-
水体富营养化导致藻类等水生浮游植物大量繁殖并形成水华,其中蓝藻属的铜绿微囊藻引起的水华最为常见,还释放藻毒素,严重威胁到其他水生生物生态功能的正常发挥,甚至影响人类的健康[1]。为此我们已经提出了许多方法来管理广泛存在的蓝藻水华,如水更新、沉积物疏浚、使用硫酸铜、高锰酸钾、氯和臭氧开发蓝藻杀菌剂,以及建造湿地和由植物、动物和微生物组成的漂浮岛屿[2]。然而,这些方法存在各种缺点,如成本高、二次污染、流域小、不切实际等,使得目前为止仍然没有一种经济有效环境友好型的治理方法[3]。因此,有必要确定具有高效、安全和经济特征的环境友好型蓝藻控制策略。化感作用的发现和利用为降低蓝藻细菌的丰度、维持生态平衡和可持续性提供了一种新的、有前途的方法[4]。
目前已经证实了化感作用可通过损害细胞微结构、细胞活力、光合作用、细胞内氧化还原平衡和酶活性,来抑制蓝藻的生长,并导致细胞程序性死亡和细胞外物质的释放[5]。高云霓等发现穗花狐尾藻种植水显著抑制铜绿微囊藻产毒株和非产毒株的生长,影响其生理过程[6]。崔莉凤[7]等通过研究证实了水生植物干燥体仍具有化感物质,穗花狐尾藻浸出液对微囊藻的生长和产毒都具有很强的抑制能力,其中对微囊藻的生长抑制率最高能够达到91.89%。朱俊英[8]等在共培养条件下研究发现铜绿微囊藻的叶绿素-a含量及叶绿素荧光参数在穗花狐尾藻分泌的化感物质作用下显著降低。高浩杰[9]以轮叶狐尾藻为实验材料,证实了轮叶狐尾藻分泌出的化感物质会破坏铜绿微囊藻的细胞膜结构从而造成细胞损伤,导致铜绿微囊藻死亡。诺加罗等[10]发现霍甫水丝蚓生物扰动能够提高反硝化菌的活性,同时增强了氨化作用。KANG等[11]也发现霍甫水丝蚓的投加有利于有机质的分解,增加碳氮比,为微生物脱氮提供了充足的碳源,同时在水丝蚓的扰动作用下水-沉积物界面处溶解氧(dissolved oxygen,DO)降低,微生物的电子受体在沉积物水平上重新分布,利于反硝化菌的生长和反硝化进程加快,刺激了微生物脱氮活性。霍甫水丝蚓可以改善水生植物根系土壤的理化性质,促进养分循环,利于植物生长,提高植物产量和抵抗外在胁迫的能力,可提高水生植物的修复效果[12]。
底栖动物是水生生态系统的重要组成部分之一,在维持生物多样性和生态平衡方面具有巨大的应用潜力。底栖动物很容易食用底栖藻类、浮游植物和陆生有机物,其本身就是鱼类食物的组成部分[13]。ZUO[14]等采用微观实验方法探讨了底栖动物对大型植物化感作用的影响,结果表明,4种代表性的底栖动物在单独培养中表现出较低的海藻抑制作用,当底栖动物与被试微藻完全混合时,其抑制率有所提高。此外,大型植物对海藻的化感抑制作用与底栖动物平均单株生物量呈显著正相关。与不含底栖动物和沉水植物的疏浚区相比,沉水植物填充区藻类生物量和密度显著降低。水底大型植物和底栖动物共同存在的区域藻华最少,这是底栖动物和大型植物协同作用的结果。因此,本研究通过霍甫水丝蚓来改变穗花狐尾藻的根际微环境,对共培种植水纯化且将其加入铜绿微囊藻中,通过对铜绿微囊藻生化指标的测定考察了水丝蚓对穗花狐尾藻化感作用的影响,为底栖动物促进大型水生植物的化感作用提供依据。
-
实验所用铜绿微囊藻(FACHB-524) 购自中国科学院水生生物研究所淡水藻种库。通过无菌BG11培养基培养,光照强度为2 000 lx,光暗周期为12 h:12 h,温度为(25±1) °C,每天手动定时摇瓶3次,每隔7 d重新接种1次,连续多代驯化培养,等待其长到对数生长期且藻类浓度约为 8×105细胞·mL−1时进行实验。穗花狐尾藻2021年采自芜湖市扁担河,栽种于实验室种植桶中,桶高为0.5 m,底部半径为0.12 m。种植桶中添加10 cm厚底泥,10%霍格兰营养液培养。选取大小较为一致,鲜质量相同的穗花狐尾藻幼苗每5株为1组栽入4个相同规格的种植桶中,加入购自花鸟市场的水丝蚓进行连续培养。当穗花狐尾藻幼苗长大后,取其种植水用于实验。超氧化物歧化酶(superoxide dismutase,SOD)、丙二醛(malonic dialdehyde,MDA)、总磷(total phosphorus,TP)分析试剂盒来自南京建成公司。
-
设置3个穗花狐尾藻处理组:高密度水丝蚓(15 000 条·m−2)处理组、中密度水丝蚓(8 300 条·m−2)处理组、低密度水丝蚓(1 600 条·m−2)处理组,零密度处理组(对照组)为穗花狐尾藻种植体系不加水丝蚓。培养体系统一使用10%霍格兰营养液,每组设置3个平行实验。每组实验取400 mL穗花狐尾藻种植水抽滤过0.22 μm孔径的滤膜,去除其中的颗粒杂质和微生物干扰后使用乙酸乙酯按水相与乙酸乙酯相体积比1:3进行旋转蒸发提纯,再用5 mL蒸馏水进行超声萃取,萃取液用于抑藻实验。种植水制备和铜绿微囊藻接种均在无菌操作台上进行,所用器皿均提前做好灭菌处理。实验在250 mL锥形瓶中进行,瓶中加入100 mL对数生长期铜绿微囊藻,暴露于5 mL种植水萃取液,藻起始密度约为8×105细胞·mL−1。连续培养7 d,每天手动摇匀3次。分别于0、2、4、6 d测定暴露的铜绿微囊藻中膜脂过氧化产物MDA浓度、SOD活性、叶绿素-a(chlorophyl,Chl-a)含量,并且每天记录铜绿微囊藻藻密度。
1)铜绿微囊藻抑制率的测量。每天定时取铜绿微囊藻1 mL,并加入9 mL蒸馏水与鲁哥氏固定液混合液进行混合固定,静置10 min。再取20 μL用血球计数板在光学显微镜(BMSG20BD,上海BM光学仪器制造有限公司)进行计数。藻细胞抑制率按式(1)计算。
式中:I为藻细胞抑制率,%;X0为对照组细胞数,105个;X为处理组细胞数,105个。
2)铜绿微囊藻叶绿素-a的测定。以加5 mL高、中、低密度水丝蚓穗花狐尾藻种植水和无水丝蚓穗花狐尾藻种植水为处理组,5 mL蒸馏水为对照组;每2 d测定1次叶绿素-a。采用90%丙酮溶液提取、分离藻细胞叶绿素-a[15],用三色法测定[16]。叶绿素-a的含量根据式(2)计算。
式中:C表示叶绿素-a的质量浓度,mg·L−1;A663、A645、A630分别为紫外可见分光光度计下663、645、630 nm波长处吸光度值。
3)铜绿微囊藻SOD活性和MDA含量的测定。取10 mL藻液于灭菌离心管中,冰浴下超声波破碎(JY92-Ⅱ型超声波细胞粉碎机-宁波新芝生物科技股份有限公司生产,超声5 s间隔5 s工作次数为24次功率600 W重复3次),按照自南京建成公司购买的SOD试剂盒、MDA试剂盒说明书所述步骤进行测定。
4)穗花狐尾藻株高、鲜质量的测定。使用直尺和分析天平测量穗花狐尾藻栽种前的株高和鲜质量;栽种后与水丝蚓进行共培,实验后再取出穗花狐尾藻对测量其株高和鲜质量。
5)化感物质定性与定量。取种植水乙酸乙酯萃取液1 mL加入15 mL离心管中,继续加入2%盐酸-甲醇溶液,3 mL氯仿甲醇溶液(体积比1∶1),10 μL十九烷酸甲醇内标。于85 ℃水浴锅中水浴1 h。等温度降至室温,在离心管中加入1 mL正己烷,振荡萃取2 min之后,静置1 h,等待分层。取上清液100 μL,用正己烷定容到1 mL。用0.45 μm滤膜过滤后采用ThermoFisher Trace1310ISQ气相色谱质谱联用仪测试[17]。
-
采用Excel 2013对实验数据进行整理,利用SPSS 19.0进行数据分析,所有处理均采用正态分布检验和方差齐性检验,图象绘制使用OriginPro 9.1。
-
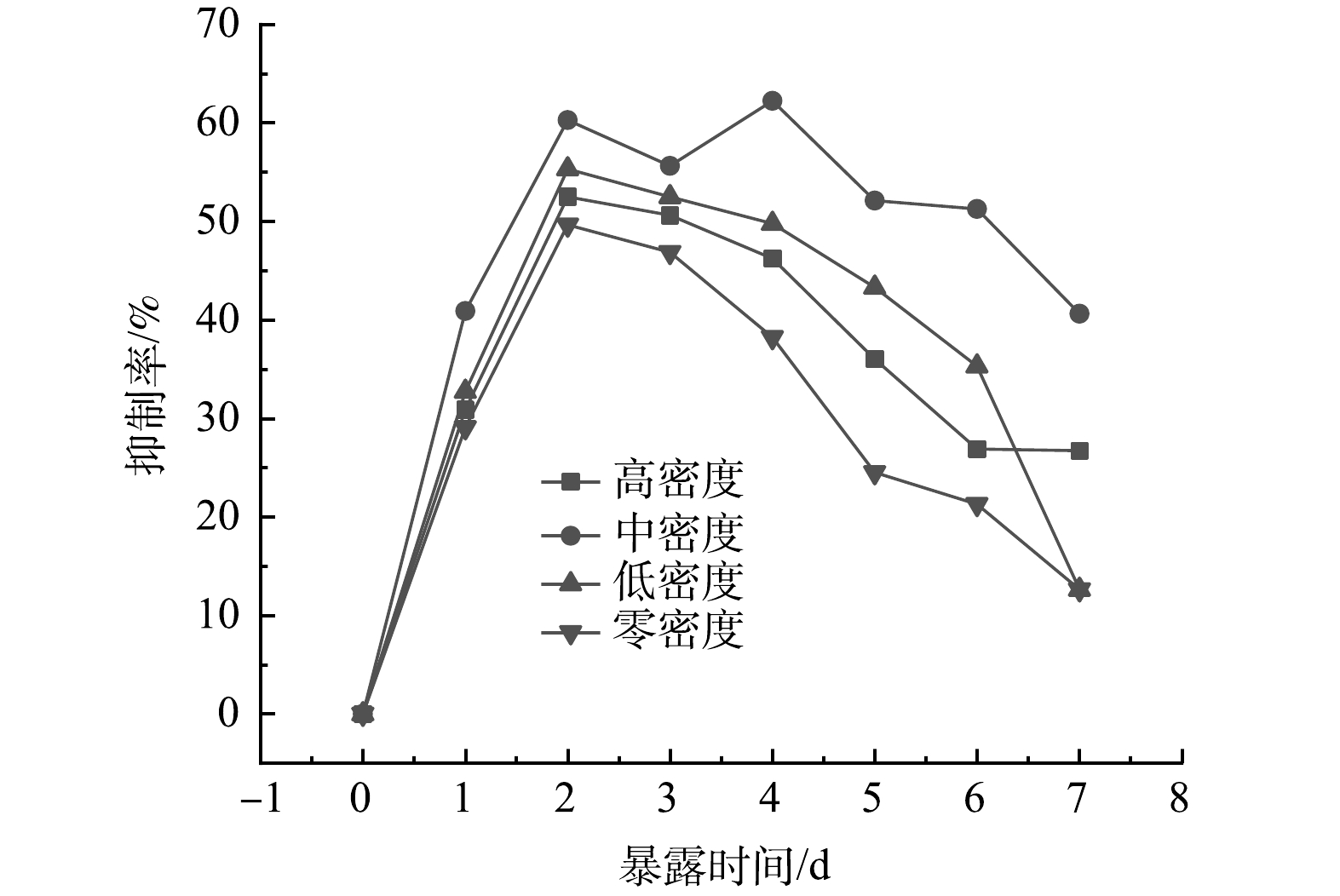
由图1可以得出,在水丝蚓的作用下,穗花狐尾藻的化感作用得到增强,对蓝藻的抑制率明显高于无水丝蚓培养下的穗花狐尾藻(P<0.05)。处理组抑制率均呈现先升高后降低的趋势,可能是铜绿微囊藻暴露于种植水后化感物质参与到藻细胞合成代谢过程中,所以抑制率开始呈现上升趋势。但由于化感物质易被藻细胞代谢分解,随着时间的延长浓度会降低,所以抑制率后呈下降趋势。
中密度水丝蚓处理组化感抑藻效应最强,于第4天抑制率达到最高62.22%,显著高于其他处理组和零密度组。这可能是因为水丝蚓的生物扰动增强穗花狐尾藻化感物质的释放,随着水丝蚓生物量的增加,扰动增强,穗花狐尾藻化感物质的释放增多;但当水丝蚓生物量达到临界值时,水丝蚓的生化作用会对穗花狐尾藻造成胁迫作用,从而使得穗花狐尾藻化感物质的释放量开始降低。
-
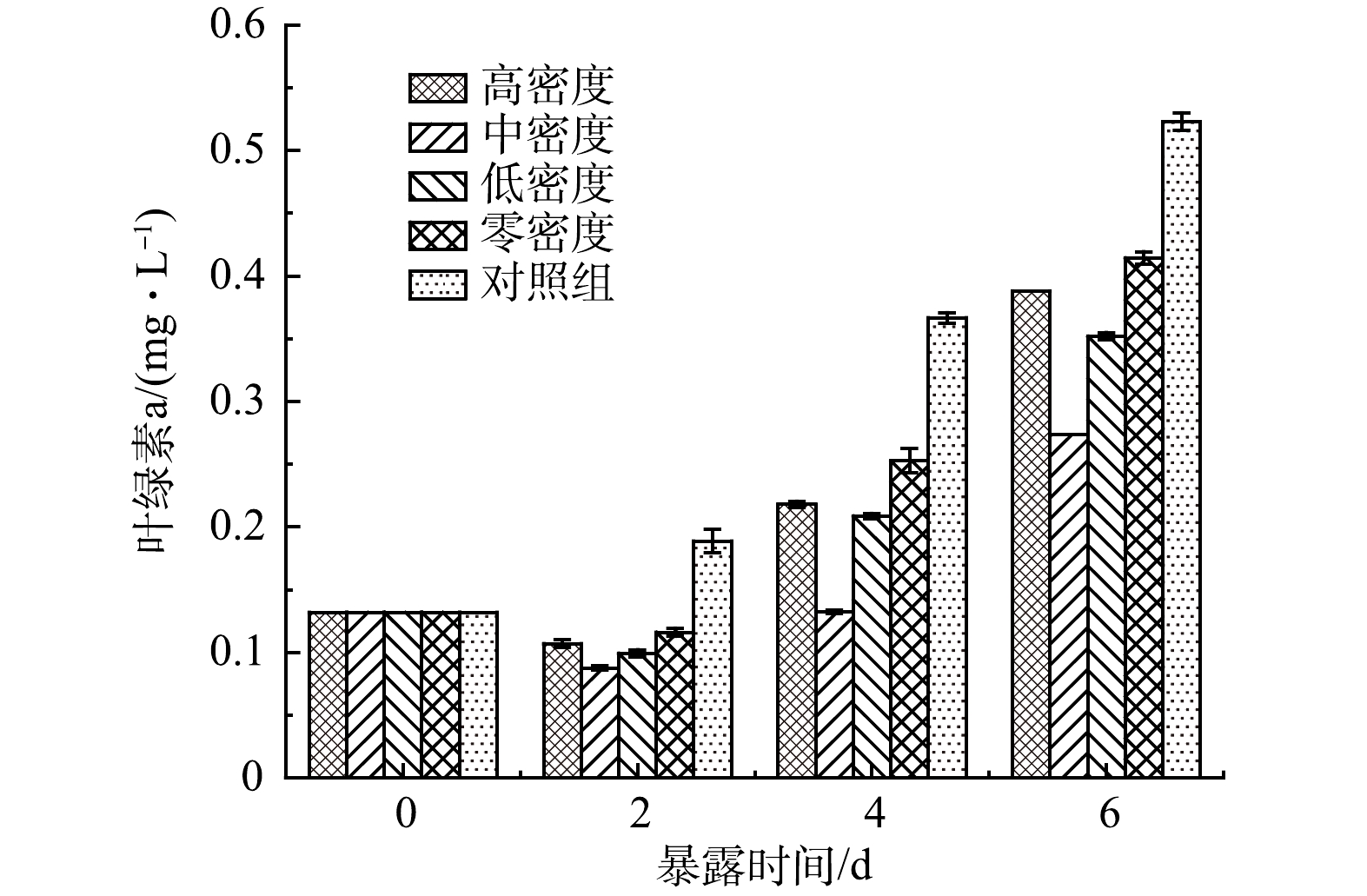
藻类和浮游植物依靠光合作用生长,而叶绿素-a是所有藻类的主要光合色素,通过测定藻类和浮游植物叶绿素-a含量可掌握水体的初级生产力情况,可将叶绿素-a作为其光合作用潜力的一个指标[18]。由图2可见,处理组中铜绿微囊藻叶绿素-a含量明显低于对照组。这进一步说明霍甫水丝蚓可促进穗花狐尾藻的抑藻效应。各实验组中铜绿微囊藻叶绿素-a含量变化趋势与图1中铜绿微囊藻生长抑制率高度一致,即处理组叶绿素-a含量低于对照组。处理组由于水丝蚓密度不同,处理效果也不同,其中中密度组对叶绿素-a含量抑藻效应最强。
-
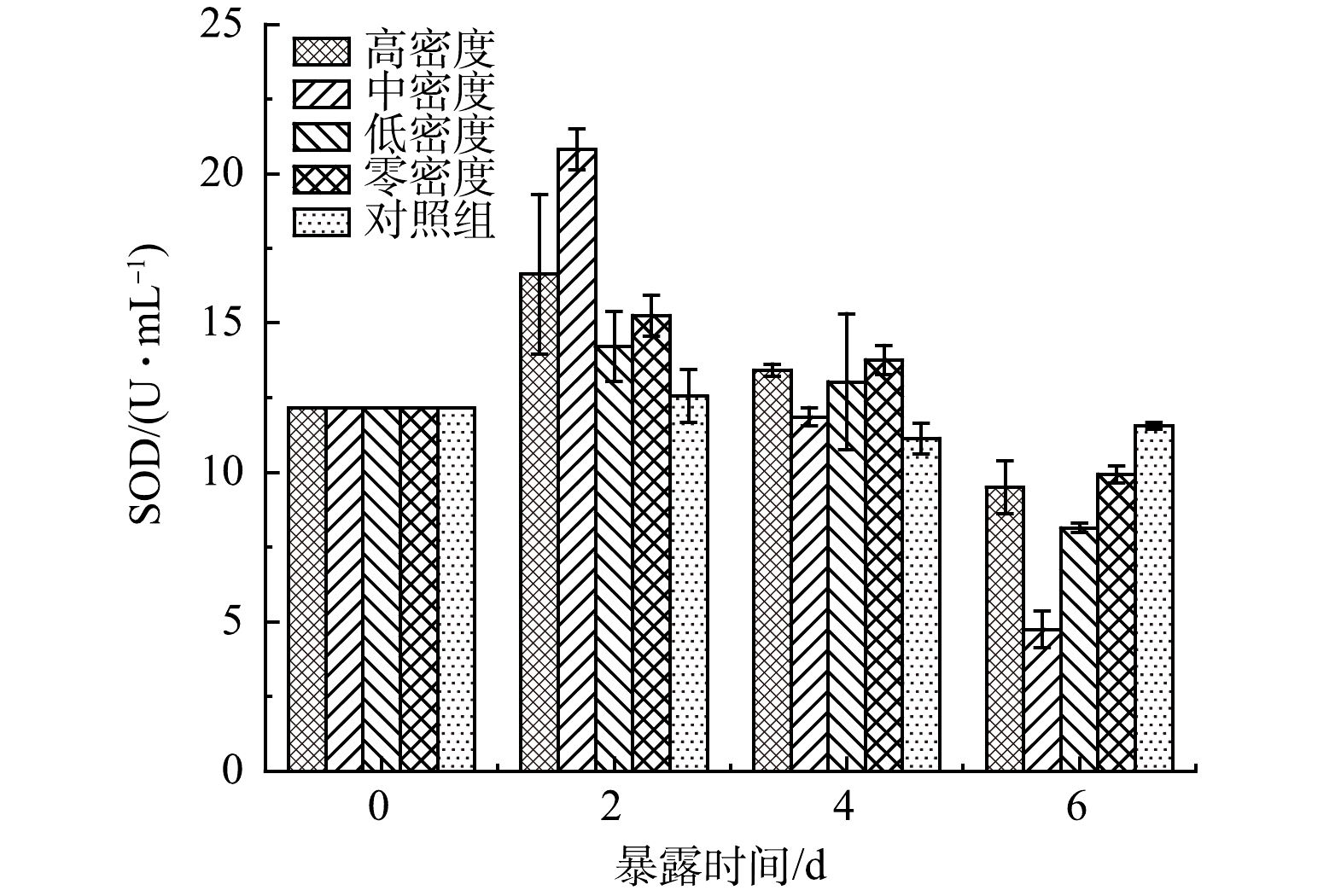
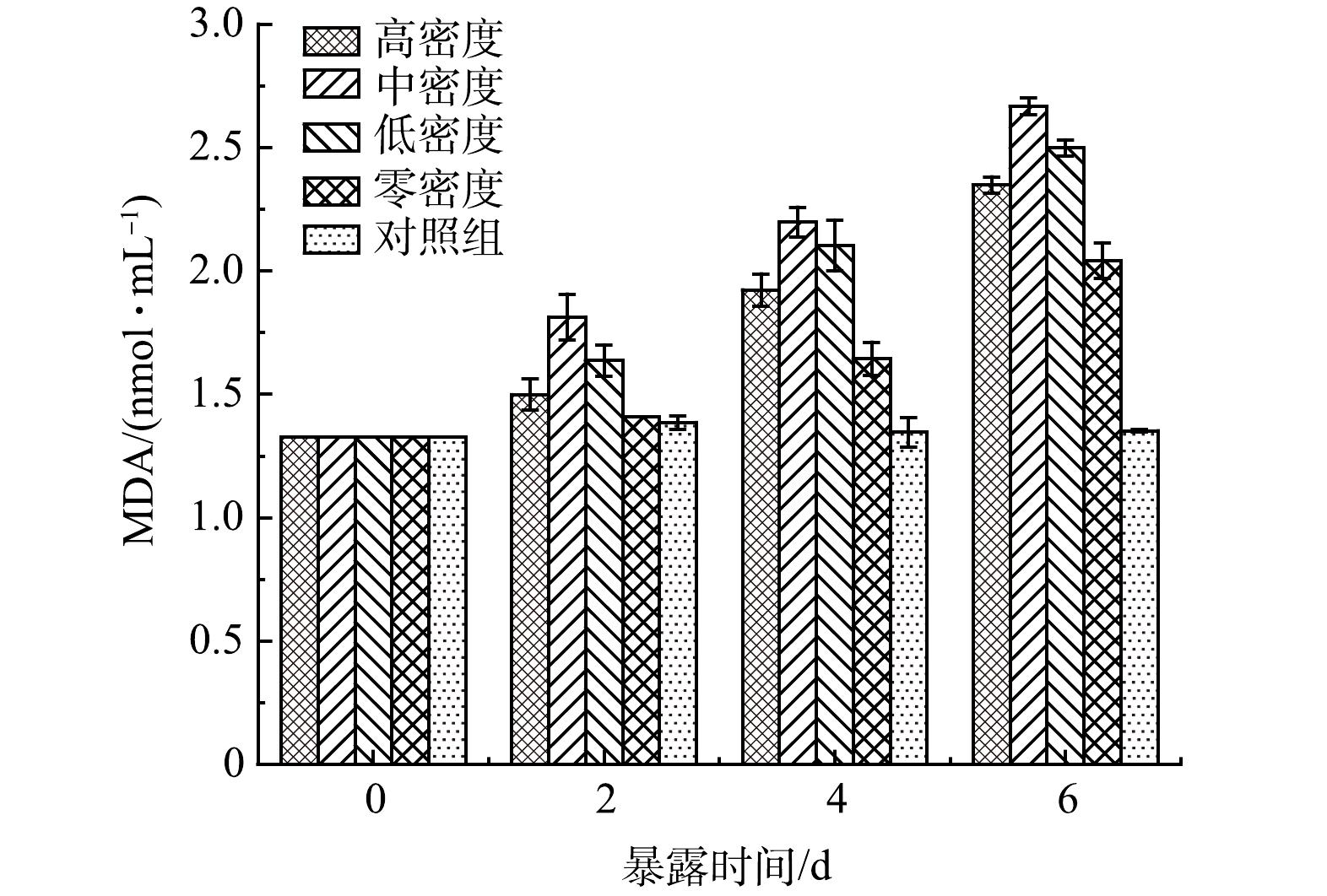
由图3可见,对照组的SOD活性在实验过程中几乎没有变化。说明对照组的藻类生长状况良好,基本上没有受到外界条件的胁迫。而实验组铜绿微囊藻细胞中SOD活性呈现先升高后降低(P<0.05)。说明原有的氧自由基的产生与清除平衡被打破。当藻细胞暴露于实验组种植水时,细胞受到胁迫作用,细胞内产生大量氧自由基,藻细胞尝试建立新的氧自由基平衡,SOD活性升高,提高抗氧化能力。但由于持续的暴露在种植水中,细胞已经受到损伤,抗氧化系统被破坏,SOD活性开始下降。这可以由图4中藻细胞的MDA值变化可以验证。实验组藻细胞MDA从开始的1.326 nmol·mL−1开始持续增高,对照组MDA数值略微变化,即实验组藻细胞受到持续伤害,对照组正常生长;藻细胞受到伤害程度按无水丝蚓组、高密度组、低密度组、中密度组顺序依次升高。由图3和图4可见,当铜绿微囊藻暴露于种植水时,藻细胞受到损伤,MDA升高,抗氧化系统迅速反应,SOD活性升高;但随着持续暴露,细胞损伤加剧,MDA持续增高,抗氧化系统遭到破坏,SOD活性降低。
-
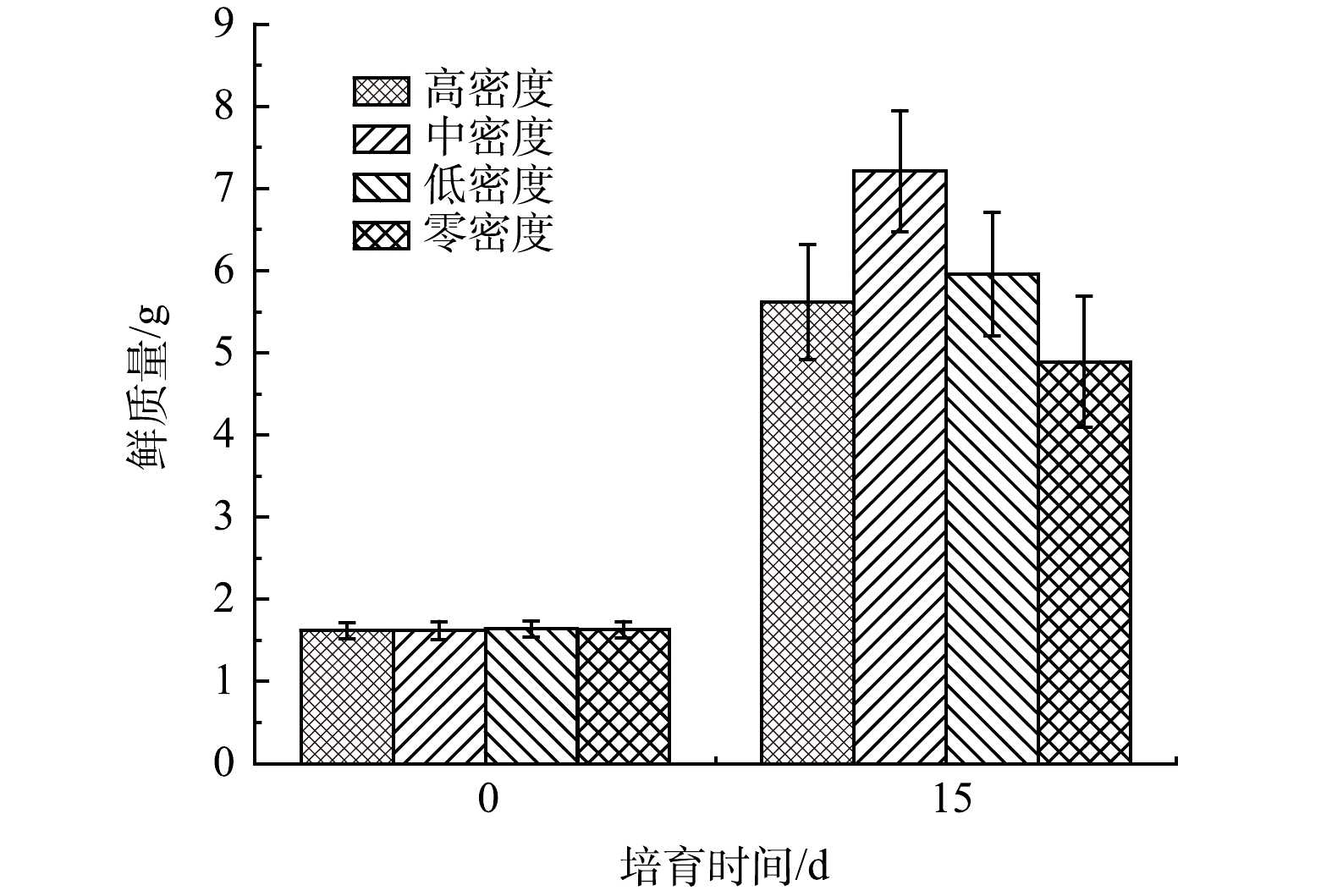
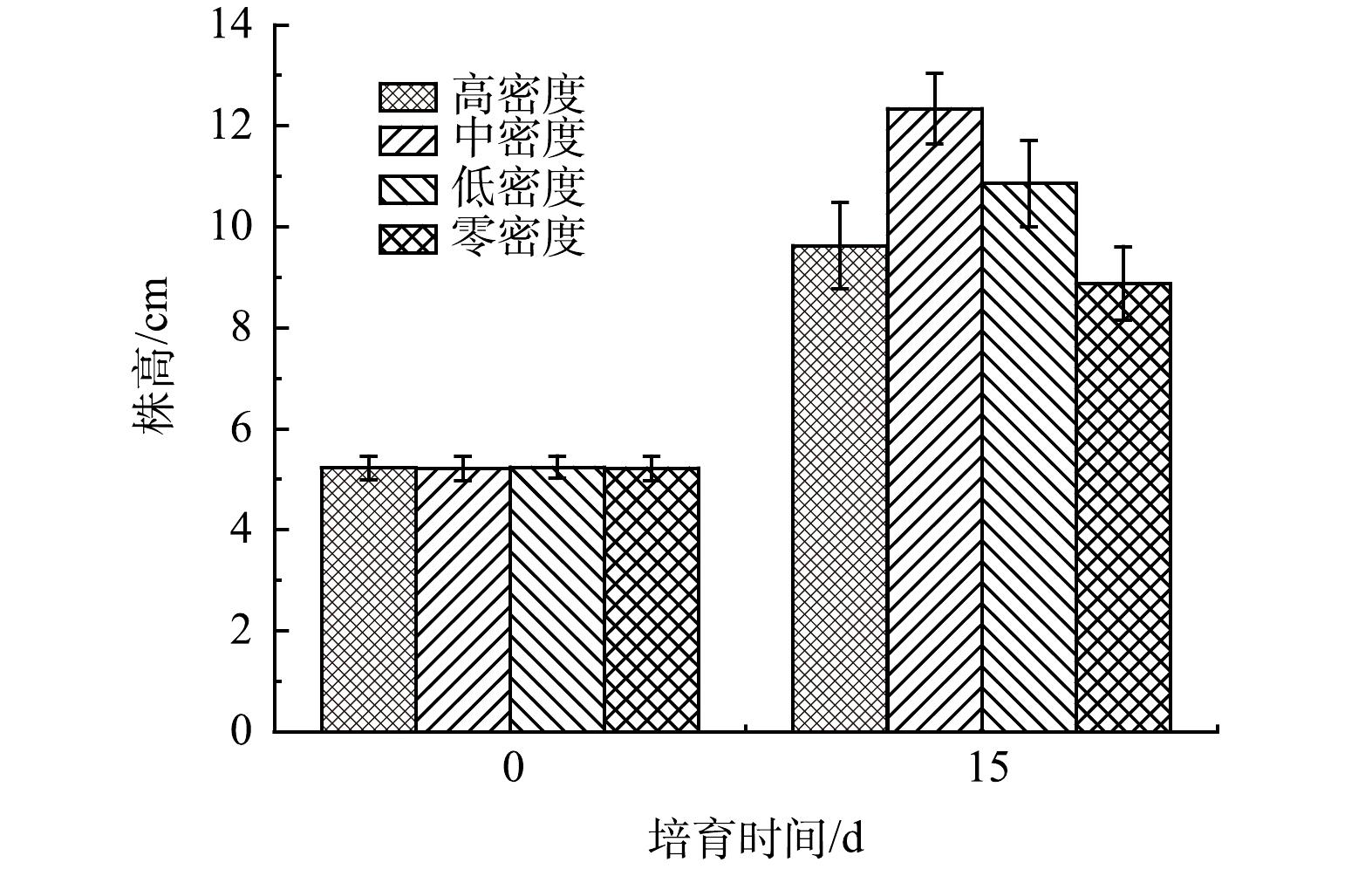
如图5和图6所示,中密度水丝蚓作用下的穗花狐尾藻生长状态最好(P<0.05);长势由高到低变化为中密度组>低密度组>高密度组>无水丝蚓组。由此可以看出水丝蚓对于穗花狐尾藻的生长是有着正面作用的。
-
穗花狐尾藻种植实验结束后对穗花狐尾藻中化感物质含量进行测定,结果见表1。藻细胞中化感物质含量按零密度组、高密度组、低密度组、中密度组顺序依次升高,说明霍甫水丝蚓可以促进穗花狐尾藻释放化感物质,促进作用与霍甫水丝蚓的密度相关,中密度促进作用最明显,低密度次之,高密度最弱。其中茉莉酸与水杨酸被认为化感效应信号分子,种植水中并未测出水杨酸,但穗花狐尾藻组织提取物中有检测到水杨酸。
-
目前,通过检测霍甫水丝蚓的肽转移总蛋白、金属硫蛋白(MTs)、血红蛋白、谷胱甘肽、三磷酸腺苷(ATP)等生物标记物,可监测污染水体中痕量重金属Al、Cd、Cr、Cu、Fe、Hg、Mn、Ni、Pb和Zn[19]。也有研究利用水丝蚓对全氟辛烷磺酰基化合物(perfluorooctane sulphonate,PFOs)进行毒性评价。LIU等[20]发现PFOs对水丝蚓有毒性效应,及其与Zn2+、Cd2+、Cu2+具有联合毒性,毒性与环境pH有关。水丝蚓对于有机氯农药亦有环境监测的作用。NIKKILA等[21]研究发现五氯酚对水丝蚓毒性作用表现为:出现回避行为,分泌粘蛋白,身体发生肿胀、脱色和裂解。一般可通过研究水丝蚓的状态来分析五氯酚是否超标。本研究主要利用水丝蚓来改变沉水植物穗花狐尾藻的根际微环境,以证明水丝蚓能够增强穗花狐尾藻的化感抑藻作用。
霍甫水丝蚓一方面可以通过摄食水体中的氮磷等营养物质,将其从溶解态变为颗粒态进入沉积物中,降低水体中的氮磷含量;另一方面其主要的排泄物中主要以氨氮(NH4+-N)和正磷酸盐(PO43−-P)为主,在水动力作用下释放到水环境中,此过程可将氮磷变为有机态,增加了水体中营养盐的浓度,加速了水华的暴发[11]。因此,有必要研究水丝蚓、穗花狐尾藻和蓝藻共培体系下蓝藻的生化状态以期求得水丝蚓在自然环境下的最佳密度。李鹏飞等[22]指出水丝蚓通过改变植物根系微环境,间接地影响植物的生长。KANG等[11]发现水丝蚓的排泄活动使NH4+-N沉降到沉积物中,同时溶解态磷通过附着到粪便中转化为颗粒态。其实底栖动物对于植物的生长作用早有大量研究。徐姗楠等[23]指出底栖动物通过扰动作用加速了红树凋落物的分解,提高了红树林生态系统的初级生产力。由图5和图6也可看出,水丝蚓对于穗花狐尾藻的生长有着促进作用,且水丝蚓的密度不同,促进程度也不相同。
-
抗氧化体系是生物体面对外界环境有害胁迫时的重要防御机制之一,其相关抗氧化酶活性的变化可反映生物体所受到的外界环境胁迫的程度[24-25]。SOD和过氧化物酶(peroxidase,POD)能使藻细胞内活性氧(reactive oxygen species,ROS)的产生与消除处于动态平衡,是重要的保护酶[8]。当藻细胞受到外界环境的胁迫时,ROS会在藻细胞中累积从而导致细胞膜脂质过氧化,抑制SOD和POD的活性。已有研究表明,酚酸类化感物质主要是通过氧化损伤来抑制蓝藻的生长[26]。SHAO[27]等研究表明,在化感物质邻苯三酚的胁迫作用下,铜绿微囊藻细胞SOD活性增强,MDA值明显高于对照组。张庭廷[28]等通过研究阿魏酸和对羟基苯甲酸对水华鱼腥藻以及蛋白核小球藻的化感抑制作用,发现藻细胞叶绿素-a含量下降,藻细胞SOD活性先上升后下降,下降的速度与细胞受损情况成正比;MDA在在实验过程中先上升,在后期开始下降,可能是化感物质导致藻细胞膜结构解体死亡。赵坤[29]利用水网藻种植水培养铜绿微囊藻时,藻细胞SOD活性出现了显著升高,达到峰值后下降至彻底失活的现象。本研究中,藻细胞SOD同样出现了显著升高,达到顶峰后下降;且不同密度水丝蚓处理组所表现出来的SOD变化趋势也不相同。具体为中密度组SOD变化最为显著,低密度组次之,高密度组再次之,无水丝蚓处理组SOD变化仅高于对照组。MDA值可作为表征生物细胞膜脂过氧化程度的重要指标,反映细胞膜完整性被破坏的程度[30]。本实验中,4个处理组MDA均逐渐升高,且升高速率与SOD变化基本吻合。说明种植水对铜绿微囊藻的生长产生了胁迫,导致其细胞膜发生了膜脂过氧化作用,生成了膜脂过氧化产物[31-32]。藻细胞受到种植水胁迫强度为中密度组>低密度组>高密度组>无水丝蚓组>对照组。
-
通过实验表明处理组蓝藻叶绿素-a含量有着显著变化,通过蓝藻的抗氧化系统变化可以推断出这主要是穗花狐尾藻释放出的化感物质作用于蓝藻,抑制了蓝藻的生长[33]。叶绿素-a作为藻类细胞重要的组成成分,也是可用来表征浮游植物生物量的最常用的指标之一[34]。研究表明化感物质可以通过多种不同的途径影响藻类正常的生长代谢,其中最常见的途径是通过抑制光系统Ⅱ(photosystem Ⅱ,PSⅡ)(叶绿素-a)活性来影响藻类的光合作用,从而抑制植物生长[35]。WANG[36]等利用调制荧光仪(Phyto-PAM)和Clark型氧电极实验表明铜绿微囊藻在焦性没食子酸和没食子酸的作用下,细胞中PSⅡ中心的电子传递链活性明显降低,导致电子传递被抑制。此外,陈国元[37]等研究表明,化感抑制作用对铜绿微囊藻具有累积效应,随着培养时间的延长,藻叶绿素-a的含量、光系统Ⅱ最大量子产量和有效产量均有所降低,藻叶绿素的合成和分解也受到影响,导致叶绿素-a含量下降。由图1和图2可以看出,随着时间的延长,各处理组种植水对藻细胞和藻细胞叶绿素的抑制率有所降低。这可能是由于植物分泌进入水体的化感物质本身较为分散、并且二次代谢产物大多不稳定,在化感消耗作用下,化感物质浓度迅速降低[38],导致叶绿素抑制率降低。化感物质主要可能是通过2个方面来影响藻类叶绿素-a含量:一方面,可能由于穗花狐尾藻种植水中存在某些具有抑藻效应的化感物质,直接阻碍了铜绿微囊藻叶绿素-a的合成,或影响了与其合成直接相关的物质的活性,使叶绿素-a的合成受到阻碍[39];另一方面,种植水中的化感物质对铜绿微囊藻细胞中一些具备捕获光能、传递光能、激发转换、维护叶绿素-a等重要辅助功能团色素如藻蓝蛋白、别藻蓝蛋白等造成了损伤,从而间接影响了叶绿素-a的合成及功能,导致藻细胞光合活性大大降低,影响其正常生长[40-41]。
-
化感作用概念的首次出现是在1937年由MOLISCH提出,直到1984年,RICE[42]明确了其定义:植物通过释放某种化学物质到环境中,从而对其他植物(包括微生物)造成有利或不利的影响。这种被释放出来的化学物质被称为化感物质。已有学者将化感物质分为14类,迄今为止,化感物质的相关研究主要是集中3种物质,分别为生物碱类、简单酚类和黄酮类[43]。NAKAI[38]等采用分析高效液相色谱(HPLC)和大气压化学电离质谱(APCI-MS)对穗花狐尾藻分泌出的化感物质进行了鉴定,结果表明,检测到4种可抑制生长的多酚类化合物,即埃拉酸、没食子酸和邻苯三酚以及(+)-儿茶素。GROSS等[44]在穗花狐尾藻种检测到的化感物质主要为没食子酸和鞣花酸。本研究通过对种植水和穗花狐尾藻组织提取物进行碳酸、烯酸、水杨酸和茉莉酸的测定,发现其主要化感物质为棕榈酸、硬脂酸、油酸、亚油酸、二十二碳二烯酸,其中含量最高的为二十二碳二烯酸,最高组含量可达0.86 mg·kg−1,最低组也达到0.6 mg·kg−1;组织提取物含量最高,可达1.31 mg·kg−1。不同密度水丝蚓作用下植物有效抑藻化感物质的含量大小顺序为中密度组>低密度组>高密度组>零密度组。而抑藻化感物质的含量能充分解释不同密度水丝蚓作用下各组种植水提取物之间抑藻率的变化趋势。
-
1)底栖动物水丝蚓对沉水植物穗花狐尾藻生长具有促进作用,且促进作用与水丝蚓的密度有关。
2)穗花狐尾藻种植水抑制藻类生长的机理可能为,通过抑制藻细胞正常生长和正常光合作用以及破坏藻细胞内部的抗氧化防御系统,进而对藻细胞产生毒害作用来达到对藻类的生长抑制。水丝蚓共培后穗花狐尾藻对藻细胞内部抗氧化系统稳定性破坏能力增强。从而初步证明水丝蚓对穗花狐尾藻抑藻机制有一定的增强作用。
3)不同密度的水丝蚓对穗花狐尾藻化感抑藻效应有着不同影响,当水丝蚓密度处于一个最适范围时,其对穗花狐尾藻化感抑藻效应最强。
不同投加密度的霍甫水丝蚓对穗花狐尾藻化感抑藻效应的影响
Effect of different density of Limnodrilus hoffmeisteri on allelopathic Myriophyllum spicatum inhibition
-
摘要: 利用沉水植物释放化感物质抑制水体中蓝藻生长是目前生态安全性极高的抑藻手段。穗花狐尾藻已被证实具有抑藻作用,为此,从外在生物作用可能会对沉水植物抑藻产生影响的角度出发,选用霍甫水丝蚓和穗花狐尾藻、铜绿微囊藻(FACHB-524)为研究对象,以低、中、高密度霍甫水丝蚓与穗花狐尾藻进行共培养,同时设置对照组(无霍甫水丝蚓投加即零密度组),并以蒸馏水代替种植水为空白组;对穗花狐尾藻种植水进行抑藻效应测定,对比分析了各组铜绿微囊藻的藻密度、SOD酶活性、MDA含量、叶绿素-a含量的变化。结果表明:处理组的抑藻效应显著高于对照组,霍甫水丝蚓对穗花狐尾藻的促进作用与霍甫水丝蚓密度相关,且霍甫水丝蚓对穗花狐尾藻化感抑藻效应存在一个最佳密度。不同密度(低、中、高)的霍甫水丝蚓的存在均可增强穗花狐尾藻化感作用。其中中密度组最为显著,其抑藻率最高可达到62.22%,各处理组叶绿素-a含量下降,中密度组最为显著。对藻细胞的生理指标进行分析,发现处理组中SOD 活性先升后降、MDA含量持续升高,化感物质造成藻细胞活性氧的过量积累可能是导致其死亡的直接原因。Abstract: The release of allelopathic substances by submerged plants to inhibit the growth of cyanobacteria in waterbody is a highly ecological safety method. Myriophyllum spicatum has been proved to have an algal inhibiting effect. In this study, Limnodrilus hoffmeisteri, Myriophyllum spicatum and Microcystis aeruginosa (FACHB-524) were chosen as the main research objects from the perspective that external biological effects might affect the algal inhibiting effect of submerged plants. High, medium, and low densities of Limnodrilus hoffmeisteri and Myriophyllum spicatum were cocultured, and control 1 (zero density group without the addition of Limnodrilus hoffmeisteri) and control 2 (control group) with distilled water instead of planting water were set up in the experments. The algal inhibiting effect of Myriophyllum spicatum planting water was determined. The variations of density, SOD activity, MDA content and chlorophyll a content of Microcystis aeruginosa in each group were measured for comparative analysis. The results showed that the algal inhibiting effect of the treatment group was significantly higher than that of the control group. The promoting effect of the Limnodrilus hoffmeisteri on the Myriophyllum spicatum was related to the density of the Limnodrilus hoffmeisteri, and there was an optimal density of the allelopathic algal inhibiting effect of the Limnodrilus hoffmeisteri on the Myriophyllum spicatum. The allelopathy of Myriophyllum spicatum was enhanced by the presence of different densities (low, medium and high) of Limnodrilus hoffmeisteri. of which the medium density group had the most significant inhibiting effect with the highest algal inhibiting rate of 62.22%. The content of chlorophyll a decreased in all treatment groups, and the medium density group was the most one. The analysis of physiological indexes of algal cells showed that SOD activity increased firstly and then decreased, and MDA content continued to increase in the treatment group. The excessive accumulation of reactive oxygen species caused by allelopathic substances might be the direct cause of algal cell death.
-
水体富营养化导致藻类等水生浮游植物大量繁殖并形成水华,其中蓝藻属的铜绿微囊藻引起的水华最为常见,还释放藻毒素,严重威胁到其他水生生物生态功能的正常发挥,甚至影响人类的健康[1]。为此我们已经提出了许多方法来管理广泛存在的蓝藻水华,如水更新、沉积物疏浚、使用硫酸铜、高锰酸钾、氯和臭氧开发蓝藻杀菌剂,以及建造湿地和由植物、动物和微生物组成的漂浮岛屿[2]。然而,这些方法存在各种缺点,如成本高、二次污染、流域小、不切实际等,使得目前为止仍然没有一种经济有效环境友好型的治理方法[3]。因此,有必要确定具有高效、安全和经济特征的环境友好型蓝藻控制策略。化感作用的发现和利用为降低蓝藻细菌的丰度、维持生态平衡和可持续性提供了一种新的、有前途的方法[4]。
目前已经证实了化感作用可通过损害细胞微结构、细胞活力、光合作用、细胞内氧化还原平衡和酶活性,来抑制蓝藻的生长,并导致细胞程序性死亡和细胞外物质的释放[5]。高云霓等发现穗花狐尾藻种植水显著抑制铜绿微囊藻产毒株和非产毒株的生长,影响其生理过程[6]。崔莉凤[7]等通过研究证实了水生植物干燥体仍具有化感物质,穗花狐尾藻浸出液对微囊藻的生长和产毒都具有很强的抑制能力,其中对微囊藻的生长抑制率最高能够达到91.89%。朱俊英[8]等在共培养条件下研究发现铜绿微囊藻的叶绿素-a含量及叶绿素荧光参数在穗花狐尾藻分泌的化感物质作用下显著降低。高浩杰[9]以轮叶狐尾藻为实验材料,证实了轮叶狐尾藻分泌出的化感物质会破坏铜绿微囊藻的细胞膜结构从而造成细胞损伤,导致铜绿微囊藻死亡。诺加罗等[10]发现霍甫水丝蚓生物扰动能够提高反硝化菌的活性,同时增强了氨化作用。KANG等[11]也发现霍甫水丝蚓的投加有利于有机质的分解,增加碳氮比,为微生物脱氮提供了充足的碳源,同时在水丝蚓的扰动作用下水-沉积物界面处溶解氧(dissolved oxygen,DO)降低,微生物的电子受体在沉积物水平上重新分布,利于反硝化菌的生长和反硝化进程加快,刺激了微生物脱氮活性。霍甫水丝蚓可以改善水生植物根系土壤的理化性质,促进养分循环,利于植物生长,提高植物产量和抵抗外在胁迫的能力,可提高水生植物的修复效果[12]。
底栖动物是水生生态系统的重要组成部分之一,在维持生物多样性和生态平衡方面具有巨大的应用潜力。底栖动物很容易食用底栖藻类、浮游植物和陆生有机物,其本身就是鱼类食物的组成部分[13]。ZUO[14]等采用微观实验方法探讨了底栖动物对大型植物化感作用的影响,结果表明,4种代表性的底栖动物在单独培养中表现出较低的海藻抑制作用,当底栖动物与被试微藻完全混合时,其抑制率有所提高。此外,大型植物对海藻的化感抑制作用与底栖动物平均单株生物量呈显著正相关。与不含底栖动物和沉水植物的疏浚区相比,沉水植物填充区藻类生物量和密度显著降低。水底大型植物和底栖动物共同存在的区域藻华最少,这是底栖动物和大型植物协同作用的结果。因此,本研究通过霍甫水丝蚓来改变穗花狐尾藻的根际微环境,对共培种植水纯化且将其加入铜绿微囊藻中,通过对铜绿微囊藻生化指标的测定考察了水丝蚓对穗花狐尾藻化感作用的影响,为底栖动物促进大型水生植物的化感作用提供依据。
1. 材料与方法
1.1 实验材料
实验所用铜绿微囊藻(FACHB-524) 购自中国科学院水生生物研究所淡水藻种库。通过无菌BG11培养基培养,光照强度为2 000 lx,光暗周期为12 h:12 h,温度为(25±1) °C,每天手动定时摇瓶3次,每隔7 d重新接种1次,连续多代驯化培养,等待其长到对数生长期且藻类浓度约为 8×105细胞·mL−1时进行实验。穗花狐尾藻2021年采自芜湖市扁担河,栽种于实验室种植桶中,桶高为0.5 m,底部半径为0.12 m。种植桶中添加10 cm厚底泥,10%霍格兰营养液培养。选取大小较为一致,鲜质量相同的穗花狐尾藻幼苗每5株为1组栽入4个相同规格的种植桶中,加入购自花鸟市场的水丝蚓进行连续培养。当穗花狐尾藻幼苗长大后,取其种植水用于实验。超氧化物歧化酶(superoxide dismutase,SOD)、丙二醛(malonic dialdehyde,MDA)、总磷(total phosphorus,TP)分析试剂盒来自南京建成公司。
1.2 实验设计
设置3个穗花狐尾藻处理组:高密度水丝蚓(15 000 条·m−2)处理组、中密度水丝蚓(8 300 条·m−2)处理组、低密度水丝蚓(1 600 条·m−2)处理组,零密度处理组(对照组)为穗花狐尾藻种植体系不加水丝蚓。培养体系统一使用10%霍格兰营养液,每组设置3个平行实验。每组实验取400 mL穗花狐尾藻种植水抽滤过0.22 μm孔径的滤膜,去除其中的颗粒杂质和微生物干扰后使用乙酸乙酯按水相与乙酸乙酯相体积比1:3进行旋转蒸发提纯,再用5 mL蒸馏水进行超声萃取,萃取液用于抑藻实验。种植水制备和铜绿微囊藻接种均在无菌操作台上进行,所用器皿均提前做好灭菌处理。实验在250 mL锥形瓶中进行,瓶中加入100 mL对数生长期铜绿微囊藻,暴露于5 mL种植水萃取液,藻起始密度约为8×105细胞·mL−1。连续培养7 d,每天手动摇匀3次。分别于0、2、4、6 d测定暴露的铜绿微囊藻中膜脂过氧化产物MDA浓度、SOD活性、叶绿素-a(chlorophyl,Chl-a)含量,并且每天记录铜绿微囊藻藻密度。
1)铜绿微囊藻抑制率的测量。每天定时取铜绿微囊藻1 mL,并加入9 mL蒸馏水与鲁哥氏固定液混合液进行混合固定,静置10 min。再取20 μL用血球计数板在光学显微镜(BMSG20BD,上海BM光学仪器制造有限公司)进行计数。藻细胞抑制率按式(1)计算。
I=X0−XX0×100% (1) 式中:I为藻细胞抑制率,%;X0为对照组细胞数,105个;X为处理组细胞数,105个。
2)铜绿微囊藻叶绿素-a的测定。以加5 mL高、中、低密度水丝蚓穗花狐尾藻种植水和无水丝蚓穗花狐尾藻种植水为处理组,5 mL蒸馏水为对照组;每2 d测定1次叶绿素-a。采用90%丙酮溶液提取、分离藻细胞叶绿素-a[15],用三色法测定[16]。叶绿素-a的含量根据式(2)计算。
C=11.64A663−2.16A645+0.1A630 (2) 式中:C表示叶绿素-a的质量浓度,mg·L−1;A663、A645、A630分别为紫外可见分光光度计下663、645、630 nm波长处吸光度值。
3)铜绿微囊藻SOD活性和MDA含量的测定。取10 mL藻液于灭菌离心管中,冰浴下超声波破碎(JY92-Ⅱ型超声波细胞粉碎机-宁波新芝生物科技股份有限公司生产,超声5 s间隔5 s工作次数为24次功率600 W重复3次),按照自南京建成公司购买的SOD试剂盒、MDA试剂盒说明书所述步骤进行测定。
4)穗花狐尾藻株高、鲜质量的测定。使用直尺和分析天平测量穗花狐尾藻栽种前的株高和鲜质量;栽种后与水丝蚓进行共培,实验后再取出穗花狐尾藻对测量其株高和鲜质量。
5)化感物质定性与定量。取种植水乙酸乙酯萃取液1 mL加入15 mL离心管中,继续加入2%盐酸-甲醇溶液,3 mL氯仿甲醇溶液(体积比1∶1),10 μL十九烷酸甲醇内标。于85 ℃水浴锅中水浴1 h。等温度降至室温,在离心管中加入1 mL正己烷,振荡萃取2 min之后,静置1 h,等待分层。取上清液100 μL,用正己烷定容到1 mL。用0.45 μm滤膜过滤后采用ThermoFisher Trace1310ISQ气相色谱质谱联用仪测试[17]。
1.3 数据处理与分析
采用Excel 2013对实验数据进行整理,利用SPSS 19.0进行数据分析,所有处理均采用正态分布检验和方差齐性检验,图象绘制使用OriginPro 9.1。
2. 结果与分析
2.1 水丝蚓增强了穗花狐尾藻对铜绿微囊藻的抑制
由图1可以得出,在水丝蚓的作用下,穗花狐尾藻的化感作用得到增强,对蓝藻的抑制率明显高于无水丝蚓培养下的穗花狐尾藻(P<0.05)。处理组抑制率均呈现先升高后降低的趋势,可能是铜绿微囊藻暴露于种植水后化感物质参与到藻细胞合成代谢过程中,所以抑制率开始呈现上升趋势。但由于化感物质易被藻细胞代谢分解,随着时间的延长浓度会降低,所以抑制率后呈下降趋势。
中密度水丝蚓处理组化感抑藻效应最强,于第4天抑制率达到最高62.22%,显著高于其他处理组和零密度组。这可能是因为水丝蚓的生物扰动增强穗花狐尾藻化感物质的释放,随着水丝蚓生物量的增加,扰动增强,穗花狐尾藻化感物质的释放增多;但当水丝蚓生物量达到临界值时,水丝蚓的生化作用会对穗花狐尾藻造成胁迫作用,从而使得穗花狐尾藻化感物质的释放量开始降低。
2.2 铜绿微囊藻叶绿素-a的变化
藻类和浮游植物依靠光合作用生长,而叶绿素-a是所有藻类的主要光合色素,通过测定藻类和浮游植物叶绿素-a含量可掌握水体的初级生产力情况,可将叶绿素-a作为其光合作用潜力的一个指标[18]。由图2可见,处理组中铜绿微囊藻叶绿素-a含量明显低于对照组。这进一步说明霍甫水丝蚓可促进穗花狐尾藻的抑藻效应。各实验组中铜绿微囊藻叶绿素-a含量变化趋势与图1中铜绿微囊藻生长抑制率高度一致,即处理组叶绿素-a含量低于对照组。处理组由于水丝蚓密度不同,处理效果也不同,其中中密度组对叶绿素-a含量抑藻效应最强。
2.3 铜绿微囊藻抗氧化系统的变化
由图3可见,对照组的SOD活性在实验过程中几乎没有变化。说明对照组的藻类生长状况良好,基本上没有受到外界条件的胁迫。而实验组铜绿微囊藻细胞中SOD活性呈现先升高后降低(P<0.05)。说明原有的氧自由基的产生与清除平衡被打破。当藻细胞暴露于实验组种植水时,细胞受到胁迫作用,细胞内产生大量氧自由基,藻细胞尝试建立新的氧自由基平衡,SOD活性升高,提高抗氧化能力。但由于持续的暴露在种植水中,细胞已经受到损伤,抗氧化系统被破坏,SOD活性开始下降。这可以由图4中藻细胞的MDA值变化可以验证。实验组藻细胞MDA从开始的1.326 nmol·mL−1开始持续增高,对照组MDA数值略微变化,即实验组藻细胞受到持续伤害,对照组正常生长;藻细胞受到伤害程度按无水丝蚓组、高密度组、低密度组、中密度组顺序依次升高。由图3和图4可见,当铜绿微囊藻暴露于种植水时,藻细胞受到损伤,MDA升高,抗氧化系统迅速反应,SOD活性升高;但随着持续暴露,细胞损伤加剧,MDA持续增高,抗氧化系统遭到破坏,SOD活性降低。
2.4 穗花狐尾藻鲜质量与株高变化
如图5和图6所示,中密度水丝蚓作用下的穗花狐尾藻生长状态最好(P<0.05);长势由高到低变化为中密度组>低密度组>高密度组>无水丝蚓组。由此可以看出水丝蚓对于穗花狐尾藻的生长是有着正面作用的。
2.5 化感物质含量测定
穗花狐尾藻种植实验结束后对穗花狐尾藻中化感物质含量进行测定,结果见表1。藻细胞中化感物质含量按零密度组、高密度组、低密度组、中密度组顺序依次升高,说明霍甫水丝蚓可以促进穗花狐尾藻释放化感物质,促进作用与霍甫水丝蚓的密度相关,中密度促进作用最明显,低密度次之,高密度最弱。其中茉莉酸与水杨酸被认为化感效应信号分子,种植水中并未测出水杨酸,但穗花狐尾藻组织提取物中有检测到水杨酸。
表 1 化感物质含量测定Table 1. Allelochemicals content determinationmg·kg−1 化感物质 高密度 低密度 中密度 零密度 组织提取物 十一碳酸(十一酸) 0.33 0.36 0.39 0.21 0.28 十六碳酸(棕榈酸) 0.21 0.23 0.26 0.18 1.78 十八碳酸(硬脂酸) 0.21 0.25 0.27 0.13 0.38 顺-9-十八碳一烯酸(油酸) 0.13 0.17 0.26 0.10 1.00 顺,顺-9,12-十八碳二烯酸(亚油酸) 0.23 0.28 0.43 0.10 6.17 顺,顺,顺-9,12,15-十八碳三烯酸(亚麻酸) 0 0 0 0 0.37 二十碳酸(花生脂酸) 0 0 0 0 0.20 顺,顺-11,14-二十碳二烯酸(花生二烯) 0 0 0 0 0.21 顺-13,16-二十二碳二烯酸(二十二碳二烯酸) 0.71 0.76 0.86 0.60 1.31 水杨酸 0 0 0 0 0.76 茉莉酸 0.42 0.55 0.71 0.33 6.23 3. 讨论
3.1 水丝蚓选择的影响意义
目前,通过检测霍甫水丝蚓的肽转移总蛋白、金属硫蛋白(MTs)、血红蛋白、谷胱甘肽、三磷酸腺苷(ATP)等生物标记物,可监测污染水体中痕量重金属Al、Cd、Cr、Cu、Fe、Hg、Mn、Ni、Pb和Zn[19]。也有研究利用水丝蚓对全氟辛烷磺酰基化合物(perfluorooctane sulphonate,PFOs)进行毒性评价。LIU等[20]发现PFOs对水丝蚓有毒性效应,及其与Zn2+、Cd2+、Cu2+具有联合毒性,毒性与环境pH有关。水丝蚓对于有机氯农药亦有环境监测的作用。NIKKILA等[21]研究发现五氯酚对水丝蚓毒性作用表现为:出现回避行为,分泌粘蛋白,身体发生肿胀、脱色和裂解。一般可通过研究水丝蚓的状态来分析五氯酚是否超标。本研究主要利用水丝蚓来改变沉水植物穗花狐尾藻的根际微环境,以证明水丝蚓能够增强穗花狐尾藻的化感抑藻作用。
霍甫水丝蚓一方面可以通过摄食水体中的氮磷等营养物质,将其从溶解态变为颗粒态进入沉积物中,降低水体中的氮磷含量;另一方面其主要的排泄物中主要以氨氮(NH4+-N)和正磷酸盐(PO43−-P)为主,在水动力作用下释放到水环境中,此过程可将氮磷变为有机态,增加了水体中营养盐的浓度,加速了水华的暴发[11]。因此,有必要研究水丝蚓、穗花狐尾藻和蓝藻共培体系下蓝藻的生化状态以期求得水丝蚓在自然环境下的最佳密度。李鹏飞等[22]指出水丝蚓通过改变植物根系微环境,间接地影响植物的生长。KANG等[11]发现水丝蚓的排泄活动使NH4+-N沉降到沉积物中,同时溶解态磷通过附着到粪便中转化为颗粒态。其实底栖动物对于植物的生长作用早有大量研究。徐姗楠等[23]指出底栖动物通过扰动作用加速了红树凋落物的分解,提高了红树林生态系统的初级生产力。由图5和图6也可看出,水丝蚓对于穗花狐尾藻的生长有着促进作用,且水丝蚓的密度不同,促进程度也不相同。
3.2 穗花狐尾藻种植水对铜绿微囊藻抗氧化体系的影响
抗氧化体系是生物体面对外界环境有害胁迫时的重要防御机制之一,其相关抗氧化酶活性的变化可反映生物体所受到的外界环境胁迫的程度[24-25]。SOD和过氧化物酶(peroxidase,POD)能使藻细胞内活性氧(reactive oxygen species,ROS)的产生与消除处于动态平衡,是重要的保护酶[8]。当藻细胞受到外界环境的胁迫时,ROS会在藻细胞中累积从而导致细胞膜脂质过氧化,抑制SOD和POD的活性。已有研究表明,酚酸类化感物质主要是通过氧化损伤来抑制蓝藻的生长[26]。SHAO[27]等研究表明,在化感物质邻苯三酚的胁迫作用下,铜绿微囊藻细胞SOD活性增强,MDA值明显高于对照组。张庭廷[28]等通过研究阿魏酸和对羟基苯甲酸对水华鱼腥藻以及蛋白核小球藻的化感抑制作用,发现藻细胞叶绿素-a含量下降,藻细胞SOD活性先上升后下降,下降的速度与细胞受损情况成正比;MDA在在实验过程中先上升,在后期开始下降,可能是化感物质导致藻细胞膜结构解体死亡。赵坤[29]利用水网藻种植水培养铜绿微囊藻时,藻细胞SOD活性出现了显著升高,达到峰值后下降至彻底失活的现象。本研究中,藻细胞SOD同样出现了显著升高,达到顶峰后下降;且不同密度水丝蚓处理组所表现出来的SOD变化趋势也不相同。具体为中密度组SOD变化最为显著,低密度组次之,高密度组再次之,无水丝蚓处理组SOD变化仅高于对照组。MDA值可作为表征生物细胞膜脂过氧化程度的重要指标,反映细胞膜完整性被破坏的程度[30]。本实验中,4个处理组MDA均逐渐升高,且升高速率与SOD变化基本吻合。说明种植水对铜绿微囊藻的生长产生了胁迫,导致其细胞膜发生了膜脂过氧化作用,生成了膜脂过氧化产物[31-32]。藻细胞受到种植水胁迫强度为中密度组>低密度组>高密度组>无水丝蚓组>对照组。
3.3 水丝蚓作用下穗花狐尾藻种植水对铜绿微囊藻叶绿素-a的影响
通过实验表明处理组蓝藻叶绿素-a含量有着显著变化,通过蓝藻的抗氧化系统变化可以推断出这主要是穗花狐尾藻释放出的化感物质作用于蓝藻,抑制了蓝藻的生长[33]。叶绿素-a作为藻类细胞重要的组成成分,也是可用来表征浮游植物生物量的最常用的指标之一[34]。研究表明化感物质可以通过多种不同的途径影响藻类正常的生长代谢,其中最常见的途径是通过抑制光系统Ⅱ(photosystem Ⅱ,PSⅡ)(叶绿素-a)活性来影响藻类的光合作用,从而抑制植物生长[35]。WANG[36]等利用调制荧光仪(Phyto-PAM)和Clark型氧电极实验表明铜绿微囊藻在焦性没食子酸和没食子酸的作用下,细胞中PSⅡ中心的电子传递链活性明显降低,导致电子传递被抑制。此外,陈国元[37]等研究表明,化感抑制作用对铜绿微囊藻具有累积效应,随着培养时间的延长,藻叶绿素-a的含量、光系统Ⅱ最大量子产量和有效产量均有所降低,藻叶绿素的合成和分解也受到影响,导致叶绿素-a含量下降。由图1和图2可以看出,随着时间的延长,各处理组种植水对藻细胞和藻细胞叶绿素的抑制率有所降低。这可能是由于植物分泌进入水体的化感物质本身较为分散、并且二次代谢产物大多不稳定,在化感消耗作用下,化感物质浓度迅速降低[38],导致叶绿素抑制率降低。化感物质主要可能是通过2个方面来影响藻类叶绿素-a含量:一方面,可能由于穗花狐尾藻种植水中存在某些具有抑藻效应的化感物质,直接阻碍了铜绿微囊藻叶绿素-a的合成,或影响了与其合成直接相关的物质的活性,使叶绿素-a的合成受到阻碍[39];另一方面,种植水中的化感物质对铜绿微囊藻细胞中一些具备捕获光能、传递光能、激发转换、维护叶绿素-a等重要辅助功能团色素如藻蓝蛋白、别藻蓝蛋白等造成了损伤,从而间接影响了叶绿素-a的合成及功能,导致藻细胞光合活性大大降低,影响其正常生长[40-41]。
3.4 水丝蚓对于穗花狐尾藻释放化感物质的影响
化感作用概念的首次出现是在1937年由MOLISCH提出,直到1984年,RICE[42]明确了其定义:植物通过释放某种化学物质到环境中,从而对其他植物(包括微生物)造成有利或不利的影响。这种被释放出来的化学物质被称为化感物质。已有学者将化感物质分为14类,迄今为止,化感物质的相关研究主要是集中3种物质,分别为生物碱类、简单酚类和黄酮类[43]。NAKAI[38]等采用分析高效液相色谱(HPLC)和大气压化学电离质谱(APCI-MS)对穗花狐尾藻分泌出的化感物质进行了鉴定,结果表明,检测到4种可抑制生长的多酚类化合物,即埃拉酸、没食子酸和邻苯三酚以及(+)-儿茶素。GROSS等[44]在穗花狐尾藻种检测到的化感物质主要为没食子酸和鞣花酸。本研究通过对种植水和穗花狐尾藻组织提取物进行碳酸、烯酸、水杨酸和茉莉酸的测定,发现其主要化感物质为棕榈酸、硬脂酸、油酸、亚油酸、二十二碳二烯酸,其中含量最高的为二十二碳二烯酸,最高组含量可达0.86 mg·kg−1,最低组也达到0.6 mg·kg−1;组织提取物含量最高,可达1.31 mg·kg−1。不同密度水丝蚓作用下植物有效抑藻化感物质的含量大小顺序为中密度组>低密度组>高密度组>零密度组。而抑藻化感物质的含量能充分解释不同密度水丝蚓作用下各组种植水提取物之间抑藻率的变化趋势。
4. 结论
1)底栖动物水丝蚓对沉水植物穗花狐尾藻生长具有促进作用,且促进作用与水丝蚓的密度有关。
2)穗花狐尾藻种植水抑制藻类生长的机理可能为,通过抑制藻细胞正常生长和正常光合作用以及破坏藻细胞内部的抗氧化防御系统,进而对藻细胞产生毒害作用来达到对藻类的生长抑制。水丝蚓共培后穗花狐尾藻对藻细胞内部抗氧化系统稳定性破坏能力增强。从而初步证明水丝蚓对穗花狐尾藻抑藻机制有一定的增强作用。
3)不同密度的水丝蚓对穗花狐尾藻化感抑藻效应有着不同影响,当水丝蚓密度处于一个最适范围时,其对穗花狐尾藻化感抑藻效应最强。
-
表 1 化感物质含量测定
Table 1. Allelochemicals content determination
mg·kg−1 化感物质 高密度 低密度 中密度 零密度 组织提取物 十一碳酸(十一酸) 0.33 0.36 0.39 0.21 0.28 十六碳酸(棕榈酸) 0.21 0.23 0.26 0.18 1.78 十八碳酸(硬脂酸) 0.21 0.25 0.27 0.13 0.38 顺-9-十八碳一烯酸(油酸) 0.13 0.17 0.26 0.10 1.00 顺,顺-9,12-十八碳二烯酸(亚油酸) 0.23 0.28 0.43 0.10 6.17 顺,顺,顺-9,12,15-十八碳三烯酸(亚麻酸) 0 0 0 0 0.37 二十碳酸(花生脂酸) 0 0 0 0 0.20 顺,顺-11,14-二十碳二烯酸(花生二烯) 0 0 0 0 0.21 顺-13,16-二十二碳二烯酸(二十二碳二烯酸) 0.71 0.76 0.86 0.60 1.31 水杨酸 0 0 0 0 0.76 茉莉酸 0.42 0.55 0.71 0.33 6.23 -
[1] ZHANG W H, ZHANG X H, ZHANG G M. Variation of microcystions in a lake forwater supply[J]. Environmental Science and Health, 2003, 38(12): 2857-2865. doi: 10.1081/ESE-120025836 [2] GAO Y N, DONG J, FU Q Q. Allelopathic effects of submerged macrophytes on phytoplankton[J]. Allelopathy Journal, 2017, 40(1): 1-22. doi: 10.26651/2017-40-1-1062 [3] ZUO S P, WANG H M, GAN L D, et al. Allelopathy appraisal of worm metabolites in the synergistic effect between Limnodrilus hoffmeisteri and Potamogeton malaianus on algal suppression[J]. Ecotoxicology and Environmental Safety, 2019, 182: 109482. doi: 10.1016/j.ecoenv.2019.109482 [4] HUISMAN J, CODD G A, PAERL H W, et al. Cyanobacterial blooms[J]. Nature Reviews Microbiology, 2018, 16(8): 471-483. doi: 10.1038/s41579-018-0040-1 [5] GROSS E M. Allelopathy of aquatic autotrophs[J]. Critical Reviews in Plant Sciences. 2003, 22 (3/4): 313-339. [6] 高云霓, 武静, 杨惠, 等. 穗花狐尾藻种植水对铜绿微囊藻的影响[J]. 水资源保护, 2021, 37(6): 162-167. doi: 10.3880/j.issn.1004-6933.2021.06.024 [7] 崔莉凤, 赵硕, 吴溶, 等. 穗花狐尾藻浸出液对铜绿微囊藻生长和产毒的影响[J]. 环境科学与技术, 2010, 33(S2): 50-54. [8] 朱俊英, 刘碧云, 王静, 等. 穗花狐尾藻化感作用对铜绿微囊藻光合效率的影响[J]. 环境科学, 2011, 32(10): 2904-2908. doi: 10.13227/j.hjkx.2011.10.010 [9] 高浩杰. 轮叶狐尾藻对镉和铜绿微囊藻污染水体的净化能力研究[D]. 雅安: 四川农业大学, 2018. [10] NOGARO G, BURGIN A J. Influence of bioturbation on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in freshwater sediments[J]. Biogeochemistry, 2014(120): 279-294. [11] KANG Y, ZHANG J, XIE H, et al. Enhanced nutrient removal and mechanisms study in benthic fauna added surface-flow constructed wetlands: The role of Tubifex tubifex[J]. Bioresource Technology, 2017(224): 157-165. [12] 邵明昊, 左胜鹏, 洪文秀. 霍甫水丝蚓(Limnodrilus hoffmeisteri)环境监测与生态修复效应研究进展[J]. 环境科学导刊, 2020, 39(2): 1-9. [13] DEVLIN S P, ZANDEN J V, Vadeboncoeur Y. Depth-specific variation in carbon isotopes demonstrates resource partitioning among the littoral zoobenthos[J]. Freshwater Biology, 2013, 58(11): 2389-2400. [14] ZUO S P, ZHANG S F, ZHOU S B, et al. Benthic Fauna Promote Algicidal Effect of Allelopathic Macrophytes on Microcystis aeruginosa[J]. Journal of Plant Growth Regulation, 2016, 35(3): 646-654. doi: 10.1007/s00344-015-9566-x [15] GREGOR J, MARSALEK B. Freshwater phytoplankton quantification by chlorophyll a: a comparative study of in vitro, in vivo and in situ methods.[J]. Water research, 2004, 38(3): 517-522. doi: 10.1016/j.watres.2003.10.033 [16] 戴荣继, 黄春, 佟斌, 等. 藻类叶绿素及其降解产物的测定方法[J]. 中央民族大学学报(自然科学版), 2004(1): 75-80. [17] ALENCAR FILHO J M T, TEIXEIRA H A P, Sampaio P A. Phytochemical analysis in Alternanthera brasiliana by LC-MS/MS and GC-MS[J]. Natural Product Research, 2019(1): 1-5. [18] 李磊, 侯文华. 荷花和睡莲种植水对铜绿微囊藻生长的抑制作用研究[J]. 环境科学, 2007(10): 2180-2187. doi: 10.3321/j.issn:0250-3301.2007.10.005 [19] OZTETIK E, CICEK A, Arslan N. Early antioxidative defence responses in the aquatic worms(Limnodrilus sp)in Porsuk Creek in Eskisehir(Turkey)[J]. Toxicology and Industrial Health, 2013, 29(6): 541-554. doi: 10.1177/0748233712442734 [20] LI C G, QU R J, CHEN J, et al. The pH-dependent toxicity of triclosan to five aquatic organisms (Daphnia magna, Photobacterium phosphoreum, Danio rerio, Limnodrilushoffmeisteri, and Carassius auratus)[J]. Environmental science and pollution research international, 2018, 25(10): 9636-9646. doi: 10.1007/s11356-018-1284-z [21] NIKKILA A, HALME A, KUKKONEN J VK. Toxicokinetics, toxicity and lethal body residues of two chlorophenols in the oligochaete worm, Lumbriculus variegatus, in different sediments[J]. Chemosphere, 2003, 51(1): 35-46. doi: 10.1016/S0045-6535(02)00791-9 [22] 李鹏飞. 底栖类动物生态湿地的构建和相关运行机制研究[D]. 济南: 山东大学, 2016. [23] 徐姗楠, 陈作志, 黄小平, 等. 底栖动物对红树林生态系统的影响及生态学意义[J]. 生态学杂志, 2010, 29(4): 812-820. doi: 10.13292/j.1000-4890.2010.0125 [24] WANG J X, XIE P. Antioxidant enzyme activities of Microcystis aeruginosa in response to nonylphenols and degradation of nonylphenols by Maeruginosa[J]. Environmental Geochemistry and health, 2007, 29(5): 375-83. doi: 10.1007/s10653-007-9081-5 [25] HONG Y, HU H Y, XIE X, et al. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa[J]. Aquatic Toxicology, 2008, 91(3): 262-269. [26] HONDA T, TAKAHASHI H, SAKO Y, et al. Gene expression of Microcystis aeruginosa during infection of cyanomyovirus Ma-LMM01[J]. Fisheries Science, 2014, 80(1): 83-91. doi: 10.1007/s12562-013-0685-7 [27] SHAO J H, WU Z X, YU G L, et al. Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): From views of gene expression and antioxidant system[J]. Chemosphere, 2009, 75(7): 924-928. doi: 10.1016/j.chemosphere.2009.01.021 [28] 张庭廷, 吴安平, 何梅, 等. 酚酸类物质对水华藻类的化感作用及其机理[J]. 中国环境科学, 2007(4): 472-476. doi: 10.3321/j.issn:1000-6923.2007.04.009 [29] 赵坤. 水网藻对铜绿微囊藻的化感抑制作用研究[D]. 北京: 华北电力大学, 2011. [30] SHI S Y, TANG D S, LIU Y D. Effects of an algicidal bacterium Pseudomonas mendocina on the growth and antioxidant system of Aphanizomenonflos-aquae[J]. Current Microbiology, 2009, 59(2): 107-112. doi: 10.1007/s00284-009-9404-0 [31] CHOUDHARY M, JETLEY U K, KHAN M A, et al. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5[J]. Ecotoxicology and Environmental Safety, 2006, 66(2): 204-209. [32] YANG W W, TANG Z P, ZHOU F Q, et al. Toxicity studies of tetracycline on Microcystis aeruginosa and Selenastrumcapricornutum[J]. Environmental Toxicology and Pharmacology, 2013, 35(2): 320-324. doi: 10.1016/j.etap.2013.01.006 [33] GROSS E M, MEYER H, SCHILLING G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum[J]. Phytochemistry, 1996, 41(1): 133-138. doi: 10.1016/0031-9422(95)00598-6 [34] 于海燕, 周斌, 胡尊英, 等. 生物监测中叶绿素a浓度与藻类密度的关联性研究[J]. 中国环境监测, 2009, 25(6): 40-43. doi: 10.3969/j.issn.1002-6002.2009.06.012 [35] 周立红. 基于化感作用的环境友好型抑藻剂的研究[D]. 厦门: 厦门大学, 2008. [36] WANG J, ZHU J Y, GAO Y N, et al. Toxicity of allelochemicals released by submerged macrophytes on phytoplankton[J]. Allelopathy Journal, 2013, 31(1): 199-210. [37] 陈国元, 李青松, 谢莆尧, 等. 共培养条件下黄菖蒲和狭叶香蒲对铜绿微囊藻光合系统的影响[J]. 环境工程学报, 2015, 9(9): 4145-4152. doi: 10.12030/j.cjee.20150909 [38] NAKAI S, INOUE Y, HOSOMI M, et al. Growth inhibition of blue–green algae by allelopathic effects of macrophytes[J]. Water Science and Technology, 1999, 39(8): 47-53. doi: 10.2166/wst.1999.0382 [39] YE C P, LIAO H P, YANG Y F. Allelopathic inhibition of photosynthesis in the red tide-causing marine alga, Scrippsiellatrochoidea (Pyrrophyta), by the dried macroalga, Gracilarialemaneiformis (Rhodophyta)[J]. Journal of Sea Research, 2014, 90: 10-15. doi: 10.1016/j.seares.2014.02.015 [40] 马建飞, 林瀚智, 秦松. 蓝藻藻胆体的体外组装研究进展[J]. 中国科学:生命科学, 2016, 46(9): 1054-1061. [41] 贾惠雁, 陈永华, 陈明利, 等. 5种水生植物对铜绿微囊藻的抑制与生理影响研究[J]. 农业现代化研究, 2019, 40(6): 1056-1064. doi: 10.13872/j.1000-0275.2019.0097 [42] RICE E. L. 天然化学物质与有害生物防治[M]. 胡敦孝, 译. 北京: 科学出版社, 1988. [43] 杨浩娜, 周成言, 邬腊梅, 等. 植物化感物质的作用机理研究进展[J]. 湖南农业科学, 2022(3): 108-112. doi: 10.16498/j.cnki.hnnykx.2022.003.025 [44] GROSS E M, SÜTFELD R. Polyphenols with algicidal activity in the submerged macrophyte Myriophyllum spicatum L.[J]. Acta Hortic, 1994(381): 710-716. doi: 10.17660/ActaHortic.1994.381.103 期刊类型引用(2)
1. 张梅,牛敏敏,杨兴丽,江润海,温绍福,朱城强,靳松,侯秀丽,常学秀. 不同种植密度苦草与铜绿微囊藻的交互作用. 环境科学与技术. 2024(01): 22-34 .  百度学术
百度学术
2. 杨硕,左胜鹏,王会梅,李怡. 穗花狐尾藻附着菌及其水质对抑藻效应的影响. 环境科学与技术. 2024(S2): 133-138 .  百度学术
百度学术
其他类型引用(1)
-






 下载:
下载: