-
伴随着我国城镇化进程的加快,市政污水的处理需求在快速上升。由于我国现代化建设起步较晚,污水排放管网建设多采用合流制[1],致使管道除了承接生活污水之外,还容纳了工业废水及雨水等[2]。当前我国市政污水总体呈现出有机污染物含量低、水质水量变化大、污染物成分复杂等特点[3],导致在污水处理中,生物脱氮的实际效果不佳,出水水质不达标。污水中的大量氮源若不能达到排放标准就进入自然水体,势必加剧河道和湖泊水体的富营养化[4]。因此,解决低碳氮比市政污水含碳量不足的问题,是提高市政污水处理高效脱氮的重要方向。
常规的生物脱氮主要由氨化作用、硝化作用和反硝化作用3个部分完成。根据氮的存在形态,功能微生物菌群在特定微环境下相互协作,共同完成脱氮过程[5]。当前生物脱氮多采用悬浮生长系统为主的缺氧/好氧、厌氧/缺氧/好氧、序批式活性污泥法、氧化沟等传统工艺。但这些传统生物脱氮工艺存在多种缺点,例如:因活性污泥在水中的形态多为絮体状,导致反应装置的水力停留时间和污泥停留时间难以控制;污泥内的自养菌生长周期长,易随出水流出;活性污泥抗环境胁迫能力较差等[6-8]。
为克服上述缺点,以填料为载体的悬浮生长系统正在被广泛采用。其中代表性工艺包括生物接触氧化、生物转盘、及生物滤池等[6,9]。不过,常规填料上附着的活性生物膜厚度一般不超过2 mm。受传质阻力和水力冲击的作用,生物膜较易脱落,因而影响水处理效能。近来有研究在单一反应器中引入改性玄武岩纤维(modified basalt fiber, MBF)做填料。这种新型生物载体可快速吸附活性污泥,形成一种有别于传统生物膜的微生物聚集体(生物巢)[10]。通常,生物巢尺寸可达10 cm以上。其生物量虽然巨大,但并不影响传质和生物活性,因而可实现不同类型污/废水的高效脱氮除碳[11]。相对于传统填料,新型改性玄武岩纤维更适合做生物载体。其能够富集大量的功能微生物菌群,尤其是生长缓慢的氨氧化细菌、亚硝酸盐氧化菌以及异养硝化-好氧反硝化(heterotrophic nitrifying aerobic denitrifying, HN-AD)菌等[12-13]。尽管从材料改性的角度可以优化功能微生物的富集,但功能微生物的生长通常受到生理条件如C/N比的限制。在传统的生物脱氮过程中,生物反硝化需要大量的有机碳源作为电子供体。而我国的市政污水的碳氮比普遍较低,导致反硝化菌生长受到抑制。为了降低出水中含氮污染物的浓度,现有工艺通常通过外加碳源来提高污水的碳氮比。不过,使用外加碳源一方面会增加污水厂的运行成本,另一方面由于实际废水进水的水质水量具有较大的波动性,碳源投加量难以精确控制[14]。
在此背景下,解决市政污水中碳源有效利用的问题就成了当前污水处理领域的研究热点之一。考虑到在偏中性和碱性环境中NO3−/NO2−电对的氧化还原电位高于Fe(II)/Fe(III),研究人员尝试在复杂菌群中投加铁氧化物介导种间电子传递,以实现有机底物氧化与硝酸盐还原的耦合。纳米级铁氧化物如赤铁矿(α-Fe2O3)、磁赤铁矿(γ-Fe2O3)、磁铁矿(Fe3O4)作为半导体和导体材料具有良好的生物亲和性。微生物可以利用这些纳米材料作为电子介体实现电子传递[15]。据报道Geobacter sulfurreducens可以氧化乙酸盐但不能还原NO3−,而Thiobacillus denitrificans能够还原NO3−但不能氧化乙酸盐。两者的共培养并不能实现有机物氧化和硝酸盐还原的耦合发生,但当添加α-Fe2O3或Fe3O4纳米颗粒时共培养体系能成功实现共代谢[16]。此外,研究还发现氢自养反硝化菌的外膜细胞色素对α-Fe2O3具有高度亲和性,借助α-Fe2O3可加速电子向胞内硝酸盐还原酶的传递,从而促进反硝化的进行[17]。因此,铁氧化物介导的种间电子传递为菌群共代谢提供了一种有效途径[18]。此外,对于一些具有跨膜电子转移能力的反硝化菌,进入细胞内部的α-Fe2O3有可能在周质空间直接介导NADH(还原性辅酶I)等电子供体与硝酸盐还原酶之间的电子传递。因此,这种胞内电子传递促进机制也为碳源有效利用提供了新的途径。但是,目前尚未有关于投加铁氧化物以解决市政污水碳源不足问题的研究报道。
为此,本研究选用MBF作为生物载体,在生物接触氧化池中培养生物巢,借助于生物巢独特的好氧-缺氧-厌氧微环境,富集各种与氮转化相关的功能微生物。同时,在池体中投加纳米氧化铁(α-Fe2O3),考察其介导种间或胞内电子转移机制对脱氮效能的刺激作用,并解析氮转化的代谢通路。本研究旨在探索α-Fe2O3介导下碳源有效利用的可行性及潜在的机制,为提高低碳氮比市政污水的高效脱氮提供一种新的策略。
-
本实验所选用的种泥均采自镇江市某污水处理厂中的好氧生化池。在曝气条件下培养种泥,每日静置30 min后的上清液用等体积培养液替换。培养液的主要成分包括5 g·L−1的胰蛋白胨、10 g·L−1的酵母菌浸出物和10 g·L−1的NaCl。培养7 d后检测污泥的MLSS和MLVSS,测得MLSS含量为4 000 mg·L−1、MLVSS含量为2 211 mg·L−1、VSS/SS为0.552 8。随后将上述污泥接种到反应器中,同时保存部分污泥(−80 ℃冰箱)中用于微生物群落检测。在通入实际污水之前,反应器的出水COD和总氮(TN)需连续稳定7 d,使用模拟污水培养反应器的运行水质稳定状况如图1所示。
-
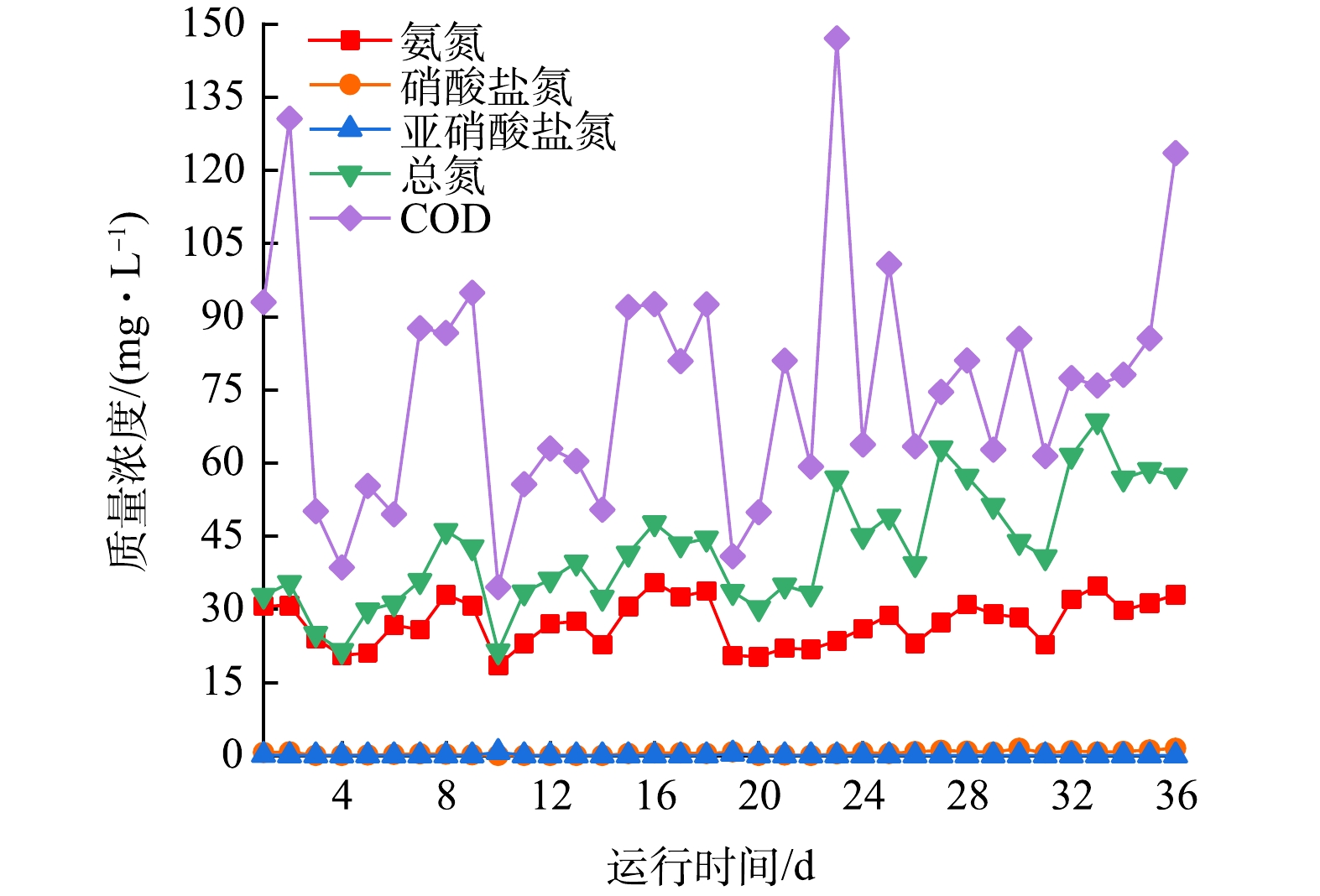
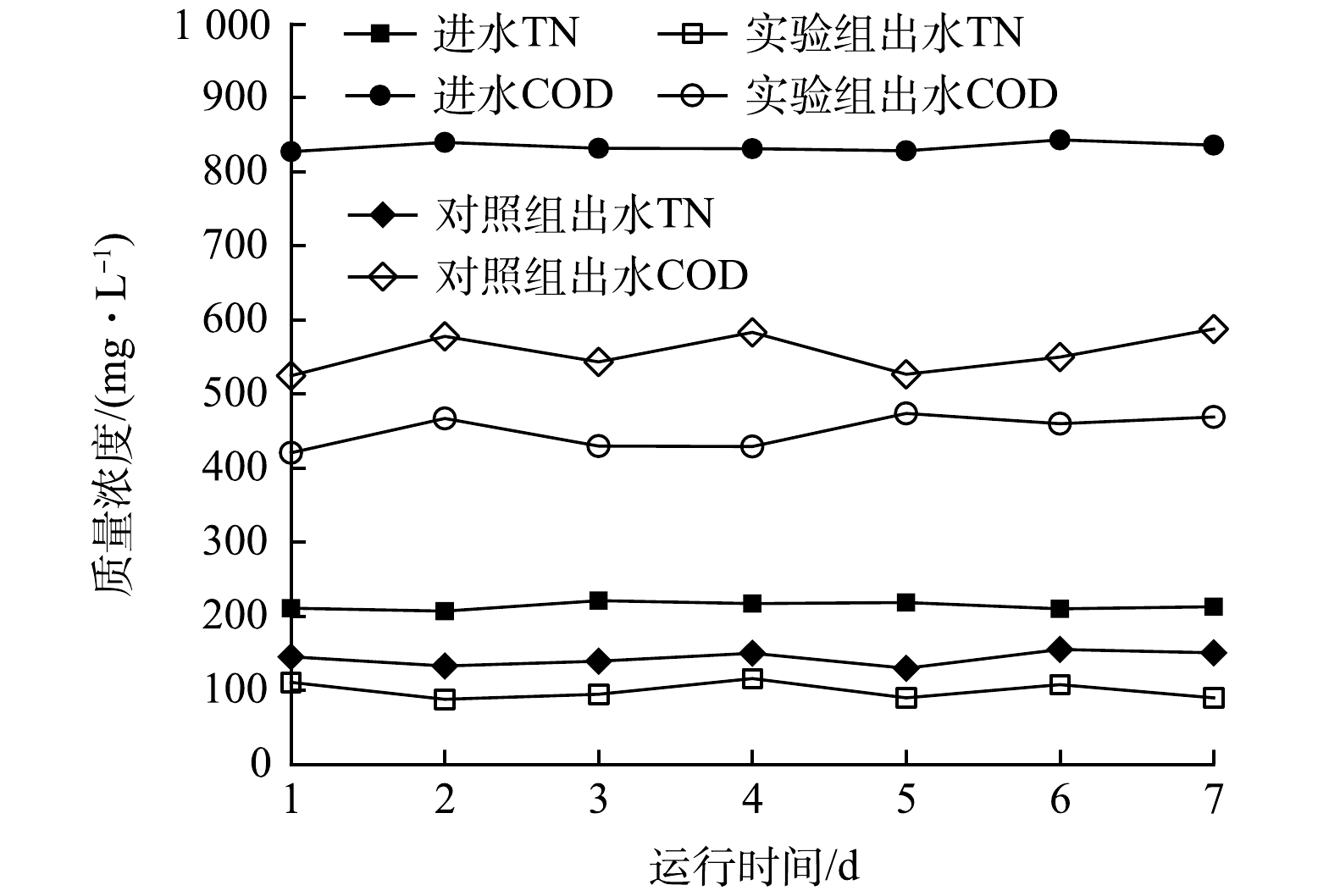
本实验所选用的低C/N(1.83±0.51)市政污水取自镇江某市政污水处理厂的夏季进水,进水有机碳源的质量浓度过低,C/N比已经接近1:1,远低于正常生物脱氮所需要的C/N,且进水水质含量受天气影响有较大的波动。实验所用污水的水质质量浓度的变化见图2。
-
本实验选用纳米α-Fe2O3作为外加铁源。该α-Fe2O3(罗恩试剂)外观形貌主要呈球形,尺寸在20 nm。活性污泥MLSS与纳米氧化铁按3:1的质量比投加入反应器中。
-
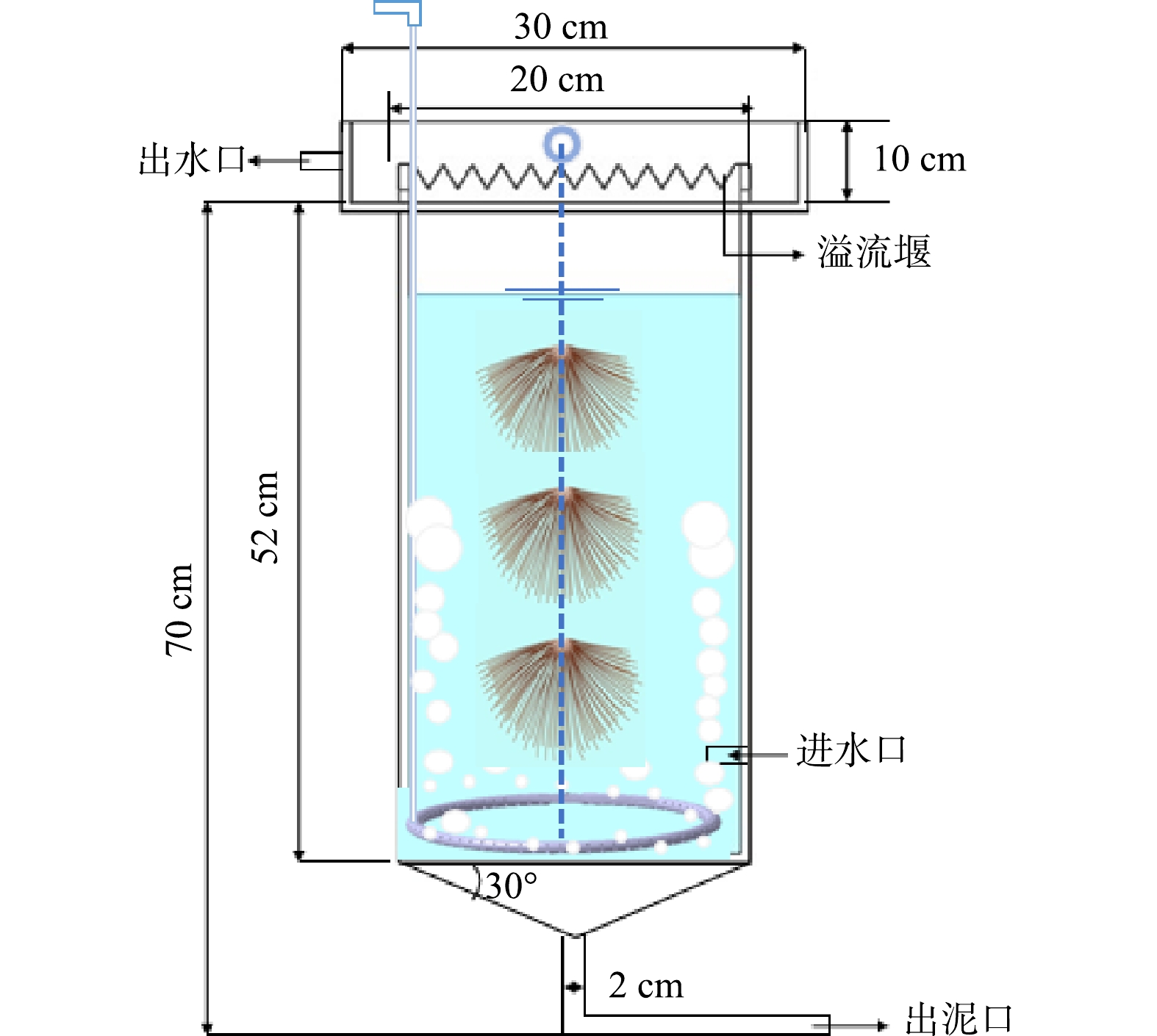
本研究所设计的反应器为小型生物接触氧化池。设计反应器池体高70 cm,直径30 cm,池体容积设计为25 L。池体顶部设置溢流堰,底部设置排泥口,整体为亚克力板构造而成。在池体底部设有环状曝气管,自下至上给反应器提供曝气功能。反应器进水口设置于池体右下侧、略高于并紧贴于曝气环。出水口设置于反应器溢流堰的左上方。每个反应器培养3座生物巢。相邻生物巢间距为5 cm,放置位置须高于底部曝气环10 cm。反应器设计水力停留时间为12 h,包括换水沉淀1 h和曝气运行11 h,以连续流的形式运行,控制流量泵的流速为35 mL·min−1。具体反应装置示意图如图3所示。
-
实验分为对照组和实验组。对照组直接将25 L活性污泥加入反应器中,而实验组除加入25 L活性污泥外,还混入33.3 α-Fe2O3。2组反应器同步运行。2组反应器均悬挂MBF(纤维长15 cm,单丝直径13 μm)填料,通过吸附活性污泥以形成稳定的生物巢。待反应器稳定后,持续运行36 d。每天定时采集进水和出水并进行水质检测。实验期间,反应器内的水温控制在25~30 ℃,溶解氧(DO)维持在3~5 mg·L−1,进水pH维持在7.0~7.5。反应器运行过程中每天采集进出水水样。统计数据采用中位数进行分析。
-
进出水中的BOD使用BOD检测仪(Lovibond BD600)检测。TN、NH4+、NO2−、NO3−和COD分别使用碱性过硫酸钾消解紫外分光光度法(GB 11894-89)、纳氏试剂分光光度法(GB 7479-87)、盐酸萘乙二胺分子吸收分光光度法(GB 7493-87)、盐酸紫外分光光度法(HJ/T 346-2007)和快速消解分光光度法(HJ/T 399-2007)进行检测。
实验结束后,对两座反应器的泥样进行宏基因组测序,以探究反应器中的微生物群落组成。采用CTAB法提取样本的DNA,利用Agilent 5400依次检测样本的DNA浓度、完整性和纯度。随后取用检测合格的样品,用Covaris超声波破碎仪将样品的DNA链打断,制备长度在350 bp左右的DNA片段,再对DNA片段进行修饰、加尾、接头、纯化、PCR扩增等[19-21]。最后,在Illumina NovaSeq高通量测序平台(深圳微科盟科技集团有限公司)上对样品DNA进行宏基因组测序,从而得到样品的宏基因组数据。最后基于RAST和KEGG数据库上对氮转化和生物电子转移相关的功能基因进行注释。
另取样品,用PBS缓冲溶液反复清洗3次并去除上清液,再用25%戊二醛溶液固定样品并置于4 ℃冰箱中12 h,随后再次使用PBS缓冲溶液清洗样品,并依次使用浓度为50%、60%、70%、80%和100%的乙醇稀释液对样品进行5次脱水处理备用[22]。最后将样品放置于硅片上,置于真空冷冻干燥箱内干燥,喷金处理后在透射电镜(TEM)上扫描拍摄,以观察样品形态及纳米Fe2O3的聚集位置。
-
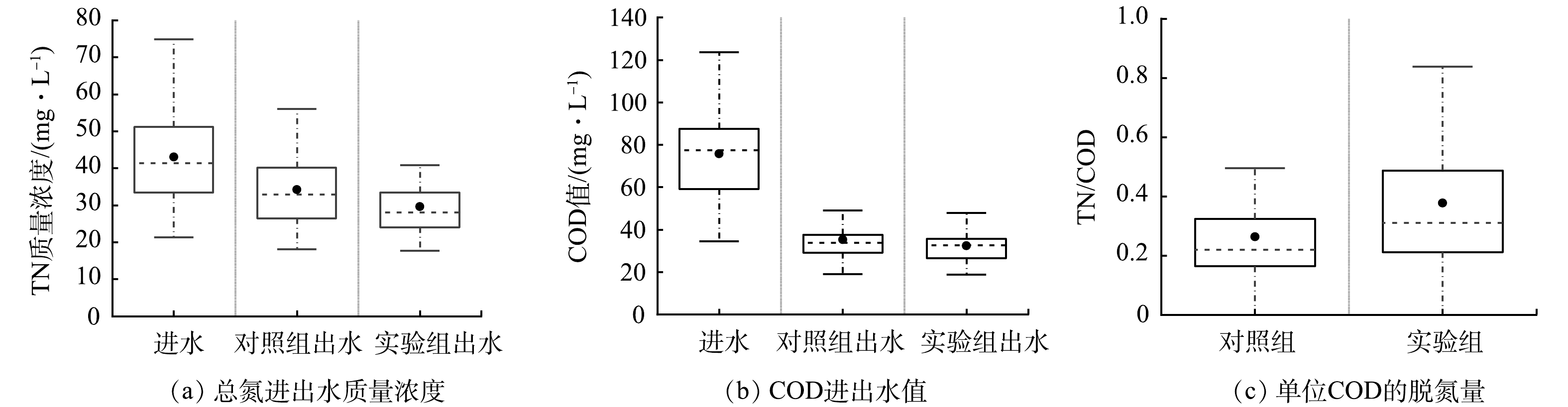
待反应器通入实际污水之后,开始测量2组反应器的进出水水质。本实验所使用的污水水质波动较大,在反应器运行后期进水中有机氮的质量浓度升高(图2),总氮的进水质量浓度由30~40 mg·L−1提升至50~60 mg·L−1,反应器的出水总氮质量浓度也随之升高。整体而言,对照组的平均总氮出水质量浓度为(34.22±9.17) mg·L−1,实验组的平均出水质量浓度则为(29.63±8.14) mg·L−1(图4(a));对照组的平均总氮去除率保持在(21.85±11.35)%左右,而实验组的平均总氮去除率则达到了(32.21到了平均总率保持。相比于对照组,实验组的总氮去除率提高了10% 左右,说明投加α-Fe2O3可以提高总氮的去除率。
实验所用污水的进水COD值在暴雨天低至40~50 mg·L−1,日常维持在80 mg·L−1,最高能达到130~150 mg·L−1(图2)。而反应器的平均出水COD值明显降低,且波动幅度减小。从COD的平均去除率来看,对照组的平均去除率为(49.84±19.37)%,而实验组的平均去除率则为(53.33±17.5)%(图4(b))。从出水COD值来看,对照组的出水COD值为(35.35±16.34) mg·L−1,实验组则为(32.43±8.17) mg·L−1。实验组的出水COD值基本低于《城镇污水处理厂污染物排放标准》一级A标准的出水指标。
整体而言,实验组中单位COD的脱氮量TN/COD约为0.370,明显高于对照组TN/COD的0.234,脱氮效率提高了58%(图4(c))。这一结果证明了在实际污水的处理中,纳米氧化铁的添加能够有效提高单位COD的利用效率。
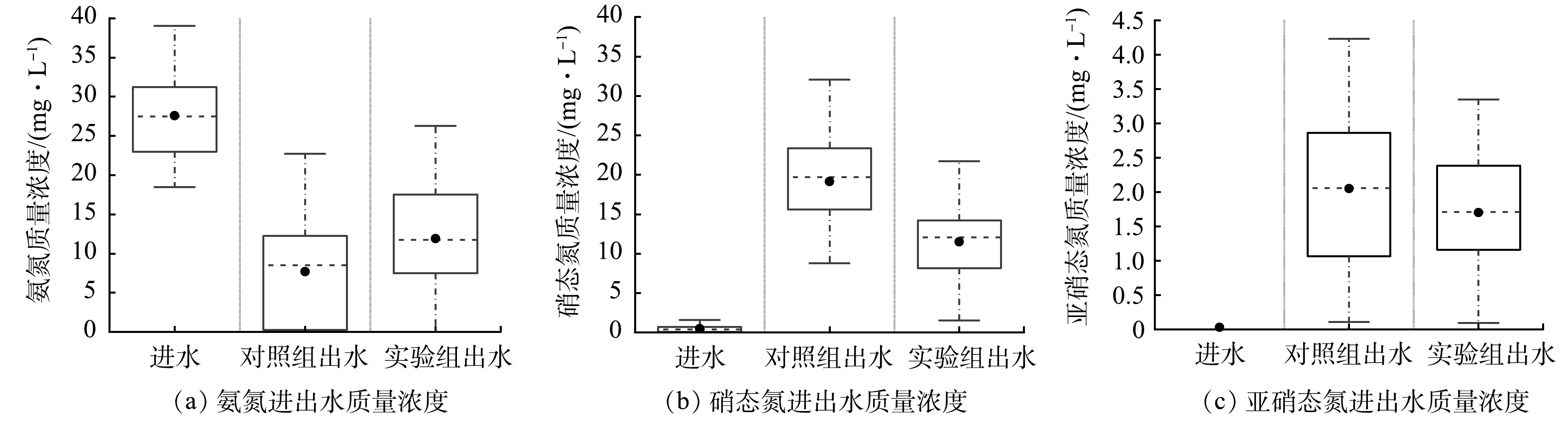
此外,在对2组反应器出水总氮组成进行检测时发现,实验组出水中有机氮的质量浓度要低于对照组。这也证明相较于对照组,α-Fe2O3能够诱导池体内的大量微生物将进水中的有机氮化成氨氮。所以结果表现为出水时实验组总氮质量浓度低于对照组,同时氨氮又高于对照组的现象,因此,即使实验组的出水氨氮质量浓度要高于对照组,也不能说明实验组的脱氮效果差。其中,对照组的出水氨氮质量浓度为(3.03±2.49) mg·L−1,而实验组的出水氨氮质量浓度则为(5.89±2.66) mg·L−1,到25 d后因水质波动分别提升至(9.83±2.82) mg·L−1和(17.27±3.86) mg·L−1(图5(a))。
该市政污水的硝态氮及亚硝态氮的质量浓度很低,平均进水质量浓度仅为0.49 mg·L−1及0.03 mg·L−1(图2)。但因为进水有机碳源不足,导致反硝化作用不充分,2组反应器的出水中硝酸盐及亚硝酸盐质量浓度均有上升。整体而言,对照组的出水硝酸盐质量浓度平均为(19.14±5.77) mg·L−1,高于实验组的(11.50±5.38) mg·L−1(图5(b));对照组的出水亚硝态氮质量浓度平均为(2.05±1.13) mg·L−1,高于实验组的(1.70±0.86) mg·L−1(图5(c))。这证明添加了α-Fe2O3的实验组在碳源不足的污水中的反硝化能力要强于对照组。结果证明添加α-Fe2O3能够提高实际市政污水处理中的生物脱氮效率。
-
原始泥样的MLSS含量为4 000 mg·L−1,MLVSS为2 211 mg·L−1,VSS/SS为0.552,显示本实验所用污泥活性较高[23]。如表1所示,2组反应器经过约40 d的培养后,对照组的污泥MLSS含量为4 125 mg·L−1,MLVSS为2 253 mg·L−1以及VSS/SS为0.553。这一结果显示,对照组的污泥生物质与原始泥样相比没有明显变化。而实验组因为额外投加了α-Fe2O3,MLSS和MLVSS含量分别显著提升至5 239 mg·L−1和2 599 mg·L−1,同时VSS/SS降至0.496,显示污泥生物质存在明显变化。其中实验组的MLSS含量仅比对照组的含量增加了1 114 mg·L−1,低于投入反应器中1 333.3 mg·L−1的α-Fe2O3,说明α-Fe2O3会随着反应器的出水少量流失。同时实验组的MLVSS比对照组高了316 mg·L−1。这说明α-Fe2O3可能具有诱导胞外聚合物分泌的能力。
-
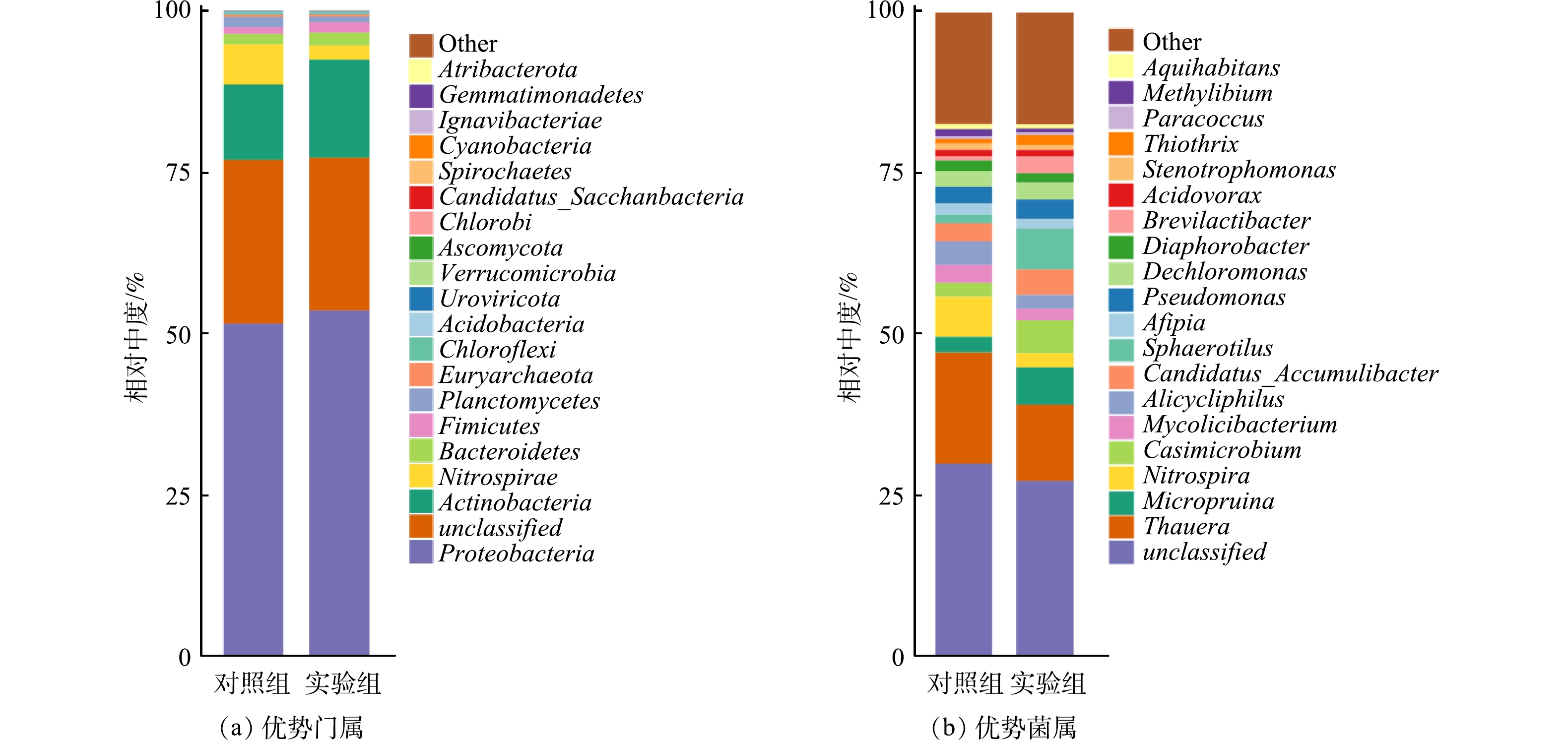
通过宏基因组对2组反应器中的微生物群落进行门和属水平上的分类并进行功能结构分析。依据宏基因组学的物种丰度聚类分析测序结果显示,2组反应器的活性污泥中总共包含42个菌门,其中未被定义的菌门分别占对照组和实验组中总菌门的25.28%和23.54%。在门水平上,在对照组和实验组反应器中占比均超过1%的有Proteobacteria (51.64%;53.69%)、Actinobacteria (11.62%;15.13%)、Nitrospirae (6.19%;2.23%)、Bacteroidetes (1.65%;2%)和Firmicutes (1.04%;1.62%) (图6(a))。其中,在2组反应器中占比较高的Proteobacteria (变形菌门)和Actinobacteria (放线菌门)是优势菌门,二者在不同反应器中占比均超过了10%。变形菌门中含有大量可以分别在好氧和厌氧的环境中生存的硝化以及反硝化菌属 [24-25]。这2个菌门在实验组中的占比均高于对照组,说明投加α-Fe2O3可以刺激反硝化菌的生长。剩余的优势菌门Nitrospirae (硝化螺旋菌门)、Bacteroidetes (拟杆菌门)和Firmicutes (厚壁菌门)中同样包含大量与氮代谢相关菌属,说明反应器的微生物群落有利于去除含氮污染物。
而属水平上的分类显示,2组反应器中未被定义的菌属分别占对照组和实验组中总菌属的29.8%和27.19%。在对照组和实验组反应器中占比均超过1 %的属分别有Thauera (17.34%;11.85%)、Nitrospira (6.19%;2.23%)、Alicycliphilus (3.62%;2.14%)、Candidatus_Accumulibacter (2.89%;3.9%)、Mycolicibacterium (2.84%;1.83%)、Pseudomonas (2.52%;2.93%)、Micropruina (2.48%;5.78%)、Dechloromonas (2.42%;2.68%)、Casimicrobium (2.11%;5.11%)、Afipia (1.79%;1.57%)、Diaphorobacter (1.71%;1.44%)、Sphaerotilus (1.31%;6.37%)(图6(b))。其中,优势菌属Thauera、Pseudomonas以及Dechloromonas是具有异养硝化-好氧反硝化功能的菌属,能在有氧条件下将不同形态的氮转化为氮气[26-28]。此外,一些HN-AD菌被证明具有尿素水解酶[29],可以将有机氨转化为氨氮。这可能是实验组反应器内氨氮质量浓度高于对照组的原因。Nitrospira是典型的亚硝酸盐氧化菌[30]。Alicycliphilus具有多样性的代谢机制,可利用硝酸盐等作为电子受体,参与污水处理中的反硝化过程[31]。Candidatus_Accumulibacter是典型的反硝化聚磷菌,在缺氧条件下以硝酸盐或亚硝酸盐为电子受体实现反硝化和聚磷[32]。Mycolicibacterium是一种革兰氏阳性菌,具有脲酶和硝酸盐还原活性[33]。Casimicrobium是市政污水处理厂一种常见的好氧菌,却能将硝酸盐还原为唯一产物亚硝酸盐[34]。Sphaerotilus是一种铁基好氧菌,能够以以Fe2+为电子供体实现自养反硝化[35],其相对丰度在实验组中增加进一步证实了α-Fe2O3参与了反硝化菌的呼吸过程。Micropruina是一种聚糖元菌,在厌氧条件下可以将糖、氨基酸等同化成末端发酵产物如乳酸盐、乙酸盐、乙醇、丙酮酸盐等(作为内源性碳)[36]。Afipia属主要参与大多数有机底物如葡萄糖、果糖、蔗糖等的氧化[37]。总之,氨氮的转化和去除是HN-AD菌、硝化菌、反硝化菌共同作用的结果。
-
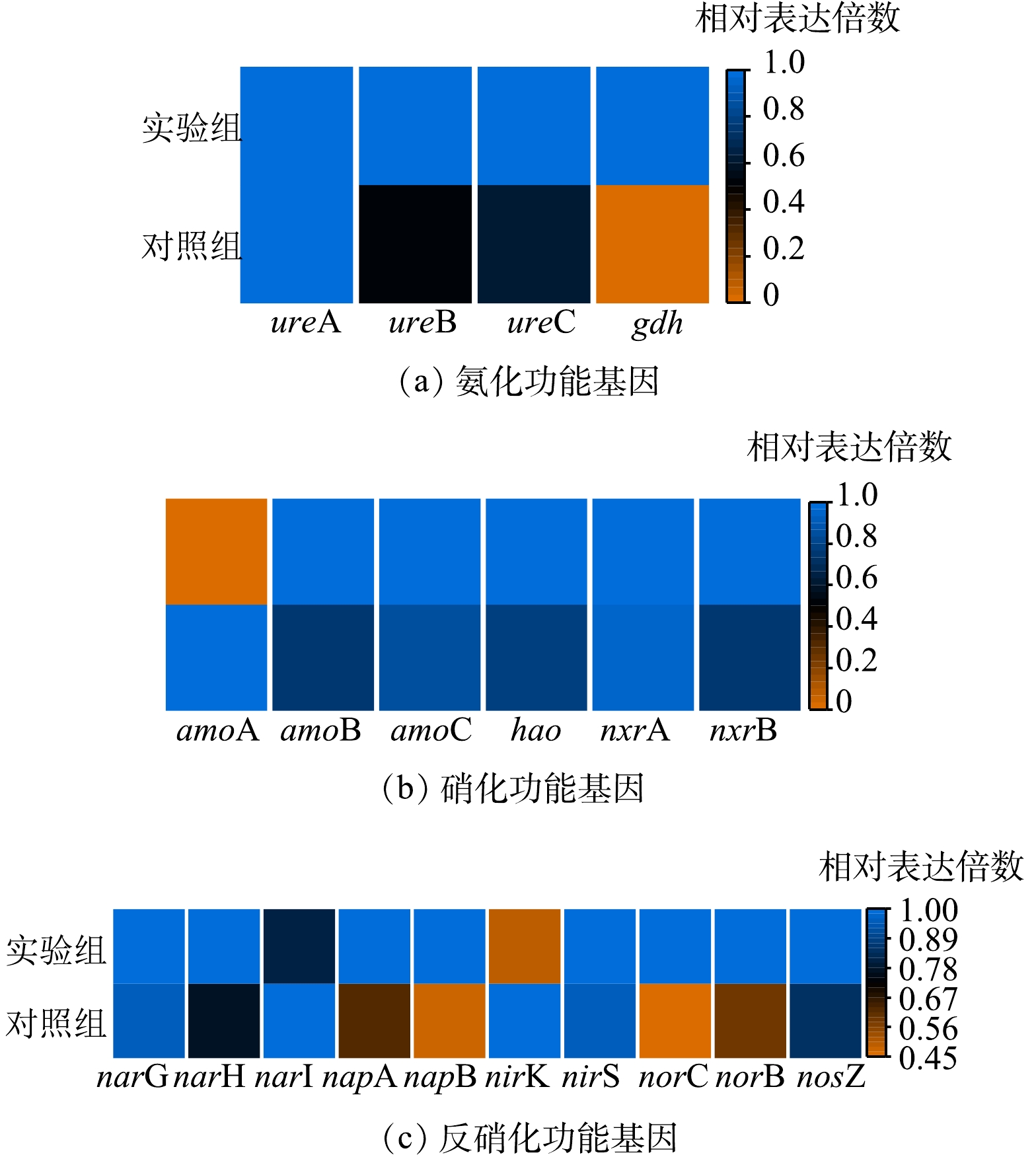
通过对比2组反应器中与氮转化相关基因,可以分析α-Fe2O3对生物脱氮的影响。首先探究了能够影响氨化过程的功能基因,研究发现检出的实验组氨化功能基因簇ureA、ureB和ureC的表达量均高于对照组[38],分别为对照组的1.58,1.89和1.03倍。此外,实验组中还检出了对照组中所没有的功能基因gdh[39](图7(a))。这说明在功能基因的层面上,α-Fe2O3确实能够诱导微生物更高效进行氨化反应。这进一步印证了对照组反应器中氨氮质量浓度较高的现象。在能够影响硝化过程功能基因中,功能基因amoB、amoC、hao、nxrA和nxrB的表达量实验组均高于对照组[40],分别为对照组的1.36、1.19、1.29、1.05和1.31倍,证明添加α-Fe2O3确实可以提高微生物的硝化潜能(图7(b))。而在影响反硝化过程的功能基因中,除narI和nirK外,实验组的narG、narH、napA、napB、nirS、norC、norB和nosZ的相对表达量均高于对照组[41],分别为对照组的1.05、1.31、1.59、2.09、1.04、2.23、1.68、1.19倍(图7(c))。功能基因相对丰度的差异表明,添加α-Fe2O3可正向调控氮转化功能基因的表达。本研究显示在单一池体的曝气条件下,生物巢可以实现同步硝化和反硝化。
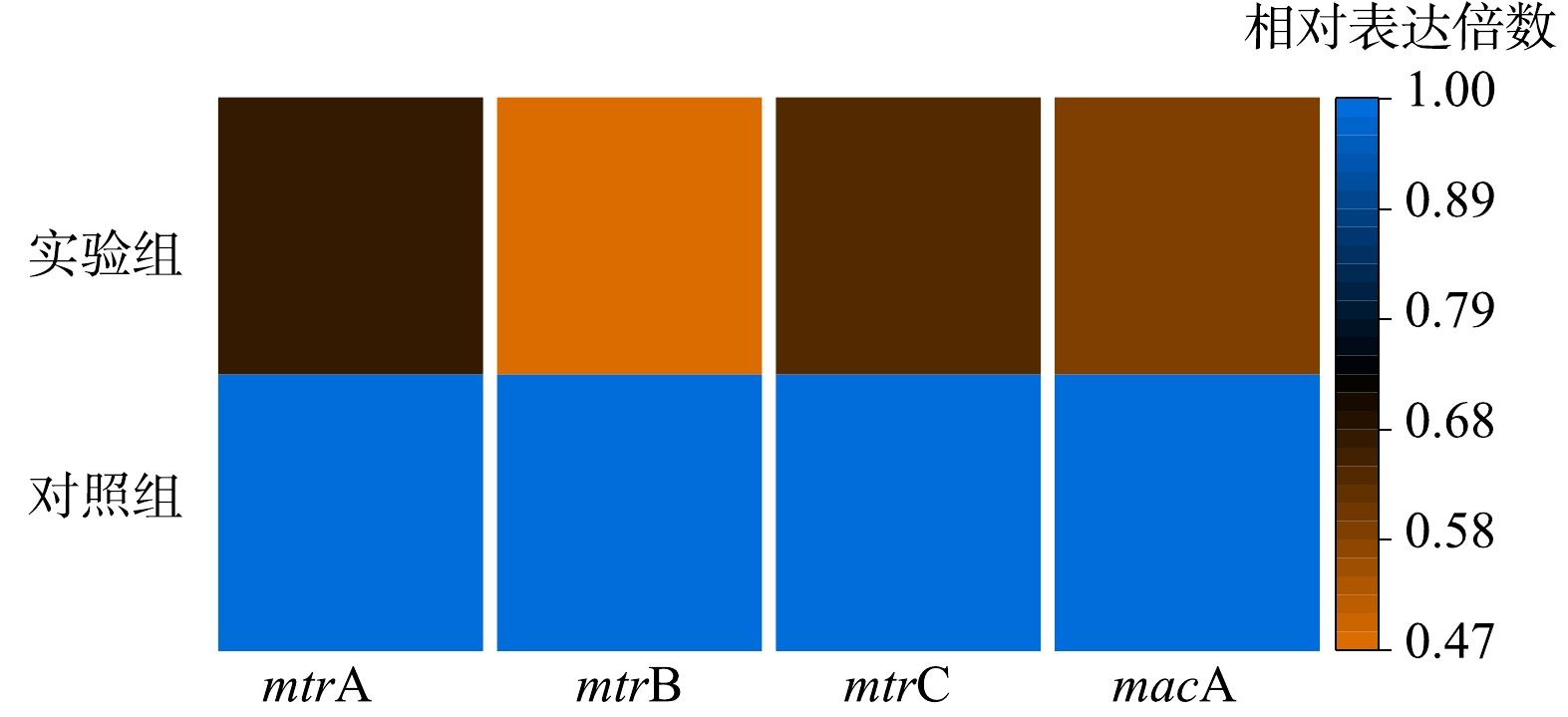
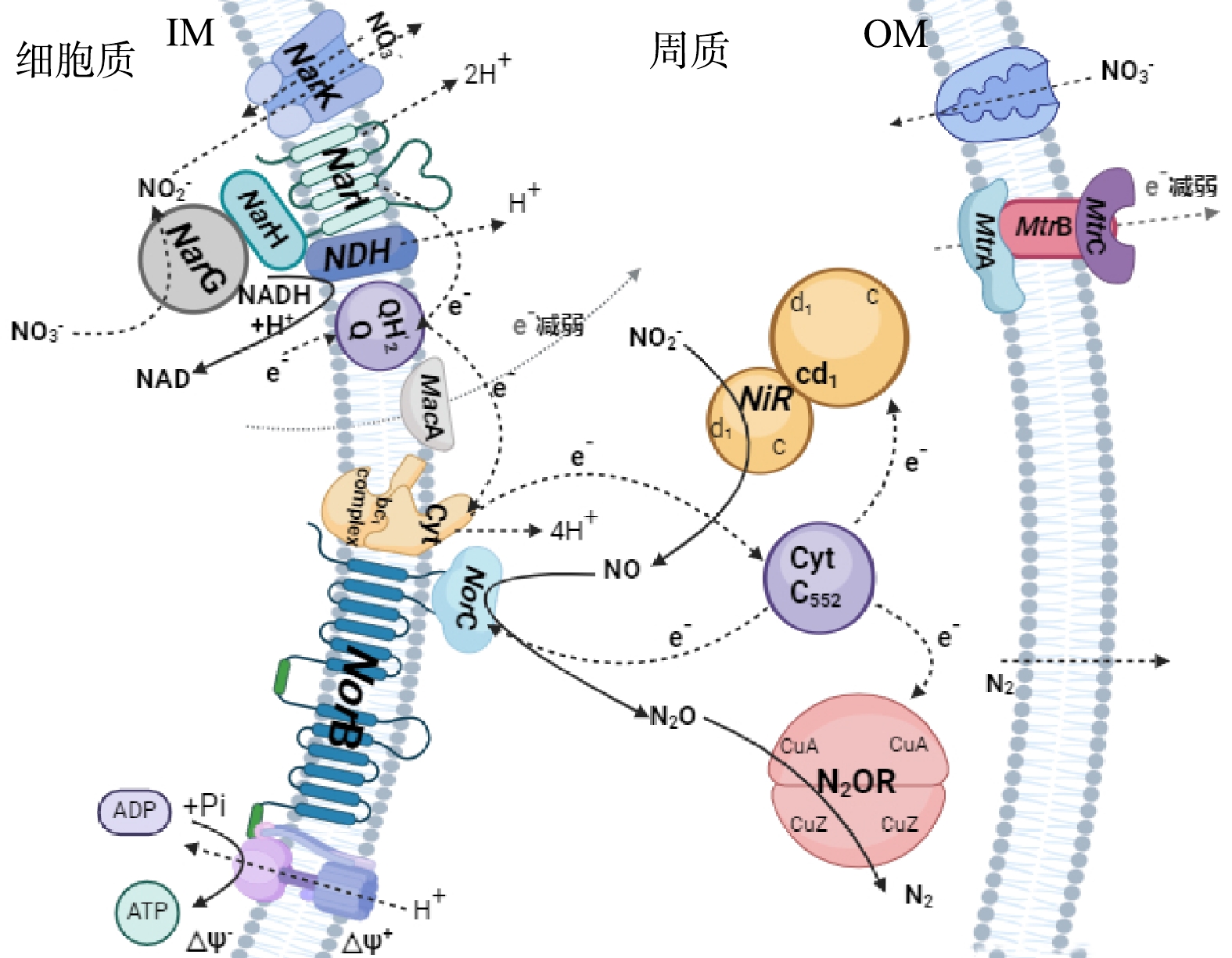
此外,本研究对跟电子转移蛋白相关的编码基因进行了研究,共检索到4个编码跨膜电子转移蛋白的功能基因,分别是mtrA、mtrB、mtrC和macA[42]。与对照组相比,实验组的4个功能基因的表达量均受到了抑制,分别为对照组的67.89%、47.59%、63.56%和58.23% (图8)。这意味着投加α-Fe2O3后细胞已不需要更多的跨膜电子转移蛋白参与电子向胞外的传输,从而导致电子转运蛋白表达量减少。
-
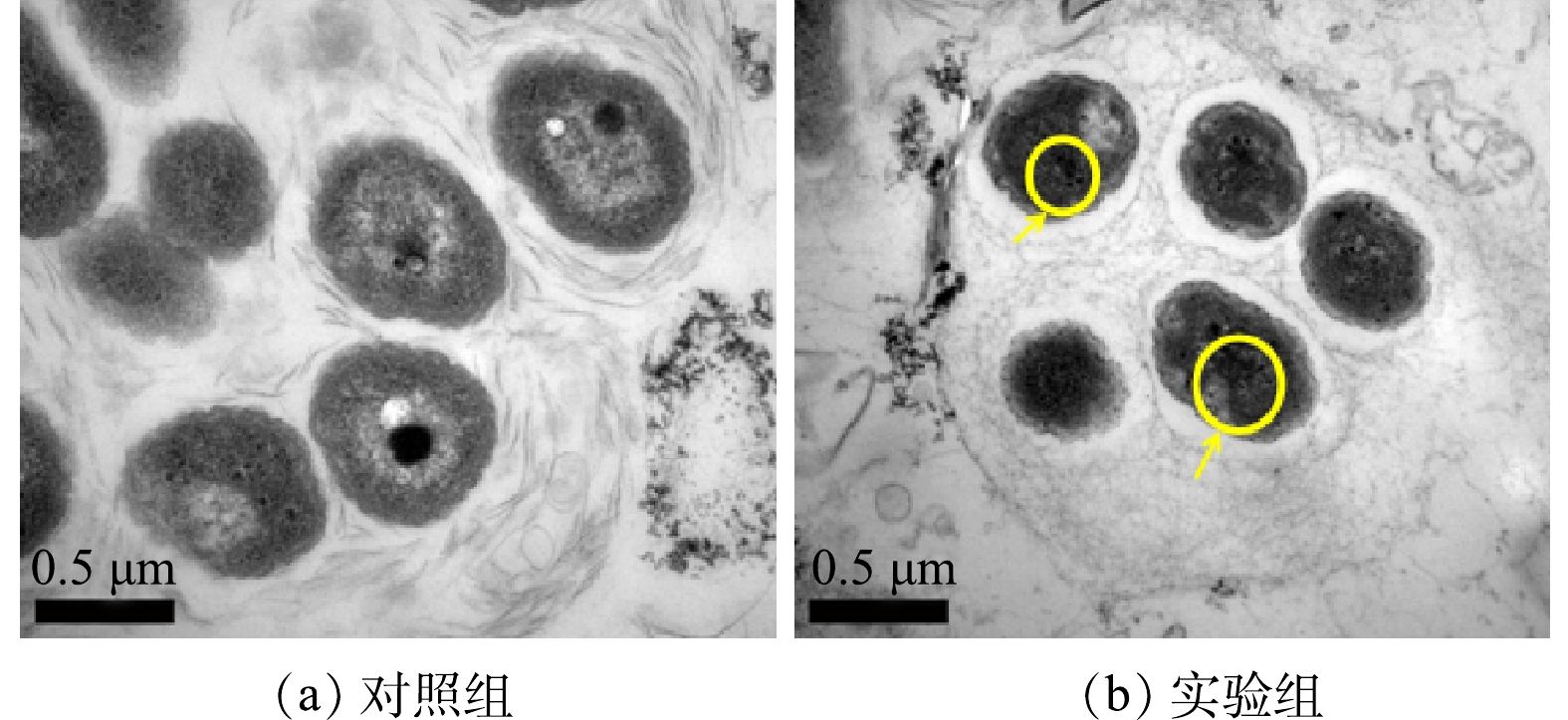
如上所述,实验组中跨膜电子转移相关基因的表达量要普遍低于对照组,这意味着更多的电子参与胞内含氮化合物尤其是硝酸盐的还原或反硝化过程。这导致了α-Fe2O3存在的情况下,消耗单位COD可以有利于去除更多的TN。外膜上电子转移蛋白表达的下调间接表明α-Fe2O3在胞内参与了电子传递与含氮化合物的转化[43-44]。此外,TEM表征结果(图9)表明,实验组中的细胞内存在大量黑色颗粒物,而未投加α-Fe2O3的对照组中则没有颗粒物的堆积。这一现象进一步证明了α-Fe2O3能够进入胞内参与电子传递(即介导了NADH等电子供体与硝酸盐还原酶之间的电子传递),保证了更多电子(或碳源)被用于反硝化过程。这解释了在α-Fe2O3存在下消耗单位COD可以去除更多TN的原因。
综上所述,α-Fe2O3一方面能够显著提高氮转化相关基因的表达水平,提高系统内氮转化的效率;另一方面能够下调微生物周质空间和外膜上电子转移蛋白的表达,减少了跨膜的电子转移,将更多的电子留存在胞内进行硝酸盐呼吸,实现碳源的有效利用。基于出水水质、微生物群落结构、功能基因结构及其表达水平,本研究推测性地提出了α-Fe2O3介导下反硝化菌胞内的氮代谢通路(图10)。
-
本研究依据外加铁源对生物脱氮性能的影响,探索性地将α-Fe2O3材料和MBF生物巢联系起来,成功构建出一种能够显著提高MBF生物巢反应器内单位COD利用效率的新工艺。具体结论如下。
1)实际污水实验中,对照组和实验组反应器对总氮的去除率分别为(21.85±11.35)%和(32.21±11.35)%,单位COD的脱氮效率(TN/COD)则分别为0.234和0.370,实验组比对照组的脱氮效率高出58%,说明α-Fe2O3能够提高MBF生物巢反应器对实际市政废水的脱氮效果。
2)宏基因组测序结果表明,α-Fe2O3的投加提高了氮转化相关基因的表达量,同时能够抑制周质与外膜上电子转移蛋白相关基因的表达。这说明α-Fe2O3能够参与胞内电子转移,促使更多电子参与胞内硝酸盐呼吸作用。
纳米Fe2O3强化MBF生物巢脱氮的机制
Augmentation of nitrogen removal by MBF bio-nests supplemented with nano-Fe2O3
-
摘要: 针对我国市政污水C/N比偏低的问题,本研究旨在探讨纳米氧化铁在MBF生物巢反应器中对低C/N比市政污水处理的强化脱氮效果及其机理。将纳米氧化铁添加到MBF生物巢反应器中,处理实际低C/N比市政污水,通过监测脱氮效率和碳源利用情况,结合微生物群落结构和功能基因表达量的分析,评价其处理效果。结果表明,纳米氧化铁的加入可显著提高反应器的脱氮效率,脱氮率提升了58%。微生物群落结构分析结果表明,纳米氧化铁影响了特定脱氮微生物的相对丰度。功能基因表达量分析结果表明,纳米氧化铁下调了微生物外膜上电子转移蛋白的表达,减少了种间电子传递,从而提高了胞内电子的有效利用率。以上结果表明,纳米氧化铁通过调节微生物群落结构和功能基因表达,可提高碳源的利用效率,进而实现对低C/N比市政污水的有效脱氮,可为市政污水处理提供技术途径参考。Abstract: Addressing the issue of low C/N ratio in municipal wastewater in China, this study aims to investigate the enhanced nitrogen removal efficacy and its mechanism of nano-iron oxide in an MBF bio-nest reactor treating municipal wastewater with low C/N ratio. As nano-iron oxide was added to the MBF bio-nest treating actual municipal wastewater with low C/N ratio, the nitrogen removal efficiency and carbon source utilization were monitored, and the microbial community structure and functional gene expression levels were also analyzed, then the performance on treating wastewater with low C/N ratio was evaluated. The results showed that addition of nano-iron oxide significantly increased the nitrogen removal efficiency of the reactor by 58%. Microbial community structure analysis revealed that nano-iron oxide affected the relative abundance of specific nitrogen-removing microorganisms. Functional gene expression analysis found that nano-iron oxide downregulated the expression of electron transfer proteins on the outer membrane of microorganisms, reducing interspecies electron transfer and thereby enhancing the effective utilization of electrons within the cells. Above results indicated that nano-iron oxide increased the efficiency of carbon source utilization and effectively removed nitrogen from municipal wastewater with low C/N ratio through regulating microbial community structure and functional gene expression. This provides a new technical approach for municipal wastewater treatment.
-
伴随着我国城镇化进程的加快,市政污水的处理需求在快速上升。由于我国现代化建设起步较晚,污水排放管网建设多采用合流制[1],致使管道除了承接生活污水之外,还容纳了工业废水及雨水等[2]。当前我国市政污水总体呈现出有机污染物含量低、水质水量变化大、污染物成分复杂等特点[3],导致在污水处理中,生物脱氮的实际效果不佳,出水水质不达标。污水中的大量氮源若不能达到排放标准就进入自然水体,势必加剧河道和湖泊水体的富营养化[4]。因此,解决低碳氮比市政污水含碳量不足的问题,是提高市政污水处理高效脱氮的重要方向。
常规的生物脱氮主要由氨化作用、硝化作用和反硝化作用3个部分完成。根据氮的存在形态,功能微生物菌群在特定微环境下相互协作,共同完成脱氮过程[5]。当前生物脱氮多采用悬浮生长系统为主的缺氧/好氧、厌氧/缺氧/好氧、序批式活性污泥法、氧化沟等传统工艺。但这些传统生物脱氮工艺存在多种缺点,例如:因活性污泥在水中的形态多为絮体状,导致反应装置的水力停留时间和污泥停留时间难以控制;污泥内的自养菌生长周期长,易随出水流出;活性污泥抗环境胁迫能力较差等[6-8]。
为克服上述缺点,以填料为载体的悬浮生长系统正在被广泛采用。其中代表性工艺包括生物接触氧化、生物转盘、及生物滤池等[6,9]。不过,常规填料上附着的活性生物膜厚度一般不超过2 mm。受传质阻力和水力冲击的作用,生物膜较易脱落,因而影响水处理效能。近来有研究在单一反应器中引入改性玄武岩纤维(modified basalt fiber, MBF)做填料。这种新型生物载体可快速吸附活性污泥,形成一种有别于传统生物膜的微生物聚集体(生物巢)[10]。通常,生物巢尺寸可达10 cm以上。其生物量虽然巨大,但并不影响传质和生物活性,因而可实现不同类型污/废水的高效脱氮除碳[11]。相对于传统填料,新型改性玄武岩纤维更适合做生物载体。其能够富集大量的功能微生物菌群,尤其是生长缓慢的氨氧化细菌、亚硝酸盐氧化菌以及异养硝化-好氧反硝化(heterotrophic nitrifying aerobic denitrifying, HN-AD)菌等[12-13]。尽管从材料改性的角度可以优化功能微生物的富集,但功能微生物的生长通常受到生理条件如C/N比的限制。在传统的生物脱氮过程中,生物反硝化需要大量的有机碳源作为电子供体。而我国的市政污水的碳氮比普遍较低,导致反硝化菌生长受到抑制。为了降低出水中含氮污染物的浓度,现有工艺通常通过外加碳源来提高污水的碳氮比。不过,使用外加碳源一方面会增加污水厂的运行成本,另一方面由于实际废水进水的水质水量具有较大的波动性,碳源投加量难以精确控制[14]。
在此背景下,解决市政污水中碳源有效利用的问题就成了当前污水处理领域的研究热点之一。考虑到在偏中性和碱性环境中NO3−/NO2−电对的氧化还原电位高于Fe(II)/Fe(III),研究人员尝试在复杂菌群中投加铁氧化物介导种间电子传递,以实现有机底物氧化与硝酸盐还原的耦合。纳米级铁氧化物如赤铁矿(α-Fe2O3)、磁赤铁矿(γ-Fe2O3)、磁铁矿(Fe3O4)作为半导体和导体材料具有良好的生物亲和性。微生物可以利用这些纳米材料作为电子介体实现电子传递[15]。据报道Geobacter sulfurreducens可以氧化乙酸盐但不能还原NO3−,而Thiobacillus denitrificans能够还原NO3−但不能氧化乙酸盐。两者的共培养并不能实现有机物氧化和硝酸盐还原的耦合发生,但当添加α-Fe2O3或Fe3O4纳米颗粒时共培养体系能成功实现共代谢[16]。此外,研究还发现氢自养反硝化菌的外膜细胞色素对α-Fe2O3具有高度亲和性,借助α-Fe2O3可加速电子向胞内硝酸盐还原酶的传递,从而促进反硝化的进行[17]。因此,铁氧化物介导的种间电子传递为菌群共代谢提供了一种有效途径[18]。此外,对于一些具有跨膜电子转移能力的反硝化菌,进入细胞内部的α-Fe2O3有可能在周质空间直接介导NADH(还原性辅酶I)等电子供体与硝酸盐还原酶之间的电子传递。因此,这种胞内电子传递促进机制也为碳源有效利用提供了新的途径。但是,目前尚未有关于投加铁氧化物以解决市政污水碳源不足问题的研究报道。
为此,本研究选用MBF作为生物载体,在生物接触氧化池中培养生物巢,借助于生物巢独特的好氧-缺氧-厌氧微环境,富集各种与氮转化相关的功能微生物。同时,在池体中投加纳米氧化铁(α-Fe2O3),考察其介导种间或胞内电子转移机制对脱氮效能的刺激作用,并解析氮转化的代谢通路。本研究旨在探索α-Fe2O3介导下碳源有效利用的可行性及潜在的机制,为提高低碳氮比市政污水的高效脱氮提供一种新的策略。
1. 材料与方法
1.1 实验用污泥培养
本实验所选用的种泥均采自镇江市某污水处理厂中的好氧生化池。在曝气条件下培养种泥,每日静置30 min后的上清液用等体积培养液替换。培养液的主要成分包括5 g·L−1的胰蛋白胨、10 g·L−1的酵母菌浸出物和10 g·L−1的NaCl。培养7 d后检测污泥的MLSS和MLVSS,测得MLSS含量为4 000 mg·L−1、MLVSS含量为2 211 mg·L−1、VSS/SS为0.552 8。随后将上述污泥接种到反应器中,同时保存部分污泥(−80 ℃冰箱)中用于微生物群落检测。在通入实际污水之前,反应器的出水COD和总氮(TN)需连续稳定7 d,使用模拟污水培养反应器的运行水质稳定状况如图1所示。
1.2 实验用污水
本实验所选用的低C/N(1.83±0.51)市政污水取自镇江某市政污水处理厂的夏季进水,进水有机碳源的质量浓度过低,C/N比已经接近1:1,远低于正常生物脱氮所需要的C/N,且进水水质含量受天气影响有较大的波动。实验所用污水的水质质量浓度的变化见图2。
1.3 外加铁源
本实验选用纳米α-Fe2O3作为外加铁源。该α-Fe2O3(罗恩试剂)外观形貌主要呈球形,尺寸在20 nm。活性污泥MLSS与纳米氧化铁按3:1的质量比投加入反应器中。
1.4 反应器设计
本研究所设计的反应器为小型生物接触氧化池。设计反应器池体高70 cm,直径30 cm,池体容积设计为25 L。池体顶部设置溢流堰,底部设置排泥口,整体为亚克力板构造而成。在池体底部设有环状曝气管,自下至上给反应器提供曝气功能。反应器进水口设置于池体右下侧、略高于并紧贴于曝气环。出水口设置于反应器溢流堰的左上方。每个反应器培养3座生物巢。相邻生物巢间距为5 cm,放置位置须高于底部曝气环10 cm。反应器设计水力停留时间为12 h,包括换水沉淀1 h和曝气运行11 h,以连续流的形式运行,控制流量泵的流速为35 mL·min−1。具体反应装置示意图如图3所示。
1.5 反应器运行
实验分为对照组和实验组。对照组直接将25 L活性污泥加入反应器中,而实验组除加入25 L活性污泥外,还混入33.3 α-Fe2O3。2组反应器同步运行。2组反应器均悬挂MBF(纤维长15 cm,单丝直径13 μm)填料,通过吸附活性污泥以形成稳定的生物巢。待反应器稳定后,持续运行36 d。每天定时采集进水和出水并进行水质检测。实验期间,反应器内的水温控制在25~30 ℃,溶解氧(DO)维持在3~5 mg·L−1,进水pH维持在7.0~7.5。反应器运行过程中每天采集进出水水样。统计数据采用中位数进行分析。
1.6 分析与检测
进出水中的BOD使用BOD检测仪(Lovibond BD600)检测。TN、NH4+、NO2−、NO3−和COD分别使用碱性过硫酸钾消解紫外分光光度法(GB 11894-89)、纳氏试剂分光光度法(GB 7479-87)、盐酸萘乙二胺分子吸收分光光度法(GB 7493-87)、盐酸紫外分光光度法(HJ/T 346-2007)和快速消解分光光度法(HJ/T 399-2007)进行检测。
实验结束后,对两座反应器的泥样进行宏基因组测序,以探究反应器中的微生物群落组成。采用CTAB法提取样本的DNA,利用Agilent 5400依次检测样本的DNA浓度、完整性和纯度。随后取用检测合格的样品,用Covaris超声波破碎仪将样品的DNA链打断,制备长度在350 bp左右的DNA片段,再对DNA片段进行修饰、加尾、接头、纯化、PCR扩增等[19-21]。最后,在Illumina NovaSeq高通量测序平台(深圳微科盟科技集团有限公司)上对样品DNA进行宏基因组测序,从而得到样品的宏基因组数据。最后基于RAST和KEGG数据库上对氮转化和生物电子转移相关的功能基因进行注释。
另取样品,用PBS缓冲溶液反复清洗3次并去除上清液,再用25%戊二醛溶液固定样品并置于4 ℃冰箱中12 h,随后再次使用PBS缓冲溶液清洗样品,并依次使用浓度为50%、60%、70%、80%和100%的乙醇稀释液对样品进行5次脱水处理备用[22]。最后将样品放置于硅片上,置于真空冷冻干燥箱内干燥,喷金处理后在透射电镜(TEM)上扫描拍摄,以观察样品形态及纳米Fe2O3的聚集位置。
2. 结果与讨论
2.1 出水水质评价
待反应器通入实际污水之后,开始测量2组反应器的进出水水质。本实验所使用的污水水质波动较大,在反应器运行后期进水中有机氮的质量浓度升高(图2),总氮的进水质量浓度由30~40 mg·L−1提升至50~60 mg·L−1,反应器的出水总氮质量浓度也随之升高。整体而言,对照组的平均总氮出水质量浓度为(34.22±9.17) mg·L−1,实验组的平均出水质量浓度则为(29.63±8.14) mg·L−1(图4(a));对照组的平均总氮去除率保持在(21.85±11.35)%左右,而实验组的平均总氮去除率则达到了(32.21到了平均总率保持。相比于对照组,实验组的总氮去除率提高了10% 左右,说明投加α-Fe2O3可以提高总氮的去除率。
实验所用污水的进水COD值在暴雨天低至40~50 mg·L−1,日常维持在80 mg·L−1,最高能达到130~150 mg·L−1(图2)。而反应器的平均出水COD值明显降低,且波动幅度减小。从COD的平均去除率来看,对照组的平均去除率为(49.84±19.37)%,而实验组的平均去除率则为(53.33±17.5)%(图4(b))。从出水COD值来看,对照组的出水COD值为(35.35±16.34) mg·L−1,实验组则为(32.43±8.17) mg·L−1。实验组的出水COD值基本低于《城镇污水处理厂污染物排放标准》一级A标准的出水指标。
整体而言,实验组中单位COD的脱氮量TN/COD约为0.370,明显高于对照组TN/COD的0.234,脱氮效率提高了58%(图4(c))。这一结果证明了在实际污水的处理中,纳米氧化铁的添加能够有效提高单位COD的利用效率。
此外,在对2组反应器出水总氮组成进行检测时发现,实验组出水中有机氮的质量浓度要低于对照组。这也证明相较于对照组,α-Fe2O3能够诱导池体内的大量微生物将进水中的有机氮化成氨氮。所以结果表现为出水时实验组总氮质量浓度低于对照组,同时氨氮又高于对照组的现象,因此,即使实验组的出水氨氮质量浓度要高于对照组,也不能说明实验组的脱氮效果差。其中,对照组的出水氨氮质量浓度为(3.03±2.49) mg·L−1,而实验组的出水氨氮质量浓度则为(5.89±2.66) mg·L−1,到25 d后因水质波动分别提升至(9.83±2.82) mg·L−1和(17.27±3.86) mg·L−1(图5(a))。
该市政污水的硝态氮及亚硝态氮的质量浓度很低,平均进水质量浓度仅为0.49 mg·L−1及0.03 mg·L−1(图2)。但因为进水有机碳源不足,导致反硝化作用不充分,2组反应器的出水中硝酸盐及亚硝酸盐质量浓度均有上升。整体而言,对照组的出水硝酸盐质量浓度平均为(19.14±5.77) mg·L−1,高于实验组的(11.50±5.38) mg·L−1(图5(b));对照组的出水亚硝态氮质量浓度平均为(2.05±1.13) mg·L−1,高于实验组的(1.70±0.86) mg·L−1(图5(c))。这证明添加了α-Fe2O3的实验组在碳源不足的污水中的反硝化能力要强于对照组。结果证明添加α-Fe2O3能够提高实际市政污水处理中的生物脱氮效率。
2.2 生物质变化
原始泥样的MLSS含量为4 000 mg·L−1,MLVSS为2 211 mg·L−1,VSS/SS为0.552,显示本实验所用污泥活性较高[23]。如表1所示,2组反应器经过约40 d的培养后,对照组的污泥MLSS含量为4 125 mg·L−1,MLVSS为2 253 mg·L−1以及VSS/SS为0.553。这一结果显示,对照组的污泥生物质与原始泥样相比没有明显变化。而实验组因为额外投加了α-Fe2O3,MLSS和MLVSS含量分别显著提升至5 239 mg·L−1和2 599 mg·L−1,同时VSS/SS降至0.496,显示污泥生物质存在明显变化。其中实验组的MLSS含量仅比对照组的含量增加了1 114 mg·L−1,低于投入反应器中1 333.3 mg·L−1的α-Fe2O3,说明α-Fe2O3会随着反应器的出水少量流失。同时实验组的MLVSS比对照组高了316 mg·L−1。这说明α-Fe2O3可能具有诱导胞外聚合物分泌的能力。
表 1 原始泥样和反应器中的MLSS和MLVSS及其比值Table 1. MLSS, MLVSS and their ratios in the raw sludge and reactors样品 MLSS MLVSS MLSS/MLVSS 原始泥样 4 000 2 211 0.552 对照组 4 125 2 283 0.553 实验组 5 239 2 599 0.496 2.3 微生物种群结构分析
通过宏基因组对2组反应器中的微生物群落进行门和属水平上的分类并进行功能结构分析。依据宏基因组学的物种丰度聚类分析测序结果显示,2组反应器的活性污泥中总共包含42个菌门,其中未被定义的菌门分别占对照组和实验组中总菌门的25.28%和23.54%。在门水平上,在对照组和实验组反应器中占比均超过1%的有Proteobacteria (51.64%;53.69%)、Actinobacteria (11.62%;15.13%)、Nitrospirae (6.19%;2.23%)、Bacteroidetes (1.65%;2%)和Firmicutes (1.04%;1.62%) (图6(a))。其中,在2组反应器中占比较高的Proteobacteria (变形菌门)和Actinobacteria (放线菌门)是优势菌门,二者在不同反应器中占比均超过了10%。变形菌门中含有大量可以分别在好氧和厌氧的环境中生存的硝化以及反硝化菌属 [24-25]。这2个菌门在实验组中的占比均高于对照组,说明投加α-Fe2O3可以刺激反硝化菌的生长。剩余的优势菌门Nitrospirae (硝化螺旋菌门)、Bacteroidetes (拟杆菌门)和Firmicutes (厚壁菌门)中同样包含大量与氮代谢相关菌属,说明反应器的微生物群落有利于去除含氮污染物。
而属水平上的分类显示,2组反应器中未被定义的菌属分别占对照组和实验组中总菌属的29.8%和27.19%。在对照组和实验组反应器中占比均超过1 %的属分别有Thauera (17.34%;11.85%)、Nitrospira (6.19%;2.23%)、Alicycliphilus (3.62%;2.14%)、Candidatus_Accumulibacter (2.89%;3.9%)、Mycolicibacterium (2.84%;1.83%)、Pseudomonas (2.52%;2.93%)、Micropruina (2.48%;5.78%)、Dechloromonas (2.42%;2.68%)、Casimicrobium (2.11%;5.11%)、Afipia (1.79%;1.57%)、Diaphorobacter (1.71%;1.44%)、Sphaerotilus (1.31%;6.37%)(图6(b))。其中,优势菌属Thauera、Pseudomonas以及Dechloromonas是具有异养硝化-好氧反硝化功能的菌属,能在有氧条件下将不同形态的氮转化为氮气[26-28]。此外,一些HN-AD菌被证明具有尿素水解酶[29],可以将有机氨转化为氨氮。这可能是实验组反应器内氨氮质量浓度高于对照组的原因。Nitrospira是典型的亚硝酸盐氧化菌[30]。Alicycliphilus具有多样性的代谢机制,可利用硝酸盐等作为电子受体,参与污水处理中的反硝化过程[31]。Candidatus_Accumulibacter是典型的反硝化聚磷菌,在缺氧条件下以硝酸盐或亚硝酸盐为电子受体实现反硝化和聚磷[32]。Mycolicibacterium是一种革兰氏阳性菌,具有脲酶和硝酸盐还原活性[33]。Casimicrobium是市政污水处理厂一种常见的好氧菌,却能将硝酸盐还原为唯一产物亚硝酸盐[34]。Sphaerotilus是一种铁基好氧菌,能够以以Fe2+为电子供体实现自养反硝化[35],其相对丰度在实验组中增加进一步证实了α-Fe2O3参与了反硝化菌的呼吸过程。Micropruina是一种聚糖元菌,在厌氧条件下可以将糖、氨基酸等同化成末端发酵产物如乳酸盐、乙酸盐、乙醇、丙酮酸盐等(作为内源性碳)[36]。Afipia属主要参与大多数有机底物如葡萄糖、果糖、蔗糖等的氧化[37]。总之,氨氮的转化和去除是HN-AD菌、硝化菌、反硝化菌共同作用的结果。
2.4 功能基因注释
通过对比2组反应器中与氮转化相关基因,可以分析α-Fe2O3对生物脱氮的影响。首先探究了能够影响氨化过程的功能基因,研究发现检出的实验组氨化功能基因簇ureA、ureB和ureC的表达量均高于对照组[38],分别为对照组的1.58,1.89和1.03倍。此外,实验组中还检出了对照组中所没有的功能基因gdh[39](图7(a))。这说明在功能基因的层面上,α-Fe2O3确实能够诱导微生物更高效进行氨化反应。这进一步印证了对照组反应器中氨氮质量浓度较高的现象。在能够影响硝化过程功能基因中,功能基因amoB、amoC、hao、nxrA和nxrB的表达量实验组均高于对照组[40],分别为对照组的1.36、1.19、1.29、1.05和1.31倍,证明添加α-Fe2O3确实可以提高微生物的硝化潜能(图7(b))。而在影响反硝化过程的功能基因中,除narI和nirK外,实验组的narG、narH、napA、napB、nirS、norC、norB和nosZ的相对表达量均高于对照组[41],分别为对照组的1.05、1.31、1.59、2.09、1.04、2.23、1.68、1.19倍(图7(c))。功能基因相对丰度的差异表明,添加α-Fe2O3可正向调控氮转化功能基因的表达。本研究显示在单一池体的曝气条件下,生物巢可以实现同步硝化和反硝化。
此外,本研究对跟电子转移蛋白相关的编码基因进行了研究,共检索到4个编码跨膜电子转移蛋白的功能基因,分别是mtrA、mtrB、mtrC和macA[42]。与对照组相比,实验组的4个功能基因的表达量均受到了抑制,分别为对照组的67.89%、47.59%、63.56%和58.23% (图8)。这意味着投加α-Fe2O3后细胞已不需要更多的跨膜电子转移蛋白参与电子向胞外的传输,从而导致电子转运蛋白表达量减少。
2.5 碳源有效利用机理分析
如上所述,实验组中跨膜电子转移相关基因的表达量要普遍低于对照组,这意味着更多的电子参与胞内含氮化合物尤其是硝酸盐的还原或反硝化过程。这导致了α-Fe2O3存在的情况下,消耗单位COD可以有利于去除更多的TN。外膜上电子转移蛋白表达的下调间接表明α-Fe2O3在胞内参与了电子传递与含氮化合物的转化[43-44]。此外,TEM表征结果(图9)表明,实验组中的细胞内存在大量黑色颗粒物,而未投加α-Fe2O3的对照组中则没有颗粒物的堆积。这一现象进一步证明了α-Fe2O3能够进入胞内参与电子传递(即介导了NADH等电子供体与硝酸盐还原酶之间的电子传递),保证了更多电子(或碳源)被用于反硝化过程。这解释了在α-Fe2O3存在下消耗单位COD可以去除更多TN的原因。
综上所述,α-Fe2O3一方面能够显著提高氮转化相关基因的表达水平,提高系统内氮转化的效率;另一方面能够下调微生物周质空间和外膜上电子转移蛋白的表达,减少了跨膜的电子转移,将更多的电子留存在胞内进行硝酸盐呼吸,实现碳源的有效利用。基于出水水质、微生物群落结构、功能基因结构及其表达水平,本研究推测性地提出了α-Fe2O3介导下反硝化菌胞内的氮代谢通路(图10)。
3. 结论
本研究依据外加铁源对生物脱氮性能的影响,探索性地将α-Fe2O3材料和MBF生物巢联系起来,成功构建出一种能够显著提高MBF生物巢反应器内单位COD利用效率的新工艺。具体结论如下。
1)实际污水实验中,对照组和实验组反应器对总氮的去除率分别为(21.85±11.35)%和(32.21±11.35)%,单位COD的脱氮效率(TN/COD)则分别为0.234和0.370,实验组比对照组的脱氮效率高出58%,说明α-Fe2O3能够提高MBF生物巢反应器对实际市政废水的脱氮效果。
2)宏基因组测序结果表明,α-Fe2O3的投加提高了氮转化相关基因的表达量,同时能够抑制周质与外膜上电子转移蛋白相关基因的表达。这说明α-Fe2O3能够参与胞内电子转移,促使更多电子参与胞内硝酸盐呼吸作用。
-
表 1 原始泥样和反应器中的MLSS和MLVSS及其比值
Table 1. MLSS, MLVSS and their ratios in the raw sludge and reactors
样品 MLSS MLVSS MLSS/MLVSS 原始泥样 4 000 2 211 0.552 对照组 4 125 2 283 0.553 实验组 5 239 2 599 0.496 -
[1] ZHEN Y Z, JIAN Z, GEORGE Z. Transformation of water resource management: A case study of the South-to-North Water Diversion project[J]. Journal of Cleaner Production, 2015, 163: 136-145. [2] 栗清静. 中国水资源利用与水环境保护探析[J]. 绿色环保建材, 2020, 160(6): 56-57. [3] 王仲杰. 探析市政污水处理存在的问题及对策[J]. 皮革制作与环保科技, 2022, 3(20): 179-181. [4] CHEN X J, YUAN L J, ZHAO B B. Capturing influent organic substrate for endogenous denitrification to enhance nitrogen removal in low C/N ratio municipal wastewater[J]. Journal of Water Process Engineering, 2022, 50: 103240. doi: 10.1016/j.jwpe.2022.103240 [5] 王冰, 杨琳, 孙冰, 等. 生物脱氮工艺的原理及研究发展[J]. 建筑与预算, 2020, 295(11): 5-7. [6] 姜瑞, 于振波, 李晶, 等. 生物接触氧化法的研究现状分析[J]. 环境科学与管理, 2013, 38(5): 61-63. doi: 10.3969/j.issn.1673-1212.2013.05.014 [7] 刘亚琴. 基于沸石吸附/再生的改良型A/O脱氮工艺研究[D]. 镇江: 江苏大学, 2018. [8] 刘平. 生活污水A/O处理系统运行优化改造研究[D]. 武汉: 武汉科技大学, 2008. [9] 徐亚同, 史家梁, 张大鹏. 废水处理 第五篇 废水好氧生物处理的方法(一)——活性污泥法[J]. 上海化工, 1998(17): 39-42. [10] NI H C, ZHOU X T, ZHANG X Y, et al. Feasibility of using basalt fiber as biofilm Carrier to construct bio-nest for wastewater treatment[J]. Chemosphere, 2018, 212: 768-776. doi: 10.1016/j.chemosphere.2018.08.136 [11] 倪慧成. 基于改性玄武岩纤维(MBF)填料的污/废水处理技术及其应用研究[D]. 镇江: 江苏大学, 2022. [12] 张倩. 新型玄武岩纤维填料在生活污水处理中的结构组合研究[D]. 镇江: 江苏大学, 2019. [13] 席海朋. 厌氧/好氧折流板反应器强化生活污水脱氮的研究[D]. 镇江: 江苏大学, 2021. [14] 廖宏翔, 肖安琪. 城镇污水处理厂外加碳源分析和可降耗措施探究[J]. 四川化工, 2023, 26(02): 49-51+56. [15] ZHI M W, WEI J L, ZHENG Q Y, et al. Denitrification mechanism in oxygen-rich aquatic environments through long-distance electron transfer[J]. Npj Clean Water, 2022, 5: 61. doi: 10.1038/s41545-022-00205-x [16] SOUICHIRO K, KAZUHITO H, KAZUYA W. Microbial interspecies electron transfer via electric currents through conductive minerals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(25): 10042-10046. [17] WANG S Y, YANG X Y, MENG H S, et al. Enhanced denitrification by nano ɑ-Fe2O3 induced self-assembled hybrid biofilm on particle electrodes of three-dimensional biofilm electrode reactors[J]. Environment International, 2019, 125: 142-151. doi: 10.1016/j.envint.2019.01.060 [18] CAROLINA V C, SIMONA R, STEFANO F, et al. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation[J]. Environmental Science Technology, 2014, 48(13): 7536-7543. doi: 10.1021/es5016789 [19] ZHANG L H, WANG S J. Bacterial community diversity on in-shell walnut surfaces from six representative provinces in China[J]. Scientific Reports, 2017, 7(1): 10054. doi: 10.1038/s41598-017-10138-y [20] LU J, BREITWIESER F P, THIELEN P, et al. Bracken: estimating species abundance in metagenomics data[J]. Peerj Computer Science, 2017, 3(1): e104. [21] COTILLARD A, KENNEDY S P, KONG L C, et al. Dietary intervention impact on gut microbial gene richness[J]. Nature, 2013, 500(7464): 585. doi: 10.1038/nature12480 [22] 郭欣. 生物透射电镜样品制备过程中常易遇到的问题以及解决办法[J]. 科技信息, 2011, 376(20): 539-540. doi: 10.3969/j.issn.1001-9960.2011.20.468 [23] LI Y, LIU S J, CHEN F M, et al. Development of a dynamic feeding strategy for continuous-flow aerobic granulation and nitrogen removal in a modified airlift loop reactor for municipal wastewater treatment[J]. Science of the Total Environment, 2020, 714: 136764. doi: 10.1016/j.scitotenv.2020.136764 [24] 郑力, 江鹰, 程晓夏. 铁屑耦合固相反硝化对低碳氮比废水中总氮的处理[J]. 环境工程学报, 2022, 16(11): 3716-3727. [25] 杨浩, 张国珍, 杨晓妮, 等. 16S rRNA高通量测序研究集雨窖水中微生物群落结构及多样性[J]. 环境科学, 2017, 38(04): 1704-1716. [26] SOLISIO C, LODI A, CONVERTI A, et al. The effect of acid pre-treatment on the biosorption of chromium(III) by Sphaerotilus natans from industrial wastewater[J]. Water Research, 2000, 34(12): 3171-3178. doi: 10.1016/S0043-1354(00)00059-2 [27] MIGUEL S, LEA W, Sara H, et al. Differential expression of clade I and II N2O reductase genes in denitrifying Thauera linaloolentis 47LolT under different nitrogen conditions[J]. FEMS Microbiology Letters, 2020, 367(24): 205. [28] XU T, YAN L Y, YONG W L, et al. Quantitative ecology associations between heterotrophic nitrification-aerobic denitrification, nitrogen-metabolism genes, and key bacteria in a tidal flow constructed wetland[J]. Bioresource Technology, 2021, 337: 125449. doi: 10.1016/j.biortech.2021.125449 [29] ZHOU X T, ZHAO L, WANG X, et al. Organic and inorganic nitrogen removals by an ureolytic heterotrophic nitrification and aerobic denitrification strain Acinetobacter sp. Z1: elucidatingits physiological characteristics and metabolic mechanisms[J]. Bioresource Technology, 2022, 362: 127792. doi: 10.1016/j.biortech.2022.127792 [30] BAYER B, SAITO M A, MCILVIN M R, et al. Metabolic versatility of the nitrite-oxidizing bacterium Nitrospira marina and its proteomic response to oxygen-limited conditions[J]. The ISME Journal, 2020, 15(4): 1025-1039. [31] GONZÁLEZ C J, LOZA-TAVERA H. Alicycliphilus: current knowledge and potential for bioremediation of xenobiotics[J]. Journal of applied microbiology, 2019, 126(6): 1643-1656. doi: 10.1111/jam.14207 [32] SONDOS A, LAURENS W, BEN A, et al. Denitrification of nitrate and nitrite by ‘Candidatus Accumulibacter phosphatis’hosphatIC[J]. Water Research, 2016, 105: 97-109. doi: 10.1016/j.watres.2016.08.061 [33] GOLUBEV S N, MURATOVA A Y. , PANCHENKO L V , et al. Mycolicibacterium sp. strain PAM1, an alfalfa rhizosphere dweller, catabolizes PAHs and promotes partner-plant growth[J]. Microbiological Research, 2021, 253: 126885. [34] SONG Y, JIANG Y C, LIANG Z L, et al. Casimicrobium huifangae gen. nov. , sp. nov. , a Ubiquitous “Most-Wanted” Core Bacterial Taxon from Municipal Wastewater Treatment Plants[J]. Applied and Environmental Microbiology, 2019, 86(4): 1183-1195. [35] LIANG Y F, PAN Z R , FENG H B, et al. Biofilm coupled micro-electrolysis of waste iron shavings enhanced iron and hydrogen autotrophic denitrification and phosphate accumulation for wastewater treatment[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108959. [36] ZHEN J Y, OEHMEN A, WEI W, et al. Synergism and physiological characteristics of glycogen accumulating organisms (GAOs) in anaerobic ammonia oxidation based (anammox-based) systems: Mechanisms and prospects[J]. Chemical Engineering Journal, 2023, 478: 147316. doi: 10.1016/j.cej.2023.147316 [37] BERNARD S L, MARIE-NOËLLE M, GRIMONT P A D, et al. Description of Afipia birgiae sp. nov. and Afipia massiliensis sp. nov. and recognition of Afipia felis genospecies A.[J]. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(5): 1773-1782. [38] NEYROLLES O, FERRIS S, BEHBAHANI N, et al. Organization of Ureaplasma urealyticum urease gene cluster and expression in a suppressor strain of Escherichia coli[J]. Journal of Bacteriology, 1996, 178(9): 2725. doi: 10.1128/jb.178.9.2725-2725.1996 [39] SPANAKI C, PLAITAKIS A. The role of glutamate dehydrogenase in mammalian ammonia metabolism.[J]. Neurotoxicity Research, 2012, 21(1): 117-127. doi: 10.1007/s12640-011-9285-4 [40] LI L , DONG Y H, QIAN G S, et al. Performance and microbial community analysis of bio-electrocoagulation on simultaneous nitrification and denitrification in submerged membrane bioreactor at limited dissolved oxygen[J]. Bioresource Technology, 2018, 258: 168-176. [41] BERGAUST L, VAN S R J M, FROSTEGÅRD Å, et al. Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR[J]. Microbiology, 2012, 158(Pt3): 826-834. [42] BJÖRN S, JULIAN S , GUNNAR S , et al. Investigation of the electron transport chain to and the catalytic activity of the diheme cytochrome c peroxidase CcpA of Shewanella oneidensis. [J]. Applied and Environmental microbiology, 2011, 77(17): 6172-80. [43] WANG Z X, WANG X P, SUN Y, et al. Fe(OH)3 induced the Anammox system to perform extracellular electron transfer for enhancement of NH4+ removal[J]. Chemical Engineering Journal, 2023, 460: 141768. doi: 10.1016/j.cej.2023.141768 [44] CHEN S T, ZHOU B H, CHEN H L, et al. Iron mediated autotrophic denitrification for low C/N ratio wastewater: A review[J]. Environmental Research, 2023, 216: 114687. doi: 10.1016/j.envres.2022.114687 -




 下载:
下载: