-
随着全球经济的快速发展,城市固体废物的排放也带来了越来越大的环境压力。据统计,我国2020年城市固体废物排放量为2.35×108 t[1]。焚烧是全球应用最广泛的固废处理方法之一,通过焚烧不仅能大幅降低固废的体积和质量[2],而且焚烧所产生的热能还可用于发电,其在中国城市生活垃圾处理中的占比已从2004年的5.55%急剧上升至2020年的62.13%。尽管焚烧在垃圾处理方面表现良好,但其会产生次级废物——垃圾焚烧飞灰。飞灰的成分复杂,且在不同时间、地点所产生的飞灰的成分差距较大[3],主要包括重金属盐、硫化物、硝酸盐、活性炭和二恶英[4]等。此外,在焚烧过程中通常需要添加熟石灰来吸附焚烧过程中的酸性气体和痕量有机物,这使飞灰具有较高的碱度[5]。飞灰中含有剧毒的二恶英和易浸出的重金属,因此为危险废物[6],必须加以谨慎处置。2021年12月10日,生态环境部等多部门联合印发了《“十四五”时期“无废城市”建设工作方案》[7],提出要强化固体废物综合利用水平和无害化处置能力,推进“无废城市”建设。研究飞灰的无害化处理和资源化利用变得尤为重要。
目前,飞灰的处理工艺和方法主要包括固化稳定化[8]、热处理[9]和分离浸出[10]。洗涤是飞灰处理常用的预处理方法,水洗可去除飞灰中高浓度的可溶性盐。HU等[11]的研究表明,在液固比为2.5~3时,用清水洗涤几乎能去除飞灰中所有的可溶性盐类,但水洗后重金属的浸出率有所升高;YANG等[12]在液固比为10时用去离子水洗涤,使飞灰中氯化物的比例由16.6%降为1.2%。LI等[13]的研究发现,洗涤能使飞灰的孔径和比表面积增大,并提高其吸附能力。水洗后,超过90%的Pb和Zn依然留存在水洗灰中,且赋存形态基本不变[14]。酸洗可溶解飞灰中的氢氧化物和碳酸化合物,从而破坏飞灰的固体结构[15]。ZHAO等[16]通过硝酸酸洗能去除飞灰中87.97%的Cd;林涛等[17]采用盐酸能洗出飞灰中95%以上的Zn、Pb、Cd和81.38%的Cu;HUANG等[18]采用水洗和酸洗联用技术,能去除飞灰中86%的Pb、98%的Zn、96%的Cd和62%的Cu;孙福成等[19]将飞灰与酸性废水联合处理,使飞灰中Pb和Cd的浸出浓度降低了90%以上。这些研究结果表明,酸洗能使飞灰中的大部分可溶性盐和重金属从固相转移至液相,便于后续的回收处理。
采用电沉积技术处理含重金属的洗涤废液,是通过废液中金属离子的电迁移,使其在阴极发生电化学还原反应而析出金属的过程,具有回收处理成本低、二次污染小的特点。彭腾等[20]采用柠檬酸浸出-电沉积联用技术处理废锂电池,使废电池中94.84%的钴得以回收;李子良等[21]应用电沉积技术处理酸性含汞废液,其中98%的汞能得到回收。陈熙等[22]认为,在电沉积过程中,阳极的氧化反应会产生氧气,溶液中氧气浓度的升高会腐蚀阴极表面沉积的重金属单质,造成重金属单质的返溶现象,从而影响重金属的处理效率。近年来,电沉积技术越来越多地被用于催化剂制备和高浓度金属废水处理,但涉及用于飞灰中重金属回收利用的研究却很少。
本研究采用酸洗-电沉积联用技术,用以去除并回收飞灰中的Zn、Pb、Cu和Cd 4种重金属。以硝酸作为酸洗试剂,确定合适的洗涤工艺,并分析飞灰酸洗前后的微观形貌、晶相、重金属存在形态等的变化。通过电沉积技术以回收酸洗废液中的Zn、Pb、Cu和Cd,进而评估酸洗-电沉积技术应用于飞灰中重金属回收的可行性,以期为飞灰无害化处理和资源回收提供参考。
-
垃圾焚烧飞灰样品由位于中国天津的一家垃圾焚烧厂提供。飞灰通过200目网筛筛分,经过烘箱105 ℃烘干,使用前保持在60 ℃。所有试剂均为分析纯。
用扫描电子显微镜 (SEM,S-4800,株式会社 日立制作所) 表征飞灰样品的表面形貌,通过X射线衍射仪 (XRD,D8-FOCUS,德国布鲁克AXS有限公司) 测量飞灰样品的晶相,并采用专业软件 (MDI Jade 6.5) 对衍射花样进行分析。飞灰中重金属离子在酸洗前后的存在形态采用BCR (European Community Bureau of Reference) 顺序提取程序[23]进行分析。通过X射线荧光光谱仪 (XRF,S4 Pioneer,日本株式会社理学公司) 测定飞灰样品的元素组成,通过电感耦合等离子体发射光谱 (ICP-OES,Thermo iCAP 7400,美国Thermo Fisher Scientific公司) 测定样品中Zn、Pb、Cu和Cd元素的质量浓度。采用王水消解法处理并测定飞灰中主要重金属的质量分数,采用醋酸缓冲溶液法 (HJ/T 300-2007) [24]评估飞灰的浸出毒性。
-
称取5.0 g干燥飞灰放入100 mL杯中,按设定的液固比和浓度加入配置好的硝酸溶液,酸洗过程中持续搅拌。待洗涤终止后,采用孔径为0.45 μm的滤膜进行过滤,收集澄清滤液备用。采用电感耦合等离子体发射光谱 (ICP-OES,Thermo iCAP 7400,美国Thermo Fisher Scientific公司) 测定浸出液中Zn、Pb、Cu和Cd元素的质量浓度,以重金属的浸出去除率 (浸出液中重金属的总质量与原始飞灰试样中重金属的总质量的比值) 表示酸洗浸出效果。探究硝酸浓度、液固比、洗涤时间对酸洗浸出效果的影响,实验条件的设计如下。
1) 浓度影响的实验条件为:在液固比为10和洗涤时间为60 min时,取硝酸浓度为0.5、1.0、1.5、2.0、2.5、3.0 mol·L−1。
2) 液固比影响的实验条件为:在硝酸浓度为2 mol·L−1和洗涤时间为60 min时,取液固比为5、10、15、20、25、30。
3) 在洗涤时间影响的实验中,选择硝酸浓度为2 mol·L−1和液固比为10,考察洗涤时间为5、30、60、120、240、360、480 min时的洗涤效果。
-
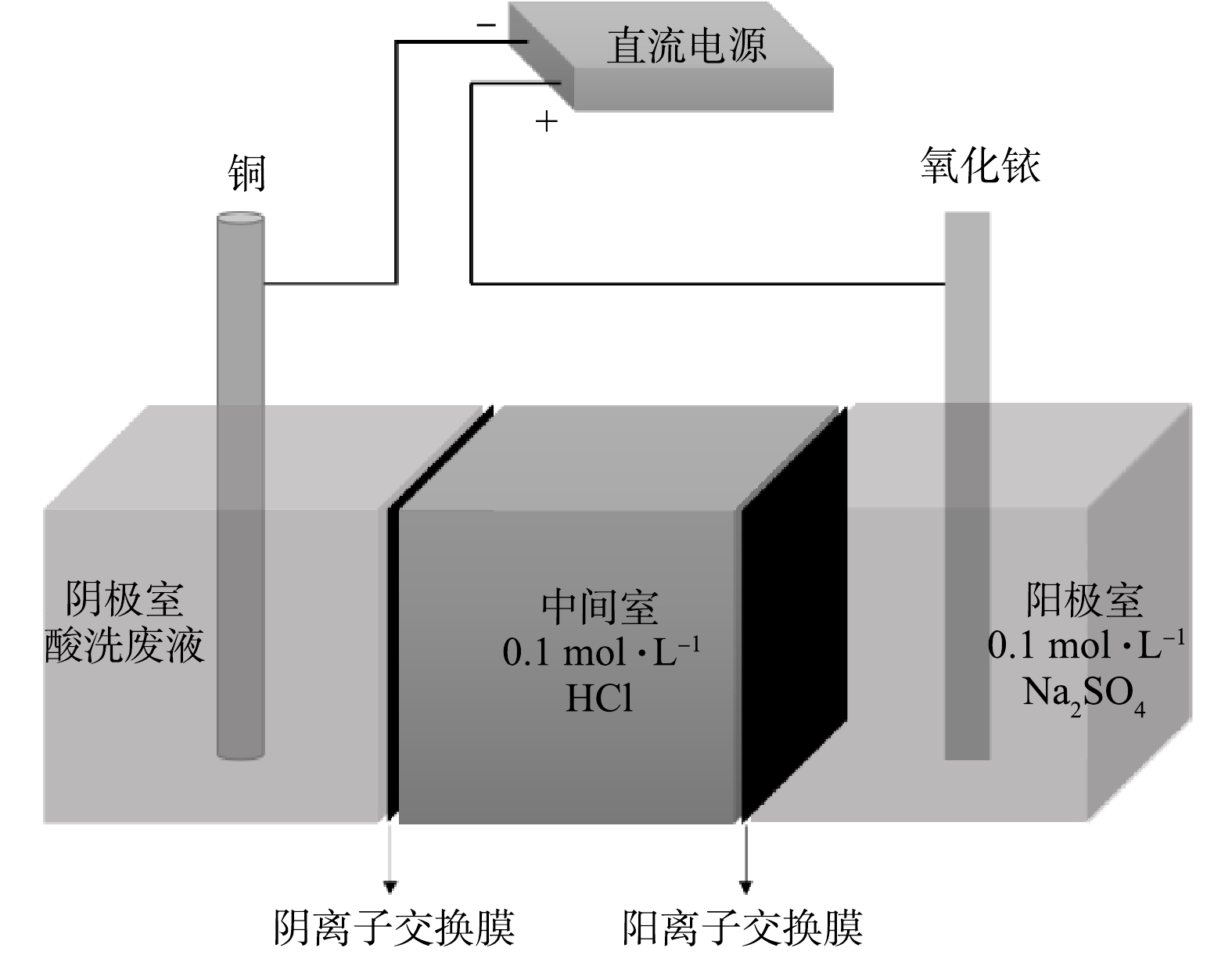
电沉积装置结构示意图如图1所示,在中间固定阴离子交换膜 (G1204,天津市兰力科化学电子高技术有限公司) 和阳离子交换膜 (G0014,天津市兰力科化学电子高技术有限公司) 将电解槽分为阴极室、中间室和阳极室。采用由阴、阳离子交换膜分隔的三室电解槽结构,其目的是为了阻隔废水中的阴离子通过离子迁移转移到阳极,从而防止飞灰中含有的大量氯盐在洗涤进入溶液后,电解时在阳极发生副反应产生氯气而污染环境;同时也能防止阳极区生成的O2迁移至阴极区而腐蚀阴极表面的金属沉积物。在电沉积处理含重金属的废水时,将酸洗滤液置入阴极室,中间室加入0.1 mol·L−1 HCl溶液,阳极室加入0.1 mol·L−1 Na2SO4溶液。在电沉积期间持续搅拌以保持传质。氧化铱板用作阳极,直径为6 mm的铜棒用作阴极。直流电源通电电解,所控制的电解参数主要包括电压和电解时间等。
-
表1所示是飞灰元素组成的分析结果。可以看出,飞灰的主要元素组成是Ca、Cl、S、K、Si、Na和Fe等。飞灰中的Ca成分主要源自为吸附焚烧烟气中的酸性气体和痕量有机物而注入的过量石灰。同时,塑料制品、厨余垃圾和其他含氯组分在焚烧过程中存在挥发-冷凝富集现象,因此飞灰中含有较多的可溶性氯盐[25]。表2为飞灰中重金属的全量消解测试结果,结合表1数据可以看出,飞灰中Zn、Pb和Cu的质量分数较高,且毒性较高的Cd也高于检出限。对飞灰中的重金属进行浸出实验的结果显示,浸出液中Zn、Pb、Cu和Cd的质量浓度分别达到9.971、1.030、0.620和0.340 mg·L−1,其中Pb和Cd明显超过《生活垃圾填埋场污染控制标准》(GB 16889-2008)[26]规定的限值0.25和0.15 mg·L−1,说明该飞灰具有较强的重金属浸出毒性。因此,本研究主要关注Zn、Pb、Cu和Cd 4种重金属的浸出情况。
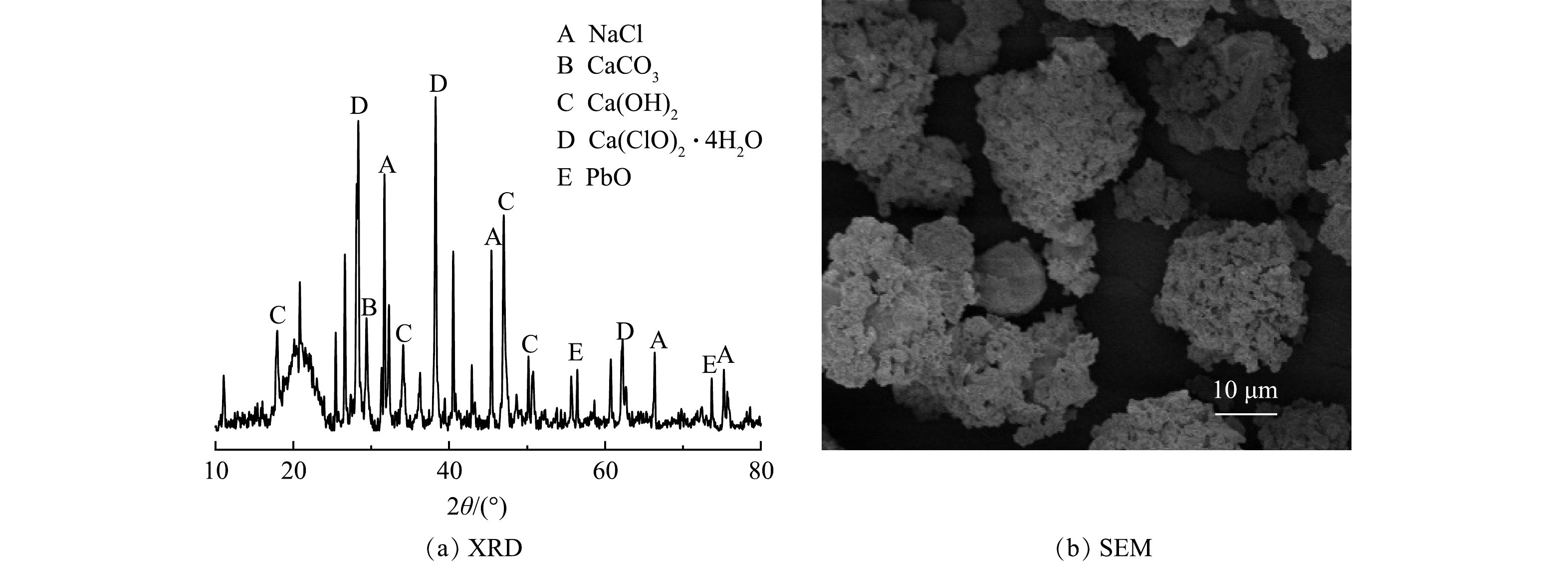
采用XRD技术对飞灰的矿物学成分进行分析,其结果如图2(a)所示。可以看出,飞灰具有CaCO3和Ca(OH)2相,这使得飞灰具有极强的酸缓冲能力。然而,飞灰中非晶态物质占比约为50%~55%[27],高占比的无定形物质增强了XRD衍射花样的噪声背景,掩盖了一些晶体衍射的特征峰。因此,通过XRD测定结果很难分析和识别飞灰中重金属的具体矿物相种类。飞灰的SEM结果如图2(b)所示。从中可看出,无定形颗粒松散地排列,颗粒大小不一,呈近似球状,表面疏松多孔,这将大大增加飞灰的比表面积,从而使飞灰中的可溶性盐和重金属具有易浸出的特性。
-
在飞灰的洗涤预处理中,常用的无机酸包括HCl、HNO3和H2SO4等。由于飞灰酸洗产生的Pb2+和大量Ca2+能与SO42−反应生成难溶物而覆盖飞灰颗粒表面,阻碍洗涤反应的进一步进行。因此,H2SO4作为浸出试剂是不适宜的。HCl和HNO3均具有较高的酸洗去除飞灰中重金属的能力,特别是高浓度的HCl,Cl−可与金属离子形成配合物,如[PbCl4]2−等,这有利于重金属浸出率的提高。但配合物通常带有较多的负电荷,在阴极表面所受到的电场排斥力较大。同时,相较于简单金属离子,配合物因具有较低的能态而更加稳定,放电时需要克服更高的活化能垒,使电沉积所需的电压升高,最终导致金属电沉积回收成本提高。因此,选用盐酸作为浸出剂对浸出废液的电沉积处理不利。而高浓度的HNO3具有一定的氧化性,可以洗出飞灰中较难溶的重金属。因此,选择硝酸作为浸出剂进行研究。
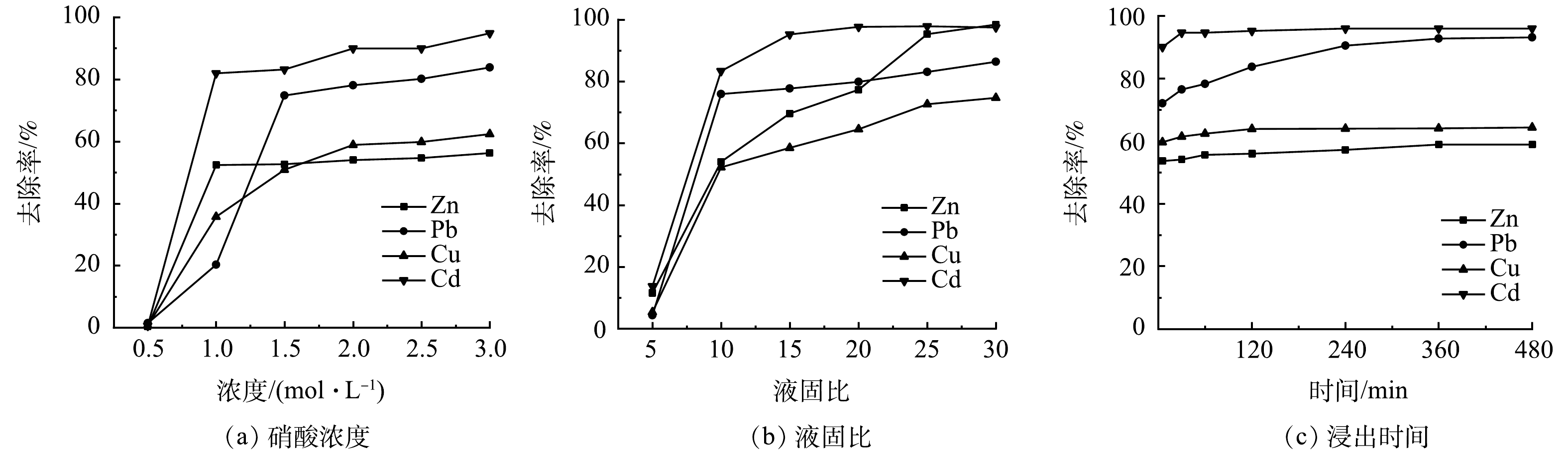
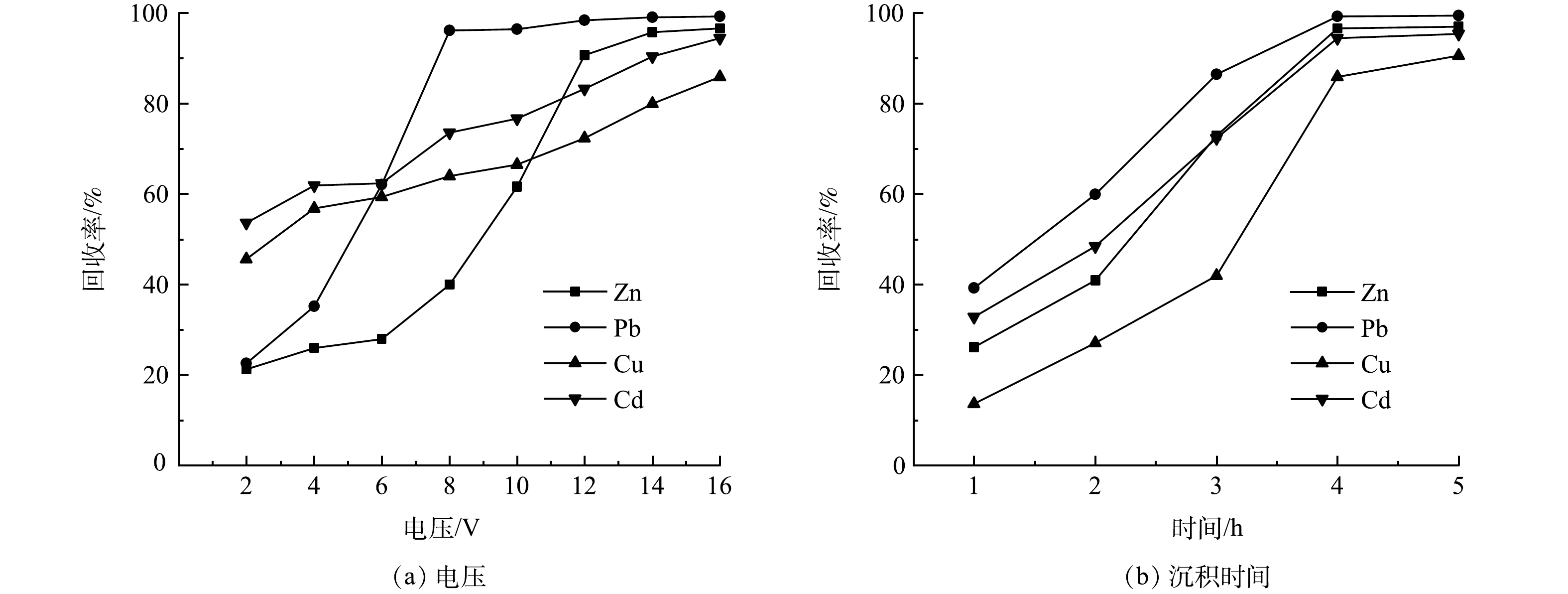
图3所示是不同酸洗条件对重金属洗涤去除率影响的测定结果。从图3(a)硝酸浓度的影响中可以看出,当用0.5 mol·L−1的HNO3浸渍洗涤时,飞灰中各重金属的浸出去除率均较低,仅为1%左右,这是因为此时所用HNO3的浓度较低。在洗涤的初期,其中的H+均已被飞灰中高质量分数的CaCO3、Ca(OH)2等所消耗,飞灰颗粒的结构未被破坏,包裹在颗粒内部的金属离子外迁浸出困难,最终导致浸出去除率较低。这一结果也说明,飞灰具有较强的酸缓冲能力,想消耗较少酸从飞灰中彻底洗出重金属几乎是不可能的。在硝酸浓度为1.0 mol·L−1时,各重金属的去除率均得到显著提高,其中Cd的去除率已达80%以上。此时飞灰颗粒的表层结构已遭到酸的溶解破坏,这对颗粒内部金属离子的外迁浸出是有利的。随着硝酸浓度的进一步提高,重金属的去除率也在不断提高,在硝酸浓度提高至2.0 mol·L−1后,重金属去除率的增速已明显变缓,再提高硝酸的浓度对飞灰中重金属的溶出已意义不大。考虑到飞灰处理的效果和硝酸的消耗量,选择浓度为2.0 mol·L−1的硝酸作为酸洗剂较为合适。
液固比是影响酸洗效果的另一重要因素。如图3(b)所示,当液固比在5~30范围内,随着液固比的增加,重金属的去除率起初急剧增加,然后缓慢增加并最终趋于平稳。当液固比超过10之后,液固比的增加对Zn浸出的影响最为明显,对Cu浸出的影响次之,而对Pb和Cd浸出的影响较小,说明Zn和Cu的浸出主要受酸溶解过程控制。在液固比为30处,Zn、Pb、Cu和Cd的去除率分别达到98.44%、86.35%、74.66%和97.46%。综合考虑重金属的浸出效果和硝酸的消耗量,液固比为25应是较为合适的洗涤浸出条件。
洗涤时间对重金属浸出去除率的影响如图3(c)所示,在洗涤时间达到60 min时,Zn、Cu和Cd的去除率已基本稳定,即其溶解基本达到平衡,而此时Pb的浸出去除率仍在显著地增加,其达到稳定的浸出时间大致在360 min之后。根据JIAO等[28]的浸出试验结果,Cu和Zn的沉淀-吸附平衡与飞灰中含钙化合物显著相关,在强酸性条件下碳酸钙等的溶解将导致Cu和Zn的快速浸出。同时,CAVIGLIA等[29]的浸出动力学研究表明,Pb的扩散过程非常缓慢,这可能是Pb浸出达到平衡需要更长时间的原因。
在硝酸浓度为2 mol·L−1、液固比为25和洗涤时间为60 min条件下,酸洗能去除飞灰中95.26%的Zn、83.06%的Pb、72.62%的Cu和97.85%的Cd。延长洗涤时间至360 min,可将Pb的去除率提升至85%以上。林涛等[17]采用盐酸洗涤飞灰,使Pb生成了配合物[PbCl4]2−,这虽能进一步提高Pb的去除率,但由于[PbCl4]2−带有负电荷且比简单金属离子更加稳定,在后续浸出废液的电沉积处理时,将极大地增加Pb在阴极表面上析出回收的难度。因此,采用硝酸洗涤更为合适。
-
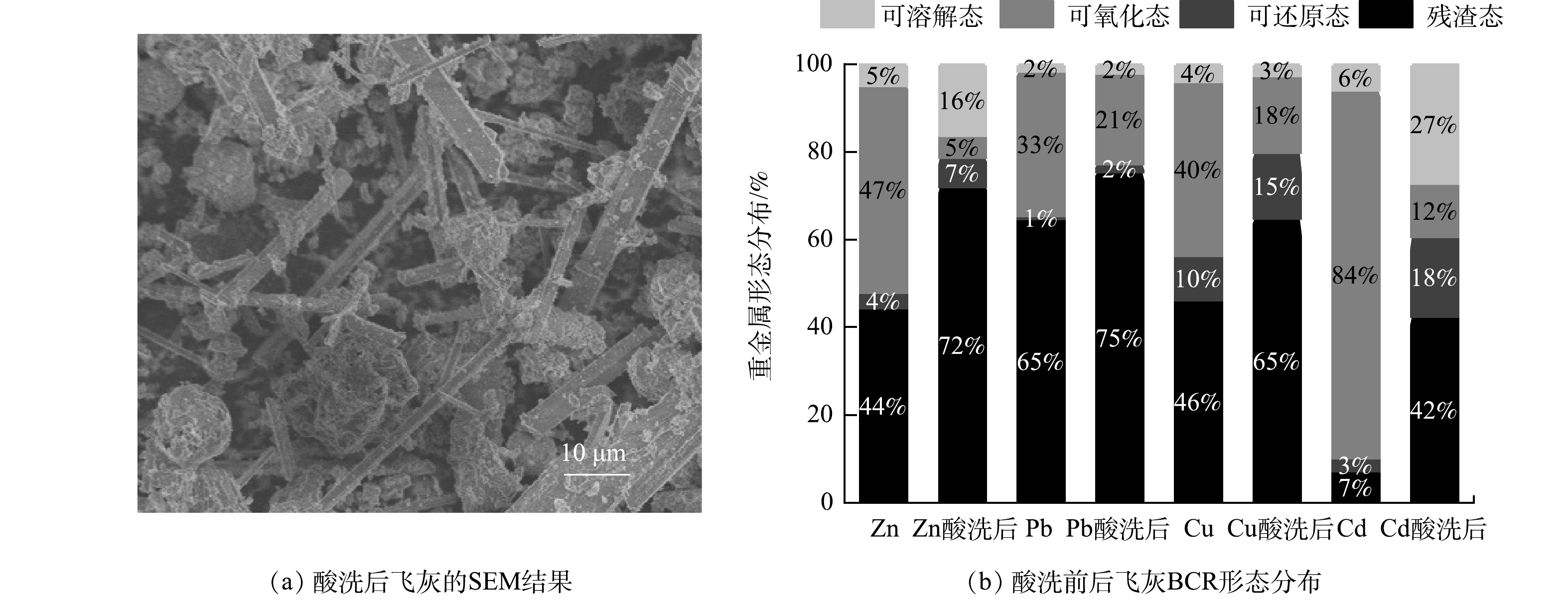
酸洗后飞灰的微观形貌如图4(a)所示,对比图2(b)酸洗前飞灰的微观形貌可以看出,酸洗后飞灰中疏松多孔的非晶态物质大部分得到溶解,粒径显著缩小,使棒状或条状晶体颗粒充分暴露出来。这进一步表明飞灰的成分较为复杂,酸洗能将包裹飞灰颗粒的CaCO3和Ca(OH)2外壳去除,同时也能去除飞灰中大量的水溶性离子 (如Cl−、Na+等) ,将飞灰中的金属组分释放出来,从而显著降低洗后飞灰的浸出毒性,为垃圾焚烧飞灰的资源化利用提供了可能。
BCR顺序提取将飞灰中重金属组分按其浸出的难易程度分为可溶解态>可氧化态>可还原态>残渣态,其中可溶解态和可还原态是对环境危害最大的形态,残渣态是最稳定的形态,也是最不易被释放出来的形态。从图4(b)所示的飞灰酸洗前后BCR形态分布图中可以看出,在酸洗前飞灰中可溶解态占比较低,这也进一步说明了飞灰具有极强的酸缓冲能力,仅靠水和弱酸很难分离飞灰中的重金属。可氧化态占了较大的比重,Zn、Pb和Cu的可氧化态占比分别为47%、33%和40%,而Cd则达到了84%。这说明未处理的飞灰极易浸出,对环境危害极大。而在酸洗处理之后,这一比重显著下降,残渣态成分明显提高,Zn、Pb、Cu和Cd的残渣态成分分别占到总量的72%、75%、65%和42%。这说明酸洗对飞灰有很强的净化效果,使得易浸出组分大幅降低,残渣态组分占比升高。易浸出组分被分离去除或转化为更加稳定的形态,使酸洗后的飞灰更具稳定性,浸出能力降低,对环境的危害减小。
酸洗前后飞灰的浸出毒性测试结果如表3所示。可以看出,酸洗前飞灰浸出液中Pb和Cd的质量浓度均显著高于《生活垃圾填埋场污染控制标准》(GB 16889-2008)[26]规定的浸出液质量浓度限值,而酸洗后飞灰中各重金属浸出质量浓度大幅降低,均低于限值。酸洗后的飞灰可直接进入生活垃圾填埋场进行填埋处理,也可作为非危废固体进行资源化开发利用。
-
为评估电沉积技术用于回收酸洗废液中重金属的可行性,将飞灰酸洗废液用于电沉积处理,所用酸洗废液的Zn、Pb、Cu和Cd质量浓度分别为95.08、50.79、13.23和5.61 mg·L−1。图5(a)是在电沉积时间为4 h时电压对金属回收率影响的测定结果。可以看出,各重金属离子的回收率与电压呈正相关,电压越高,回收率也越高。由于飞灰的组成较为复杂,其酸洗废液也具有较复杂的组成,酸洗废液中往往含有影响重金属回收率的杂质离子,如溶液中Cl−浓度过高会使氧化还原电位升高,并抑制Zn的析出[30],这将导致在低电压时Zn的回收率极低,如在电压为6 V时仅能回收27.89%。在酸洗废液中含有大量的H+,并且H+的析出能力较强,其会与重金属离子在阴极竞争电子而不利于金属的析出,这也会降低重金属的回收率。随着电沉积反应的进行,酸洗废液中金属离子的质量浓度不断降低,致使析出电位不断提高,当施加电压不足以继续析出金属时,反应达到平衡,只有进一步提高电压,电沉积反应才能继续进行。在电压为8 V时,Pb的回收率可高达96.15%,而后基本保持平稳。Zn在电压为6~12 V区间内回收率迅速升高,从27.89%提高至90.68%,这表明电压提高后,在较强电场作用下金属离子的电迁移速率加快,克服了Cl−对Zn析出的抑制作用。在电压为14 V时,Zn、Pb、Cu和Cd的回收率分别达到了95.80%、99.04%、79.95%和90.37%,处理后废液中Zn、Pb、Cu和Cd的残余质量浓度分别为3.99、0.49、2.65和0.54 mg·L−1,仍不能满足排放要求,若继续提高电压进行更长时间的电沉积将导致处理费用急剧增加,可行的方案是将处理后的废液用于酸洗剂的配制,进入下一批次的飞灰洗涤而得到循环利用。
在电压为16 V时,电沉积时间对重金属离子回收率的影响如图5(b)所示。可以看出,重金属离子回收率均随沉积时间的延长而升高,在电沉积时间为4 h时基本到达稳定,表明析出反应已基本达到平衡。电沉积反应刚开始时,废液中重金属离子质量浓度较高,电极表面的活性位点也较多,这有利于金属离子电沉积的进行。但实际情况中回收率均不高,一方面原因是沉积反应时间较短,另一方面可能是因为酸洗废液中含有大量的H+。H+扩散传质速率较快,还原电位较低,析氢副反应与重金属离子的析出反应存在竞争,导致重金属离子还原析出缓慢。随着时间的延长,由于H2的析出而使溶液中H+浓度逐渐降低,析氢副反应减少,重金属离子的沉积反应速率得到提高,这与在2~4 h时间范围内重金属离子回收率的增加随时间的延长而大致是加快的结果是一致的。
-
1) 硝酸洗涤能有效去除飞灰中的重金属,其去除率随硝酸浓度、液固比、洗涤时间的增加而提高。在硝酸浓度为2 mol·L−1、液固比为25和浸出洗涤时间为60 min时,酸洗能去除飞灰中95.26%的Zn、83.06%的Pb、72.62%的Cu和97.85%的Cd。
2) 酸洗使飞灰中的重金属浸出毒性降低1~2个数量级,使其满足《生活垃圾填埋场污染控制标准》(GB 16889-2008)要求,可以直接进入生活垃圾填埋场进行填埋,也可作为非危废固体进一步资源化开发利用,这对“无废城市”的建设和循环经济都具有重要意义。
3) 采用三室电解槽体系,在电压为14 V时,电沉积4 h可回收酸洗废液中95.80%的Zn、99.04%的Pb、79.95%的Cu和90.37%的Cd。处理后的废液仍含有一定质量浓度的重金属,其可用于酸洗剂的配制而得到循环利用。
酸洗-电沉积联用技术回收垃圾焚烧飞灰中的重金属
Recovery of heavy metals from municipal solid waste incineration fly ash by the combined acid washing and electrodeposition technology
-
摘要: 垃圾焚烧飞灰中的重金属浸出能力强、毒性大,属于危险废物,若处置不当将会对环境造成严重污染。采用酸洗-电沉积联用技术,考察硝酸浓度、液固比和酸洗时间对重金属去除率的影响,研究电沉积电压和时间对酸洗废液中重金属回收率的影响,同时评估处理后飞灰的浸出毒性。结果表明,在浓度为2 mol·L−1、液固比为25的硝酸中浸渍洗涤60 min条件下,酸洗能成功去除飞灰中95.26%的Zn、83.06%的Pb、72.62%的Cu和97.85%的Cd;在电压为14 V,电沉积4 h的条件下可回收酸洗废液中95.80%的Zn、99.04%的Pb、79.95%的Cu和90.37%的Cd。对Zn、Pb、Cu、Cd的连续提取和浸出毒性测试表明,处理后飞灰易浸出组分占比降低,残余态物质占比提高,重金属浸出质量浓度符合《生活垃圾填埋场污染控制标准》(GB 16889-2008)要求,可直接进行填埋或作为非危废固体进行资源化利用。本研究结果可为飞灰中重金属脱除和回收利用提供参考。Abstract: The municipal solid waste incineration fly ash is classified as hazardous waste and will cause serious pollution to the environment if disposed improperly, owing to its strong leaching ability and high toxicity of heavy metals. Herein, the combined acid washing and electrodeposition technology was used to study the effects of the nitric acid concentration, liquid-solid mass ratio, acid washing time on the removal rate of heavy metals, and the effects of electrodeposition voltage and time on the recovery rate of the heavy metals from the acid washing waste solution, as well as to evaluate the leaching toxicity of the treated fly ash. The results showed that 95.26% of Zn, 83.06% of Pb, 72.62% of Cu and 97.85% of Cd were successfully removed from fly ash after immersing and washing for 60 min in nitric acid with concentration of 2 mol·L−1 and liquid-to-solid ratio of 25. Meanwhile, 95.80% of Zn, 99.04% of Pb, 79.95% of Cu and 90.37% of Cd in the acid washing waste solution were recovered at a voltage of 14 V and 4 h of electrodeposition. The continuous extraction and leaching toxicity tests of Zn, Pb, Cu and Cd demonstrated that the content of easily leachable components of the washed fly ash decreased, the proportion of residual state materials increased, and the leaching mass concentration of heavy metals met the requirements of “Pollution Control Standards for Domestic Waste Landfills” (GB 16889-2008), as thus, the acid washed fly ash can be directly landfilled or used as a non-hazardous waste solid for resource utilization. This study can provide a reference for the removal and recovery of heavy metals from fly ash.
-
蓝藻水华在世界各地许多湖库水体频繁发生,其代谢产生的微囊藻毒素(microcystins,MCs)引发的水源地和饮用水安全问题长期以来受到各地政府和学者的持续关注,至今已报道检测出百余种MCs的异构体。其中,Microcystin-LR是一类具强致癌效应的肝毒素,天然水体中Microcystin-LR大部分以细胞结合态、少部分以溶解态存在,一般条件下极难降解,可通过水生生物富集和食物链进入人体,对健康造成严重威胁。目前,在世界卫生组织(WHO)和我国《生活饮用水卫生标准》(GB 5749-2012)中,Microcystin-LR是唯一被明确管制的MCs指标,其最高浓度限值均为1 μg·L−1,因此,对水中Microcystin-LR的去除或无害化降解成为研究热点。已报道水中Microcystin-LR的去除方法包括物理吸附法[1-2]、化学氧化法[3-4]、微生物降解法等[5-6]。物理吸附(如活性炭)对于低浓度污染去除效率高,但无法从根本上去除Microcystin-LR,并存在二次污染的潜在风险;化学氧化法(如臭氧、高锰酸钾等)反应速度快,但矿化率低,反应过程可能产生具诱发癌变的基因毒性中间副产物;微生物降解法温和安全,但反应时间长、稳定可控性差。近十多年来对于水中难降解微污染有机物的研究,光催化技术因其具有降解高效彻底、环境友好等优点在水处理领域发展迅速[7],包括用于降解水中Microcystin-LR的研究也逐渐深入。
自1972年起,FUJISHIMA [8]发现,在TiO2电极上将水光解产生氢气的现象后,TiO2日益成为最具发展前景的半导体光催化剂,引发了广泛研究兴趣,并在环境污染物降解、清洁能源制取等多领域取得良好的研究进展[7,9]。但由于TiO2光谱响应范围窄(仅对紫外光响应)、光生电子-空穴对(e−-h+)易复合等缺点的制约,无法满足其在环保领域提升高效节能处理技术的发展需求。为促进TiO2光催化响应活性和效率,学者们对TiO2开展大量相关的掺杂元素(氮、硫等非金属,铜、铋等金属)改性与负载复合材料(硅藻土、活性炭)等研究,结果表明可不同程度提高可见光降解处理效率[10]。特别是氮元素掺杂对提高TiO2的可见光响应活性已从理论到对多种有机污染物降解实验效果被反复验证有效[11]。氧化石墨烯(GO)作为一种单层二维碳纳米材料,是碳原子以sp2杂化轨道组成的六角型平面结构,具有比表面积大、吸附性能好、表面富含多类含氧官能团,电荷迁移率高等特性,在光催化领域将其作为负载材料引入成为近年来关注热点。已证实GO负载不仅增强了材料的分散性、减缓了团聚现象,还能促进价带电子激发跃迁至导带,提高光生载流子的迁移速率,有效降低e−-h+的复合率,使光催化活性得到提高[12-13]。
目前关于GO负载改性TiO2的降解研究主要集中在染料废水的处理,在净水领域对微污染有机物的处理研究还较少见。因此,本研究基于氮掺杂改性TiO2(N-TiO2)在可见光下降解Microcystin-LR的前期研究基础[14],通过GO负载N-TiO2一步水热法合成N-TiO2/rGO复合材料,探究了二者协同效应进一步提高可见光催化Microcystin-LR的降解减毒和反应机理,深化改性TiO2的可见光催化方法理论,以期为蓝藻水华发生期水源地与水厂原水绿色高效去除Microcystin-LR污染提供参考。
1. 材料与方法
1.1 试剂与仪器
钛酸丁酯(分析纯,阿拉丁),氧化石墨烯(GO≥96.6%,图灵进化科技),无水乙醇、浓盐酸、尿素(分析纯,西陇化工股份有限公司),Microcystin-LR标样(Microcystin-LR>95%,中国科学院水生生物研究所),三氟乙酸、对苯二甲酸、乙二胺四乙酸二钠(分析纯,国药集团化学试剂有限公司),甲醇(色谱纯,国药集团化学试剂有限公司)。
场发射扫描电子显微镜(Regulus 8100,HITACHI),氮气吸附-脱附仪(BELSORP-Mini Ⅱ,MicrotracBEL),X射线粉末衍射仪(D8 Advance,Bruker),显微共聚焦拉曼光谱仪(DXR2xi,Thermo Scientific),傅里叶变换红外光谱仪(Nicolet iS10,Thermo Scientific),紫外-可见漫反射光谱仪(U-4100,HITACHI),高效液相色谱仪(Agilent 1260,Agilent),总有机碳分析仪(TOC-VCPH,Shimadzu)。
1.2 N-TiO2/rGO复合材料制备
取适量GO置于无水乙醇中,超声15 min使其分散均匀;将10 mL钛酸丁酯和50 mL无水乙醇缓慢滴入其中,搅拌混合均匀,再滴入适量尿素溶液,搅拌30 min后,滴加浓盐酸调节pH至2;再将10 mL H2O缓慢滴入,持续搅拌1 h。将混合液转移至100 mL水热反应釜中,置于电热鼓风干燥箱180℃反应12 h,冷却至室温,并用去离子水、无水乙醇洗涤,80 ℃干燥,研磨备用。另外,实验中用于对比的纯TiO2(不添加GO及尿素)和N-TiO2(不添加GO)的制备条件及流程与上述一致。
1.3 表征方法
采用扫描电子显微镜(SEM)观察材料微观形貌、颗粒结构与尺寸,扫描电压为5.0 kV,样品的真空度小于2.7×10−5 Pa;采用氮气吸附-脱附仪(BET)分析材料比表面积、孔容和孔径等信息;采用X射线粉末衍射仪(XRD)分析材料晶型结构,使用Cu-Kα射线(λ=0.154 06 nm),扫描范围为5°~80°;显微共聚焦拉曼光谱(Raman)用于研究材料的分子振动和分子结构;傅里叶变换红外光谱(FT-IR)用于分析材料的化学键和官能团结构,设置波长为400~4 000 cm−1,分辨率4 cm−1,扫描32次;紫外-可见漫反射光谱仪(UV-vis DRS)用于测试材料在紫外-可见光谱频区的吸收性能曲线,扫描波长范围200~800 nm,以BaSO4作参比。
1.4 光催化降解实验
1)不同材料光催化降解Microcystin-LR实验。为比较不同材料对Microcystin-LR降解效果的影响,实验设置TiO2、6%N-TiO2(N-TiO2)和6%N-TiO2/5%GO(N-TiO2/rGO)可见光下催化降解Microcystin-LR,其中光催化材料投加量为1 g·L−1,Microcystin-LR溶液初始浓度为1 mg·L−1。暗反应30 min达到吸附-脱附平衡后在氙灯可见光(300 W,420 nm<λ<800 nm)下反应90 min,期间每隔15 min取样,3 000 r·min−1离心5 min,上清液过0.22 μm滤膜后用高效液相色谱测定。
检测条件:色谱柱 SB-C18 ( 5 μm,4.6 mm×150 mm,Agilent 1260);流动相为 V (体积分数为0.1%三氟乙酸溶液):V (甲醇) = 30:70;柱温40℃;流速0.8 mL·min−1;检测波长239 nm;进样量10 μL。
2)自由基捕获实验。通过自由基捕获实验探究Microcystin-LR光催化降解过程中起主要作用的活性物种,分析降解机理。羟基自由基(·OH)清除剂为1 mmol·L−1的对苯二甲酸(PTA),光生空穴(h+)清除剂为1 mmol·L−1的乙二胺四乙酸二钠(EDTA-2Na)。通过比较含有清除剂和不含清除剂的三组实验中Microcystin-LR去除效果的差异来判断两种主要活性物种发挥的作用,以自由基的贡献率(式(1))来表示。
η=(η1−η2)/η1×100% (1) 式中:ƞ1为不含清除剂的Microcystin-LR降解率,%;ƞ2为含有清除剂(PTA或EDTA-2Na )的Microcystin-LR降解率,%;ƞ为贡献率,%。
3)降解动力学分析。采用L-H动力学模型(式(2))拟合Microcystin-LR的可见光催化降解过程,探究不同材料对反应速率的影响。
lnC=−kt+lnC0 (2) 式中:C0为Microcystin-LR溶液的初始质量浓度,mg·L−1;C为Microcystin-LR溶液的实时质量浓度,mg·L−1;k为降解速率常数,min−1;t为光催化反应时间,min。
4)降解矿化率分析。通过测定反应过程中总有机碳(TOC)的浓度变化,探究Microcystin-LR在光降解过程中的深度氧化矿化水平,以矿化率θ (式(3))来表示其矿化程度。
θ=(C0−C)/C0×100% (3) 式中:θ为矿化率,%;C0为Microcystin-LR溶液的初始TOC含量,mg·L−1;C为Microcystin-LR溶液的实时TOC含量,mg·L−1。
2. 结果与讨论
2.1 催化材料表征分析
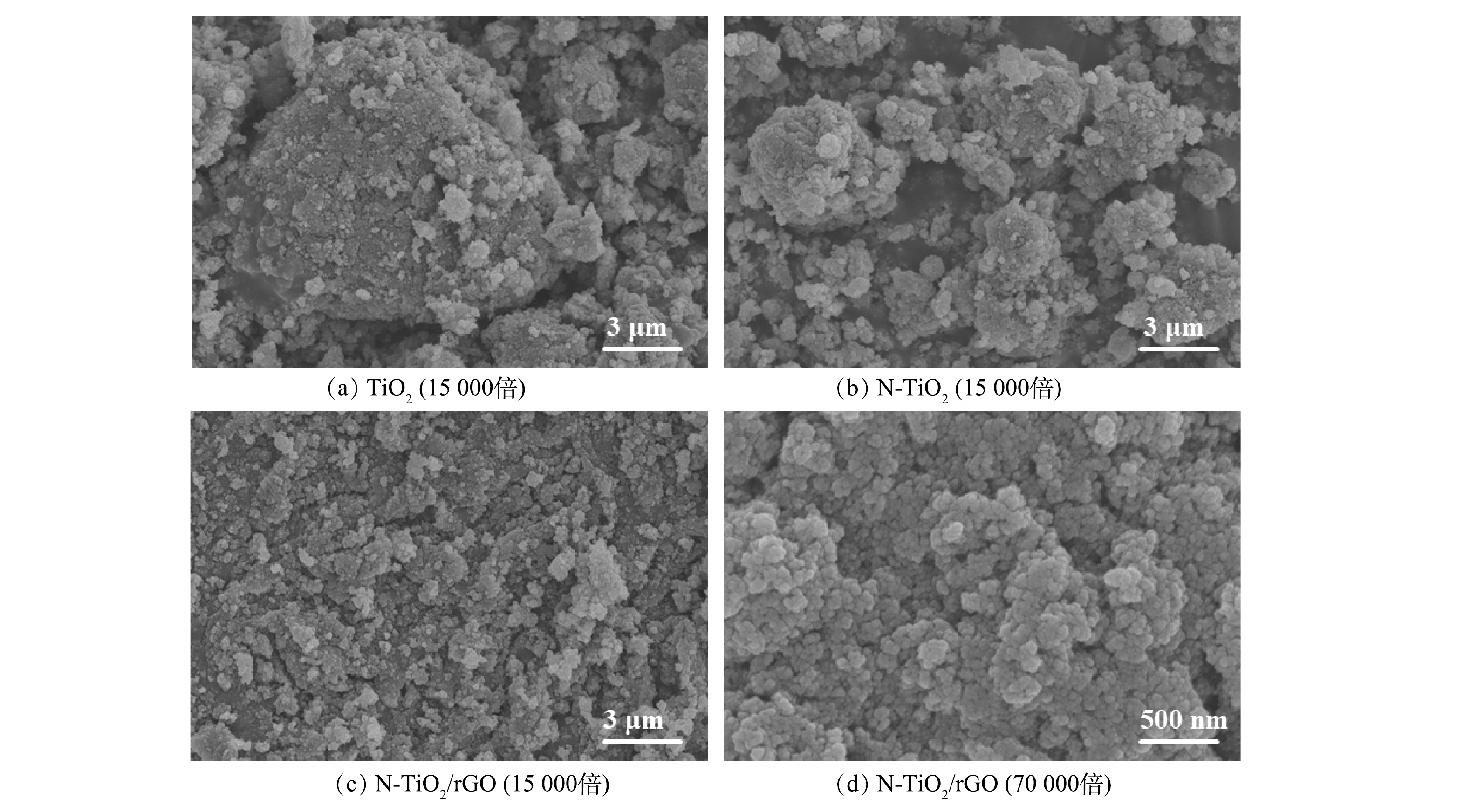
1) SEM分析。图1是不同放大倍数下TiO2、N-TiO2和N-TiO2/rGO的扫描电镜图。由图1(a)可见,纯TiO2形状不规则、颗粒粒径较大,大小不均的TiO2颗粒附着多呈块状且相互粘结集聚,团聚现象明显; N掺杂后的N-TiO2颗粒粒径减小,颗粒大小差异也显著缩小,材料整体分散性提高(图1(b))。GO载体表面原有的含氧官能团提供活性位点利于N-TiO2粒子与其键合,促进N-TiO2粒子的分散与生成。因此,负载后的N-TiO2/rGO(图1(c)~(d))颗粒更加均匀细小,N-TiO2粒子紧密附着在GO片层表面,分散较均匀且轮廓分明,证明N-TiO2粒子成功负载。相比于TiO2和 N-TiO2,N-TiO2/rGO颗粒之间分布较疏松均匀,几乎没有出现块状或团聚态颗粒。一方面,这是由于N的掺杂会部分替换晶格氧,消耗TiO2晶体生长时所需的能量,从而使颗粒粒径减小;另一方面,GO二维六角型平面结构良好的导电性促使颗粒间的静电作用力减弱[15],其平面晶格含有大量褶皱也同时为TiO2提供丰富附着点位[16],可有效克服晶体团聚,进而增强分散性、增大比表面积,易于有机污染物在N-TiO2/rGO复合材料上充分吸附,通过促进光生电子和空穴的产生与转移,强化其在纳米复合材料上的光催化降解反应进程和效率[17]。
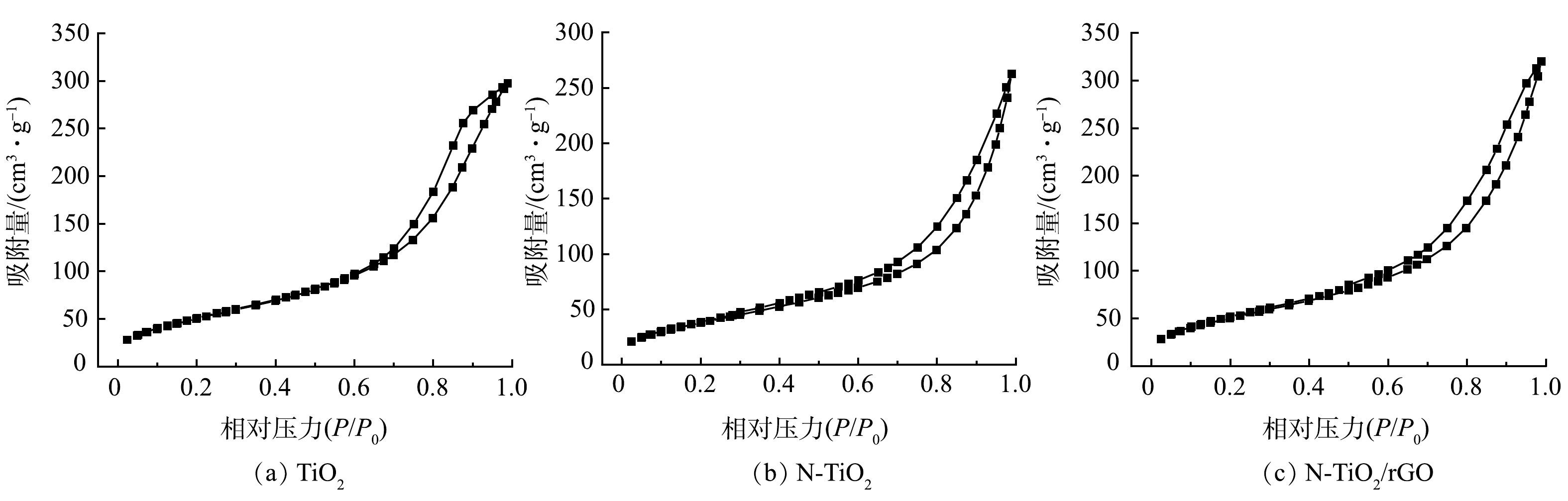
2) BET分析。采用氮气吸附-脱附法测试光催化材料的孔径结构变化,结果如图2所示。TiO2、N-TiO2和N-TiO2/rGO 3种光催化材料的比表面积、孔容及孔径见表1。分析图2,TiO2、N-TiO2和N-TiO2/rGO的吸附等温线均属于Ⅳ型等温线,为中孔材料,有滞后环伴随出现。等温线在末端出现吸附回滞环,说明在孔中会发生毛细凝聚现象,毛细凝聚会使其吸附中止,阻止更深入层次的吸附。图2(a)中TiO2在等温线末端的回滞环凸起更加明显,这也是其吸附性能较差从而导致光催化活性低的原因之一[18]。
表 1 GO、TiO2、N-TiO2和N-TiO2/rGO的比表面积、孔容和孔径Table 1. Specific surface area, pore volume and pore size of GO, TiO2, N-TiO2 and N-TiO2/rGO样品 比表面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/nm GO 6.125 0.019 12.421 TiO2 101.055 0.173 3.939 N-TiO2 200.127 0.427 6.874 N-TiO2/rGO 265.124 0.561 7.822 相比GO、TiO2和N-TiO2,N-TiO2/rGO的比表面积、孔容和孔径均有所增大(表1)。GO负载后,N-TiO2/rGO的比表面积从101.055 m2·g−1提升至265.124 m2·g−1,增加了1.6倍。这表明N和GO的加入减小了TiO2纳米微粒的团聚程度,这也是N-TiO2/rGO具有更大比表面积的原因,进一步验证了前述SEM的表征结果。此外,N-TiO2/rGO相对于纯TiO2,孔容由0.173 cm3·g−1增至0.561 cm3·g−1,孔径由3.939 nm增至7.822 nm,这可能是N-TiO2与GO水热过程脱除部分氧官能团促进了孔隙的生成。N-TiO2/rGO更加丰富的介孔结构,能增加吸附容量并提供更多反应活性位点,更大的比表面可有效提高反应体系分子的传递能力和载流子的迁移率[19],从而提高光催化反应速率。
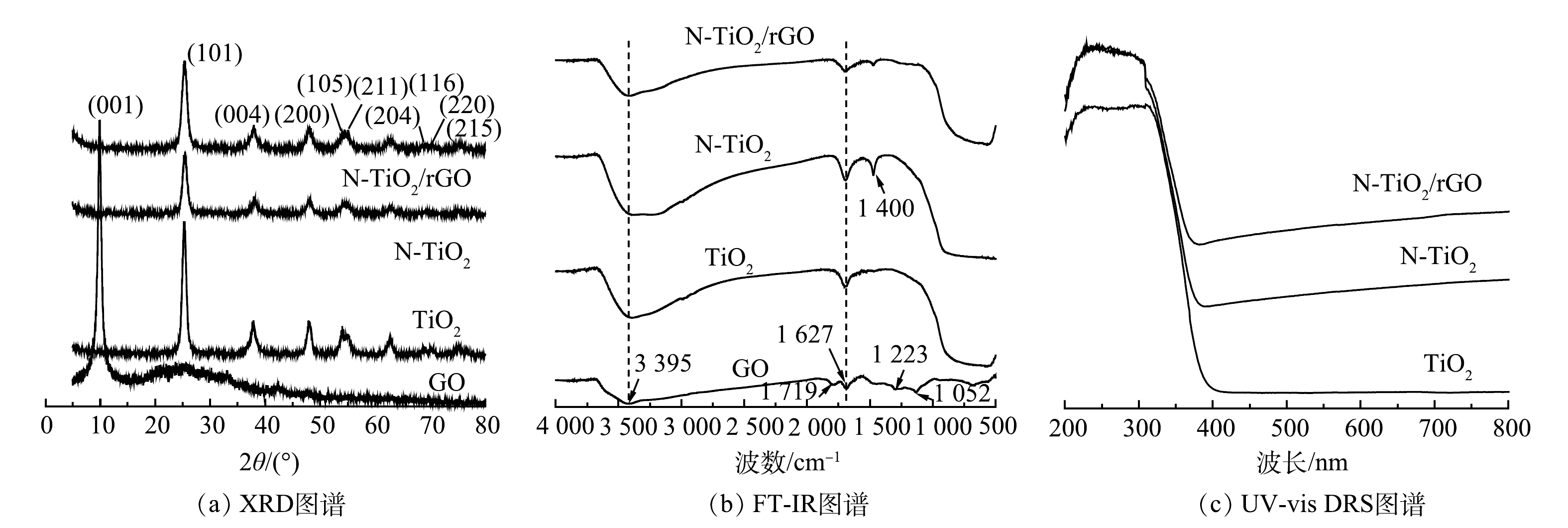
3) XRD、FT-IR、 UV-vis DRS光谱分析。水热合成纯TiO2、N-TiO2和N-TiO2/rGO样品的XRD、FT-IR、UV-vis DRS分析结果如图3。由图3(a)对比锐钛矿型标准卡片(JCPDS No.21-1272)可知,三者在25.39°处均出现主衍射峰,对应(101)晶面,同时在37.98°(004)、48.09°(200)、54.42°(105)、55.05°(211)、63.01°(204)、69.22°(116)、69.85°(220)、75.32°(215)出现一系列特征衍射峰,未发现TiO2其他相的衍射峰,表明3种催化剂均为锐钛矿型[20],N掺杂与GO负载对TiO2晶型结构没有明显影响。GO负载TiO2后,其在9.97°的特征衍射峰(001)消失,表明此时GO已经被还原,大部分含氧官能团消失。这进一步证实GO的负载没有破坏TiO2 的原物相结构,并且未出现新的衍射峰,其原因可能是GO在水热处理过程中材料组成遭到破坏,被还原成rGO,而其在24.5°处的(002)特征衍射峰又被TiO2锐钛矿相在25.39°处(101)特征衍射峰所掩盖[21]。图中均未出现明显N的特征峰,推测是由于材料中N组分的含量较少,且N原子的离子半径与O原子的较为接近,可能以置换的方式与TiO2晶体相结合,故未被检测出来。
图3(b)显示了GO、TiO2、N-TiO2和N-TiO2/rGO纳米复合物的FT-IR光谱。4种催化剂在1 627 cm−1和3 395 cm−1处均出现较明显的特征峰,归属于吸附在TiO2表面水分子的-OH弯曲和伸缩振动峰,同时1 627 cm−1处特征峰也是GO的sp2结构的C=C骨架伸缩振动峰。从GO图谱还可以看到,1 719 cm−1处出现清晰的C=O伸缩振动峰,来自于GO片层边缘羧基和羰基;1 052 cm−1和1 223 cm−1处的特征峰分别对应烷氧基和环氧基的C—O伸缩振动[11],表明GO含有大量含氧官能团。TiO2、N-TiO2和N-TiO2/rGO在400~900 cm−1处出现TiO2的Ti—O—Ti伸缩振动[22],说明TiO2自身没有发生明显变化;对比发现掺杂N后在1 400 cm−1处出现新的特征峰,可能是—NH2基团吸收峰,结合XRD分析,推测N原子进入TiO2晶格内取代部分O,在400~900 cm−1处形成Ti—O—N结构,可增强对可见光的吸收阈值。与GO相比,N-TiO2/GO在1 719 cm−1处的C=O伸缩振动、1 052 cm−1处烷氧基及1 223 cm−1处环氧基的C—O伸缩振动峰几乎都消失,说明GO在水热过程中发生了一定还原,大量含氧官能团被移除;同时,表面残余的部分含氧官能团有助于TiO2纳米颗粒与GO片层的结合,形成Ti—O—C键[23],与Ti—O—Ti同时存在。以上都表明,GO与N-TiO2已成功形成复合材料。
从TiO2、N-TiO2和N-TiO2/rGO的紫外-可见漫反射的吸收光谱(图3(c))中可明显看出,TiO2仅在紫外光区有明显吸收,在可见光区域几乎没有响应,而N-TiO2和N-TiO2/rGO的光吸收阈值显著增大,吸收边带发生明显红移。结合XRD和FT-IR的表征结果分析其原因:一方面,N原子进入晶体内部形成Ti—O—N结构,有利于产生氧空位,缩窄带隙,从而可减小电子发生跃迁时所需能量;另一方面,负载的GO片层可与TiO2结合形成Ti—O—C键[24-25],可加快光生电子的转移。通过UV vis-DRS一级积分曲线分别得到TiO2、N-TiO2和N-TiO2/rGO材料光吸收阈值λ为385、400和418 nm,根据Eg(eV)=1 240/λ计算得到相应的带隙值分别为3.22、3.10和2.97 eV。N-TiO2/rGO复合材料带隙变窄归因于N掺杂和GO的引入,使得TiO2的可见光响应范围拓宽。
4) Raman分析。为比较TiO2及其改性后复合材料的分子振动,研究碳原子在GO表面的无序排列和缺陷,对TiO2、N-TiO2和GO、N-TiO2/rGO材料进行了拉曼光谱表征分析(图4)。在143(Eg)、397(B1g)、517(A1g/B1g)和638(Eg) cm−1处出现的4个典型峰对应于锐钛矿相TiO2的特征峰[24],表明三者存在锐钛矿相,N掺杂未使TiO2的特征峰发生偏移,印证了XRD的表征结果(图3(a))。GO和N-TiO2/rGO在1 350 cm−1和1 580 cm−1处显示出GO的特征D峰和G峰,分别与sp3和sp2杂化碳原子的Raman振动峰相一致[26],这表明N-TiO2与GO已很好地结合。D峰表示GO边缘缺陷引起的无定型结构,G峰对应GO结构的有序状态,两峰的比值ID/IG反映结构的缺陷程度[27]。计算得出,GO和N-TiO2/rGO的ID/IG分别为0.98和1.01,与GO相比,N-TiO2/rGO的ID/IG更高。这表明GO与TiO2复合引入了sp3缺陷,GO的sp3杂化碳原子增加,推测这是GO与TiO2界面间的强相互作用(如Ti-O-C键)所致,即GO水热反应后被还原,大部分含氧官能团被移除,碳原子的化学状态发生了显著变化[24,26,28]。
2.2 N-TiO2/rGO催化降解Microcystin-LR的性能与机理
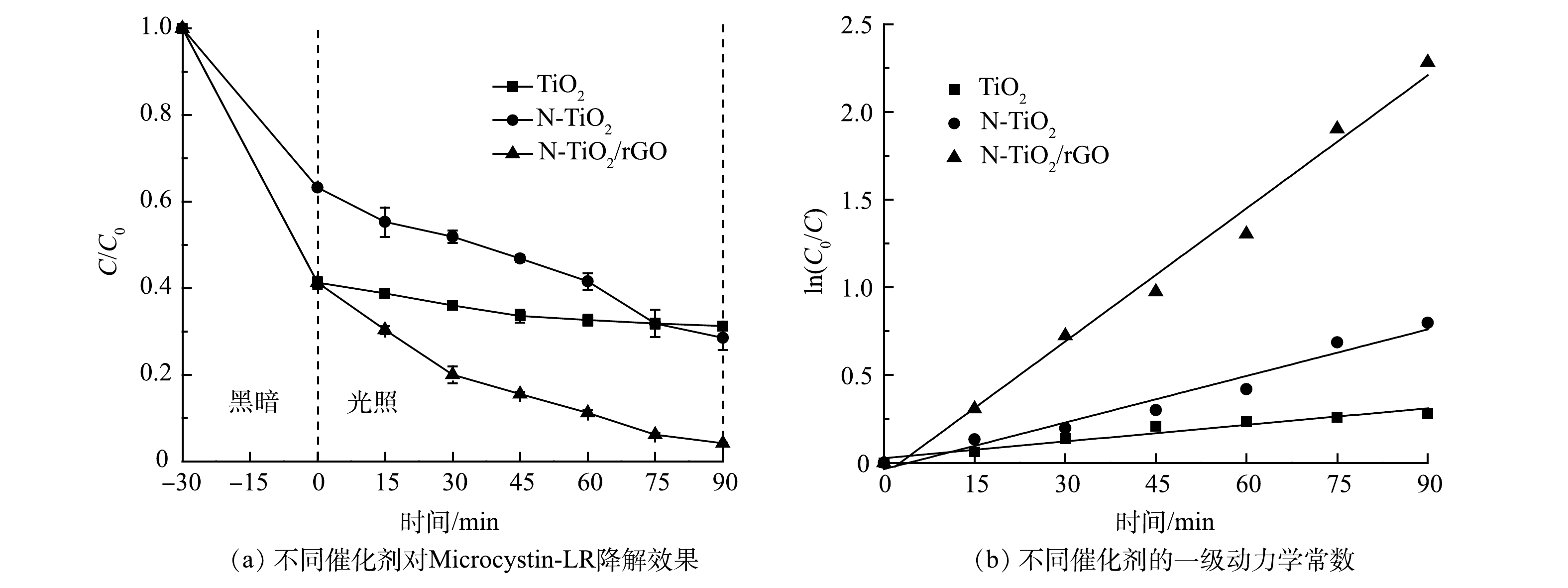
1)不同材料可见光催化降解Microcystin-LR及其动力学分析。由图5(a)可见,纯TiO2对Microcystin-LR光反应90 min后,去除率仅有24.2%;与纯TiO2相比,N-TiO2和N-TiO2/rGO对Microcystin-LR的降解效果均有显著提升,降解率分别达到54.9%和89.8%。根据UV-Vis DRS表征结果,N-TiO2/rGO的禁带宽度最小,光子利用率高,同时由于GO的负载,生成的光生电子-空穴对可以更快传递到光催化材料表面,与Microcystin-LR溶液反应生成·OH和O2−(式4),随后·OH与h+将Microcystin-LR氧化,生成中间产物或最终产物(式5)[29]。结合BET分析结果可知,N-TiO2/rGO的比表面积为265.1 m2·g−1,远大于N-TiO2(200.1 m2·g−1)和TiO2(101.0 m2·g−1),而较大的比表面积能够将Microcystin-LR有效吸附在材料表面,促进界面反应的进行。因界面反应过程是光催化反应过程的速率控制步骤,故N-TiO2/rGO对Microcystin-LR有较为优异的降解效果。
h++OH−→⋅OH,e−+O2→⋅O−2+H+→H2O2+e−→⋅OH (4) ⋅OH+MC−LR→MC−LR′+H2Oh++MC−LR→MC−LR′→中间产物/最终降解产物 (5) 图5(b)的拟合结果说明,Microcystin-LR光降解反应过程的动力学拟合曲线符合准一级动力学方程。采用L-H模型拟合动力学方程,结果见表2。可见,TiO2光降解Microcystin-LR反应速率常数k仅为0.003 2 min−1,N-TiO2和N-TiO2/rGO分别提高1.78倍和6.91倍,达到0.008 9 min−1和0.025 3 min−1。结合BET分析,N-TiO2/rGO拥有更大的比表面积,能够提供更多的活性吸附位点;同时,反应体系中·OH和·O2−等具有强氧化性的活性物质浓度增大,使界面反应的效率得到提高,从而促进光催化效率。
表 2 不同材料可见光催化降解Microcystin-LR的动力学拟合方程Table 2. Kinetic fitting equations of visible-light photocatalytic degradation of Microcystin-LR by different materials材料 准一级动力学方程 R2 k/min-1 TiO2 ln(C0/C)=0.003 2x+0.026 6 0.926 4 0.003 2 N-TiO2/rGO ln(C0/C)=0.008 9x−0.036 1 0.957 9 0.008 9 N-TiO2/rGO ln(C0/C)=0.025 3x−0.067 0 0.986 1 0.025 3 2) Microcystin-LR降解矿化率分析。由图6可见,当N-TiO2/rGO投加量为1 g·L−1、Microcystin-LR初始质量浓度为1 mg·L−1时,光催化90 min后Microcystin-LR的降解率为89.8%,矿化率为81.8%。这表明,在光催化降解过程中,N-TiO2/rGO能够将大部分Microcystin-LR分子矿化为CO2、H2O以及少量的小分子有机化合物。
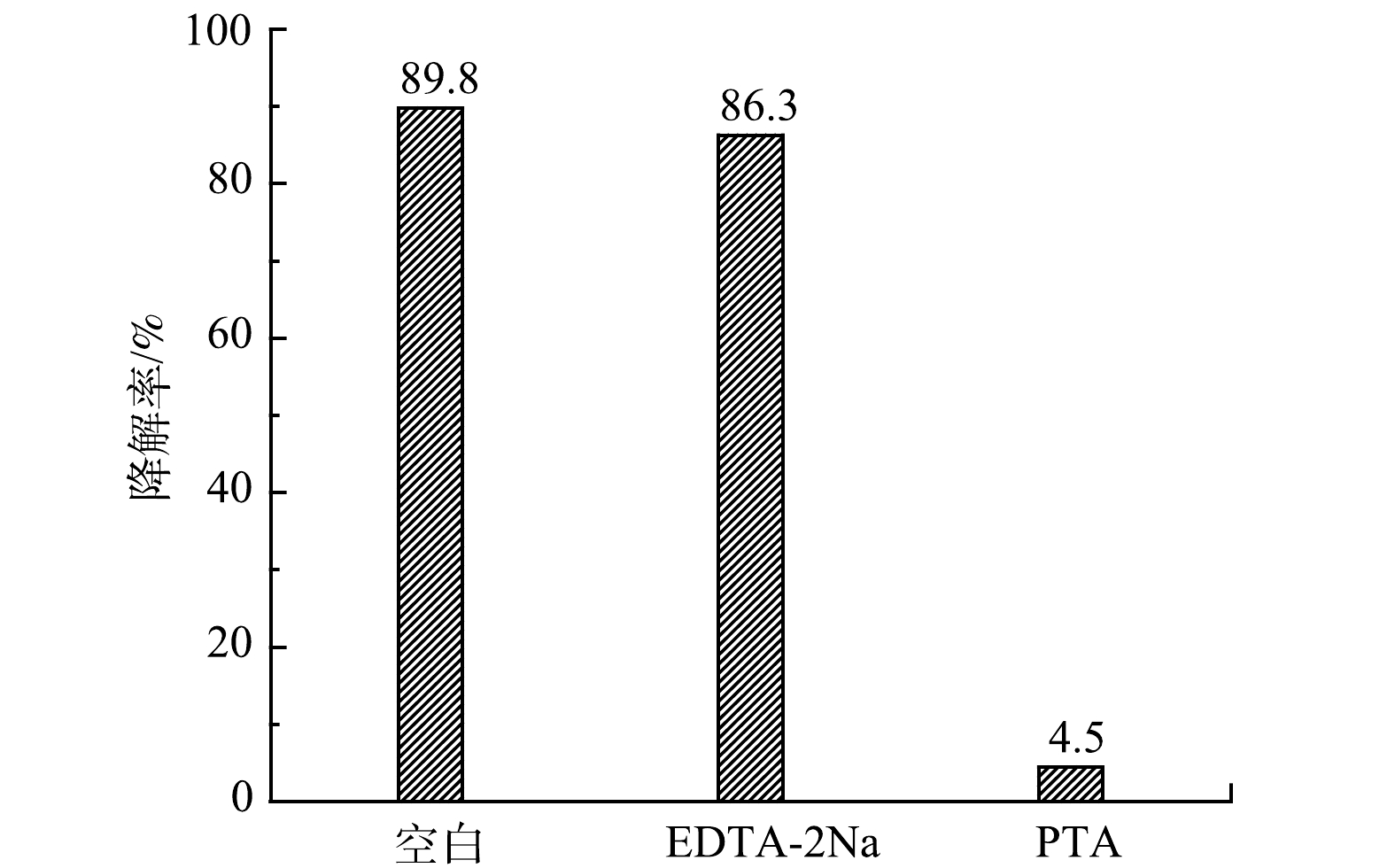
3) N-TiO2/rGO光催化降解Microcystin-LR的机理分析。N-TiO2/rGO复合光催化材料价带上的e−被激发跃迁到导带生成光生电子,同时在价带生成对应的光生空穴(h+),通过与水中O2氧化反应生成超氧自由基(·O2−),并进一步反应生成强氧化性的羟基自由基(·OH)。因此,光催化过程对有机物起主要作用的活性物种为羟基自由基(·OH)、空穴(h+)和超氧自由基(·O2−)等。为探究N-TiO2/rGO对Microcystin-LR光催化降解过程各类活性物种的贡献程度,采用竞争动力学实验,以对苯二甲酸(PTA)和乙二胺四乙酸二钠(EDTA-2Na)分别为·OH、h+的清除剂[30],比较含有与不含清除剂反应体系中Microcystin-LR的降解效果以及·OH/h+与Microcystin-LR竞争反应的影响程度。PTA是·OH的高效清除剂,反应体系中加入过量PTA,将优先捕获体系中生成的·OH并形成几乎没有氧化能力的二羟基-PTA,阻断·OH对Microcystin-LR的氧化降解;反应体系中过量EDTA-2Na快速将 h+还原,抑制h+氧化Microcystin-LR的反应,由此可间接获得相应活性氧物种在反应体系中的贡献。如图7所示,PTA的加入使得Microcystin-LR的降解率由89.8%锐减至4.5%;而 EDTA-2Na对光降解的抑制效果不明显,反应中加入捕获h+的EDTA-2Na后,Microcystin-LR的降解率仍达86.3%。由此,根据清除剂加入前后Microcystin-LR的光降解率差异推算·OH和h+对Microcystin-LR可见光催化降解的贡献率分别为95.0%和3.9%。此外,反应中间过程产生的超氧自由基(·O2−)等因存在时间短,氧化性较·OH和h+明显弱,贡献率仅约1%。因此,在TiO2/rGO复合光催化Microcystin-LR过程中,·OH起了主导性的贡献作用。许文泽等[31]在采用二氧化钛光催化氧化阿散酸中也证实,·OH是发挥主要氧化作用的物种。
有研究报道[32],光激发诱导产生的·OH通过攻击Microcystin-LR分子Adda支链上共轭双键及肽环结构等降解复杂的大分子,并进一步发生裂环分解成多个小分子物质,最后矿化为H2O和CO2。其中Adda支链共轭双键、苯环和甲氧基优先被·OH攻击,C4-C5和C6-C7共轭双键断裂氧化分别生成醛和酮;C9位甲氧基被氧化成甲酸,在酸性环境中可以水解,将Adda侧链从肽链中分离出来[33-35],因此,通过·OH的氧化分解可实现Microcystin-LR的降解减毒。
3. 结论
1)采用水热法一步还原制备N-TiO2/rGO光催化复合材料,最优制备条件为:水热反应温度180℃、掺N量6%、GO负载量5%。相较TiO2、N-TiO2,复合材料N-TiO2/rGO颗粒粒径小,分散性、层次性、多孔性优,晶型仍以锐钛矿相为主,光生载流子分离效率高,可见光催化活性好。
2)投加1 g·L−1的N-TiO2/rGO复合光催化剂,可见光照射90 min后,对初始质量浓度为1 mg·L−1的Microcystin-LR降解率达 89.8%,反应符合准一级动力学方程,降解速率常数为0.025 3 min−1。
3) ·OH 是Microcystin-LR光催化降解的主要活性物种,其对降解的贡献率达95.0%,而h+的贡献率仅为3.9%;·OH通过对Microcystin-LR分子Adda侧链共轭双键、苯环和甲氧基的攻击,实现降解和减毒。
-
表 1 垃圾焚烧飞灰的XRF分析结果
Table 1. XRF analysis results of the MSWI fly ash
% (质量分数) Ca Cl S K Si Na Fe Mg Zn Al Pb Ti 36.23 15.82 8.77 4.21 2.21 1.17 1.1 0.762 0.44 0.41 0.21 0.21 P Cr Br Cu Ba Mn Sb Sr Sn Rb Cd Ni 0.18 0.08 0.61 0.059 0.058 0.041 0.037 0.032 0.029 0.011 0.007 0.004 表 2 飞灰中重金属质量分数
Table 2. Mass fraction of heavy metals in MSWI fly ash
mg·kg−1 Zn Pb Cu Cd 2872 1166 588 107 表 3 酸洗前后飞灰浸出液的重金属质量浓度
Table 3. Mass concentrations of heavy metal in fly ash leaching solution before and after acid washing
mg·L−1 -
[1] 中华人民共和国国家统计局. 2020中国统计年鉴[M]. 北京: 中国统计出版社, 2020. [2] LI G L, WU Q R, WANG S X, et al. The influence of flue gas components and activated carbon injection on mercury capture of municipal solid waste incineration in China[J]. Chemical Engineering Journal, 2017, 326: 561-569. doi: 10.1016/j.cej.2017.05.099 [3] 蒋旭光, 段茵, 吕国钧, 等. 垃圾焚烧飞灰中重金属固化稳定机理及系统评价方法的研究进展[J]. 环境工程学报, 2022, 16(1): 10-19. doi: 10.12030/j.cjee.202105098 [4] BAI R B, SUTANTO M, The practice and challenges of solid waste management in Singapore[J]. Waste Management, 2002, 22: 557-567. [5] SHI H S, KAN L L, Characteristics of municipal solid wastes incineration (MSWI) fly ash–cement matrices and effect of mineral admixtures on composite system[J]. Construction and Building Materials, 2009, 23(6): 2160-2166. [6] LUO H W, HE D Q, ZHU W P, et al. Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu(II) ions[J]. Waste Management, 2019, 84: 83-90. doi: 10.1016/j.wasman.2018.11.037 [7] 生态环境部办公厅. 《“十四五”时期“无废城市”建设工作方案》[EB/OL][2021-12-15]. https://www.mee.gov.cn/xxgk2018/xxgk/xxgk03/202112/W020211215761126730366.pdf [8] ZOU D A, CHI Y, FU C, et al. Co-destruction of organic pollutants in municipal solid waste leachate and dioxins in fly ash under supercritical water using H2O2 as oxidant[J]. Journal of Hazardous Materials, 2013, 248-249: 177-184. doi: 10.1016/j.jhazmat.2013.01.005 [9] QUINA M J, BONTEMPI E, BOGUSH A, et al. Technologies for the management of MSW incineration ashes from gas cleaning: new perspectives on recovery of secondary raw materials and circular economy[J]. Science of the Total Environment, 2018, 635: 526-542. doi: 10.1016/j.scitotenv.2018.04.150 [10] LINDBERG D, MOLIN C, HUPA M. Thermal treatment of solid residues from WtE units: a review[J]. Waste Management, 2015, 37: 82-94. doi: 10.1016/j.wasman.2014.12.009 [11] HU Y Y, ZHANG P F, CHEN D Z, et al. Hydrothermal treatment of municipal solid waste incineration fly ash for dioxin decomposition[J]. Journal of Hazardous Materials, 2012, 207-208: 79-85. doi: 10.1016/j.jhazmat.2011.05.068 [12] YANG Z Z, JI R, LIU L L, et al. Recycling of municipal solid waste incineration by-product for cement composites preparation[J]. Construction and Building Materials, 2018, 162: 794-801. doi: 10.1016/j.conbuildmat.2017.12.081 [13] LI M, Chen J, Lin X, et al. Study on three-stage counter-current water washing desalination characteristics and mechanism of high chlorine waste incineration fly ash[J]. Processes, 2022, 10: 2540. doi: 10.3390/pr10122540 [14] 董光辉, 左武, 赵润博, 等. 水泥窑协同处置生活垃圾焚烧飞灰过程中Pb和Zn的迁移转化特性[J]. 环境工程学报, 2023, 17(1): 250-258. doi: 10.12030/j.cjee.202210043 [15] HU Y Y, ZHANG P F, LI J P, et al. Stabilization and separation of heavy metals in incineration fly ash during the hydrothermal treatment process[J]. Journal of Hazardous Materials, 2015, 299: 149-157. doi: 10.1016/j.jhazmat.2015.06.002 [16] ZHAO X, WANG L A, WANG L, et al. Distribution of remaining Cd in MSWI fly ash washed with nitric acid[J]. Journal of Material Cycles and Waste Management, 2017, 19: 1415-1422. doi: 10.1007/s10163-016-0535-7 [17] 林涛, 谢巧玲, 陈福明, 等. 基于重金属提取的垃圾焚烧飞灰无害化处理[J]. 环境工程学报, 2018, 12(9): 2642-2649. doi: 10.12030/j.cjee.201804067 [18] HUANG K, INOUE K, HARADA H, et al. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants[J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1422-1427. doi: 10.1016/S1003-6326(11)60876-5 [19] 孙福成, 丁慧敏, 柯伟, 等. 城市生活垃圾焚烧飞灰与矿山废水共处置技术研究与工程应用[J]. 环境工程学报, 2016, 10(4): 2151-2156. doi: 10.12030/j.cjee.20160490 [20] 彭腾, 冉雪玲, 杨宁, 等. 采用柠檬酸浸出—电沉积法回收废锂电池中的钴[J]. 湿法冶金, 2021, 40(3): 196-201. doi: 10.13355/j.cnki.sfyj.2021.03.005 [21] 李子良, 徐志峰, 张溪, 等. 酸性含汞溶液中电沉积回收汞的研究[J]. 工程科学学报, 2020, 42(8): 999-1006. [22] 陈熙, 徐新阳, 赵冰, 等. 喷射床电沉积法处理铜镍混合废水[J]. 化工学报, 2015, 66(12): 5060-5066. doi: 10.11949/j.issn.0438-1157.20151074 [23] RAURET G, LOPEZ S J F, SAHUQUILLO A, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials[J]. Journal of Environmental Monitoring, 1999, 1(1): 57-61. doi: 10.1039/a807854h [24] 环境保护部. 固体废物浸出毒性浸出方法醋酸缓冲溶液法: HJ/T 300-2007[S]. 北京: 中国环境科学出版社, 2007. [25] 韩大健, 王文祥, 孙水裕, 等. 城市生活垃圾焚烧飞灰中钾盐浸出研究[J]. 环境科学学报, 2017, 37(6): 2223-2231. doi: 10.13671/j.hjkxxb.2016.0420 [26] 环境保护部. 生活垃圾填埋场污染控制标准: GB16889-2008[S]. 北京: 中国环境科学出版社, 2008. [27] TIAN Y X, THEMELIS N. J, BOURTSALAS A. C. T, et al. Systematic study of the formation and chemical/mineral composition of waste-to-energy (WTE) fly ash[J]. Materials Chemistry and Physics, 2023, 293: 126849. doi: 10.1016/j.matchemphys.2022.126849 [28] JIAO F, ZHANG L, DONG Z, et al. Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior[J]. Fuel Processing Technology, 2016, 152: 108-115. doi: 10.1016/j.fuproc.2016.06.013 [29] CAVIGLIA C, DESTEFANIS E, PASTERO L, et al. MSWI fly ash multiple washing: Kinetics of dissolution in water, as function of time, temperature and dilution[J]. Minerals, 2022, 12: 742. [30] 朱军, 高首坤, 赵奇, 等. F−和Cl−浓度对电沉积锌电化学过程的影响[J]. 材料保护, 2018, 51(1): 28-30,110. -




 下载:
下载: