-
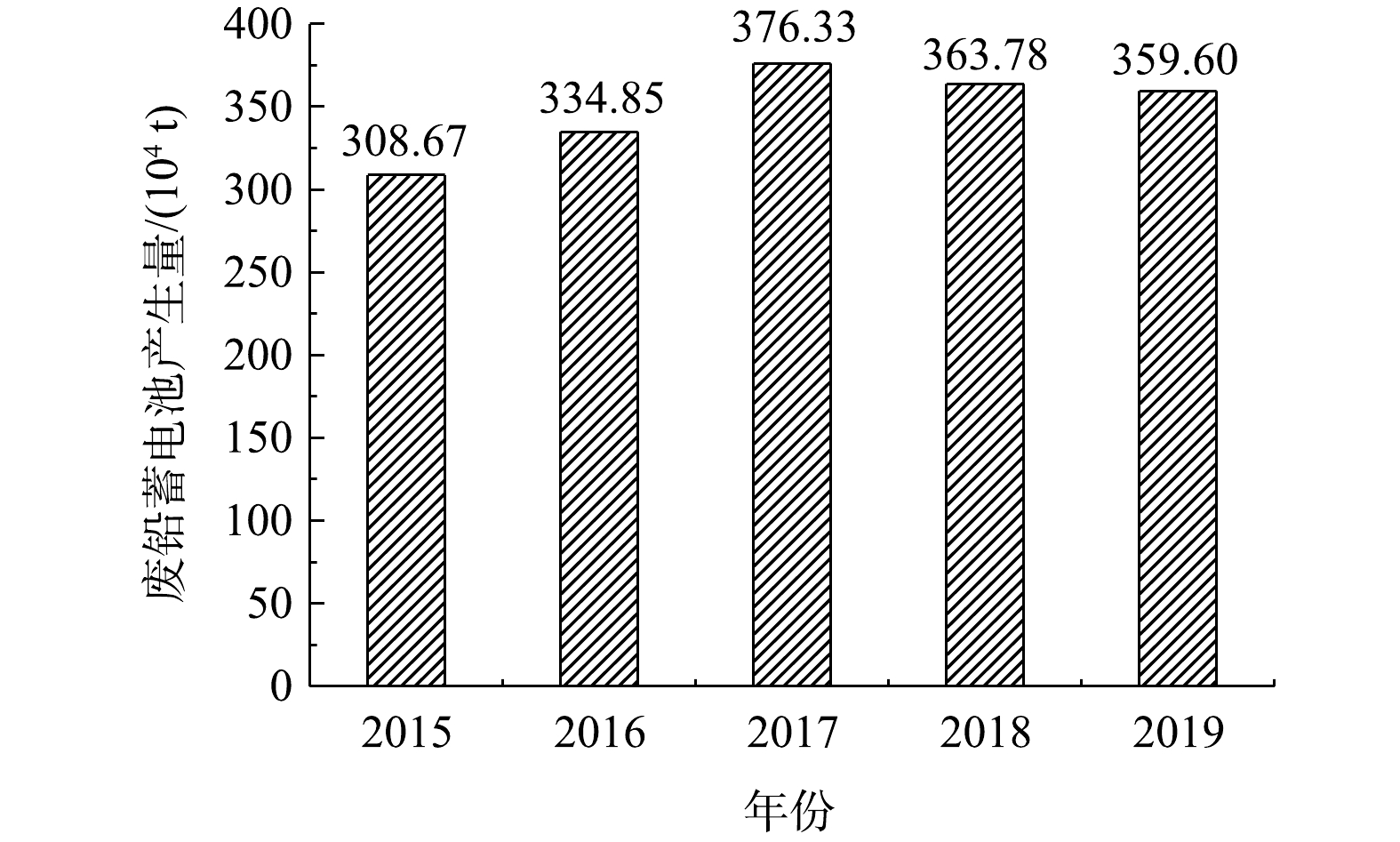
20世纪90年代以来,铅蓄电池相关领域工业迅速发展带来铅消费量不断攀高,全球精铅产量已从1999年的630×104 t增加到2022年的1 481×104 t[1-2]。2004年开始,中国跃居为世界最大精铅生产国及消费国,其中超过77%的铅应用于蓄电池领域[3]。随着碳达峰和碳中和持续推进,世界各国对新能源产业大力扶持,还将为价格低廉、工艺技术成熟的铅蓄电池行业带来新一轮增长空间[4]。铅蓄电池的大范围应用必然产生数量庞大的废铅蓄电池[5-6]。据统计,2015—2020年,我国废铅蓄电池产生量始终维持在300×104 t以上,2019年达到360×104 t,其中主要来源于汽车、电动自行车、电动三轮车和四轮车、通信基站等应用领域 (图1) [7]。一方面,这些数量庞大的废铅蓄电池中含有大量重金属铅和硫酸溶液,如不进行有效回收和规范处理,将对生态环境安全造成严重威胁;另一方面,废铅蓄电池除了具有危害性还具有资源价值,其回收利用可以减少对原生矿产资源的依赖程度。2011年起,国家陆续出台了一系列再生铅产业政策及配套措施,促使企业不断进行工艺技术创新,使我国规模以上企业再生铅冶炼技术总体达到了世界一流水平,铅资源的回收率可达98%以上[8]。然而,废铅蓄电池中含量多且容易造成环境污染的硫,目前对其资源化利用技术和全流程污染防治的关注还有待进一步提升。
根据国家统计局公布的数据,2019年全国工业源SO2排放量为400×104 t,按我国工业SO2排放行业结构估算,有色金属行业SO2排放量约为24×104 t[9],主要来自于铅、锌、铜的生产。由于SO2排放后会形成酸雨,使水质和土壤酸化,严重破坏生态系统;被人类吸入后容易生成具有腐蚀性的亚硫酸、硫酸和硫酸盐,极大损害人体健康。有效控制大气中SO2污染非常必要且刻不容缓,未来几年,环保部门将长期以降低SO2排放总量为重点推进大气污染防治。因此,再生铅行业含硫污染物治理及管控仍需进一步加强。
近年来,赵丽娟[10]、张俊丰[11]、汪振忠[12]、宋金超[13]、黄朝冉[14]等从不同角度对废铅膏预脱硫技术进行了详细的论述,包括常用的预脱硫技术、不同预脱硫技术的原理以及未来发展方向预测等,研究结果充分揭示了不同技术的特色与不足。然而,一方面废铅蓄电池处理造成的硫污染是以多种形式排放的,其排放水平除了取决于废铅膏预处理技术,还取决于废铅蓄电池处理企业采取的全流程硫污染控制技术;另一方面,伴随技术改造和设备更新,也出现了一些新形式的硫资源化利用技术。因此,有必要弄清废铅蓄电池处理全过程硫污染物产生情况,总结当前主要的硫综合利用和处理措施,为未来二氧化硫进一步减排提供详实全面的环境信息。
-
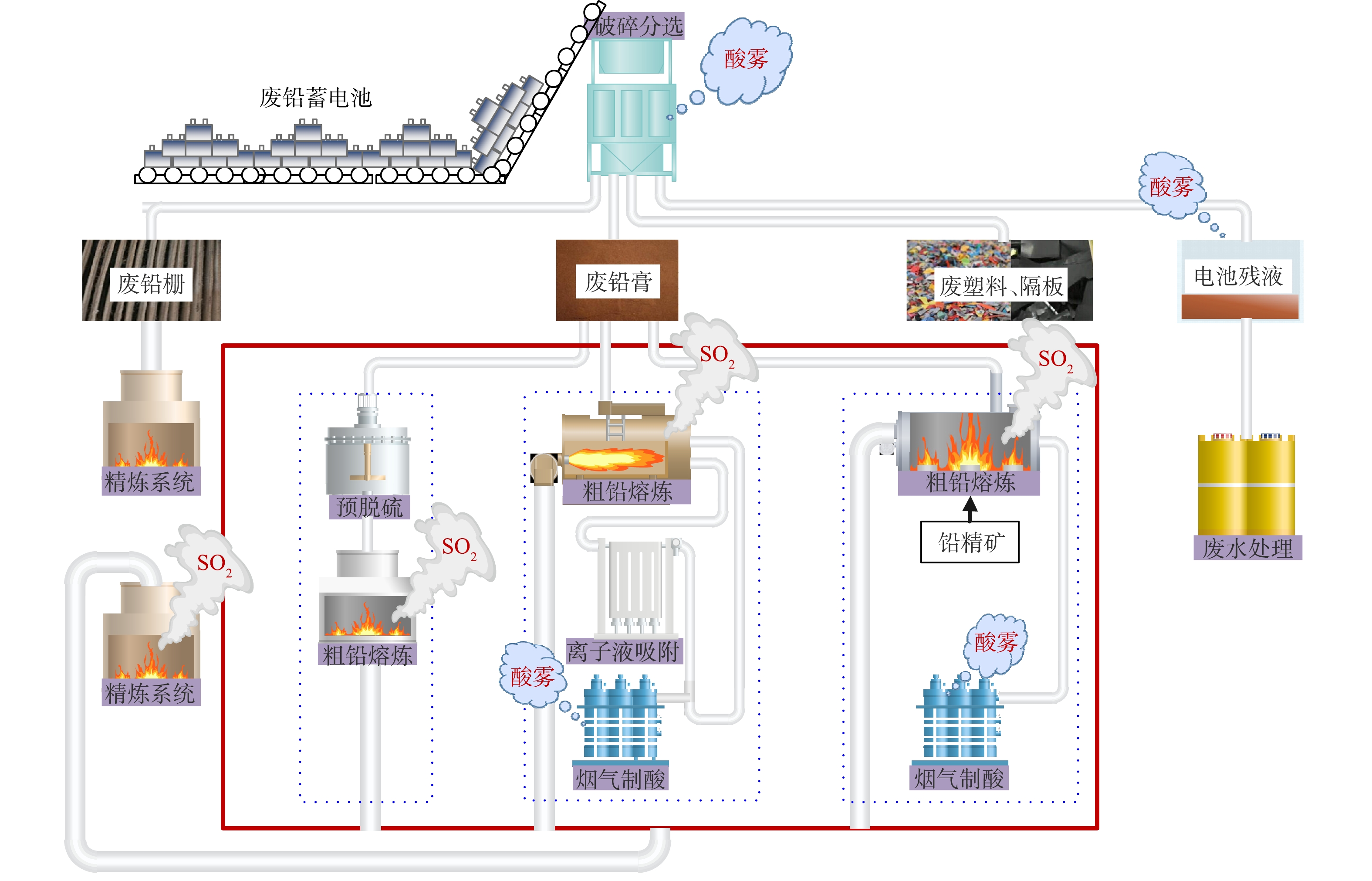
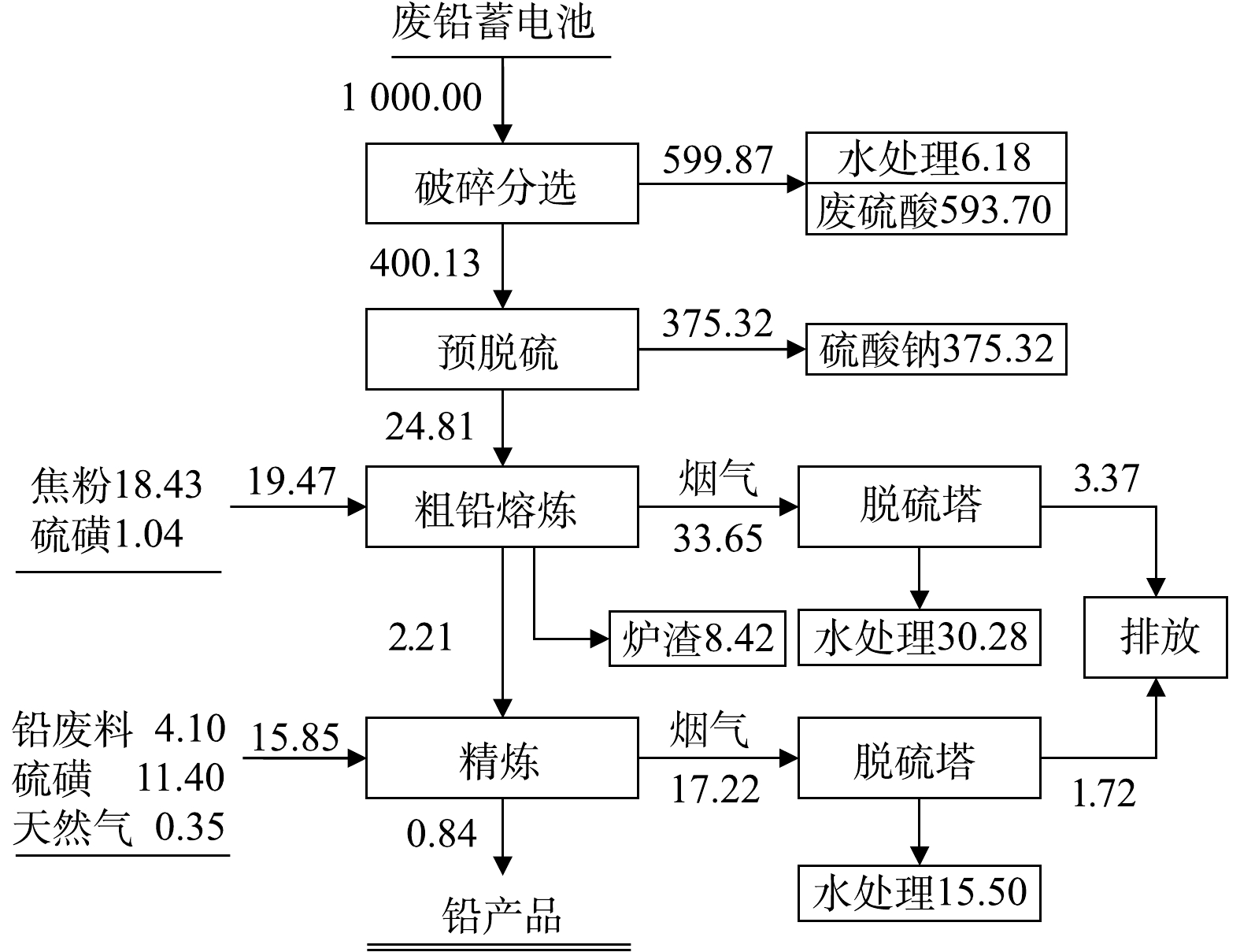
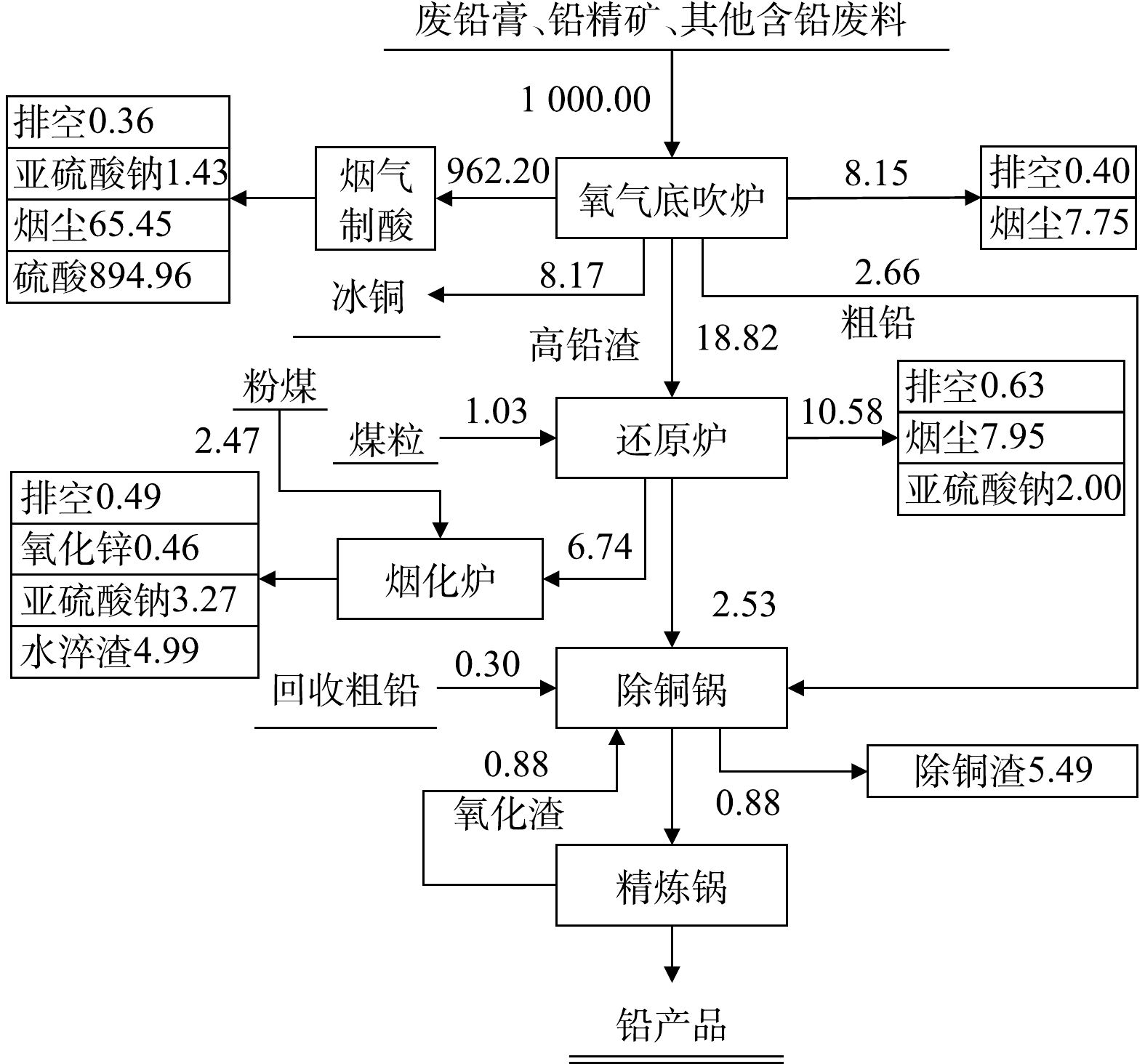
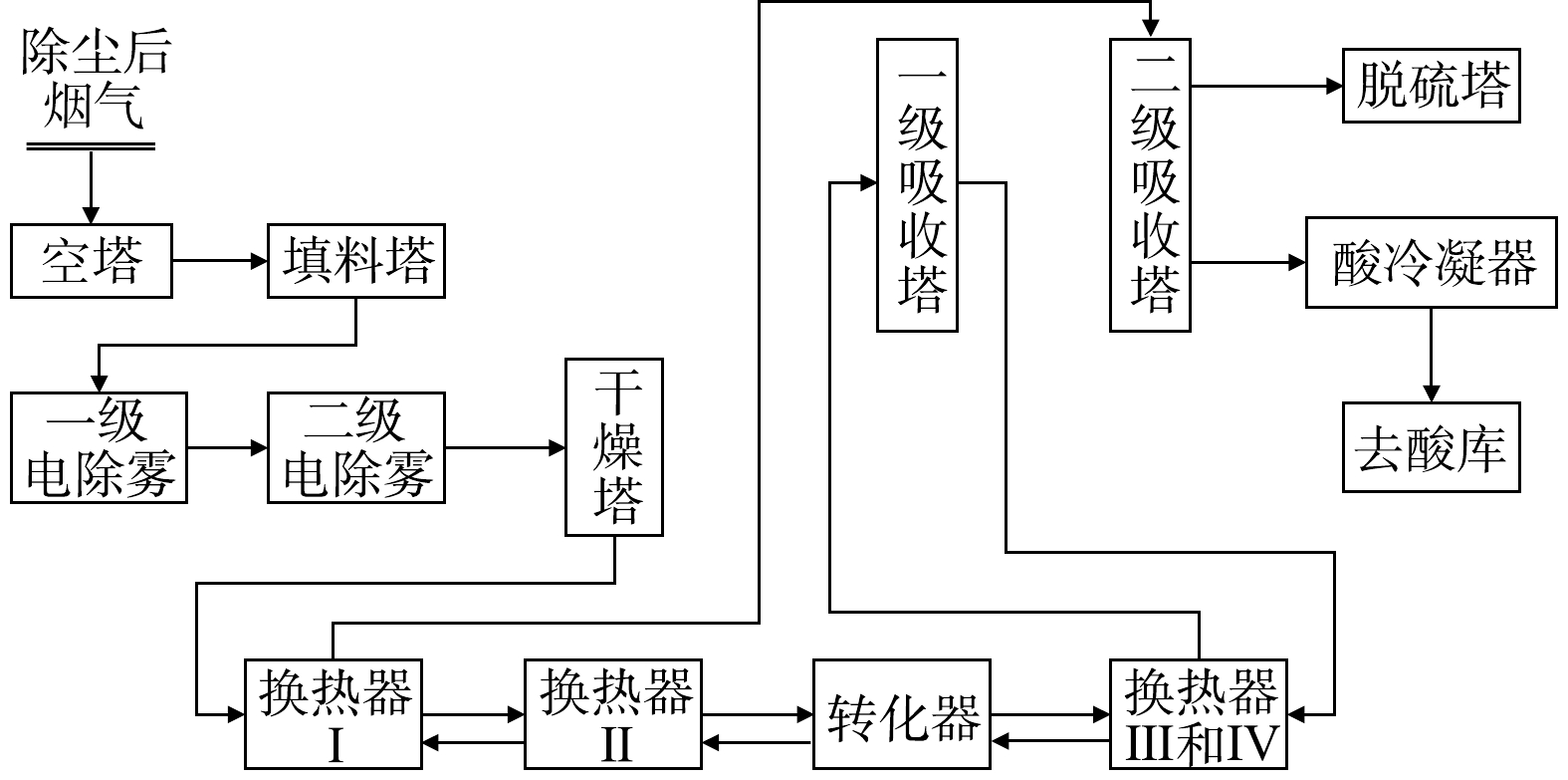
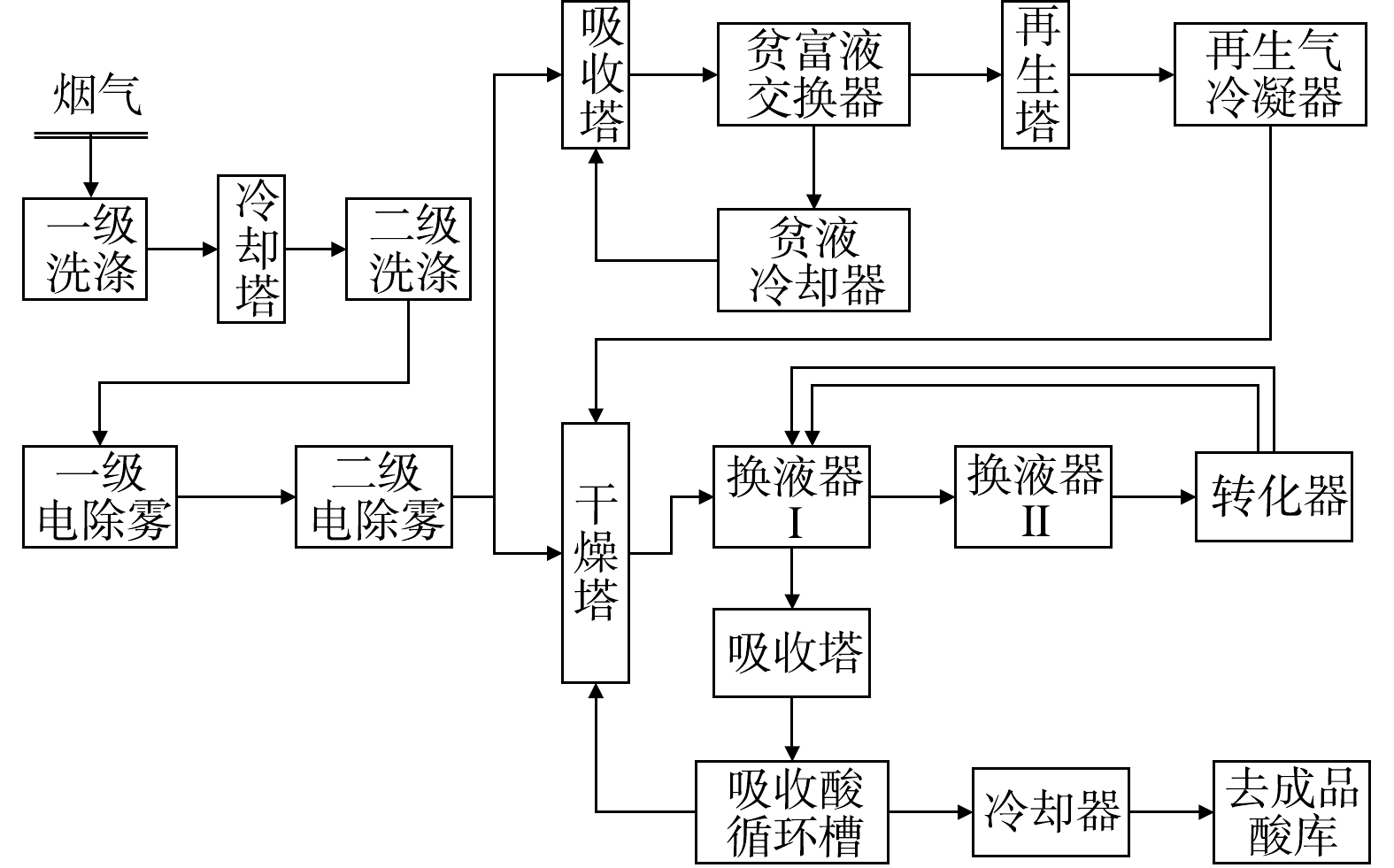
废铅蓄电池处理技术常按废铅膏的处理方式分为预脱硫-还原熔炼技术、再生铅-铅精矿混合熔炼技术和直接熔炼-烟气制酸技术。但不论哪种技术,均包括破碎分选工序、铅栅熔炼工序、废铅膏冶炼工序,其中部分技术包括废铅膏预脱硫工序。因此,首先从工序组成来分析生产过程污染物的排放情况,如图2至图4所示。
破碎分选工序包括废酸分离、酸液净化或处置、破碎分选及压滤等流程,其中部分硫以硫酸雾形式挥发;预脱硫转化工序包括一次脱硫、二次脱硫、压滤及蒸发结晶等流程,其中90%以上硫以硫酸盐形式回收。废铅膏冶炼常根据是否进行预脱硫分为3种:第一种,脱硫铅膏直接进入还原炉熔炼产出粗铅,粗铅进入精炼系统产出精炼铅,其中硫以烟气形式进入脱硫塔;第二种,未脱硫铅膏搭配铅精矿混合熔炼制取粗铅,并对粗铅电解制取精铅,其中含硫烟气直接用于硫酸生产原料,同时排出SO2烟气和硫酸雾;第三种,未脱硫铅膏直接进入富氧熔炼炉产出粗铅,粗铅经电解精炼产出电解铅,所产生的含硫烟气经离子液脱硫与一转一吸共同处理完成硫的回收,同时排出SO2烟气和硫酸雾。
综上所述,根据含硫污染物的产生阶段,SO2全流程控制途径可分为硫酸雾处理工序、前端预脱硫转化工序或后端烟气预转化工序、以及尾气脱硫工序。
-
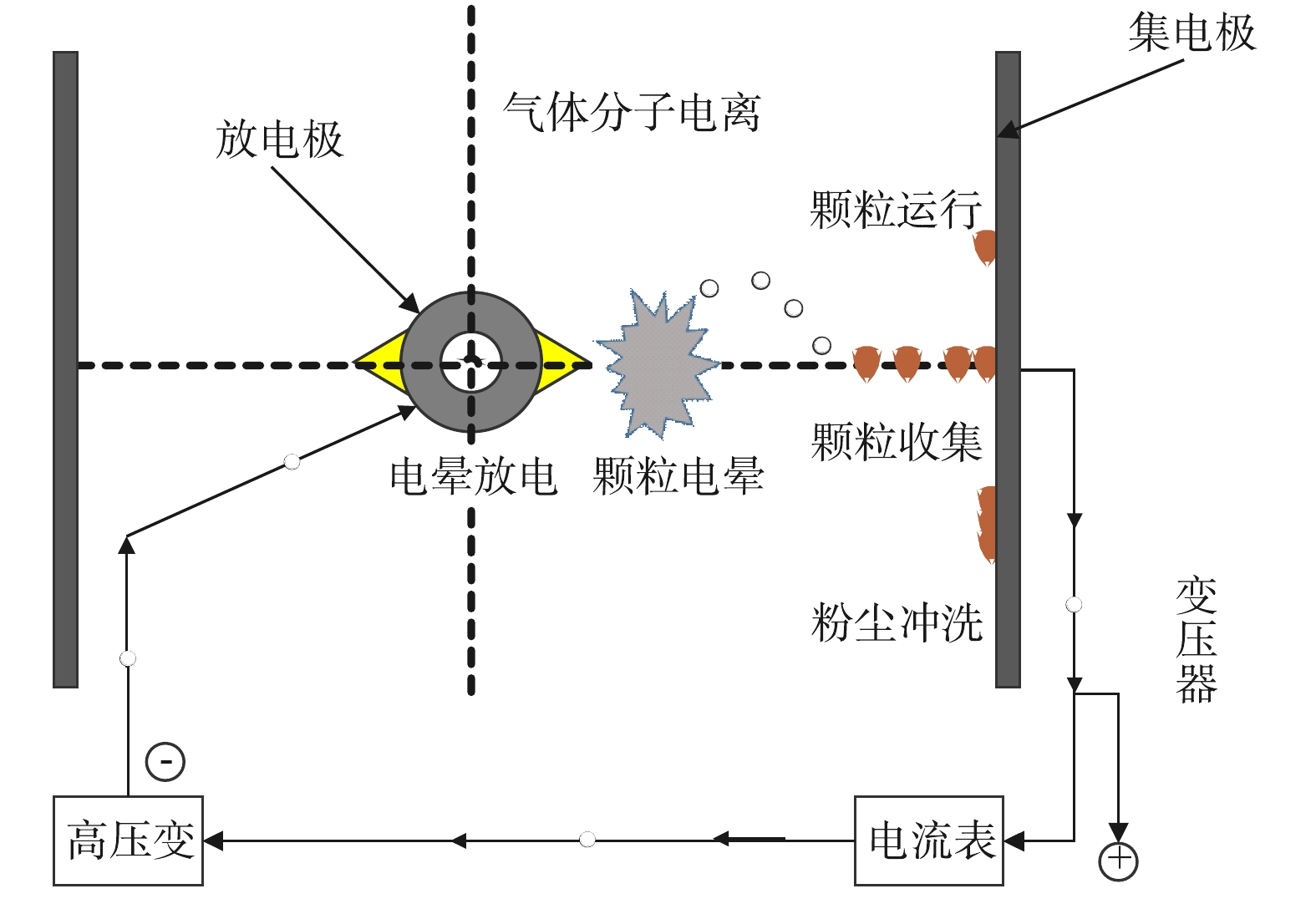
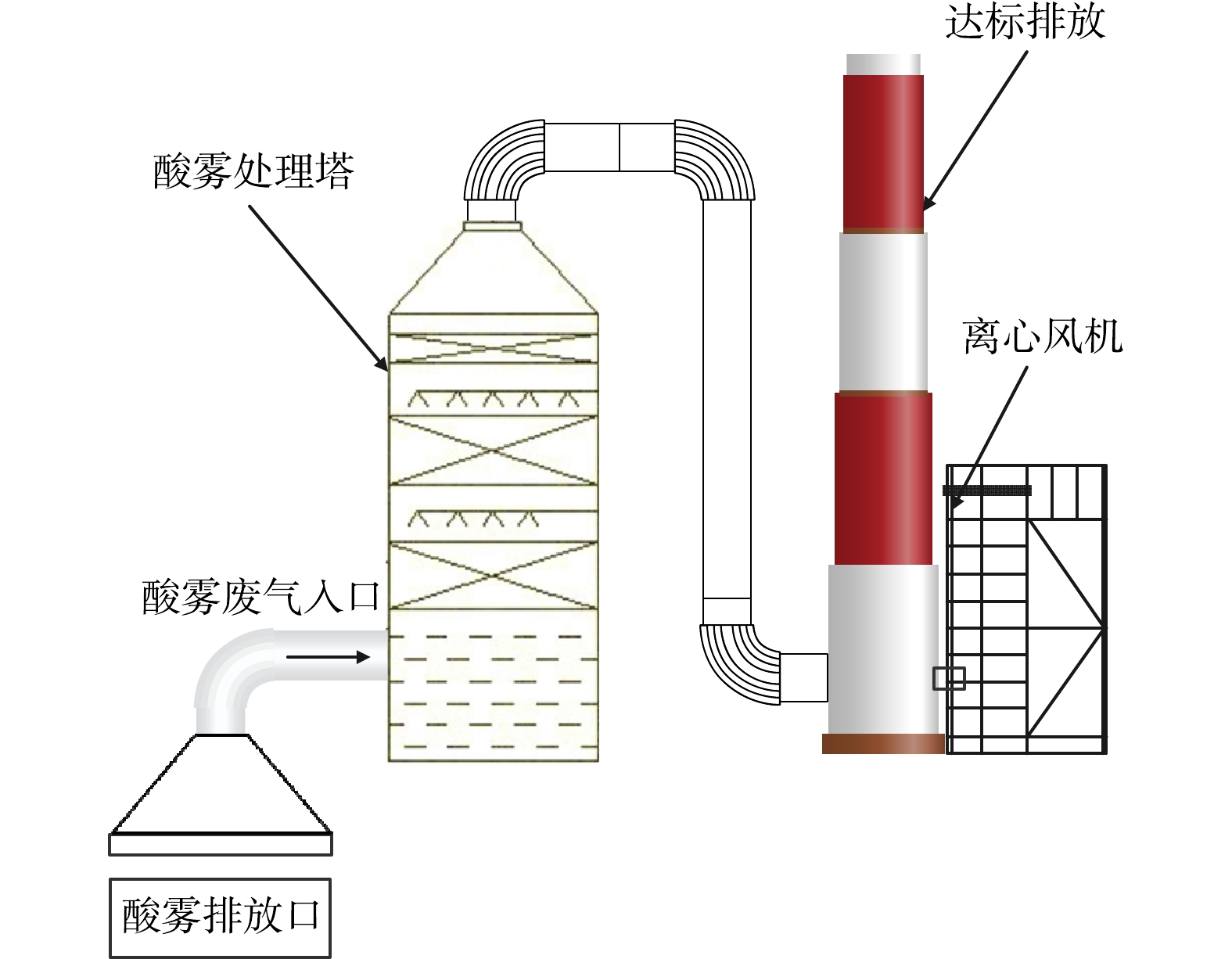
硫酸属于不易挥发的液体,但在破碎分选和烟气制酸过程中,仍会有少量硫酸雾挥发。硫酸雾在空气中颗粒很小 (粒径0.1~10 µm) ,且比烟的湿度高,是介于烟气与水雾之间的物质,具有较强的腐蚀性,因此需要对其进行捕集处理后排放。常用的硫酸雾的处理技术有静电除雾法和液体吸收法。
-
如图5所示,该技术首先在电晕线 (阴极) 和酸雾捕集极板 (阳极) 之间形成强电场,使空气分子瞬间被电离而产生大量电子和正、负离子,这些电子及离子在电场作用下作定向运动,形成捕集酸雾的媒介;而后使酸雾微粒荷电作定向运动,抵达到捕集酸雾的阳极板上并释放电子,完成酸雾捕集[15]。该技术较为成熟,可达到98%以上的硫酸雾吸收率,但是设备复杂、酸雾吸收效果受气体温度、湿度影响较大[16]。而且近年来研究发现,当硫酸气溶胶被引入湿式静电除尘器时,液滴的生长和团聚可以因碰撞频率增加而得以增强[17],但偶尔也会因为存在细颗粒出现电晕抑制[18],因此了解静电电除尘器中的电气控制特性对于提供适当的改造技术指导至关重要。基于此,多个学者建立了不同的数值模型来研究不同电极配置下的电场和离子密度分布[19-20],如WANG等[21]针对烟气具有高污染物浓度、高气体速度和高湿度的特点,设计了一种新的电流测量系统,用于自动扫描记录沿气流方向的电流密度分布,并初步探索了不同SO3浓度、气体速度和电极配置下的电晕放电,提出可通过加强入口电晕放电和促进液滴生长来缓解电晕抑制作用。
-
如图6所示,该技术首先使排气罩捕集的含酸雾废气进入吸附设备,而后使流体吸收液与废气充分接触、酸碱中和,达到净化酸雾的目的。常用的吸收剂有10%的Na2CO3、4%~6%的NaOH和NH3的水溶液,所采用净化废气处理设备主要有喷淋塔、填料塔和筛板塔等。该技术的吸收率可达95%、设备投资也低,但是存在能耗大、成本高和北方冬季应用受限的问题。因此近年来不断有人研究适用于宽泛温度范围的低成本硫酸雾处理方法,如SHU等[22]以粉煤灰为原料,通过NaOH、CaO和H2O活化,制备了用于除硫酸雾的吸附剂,并且研究了吸附剂制备过程中温度、时间、CFA/活化剂和水/固体配比等参数的影响,发现与原始粉煤灰和CaO本身相比,所产生的吸附剂具有较高的比表面积,对硫酸雾的去除效率更高。
-
考虑到占废铅膏含量40%以上的PbSO4冶炼温度高、能耗大、硫污染物严重[23],江苏新春兴再生资源有限责任公司、重庆吉鑫再生资源有限公司、贵州火麒麟能源科技有限公司等企业在铅膏熔炼前对其进行预脱硫转化,有效降低了熔炼过程SO2的释放。预脱硫技术常根据所用脱硫剂分为:碳酸盐体系脱硫、氢氧化钠体系脱硫、氯盐体系脱硫和其他新方法脱硫。
-
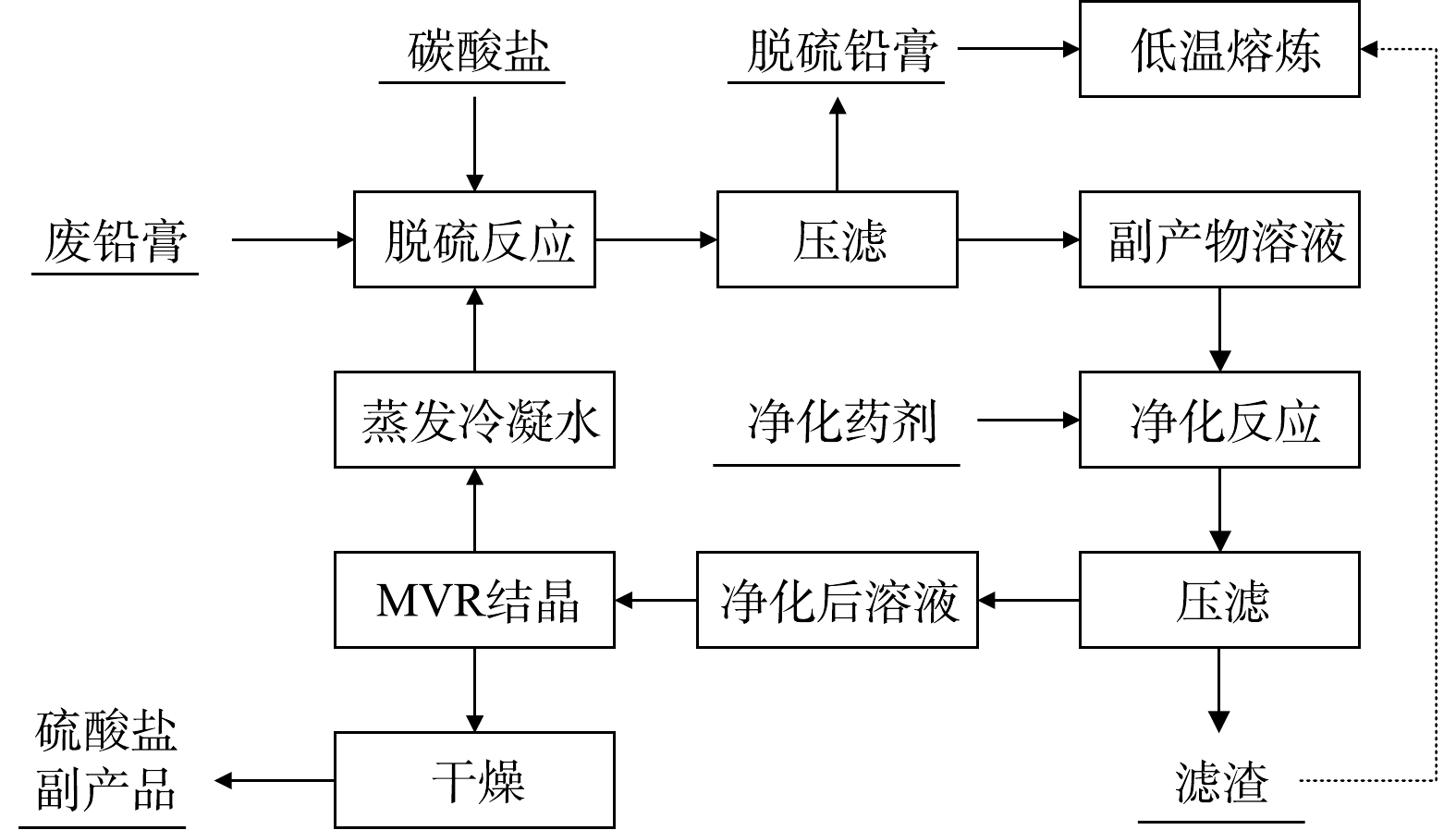
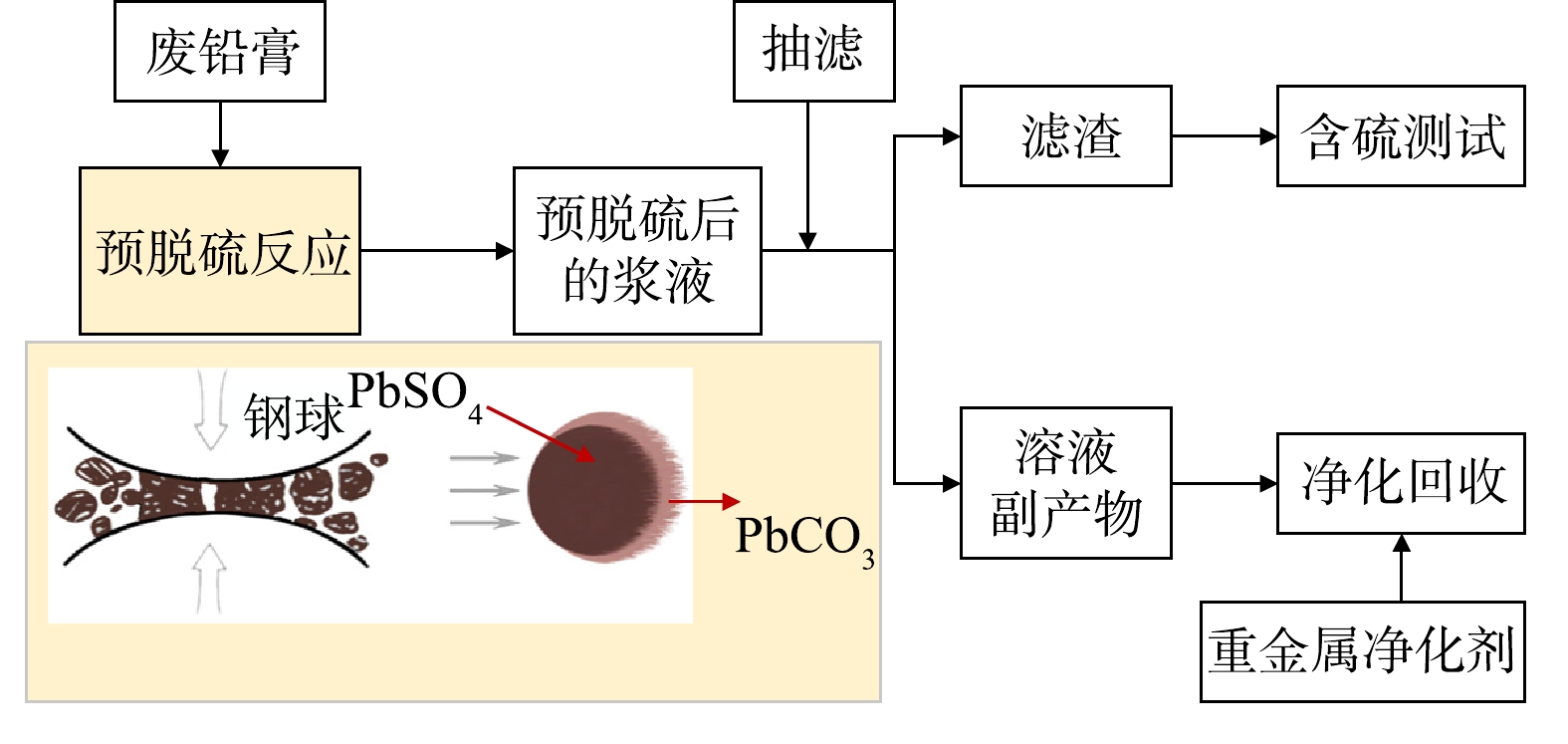
碳酸盐体系脱硫在早期预脱硫企业中应用最广泛。该方法依据PbCO3的Ksp (7.4×10−14) 远远小于硫酸铅的Ksp (1.6×10−8) 的原理,通过单独添加Na2CO3、NaHCO3、(NH4)2CO3、NH4HCO3或按比例混合添加上述碳酸盐,将PbSO4转化成PbCO3,并将溶液净化后得到硫酸盐副产品,具体流程如,并将溶液净化后得到硫酸盐副产品,具体流程如图7所示。关于碳酸盐体系脱硫的研究进展如表1所示[24-26]。LIU等[27]分别研究了Na2CO3、NH4HCO3和(NH4)2CO3这3种脱硫剂,结果显示Na2CO3脱硫率可达98.10%,但存在原料价格高、脱硫产物含有杂质相且在后续焙烧过程难以去除残留钠、副产品Na2SO4销量差等问题;NH4HCO3虽然能避免在脱硫产物中形成NaPb2(CO3)2OH杂质相,但反应过程将CO2引入脱硫铅膏中,导致操作困难且可控性低,最终造成脱硫率较低,仅为93.43%;(NH4)2CO3脱硫率为97.87%,略低于Na2CO3,其脱硫副产品(NH4)2SO4作为氨肥具有较好的市场前景,但目前也是存在原料成本较高和副产品销量差的问题。俞小花等[28]考虑到(NH4)2CO3成本高的问题,采用NH4HCO3+NH3·H2O混合液进行脱硫,结果显示整个反应过程快,但由于碳酸铅固体包裹硫酸铅物料导致反应进行并不彻底,脱硫率仅为88.00%。
可见,碳酸盐体系脱硫整体上反应比较简单,但是原料成本高和反应过程碳酸铅包裹阻碍反应速率的问题很难同时得到解决,这也直接导致近几年来采用此工艺的企业纷纷放弃此类技术,几乎不再进行延续性技术革新。
-
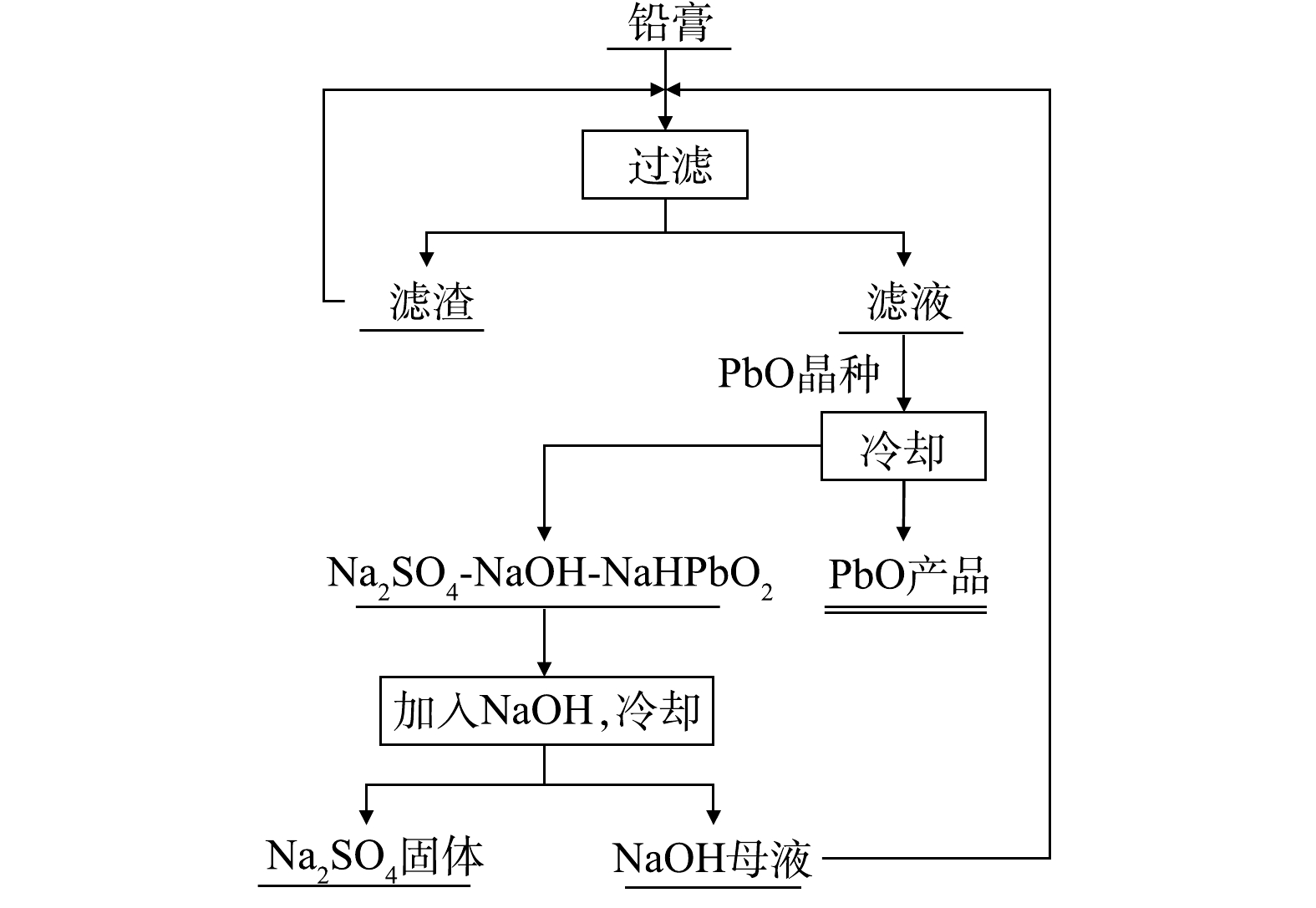
氢氧化钠体系预脱硫过程可将PbSO4转化为PbO或Pb(OH),当碱过量时也会转化为NaHPbO2,该预脱硫转化方法常与电解沉积联用,反应原理如式 (1)~式 (3) 所示。
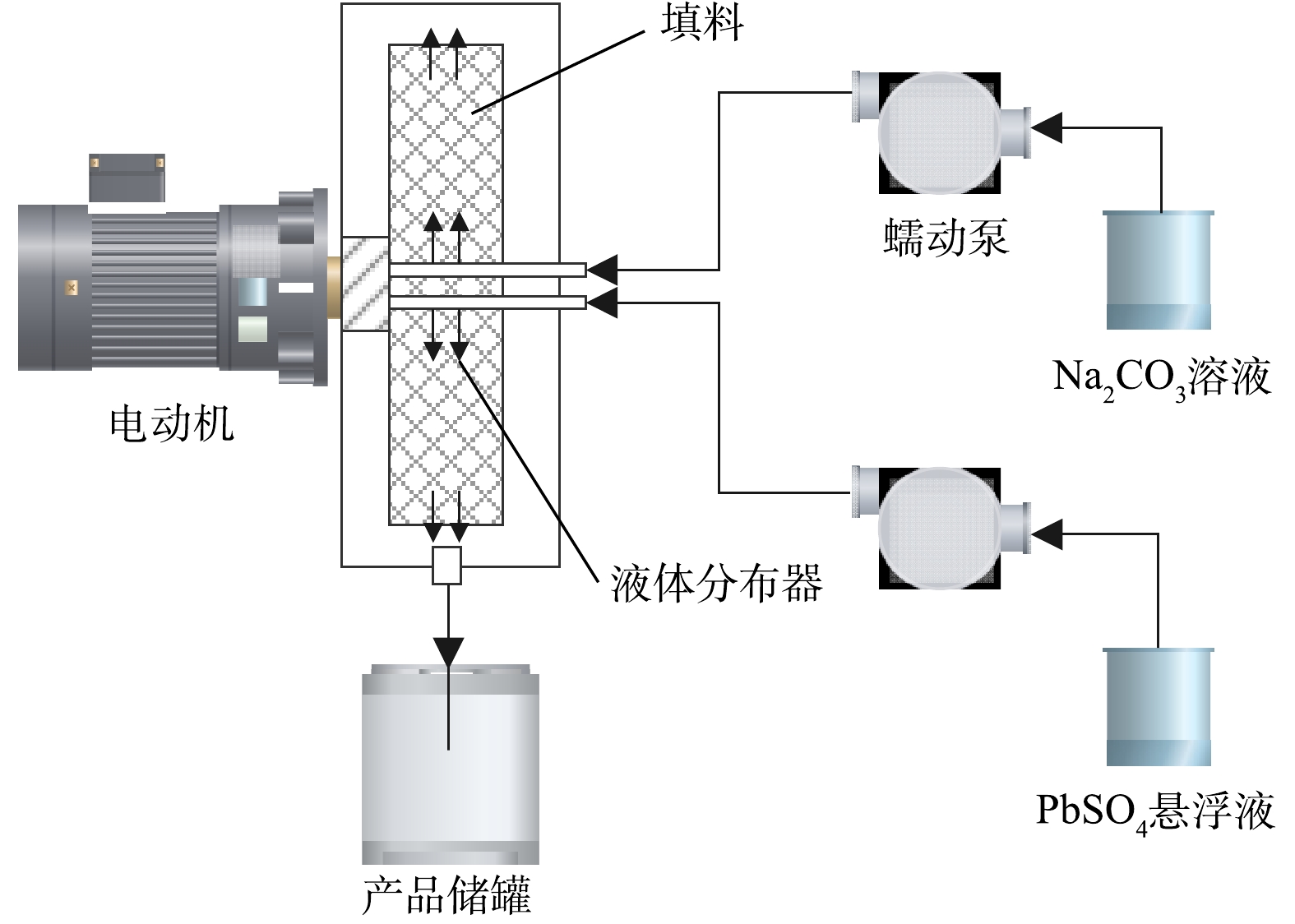
熊浩宇[29]以NaOH为脱硫剂对废铅膏进行预脱硫,在最佳条件下脱硫率可达95.40%;潘军青等[30-31]以NaOH为脱硫剂开发了废铅膏循环脱硫-结晶技术,脱硫率可达98.50%以上,同时在硫酸钠结晶过程中回收NaOH母液,实现脱硫母液循环利用,如图8所示。该体系可以实现较低的铅损失,铅产物纯度高,但存在脱硫时间较长,而且脱硫后必须在电解条件下才能得到高纯度铅单质,操作相对复杂,经济效益低。
-
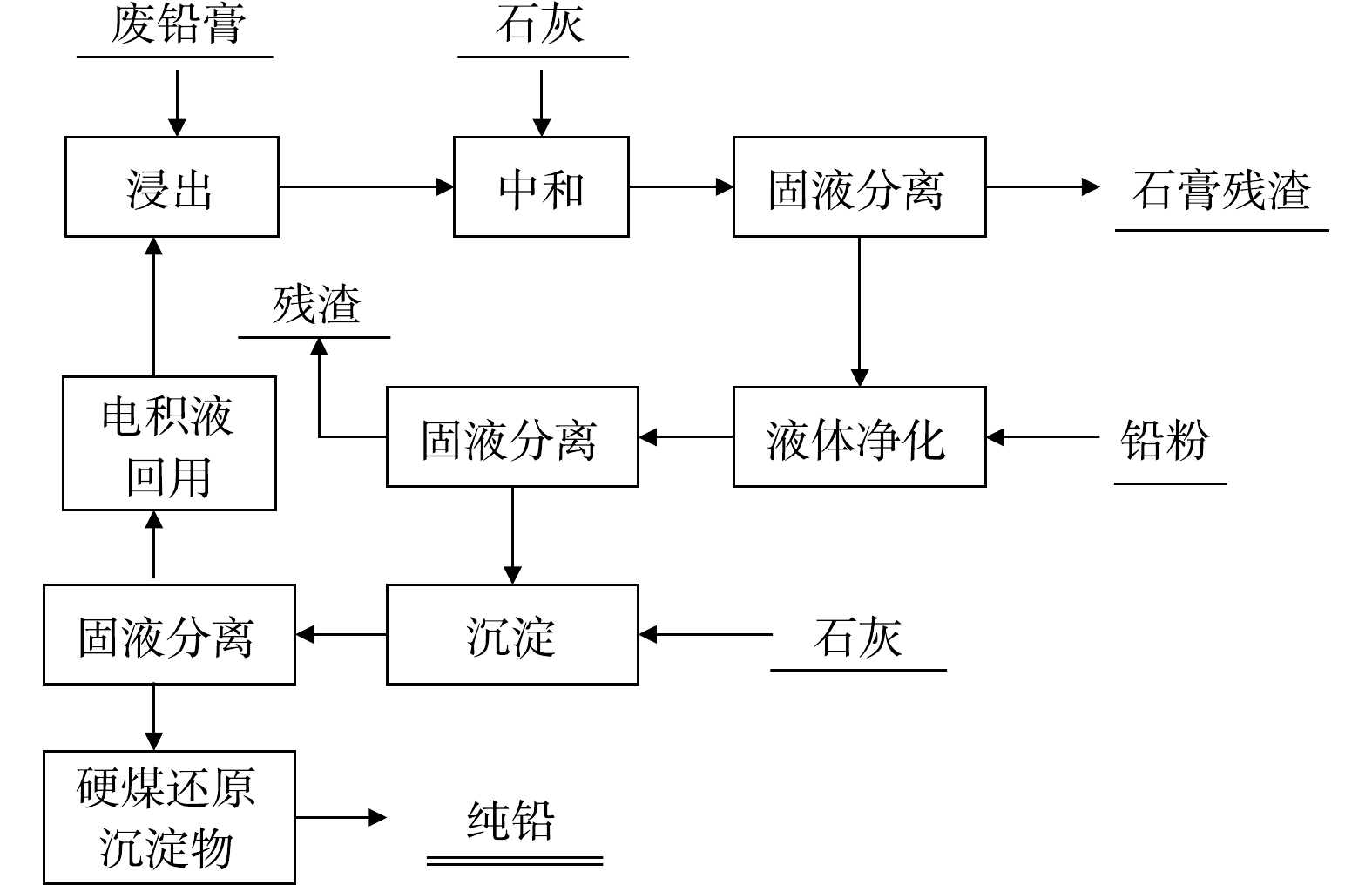
该体系利用铅膏中的铅、氧化铅以及二氧化铅溶于盐酸,硫酸铅可溶于高浓度的热氯化钠溶液中以配合物形式浸出,最终达到脱硫目的,主要反应如式 (4)~式 (7) 所示。
氯盐体系的代表工艺是Placid工艺,该工艺将铅膏与其他含铅氧化物料一并用热HCl-NaCl溶液浸出,生成可溶性PbCl2,而后转移至阳离子交换隔膜电解池阴极室,在1 000~1 200 A·m−2高电流密度下进行电解沉积。后期考虑到操作过程能耗高达1 300 kWh·tPb−1,又开发了Plint工艺 (如图9所示) ,实验过程主张采用石灰石沉淀代替电解沉积,其脱硫转化过程与Placid工艺相同,但该工艺会产生含重金属的硫酸钙堆积,对环境产生新的污染。
氯盐体系处理废铅膏属于湿法冶金,因此与反应过程产物的最大可能产出量和产物纯度相比,学者们更关注的是反应速率。齐美富[32]、桂双林[33]等用该体系处理废铅膏,浸出率可达到94.86%,说明该体系整体脱硫效率较低,而且该浸出体系氯盐浓度高、腐蚀性强,这也成为近年来该工艺进一步扩大化应用的最大瓶颈。
-
边文景等[34-35]提出铅膏预脱硫“表面更新”模式,即以Na2CO3为脱硫剂,在搅拌釜中添加粒子作为碰撞研磨介质,反应浆液与粒子在反应器内旋转,对反应颗粒进行碰撞、研磨,即时破坏产物碳酸铅包裹层,实现反应颗粒表面更新,最终可使反应后固体含硫率小于0.5%,如图10所示。
NING等[36]考虑到传统反应器中较低的液固传质速率对湿法冶金铅膏脱硫的工业应用有很大限制,于是提出了一种在旋转填充床反应器 (rotating packed bed,RPB) 中用Na2CO3溶液进行铅膏脱硫的传质强化方法,并详细探讨了各种操作变量对脱硫程度的影响,如图11所示。结果表明在RPB反应器中,PbSO4在很短的接触时间内,仅持续一个循环的液固接触,脱硫率即可达到99.70%,因此认为RPB反应器在铅膏脱硫的工业应用中表现出巨大的潜力。
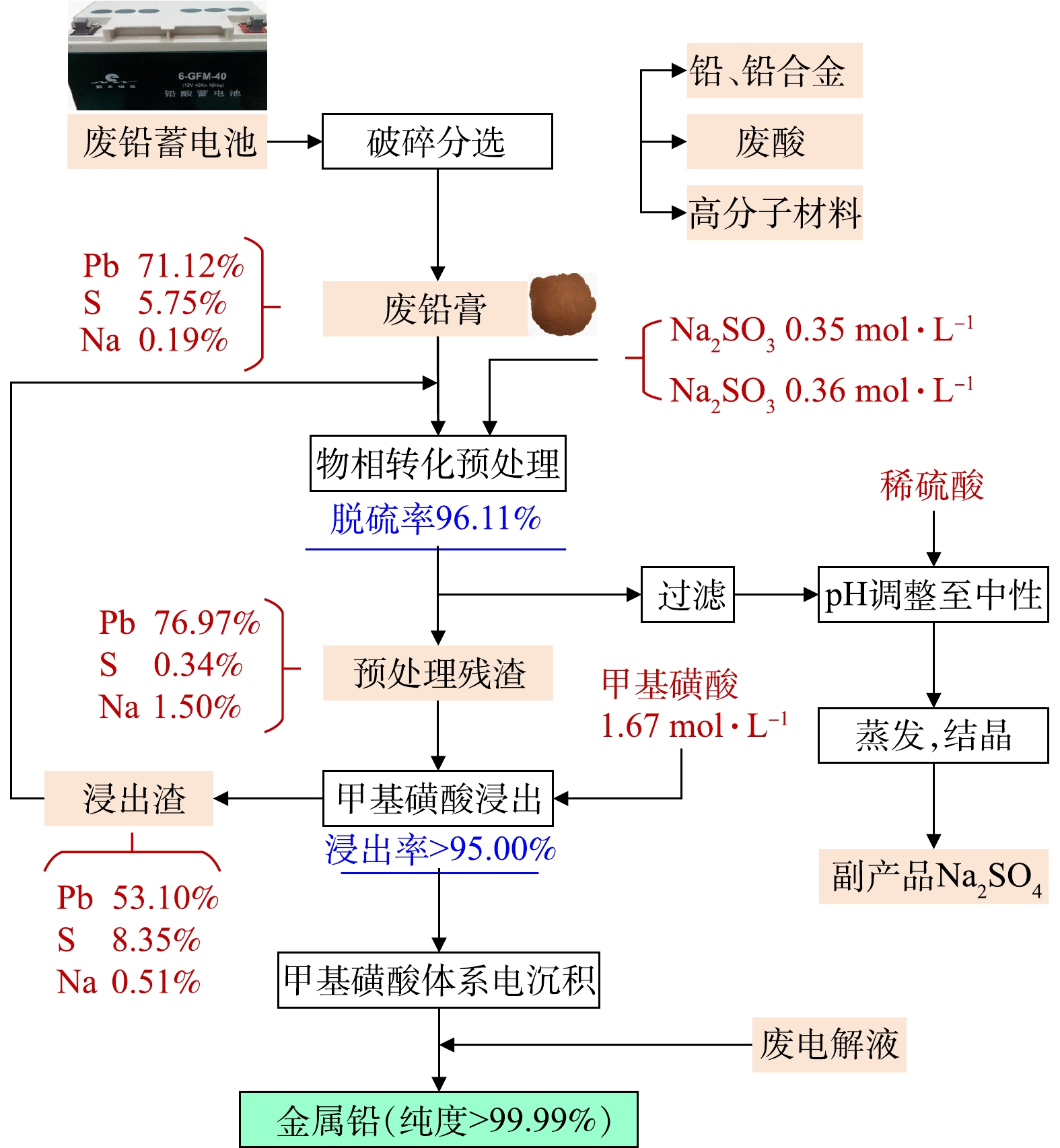
CHANG等[37]为了显著提高铅的浸出效率,以Na2CO3-Na2SO4为脱硫剂,将铅膏中的PbSO4首先转化为酸溶性Pb3(CO3)2(OH)2或NaPb2(CO3)2OH,然后将预处理的产物在甲基磺酸溶液中浸出以获得含铅浸出液,该浸出液被最终电沉积以产生铅沉积物并再生甲基磺酸,如图12所示。研究结果显示,铅膏中95%以上的PbSO4可在50 min内可转化为酸溶性Pb3(CO3)2(OH)2或NaPb2(CO3)2OH。
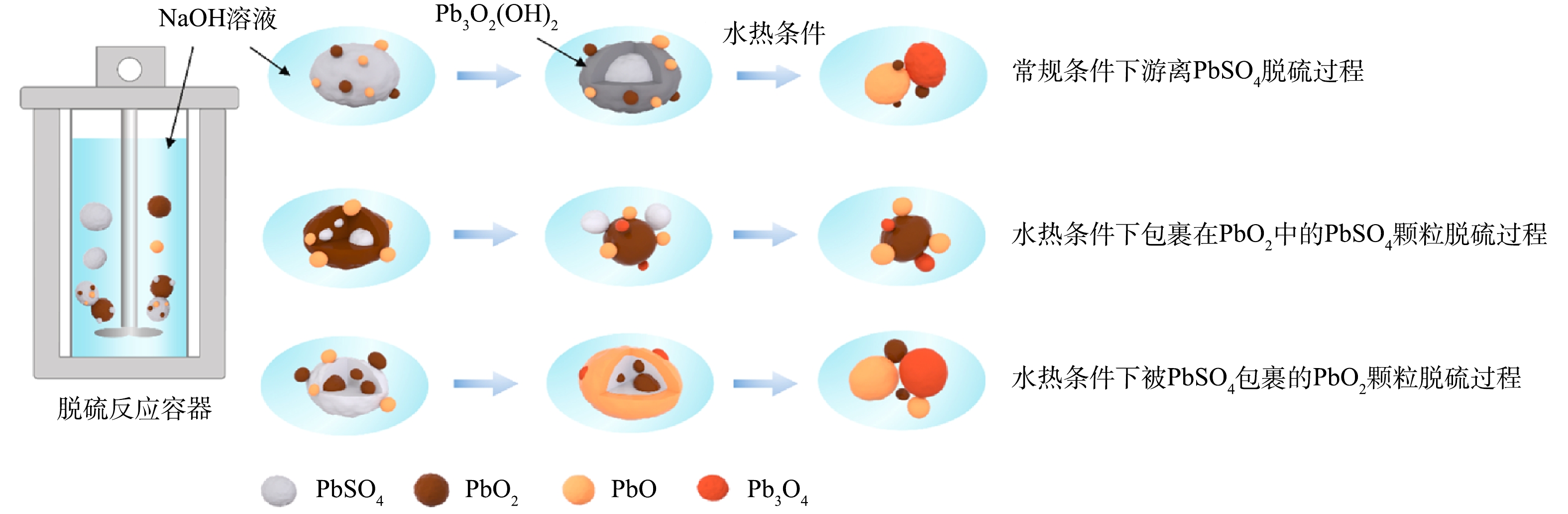
DENG等[38]为解决硫酸铅在氧化铅中扩散并相互混合导致预处理过程中脱硫不完全和试剂消耗大的问题,以NaOH为脱硫剂在水热条件下强化PbSO4的脱硫反应,使PbO和PbO2反应形成Pb3O4,这种配比反应破坏了固体产物层的包封,有利于脱硫反应的进行,在优化的还原条件下 (即时间60 min,氢氧化钠系数1.1,温度150 ℃、 液固比10 mL·g−1,搅拌速度600 r·min−1) ,脱硫率达到98.95%,如图13所示。
-
考虑到废铅膏预脱硫处理成本高、副产品堆存严重,近5年来越来越多的企业开始淘汰铅膏预脱硫转化工序,选择后端烟气预转化制酸工艺。冶炼烟气预转化制酸的效果好坏主要取决于SO2转化为 SO3的转化过程,此过程转化率高则硫回收率高,且所得硫酸品质高。反之,转化率低,则硫回收率低,对环境的污染较大。不同的出炉SO2浓度将会显著影响转换过程和转化率,因此,对于再生铅-铅精矿混合熔炼的企业,其烟气中SO2浓度较高 (6%~12%),可直接采用直接制酸工艺生产硫酸 (如两转两吸法) ;对于单独的再生铅企业,由于烟气中SO2浓度较低 (<6%) ,多采用间接制酸工艺。
-
烟气直接制酸转化过程如图14所示,由富氧底吹炉来的烟气,经余热锅炉及电除尘后分别进入一级洗涤器和二级洗涤器,完成洗涤、增湿、降温除尘,使烟气冷却至39 ℃左右;而后进入电除雾器,使烟气中酸雾及残余的烟尘得到进一步去除后进入干吸系统;从电除雾器出来的SO2烟气经干燥后,直接送往转化工段进行第一次转化,转化后排出的烟气则再次进入转化工段进行二次转化,烟气则送往脱硫系统进一步处理,转化率可达到99.80%;成品酸经冷却后流入地下槽,并用泵送到酸罐,定期外运销售[39]。
烟气制酸是一个复杂的非线性过程,具有多个变量和强耦合性。而运行数据是状态监测、最优控制和故障诊断的重要依据。针对烟气制酸环境复杂,设备众多,检测设备获取的数据容易出现数据污染、丢失、异常值等异常现象的问题,LI等[40]提出了一种基于改进随机森林的数据清理方法,能够准确地消除和补偿烟气制酸过程中的异常数据,利用清洗后的数据,可以建立更准确的模型。考虑到生产过程中参数变化和工况波动 (如初始SO2浓度、气体流速、催化剂活性等) ,现有的SO2转化率优化方法很难达到最佳效果,JIA等[41]提出了一种具有调节因子的自适应策略,用于SO2转化过程的实时优化,仿真结果表明当模型参数与生产过程不匹配,或者工作条件发生变化时,经过多次迭代,算法可以收敛到实际工业过程的最优点附近。
-
间接制酸工艺是通过物理或者化学方法将低浓度SO2烟气转化为高浓度SO2气体,再采用传统技术制备硫酸[42-43],目前离子液法脱硫联合一转一吸制酸工艺已在工厂规模化使用,如图15所示。富氧侧吹炉的烟气经过两级洗涤塔-两级电除雾器净化后,达到降温和除尘目的;而后经过1次干燥、1次吸收,并对净化后的烟气进行合理分配,约60%的烟气直接进离子液脱硫系统,40%的烟气与离子液脱硫后的SO2气体混合后进入干燥塔;干燥后的气体被送至转化工序,经换热、催化氧化后得到SO3气体,经换热器后送至吸收塔,SO2转化率可达到99%以上,烟气排放ρ(SO2)<100 mg·m−3。该工艺有效解决了单独再生铅冶炼过程因烟气硫含量低而无法高效脱硫制酸的问题,但侧吹炉冶炼还原过程,烟气中SO2浓度较低,容易造成转化器中温度不稳定,影响后续转化过程,未来还需要进一步精准掌握出炉烟气中SO2浓度,同时不断开发更高效的SO2捕获剂[44]。如JIANG等[45]基于Langmuir模拟、理论计算和量子相结合的定量计算,设计并制备了5种不同阴离子的咪唑型离子液体,研究了它们对SO2的吸收能力、解吸残留物和有效吸收能力的行为,发现通过预测方法获得的理想离子液体对于捕获2 000 ug·mL−1的SO2具有优异的吸收能力。
通过后端烟气制酸对含硫烟气进行转化,可有效地降低脱硫处理费用,同时生产出产品级浓硫酸,真正实现了废物再利用过程零产废。但后端烟气制酸也存在一些问题,如需通过定期更换催化剂,控制好热平衡、硫平衡、水平衡方能实现稳定运行;前端烟气温度控制不好,会导致硫酸中氮氧化物过高;脱硫系统长期运行,导致盐含量过高,天冷易造成管道结晶,需增加冰冷系统,但成本高;制酸操作人员对前端熔炼数据不关注,尚无法根据输入数据变化及时做出调整以维持制酸系统的稳定性等。
-
我国铅冶炼行业含有的大量硫元素常采用前端预脱硫转化或后端烟气制酸进行处理,对于末端低浓度SO2烟气,以往主要通过烟气稀释的方式使SO2浓度满足标准要求进行高空排放。近年来,国家相继出台了多个限制标准,从排放浓度和排放总量2方面进行规范化要求[46],如《排污许可证申请与核发技术规范 有色金属工业 再生金属》 (HJ 863.4-2018) [47]要求再生铅排污单位熔炼炉尾气烟囱基准烟气量3 000 m3·t−1,熔炼炉环境集烟烟囱基准烟气量3 000 m3·t−1,精炼锅集烟烟囱基准烟气量4 000 m3·t−1;《再生铜、铝、锌工业污染物排放标准》 (GB31574-2015) [48]要求SO2排放限值150 mg·m−3,基准排气量10 000 m3·t−1。因此,各个企业为了达到标准规定的污染物排放控制要求,也都相应的采取必要的尾气脱硫措施。经过百余年的探索研究,国内外学者开发出200多种脱硫方法,在铅冶炼行业相对成熟可推广的技术主要包括石灰-石膏法、钠碱法和氨吸收法。
-
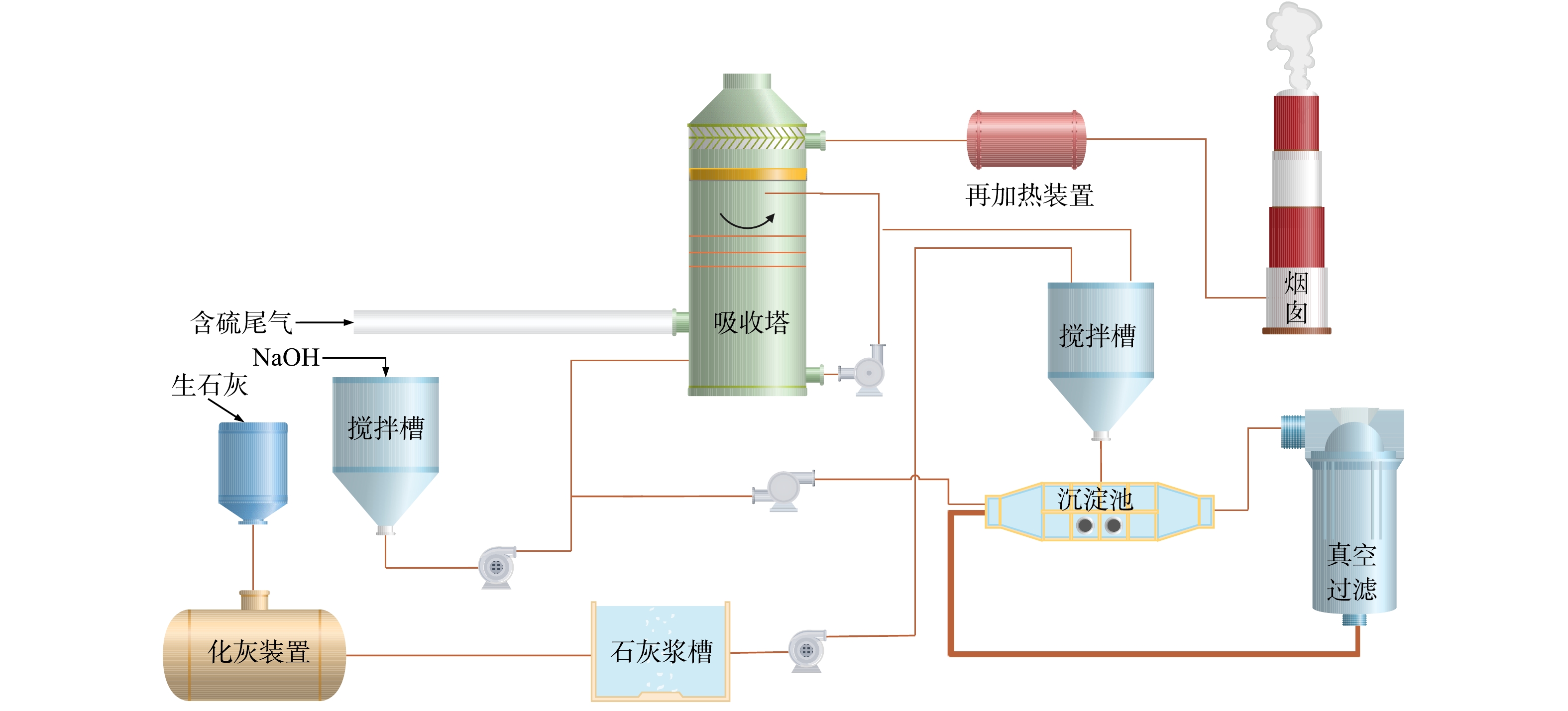
该技术是将石灰或石灰石粉与水混合制成吸收浆料,而后经泵打入吸收塔中与烟气中SO2充分混合,使烟气中的SO2与浆液中的CaCO3反应生成CaSO3,而后从吸收塔下部浆池鼓入氧化空气使CaSO3氧化成CaSO4,当CaSO4达到一定饱和度后结晶形成生石膏[49-51],脱硫原理如式 (8)~(11) 所示。
该技术所需装备结构简单,吸收剂价格便宜,SO2吸收率可达90%,但运行过程也还需要不断针对存在的问题进行合理改进,如吸收剂消耗大、pH值难以稳定控制、系统阻力大、管道转弯部位易结垢腐蚀、渣多难处理等。针对关键指标控制问题,崔名双等[52]认为脱硫效率主要取决于SO2初始浓度、烟气量、钙硫比、石灰浆料的pH值、吸收塔再循环浆料与吸收塔出口烟气比值等。针对pH控制方面,刘永龙等[53]认为随着循环浆液对SO2的不断吸收,系统pH值不断下降,如按设定程序在pH<5.5时补充石灰乳,则会因为pH检测和pH变化的延时性影响系统正常运行,因此需要优化pH计的安装位置并提高其灵敏性。针对除雾器堵塞问题,江立振等[54]认为脱硫过程不能盲目追求高脱硫率而大量注入石灰浆料,否则容易导致脱硫塔中的烟气携带一部分石膏晶体黏附在除雾器上造成堵塞。对于脱硫塔腐蚀问题,应经常调整除雾器喷头方向,减少冲洗盲区,并且在每次大修时认真检查腐蚀部位进行及时的补焊。针对成本高的问题,认为石灰石活性低,因此脱硫过程需要增大吸收液喷淋量,直接提升了运行费用,提出可尝试用石灰粉替代石灰石。QIAO等[55]建立了WFGD的数学动力学模型,用于描述和优化反应系统的脱硫性能和脱硫系统的运行条件,确定了脱硫的最佳工艺条件。上述研究也表明该技术目前的主要研究方向包括设计新型的喷淋装置,增加气液瞬间接触面积;根据烟气中SO2浓度,精确调整浆液的pH值;通过添加钙基工业废渣合成新的吸收剂,以废治废的同时降低成本;研发基于大数据的智能参数调控系统,根据前后段工艺参数波动及时调整吸收剂参数,从而使脱硫效率能够发挥至最理想状态等。
-
钠碱法脱硫工艺是为了克服石灰-石膏法容易结垢的缺点而发展起来的。该技术采用可溶性碱 (NaOH或Na2CO3) 作为脱硫剂吸收烟气中的SO2,并利用Ca(OH)2对排入再生池内的脱硫产物进行还原再生,再生出的钠基脱硫剂再被打回脱硫塔循环使用[56],如图16所示。以NaOH-Ca(OH)2为例,其在吸收塔中与SO2接触后,常发生如下反应 (式 (12)~式 (16) ) 。可见,吸收过程所生成的NaHSO3对SO2没有吸收能力,当NaHSO3数量增多后,吸收液的吸收能力会下降,需要进一步补充碱液,使NaHSO3转化为Na2SO3。实际上,NaOH仅仅是一种启动碱,理论上启动后可循环使用并不消耗,实际运行过程因清渣会有少量损耗。
钠碱法吸收效率可达90%~95%、无废水排放、生产运行安全可靠,且不存在管道结垢堵塞问题,在众多的脱硫技术中,始终占据主导地位。但在实际工程中,也还存在一些小问题[57-59],如置换过程pH值的及时调整和精准控制,才能保证置换反应充分进行并且钙离子不会大量过剩;置换过程中亚硫酸钙结晶颗粒粒径很小 (<10 μm) ,很难通过简单沉淀完成分离,最终会因硫酸钠的存在降低石膏质量;钠碱法实际上并没有从根本上解决结垢问题,在脱硫过程仍然必须严格控制关键工艺参数,如监测亚硫酸盐浓度保证钙离子保持在较低浓度范围;动态调整pH值防止碳酸盐沉淀结垢等;高价值的NaOH等物质转化为低价值的Na2SO4,脱硫成本高;NaOH对管道设备腐蚀较严重;脱硫副产物综合利用相对复杂等也在一定程度上限制了其发展。因此,为了解决上述问题并提高对烟气的净化效果,将钠碱溶液与过硫酸盐、紫外线和催化剂等条件的结合进行烟气的脱硫,逐渐成为近年烟气净化的研究方向之一。
-
该技术也是世界上商业化应用的脱硫方法之一,其是以氨基物质 (氨或者氨水) 作吸收剂脱除烟气中的SO2并回收副产物 (如硫酸铵等) 的过程。主要包括SO2被吸收形成亚硫酸铵和亚硫酸铵被氧化成硫酸铵的两个反应过程,如式 (17)~式 (20) 所示。
该技术SO2吸收率可达95%,所用设备结构简单、运行成本低、运行过程气-液传质效率高且无废水废渣产生,被列为发展前景最好的资源回收型脱硫技术。但是良好的吸收效果仍然取决于多种关键因素,如喷淋塔内气-液传质效果、温度场、流场、反应物浓度场、反应过程氨逃逸的控制等[60-61]。因此,当前研究多围绕如何对关键参数进行精细化控制方面[62]。如JIA等[63]建立了氨基烟气脱硫系统储罐中总亚硫酸盐氧化的数学模型,可预测不同操作条件下总亚硫酸盐的氧化率;LI等[64]根据喷淋散射塔的结构和流动特性,分别建立了喷淋区和气泡区的流动、传热和传质模型,研究了液气比、入口SO2浓度、散射管浸入深度和烟气流量等操作参数对整体脱硫效率的影响;YAN等[65]考虑到脱硫系统中氨挥发会产生气溶胶问题,研究了氨湿法烟气脱硫中气溶胶的形成机理和排放特性,以及减少气溶胶排放的工艺条件。
-
本文基于从过程管控到末端治理的全过程管控思路,结合再生铅行业含硫污染物控制实践经验,对再生铅行业超低SO2排放所采用的关键技术、具体举措、技术发展趋势进行了较为详细的论述。研究发现前端预脱硫转化技术很难同时解决原料成本高和反应过程碳酸铅包裹阻碍反应速率的问题,而且硫酸盐副产品的后处理非常困难,未来将会被逐步淘汰。硫酸雾处理技术、后端烟气制酸转化技术和末端尾气脱硫技术均得到了长足发展,在不同类型企业得到了广泛的应用,但目前仍然存在脱硫成本较高、对复杂工况下的SO2烟气的脱除控制较差的问题,因此认为未来应从以下方面寻求突破。
1) 降低脱硫成本。国内常用烟气脱硫技术普遍存在运行成本高,脱硫副产物处理难的问题。烟气脱硫过程中脱硫剂消耗量较大是不争的事实,一方面要着力寻求更廉价的高效脱硫剂;另一方面,只有不断突破技术瓶颈,提升脱硫产物品质使其可以得到利用,才能够缓解脱硫剂的成本压力,同时减少脱硫产物处理开支。
2) 基于大数据驱动模型的工艺优化技术研究。因在复杂冶炼系统建模时,传统的机理建模基于平均场理论,与实际生产现场差距较大,具有明显的局限性。为了有效处理冶炼过程表现出强烈的非线性、多模式多时段特性,可尝试引入数据驱动的复杂系统建模方法,精确建立生产过程以及运行指标与被控变量之间的关系,精准预测未来数分钟的SO2排放浓度,为运行人员提前优化运行、保证 SO2的超低排放创造空间。
-
再生铅行业硫污染物的进一步减排,同时需要政策的配套支持,以便于从全流程角度减轻硫污染物的产生和排放。如加大推进硫污染控制技术的升级改造;及时根据国家产业结构调整目录,淘汰落后生产工艺装备;做好生产企业污染源在线监测设备与各级生态环境部门联网;鼓励研发污染控制过程所得产物的“资源化”技术以减少二次污染等。
再生铅行业硫污染及其控制技术研究进展
Research progress on sulfur pollution and its control technology in the secondary lead industry
-
摘要: 废铅蓄电池处理生产线十分庞大,多个设备或生产环节中都会产生含硫污染物,如不合理处置,将会对环境造成严重污染,也会造成硫资源浪费。从全流程角度出发,梳理了废铅蓄电池处理过程硫污染物产生环节和存在形式;总结了不同环节所用脱硫技术的原理、适用范围、实施过程及优缺点;最后,提出了再生铅行业硫污染控制技术的未来发展前景。结果表明:前端预脱硫转化技术很难同时解决碳酸铅包裹阻碍反应速率和成本高的问题,而且硫酸盐副产物销量差。硫酸雾处理技术、后端烟气制酸转化技术和末端尾气脱硫技术均得到了长足发展,在不同类型企业得到了广泛应用,但目前仍然存在脱硫成本较高、对复杂工况下的SO2烟气的脱除控制较差等问题,未来应从进一步降低脱硫成本和探索基于大数据驱动模型的工艺优化技术方面寻求突破。Abstract: The production line for treating waste lead-acid batteries is very large, with multiple equipment or production processes producing sulfur pollutants. If not properly disposed of, it will cause serious environmental accidents and waste sulfur resources. From the perspective of the entire process, the author analyzed the processes and forms of sulfur pollutants generated during the treatment of waste lead batteries; and summarized the principles, scope of application, implementation process, advantages and disadvantages of desulfurization technologies used in different stages; finally, the future development prospects of sulfur pollution control technology in the secondary lead industry were proposed. The results indicated that pre-desulfurization technology was difficult to simultaneously solve the problem of reduced reaction rate caused by lead carbonate and high cost caused by raw materials, and the sales volume of sulfate by-products was poor. The sulfuric acid mist treatment technology, flue gas preparation sulfuric acid technology, and tail gas desulfurization technology had all made significant progress and had been widely applied in different types of enterprises. However, there were still problems such as high desulfurization costs and poor control of SO2 flue gas removal under complex operating conditions. Therefore, in the future, breakthroughs should be sought in further reducing desulfurization costs and exploring process optimization technologies based on big-data-driven models.
-
表 1 碳酸盐体系脱硫情况
Table 1. The situation of desulfurization in carbonate system
脱硫剂 反应原理 脱硫率/% Na2CO3 PbSO4+Na2CO3→PbCO3+Na2SO4 98.10 NH4HCO3 PbSO4+2NH4HCO3→PbCO3+(NH4)2SO4+CO2+H2O 93.43 (NH4)2CO3 PbSO4+(NH4)2CO3→PbCO3+(NH4)2SO4 97.84 NH4HCO3和NH3·H2O PbSO4+NH3+H2O→NH4[Pb(OH)SO4] PbSO4+NH4HCO3+NH3⋅H2O→PbCO3+(NH4)2SO4+H2O 88.00 -
[1] 世界金属统计局. 全球精炼铅市场供应[EB/OL]. [2023-05-09].https://pb.iyunhui.com/news-776758/, 2023. [2] 任柏峰. 2000年铅价将稳中看涨——1999年国内外铅市回顾及2000年展望[J]. 有色冶炼, 2000, 29(1): 52-54. [3] CHEN S, LIAN Z, LI S, et al. The environmental burdens of lead-acid batteries in China: insights from an integrated material flow analysis and life cycle assessment of lead[J]. Energies, 2017, 10(12): 1969. doi: 10.3390/en10121969 [4] 田西. 再生资源产业绿色发展研究[M]. 北京: 社会科学文献出版社, 2019: 25-57. [5] HUANG K, LIU H, DONG H, et al. A novel approach to recover lead oxide from spent lead acid batteries by desulfurization and crystallization in sodium hydroxide solution after sulfation[J]. Resources Conservation and Recycling, 2021, 167: 105385. doi: 10.1016/j.resconrec.2020.105385 [6] JIANG S, SONG Z. A review on the state of health estimation methods of lead-acid batteries[J]. Journal of Power Sources, 2022, 517: 230710. doi: 10.1016/j.jpowsour.2021.230710 [7] 何艺, 郑洋, 何叶, 等. 中国废铅蓄电池产生及利用处置现状分析[J]. 电池工业, 2020(4): 216-224. doi: 10.3969/j.issn.1008-7923.2020.04.009 [8] 中华人民共和国生态环境部. 废铅蓄电池处理污染控制技术规范[EB/OL]. [2023-05-09]. https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/qtxgbz/202005/t20200521_780199.shtml, 2020. [9] 华经情报网. 行业重点需求转移, 2020年工业烟气处理市场内生增长强劲[EB/OL]. [2023-05-09].https://www.huaon.com/channel/trend/659354.html, 2020. [10] 赵丽娟, 谭哲, 张晓光, 等. 废铅膏湿法脱硫工艺和利用技术研究进展[J]. 有色金属:冶炼部分, 2022(1): 118-126. [11] 张俊丰, 陈彪, 黄妍, 等. 废铅酸蓄电池资源回收行业技术与发展[J]. 中国有色冶金, 2023, 52(1): 2-15. doi: 10.19612/j.cnki.cn11-5066/tf.2023.01.001 [12] 汪振忠, 柯昌美, 王茜. 废铅酸蓄电池铅膏脱硫工艺的研究进展[J]. 无机盐工业, 2013, 45(1): 60-62. doi: 10.3969/j.issn.1006-4990.2013.01.020 [13] 宋金超. 废铅蓄电池资源再生行业硫污染防治研究[D]. 湘潭: 湘潭大学, 2021. [14] 黄朝冉, 黄魁, 黄荃莅, 等. 预脱硫-火法冶炼回收废铅膏中的铅[J]. 电源技术, 2020, 44(12): 1748-1751. doi: 10.3969/j.issn.1002-087X.2020.12.010 [15] 闫君. 湿式静电除雾器脱除烟气中酸雾的试验研究[D]. 山东: 山东大学, 2010. [16] CORDOBA P. Status of flue gas desulphurisation (FGD) systems from coal-fired power plants: overview of the physic-chemical control processes of wetlimestone FGDs[J]. Fuel, 2015, 144: 274-286. doi: 10.1016/j.fuel.2014.12.065 [17] YANG Z, ZHENG C, LI Q, et al. Fast evolution of sulfuric acid aerosol activated by external fields for enhanced emission control[J]. Environmental Science and Technology, 2020, 54(5): 2022-3031. [18] RIEBEL U, RADTKE R, LOOS R. An experimental investigation on corona quenching[J]. Journal of Electrostatics, 2002, 54(2): 159-165. doi: 10.1016/S0304-3886(01)00175-9 [19] ARIF S, BRANKEN D J, EVERSON R C, et al. CFD modeling of particle charging and collection in electrostatic precipitators[J]. Journal of Electrostatics, 2016, 84: 10-22. doi: 10.1016/j.elstat.2016.08.008 [20] LUO K, YAN L, ZHENG C, et al. Numerical simulation of temperature effect on particles behavior via electrostatic precipitators[J]. Applied Thermal Engineering, 2015, 88: 127-139. doi: 10.1016/j.applthermaleng.2014.11.078 [21] WANG Y F, GAO W C, ZHANG X F, et al. Exploring the role of sulfuric acid aerosol in corona discharge through a honeycomb wet electrostatic precipitator[J]. Process Safety and Environmental Protection, 2021, 146: 763-769. doi: 10.1016/j.psep.2020.12.018 [22] SHU Y, WEI X, FANG Y, et al. Removal of sulfuric acid mist from lead-acid battery plants by coal fly ash-based sorbents[J]. Journal of Hazardous Materials, 2015, 286: 517-524. doi: 10.1016/j.jhazmat.2015.01.014 [23] 朱新锋, 杨丹妮, 胡红云, 等. 废铅酸蓄电池铅膏性质分析[J]. 环境工程学报, 2012, 6(9): 3259-3262. [24] YANAKIEVA V P, HARALAMPIEV G A, LYAKOV N K. Desulphurization of the damped lead battery paste with potassium carbonate[J]. Journal of Power Sources, 2000, 85(1): 178-180. doi: 10.1016/S0378-7753(99)00398-5 [25] 刘辉, 银星宇, 覃文庆, 等. 铅膏碳酸盐化转化过程的研究[J]. 湿法冶金, 2005, 24(3): 146-149. doi: 10.3969/j.issn.1009-2617.2005.03.008 [26] 孙晓娟, 李卉, 朱新锋, 等. 复合脱硫剂对废铅酸蓄电池铅膏脱硫影响的研究[J]. 蓄电池, 2013, 50(4): 148-152. doi: 10.16679/j.cnki.21-1121.2013.04.001 [27] LIU W K, QIN Q W, LI D Q, et al. Lead recovery from spent lead acid battery paste by hydrometallurgical conversion and thermal degradation[J]. Waste Management and Research, 2019, 38(3): 263-270. [28] 俞小花, 杨大锦, 谢刚, 等. 含硫酸铅物料的碳酸盐转化试验研究[J]. 中国稀土学报, 2010, 28: 457-461. [29] 熊浩宇. 废铅膏湿法浸出-液相合成回收PbO的工艺探究[D]. 广西: 广西大学, 2021. [30] 潘斌. 开启铅酸蓄电池的节能环保新时代——五院士首肯"原子经济法铅回收技术"达到世界领先水平[J]. 世界有色金属, 2014(5): 22-27. [31] WANG P, PAN J, GONG S, et al. A green preparation method of battery grade α-PbO based on Pb-O2 fuel cell[J]. Journal of Power Sources, 2017, 360: 324-327. doi: 10.1016/j.jpowsour.2017.05.107 [32] 齐美富, 郑园芳, 桂双林. 废铅酸蓄电池中铅膏氯盐体系浸取铅的动力学研究[J]. 矿冶工程, 2010, 30(6): 61-64. doi: 10.3969/j.issn.0253-6099.2010.06.016 [33] 桂双林. 废铅蓄电池中铅泥浸出特性及氯盐法浸出条件研究[D]. 南昌: 南昌大学, 2008. [34] 边文璟. 基于粒子研磨作用的废铅膏脱硫新方法实验研究[D]. 湘潭: 湘潭大学, 2016. [35] ZHANG J, YI L, YANG L, et al. A new pre-desulphurization process of damped lead battery paste with sodium carbonate based on a "surface update" concept[J]. Hydrometallurgy, 2016, 160: 123-128. doi: 10.1016/j.hydromet.2015.12.016 [36] NING P, PAN J Q, LI X, et al. Accelerated desulphurization of waste lead battery paste in a high-gravity rotating packed bed[J]. Chemical Engineering and Processing Process Intensification, 2016, 104: 148-153. doi: 10.1016/j.cep.2016.03.007 [37] CHANG C, YANG S H, LI Y G, et al. Green hydrometallurgical extraction of metallic lead from spent lead paste in the methanesulfonic acid system[J]. Separation and Purification Technology, 2023, 306(Part A): 122592. [38] DENG X, LIU W, ZHANG D, et al. Hydrothermal desulfurization of spent lead paste based on comproportionation reaction[J]. Separation and Purification Technology, 2020, 259: 118115. [39] 李晓理, 刘明华, 王康, 等. 冶炼烟气制酸技术及控制研究进展[J]. 北京工业大学学报, 2023(4): 475-484. doi: 10.11936/bjutxb2022080005 [40] LI X L, LIU M H, WANG K, et al. Data cleaning method for the process of acid production with flue gas based on improved random forest[J]. Chinese Journal of Chemical Engineering, 2023(In press). [41] JIA M, CHEN C, KOU W, et al. Real-time optimization of converter inlet temperature in acid production with flue gas[J]. Chemical Engineering Research and Design, 2017, 122: 226-232. doi: 10.1016/j.cherd.2017.04.002 [42] 艾新桥, 杨德鑫, 杨晶丽, 等. 离子液脱硫技术在冶炼烟气制酸中的应用[J]. 硫酸工业, 2016(4): 31-34. doi: 10.3969/j.issn.1002-1507.2016.04.007 [43] 李燕梅, 瞿尚君, 唐照勇. 低浓度冶炼烟气脱硫工艺运行实践浅析[J]. 硫酸工业, 2015(4): 15-19. [44] 舒绍明, 刘鹏, 柯顺, 等. 再生铅冶炼烟气制酸系统设计运行总结[J]. 硫酸工业, 2020(7): 25-28. [45] JIANG L, MEI K, CHEN K, et al. Design and prediction for highly efficient SO2 capture from flue gas by imidazolium ionic liquids[J]. Green Energy and Environment, 2020, 7(1): 130-136. [46] 何艺, 王维, 丁鹤, 等. 《废铅蓄电池处理污染控制技术规范》(HJ 519-2020)解读及实施建议[J]. 环境工程学报, 2021, 15(6): 2018-2026. doi: 10.12030/j.cjee.202009084 [47] 中华人民共和国生态环境部. 排污许可证申请与核发技术规范有色金属工业-再生金属: HJ 863.4-2018 [S]. 北京: 中国环境科学出版社出版, 2018. [48] 中华人民共和国环境保护部. 再生铜、铝、铅、锌工业污染物排放标准: GB 31574-2015 [S]. 北京: 中国环境科学出版社出版, 2015. [49] 李晶. 有色金属冶炼厂尾气脱硫技术比较[J]. 山西冶金, 2023, 46(1): 52-53. doi: 10.16525/j.cnki.cn14-1167/tf.2023.01.019 [50] 姜秀玲. 石灰-石膏法烟气脱硫技术实践[J]. 一重技术, 2017(1): 24-26. doi: 10.3969/j.issn.1673-3355.2017.01.005 [51] 何启贤. 石灰-石膏法在回转窑烟气脱硫中的应用[J]. 广州化工, 2016, 44(4): 124-126. doi: 10.3969/j.issn.1001-9677.2016.04.047 [52] 崔名双. 石灰-石膏湿法烟气脱硫影响因素分析[C]//中国煤炭学会煤粉锅炉专业委员会成立大会暨第一届学术交流会[C]. 济南, 2021: 98-102. [53] 刘永龙, 郑小娜. 冶炼烟气制酸石灰石膏法脱硫系统运行实践与改造[J]. 有色设备, 2019(5): 45-48. doi: 10.3969/j.issn.1003-8884.2019.05.011 [54] 江立振, 胡明意, 方俊杰, 等. 石灰—石膏湿法烧结脱硫问题治理[J]. 河南冶金, 2018, 26(5): 26-28. doi: 10.3969/j.issn.1006-3129.2018.05.009 [55] QIAO Z, WANG X, GU H, et al. An investigation on data mining and operating optimization for wet flue gas desulfurization systems[J]. Fuel, 2019, 258: 116171-116178. [56] 魏后超, 刘宏林, 缪云阳. 钠碱法脱硫工艺及应用[J]. 江苏建材, 2021(5): 5-7. doi: 10.3969/j.issn.1004-5538.2021.05.002 [57] 张伟明, 秦茜, 宋舟, 等. 钠碱法脱硫问题的研究与探讨[J]. 硫酸工业, 2022(6): 33-37. [58] 徐国主. 钠碱法脱硫循环液pH值的自动控制与效益影响[J]. 硫磷设计与粉体工程, 2020(4): 13-15. doi: 10.16341/j.cnki.spbmh.2020.04.004 [59] 陈立新. 钠碱法脱硫实现硫酸装置SO2超低排放[J]. 石油石化绿色低碳, 2017, 2(2): 37-41. [60] 张安贵, 刘吉平, 朱中华, 等. 氨法脱硫装置生产过程中存在问题及解决方案[J]. 能源科技, 2023, 21(1): 67-70. [61] 孙伟. 氨法脱硫技术在燃煤锅炉烟气减排中的高效运用[J]. 化工设计通讯, 2022(9): 4-6. doi: 10.3969/j.issn.1003-6490.2022.09.002 [62] 杨国华, 吴迪, 苟远波, 等. 基于气-液传质氨法脱硫喷淋吸收CFD仿真模拟[J]. 中国环境科学, 2023, 43(4): 1519-1527. doi: 10.19674/j.cnki.issn1000-6923.2023.0054 [63] JIA Y, YIN L G, XU Y L, et al. A model for performance of sulfite oxidation of ammonia-based flue gas desulfurization system[J]. Atmospheric Pollution Research, 2015, 6(6): 997-1003. doi: 10.1016/j.apr.2015.05.005 [64] LI X, DONG M, LI S, et al. A numerical study of the ammonia desulfurization in the spray scattering tower[J]. Chemical Engineering and Processing, 2020, 155: 108069. doi: 10.1016/j.cep.2020.108069 [65] YAN J, BAO J, YANG L, et al. The formation and removal characteristics of aerosols in ammonia-based wet flue gas desulfurization[J]. Journal of Aerosol Science, 2011, 42(9): 604-614. doi: 10.1016/j.jaerosci.2011.05.005 -




 下载:
下载: