-
人工湿地技术是通过过滤、吸附、沉淀、植物吸收和生物降解等过程,实现对城市生活污水的高效处理,其建设成本和能耗较低,环境美化效果好[1]。人工湿地中所含大量的养分负荷会刺激微生物生长代谢。与天然湿地相比,温室气体CH4[2]、N2O和CO2[3]的排放通量更高,因此,亟需探究如何规模化实现该工艺的温室气体减排。
人工湿地实现污染物去除的主要部分为基质填料。填料材料通过物理、化学和生物的作用完成对污染物的去除。由于单一基质类型的人工湿地无法同时达到高效脱氮、除碳的目的[4]。组合填料所发挥的协同作用可高效去除污水中的多种污染物质[5],已被越来越多地运用到人工湿地中。然而,在实际运用中,组合填料的种类、填充方式、孔径和含碳量等条件均会影响湿地系统的复氧能力及微生物的代谢活动,从而间接影响系统污染物的去除能力[5]。
SHEN等[6]研究铁碳微电解填料时发现,以铁为阳极、碳为阴极会形成大量微观原电池,可将NO3−/NO2−直接还原为N2,因此,铁碳含量改变会影响NO3−/NO2−还原为N2过程的进行,进而影响N2O的排放。 WANG[7]等发现沸石具备良好的吸附功能,具有与普通材料相似的均一孔隙,故其性质与分子筛类物质相似,选择吸附性能优异。该材料可有效吸附系统产生的CH4,当沸石占比增大时,可能更加有利于减少CH4的减排。赵仲婧等[1]发现,采用铁碳和沸石作为基质组合填料的间歇曝气人工湿地系统可明显提高污水处理效率和温室气体减排效果。铁碳微电解材料与沸石的粒径、孔隙度均不相同,因此,当二者间填充顺序不同时,可通过影响溶解氧 (dissolved oxygen,DO) 环境来构成不同微生物群落结构,优化硝化、反硝化过程,以降低温室气体排放量。
基于此,本研究以铁碳和沸石组合填料为研究对象,通过改变二者的填充顺序和填充配比,探究基质填充方式对人工湿地对污染物去除过程中温室气体减排效果的影响,以期为实现人工湿地技术的减污降碳目标提供参考。
-
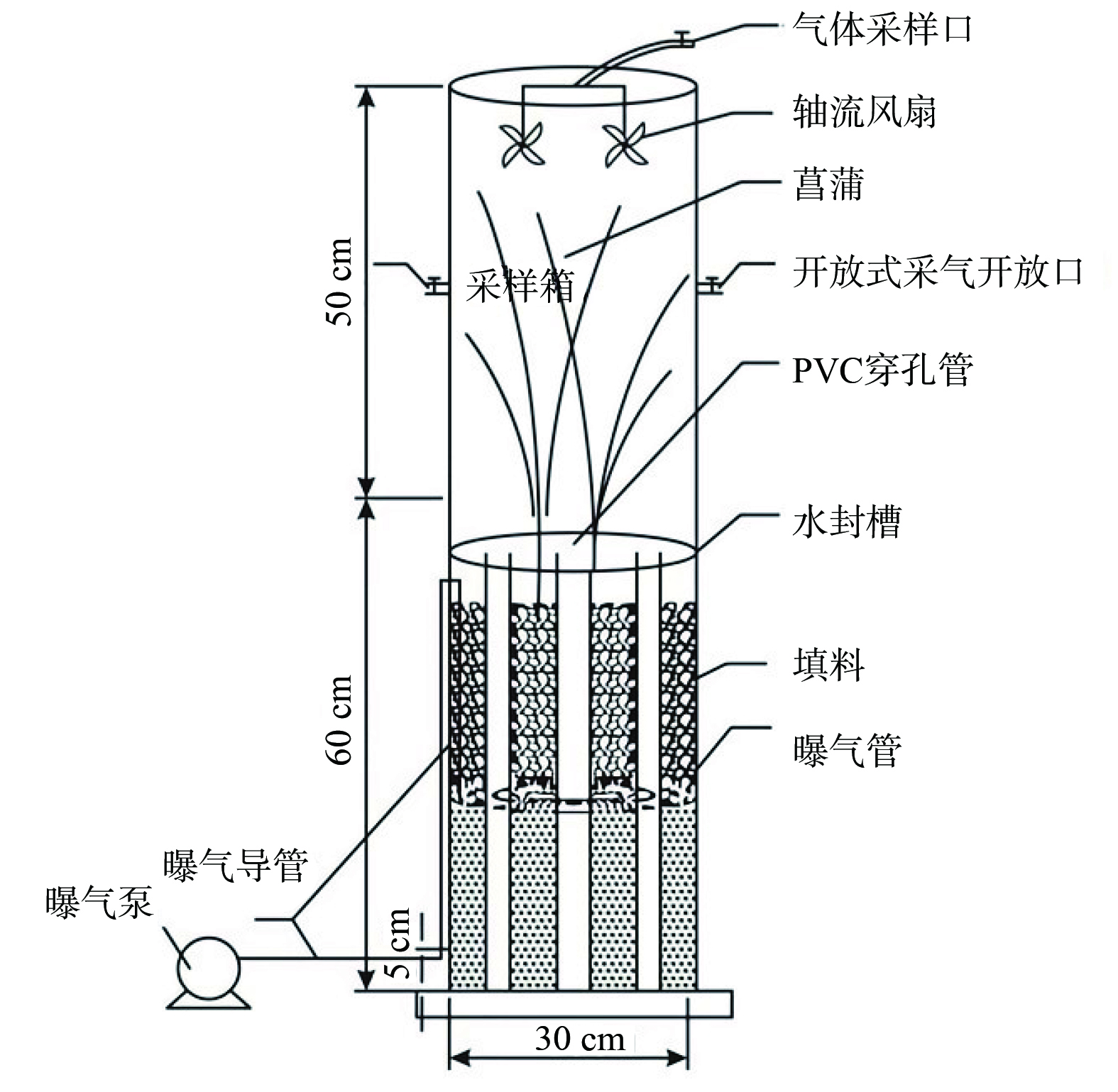
实实验装置位于西南大学某玻璃温室大棚。该装置具备良好的通风性,且自然照射充足。图1 为实验用到的仿真垂直潜流式人工湿地系统,采用 PVC材质制作的筒状容器,其底部直径30 cm,高60 cm,上部边缘设有3 cm的矩形水密封凹槽。中心有3个4 cm宽,60 cm长的 PVC穿孔管,最中间一根用于进水、虹吸排水和收集水样本,两侧2根内部装有和人工湿地系统相同且等高的填料,用于提取湿地基质及微生物。距装置底部5 cm处设置微孔曝气管。
装置中种植的植物为野生菖蒲 (Acorus calamus L.) ,取自北碚区某水库。菖蒲采回后,先将其根部清洗干净,放入有培养液的装置中,置于光照培养箱中进行驯化培养。培养温度为 (25±2) ℃,光照强度为 (3 000±300) lux,光暗时间比为12 h:12 h,每隔2 d更换一次进水,经过30 d的人工培育,将生长状况好、体形相似的植物移植到人工湿地设备中,栽种密度为每平方米30株。
实验所选用的沸石是从河南景盈建材有限责任公司采购的斜发沸石,沸石颗粒的直径为5~10 mm。使用纯水清洗干净,经风干、称重后填入湿地装置之中。铁碳微电解填料主要由废铁屑和活性炭制成,粒径为10~30 mm,均由郑州众邦水处理有限公司提供。
培养微生物所需接种的活性污泥取自北碚区污水处理厂二沉池,采用人工配制的污水进行驯化。驯化3周后将活性污泥接种到人工湿地系统中,接种污泥的质量浓度为1 000 mg·L−1。
-
实验装置按铁碳和沸石的填充方式分为2组。湿地组1铁碳填充在表层,沸石在底层;湿地组2沸石填充在表层,铁碳在底层。以添加100%沸石的人工湿地作为对照组。沸石、铁碳分别用F、T表示,且用x、y表示沸石和铁碳的占比。每组设置3个装置,组1分别添加20%铁碳+80%沸石 (T2F8) 、40%铁碳+60%沸石 (T4F6) 、60%铁碳+40%沸石 (T6F4) ;组2分别添加20%沸石+80%铁碳 (F2T8) 、40%沸石+60%铁碳 (F4T6) 、60%沸石+40%铁碳 (T6F4) 。
实验人工湿地系统设置水力停留时间 (hydraulic retention time,HRT) 为2 d,进水采用人工配置的模拟污水,固定进水的碳氮比 (COD/N) 为5:1,具体成分参照文献[7]。向模拟污水中投入蔗糖、NH4Cl 和KNO3提供碳源和氮源。模拟污水的 COD、NH4+-N和NO3−-N分别为300、40和20 mg·L−1。其他盐类或物质(每升水中添加的量)为 :KH2PO4 (22.50 mg) 、MgSO4·7H2O (97.56 mg) 、CaCl2 (58.28 mg) 、蛋白胨 (10.00 mg) ,以及微量元素溶液1 mL。其中,微量元素溶液(每升中添加的量)中又包含盐类有:H3BO3 (0.17 mg) 、MnCl2·4H2O (0.11 mg) 、ZnSO4·7H2O (0.13 mg) 、CuSO4·5H2O (0.04 mg) 和H2MoO4·4H2O (0.004 9 mg) 。微量元素溶液的pH为 (7.09±0.01) 。
该系统每天曝气2 h,采用机械式间歇曝气方式进行。时间段为每天00:00—01:00及12:00—13:00[8]进行。该系统中的DO控制为约3 mg·L−1。 湿地系统于2021年5月开始运行,并在5月进行第一次气体测定,运行180 d,在2021年11月停止运行。本研究选取5月、6月、7月这3个月的数据进行计算分析。
-
(1) 水样的采集与测定。在系统正常运行后,每2 d进行一次常规水样收集并检测。取样时间为09:00—10:00。根据饮用水及污水的国家标准分析方法,对COD、NH4+-N、NO3−-N、NO2−-N和TN等指标进行测定。水样的原位指标包括DO、氧化还原电位 (oxidation-reduction potential,ORP) Eh、水温和pH,均利用多参数测定仪 (SG98型梅特勒-托利多,瑞士) 进行测定。
(2) 气样的采集与测定。本研究中主要对CH4和N2O这两种气体进行采集分析。气体采样箱 (见图1上半部分) 是PVC材质的圆柱体,由顶箱 (直径30 cm,高50 cm) 和延长箱 (直径30 cm,高70 cm) 组成,延长箱可在植物生长高度超过50 cm时使用。采样箱内有2个轴流风扇。在人工湿地系统稳定运行期间,每月采气3~4次,采样时间为上午9:00—11:00。在每个周期内进行温室气体的采样,并分析其排放规律。在1个典型周期中,设定13个不同停留时间,分别为0、2、6、12、14、18、24、26、3、36、38、42和48 h。其中,在典型水力停留期间,对人工湿地的排放进行了模拟,并对其进行了采集与分析。
气体样品的采集方式分3种:非曝气段采样、曝气段采样和溶解态CH4和 N2O的采集3种情况。其中,非曝气段的气体排放通量计算式[9]为式 (1) 。该公式是以气体样品中温室气体质量浓度随时间变化的速率计。
曝气段的气体排放通量计算式[10]为式 (2) 。
利用计算所得的气体排放通量根据内插累加法求得CH4和 N2O的累积排放量,计算式见式 (3)。
溶解态温室气体的浓度计算式见式 (4) 。
式中:F为气体 (CH4和N2O) 排放通量,μg·(m2h)−1;H为箱内高度,m;T为采样箱内平均气温,K;P即采样时的大气压力,Pa;P0是校准条件下的大气压力,Pa;
ρ 为某一被测气体的密度 (摩尔质量/标准状态下的气体摩尔体积,g·L−1) ;dc/dt为采样期间采样箱内某一被测气体的浓度变化速率,其中CH4的浓度变化速率单位为cm3·(m3h)−1;N2O体积浓度变化速率单位为mm3·(m3h)−1;Q为人工湿地曝气量,L·min−1;ϕ 为测得气样中的气体体积分数,%;R表示理想气体常数,即8 308.65 L Pa·(mol·K)−1;M为气体摩尔质量,g·mol−1;S为采气箱覆盖面积,m2;cdis 为单位体积水样中溶解的气体质量浓度,μg·L−1;K0为 CH4或N2O的亨利常数,mol·(L1Pa)−1;β 为取样瓶上部空间与水样的体积比;ω 为测得的上部空间气体体积分数。基于全球增温潜能值 (Global Warming Potential,GWP) 的概念,通过比较各种人工湿地的温室气体排放情况,将CH4和N2O的排放量换算成CO2当量 (CO2-eq) 。各组人工湿地的综合GWP计算式[11]见 (5) 。
-
利用 Origin 8.5绘制数据图;显著性检验分析及相关性分析采用SPSS19.0软件;显著性检验采用One-way ANOVA方法 (P<0.05、P<0.01表示达到显著水平) 。
-
每月对不同人造湿地的CH4排放情况进行统计,结果见表1。在系统稳定的工作状态下,对照组中F的CH4平均排放通量为 (0.33±0.02) g·(m2·h)−1。湿地组1中,T4F6和T6F4的CH4排放通量在添加铁碳后增加,仅有T2F8有减排效果,其CH4的排放通量相较于F减少了5.16% (P<0.05) 。湿地组2中,相较于对照组,F8T2、F6T4的CH4排放量分别减少了22.59%~42.86%、0~40% (P<0.05),而F4T6并无CH4减排效果。通过比较两组湿地的CH4月排放通量发现,沸石在上、铁碳在下填充基质的湿地组2更有利于CH4减排;且适当添加铁碳有利于CH4的减排作用,但铁碳的占比不宜超过沸石,沸石/铁碳为8:2的湿地对CH4减排效果会更加明显。
以上现象表明,表面分子筛的孔隙结构可以对CH4进行吸收和储存,从而提高了装置内的氧气浓度,促使CH4氧化[12-13]。当系统中添加铁碳填料时,铁屑和活性炭颗粒充当电极材料,产生明显微电场,使系统更易形成微电解系统[14]。而这个铁碳微电解系统置于底部时,更加接近植物根系。在电解过程中,阳极产生的Fe2+、Fe3+会参与微生物生命活动的电子传递过程,从而提升根系微生物活力,进而使根系微生物与产甲烷菌竞争加剧,产甲烷菌将无法获得足够碳源与电子[15],从而活性受到抑制。另外,铁氧化物在根部厌氧体系中可能存在异化铁还原过程[16],异化铁还原菌和产甲烷菌之间存在底物竞争与热力学反应的优先顺序[17],当Fe3+质量浓度上升时,有利于体系中异化铁还原过程的进行,使产甲烷菌活性被抑制。
-
两组人工湿地中N2O平均排放通量如表2所示。在系统稳定运行期间,对照组F的N2O平均排放通量为 (651.51±88.53) μg·(m2h)−1。在湿地组1中,,与F相比,湿地组1中的T2F8、T4F6的N2O排放量分别减少了26.22%~70.62%、26.32%~56.62%、8.49%~42.30% (P<0.05) 。在湿地组2中,湿地组2中的F8T2、F6T4和F4T6相较于F分别减少了61.33%~84.29%、52.98%~75.61%、0~16.87% (P<0.05) 。以上结果表明,添加铁碳有利于N2O的减排。两组湿地的N2O月排放通量均随着铁碳占比的降低而明显减少;当铁碳-沸石体积比为2:8时减排效果最佳。通过进一步对湿地组1和2的N2O月平均排放通量进行比较,湿地组2的N2O减排效果优于湿地组1。
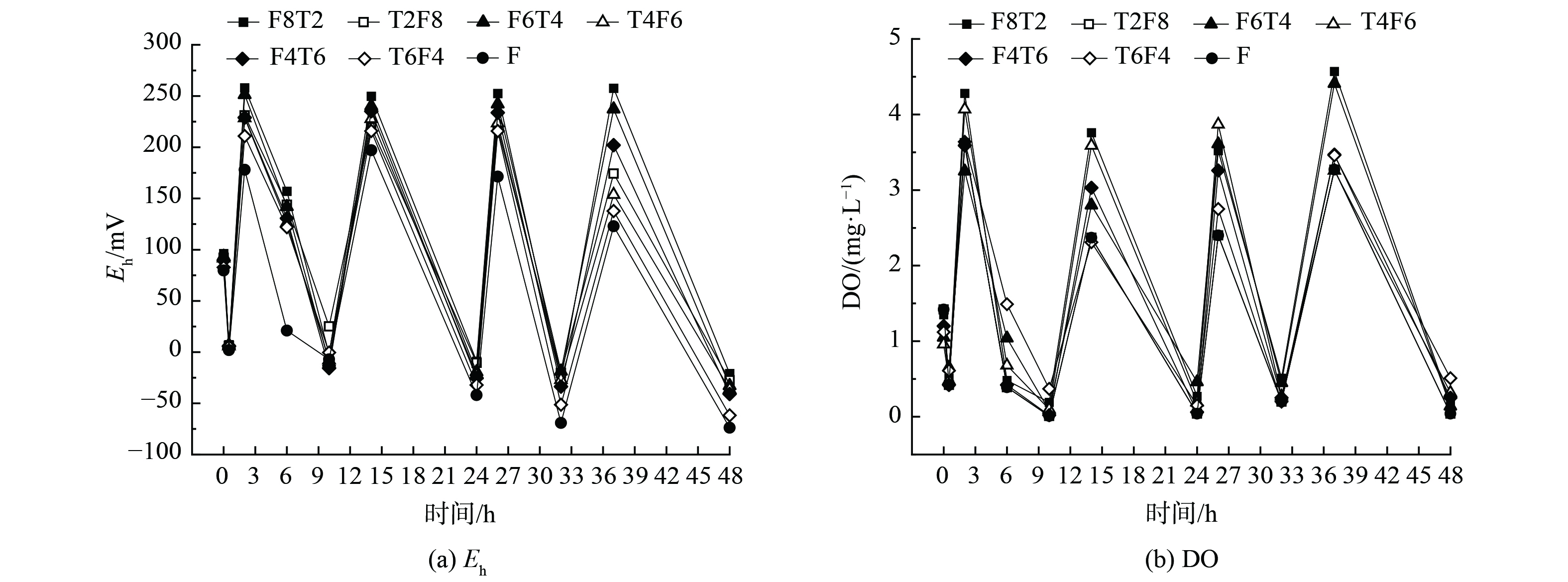
分析其原因可能是,N2O既是硝化反应副产物,亦为反硝化反应的中间体[18]。随着铁碳填料增多,铁碳微电解填料中存在的高水平Fe3+可能会抑制N2O还原酶的活性,使得N2O 作为中间产物逸出。另外,铁碳的存在会改变系统溶解氧环境,铁碳占比更少的湿地系统DO更高,氨氧化细菌 (ammonia-oxidizing bacteria,AOB) 的好氧反硝化过程被抑制,会进一步减少N2O的释放[1],且有利于N2O还原为N2;铁碳的加入会导致环境中氧化还原电位Eh升高,且随着铁碳占比减少Eh升高 (如图6) ,可能会降低硝酸还原酶的活性,从而减弱反硝化作用[19],减少N2O释放。当把铁碳填料置于底部时,能有效改善底层微生物的反应环境、促进其对碳源的利用,进而强化异养脱氮反应的效果,使得反硝化反应更顺利地进行;且将铁碳填料置于底层,会更加接近植物根系,能有效促进植物根系泌氧[20],为N2O还原酶提供好氧环境,使得一些细菌在一定氧浓度下能还原N2O[21]。此外,铁作为电子供体实现了微生物的自养反硝化[22],能减少N2O产生。
-
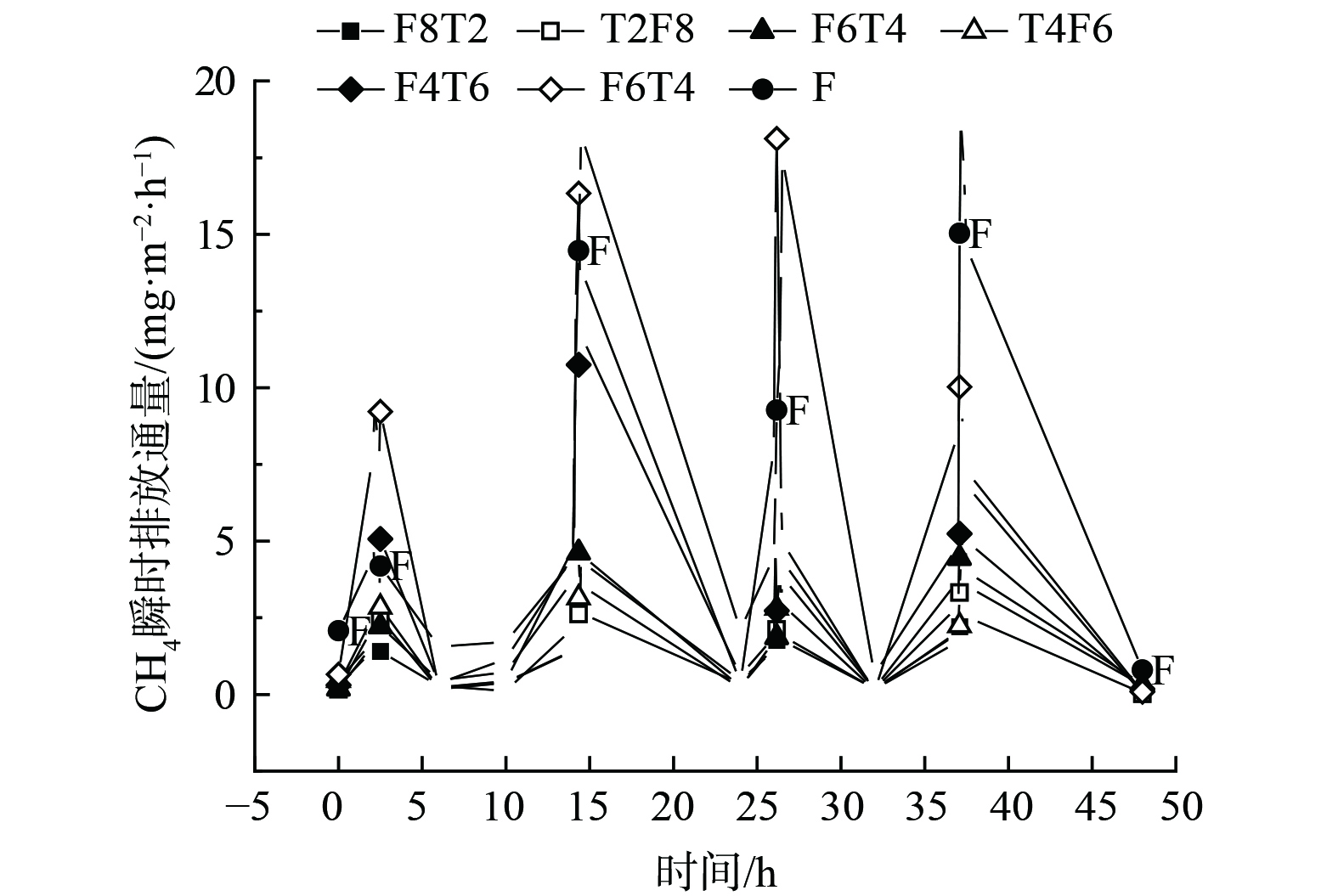
图2展现了典型周期内两组人工湿地CH4瞬时排放通量。以48 h为一个典型周期,各湿地中的CH4排放通量在曝气阶段迅速升高,曝气结束后又迅速下降,且每一曝气时段CH4最低瞬时排放通量都出现在铁碳-沸石体积比为2:8的实验组。另外,当沸石/铁碳体积比相同、而沸石、铁碳的填充顺序不同时,湿地组2的CH4瞬时排放量明显低于湿地组1。
图3为典型周期内曝气段和非曝气段末端溶解态CH4的质量浓度,不同于CH4的排放通量,溶解态CH4质量浓度在非曝气条件下,要明显高于曝气段。在曝气阶段,CH4的质量浓度分别为14.53、23.26、30.68 μg·L−1,而在非曝气阶段,CH4的质量浓度分别达到了40.96、59.51、30.68 μg·L−1。然而,CH4的生成大多发生在厌氧段,该反应段的Eh通常低于−150 mV。此时,产甲烷菌的活力会显著提升,进而使得CH4的生成量增加。当湿地的Eh高于50 mV时,会停止产生CH4[23]。结合图4可知,两组湿地非曝气段最低Eh均低于−20 mV,曝气段Eh峰值均超过100 mV,在非曝气阶段时,随着湿地Eh降低,平均CH4排放通量会升高。湿地组1中的非曝气段最低Eh分别为−27.5、−36.3、−61.7 mV,曝气段Eh均超过100 mV;湿地组2中的非曝气段最低Eh分别为−26.5、−32.81、−40.9 mV,曝气段Eh均超过100 mV。
上述结果表明,CH4的生成多发生在曝气段以外,曝气段会以曝气方式将CH4吹出[24]。在曝气段,湿地系统本身是不会产生CH4的,而是将之前积累的CH4排入大气,从而使系统出现CH4排放通量迅速增大,并达到高峰,最后在曝气段结束后又出现显著下降的现象。另外,使用不同孔隙率的人工湿地填料可改变其溶解氧供应,从而改善湿地溶解氧条件。铁碳微电解填充物主要对CH4的生成和CH4催化起主要作用。随着Fe3+的加入,系统中原有的大量铁氧体,即铁还原菌,会参与产甲烷菌的反应,从而会与其共同竞争有机酸或氢气等底物,最终对CH4产生起到阻碍的作用。沸石的添加则直接减少了产甲烷古菌的数量,阻碍了CH4产生[25-26]。此外,极高的氧化还原电位,使CH4更易实现厌氧氧化,做为唯一的电子供体且有合适的电子受体,CH4被氧化为CO2[27-29]。但铁碳/沸石比例过高时,过量铁屑可能会将Fe3+还原为Fe2+。CH4的电子受体减少使CH4转化为CO2过程受阻,导致CH4减排效果变差。
-
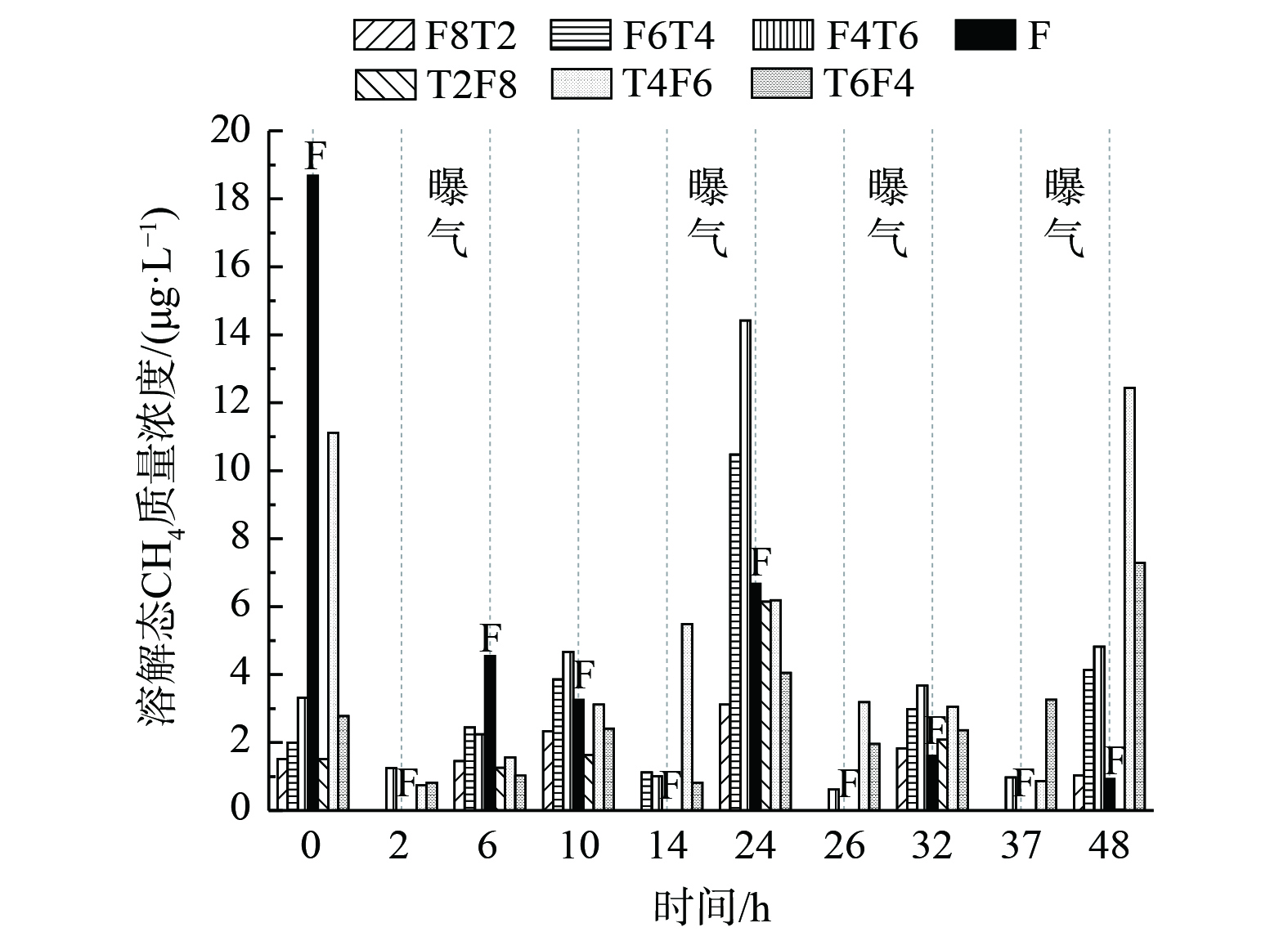
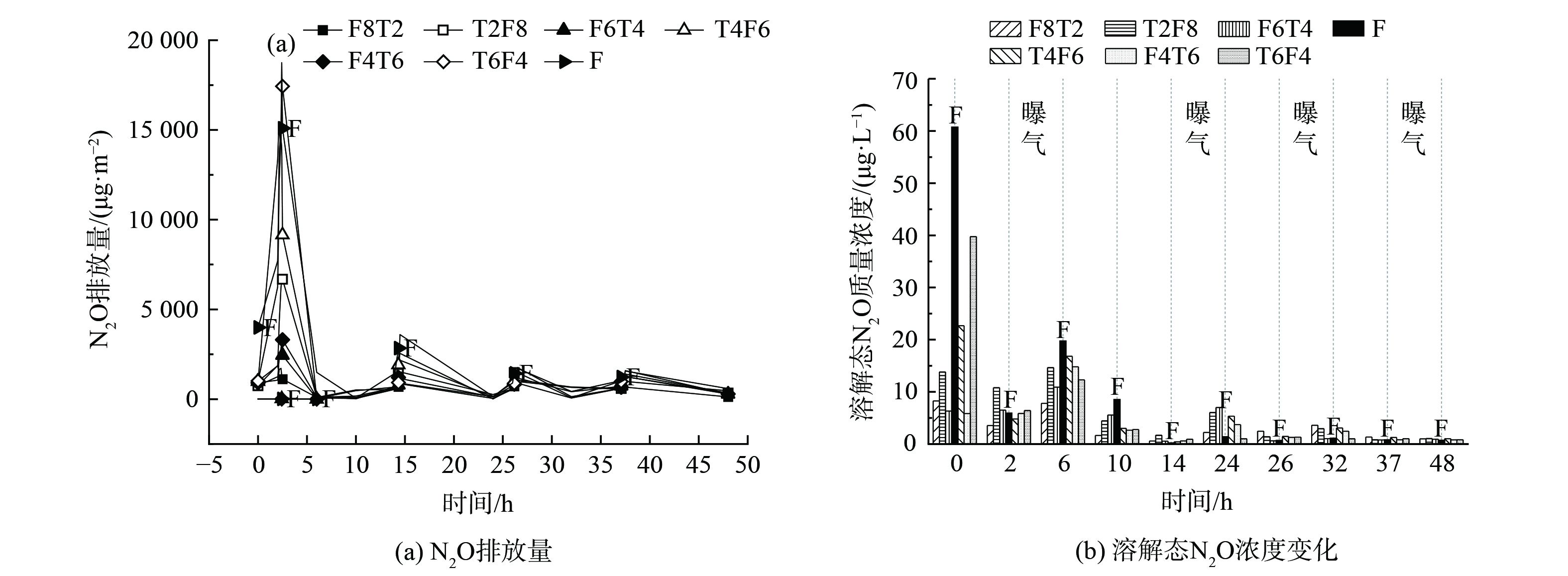
图5 (a) 为典型周期内不同人工湿地N2O瞬时排放通量。以48 h为一个典型周期,湿地F在此周期内的N2O的累积排放量为 (100.04±18.84) μg·m−2。由表3可知与湿地F相比,湿地组1和湿地组2的N2O累积排放量均有下降,且湿地组2的N2O累计排放量低于湿地组1。当沸石/铁碳体积比相同但二者填充顺序不同时,铁碳在底层湿地组2比湿地组1的N2O减排效果更为明显。从铁碳-沸石填充体积比来看,铁碳占比越少,N2O减排效果越好。曝气段N2O排放通量明显高于非曝气段,各人工湿地系统中N2O排放通量和溶解态N2O均随着曝气次数的增加而逐渐降低。一方面,由于曝气吹脱作用把溶解态N2O排入大气环境中[1];同时,曝气段DO迅速上升会影响氧化亚氮还原酶(Nos)的活力,进而促使N2O生成并大量排放[30]。
图5 (b) 为一个典型周期内的曝气和非曝气两阶段中N2O 的最终浓度变化。在循环初期和非曝气段,N2O的质量浓度均很高,但在反应进行8 h后,N2O的质量浓度出现明显降低。在4个人工湿地系统中,每个周期的前24 h,其溶解态N2O质量浓度均为(4.01-3.27)~(16.11-22.96)μg·L−1。而反应进行24 h后,N2O质量浓度则出现明显降低,仅为(0.84+0.18) ~(2.10+1.18)μg·L−1。总体来说,在曝气段N2O的质量浓度比非曝气段要低。这是因为在反应前期,微生物会发生好氧降解有机质的反应,该过程使系统内DO迅速降低,而NO3−在此时被还原,从而导致NO2−的累积,促进了溶解态N2O生成。由于系统内硝化与反硝化反应不断进行,从而使得底物的TN指标降低,曝气段N2O最高值和溶解态N2O质量浓度也不断降低。[1]。
-
全球增温潜能值 (GWP) 可反映温室气体对温室效应的强化能力[27]。如表4所示,从铁碳-沸石不同填充顺序来看,铁碳填充在底层湿地组2的GWP均明显低于铁碳填充在表层湿地1;从铁碳-沸石不同填充体积比来看,铁碳占比越少GWP越低。其中,GWP最低的是F8T2,比起对照组F的综合GWP降低了79.51% (P<0.05) ;而GWP最高的T6F4相较于F也下降了13.86% (P<0.05) 。N2O对综合GWP贡献显著大于CH4,达到了69.71%~88.92%,而CH4贡献率仅为11.08%~30.29%。由此可见,典型周期内铁碳在底层的湿地F8T2所排放的CH4和N2O均最少 (P<0.05) ,且综合GWP仅为 (16.94±1.45) g·m−2 (以CO2-eq计) ,其综合减排效果最好。
-
在铁碳-沸石为基质的人工湿地中,铁碳在底层,沸石在顶层的填充顺序下,CH4和N2O减排效果均优于铁碳在表层,沸石在底层的湿地系统。在填充顺序一定的情况下,基质中沸石/铁碳的填充体积比对CH4和N2O减排有一定影响。当沸石与铁碳体积比为8:2时,综合GWP最低,湿地在水质净化与温室气体减排方面均有明显效果,为本实验中最佳组合人工湿地。
基质中铁碳-沸石填充方式对人工湿地温室气体排放的影响
Influence of substrate filling method and volume ratio on greenhouse gases emissions from iron carbon-zeolite constructed wetlands
-
摘要: 人工湿地在污水治理中已获得广泛应用,但其温室气体排放通量是自然湿地的 2~10倍,对人工湿地温室气体的减排已是亟待解决的问题。通过在温室构建潜流人工湿地系统装置,均为间歇曝气,设立不同填料的填充配比和填充顺序,分别为:添加20%铁碳+80%沸石 (其中铁碳在上层沸石在下层记为T2F8,铁碳在下层沸石在上层记为F8T2,下同) 、40%铁碳+60%沸石 (记为T4F6和F6T4) 、60%铁碳+40%沸石 (记为T6F4和F4T6) 、以添加100%沸石为对照组 (F) ,探究了基质的填充方式对人工湿地系统中温室气体排放的影响。结果表明,与铁碳填充在表层的湿地组1 (T2F8、T4F6、T6F4) 相比,铁碳填充在底层的湿地组2 (F8T2、F6T4、F4T6) 均显著实现了人工湿地中CH4、N2O的减排 (P<0.05) ;同时,铁碳-沸石体积比对CH4、N2O减排效果影响显著,且铁碳占比越低CH4和N2O排放越少 (P<0.05) ;典型周期中曝气段出现CH4和N2O排放峰值,湿地F8T2的排放峰值均显著低于其他湿地 (P<0.05) ,其综合GWP最大减排值达到75.82%。铁碳填充在底层及铁碳-沸石体积比为2:8铁碳-沸石人工湿地 (F8T2) 的综合GWP最低,温室气体的减排效果最好。本研究结果可为人工湿地温室气体减排的实践提供参考。
-
关键词:
- 铁碳-沸石基质填充方式 /
- 人工湿地 /
- 甲烷 /
- 氧化亚氮
Abstract: Constructed wetlands have been widely used in wastewater treatment, but the greenhouse gas flux from constructed wetlands is two to ten times higher than that from natural wetlands, so there is an urgent need to reduce greenhouse gas emissions from constructed wetlands. In this study, a submerged wetland system was constructed in a greenhouse with different filler ratios and filling sequences: 20% Fe carbon filler+80% zeolite filler+intermittent aeration (where Fe carbon in the upper zeolite layer is denoted as T2F8 and Fe carbon in the lower zeolite layer is denoted as F8T2, hereinafter), 40% Fe carbon filler+60% zeolite filler+intermittent aeration (denoted as T4F6 and F6T4), 60% FeC filler+40% zeolite filler+intermittent aeration (denoted as T6F4 and F4T6), and 100% zeolite filler+intermittent aeration as the control group (F), to investigate the effect of substrate filling method on greenhouse gas emissions in the constructed wetland system. The results showed that compared with the iron carbon filled wetland group 1 (T2F8, T4F6, T6F4), the iron carbon filled wetland group 2 (F8T2, F6T4, F4T6) significantly reduced the CH4 and N2O emissions in the constructed wetland (P<0.05). The lower the proportion of iron carbon, the lower the CH4 and N2O emission (P<0.05); the peak of CH4 and N2O emission in the aeration section in a typical cycle, the peak of emission in wetland F8T2 is significantly lower than other wetlands (P<0.05), and the maximum reduction of its integrated GWP reaches 75.82%. In total, the FeC-filled substrate and the FeC-zeolite constructed wetland with a 2:8 FeC-zeolite volume ratio (F8T2) had the lowest integrated GWP and the best GHG reduction.-
Key words:
- greenhouse gases /
- constructed wetlands /
- methane /
- nitrous oxide
-
国内外的饮用水中均检测出医药品污染物,其浓度通常处于ng·L−1或更低水平. 风险评价研究显示,饮用水中微量的医药品污染物对于健康的成年人影响较小,但是敏感性人群特别是孕妇及新生儿暴露于环境中的风险需要加以考虑[1]. 布洛芬(IBU)属于抗炎药物,据估计全球年产量达数千吨,其在地表水和废水中检测到的浓度范围为ng·L−1至低μg·L−1水平[2];传统的水处理技术无法对持久性污染物IBU有效去除,研究发现IBU能对淡水环境中的无尾类动物的胚胎发育产生影响[3]. 非均相催化臭氧氧化技术是一种能够在室温和常压下,将那些难以通过臭氧单独氧化的有机污染物高效分解的方法[4-5]. 臭氧在催化剂表面有效分解产生羟基自由基等活性氧来降解水中有机污染物[6-7]. 由于不同催化剂的属性差异较大,其促进臭氧有效分解的机理还不清楚,需要深入研究催化臭氧氧化水中有机污染物的作用机制[8-9].
本文采用固体酸γ-Al2O3和多价态的钛活性组份负载法制备了TiO2/γ-Al2O3介孔催化剂. 由于TiO2的Lewis酸性强于Al2O3,通过调节钛的负载量来控制γ-Al2O3的酸量,从而更深入的研究Lewis酸性位对布洛芬去除的影响,并通过吡啶红外光谱、电子自旋共振和原位激光显微拉曼光谱等测试手段来探究催化反应机理.
1. 实验部分(Experimental section)
1.1 材料与试剂
布洛芬(IBU)从东京化成工业株式会社购买. 自由基捕捉剂BMPO从东仁化学科技(上海)有限公司购买. 实验用超纯水(电阻率为18.2 MΩ·cm)是用Milli-Q超纯水仪制备的. 异丙醇铝(Al[OCH(CH3)2]3)购买于Sigma-Aldrich公司. 葡萄糖、钛酸四正丁酯(TBOT)、盐酸(HCl)、硝酸(HNO3)和氢氧化钠(NaOH)在国药集团化学试剂北京有限公司采购. 全部化学试剂均是分析纯. 溶液pH由2 mol·L−1盐酸或者氢氧化钠溶液进行调节.
1.2 催化剂的制备
γ-Al2O3制备[10]. 称取16.8 g异丙醇铝和14.4 g葡萄糖溶于216 mL水中,在35 ℃恒温条件下搅拌6 h,用硝酸溶液(质量分数为10%)将pH调到5.5,继续搅拌24 h,将其在100 ℃下烘干,然后用马弗炉在600 ℃下煅烧6 h得到γ-Al2O3.
TiO2/γ-Al2O3制备. 称取6 g γ-Al2O3和适量TBOT(Al/Ti = 75)溶于200 mL水中,在35 ℃条件下搅拌24 h后在100 ℃烘干,然后用马弗炉600 ℃煅烧6 h得到TiO2/γ-Al2O3. 经前期实验优化,当Al/Ti = 75时,TiO2/γ-Al2O3催化臭氧氧化水中布洛芬具有最佳催化活性.
1.3 催化剂的表征
催化剂晶相结构由Scintag-XDS-2000型X射线衍射仪(XRD)测试分析,用Cu Kα(λ = 0.154 nm)辐射为激发源,操作电压为40 kV,操作电流为100 mA. 催化剂的比表面积、孔径和孔容采用美国Micromeritics公司的ASAP-2020比表面积仪测定. 孔隙度是基于氮气吸附-解吸等温线及Barrett-Joyner-Halenda(BJH)法获得的. 利用日本Hitachi公司 SU8020 FESEM电子扫描电镜对催化剂形貌和结构进行了观察. 用马尔文Nano-ZS90型Zeta电位计测定了催化剂的表面零电荷点(pHpzc). 利用Nicolet 6700红外光谱(Pyridine-FTIR)对吡啶吸附的催化剂酸性位进行了表征. 具体方法:压片后的催化剂放入红外的样品池中,将样品在常温真空处理30 min,获得样品背景谱图,该仪器的分辨率为4 cm−1. 再分别于20 ℃,150 ℃和350 ℃解吸得到样品的吡啶红外谱图. 因红外谱图峰高正比于催化剂的酸量. 依此,可对催化剂的表面酸性作定性或半定量的分析[11].
1.4 实验装置与分析方法
1.4.1 实验装置
催化臭氧氧化试验是在一个1.2 L鼓泡式玻璃反应器内进行的. 利用高纯度氧气作为气源,通过臭氧发生器(购于北京同林科技有限公司)进行放电生成臭氧,流量计测量后送入玻璃反应器底部微孔砂心布气头,经过磁力搅拌器搅拌后臭氧可均匀的分布于水中. 具体实验参数:在20 ℃条件下,将1 L浓度为10 mg·L−1的布洛芬水溶液为模型化合物和前期优化确定的1.5 g催化剂加入到反应器中,利用盐酸或氢氧化钠调节pH到7.0,然后通入臭氧浓度为30 mg·L−1的O3/O2混合气体,气体的流速为200 mL·min−1. 反应后残余的臭氧用碘化钾溶液吸收. 每隔一段时间采样1次,所取样品立即加几滴0.1 mol·L−1硫代硫酸钠以终止溶液中残余臭氧,然后用0.45 μm醋酸纤维滤膜滤出样品待测.
1.4.2 分析方法
水溶液中布洛芬的浓度采用高效液相色谱(1200 series;Agilent,Santa Clara,CA)测定,色谱柱为Eclipse XDB-C18 column(5 μm,4.6 mm×150 mm;Agilent). 分析条件为:流动相为60:40(V/V)乙腈:磷酸盐缓冲液(20 mmol·L−1,pH = 2.5)溶液,柱温40 ℃,进样量20 μL,流量为1 mL·min−1,紫外检测波长220 nm. 总有机碳分析(TOC)采用岛津TOC-VCPH型总有机碳分析仪测定. 利用电感耦合等离子体原子发射光谱分析(ICP-OES)分析溶液中金属离子浓度. 气相臭氧浓度采用IDEAL-2000型臭氧浓度检测仪进行检测. 用靛蓝法测定了水中溶解臭氧的浓度[12]. 利用BMPO作为自由基捕捉获剂,利用德国布鲁克公司ESP300E型电子顺磁共振波谱仪(EPR)对反应溶液中的羟基自由基及超氧自由基进行直接测定,微波频率为9.79 GHz,功率为5.05 mW. 原位激光显微拉曼光谱分析利用Lab RAM HR Evolution型共聚焦激光显微拉曼光谱仪进行测定. 激光激发波长633 nm、分辨率为1 cm−1、持续时间为100 s. 样品制备方法:将0.1 g催化剂与2 mL超纯水或者饱和臭氧水摇匀后,快速滴入载玻片,调整视野开始扫描测定.
2. 结果与讨论 (Results and discussion)
2.1 催化剂表征
图1是γ-Al2O3和TiO2/γ-Al2O3的小角和广角XRD图谱. 在小角范围,γ-Al2O3和TiO2/γ-Al2O3在1o附近都显示出一个很强的衍射峰,说明这两种催化剂都具有介孔结构;在广角范围,Al2O3显示出典型的γ相(JCPD no. 00-046-1131)[10, 13]. TiO2/γ-Al2O3样品除了出现γ-Al2O3的特征峰,同时在25.3o出现强的TiO2的特征峰,说明TiO2负载于γ-Al2O3表面.
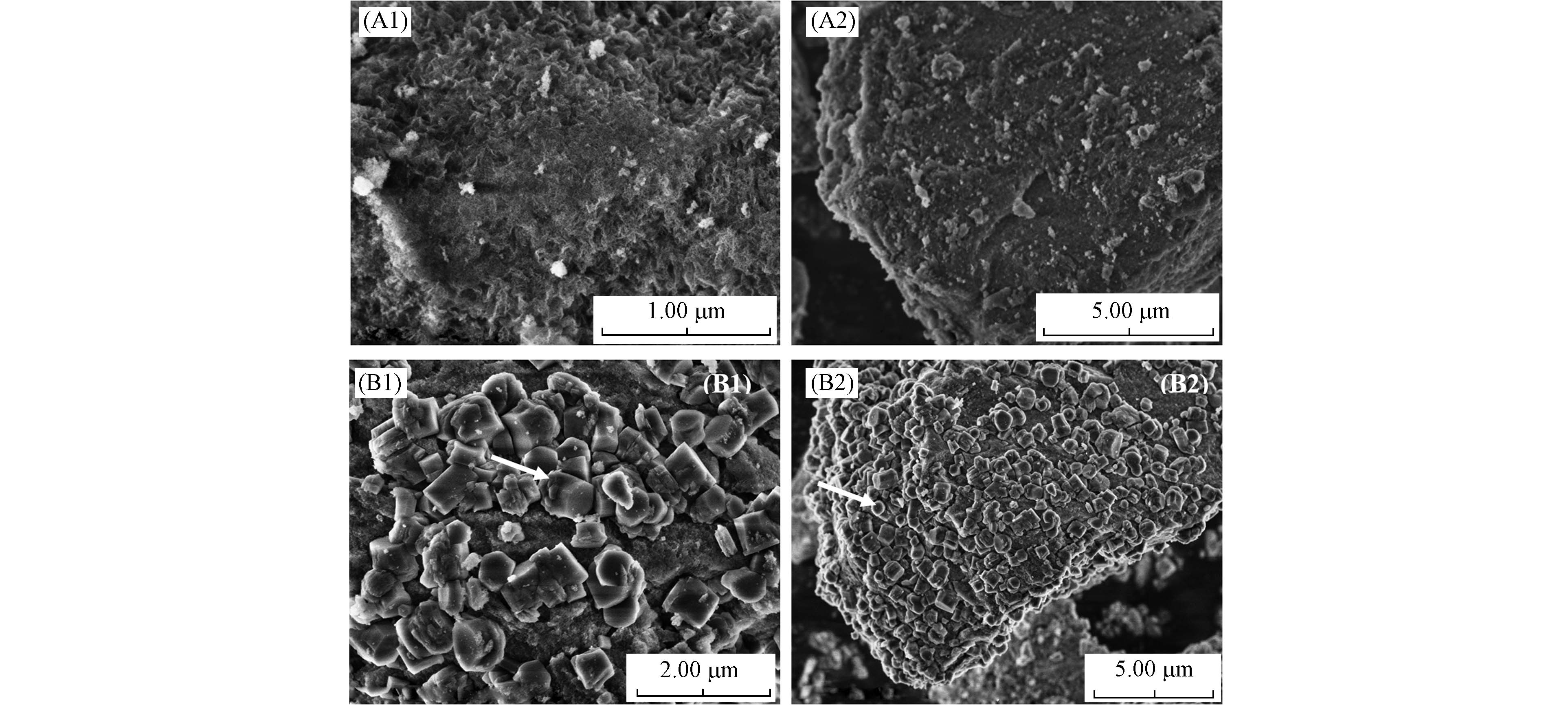
γ-Al2O3和TiO2/γ-Al2O3的扫描电镜照片见图2,γ-Al2O3显示出海绵结构,而对于TiO2/γ-Al2O3大的TiO2颗粒出现在γ-Al2O3表面,证实了TiO2负载于γ-Al2O3表面.
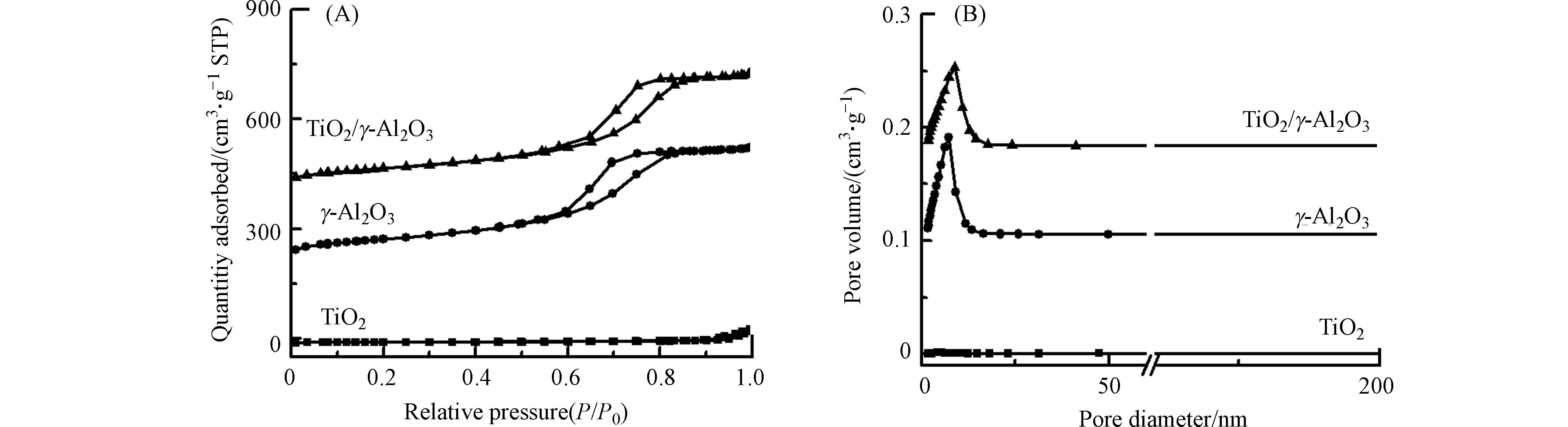
图3是γ-Al2O3和TiO2/γ-Al2O3的氮气吸附脱附等温线和孔径、孔容分布图. 所有样品都符合第Ⅳ类吸附等温线特征,说明这两种催化剂都有介孔结构,都表现出集中的孔径分布. 另外从表1可以看出,与γ-Al2O3相比,TiO2/γ-Al2O3的比表面积、孔径、孔容下降,这是由于TiO2负载于γ-Al2O3表面.
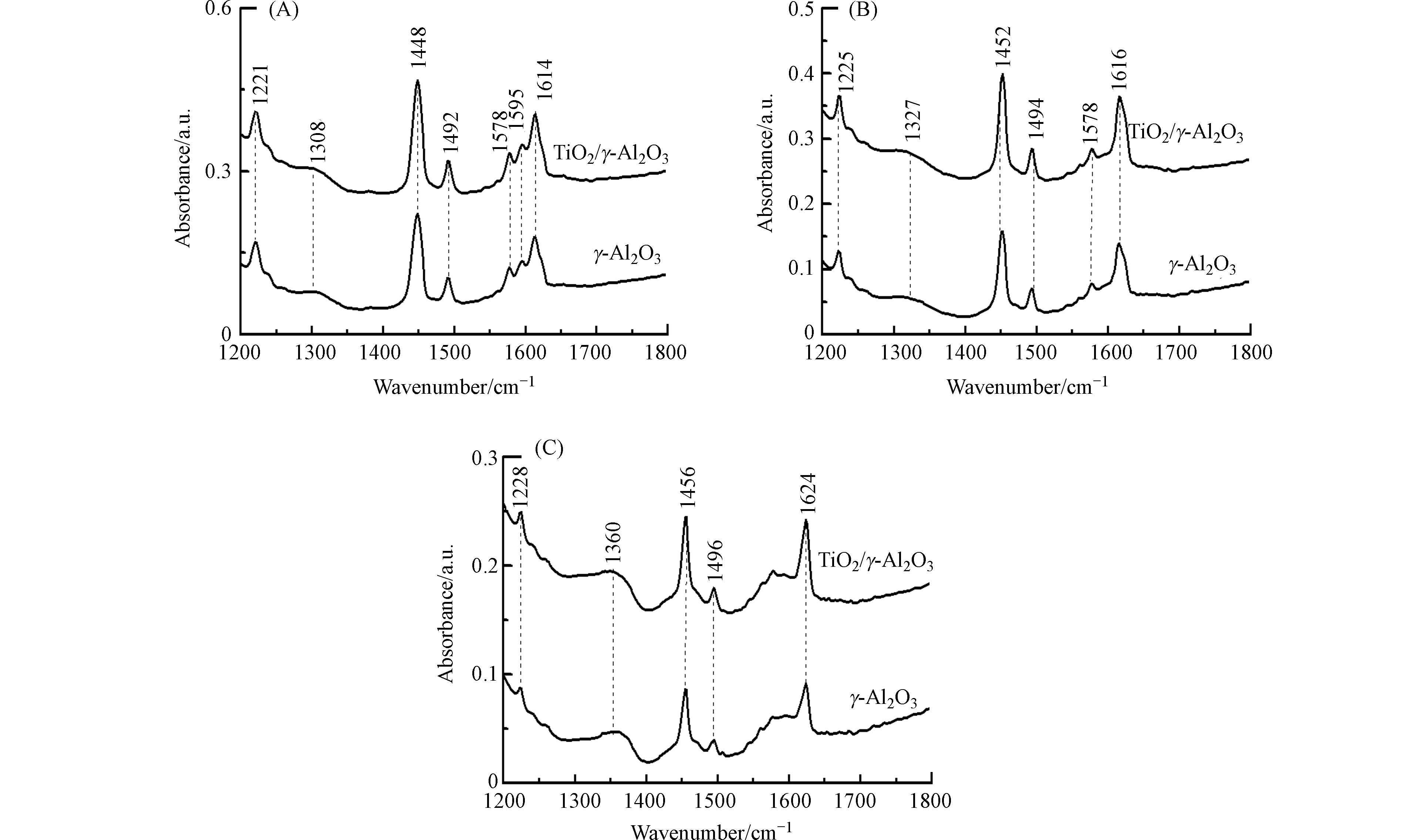
表 1 催化剂的比表面、孔径、孔容和孔壁Table 1. Specific surface area, pore diameter, pore volume, and pore wall of catalysts催化剂 Catalyst 比表面积/ (m2·g−1) S BET 孔径/ nm Pore diameter 孔容/(cm3·g−1) Pore volume γ-Al2O3 252.81 0.50 6.13 TiO2/γ-Al2O3 221.97 0.47 5.86 利用吡啶-红外光谱技术,对γ-Al2O3和TiO2/γ-Al2O3催化剂表面的酸性位进行了分析. 图4为催化剂在20 ℃、150 ℃和350 ℃脱附的吡啶-红外光谱. 在1200—1800 cm−1范围的吡啶-红外光谱代表吡啶环υ(C—N)和υ(C—C)振动[14]. 在20 ℃条件下脱附,γ-Al2O3和TiO2/γ-Al2O3催化剂在1221、1308、1448、1492、1578、1595、1614 cm−1位置都有吸收,TiO2/γ-Al2O3具有比γ-Al2O3更强的吸收强度. 吡啶-红外光谱在1221、1308、1448、1492、1614 cm−1处的吸收峰,代表吡啶被催化剂Lewis酸性位所吸附[15]. 150 ℃解吸时,1595 cm−1处的吸收峰消失表明吡啶和催化剂表面之间的弱相互作用,代表吡啶在催化剂表面上吸附氢键的位置. 350 ℃脱附过程中,1578 cm−1吸收峰逐渐消失,表明吡啶在中强的Lewis酸性位上发生了吸附. 随脱附温度升高,吡啶的吸收峰迁移到较高频位,表明吡啶在强Lewis酸性位发生了吸附. 基于不同温度条件下催化剂脱附曲线,进一步定量分析了γ-Al2O3和TiO2/γ-Al2O3表面的Lewis酸量[11]. 从表2可见,TiO2/γ-Al2O3与γ-Al2O3相比Lewis酸量略有增加. 这些结果说明钛负载于γ-Al2O3表面能够增大γ-Al2O3的Lewis酸量.
表 2 吡啶-红外光谱测定的不同催化剂的Lewis酸量Table 2. Amount of Lewis acid sites of different catalysts determined by Pyridine-FTIR催化剂 Catalyst 总酸量/(μmol·g−1) Total acid 中强酸量/(μmol·g−1) Medium acid 强酸量/(μmol·g−1) Strong acid γ-Al2O3 540.1 197.4 61.1 TiO2/γ-Al2O3 653.8 222.9 85.4 2.2 催化剂催化活性评价
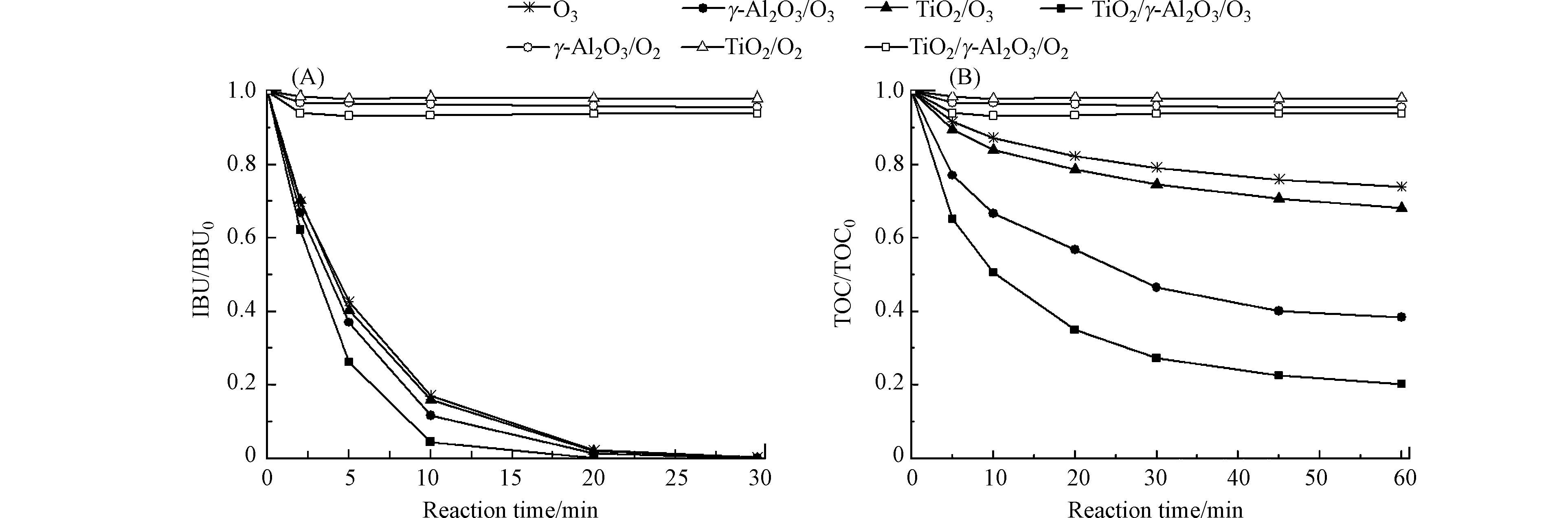
图5是不同工艺对布洛芬的催化性能. 由图5可知,TiO2/γ-Al2O3/O2、γ-Al2O3/O2和TiO2/O2过程对布洛芬的吸附去除率分别为6%、4%和2%;催化臭氧氧化比单独臭氧氧化更有利于布洛芬降解;TiO2/γ-Al2O3/O3工艺具有最高的催化活性. 反应30 min时,不同工艺过程均将布洛芬完全降解,催化臭氧氧化过程可极大提高布洛芬的矿化率. 反应60 min,TiO2/γ-Al2O3对布洛芬的TOC去除率达到80%,γ-Al2O3、TiO2及单独臭氧氧化过程中TOC去除率分别达62%、32%及26%. 由于布洛芬在TiO2/γ-Al2O3表面的吸附去除率只有6%,表明TiO2/γ-Al2O3与臭氧之间具有良好的协同作用. 通过对比表2和图5,可以发现催化剂表面的Lewis酸量与其催化活性呈正相关,TiO2/γ-Al2O3最大Lewis酸量使其具备最高的催化活性.
2.3 催化臭氧氧化机理
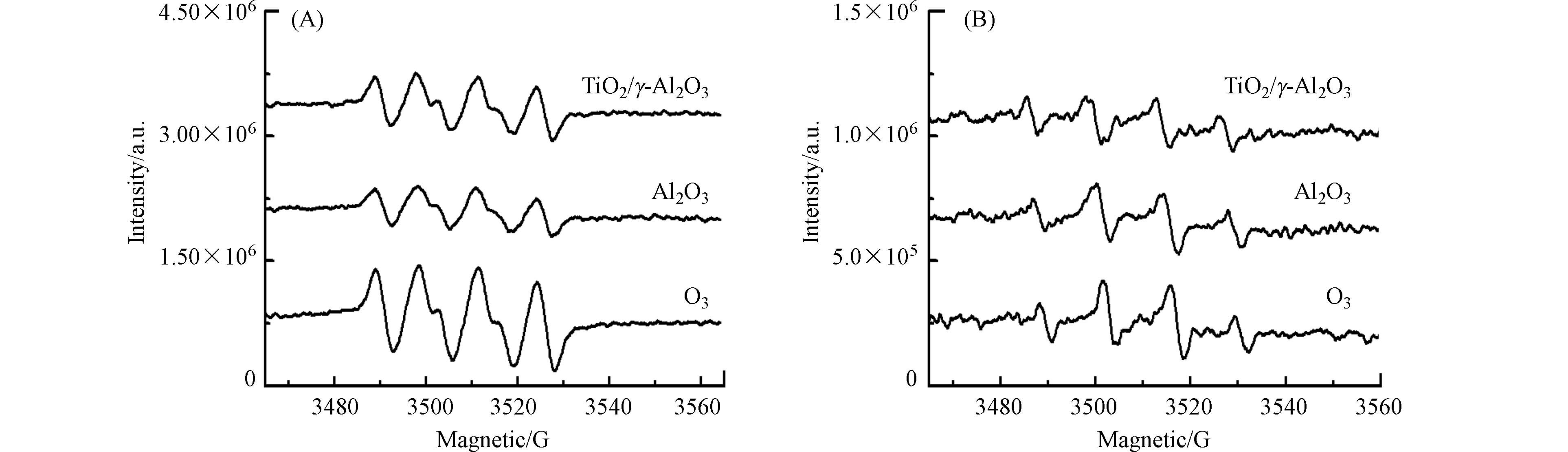
催化臭氧氧化过程,催化剂的表面属性与活性位共同决定了臭氧的吸附与高效分解. 文献报道臭氧可以在Lewis酸性位替代羟基基团与水分子结合,进行有效分解成活性氧物种[16]. 利用电子顺磁共振光谱对反应体系产生的羟基自由基及超氧自由基进行了考察. 由图6可见,在γ-Al2O3和TiO2/γ-Al2O3的臭氧悬浆,羟基自由基和超氧自由基的信号比单独臭氧溶液的要低,说明吸附于γ-Al2O3和TiO2/γ-Al2O3催化剂表面的臭氧没有分解产生羟基自由基和超氧自由基.
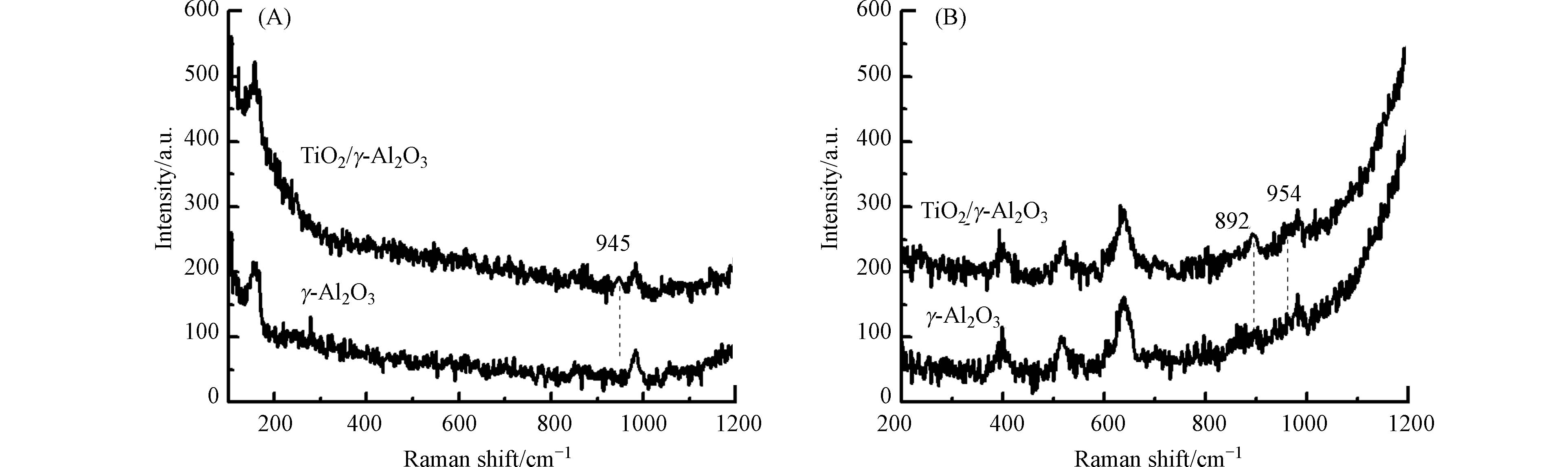
原位激光显微拉曼光谱用于研究γ-Al2O3及TiO2/γ-Al2O3催化剂上形成的活性氧物种(图7). γ-Al2O3的臭氧悬浆中,γ-Al2O3的拉曼光谱在945 cm−1有一个新的吸附峰,表明γ-Al2O3催化剂上有活性原子氧生成(≡Al—*O)[16-17];TiO2/γ-Al2O3的臭氧悬浆在892 cm−1和954 cm−1均出现了一个新吸收峰,表明TiO2/γ-Al2O3催化剂上既生成了过氧物种(≡Ti4+—*O2)又生成了活性原子氧(≡Al3+—*O). 通过与γ-Al2O3催化剂的原位激光显微拉曼光谱进行比较,发现TiO2/γ-Al2O3的拉曼光谱中活性氧物种吸收峰的位置有所偏移. 这是由于钛负载于γ-Al2O3表面改变了钛原子和铝原子对表面吸附的活性氧原子和过氧基团的键强度引起的[18-20]. 原位激光显微拉曼光谱证实了活性原子氧和过氧物种是TiO2/γ-Al2O3催化臭氧氧化反应的活性氧物种. 综合以上研究结果可得:TiO2/γ-Al2O3催化剂表面的Lewis酸性位为臭氧分解的活性位,当臭氧在γ-Ti-Al2O3催化剂表面分解时,会生成相对稳定的活性原子氧和过氧物种,这有助于有机物的吸附矿化.
2.4 催化剂的活性与稳定性
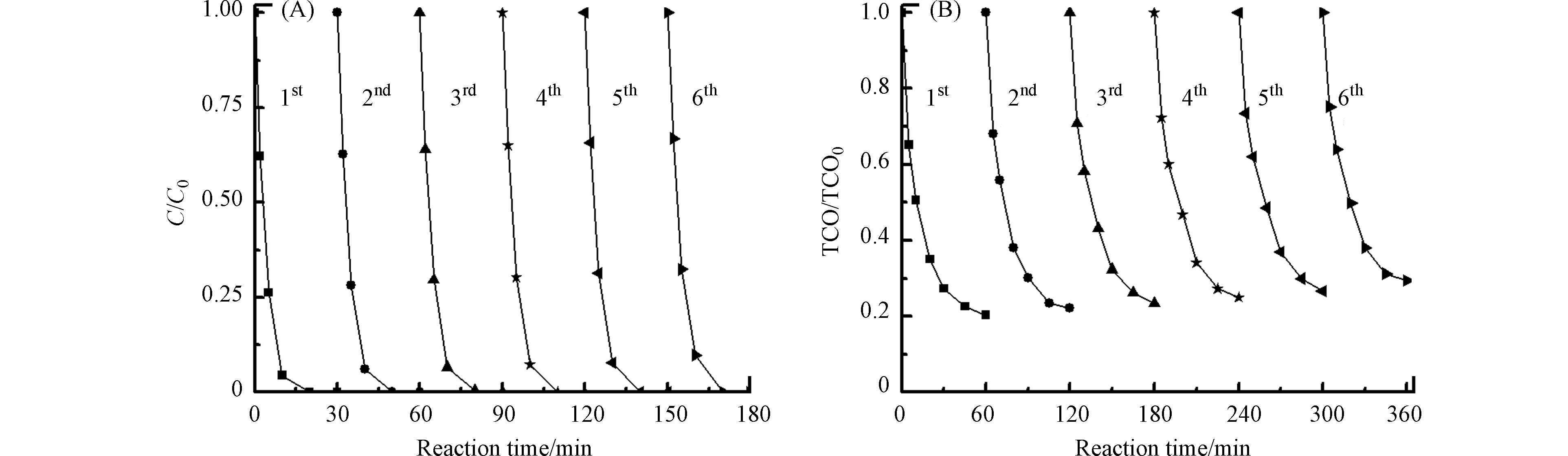
图8是TiO2/γ-Al2O3催化剂的活性与稳定性实验. 催化剂重复使用6次后,布洛芬的降解速率几乎不变,TOC的去除率重复3次后就基本稳定,仅下降6%,并且反应后溶液中未检测到Ti、Al的溶出,这说明TiO2/γ-Al2O3催化剂具有很好的活性与稳定性.
3. 结论(Conclusion)
成功制备了钛负载γ-Al2O3介孔分子筛催化剂. XRD、SEM、氮气吸附脱附和吡啶红外光谱表征结果表明,TiO2/γ-Al2O3催化剂具有大的比表面积,更多的Lewis酸量,二氧化钛高度分散于γ-Al2O3表面. TiO2/γ-Al2O3催化臭氧氧化布洛芬结果表明,该催化剂具有良好的催化活性与稳定性,反应60 min时,TOC的去除率为80%. 单独臭氧氧化过程中TOC的去除率仅为26%. 电子顺磁共振和原位激光显微拉曼光谱实验结果表明,TiO2/γ-Al2O3催化剂表面的Lewis酸性位是催化臭氧氧化反应的活性位,臭氧分解产生活性原子氧和过氧物种有利于有机物的矿化,从而表现出最高的催化活性. TiO2/γ-Al2O3是一种有前景的臭氧化催化剂.
-
表 1 不同湿地组各月份的CH4平均排放通量
Table 1. Average CH4 emission fluxes of constructed wetlands in different treatments by month
mg·(m2·h)−1 湿地组别 填充方式 5月 6月 7月 月平均值 1 T2F8 0.362±0.028 0.298±0.031 0.277±0.042 0.31±0.03 1 T4F6 0.647±0.015 0.454±0.028 0.203±0.041 0.44±0.03 1 T6F4 0.917±0.015 0.873±0.024 0.570±0.018 0.79±0.02 2 F8T2 0.154±0.014 0.257±0.021 0.251±0.035 0.22±0.02 2 F6T4 0.242±0.116 0.366±0.025 0.181±0.013 0.26±0.05 2 F4T6 0.613±0.021 0.537±0.051 0.396±0.045 0.52±0.04 对照组 F 0.407±0.018 0.260±0.024 0.320±0.016 0.33±0.02 表 2 不同湿地组各月份的NO2平均排放通量
Table 2. Average NO2 emission fluxes of artificial wetlands in different treatments for each month μg·(m2·h)−1
湿地组别 填充方式 5月 6月 7月 月平均值 1 T2F8 467.26±66.43 216.95±104.5 264.67±126.3 316.29±99.08 1 T4F6 73.41±13.2 226.10±53.2 154.18±74.2 367.90±46.87 1 T6F4 931.50±65.7 293.18±53.2 365.01±95.2 529.90±71.37 2 F8T2 215.69±105 187.03±32.4 98.08±14.6 166.93±50.67 2 F6T4 412.80±65.4 133.12±33.4 121.67±27.3 222.53±42.03 2 F4T6 898.87±47.3 265.36±125.4 355.37±153.2 506.53±108.63 对照组 F 1047.48±68.2 508.14±107.9 398.91±89.5 651.51±88.53 表 3 典型周期内不同湿地组 累计排放量
Table 3. Cumulative NO2 emissions from different wetland groups in a typical cycle μg·m−2
湿地组别 填充方式 NO2累计排放量 1 T2F8 57.70±5.38 1 T4F6 59.89±7.55 1 T6F4 67.39±12.17 2 F8T2 32.45±2.71 2 F6T4 40.22±3.69 2 F4T6 38.85±4.31 对照组 F 100.04±18.84 表 4 典型周期内人工湿地CH4及N2O的排放量及综合GWP
Table 4. CH4 and N2O emissions and integrated GWP in the typical cycle
湿地组名称 填充方式 CH4/(mg·m−2) GWP-CH4/(g·m−2) N2O/(mg·m−2) GWP-N2O/(g·m−2) GWP (CH4+N2O) /(g·m−2) 1 T2F8 155.44±0.76d 5.28±0.03 99.05±3.27d 29.52±0.09 34.80±2.71 2 F8T2 101.95±0.53f 3.47±0.02 45.21±1.35f 13.47±0.04 16.94±1.45f 1 T4F6 164.73±0.87d 5.60±0.03 150.88±3.68c 44.96±0.11 50.56±3.04c 2 F6T4 150.06±0.85ed 5.10±0.03 67.11±2.93e 20.00±0.08 25.10±2.14ed 1 T6F4 440.05±0.97b 14.96±0.04 188.81±6.37b 55.87±0.19 71.23±2.46b 2 F4T6 271.25±0.75c 9.22±0.02 71.20±4.83e 21.22±0.14 30.44±2.95d 对照 F 559.41±1.09a 19.02±0.04 213.66±7.21a 63.67±0.21 82.69±3.17a 注:各种温室气体的GWP以CO2当量 (CO2−eq) 计。 -
[1] 赵仲婧, 郝庆菊, 涂婷婷, 等. 铁碳微电解填料对人工湿地温室气体排放的影响[J]. 环境科学, 2021, 42(7): 3482-3493. doi: 10.13227/j.hjkx.202011248 [2] HUANG G H, LI X Z, HU Y M, et al. Methane (CH4) emission from a natural wetland of northern China[J]. Journal of Environmental Science and Health, Part A. Toxic/Hazardous Substances & Environmental Engineering, 2005, 40(6/7): 1227-1238. [3] MALTAIS-LANDRY G, MARANGER R, BRISSON J, et al. Greenhouse gas production and efficiency of planted and artificially aerated constructed wetlands, Environmental Pollution, 2009, 157(3): 748-754. [4] 宋长友. 不同基质人工湿地对氨氮及硝酸盐氮净化效果的研究[J]. 山东化工, 2022, 51(2): 201-203. doi: 10.3969/j.issn.1008-021X.2022.02.065 [5] 孙鹤洲, 刘骅, 田甜, 等. 人工湿地基质处理效果分析及发展趋势研究[J]. 绿色科技, 2022, 24(2): 156-158. doi: 10.3969/j.issn.1674-9944.2022.02.039 [6] SHEN Y H, ZHUANG L L, ZHANG J, et al. A study of ferric-carbon micro-electrolysis process to enhance nitrogen and phosphorus removal efficiency in subsurface flow constructed wetlands[J]. Chemical Engineering Journal, 2019, 359: 706-712. doi: 10.1016/j.cej.2018.11.152 [7] WANG K, LIU S, ZHANG Q, et al. Pharmaceutical wastewater treatment by internal micro-electrolysis-coagulation, biological treatment and activated carbon adsorption[J]. Environmental Technology, 2009, 30(13): 1469-1474. doi: 10.1080/09593330903229164 [8] ZHOU X, GAO L, ZHANG H, et al. Determination of the optimal aeration for nitrogen removal in biochar-amended aerated vertical flow constructed wetlands[J]. Bioresource Technology, 2018, 261: 461-464. doi: 10.1016/j.biortech.2018.04.028 [9] HAO Q J, JIANG C S, CHAI X S, et al. Drainage, no-tillage and crop rotation decreases annual cumulative emissions of methane and nitrous oxide from a rice field in Southwest China[J]. Agriculture, Ecosystems&Environment, 2016, 233: 270-281. [10] 王宁, 黄磊, 罗星, 等. 生物炭添加对曝气人工湿地脱氮及氧化亚氮释放的影响[J]. 环境科学, 2018, 39(10): 4505-4511. doi: 10.13227/j.hjkx.201801302 [11] IPCC (Intergovernmental Panel on Climate Change). Special report on emissions scenarios, a special report of Working Group III of the intergovernmental panel on climate change[M]. Cambridge: Cambridge University Press, 2013. [12] 任延刚. A/A/O工艺处理城市污水过程中温室气体的释放研究[D]. 济南: 山东大学, 2013. [13] ERMOSHIN V A, ENGEL V. Construction of a potential energy surface for molecular dynamics studies of methane adsorbed in zeolites[J]. The Journal of Physical Chemistry A, 1999, 103(26): 5116-5122. doi: 10.1021/jp9843860 [14] ZHANG W X, LI X M, YANG Q, et al. Pretreatment of landfill leachate in near-neutral pH condition by persulfate activated Fe-C micro-electrolysis system[J]. Chemosphere, 2019, 216: 749-756. doi: 10.1016/j.chemosphere.2018.10.168 [15] JIA W L, WANG Q, ZHANG J, et al. Nutrients removal and nitrous oxide emission during simultaneous nitrification, denitrification, and phosphorus removal process: effect of iron[J]. Environmental Science and Pollution Research, 2016, 23(15): 15657-15664. doi: 10.1007/s11356-016-6758-2 [16] 朱剑锋, 王艳琼, 王红武. 铁氧化物促进微生物直接种间电子传递的机理及其研究现状[J]. 环境化学, 2022, 41(4): 1-13. doi: 10.7524/j.issn.0254-6108.2021112501 [17] WU Y, WANG S, LIANG D, et al. Conductive materials in anaerobic digestion: From mechanism to application[J]. Bioresource Technology, 2019, 298: 122403. [18] 邹旭青, 郝庆菊, 赵茂森, 等. 铁矿石和生物炭添加对潜流人工湿地污水处理效果及温室气体排放的影响[J]. 环境工程学报, 2021, 15(2): 588-598. doi: 10.12030/j.cjee.202005025 [19] 程施艺. 含铁/锰基质人工湿地系统净水效果及温室气体排放研究[D]. 青岛: 山东大学, 2021. [20] 杨睿, 袁林江. 氧化亚氮还原酶对生物脱氮过程的好氧阶段中氧化亚氮的消耗和累积的调控机制研究[A]. 中国环境科学学会. 2020中国环境科学学会科学技术年会论文集(第二卷)[C]. 中国环境科学学会: 中国环境科学学会, 2020: 823-831. [21] DENG S, LI D, YANG X, et al. Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition[J]. Bioresource Technology, 2016, 219: 677-686. doi: 10.1016/j.biortech.2016.08.014 [22] 何起利, 梁威, 贺锋, 等. 人工湿地氧化还原特征及其与微生物活性相关性[J]. 华中农业大学学报, 2007, 26(6): 844-849. [23] 马宏璞. 锰矿物驱动湿地甲烷消减及甲烷厌氧氧化研究[D]. 重庆: 重庆大学, 2019. [24] 闵航, 谭玉龙, 吴伟祥, 等. 一个厌氧甲烷氧化菌菌株的分离、纯化和特征研究[J]. 浙江大学学报(农业与生命科学版), 2002, 28(6): 32-37. [25] 张馨文. 尾气增氧人工湿地污染物强化去除机制及其氧化亚氮减排效能研究[D]. 青岛: 山东大学, 2018. [26] LIU Y, CHENG X, LUN X X, et al. CH4 emission and conversion from A2O and SBR processes in full-scale wastewater treatment plants[J]. Journal of Environmental Science, 2014, 26(1): 224-230. doi: 10.1016/S1001-0742(13)60401-5 [27] 郑婧. 铁碳微电解处理高浓度酒精废水研究[D]. 哈尔滨: 哈尔滨工业大学, 2007. [28] CAKIR F Y, STENSTROM M K. Greenhouse gas production: A comparison between aerobic and anaerobic wastewater treatment technology[J]. Proceedings of the Water Environment Federation, 2004, 15: 566-580. [29] 刘聪敏. 吸附法浓缩煤层气甲烷研究[D]. 天津: 天津大学, 2010. [30] 李秀娟. 反硝化除磷脱氮工艺中N2O的产生及减量化控制[D]. 济南: 山东大学, 2012. -





 下载:
下载: