-
诺氟沙星(1-乙基-6-氟-1,4-二氢-4-氧代-7-(1-哌嗪基)-3-喹啉羧酸,NOF)是抑菌范围广,活性强且安全性、稳定性高的喹诺酮类抗生素药物,通常被广泛应用于治疗尿道、呼吸道感染、皮肤感染和其他疾病。NOF广泛存在于自然界的水环境和土壤中,可以引发人体常见的头疼头晕等神经毒性反应以及腹痛腹泻等肠胃不良反应。自然环境中的NOF会被植物吸收和富集,影响其生长,而且会使土壤中的部分益生菌死亡,扰乱土壤内部环境。许多研究表明,NOF及其代谢物已普遍存在于自然水体和土壤中,其可通过食物链进入到人体,从而对人体造成危害。ZHOU等[1]使用GC-MS技术检测辽河、黄河和海河沉积物常用抗生素(大环内酯类、磺胺类、四环素类、和喹诺酮类)的存在情况,结果表明,海河沉积物中抗生素含量高于其他河流,其中NOF高达5 770 ng·g−1。NA等[2]在黄海海域检测出NOF含量高达108.8 ng·L−1。LI等[3]在以畜禽粪便为肥料的种植的蔬菜中检测出658.3 µg·kg−1的NOF残留。此外,残留在环境中的NOF还将使细菌产生抗药性,进而大量繁殖,对人类、生物乃至生态环境造成危害[4-5]。因此,选择合适的方法降解NOF是必不可少的。在众多方法中,被认为是最有效并且降解效率最高的方法是高级氧化法[6-9]。常见的高级氧化法有臭氧氧化法[10-11] 、催化湿式氧化法[12-13] 、电化学氧化法[14-15] 、Fenton氧化法[16-17]等。其中,Fenton氧化法主要是利用Fe2+与H2O2反应,催化生成具有强氧化性的·OH,可以氧化各种难生物降解的有机化合物,从而达到去除污染物的目的。
目前,对NOF残留的去除研究主要包括了混凝、吸附、膜分离及高级氧化等。其中,混凝和吸附不利于去除低质量浓度条件下的污染物,且废水中的其他污染物对其影响较大。膜分离技术需要高压驱动,膜成本偏高,难以大规模推广。非均相Fenton是一种新型的高级氧化技术,降解效率高,而且能同时去除多种污染物,在抗生素废水治理领域有良好的应用前景。已有大量文献将Fenton技术用于去除NOF的研究,CHAO等[18]采用HA-Fenton体系降解NOF,结果表明,HA-Fenton体系在3.0~9.0的较宽pH范围内均能有效降解,是Fenton体系的10.9倍。作为Fenton技术之一的铁基催化剂介导技术,虽然在去除水中有机污染物方面起着不可或缺的作用,但其在当前的应用中仍存在着局限性。例如,有磁性的铁基催化剂(Fe0、Fe3O4等)仅能在酸性条件具有优异的活化H2O2能力,其对pH耐受性方面的研究还有待完善,并且在高效活化H2O2方面的技术还有待提高[19-21];铁的硫化物(二硫化亚铁、硫化亚铁和三硫化二铁等)虽然能够高效活化H2O2且具有较宽的pH适用性,但其回收利用的过程非常困难,极易造成再次污染[22- 23]。由上述分析可知,Fe0和Fe3O4等传统Fe基催化剂和铁的硫化物在活化H2O2方面具有明显的互补性。因此,我们推断,若能将二者结合构建复合Fe基催化剂不仅可以提高Fe基催化剂的pH适用性,还能实现催化剂的快速回收。
基于此,本文以NOF为目标污染物,采用水热法制备新型铁基催化剂(Fe0/FeS2),研究其对H2O2的催化效果,并在铁基催化剂非均相芬顿体系的基础上,对NOF的降解路径及机理做出探讨。本实验选用XRD、VSM、XPS、SEM-Mapping等方法对所制备的新型铁基催化剂(Fe0/FeS2)进行表征,综合研究不同污染物初始质量浓度、pH、H2O2质量浓度、催化剂投加量对其活化H2O2降解NOF的影响,以期为非均相芬顿体系催化降解新型有机污染物提供参考。
-
主要试剂:诺氟沙星(NOF)、还原铁粉、邻菲罗啉、无水乙醇、升华硫、油胺、C4H6FeO4、CH2O2、C3H6O、H2O2、NaOH、NH3OHCl、CH3COONa、HCl(aq)、CH4O、Na2SO4、NaCl、Na2HPO4·12H2O、NaNO3、NaHCO3、Na2CO3,实验用水均为超纯水。
主要仪器:Hitachi S4800型冷场发射扫描电镜显微镜(日本日立公司)、MPMS (SQUID) XL型磁学测量系统(美国 Quantum Design 公司)、Miniflex 600型X-射线粉末衍射仪(日本株式会社)、KQ5200DE 型Rigaku 超声波清洗器(昆山市超声仪器有限公司)、H1850型台式高速离心机(湖南湘仪开发有限公司)、DHG-9053A型电热恒温鼓风干燥箱(上海精宏实验设备有限公司)、ESCALAB 250XI型X-射线光电子能谱仪(美国赛默飞世尔科技)、BSA124S-CW型电子分析天平(赛多利斯科学仪器有限公司)、Invia Reflex型激光显微拉曼光谱仪(英国雷尼绍公司)、LC-20AT型高效液相色谱仪(日本岛津公司)、G6520B型液相色谱-四级杆飞行时间串联质谱(美国 Agilent 公司)、JES FA200型电子顺磁共振波谱仪(日本电子株式会社)、Color squid型磁力搅拌器(德国 IKA)、TOC-L型总有机碳分析仪(日本岛津有限公司)、PHSJ-4F型pH计(上海仪电科学仪器有限公司)、UV-1780型紫外分光光度计(日本岛津有限公司)、JES FA200型电子顺磁共振波谱仪(日本株式会社)。
-
1) Fe0 /FeS2的制备。准备40 mL乙醇和20 mL油胺,将0.87 g醋酸亚铁、0.45 g还原铁粉和0.96 g升华硫加入其中。超声分散20 min后置于密封的水热反应釜中,在220 ℃下反应10 h。待反应釜冷却后,在100 00 r·min−1转速下离心5 min,固液分离后得到黑色固体。用丙酮和乙醇分别洗涤数次后于60 ℃真空干燥 12 h,得到黑色固体即为Fe0 /FeS2。重复上述操作,不投加 Fe0时得到对照样品FeS2[24]。
2) Fe0 /FeS2的表征。样品晶型用X射线粉末衍射仪进行分析,样品表面形貌用冷场发射扫描电镜显微镜观察并结合 Mapping 进行分析,选用X射线光电子能谱仪对样品表面成分、元素价态及半定量进行分析,材料磁强度用磁学测量系统进行分析,采用激光显微拉曼光谱仪对Fe0、FeS2、Fe0/FeS2材料物相组成进行分析。
-
准备的50 mL的NOF溶液(质量浓度为20 mg·L−1),将10 mg Fe0/FeS2加入其中。以500·min−1的转速搅拌,并同时加入质量浓度为33.28 g·L−1的H2O2溶液76.5 µL,每过一段时间用2 mL注射器取样,经0.22 µL玻璃纤维滤膜过滤后置于预先装有一定量自由基淬灭剂(甲醇)的液相瓶中,采用HPLC分析NOF残留质量浓度。
-
1) NOF的定量分析实验。NOF质量浓度选用日本岛津LC-20AT型HPLC进行检测,色谱柱选用C18柱(Agilent5HC-C18250×4.6mm)。检测方法:流动相设为甲醇:35%和甲酸溶液(含甲酸 0.1%,质量浓度):65%,进样量10 µL,流速0.5 mL·min−1,紫外检测波长278 nm。
2) NOF的降解产物分析。采用LC-QTOF-MS对NOF降解产物进行检测,色谱柱选用GL Sciences C18柱(4.6 mm×250 mm)。液相条件:流动相配比为甲酸(0.1%):甲醇=65:35,流速为0.8 mL·min−1,检测器波长为278 nm,进样量为10 µL。质谱条件:Dual ESI 源,正离子模式;采集模式2 GHz动态拓展模式;扫描范围为 100~400 。以纯度>99.9%的氮气作为载气,纯度>99.999%氮气作为碰撞气。
3)离子浓度分析。Fe2+和 Fe3+的测定:通过邻菲罗啉分光光度法测定溶液中 Fe2+的含量变化[25]。在测定总溶解性Fe之前,先加入过量盐酸羟胺使溶液中Fe3+全部转化成 Fe2+。Fe3+浓度=总Fe-Fe2+浓度。SO42−的测定:采用离子色谱对SO42−的含量进行测定,流动相Na2CO3/NaHCO3 (2.4 mmol·L−1/6.4 mmol·L−)溶液为流动相,抑制器电流45 mA,流速1.5 mL·min−1,进样量100 µL。
4) NOF的TOC分析。TOC检测选用TOC分析仪进行检测(日本岛津TOC-L型)。TOC去除率的计算公式如式(1)所示。
式中:R为TOC的去除率,%,C0为NOF溶液初始TOC值,mg·L−1,Ct代表NOF溶液反应t时刻的TOC值,mg·L−1。
5) Fe0 /FeS2的元素定量分析。采用元素分析仪对Fe0 /FeS2中的C、N、O和S进行定量分析。采用XSERIES2型电感耦合等离子体质谱仪对Fe0 /FeS2中的Fe元素进行定量分析。Fe0 /FeS2预先经5%的稀硝酸消解60 min,之后过滤膜后进行ICP分析。
6)自由基测定。采用JESFA200型电子顺磁共振波谱仪对催化剂反应时产生的自由基进行电子自旋共振(ESR)分析,仪器参数设定:中心场为250 mT,扫场宽度250 mT,测量时长1 min,微波频率9 054 MHz,调制频率100 kHz,微波功率0.998 mW。
-
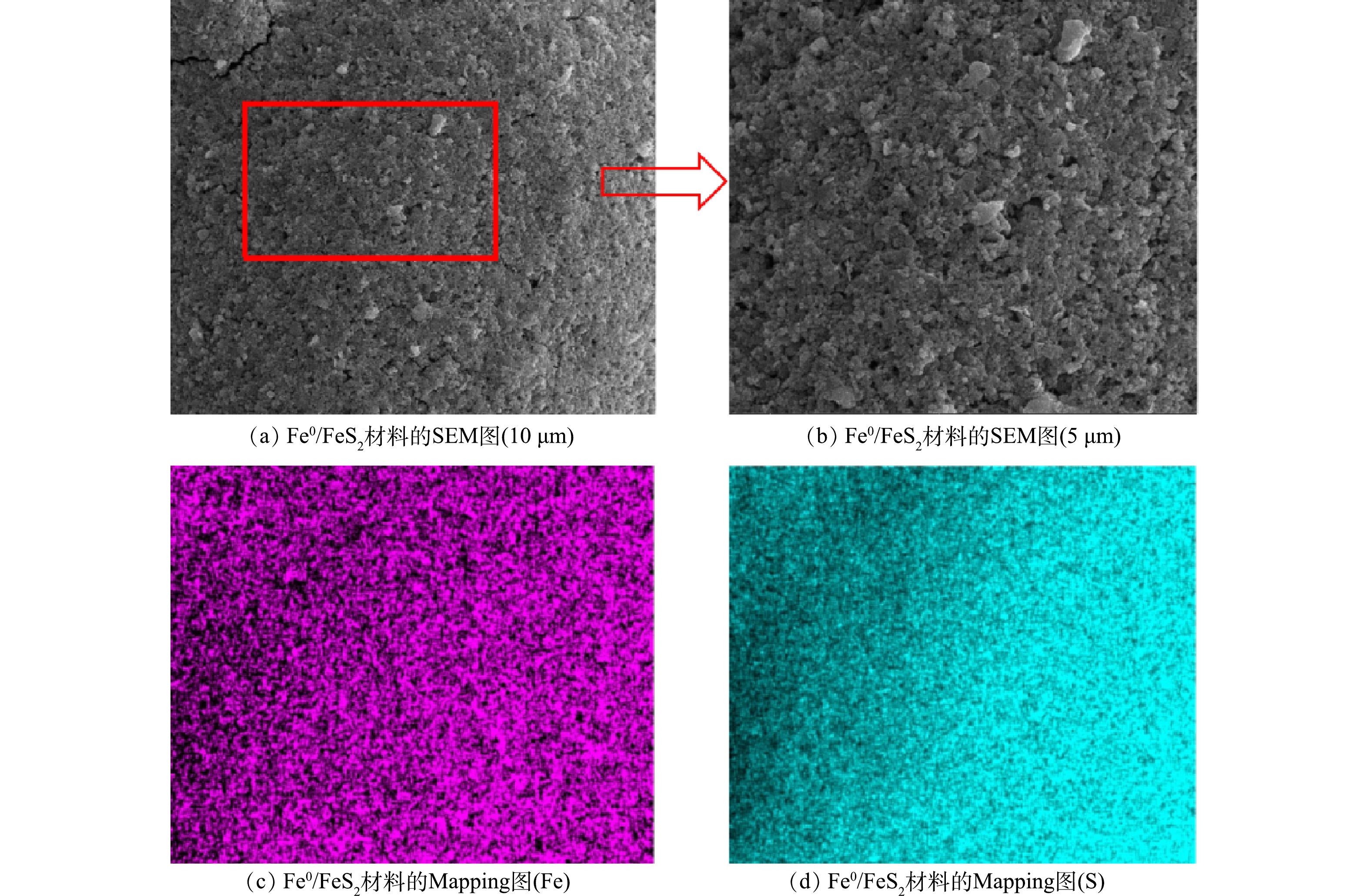
1)表面形貌及能谱分析。由图1(a)~(b)可以看出,Fe0/FeS2复合材料为大小不一的颗粒状,且颗粒间具有明显团聚现象,究其原因是磁性颗粒之间具有强吸引力。由图1(c)~(d)可以看出,新型铁基催化剂表面存在铁和硫2种元素,并且铁和硫这2种元素均匀地分散于样品表面。此外,为进一步分析Fe0/FeS2复合材料的组成,本论文采用元素分析和ICP分别对复合材料中的C、N、O、S和Fe进行了定量分析。结果表明,Fe0/FeS2复合材料中C、N、O、S和Fe的具体质量分数分别为1.8%、1.2%、6.7%、30.7%和59.6%。这表明制备的Fe0/FeS2复合材料主要是由Fe和S两种元素组成。
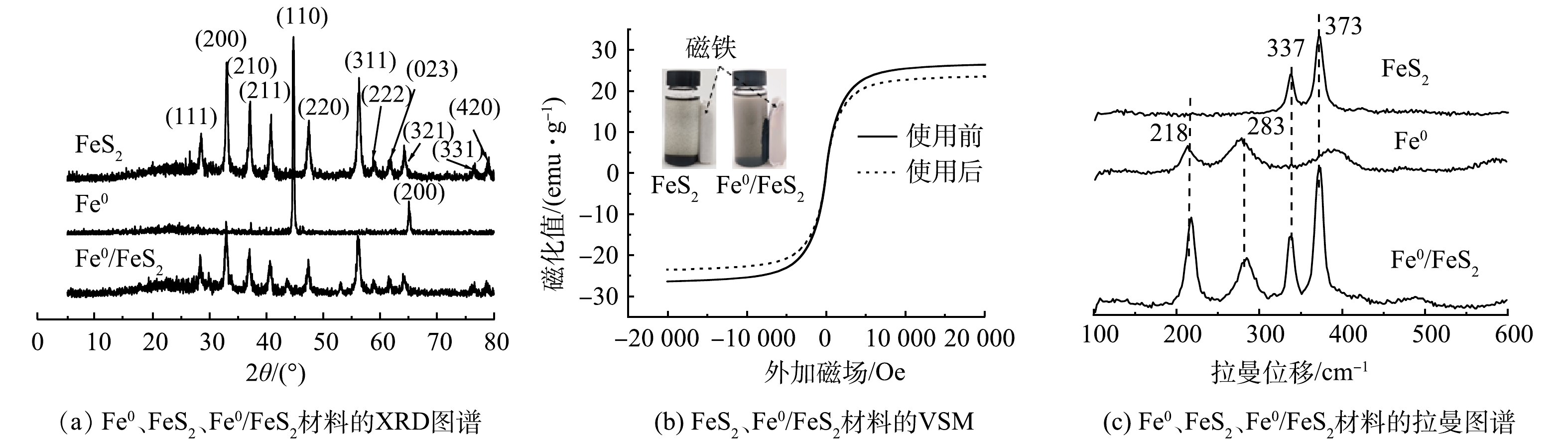
2)物相结构和磁性分析。材料的晶相结构可以由XRD图谱反映出来。图2(a)为Fe0、FeS2和Fe0/FeS2复合材料的XRD图谱。由图2(a)中可以看出,Fe0 的XRD图谱在2θ为44.66°、65.00°有2个明显的衍射峰,分别对应Fe0的(110)、(200)晶面。FeS2的XRD图谱分别在2θ为28.51°、33.01°、37.13°、40.76°、47.48°、56.31°、58.97°、61.79°、64.28°、76.53°和78.97°有明显的衍射峰,分别对应FeS2的(111)、(200)、(210)、(211)、(220)、(311)、(222)、(023)、(321)、(331)、(420)晶面。Fe0/FeS2在2θ为28.50°、33.03°、37.06°、40.75°、44.19°、47.41°、56.26°、58.99°、61.66°、64.26°、76.57°和78.94°出现了FeS2特征衍射峰。然而,复合材料中Fe0的特征衍射峰并不明显,这可能是由于Fe0被FeS2包裹或Fe0表面钝化造成的。
由于Fe0具有磁性而FeS2没有磁性,为进一步验证Fe0的存在,本论文采用振动样品磁强计(vibrating sample magnetometer, VSM)对样品的磁性进行了分析。由图2(b)可知,使用前Fe0 /FeS2磁滞回线呈S型,磁滞回线窄,剩磁和矫顽力小,其饱和磁化(Ms)值为26.38 emu·g−1,使用后,Fe0 /FeS2的磁滞回线也呈S型,磁滞回线变窄,剩磁和矫顽力变小,其饱和磁化(Ms)值变为23.50 emu·g−1。此外,从图中也可以直观地观察到,FeS2没有磁性,无法被磁铁吸住,而 Fe0 /FeS2 则能够被磁铁吸住。结合VSM分析及磁铁实验可知,Fe0 /FeS2反应前后均具有磁性,验证了Fe0的存在,并且Fe0 /FeS2在使用过程中可在外加磁场的作用下实现回收利用。
与此同时,为进一步分析Fe0/FeS2复合材料的物相组成,本论文采用拉曼光谱对Fe0、FeS2、Fe0/FeS2材料物相组成进行分析,结果如图2(c)所示。由图可知,Fe0/FeS2复合材料分别在218、283和337、373 cm−1处出现了Fe0和FeS2物相的特征峰,表明制备的Fe0/FeS2复合材料由Fe0和FeS2 2种物相构成。
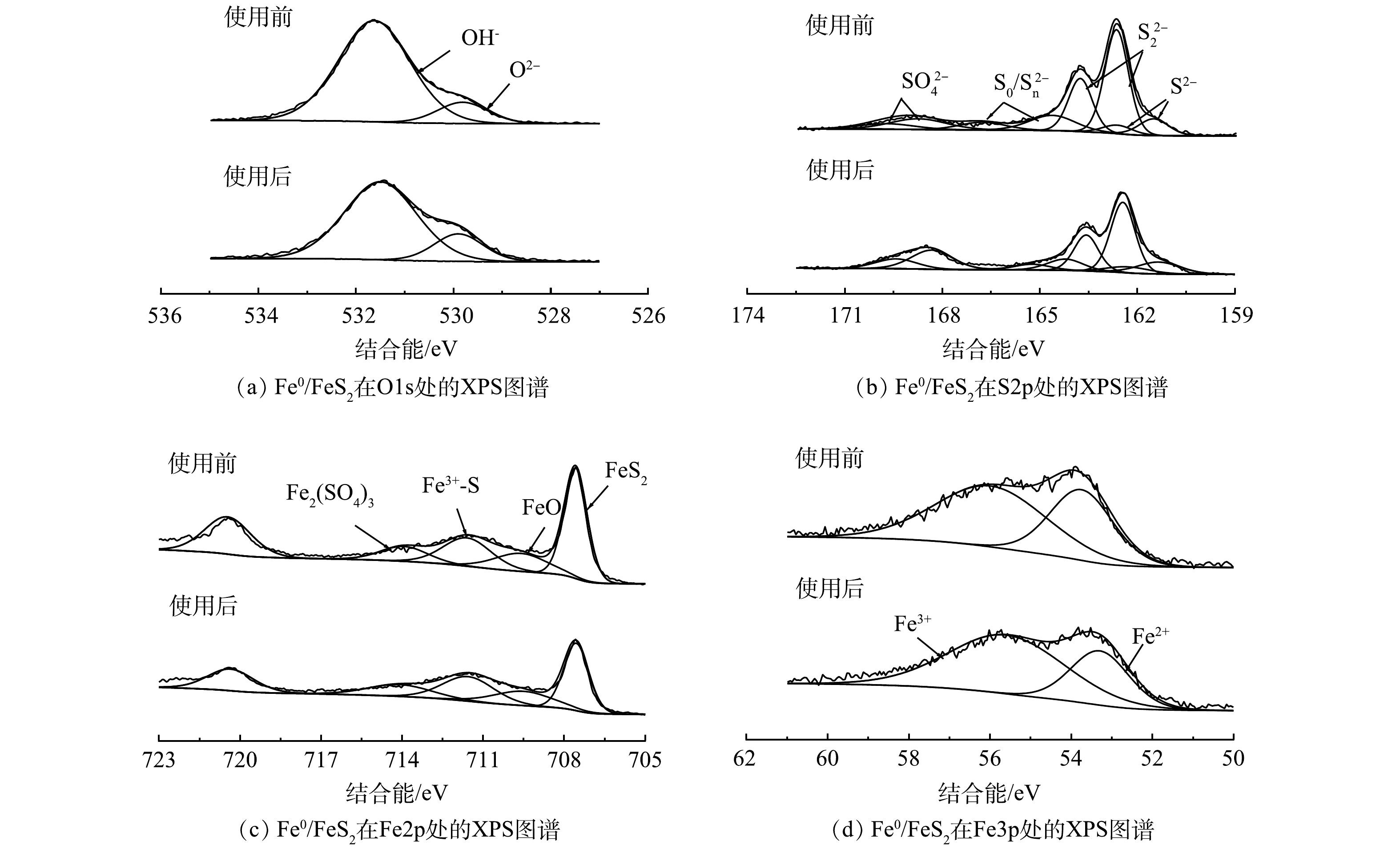
3) XPS分析。采用XPS分析制备的Fe0/FeS2,结果如图3所示。从图3(a)可以看出,在结合能为529.82 eV处的峰为O2−,在结合能为531.63 eV处的峰为OH−[26],其中,O2−主要来自于表面吸附的CO2或Fe在干燥、存储以及送样过程中因氧气局部氧化在表面形成的铁氧化物,而OH−主要来自于表面吸附的水蒸气或Fe氧化形成的氢氧化物。在S2p的图谱中有2个峰值区:一个是硫离子的162~164 eV左右,另一个是硫酸盐的167~170 eV左右[27]。观察S2p的XPS高分辨图谱(图3(b))可以知道,在结合能为162.44 eV和168.34 eV处的峰分别对应的是S22−和SO42-[8,28],其中,S22−峰的出现表明材料中含有FeS2的存在。在结合能161.34 eV和164.21 eV处的峰分别为S2−和S0/Sn2-[8,27]。由Fe2p的XPS高分辨图谱(图3(c))可以得知,在结合能为707.55 eV处出现了的特征峰为FeS2的铁原子[7],结合能为709.32 eV可能为FeO[29],在结合能为711.55 eV的峰可能为材料表面氧化产生的Fe3+-S或Fe3+-O[30]。在结合能为714.0 eV的峰值归属于FeSO4或Fe2(SO4)3中的铁原子。这是因为硫酸铁是在水热条件下合成黄铁矿样品的前驱体,因此在光谱中可能会有硫酸铁的存在[31]。但在XRD谱图上并没有明显的硫酸铁特征峰,表明其含量在样品中很低。在Fe3p的XPS高分辨图谱(图3(d))中,结合能为53.42 eV和55.90 eV处的峰为Fe(Ⅱ)和Fe(Ⅲ)[32]。从XPS高分辨图谱可以看出材料表面包含二硫化铁和少量的铁氧化物。
综合SEM、XRD、VSM、XPS、拉曼光谱和元素分析结果可知,制得的Fe0/FeS2复合材料主要是由Fe0和FeS2构成,表面存在少量的Fe氧化物。
-
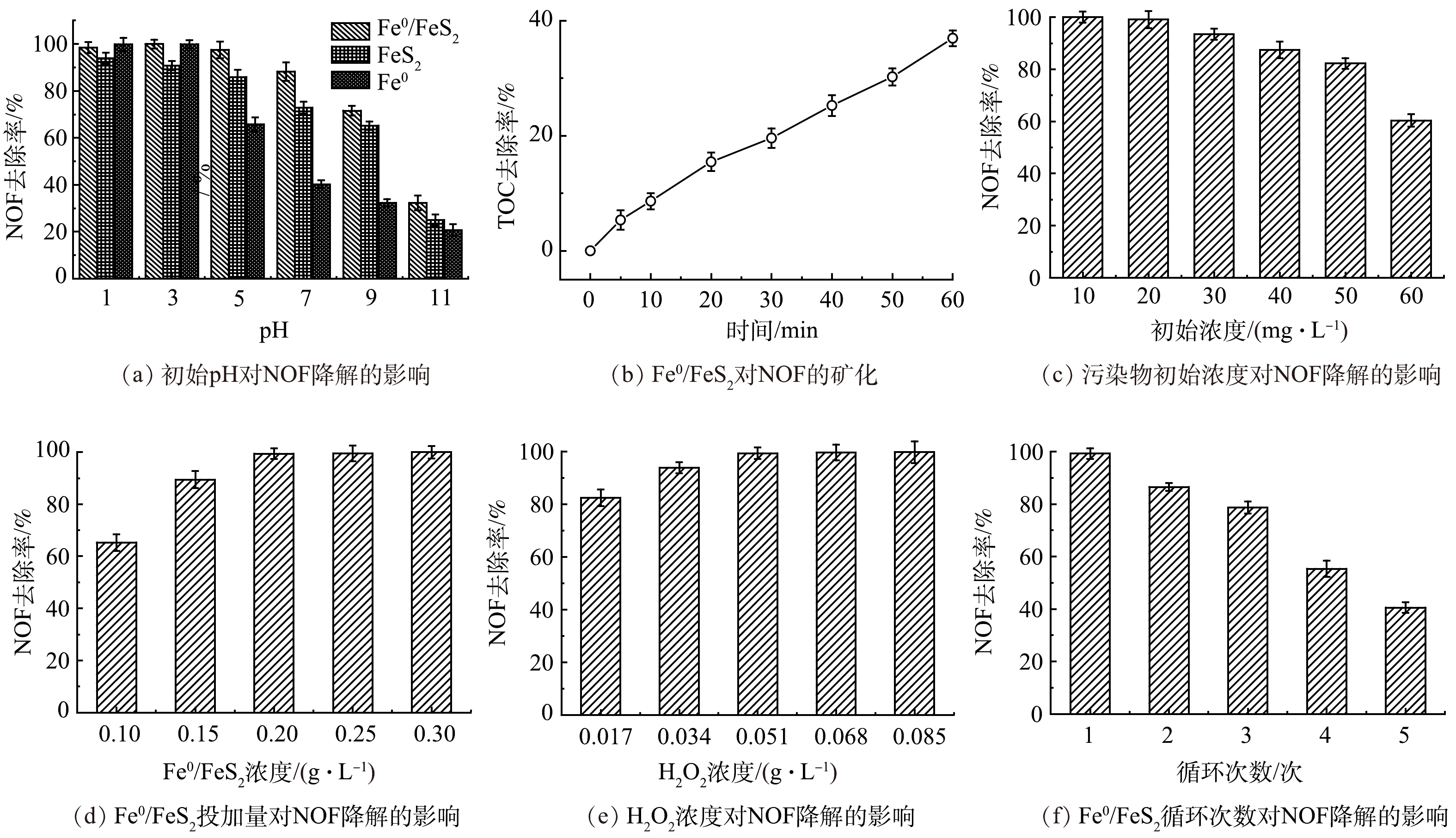
1)不同体系对NOF的去除率分析。通过对比不同催化剂体系(FeS2、Fe0和Fe0/FeS2)对NOF的降解效果来验证Fe0/FeS2介导的非均相Fenton体系为降解NOF的最优体系。本实验以NOF的去除率作为评价指标,降解条件如下:NOF的初始质量浓度为20 mg·L−1,H2O2质量浓度为0.034 g·L−1,催化剂的投加量为0.2 g·L−1。由图4(a)可以得知,FeS2、Fe0和Fe0/FeS2体系均能有效活化H2O2降解NOF,而在强酸性条件下(pH<3),3个体系都能有效降解90%以上的NOF,而随着pH的上升,3个催化剂体系对NOF的降解率都不断降低。其中,Fe0/H2O2体系的下降趋势最为明显,在pH为7时,NOF的去除率下降到(40.15±1.78)%,此时FeS2/H2O2和Fe0/FeS2/H2O2的去除率分别为(72.87±2.56)%和(88.23±4.01)%,表明Fe0/FeS2在中性条件仍能保持良好的活化能力。在pH为9时,Fe0/H2O2体系对NOF的去除率仅为(32.31±1.65)%,此时Fe0/FeS2/H2O2的去除率仍可以达到70%以上,也高于FeS2/H2O2体系,表明Fe0/FeS2在弱碱性条件下仍能活化H2O2。由此可知,与传统的纯Fe0相比,Fe0/FeS2材料具有较广的pH范围适用性。与此同时,由图2(b)可以得知,FeS2材料不具有磁性,而Fe0/FeS2复合材料具有磁性,可实现催化剂的回收重复利用,避免催化剂进入水体引起二次污染。总有机碳(TOC)是指水中溶解性和悬浮性有机物的总含碳,是评价污染物矿化情况的重要指标之一。由图4(b)可以看出,反应30 min后,Fe0/FeS2/H2O2体系中总有机碳的去除率可以提升到(36.96±1.39)%,说明体系能够矿化NOF。
2)不同因素对NOF去除的影响。在不同NOF初始质量浓度、初始pH、H2O2投加量和催化剂投加量的条件下,以NOF的去除率为评价指标,探究不同因素对Fe0/FeS2/H2O2介导的非均相芬顿体系降解NOF的影响。本文在NOF的初始质量浓度为20 mg·L−1,H2O2质量浓度为0.034 g·L−1,催化剂的投加量为0.2 g·L−1的条件下,通过氯化钠和盐酸来调节反应体系的pH,分析溶液在不同pH条件下对NOF的去除率,结果如图4(a)所示。经过20 min的反应,Fe0/FeS2/H2O2体系在不同初始pH条件下对NOF的去除率存在着显著差异。Fe0/FeS2/H2O2体系对NOF的去除率在初始溶液pH小于3时,随着pH的上升有逐渐上升的趋势;在初始溶液pH等于3时,NOF的去除率最高,达到(99.94±1.90)%;在初始溶液pH大于3时,NOF的去除率随着pH的升高呈逐步下降的趋势;在pH为7时,NOF的去除率仍能达到(88.23±4.01)%;在pH为9时,NOF去除率下降至(71.56±2.08)%,当体系的pH为11时的去除率仅为(32.30±3.14)%。由此可知,强碱环境不利于NOF的去除,这是因为在强碱性条件下,溶液中的H+浓度非常低,阻碍了自由基的产生。在酸性条件下(pH<5),Fe0/FeS2/H2O2体系对NOF的去除率均高达95%,在中性条件下(pH=7),Fe0/FeS2/H2O2体系对NOF的去除率大于88%,在弱碱条件下(pH=9),Fe0/FeS2/H2O2体系对NOF的去除率大于70%,表明本实验制备的Fe0/FeS2催化剂具有较广的pH适用性。考虑到基于pH为5的反应条件更加温和,NOF的去除率也大于95%。因此,选择初始pH为5作为Fe0/FeS2复合材料降解NOF体系的最佳pH。
其次,污染物的初始质量浓度也是影响降解效率的重要因素[33]。本实验在溶液初始pH为5,H2O2质量浓度为0.034 g·L−1,催化剂(Fe0/FeS2复合材料)的投加量为0.2 g·L−1的条件下,探究不同初始质量浓度的NOF对NOF的去除率,结果如图4(c)所示。从图4(c)中可知,经过20 min的反应,不同NOF初始质量浓度对Fe0/FeS2/H2O2体系降解NOF的过程存在明显的影响。在NOF的质量浓度为10 mg·L−1时,Fe0/FeS2/H2O2体系对NOF的去除率最高,为(99.94±2.13)%。随着NOF初始质量浓度的升高,去除率呈下降趋势,在NOF的初始质量浓度由10 mg·L−1上升至60 mg·L−1的过程中,NOF的去除率由(99.94±2.13)%下降至(60.32±2.35)%。这是因为随着污染物质量浓度的增加,催化剂吸附的污染物分子也会变多,这不利于H2O2的分解,不利于·OH的生成[34]。因此,污染物的去除效率会随着污染物初始质量浓度的增加而降低。值得注意的是,在NOF初始质量浓度为20 mg·L−1时,NOF的去除率仍能高达99%,因此,选择初始质量浓度为20 mg·L−1作为Fe0/FeS2复合材料降解NOF体系的最佳初始质量浓度。
催化剂(Fe0/FeS2)投加量也是会影响污染物的降解效率的因素之一[35]。本文在溶液初始pH=5,H2O2为为0.034 g·L−1,NOF初始质量浓度为20 mg·L−1的条件下,考察不同质量浓度下Fe0/FeS2对NOF的去除率,结果如图4(d)所示。NOF的去除率随着Fe0/FeS2投加量增加逐步升高。当Fe0/FeS2投加量为0.1 g·L−1时,NOF的去除率最低,仅为(65.27±3.17)%。当Fe0/FeS2投加量为增加至0.2 g·L−1时,NOF的去除率大于99%,继续增加Fe0/FeS2投加量,NOF的去除率提升速度减缓,去除率均可以达99%以上。
H2O2投加量的大小是决定NOF去除率的关键因素,这是因为H2O2在Fenton体系中分解产生羟基自由基,因此,在溶液初始pH为5,NOF初始质量浓度为20 mg·L−1,催化剂(Fe0/FeS2复合材料)的投加量为0.2 g·L−1的条件下,考察不同H2O2质量浓度(0.017、0.034、0.051、0.068、0.085 g·L−1)对NOF降解效率的影响由图4(e)可知,反应10 min后,不同质量浓度的H2O2对Fe0/FeS2/H2O2体系降解NOF的过程产生了明显的差异。随着H2O2投加量增加,NOF的去除率也不断上升。当H2O2投加量为0.085 g·L−1时,NOF的去除率达到最高,为(99.75±4.11)%,而当H2O2投加量大于0.051 g·L−1后NOF的去除率均大于99%。因此,选H2O2投加量为0.051 g·L−1作为Fe0/FeS2/H2O2体系降解NOF的最佳投加量。
综上可知,Fe0/FeS2/H2O2体系降解NOF的最佳条件如下:初始pH=5,NOF初始质量浓度为20 mg·L−1,H2O2为51 mg·L−1,Fe0/FeS2为200 mg·L−1。在上述条件下,体系在10 min时NOF的降解率可以达到(99.27±2.24)%。由图4(f)可知,Fe0/FeS2在使用3次后对NOF的降解效率仍然可以保持在75%以上,在重复利用5次后降解效率仍有40.56%,这表明Fe0/FeS2能够较好地循环再利用。由图2(b)可以看出, Fe0/FeS2在经过重复使用后,Ms值由26.38 emu·g−1降至23.50 emu·g−1,但其依然具备磁性,可快速与溶液分离,达到回收再利用的目的。
-
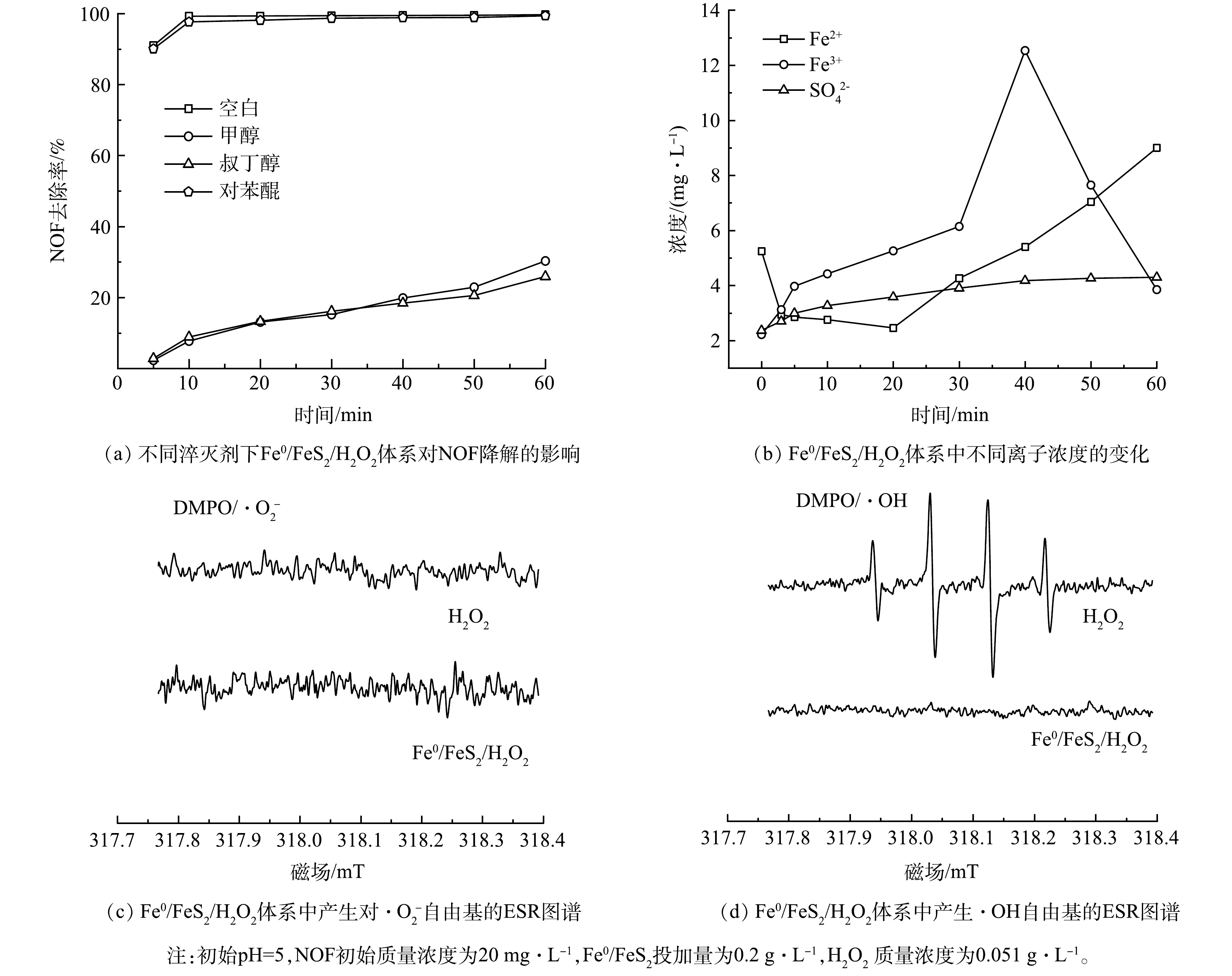
1)自由基分析。为了进一步探究Fe0/FeS2/H2O2体系中降解NOF的主要活性物种,本论文进行了自由基淬灭实验,结果如图5(a)所示。以甲醇作为·OH和SO4·−淬灭剂,叔丁醇作为·OH淬灭剂,苯醌作为·O2−淬灭剂,通过分析不同自由基淬灭剂作用下NOF降解效率的变化分析Fe0/FeS2/H2O2体系中不同自由基(·OH、SO4·−和·O2−)对NOF降解的贡献。由图5(a)可知,在不添加任何淬灭剂的情况下,经过10 min的反应,NOF的去除率达到(99.27±2.24)%,在加入对苯醌之后,NOF的降解趋势和不添加淬灭剂的趋势相同,去除率为(97.68±2.01)%,说明·O2−自由基在NOF的降解过程中不发挥明显作用,不是该体系主要的反应活性基团。当在体系中加入甲醇,NOF在10 min内的去除率为(7.71±1.29)%;与此同时,添加叔丁醇后,NOF在10 min内的去除率为(8.86±2.36)%。由于投加叔丁醇和甲醇对Fe0/FeS2/H2O2体系降解NOF的去除率无显著影响,这说明Fe0/FeS2/H2O2体系内起最关键作用的活性基团是·OH。此外,电子自旋共振光谱(ESR)(图5(c)~(d))也直接证实了体系中的主要自由基是·OH,而·O2−可以忽略。
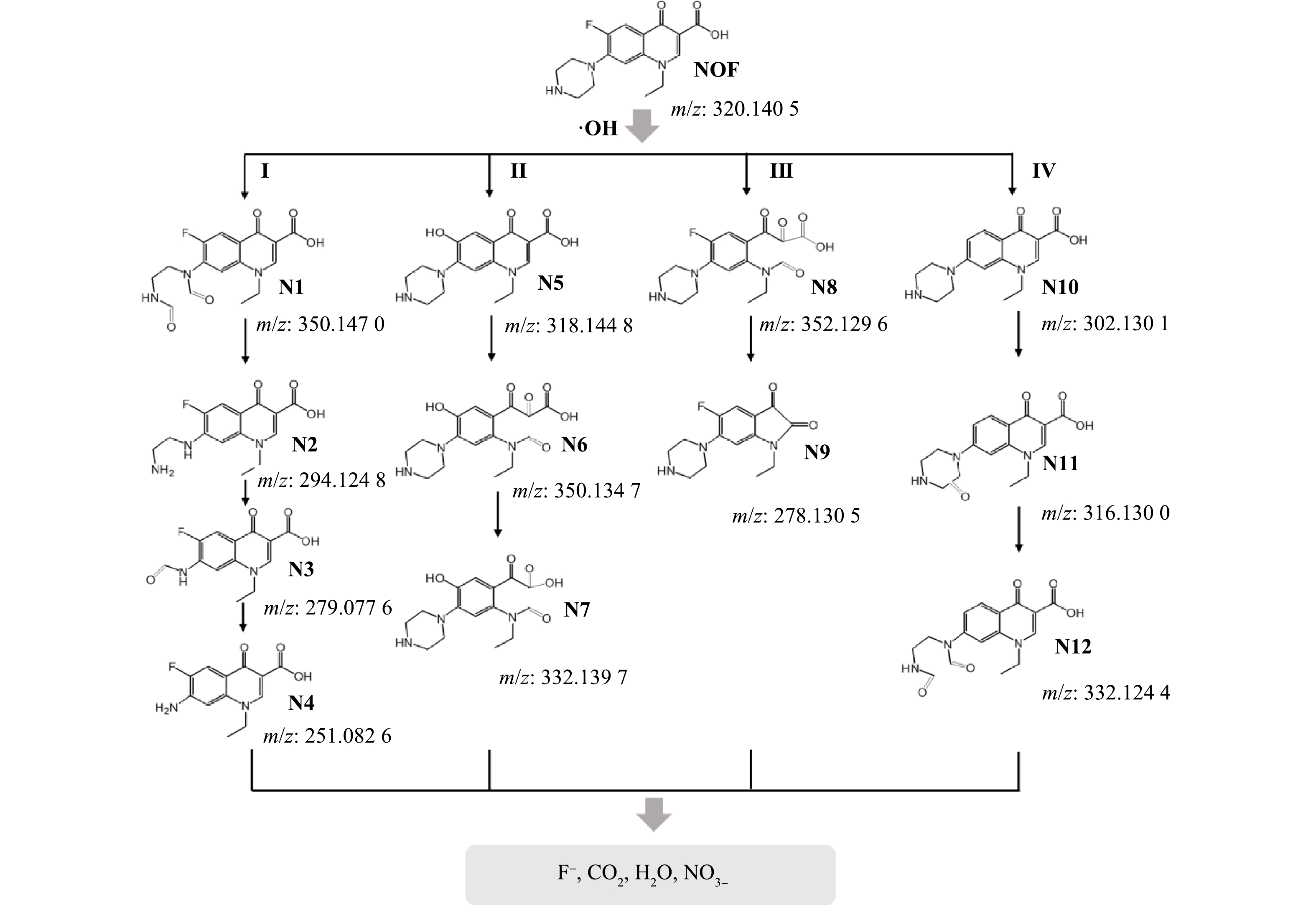
2)降解产物分析。利用LC-QTOF-MS对复合材料Fe0/FeS2活化H2O2降解NOF的中间产物进行测定,定性检测并鉴定了降解过程中产生的12种中间体,将其命名为N1-N12,其中m/z的数值如表1所示。根据液相质谱结果可以推测出NOF降解产物的元素组成,将其与相关研究进行对比[35-37],可以分析出NOF降解产物可能的分子式和结构式。
由图5(b)可知,加入H2O2之前,体系中Fe2+和Fe3+质量浓度分别为(5.25±0.54) mg·L−1和(2.22±0.32) mg·L−1。在投加H2O2之后,反应的前20 min之内,Fe2+的质量浓度随着反应的进行而逐渐降低,最低质量浓度为(2.46±0.35) mg·L−1;而后随着反应的进行,Fe2+质量浓度逐渐升高,这可能是由于反应中的Fe3+会转化为Fe2+。与之相对应的,体系Fe3+的质量浓度随着反应的进行而逐渐升高,体系中Fe3+质量浓度最高为(12.53±0.50) mg·L−1,之后呈下降趋势。这可能是由于体系中有部分Fe3+被Fe0转化成Fe2+。此外,本实验也对NOF降解过程中SO42−质量浓度进行了测试,由图5(b)可知,随着反应的进行,溶液中的SO42−质量浓度呈现上升趋势,最终的质量浓度为4.3 mg·L−1,表明Fe0/FeS2中的S也参与了H2O2的活化。
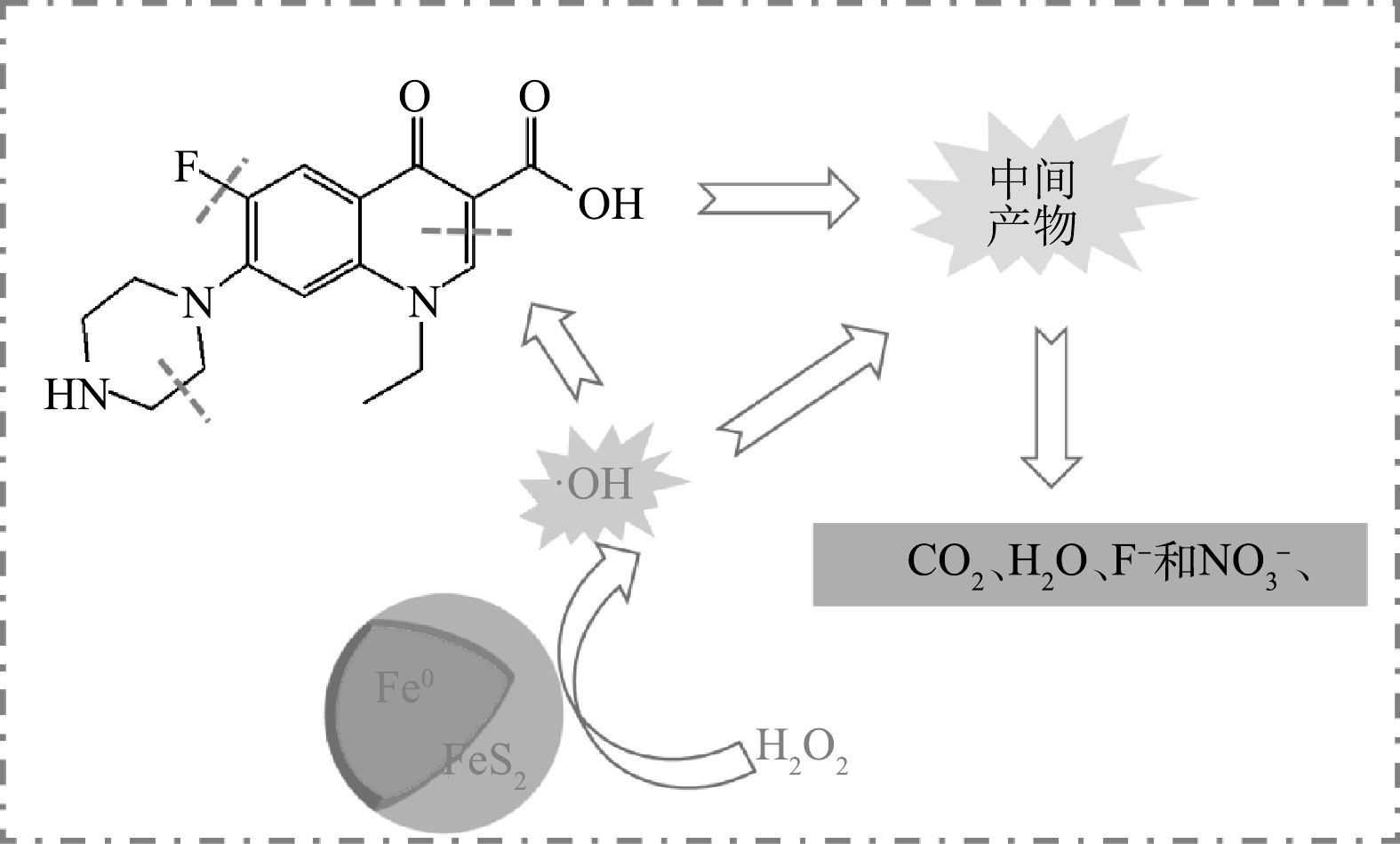
3) NOF降解途径。基于对降解产物、自由基捕获实验和XPS高分辨图谱的分析结果,本文尝试提出NOF可能的降解途径有以下4种(图6)。途径1:NOF的哌嗪环在强氧化性羟基自由基(·OH)的进攻下引发开环反应生成中间体N1(m/z=350.147 0),然后进一步被氧化失去2个-CO生成中间体N2(m/z=294.124 8),再经过氨基醛化生成中间体N3(m/z=279.077 6);最后再经过氧化损失-CO生成中间体N4(m/z=251.082 6);途径2:NOF的C-F键在·OH攻击下断裂,然后氟离子被羟基取代生成中间体N5(m/z=318.144 8),N5被强氧化性基团氧化,喹诺酮环断裂生成中间体N6(m/z=350.134 7),然后再氧化脱去-CO生成中间体N7(m/z=322.139 7);途径3:喹诺酮基被毗邻羧基的C=C双键上的·OH攻击形成中间体N8(m/z=352.129 6)和N9(m/z=278.130 5)[38];途径4:这个过程从脱氟开始,然后·OH攻击哌嗪环上的碳原子,生成N10(m/z=302.130 1)和N11(m/z=316.130 0)。中间体N11的哌嗪环断裂生成产物N12(m/z=332.123 8)。最后上述中间体均被·OH氧化NO3−,F−,H2O和CO2等为小分子物质。之前的研究也表明NOF的降解转化产物可以被氧化为低分子的有机和无机产物,如CO2、H2O、F−和NO3−[37, 39]。
4)降解机理。Fe0/FeS2使用后的XPS高分辨图谱如图3所示。由图3(a)可知,O2−的比例由35.04%下降到34.41%,OH−的比例由64.96%提高到65.69%。这说明反应过程中有氧化物的消耗和氢氧化物的生成。由图3(b)能够看出,反应后材料中S22−由58.08%下降到47.63%,SO42−由14.23%提高到24.29%,S2−由11。54%上升到15.77%,S0/Sn2−由16.15%下降到12.30%,说明S22−参与了反应。由图3(c)能够看出,FeS2由35.21%下降到30.77%,FeO由13.03%上升到15.38%,Fe3+-S由18.66%上升到21.85%,Fe2(SO4)3由9.50%上升到13.85%,说明了FeS2中的Fe2+参与反应可能部分转化为了FeO、Fe3+-S和Fe2(SO4)3。结合Fe3p的XPS高分辨图谱(图3(d))中Fe2+由39.76%下降为31.51%,Fe(Ⅲ)由60.24%上升到68.49%,进一步验证了Fe2+参与了反应。
结合自由基验证、催化剂表面元素变化及降解产物分析,Fe0/FeS2催化降解NOF的机理如图7所示。
下述为Fe0/FeS2介导的非均相芬顿体系中涉及到的相关反应方程[40-43]。结合相关文献[43],在pH小于7的情况下,二硫化亚铁产生二价铁离子的方式2种:一种为二硫化亚铁与水中的氧气在催化剂表面反应(式(2))生成二价铁离子、硫酸根离子和氢离子;另一种由2个连续反应构成(式(3)和式(4)),二硫化亚铁先与H2O2反应生成三价铁离子、硫酸根离子、氢离子和水,接着三价铁离子在材料表面与二硫化亚铁继续反应生成二价铁离子。第2种路径为二价铁离子主要的生成方式。二价铁生成后与H2O2反应生成氢氧根离子(式(5))。同时[36],零价铁可与溶液中的氧气和氢离子在催化剂表面反应生成二价铁离子和H2O2(式(6)),H2O2将零价铁氧化成二价铁离子(式(7)),二价铁离子与H2O2反应生成羟基自由基,并将羟基自由基释放到水中(式(8)),而式(2)、式(3)、式(4)中产生的氢离子则有助于零价铁反应生成二价铁离子,进而促进H2O2的活化。羟基自由基是一种氧化性极强的自由基,能够快速氧化NOF,造成脱氟喹诺酮环开环和哌嗪环开环,生成氟离子、水、二氧化碳和硝酸根离子(式(9))。
-
1)本文利用水热法制备了能够高效活化H2O2且重复利用率较高的新型铁基(Fe0/FeS2)催化剂。该催化主要是由Fe0和FeS2组成,并含有少量的Fe氧化物,具有良好的磁性,能够实现催化的快速回收。
2)与传统的Fe0催化剂相比,Fe0/FeS2不仅具有优异的活化H2O2的能力,还具有更广的pH(3~9)适用性,其介导的非均相Fenton体系能够实现NOF的快速降解。在最佳条件下(初始pH为5,NOF初始质量浓度为20 mg·L−1,H2O2质量浓度为51 mg·L−1,Fe0/FeS2投加量为200 mg·L−1),Fe0/FeS2介导的非均相芬顿体系可以在10 min内实现对NOF的完全降解,并进一步实现其矿化。
3)在Fe0/FeS2/H2O2体系降解NOF过程中,主要的活性自由基为·OH。在·OH的攻击下,NOF的降解过程涉及C-F键断裂、哌嗪环和喹诺酮环的开环作用,并最终矿化成F−、H2O、CO2和NO3−等物质。
Fe0/FeS2活化H2O2快速降解诺氟沙星
Rapid degradation of norfloxacin by Fe0/FeS2 activated H2O2
-
摘要: 通过水热法成功制备具有磁性的Fe0/FeS2复合铁基催化剂,并将其用于构建非均相芬顿体系降解典型的喹诺酮类抗生素(诺氟沙星,NOF)。SEM-Mapping 结果显示,制备的Fe0/FeS2复合材料由Fe和S两种元素组成,形态为颗粒状且尺寸不一。XRD、XPS、Raman和磁学测量系统(VSM)等表征结果表明进一步证明Fe0/FeS2复合材料的成功制备且具有良好的磁性。通过实验得到 Fe0/FeS2/H2O2体系降解NOF最优的降解体系为初始pH为5,NOF起始质量浓度20 mg·L−1,Fe0/FeS2 投加量为0.2 g·L−1,H2O2质量浓度0.051 g·L−1。Fe0/FeS2介导的非均相芬顿体系可以快速降解NOF,10 min后的降解率为99.27%,且具有良好的重复利用性,使用 3 次后,NOF 的降解效率仍超过75%。NOF在羟基自由基(·OH)的作用下可能破坏C-F键以及实现哌嗪环和喹诺酮环的开环,最终生成一些小分子物质,如 F−、H2O、CO2和NO3−等。Abstract: The magnetic Fe0/FeS2 composite iron-based catalyst was successfully prepared by hydrothermal method, and was employed to mediate a heterogeneous Fenton system for the remediation of a typical Fluoroquinolones antibiotic (norfloxacin, NOF). The SEM-Mapping results showed that the Fe0/FeS2 composite was composed of Fe and S elements, and displayed granular structure with different sizes. The characterization results of XRD, XPS, Raman and Magnetic Measurement System (VSM) showed that the Fe0/FeS2 composite material had been successfully prepared with good magnetic properties. The results of NOF degradation experiments showed that the optimal conditions of Fe0/FeS2/H2O2 system for norfloxacin degradation were initial pH=5, norfloxacin initial concentration of 20 mg·L−1, Fe0/FeS2 dosage of 0.2 g·L−1, H2O2 concentration of 0.051 g·L−1. The heterogeneous Fenton system could instantaneously degrade 99.27% of NOF in 10 min, and the Fe0/FeS2 exhibited a good reusability. After 3 runs, the degradation efficiency of NOF was still over 75%. Under the attack of ·OH, norfloxacin may break the C-F bond and open the ring of piperazine and quinolone, and finally small molecular substances such as F−, H2O, CO2 and NO3− will be generated.
-
Key words:
- Fe0/FeS2 /
- H2O2 /
- norfloxacin /
- heterogeneous Fenton /
- degradation
-
表 1 NOF 降解过程中的中间产物
Table 1. Identification of the intermediates during NOF degradation
代谢物 m/z 分子式 诺氟沙星 320.140 5 C16H18FN3O3 N1 350.147 C16H16FN3O5 N2 294.124 8 C14H16FN3O3 N3 279.077 6 C13H11FN2O4 N4 251.082 6 C12H11FN2O3 N5 318.144 8 C16H19N3O4 N6 350.134 7 C16H19N3O6 N7 322.139 7 C15H19N3O5 N8 352.129 6 C16H17FN3O5 N9 278.130 5 C16H16FN3O2 N10 302.130 1 C16H20N3O3 N11 316.130 0 C16H18N3O4 N12 332.124 4 C16H18N3O5 -
[1] ZHOU L J, YING G G, ZHAO J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China[J]. Environmental Pollution, 2011, 159(7): 1877-1885. doi: 10.1016/j.envpol.2011.03.034 [2] NA G, GU J, GE L, et al. Detection of 36 antibiotics in coastal waters using high performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Oceanology and Limnology, 2011, 29(5): 1093-1102. doi: 10.1007/s00343-011-0225-1 [3] LI, Y W, WU, X L, MO, C H, et al. Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, southern China[J]. Journal of agricultural and food chemistry, 2011, 59(13): 7268-7276. doi: 10.1021/jf1047578 [4] ALLEN H K, DONATO J, WANG H H, et al. Call of the wild: Antibiotic resistance genes in natural environments[J]. Nature Reviews Microbiology, 2010, 8(4): 251-259. doi: 10.1038/nrmicro2312 [5] 张娣, 王懿萱, 牛红云, 等. 纳米Fe3O4/H2O2降解诺氟沙星[J]. 环境科学, 2011, 32(10): 2943-2948. [6] CHEN S, DENG J, YE C, et al. Simultaneous removal of para-arsanilic acid and the released inorganic arsenic species by CuFe2O4 activated peroxymonosulfate process[J]. Science of the Total Environment, 2020, 742:140587. [7] ZHENG S, JIANG W, CAI Y, et al. Adsorption and photocatalytic degradation of aromatic organoarsenic compounds in TiO2 suspension[J]. Catalysis Today, 2014, 224: 83-88. doi: 10.1016/j.cattod.2013.09.040 [8] ZHAO Z, PAN S, YE Y, et al. FeS2/H2O2 mediated water decontamination from p- arsanilic acid via coupling oxidation, adsorption and coagulation: Performance and mechanism[J]. Chemical Engineering Journal, 2020, 381: 122667. doi: 10.1016/j.cej.2019.122667 [9] XIE X, ZHAO W, HU Y, et al. Permanganate oxidation and ferric ion precipitation (KMnO4 -Fe (III)) process for treating phenylarsenic compounds[J]. Chemical Engineering Journal, 2019, 357: 600-610. doi: 10.1016/j.cej.2018.09.194 [10] SNYDER S A, WERT E C, REXING D J, et al. Ozone oxidation of endocrine disruptors and pharmaceuticals in surface water and wastewater[J]. Ozone:Science & Engineering, 2007, 28(6): 445-460. [11] 马富军, 李新洋, 宗博洋, 等. 电-多相臭氧催化技术处理金刚烷胺制药废水[J]. 中国环境科学, 2018, 38(10): 3713-3719. doi: 10.3969/j.issn.1000-6923.2018.10.014 [12] MISHRA V S, MAHAJANI V V, JOSHI J B. Wet air oxidation[J]. Industrial & Engineering Chemistry Research, 1995, 34(1): 2-48. [13] 张宣娇, 孙羽, 刘明, 等. CeO2形貌结构对催化湿式空气氧化苯酚性能的影响[J]. 中国环境科学, 2020, 40(10): 4330-4334. doi: 10.3969/j.issn.1000-6923.2020.10.016 [14] SIMOND O, SCHALLER V, COMNINELLIS C. Theoretical model for the anodic oxidation of organics on metal oxide electrodes[J]. Electrochimica Acta, 1997, 42(13-14): 2009-2012. doi: 10.1016/S0013-4686(97)85475-8 [15] 周玉莲, 于永波, 黄湾, 等. 氧化石墨烯电催化高效降解有机染料RBk5[J]. 中国环境科学, 2019, 39(11): 4653-4659. doi: 10.3969/j.issn.1000-6923.2019.11.021 [16] RODOPULO A. K. Oxidation of tartaric acid in wine in the presence of heavy metal salts (activation of oxygen by iron)[J]. Izvestiia Akademii nauk SSSR. Seriia biologicheskaia, 1951, 3: 115-128. [17] 杨远秀, 姚创, 刘晖, 等. 磁性Fen+@GO非均相Fenton催化氧化亚甲基蓝[J]. 中国环境科学, 2018, 38(5): 1719-1726. doi: 10.3969/j.issn.1000-6923.2018.05.014 [18] CHAO W, GUO C Y, HAI C, et al. Degradation of norfloxacin by hydroxylamine enhanced fenton system: Kinetics, mechanism and degradation pathway[J]. Chemosphere, 2021, 270: 129408. doi: 10.1016/j.chemosphere.2020.129408 [19] WAN Z, WANG J L. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst[J]. Journal of hazardous materials, 2017, 324(B): 653-664. [20] LI J, LI X, HAN J, et al. Mesoporous bimetallic Fe/Co as highly active heterogeneous Fenton catalyst for the degradation of tetracycline hydrochlorides[J]. Scientific Reports, 2019, 9(1): 1-11. doi: 10.1038/s41598-018-37186-2 [21] LIU J, DU Y, SUN W, et al. et al., Preparation of new adsorbent-supported Fe/Ni particles for the removal of crystal violet and methylene blue by a heterogeneous Fenton-like reaction[J]. RSC Advances, 2019, 9(39): 22513-22522. doi: 10.1039/C9RA04710G [22] WU D, FENG Y, MA L. Oxidation of azo dyes by H2O2 in presence of natural pyrite[J]. Water, Air, & Soil Pollution, 2013, 224(2): 1-11. [23] CHEN H, ZHANG Z, YANG Z, et al. Heterogeneous fenton-like catalytic degradation of 2, 4-dichlorophenoxyacetic acid in water with FeS[J]. Chemical Engineering Journal, 2015, 273: 481-489. doi: 10.1016/j.cej.2015.03.079 [24] GUO C, TONG X, GUO X Y. Solvothermal synthesis of FeS2 nanoparticles for photoelectrochemical hydrogen generation in neutral water[J]. Materials Letters, 2015, 161: 220-223. doi: 10.1016/j.matlet.2015.08.112 [25] STUCKI, J. W. The quantitative assay of minerals for Fe2+ and Fe3+ using 1, 10-phenanthroline: II. A photochemical method[J]. Soil Science Society of America Journal, 1981, 45(3): 638-641. doi: 10.2136/sssaj1981.03615995004500030040x [26] LI D, ZHU X, ZHONG Y, et al. Abiotic transformation of hexabromocyclododecane by sulfidated nanoscale zerovalent iron: Kinetics, mechanism and influencing factors[J]. Water Research, 2017, 121: 140-149. doi: 10.1016/j.watres.2017.05.019 [27] MORALES-GALLARDO M V, AYALA A M, MOU P, et al. Synthesis of pyrite FeS2 nanorods by simple hydrothermal method and its photocatalytic activity[J]. Chemical Physics Letters, 2016, 660: 93-98. doi: 10.1016/j.cplett.2016.07.046 [28] WANG Z. , DU Y., ZHOU P., et al. Strategies based on electron donors to accelerate Fe (III)/Fe (II) cycle in Fenton or Fenton-like processes[J]. Chemical Engineering Journal, 2023, 454: 140096. doi: 10.1016/j.cej.2022.140096 [29] 曾令玉. 黄铁矿(FeS2)异相 Fenton 反应催化氧化对硝基酚的研究[D]. 武汉: 华中科技大学, 2019. [30] EGGLESTON, CARRICK M, EHRHARDT, et al. Surface structural controls on pyrite oxidation kinetics: An XPS-UPS, STM, and modeling study[J]. American Mineralogist, 2015, 81(9-10): 1036-1056. [31] CAI Y F, PAN Y G, XUE J Y, et al. Comparative XPS study between experimentally and naturally weathered pyrites[J]. Applied Surface Science, 2010, 255(21): 8750-8760. [32] 吕源财. 纳米零价铁钯/微生物联合体系降解2, 2’, 4, 4’-四溴联苯醚的研究[D]. 广州: 华南理工大学, 2016. [33] ZHANG Y, ZHOU Z, WEN F, et al. A flower-like MoS2 decorated MgFe2O4 nanocomposite: Mimicking peroxidase and colorimetric detection of H2O2 and glucose[J]. Sensors and Actuators B-Chemical, 2018, 275: 155-162. doi: 10.1016/j.snb.2018.08.051 [34] REN B, MIAO J F, XU Y L, et al. A grape-like N-doped carbon/CuO-Fe2O3 nanocomposite as a highly active heterogeneous Fenton-like catalyst in methylene blue degradation[J]. Journal of Cleaner Production, 2019, 240: 118143. doi: 10.1016/j.jclepro.2019.118143 [35] WEN X J, NIU C G, HUANG D W, et al. Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst[J]. Journal of Catalysis, 2017, 355: 73-86. doi: 10.1016/j.jcat.2017.08.028 [36] HUBICKA, URSZULAA, ŻMUDZKI P, et al. Photodegradation assessment of ciprofloxacin, moxifloxacin, norfloxacin and ofloxacin in the presence of excipients from tablets by UPLC-MS/MS and DSC[J]. Springer International Publishing, 2013, 7(1): 133. [37] WANG G, ZHAO D Y, KOU F Y, et al. Removal of norfloxacin by surface Fenton system (MnFe2O4 /H2O2): Kinetics, mechanism and degradation pathway[J]. Chemical Engineering Journal, 2018, 351: 747-755. doi: 10.1016/j.cej.2018.06.033 [38] LIU C, NANABOINA V, KORSHIN G V, et al. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater[J]. Water Research, 2012, 46(16): 5235-5246. doi: 10.1016/j.watres.2012.07.005 [39] ZHOU T, ZOU X L, WU X H, et al. Synergistic degradation of antibiotic norfloxacin in a novel heterogeneous sonochemical Fe0/tetraphosphate Fenton-like system[J]. Ultrasonics Sonochemistry, 2017, 37(1): 320-327. [40] WU D, CHEN Y, ZHANG Y, et al. Ferric iron enhanced chloramphenicol oxidation in pyrite (FeS2) induced Fenton-like reactions[J]. Separation and Purification Technology, 2015, 154: 60-67. doi: 10.1016/j.seppur.2015.09.016 [41] 周 洋. 基于黄铁矿的非均相类-Fenton反应高效降解邻苯二甲酸二乙酯的机制研究[D]. 芜湖: 安徽师范大学, 2019. [42] ZHANG W J, GAO H Y, HE J J, et al. Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: Optimization of operating conditions and degradation pathway[J]. Separation and Purification Technology, 2017, 172: 158-167. doi: 10.1016/j.seppur.2016.08.008 [43] CHE H, BAE S, LEE W, et al. Degradation of trichloroethylene by Fenton reaction in pyrite suspension[J]. Journal of Hazardous Materials, 2011, 185(2/3): 1355-1361. -




 下载:
下载: