-
水体磷污染问题由来已久[1-2]。进入水体中的磷可通过一系列物理化学作用蓄积在沉积物中,而在环境因子或水动力条件发生改变时,沉积物中的磷又会重新回到水中,从而诱发河湖富营养化[3-6]。沉积物对磷的吸附过程,是水体磷循环的关键环节[7-9]。沉积物对磷的吸附能力,反映了河流、湖泊和湿地对于外源磷污染的净化和贮存能力。近年来,已有研究人员对黄河三角洲湿地[10]、黄河兰州段消落带[11]和洱海入湖河口湿地[12-13]等水生态系统的沉积物进行了分析,证实其均具有缓冲外源磷污染的能力。吸附模型拟合也成为了描述沉积物-水界面磷交换行为的重要方法。如使用线性模型拟合吸附等温线得到的沉积物磷吸附-解吸平衡浓度 (EPC0) 是判断沉积物是否发生磷释放的关键指标。当上覆水中磷质量浓度大于EPC0时,沉积物会吸附磷,反之则会释放磷[14-17]。
新河地处我国北方半湿润半干旱季风气候区[18],发源自秦岭浅山区,是渭河支流之一。除洪水期外,新河天然径流严重不足[19]。同时,流域面源污染和沿岸城镇排污也导致新河水质出现恶化,氮和磷质量浓度峰值为25.08和2.94 mg·L−1,远超V类标准[20]。目前,有关沉积物磷吸附特征的研究多集中在清洁或微污染水体中,对具有较高磷本底值的受污染河道较少[7],故沉积物中超量累积的磷对其吸附行为的影响尚不清楚。
采集新河3个典型点位在不同季节的沉积物样品,测定其pH、粒径、化学组成、有机质、总磷含量和磷形态分级等多个理化指标,通过吸附实验和吸附模型研究其磷吸附行为,分析其影响因素。本研究结果将有助于掌握新河沉积物磷污染状况,并为制定相关生态修复策略提供参考。
-
分别选取西户铁路新河桥 (点位1#:坐标34o8′30.598 8″N、108°39′29.253 6″E) 、银榕公路新河桥 (点位2#:34°13′34.95″N、108°39′57.672″E) 和新渭沙湿地公园 (点位3#:34°15′41.85″N、108°40′34.712 4″E) 为采样点 (图1) 。采样点1#为城乡过渡区,点位2#和3#流经市区。3个采样点附近的土地利用类型分别为农用地、建设用地和生态用地。分别于2021年8月 (夏季) 、2021年11月 (秋季) 和2022年4月 (春季) 使用彼得森取泥器采集河底表层5 cm处的沉积物样品,运回实验室冻干后过80目筛保存备用。
-
沉积物的平均粒径使用激光粒度仪 (Mastersizer 3000,德国Malvern Panalytical公司) 测定;pH通过向冻干样品中添加纯水,在固液比1∶10下测定;元素组成使用X射线荧光光谱仪 (PANalytical Axios,德国Malvern Panalytical公司) 测定;有机质含量使用低温外热重铬酸钾氧化法测定[21];总磷使用K2S2O8-H2SO4消解法测定[22]。磷形态分级使用Rydin等提出的逐级提取法[23]:1) 向1 g冻干沉积物中加入浓度为1 mol∙L−1的NH4Cl溶液,反应0.5h后提取出松结合态磷 (LB-P) ;2) 向残渣中加入浓度为0.11 mol∙L−1的BD 溶液 (NaHCO3/Na2S2O4) ,反应1h后提取出铁锰螯合态磷 (BD-P) ;3) 继续加入浓度为0.1 mol∙L−1的NaOH溶液,反应16 h,一部分上清液直接测定磷含量,得到铝结合态磷 (NaOH-P) ,另一部分加入质量分数为5% 的K2S2O4,121 ℃下消解40 min后得到NaOH-TP,而NaOH-TP与NaOH-P的差值即为有机质结合态磷 (Org-P) ;4) 再向残渣加入浓度为0.5 mol∙L−1 的HCl溶液,反应16h后提取出钙结合态磷 (HCl-P) ;5) 将固体残渣转移至比色管中,加入质量分数为8% 的K2S2O4及质量分数为30% H2SO4溶液,121 ℃消解40 min后得到残渣态磷 (Res-P) 。松结合态磷 (LB-P) 、铁锰螯合态磷 (BD-P) 、铝结合态磷 (NaOH-P) 和有机质结合态磷 (Org-P) 在环境条件发生变化时容易向水中释放,统称为易释放态磷。
使用磷富集系数对新河沉积物中磷污染情况进行评价,计算见式 (1) [13]。
式中:PI为沉积物磷富集系数,无量纲;Ci为沉积物中总磷含量的实测值,mg·kg−1;Cs为沉积物中总磷的背景值,mg·kg−1;本研究使用陕西省关中地区土壤磷背景含量值[24]。当PI<0.5时,为I级风险,沉积物属于清洁类型;当0.5≤PI<1时,为II级风险,沉积物属于轻度污染类型;当1≤PI<1.5时,为III级风险,沉积物属于中度污染类型;当PI≥1.5时,为IV级风险,沉积物属于重度污染类型[13]。
-
取0.4 g冻干沉积物样品与40 mL不同质量浓度的磷溶液在50 mL离心管中混合,分别进行吸附动力学和吸附等温线实验。在25 ℃恒温摇床中,以180 r·min−1振荡反应;使用浓度为0.1 mol·L−1的HCl和NaOH溶液调节混合液pH为7~8。一定时间后,取出离心管在3 000 r·min−1条件下离心10 min,采用钼酸铵分光光度法[25]测定上清液中的磷浓度。磷的吸附量采用式 (2) 计算。
式中:q为沉积物的磷吸附量,mg·kg−1;V为溶液体积,L;C0和Ce为吸附前后溶液中磷质量浓度,mg·L−1;m是沉积物的干重,kg。
1) 吸附动力学
吸附动力学实验使用质量浓度为5 mg·L−1的磷溶液与沉积物进行反应,时间分别为0.5、1、2、4、8、12、24和48 h。每个反应时间均进行3个平行实验,结果选用标准误差修订后的平均值。使用准一级和准二级动力学模型拟合吸附动力学数据,计算见式 (3) 和式 (4) [26]。
式中:qt和qe是t时刻和吸附平衡时的吸附量,mg·kg−1;k1是准一级吸附速率常数,h−1;k2是准二级吸附速率常数,kg∙(mg∙h)−1。
2) 吸附等温线
采用质量浓度为0~10 mg·L−1的磷溶液与沉积物进行反应,时间为48 h。采用线性模型拟合低浓度吸附等温线,计算见式 (5) 和式 (6) [27]。
式中:K为线性模型系数,L∙kg−1;NAP为沉积物本底磷吸附量,mg·kg−1;EPC0为沉积物的磷吸附-解吸平衡质量浓度,mg·L−1。
采用Langmuir和Freundlich模型拟合高浓度吸附等温线,计算见式 (7)~(9) [28]。
式中:qmax为根据Langmuir模型计算出的沉积物最大磷吸附量,mg·kg−1;KL为Langmuir常数,L·mg−1;KF为Freundlich常数,(mg∙kg−1)∙(mg∙L−1)−1/n;1/n为吸附密度;MBC为沉积物磷最大缓冲容量,L·kg−1。
根据WANG等[22]提出的方法,计算表层沉积物的磷吸附能力,计算见式 (10) 和式 (11) 。
式中:C为单位面积沉积物磷吸附能力,mg∙m−2;M为单位面积沉积物质量,kg∙m−2。S为单位面积,取1 m2;H为沉积物“活跃层”厚度,m。ρ为沉积物固相密度,g∙cm−3。
采用D-R模型拟合全部吸附等温线数据,计算见式 (12)~(14) [12]。
式中:qe和qmax (式 (12) ) 分别为平衡吸附量和饱和吸附量,mol·g−1;KD为与吸附过程平均自由能有关的吸附常数,mol2·J−2;
ε 为吸附势,J·mol−1;R为理想气体常数8.314 J·(mol·K)−1;T为绝对温度,K;Ce (式 (13) ) 为平衡浓度,mol·L−1;E是吸附自由能,kJ·mol−1。 -
采用美国IBM公司的SPSS 19.0软件,分析吸附模型拟合得到的参数与沉积物环境因子之间的关系。
-
沉积物的理化性质如表1所示。不同季节各点位沉积物的pH为7.64~8.88,呈现弱碱性,大于顾艳林等[12]对洱海沉积物的测定值,而接近XU等[10]在黄河三角洲测定的结果。沉积物pH较高主要是由于该区域土壤类型为石灰型土壤,碳酸钙的浓度较高[29]。沉积物的平均粒径在秋季小于夏季和春季,这可能是由于新河属于雨源型河流[30],当深秋和冬季降雨量较小时河流流速变缓,粒度较小的悬移质沉降使得沉积物平均粒径变小[31-32]。XRF (X射线荧光光谱分析) 结果表明,样品中氧化硅的质量分数达50%以上;氧化铝、氧化铁和氧化钙质量分数均为约10%左右。对比顾艳林等[12]在云南洱海河口湿地中的研究结果,新河沉积物中铁、铝氧化物浓度较少。有机质的质量分数在1.56%~2.48%,点位1#的有机质浓度明显低于点位2#和3#,这说明河道沉积物中有机物的累积主要出现在进入城区之后。
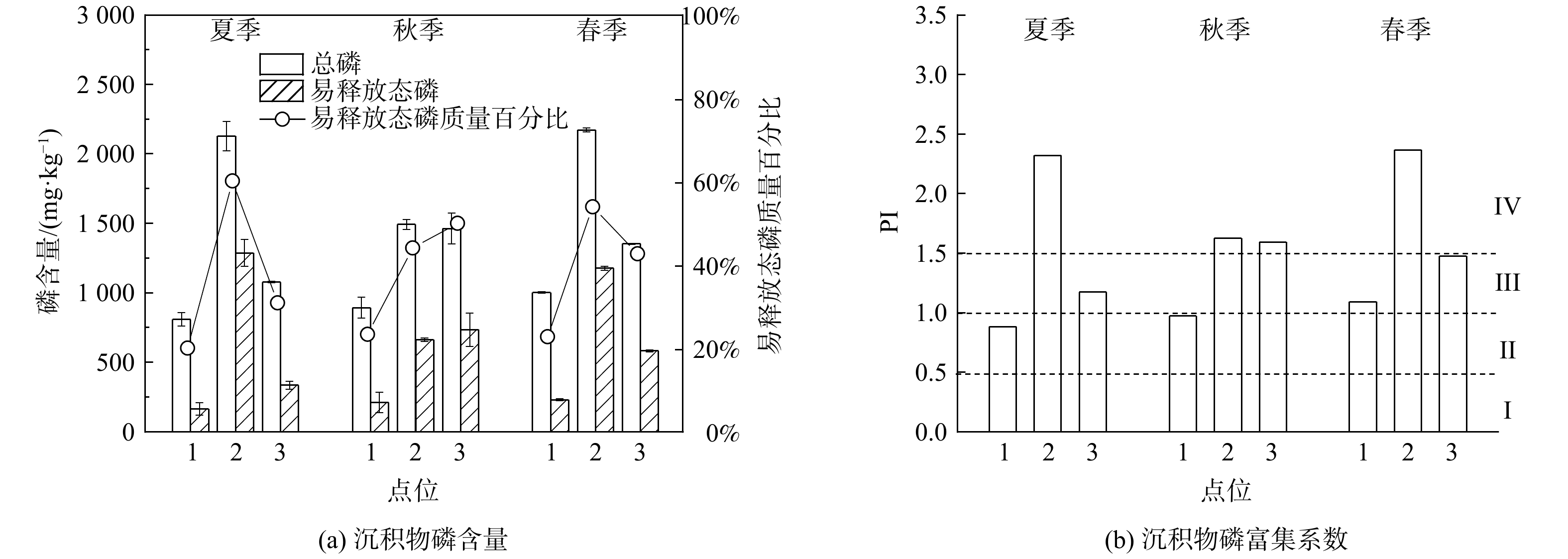
如图2 (a) 所示,新河中不同季节的3个点位的沉积物TP为870.00~2 173.00 mg·kg−1,点位2#>点位3#>点位1#。新河自点位1#流至点位2#后,沉积物中出现显著的磷累积,这与点位2#附近高强度的城市开发有密切关系。点位3#相比点位2#磷累积量较低,这可能是由于该点位地处新渭沙湿地公园,丰富的水生植被利用了沉积物中生物有效磷[33]。对比前人研究,新河沉积物中TP远超过同区域的城市湖泊汉城湖的水平 (655.38~809.38 mg·kg−1) [34]。对沉积物中磷的形态进行分级提取发现,点位2#和3#的易释放态磷 (LB-P、BD-P、NaOH-P和Org-P) 的质量分数及相应在总磷中所占的质量百分比,明显高于点位1#。沉积物中钙元素和有机质对其磷累积有正向作用[35-36],沉积物的平均粒径则与磷累积量呈反比[8]。分析不同点位沉积物的理化性质发现,点位2#的沉积物中平均粒径较大,且钙相对含量最低而有机质质量分数最高。因此,可推测点位2#较高的有机质质量分数促进了磷累积。从季节角度来看,除点位2#以外沉积物在春季和秋季磷累积量较高,夏季磷累积量较低。这可能是由于新河秋冬季水质较差[18]蓄积了较多的磷,而夏季新河所在的陕西关中地区降雨频繁,形成的洪峰会反复冲刷河道,将沉积物中蓄积的磷冲走[37-38]。
通过磷富集系数评价沉积物中磷污染状况,结果如图2 (b) 所示。以研究区域所在地陕西省关中地区土壤磷质量分数 (917.70 mg·kg−1) [24]作为背景值时,点位2#在不同季节的污染风险均达到了IV级;点位3#在秋季达到了IV级。通过新河3个点位沉积物中总磷和磷形态的测定结果,结合沉积物磷富集系数的评价,可看出新河流经市区后沉积物中存在明显的磷富集现象。
-
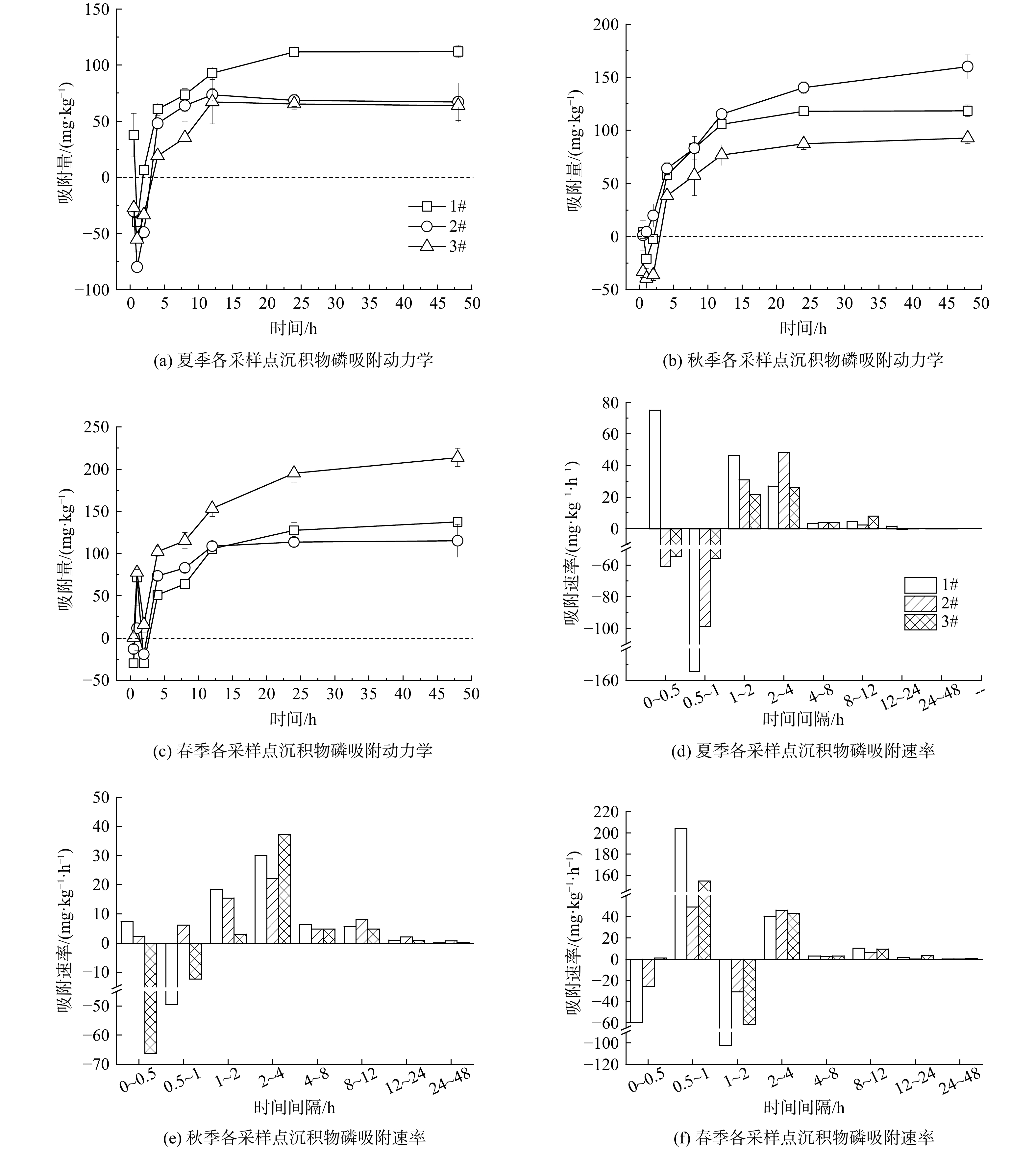
不同季节各点位的磷吸附动力学过程如图3 (a)~(c) 所示。吸附动力学过程可划分为3个阶段:第1阶段为0~2 h,此时吸附量在正负值间波动,这可能是由于沉积物中总磷含量非常高,在剧烈振荡的实验条件下出现了快速的释磷现象;第2阶段为2~4 h,沉积物对磷的吸附作用逐渐加强并超过了释放,吸附量迅速增加并变为正值,进入了快速吸附阶段;第3阶段为4~48 h,磷的吸附量继续增加但增速减缓,为慢速吸附阶段,并逐渐达到平衡。TANG等[39]发现丹江口水库沉积物在最初1 h内可快速吸附质量浓度为10 mg·L−1的磷,之后吸附量增加但逐渐减缓并最终平衡。PENG等[40]研究了污水稳定塘沉积物对质量浓度为5 mg·L−1的磷的吸附动力学过程,也发现了类似现象。本研究在0~2 h出现了吸附量正负波动,推测主要是由于新河沉积物本底磷含量远超上述2项研究,这导致反应初期磷释放抑制了吸附,呈现出“净”释放的效果。
进一步计算沉积物对磷的吸附速率,结果如图3 (d)~(f) 所示。磷的吸附速率在最初2 h呈现正负波动的状态;在2~4 h最大,在不同季节分别可达到22.14~48.46 mg·(kg∙h)−1;随后逐渐降低,24~48 h的吸附速率仅为2~4 h的0.03%~3.72%,此时反应基本结束,磷的吸附速率趋于稳定。沉积物对磷的吸附速率随时间的变化曲线说明对吸附阶段的划分是合理的。LIAO等[7]计算了北运河沉积物的磷吸附速率,得到的结果比本研究要大一个数量级,其原因应为其使用的磷溶液的质量浓度较高 (400 mg·L−1) 。
使用准一级动力学模型和准二级动力学模型拟合了沉积物吸附动力学过程,结果如表2所示。准一级和准二级动力学模型的相关系数R2均较高,但准二级动力学模型拟合得到的平衡吸附量qe更接近实际的结果,这说明准二级动力学模型可以更好地描述吸附反应过程。沉积物对磷的吸附,主要通过磷酸盐与沉积物中的钙、铁和铝等元素生成磷酸盐沉淀实现。因此,适用于化学和离子交换反应的准二级动力学模型能较好地描述沉积物对磷的吸附过程[26]。
-
不同季节各点位沉积物对磷的吸附等温线见图4。低平衡质量浓度范围内 (0~2.5 mg·L−1) 磷吸附量线性增长 (图4 (a)~(c) ) ,而在高平衡质量浓度范围 (>2.5mg·L−1) 吸附量的增加呈现非线性趋势 (图4 (d)~(f) ) 。
分别使用线性和非线性模型拟合,结果如表3所示。使用线性模型拟合得到的沉积物本底磷吸附量NAP,即沉积物中自带的可解吸的磷。点位2#的NAP值最高,特别在夏季为点位1#的7.76倍,达到185.35 mg·kg−1。这一水平远超过上海滴水湖入湖河道[41],而与苏州河网区农村河道水平相近[17]。对照图2发现,点位2#较高的NAP值可能与该点位较高的总磷和易释放态磷含量有关[8, 15]。
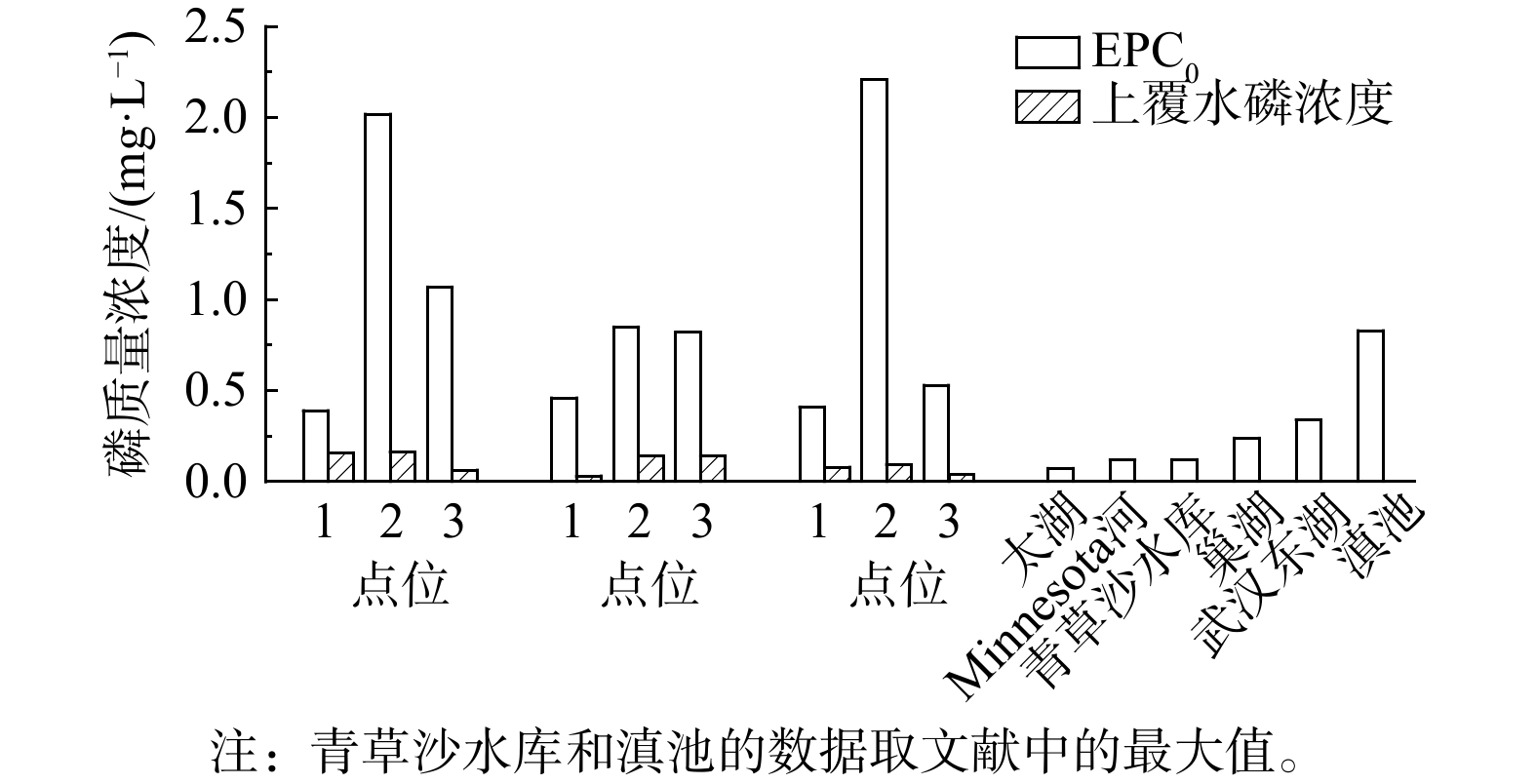
吸附-解吸平衡浓度 (EPC0) 是判别沉积物是否处于磷释放状态的重要参数。当上覆水中磷浓度低于EPC0时,沉积物中的磷被释放;反之,高于EPC0时,沉积物吸附水中的磷[27]。因此,EPC0越小,沉积物中磷的释放趋势越弱。本研究中,各点位的EPC0为0.39~2.21 mg·L−1,远高于同一时段内上覆水中磷质量浓度 (图5) ,这说明在研究时间范围内各点位沉积物始终处于磷释放状态,造成严重的内源污染。与国内外多个河流湖泊的EPC0对比[27, 42-46],新河沉积物的EPC0远高于水质较好水体 (如青草沙水库) ,与一些水质恶化、水华频繁爆发的水体 (如巢湖、东湖和滇池) 相当或更高。点位2#的EPC0为3个点位最高,应该与该点位沉积物本底磷含量较高有关。
高浓度吸附等温线拟合结果表明,与Langmuir模型相比,高平衡浓度下吸附等温线更适合被Freundlich模型拟合。拟合结果表明,在夏季和秋季,沉积物对磷的Langmuir最大吸附量分别为833.23~1 428.57 mg·kg−1和1 250.00~2 500.00 mg·kg−1,明显高于春季 (416.67~555.56 mg·kg−1) 。沉积物的磷吸附能力对于河水中磷的截留有重要意义。一般认为,表层0~10 cm沉积物可与上覆水之间发生磷交换,称之为“活跃层”[22]。取“活跃层”厚度为5 cm,沉积物固体密度为2.65 g∙cm−3。根据式 (10)~(11) ,可得到表层沉积物的磷吸附能力为110.42~189.29 g∙m−2 (夏季) 、165.63~331.25 g∙m−2 (秋季) 和55.21~73.61 g∙m−2 (春季) 。根据同期开展的水质分析结果,新河夏季、秋季和春季河水中溶解性正磷酸盐的平均质量浓度分别为0.13、0.10和0.07 mg∙L−1;基本上与关中地区河流的丰水期、枯水期和平水期相对应[19]。虽然夏季河流中磷的总量应该是3个季节中最高的,然而表层沉积物单位面积的磷吸附能力并非所有季节中最大的,这可能会导致新河在夏季输入渭河的磷负荷相较其他季节偏高。
磷最大缓冲容量 (MBC) 为综合考虑最大吸附量和吸附亲和力的参数。由于夏季的吸附亲和力较弱 (KL较小) ,故夏季沉积物MBC低于秋季,仅为秋季的31.04%~71.43%,吸附后容易向上覆水再释放。通过Freundlich模型拟合得到的常数KF也呈现出秋季>>夏季>春季的规律,与MBC值类似。秋季沉积物之所以有较高的磷吸附量和亲和力,推测是由于秋季沉积物的粒径较小,比表面积大,黏土矿物含量较高[26, 47]。监测表明,新河春季和秋季上覆水pH 为7.39~7.95,呈微弱碱性;但是夏季时,会上升到8.36~8.46。已有研究证实,上覆水pH增大会抑制沉积物中铁铝氧化物对磷的吸附[8, 12]。本研究的吸附等温线实验均在纯水配制的磷溶液中进行,没有考虑不同季节上覆水pH的差异。因此,实际环境下夏季沉积物的磷吸附能力会比本研究得到的结果更低,这对河流消纳由夏季暴雨径流导致的面源磷污染十分不利。
使用D-R模型计算了沉积物对磷的吸附能,结果表明除夏季点位2#以外其余样品对磷的吸附能为8.45~11.18 kJ·mol−1。已有研究表明,吸附能为8~16 kJ·mol−1时吸附机理为离子交换[48]。因此,本实验中绝大多数沉积物样品的吸附机制应该为离子交换。夏季点位2#的沉积物吸附能小于8 kJ·mol−1,这说明此实验有物理吸附机制的存在。这也解释了上文中夏季2#点位沉积物磷吸附量高、吸附亲和力低的形成原因,即夏季沉积物存在不稳定的物理吸附作用。
-
将吸附模型拟合参数与沉积物理化指标进行了相关分析,结果如表4所示。如吸附模型参数与沉积物任意一个理化指标的相关关系均不显著 (P>0.05) ,则不列出该参数。吸附速率常数与沉积物中钙的相对含量存在显著的正相关关系,与硅和铁的相对含量显著负相关。一般来说,沉积物中游离的钙和无定形铁铝氧化物是决定其磷吸附能力的主要因素[39],可通过与磷生成磷酸盐沉淀降低水中磷浓度。与前人研究相比,本研究发现沉积物吸附速率常数仅与钙的相对含量显著正相关,而与铁的相对含量显著负相关 (P<0.05) 。张潆元等[32]在长江三峡库区沉积物中也发现了类似现象,并认为其原因在于沉积物中的铁铝元素来自于成岩基质,活性较低,因而对磷吸附的贡献较小。DELAUNE等[35]发现,沉积物pH小于6.5时,铁铝元素对磷吸附的影响较为显著;pH大于7.5,则钙镁元素对磷吸附的影响占到主导作用。pH均大于7.5,因而沉积物对磷的吸附受钙元素的影响更大。 如前所述,作为一条典型的石灰型土壤地区河道,新河沉积物中钙浓度高于其他类似河道,这也映证了上文分析的磷吸附机制。
沉积物的吸附-解吸平衡浓度 (EPC0) 是决定其是否发生磷释放的重要指标。本研究中,EPC0与沉积物的总磷和易释放态磷极显著正相关 (P<0.01) ,这说明沉积物中本底 (非稳定形态) 磷含量是影响沉积物磷吸附-解吸行为最为关键的因素。JARVIE等[15]在对英国城市河流Hampshire Avon和Herefordshire Wye的研究中发现,城市污水厂下游沉积物中蓄积了大量磷,使其EPC0明显升高,磷释放风险加大。肖文娟等[49]也发现太湖沉积物中较高的总磷含量会增加磷释放风险。沉积物EPC0和总磷和易释放态磷的相关关系,这说明其磷释放风险主要源自于自身磷的过度蓄积。如要降低新河沉积物内源磷释放风险,应及时采取工程措施 (如环保疏浚等) 进行治理。
表3表明,EPC0与沉积物平均粒径和有机质显著正相关,而与pH显著负相关。沉积物平均粒径越大,比表面积越小,EPC0也会随之升高[14]。LABOSKI等[50]发现施加有机肥会降低土壤吸附磷的吸附能,从而促进磷释放。此外,李如忠等[51]提出了一种基于生态计量化学的解释,即沉积物中有机质含量升高会改变生源要素碳、氮和磷的比例,可能导致可吸收磷的微生物代谢活性下降,进而使磷释放量增加。沉积物pH与EPC0之间的负相关关系,应是由于沉积物中钙与磷的相互作用机制引起的。钙元素的固磷模式是与磷酸盐形成磷酸钙沉淀,如磷酸三钙、磷酸八钙和羟基磷灰石等。由于在高pH环境下易生成磷酸钙沉淀,因而EPC0与pH成反比[52]。
-
1) 新河沉积物TP为870~2 173 mg·kg−1,磷富集系数显示沉积物处于严重污染水平。沉积物吸附磷的动力学过程分为0~2 h的波动期、2~4 h快速吸附期和4~48 h逐渐平衡期,吸附速率在2~4 h时最大。
2) 新河沉积物的EPC0为0.39~2.21 mg·L−1,高于同时段上覆水中磷浓度,具有高释放风险;磷吸附缓冲容量MBC和Freundlich常数具有秋季>>夏季>春季的趋势;吸附机理应为离子交换。
3) 沉积物的磷吸附速率常数与钙的相对含量显著正相关;沉积物EPC0与其总磷和易释放态磷含量极显著正相关。
4) 沉积物的磷释放风险主要源于自身的超量磷累积。对流经市区的重污染河段,应及时采取环保疏浚等工程措施。对流入市区前、磷累积量较少的河段,则可使用更加生态友好的水生植物和微生物菌剂等进行治理。
受污染城市河道沉积物磷吸附特征及其影响因素
Characteristics and influencing factors of phosphorus adsorption in polluted urban river sediments
-
摘要: 采集了典型受污染城市河道——陕西省新河不同季节的沉积物,研究其磷吸附特征及影响因素,并考察其缓冲外源污染的能力,评估其磷释放风险。结果显示,沉积物平均粒径在秋季小于夏季和春季,pH为7.64~8.88;沉积物总磷 (TP) 为870~2 173 mg·kg−1;计算了沉积物磷富集系数,明确了新河部分点位为重度污染类型。吸附动力学研究结果表明:0~2 h沉积物对磷的吸附量在正负之间波动;2~4 h吸附量迅速增加,吸附速率也达到了峰值;之后吸附量增加趋势变缓,并在48 h达到平衡。绘制吸附等温线,可知沉积物的磷吸附-解吸平衡质量浓度 (EPC0) 为0.39~2.21 mg·L−1,高于同时段上覆水中磷的质量浓度,具有释放风险;磷最大缓冲容量MBC和Freundlich常数KF呈现秋季>>夏季>春季的规律;经D-R模型拟合,绝大多数样品的吸附能为8.45~11.18 kJ·mol−1,说明吸附机制为离子交换。相关性分析结果表明,沉积物的磷吸附速率常数与钙的相对含量呈显著相关 (P<0.05) 。EPC0与沉积物的总磷和易释放态磷含量呈显著相关 (P<0.01) ,与沉积物平均粒径和有机质显著相关 (P<0.05) ,与pH显著相关 (P<0.05) 。本研究结果可为受污染城市河道治理提供参考。Abstract: The sediment samples of Xinhe River were collected, which was a typical polluted urban river in Shaanxi Province. The phosphate immobilization capacities of the sediment samples were studied through adsorption experiments, and the influencing factors were also analyzed though a statistical method. The results showed that the pH of sediments was between 7.64 and 8.88, and their average particle size in autumn was smaller than that in summer and spring. The total phosphorus contents of the sediment were between 870.00 and 2 173.00 mg·kg−1. The sediment phosphorus enrichment coefficient was calculated, and some sites of Xinhe River were identified as the type of heavy pollution. The adsorption kinetics showed that the phosphate adsorption amount of the sediment fluctuated between positive and negative in the first 2 hours, and then increased rapidly in 2 to 4 h, and the adsorption rate reached the peak. Subsequently, the adsorption amount increased slowly and reached equilibrium at 48 h. The adsorption isotherms showed that the phosphate adsorption-desorption equilibrium concentration (EPC0) of the sediment ranged from 0.39 to 2.21 mg·L−1, which was higher than the phosphate concentration in the overlying water, and had the risk of release. The maximum phosphorus buffer capacity (MBC) and Freundlich constant KF showed the tendency of autumn>summer>spring. For most of the samples, adsorption energy was between 8.45 and 11.18 KJ·mol−1, indicating that the adsorption mechanism was ion exchange. The correlation analysis showed that the phosphate adsorption rate constant was significantly positively correlated with the calcium content of the sediment (P<0.05). The EPC0 value was highly significantly positively correlated with the total phosphorus and easily released phosphorus content (P<0.01), and was significantly positively correlated with the average particle size and organic matter (P<0.05). Nevertheless, EPC0 was negatively correlated with pH (P<0.05). This study could offer reference for the remediation of polluted urban river.
-
Key words:
- phosphate /
- sediment /
- adsorption /
- polluted river.
-
水体磷污染问题由来已久[1-2]。进入水体中的磷可通过一系列物理化学作用蓄积在沉积物中,而在环境因子或水动力条件发生改变时,沉积物中的磷又会重新回到水中,从而诱发河湖富营养化[3-6]。沉积物对磷的吸附过程,是水体磷循环的关键环节[7-9]。沉积物对磷的吸附能力,反映了河流、湖泊和湿地对于外源磷污染的净化和贮存能力。近年来,已有研究人员对黄河三角洲湿地[10]、黄河兰州段消落带[11]和洱海入湖河口湿地[12-13]等水生态系统的沉积物进行了分析,证实其均具有缓冲外源磷污染的能力。吸附模型拟合也成为了描述沉积物-水界面磷交换行为的重要方法。如使用线性模型拟合吸附等温线得到的沉积物磷吸附-解吸平衡浓度 (EPC0) 是判断沉积物是否发生磷释放的关键指标。当上覆水中磷质量浓度大于EPC0时,沉积物会吸附磷,反之则会释放磷[14-17]。
新河地处我国北方半湿润半干旱季风气候区[18],发源自秦岭浅山区,是渭河支流之一。除洪水期外,新河天然径流严重不足[19]。同时,流域面源污染和沿岸城镇排污也导致新河水质出现恶化,氮和磷质量浓度峰值为25.08和2.94 mg·L−1,远超V类标准[20]。目前,有关沉积物磷吸附特征的研究多集中在清洁或微污染水体中,对具有较高磷本底值的受污染河道较少[7],故沉积物中超量累积的磷对其吸附行为的影响尚不清楚。
采集新河3个典型点位在不同季节的沉积物样品,测定其pH、粒径、化学组成、有机质、总磷含量和磷形态分级等多个理化指标,通过吸附实验和吸附模型研究其磷吸附行为,分析其影响因素。本研究结果将有助于掌握新河沉积物磷污染状况,并为制定相关生态修复策略提供参考。
1. 研究方法
1.1 样品采集
分别选取西户铁路新河桥 (点位1#:坐标34o8′30.598 8″N、108°39′29.253 6″E) 、银榕公路新河桥 (点位2#:34°13′34.95″N、108°39′57.672″E) 和新渭沙湿地公园 (点位3#:34°15′41.85″N、108°40′34.712 4″E) 为采样点 (图1) 。采样点1#为城乡过渡区,点位2#和3#流经市区。3个采样点附近的土地利用类型分别为农用地、建设用地和生态用地。分别于2021年8月 (夏季) 、2021年11月 (秋季) 和2022年4月 (春季) 使用彼得森取泥器采集河底表层5 cm处的沉积物样品,运回实验室冻干后过80目筛保存备用。
1.2 指标测定与评价
沉积物的平均粒径使用激光粒度仪 (Mastersizer 3000,德国Malvern Panalytical公司) 测定;pH通过向冻干样品中添加纯水,在固液比1∶10下测定;元素组成使用X射线荧光光谱仪 (PANalytical Axios,德国Malvern Panalytical公司) 测定;有机质含量使用低温外热重铬酸钾氧化法测定[21];总磷使用K2S2O8-H2SO4消解法测定[22]。磷形态分级使用Rydin等提出的逐级提取法[23]:1) 向1 g冻干沉积物中加入浓度为1 mol∙L−1的NH4Cl溶液,反应0.5h后提取出松结合态磷 (LB-P) ;2) 向残渣中加入浓度为0.11 mol∙L−1的BD 溶液 (NaHCO3/Na2S2O4) ,反应1h后提取出铁锰螯合态磷 (BD-P) ;3) 继续加入浓度为0.1 mol∙L−1的NaOH溶液,反应16 h,一部分上清液直接测定磷含量,得到铝结合态磷 (NaOH-P) ,另一部分加入质量分数为5% 的K2S2O4,121 ℃下消解40 min后得到NaOH-TP,而NaOH-TP与NaOH-P的差值即为有机质结合态磷 (Org-P) ;4) 再向残渣加入浓度为0.5 mol∙L−1 的HCl溶液,反应16h后提取出钙结合态磷 (HCl-P) ;5) 将固体残渣转移至比色管中,加入质量分数为8% 的K2S2O4及质量分数为30% H2SO4溶液,121 ℃消解40 min后得到残渣态磷 (Res-P) 。松结合态磷 (LB-P) 、铁锰螯合态磷 (BD-P) 、铝结合态磷 (NaOH-P) 和有机质结合态磷 (Org-P) 在环境条件发生变化时容易向水中释放,统称为易释放态磷。
使用磷富集系数对新河沉积物中磷污染情况进行评价,计算见式 (1) [13]。
PI=Ci/Cs (1) 式中:PI为沉积物磷富集系数,无量纲;Ci为沉积物中总磷含量的实测值,mg·kg−1;Cs为沉积物中总磷的背景值,mg·kg−1;本研究使用陕西省关中地区土壤磷背景含量值[24]。当PI<0.5时,为I级风险,沉积物属于清洁类型;当0.5≤PI<1时,为II级风险,沉积物属于轻度污染类型;当1≤PI<1.5时,为III级风险,沉积物属于中度污染类型;当PI≥1.5时,为IV级风险,沉积物属于重度污染类型[13]。
1.3 磷吸附测定
取0.4 g冻干沉积物样品与40 mL不同质量浓度的磷溶液在50 mL离心管中混合,分别进行吸附动力学和吸附等温线实验。在25 ℃恒温摇床中,以180 r·min−1振荡反应;使用浓度为0.1 mol·L−1的HCl和NaOH溶液调节混合液pH为7~8。一定时间后,取出离心管在3 000 r·min−1条件下离心10 min,采用钼酸铵分光光度法[25]测定上清液中的磷浓度。磷的吸附量采用式 (2) 计算。
q=(C0−Ce)×Vm (2) 式中:q为沉积物的磷吸附量,mg·kg−1;V为溶液体积,L;C0和Ce为吸附前后溶液中磷质量浓度,mg·L−1;m是沉积物的干重,kg。
1) 吸附动力学
吸附动力学实验使用质量浓度为5 mg·L−1的磷溶液与沉积物进行反应,时间分别为0.5、1、2、4、8、12、24和48 h。每个反应时间均进行3个平行实验,结果选用标准误差修订后的平均值。使用准一级和准二级动力学模型拟合吸附动力学数据,计算见式 (3) 和式 (4) [26]。
ln(qe−qt)=lnqe−k12.303t (3) tqt=1k2q2e+tqe (4) 式中:qt和qe是t时刻和吸附平衡时的吸附量,mg·kg−1;k1是准一级吸附速率常数,h−1;k2是准二级吸附速率常数,kg∙(mg∙h)−1。
2) 吸附等温线
采用质量浓度为0~10 mg·L−1的磷溶液与沉积物进行反应,时间为48 h。采用线性模型拟合低浓度吸附等温线,计算见式 (5) 和式 (6) [27]。
qe=KCe−NAP (5) EPC0=NAP/K (6) 式中:K为线性模型系数,L∙kg−1;NAP为沉积物本底磷吸附量,mg·kg−1;EPC0为沉积物的磷吸附-解吸平衡质量浓度,mg·L−1。
采用Langmuir和Freundlich模型拟合高浓度吸附等温线,计算见式 (7)~(9) [28]。
qe=qmaxKLCeKLCe+1 (7) qe=KFC1/ne (8) MBC=qmaxKL (9) 式中:qmax为根据Langmuir模型计算出的沉积物最大磷吸附量,mg·kg−1;KL为Langmuir常数,L·mg−1;KF为Freundlich常数,(mg∙kg−1)∙(mg∙L−1)−1/n;1/n为吸附密度;MBC为沉积物磷最大缓冲容量,L·kg−1。
根据WANG等[22]提出的方法,计算表层沉积物的磷吸附能力,计算见式 (10) 和式 (11) 。
C=M⋅qmax (10) M=S⋅H⋅ρ (11) 式中:C为单位面积沉积物磷吸附能力,mg∙m−2;M为单位面积沉积物质量,kg∙m−2。S为单位面积,取1 m2;H为沉积物“活跃层”厚度,m。ρ为沉积物固相密度,g∙cm−3。
采用D-R模型拟合全部吸附等温线数据,计算见式 (12)~(14) [12]。
qe=qmaxexp(−KDε2) (12) ε=RTln(1+1/Ce) (13) E=1(2KD)0.5 (14) 式中:qe和qmax (式 (12) ) 分别为平衡吸附量和饱和吸附量,mol·g−1;KD为与吸附过程平均自由能有关的吸附常数,mol2·J−2;
ε 1.4 统计分析
采用美国IBM公司的SPSS 19.0软件,分析吸附模型拟合得到的参数与沉积物环境因子之间的关系。
2. 结果与讨论
2.1 沉积物理化性质和磷污染风险评价
沉积物的理化性质如表1所示。不同季节各点位沉积物的pH为7.64~8.88,呈现弱碱性,大于顾艳林等[12]对洱海沉积物的测定值,而接近XU等[10]在黄河三角洲测定的结果。沉积物pH较高主要是由于该区域土壤类型为石灰型土壤,碳酸钙的浓度较高[29]。沉积物的平均粒径在秋季小于夏季和春季,这可能是由于新河属于雨源型河流[30],当深秋和冬季降雨量较小时河流流速变缓,粒度较小的悬移质沉降使得沉积物平均粒径变小[31-32]。XRF (X射线荧光光谱分析) 结果表明,样品中氧化硅的质量分数达50%以上;氧化铝、氧化铁和氧化钙质量分数均为约10%左右。对比顾艳林等[12]在云南洱海河口湿地中的研究结果,新河沉积物中铁、铝氧化物浓度较少。有机质的质量分数在1.56%~2.48%,点位1#的有机质浓度明显低于点位2#和3#,这说明河道沉积物中有机物的累积主要出现在进入城区之后。
表 1 沉积物理化性质Table 1. Physical and chemical characteristics of the sediments季节 点位 平均粒径/μm pH 化学组成 有机质 SiO2 CaO Al2O3 Fe2O3 其他 夏季 1# 25.79 8.88 57.88% 10.54% 13.62% 8.14% 9.82% 1.56% 2# 46.25 7.64 59.14% 5.74% 14.64% 8.69% 11.79% 2.42% 3# 54.13 8.13 61.08% 8.56% 13.29% 6.61% 10.46% 2.07% 秋季 1# 22.63 8.63 58.36% 9.34% 14.08% 8.29% 9.93% 1.82% 2# 33.06 7.98 59.64% 6.99% 14.33% 8.39% 10.65% 2.33% 3# 48.16 8.1 61.03% 8.43% 13.33% 6.82% 10.39% 2.33% 春季 1# 56.33 8.24 60.9% 6.92% 13.66% 8.03% 10.49% 1.56% 2# 96.19 7.89 57.63% 6.72% 15.77% 8.76% 11.12% 2.49% 3# 50.10 8.1 60.35% 7.12% 14.11% 7.76% 10.66% 2.17% 如图2 (a) 所示,新河中不同季节的3个点位的沉积物TP为870.00~2 173.00 mg·kg−1,点位2#>点位3#>点位1#。新河自点位1#流至点位2#后,沉积物中出现显著的磷累积,这与点位2#附近高强度的城市开发有密切关系。点位3#相比点位2#磷累积量较低,这可能是由于该点位地处新渭沙湿地公园,丰富的水生植被利用了沉积物中生物有效磷[33]。对比前人研究,新河沉积物中TP远超过同区域的城市湖泊汉城湖的水平 (655.38~809.38 mg·kg−1) [34]。对沉积物中磷的形态进行分级提取发现,点位2#和3#的易释放态磷 (LB-P、BD-P、NaOH-P和Org-P) 的质量分数及相应在总磷中所占的质量百分比,明显高于点位1#。沉积物中钙元素和有机质对其磷累积有正向作用[35-36],沉积物的平均粒径则与磷累积量呈反比[8]。分析不同点位沉积物的理化性质发现,点位2#的沉积物中平均粒径较大,且钙相对含量最低而有机质质量分数最高。因此,可推测点位2#较高的有机质质量分数促进了磷累积。从季节角度来看,除点位2#以外沉积物在春季和秋季磷累积量较高,夏季磷累积量较低。这可能是由于新河秋冬季水质较差[18]蓄积了较多的磷,而夏季新河所在的陕西关中地区降雨频繁,形成的洪峰会反复冲刷河道,将沉积物中蓄积的磷冲走[37-38]。
通过磷富集系数评价沉积物中磷污染状况,结果如图2 (b) 所示。以研究区域所在地陕西省关中地区土壤磷质量分数 (917.70 mg·kg−1) [24]作为背景值时,点位2#在不同季节的污染风险均达到了IV级;点位3#在秋季达到了IV级。通过新河3个点位沉积物中总磷和磷形态的测定结果,结合沉积物磷富集系数的评价,可看出新河流经市区后沉积物中存在明显的磷富集现象。
2.2 吸附动力学
不同季节各点位的磷吸附动力学过程如图3 (a)~(c) 所示。吸附动力学过程可划分为3个阶段:第1阶段为0~2 h,此时吸附量在正负值间波动,这可能是由于沉积物中总磷含量非常高,在剧烈振荡的实验条件下出现了快速的释磷现象;第2阶段为2~4 h,沉积物对磷的吸附作用逐渐加强并超过了释放,吸附量迅速增加并变为正值,进入了快速吸附阶段;第3阶段为4~48 h,磷的吸附量继续增加但增速减缓,为慢速吸附阶段,并逐渐达到平衡。TANG等[39]发现丹江口水库沉积物在最初1 h内可快速吸附质量浓度为10 mg·L−1的磷,之后吸附量增加但逐渐减缓并最终平衡。PENG等[40]研究了污水稳定塘沉积物对质量浓度为5 mg·L−1的磷的吸附动力学过程,也发现了类似现象。本研究在0~2 h出现了吸附量正负波动,推测主要是由于新河沉积物本底磷含量远超上述2项研究,这导致反应初期磷释放抑制了吸附,呈现出“净”释放的效果。
进一步计算沉积物对磷的吸附速率,结果如图3 (d)~(f) 所示。磷的吸附速率在最初2 h呈现正负波动的状态;在2~4 h最大,在不同季节分别可达到22.14~48.46 mg·(kg∙h)−1;随后逐渐降低,24~48 h的吸附速率仅为2~4 h的0.03%~3.72%,此时反应基本结束,磷的吸附速率趋于稳定。沉积物对磷的吸附速率随时间的变化曲线说明对吸附阶段的划分是合理的。LIAO等[7]计算了北运河沉积物的磷吸附速率,得到的结果比本研究要大一个数量级,其原因应为其使用的磷溶液的质量浓度较高 (400 mg·L−1) 。
使用准一级动力学模型和准二级动力学模型拟合了沉积物吸附动力学过程,结果如表2所示。准一级和准二级动力学模型的相关系数R2均较高,但准二级动力学模型拟合得到的平衡吸附量qe更接近实际的结果,这说明准二级动力学模型可以更好地描述吸附反应过程。沉积物对磷的吸附,主要通过磷酸盐与沉积物中的钙、铁和铝等元素生成磷酸盐沉淀实现。因此,适用于化学和离子交换反应的准二级动力学模型能较好地描述沉积物对磷的吸附过程[26]。
表 2 不同模型对吸附动力学的拟合结果Table 2. Fitting results of adsorption kinetics by different models季节 点位 准一级动力学模型 准2级动力学模型 k1 qe R2 k2 qe R2 夏季 1# 0.25 161.14 0.92 0.002 7 119.05 0.99 2# 0.06 62.60 0.56 0.011 2 64.94 0.99 3# 0.08 77.75 0.75 0.022 5 64.10 0.92 秋季 1# 0.24 169.82 0.98 0.000 7 151.52 0.90 2# 0.09 146.99 0.98 0.000 4 204.08 0.96 3# 0.14 126.68 0.95 0.041 8 89.29 0.94 春季 1# 0.11 140.60 0.88 0.012 0 133.33 0.88 2# 0.20 129.03 0.95 0.009 3 111.11 0.87 3# 0.09 166.07 0.98 0.000 8 232.56 0.99 2.3 吸附等温线
不同季节各点位沉积物对磷的吸附等温线见图4。低平衡质量浓度范围内 (0~2.5 mg·L−1) 磷吸附量线性增长 (图4 (a)~(c) ) ,而在高平衡质量浓度范围 (>2.5mg·L−1) 吸附量的增加呈现非线性趋势 (图4 (d)~(f) ) 。
分别使用线性和非线性模型拟合,结果如表3所示。使用线性模型拟合得到的沉积物本底磷吸附量NAP,即沉积物中自带的可解吸的磷。点位2#的NAP值最高,特别在夏季为点位1#的7.76倍,达到185.35 mg·kg−1。这一水平远超过上海滴水湖入湖河道[41],而与苏州河网区农村河道水平相近[17]。对照图2发现,点位2#较高的NAP值可能与该点位较高的总磷和易释放态磷含量有关[8, 15]。
表 3 不同模型对吸附等温线的拟合结果Table 3. Fitting results of adsorption isotherms by different models季节 点位 线性模型 Langmuir模型 Freundlich模型 D-R模型 NAP EPC0 R2 Qmax KL R2 MBC n KF R2 E R2 夏季 1# 23.90 0.39 0.98 1 000.00 0.10 0.92 98.04 0.72 102.47 0.98 8.45 0.98 2# 185.35 2.02 1.00 1 428.57 0.05 0.95 68.97 0.91 63.08 0.98 7.45 0.98 3# 71.19 1.07 1.00 833.33 0.11 0.81 95.24 0.68 102.66 0.93 9.13 0.93 秋季 1# 75.07 0.46 0.99 1 666.67 0.09 0.97 153.85 0.68 176.44 0.99 9.13 0.99 2# 174.13 0.85 0.97 1 250.00 0.18 0.87 222.22 0.54 252.12 0.93 10.00 0.93 3# 126.75 0.82 0.98 2 500.00 0.05 0.87 133.33 0.78 149.42 0.99 8.45 0.99 春季 1# 41.44 0.41 0.95 416.67 0.15 0.95 64.10 0.62 66.28 0.95 9.13 0.96 2# 241.05 2.21 0.96 526.32 0.11 0.81 55.87 0.64 15.21 0.92 9.13 0.92 3# 143.86 0.53 0.99 555.56 0.31 0.97 169.49 0.50 149.45 0.97 11.18 0.98 吸附-解吸平衡浓度 (EPC0) 是判别沉积物是否处于磷释放状态的重要参数。当上覆水中磷浓度低于EPC0时,沉积物中的磷被释放;反之,高于EPC0时,沉积物吸附水中的磷[27]。因此,EPC0越小,沉积物中磷的释放趋势越弱。本研究中,各点位的EPC0为0.39~2.21 mg·L−1,远高于同一时段内上覆水中磷质量浓度 (图5) ,这说明在研究时间范围内各点位沉积物始终处于磷释放状态,造成严重的内源污染。与国内外多个河流湖泊的EPC0对比[27, 42-46],新河沉积物的EPC0远高于水质较好水体 (如青草沙水库) ,与一些水质恶化、水华频繁爆发的水体 (如巢湖、东湖和滇池) 相当或更高。点位2#的EPC0为3个点位最高,应该与该点位沉积物本底磷含量较高有关。
高浓度吸附等温线拟合结果表明,与Langmuir模型相比,高平衡浓度下吸附等温线更适合被Freundlich模型拟合。拟合结果表明,在夏季和秋季,沉积物对磷的Langmuir最大吸附量分别为833.23~1 428.57 mg·kg−1和1 250.00~2 500.00 mg·kg−1,明显高于春季 (416.67~555.56 mg·kg−1) 。沉积物的磷吸附能力对于河水中磷的截留有重要意义。一般认为,表层0~10 cm沉积物可与上覆水之间发生磷交换,称之为“活跃层”[22]。取“活跃层”厚度为5 cm,沉积物固体密度为2.65 g∙cm−3。根据式 (10)~(11) ,可得到表层沉积物的磷吸附能力为110.42~189.29 g∙m−2 (夏季) 、165.63~331.25 g∙m−2 (秋季) 和55.21~73.61 g∙m−2 (春季) 。根据同期开展的水质分析结果,新河夏季、秋季和春季河水中溶解性正磷酸盐的平均质量浓度分别为0.13、0.10和0.07 mg∙L−1;基本上与关中地区河流的丰水期、枯水期和平水期相对应[19]。虽然夏季河流中磷的总量应该是3个季节中最高的,然而表层沉积物单位面积的磷吸附能力并非所有季节中最大的,这可能会导致新河在夏季输入渭河的磷负荷相较其他季节偏高。
磷最大缓冲容量 (MBC) 为综合考虑最大吸附量和吸附亲和力的参数。由于夏季的吸附亲和力较弱 (KL较小) ,故夏季沉积物MBC低于秋季,仅为秋季的31.04%~71.43%,吸附后容易向上覆水再释放。通过Freundlich模型拟合得到的常数KF也呈现出秋季>>夏季>春季的规律,与MBC值类似。秋季沉积物之所以有较高的磷吸附量和亲和力,推测是由于秋季沉积物的粒径较小,比表面积大,黏土矿物含量较高[26, 47]。监测表明,新河春季和秋季上覆水pH 为7.39~7.95,呈微弱碱性;但是夏季时,会上升到8.36~8.46。已有研究证实,上覆水pH增大会抑制沉积物中铁铝氧化物对磷的吸附[8, 12]。本研究的吸附等温线实验均在纯水配制的磷溶液中进行,没有考虑不同季节上覆水pH的差异。因此,实际环境下夏季沉积物的磷吸附能力会比本研究得到的结果更低,这对河流消纳由夏季暴雨径流导致的面源磷污染十分不利。
使用D-R模型计算了沉积物对磷的吸附能,结果表明除夏季点位2#以外其余样品对磷的吸附能为8.45~11.18 kJ·mol−1。已有研究表明,吸附能为8~16 kJ·mol−1时吸附机理为离子交换[48]。因此,本实验中绝大多数沉积物样品的吸附机制应该为离子交换。夏季点位2#的沉积物吸附能小于8 kJ·mol−1,这说明此实验有物理吸附机制的存在。这也解释了上文中夏季2#点位沉积物磷吸附量高、吸附亲和力低的形成原因,即夏季沉积物存在不稳定的物理吸附作用。
2.4 沉积物磷吸附特征的影响因素分析
将吸附模型拟合参数与沉积物理化指标进行了相关分析,结果如表4所示。如吸附模型参数与沉积物任意一个理化指标的相关关系均不显著 (P>0.05) ,则不列出该参数。吸附速率常数与沉积物中钙的相对含量存在显著的正相关关系,与硅和铁的相对含量显著负相关。一般来说,沉积物中游离的钙和无定形铁铝氧化物是决定其磷吸附能力的主要因素[39],可通过与磷生成磷酸盐沉淀降低水中磷浓度。与前人研究相比,本研究发现沉积物吸附速率常数仅与钙的相对含量显著正相关,而与铁的相对含量显著负相关 (P<0.05) 。张潆元等[32]在长江三峡库区沉积物中也发现了类似现象,并认为其原因在于沉积物中的铁铝元素来自于成岩基质,活性较低,因而对磷吸附的贡献较小。DELAUNE等[35]发现,沉积物pH小于6.5时,铁铝元素对磷吸附的影响较为显著;pH大于7.5,则钙镁元素对磷吸附的影响占到主导作用。pH均大于7.5,因而沉积物对磷的吸附受钙元素的影响更大。 如前所述,作为一条典型的石灰型土壤地区河道,新河沉积物中钙浓度高于其他类似河道,这也映证了上文分析的磷吸附机制。
表 4 吸附模型参数与沉积物理化指标相关分析结果Table 4. Correlation analysis results between adsorption model parameters and physicochemical indexes of sediments参数 平均粒径 pH 有机质 SiO2 Al2O3 CaO Fe2O3 TP Liable-P k1 −0.153 0.753 −0.42 −0.71* 0.116 0.703* 0.249 −0.342 −0.345 k2 0.247 −0.248 0.249 0.568 −0.41 0.034 −0.713* 0.116 0.157 EPC0 0.67* −0.741* 0.754* −0.39 0.743* −0.604 0.361 0.913** 0.898** KF −0.685* 0.192 0.016 0.214 −0.348 0.229 −0.169 −0.357 −0.332 注:*和**分别表示在 P<0.05 和 P<0.01 水平下显著相关;双尾检验。 沉积物的吸附-解吸平衡浓度 (EPC0) 是决定其是否发生磷释放的重要指标。本研究中,EPC0与沉积物的总磷和易释放态磷极显著正相关 (P<0.01) ,这说明沉积物中本底 (非稳定形态) 磷含量是影响沉积物磷吸附-解吸行为最为关键的因素。JARVIE等[15]在对英国城市河流Hampshire Avon和Herefordshire Wye的研究中发现,城市污水厂下游沉积物中蓄积了大量磷,使其EPC0明显升高,磷释放风险加大。肖文娟等[49]也发现太湖沉积物中较高的总磷含量会增加磷释放风险。沉积物EPC0和总磷和易释放态磷的相关关系,这说明其磷释放风险主要源自于自身磷的过度蓄积。如要降低新河沉积物内源磷释放风险,应及时采取工程措施 (如环保疏浚等) 进行治理。
表3表明,EPC0与沉积物平均粒径和有机质显著正相关,而与pH显著负相关。沉积物平均粒径越大,比表面积越小,EPC0也会随之升高[14]。LABOSKI等[50]发现施加有机肥会降低土壤吸附磷的吸附能,从而促进磷释放。此外,李如忠等[51]提出了一种基于生态计量化学的解释,即沉积物中有机质含量升高会改变生源要素碳、氮和磷的比例,可能导致可吸收磷的微生物代谢活性下降,进而使磷释放量增加。沉积物pH与EPC0之间的负相关关系,应是由于沉积物中钙与磷的相互作用机制引起的。钙元素的固磷模式是与磷酸盐形成磷酸钙沉淀,如磷酸三钙、磷酸八钙和羟基磷灰石等。由于在高pH环境下易生成磷酸钙沉淀,因而EPC0与pH成反比[52]。
3. 结论
1) 新河沉积物TP为870~2 173 mg·kg−1,磷富集系数显示沉积物处于严重污染水平。沉积物吸附磷的动力学过程分为0~2 h的波动期、2~4 h快速吸附期和4~48 h逐渐平衡期,吸附速率在2~4 h时最大。
2) 新河沉积物的EPC0为0.39~2.21 mg·L−1,高于同时段上覆水中磷浓度,具有高释放风险;磷吸附缓冲容量MBC和Freundlich常数具有秋季>>夏季>春季的趋势;吸附机理应为离子交换。
3) 沉积物的磷吸附速率常数与钙的相对含量显著正相关;沉积物EPC0与其总磷和易释放态磷含量极显著正相关。
4) 沉积物的磷释放风险主要源于自身的超量磷累积。对流经市区的重污染河段,应及时采取环保疏浚等工程措施。对流入市区前、磷累积量较少的河段,则可使用更加生态友好的水生植物和微生物菌剂等进行治理。
-
表 1 沉积物理化性质
Table 1. Physical and chemical characteristics of the sediments
季节 点位 平均粒径/μm pH 化学组成 有机质 SiO2 CaO Al2O3 Fe2O3 其他 夏季 1# 25.79 8.88 57.88% 10.54% 13.62% 8.14% 9.82% 1.56% 2# 46.25 7.64 59.14% 5.74% 14.64% 8.69% 11.79% 2.42% 3# 54.13 8.13 61.08% 8.56% 13.29% 6.61% 10.46% 2.07% 秋季 1# 22.63 8.63 58.36% 9.34% 14.08% 8.29% 9.93% 1.82% 2# 33.06 7.98 59.64% 6.99% 14.33% 8.39% 10.65% 2.33% 3# 48.16 8.1 61.03% 8.43% 13.33% 6.82% 10.39% 2.33% 春季 1# 56.33 8.24 60.9% 6.92% 13.66% 8.03% 10.49% 1.56% 2# 96.19 7.89 57.63% 6.72% 15.77% 8.76% 11.12% 2.49% 3# 50.10 8.1 60.35% 7.12% 14.11% 7.76% 10.66% 2.17% 表 2 不同模型对吸附动力学的拟合结果
Table 2. Fitting results of adsorption kinetics by different models
季节 点位 准一级动力学模型 准2级动力学模型 k1 qe R2 k2 qe R2 夏季 1# 0.25 161.14 0.92 0.002 7 119.05 0.99 2# 0.06 62.60 0.56 0.011 2 64.94 0.99 3# 0.08 77.75 0.75 0.022 5 64.10 0.92 秋季 1# 0.24 169.82 0.98 0.000 7 151.52 0.90 2# 0.09 146.99 0.98 0.000 4 204.08 0.96 3# 0.14 126.68 0.95 0.041 8 89.29 0.94 春季 1# 0.11 140.60 0.88 0.012 0 133.33 0.88 2# 0.20 129.03 0.95 0.009 3 111.11 0.87 3# 0.09 166.07 0.98 0.000 8 232.56 0.99 表 3 不同模型对吸附等温线的拟合结果
Table 3. Fitting results of adsorption isotherms by different models
季节 点位 线性模型 Langmuir模型 Freundlich模型 D-R模型 NAP EPC0 R2 Qmax KL R2 MBC n KF R2 E R2 夏季 1# 23.90 0.39 0.98 1 000.00 0.10 0.92 98.04 0.72 102.47 0.98 8.45 0.98 2# 185.35 2.02 1.00 1 428.57 0.05 0.95 68.97 0.91 63.08 0.98 7.45 0.98 3# 71.19 1.07 1.00 833.33 0.11 0.81 95.24 0.68 102.66 0.93 9.13 0.93 秋季 1# 75.07 0.46 0.99 1 666.67 0.09 0.97 153.85 0.68 176.44 0.99 9.13 0.99 2# 174.13 0.85 0.97 1 250.00 0.18 0.87 222.22 0.54 252.12 0.93 10.00 0.93 3# 126.75 0.82 0.98 2 500.00 0.05 0.87 133.33 0.78 149.42 0.99 8.45 0.99 春季 1# 41.44 0.41 0.95 416.67 0.15 0.95 64.10 0.62 66.28 0.95 9.13 0.96 2# 241.05 2.21 0.96 526.32 0.11 0.81 55.87 0.64 15.21 0.92 9.13 0.92 3# 143.86 0.53 0.99 555.56 0.31 0.97 169.49 0.50 149.45 0.97 11.18 0.98 表 4 吸附模型参数与沉积物理化指标相关分析结果
Table 4. Correlation analysis results between adsorption model parameters and physicochemical indexes of sediments
参数 平均粒径 pH 有机质 SiO2 Al2O3 CaO Fe2O3 TP Liable-P k1 −0.153 0.753 −0.42 −0.71* 0.116 0.703* 0.249 −0.342 −0.345 k2 0.247 −0.248 0.249 0.568 −0.41 0.034 −0.713* 0.116 0.157 EPC0 0.67* −0.741* 0.754* −0.39 0.743* −0.604 0.361 0.913** 0.898** KF −0.685* 0.192 0.016 0.214 −0.348 0.229 −0.169 −0.357 −0.332 注:*和**分别表示在 P<0.05 和 P<0.01 水平下显著相关;双尾检验。 -
[1] LE MOAL M, GASCUEL-ODOUX C, MéNESGUEN A, et al. Eutrophication: A new wine in an old bottle?[J]. Science of The Total Environment, 2019, 651: 1-11. doi: 10.1016/j.scitotenv.2018.09.139 [2] XU J, MO Y, TANG H, et al. Distribution, transfer process and influence factors of phosphorus at sediment-water interface in the Huaihe River[J]. Journal of Hydrology, 2022, 612: 128079. doi: 10.1016/j.jhydrol.2022.128079 [3] YAN Q, CHENG T, SONG J, et al. Internal nutrient loading is a potential source of eutrophication in Shenzhen Bay, China[J]. Ecological Indicators, 2021, 127: 107736. doi: 10.1016/j.ecolind.2021.107736 [4] XIA L, VERBEECK M, BERGEN B, et al. Effect of external and internal loading on source-sink phosphorus dynamics of river sediment amended with iron-rich glauconite sand[J]. Journal of Environmental Management, 2023, 332: 117396. doi: 10.1016/j.jenvman.2023.117396 [5] 高敏, 姜晓霞, 储茵. 不同水文条件下巢湖典型河流磷形态研究[J]. 水文, 2023, 43(1): 90-95. [6] 黄镁宁, 宁寻安, 张建易, 等. 漫水河清远流域磷污染特征及富里酸对沉积物释磷的影响[J]. 环境工程学报, 2022, 16(5): 1549-1557. doi: 10.12030/j.cjee.202112023 [7] LIAO R, HU J, LI Y, et al. Phosphorus transport in riverbed sediments and related adsorption and desorption characteristics in the Beiyun River, China[J]. Environmental Pollution, 2020, 266: 115153. doi: 10.1016/j.envpol.2020.115153 [8] 鲍林林, 李叙勇. 河流沉积物磷的吸附释放特征及其影响因素[J]. 生态环境学报, 2017, 26(2): 350-356. doi: 10.16258/j.cnki.1674-5906.2017.02.023 [9] NGUYEN T T N, NEMERY J, GRATIOT N, et al. Phosphorus adsorption/desorption processes in the tropical Saigon River estuary (Southern Vietnam) impacted by a megacity[J]. Estuarine Coastal and Shelf Science, 2019, 227: 106321. doi: 10.1016/j.ecss.2019.106321 [10] XU G, SONG J, ZHANG Y, et al. Enhancement of phosphorus storage capacity of sediments by coastal wetland restoration, Yellow River Delta, China[J]. Marine Pollution Bulletin, 2020, 150: 110666. doi: 10.1016/j.marpolbul.2019.110666 [11] 王若凡, 田甜, 刘骅, 等. 黄河兰州段消落带表层沉积物对磷的吸附[J]. 环境工程学报, 2023, 17(1): 343-350. doi: 10.12030/j.cjee.202205138 [12] 顾艳林, 宝冬润, 侯磊, 等. 洱海河口湿地沉积物对磷的吸附特征及影响因素[J]. 环境科学研究, 2021, 34(10): 2358-2368. doi: 10.13198/j.issn.1001-6929.2021.05.37 [13] 王书锦, 刘云根, 王妍, 等. 洱海入湖河口湿地干湿季沉积物氮、磷、有机质垂向分布特征及污染风险差异性[J]. 环境科学, 2016, 37(12): 4615-4625. doi: 10.13227/j.hjkx.201605193 [14] PALMER-FELGATE E J, BOWES M J, STRATFORD C, et al. Phosphorus release from sediments in a treatment wetland: Contrast between DET and EPC0 methodologies[J]. Ecological Engineering, 2011, 37(6): 826-832. doi: 10.1016/j.ecoleng.2010.12.024 [15] JARVIE H P, JüRGENS M D, WILLIAMS R J, et al. Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye[J]. Journal of Hydrology, 2005, 304(1): 51-74. [16] 杨文澜, 蒋功成, 王兆群, 等. 洪泽湖不同湖区表层沉积物对磷的吸附特征[J]. 地理学报, 2012, 67(7): 985-991. doi: 10.11821/xb201207011 [17] 魏荣菲, 庄舜尧, 杨浩, 等. 苏州河网区河道沉积物磷的吸附释放特征研究[J]. 水土保持学报, 2010, 24(3): 232-237. doi: 10.13870/j.cnki.stbcxb.2010.03.004 [18] 董雯, 王瑞琛, 李怀恩, 等. 渭河西咸段水质时空变异特征分析[J]. 水力发电学报, 2020, 39(11): 80-89. [19] 潘文学, 孙淑侠, 薛晨亮, 等. 沣西新城新河治理思路研究[J]. 陕西水利, 2018(6): 291-292. doi: 10.16747/j.cnki.cn61-1109/tv.2018.06.108 [20] 国家环境保护总局, 国家质量监督检验检疫总局. GB3838-2002地表水环境质量标准[S]. 北京, 中国标准出版社, 2002. [21] 鲁如坤. 土壤农业化学分析方法 [M]. 北京: 中国农业科技出版社, 2000. [22] WANG Z, LU S, WU D, et al. Control of internal phosphorus loading in eutrophic lakes using lanthanum-modified zeolite[J]. Chemical Engineering Journal, 2017, 327: 505-513. doi: 10.1016/j.cej.2017.06.111 [23] EMIL RYDIN. Potentially mobile phosphorus in Lake Erken sediment [J]. Water Research, 2000, 34(7); 2037-2042. [24] 任蕊, 王会锋, 卢婷, 等. 关中平原土壤地球化学基准值与背景值研究[J]. 西北大学学报(自然科学版), 2013, 43(5): 742-748. doi: 10.16152/j.cnki.xdxbzr.2013.05.010 [25] 国家环境保护总局《水和废水监测分析方法》编委会. 水和废水监测分析方法 [M]. 第四版. 北京: 中国环境科学出版社, 2002. [26] 胡洁蕴, 李淑芹, 宋歌, 等. 北京市北运河沉积物对氮、磷的吸附/解吸动力学特征[J]. 湖泊科学, 2018, 30(3): 650-659. doi: 10.18307/2018.0307 [27] LI X, XIE Q, CHEN S, et al. Inactivation of phosphorus in the sediment of the Lake Taihu by lanthanum modified zeolite using laboratory studies[J]. Environmental Pollution, 2019, 247: 9-17. doi: 10.1016/j.envpol.2019.01.008 [28] LIN J, ZHAO Y, ZHANG Z, et al. Immobilization of mobile and bioavailable phosphorus in sediments using lanthanum hydroxide and magnetite/lanthanum hydroxide composite as amendments[J]. Science of the Total Environment, 2019, 687: 232-243. doi: 10.1016/j.scitotenv.2019.06.042 [29] 李超, 张凤荣, 张天柱, 等. 土壤的石灰反应强度估测CaCO3含量和pH研究——以山西省黄土性母质土壤为例[J]. 土壤学报, 2017, 54(6): 1369-1376. [30] 李青. 基于河流地貌学的新河河道地形设计研究 [D]. 西安: 西安建筑科技大学, 2020. [31] 范亚宁, 刘康, 陈姗姗, 等. 秦岭北麓陆地生态系统水源涵养功能的空间格局[J]. 水土保持通报, 2017, 37(2): 50-56. doi: 10.13961/j.cnki.stbctb.2017.02.008 [32] 张潆元, 黑鹏飞, 杨静, 等. 本底吸附物对长江沉积物磷吸附容量的影响[J]. 环境科学研究, 2017, 30(4): 545-551. doi: 10.13198/j.issn.1001-6929.2017.01.78 [33] 向速林, 吴涛哲, 龚聪远, 等. 去除有机质对城市浅水湖泊氮磷释放特征的影响[J]. 水土保持通报, 2021, 41(5): 9-14+74. doi: 10.13961/j.cnki.stbctb.2021.05.002 [34] 于佳真, 王晓昌, 薛涛, 等. 不同温度下西安汉城湖沉积物吸附、释放特性和磷形态[J]. 环境工程学报, 2016, 10(11): 6275-6282. doi: 10.12030/j.cjee.201506085 [35] REDDY K R, DELAUNE R D. Biogeochemistry of Wetlands : Science and Applications [M]. Taylor and Francis Group, Boca Raton : Crc Press, 2008. [36] 赵海超, 王圣瑞, 张莉, 等. 有机质含量及其组分对洱海沉积物磷吸附-释放影响[J]. 环境科学学报, 2014, 34(9): 2346-2354. doi: 10.13671/j.hjkxxb.2014.0599 [37] PULLEY S, FOSTER I, ANTUNES P. The dynamics of sediment-associated contaminants over a transition from drought to multiple flood events in a lowland UK catchment[J]. Hydrological Processes, 2016, 30(5): 704-719. doi: 10.1002/hyp.10616 [38] ZHANG K, CHENG P D, ZHONG B C, et al. Total phosphorus release from bottom sediments in flowing water[J]. Journal of Hydrodynamics, 2012, 24(4): 589-594. doi: 10.1016/S1001-6058(11)60281-3 [39] TANG X, WU M, DAI X, et al. Phosphorus storage dynamics and adsorption characteristics for sediment from a drinking water source reservoir and its relation with sediment compositions[J]. Ecological Engineering, 2014, 64: 276-284. doi: 10.1016/j.ecoleng.2014.01.005 [40] PENG J-F, WANG B-Z, SONG Y-H, et al. Adsorption and release of phosphorus in the surface sediment of a wastewater stabilization pond[J]. Ecological Engineering, 2007, 31(2): 92-97. doi: 10.1016/j.ecoleng.2007.06.005 [41] 诸葛祥真. 上海市不同类型河岸带土壤磷的环境地球化学特征 [D]. 上海: 华东师范大学, 2014. [42] JAMES W F, LARSON C E. Phosphorus dynamics and loading in the turbid Minnesota River (USA): controls and recycling potential[J]. Biogeochemistry, 2008, 90(1): 75-92. doi: 10.1007/s10533-008-9232-5 [43] 金晓丹, 吴昊, 陈志明, 等. 长江河口水库沉积物磷形态、吸附和释放特性[J]. 环境科学, 2015, 36(2): 448-456. doi: 10.13227/j.hjkx.2015.02.011 [44] YIN H, HAN M, TANG W. Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation[J]. Chemical Engineering Journal, 2016, 285: 671-678. doi: 10.1016/j.cej.2015.10.038 [45] ZHANG F, YAN J, FANG J, et al. Sediment phosphorus immobilization with the addition of calcium/aluminum and lanthanum/calcium/aluminum composite materials under wide ranges of pH and redox conditions[J]. Science of The Total Environment, 2023, 863: 160997. doi: 10.1016/j.scitotenv.2022.160997 [46] XIE F, DAI Z, ZHU Y, et al. Adsorption of phosphate by sediments in a eutrophic lake: Isotherms, kinetics, thermodynamics and the influence of dissolved organic matter[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 562: 16-25. [47] 黄慧倩, 胡浩鹏, 杨斌, 等. 亚热带海湾表层沉积物对磷的吸附解析特征研究[J]. 环境科学研究, 2023, 36(2): 363-372. [48] 何宗健, 刘文斌, 王圣瑞, 等. 洱海表层沉积物吸附磷特征[J]. 环境科学研究, 2011, 24(11): 1242-1248. doi: 10.13198/j.res.2011.11.43.hezj.015 [49] 肖文娟, 曹秀云, 宋春雷, 等. 太湖不同营养类型湖区沉积物磷的形态与吸附行为的比较[J]. 环境工程学报, 2015, 9(7): 3525-3530. doi: 10.12030/j.cjee.20150772 [50] LABOSKI C, LAMB J A. Impact of manure application on soil phosphorus sorption characteristics and subsequent water quality implications[J]. Soil Science, 2004, 169(6): 440-448. doi: 10.1097/01.ss.0000131229.58849.0f [51] 李如忠, 宋敏, 杨继伟. 城市新城区公园沟塘沉积物磷释放风险及影响因素分析[J]. 环境科学, 2021, 42(9): 4287-4295. doi: 10.13227/j.hjkx.202101015 [52] 郝晓地, 王崇臣, 金文标. 磷危机概观与磷回收技术 [M]. 北京: 高等教育出版社, 2011. -





 下载:
下载: