-
氟(F)是卤族元素中电负性最强的元素,化学性质极为活泼。自然界中的氟广泛分布于岩石、土壤、空气和水体内[1],水体中的氟通常以F−的形态存在[2]。
近几十年来,水体氟污染逐渐成为受到全球性关注的重点问题[3]。氟是人体必需的微量元素[4],摄入少量的氟(0.05~0.07 mg·L−1)可帮助硬化牙釉质,有效防止蛀牙的发生[5-6]。但由于天然来源如富氟矿物的溶解,以及人类活动加剧环境水体中氟的持续输入[7],水体氟化物的超标正威胁着居民饮用水水质安全[8]。过量地摄入氟不仅会引起氟斑牙和氟骨症等疾病[9],而且会对人体的免疫系统、肾脏、胃肠道等产生不利影响[10]。我国严格限制水体中氟化物质量浓度[11],《生活饮用水卫生标准》(GB 5749-2006)和《地表水环境质量标准》(GB 3838-2002)中Ⅲ类水的氟化物(以F−计)标准限值均为1.0 mg·L−1。
山东省胶莱盆地为典型的高氟区,根据1980年山东省地方病研究所对胶莱盆地的地下水氟含量普查结果,沿胶莱河南岸一带地下水氟含量普遍高于4 mg·L−1[12]。虽然自20世纪80年代开始山东省采取了引水和理化除氟等措施改水降氟,但至今仍有不少地区遭受高氟的危害[13]。氟化物浓度过高可能对河流内动植物[14]和周围居民的生活造成危害,对地方水污染治理造成了较大挑战,也是目前水环境修复面临的主要难题[15]。因此,提供有效防止地表水体氟污染的技术具有必要性和迫切性[16]。
目前常用的除氟方法包括化学沉淀法、吸附法、膜过滤和离子交换法等[17]。其中吸附法往往再生后除氟效果不理想[18];膜过滤和离子交换法成本高,维护与管理困难[19]。比较成熟的石灰沉淀法(CaO)会使pH明显升高而呈强碱性[20],不宜用于自然水体除氟;CaCl2作为可溶性钙盐可提供大量Ca2+,理论上会促进CaF2沉淀的生成,采用石灰和CaCl2联合处理法可在控制pH的同时保证有足够的Ca2+[21];根据CaCO3 (Ksp=2.9×10−9)和CaF2 (Ksp=2.7 ×10−11)的溶度积差异,使用CaCO3可选择性地将F−转化为CaF2[22];普通硅酸盐水泥(简称水泥)外观为灰色粉末,其主要原材料包括CaO、SiO2和少量的Fe2O3和Al2O3等[23]。有研究表明,水化普通硅酸盐水泥[24]和水化铝酸盐水泥[25]是有效的除氟材料,而未经过水化、凝结、硬化的水泥粉末也同样具有除氟效果,TARALI等[26]在模拟含氟溶液中加入10%的水泥粉末能够使氟离子质量浓度从30 mg·L−1降低至11.67 mg·L−1 (去除率为61.1%),KANG等[27]在氟离子质量浓度为100 mg·L−1的模拟含氟溶液中投加1%的水泥粉末后可将质量浓度降低至52.7 mg·L−1 (去除率为47.3%)。水泥曾被应用于贵州省酸性含铁废水处理[28]和城市富营养水体[29]除磷,证明水泥应用于地表水污染治理具有可行性且未出现负面影响。
大量除氟技术的相关研究均采用实验室配制的模拟含氟溶液[17,24,26-27],杂质较少,与自然水体有较大差异,这也解释了现行的除氟技术在自然水体中除氟效果不明显,无法大范围推广的原因[18,30]。本研究以真实的地表水样为研究对象,选取了CaCO3、CaCl2和水泥3种材料,考察了其在不同投加条件下对水样中氟离子去除效果的影响,同时对表现出除氟效果的原材料和处理水样后形成的沉淀产物进行了表征分析,探究了可能的除氟机理,为水体氟污染控制提供参考和依据。
-
实验水样于2021年12月取自山东青岛胶州市的南胶莱河(图1),近年来该河流水体的氟离子质量浓度常处于劣V类水平(>1.5 mg·L−1)。南胶莱河发源于青岛平度市姚家村,经胶州市胶东街道店口村汇入大沽河,全长30 km,呈东南—西北走向,流域面积1 500 km2[31]。河流平均水深约1.5~2.0 m,流量约60 000~80 000 m3·d−1。
水样运输至实验室后测定基础水质指标(表1)并立即开展实验。对比《地表水环境质量标准》(GB 3838-2002)中标准限值可知,实验水样的初始氟离子质量浓度高于Ⅴ类水标准限值,本研究除氟目标为(在原位治理中)降低地表水氟离子质量浓度。
-
1)除氟材料。钙盐选用碳酸钙(AR)、无水氯化钙(AR)两种盐类,均购买自成都市科隆化学品有限公司;水泥(P.O 42.5R)购买自重庆市东方希望水泥有限公司。
2)实验试剂。氟化钠(GR)、硝酸钠(AR)、二水柠檬酸三钠(AR)、溴甲酚紫(IND)、氢氧化钠(AR)均购买自成都市科隆化学品有限公司;盐酸(36%~38%)购买自成都市科龙化工试剂厂。所有溶液均使用超纯水(18.25 MΩ·cm)配制。
3)实验仪器。磁力搅拌器JB-10型、离子计PXSJ-270F型、氟离子复合电极PF-202型、便携式pH计PHB-4型和电导率仪DDSJ-308A型均购买自上海仪电科学仪器股份有限公司;六联电动搅拌器JJ-4A型来自常州市金坛科兴仪器厂;超纯水机UPR-Ⅱ-40L型来自四川优普超纯科技有限公司。
4)材料表征。采用X射线衍射仪(EMPYREAN,荷兰纳帕科公司)分析处理前后材料物相组成的变化;红外光谱仪(NEXUS 670,美国赛默飞世尔科技公司)测定官能团变化;扫描电子显微镜(Helios G4 UC,美国赛默飞世尔科技公司)用于观察微观结构,能谱仪(Symmetry,英国牛津仪器)用于观察微区元素组成变化。
-
氟离子质量浓度参考《水质氟化物的测定 离子选择电极法》(GB 7484-87)中标准曲线法测定,0.2 mol·L−1二水柠檬酸三钠和1 mol·L−1硝酸钠作为总离子强度缓冲溶液。氟离子选择电极在实验前按照使用说明进行活化和清洗。测定溶液pH=5~8,需使用盐酸调节pH,利用溴甲酚紫指示剂判断溶液pH,判断依据为溶液由蓝紫色突变为黄色。待测水样倒入塑料烧杯中并置于磁力搅拌器上,读数稳定后读取电位响应值(mV),通过标准曲线得到氟离子质量浓度。
总磷(TP)测定参考《水质 总磷的测定 钼酸铵分光光度法》(GB 11893-1989);总氮(TN)测定参考《水质 总氮的测定 碱性过硫酸钾消解紫外分光光度法》(HJ 636-2012);氨氮(NH3-N)测定参考《水质 氨氮的测定 纳氏试剂分光光度法》(HJ 538-2009);高锰酸盐指数(CODMn)测定参考《水质 高锰酸盐指数的测定》(GB 11892-89);pH值测定采用玻璃电极法;电导率(EC)测定采用电导仪法。
-
通过系列实验研究不同静置时间、材料投加量、扰动程度和组合材料对除氟效果的影响。
1)不同静置时间对除氟效果的影响。向1 L水样中投加用量为1.5 g·L−1的水泥粉末,在100 r·min−1的转速下搅拌5 min后,分别于0、12、24和36 h后取上层液体测定氟离子质量浓度、pH和EC。
2) CaCO3、CaCl2和水泥在不同投加量处理下对除氟效果的影响。将3种除氟材料以0.5、1.0、1.5 g·L−1的用量分别投加至1 L水样中,对照组(CK)不投加任何材料,在100 r·min−1的转速下搅拌5 min,静置24 h后取上清液测定氟离子质量浓度、pH和EC。
3)不同扰动程度对除氟效果的影响。向1 L水样中投加用量为1.5 g·L−1的水泥粉末,分别改变搅拌速度(0、100、200、300 r·min−1)和搅拌时间(0、5、10、15 min),静置24 h后取上清液测定氟离子质量浓度、pH和EC。
4) CaCO3、水泥不同组合比例对除氟效果的影响。固定CaCO3的投加量为1.0 g·L−1,水泥以0.5、1.0、1.5 g·L−1 3种投加比例与CaCO3组合加入1 L水样中,即CaCO3与水泥的质量比分别为2∶1、1∶1、2∶3。对照组(CK)为不投加任何材料的处理,在100 r·min−1的转速下搅拌5 min,静置24 h后取上清液测定氟离子质量浓度、pH和EC。
-
实验中各处理组均设置3次重复,数据以均值±标准差(SD)形式表示。各组处理的氟离子质量浓度均值间的多重比较采用Dunnett’s T3法,显著性水平为0.05,不同的英文字母代表数据均值间差异显著。使用SPSS 25.0进行统计分析。采用Origin 2018绘制图表。
-
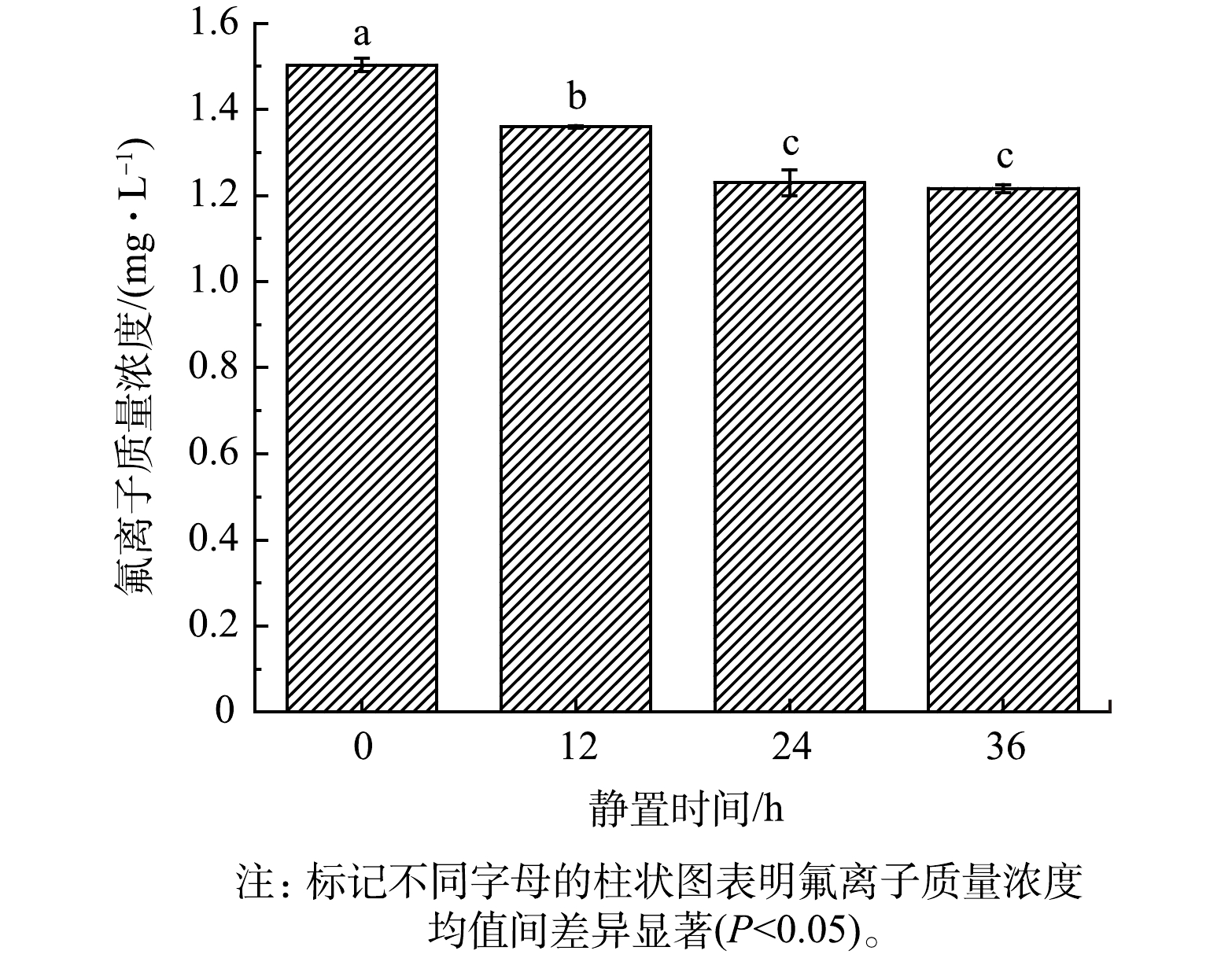
在0~24 h内,随着静置时间的增加,水样中的氟离子质量浓度不断降低,由1.50 mg·L−1降至1.23 mg·L−1(见图2)。根据统计分析结果,静置0、12和24 h后氟离子质量浓度均值间差异显著,而静置24 h和36 h后氟离子质量浓度均值间差异不显著。因此,之后的实验均静置24 h后取上清液测定水样中的氟离子质量浓度。
-
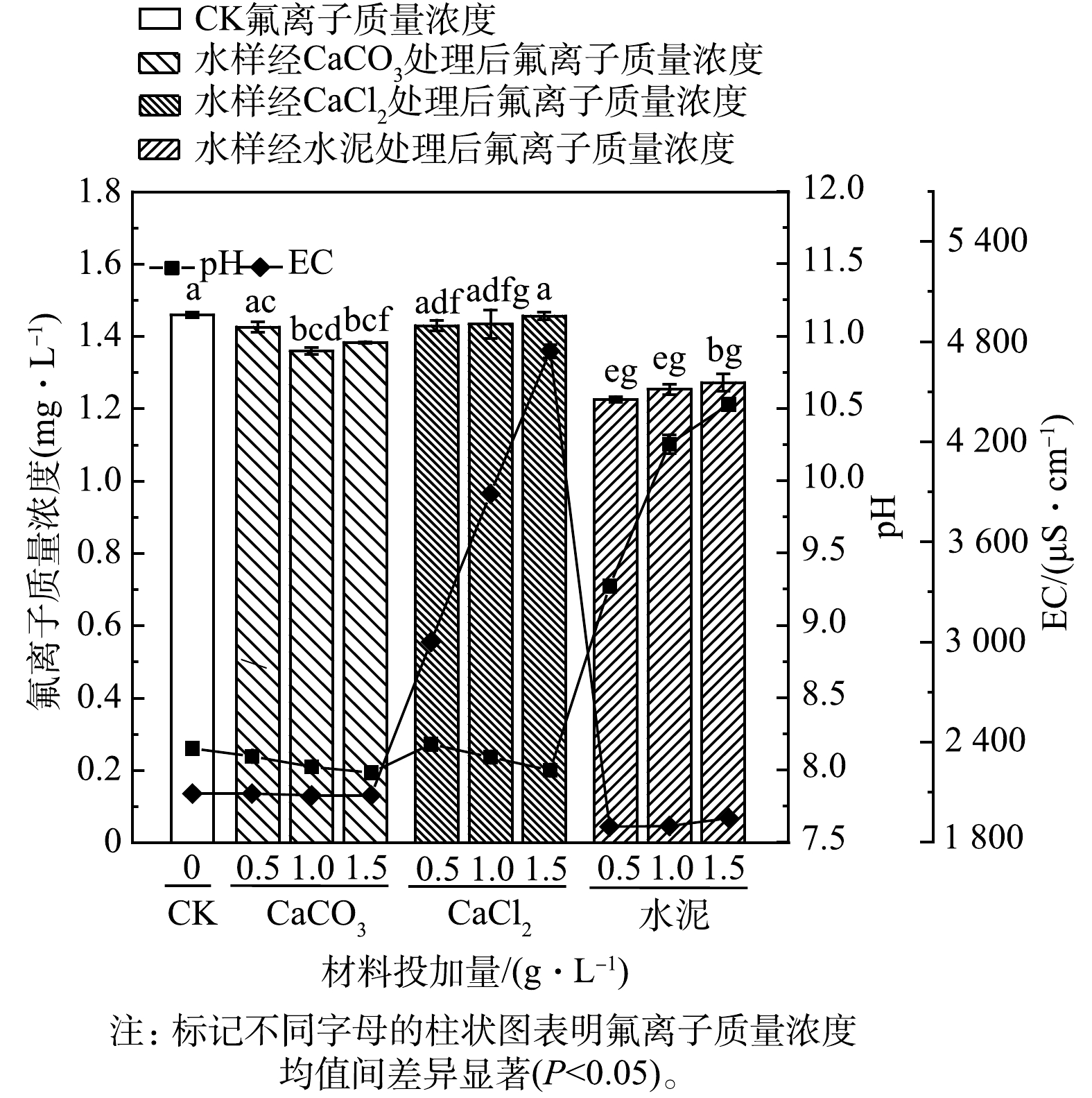
根据氟离子质量浓度的统计分析结果(图3)可知,CaCl2在3种投加量处理下的氟离子质量浓度均值与对照组差异不显著;当在水样中投加1.0 g·L−1和1.5 g·L−1的CaCO3时,氟离子质量浓度均值与对照组差异显著,表现为低于对照组,分别由1.46 mg·L−1降至1.36 mg·L−1和1.38 mg·L−1;水泥在3种投加量处理下的氟离子质量浓度均值显著地低于对照组和CaCO3处理组,相较于对照组,在水样中投加0.5、1.0和1.5 g·L−1的水泥处理后氟离子质量浓度由1.46 mg·L−1分别降至1.23、1.25和1.27 mg·L−1,在3种水泥投加量下氟离子质量浓度均值间差异不显著。
当3种材料分别用于地表水样除氟时,CaCl2未表现出除氟能力,CaCO3、水泥可显著降低水样中的氟离子质量浓度,水泥的除氟效果优于CaCO3,在水样中投加0.5 g·L−1的水泥可达到16%的除氟率,而投加1.0 g·L−1的CaCO3后除氟率仅为7%。CaCl2易溶于水,可解离出大量Ca2+和Cl−,大量的Cl−会产生一定的竞争吸附,抑制除氟效果[32],地表水样相较于模拟含氟水溶液往往含有较多其他共存的阴离子,同样的材料通常在天然高氟水中除氟效果明显降低[33]。
在水样中加入CaCO3和CaCl2对pH没有明显的影响。加入水泥后,水样pH明显升高,且pH随着投加量的增加而增大,在1.0 g·L−1和1.5 g·L−1的水泥投加量下pH已分别达到了10.25和10.53。投加水泥后出现的pH大幅增升高可认为是水化反应形成的Ca(OH)2引起的,同时游离的Ca2+可与F−反应生成CaF2沉淀,降低水样中氟离子质量浓度[34]。
根据EC测定结果可知,在水样中投加CaCl2后会引起EC剧增,投加CaCO3和水泥后,水样EC均有不同程度的下降,水泥引起的EC下降相较CaCO3更明显。与对照组相比,投加0.5、1.0和1.5 g·L−1的水泥后,水样的EC由2 090 μS·cm−1降低至1 892、1 896、1 943 μS·cm−1。EC可反映水体中总溶解离子的含量[35],CaCl2作为溶解度高的离子化合物,溶于水后总溶解离子含量的升高使得水样的EC呈上升趋势;而投加CaCO3和水泥后水样中氟离子质量浓度降低,意味着游离的氟离子在处理后被固定,总溶解离子含量降低,从而导致EC降低。
-
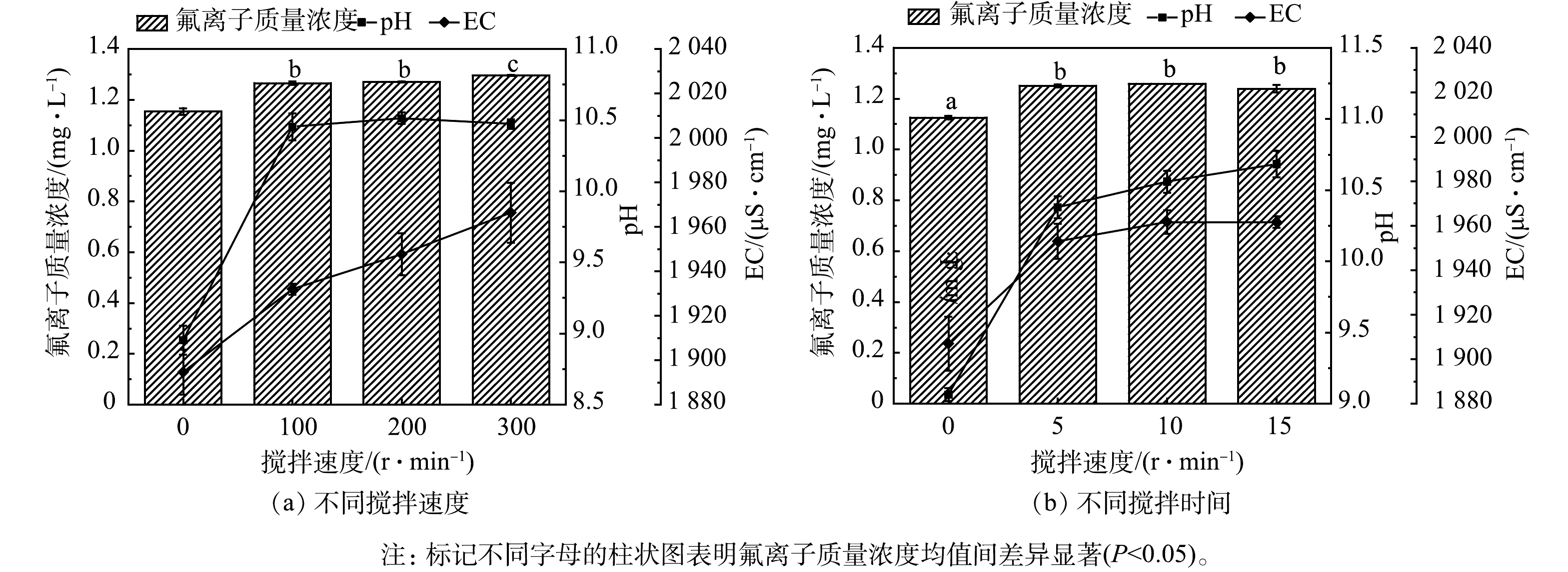
通过改变搅拌时间和搅拌速度可改变水样的扰动程度,相关影响见图4。在水泥加入量(1.5 g·L−1)相同的情况下,搅拌速度为0 r·min−1时,水样的氟离子质量浓度均值(1.15 mg·L−1)显著地低于搅拌速度为100、200、300 r·min−1的处理;搅拌时间为0 min时,水样的氟离子质量浓度均值(1.12 mg·L−1)也显著地低于搅拌时间为5、10、15 min的处理。搅拌速度为0 r·min−1、搅拌时间为0 min时,pH分别为8.95和9.06,而经过搅拌后,pH均达到了10.00以上。随着搅拌速度和搅拌时间的增加,EC也随之增加。
与工业含氟废水相比,氟污染地表水中氟离子质量浓度较低,现行的除氟技术对自然水体中较低含量的氟化物去除效果不明显[30]。沉淀的形成需要先由构晶离子形成晶核,然后晶核成长为沉淀微粒,在较低氟浓度条件下很难诱导沉淀形成晶核[36]。在沉淀颗粒成核生长过程中,若受到较大扰动,晶粒难以成长,粒度减小,沉淀物溶解度增加[37]。
适当搅拌可使构晶离子充分接触,形成沉淀微晶体。本研究中,在投加相同量的水泥的情况下,不经过搅拌处理的水样中氟离子质量浓度和EC更低,说明在搅拌速度为100 r·min−1和搅拌时间为5 min的处理下,部分形成的晶体结构已被破坏,使得氟离子重新释放进入溶液中[38]。
-
根据结果2.2可知CaCO3和水泥分别表现出了一定的降氟效果,将CaCO3、水泥组合,研究这2种材料以不同的组合比例混合使用对除氟效果的影响,结果如图5所示。
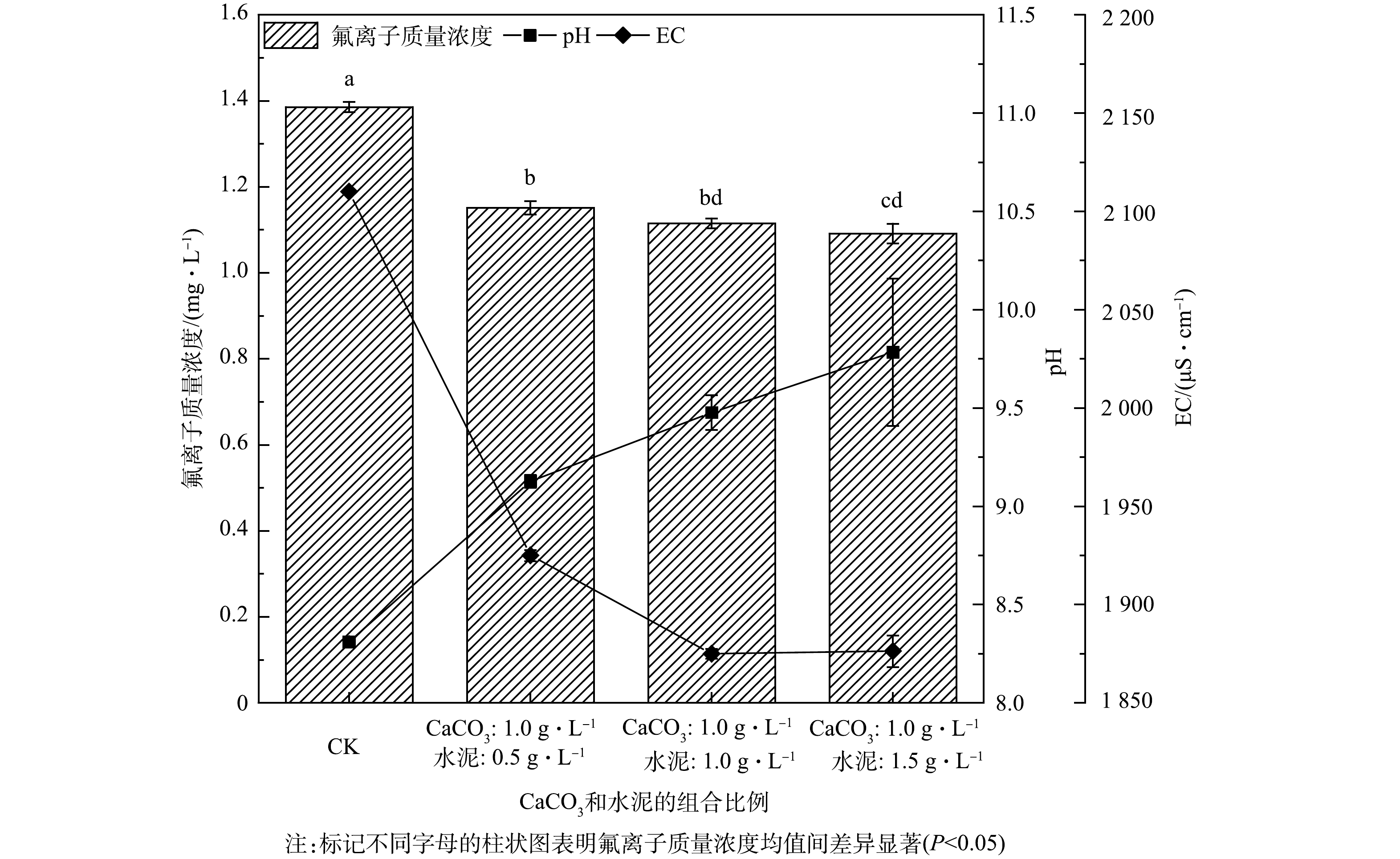
根据分析结果可知,投加组合材料后氟离子质量浓度均值显著地低于对照组(1.39 mg·L−1)。随着水泥比例的升高,氟离子质量浓度呈下降趋势,分别达到1.15、1.11、1.09 mg·L−1,但水泥投加量为1.5 g·L−1时的氟离子质量浓度均值与投加量为1.0 g·L−1时的氟离子质量浓度差异不显著。
同样,随着水泥比例增加,EC呈现下降趋势。对照组的EC为2 110 μS·cm−1,投加水泥1.0 g·L−1、1.5 g·L−1时,EC可分别降至1 875 μS·cm−1、1 876 μS·cm−1。
此外,随着组合材料中水泥比例的增加,水样的pH随之升高。对照组的pH为8.31,投加水泥0.5、1.0、1.5 g·L−1时,pH分别为9.12、9.47和9.78。
2种除氟材料组合处理组比单独处理的除氟率更高。当CaCO3投加量为1.0 g·L−1,水泥投加量为1.5 g·L−1时,组合材料对水样中氟离子的去除率达到22%,而在水样中单独投加1.0 g·L−1的CaCO3和1.5 g·L−1的水泥,除氟率分别为7%和13%。除提升除氟率以外,组合处理组可较好地控制水样的pH,这在自然地表水体除氟中尤为重要。在CaCO3、水泥的3种组合比例下,水样的pH保持在9~10,pH升高幅度小于单独水泥处理。CaCO3、水泥组合使用后pH升幅降低,这有利于地表水除氟,其机理值得在未来进行进一步的研究。在实验规模为1 L地表水样的小型实验中,水泥造成的pH大幅升高不可避免,而在实际的地表水中,碳酸及碳酸盐缓冲系统、硅酸和硅酸盐以及有机酸和有机酸盐等多种缓冲系统可发挥作用,降低pH的变化[39]。
-
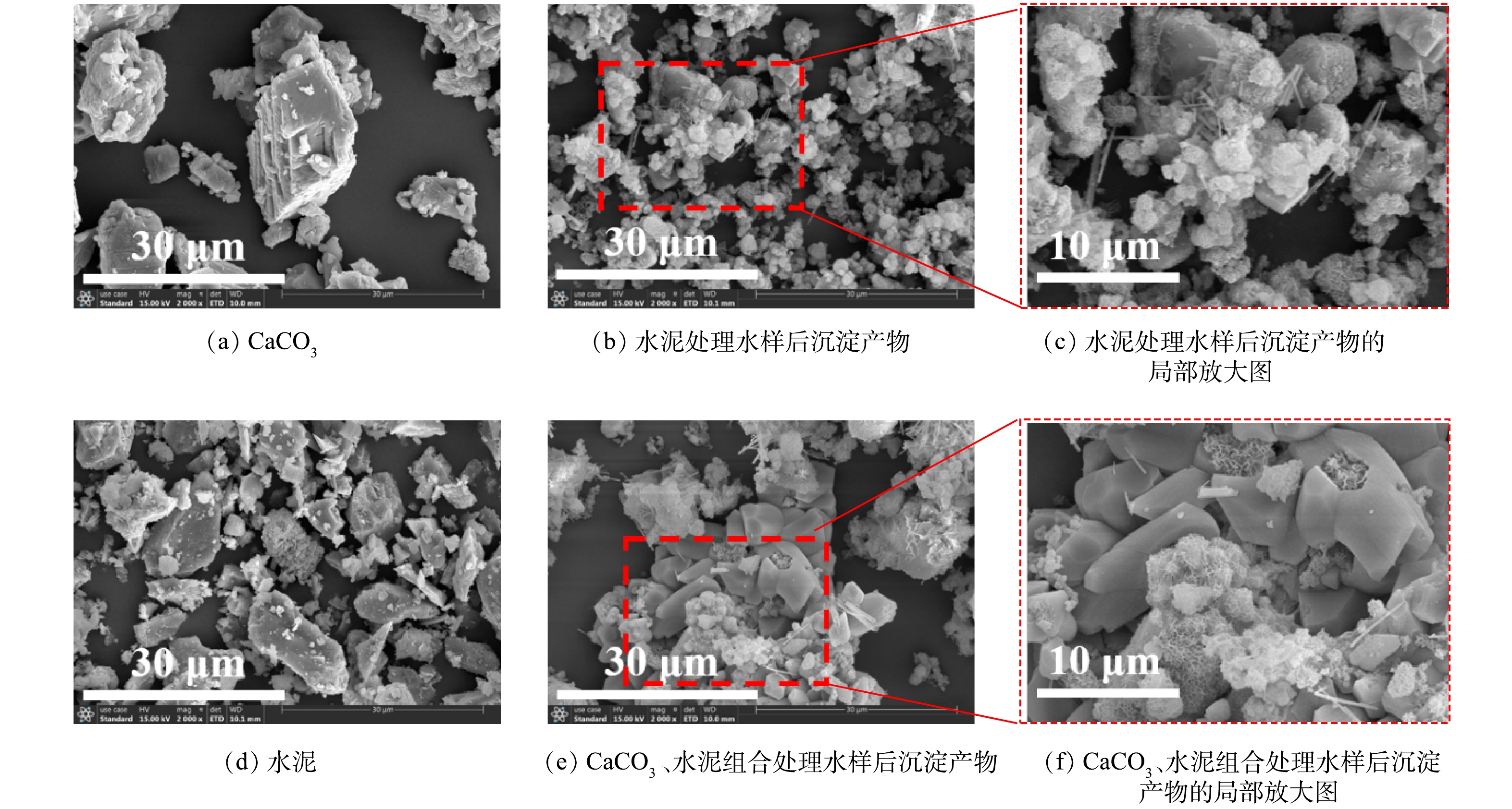
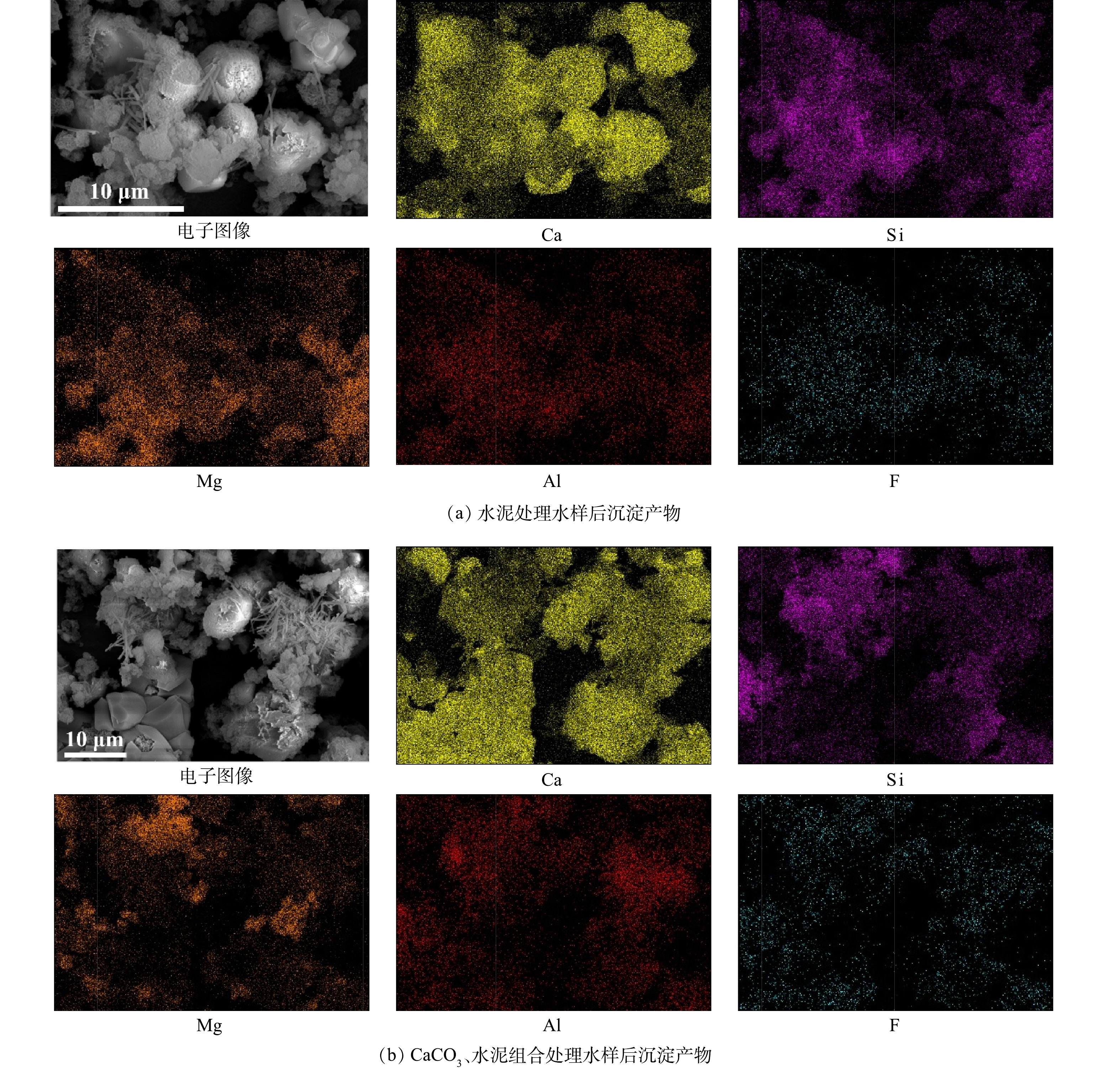
1)扫描电镜(SEM)+能谱仪(EDS)。通过SEM可观察到处理前CaCO3颗粒整体呈立方体状(图6 (a))。水泥由粒径范围较大(粒径不均匀)的颗粒组成(图6 (d)),颗粒外观不规则。无论是单独投加水泥还是CaCO3、水泥组合应用于水样除氟后,在底部沉淀产物中均可观察到大量新生成的球状颗粒和针棒状结构。化学沉淀法的除氟效果与沉淀颗粒的大小有关,沉淀颗粒越大,出水氟离子质量浓度越低[40]。相较于单独投加水泥,CaCO3、水泥组合处理中由于有相对大尺寸颗粒CaCO3的存在,氟离子与投加的材料反应生成的沉淀会更容易随着CaCO3而沉降,从而使处理后水样的氟离子质量浓度更低。在CaCO3、水泥组合处理水样的沉淀产物中,粒径较小的球状颗粒和针棒状结构附着于粒径较大的CaCO3周围(图6 (e)),证明了CaCO3的存在有利于沉淀产物的沉降。
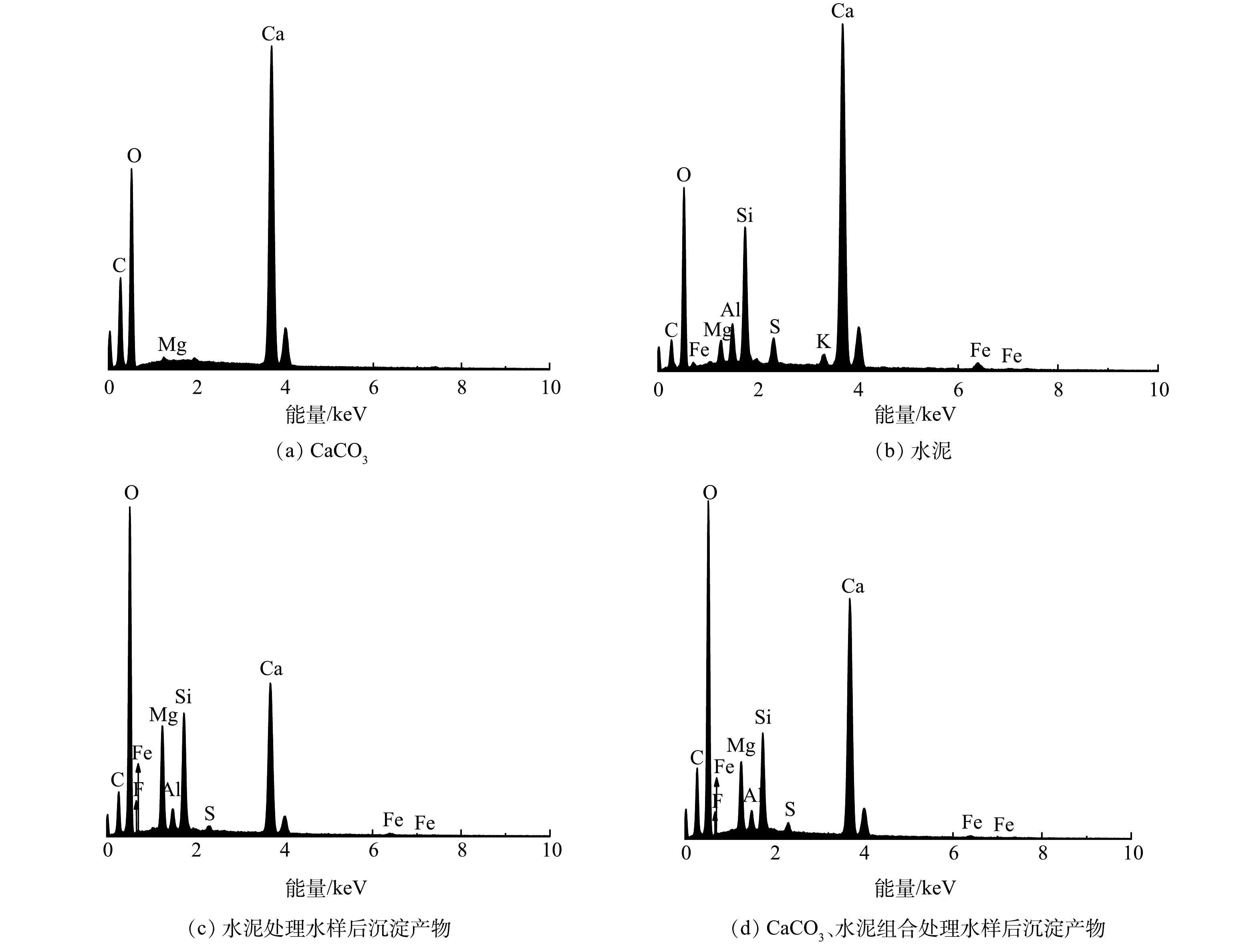
图7为处理前CaCO3、水泥和处理后沉淀产物的EDS图谱,处理前CaCO3和水泥中未检测到含有氟元素,而处理后沉淀产物中出现氟元素峰(水泥处理水样后沉淀产物和CaCO3、水泥组合处理水样后沉淀产物的氟元素质量百分比分别为0.06%、0.24%),可认为水样中游离的氟离子已经被固定至沉淀物中。根据EDS面扫描结果(图8),利用水泥和CaCO3、水泥组合处理水样后形成的沉淀产物中氟元素分布范围广,与钙、镁、铝元素的分布区域存在重合部分,说明氟元素可能通过与这3种元素形成难溶的化合物的方式被固定于沉淀物中。利用CaCO3、水泥组合处理水样后沉淀产物中硅、镁、铝元素的分布集中在钙元素周围,而单独利用水泥处理水样后沉淀产物中硅、镁、铝元素的分布更均匀。硅、镁、铝3种元素主要来自水泥(图7 (b)),这也说明了CaCO3、水泥组合处理下,水泥与水样反应后形成的产物会附着于CaCO3周围,从而可以观察到硅、镁、铝元素分布区域的差异。
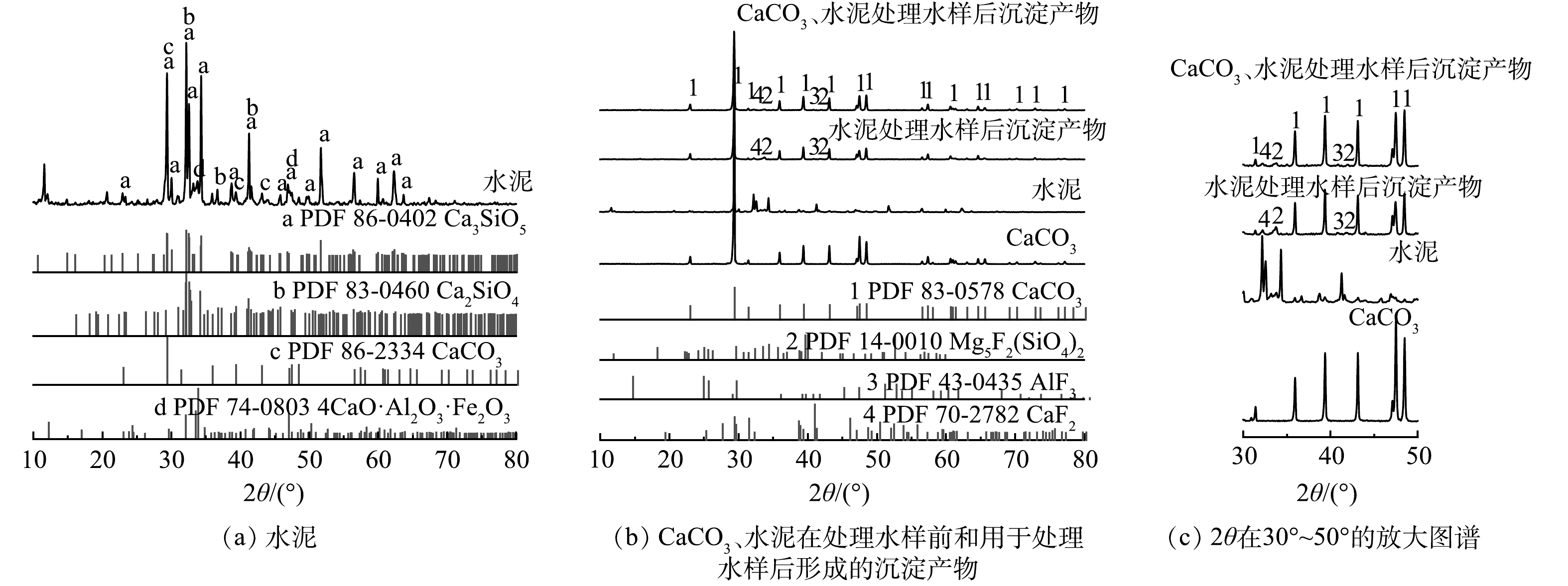
2) X射线衍射(XRD)。水泥的XRD图谱如图9(a)所示,其主要组成成分为硅酸三钙(Ca3SiO5,C3S)、硅酸二钙(Ca2SiO4,C2S)、CaCO3和铁铝酸四钙(4CaO·Al2O3·Fe2O3)[41]。图9 (b)为CaCO3、水泥在处理水样前和用于处理水样后形成的沉淀产物的XRD图谱,图9 (c)为2θ在30°~50°内的放大图谱。除水泥外,3种物质均出现明显的方解石(CaCO3)特征峰,由图9 (c)可以看出,2θ为33.510°和42.008°对应Mg5F2(SiO4)2,2θ为40.816°对应AlF3,2θ为32.341°对应CaF2的存在,这些新出现的衍射峰强度较低。水样本身的氟离子质量浓度较低,使得XRD图谱中含氟化合物的衍射峰信号较弱,但新衍射峰的出现证明了处理后产物中确实产生了含氟化合物沉淀。
XRD表征结果表明,无论是CaCO3、水泥单独处理还是组合处理,沉淀产物的主要组分均为CaCO3。硅酸盐矿物易发生碳酸化反应生成CaCO3[42]。对于水泥来说,碳酸化反应的主要过程包括3条路径:1)有水分存在的情况下,水泥中C3S和C2S水化生成Ca(OH)2和C—S—H凝胶[43];2) CO2的吸收和水中CO32−形成;3)化学反应形成CaCO3沉淀[44-45]。Ca2+除了可以由Ca(OH)2提供外,还可以通过C—S—H、钙矾石和未水化的C3S和C2S脱钙提供[44,46]。
氟是非金属中化学性质最活泼的元素,许多金属氟化物易溶于水[47],但F−与Ca2+、Mg2+、Al3+反应生成的氟化物难溶于水[3,10,48]。Ca2+与F−反应生成溶度积极低的CaF2 (Ksp=2.7 ×10−11)是固定氟的主要途径之一[49]。利用CaCO3处理水样时,氟离子通过形成CaF2被去除(式(1)),该反应涉及CaCO3上CaF2晶体的外延生长,晶体结构中Ca2+的位置不变[50]。
利用水泥处理水样时,水样中的氟离子通过与水泥反应形成CaF2、AlF3和Mg5F2(SiO4)2沉淀而被去除。因此,水泥单独处理水样和CaCO3、水泥组合处理水样后沉淀产物的组分是一致的。在使用水化普通硅酸盐水泥吸附氟化物的研究中,吸附后水化普通硅酸盐水泥的主要组分为CaCO3、CaF2 [24]。在使用热活化高铝水泥颗粒吸附氟化物的研究中,吸附后颗粒的XRD图谱出现新衍射峰,表明有AlF3和CaF2生成[25]。以上研究中的XRD表征结果与本研究结果一致。AlF3和CaF2可能源自水泥中金属氧化物的羟基化对水中的F−产生了吸附凝聚作用,吸附过程可能为MOH+F−=MF+OH−(M为Ca、Al等金属元素)[25]。
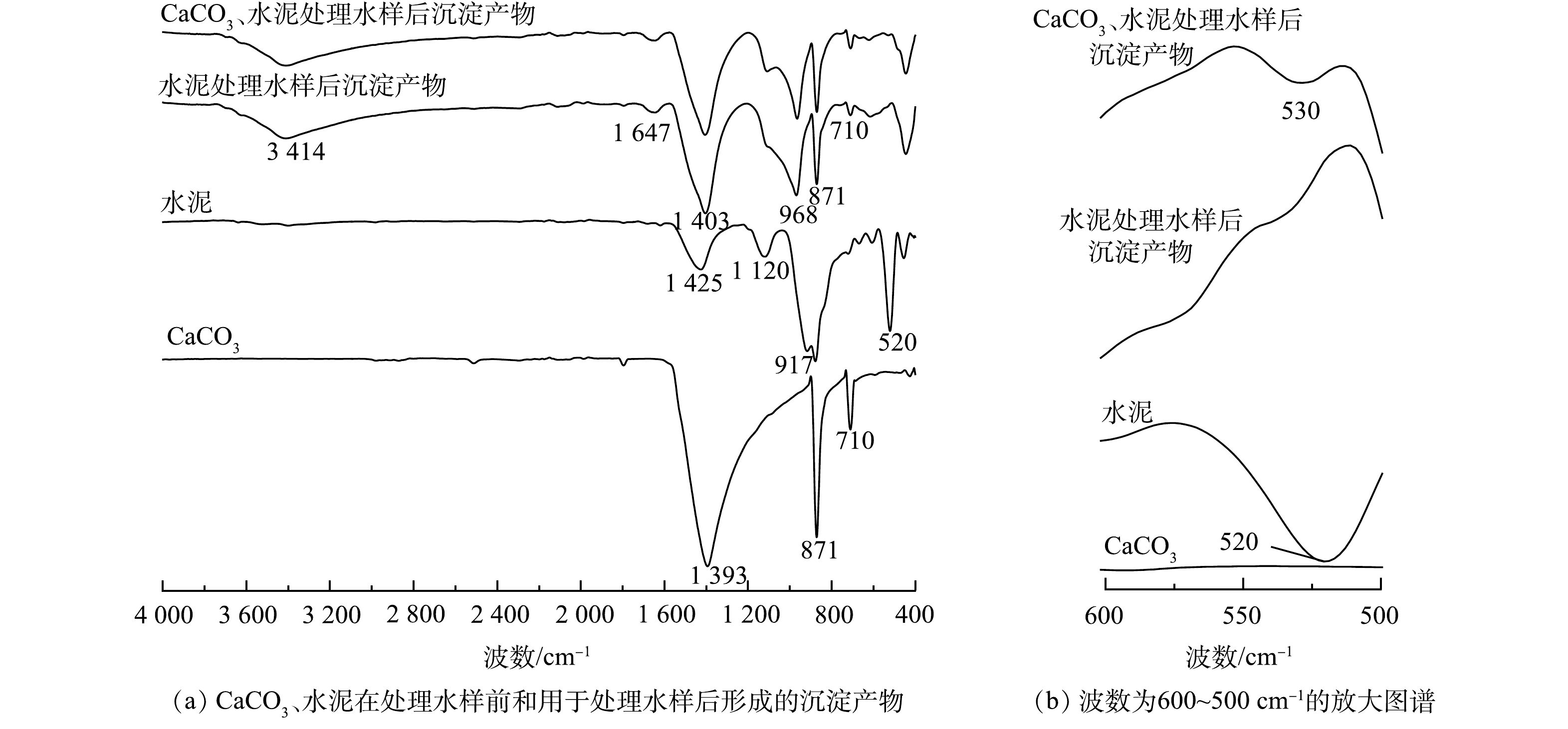
3)傅立叶变换红外光谱(FT-IR)。通过FT-IR图谱(图10)可对比CaCO3、水泥处理前后沉淀产物的化学键差异。在处理后的沉淀产物中,于3 414 cm−1和1 647 cm−1处出现的新峰可分别对应羟基伸缩振动峰和弯曲振动峰。图10反映了红外光谱局部窄谱图(600~500 cm−1),530 cm−1处出现的新峰可归属于Al—F键的伸缩振动[25,51]。在水泥的红外谱线中917 cm−1和处理后沉淀产物红外谱线中968 cm−1处的峰可归属于SiO44−基团[52];位于710、871、1 403和1 425 cm−1的峰可归属于CaCO3中的CO32−特征吸收峰[53],其中1 403 cm−1、1 425 cm−1处可对应C—O键的伸缩振动;871 cm−1处可对应C-O键的面内弯曲振动;710 cm−1处可对应C—O键的面外弯曲振动[54]。
根据FT-IR分析,处理后沉淀产物中发现可归属于CaCO3的CO32−特征吸收峰,进一步证实了CaCO3是沉淀产物的主要组分。对比处理前的材料和处理后的沉淀产物的图谱,沉淀产物出现—OH基团可归属于水泥水化反应生成的Ca(OH)2或金属氧化物的羟基化,F−相较于OH−更具亲核性,在含氟溶液中F−可取代OH−[55],这与XRD分析的结果具有一致性。CaCO3、水泥组合处理后的沉淀产物中出现Al—F键,说明金属离子可通过与氟离子发生化学反应形成难溶的金属氟化物而将氟离子固定在沉淀产物中[25]。
-
1)在实际地表水中较低的初始氟离子质量浓度下,在3种除氟材料中,硅酸盐水泥的除氟效果普遍优于CaCO3和CaCl2,但单独使用该材料时引起的pH升高不可忽视,且水泥的除氟效果没有随着投加量的增加而提升;CaCO3除氟效果较弱,但不会引起水样pH的剧烈变化;CaCl2未表现出除氟作用。组合2种具有除氟效果的材料(CaCO3、水泥)不仅可以实现比单独使用1种材料更高的除氟率,而且对pH的影响更小,有利于应用至地表水除氟中。
2)投加水泥或CaCO3与水泥的组合材料后,沉淀产物的主要组分均为CaCO3、Mg5F2(SiO4)2、AlF3和CaF2,利用CaCO3、水泥组合处理水样可同时发挥CaCO3对氟离子的置换作用和水泥的化学沉淀作用。化学沉淀法的除氟效果与沉淀颗粒的大小呈正相关, CaCO3、水泥组合处理水样后的沉淀产物粒径大于水泥单独处理后沉淀产物粒径,CaCO3、水泥的组合处理在发挥各自除氟作用的同时促进了沉淀产物的沉降,进一步提升了除氟率。
3)当水样受到的扰动程度较大时,沉淀颗粒的晶核难以形成,沉淀物溶解度增加,除氟效果减弱。将来利用水泥进行地表水除氟时,应选择对水体扰动较小的材料投加方式,如使用人工方法或机械手段从水面撒施。
普通硅酸盐水泥和钙盐对氟污染地表水的除氟效果
Fluoride removal effect of portland cement and calcium salts on fluoride-contaminated surface water
-
摘要: 水体氟污染问题受到了广泛关注,现行的除氟技术对自然水体中氟化物的去除效果不明显,无法大范围推广应用。选择碳酸钙(CaCO3)、氯化钙(CaCl2)2种钙盐和普通硅酸盐水泥(简称水泥)对山东省胶州市南胶莱河水样进行了处理,考察了3种材料及其相应的组合对氟离子的去除效果,并筛选出适用于地表水体除氟的材料;通过改变搅拌时间和搅拌速度研究了不同扰动程度对除氟效果的影响;通过扫描电镜、能谱仪、X射线衍射分析和傅里叶红外光谱分析讨论除氟机理。结果表明,当这3种材料单独使用时,CaCO3和水泥表现出除氟效果,水泥除氟效果优于CaCO3,在添加0.5 g·L−1水泥的处理中,水样中的氟离子质量浓度由1.46 mg·L−1降至1.23 mg·L−1;CaCO3和水泥组合的除氟率高于单独CaCO3或水泥,可将水样的氟离子质量浓度由1.39 mg·L−1降至1.09 mg·L−1。改变搅拌时间和速度的实验结果表明,扰动程度增加,除氟率随之下降。表征分析结果表明,水样中氟离子主要依靠化学反应形成沉淀去除。使用水泥用于地表水除氟的步骤简单、效果明显,考虑到水泥与CaCO3组合使用表现出的协同效果以及扰动程度对除氟效果的影响,未来采用水泥处理氟污染地表水时,建议与CaCO3组合使用并采取撒施等对水体扰动较小的施工方式。Abstract: The problem of fluoride contamination in water bodies has received widespread concern around the world, and the existing fluoride removal techniques are ineffective in fluoride removal from natural water bodies and cannot be applied in a large scale. Calcium carbonate (CaCO3), calcium chloride (CaCl2), and portland cement (hereafter referred to as cement) were selected to treat water samples from South Jiaolai River in Jiaozhou City, Shandong Province, and the materials suitable for fluoride removal from surface water bodies were screened by studying the removal effects of the three materials and their combinations; the effects of different degrees of disturbance on the fluoride removal effects were also studied by varying the mixing time and speed; scanning electron microscopy, energy dispersive spectroscopy, X-ray diffraction analysis, and Fourier infrared spectroscopy were used to investigate the mechanism of fluoride removal. The results showed that only CaCO3 and cement could remove fluoride when the three materials were used separately, and the cement showed better fluoride removal than CaCO3. The fluoride concentration in the water sample decreased from 1.46 mg·L−1 to 1.23 mg·L−1 after the addition of 0.5 g·L−1 of cement; a mixture of CaCO3 and cement resulted in a higher fluoride removal rate than CaCO3 or cement alone, and could reduce the fluoride concentration in the water sample from 1.39 mg·L−1 to 1.09 mg·L−1. In the experiments with different mixing time and speed, the fluoride removal rate decreased with the increase of mixing time and speed. Characterization analysis revealed that fluoride ions in the water samples were mainly removed by chemical reactions via forming precipitation. Considering the synergistic effect of mixing cement with CaCO3 and the effect of disturbance on fluoride removal, it is suggested that cement be mixed with CaCO3 and applied in a less disruptive way such as dispersion to remove fluoride from the surface water in the future.
-
Key words:
- fluoride /
- surface water /
- portland cement /
- CaCO3
-
随着我国工业化和城镇化进程的加速发展,工业结构调整和空间格局重构导致大批污染企业搬迁、改造或关闭,在城市及其周边地区遗留千万公顷污染场地,严重威胁居民健康和环境安全[1-4]。由于氯代烃、石油烃等有机污染物的溶解度较低,通常以非水相液体 (NAPLs) 存在于地下土水环境中并迁移形成污染羽。当存在有低渗透地层时,会在低渗透地层上形成污染池,并通过扩散作用或者天然裂隙侵入低渗透地层,形成持久性污染源[5]。现有抽提、氧化等原位修复技术[6-8]可以有效修复低渗透介质层上的污染池,但受限于物质传输速率,低渗透介质内的污染物难以被去除,导致修复反弹[5, 9-10]。因此,低渗透污染地层的修复是当前污染场地修复的重大挑战。
为了提升低渗透污染地层的渗透性,可以引入石油开采行业的压裂技术形成缝网结构,提升物质传输速率[11-13],与多相抽提技术形成协同工作模式,实现高效去除[14-16]。目前,国内外学者已针对压裂协同抽提修复技术开展了一系列研究。MURDOCH等[17]以未压裂抽提井为空白对照,设置2个压裂抽提井,发现压裂前后抽提流量提高了13~31倍,随着流动相和非流动相之间物质传质速率的增大,污染物去除率提高675%[18],论证了水力压裂与抽提协同的有效性。为了进一步揭示压裂对气相抽提的影响机制,SCHULENBERG和REEVES[19]建立了压裂协同气相抽提轴对称模型,发现裂隙长度对污染物去除率的敏感性不如裂隙渗透率;CHEN等[20]利用TMVOC代码和MINC方法,建立了双孔隙介质压裂协同热强化抽提数值模型,同样发现基质渗透率的敏感性远大于抽提压力、裂隙间距和孔径;BRANDNER和MURDOCH[21]利用数值模型,参考石油行业开采井的标量评价指标流动速率,定义裂隙有效流通率,量化评价裂隙对污染物去除率的影响。在试验方面,TZOVOLOU等[22]以砂层等效水力压裂裂缝,利用模型试验论证了裂缝中对蒸汽修复效率的强化作用,去除率可达77%,同时他们也在现场试验中论证了压裂协同生物通风修复低渗透污染土的技术有效性和经济性[23]。针对埋深9~12 m的污染低渗冰川土,CUSHMAN等[24]通过试验发现压裂后的多相抽提效率是压裂前的6倍;NILSSON等[25]则通过低渗污染场地的压裂协同抽提中试试验,发现气/水/非水相污染物均被负压抽提,而总NAPL的质量去除率达到30%。虽然,目前污染场地的压裂协同抽提修复的技术有效性均得到了试验和数值仿真的论证,但是现有研究仍主要以尝试为主,并将污染物去除率作为主要评价指标,缺乏对压裂增渗强化污染物去除机理的深入研究,特别是孔隙-裂隙介质中的优势渗流规律。
为此,本研究首先建立孔隙-裂隙介质中多相抽提数值模型,利用COMSOL MULTIPHYSICS软件进行求解,以低渗透污染地层污染程度、压裂缝网特征参数 (裂隙长度、厚度和渗透率) 开展单因素和多因素参数化分析,探究压裂缝网对低渗透NAPL污染地层多相抽提效率的影响机制。
1. 孔隙-裂隙介质中多相抽提数学模型
1.1 数学模型的建立
饱和态孔隙-裂隙介质的多相抽提主要涉及2个过程,一是抽提作用下自由相和水相迁移,二是溶解相对流-弥散作用,由于自由相的浓度远高于溶解相,本研究主要考虑自由相污染物去除,因此忽略污染物的相间传质,并假设地层中初始污染物均匀分布。模型控制方程由孔隙介质与裂隙介质中的水相和NAPL相质量守恒方程、水力本构方程、溶解相污染物运移方程组成。
孔隙-裂隙介质中基质部分的水相 (w) 和NAPL相 (nw) 质量守恒方程[26]见式(1)。
stringUtils.convertMath(!{formula.content}) (1) 式中i =w、j= nw或者i =nw、j= w;ρ是流体密度 (kg·m−3) ;μ是流体粘度 (Pa·s) ;Cp是比容 (Pa−1) ;k是孔隙基质的固定渗透率 (m2) ;kr是流体相的相对渗透率;u是流体达西速度 (m·s−1) ;p是流体的达西场压力 (Pa) ;g是重力加速度 (m·s−2) ;D是竖向标高 (m) 。
毛细压力 pc 通常定义为NAPL相和水相之间的压差,即式(2)。
stringUtils.convertMath(!{formula.content}) (2) 多孔介质的孔隙空间可以完全填充为NAPL相或水相,也可以同时存在NAPL相和水相,两者有效饱和度满足式(3)。
stringUtils.convertMath(!{formula.content}) (3) 水相比容Cpw取决于水相饱和度相对于毛细压力的变化关系,定义为式(4)。
stringUtils.convertMath(!{formula.content}) (4) 根据式(3)和式(4)可以将NAPL相比容Cpnw定义为式(5)。
stringUtils.convertMath(!{formula.content}) (5) 由于水相比容和NAPL相比容,仅仅相差一个负号,因此,统一定义为比容Cp。
毛细管压力、相对渗透率和相饱和度通过VAN GENUCHTEN水力本构 (式(6)~式(9)) 建立K-S-P关系[27],基于水力本构方程可以推导
Cp stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) stringUtils.convertMath(!{formula.content}) (8) stringUtils.convertMath(!{formula.content}) (9) stringUtils.convertMath(!{formula.content}) (10) 式中α、m为VG模型参数;θsw是总孔隙率或饱和体积分数;θrw是残余水相体积分数;Srw为残余水相饱和度;Sew为水相有效饱和度;ε为基质孔隙率。
孔隙-裂隙介质中裂隙部分的水相 (w) 和NAPL相 (nw) 质量守恒方程[26]见式(11)。
stringUtils.convertMath(!{formula.content}) (11) 式中:
df kf 孔隙-裂隙介质中基质部分溶解相污染物运移方程见式(12)。
stringUtils.convertMath(!{formula.content}) (12) 式中:Sw为水相饱和度,Sw = Srw+Sew(1‒Srw‒Srnw);Dw为水动力弥散系数 (m2·s−1) ,包括机械弥散和分子扩散,二维水动力弥散系数计算见式(13)。
stringUtils.convertMath(!{formula.content}) (13) 式中:Dew为溶解相污染物分子扩散系数 (m2·s−1) ;αLw和αTw为溶解相污染物的纵向和横向弥散系数 (m) 。
孔隙-裂隙介质中裂隙部分溶解相污染物运移方程为式(14)。
stringUtils.convertMath(!{formula.content}) (14) 式中:
εf 孔隙-裂隙多孔介质多相抽提修复效率的评价指标是污染物去除率PR,总NAPL去除率、自由相NAPL去除率和溶解相NAPL去除率可以分别定义为式(15)~式(17)。
stringUtils.convertMath(!{formula.content}) (15) stringUtils.convertMath(!{formula.content}) (16) stringUtils.convertMath(!{formula.content}) (17) 式中:Sn0 、Sn为NAPL相初始饱和度和终止饱和度;Sw0、Sw为水相初始饱和度和终止饱和度;C0、C是溶解相NAPL初始浓度和终止浓度 (mol·m−3) ;ρn 为NAPL相密度 (kg·m−3) ;M为NAPL相摩尔分子质量 (g·mol−1) 。
1.2 数学模型的求解
采用大型多物理场耦合仿真软件COMSOL MULTIPHYSICS进行求解,采用双达西定律、多孔介质中稀物质传递模块和裂隙流模块模拟饱和态低渗介质中NAPL去除。该两相流体运移模型先前已通过水气蒸发土壤干燥[28]、气相抽提[29]、热强化抽提[29]涉及的气、水两相流验证,本研究在此基础上,与HOPMANS的水、NAPL两相流试验[30]验证良好 (图1) ,验证了模型的正确性和可靠性。
2. 孔隙-裂隙介质多相抽提模型及基本参数
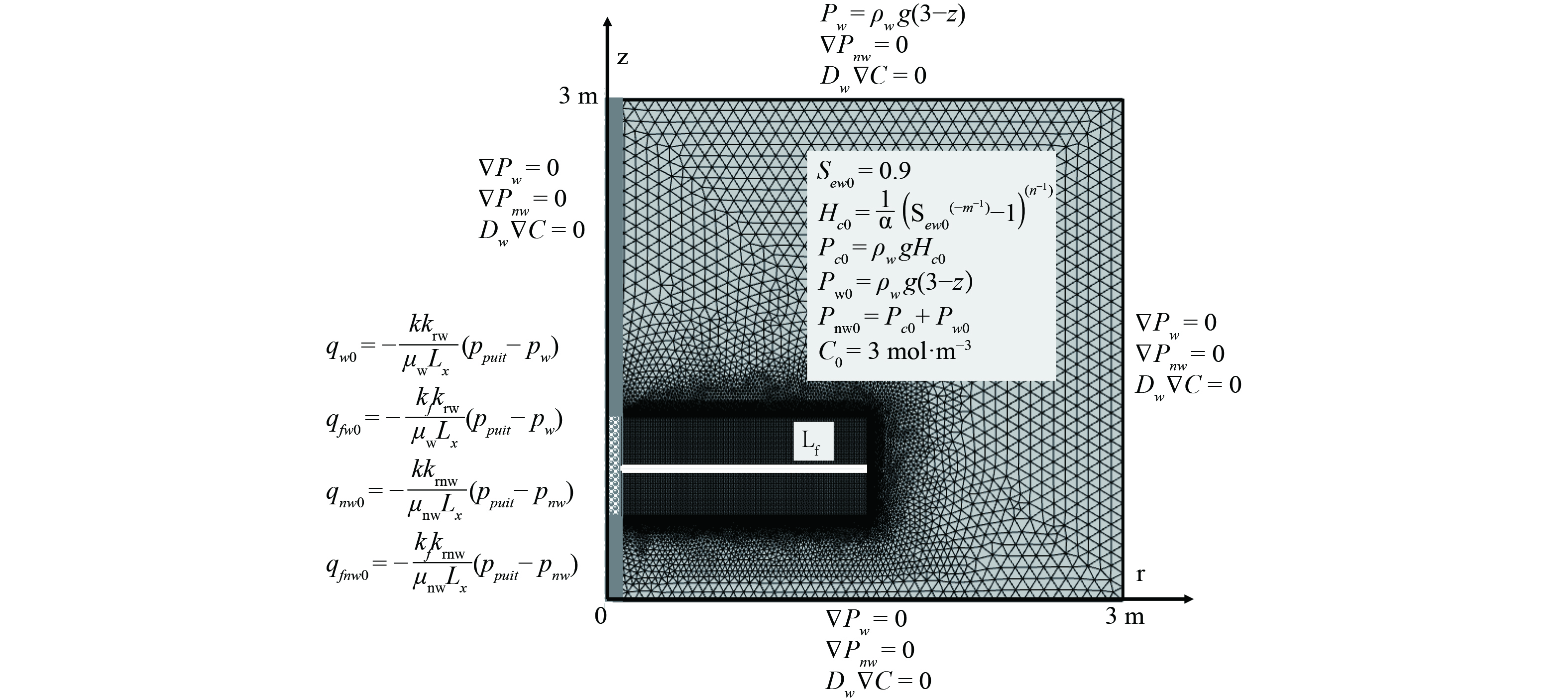
2.1 模型设置与初始值、边界条件
含水层中低渗污染土压裂协同抽提修复的概化模型如图2所示,模拟尺寸为3 m×3 m,是以抽提井管为中轴线的二维轴对称模型,井管筛口处施加负压并在中心位置生成水平向裂隙。图2也详细介绍了水相达西场、NAPL相达西场、溶解相传递场的初始值和边界条件。
2.2 模型基本参数
模型中采用的参数值,如表1所示,包括土壤、水和NAPL的特征参数。
表 1 模型相关参数Table 1. Parameters used in the numerical model模型参数名称 符号 取值 单位 土体孔隙率 ε 0.32 1 土体固有渗透率 k 1×10−13 m2 土体干密度 ρ 1 600 kg·m−3 水的密度 ρw 1 000 kg·m−3 水的粘度 μw 1×10−3 Pa·s 水的残余饱和度 Srw 0.007 2 1 NAPL的密度 ρnw 1 460 kg·m−3 NAPL的粘度 μnw 5.8×10−3 Pa·s NAPL的相对分子质量 M 131 g·mol−1 NAPL的残余饱和度[31] Srnw 0 1 NAPL溶解相分子扩散系数[32] Dwe 1.31×10−6 cm2·s−1 NAPL溶解相的纵向弥散系数 αwL 0.5 m NAPL溶解相的横向弥散系数 αwT 0.05 m K-S-P本构参数 α 3.58 m−1 K-S-P本构参数 l 0.5 1 K-S-P本构参数 n 3.136 5 1 K-S-P本构参数 m 1-1/n 1 裂隙的孔隙率 εf 0.5 1 裂隙的固有渗透率 kf/k 100 1 裂隙的厚度 df 0.03 m 裂隙的长度 Lf 1.5 m 抽提压力 ppuit −50 kPa 筛口过滤的等效流动阻力[16] Lx 5 cm 3. 孔隙-裂隙介质多相抽提规律与讨论
3.1 压裂增渗对污染物去除率的影响
单一水力压裂缝网采用筛口中心的水平向裂隙进行表征,本节分别模拟了筛口抽提 (仅设置基质抽提边界
qw0 qnw0 qfw0 qfnw0 3.2 裂缝特征参数单因素分析
采用第150 d的污染物去除率表征低渗透污染场地中前期的修复效率,本节分别研究了污染程度 (Sn) 、裂缝渗透率 (kf/k) 、裂隙厚度 (df) 、裂隙长度 (Lf) 等因素的影响规律 (抽提负压为50 kPa) 。由图4可知,在单因素作用下,污染物去除率随着各自变量 (Sn、kf/k、df、Lf) 的增加而增大,变化规律均可拟合为PR = A(1‒exp(‒x/B))分布,参数A表征单因素条件下污染物的最大去除率,参数B表征单因素的影响权重。可以发现,污染程度对污染物最大去除率的影响最大,高浓度污染时去除率最大,且裂隙渗透率是影响去除效率的显著特征参数。随着裂隙渗透率的增加,污染物去除率的增加速率先快后慢,kf/k≤100时,裂隙对抽提效率的提升效果最佳。
3.3 裂缝特征参数多因素分析
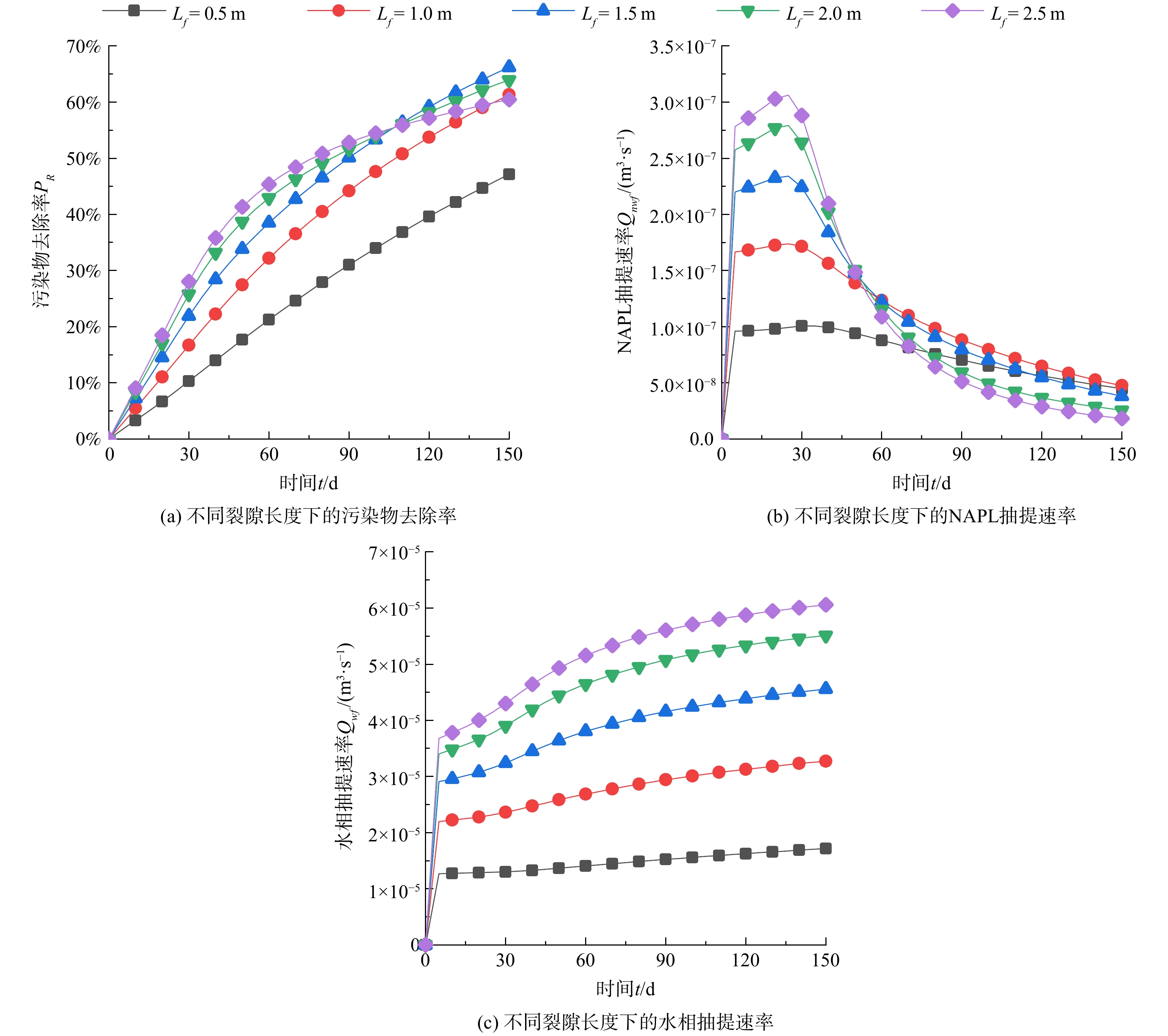
本节设计了不同污染程度、裂缝渗透率、裂隙厚度、裂隙长度组合的1715组工况,综合分析了多因素共同作用下污染物去除率的变化规律。以NAPL初始饱和度为0.1为例,图5给出了裂缝特征参数对污染物去除率的耦合影响,可以发现在不同裂隙长度和裂隙渗透率条件下,增大裂隙厚度df都对污染物去除率有促进作用。同时,随着裂缝长度的增加,当裂隙渗透率kf/k≤100时污染物去除率逐渐缓慢增加;当裂隙渗透率kf/k>100时,污染物去除率呈先增加后波动降低的趋势,裂隙长度为1.5 m时效果最佳。
以kf/k = 1 000、df = 0.03 m为例,图6(a)进一步展示了污染物去除率随裂隙长度和修复时间的变化规律。可以发现,在修复前期 (0~40 d) ,污染物去除率的增大速率随着裂缝长度的增大而增大;但是,在修复中后期 (40~150 d) ,当裂隙长度大于1.5 m时,污染物去除率的增大速率发生减缓。图6(b)和(c)给出了裂隙口的NAPL相和水相抽提速率的变化曲线,在第40 d至60 d期间,NAPL相抽提速率发生陡降,且裂隙越长,下降越快,不同裂隙长度条件下抽提速率的大小关系发生改变;但是,水相抽提速率仍持续增大,且大小关系没有改变。因此,当裂隙长度大于1.5 m时,污染物去除率下降主要是因为NAPL抽提速率的下降和水相抽提速率的增加。由图7的NAPL相饱和度分布可知,随着修复时间逐渐从40 d增加到60 d,裂隙标高以上的NAPL相污染物逐渐减少,当裂隙长度大于1.5 m时,这部分污染物基本上被完全去除。因此,当裂隙标高上存在NAPL相污染物时,裂隙长度越长,修复效率越高,裂缝可以完全发挥作用;当裂隙标高以上的NAPL相污染物被去除后,污染物去除效率会下降,裂隙长度的优势也会被逐渐被削弱,无法完全发挥强化修复的作用。
4. 结论
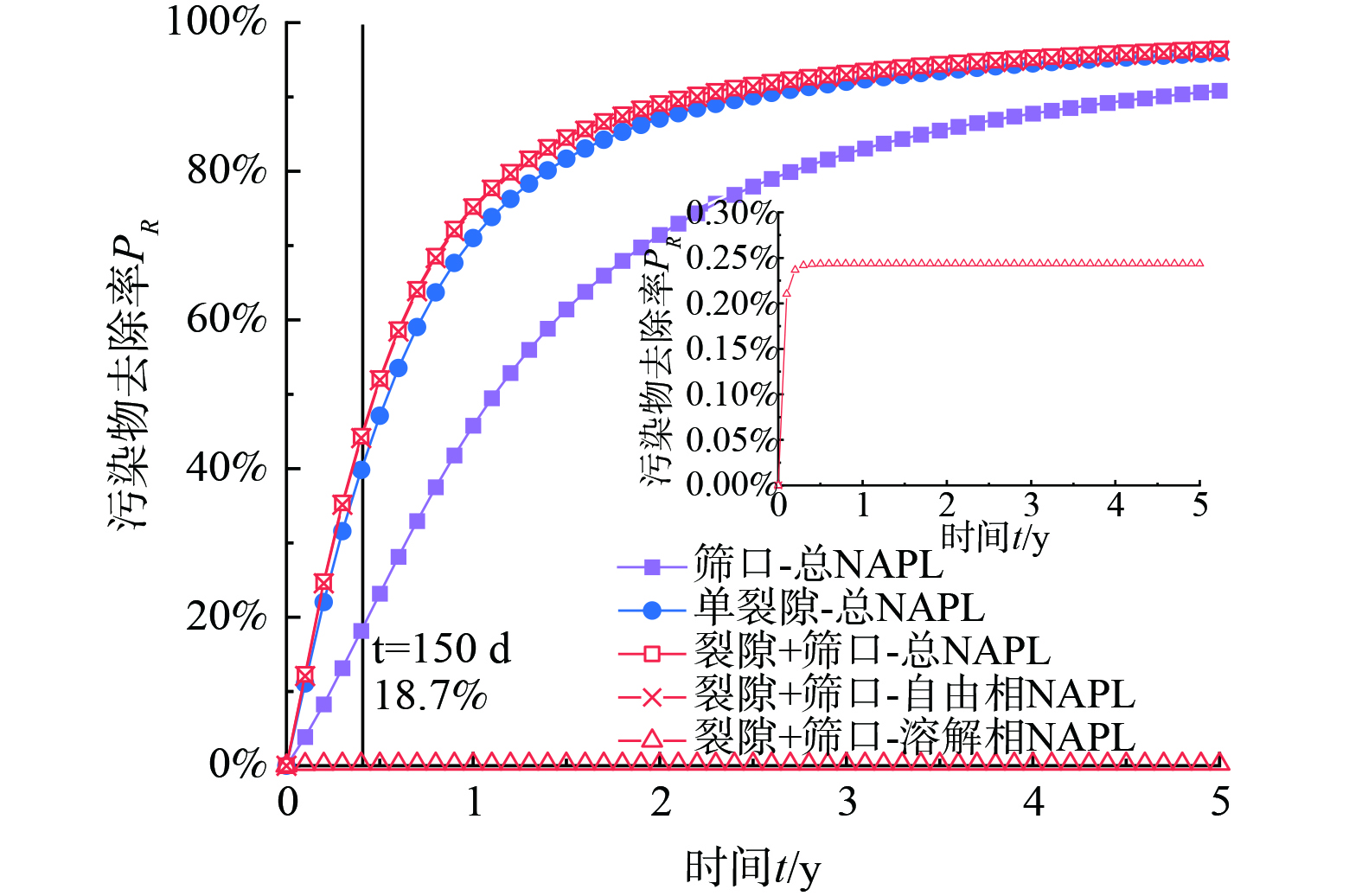
1) 多相抽提去除主要以NAPL自由相为主,溶解相为辅,且当裂隙存在时,污染物去除率显著增大;同时,裂隙强化抽提效率主要作用于修复中前期。
2) 在单因素作用下,污染物去除率随着各自变量 (Sn、kf/k、df、Lf) 的增加而增大,变化规律均可拟合为PR = A(1‒exp(‒x/B))分布;污染程度对污染物最大去除率的影响最大,高浓度污染时去除率最大,且裂隙渗透率是影响去除效率的显著特征参数。
3) 在多因素耦合作用下,当裂隙渗透率kf/k大于100时,污染物去除率呈先增加后波动降低的趋势,且当裂隙长度为1.5 m时,去除效果最佳,这是因为当裂隙上层污染物被去除后,裂隙长度的促进作用会大幅衰减。因此,当裂隙设置于污染地层的下部,更有利于污染物的去除。
-
表 1 实验水样的水质检测结果
Table 1. The water quality of experimental water samples
mg·L−1 水质及标准 TP TN NH3-N CODMn 氟化物(以F−计) GB3838-2002Ⅴ类水标准 ≤0.4 — ≤2.0 ≤15 ≤1.5 实验水样水质 0.06 9.76 0.08 9.77 1.52 注:河流不考核总氮指标。 -
[1] 陈显赫, 吴春锋, 张海涛. 氟斑牙研究现状[J]. 中国地方病防治, 2020, 35(3): 208-211. [2] 聂文博, 郭敏, 李林霜, 等. 高氟水处理吸附剂的研究进展[J]. 广州化工, 2015, 43(20): 1-6. doi: 10.3969/j.issn.1001-9677.2015.20.001 [3] ALI S, THAKUR S K, SARKAR A, et al. Worldwide contamination of water by fluoride[J]. Environmental Chemistry Letters, 2016, 14(3): 291-315. doi: 10.1007/s10311-016-0563-5 [4] 马勇, 刘东阳, 陈慧英, 等. 介电泳增强吸附法去除饮用水中的氟离子[J]. 环境工程学报, 2020, 14(5): 1245-1251. doi: 10.12030/j.cjee.201907102 [5] AGHAPOUR S, BINA B, TARRAHI M J, et al. Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS[J]. Environmental Monitoring and Assessment, 2018, 190(3): 137. doi: 10.1007/s10661-018-6467-z [6] LAXMANKUMAR D, SATYANARAYANA E, DHAKATE R, et al. Hydrogeochemical characteristics with respect to fluoride contamination in groundwater of Maheshwarm Mandal, RR District, Telangana State, India[J]. Groundwater for Sustainable Development, 2019, 8: 474-483. doi: 10.1016/j.gsd.2019.01.008 [7] 涂成龙, 何令令, 崔丽峰, 等. 氟的环境地球化学行为及其对生态环境的影响[J]. 应用生态学报, 2019, 30(1): 21-29. doi: 10.13287/j.1001-9332.201901.004 [8] 郝春明, 张伟, 何瑞敏, 等. 神东矿区高氟矿井水分布特征及形成机制[J]. 煤炭学报, 2021, 46(6): 1966-1977. doi: 10.13225/j.cnki.jccs.ST21.0160 [9] 陈媛, 熊传龙, 张琦, 等. 氟中毒暴露途径及健康效应研究进展[J]. 环境与健康杂志, 2016, 33(1): 84-87. doi: 10.16241/j.cnki.1001-5914.2016.01.024 [10] OZSVATH D L. Fluoride and environmental health: A review[J]. Reviews in Environmental Science and Bio/Technology, 2008, 8(1): 59-79. [11] 赵迎新, 宋倩, 马同宇, 等. 改性/新型氟吸附材料的研究进展[J]. 工业水处理, 2018, 38(5): 9-14. doi: 10.11894/1005-829x.2018.38(5).009 [12] 王俊兰, 魏海红, 毕雯雯. 浅析山东省胶莱盆地地方性氟中毒与地质环境的关系[J]. 山东国土资源, 2013, 29(9): 103-105. doi: 10.3969/j.issn.1672-6979.2013.09.025 [13] 刘春华, 王威, 杨丽芝, 等. 山东省地下水氟富集规律及其驱动机制[J]. 地质学报, 2021, 95(6): 1962-1972. doi: 10.3969/j.issn.0001-5717.2021.06.020 [14] 君珊, 张博, 王鹏飞, 等. 呼伦湖水体氟化物演变特征及其影响因素[J]. 环境科学研究, 2021, 34(4): 841-848. doi: 10.13198/j.issn.1001-6929.2021.02.08 [15] 段平洲, 贾晓波, 后希康, 等. 磁性铝基MOF的表征和对水体中氟化物吸附性能研究[J]. 环境科学研究, 2021, 34(5): 1139-1147. doi: 10.13198/j.issn.1001-6929.2020.10.25 [16] 陈乐, 王晓丽. 内蒙古部分河段表层沉积物对氟的吸附特征[J]. 土壤通报, 2019, 50(6): 1478-1483. doi: 10.19336/j.cnki.trtb.2019.06.29 [17] KHATIBIKAMAL V, TORABIAN A, JANPOOR F, et al. Fluoride removal from industrial wastewater using electrocoagulation and its adsorption kinetics[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 276-280. [18] 陈东. 饮用水除氟技术研究综述[J]. 山东化工, 2021, 50(2): 261-262. doi: 10.3969/j.issn.1008-021X.2021.02.104 [19] 段誉梅, 潘洪义. 水葫芦对氟化物的净化效果及生理特性变化[J]. 北方园艺, 2018(2): 90-96. doi: 10.11937/bfyy.20171617 [20] 尹国勋, 郑明凯, 朱利霞. CaO+KH2PO4在高氟地下水水质处理中的应用[J]. 水资源保护, 2007(4): 82-84. doi: 10.3969/j.issn.1004-6933.2007.04.022 [21] 李雪玲, 刘俊峰, 李培元. 石灰沉淀法除氟的应用[J]. 水处理技术, 2000(6): 359-361. doi: 10.3969/j.issn.1000-3770.2000.06.012 [22] 邓慧东, 李大炳, 康绍辉, 等. 用碳酸钙从碱性含氟废水中置换除氟[J]. 铀矿冶, 2021, 40(4): 317-320. doi: 10.13426/j.cnki.yky.2021.04.010 [23] BIER T H A. Influence of type of cement and curing on carbonation progress and pore structure of hydrated cement pastes[J]. MRS Proceedings, 2011, 85: 123. [24] 曹艳, 任勇翔, 黄廷林, 等. 水化普通硅酸盐水泥吸附水中氟化物的动力学与热力学解析[J]. 环境科学研究, 2012, 25(2): 200-206. doi: 10.13198/j.res.2012.02.78.caoy.009 [25] 任勇翔, 钱壮, 赵春玲, 等. 热活化水化高铝水泥颗粒吸附氟化物行为研究[J]. 建筑材料学报, 2020, 23(2): 421-429. [26] TARALI S V, HOOLIKANTIMATH N P, KULKARNI N, et al. A novel cement-based technology for the treatment of fluoride ions[J]. Sn Applied Sciences, 2020, 2(7): 1205. doi: 10.1007/s42452-020-2986-7 [27] KANG W H, KIM E I, PARK J Y. Fluoride removal capacity of cement paste[J]. Desalination, 2007, 202(1/2/3): 38-44. [28] LIU L, LIU B H, LI W, et al. An effective way to treat the iron-rich acid mine drainage from coal mining in Guizhou's mountainous areas[J]. Journal of Mountain Science, 2020, 17(6): 1345-1359. doi: 10.1007/s11629-020-5998-2 [29] LIU B H, LIU L, LI W. Effective removal of phosphorus from eutrophic water by using cement[J]. Environmental Research, 2020, 183: 109218. doi: 10.1016/j.envres.2020.109218 [30] 李洋, 张玄, 张丽君, 等. 白洋淀氟化物污染、迁移规律及其治理对策研究[J]. 节能, 2019, 38(3): 74-76. doi: 10.3969/j.issn.1004-7948.2019.03.025 [31] 贾春伟, 王振厅, 时青. 青岛南胶莱河流域干流设计洪水计算分析[J]. 山东水利, 2021(1): 25-26. doi: 10.16114/j.cnki.sdsl.2021.01.009 [32] 温元波, 张陆军, 王宁宁, 等. 水化氯铝酸钙去除水中氟及其动力学研究[J]. 应用化工, 2021, 50(2): 311-315. doi: 10.3969/j.issn.1671-3206.2021.02.008 [33] 贾翠萍, 杨梦圆, 薛鑫. Ca-Fe-Mg复合材料制备及其对矿区高氟水氟去除性能研究[J]. 中国矿业, 2018, 27(9): 158-161. doi: 10.12075/j.issn.1004-4051.2018.09.024 [34] 郭朝斌, 孙婷婷, 郑炎松, 等. 改性灰岩除氟的实验研究[J]. 环境科学与技术, 2011, 34(8): 89-94. doi: 10.3969/j.issn.1003-6504.2011.08.020 [35] 李林. 塔里木河流域地表水和地下水的转化关系[J]. 水土保持通报, 2021, 41(6): 23-28. doi: 10.13961/j.cnki.stbctb.2021.06.004 [36] 王而力, 王晓锋, 钱凤国, 等. 氟化钙晶核在处理低浓度含氟废水中的作用[J]. 辽宁城乡环境科技, 2001(1): 21-24. [37] 付国燕, 王玮玮, 刘召波, 等. 氢氧化物沉淀法制备层状结构氧化钪的研究[J]. 材料导报, 2020, 34(S2): 1164-1167. [38] 程浩铭, 张翠玲, 任昊晔, 等. 化学沉淀法处理高氟废水的工艺条件优化[J]. 兰州交通大学学报, 2018, 37(5): 80-84. doi: 10.3969/j.issn.1001-4373.2018.05.014 [39] 沈青. 地表水中藻类代谢对pH和含氧量影响分析[J]. 环境科学与技术, 2011, 34(S2): 261-262. [40] 姜科, 周康根, 李程文. 粒径对CaF2沉淀-溶解平衡的影响[J]. 中国有色金属学报, 2011, 21(12): 3195-3201. [41] 邓磊, 蔡攀. XRD分析在出厂水泥质量控制中的应用[J]. 水泥, 2019(S1): 115-117. doi: 10.13739/j.cnki.cn11-1899/tq.2019.S1.036 [42] 梁晓杰. 碳酸化对普通硅酸盐水泥水化性能的影响[J]. 水泥工程, 2021(3): 1-4. doi: 10.13697/j.cnki.32-1449/tu.2021.03.001 [43] 王亚琛, 陈小亮, 朱南文, 等. 复合水泥对MBR工艺渗滤液尾水深度处理的协同效应[J]. 净水技术, 2013, 32(2): 30-35. doi: 10.3969/j.issn.1009-0177.2013.02.007 [44] GARCIA-GONZALEZ C A, HIDALGO A, ANDRADE C, et al. Modification of composition and microstructure of portland cement pastes as a result of natural and supercritical carbonation procedures[J]. Industrial & Engineering Chemistry Research, 2006, 45(14): 4985-4992. [45] MO L W, PANESAR D K. Effects of accelerated carbonation on the microstructure of portland cement pastes containing reactive MgO[J]. Cement and Concrete Research, 2012, 42(6): 769-777. doi: 10.1016/j.cemconres.2012.02.017 [46] BORGES P H R, COSTA J O, MILESTONE N B, et al. Carbonation of CH and C-S-H in composite cement pastes containing high amounts of BFS[J]. Cement and Concrete Research, 2010, 40(2): 284-292. doi: 10.1016/j.cemconres.2009.10.020 [47] 张博, 郭云艳, 陈俊伊, 等. 岱海沉积物氟化物赋存特征及其释放风险[J]. 中国环境科学, 2020, 40(04): 1748-1756. doi: 10.3969/j.issn.1000-6923.2020.04.043 [48] SKJELKVALE B L. Factors influencing fluoride concentrations in norwegian lakes[J]. Water Air and Soil Pollution, 1994, 77(1/2): 151-167. [49] 于波, 任桐, 都兴红, 等. 含氟废水处理工艺研究[J]. 中国资源综合利用, 2020, 38(11): 192-195. doi: 10.3969/j.issn.1008-9500.2020.11.056 [50] YANG M, HASHIMOTO T, HOSHI N, et al. Fluoride removal in a fixed bed packed with granular calcite[J]. Water Research, 1999, 33(16): 3395-3402. doi: 10.1016/S0043-1354(99)00052-4 [51] WU S B, ZHANG K S, HE J Y, et al. High efficient removal of fluoride from aqueous solution by a novel hydroxyl aluminum oxalate adsorbent[J]. Journal of Colloid and Interface Science, 2016, 464: 238-245. doi: 10.1016/j.jcis.2015.10.045 [52] 王敬尊, 王霆. 如何解释红外谱图[J]. 大学化学, 2016, 31(6): 90-97. doi: 10.3866/pku.DXHX201504001 [53] WANG H L, LIU H B, XIE J J, et al. An insight into the carbonation of calcined clayey dolomite and its performance to remove Cd (II)[J]. Applied Clay Science, 2017, 150: 63-70. doi: 10.1016/j.clay.2017.09.012 [54] 王凌凯, 陈冬, 刘海波, 等. 碳酸化对白云石热分解产物联合除磷除氟的影响[J]. 硅酸盐通报, 2021, 40(9): 3053-3063. doi: 10.16552/j.cnki.issn1001-1625.20210604.004 [55] 童庆, 徐慧, 樊华, 等. Al13改性羟基磷灰石的除氟性能研究[J]. 环境科学学报, 2021, 41(7): 2748-2757. -











 下载:
下载: