-
汞 (Hg) 是环境中生物毒性很强的金属污染物,具有持久性、易迁移性和高度的生物富集性等特点[1]。汞可通过地球化学循环和食物链富集,给人类和生态环境造成极大危害[2]。目前,燃煤电厂排放的汞是最大的人为汞排放源。作为以煤为主的能源消费大国,我国汞污染较为严重[3-5],每年煤炭燃烧向大气中排放810 t汞,占各种人为源汞排放总量的35%[6]。2013年,《关于汞的水俣公约》规定了汞的长期减排控制措施。我国现行的《火电厂大气污染物排放标准》也对汞的排放控制提出了明确限值[7],即自2015年起全面执行火电厂汞排放质量浓度不超过30 μg·m−3的规定。煤炭燃烧产生的汞有单质汞 (Hg0) 、氧化汞 (Hg2+) 和颗粒汞 (Hgp) 3种形态。Hg2+易溶于水,故可通过湿法脱硫设备去除[8];HgP易吸附在尘粒、飞灰颗粒表面,可通过除尘装置捕获去除。然而,Hg0因具有较高的挥发性 (2.46×10−1 Pa,25 ℃) 和较低的水溶性 (6×10−5 g·L−1,25 ℃) ,极易在大气中通过长距离运输而造成全球性汞污染,为最难控制的汞形态[9]。因此,有效控制Hg0是实现汞污染减排的关键。
催化脱除Hg0是一种行之有效的方法。传统选择性催化还原 (selective catalytic reduction,SCR) 催化剂 (V2O5-MoO3/TiO2和V2O5-WO3/TiO2) 在催化还原NOx的同时,可将Hg0氧化为Hg2+,并进一步利用后续脱硫装置进行协同脱除,从而提高设备经济性,因此被认为是应用前景良好的控制技术[10-11]。然而,SCR脱硝系统通常布置在高温、高尘、高酸性气体环境中,会降低催化剂的使用寿命[12]。由于其较高的工作温度,因而适用于非电行业的中低温催化氧化技术受到关注。目前,中低温钒钛SCR催化剂催化氧化Hg0,易受烟气组分 (如O2、NO、NH3、HCl、SO2、H2O) 和温度影响[13-16]。烟气中O2和NO可提供活性氧物种,从而促进Hg0的氧化。NH3会与Hg0竞争吸附催化剂活性位点,进而抑制Hg0氧化[17-20]。然而,烟气中SO2对Hg0氧化的影响机理还存在较大争议。有研究表明,SO2对Hg0的氧化表现为促进作用[21-22]。一方面,在O2存在的情况下,低浓度SO2会氧化生成SO3,与Hg0反应生成HgSO4;另一方面,SO2吸附在催化剂表面生成硫酸盐,可为Hg0氧化提供活性中心。然而,在某些情况下,SO2会与催化剂表面晶格氧反应生成硫酸盐和亚硫酸盐,使得催化剂表面活性氧位点减少,从而抑制Hg0氧化[23-26]。除此之外,多烟气组分共存时,Hg0的脱除机理尚不明确。
现有研究中,Cu作为活性组分,具有良好的氧化还原性[27]和抗硫性[28],添加到催化剂表面可极大地提升Hg0氧化效率。本研究以低温V2O5-MO3/TiO2脱硝催化剂为基础配方、Cu2O为改性组分,采用浸渍法制备Cu2O-V2O5-MoO3/TiO2催化剂,通过固定床反应器考察O2、NO、NH3、HCl、SO2、H2O等烟气组分对Hg0氧化性能的影响;并在此基础上,进一步探讨多烟气组分共存条件下Hg0的脱除机理,以期为SCR脱硝催化剂协同汞氧化提供参考。
-
所用催化剂由质量分数为3%的V2O5、质量分数为6% MoO3、Cu2O (质量分数为0~10%) 和TiO2组成。催化剂的制备步骤:称取定量偏钒酸铵、草酸、磷酸铵、钼酸铵、氧化亚铜和钛白粉溶于50 mL去离子水中,恒温水浴搅拌2 h,所得浆液置于105 ℃烘箱中3 h烘干水分。随后,将样品放入马弗炉中,在250 ℃空气气氛下焙烧1 h,之后在490 ℃下焙烧3 h,得到Cu2O负载量分别为0、1%、2%、6%、10%的Cu2O-V2O5-MoO3/TiO2催化剂,将其分别标记为0CuVMT、1CuVMT、2CuVMT、6CuVMT、10CuVMT。所有样品过60~80目 (0.180~0.250 mm) 筛备用。
-
使用美国Micromeritics公司生产的ASAP2020低氮吸附仪测定催化剂比表面积、孔容和孔径。其中,比表面积通过Brunauer-Emmett-Teller (BET)方法计算获得,孔容和孔径采用Barret-Joyner-Halenda (BJH)方法计算获得。
使用美国赛默飞世尔生产的Thermo Scientific K-Alpha型X射线光电子能谱仪进行X射线光电子能谱(XPS)分析,射线光源采用单色化AlKa源 (Mono AlKa,能量为1486.6eV) 。
H2程序升温还原(H2-TPR)实验在AutoChem II 2920型化学吸附仪(Micrometritics Co.)上进行。实验步骤如下:取50 mg样品(40~60目),在纯氧气氛下400 ℃预处理30 min;降至室温后用He吹扫15 min;然后通入体积分数为10% H2/Ar混合气,待仪器基线平稳后,以10 ℃·min−1速率升温至600 ℃,采用TCD检测耗氢量。
-
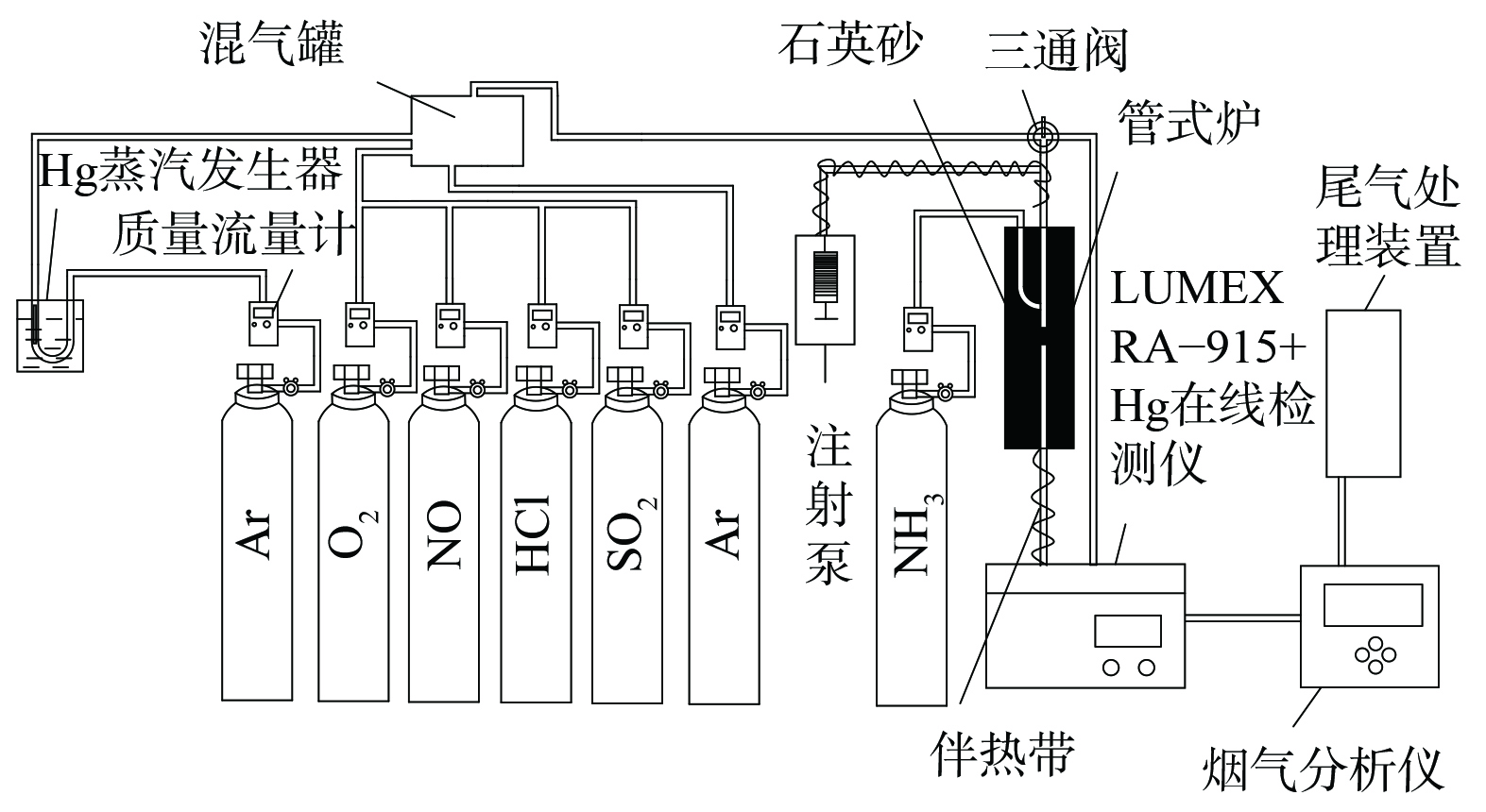
催化剂性能评价装置主要由模拟烟气、固定床反应装置、汞检测系统和尾气处理4部分组成 (见图1) 。其中,模拟烟气包括Hg0、O2、NO、NH3、HCl、SO2及平衡气Ar,烟气总流量为1 L·min−1。气体流量采用质量流量计精确控制。汞蒸气由置于恒温水浴锅中的汞渗透管产生,经载气Ar带出。固定床反应器使用内径4 mm石英管,中层添加石英砂作为支撑,采用管式电炉控制催化剂层反应温度。进出口Hg0浓度使用测汞仪 (RA-915+、LUMEX、美国) 进行监测,进出口NO和N2O体积分数由质谱仪 (DECRA、Hiden Analytical Ltd.,英国) 进行监测,SO2体积分数采用Testo 350烟气分析仪(Testo Co., Germany)检测。实验尾气经净化装置处理后排空。为避免汞蒸气沉积于管壁及水蒸气冷凝,实验管路均使用聚四氟乙烯管连接,并用伴热带加热至120 ℃。
活性评价实验过程:称取50 mg催化剂置于石英管内,用石英棉固定两端;使用Ar吹扫管路,待基线稳定后,将模拟烟气切换至旁路,检测反应器进口Hg0的初始浓度;15 min后将模拟烟气切换至反应器,检测出口Hg0浓度。Hg0氧化率(EHg)和NO转化率(ENO)计算方法见式 (1)~(2) 。
式中:[Hg0]in和[Hg0]out分别为固定床反应器进口和出口Hg质量浓度,μg·m−3;[NO]in和[NO]out分别表示固定床反应器进口和出口NO的体积分数,%。
-
不同Cu2O负载量催化剂的NO转化率和Hg0氧化率见图2。由于实际工业烟气中Hg0质量浓度较低,SCR净化装置空速为3 000~8 000 h−1。为缩短Hg0和NO在模拟烟气中达到反应平衡的时间,本研究将脱硝实验的空速设置为30 000 h−1,汞氧化实验的空速设置为1 600 000 h−1。图2表明,在200 ℃时,不同Cu2O负载量的CuVMT催化剂的NO转化率表现为:0CuVMT(94.1%)>1CuVMT(91.2%)>2CuVMT(90.9%)>6CuVMT(88.5%)>10CuVMT(76.7%),而Hg0氧化率表现为:0CuVMT(64.1%)<1CuVMT(96.1%)<2CuVMT(99.9%)~10CuVMT(99.9%)。与0CuVMT和1CuVMT催化剂相比,2CuVMT催化剂的NO转化率分别降低了3.4%和0.32%,而N2O生成量没有明显提升,与此同时,Hg0氧化率分别提升了35.8%和3.8%。当Cu2O负载量超过2%之后,NO转化率继续降低,N2选择性也出现不同程度下降,而Hg0氧化率保持不变。因此,综合催化剂的NO转化率、Hg0氧化率、N2选择性及制备成本等几方面因素,最终选定2CuVMT催化剂作为脱硝协同汞氧化的最优配方。
-
工业烟气组分复杂,与Hg0相比,各烟气组分在气体浓度及分子偶极等方面占据优势,会优先与催化材料活性位点发生键合,进而严重影响Hg0的氧化。因此,有必要深入研究不同烟气组分对2CuVMT催化剂脱除Hg0性能的影响,进而阐明复杂组分下Hg0的脱除机理,所有反应时长均为10 h。
-
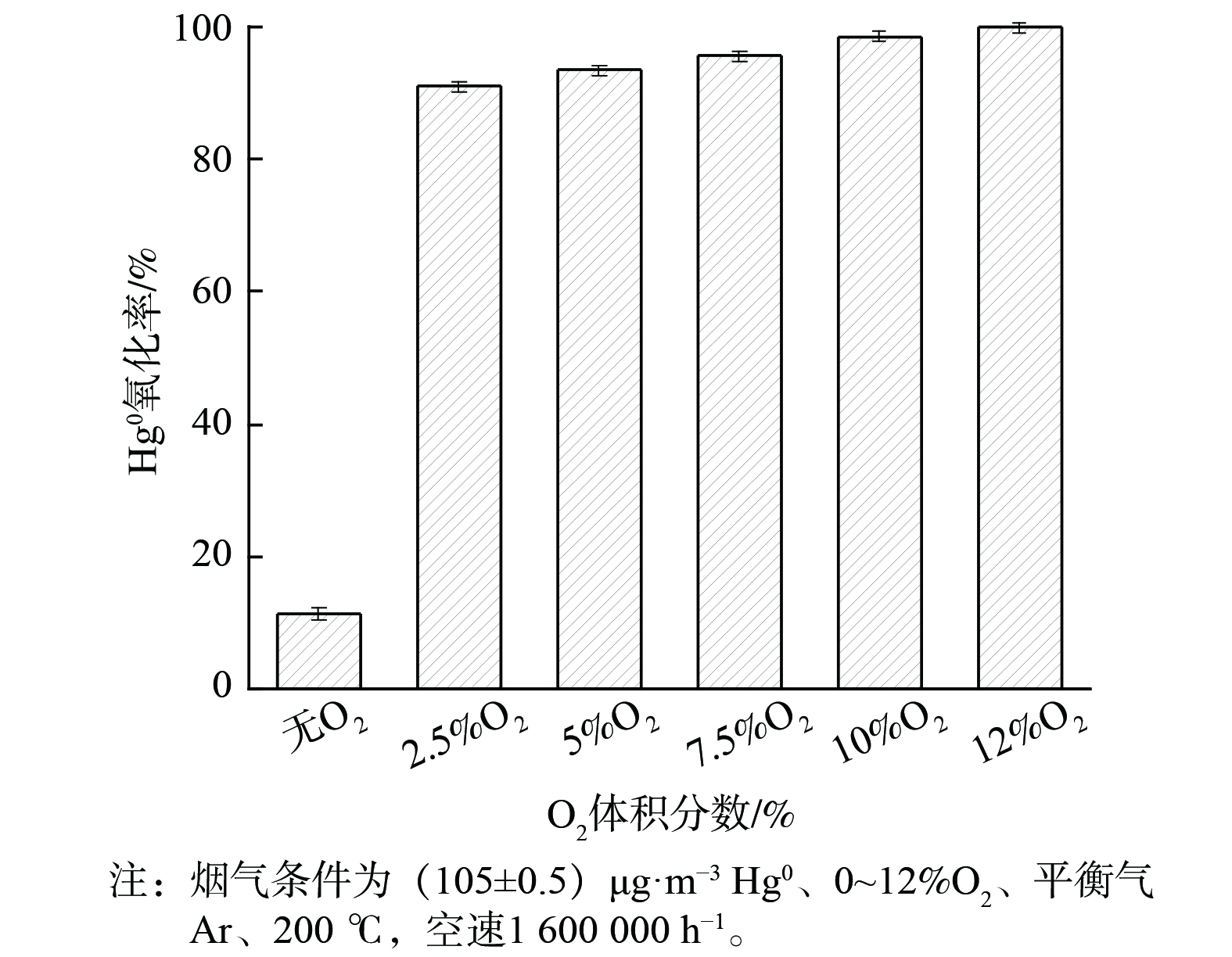
在200 ℃下,O2体积分数对2CuVMT催化剂Hg0氧化率的影响见图3。在无氧条件下,2CuVMT催化剂的Hg0氧化率仅为11.3%。此时,Hg0处于氩气惰性气氛下,物理吸附态的Hg0会与催化剂表面晶格氧结合[14,29],生成HgO。但由于晶格氧含量有限,Hg0氧化性能不高。当向反应体系通入2.5%的O2后,催化剂对Hg0的氧化性能显著提升,达到91.5%。这是由于O2可再生催化剂表面吸附氧和晶格氧,生成新的含氧活性位点,从而促进Hg0的氧化[30]。随着O2体积分数继续增至12%,Hg0氧化率也逐渐增至99.9%。
-
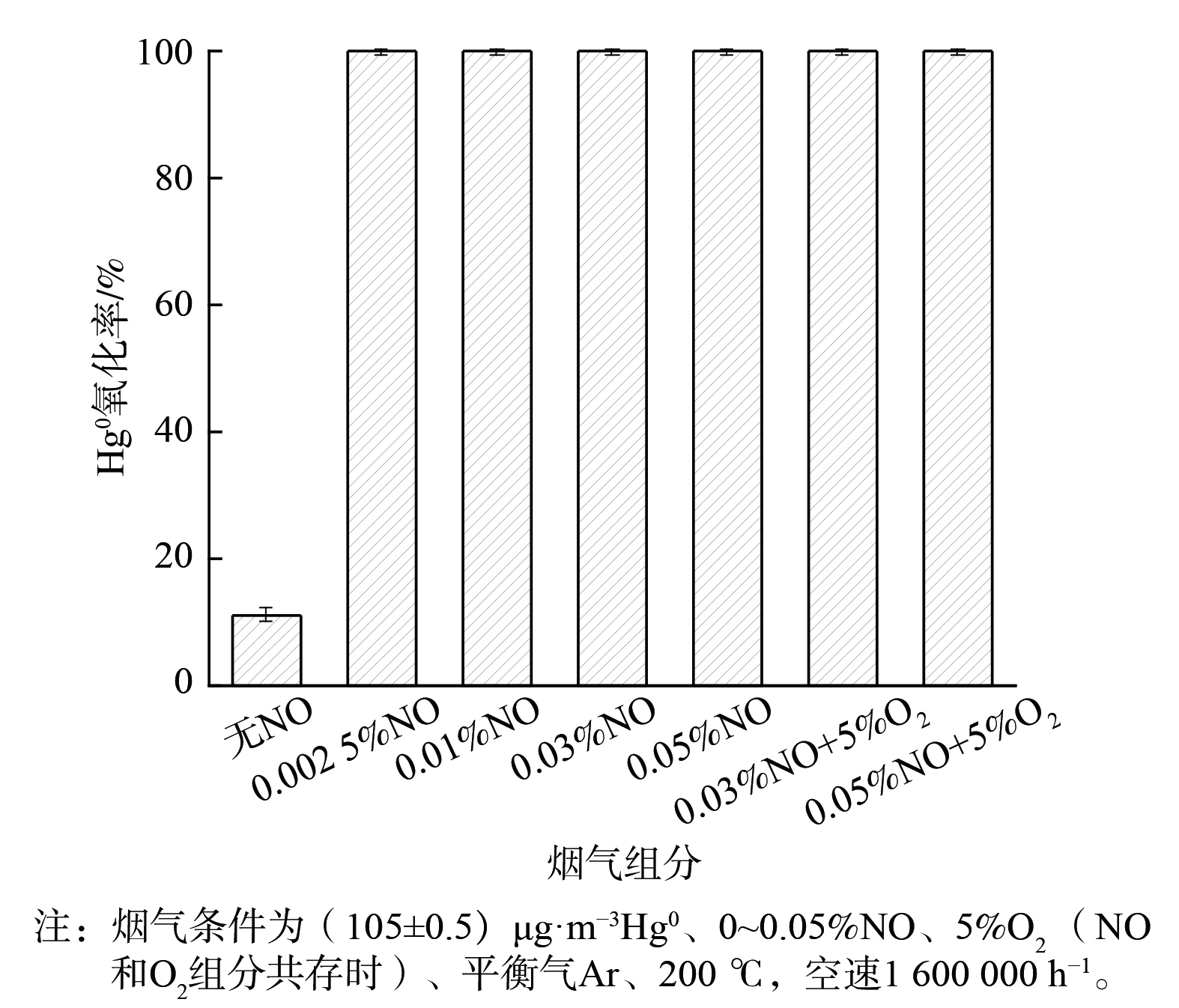
NO体积分数对2CuVMT催化剂Hg0氧化率的影响见图4。当0.002 5% NO通入反应体系后,Hg0氧化率立即升至99.9%;当NO体积分数增至0.05%时,Hg0氧化率依然稳定在99.9%。这可能是由于吸附态NO与催化剂表面活性氧反应生成具有氧化性的NO+、NO2一、NO2和NO3一等活性中间体,可将Hg0氧化为Hg(NO3)2[14,31],进而促进了Hg0氧化。当反应体系中继续添加5% O2后,O2可补充NO消耗掉的催化剂表面晶格氧[32],故Hg0的氧化率依然保持在99.9%。
-
NH3体积分数对2CuVMT催化剂Hg0氧化率的影响见图5。当烟气中加入0.005% NH3后,Hg0氧化率基本降至0。继续增加NH3体积分数至0.05%时,Hg0氧化率依然为0。这表明NH3对Hg0的氧化有强烈抑制作用。NH3会与Hg0发生强烈的竞争吸附[33],消耗催化剂表面晶格氧,从而抑制Hg0在催化剂表面发生氧化反应。反应过程如式 (3) 所示。
为探究SCR气氛条件对Hg0氧化率的影响,在Ar+NH3的气氛下,考察向烟气中添加体积分数为5%的O2,或添加混合气体 (含体积分数为5%的O2+体积分数为0.05%的NO) 对催化剂Hg0氧化率的影响。当体积分数为5%的O2加入烟气中时,2CuVMT催化剂Hg0氧化率显著提升。这说明O2补充了NH3消耗的催化剂晶格氧,并部分抵消了NH3对Hg0氧化的负面影响[34]。
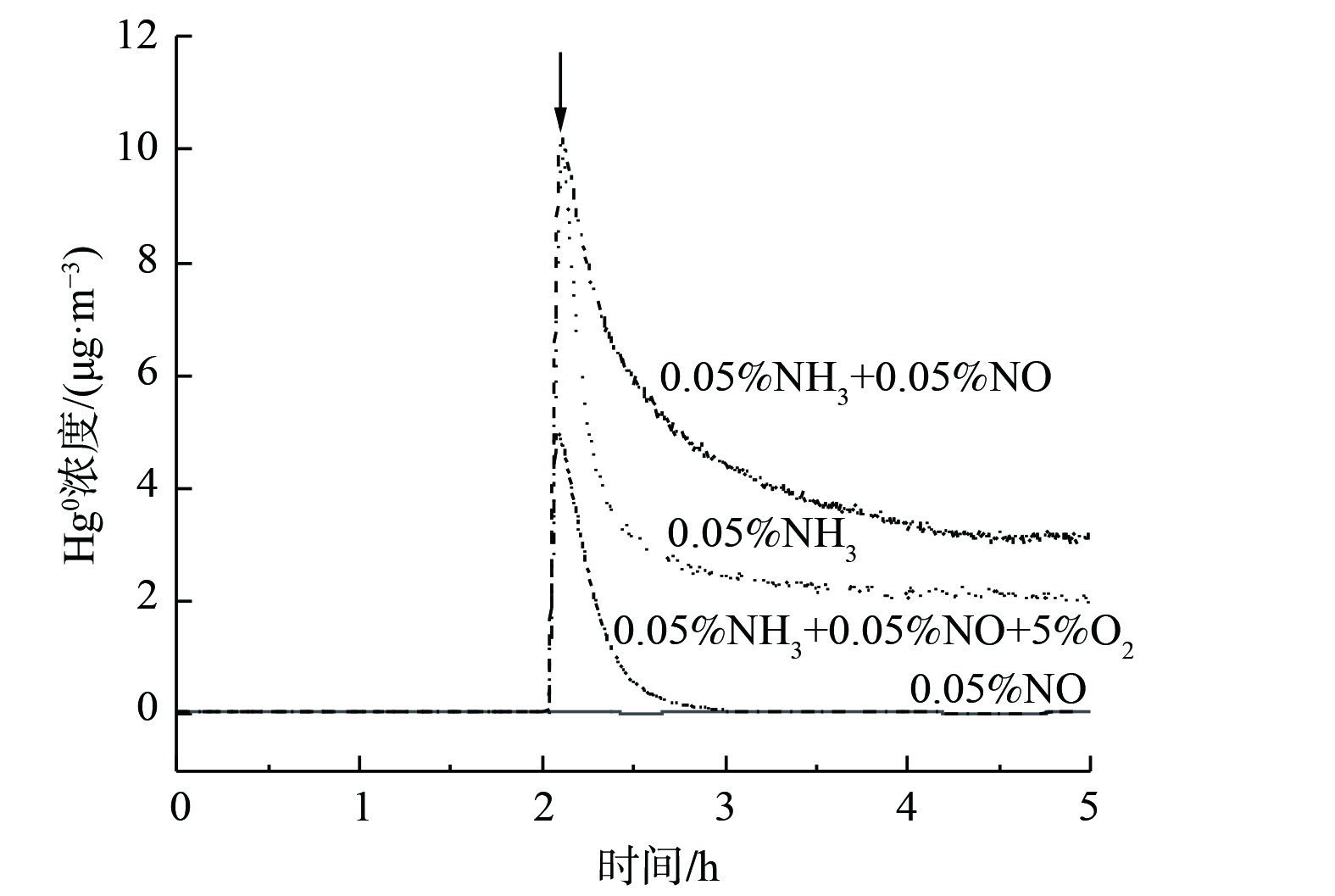
当NO、NH3和O2中的2种或3种组分以不同体积分数加入烟气时,各种添加方式下Hg0的氧化率表现为: (0.05% NO+5% O2) > (0.05% NH3+5% O2) > (0.05% NO+0.05% NH3+5% O2) > (0.05% NO+0.05% NH3) 。当烟气中通入混合气体 (0.05% NH3+0.05% NO) 时,Hg0氧化率为0。这表明此时NH3的抑制作用占主导地位,继续向烟气中加入5% O2后,Hg0氧化率升至57.3%。对比 (NH3+O2) 和 (NO+NH3+O2) 2种混合气体,反应体系中通入NO后,Hg0氧化率反而下降。这表明在此气氛下,发生了Hg2+被还原为Hg0的副反应。为探究副反应的发生机制,在200 ℃下通入混合气体 (10% O2+105 μg·m−3 Hg0+Ar) 3 h,使得催化剂表面沉积HgOx。之后用Ar吹扫,当Hg0质量浓度降为0后 (图6中箭头处) ,分别在烟气中通入0.05% NO、0.05% NH3、 (0.05% NH3+0.05% NO) 和 (0.05% NH3+0.05% NO+5% O2) 。这表明NH3和 (NO+NH3) 对HgOx存在还原作用,并且NO+NH3的还原性更强,而加入O2可在一定程度上抑制HgOx还原反应。因此,烟气中通入混合气体 (0.05% NH3+0.05% NO+5% O2) 后,仅有少量HgOx被还原。反应过程如式 (4)~(5) 所示。其中,x为1或1/2。
-
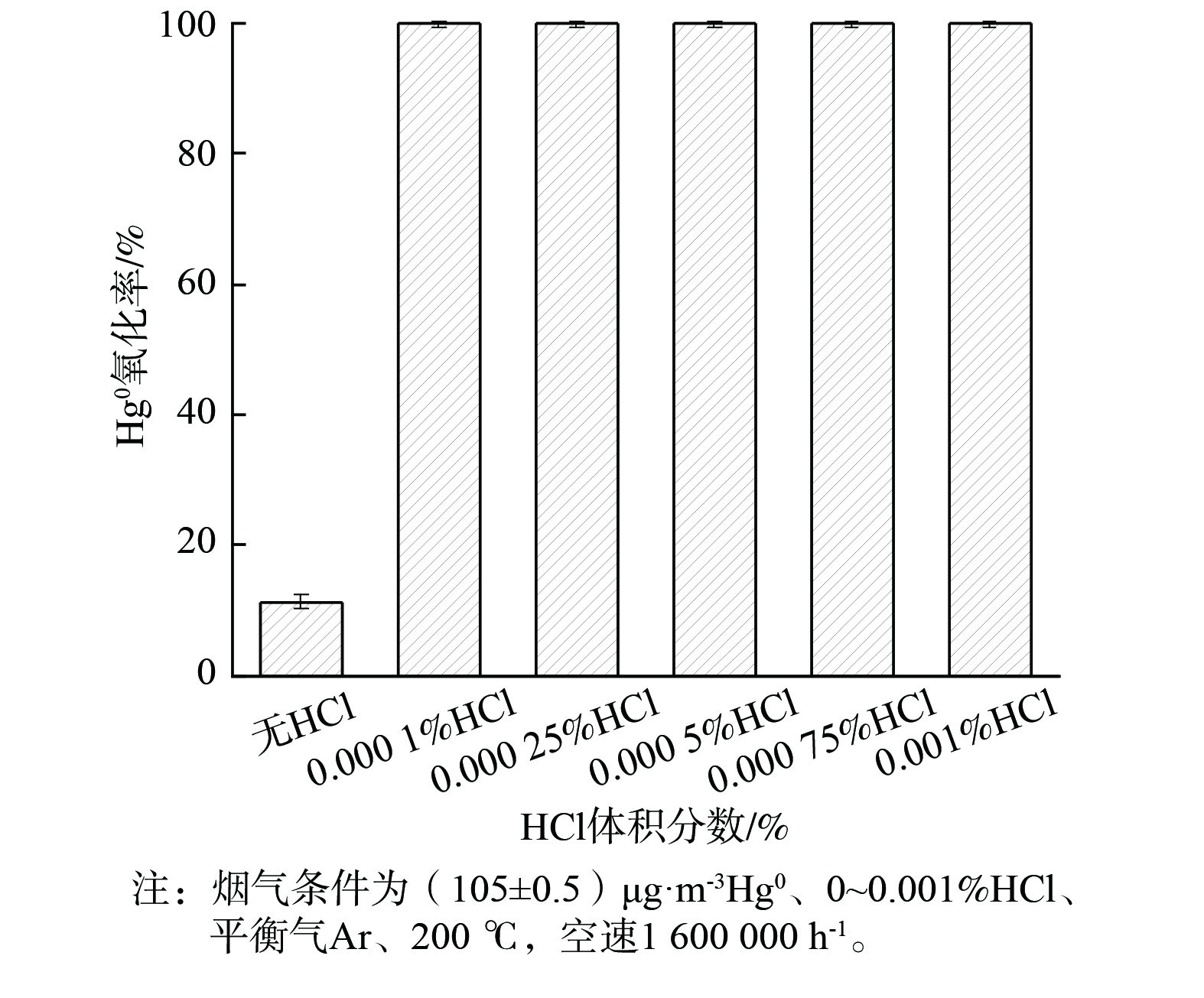
HCl是影响Hg0氧化反应的重要因素[35]。HCl的体积分数对2CuVMT催化剂Hg0氧化率的影响见图7。当体积分数为0.000 1%的HCl添加到烟气中时,Hg0氧化率即可达到99.9%。随着HCl体积分数逐渐增至0.001%时,Hg0氧化率稳定在99.9%。这表明HCl对Hg0氧化有明显促进作用。这可能是由于HCl在催化剂表面形成化学吸附态的活性Cl物种,可直接将Hg0氧化生成HgCl2[36]。
-
SO2和H2O对2CuVMT催化剂Hg0氧化率的影响见图8。当烟气中添加0.001% SO2时,Hg0氧化率可达到37.5%。随着SO2体积分数增至0.01%时,Hg0氧化率提升至52.1%。这表明SO2对Hg0氧化有一定促进作用。这可能是由于SO2和Hg0在催化剂表面发生竞争吸附的同时,在晶格氧的作用下,SO2和O* (晶格氧) 反应生成SO3和硫酸根,进而与吸附态Hg0反应生成HgSO3或HgSO4[23-24],从而有利于Hg0的去除。当烟气中同时存在SO2和O2时,Hg0氧化率明显下降,此时,SO2和O2反应生成SO3,加快了与催化剂活性组分生成CuSO4,使得催化剂硫酸化,最终导致催化剂活性位点数量减少、Hg0氧化率下降[27-28]。在实际SCR工况下,体系还会含有一定量水分。因此,考察在烟气中 (SCR条件:Ar+5% O2+0.05% NO+0.05% NH3+0.001%HCl) 加入H2O和SO2后对2CuVMT催化剂Hg0氧化率的影响。当烟气中加入体积分数为5%的H2O后,Hg0的氧化效率由99.9%降为97.3%。这是由于H2O和Hg0会竞争吸附催化剂表面活性位点,导致氧化率下降。继续向烟气中添加0.05% SO2后,催化剂Hg0的氧化效率降至95.1%。
-
反应温度是影响催化剂活性的重要因素。不同温度 (150~350 ℃) 对2CuVMT催化剂Hg0氧化率的影响见图9。随着反应温度的升高,2CuVMT催化剂Hg0氧化率呈现先平稳后降低的趋势。在150~250 ℃烟温范围内,Hg0氧化率保持在99.9%。然而,当温度升至300 ℃时,Hg0氧化率开始下降;在350 ℃时,Hg0氧化率进一步降至64.1%。参考文献[37]的研究结果,低温条件有利于Hg0吸附在催化剂表面参与氧化反应,而高温 (≥300 ℃) 则不利于Hg0在催化剂表面吸附,会使得参与氧化反应的Hg0质量浓度降低,从而导致Hg0的氧化被抑制。
-
1) BET分析。0CuVMT和2CuVMT催化剂的比表面积、孔容和介孔平均孔径见表1。在负载Cu2O后,催化剂的比表面积和孔容均呈下降趋势。其中,比表面积由0CuVMT的68.4 m2·g−1降至2CuVMT的57.3 m2·g−1,孔容由0.36 cm3·g−1 (0CuVMT) 略微降至0.33 cm3·g−1 (2CuVMT)。这表明添加Cu2O会堵塞催化剂部分孔道,导致催化剂比表面积和孔容降低。由于Cu2O堵塞了催化剂微孔,催化剂介孔平均孔径由0CuVMT催化剂的19 nm增至2CuVMT催化剂的21.3 nm。XU等[38]的研究结果表明,CuO/TiO2催化剂的比表面积、孔容、孔径与催化剂活性没有明显相关关系。
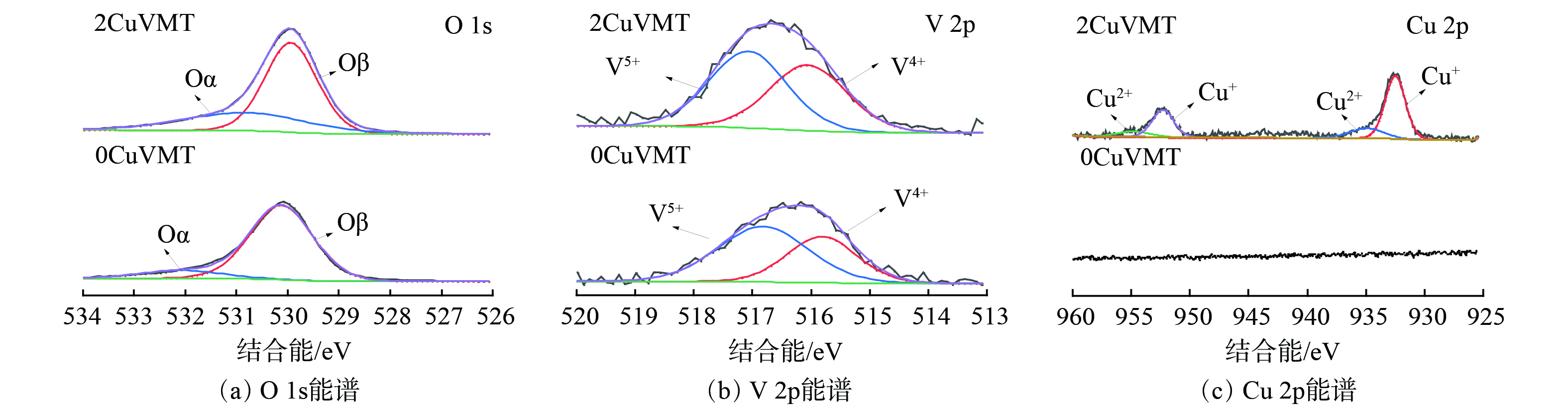
2) XPS表征。为确定催化剂表面O、Cu和V元素的化学价态,对CuVMT催化剂进行了XPS光谱分析,测定结果见图10。不同催化剂反应前后样品的O 1s XPS光谱 (图10 (a) ) 显示出2种特征峰。其中,结合能为530~530.3 eV的特征峰为晶格氧 (Oβ) [39],而在532~532.3 eV的特征峰归属于化学吸附氧 (Oα) [40]。当负载2%Cu2O后,催化剂表面的化学吸附氧 (Oα) 占比 (Oα/ (Oα+Oβ) ) 由31.9%逐渐升至36.5%。这表明化学吸附氧对于Hg0的氧化具有更高的活性[41]。
CuVMT催化剂样品的V 2p XPS光谱 (图10 (b) ) 可分为516.4 eV和517.3 eV两个峰,分别对应V4+和V5+的特征峰[42-43]。随着Cu负载量的增加,催化剂中V5+/V4+的比例由155% (0CuVMT) 降至117% (2CuVMT) 。这说明Cu2O改性提高了催化剂表面V4+的含量。这可能是由于Cu2O将部分V5+还原为了V4+。
CuVMT催化剂反应前后样品的Cu2p XPS光谱 (图10 (c) ) 表明,催化剂表面Cu的主要价态为Cu+和Cu2+。其中,Cu+为Cu元素主要的存在形态,其对应的结合能为932.5eV和952.2eV,而结合能在935.7eV和954.6eV的特征峰归属于Cu2+[44]。结合V 2p XPS结果,Cu2O改性使得催化剂表面V4+增加。这说明在制备过程中催化剂表面的Cu+和V5+确实存在相互作用,发生反应V4++Cu2+↔V5++Cu+,使得催化剂表面产生不饱和化学键和氧空位[41],可有效增加催化剂表面化学吸附氧含量,与O 1s XPS结果相一致。
3) H2-TPR表征。为阐明Cu改性对催化剂氧化还原性能的影响,对CuVMT催化剂进行了H2-TPR表征分析,测定结果见图11。0CuVMT催化剂在400~600 ℃出现了V5+和V4+的还原峰[45-46]。添加体积分数为2%的Cu2O改性后,催化剂原先V物种的还原峰大幅向低温方向偏移,且在154~263 ℃和300~400 ℃出现新的还原峰。其中,154~263 ℃的峰归属于Cu2+和Cu+的还原[47-48],300~400 ℃的峰为Cu-O-V物种的还原[47]。这表明添加Cu物种可增强催化剂表面Cu与V物种的交互作用,这与XPS结果相一致。总体来看,Cu改性后催化剂的还原峰整体向低温区迁移,H2的消耗量由0.036 mmol·g−1 (0CuVMT) 增至0.054 mmol·g−1 (2CuVMT) ,使得催化剂的氧化还原性能提高。
-
基于上述研究结果,本研究中Hg0的脱除过程主要分为吸附和催化氧化2个阶段。在吸附阶段,气态Hg0物理吸附在催化剂表面,反应过程如式 (6) 所示。Ads表示物质的吸附态。
Hg0(ads)与催化剂活性组分 (CuOx、V2O5) 中的晶格氧发生反应,生成HgO(ads)。与此同时,催化剂表面形成可由氧气补充的氧空位,反应过程如式 (7)~(8) 所示(M表示Cu或V)。
当反应体系中通入NO时,NO可与催化剂晶格氧反应生成NO2等具有氧化性的活性中间产物,从而促进Hg0的氧化。反应过程如式 (9)~(12) 所示。
当HCl通入反应体系时,HCl与催化剂活性组分反应生成活性Cl物种,进而与催化剂表面吸附态的Hg0(ads)反应,生成HgCl2。反应过程如式 (13)~(17) 所示。
当反应体系中存在SO2时,SO2可与晶格氧反应生成SO3,进而在催化剂表面与Hg0 (ads)发生催化氧化反应,生成HgSO4。反应过程如式 (18)~(20) 所示。
-
1) 通过对传统V2O5-MoO3/TiO2催化剂掺杂Cu2O改性,提高了催化剂在低温条件下Hg0的氧化率,Cu2O负载量为2%时,催化剂具有较好的脱硝协同氧化Hg0性能。
2) 不同烟气组分对Hg0氧化率的影响分析发现,O2、NO、HCl、SO2对Hg0的氧化具有促进作用,而NH3会消耗催化剂表面晶格氧,从而抑制Hg0的氧化。在多组分烟气条件下,Hg0氧化率表现为ENO+O2>ENH3+O2>ENO+NH3+O2>ENO+NH3。在NH3和 (NO+NH3) 两种气氛下,会将HgOx还原为Hg0,使得Hg0氧化率降低。
3) 随着温度由150 ℃升至250 ℃,Hg0氧化率一直维持在99.9%,进一步升温至350 ℃,Hg0氧化率则大幅下降。
4) 结合BET、XPS和H2-TPR分析,经过Cu2O改性后部分催化剂表面微孔被堵塞,催化剂表面存在Cu和V的相互作用,使得催化剂表面产生不饱和化学键和氧空位,有效提升催化剂表面化学吸附氧含量和低温氧化还原性能,促进Hg0氧化。此时催化剂表面的氧化反应遵循Mars-Maessen机理,即Hg0优先吸附在催化剂表面与晶格氧发生反应。
不同烟气组分对Cu2O改性V2O5-MoO3/TiO2脱硝催化剂汞氧化性能的影响
Effect of different flue gas components on mercury oxidation performance of Cu2O modified V2O5-MoO3/TiO2 De-NOx catalyst
-
摘要: 为提高传统选择性催化还原 (Selective Catalytic Reduction,SCR) 催化剂的低温汞氧化效率,采用Cu2O对钒钛催化剂进行改性,通过浸渍法制备了系列Cu2O-V2O5-MoO3/TiO2催化剂,利用固定床反应器研究催化剂在不同烟气组分条件下对单质汞的氧化特性。结果表明,在200 ℃时,2%Cu2O-V2O5-MoO3/TiO2催化剂的Hg0氧化率稳定在99.9%,NO转化率保持在90.9%,具有较好的脱硝协同汞氧化性能。单独的烟气组分如O2、NO、HCl、SO2均有利于Hg0的氧化,而NH3和NO+NH3会抑制Hg0氧化为Hg2+。随着反应温度升高,Hg0氧化率呈现先平稳后降低的趋势,在350 ℃时,Hg0氧化率仅为64.1%。比表面积测试法 (BET) ,X射线光电子能谱技术 (XPS) 和H2程序升温还原 (H2-TPR) 分析表明,Cu2O改性后的V2O5-MoO3/TiO2催化剂,表面Cu和V存在相互作用,使催化剂表面产生不饱和化学键和氧空位,有利于化学吸附氧的增加,从而促进Hg0的氧化。本研究可为提升SCR脱硝催化剂对汞的协同氧化性能提供参考。
-
关键词:
- Cu2O-V2O5-MoO3/TiO2催化剂 /
- 低温选择性催化还原(SCR) /
- Hg0氧化 /
- 烟气组分
Abstract: To improve the mercury oxidation efficiency over conventional traditional Selective Catalytic Reduction (SCR)catalysts at low temperatures, the vanadium-titanium catalysts were modified by Cu2O. A series of Cu2O-V2O5-MoO3/TiO2 catalysts were prepared by the impregnation method. The effects of different flue gas components on the oxidation of mercury over the catalysts were investigated by using a fixed-bed reactor. The results suggested that the Hg0 oxidation efficiency was stabilized at 99.9% and the NO conversion efficiency was maintained at 90.9% over 2%Cu2O-V2O5-MoO3/TiO2 catalyst at 200 ℃, which showed a good performance of synergistic De-NOx and mercury oxidation. The individual flue gas components, such as O2, NO, HCl, and SO2 were conductive to the oxidation of Hg0, while NH3 and the coexistence of NO and NH3 inhibited the oxidation of Hg0 to Hg2+. With the increase of reaction temperature, the Hg0 oxidation efficiency presented a trend of stability and then decrease.The oxidation rate was only 64.1% when the reaction temperature reached 350 ℃. Specific surface area testing (Brunauer-Emmett-Teller, BET), X-ray photoelectron spectroscopy (XPS) and H2 temperature programmed reduction (H2-TPR) analysis demonstrated that when loaded with Cu2O, the interaction between Cu and V existed over the Cu2O-V2O5-MoO3/TiO2 catalyst surface produced unsaturated chemical bonds and oxygen vacancies on the surface of the catalyst,which was conductive to the increase of chemisorbed oxygen, thus promoting the oxidation of Hg0. This study can provide a reference for improving the co-oxidation performance of SCR denitrification catalyst for mercury. -
表 1 CuVMT催化剂的比表面积及孔道结构
Table 1. Specific surface area and pore structure of CuVMT catalysts
催化剂 比表面积/ (m2·g−1) 孔容/ (cm3·g−1) 介孔平均孔径/nm 0CuVMT 68.4 0.36 19.0 2CuVMT 57.3 0.33 21.3 -
[1] HU Y A, CHENG H F. Control of mercury emissions from stationary coal combustion sources in China: current status and recommendations[J]. Environmental Pollution, 2016, 218: 1209-1221. doi: 10.1016/j.envpol.2016.08.077 [2] GOLDING G R, KELLY C A, SPARLING R, et al. Evaluation of mercury toxicity as a predictor of mercury bioavailability[J]. Environmental Science & Technology, 2007, 41(16): 5685-5692. [3] CHIU C H, KUO T H, CHANG T C, et al. Multipollutant removal of Hg0/SO2/NO from simulated coal-combustion flue gases using metal oxide/mesoporous SiO2 composites[J]. International Journal of Coal Geology, 2017, 170: 60-68. doi: 10.1016/j.coal.2016.08.014 [4] 左朋莱, 王晨龙, 佟莉, 等. 小型燃煤机组烟气重金属排放特征研究[J]. 环境科学研究, 2020, 33(11): 2599-2604. doi: 10.13198/j.issn.1001-6929.2020.06.07 [5] 魏忠秋, 刁永发, 姚跃辉. 活性焦配方型吸附剂脱除模拟烟气中Hg0[J]. 环境工程学报, 2017, 11(2): 1003-1008. doi: 10.12030/j.cjee.201509220 [6] STREETS D G, HAO J M, WU Y, et al. Anthropogenic mercury emissions in China[J]. Atmospheric Environment, 2005, 39(40): 7789-7806. doi: 10.1016/j.atmosenv.2005.08.029 [7] 国家质量监督检验检疫总局, 中国国家标准化管理委员会. 火电厂大气污染物排放标准: GB 13223—2011[S]. 北京: 中国环境科学出版社, 2012. [8] 鹿存房, 刘清才, 全学军. 利用污泥脱除燃煤电厂烟气中的汞[J]. 环境工程学报, 2017, 11(10): 5559-5564. doi: 10.12030/j.cjee.201611166 [9] 黄永健. 大气气溶胶汞污染研究[D]. 成都: 成都理工大学, 2002. [10] ESWARAN S, STENGER H G. Understanding mercury conversion in selective catalytic reduction (SCR) catalysts[J]. Energy & Fuels, 2005, 19(6): 2328-2334. [11] SCHWÄMMLE T, BERTSCHE F, HARTUNG A, et al. Influence of geometrical parameters of honeycomb commercial SCR-DeNOx-catalysts on DeNOx-activity, mercury oxidation and SO2/SO3-conversion[J]. Chemical Engineering Journal, 2013, 222: 274-281. doi: 10.1016/j.cej.2013.02.057 [12] 陈进生. 火电厂烟气脱硝技术: 选择性催化还原法[M]. 北京: 中国电力出版社, 2008. [13] WAN Q, YAO Q, DUAN L, et al. Comparison of elemental mercury oxidation across vanadium and cerium based catalysts in coal combustion flue gas: catalytic performances and particulate matter effects[J]. Environmental Science & Technology, 2018, 52(5): 2981-2987. [14] ZHAO L K, LI C T, ZHANG J, et al. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas[J]. Fuel, 2015, 153: 361-369. doi: 10.1016/j.fuel.2015.03.001 [15] MEI J, SUN P X, XIAO X, et al. Influence mechanism of the compositions in coal-fired flue gas on Hg0 oxidation over commercial SCR catalyst[J]. Journal of Industrial and Engineering Chemistry, 2019, 75: 130-137. doi: 10.1016/j.jiec.2019.03.013 [16] 陶莉, 张旭楠, 李彩亭, 等. 选择性催化还原催化剂氧化脱除烟气中单质汞[J]. 环境工程学报, 2015, 9(6): 2925-2932. doi: 10.12030/j.cjee.20150664 [17] STOLLE R, KOESER H, GUTBERLET H. Oxidation and reduction of mercury by SCR DeNOx catalysts under flue gas conditions in coal fired power plants[J]. Applied Catalysis B:Environmental, 2014, 144: 486-497. doi: 10.1016/j.apcatb.2013.07.040 [18] LI Y, MURPHY P D, WU C Y, et al. Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas[J]. Environmental Science & Technology, 2008, 42(14): 5304-5309. [19] YANG J, YANG Q, SUN J, et al. Effects of mercury oxidation on V2O5-WO3/TiO2 catalyst properties in NH3-SCR process[J]. Catalysis Communications, 2015, 59: 78-82. doi: 10.1016/j.catcom.2014.09.049 [20] BERETTA A, USBERTI N, LIETTI L, et al. Modeling of the SCR reactor for coal-fired power plants: impact of NH3 inhibition on Hg0 oxidation[J]. Chemical Engineering Journal, 2014, 257: 170-183. doi: 10.1016/j.cej.2014.06.114 [21] YANG B, LI Z, HUANG Q, et al. Synergetic removal of elemental mercury and NO over TiCe0.25Sn0.25Ox catalysts from flue gas: performance and mechanism study[J]. Chemical Engineering Journal, 2019, 360: 990-1002. doi: 10.1016/j.cej.2018.09.193 [22] CHIU C H, HSI H C, LIN H P, et al. Effects of properties of manganese oxide-impregnated catalysts and flue gas condition on multipollutant control of Hg0 and NO[J]. Journal of Hazardous Materials, 2015, 291: 1-8. doi: 10.1016/j.jhazmat.2015.02.076 [23] SUN X M, WU J, TIAN F G, et al. Synergistic effect of surface defect and interface heterostructure on TiO2/BiOIO3 photocatalytic oxide gas-phase mercury[J]. Materials Research Bulletin, 2018, 103: 247-258. doi: 10.1016/j.materresbull.2018.03.040 [24] WANG T, YANG Y H, WANG J W, et al. Preadsorbed SO3 inhibits oxygen atom activity for mercury adsorption on Cu/Mn doped CeO2(110) surface[J]. Energy & Fuels, 2020, 34(4): 4734-4744. [25] 宗晨曦, 纪蕾朋, 陈奎续, 等. Cu/SAPO-34对模拟烟气中零价汞的脱除性能[J]. 环境工程学报, 2018, 12(6): 1691-1701. doi: 10.12030/j.cjee.201710163 [26] 范红兵, 刁永发, 李攀, 等. 烟气成分对负载V2O5-WO3/TiO2聚苯硫醚纤维脱除烟气中Hg0的影响[J]. 环境工程学报, 2014, 8(7): 2957-2962. [27] YAMAGUCHI A, AKIHO H, ITO S. Mercury oxidation by copper oxides in combustion flue gases[J]. Powder Technology, 2008, 180(1/2): 222-226. [28] LIU Y, WANG Y J, WANG H Q, et al. Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by Sol-gel method[J]. Catalysis Communications, 2011, 12(14): 1291-1294. doi: 10.1016/j.catcom.2011.04.017 [29] ZHAO L K, LI C T, WANG Y, et al. Simultaneous removal of elemental mercury and NO from simulated flue gas using a CeO2 modified V2O5-WO3/TiO2 catalyst[J]. Catalysis Science & Technology, 2016, 6(15): 6076-6086. [30] ZHANG X N, LI C T, ZHAO L K, et al. Simultaneous removal of elemental mercury and NO from flue gas by V2O5-CeO2/TiO2 catalysts[J]. Applied Surface Science, 2015, 347: 392-400. doi: 10.1016/j.apsusc.2015.04.039 [31] 李海龙. 新型SCR催化剂对汞的催化氧化机制研究[D]. 武汉: 华中科技大学, 2011. [32] SHEN M Q, LI C X, WANG J Q, et al. New insight into the promotion effect of Cu doped V2O5/WO3-TiO2 for low temperature NH3-SCR performance[J]. RSC Advances, 2015, 5(44): 35155-35165. doi: 10.1039/C5RA04940G [33] LI H L, ZHAO J X, ZHANG W L, et al. NH3 inhibits mercury oxidation over low-temperature MnOx/TiO2 SCR catalyst[J]. Fuel Processing Technology, 2018, 176: 124-130. doi: 10.1016/j.fuproc.2018.03.022 [34] LI H L, WU S K, WU C Y, et al. SCR atmosphere induced reduction of oxidized mercury over CuO-CeO2/TiO2 catalyst[J]. Environmental Science & Technology, 2015, 49(12): 7373-7379. [35] SENIOR C L, SAROFIM A F, ZENG T F, et al. Gas-phase transformations of mercury in coal-fired power plants[J]. Fuel Processing Technology, 2000, 63(2/3): 197-213. [36] ZHAO L K, LI C T, ZHANG X N, et al. A review on oxidation of elemental mercury from coal-fired flue gas with selective catalytic reduction catalysts[J]. Catalysis Science & Technology, 2015, 5(7): 3459-3472. [37] 胡鹏, 段钰锋, 陈亚南, 等. Mo-Mn/TiO2催化剂的协同脱硝脱汞特性[J]. 中国环境科学, 2018, 38(2): 523-531. doi: 10.3969/j.issn.1000-6923.2018.02.014 [38] XU W Q, WANG H R, ZHOU X, et al. CuO/TiO2 catalysts for gas-phase Hg0 catalytic oxidation[J]. Chemical Engineering Journal, 2014, 243: 380-385. doi: 10.1016/j.cej.2013.12.014 [39] LI H L, WU C Y, LI Y, et al. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas[J]. Environmental Science & Technology, 2011, 45(17): 7394-7400. [40] HUANG W J, XU H M, QU Z, et al. Significance of Fe2O3 modified SCR catalyst for gas-phase elemental mercury oxidation in coal-fired flue gas[J]. Fuel Processing Technology, 2016, 149: 23-28. doi: 10.1016/j.fuproc.2016.04.007 [41] CHI G L, SHEN B X, YU R R, et al. Simultaneous removal of NO and Hg0 over Ce-Cu modified V2O5/TiO2 based commercial SCR catalysts[J]. Journal of Hazardous Materials, 2017, 330: 83-92. doi: 10.1016/j.jhazmat.2017.02.013 [42] HE S, ZHOU J S, ZHU Y Q, et al. Mercury oxidation over a vanadia-based selective catalytic reduction catalyst[J]. Energy & Fuels, 2009, 23(1): 253-259. [43] ZHAO L K, LI C T, LI S H, et al. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst[J]. Applied Catalysis B:Environmental, 2016, 198: 420-430. doi: 10.1016/j.apcatb.2016.05.079 [44] ZHANG Q L, XU L S, NING P, et al. Surface characterization studies of CuO-CeO2-ZrO2 catalysts for selective catalytic reduction of NO with NH3[J]. Applied Surface Science, 2014, 317: 955-961. doi: 10.1016/j.apsusc.2014.09.017 [45] LEE S. M, HONG S. C. Promotional effect of vanadium on the selective catalytic oxidation of NH3 to N2 over Ce/V/TiO2 catalyst[J]. Applied Catalysis B:Environmental, 2015, 163: 30-39. doi: 10.1016/j.apcatb.2014.07.043 [46] ZHAO X, HUANG L, LI H, et al. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3[J]. Chinese Journal of Catalysis, 2015, 36(11): 1886-1899. doi: 10.1016/S1872-2067(15)60958-5 [47] DONG L, ZHANG L, SUN C, et al. Study of the properties of CuO/VOx/Ti0.5Sn0.5O2 catalysts and their activities in NO+CO reaction[J]. Acs Catalysis, 2011, 1(5): 468-480. doi: 10.1021/cs200045f [48] CHEN B, XU R, ZHANG R, et al. Economical way to synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOx by ammonia[J]. Environmental Science & Technology, 2014, 48(23): 13909-13916. -






 下载:
下载: