-
饮用水安全与人类健康密切相关[1],随着经济的发展与生活水平的不断提高,饮用水安全问题受到人们的广泛关注[2-3]。有研究表明,部分地区饮用水中含有多种微污染物,包括消毒副产物(disinfection by-products, DBPs)[4-5]、药品及个人护理品(pharmaceutical and personal care products, PPCPs)[6-9]、内分泌干扰物(endocrine disrupting chemicals, EDCs)[10-12]等。如WANG等研究发现饮用水源中抗生素质量浓度能够达到364~580 ng·L−1 [6]。此外,多地自来水厂出水中卤代甲烷、卤乙酸等DBPs的含量超标[13-14]。这些微污染物即使在低浓度下也会对人体健康和生态环境造成危害,长期饮用含有这些微污染物的饮用水有致癌、致畸、致突变等潜在危害[12, 15-18]。因此对饮用水进行深度处理具有重要意义。
吸附是一种常用的饮用水深度处理技术[8, 17-18]。活性炭是一种常用吸附剂,被广泛应用于饮用水处理中[15-16]。但活性炭对有些污染物吸附能力较差,有研究表明,多种市售活性炭对DBPs的吸附容量仅有7.4~98.8 µg·g−1 [19],这使得活性炭的使用寿命较低。市售活性炭滤芯的建议寿命一般为3~9个月,但有调查显示66.7%的家用净水器中的活性炭滤芯未能及时更换[20]。这些滤芯在长时间使用情况下无法有效去除饮用水中的微污染物,危害人体健康。因此,设法延长活性炭滤芯的使用寿命受到人们的关注。
电吸附技术是通过施加电场使水中带电物质在电场作用下向带相反电荷的电极(吸附剂)表面移动,形成双电层[6, 21],实现吸附质在吸附剂上的富集,从而去除水中污染物。通过施加外电场,可以增强吸附过程中吸附剂与吸附质之间的静电吸引力,使吸附过程的吸附速率与吸附容量大幅提升,进而延长吸附剂的使用寿命[22-23]。如LI等[21]制备了碳纳米管电极,在0.6 V电压下全氟烷酸化合物的初始吸附速率相比不加电的条件下提高了41~60倍,最大吸附容量相比不加电下提高了50~94倍。WANG等[6]发现在外加电压辅助下活性炭纤维对3种抗生素的吸附容量能达到不加电下的约5倍。HAN等[24]考察了0.6 V外加电压对活性炭纤维(ACF)吸附苯胺性能的影响,结果表明,ACF对苯胺的最大吸附容量比不加电时提升了约3倍。YU等[22]制备了氧化石墨烯/聚吡咯修饰的多孔铜镍泡沫电极并发现电辅助下该电极对罗丹明B的吸附能力对比不加电时提高了1.8倍。由此可见,在活性炭滤芯上施加电压,通过电增强的方法有望提高活性炭滤芯的吸附速率与吸附容量,延长滤芯的使用寿命。
本研究从10种市售活性炭滤芯中筛选出比表面积大、导电性良好的电吸附滤芯,考察了电增强对这些活性炭滤芯吸附饮用水中3种典型微污染物二氯乙酸(dichloroacetic acid, DCAA)、左氧氟沙星(levofloxacin, LVFX)和环丙沙星(ciprofloxacin, CIP)效果的影响。其中,DCAA是一种饮用水中常见的含氯消毒副产物,过量摄入对人体具有致癌作用[25-26],在《生活饮用水卫生标准》(GB 5749-2022)中的限值为50 µg·L−1;LVFX和CIP是饮用水源中常被检出的氟喹诺酮类抗生素[6, 8],在2023年施行的《重点管控新污染物清单(2023年版)》中被列入了重点管控范围。通过测试活性炭滤芯在不同电压下对3种微污染物的吸附动力学与吸附等温线,研究外加电压对活性炭滤芯吸附3种微污染物的影响,并进一步考察活性炭滤芯在流动态下对水中DCAA的电增强吸附效果,测定出水水质与处理水量,评估其在饮用水处理中的应用前景。
-
10种市售活性炭滤芯;DCAA、LVFX、CIP和磷酸二氢钾(分析纯),购自上海阿拉丁试剂有限公司;硫酸钠、磷酸和草酸(分析纯),购自天津大茂化学试剂厂;甲醇和乙腈(色谱纯),购自百灵威化学试剂有限公司;高纯水(18.2 MΩ·cm,Milli-Q超纯水机)。
实验仪器:高效液相色谱(HPLC 2695,美国Waters公司);扫描电子显微镜(S4800,日本日立公司);万能测试仪(SHIMADZU,日本岛津公司);泡点测试仪(POROLUX 1000,德国Porolux公司);全自动比表面积及孔径分析仪(Quadrasorb S14,美国康塔公司);纳米粒度及Zeta电位分析仪(ZS90,英国马尔文仪器有限公司);电化学工作站(CHI660,上海辰华仪器有限公司);电化学工作站(Parstat2273,美国阿美特克公司)。
-
测定所购10种活性炭滤芯的密度;使用全自动比表面积及孔径分析仪测定各滤芯的比表面积与孔径;使用电阻率仪测试各滤芯的电阻率,确定其电导率;使用万能测试仪测试10种滤芯的压缩强度;过滤精度是指能够通过滤芯的最大颗粒尺寸,活性炭滤芯的过滤精度可以表示活性炭颗粒间网络状通道孔的大小,本研究通过泡点测试仪测定各滤芯的过滤精度;采用扫描电子显微镜(SEM)观察活性炭滤芯的表面形貌。10种滤芯的相关参数见表1。
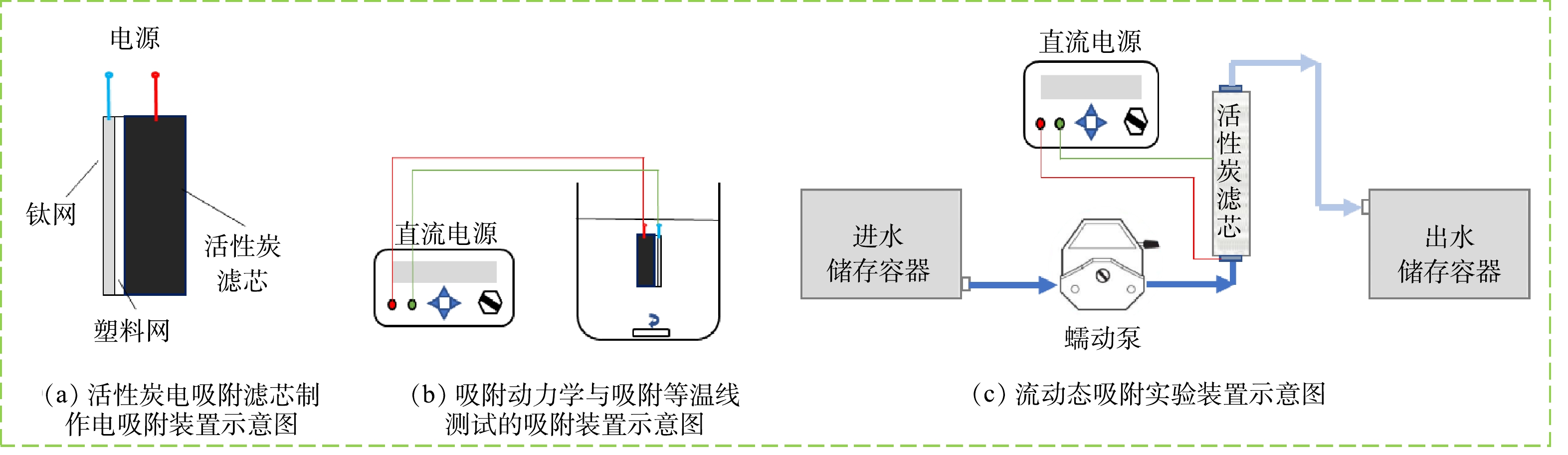
对比表1中10种滤芯的基本性质,初步筛选出比表面积较大或导电性较好的2号、6号与9号滤芯。在这3种滤芯上分别截取相同质量(0.4 g)的滤芯块体进行吸附和电吸附实验。其中吸附实验是将取下的滤芯置于含1 mmol·L−1 Na2SO4电解质的中性DCAA溶液(40 mg·L−1)中进行吸附;电吸附实验是在按图1(a)所示的电吸附滤芯上施加2.0 V电压,并将滤芯置于含1 mmol·L−1 Na2SO4的中性DCAA溶液(40 mg·L−1)中开展实验。反应过程中不断搅拌,吸附平衡后(7 d)取样检测溶液中DCAA质量浓度。比较各滤芯的吸附量,筛选出合适的滤芯用于后续实验。
以筛选出的滤芯作为工作电极,Ag/AgCl电极作为参比电极,铂丝电极作为对电极,分别在含1 mmol·L−1 Na2SO4的中性DCAA溶液(100 mg·L−1)、LVFX (300 mg·L−1)和CIP (30 mg·L−1)溶液中,以10 mV·s−1的扫描速率扫描活性炭滤芯的循环伏安曲线,扫描电压为-1.0 V~1.0 V。将筛选出的滤芯研磨后混合到1 mmol·L−1 Na2SO4溶液中后,使用纳米粒度及Zeta电位分析仪测定滤芯在不同pH (2.5~10)下的Zeta电位,将pH和Zeta电位作图,可获得滤芯表面电荷为零的pH,即等电点pHiep。使用电化学工作站采用浸没电势法测量活性炭滤芯电极的零电荷电势EPZC。
-
在筛选出的活性炭滤芯上截取0.4 g,按图1(a)所示制成电吸附滤芯。将电吸附滤芯置于不断搅拌的溶液中,连接直流电源,进行电吸附实验,电吸附动力学实验装置见图1(b)。在−1.0、0(不加电条件)、1.0、1.5和2.0 V条件下对40 mg·L−1的DCAA溶液(内含1 mmol·L−1 Na2SO4,调节pH=7)进行吸附;此外,在−2.0、−1.0、0和1.0 V条件下对300 mg·L−1的LVFX溶液和30 mg·L−1的CIP溶液(内含1 mmol·L−1 Na2SO4,调节pH=7)进行吸附。定时取样并测量溶液浓度。分别采用准一级动力学模型(式(1))和准二级动力学模型(式(2))拟合3种微污染物在不同电压下的吸附动力学实验数据。
其中:qt和qe分别为t时刻和吸附平衡时污染物在活性炭滤芯上的吸附量,mg·g−1;k1为准一级吸附常数,h−1;k2为准二级吸附常数,g·(mg·h)−1;v0是初始吸附速率,mg·(g·h)−1。
-
在不同外加电压下进行DCAA (−1.0、0、1.0和2.0 V)、LVFX和CIP (−2.0、−1.0、0和1.0 V)的吸附等温线实验,实验方法与(2)中相同,吸附质质量浓度范围分别为20~100 mg·L−1 (DCAA),100~500 mg·L−1 (LVFX)和10~30 mg·L−1 (CIP)。吸附等温线数据分别用Langmuir模型(式(3))和Freundlich模型(式(4))进行拟合。
式中:qe为达到吸附平衡时吸附剂的吸附量,mg·g−1;ce为吸附平衡时溶液中吸附质质量浓度,mg·L−1;qm为最大吸附容量,mg·g−1;b为Langmuir吸附平衡常数;KF和n分别为与吸附容量和吸附强度有关的Freundlich模型常数。
-
按图1(a)制备电吸附滤芯,活性炭滤芯的体积为1.4 mL (即1个床体积为1.4 mL),使用制备的电吸附活性炭滤芯对100 µg·L−1的含1 mmol·L−1 Na2SO4的中性DCAA溶液(2倍于《生活饮用水卫生标准》GB 5749-2022中DCAA限值50 µg·L−1)进行电吸附处理,实验装置如图1(c)所示。用蠕动泵控制DCAA溶液流速为2.4 mL·min−1,使溶液流过滤芯并被滤芯吸附处理。分别在0 V和2.0 V电压下进行电增强吸附实验,前期每30床体积取样检测1次,200床体积后每60床体积取样检测1次。测定出水中DCAA质量浓度,出水中DCAA质量浓度超过50 µg·L−1时,达到《生活饮用水卫生标准》(GB 5749-2022)中DCAA的限值,认为滤芯达到使用寿命,停止实验,确定滤芯处理的水量。
-
3种污染物DCAA、LVFX和CIP的结构式、相对分子质量、溶解度以及pKa见表2。采用液相色谱法对3种污染物进行定量分析。色谱柱为Ultimate XB-C18 (4.6 mm×250 mm,5 µm),柱温30 ℃,流动相流速为1 mL·min−1。DCAA的流动相为乙腈/磷酸溶液(pH=2) (20:80,V:V),进样量20 µL,紫外检测器,检测器波长214 nm。LVFX的流动相组成是乙腈/0.01 mol·L−1草酸溶液/甲醇(35:45:20,V:V:V),进样量10 µL,紫外检测器,检测波长为293 nm。CIP的流动相是乙腈/0.02 mol·L−1磷酸溶液(22:78,V:V),进样量10 µL,荧光检测器,检测器的激发波长(Ex)为284 nm,发射波长(Em)为445 nm。
-
如图2(a)所示,活性炭滤芯为空心圆筒状,滤芯外径尺寸为65 mm,内径为28 mm,高度为254 mm。通过扫描电镜观察滤芯的表面形态。由图2(b)可见,活性炭滤芯是由粘结剂将颗粒活性炭粘结而成。颗粒活性炭直径约为200~400 µm,并且颗粒活性炭上含有丰富的孔道结构(图2(c)孔道Ⅰ)。
由表1可见除6号滤芯是果木活性炭,其他9种滤芯均为椰壳活性炭。各滤芯的比表面积差别较大,1号、7号和10号滤芯比表面积小于100 m2·g−1,而2号、4号、8号和9号比表面积大于400 m2·g−1。活性炭的孔道以微孔为主,大的比表面积与丰富的微孔结构有利于滤芯对污染物的吸附。10种活性炭滤芯均具有导电性,其中2号和6号滤芯的电导率分别为14.7 S·m−1和32.2 S·m−1,明显优于其他活性炭滤芯(0.4~4.1 S·m−1)。10种滤芯的密度差别不大,均在0.6~0.8 g·cm−3。此外,滤芯的压缩强度在1.8~6.7 MPa。家庭用水的水压一般不超过0.4 MPa,因此,10种活性炭滤芯均能满足家用水压的要求。活性炭滤芯的过滤精度可以表现活性炭颗粒之间的网络状通道孔(图2(b)中的孔道Ⅱ)的结构尺寸,测得10种滤芯的过滤精度在6.1~80.6 µm,其中2号滤芯(6.1 µm)过滤精度最小。
-
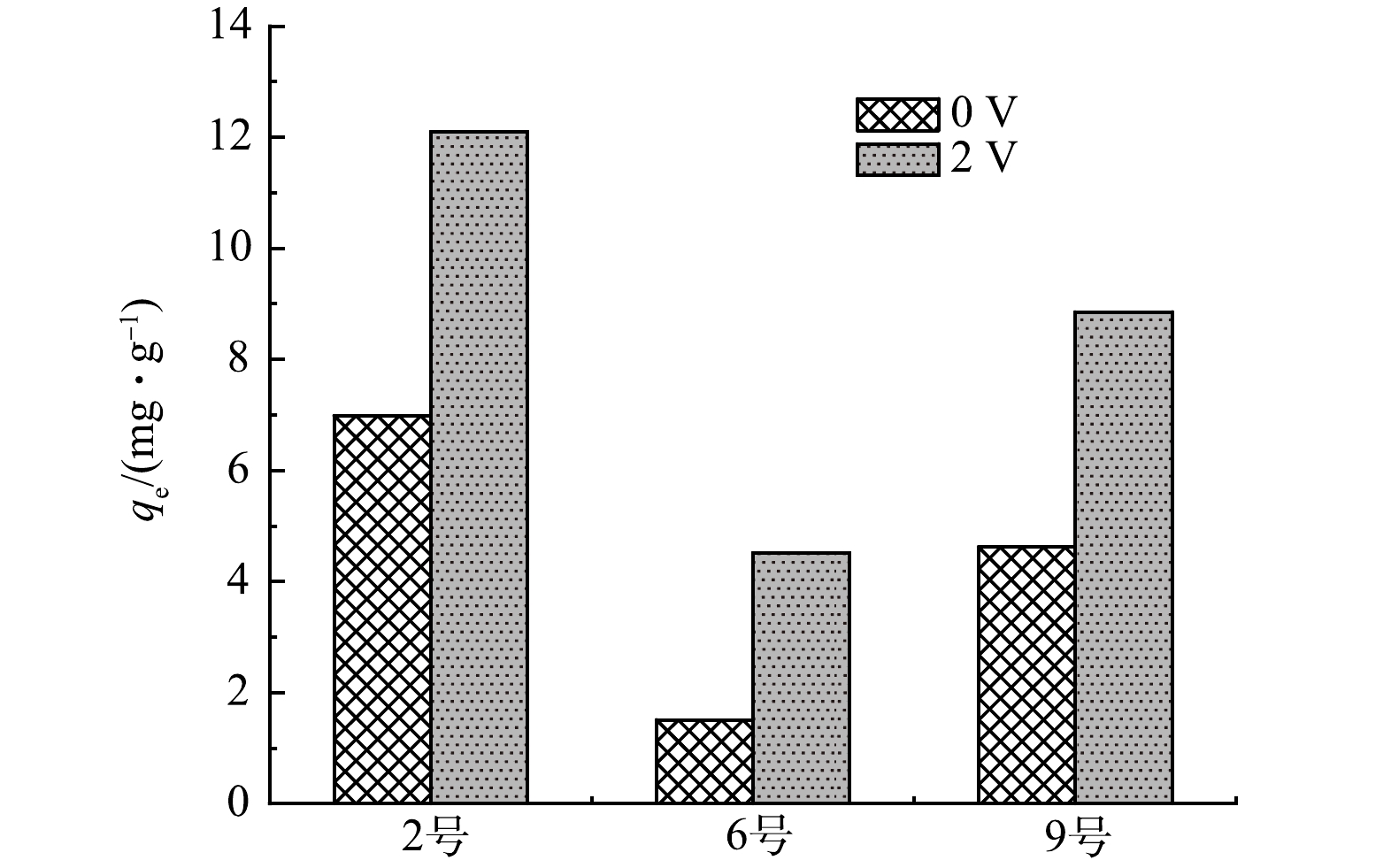
为满足电吸附过滤的要求,所采用的活性炭滤芯需具有良好的导电性、比表面积与吸附性能。对比10种活性炭滤芯可以发现,2号(441 m2·g−1,14.7 S·m−1)、6号(114.7 m2·g−1,32.2 S·m−1)与9号(603.6 m2·g−1,1.5 S·m−1)滤芯具有相对较好的比表面积或导电性能。此外,进一步测试了3种滤芯在0 V和2.0 V条件下对40 mg·L−1的DCAA溶液的吸附量。如图3所示,2号滤芯对DCAA具有最好的电吸附性能,在电压为2.0 V下吸附量达到12.1 mg·g−1,高于6号滤芯的4.5 mg·g−1和9号滤芯的8.9 mg·g−1,因此,选择2号滤芯作为后续研究对象。
-
图4是2号滤芯的循环伏安曲线。可以看出,在-0.75~0.75 V (vs Ag/AgCl)内滤芯不会发生水的电解反应,而且在此范围内滤芯也未与DCAA、LVFX和CIP发生电化学氧化还原反应。通过电化学工作站测量可知,−0.75~0.75 V相当于实验中的直流电源所施加的−2.2~2.2 V。因此,后续实验中选择外加电压为−2.0~2.0 V。
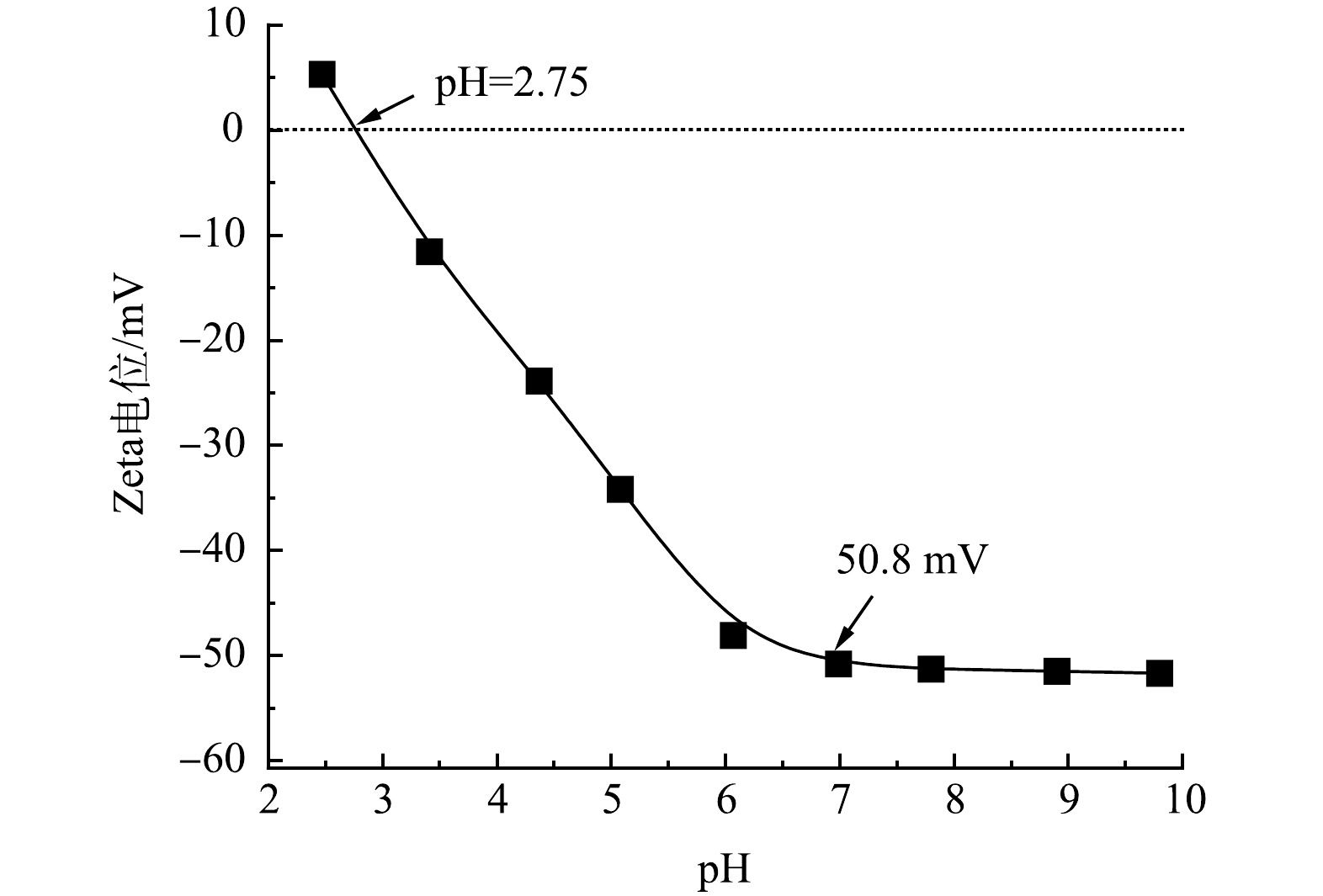
可用活性炭滤芯的Zeta电位表征活性炭颗粒的表面电荷特征,2号滤芯的Zeta电位随pH变化曲线如图5所示。滤芯的等电点pHiep=2.75,当pH>2.75时,滤芯表面带负电,中性条件下滤芯表面电势为−50.8 mV。活性炭滤芯电极的零电荷电势EPZC为43 mV,表明活性炭滤芯带弱的负电性,当外加电势为43 mV时,滤芯表现为零电荷。当施加与污染物电荷属性相反的外电压时,滤芯表面与溶液中污染物之间的静电吸引作用增强;反之,施加与污染物电荷属性相同的外电压时,静电排斥作用增强。
-
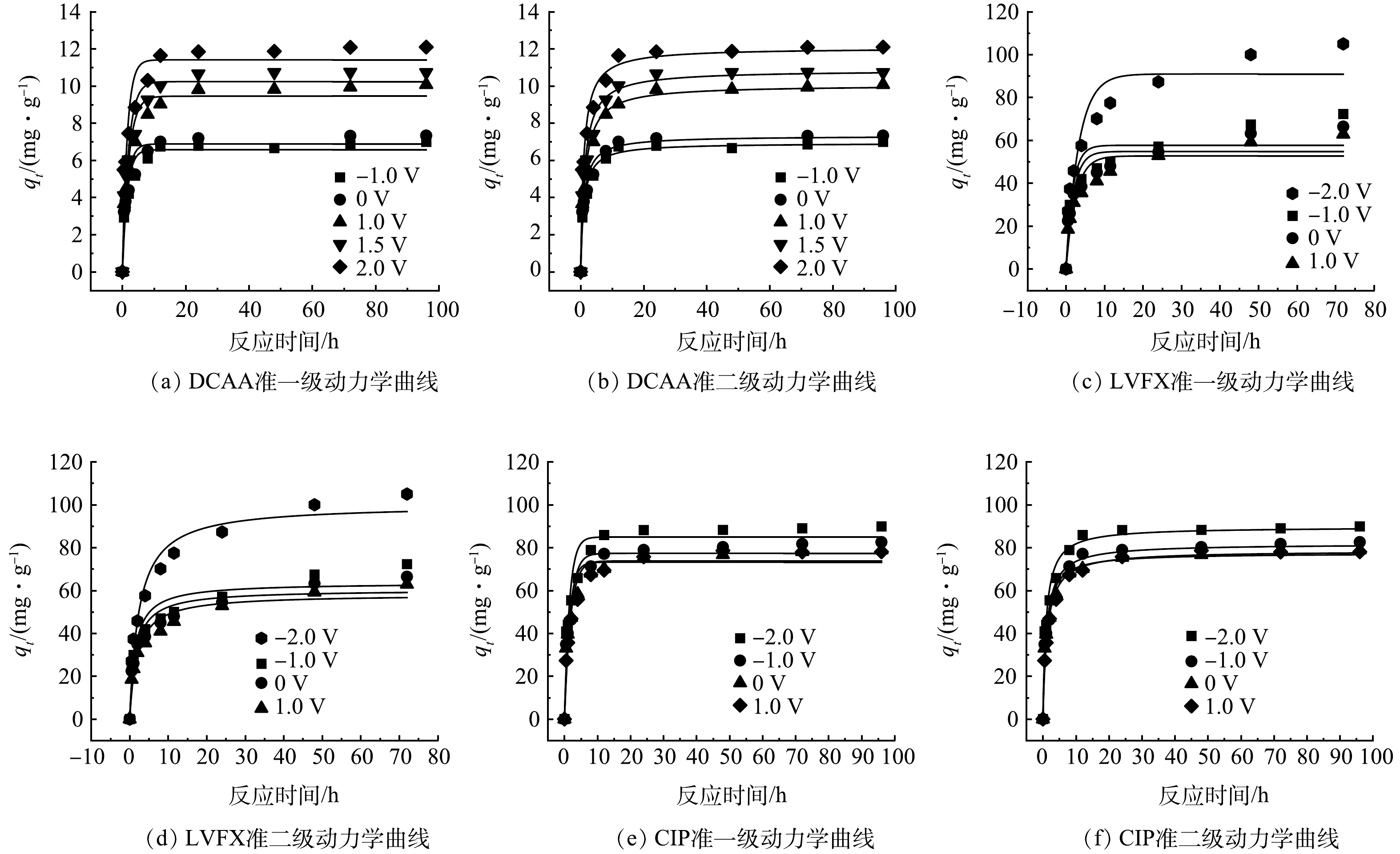
在不同电压下开展活性炭滤芯电增强吸附动力学实验。3种污染物吸附量随时间的变化如图6所示,前10 h是污染物的快速吸附期,这是3种污染物向吸附剂表面富集的过程。DCAA和CIP在20 h左右达到吸附平衡,而LVFX则在约50 h才达到吸附平衡。
采用准一级动力学与准二级动力学模型拟合吸附动力学数据,拟合曲线如图6所示,相关模型参数见表3。3种污染物的准二级动力学模型的相关系数R2均大于准一级动力学模型,这意味着吸附过程更符合准二级动力学模型,表明吸附过程存在着化学吸附,并且污染物的吸附容量与活性炭上的吸附位点有关[21]。DCAA属于有机酸,其pKa为1.26 (表2),在中性条件下以阴离子形式存在。滤芯的等电点pHiep=2.75,在pH=7时滤芯表面带负电荷,滤芯与DCAA阴离子静电排斥,不利于DCAA的吸附。但在实验中的外加正电压的影响下,滤芯表面带正电,与DCAA静电吸引。此外,随着外加电压由−1.0 V逐渐增加到2.0 V,DCAA与活性炭滤芯之间的静电吸引作用逐渐增强,吸附速率逐渐提高,顺序为:2.0 V>1.5V>1.0 V>0 V>−1.0 V。2.0 V下的初始吸附速率v0达到12.4 mg·(g·h)−1,相比不加电时提升了1.7倍。此外,LVFX和CIP是2种常用的氟喹诺酮类抗生素,其中LVFX含有胺基和羧基2种可解离基团,而CIP分子则含有羧基、胺基和哌嗪基等可解离基团,2种抗生素均属于两性分子。根据2种抗生素的pKa (表2)可知,在中性条件下LVFX和CIP均带正电。由于静电引力的作用,在负电压下LVFX和CIP的吸附速率增加,而在正电压下吸附速率降低,吸附速率的大小按如下规律排列:−2.0 V>−1.0 V>0 V>1.0 V。在−2.0 V电压下LVFX和CIP的v0分别达到45.3 mg·(g·h)−1和93.1 mg·(g·h)−1,比不加电时分别提升了1.2倍和1.3倍。
-
DCAA、LVFX和CIP的吸附等温线分别用Langmuir和Freundlich模型进行拟合,结果如图7所示,拟合后的相关模型参数见表4。Langmuir模型的相关系数R2更接近1,这表明Langmuir模型更符合3种污染物在活性炭滤芯上的吸附,吸附过程主要为单层吸附[21, 27]。在中性条件下DCAA以阴离子形式存在,而活性炭滤芯在中性条件下也带负电,与DCAA静电排斥。当实验过程施加正电压时,吸附剂与DCAA之间的静电引力增强,吸附量增大。在2.0 V电压下DCAA的最大吸附量qm达到26.3 mg·g−1,是不加电下的3.2倍。与DCAA相反,LVFX和CIP在中性条件下带正电,在外加负电压时,活性炭滤芯与LVFX和CIP之间的静电引力增强,吸附容量提高,负电压越大,吸附量则越大。在-2.0 V下LVFX和CIP的qm分别达到207.9 mg·g−1和106.1 mg·g−1,分别为不加电压时的1.5倍和1.2倍。
-
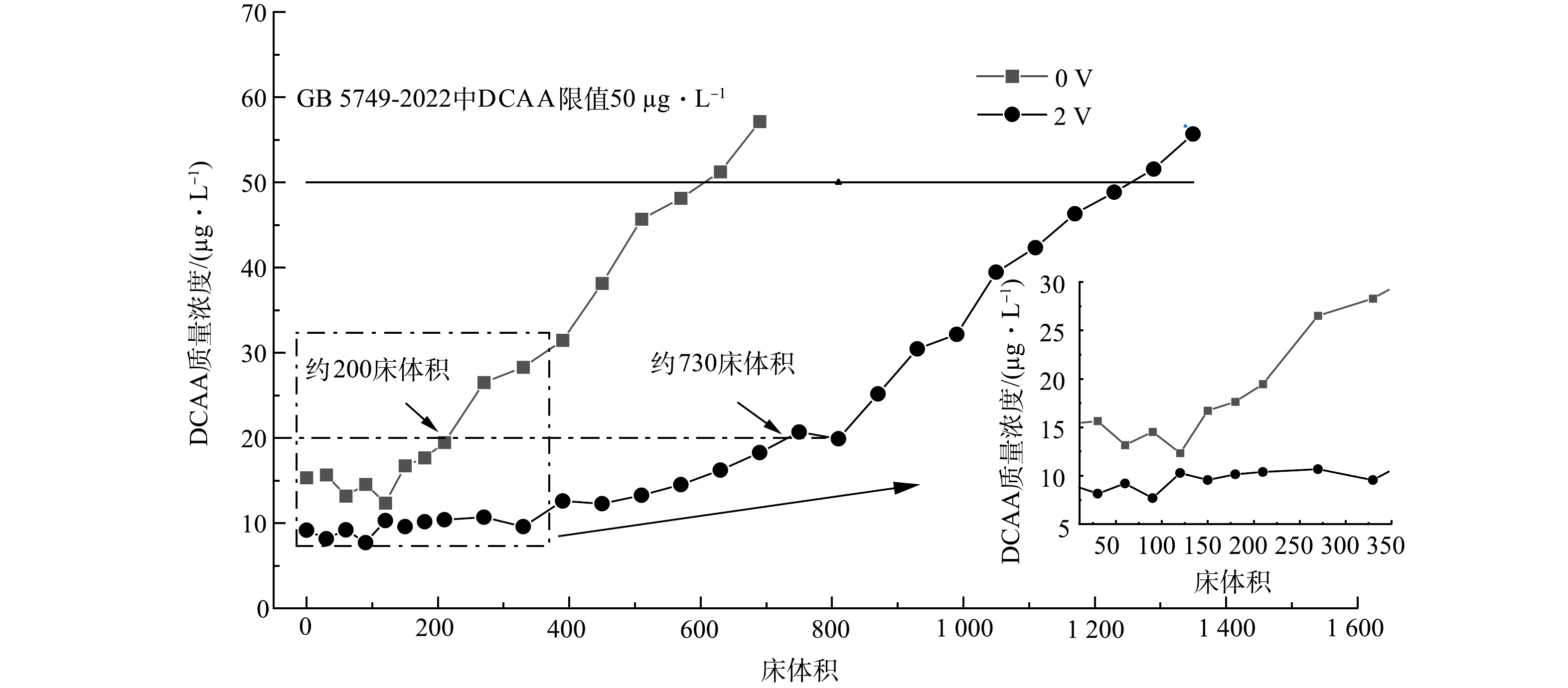
采用2号活性炭滤芯作为研究对象,进一步考察在流动态运行条件下电增强活性炭滤芯对DCAA的吸附性能。如图8所示,在2.0 V电压下,初始出水中DCAA质量浓度<8 µg·L−1,DCAA去除率大于92%,而0 V电压下初始出水中DCAA质量浓度>16 µg·L−1,去除率小于84%。随着处理水量的增加,0 V和2.0 V下的出水中DCAA的质量浓度均逐渐上升,但2.0 V电压下的出水质量浓度始终小于0 V的出水质量浓度,这表明2.0 V下出水DCAA质量浓度更低,水质更好。
施加0 V电压时,在处理水量约为200床体积以内时出水质量浓度增长较慢,维持在16~20 µg·L−1;在200床体积后,出水质量浓度超过20 µg·L−1并快速上升。在约600床体积时出水质量浓度便超过《生活饮用水卫生标准》 (GB 5749-2022)中DCAA的质量浓度上限50 µg·L−1。相比之下,施加2.0 V电压时,在约730床体积内出水质量浓度(8~20 µg·L−1)均较低。然后,出水DCAA质量浓度缓慢提升,并在1 300床体积后出水质量浓度才超过50 µg·L−1。受外加电压产生的静电引力的影响,在2.0 V下滤芯吸附DCAA的能力增强,活性炭滤芯的处理水量达到1 300床体积,相比于不加电时提高了2.2倍,相应的其使用寿命也延长了2.2倍。
-
本研究以市售活性炭滤芯为研究对象,选取比表面积为441.0 m2·g−1、导电性为14.7 S·m−1的活性炭滤芯,考察了其对水中DCAA、LVFX和CIP 3种微污染物的电增强吸附效果。
1)施加与污染物所带电荷相反的外加电压能有效提升活性炭滤芯对3种微污染物的吸附速率与吸附容量。电增强吸附动力学符合准二级动力学模型,在施加电压为2 V时DCAA的初始吸附速率v0达到12.4 mg·(g·h)−1,是不加电时的1.7倍;在施加电压为−2 V时LVFX和CIP的初始吸附速率v0分别达到45.3 mg·(g·h)−1和93.1 mg·(g·h)−1,分别是不加电时的1.2和1.3倍。
2) 3种污染物的电增强吸附等温线均符合Langmuir模型。DCAA在中性条件下带负电,其在2.0 V电压下的最大吸附容量qm达到26.3 mg·g−1,是未加电时的3.2倍;而LVFX和CIP在中性条件下带正电,在−2.0 V电压下的qm分别达到207.9 mg·g−1和106.1 mg·g−1,分别是未加电条件下的1.5倍和1.2倍。
3)电压为2.0 V下的电增强吸附实验出水中DCAA质量浓度相对0 V更低,出水水质更佳。相比于0 V的600个床体积的处理水量,2.0 V下的处理水量达到了1 300个床体积,处理水量增加了2.2倍,表明活性炭滤芯在电增强吸附条件下的使用寿命延长了2.2倍。
4)本研究结果表明在商业活性炭滤芯上施加外电压,通过电增强吸附技术可以有效提高滤芯对水中带电微污染物的吸附去除性能,该技术在深度处理饮用水中微污染物方面有较好的应用前景。
电增强活性炭滤芯吸附去除水中微污染物
Electrochemically assisted adsorption and removal of micropollutants in water on activated carbon filter
-
摘要: 选取了10种市售活性炭滤芯并测试其相关理化性质,筛选出比表面积高、导电性好的滤芯用于电增强吸附二氯乙酸(DCAA)、左氧氟沙星(LVFX)和环丙沙星(CIP) 3种典型饮用水中的微污染物。3种污染物的电增强吸附符合二级动力学模型,电增强活性炭滤芯吸附DCAA、LVFX和CIP的最佳初始吸附速率v0分别达到12.4 mg·(g·h)−1 (2 V)、45.3 mg·(g·h)−1 (−2 V)和93.1 mg·(g·h)−1 (−2 V),相比不加电情况下提高了1.2~1.7倍;3种污染物的电增强吸附等温线符合Langmuir模型,电辅助下DCAA、LVFX和CIP的最大吸附容量qm可达到26.3 mg·g−1 (2 V)、207.9 mg·g−1 (−2 V)和106.1 mg·g−1 (−2 V),相比于不加电时提升了1.2~3.2倍。在流动态吸附实验中,活性炭滤芯在电增强吸附下的出水的DCAA质量浓度比不加电时更低,出水水质更佳。电增强吸附下的处理水量达到1 300床体积,比不加电条件下提升了2.2倍。以上研究结果表明活性炭滤芯的电增强吸附在饮用水深度处理中有着较好的应用前景。Abstract: In this study, 10 kinds of commercial activated carbon filters were selected and their relevant physicochemical properties were tested. The filters with high specific surface area and good electrical conductivity were selected for the electrochemically assisted adsorption of three types of typical micropollutants in drinking water of dichloroacetic acid (DCAA), levofloxacin (LVFX) and ciprofloxacin (CIP). The adsorption kinetic curves of 3 types of pollutants under electrochemical assistance were consistent with the pseudo second-order model. The optimal initial adsorption rates v0 for DCAA, LVFX and CIP reached 12.4 mg·g−1·h−1 (2.0 V), 45.3 mg·g−1·h−1 (−2.0 V) and 93.1 mg·g−1·h−1 (−2.0 V), respectively, which were 1.2~1.7 times higher than those without electrochemical assistance. The electrochemically assisted adsorption isotherms of three types of pollutants were well fitted by the Langmuir model. The maximum adsorption capacities qm of DCAA, LVFX and CIP under electrochemical assistance could reach 26.3 mg·g−1 (2.0 V), 207.9 mg·g−1 (−2.0 V), and 106.1 mg·g−1 (−2.0 V), respectively, which were enhanced by 1.2~3.2 times compared with those without electrochemical assistance. In the flow-mode of adsorption experiment, the DCAA concentration of the effluent under electrochemical assistance was lower than that without electricity, presenting a better effluent quality. The treated water quantity under electrically enhanced adsorption reached 1 300 bed volumes, which was a 2.2-fold improvement over that without electrochemical assistance. The study demonstrates that the electrochemically assisted adsorption of activated carbon filter is promising in the advanced treatment of drinking water.
-
Key words:
- activated carbon filters /
- electrochemical assistance /
- adsorption /
- micropollutants
-
在废水生物处理系统中,微生物是污染物降解的主体。一般而言,活性污泥处理系统中微生物生长繁殖需要的营养物质含量具有一个经验比例,即化学需氧量∶氮∶磷(COD∶N∶P)为100∶5∶1。当处理废水中营养物质含量达不到该比例时,如耗氧有机污染物(以COD计)浓度过低,或者氮、磷浓度比例过低,则该废水被称为贫营养废水。在贫营养水质条件下,系统营养物质比例失调,生物系统中活性污泥微生物由于缺乏某类营养物质而活性降低,甚至大量死亡,导致处理系统失效。WANG等[1]发现,在脱氮除磷系统中,当有机物浓度过低时,微生物细胞内合成和贮存的PHA不足,系统的脱氮除磷效率将大幅降低。PENG等[2]的研究表明,进水底物中缺乏氮或磷会刺激活性污泥中丝状菌的增殖,从而发生丝状污泥膨胀而使系统处理性能降低。为解决贫营养废水处理效率低的难题,目前大多数污水处理厂通常采用投加有机物或氮磷等营养物质的方式调整系统进水营养比例,以满足活性污泥微生物生长需求,保证系统稳定运行。
近年来,随着微生物测序技术的飞速发展,基于16SrRNA高通量测序技术的微生物检测被广泛应用于污水处理厂活性污泥微生物群落结构的分析中,大大提高了人们对污泥微生物群落及其功能的了解。目前,对污水处理系统内主要微生物群落的研究已有大量报道。JIN等[3]的研究表明,在门水平上,活性污泥中的细菌主要集中在变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)4种。ZHANG等[4]对滁州市污水处理系统5个活性污泥微生物群落结构的研究中发现,在所有活性污泥样品中,除了上述4种菌门,绿弯菌门(Chloroflexi)和酸杆菌门(Acidobacteria)也是优势菌。在这些优势菌门中,变形菌门(Proteobacteria)是活性污泥系统中最常见的原生门类,也是传统废水生物处理过程中的关键细菌群[5]。有研究[6]表明,该菌门与污水生物处理过程中的有机物降解和脱氮除磷密切相关,而隶属于该门的Zoogloea属是构成活性污泥菌胶团的重要组成菌属,在多种有机物的降解过程中发挥着重要作用[7]。拟杆菌门(Bacteroidetes)是异养微生物的另一类重要门类,能参与有机碳和蛋白类物质的循环[8]。该门同时也是厌氧消化过程中常见的产酸菌[9],其部分菌属能降解长链脂肪酸,并可水解发酵多糖类有机化合物[10-11]。有研究[12-13]表明,拟杆菌门中的拟杆菌属(Bacteroidales)能够利用纤维素和多糖,并可将纤维素、二糖、葡萄糖和甘露糖等有机物降解为醋酸酯或琥珀酸。绿弯菌门(Chloroflexi)作为污水处理厂常见的菌群,具有较高的有机负荷承受能力[14],并且对糖类有较好的降解能力,可产生乙酸[15]。厚壁菌门(Firmicutes)广泛分布在厌氧环境中,包含梭菌纲(Clostridium)、芽孢杆菌纲(Bacillibacteria)等多种功能菌纲,其对有机物的水解和酸化有重要作用[16]。此外,酸杆菌门(Acidobacteria)和放线菌门(Actinobacteria)也是污水处理厂厌氧过程中重要的功能菌群,是厌氧消化过程中的主要产酸菌,起水解酸化作用[17-18]。以上这些污水处理厂常见主要菌群的研究结论,大多是基于普通的污水生物处理过程,即微生物生长所需营养物质能够得到满足的条件下得到的结果。然而,当污水处理系统中氮、磷等营养受限时,微生物群落的结构变化和种群分布规律还有待进一步研究。
云南省昆明市某制药厂废水处理系统中,进水耗氧有机污染物(以COD计)和悬浮物浓度高,而氮磷浓度极低,进水营养物质含量比例达不到常规微生物生长需求,COD∶N∶P仅为100∶0.4∶0.07。然而系统长期运行状况表明,在氮磷营养贫乏的条件下,该系统运行稳定,污水处理效果良好,出水水质均达到了国家《污水综合排放标准》(GB 8978-1996)的一级标准。为此,本研究对该贫营养废水处理系统中关键环节的污染物降解情况进行了监测,并对其活性污泥中微生物进行了高通量测序分析。本研究旨在从系统运行及污泥微生物的角度解释该制药厂贫营养废水处理系统长期稳定运行的原因,为营养不均衡的高浓度有机废水的处理及系统维护提供有益指导。
1. 工艺系统概况
1.1 水质水量概况
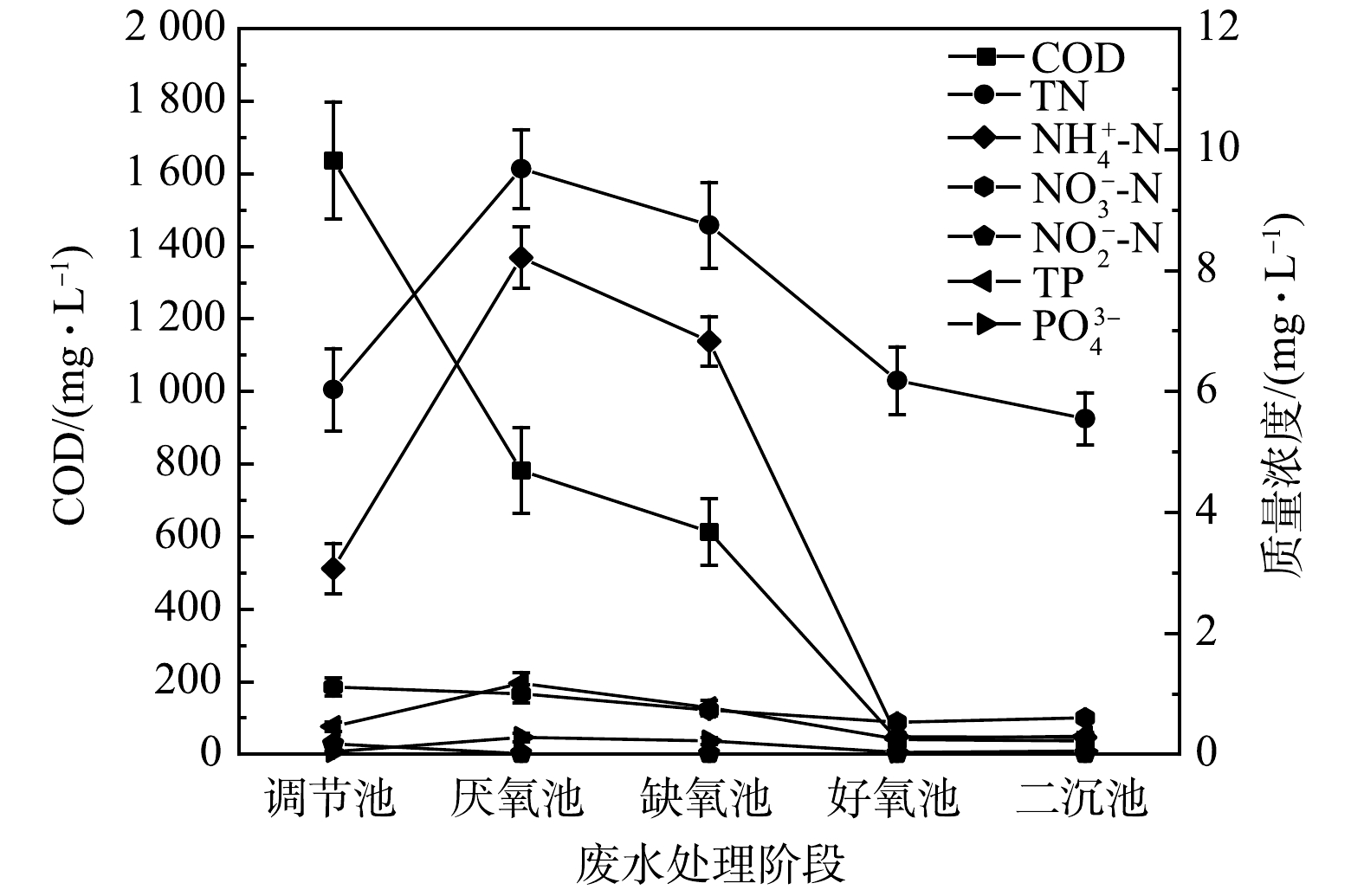
云南省昆明市某制药厂污水处理站总处理水量约为1 000 m3·d−1,进水分别来源于生产废水和生活污水。生产废水主要来自车间洗泡蒸煮药材、冲洗、制剂等生产过程,该废水的特点为:废水COD值高、波动范围较大,耗氧有机污染物(以COD计)质量浓度通常在1 000~2 000 mg·L−1波动;废水中氮磷等营养元素含量较低(TN<9 mg·L−1、TP<1 mg·L−1),加大了生物处理的难度。工艺好氧段曝气池MLSS为3 000~4 000 mg·L−1,MLVSS为1 500~2 000 mg·L−1,SVI为100~150 mL·g−1。废水经污水处理站处理后,部分废水排入市政管网,部分经过深度处理后作为该厂中水进行回用。
1.2 工艺流程
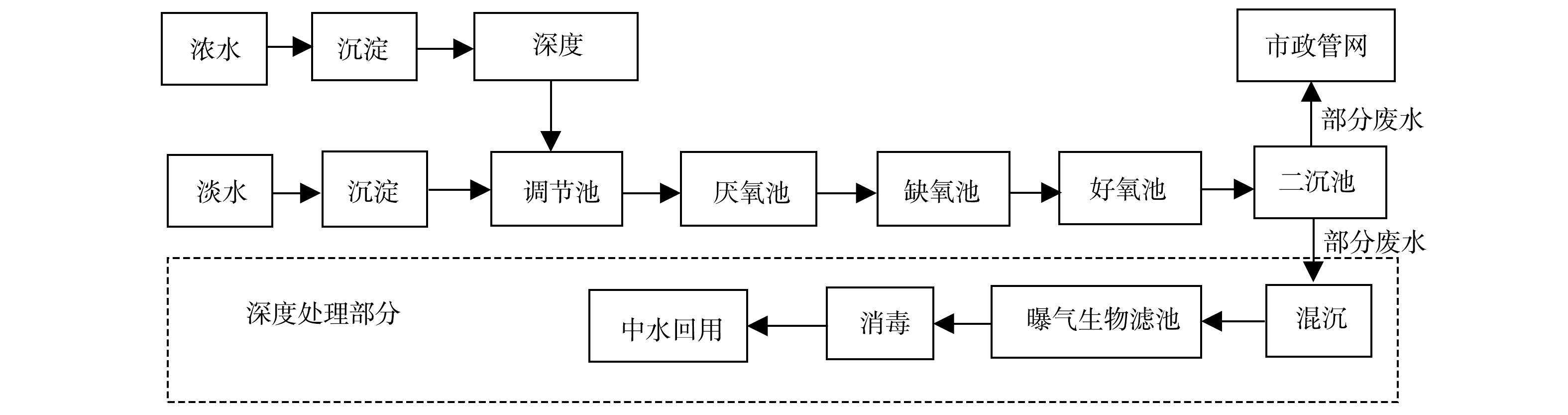
系统处理工艺为A2O工艺,厌氧过程采用IC工艺,好氧过程采用活性污泥完全混合处理工艺。为了适应不同的进水水质,在生化处理工艺的前端分别设置了2组预处理工艺。污染物浓度较高的生产废水(浓废水),首先经过沉淀作用去除水中部分悬浮物,再进入浓废厌氧池进行部分有机污染物的降解,而后流入调节池调节水质水量。经过这样预处理后,浓废水才能进入后续的主体生化系统进行处理;污染物浓度较低的生活污水(淡废水),经沉淀后直接流入调节池进行后续的生化处理。混合后的调节池出水从厌氧污泥池底部进入,与堆积在池子底部的污泥混合接触,经污泥中微生物对废水中的有机物进行充分降解后,出水自厌氧池上部出水口流入缺氧池进行反硝化脱氮。缺氧池出水继而流入好氧池,在大量好氧异养菌的作用下,有效地去除剩余有机污染物。好氧池出水经过二沉池泥水分离后,部分出水排入市政管网,剩下的部分废水经过混凝沉淀、曝气生物滤池、消毒等深度处理工艺处理后作为中水回用。系统详细工艺流程图如图1所示。
2. 实验与分析方法
2.1 分析项目及方法
分别取调节池和生化处理单元各反应池末端的出水进行水质检测。采用标准方法[19]对COD、氨氮、硝态氮、TN、TP、SS、SVI、MLSS、MLVSS等指标进行测定;DO、pH、水温等用雷磁多参数水质分析仪测定。
2.2 微生物群落结构及特征分析
在工艺系统运行过程中,分别收集厌氧池、曝气池的污泥微生物样品。采用CTAB或SDS方法对污泥样品的基因组DNA进行提取,提取样品总DNA后,根据全长引物序列合成带有Barcode的特异引物,进行PCR扩增并对其产物进行纯化、定量和均一化,形成测序文库(SMRT Bell),建好的文库先进行文库质检,质检合格的文库用PacBio Sequel进行上机测序。使用USEARCH(10.0版)在相似性97%(默认)的水平上对有效的标签序列进行聚类,默认以测序所有序列数的0.005%作为阈值过滤OTUs。以silva为参考数据库,使用朴素贝叶斯分类器对OTUs代表序列进行分类学注释,得到物种分类学信息,进而统计各个样品在门、纲、目、科、属和种水平上的群落组成。利用QIIME软件计算各样品在97%相似度水平下的ACE、Chao、Shannon及Simpson指数。将数据批量导入QIIME软件,对OTUs表进行组间差异性分析,生成不同分类水平上的物种丰度表,再利用R语言工具绘制成样品各分类学水平下的群落结构图。
3. 结果与讨论
3.1 系统长期运行状况分析
系统在近10年的运行过程中,处理效果稳定,出水有机污染物及氮、磷浓度低,达到国家《污水综合排放标准》(GB 8978-1996)和《污水排入城市下水道水质标准》(CJ 3082-1999)。其中,COD和BOD的去除率均高达90%以上;氮磷去除率也达到70%左右。表1显示了近期系统出水指标的具体监测结果。
表 1 中药废水处理系统出水指标监测结果Table 1. Status of the effluent from the Chinese medicine wastewater treatment system检测单元 pH 其他水质参数浓度/(mg·L−1) 溶解氧 SS COD BOD5 氨氮 TN TP 阴离子表面活性剂 调节池 9.05 1.01 47.00 1296.67 931.33 4.05 8.05 0.38 0.95 二沉池出水 7.46 3.67 13.00 81.33 12.67 0.47 2.33 0.12 0.03 去除率/% — — 72 94 99 88 71 68 97 3.2 系统各阶段处理状况分析
为探查系统在贫营养条件下各阶段的处理性能,本实验对系统进水及各反应器末端出水水质进行了取样检测。系统中各处理构筑物末端出水污染物平均浓度变化如图2所示。水质检测结果表明,贫营养水质并未对系统各处理阶段的处理性能产生明显影响;有机物在系统厌氧、缺氧和好氧过程中都得到了很好的降解,氮、磷在厌氧过程中略有积累,而在缺氧和好氧过程中得到很好的降解。厌氧池的COD去除率为52%左右,好氧池的COD去除率为93%,系统COD总去除率高达98%,这表明系统具有良好的有机物降解效果。TN和氨氮的降解主要在缺氧段和好氧段,缺氧段的去除率为9.61%和16.81%,好氧段为29.37%和95.75%。厌氧过程中氮磷浓度略有上升,这可能与其过长的SRT有关。有研究表明,厌氧条件下氮磷的释放受SRT影响[20],过长的SRT会导致氮磷的释放量增加[21]。本系统中厌氧池污泥长时间停留在反应器内,其SRT长达78 d左右,有可能导致其氮磷浓度增加。此外,有研究[22]表明,当活性污泥系统同时缺乏氮磷时,部分污泥将发生解体。因此,本研究中由于氮磷缺乏而导致的部分微生物死亡解体,从而释放氮磷,也可能是造成厌氧池氮磷浓度升高的原因。
3.3 贫营养条件下系统微生物群落分析
在污水生物处理系统中,微生物是污染物去除的主要执行者,系统中微生物的种类、数量及分布等状况,从根本上决定着系统处理性能及效果。为揭示贫营养水质条件对系统优势菌群的影响,本研究对该污水处理系统中的厌氧池和好氧曝气池的污泥样品进行了高通量测序,考察两生化反应池与正常营养水平废水处理系统中微生物群落的差异。
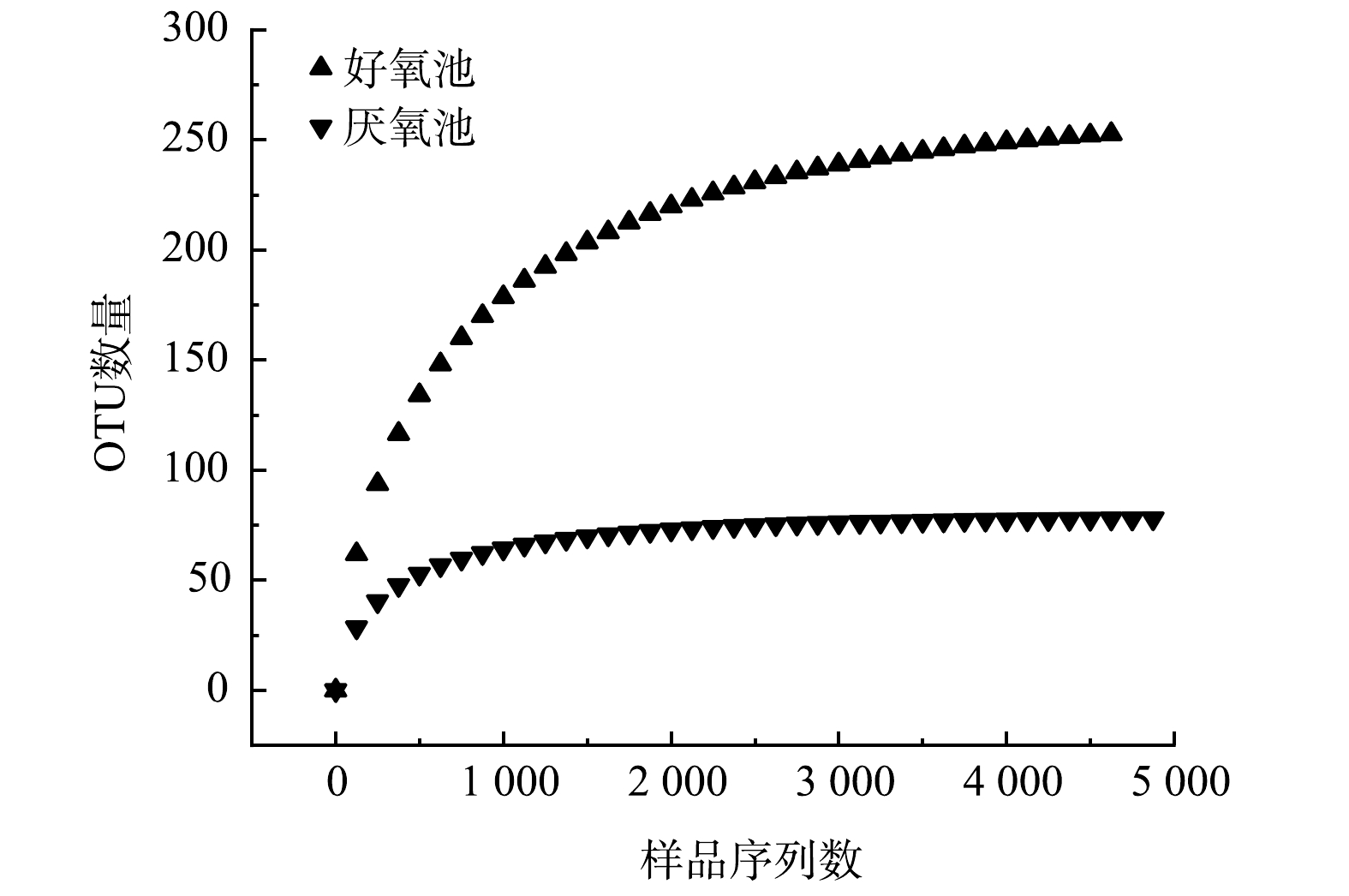
1)高通量测序数据结果表明,该系统中好氧池和厌氧池的有效序列数分别为4 625和4 875;在97%的相似度水平上对有效序列归类OTUs,好氧池和厌氧池的OUTs数量分别为253和78。好氧池的OUT数量较高,表明好氧池具有较多的微生物种类。好氧池的细菌群落具有19门、24纲、55目、62科、70属;厌氧池细菌群落具有18门、27纲、35目、44科、51属。
OUTs稀释曲线能够验证测序数据量是否足以反映样品中的物种多样性,并间接反映样品中物种的丰富程度。图3所示的OUTs稀释曲线反映了在持续抽样下系统新物种出现的速率。结果表明,在97%的相似度水平上,当好氧池、厌氧池的样品序列数分别在0~1 500和0~1 000时,曲线表现为急剧上升的趋势,表明群落中有大量物种被发现;而当样品序列数分别大于4 500和3 625后,曲线趋于平缓,表明此环境中的物种并不会随测序数量的增加而显著增多,基本达到饱和。该曲线说明该测序结果能够反映2生化池微生物群落的多样性。
Alpha多样性(Alpha diversity)反映的是单个样品的物种丰度及物种多样性,一般有多种衡量指标,如Chao、ACE、Shannon和Simpson。Chao和ACE指数用于衡量物种丰度即物种数量的多少;Shannon和Simpson指数用于衡量物种多样性,受样品群落中物种丰度和物种均匀度(community evenness)的影响。在相同物种丰度的情况下,群落中各物种均匀度越大,群落多样性越高;Shannon和Simpson指数值越大,样品的物种多样性越高。厌氧池、好氧池所取污泥微生物样品的Alpha多样性分析结果如表2所示。
表 2 好氧池、厌氧池的微生物群落丰富度和多样性指数Table 2. Richness and diversity indexes of microbial community in the aerobic tank and anaerobic tank检测单元 OTUs Chao ACE Shannon Simpson 覆盖率/% 好氧池 253 259.2432 262.2059 6.4124 0.9751 99.53 厌氧池 78 78.3333 78.5846 3.9206 0.8622 99.96 由表2可知,好氧池的OTUs、Chao指数和ACE指数明显高于厌氧池,说明好氧池的细菌群落更加丰富。同时,好氧池的Shannon指数和Simpson指数也略高于厌氧池,表明好氧池比厌氧池具有更高的群落多样性。测序实验还统计了样品覆盖率(coverage指数),以鉴定测试结果是否代表样品微生物的真实情况。该指数越高,则样本中物种被测出的概率越高。2个反应池的样品覆盖率均大于0.99,说明测序结果能够反映样品微生物的真实情况。
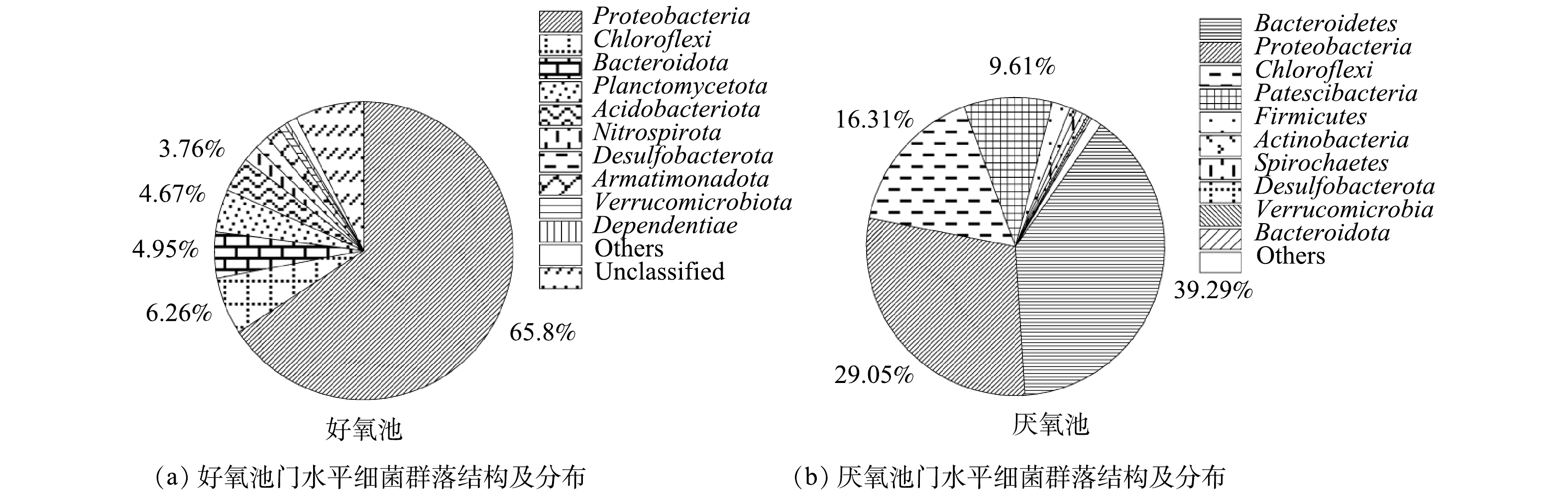
2)好氧池、厌氧池在门水平上的细菌群落分布如图4所示。从门水平上的差异可看出,好氧池的优势菌群为变形菌门(Proteobacteria,65.8%)、绿弯菌门(Chloroflexi,6.26%)、拟杆菌门(Bacteroidota,4.95%)、浮霉菌门(Planctomycetota,4.67%)以及酸杆菌门(Acidobacteriota,3.76%)。这些检测结果与WANG等[23]对中国14个污水处理系统细菌多样性的焦磷酸测序分析结果基本一致。在好氧池还检测到了硝化螺旋菌门(Nitrospirota),相对丰度占1.76%,表明好氧池具有良好的硝化作用。此外,脱硫杆菌门(Desulfobacterota)和Armatimonadota门分别占1.61%和1.57%。ANTUNES等[24]的研究表明,Armatimonadota门与废水中耗氧有机物(以COD计)、TOC、TDS和SS浓度呈显著的正相关。因此,系统中的高有机浓度有助于Armatimonadota门的生长。此外,在其他研究[3]中常发现占优势的厚壁菌门(Firmicutes)和放线菌门(Actinobacteria),在本研究中的数量却较少,存在这一差异的原因有待进一步探究。
相比之下,厌氧池丰度最高的优势菌门是污水处理系统中常见的拟杆菌门(Bacteroidetes),占总群落的39.29%,其次是变形菌门(Proteobacteria,29.05%)和绿弯菌门(Chloroflexi,16.31%)。拟杆菌门在厌氧池中占主导地位,说明厌氧池中发生了良好的水解和酸化作用。另外,Patescibacteria门在厌氧池中占9.61%,厚壁菌门(Firmicutes)占1.98%。除Patescibacteria外,以上几种优势菌门与AHRING[25]所报道的厌氧系统中常见菌门一致。在以往的研究中,关于厌氧系统中Patescibacteria的研究报道还比较少。Patescibacteria门是新定义的超级门,其普遍存在于含水层环境中,如营养物质较少、DO浓度较低的地下水中[26]。由于这一门具有超小的细胞体积,使其具有较高的表容比,能最大限度地利用营养物质[27-28]。由此可推测,该门在本系统厌氧池中的分布与贫营养水体环境的选择作用存在一定的关联。
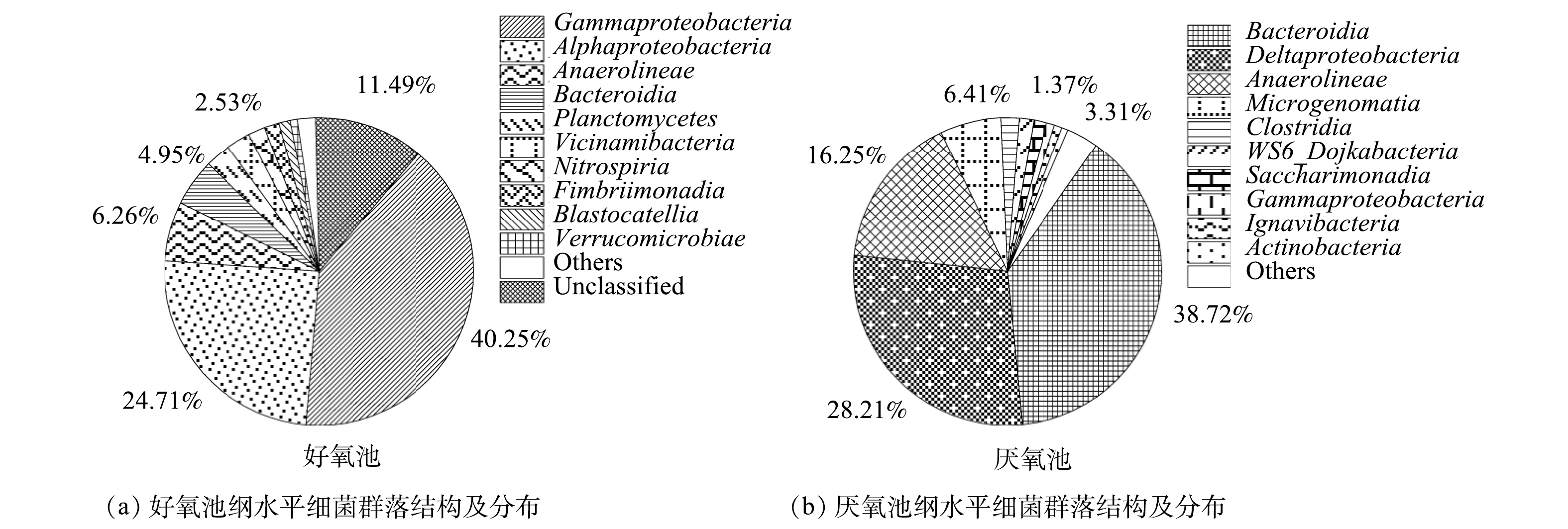
3)从2生化池细菌群落在纲水平上的分布来看(图5),好氧池的优势菌纲主要包括γ-变形菌纲(Gammaproteobacteria,40.25%)、α-变形菌纲(Alphaproteobacteria,24.71%)、厌氧绳菌纲(Anaerolineae,6.26%)、拟杆菌纲(Bacteroidia,4.95%)、浮霉菌纲(Planctomycetes,2.68%)以及Vicinamibacteria菌纲(2.53%)。此外,还发现了硝化螺旋菌纲(Nitrospiria,1.76%)以及不常见的Fimbriimonadia菌纲(1.55%)和Blastocatellia菌纲(1.19%)。与通常的研究结果不同[4],好氧池的优势菌纲中出现了厌氧绳菌纲(Anaerolineae),该菌纲是发酵分解糖类的厌氧细菌纲[29]。其能够在好氧池中富集的原因可能是Anaerolineae纲的一些菌种是半共营养型微生物[30],可与氢营养型产甲烷菌等细菌协同降解糖类[31]。另外,隶属于酸杆菌门(Acidobacteriota,3.76%)的Vicinamibacteria菌纲和Blastocatellia菌纲相对丰度之和为3.72%,表明二者是酸杆菌门的主要贡献者。Vicinamibacteria为好氧有机异养菌,能耐受广泛的pH范围,对不同的糖类、复杂的蛋白质化合物具有良好的代谢效果[32];而Blastocatellia菌纲的细菌偏好缺乏营养的生长条件[33],能够在干旱和营养限制的条件下生存[34]。Fimbriimonadia是一类严格需氧的非运动性杆状细菌,这类细菌大多生长在低营养介质中,绝大部分有机底物都不能作为碳源而被其利用[35]。
厌氧池的优势菌纲则主要为拟杆菌纲(Bacteroidia)、δ-变形菌纲(Deltaproteobacteria)以及厌氧绳菌纲(Anaerolineae),相对丰度分别为38.72%、28.21%和16.25%。其次,Microgenomatia菌纲占比为6.41%、梭状芽孢杆菌纲(Clostridia)为1.90%,最后是WS6_Dojkabacteria菌纲和Saccharimonadia菌纲,相对丰度分别为1.53%和1.37%。大多数厌氧绳菌纲(Anaerolineae)的物种都能进行发酵代谢,有助于在厌氧条件下糖类和蛋白质的降解,是厌氧系统中一个重要的发酵种群[15]。与AHRING[25]的研究结果不同,本系统的厌氧池中出现了2种不常见的菌群,即Microgenomatia和Saccharimonadia。Microgenomatia和Saccharimonadia均属于Patescibacteria门,他们不仅能够在厌氧条件下进行广泛的有机底物代谢,而且能够在营养物质匮乏的贫营养水体中生长[36]。Microgenomatia菌纲能水解发酵纤维素,Saccharimonadia菌纲中的一些菌属被发现有助于降解烃类物质[37-38]。
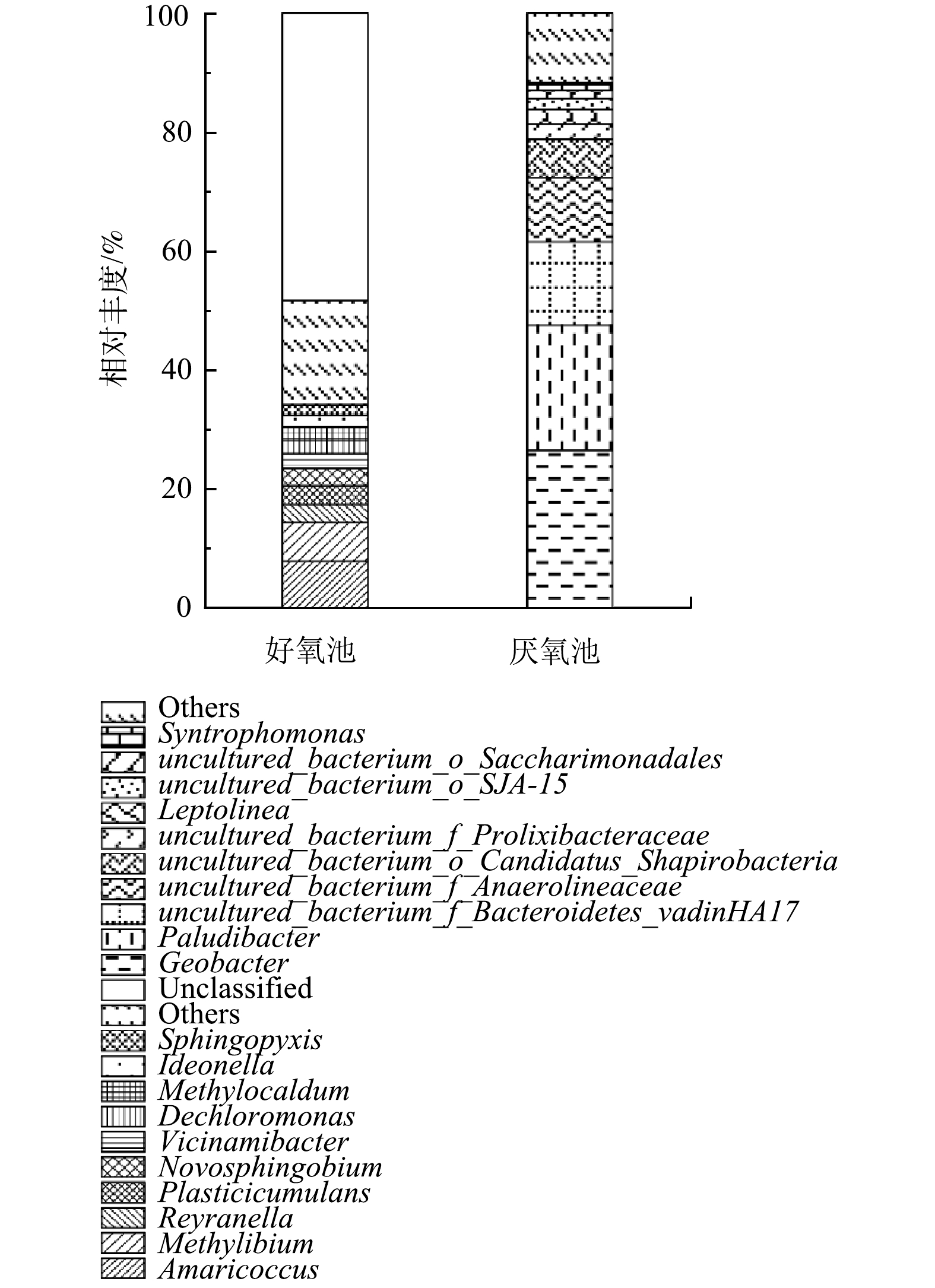
4)图6所示的百分比柱状堆积图反映了好氧池和厌氧池的微生物群落分别在属水平上的分布情况。微生物群落作为生物反应器的主体,决定了生物反应器的性能,而在一个污水生物处理过程中,微生物群落的形成又受当地气候条件、工艺类型和被处理废水的性质等因素的影响。本研究中,高有机物浓度、低氮磷的贫营养条件无疑是一个最重要的影响因素,此条件极有可能为系统中微生物的生长施加选择压。实际结果也表明,本系统的好氧池微生物菌属与常规的好氧活性污泥系统菌属有很大差别。在一般的生活污水处理厂中,活性污泥优势菌属主要为动胶菌属(Zoogloea)、不动杆菌属(Acinetobacter)、丛毛单胞菌属(Comamonas)和黄杆菌属(Flavobacterium)以及硝化螺旋菌属(Nitrospira)等[39];而在本研究中,好氧池的优势菌属包括Amaricoccus(8.03%)、Methylibium(6.50%)、Reyranella(3.1%)、Plasticicumulans(3.06%)、新鞘氨醇杆菌属(Novosphingobium,2.91%)、Vicinamibacter(2.46%)、脱氯单胞菌属(Dechloromonas,2.31%)、甲基暖菌属(Methylocaldum,2.17%)、Ideonella(1.97%)和Sphingopyxis(1.78%)。其中,丰度最高的属Amaricoccus是一种贫营养(氮、磷营养不足)增殖的好氧细菌,属于变形菌门(Proteobacteria),α-变形菌纲(Alphaproteobacteria),革兰氏阴性,能以多糖为底物降解多糖[40]。在氮、磷等营养元素受限制的情况下,他们能大量地储存胞内PHB,并合成胞外荚膜物质[41],因此,能在N、P受限制的条件下增殖[42]。Plasticicumulans属是专性好氧异养菌,能够利用多种有机化合物作为碳源和能源,如醋酸盐、乙醇和乳酸盐等[43]。该属能在超过85%的细胞干重中积累92%的PHB,并利用氨、硝酸盐和复合有机氮作为氮源[44]。通常认为,PHB是微生物在营养不均衡的条件下,如碳源过剩、而其他如氮、磷、硫等营养限制,积累在体内作为其营养和能量储存物质,并参与细胞代谢的天然产物[45]。Plasticicumulans积累PHB的性质使其得以在该系统的贫营养环境中生长。以上这些菌属在好氧池中占主导地位,表明在好氧池中出现了适应贫营养条件的优势菌属,这些菌属能够有效地降解含有大量生物难降解有机物并缺乏氮磷营养物质的中药废水,达到良好的去除效果,以此来保证系统稳定运行。
厌氧池的优势菌属主要集中在地杆菌属(Geobacter,26.62%)、帕卢迪杆菌属(Paludibacter,20.98%)、uncultured_bacterium_f_Bacteroidetes_vadinHA17(14%)、未培养的厌氧菌属(uncultured_bacterium_f_Anaerolineaceae,10.82%)等4种。此外,还发现了uncultured_bacterium_o_Candidatus_Shapirobacteria(6.41%)、uncultured_bacterium_f_Prolixibacteraceae(2.51%)和纤绳菌属(Leptolinea,2.49%)。最后是uncultured_bacterium_o_Saccharimonadales和互营单胞菌属(Syntrophomonas),相对丰度分别占1.37%和1.31%。
地杆菌属(Geobacter)在厌氧池丰度最高,占26.62%。地杆菌属(Geobacter)为变形菌门、δ-变形菌纲;革兰氏阴性、杆状、严格厌氧[46]。这类菌群显著的代谢特征是能将有机物的氧化与Fe(Ⅲ)的还原结合起来,在厌氧条件下以乙酸盐、芳香烃、有机氯化物等多种有机污染物作为电子供体和碳源,还原可溶性或不溶性的Fe(Ⅲ)[47-48]。地杆菌属(Geobacter)对生长环境的适应能力比较强,因而在被石油污染的含水层、被垃圾渗滤液污染的地下水、生活污水和各种废水等环境中均能发现其存在[49]。据以往的研究,地杆菌属(Geobacter)在参与厌氧消化方面有很大的潜力,可以广泛地利用有机物作为底物,如挥发性脂肪酸(VFAs)、醇、酚和苯等[50]。不仅如此,地杆菌属(Geobacter)还可以与其他物种(如产甲烷菌等)建立直接电子连接,即直接种间电子转移,例如,金属还原地杆菌(Geobacter metallireducens)可与甲烷丝菌属(Methanosaeta)或甲烷八叠球菌属(Methanosarcina)等产甲烷菌共培养[51-52]。地杆菌属(Geobacter)在本废水处理系统厌氧池占主导地位,说明厌氧池发生了良好的水解酸化作用,同时也有利于厌氧消化中的产甲烷过程。同时,其它的优势菌属,如帕卢迪杆菌属(Paludibacter)是β-拟杆菌纲(Betabacteroidetes)、紫单胞菌科(Porphyromonadaceae)下的一种严格厌氧菌,该菌能发酵多种单糖和二糖产生乙酸、丙酸和少量丁酸,是一种产酸菌[53-54]。
纤绳菌属(Leptolinea)在厌氧条件下能将多糖等有机物降解转化为有机酸和脂肪酸[55],有利于糖类等有机物的水解酸化。值得注意的是,纤绳菌属(Leptolinea)属于营养匮乏的绿弯菌亚门I(Chloroflexi subphylum I)中的厌氧菌纲,需要与其他微生物结合才能有效生长[30]。uncultured_bacterium_o_Saccharimonadales(1.37%)系超级门Patescibacteria门,具有非常小的基因组和细胞大小,通常与其他微生物共存、依赖共代谢生长[56];一些该细菌被证明能够吸收油酸并具有脂肪酶和其他外酶活性[57];可在发酵途径中将丙酮酸转化为乳酸。互营单胞菌属(Syntrophomonas)是厌氧发酵中常见的微生物,可以降解丁酸,是厌氧活性污泥的功能菌属之一[58]。Syntrophomonas可与氢化产甲烷菌共同消耗长链和短链脂肪酸,是厌氧消化中重要的共营养细菌,具有产生氢或甲酸作为甲烷菌电子载体的功能[59]。Syntrophomonas属在厌氧反应池中的富集,可能也有助于提高COD去除率和产甲烷量。
由上述分析可知,贫营养进水条件下,厌氧池中出现了适应该环境条件并能降解各种复杂有机物的优势菌属Gobacter和Paludibacter,Gobacter主要参与厌氧过程的水解发酵,Paludibacter参与产酸过程。其次,氮磷营养元素的限制为起水解酸化作用的贫营养菌属Leptolinea的生长提供了有利条件。以Leptolinea和Syntrophomonas为代表的优势菌属与其他功能菌属的共代谢生长有利于厌氧消化过程的顺利进行。其中,Syntrophomonas与产甲烷菌的共营养生长不仅促进了产氢产乙酸过程,还有利于厌氧产甲烷。总之,以上这些优势菌属在厌氧池的稳定生长及分工合作是厌氧池能够长期稳定运行的有力保障。
4. 结论
1)该贫营养系统运行状况表明,系统能在TN、TP分别低至约10 mg·L−1和1 mg·L−1的条件下长期稳定运行,且系统处理效果良好。
2)中药废水的特殊性质(高COD、低N、P营养)对微生物群落的生长施加了选择压,即大量有机物的存在为具有高有机物降解能力的异养菌提供了微生物选择。其中,厌氧池中表现为Geobacter(26.62%),好氧池中表现为Amaricoccus(8.03%)。
3)贫营养环境和工艺运行条件促进了贫营养需求菌的优势增长,如厌氧池中的纤绳菌属(Leptolinea)、好氧池中的Amaricoccus属和Plasticicumulans属,这些优势菌本身具有很强的有机物降解能力,并在处理统系统中各个阶段分工合作,使系统能够长期稳定运行,达到良好的处理效果。
4)一些优势菌与其他菌种的共营养生存,使系统在污染物降解的各个阶段能够有序地进行,保障了该系统在长期处理过程中的稳定运行。其中厌氧绳菌纲(Anaerolineae)、地杆菌属(Geobacter)、纤绳菌属(Leptolinea)及互营单胞菌属(Syntrophomonas)是典型的代表。
-
表 1 活性炭滤芯的基本性质
Table 1. Basic properties of activated carbon filter element
编号 种类 比表面积/(m2·g−1) BET平均孔径/nm 电导率/(S·m−1) 密度/(g·cm−3) 压缩强度/MPa 过滤精度/µm 1 椰壳炭 31.0 7.6 1.6 0.8 3.2 47.6 2 椰壳炭 441.0 2.8 14.7 0.6 5.1 6.1 3 椰壳炭 179.0 4.4 3.2 0.7 3.2 89.8 4 椰壳炭 720.0 1.4 0.4 0.6 6.3 20.3 5 椰壳炭 341.9 1.6 3.7 0.7 9.2 10.0 6 果木炭 144.7 2.3 32.2 0.7 6.7 7.3 7 椰壳炭 36.3 3.9 1.4 0.7 2.1 62.1 8 椰壳炭 495.3 1.4 2.0 0.6 1.8 80.6 9 椰壳炭 603.6 1.5 4.1 0.6 2.2 27.3 10 椰壳炭 22.3 4.5 1.4 0.8 2.8 67.8 表 2 3种污染物基本理化性质
Table 2. Physical and chemical properties of three types of pollutants
污染物 结构式 相对分子质量/Da 溶解度/(mg·L−1) pKa 二氯乙酸(DCAA) 128.9 1 000 000 1.26 左氧氟沙星(LVFX) 361.4 1 120 pKa1=6.25pKa2=8.20 环丙沙星(CIP) 331.3 <1 000 pKa1=3.01pKa2=6.14pKa3=8.70pKa4=10.58 表 3 DCAA、LVFX和CIP的吸附动力学参数
Table 3. Adsorption kinetic parameters of DCAA, LVFX and CIP
污染物 电压/V 准一级动力学参数 准二级动力学参数 qe/(mg·g−1) k1/h−1 R2 qe/(mg·g−1) k2/(g·(mg·h)−1) v0/(mg·(g·h)−1) R2 DCAA 2.0 11.4 1.5 0.921 12.1 0.09 12.4 0.975 1.5 10.1 1.3 0.933 10.9 0.07 8.7 0.978 1.0 9.4 1.2 0.942 10.1 0.07 7.6 0.986 0 6.9 1.5 0.918 7.3 0.14 7.5 0.974 −1.0 6.9 1.5 0.939 6.9 0.15 7.0 0.982 LVFX 1.0 54.7 0.9 0.870 58.2 0.009 30.6 0.949 0 54.8 1.2 0.855 60.3 0.011 39.3 0.937 −1.0 57.7 1.3 0.826 63.6 0.011 42.0 0.916 −2.0 91.0 0.7 0.900 100.0 0.004 45.3 0.965 CIP 1.0 73.8 1.3 0.951 78.5 0.010 62.9 0.992 0 73.2 1.3 0.934 77.5 0.011 73.8 0.984 −1.0 77.3 1.5 0.919 81.7 0.011 78.6 0.973 −2.0 84.9 1.5 0.926 89.6 0.013 93.1 0.977 表 4 DCAA、LVFX和CIP吸附等温线模型参数
Table 4. Adsorption isotherm model parameters of DCAA, LVFX and CIP
污染物 电压/V Langmuir 参数 Freundlich 参数 qm/(mg·g−1) b/(L·mg−1) R2 KF/(mg1-n·Ln·g−1) n R2 DCAA 2.0 26.3 0.2 0.979 7.6 0.3 0.939 1.0 16.2 0.6 0.934 7.6 0.2 0.857 0 8.2 0.5 0.917 5.3 0.1 0.866 −1.0 6.3 0.6 0.967 4.7 0.1 0.766 LVFX 1.0 122.3 0.009 0.998 9.4 0.4 0.974 0 137.6 0.007 0.997 8.2 0.4 0.970 −1.0 195.4 0.006 0.947 6.3 0.5 0.896 −2.0 207.9 0.006 0.924 6.7 0.5 0.880 CIP 1.0 87.4 1.1 0.959 44.9 0.3 0.936 0 88.0 1.2 0.982 46.7 0.3 0.947 −1.0 91.2 1.4 0.986 58.3 0.4 0.985 −2.0 106.1 1.5 0.974 49.9 0.3 0.975 -
[1] SALEHI M. Global water shortage and potable water safety: Today’s concern and tomorrow’s crisis[J]. Environment International, 2022, 106936: 158-165. [2] JIANG J, HAN J, ZHANG X. Nonhalogenated aromatic DBPs in drinking water chlorination: A gap between nom and halogenated aromatic DBPs[J]. Environmental Science & Technology, 2020, 54(3): 1646-1656. [3] HAN J, ZHANG X, JIANG J, et al. How much of the total organic halogen and developmental toxicity of chlorinated drinking water might be attributed to aromatic halogenated DBPs?[J]. Environmental Science & Technology, 2021, 55(9): 5906-5916. [4] 郭亚萍, 全燮, 陈硕, 等. 水中氯仿的活性炭电增强吸附特性[J]. 环境科学学报, 2003, 23(1): 84-87. doi: 10.3321/j.issn:0253-2468.2003.01.017 [5] LIU D Y, WANG X M, XIE Y F, et al. Effect of capacitive deionization on disinfection by-product precursors[J]. Science of the Total Environment, 2016, 568: 19-25. doi: 10.1016/j.scitotenv.2016.05.219 [6] WANG S T, LI X N, ZHAO N M, et al. Enhanced adsorption of ionizable antibiotics on activated carbon fiber under electrochemical assistance in continuous-flow modes[J]. Water Research, 2018, 134: 162-169. doi: 10.1016/j.watres.2018.01.068 [7] ZHAO W, JIA B, ZHANG Y, et al. Study on electro-sorption of heavy metals and sulfamethoxazole on activated carbon fibers[J]. Journal of Electrochemistry, 2019, 25(6): 669-681. [8] XIANG Y X, Z. WEI, Y. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors[J]. Journal of Environmental Management, 2019, 237: 128-138. [9] HUANG Q, DENG S B, SHAN D N, et al. Enhanced adsorption of diclofenac sodium on the carbon nanotubes-polytetrafluorethylene electrode and subsequent degradation by electro-peroxone treatment[J]. Journal of Colloid and Interface Science, 2017, 488: 142-148. doi: 10.1016/j.jcis.2016.11.001 [10] DAVOUD B, FERFOS K M, SEUNG M L , et al. Adsorption of bisphenol A using dried rice husk: Equilibrium, kinetic and thermodynamic studies[J]. Applied Chemistry for Engineering, 2019, 30(3): 316-323. [11] DONG Y, WU D, CHEN X, et al. Adsorption of bisphenol A from water by surfactant-modified zeolite[J]. Journal of Colloid and Interface Science, 2010, 348(2): 585-590. doi: 10.1016/j.jcis.2010.04.074 [12] LIU G F, MA J, LI X C, et al. Adsorption of bisphenol A from aqueous solution onto activated carbons with different modification treatments[J]. Journal of Hazardous Materials, 2009, 164(2-3): 1275-1280. doi: 10.1016/j.jhazmat.2008.09.038 [13] 蔡婧. 石河子市饮用水中消毒副产物的污染水平研究及风险评估[D]. 石河子: 石河子大学, 2018. [14] 栗旸, 李建云, 张旭辉, 等. 云南省k市水厂出厂水中卤乙酸的健康风险评估[J]. 实用预防医学, 2021, 28(5): 577-580. doi: 10.3969/j.issn.1006-3110.2021.05.010 [15] ANIA C O, BEGUIN F. Mechanism of adsorption and electrosorption of bentazone on activated carbon cloth in aqueous solutions[J]. Water Research, 2007, 41(15): 3372-3380. doi: 10.1016/j.watres.2007.03.031 [16] CHOI K J, KIM S G, KIM C W, et al. Effects of activated carbon types and service life on removal of endocrine disrupting chemicals: Amitrol, nonylphenol, and bisphenol-A[J]. Chemosphere, 2005, 58(11): 1535-1545. doi: 10.1016/j.chemosphere.2004.11.080 [17] HU X, JIA L, CHENG J, et al. Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: Preparation, characterization and adsorption mechanism[J]. Journal of Hazardous Materials, 2019, 362: 1-8. doi: 10.1016/j.jhazmat.2018.09.003 [18] GUILLOSSOU R, LE R J, MAILLER R, et al. Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption[J]. Water Research, 2020, 172: 115487. doi: 10.1016/j.watres.2020.115487 [19] 贺斯佳, 张硕, 孙昊, 等. 活性炭吸附饮用水中三卤甲烷的实验研究[J]. 浙江大学学报(理学版), 2022, 49: 489-497. [20] 王科霖, 刘洋洋, 姜仔璇, 等. 2020年烟台高新区城乡居民生活饮用水卫生监测结果分析[J]. 分析检测, 2021, 54(7): 197-199. [21] LI X N, CHEN S, QUAN X, et al. Enhanced adsorption of PFOA and PFOS on multiwalled carbon nanotubes under electrochemical assistance[J]. Environmental Science & Technology, 2011, 45(19): 8498-8505. [22] YU H B, CHE M, ZHAO B, et al. Enhanced electrosorption of rhodamine b over porous copper-nickel foam electrodes modified with graphene oxide/polypyrrole[J]. Synthetic Metals, 2020: 262-270. [23] LI N, YANG S, CHEN J, et al. Electro-adsorption of tetracycline from aqueous solution by carbonized pomelo peel and composite with aniline[J]. Applied Surface Science, 2016, 386: 460-466. doi: 10.1016/j.apsusc.2016.05.173 [24] HAN Y H, QUAN X, CHEN S, et al. Electro-chemically enhanced adsorption of aniline on activated carbon fibers[J]. Separation and Purification Technology, 2006, 50: 365-372. doi: 10.1016/j.seppur.2005.12.011 [25] JIANG J, ZHANG X, ZHU X, et al. Removal of intermediate aromatic halogenated DBPs by activated carbon adsorption: A new approach to controlling halogenated DBPs in chlorinated drinking water[J]. Environmental Science & Technology, 2017, 51(6): 3435-3444. [26] LIU J, ZHANG X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: Halophenolic DBPs are generally more toxic than haloaliphatic ones[J]. Water Research, 2014, 65: 64-72. doi: 10.1016/j.watres.2014.07.024 [27] QU J, WANG Y, TIAN X, et al. Koh-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration[J]. Journal of Hazardous Materials, 2021, 401: 123292. doi: 10.1016/j.jhazmat.2020.123292 -




 下载:
下载: