-
矿山开发过程中会产生大量的固体废弃矿物,在自然环境中风化和微生物的氧化过程中伴随着有毒有害元素的释放,并逐渐形成了高硫酸根、高铁浓度的极端酸性矿山废水 (acid mine drainage,AMD) ,造成了矿区附近流域水体及土壤重金属的污染,严重威胁矿区生态系统安全及人群健康[1-4]。因此,AMD所引起的环境污染已成为国内外研究热点。
以广东省韶关市大宝山金属硫化物矿区为例,ZHUANG等[5]发现,受矿区酸性废水的长期影响,大宝山矿区附近土壤的pH多低于5,土壤中铜、铅、锌和镉的平均质量分数分别为502 mg·kg−1、278 mg·kg−1、498 mg·kg−1和3.92 mg·kg−1,远超过国家标准 (GB15618-2018) 限值。而AMD表层沉积物中的重 (类) 金属,如Pb、As和Cr的质量分数高达3 100、1 896和170 mg·kg−1[6-7]。据沉积物矿物学特征调查可知,AMD污染的流域中易形成大量特征性的含铁羟基硫酸盐次生矿物[8-10]。例如,当pH为2.8~4.5时,AMD环境中的三价铁和硫酸根离子共沉淀形成典型的施氏矿物 (schwertmannite) ;当体系中存在一价阳离子且pH为1.5~3时,易形成以黄铁矾类为主的矿物;当pH>5时,Fe3+水解产生的矿物主要为水铁矿[11-12]。由于类质同象作用,这些含铁羟基硫酸盐次生矿物容易吸持AMD中的重 (类) 金属离子,使其演变成为重 (类) 金属离子的沉淀库。同时,含铁硫酸盐次生矿物容易在环境因子 (pH、还原性物质、溶解性有机质 (dissolved organic matter,DOM) 和共存离子浓度等) 改变时,发生溶解重结晶,使其吸附和共沉淀的重 (类) 金属再次释放到相应的水体流域中,造成二次生态风险[13-16]。然而,目前关于大宝山矿区环境中重金属环境风险的研究主要针对矿区土壤中重金属的迁移。
在AMD环境中,可溶性S(-II)可由硫酸盐还原菌还原硫酸根产生,并通过还原、沉淀和络合等途径对矿区中重 (类) 金属的迁移转化起制约作用[17]。例如,在厌氧环境下,S(-II)可还原溶解铁 (氢) 氧化物等矿物且自身形态发生改变,亦引起了所吸持的重 (类) 金属再次释放[18-20]。然而,关于可溶性S(-II)与矿区沉积物中次生矿物的稳定性及重 (类) 金属的迁移转化动态关系的报道还甚少。
基于此,本研究围绕大宝山矿区拦泥库沉积物,采用流动柱进行模拟淋溶实验,模拟还原大宝山矿区拦泥库沉积物中的重 (类) 金属在还原性S(-II)介导下的垂直迁移特征,以探究S(-II)对拦泥库沉积物次生矿物的稳定性影响与重金属环境行为的关系,以期为金属硫化物矿区的环境修复与污染防治提供参考。
-
主要试剂或药品:硫化钠 (Na2S,GR) 购自上海阿拉丁生化科技有限公司,硝酸 (HNO3,AR) 和氢氧化钠 (NaOH,AR) 购买于西陇科学股份有限公司;重 (类) 金属标准溶液购自于国家有色金属及电子材料分析测试中心,以5%的硝酸定容;实验用水为去离子水。
-
沉积物样品于2018年8月采集于大宝山矿区拦泥库 (经度:113°43′4.75″E,纬度:24°31′27.57″N) ,沉积物样品采集的深度为10 cm,沉积物样品呈现赭黄色。重金属质量分数分别为Pb 2 450 mg kg−1,Zn 1 009 mg kg−1,Mn 1 211 mg kg−1,Cu 680 mg kg−1,Cr 95 mg kg−1,Cd 16 mg kg−1,Mo 68 mg kg−1,以及As 760 mg kg−1。经采集的样品于干燥后压碎并混匀研磨过100目筛后进行密封保存,供沉积物流动柱实验和样品表征。
-
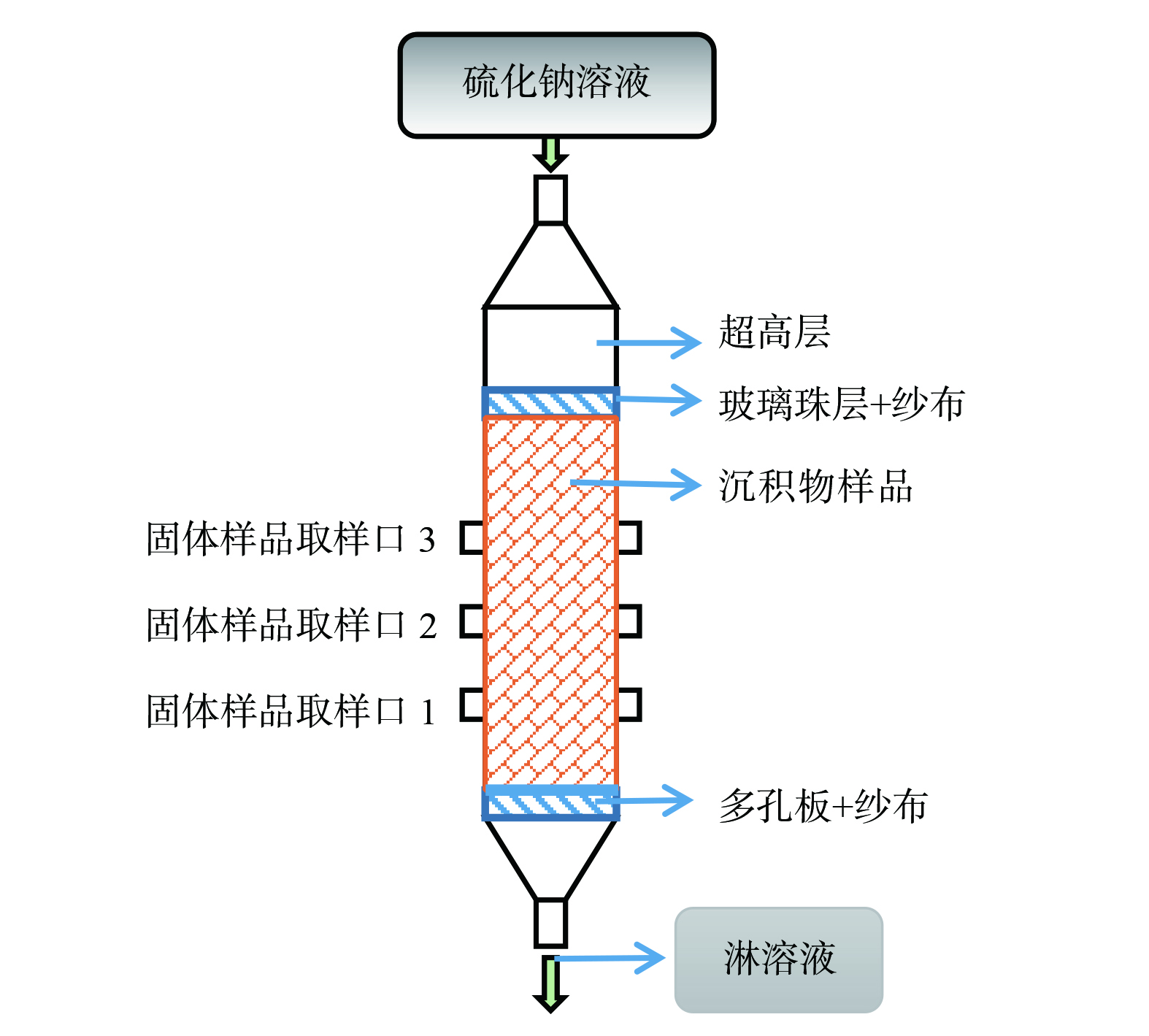
实验装置如图1所示。淋滤柱体由有机玻璃制作而成,其内径为50 mm,高度为200 mm,在两侧各有3个直径10 mm的取样孔,其间距均为20 mm。在柱体底部放置一张滤网,然后平铺一层粒径为2 mm的石英砂。将粉末状样品填入柱体中,填充高度约为15 cm,轻微震荡后依靠重力使样品压实。在样品顶层放置一张滤网,并平铺一层粒径为2 mm的石英砂,避免水力作用对样品产生冲刷,模拟自然条件下的重力渗透过程。组装其他部件,将柱体固定在铁架上。所填沉积物样品的质量约为258.61 g。先向柱体通入初始pH为2.5的模拟AMD溶液以充分润湿沉积物并使孔隙饱和。其中,AMD溶液中各类重 (类) 金属的质量浓度分别约为SO42− 540.000 mg·L−1、Zn2+ 101.290 mg·L−1、Cu2+ 3.355 mg·L−1、AsO42− 0.024 mg·L−1、Cd2+ 0.170 mg·L−1、Pb2+ 0.349 mg·L−1、CrO42− 0.022 mg·L−1、MoO42− 0.011 mg·L−1及Mn2+ 80.620 mg·L−1。然后,每隔24 h分别添加30 mL质量浓度分别为10 mg·L−1和100 mg·L−1的Na2S溶液进行淋滤,空白对照组为去离子水。每日收集淋出液,约30 mL。实验周期为31 d。
-
液体样品用一次性取样针进行采集。经0.45 μm的水系滤膜过滤后,用pH计 (上海雷磁,PHS-3C) 测定样品pH,随后滴加1滴5%硝酸进行酸化,置于4 ℃下保存。使用电感耦合等离子体发射光谱仪 (ICP,日本岛津) 测定溶液中重金属离子浓度,并使用Origin 2018软件进行绘图。ICP测定过程中通过标准样品的回收率 (< 5%) 进行质量控制。
当淋溶实验结束后,在柱体3个取样口的沉积物分别混合均匀后各取约2 g沉积物样品。另取2 g未进行淋溶实验的沉积物样品作为对照,烘干后用聚乙烯离心管密封保存。使用X射线衍射仪 (XRD,德国布鲁克,D8 Advance) 分析样品的晶型结构。X射线为Cu靶Kα射线 (λ= 0.154 18 nm) ,管电压和管电流分别为40 V和40 mA,扫描区域为10°~80°,扫描速度为10°·min−1。利用场发射扫描电镜 (SEM,FEI Nova Nano SEM 450) 检测反应前后的固体颗粒样品的形貌。同时,采用0.2 mol·L−1草酸铵 (pH=3) 对固体样品进行避光萃取4 h,以验证重金属在还原态矿物相中的相对含量。
-
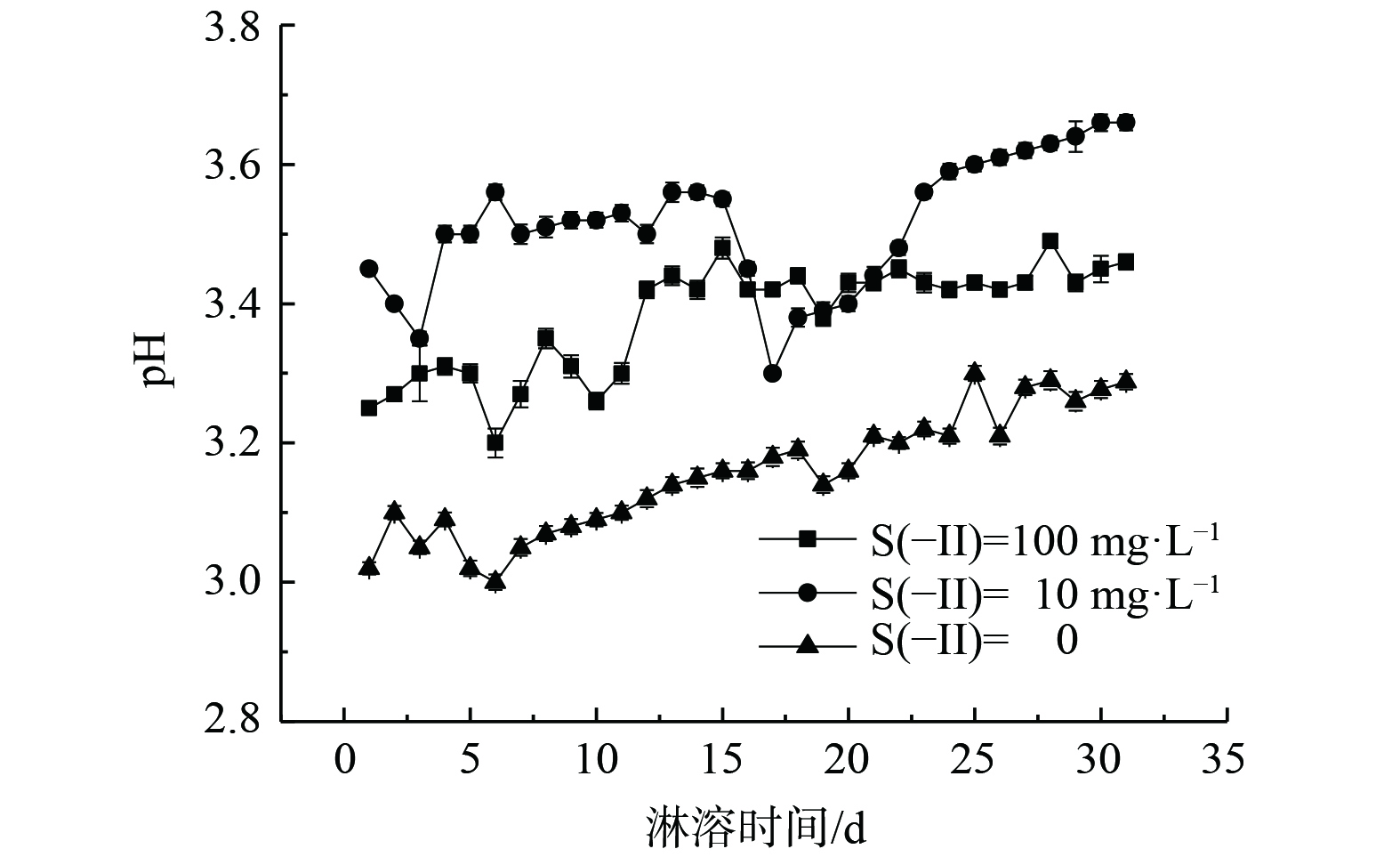
如图2所示,在整个淋滤实验过程中,各组淋出液pH总体略呈上升趋势。其中,空白对照组的pH从最低3.00增至最高3.30,增幅最大,在7~18 d时,表现出稳步上升,前期和后期均出现一定波动。可见在没有S(-II)影响下,沉积物中的淋滤液pH呈现酸性且略为上升。质量浓度为10 mg·L−1的Na2S处理组pH从初始3.45增至最终的3.68,而质量浓度为100 mg·L−1 Na2S处理组的pH从最低3.20增至最高3.49。Na2S溶液呈碱性,沉积物本身为强酸性 (pH约为2.55) ,然而在通过沉积物淋溶后的淋溶液却呈现酸性,且质量浓度较高的S(-II)反而总体pH较低。原因可能是由于碱性溶液通过沉积物而使得沉积物中介稳定的矿物如施氏矿物溶解[6] (如式 (1) ) ,部分重金属和硫酸根的释放溶出,引起H+释放,从而使pH降低。然而,整个实验过程中3个实验组的pH变化较小,呈现酸性,其有利于重金属从结构态转变为游离态[21]并促进其迁移能力。
-
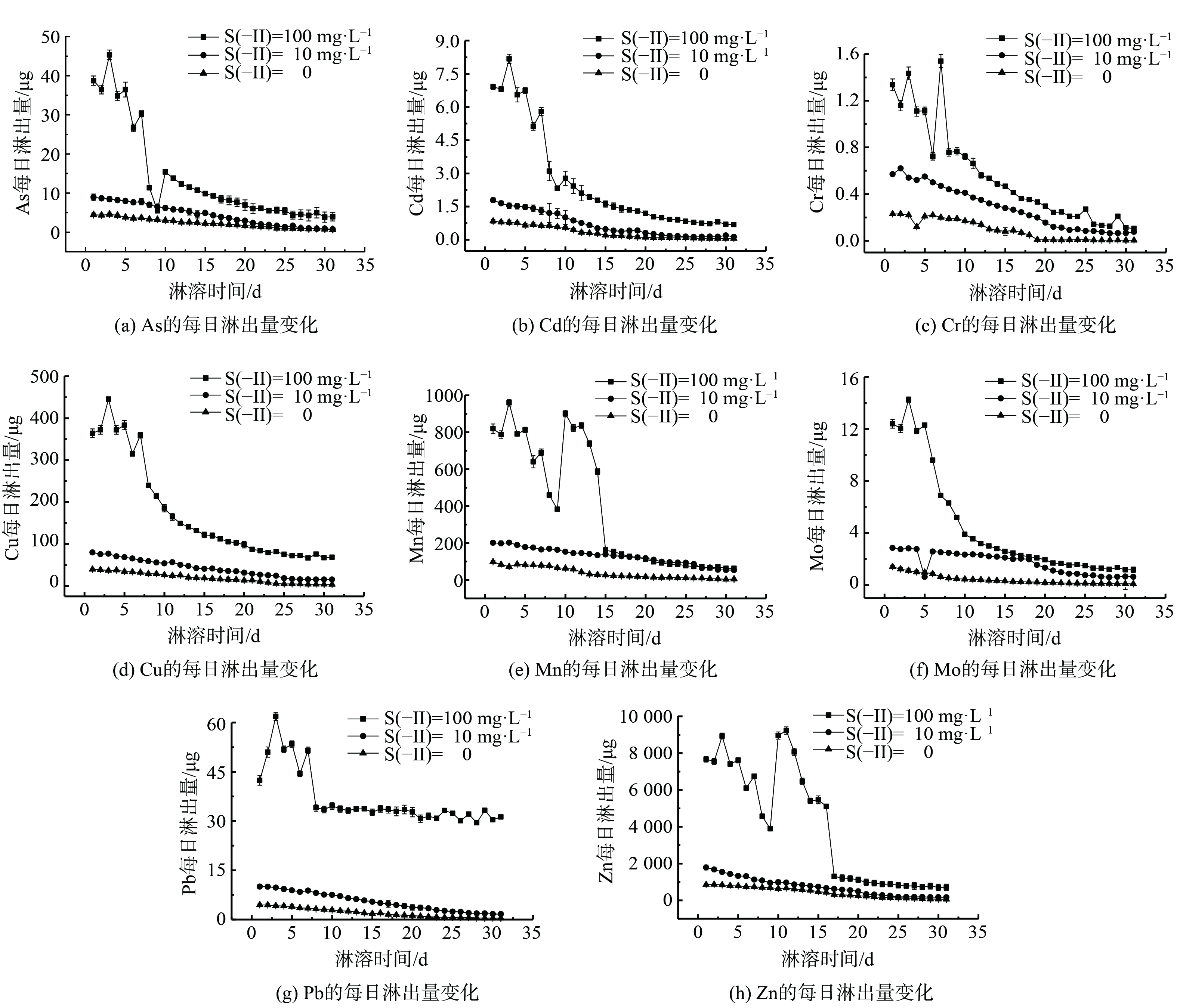
重金属每日淋出量随淋溶时间的变化趋势如图3所示。在本实验条件下,S(-II)的淋溶对于沉积物中重金属的释放有相对的促进作用。质量浓度为10 mg·L−1 Na2S处理组第1天的淋滤液中重 (类) 金属As、Cd、Cr、Cu、Mn、Mo、Pb和Zn的淋出量分别比空白高了约1.993、2.131、2.478、2.027、2.051、2.028、2.247、和2.095倍,且随着硫离子质量浓度的增加,重金属的释放量增加。
8种重金属元素的释放规律类似。1~3 d为重金属快速释放时期,各元素每日淋出量较高,且出现了一定的上升。但在此后5~10 d,各元素淋出量迅速下降。如Mo的每日淋出量从12.29 μg降至3.91 μg,降幅最大,为68.20 %。在10 d之后,各元素淋出量开始缓慢而稳定地下降,各元素的最终淋出量均小于2.09 μg·L−1。其中,Pb元素的变化趋势略有不同,每日淋出量虽在1~3 d时出现上升后下降,但其淋出量在8 d时便降至平稳阶段,其降幅相比As、Cu、Cr、Cd和Mo较小,此后一直稳定在约33.50 μg。从整体上看,Pb的每日淋出量从最高值61.88 μg降至最低值29.48 μg,变化幅度较小,但持续淋出量较高。这可能是由于拦泥库沉积物中Pb的质量分数最高 (2 450 mg·kg−1) ,这与文献[22]报道的结果一致。
Cu、Mn、Zn与Cd等其他元素在1~5 d时,同样呈现出先增长后回落的形势。Cu在7 d后稳步平滑下降。而Mn、Zn同时在第9天出现大幅异常增长后呈阶梯快速下降,最后分别于15 d和17 d转为平滑下降。从总体上看,Cu、Mn和Zn初始淋出量较高,分别为364.00、819.00 和7 670.00 μg,且Zn淋出量较Cu和Mn高出1个数量级。大宝山矿床主体为钼矿、褐铁矿、铜硫矿和铅锌矿等多金属共生或伴生矿体[23],矿区长期采矿和洗矿过程中产生的大量重金属污染,且研究区域的沉积物中重金属含量顺序为Zn>Mn>Cu>Cd。其中,Cd和As在9~11 d出现每日淋出量的大幅度快速增长。这表明各元素间可能具有一定的相互促进或抑制迁移作用。除了与沉积物中重金属的存在形态有关之外,也与淋滤液中硫离子的作用有关。研究表明,S2−离子的还原性会影响沉积物中矿物的稳定性,进而影响被沉积物固定的重金属,影响重金属的二次释放[24-26]。此外,S2−又会与重金属反应结合,生成较为稳定不易迁移的硫化物等沉淀[18, 27]。因此,S2−在整个淋滤过程中表现出前期促进而后期缓解抑制的作用。但Cu、Mn、Zn在淋滤过程中每日淋出量下降较快,或受到S2−的抑制作用。各重金属的淋溶特点均表明沉积物对环境造成的污染具有持续性特点,对于生态环境具有潜在影响[28]。
-
沉积物中重金属的迁移过程受到水分、温度、氧化还原电位等多重因素的影响,是一个多因素相互作用的动态变化反应过程。为进一步研究这一过程,借助化学动力学和数学模型进行分析,通过拟合S(-II)介导下的实验结果以了解重金属迁移过程中的影响因素和各类重金属的迁移特点[29]。通过文献调研选取了一级动力学方程、双常数方程和Elovich方程 (式 (2)~(5) ) 。其中,Elovich方程是一个经验式,其主要描述一系列反应机制的过程,如溶质的本体、界面扩散、表面的活化与去活化作用等[30],其对重金属在土壤或沉积物界面上的迁移过程较为适用。而反应条件和反应过程较复杂的化学动力学过程则适合用双常数方程进行描述。其中,各类重金属累积释放量曲线如图4所示,拟合分析结果如表1所示。
淋出液中重金属累积释放量的计算公式见式 (2) ,其一级动力学方程为式 (3) ,双常数方程为式 (4) ,Elovich方程为式 (5) 。
式中:q为沉积物样品中重金属的累积释放量,μg·kg−1;t为淋溶时间,d;a, b为常数;
Ci为第i次采样的淋溶液中重金属浓度,μg·L−1;V为第i次采样的淋溶液体积,L;m为沉积物样品质量,kg。
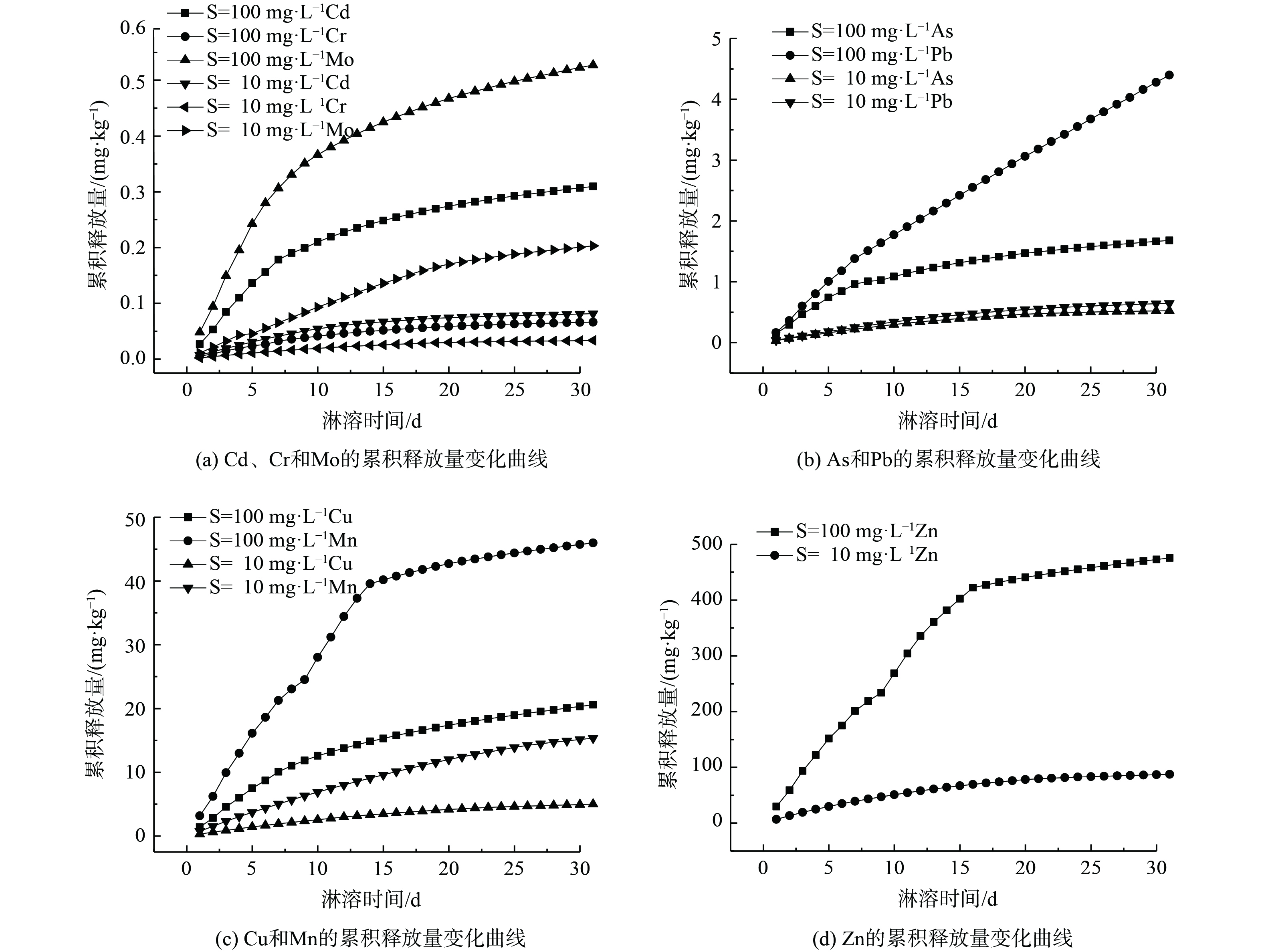
如图4所示,高浓度处理组中Cd、Mo、As和Cu的累积释放量在1~8 d增长较快,在9 d之后的增长趋于平缓;Pb的增长则呈现斜直线;Mn和Zn在1~14 d快速增长,在14 d后趋于平缓,Pb、Mn和Zn大致呈线性增长。而Cr的增长幅度不大,趋势不明显。而对于低浓度处理组,除Mn,Cd、Mo、Cr、Zn、Cu、As和Pb的累积释放量在20 d后趋于缓和,相比于高浓度处理组,累积释放量均在一定程度上下降了。
由表1拟合结果可知,对于一级动力学方程,在高浓度处理组中除Pb和Cu外,其他重金属的决定系数在0.85~0.90。这表明一级动力学对多数重金属元素拟合效果较为一般。一级动力学方程能体现出反应物浓度与反应速率之间的线性关系。这说明重金属在沉积物中吸附和分布并不是理想中的均匀状态,各吸附点位对于重金属的亲和力并不相等。但从个体来看,一级动力学方程对于Pb的拟合效果较好。而在低浓度处理组中,除了Cd,其他的决定系数都高于0.90。对于双常数方程而言,所有重金属决定系数均大于0.90。这表明双常数方程对于多数重金属拟合效果较好,且低浓度处理组的决定系数均高于0.94。其中,Pb的决定系数分别为0.98和0.99,这表明Pb的释放过程适合用双常数方程进行描述。对于Elovich方程而言,高浓度处理组中除Pb外,其余重金属决定系数均在0.95以上。这说明该方程具有较好的拟合效果。而在低浓度处理组中除As外,其余重金属决定系数均在0.92以上。这表明Elovich方程的拟合效果比双常数方程更好。
上述分析表明,低浓度处理组中所有重金属的双常数方程拟合度最优。而高浓度处理组中双常数方程能较好地描述Pb的迁移过程,这表明Pb在沉积物颗粒中的吸附和分布可能相对较为均匀,亦可能受到的影响因素较为单一。其他重金属As、Cd、Cr、Cu、Mn、Mo和Zn的Elovich方程拟合度最优。这表明多数重金属在沉积物中的吸附和解吸过程受到多种因素的制约和影响[31],如DOM、还原性物质、共存离子和pH等。
-
通过对高浓度S(-II)处理组反应前后沉积物中水溶态、吸附和可交换态及易还原矿物相中重金属的萃取结果可知,被淋滤下来的重金属主要来源于沉积物中的水溶态、吸附和可交换态及易还原矿物相。其中,Zn和Mn的释放量为8种重金属中最高(见表2)。这可能是Zn和Mn在实验所测的8种重金属中含量较高,且其金属硫化物溶解度相对较高。此外,Zn所累积的释放量高于反应前水溶态、吸附和可交换态,以及易还原矿物相中Zn的含量。这说明淋滤的部分Zn来源较为稳定的矿物相。此外,Cd、Mo和Cr的累积释放量较低,源于其在沉积物中相对含量较低。而Pb和As在沉积物中的相对含量较高,但是被淋滤所累积的释放量却相反。这可能是由于Pb和As的硫化物溶解度较低,在一定程度上抑制了Pb和As的释放。因此,在AMD体系中,重金属的迁移转化受控于还原性物质等环境因子。
-
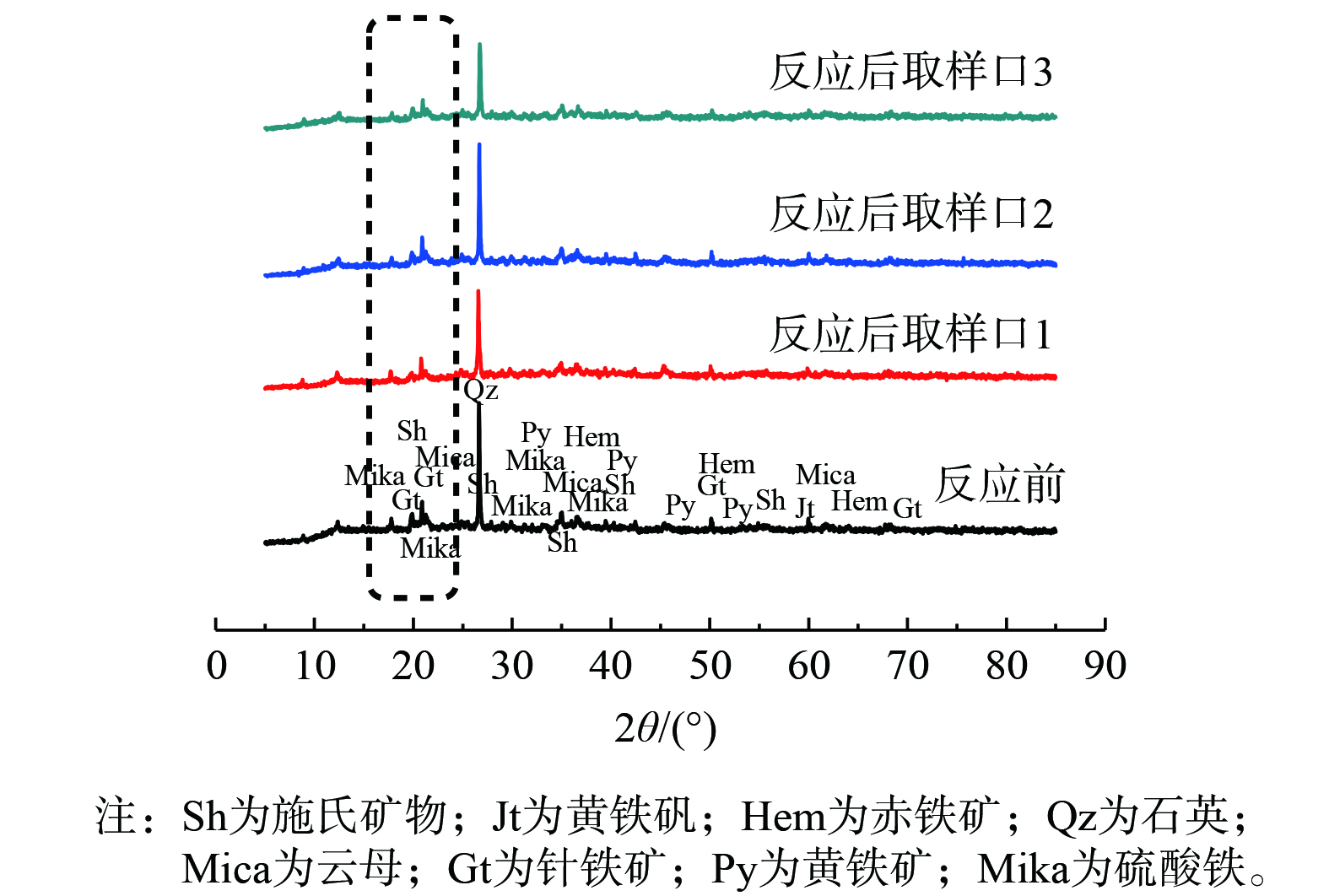
为进一步确定重金属的来源,采用了XRD对质量浓度为100 mg·L−1 Na2S处理组反应前后矿物组成进行分析,结果如图5所示。沉积物中主要矿物成分为:施氏矿物、黄铁矾、赤铁矿、石英、云母、针铁矿、黄铁矿、硫酸铁等含铁 (硫) 矿物及一些重金属与矿物形成的复合体。图5可观察到反应前后相对的区别,在峰位约20°可看到峰型的相对改变,即反应前峰型较宽,衍射强度更大,反应后衍射强度减弱,且在采样口3的位置处,无定型的矿物峰减弱最多。分析其原因,可能是S(-II)最先接触采样口3的沉积物,故采样口3的位置接触到的硫离子浓度最高。随着深度的增加,介稳定性矿物受淋溶液的影响较小,此时接触的硫离子质量浓度也低、厌氧程度加大,故出现了硫酸亚铁的峰位。因此,XRD结果表明沉积物中介稳定的矿物相对含量的变化,即淋溶液中重金属可能主要来源于沉积物中可还原态的矿物,如介稳定的施氏矿物和黄铁矾类矿物,淋溶后剩下了较为稳定的矿物相如针铁矿等。
-
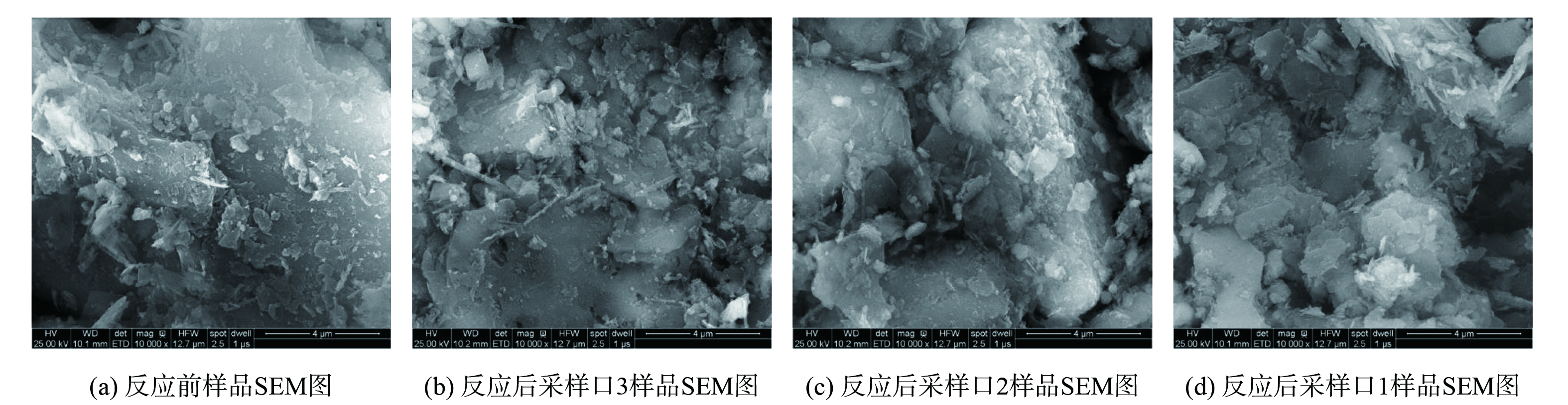
采用SEM观察高浓度S(-II)处理组反应前后沉积物表观形貌,结果如图6所示。未淋溶前沉积物样品 (图6(a)) 表面相对较为光滑完整,且附着有页状形貌矿物。经过硫化钠溶液淋滤之后,柱体上层的沉积物 (图6(b)) 表面呈现附着有较多叠加的薄片状和少数杆状形貌的物质。柱体中层的沉积物 (图6(c)) ,颗粒表面较为粗糙,附着较小的片状、球状和少数米粒状的矿物。而柱体底层的沉积物 (图6(d)) 样品显示其表面附着的细小片状、针状矿物增多。这些片状、球状、针状的物质可能对应为施氏矿物、黄铁矾、赤铁矿、针铁矿等[32]。这表明在硫化钠溶液的淋溶作用下的沉积物中,从柱体上层至下层呈现不同的形貌变化,结合重金属萃取和XRD结果可知,沉积物中垂直迁移的重金属主要来源于沉积物相中可还原的矿物相,其迁移过程表现为“迁移—稳定”的模式[33]。
-
可溶性的二价硫离子具有还原、络合和沉淀重金属的作用,因此,在一定程度上制约着酸性矿山废水中重金属的迁移转化。本研究中的沉积物样品采集于大宝山拦泥库,含有的羟基含铁硫酸盐次生矿物质量分数约为24% [6],在环境因子pH改变和还原性物质存在时会导致矿物溶解重结晶的过程中吸附和共沉淀的重金属再次释放到环境中。在本研究的条件下,S(-II)可能会使环境中原有的铁矿物发生还原溶解,产生的内源二价铁对矿物具有催化作用,能加速含铁矿物的溶解重结晶。如ZHANG等[34]的研究结果,在S(-II)作用下,体系产生Fe(II)引发催化作用下,使得As(V)-施氏矿物会转化为Magnetite等次生矿物;但同时硫离子会与重金属离子形成硫化物沉淀;其次,硫化钠溶液本身呈现碱性,对矿物的溶解有促进作用。在复杂的反应过程中,矿物的溶解占据一定优势,故可观察到前期重金属的释放量相对较大。在反应的后期,由于S(-II)被氧化后或与沉积物中的重金属或Fe形成沉淀或新的矿物,使得被淋出的重金属再次被共沉淀和吸附,导致后期重金属淋出量减少。
-
金属硫化物矿区AMD污染流域中的沉积物是矿区有毒有害金属元素的主要汇,且沉积物中存在大量的含铁硫酸盐次生矿物。这些次生矿物处于介稳定的状态,一旦环境条件改变 (还原性物质和共存离子等) 时,很容易发生溶解和相转变,从而引起沉积物中重金属的二次释放和再次分配,存在一定的环境风险。因此,基于以上的研究结果,在进行矿区AMD污染流域环境原位修复治理时,可对沉积物底泥进行清淤或者采用重金属吸附剂或钝化剂进行固定,但应避免选用还原性的吸附剂或钝化剂,并规避因流域pH变化引起固持的重金属存在二次释放的风险。
-
1) 在S(-II)介导,即在硫化钠溶液的淋滤作用下,大宝山拦泥库沉积物中重金属的垂直迁移特征,即沉积物中各重金属元素释放量的大小顺序为:Zn>Mn>Cu>Pb>As>Mo>Cd>Cr。
2) S(-II)在反应前期对重金属的释放具有较明显的促进作用,且释放的部分重金属主要来源于沉积物中的易还原矿物相,其释放过程可用双常数和Elovich模型进行描述拟合。
3) 在AMD环境下,沉积物中重金属的环境行为与介稳态矿物相的溶解-重结晶有关,存在着释放的潜在生态风险。因此,在AMD污染流域环境修复治理时应注意重金属的再次释放。
可溶性硫化物介导对矿区拦泥库沉积物中重金属垂直迁移特征的影响
Effects of soluble sulfide on the vertical migration of heavy metals in the sediment of mine retaining reservoir
-
摘要: 在金属硫化物矿区中,Fe/S循环过程制约着重 (类) 金属的迁移转化。采用流动柱进行模拟自然淋溶实验,结合释放动力学及XRD、SEM表征探究了可溶性硫化物 (S(-II)) 对大宝山沉积物中重金属垂直迁移规律的影响。结果表明,S(-II)对沉积物中重金属的释放具有相对的促进作用,各重金属元素释放量的大小顺序为Zn>Mn>Cu>Pb>As>Mo>Cd>Cr,呈现前期快速释放随后缓慢的过程。在质量浓度为100 mg·L−1的Na2S溶液作用下,As、Cd、Cr、Cu、Mn、Mo、Pb和Zn的平均淋出浓度比10 mg·L−1质量浓度处理组分别高了3.182、3.784、1.973、4.098、2.979、2.582、6.786和5.408倍。释放动力学拟合结果表明,重金属的释放过程符合双常数和Elovich模型。重金属萃取实验和XRD结果证明释放的部分重金属主要来源于沉积物中的易还原矿物相。本研究结果可为金属硫化物矿区重 (类) 金属污染控制与修复提供参考。Abstract: The Fe/S cycle process restricts the migration and transformation of heavy metals (metalloids) in the metal sulfide mines. In this paper, the release kinetics combined with XRD and SEM was used to investigate the effects of S(-II) on vertical migration characteristics of heavy metals in sediments of Dabaoshan Mine. The results showed that S(-II) was beneficial to the release of heavy metals in sediments to a certain extent. The release of heavy metals was in the order of Zn>Mn>Cu>Pb>As>Mo>Cd>Cr, showing a rapid release in the early stage followed by a slow release process. When the concentration of S(-II) increased to 100 mg·L-1, the average leaching concentrations of As, Cd, Cr, Cu, Mn, Mo, Pb and Zn were 3.182, 3.784, 1.973, 4.098, 2.979, 2.582, 6.786 and 5.408 times higher than those of the 10 mg·L-1 group, respectively.. Results of release kinetics fitting revealed that the release of heavy metals fitted well on Double Constant model and Elovich model. And the partly released heavy metals were mainly from easily reducible minerals in the sediments which were characterized by XRD and extraction. The results of this research could provide theoretical basis for the remediation of heavy metals in metal sulfide mining areas.
-
Key words:
- metal sulfide mining area /
- sediment /
- sulfur ion /
- heavy metals /
- migration
-
矿山开发过程中会产生大量的固体废弃矿物,在自然环境中风化和微生物的氧化过程中伴随着有毒有害元素的释放,并逐渐形成了高硫酸根、高铁浓度的极端酸性矿山废水 (acid mine drainage,AMD) ,造成了矿区附近流域水体及土壤重金属的污染,严重威胁矿区生态系统安全及人群健康[1-4]。因此,AMD所引起的环境污染已成为国内外研究热点。
以广东省韶关市大宝山金属硫化物矿区为例,ZHUANG等[5]发现,受矿区酸性废水的长期影响,大宝山矿区附近土壤的pH多低于5,土壤中铜、铅、锌和镉的平均质量分数分别为502 mg·kg−1、278 mg·kg−1、498 mg·kg−1和3.92 mg·kg−1,远超过国家标准 (GB15618-2018) 限值。而AMD表层沉积物中的重 (类) 金属,如Pb、As和Cr的质量分数高达3 100、1 896和170 mg·kg−1[6-7]。据沉积物矿物学特征调查可知,AMD污染的流域中易形成大量特征性的含铁羟基硫酸盐次生矿物[8-10]。例如,当pH为2.8~4.5时,AMD环境中的三价铁和硫酸根离子共沉淀形成典型的施氏矿物 (schwertmannite) ;当体系中存在一价阳离子且pH为1.5~3时,易形成以黄铁矾类为主的矿物;当pH>5时,Fe3+水解产生的矿物主要为水铁矿[11-12]。由于类质同象作用,这些含铁羟基硫酸盐次生矿物容易吸持AMD中的重 (类) 金属离子,使其演变成为重 (类) 金属离子的沉淀库。同时,含铁硫酸盐次生矿物容易在环境因子 (pH、还原性物质、溶解性有机质 (dissolved organic matter,DOM) 和共存离子浓度等) 改变时,发生溶解重结晶,使其吸附和共沉淀的重 (类) 金属再次释放到相应的水体流域中,造成二次生态风险[13-16]。然而,目前关于大宝山矿区环境中重金属环境风险的研究主要针对矿区土壤中重金属的迁移。
在AMD环境中,可溶性S(-II)可由硫酸盐还原菌还原硫酸根产生,并通过还原、沉淀和络合等途径对矿区中重 (类) 金属的迁移转化起制约作用[17]。例如,在厌氧环境下,S(-II)可还原溶解铁 (氢) 氧化物等矿物且自身形态发生改变,亦引起了所吸持的重 (类) 金属再次释放[18-20]。然而,关于可溶性S(-II)与矿区沉积物中次生矿物的稳定性及重 (类) 金属的迁移转化动态关系的报道还甚少。
基于此,本研究围绕大宝山矿区拦泥库沉积物,采用流动柱进行模拟淋溶实验,模拟还原大宝山矿区拦泥库沉积物中的重 (类) 金属在还原性S(-II)介导下的垂直迁移特征,以探究S(-II)对拦泥库沉积物次生矿物的稳定性影响与重金属环境行为的关系,以期为金属硫化物矿区的环境修复与污染防治提供参考。
1. 材料与方法
1.1 实验主要试剂
主要试剂或药品:硫化钠 (Na2S,GR) 购自上海阿拉丁生化科技有限公司,硝酸 (HNO3,AR) 和氢氧化钠 (NaOH,AR) 购买于西陇科学股份有限公司;重 (类) 金属标准溶液购自于国家有色金属及电子材料分析测试中心,以5%的硝酸定容;实验用水为去离子水。
1.2 沉积物样品的采集与预处理
沉积物样品于2018年8月采集于大宝山矿区拦泥库 (经度:113°43′4.75″E,纬度:24°31′27.57″N) ,沉积物样品采集的深度为10 cm,沉积物样品呈现赭黄色。重金属质量分数分别为Pb 2 450 mg kg−1,Zn 1 009 mg kg−1,Mn 1 211 mg kg−1,Cu 680 mg kg−1,Cr 95 mg kg−1,Cd 16 mg kg−1,Mo 68 mg kg−1,以及As 760 mg kg−1。经采集的样品于干燥后压碎并混匀研磨过100目筛后进行密封保存,供沉积物流动柱实验和样品表征。
1.3 沉积物柱实验模拟研究实验及装置
实验装置如图1所示。淋滤柱体由有机玻璃制作而成,其内径为50 mm,高度为200 mm,在两侧各有3个直径10 mm的取样孔,其间距均为20 mm。在柱体底部放置一张滤网,然后平铺一层粒径为2 mm的石英砂。将粉末状样品填入柱体中,填充高度约为15 cm,轻微震荡后依靠重力使样品压实。在样品顶层放置一张滤网,并平铺一层粒径为2 mm的石英砂,避免水力作用对样品产生冲刷,模拟自然条件下的重力渗透过程。组装其他部件,将柱体固定在铁架上。所填沉积物样品的质量约为258.61 g。先向柱体通入初始pH为2.5的模拟AMD溶液以充分润湿沉积物并使孔隙饱和。其中,AMD溶液中各类重 (类) 金属的质量浓度分别约为SO42− 540.000 mg·L−1、Zn2+ 101.290 mg·L−1、Cu2+ 3.355 mg·L−1、AsO42− 0.024 mg·L−1、Cd2+ 0.170 mg·L−1、Pb2+ 0.349 mg·L−1、CrO42− 0.022 mg·L−1、MoO42− 0.011 mg·L−1及Mn2+ 80.620 mg·L−1。然后,每隔24 h分别添加30 mL质量浓度分别为10 mg·L−1和100 mg·L−1的Na2S溶液进行淋滤,空白对照组为去离子水。每日收集淋出液,约30 mL。实验周期为31 d。
1.4 样品收集及测定
液体样品用一次性取样针进行采集。经0.45 μm的水系滤膜过滤后,用pH计 (上海雷磁,PHS-3C) 测定样品pH,随后滴加1滴5%硝酸进行酸化,置于4 ℃下保存。使用电感耦合等离子体发射光谱仪 (ICP,日本岛津) 测定溶液中重金属离子浓度,并使用Origin 2018软件进行绘图。ICP测定过程中通过标准样品的回收率 (< 5%) 进行质量控制。
当淋溶实验结束后,在柱体3个取样口的沉积物分别混合均匀后各取约2 g沉积物样品。另取2 g未进行淋溶实验的沉积物样品作为对照,烘干后用聚乙烯离心管密封保存。使用X射线衍射仪 (XRD,德国布鲁克,D8 Advance) 分析样品的晶型结构。X射线为Cu靶Kα射线 (λ= 0.154 18 nm) ,管电压和管电流分别为40 V和40 mA,扫描区域为10°~80°,扫描速度为10°·min−1。利用场发射扫描电镜 (SEM,FEI Nova Nano SEM 450) 检测反应前后的固体颗粒样品的形貌。同时,采用0.2 mol·L−1草酸铵 (pH=3) 对固体样品进行避光萃取4 h,以验证重金属在还原态矿物相中的相对含量。
2. 结果与讨论
2.1 淋滤液pH的变化规律
如图2所示,在整个淋滤实验过程中,各组淋出液pH总体略呈上升趋势。其中,空白对照组的pH从最低3.00增至最高3.30,增幅最大,在7~18 d时,表现出稳步上升,前期和后期均出现一定波动。可见在没有S(-II)影响下,沉积物中的淋滤液pH呈现酸性且略为上升。质量浓度为10 mg·L−1的Na2S处理组pH从初始3.45增至最终的3.68,而质量浓度为100 mg·L−1 Na2S处理组的pH从最低3.20增至最高3.49。Na2S溶液呈碱性,沉积物本身为强酸性 (pH约为2.55) ,然而在通过沉积物淋溶后的淋溶液却呈现酸性,且质量浓度较高的S(-II)反而总体pH较低。原因可能是由于碱性溶液通过沉积物而使得沉积物中介稳定的矿物如施氏矿物溶解[6] (如式 (1) ) ,部分重金属和硫酸根的释放溶出,引起H+释放,从而使pH降低。然而,整个实验过程中3个实验组的pH变化较小,呈现酸性,其有利于重金属从结构态转变为游离态[21]并促进其迁移能力。
stringUtils.convertMath(!{formula.content}) (1) 2.2 淋滤液中重金属含量的变化
重金属每日淋出量随淋溶时间的变化趋势如图3所示。在本实验条件下,S(-II)的淋溶对于沉积物中重金属的释放有相对的促进作用。质量浓度为10 mg·L−1 Na2S处理组第1天的淋滤液中重 (类) 金属As、Cd、Cr、Cu、Mn、Mo、Pb和Zn的淋出量分别比空白高了约1.993、2.131、2.478、2.027、2.051、2.028、2.247、和2.095倍,且随着硫离子质量浓度的增加,重金属的释放量增加。
8种重金属元素的释放规律类似。1~3 d为重金属快速释放时期,各元素每日淋出量较高,且出现了一定的上升。但在此后5~10 d,各元素淋出量迅速下降。如Mo的每日淋出量从12.29 μg降至3.91 μg,降幅最大,为68.20 %。在10 d之后,各元素淋出量开始缓慢而稳定地下降,各元素的最终淋出量均小于2.09 μg·L−1。其中,Pb元素的变化趋势略有不同,每日淋出量虽在1~3 d时出现上升后下降,但其淋出量在8 d时便降至平稳阶段,其降幅相比As、Cu、Cr、Cd和Mo较小,此后一直稳定在约33.50 μg。从整体上看,Pb的每日淋出量从最高值61.88 μg降至最低值29.48 μg,变化幅度较小,但持续淋出量较高。这可能是由于拦泥库沉积物中Pb的质量分数最高 (2 450 mg·kg−1) ,这与文献[22]报道的结果一致。
Cu、Mn、Zn与Cd等其他元素在1~5 d时,同样呈现出先增长后回落的形势。Cu在7 d后稳步平滑下降。而Mn、Zn同时在第9天出现大幅异常增长后呈阶梯快速下降,最后分别于15 d和17 d转为平滑下降。从总体上看,Cu、Mn和Zn初始淋出量较高,分别为364.00、819.00 和7 670.00 μg,且Zn淋出量较Cu和Mn高出1个数量级。大宝山矿床主体为钼矿、褐铁矿、铜硫矿和铅锌矿等多金属共生或伴生矿体[23],矿区长期采矿和洗矿过程中产生的大量重金属污染,且研究区域的沉积物中重金属含量顺序为Zn>Mn>Cu>Cd。其中,Cd和As在9~11 d出现每日淋出量的大幅度快速增长。这表明各元素间可能具有一定的相互促进或抑制迁移作用。除了与沉积物中重金属的存在形态有关之外,也与淋滤液中硫离子的作用有关。研究表明,S2−离子的还原性会影响沉积物中矿物的稳定性,进而影响被沉积物固定的重金属,影响重金属的二次释放[24-26]。此外,S2−又会与重金属反应结合,生成较为稳定不易迁移的硫化物等沉淀[18, 27]。因此,S2−在整个淋滤过程中表现出前期促进而后期缓解抑制的作用。但Cu、Mn、Zn在淋滤过程中每日淋出量下降较快,或受到S2−的抑制作用。各重金属的淋溶特点均表明沉积物对环境造成的污染具有持续性特点,对于生态环境具有潜在影响[28]。
2.3 沉积物中重金属的释放动力学
沉积物中重金属的迁移过程受到水分、温度、氧化还原电位等多重因素的影响,是一个多因素相互作用的动态变化反应过程。为进一步研究这一过程,借助化学动力学和数学模型进行分析,通过拟合S(-II)介导下的实验结果以了解重金属迁移过程中的影响因素和各类重金属的迁移特点[29]。通过文献调研选取了一级动力学方程、双常数方程和Elovich方程 (式 (2)~(5) ) 。其中,Elovich方程是一个经验式,其主要描述一系列反应机制的过程,如溶质的本体、界面扩散、表面的活化与去活化作用等[30],其对重金属在土壤或沉积物界面上的迁移过程较为适用。而反应条件和反应过程较复杂的化学动力学过程则适合用双常数方程进行描述。其中,各类重金属累积释放量曲线如图4所示,拟合分析结果如表1所示。
表 1 各重金属元素拟合结果Table 1. Fitting results of each heavy metal elements重 (类) 金属元素种类 决定系数R2 一级动力学方程 双常数方程 Elovich方程 低浓度 高浓度 低浓度 高浓度 低浓度 高浓度 As 0.915 6 0.875 9 0.968 4 0.968 4 0.713 3 0.991 9 Cd 0.854 3 0.852 5 0.948 9 0.957 0 0.977 2 0.991 7 Cr 0.906 1 0.892 7 0.967 2 0.967 2 0.965 1 0.977 4 Cu 0.950 0 0.905 1 0.985 4 0.974 9 0.948 7 0.982 4 Mn 0.978 4 0.840 4 0.994 1 0.927 4 0.920 1 0.954 5 Mo 0.959 1 0.842 3 0.981 3 0.953 7 0.921 2 0.992 7 Pb 0.941 7 0.993 5 0.981 5 0.999 1 0.952 5 0.898 3 Zn 0.911 3 0.864 8 0.972 6 0.934 8 0.966 7 0.946 7 淋出液中重金属累积释放量的计算公式见式 (2) ,其一级动力学方程为式 (3) ,双常数方程为式 (4) ,Elovich方程为式 (5) 。
stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:q为沉积物样品中重金属的累积释放量,μg·kg−1;t为淋溶时间,d;a, b为常数;
Ci为第i次采样的淋溶液中重金属浓度,μg·L−1;V为第i次采样的淋溶液体积,L;m为沉积物样品质量,kg。
如图4所示,高浓度处理组中Cd、Mo、As和Cu的累积释放量在1~8 d增长较快,在9 d之后的增长趋于平缓;Pb的增长则呈现斜直线;Mn和Zn在1~14 d快速增长,在14 d后趋于平缓,Pb、Mn和Zn大致呈线性增长。而Cr的增长幅度不大,趋势不明显。而对于低浓度处理组,除Mn,Cd、Mo、Cr、Zn、Cu、As和Pb的累积释放量在20 d后趋于缓和,相比于高浓度处理组,累积释放量均在一定程度上下降了。
由表1拟合结果可知,对于一级动力学方程,在高浓度处理组中除Pb和Cu外,其他重金属的决定系数在0.85~0.90。这表明一级动力学对多数重金属元素拟合效果较为一般。一级动力学方程能体现出反应物浓度与反应速率之间的线性关系。这说明重金属在沉积物中吸附和分布并不是理想中的均匀状态,各吸附点位对于重金属的亲和力并不相等。但从个体来看,一级动力学方程对于Pb的拟合效果较好。而在低浓度处理组中,除了Cd,其他的决定系数都高于0.90。对于双常数方程而言,所有重金属决定系数均大于0.90。这表明双常数方程对于多数重金属拟合效果较好,且低浓度处理组的决定系数均高于0.94。其中,Pb的决定系数分别为0.98和0.99,这表明Pb的释放过程适合用双常数方程进行描述。对于Elovich方程而言,高浓度处理组中除Pb外,其余重金属决定系数均在0.95以上。这说明该方程具有较好的拟合效果。而在低浓度处理组中除As外,其余重金属决定系数均在0.92以上。这表明Elovich方程的拟合效果比双常数方程更好。
上述分析表明,低浓度处理组中所有重金属的双常数方程拟合度最优。而高浓度处理组中双常数方程能较好地描述Pb的迁移过程,这表明Pb在沉积物颗粒中的吸附和分布可能相对较为均匀,亦可能受到的影响因素较为单一。其他重金属As、Cd、Cr、Cu、Mn、Mo和Zn的Elovich方程拟合度最优。这表明多数重金属在沉积物中的吸附和解吸过程受到多种因素的制约和影响[31],如DOM、还原性物质、共存离子和pH等。
2.4 固体样品的分析
2.4.1 对重金属的萃取
通过对高浓度S(-II)处理组反应前后沉积物中水溶态、吸附和可交换态及易还原矿物相中重金属的萃取结果可知,被淋滤下来的重金属主要来源于沉积物中的水溶态、吸附和可交换态及易还原矿物相。其中,Zn和Mn的释放量为8种重金属中最高(见表2)。这可能是Zn和Mn在实验所测的8种重金属中含量较高,且其金属硫化物溶解度相对较高。此外,Zn所累积的释放量高于反应前水溶态、吸附和可交换态,以及易还原矿物相中Zn的含量。这说明淋滤的部分Zn来源较为稳定的矿物相。此外,Cd、Mo和Cr的累积释放量较低,源于其在沉积物中相对含量较低。而Pb和As在沉积物中的相对含量较高,但是被淋滤所累积的释放量却相反。这可能是由于Pb和As的硫化物溶解度较低,在一定程度上抑制了Pb和As的释放。因此,在AMD体系中,重金属的迁移转化受控于还原性物质等环境因子。
表 2 重金属萃取前后的对比Table 2. Comparison of heavy metals before and after extraction重金属种类 累积释放量/ (mg·kg−1) 重金属的质量分数/ (mg·kg−1) 反应前 反应后 Pb 4.40 544.55 540.20 Zn 475.53 268.43 23.49 Mn 45.97 215.12 206.15 Cu 20.60 174.40 153.70 Cr 0.07 45.32 45.26 Cd 0.31 3.93 3.64 Mo 0.53 44.01 43.52 As 1.68 540.11 538.58 注:反应前后重金属质量分数数据为水溶性、吸附和可交换态及易还原矿物相中重金属之和。 2.4.2 XRD表征
为进一步确定重金属的来源,采用了XRD对质量浓度为100 mg·L−1 Na2S处理组反应前后矿物组成进行分析,结果如图5所示。沉积物中主要矿物成分为:施氏矿物、黄铁矾、赤铁矿、石英、云母、针铁矿、黄铁矿、硫酸铁等含铁 (硫) 矿物及一些重金属与矿物形成的复合体。图5可观察到反应前后相对的区别,在峰位约20°可看到峰型的相对改变,即反应前峰型较宽,衍射强度更大,反应后衍射强度减弱,且在采样口3的位置处,无定型的矿物峰减弱最多。分析其原因,可能是S(-II)最先接触采样口3的沉积物,故采样口3的位置接触到的硫离子浓度最高。随着深度的增加,介稳定性矿物受淋溶液的影响较小,此时接触的硫离子质量浓度也低、厌氧程度加大,故出现了硫酸亚铁的峰位。因此,XRD结果表明沉积物中介稳定的矿物相对含量的变化,即淋溶液中重金属可能主要来源于沉积物中可还原态的矿物,如介稳定的施氏矿物和黄铁矾类矿物,淋溶后剩下了较为稳定的矿物相如针铁矿等。
2.4.3 SEM表征
采用SEM观察高浓度S(-II)处理组反应前后沉积物表观形貌,结果如图6所示。未淋溶前沉积物样品 (图6(a)) 表面相对较为光滑完整,且附着有页状形貌矿物。经过硫化钠溶液淋滤之后,柱体上层的沉积物 (图6(b)) 表面呈现附着有较多叠加的薄片状和少数杆状形貌的物质。柱体中层的沉积物 (图6(c)) ,颗粒表面较为粗糙,附着较小的片状、球状和少数米粒状的矿物。而柱体底层的沉积物 (图6(d)) 样品显示其表面附着的细小片状、针状矿物增多。这些片状、球状、针状的物质可能对应为施氏矿物、黄铁矾、赤铁矿、针铁矿等[32]。这表明在硫化钠溶液的淋溶作用下的沉积物中,从柱体上层至下层呈现不同的形貌变化,结合重金属萃取和XRD结果可知,沉积物中垂直迁移的重金属主要来源于沉积物相中可还原的矿物相,其迁移过程表现为“迁移—稳定”的模式[33]。
2.5 沉积物中重金属的可能释放机理
可溶性的二价硫离子具有还原、络合和沉淀重金属的作用,因此,在一定程度上制约着酸性矿山废水中重金属的迁移转化。本研究中的沉积物样品采集于大宝山拦泥库,含有的羟基含铁硫酸盐次生矿物质量分数约为24% [6],在环境因子pH改变和还原性物质存在时会导致矿物溶解重结晶的过程中吸附和共沉淀的重金属再次释放到环境中。在本研究的条件下,S(-II)可能会使环境中原有的铁矿物发生还原溶解,产生的内源二价铁对矿物具有催化作用,能加速含铁矿物的溶解重结晶。如ZHANG等[34]的研究结果,在S(-II)作用下,体系产生Fe(II)引发催化作用下,使得As(V)-施氏矿物会转化为Magnetite等次生矿物;但同时硫离子会与重金属离子形成硫化物沉淀;其次,硫化钠溶液本身呈现碱性,对矿物的溶解有促进作用。在复杂的反应过程中,矿物的溶解占据一定优势,故可观察到前期重金属的释放量相对较大。在反应的后期,由于S(-II)被氧化后或与沉积物中的重金属或Fe形成沉淀或新的矿物,使得被淋出的重金属再次被共沉淀和吸附,导致后期重金属淋出量减少。
2.6 对环境治理的意义
金属硫化物矿区AMD污染流域中的沉积物是矿区有毒有害金属元素的主要汇,且沉积物中存在大量的含铁硫酸盐次生矿物。这些次生矿物处于介稳定的状态,一旦环境条件改变 (还原性物质和共存离子等) 时,很容易发生溶解和相转变,从而引起沉积物中重金属的二次释放和再次分配,存在一定的环境风险。因此,基于以上的研究结果,在进行矿区AMD污染流域环境原位修复治理时,可对沉积物底泥进行清淤或者采用重金属吸附剂或钝化剂进行固定,但应避免选用还原性的吸附剂或钝化剂,并规避因流域pH变化引起固持的重金属存在二次释放的风险。
3. 结论
1) 在S(-II)介导,即在硫化钠溶液的淋滤作用下,大宝山拦泥库沉积物中重金属的垂直迁移特征,即沉积物中各重金属元素释放量的大小顺序为:Zn>Mn>Cu>Pb>As>Mo>Cd>Cr。
2) S(-II)在反应前期对重金属的释放具有较明显的促进作用,且释放的部分重金属主要来源于沉积物中的易还原矿物相,其释放过程可用双常数和Elovich模型进行描述拟合。
3) 在AMD环境下,沉积物中重金属的环境行为与介稳态矿物相的溶解-重结晶有关,存在着释放的潜在生态风险。因此,在AMD污染流域环境修复治理时应注意重金属的再次释放。
-
表 1 各重金属元素拟合结果
Table 1. Fitting results of each heavy metal elements
重 (类) 金属元素种类 决定系数R2 一级动力学方程 双常数方程 Elovich方程 低浓度 高浓度 低浓度 高浓度 低浓度 高浓度 As 0.915 6 0.875 9 0.968 4 0.968 4 0.713 3 0.991 9 Cd 0.854 3 0.852 5 0.948 9 0.957 0 0.977 2 0.991 7 Cr 0.906 1 0.892 7 0.967 2 0.967 2 0.965 1 0.977 4 Cu 0.950 0 0.905 1 0.985 4 0.974 9 0.948 7 0.982 4 Mn 0.978 4 0.840 4 0.994 1 0.927 4 0.920 1 0.954 5 Mo 0.959 1 0.842 3 0.981 3 0.953 7 0.921 2 0.992 7 Pb 0.941 7 0.993 5 0.981 5 0.999 1 0.952 5 0.898 3 Zn 0.911 3 0.864 8 0.972 6 0.934 8 0.966 7 0.946 7 表 2 重金属萃取前后的对比
Table 2. Comparison of heavy metals before and after extraction
重金属种类 累积释放量/ (mg·kg−1) 重金属的质量分数/ (mg·kg−1) 反应前 反应后 Pb 4.40 544.55 540.20 Zn 475.53 268.43 23.49 Mn 45.97 215.12 206.15 Cu 20.60 174.40 153.70 Cr 0.07 45.32 45.26 Cd 0.31 3.93 3.64 Mo 0.53 44.01 43.52 As 1.68 540.11 538.58 注:反应前后重金属质量分数数据为水溶性、吸附和可交换态及易还原矿物相中重金属之和。 -
[1] 周永章, 付善明, 张澄博, 等. 华南地区含硫化物金属矿山生态环境中的重金属元素地球化学迁移模型——重点对粤北大宝山铁铜多金属矿山的观察[J]. 地学前缘, 2008, 15(5): 248-255. doi: 10.3321/j.issn:1005-2321.2008.05.027 [2] VITHANA C L, SULLIVAN L A, BURTON E D, et al. Stability of schwertmannite and jarosite in an acidic landscape: Prolonged field incubation[J]. Geoderma:A Global Journal of Soil Science, 2015: 239-240. doi: 10.1016/j.geoderma.2014.09.022 [3] GAGLIANO W B, BRILL M R, BIGHAM J M, et al. Chemistry and mineralogy of ochreous sediments in a constructed mine drainage wetland 1 1 Associate editor: P. A. Maurice[J]. Geochimica et Cosmochimica Acta, 2003, 68(9): 2119-2128. [4] QU L, XIE Y Y, LU G N, et al. Distribution, fractionation, and contamination assessment of heavy metals in paddy soil related to acid mine drainage[J]. Paddy and Water Environment, 2017, 15(3): 553-562. doi: 10.1007/s10333-016-0572-9 [5] ZHUANG P. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health[J]. Environmental Geochemistry & Health, 2009, 31(31): 707-715. [6] XIE Y Y, LU G N, YANG C F, et al. Mineralogical characteristics of sediments and heavy metal mobilization along a river watershed affected by acid mine drainage[J]. PloS one, 2018, 13(1): e0190010. doi: 10.1371/journal.pone.0190010 [7] CHEN M Q, Lu G N, GUO C L, et al. Sulfate migration in a river affected by acid mine drainage from the Dabaoshan mining area, South China.[J]. Chemosphere, 2015, 119: 734-743. doi: 10.1016/j.chemosphere.2014.07.094 [8] 邹琦, 陈莹, 刘奇缘, 等 广东大宝山铁龙AMD中赭色沉积物的含铁次生矿物研究[J]. 高校地质学报, 2017, 23(3): 442-451. [9] 周立祥. 酸性矿山废水中生物成因次生高铁矿物的形成及环境工程意义[J]. 地学前缘, 2008, 15(6): 74-82. doi: 10.3321/j.issn:1005-2321.2008.06.010 [10] PAIKARAY S, PEIFFER S. Dissolution kinetics of sulfate from schwertmannite under variable pH conditions[J]. Mine Water and the Environment, 2010, 29(4): 263-269. doi: 10.1007/s10230-010-0118-0 [11] BURTON E D, BUSH R T, SULLIVAN L A, et al. Schwertmannite transformation to goethite via the Fe(II) pathway: Reaction rates and implications for iron–sulfide formation[J]. Geochimica et Cosmochimica Acta, 2008, 72(18): 4551-4564. doi: 10.1016/j.gca.2008.06.019 [12] FRIERDICH A J, CATALANO J G. Controls on Fe(II)-activated trace element release from goethite and hematite[J]. Environmental science & technology, 2012, 46(3): 1519-1526. [13] XIE Y Y, LU G N, YE H, et al. Fulvic acid induced the liberation of chromium from CrO42−-substituted schwertmannite[J]. Chemical Geology, 2017, 475: 52-61. doi: 10.1016/j.chemgeo.2017.10.031 [14] BURTON E D, JOHNSTON S G, WATLING K, et al. Arsenic effects and behavior in association with the Fe(II)-catalyzed transformation of schwertmannite[J]. Environmental science & technology, 2010, 44(6): 2016-2021. [15] ACERO P, AYORA C, TORRENTÓ C, et al. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite - ScienceDirect[J]. Geochimica et Cosmochimica Acta, 2006, 70(16): 4130-4139. doi: 10.1016/j.gca.2006.06.1367 [16] LI J F, XIE Y Y, LU G N, et al. Effect of Cu(II) on the stability of oxyanion-substituted schwertmannite[J]. Environmental Science and Pollution Research, 2018, 25(16): 15492-15506. doi: 10.1007/s11356-018-1773-0 [17] BAO Y P, GUO C L, LU G N, et al. Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine [J]. The Science of the total environment. 2018, 616-617: 647-657. [18] HUANG F G, JIA S Y, LIU Y, et al. Reductive dissolution of ferrihydrite with the release of As(V) in the presence of dissolved S(-II)[J]. Journal of Hazardous Materials, 2015, 286: 291-297. doi: 10.1016/j.chemgeo.2015.11.020 [19] PEIFFER S, BEHRENDS T, HELLIGE K, et al. Pyrite formation and mineral transformation pathways upon sulfidation of ferric hydroxides depend on mineral type and sulfide concentration[J]. Chemical Geology, 2015, 400: 44-55. doi: 10.1016/j.chemgeo.2015.01.023 [20] KUMAR N, PACHECO J L, VINCENT N, et al. Sulfidation Mechanisms of Fe(III)-(oxyhydr) oxide Nanoparticles: A Spectroscopic Study[J]. Environmental Science:Nano, 2018, 5(4): 1012-1026. doi: 10.1039/C7EN01109A [21] CAPPUYNS V, ALIAN V, VASSILIEVA E, et al. pH dependent leaching behavior of Zn, Cd, Pb, Cu and As from mining wastes and slags: Kinetics and mineralogical control[J]. Waste and Biomass Valorization, 2014, 5(3): 355-368. doi: 10.1007/s12649-013-9274-3 [22] 魏焕鹏, 党志, 易筱筠, 等. 大宝山矿区水体和沉积物中重金属的污染评价[J]. 环境工程学报, 2011, 5(9): 1943-1949. [23] 陈梅芹. 酸根在金属硫化物矿区AMD污染河流中的迁移过程及其作用机制[D]. 广州: 华南理工大学, 2015. [24] 郑顺安, 郑向群, 张铁亮, 等. 污染紫色土重金属的淋溶特征及释放动力学研究[J]. 水土保持学报, 2011, 25(4): 253-256. doi: 10.13870/j.cnki.stbcxb.2011.04.003 [25] JIN Z S, LIU T Z, YANG Y G, et al. Leaching of cadmium, chromium, copper, lead, and zinc from two slag dumps with different environmental exposure periods under dynamic acidic condition[J]. Ecotoxicology and environmental safety, 2014, 104: 43-50. doi: 10.1016/j.ecoenv.2014.02.003 [26] 林美群, 马少健, 王桂芳, 等. 环境因素对硫化矿尾矿重金属溶出影响的模拟试验[J]. 金属矿山, 2008(06): 108-111. doi: 10.3321/j.issn:1001-1250.2008.06.030 [27] POULTON S W. Sulfide oxidation and iron dissolution kinetics during the reaction of dissolved sulfide with ferrihydrite[J]. Chemical Geology, 2003, 202(1/2): 79-94. [28] 刘玲, 刘海卿, 韩亮, 等. 铬渣动态淋滤重金属铬溶解释放规律研究[J]. 地球与环境, 2016, 44(5): 581-585. doi: 10.14050/j.cnki.1672-9250.2016.05.014 [29] GAO Y, HE J, LING W, et al. Effects of organic acids on copper and cadmium desorption from contaminated soils[J]. Environment International, 2003, 29(5): 613-618. doi: 10.1016/S0160-4120(03)00048-5 [30] CHIEN S H, CLAYTON W R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils 1[J]. Soil Science Society of America Journal, 1980, 44(2): 265-268. doi: 10.2136/sssaj1980.03615995004400020013x [31] FONSECA B, MAIO H, QUINTALAS C, et al. Retention of Cr(VI) and Pb(II) on a loamy sand soil[J]. Chemical Engineering Journal, 2009, 152(1): 212-219. doi: 10.1016/j.cej.2009.04.045 [32] AZZALI E, MARESCOTTI P, FRAU F, et al. Mineralogical and chemical variations of ochreous precipitates from acid sulphate waters (asw) at the Roia Montan gold mine (Romania)[J]. Environmental Earth Sciences, 2014, 72(9): 3567-3584. doi: 10.1007/s12665-014-3264-z [33] 曹云全, 张双圣, 刘汉湖, 等. 煤矸石中重金属动态淋滤和静态浸泡溶出特征研究[J]. 河北工程大学学报(自然科学版), 2010, 27(1): 76-80. [34] ZHANG S L, JIA S Y, YU B, et al. Sulfidization of As(V)-containing schwertmannite and its impact on arsenic mobilization[J]. Chemical Geology, 2016, 420: 270-279. -




 下载:
下载: