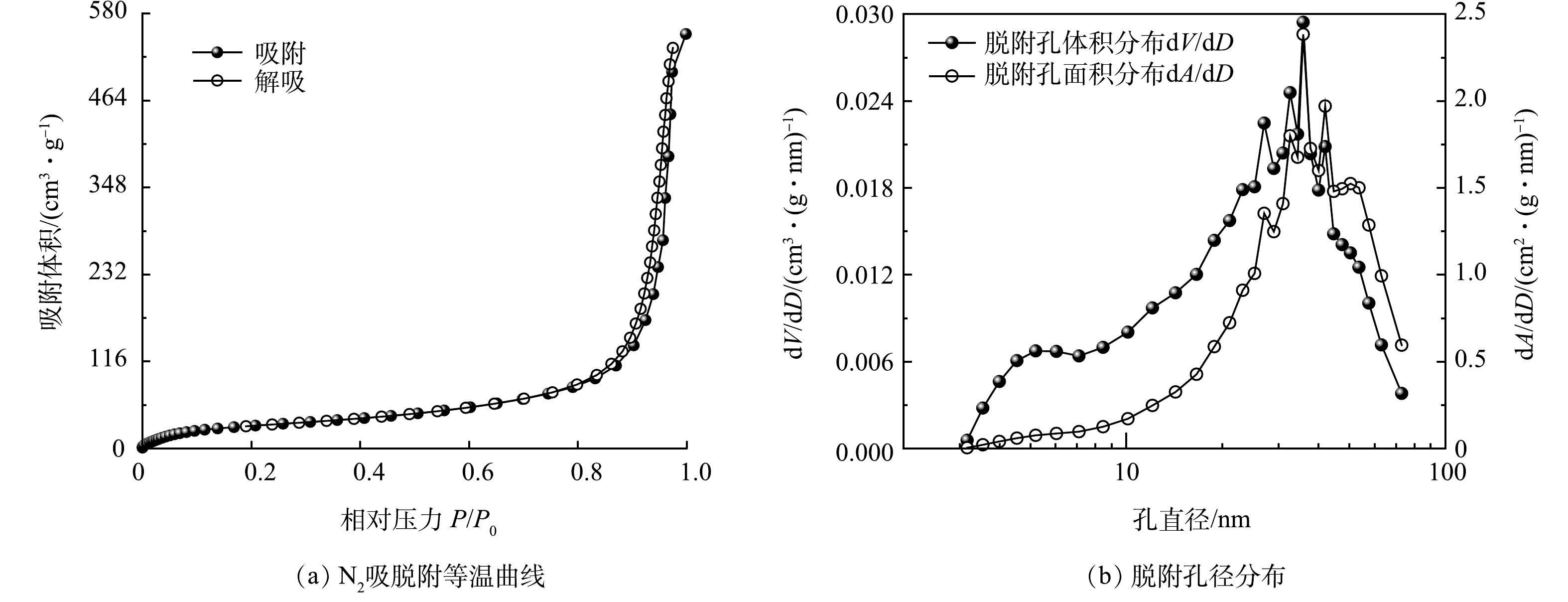
1)BET表面分析。对合成的CNT/TBAQ电极在120 ℃下进行吸附和解吸测试,并分析制备电极的表面积和吸附特性。如图2(a)所示,CNT/TBAQ的等温线属于具有滞后环的Ⅳ型等温线,在高压区出现毛细凝聚,吸脱附曲线不重合,产生吸附滞后现象,这与电极表面的介孔有关。根据计算结果,CNT/TBAQ电极单点总孔体积为0.897 4 cm3·g−1,平均孔半径为16.46 nm,相比于未改性的CNT(单点总孔体积为0.854 4 cm3·g−1和孔半径为14.89 nm)略有提升,具有更高的电容性能。但CNT/TBAQ电极的比表面积略有下降,改性后的电极比表面积从114.80 m2·g−1降至109.02 m2·g−1,这可能是由于聚合引起的孔应变。但CNT/TBAQ电极的孔径分布基本均在介孔尺寸范围内,这可以增加电极与电解质的界面面积,有利于增强电化学氧化还原反应。
2)表面形貌分析。高倍电子扫描显微镜下电极表面形貌如图3(a)~(b)所示。可见,未修饰的CNT表面管形清晰光滑但长度较短,是由于制备过程中的压制、煅烧等工序导致碳纳米管断裂。CBT/TBAQ电极表明粘附了大小不一的块状物,这表明蒽醌已经负载到碳纳米管的表面,而且经蒽醌修饰后的碳纳米管具有更好的柔性和可延展性,所以在压制、煅烧过程中不易断裂,保留着较长的碳纳米管[11]。由于碳纳米管自身的电阻比管间界面电阻小的多,因此,延长碳纳米管的长度可以有效减少电子在单位长度管道内跳跃传输,提高管间电荷传输效率[12]。电极的CB扩散层较为平整,不易发生电解液渗入问题(图3(c))。CNT/TBAQ电极剖面如图3(d)所示,可以清晰地看到电极由上而下分为催化层、支撑层和扩散层。
-
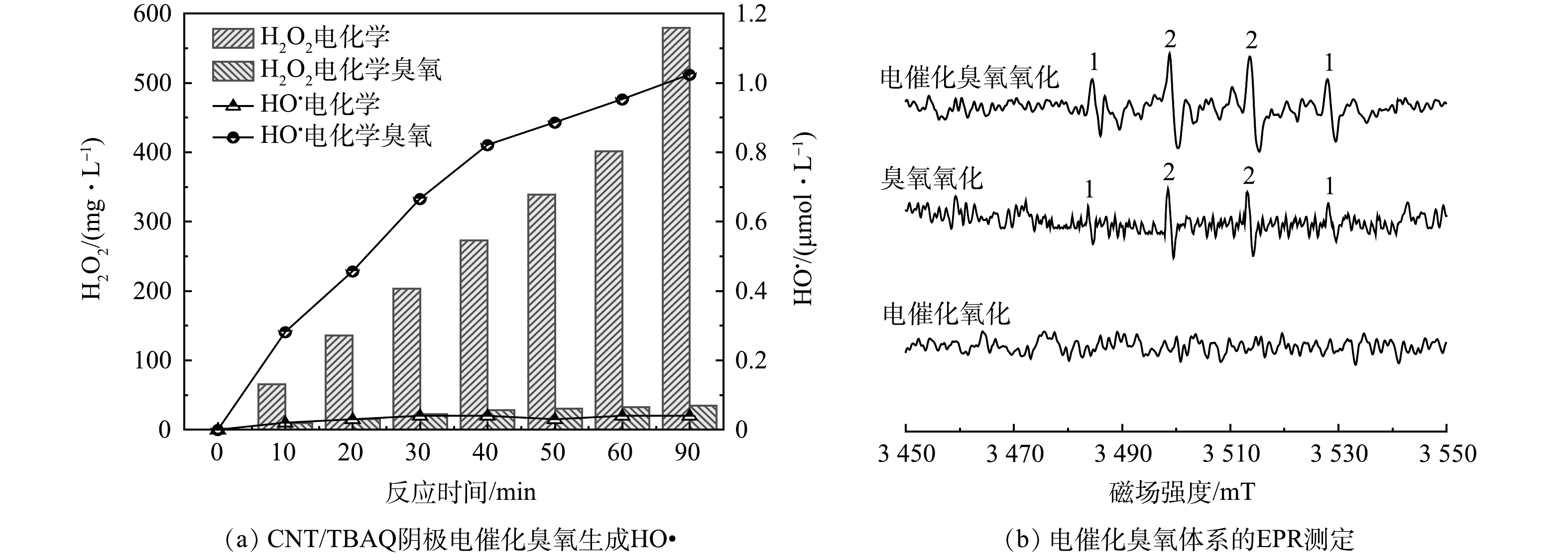
使用 CNT/TBAQ 电极作为阴极,测定了电催化臭氧系统产生HO•的能力。采用对苯二甲酸(TA)捕获电催化臭氧体系中产生的HO•,生成具有荧光特性的2-羟基对苯二甲酸(HTA),间接测试体系中HO•的产生量,结果如图4(a)所示。在电流密度为7.5 mA·cm−2下,向电解质溶液中仅通入氧气时,90 min后体系中H2O2的质量浓度达到了579.32 mg·L−1,表明 CNT/TBAQ 阴极还原 O2 有效地产生了 H2O2,此时溶液中几乎检测不到HTA。作为对比,向电解质溶液中通入混合的臭氧和氧气,90 min后电解质溶液中H2O2的质量浓度仅有34.82 mg·L−1,同时,能明显的检测到HTA的生成,溶液中的HO•浓度从 0.04 µmol·L−1 增加到 1.024 µmol·L−1,说明在电催化臭氧过程中通入臭氧后,CNT/TBAQ电极电还原产生的H2O2几乎被完全反应生成了HO• [13]。
采用电子顺磁波谱仪(EPR)直接测定溶液中的HO•的产生情况,在取样口取20 μL待测溶液,立即和10 μL 100 mmol·L−1的DMPO捕获剂混合均匀,放入样品管后插入EPR的共振腔中进行测定,测定结果如图4(b)所示。在单独电解条件下,几乎检测不到HO•的特征峰,即电解过程中几乎不产生HO•;在通入臭氧条件下的电催化臭氧系统中,能明显的检测到HO•的特征峰(峰强比为1:2:2:1),表明电催化臭氧过程中产生了较多的强氧化性的HO• [9];单独臭氧条件下,也会产生HO•,但是HO•的产生量较少。以上结果与TA捕获HO•的实验结果一致,表明电催化臭氧过程产生了大量HO•。
-
为确定电催化臭氧过程中污染物去除的机理,研究了以CNT/TBAQ为阴极的电化学技术、直接电解作用、臭氧技术和电催化臭氧技术对西玛津和TOC的去除效果。当反应电压为9 V,电流密度为7.5 mA·cm−2,臭氧质量浓度为10 mg·L−1,西玛津初始质量浓度为5 mg·L−1时,如图5(a)所示,电催化臭氧技术处理西玛津溶液6 min后,西玛津去除率达到99%,单独臭氧、单独电解和直接电解处理10 min后,西玛津的去除率分别为98%、46%和31.46%。图5(b)比较了3种技术对污染物的矿化效果,初始TOC质量浓度为1.326 mg·L−1,降解120 min后,电催化臭氧、单独臭氧和单独电解对TOC的去除率分别为62.25%、18.28%和9.04%。这一结果表明,结合了臭氧氧化技术和电化学氧化技术的电催化臭氧系统对快速降解废水中的西玛津并矿化具有显著的协同效应。产生这种协同效应的原因是,在电催化臭氧过程中引入的臭氧分子与阴极原位电化学生成的H2O2发生臭氧化反应(式(2)),生成的HO•可以快速与西玛津发生反应,从而提高污染物的矿化度[14]。通过式(3)~(6)计算了不同技术降解西玛津的能耗,可以看出,虽然臭氧氧化和电化学氧化的协同作用加快了西玛津的降解,但并没有降低污染物去除的能耗。但由于电催化臭氧技术极大地提高了西玛津的矿化程度,因此,电催化臭氧矿化能耗分别比电催化技术和臭氧技术分别降低了55%和31%。上述结果表明,以CNT/TBAQ为阴极在电催化臭氧系统在污染物矿化方面有很大的应用潜力。
从气相到液相的O3传质质量被证明是O3氧化过程中的限速步骤[15]。如图5(c)所示,当臭氧质量浓度由0增加到15 mg·L−1时,西玛津的去除率由45.28%增加到99.58%。根据传质理论,气相中O2、O3浓度的增加可以促进O2、O3从气相向液相的转化,从而提高液相的气体浓度和传质效率,加快O2发生双电子还原反应原位生成H2O2。H2O2在阴极迅速扩散并与不断鼓入的O3反应生成大量强氧化性HO•,而O3自身分解又会加快污染物的降解速度[16]。因此,从理论上讲,O3浓度越高,电催化臭氧技术处理污染物的能力就越强。但根据实际研究结果发现,当臭氧质量浓度从10 mg·L−1上升至15 mg·L−1时,西玛津的去除率并没有很大的提升,这可能是因为液相臭氧浓度已经达到饱和,臭氧与西玛津的反应达到了最大反应速率[17]。综合考虑污染物去除效率和能耗,臭氧质量浓度为10 mg·L−1时为最佳工况。
电流密度对电催化臭氧技术降解西玛津废水效果的影响如图5(d)所示,相比于臭氧浓度,电流密度对西玛津去除率的影响较小。当电流密度从2.5 mA·cm−2增加到7.5 mA·cm−2时,西玛津的去除率逐渐增加,反应速率也逐渐加快。当电流密度超过7.5 mA·cm−2时,西玛津去除率增幅很小。这是因为CNT/TBAQ具有较高的电化学H2O2生成活性,随着电流密度的增加,CNT/TBAQ电极产生的H2O2浓度达到与O3耦合的浓度极限[18]。此外,H2O2既是HO•的前体,也是HO•的捕获剂;随着电流密度的持续增加,H2O2的浓度将继续累积上升,过量的H2O2将捕获HO•,从而导致降解速度减慢[19]。
-
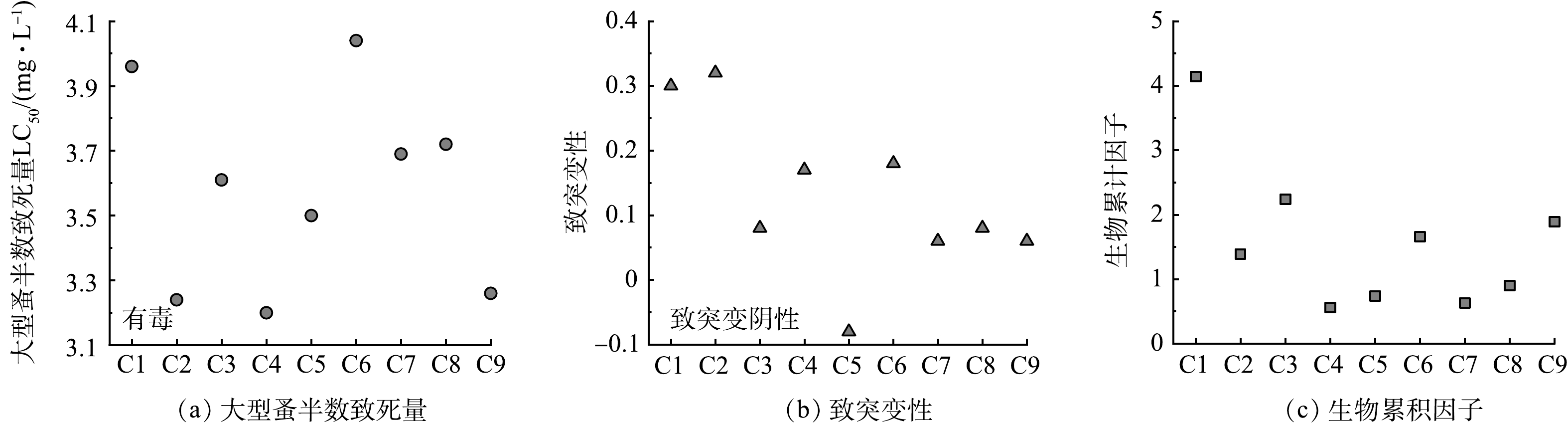
西玛津的降解和矿化速率证明电催化臭氧技术可明显提高污染物去除速率,但氧化反应过程中产生的中间体毒性也不可忽略。在先前的研究中已经给出了西玛津的降解路径,降解中间体如表1所示[8]。采用基于定量构效关系预测的T.E.S.T.软件对西玛津及其降解中间产物的大型蚤半数致死量(LC50)、致突变性和生物累积因子进行分析,评价结果如图6所示。
由图6可以看出,电催化臭氧体系降解西玛津时产生的中间体毒性变化不大,和原始西玛津(C1)毒性处于相近位置。图6(a)显示48 h大型蚤半数致死量LC50为3.96 mg·L−1,属于“有毒”(0~1属于非常毒,1~10属于有毒,10~100属于有害,100以上属于无害),随着降解的进行,其他中间体的毒性也维持在3.2~4.04 mg·L−1,急性毒性变化不大。如图6(b)所示,初始西玛津的致突变性属于阴性(大于0.5属于致突变阳性,小于0.5属于致突变阴性),但数值较高,经电催化臭氧技术降解后,没有中间体呈现阳性,而且大多数中间体的致突变性均有所降低。图6(c)显示的生物累计因子变化较大,由初始的4.14降至2以下,下降了46%~86%不等。由于本研究的毒性评价是定性分析,因此,西玛津降解的中间体毒性与原始毒性差距不大,说明使用电催化臭氧技术降解西玛津是可行的。而且根据2.3节矿化结果可知,该技术的TOC去除率可以达到60%以上,半数以上的有毒物质可被彻底矿化为CO2和H2O,表明该技术在降低污染物的毒性方面也有很广泛的应用前景。
-
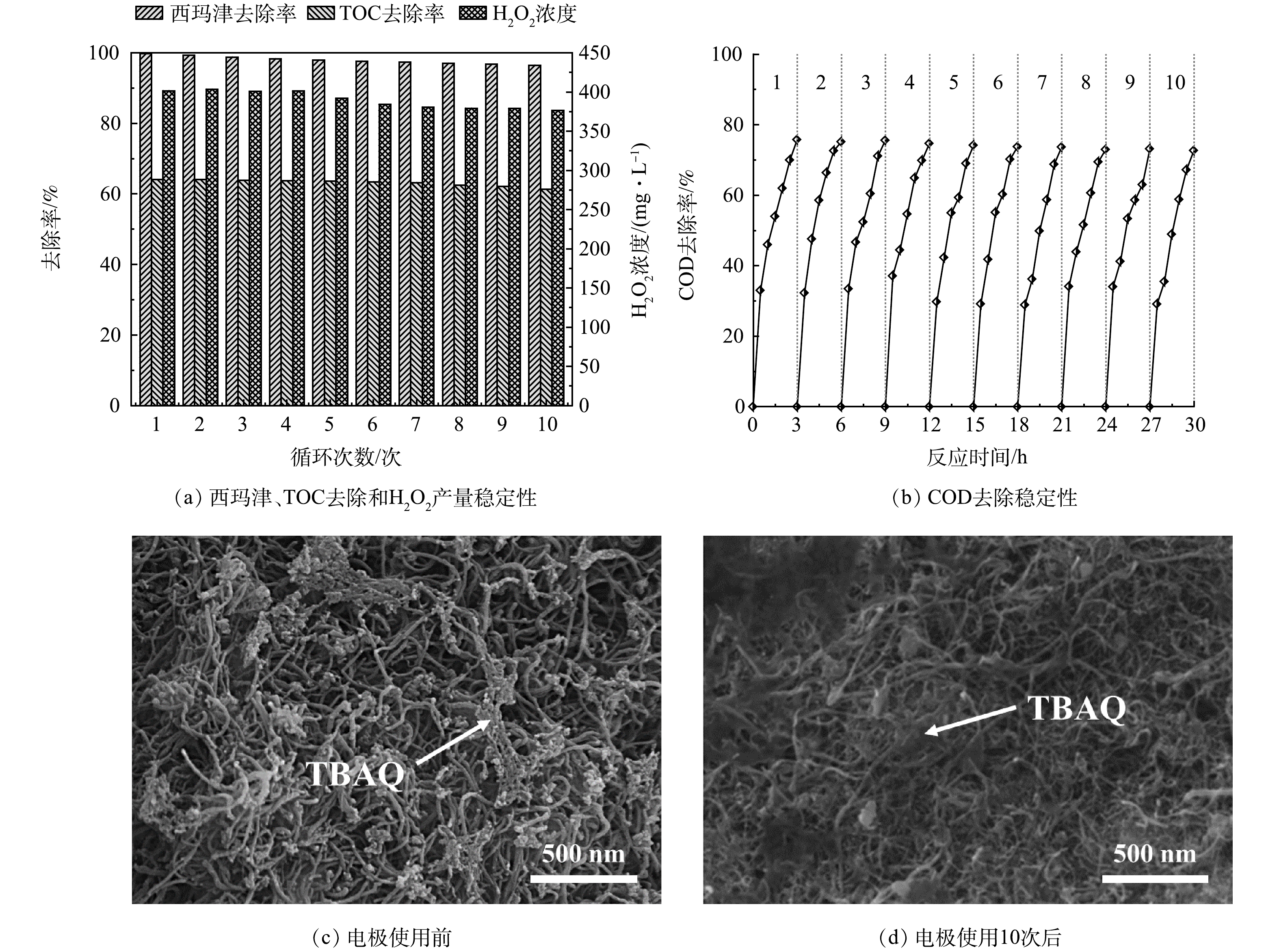
电极的稳定性是证明电极材料是否具有良好使用价值的重要指标,通过重复使用实验来评估电极的稳定性。图7(a)显示了电极经过10次循环后的污染物去除和产H2O2的效果。可以看出,电极的电化学性能基本稳定,H2O2的产率仅下降了约5.5%,西玛津的去除率下降了3.27%,TOC的去除率下降了2.75%。为了测试该系统在实际应用中的可行性,利用该系统降解了来自于西安某地喷洒农药后的农田地表水,原水COD值约为(785±79) mg·L−1。如图7(b)所示,180 min后废水中COD的去除率为76%,10次循环使用后(每次循环使用3h)COD的去除率下降了5.7%。由图7(c)~(d)可以看出,电极使用10次后,表面形貌基本没有变化。上述研究结果表明,CNT/TBAQ电极具有良好的电化学稳定性,在废水处理的实际应用中具有一定的潜力。
-
1)采用蒽醌修饰碳纳米管作为阴极的电催化臭氧技术,可以利用原位生成的H2O2与O3反应生成大量HO•,提高污染物去除和矿化效率。
2)臭氧浓度是决定以CNT/TBAQ为阴极的电催化臭氧体系降解污染物速率的主要因素,该反应体系可以实现西玛津的完全降解,其矿化能力分别是臭氧氧化和电催化氧化的3.39倍和6.88倍,能耗也有大幅下降。
3)电催化臭氧体系对西玛津的降解后产生的中间产物毒性有一定的降低,但并没有把生物致死量从有害降为无害。结合电催化臭氧体系对西玛津的矿化度为62%,该技术在降解高浓度有机废水方面有一定的应用前景。
碳纳米管电极强化电催化臭氧降解西玛津
Enhancement of electro-peroxone degradation of simazine by carbon nanotube electrodes
-
摘要: 传统的电催化技术受限于阴极原位电生H2O2的效率,且对某些特定结构污染物的降解能力较差。为提升电极对污染物降解性能和稳定性,使用压片法制备了蒽醌修饰碳纳米管(CNT/TBAQ)电极,构建了一种基于浸没电极的电催化臭氧反应器,并鉴定了反应体系内的活性物质及对西玛津的降解性能。结果表明,当气体流量为0.2 L·min−1,电流密度为7.5 mA·cm−2时,HO•生成量为1.024 µmol·L−1。与单独电催化和单独臭氧技术相比,电催化臭氧技术可以在6 min内完全去除初始质量浓度为5 mg·L−1的西玛津。当臭氧质量浓度10 mg·L−1,电流密度7.5 mA·cm−2时,电催化臭氧技术的矿化效率最高,120 min后TOC去除率为62.25%,相比于电催化氧化、臭氧氧化能耗分别下降了55%和31%,但电催化臭氧技术没有明显降低西玛津中间产物的毒性。经过10次循环使用后,CNT/TBAQ 阴极仍然保持对污染物的去除能力。以上结果表明,以CNT/TBAQ电极为阴极的电催化臭氧技术可以有效提高污染物降解效率,为微量污染物去除提供了一种有前景的技术。Abstract: Conventional electrocatalytic technology is limited by the efficiency of in-situ electrogenerated H2O2 over the cathode, and poor degradability for some pollutants with specific structures. In order to increase the pollutant degradation performance and the stability of electrodes, a self-made anthraquinone-modified carbon nanotube (CNT/TBAQ) electrode was taken as the cathode, and an electro-peroxone reactor based on the submerged and aerated electrode was constructed, which performance on the degradation of simazine by the active substances in the electro-peroxone system was studied. The results showed that the HO· generation was 1.024 µmol·L−1 at the gas flow rate of 0.2 L·min−1 and the current density of 7.5 mA·cm−2. Compared with the technology of electrocatalysis or ozone alone, the electro-peroxone technology could completely remove simazine with an initial concentration of 5 mg·L−1 within 6 min. When the O3 concentration was 10 mg·L−1 and the current intensity was 7.5 mA·cm−2, the mineralization efficiency of electro-peroxone technology was the highest, with the TOC removal rate of 62.25% after 120 min, and the energy consumption was reduced by 55% and 31% compared with electro-catalytic oxidation and ozonation, respectively. However, the electro-peroxone technology did not significantly reduce the toxicity of simazine intermediates. The cathode of the CNT/TBAQ still retained the pollutant removal capacity after ten cycles of use. These results showed that electro-peroxone technology using CNT/TBAQ electrode as the cathode could effectively increase the pollutant degradation efficiency, and provide a promising technology for the removal of trace pollutants.
-
Key words:
- anthraquinone /
- pesticide /
- hydroxyl radical /
- bio-toxicity /
- electro-peroxone
-
21世纪以来,随着农业、医疗、工业的飞速发展,产生大量难降解的污染物。因环保意识和管理制度的欠缺,我国每年有80%以上的工业废水和生活污水未经完善的处理就直接排入河流[1]。随着我国对污染物处理标准的不断完善,污水处理厂的规模和标准也在不断扩大和提高,但传统的污水处理技术难以降解工业废水和特殊行业废水中的微量污染物。因此,研发高效的新型水处理技术具有重要意义。
电催化臭氧技术(electro-peroxone)耦合了O3和电化学技术,克服了传统臭氧氧化和电化学氧化对污染物降解的局限性,并有效利用了产O3过程中逸散的O2,通过两电子还原反应在阴极原位电产H2O2(式(1)),与产生的O3发生臭氧化反应生成HO•(式(2)),并利用体系中的强氧化性物质(HO•、O3)实现有机污染物的快速降解和矿化[2]。有研究[3]表明,在含氯和含溴废水中,电催化臭氧技术可以在有效提高污染物去除的同时,阴极产生的H2O2还可有效淬灭阳极地表水中氯离子和溴离子氧化产生的次氯酸盐和溴酸盐,从而抑制含氯和含溴副产物的生成。与传统臭氧氧化和电化学氧化相比,电催化臭氧工艺可显著提升对二次出水中TOC和COD的去除效果[4],消毒效果也高于臭氧和电化学的总和。外加添加MnO2和H2O2或电化学产生的H2O2可以不同程度地促进O3向HO•的转化[5]。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) 碳纳米管(carbon nanotubes,CNTs)具有特殊的中空管状结构,与其他多孔材料相比,具有更高的比表面积和电导率,可以有效降低电子传递的内阻[6]。蒽醌类物质常用于产H2O2,其自身的氧化还原能力可以促进分子氧的催化还原,但由于蒽醌的电导率较低,极大地限制了其应用[7]。有研究[8]表明,蒽醌类与碳材料掺杂可以提高H2O2的产量。由于碳纳米管电极吸附能力较强,长时间电解后表面会附着污染物,从而导致电极电催化效果降低。采用聚四氟乙烯作为电极粘合剂,可以提高电极的疏水性,克服电极长时间电解易被污染的问题[9]。
西玛津是一种三嗪类含氮芳香族除草剂,具有一定的毒性,可干扰生物体的内分泌系统和免疫神经系统,被广泛用于防除农林业杂草,在亚洲、欧洲、美洲和澳大利亚的自然环境中常被检测到[10]。传统的生物法或物化法对西玛津去除效率较低,且需要投加药剂。因此,本研究探究了电催化臭氧技术对西玛津的降解效果,以聚四氟乙烯为粘结剂制备碳纳米管/蒽醌修饰(CNT/TBAQ)浸没曝气电极,探究了电极的形貌特征和降解西玛津的性能,在此基础上,考察了电催氧化臭氧技术用于实际有机废水处理的可行性,以期为微污染物的高效减排提供一种有应用前景的技术。
1. 材料与方法
1.1 试剂与材料
主要试剂与材料:西玛津、聚四氟乙烯乳液、2-叔丁基蒽醌、草酸钛钾、无水硫酸钠购自上海麦克林生化科技公司,均为分析纯。多壁碳纳米管和炭黑购自苏州碳丰科技,镍网和铂片购自腾丰金属材料。
主要仪器:直流稳压稳流电源(DH1719A-5)、臭氧发生器(3S-A5)、臭氧检测器(UV-300B)、管式炉(OTF-1200X)。
1.2 电极制备
CNT/TBAQ电极是以镍网为支撑层,炭黑(CB)为扩散层,碳纳米管(CNT)为催化层,制备方法如下:1)将镍网剪成2 cm×5 cm,分别在丙酮、乙醇和超纯水中超声10 min后烘干;2)称取0.18 g碳黑于50 mL的烧杯中,加入10 mL 无水乙醇,铝箔纸封口超声10 min,加入1 mL 60%(质量百分比)聚四氟乙烯乳液后封口超声20 min;将混合乳液置于电炉上80 ℃加热,玻璃棒不断搅拌成膏状,压成0.5 mm 厚的薄片贴在镍网一侧,为扩散层;3)称取0.20 g碳纳米管和4.0 mg 叔丁基蒽醌于50 mL的烧杯中,加入10 mL无水乙醇,铝箔纸封口超声10 min,加入113 μL 60%聚四氟乙烯乳液封口超声20 min;将混合乳液置于电炉上80 ℃加热,玻璃棒不断搅拌成膏状,压成0.5 mm厚的薄片贴在镍网的另一侧,为催化剂层;4)使用压片机,20 MPa下保持1 min将电极压成0.55 mm的薄片,放入管式炉内350 ℃下煅烧 60 min,自然降温得到CNT/TBAQ电极。
1.3 电催化臭氧体系构建
电催化臭氧技术降解西玛津实验采用通臭氧的圆形玻璃反应器完成,装置组成如图1所示,主要包括:玻璃反应器(内径7 cm,高 8.5 cm,反应液体积为200 mL)、铂片阳极、CNT/TBAQ阴极、磁力搅拌器、臭氧发生器、直流稳压稳流电源、臭氧检测器等。通过接通或断开直流稳压稳流电源和臭氧发生器即可实现单独臭氧技术、单独电化学技术、电催化臭氧技术的研究。
1.4 分析方法
反应器进口臭氧质量浓度使用臭氧检测仪进行实时检测,西玛津质量浓度采用液相色谱仪检测,总有机碳(TOC)质量浓度采用岛津TOC分析仪检测,H2O2质量浓度采用草酸钛钾分光光度法测定,HO•采用对二苯甲酸(TA)捕获间接检测。西玛津降解中间体采用 Ultimate 3 000 UHPLC-Q Exactive 液质联用仪(LC-MS,美国 Thermo Scientific 公司)鉴定。
电催化氧化、臭氧氧化和电催化臭氧技术矿化污染物过程中的能量消耗(E)如式(3)~(5)所示,污染物降解过程中臭氧的消耗量根据式(6)计算。
stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) stringUtils.convertMath(!{formula.content}) (6) 式中:E为处理每千克目标污染物所消耗的能量,kWh·kg−1;V为反应液体积,0.2 L;I为反应电流,A;U为电压,V;t为反应时间,min;r为每千克O3生成所消耗的能量,15 kWh·kg−1 [8];C1为消耗的O3量,g;C0为溶液初始污染物质量浓度,mg·L−1;Ct是t时刻溶液的污染物质量浓度,mg·L−1。Qg为气体流量,L·min−1;C1与C2分别为O3进出口的质量浓度,mg·L−1。
2. 结果与讨论
2.1 CNT/TBAQ电极性能
1)BET表面分析。对合成的CNT/TBAQ电极在120 ℃下进行吸附和解吸测试,并分析制备电极的表面积和吸附特性。如图2(a)所示,CNT/TBAQ的等温线属于具有滞后环的Ⅳ型等温线,在高压区出现毛细凝聚,吸脱附曲线不重合,产生吸附滞后现象,这与电极表面的介孔有关。根据计算结果,CNT/TBAQ电极单点总孔体积为0.897 4 cm3·g−1,平均孔半径为16.46 nm,相比于未改性的CNT(单点总孔体积为0.854 4 cm3·g−1和孔半径为14.89 nm)略有提升,具有更高的电容性能。但CNT/TBAQ电极的比表面积略有下降,改性后的电极比表面积从114.80 m2·g−1降至109.02 m2·g−1,这可能是由于聚合引起的孔应变。但CNT/TBAQ电极的孔径分布基本均在介孔尺寸范围内,这可以增加电极与电解质的界面面积,有利于增强电化学氧化还原反应。
2)表面形貌分析。高倍电子扫描显微镜下电极表面形貌如图3(a)~(b)所示。可见,未修饰的CNT表面管形清晰光滑但长度较短,是由于制备过程中的压制、煅烧等工序导致碳纳米管断裂。CBT/TBAQ电极表明粘附了大小不一的块状物,这表明蒽醌已经负载到碳纳米管的表面,而且经蒽醌修饰后的碳纳米管具有更好的柔性和可延展性,所以在压制、煅烧过程中不易断裂,保留着较长的碳纳米管[11]。由于碳纳米管自身的电阻比管间界面电阻小的多,因此,延长碳纳米管的长度可以有效减少电子在单位长度管道内跳跃传输,提高管间电荷传输效率[12]。电极的CB扩散层较为平整,不易发生电解液渗入问题(图3(c))。CNT/TBAQ电极剖面如图3(d)所示,可以清晰地看到电极由上而下分为催化层、支撑层和扩散层。
2.2 电催化臭氧产HO•性能
使用 CNT/TBAQ 电极作为阴极,测定了电催化臭氧系统产生HO•的能力。采用对苯二甲酸(TA)捕获电催化臭氧体系中产生的HO•,生成具有荧光特性的2-羟基对苯二甲酸(HTA),间接测试体系中HO•的产生量,结果如图4(a)所示。在电流密度为7.5 mA·cm−2下,向电解质溶液中仅通入氧气时,90 min后体系中H2O2的质量浓度达到了579.32 mg·L−1,表明 CNT/TBAQ 阴极还原 O2 有效地产生了 H2O2,此时溶液中几乎检测不到HTA。作为对比,向电解质溶液中通入混合的臭氧和氧气,90 min后电解质溶液中H2O2的质量浓度仅有34.82 mg·L−1,同时,能明显的检测到HTA的生成,溶液中的HO•浓度从 0.04 µmol·L−1 增加到 1.024 µmol·L−1,说明在电催化臭氧过程中通入臭氧后,CNT/TBAQ电极电还原产生的H2O2几乎被完全反应生成了HO• [13]。
采用电子顺磁波谱仪(EPR)直接测定溶液中的HO•的产生情况,在取样口取20 μL待测溶液,立即和10 μL 100 mmol·L−1的DMPO捕获剂混合均匀,放入样品管后插入EPR的共振腔中进行测定,测定结果如图4(b)所示。在单独电解条件下,几乎检测不到HO•的特征峰,即电解过程中几乎不产生HO•;在通入臭氧条件下的电催化臭氧系统中,能明显的检测到HO•的特征峰(峰强比为1:2:2:1),表明电催化臭氧过程中产生了较多的强氧化性的HO• [9];单独臭氧条件下,也会产生HO•,但是HO•的产生量较少。以上结果与TA捕获HO•的实验结果一致,表明电催化臭氧过程产生了大量HO•。
2.3 降解性能
为确定电催化臭氧过程中污染物去除的机理,研究了以CNT/TBAQ为阴极的电化学技术、直接电解作用、臭氧技术和电催化臭氧技术对西玛津和TOC的去除效果。当反应电压为9 V,电流密度为7.5 mA·cm−2,臭氧质量浓度为10 mg·L−1,西玛津初始质量浓度为5 mg·L−1时,如图5(a)所示,电催化臭氧技术处理西玛津溶液6 min后,西玛津去除率达到99%,单独臭氧、单独电解和直接电解处理10 min后,西玛津的去除率分别为98%、46%和31.46%。图5(b)比较了3种技术对污染物的矿化效果,初始TOC质量浓度为1.326 mg·L−1,降解120 min后,电催化臭氧、单独臭氧和单独电解对TOC的去除率分别为62.25%、18.28%和9.04%。这一结果表明,结合了臭氧氧化技术和电化学氧化技术的电催化臭氧系统对快速降解废水中的西玛津并矿化具有显著的协同效应。产生这种协同效应的原因是,在电催化臭氧过程中引入的臭氧分子与阴极原位电化学生成的H2O2发生臭氧化反应(式(2)),生成的HO•可以快速与西玛津发生反应,从而提高污染物的矿化度[14]。通过式(3)~(6)计算了不同技术降解西玛津的能耗,可以看出,虽然臭氧氧化和电化学氧化的协同作用加快了西玛津的降解,但并没有降低污染物去除的能耗。但由于电催化臭氧技术极大地提高了西玛津的矿化程度,因此,电催化臭氧矿化能耗分别比电催化技术和臭氧技术分别降低了55%和31%。上述结果表明,以CNT/TBAQ为阴极在电催化臭氧系统在污染物矿化方面有很大的应用潜力。
从气相到液相的O3传质质量被证明是O3氧化过程中的限速步骤[15]。如图5(c)所示,当臭氧质量浓度由0增加到15 mg·L−1时,西玛津的去除率由45.28%增加到99.58%。根据传质理论,气相中O2、O3浓度的增加可以促进O2、O3从气相向液相的转化,从而提高液相的气体浓度和传质效率,加快O2发生双电子还原反应原位生成H2O2。H2O2在阴极迅速扩散并与不断鼓入的O3反应生成大量强氧化性HO•,而O3自身分解又会加快污染物的降解速度[16]。因此,从理论上讲,O3浓度越高,电催化臭氧技术处理污染物的能力就越强。但根据实际研究结果发现,当臭氧质量浓度从10 mg·L−1上升至15 mg·L−1时,西玛津的去除率并没有很大的提升,这可能是因为液相臭氧浓度已经达到饱和,臭氧与西玛津的反应达到了最大反应速率[17]。综合考虑污染物去除效率和能耗,臭氧质量浓度为10 mg·L−1时为最佳工况。
电流密度对电催化臭氧技术降解西玛津废水效果的影响如图5(d)所示,相比于臭氧浓度,电流密度对西玛津去除率的影响较小。当电流密度从2.5 mA·cm−2增加到7.5 mA·cm−2时,西玛津的去除率逐渐增加,反应速率也逐渐加快。当电流密度超过7.5 mA·cm−2时,西玛津去除率增幅很小。这是因为CNT/TBAQ具有较高的电化学H2O2生成活性,随着电流密度的增加,CNT/TBAQ电极产生的H2O2浓度达到与O3耦合的浓度极限[18]。此外,H2O2既是HO•的前体,也是HO•的捕获剂;随着电流密度的持续增加,H2O2的浓度将继续累积上升,过量的H2O2将捕获HO•,从而导致降解速度减慢[19]。
2.4 降解中间产物毒性分析
西玛津的降解和矿化速率证明电催化臭氧技术可明显提高污染物去除速率,但氧化反应过程中产生的中间体毒性也不可忽略。在先前的研究中已经给出了西玛津的降解路径,降解中间体如表1所示[8]。采用基于定量构效关系预测的T.E.S.T.软件对西玛津及其降解中间产物的大型蚤半数致死量(LC50)、致突变性和生物累积因子进行分析,评价结果如图6所示。
表 1 电催化臭氧体系降解西玛津的中间产物Table 1. Intermediate products of simazine in the electro-peroxone system序号 分子式 结构式 序号 分子式 结构式 C1 C7H12ClN5 
C2 C7H10ClN5O 
C3 C6H10N5COOH 
C4 C5H8N5Cl 
C5 C5H9N5OH 
C6 C3H4N5Cl 
C7 C3H4N5OH 
C8 C3H2N4(OH)2 
C9 C3N3(OH)3 
由图6可以看出,电催化臭氧体系降解西玛津时产生的中间体毒性变化不大,和原始西玛津(C1)毒性处于相近位置。图6(a)显示48 h大型蚤半数致死量LC50为3.96 mg·L−1,属于“有毒”(0~1属于非常毒,1~10属于有毒,10~100属于有害,100以上属于无害),随着降解的进行,其他中间体的毒性也维持在3.2~4.04 mg·L−1,急性毒性变化不大。如图6(b)所示,初始西玛津的致突变性属于阴性(大于0.5属于致突变阳性,小于0.5属于致突变阴性),但数值较高,经电催化臭氧技术降解后,没有中间体呈现阳性,而且大多数中间体的致突变性均有所降低。图6(c)显示的生物累计因子变化较大,由初始的4.14降至2以下,下降了46%~86%不等。由于本研究的毒性评价是定性分析,因此,西玛津降解的中间体毒性与原始毒性差距不大,说明使用电催化臭氧技术降解西玛津是可行的。而且根据2.3节矿化结果可知,该技术的TOC去除率可以达到60%以上,半数以上的有毒物质可被彻底矿化为CO2和H2O,表明该技术在降低污染物的毒性方面也有很广泛的应用前景。
2.5 电极稳定性
电极的稳定性是证明电极材料是否具有良好使用价值的重要指标,通过重复使用实验来评估电极的稳定性。图7(a)显示了电极经过10次循环后的污染物去除和产H2O2的效果。可以看出,电极的电化学性能基本稳定,H2O2的产率仅下降了约5.5%,西玛津的去除率下降了3.27%,TOC的去除率下降了2.75%。为了测试该系统在实际应用中的可行性,利用该系统降解了来自于西安某地喷洒农药后的农田地表水,原水COD值约为(785±79) mg·L−1。如图7(b)所示,180 min后废水中COD的去除率为76%,10次循环使用后(每次循环使用3h)COD的去除率下降了5.7%。由图7(c)~(d)可以看出,电极使用10次后,表面形貌基本没有变化。上述研究结果表明,CNT/TBAQ电极具有良好的电化学稳定性,在废水处理的实际应用中具有一定的潜力。
3. 结论
1)采用蒽醌修饰碳纳米管作为阴极的电催化臭氧技术,可以利用原位生成的H2O2与O3反应生成大量HO•,提高污染物去除和矿化效率。
2)臭氧浓度是决定以CNT/TBAQ为阴极的电催化臭氧体系降解污染物速率的主要因素,该反应体系可以实现西玛津的完全降解,其矿化能力分别是臭氧氧化和电催化氧化的3.39倍和6.88倍,能耗也有大幅下降。
3)电催化臭氧体系对西玛津的降解后产生的中间产物毒性有一定的降低,但并没有把生物致死量从有害降为无害。结合电催化臭氧体系对西玛津的矿化度为62%,该技术在降解高浓度有机废水方面有一定的应用前景。
-
表 1 电催化臭氧体系降解西玛津的中间产物
Table 1. Intermediate products of simazine in the electro-peroxone system
序号 分子式 结构式 序号 分子式 结构式 C1 C7H12ClN5 C2 C7H10ClN5O C3 C6H10N5COOH C4 C5H8N5Cl C5 C5H9N5OH C6 C3H4N5Cl C7 C3H4N5OH C8 C3H2N4(OH)2 C9 C3N3(OH)3 -
[1] 高荣伟. 我国水资源污染现状及对策分析[J]. 资源与人居环境, 2018(11): 44-51. doi: 10.3969/j.issn.1672-822X.2018.11.009 [2] JIANG Y, NI P, CHEN C, et al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Advanced Energy Materials, 2018, 8(31): 1801909. doi: 10.1002/aenm.201801909 [3] YAO W, FU J, YANG H, et al. The beneficial effect of cathodic hydrogen peroxide generation on mitigating chlorinated by-product formation during water treatment by an electro-peroxone process[J]. Water Research, 2019, 157: 209-217. doi: 10.1016/j.watres.2019.03.049 [4] ZHANG Y, ZUO S, ZHANG Y, et al. Simultaneous removal of tetracycline and disinfection by a flow-through electro-peroxone process for reclamation from municipal secondary effluent[J]. Journal of Hazardous Materials, 2019, 368: 771-777. doi: 10.1016/j.jhazmat.2019.02.005 [5] GUO Y, ZHAO E, WANG J, et al. Comparison of emerging contaminant abatement by conventional ozonation, catalytic ozonation, O3/H2O2 and electro-peroxone processes[J]. Journal of Hazardous Materials, 2020, 389: 121829. doi: 10.1016/j.jhazmat.2019.121829 [6] LU Z, CHEN G, SIAHROSTAMI S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis, 2018, 1(2): 156-162. doi: 10.1038/s41929-017-0017-x [7] GAO Y, ZHU W, WANG C, et al. Enhancement of oxygen reduction on a newly fabricated cathode and its application in the electro-Fenton process[J]. Electrochimica Acta, 2020, 330: 135206. doi: 10.1016/j.electacta.2019.135206 [8] VALIM R B, REIS R M, CASTRO P S, et al. Electrogeneration of hydrogen peroxide in gas diffusion electrodes modified with tert-butyl-anthraquinone on carbon black support[J]. Carbon, 2013, 61: 236-244. doi: 10.1016/j.carbon.2013.04.100 [9] ZHAO Q, AN J, WANG S, et al. Superhydrophobic air-breathing cathode for efficient hydrogen peroxide generation through two-electron pathway oxygen reduction reaction[J]. ACS Applied Materials & Interfaces, 2019, 11(38): 35410-35419. [10] WANG Z, ZHU W, XU Y, et al. Effects of simazine and food deprivation chronic stress on energy allocation among the costly physiological processes of male lizards (Eremias argus)[J]. Environmental Pollution, 2021, 269: 116139. doi: 10.1016/j.envpol.2020.116139 [11] 王健, 胡稳茂, 王庚超. 功能化碳纳米管膜负载聚(2, 5-二羟基-羟, 4-苯醌硫)柔性电极的制备及在柔性非对称超级电容器中的应用[J]. 功能高分子学报, 2019, 32(2): 184-191. [12] WU K, ZHANG Y, YONG Z, et al. Continuous preparation and performance enhancement techniques of carbon nanotube fibers[J]. Acta Physico-Chimica Sinica, 2022, 38: 2106034. [13] 李新洋, 李燕楠, 祁丹阳, 等. 电-多相臭氧催化工艺深度处理焦化废水[J]. 中国环境科学, 2020, 40(10): 4354-4361. doi: 10.3969/j.issn.1000-6923.2020.10.020 [14] KATSOYIANNIS I A, CANONICA S, VON GUNTEN U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2[J]. Water Research, 2011, 45(13): 3811-3822. doi: 10.1016/j.watres.2011.04.038 [15] CORTEZ S, TEIXEIRA P, OLIVEIRA R, et al. Ozonation as polishing treatment of mature landfill leachate[J]. Journal of Hazardous Materials, 2010, 182(1/2/3): 730-734. [16] 朱瑾, 汪文强, 季献华, 等. 原位产H2O2催化臭氧饮用水深度处理中试[J]. 净水技术, 2023, 42(9): 74-79. [17] BAKHEET B, YUAN S, LI Z, et al. Electro-peroxone treatment of Orange II dye wastewater[J]. Water Research, 2013, 47(16): 6234-6243. doi: 10.1016/j.watres.2013.07.042 [18] YUAN S, LI Z, WANG Y. Effective degradation of methylene blue by a novel electrochemically driven process[J]. Electrochemistry Communications, 2013, 29: 48-51. doi: 10.1016/j.elecom.2013.01.012 [19] YAO W, WANG X, YANG H, et al. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process[J]. Water Research, 2016, 88: 826-835. doi: 10.1016/j.watres.2015.11.024 -





 下载:
下载: