-
我国部分地区农田土壤中的重金属累积量远远高于土壤背景值,对农产品安全和人群健康构成了严重危害[1-2]。通过降低农田土壤重金属的生物有效性,从而减少重金属通过农作物吸收及食物链进入人畜体内一直是有效、廉价的治理途径之一[3]。有研究表明,磷肥中的磷酸盐对土壤重金属具有较好的钝化效果[4-5]。但是,为了增加土壤重金属的钝化效果往往会过量施用磷肥,从而造成磷在农田土壤不同深度上的累积和对地下水的污染[6-8]。
目前,有关磷肥最佳施用量的研究结果存在一定的分歧。一些研究者认为,磷与重金属的摩尔比为3∶5较为合适[9-10],而另一些研究者认为,磷与重金属的摩尔比为4∶1较好[11-12]。有研究发现,磷肥加入单一Pb、Cu、Zn污染土壤时,均会使Pb、Cu、Zn的有效性降低[13-14];而向Pb、Cu、Zn复合污染土壤中添加磷肥时,则会使Pb的有效性降低,Cu、Zn的有效性略微增加[15]。施尧等[16]运用水溶性磷肥重过磷酸钙和难溶性磷肥磷灰石矿尾料(施磷量7 g·kg−1)钝化修复Pb、Cu和Zn复合污染土壤,发现重过磷酸钙相比于磷灰石矿尾料对重金属的钝化效果较好,但重过磷酸钙相比于磷灰石矿尾料更易导致下层土壤中磷的质量分数升高。姚臻晖等[17]在田间实验中施用钙镁磷肥(750 kg·hm−2)使农田土壤重金属Cd的钝化率达到46.90%,当磷肥施用量增加到1 500 kg·hm−2时,对Cd的钝化率仅为54.50%,且随着磷肥施用量的成倍增加而显著增加了土壤60~90 cm深度渗滤水中磷的质量浓度。陈世宝等[18]通过土柱淋溶实验,分别向污染土壤中施加5 g·kg−1不同溶解性的磷肥,会使土壤有效态Pb质量分数下降81.10%~89.70%;同时,水溶性磷肥相较于枸溶性磷肥也更易导致土壤表层以下(<70 cm)有效磷和全磷质量分数增加。MIGNARDI等[19]发现,磷肥能有效降低重金属复合污染土壤中的重金属有效性,但施用高溶解性磷肥也可能造成水体富营养化的风险。可见,施用不同溶解性磷肥及不同用量治理重金属或多种重金属复合污染时,重金属钝化效果和磷肥迁移流失风险存在较大关系。因此,迫切需要理清不同溶解性磷肥及用量对复合污染土壤中不同重金属的钝化效果和磷肥的迁移流失风险,并结合磷肥的钝化效果和磷肥的迁移流失风险合理选择磷肥的类型和用量。
根据前人通过不同磷肥钝化土壤重金属效果的研究[12-13],本研究选取2种溶解性不同且钝化效果较好的常用磷肥作为钝化剂,通过土培实验和土柱淋溶实验,研究其不同施用量对Pb、Cd、Cu、Zn复合污染土壤中不同重金属的钝化效果,并对水溶性、枸溶性磷肥的重金属钝化性能与迁移流失特征之间可能存在的相关性进行探讨,以期为施用磷肥修复重金属污染土壤及其二次污染防控提供参考。
-
供试土壤(潴育型水稻土)采自安徽铜陵某矿区周边水稻田,经过自然风干、去除石子残渣、过2 mm筛备用。按照标准方法[20]测定土壤基本理化性质(表1)。供试磷肥磷酸二氢钾(KH2PO4)为西陇公司分析纯试剂,钙镁磷肥为云南瑞磷丰化工有限公司商品性磷肥,基本性质见表2。
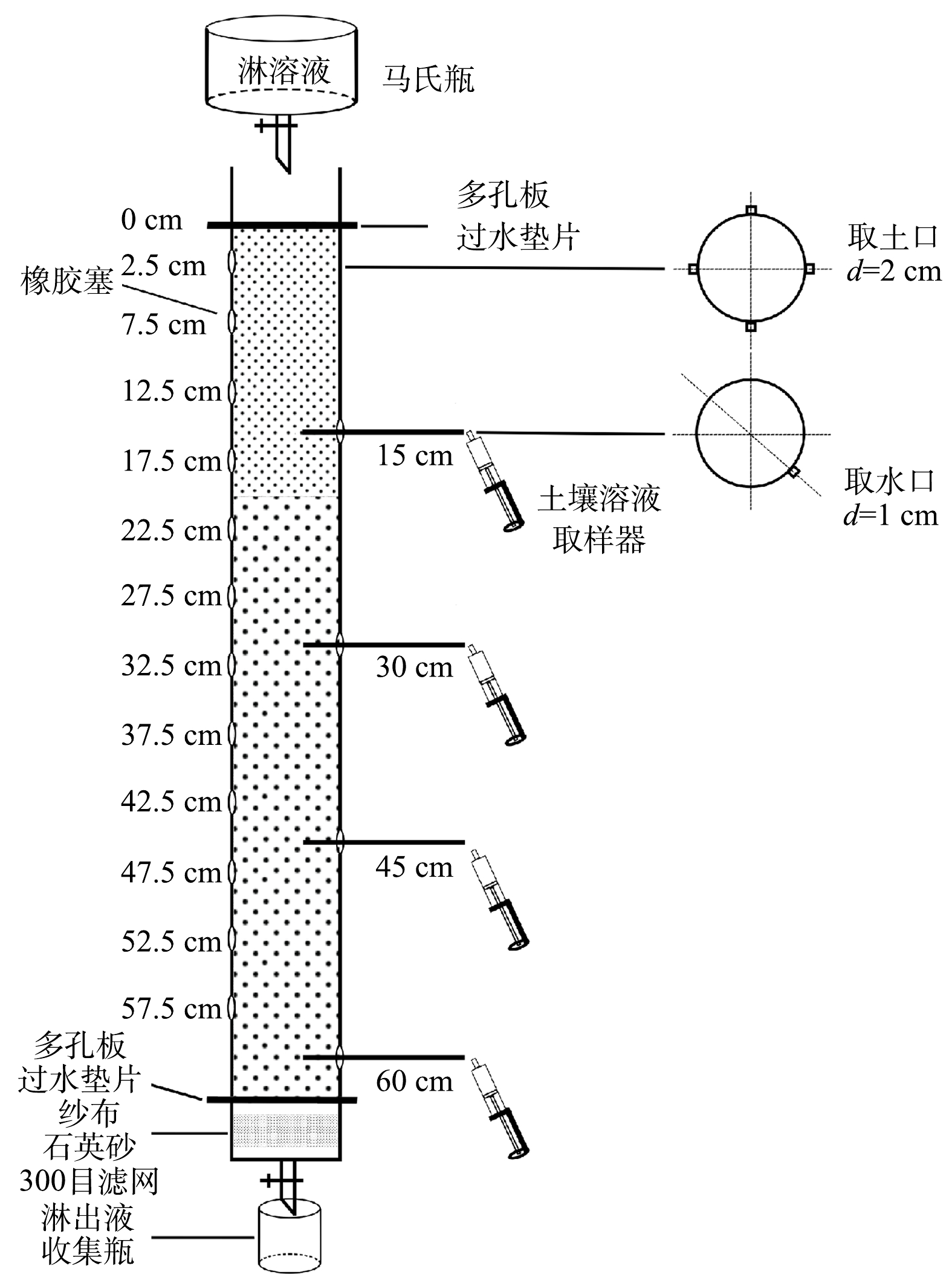
土柱实验装置如图1,马氏瓶和土柱采用亚克力有机玻璃制作,淋溶液通过塑料导管由马氏瓶淋溶进入土柱上部,土柱整体高75 cm、内径8 cm、壁厚0.5 cm,主体部分65 cm(填装土层),上下分别为高5 cm延长部分,通过多孔板和过水垫片连接。底部延长部分装有纱布、石英砂(酸洗)和300目滤网。土柱主体部分自上部2.5 cm处往下每隔5 cm位置1周4个方向设直径2 cm取土口,合计48个,用橡胶软塞塞住。另在柱体15、30、45和60 cm深度(与取土孔成45°夹角)设4个直径1 cm取水口,分别安装Rhizon土壤溶液取样器[21],底部放有收集瓶。
-
本实验分为土壤培养和土柱淋溶2部分,钙镁磷肥(CMP)和磷酸二氢钾(MPP)以5种施磷水平n(P) / n(Cd + Pb + Cu + Zn)摩尔比为0、0.5∶1、1∶1、2∶1和4∶1加入污染土壤(分别加入0、1、2、4和8 g·kg−1,以每kg土中P2O5质量分数计算,计为CK、CMP0.5、CMP1、CMP2、CMP4、MPP0.5、MPP1、MPP2、MPP4),共计9种处理。
土培实验。分别称取污染土壤500 g,加水至最大田间持水量(24%)的60%(称重法),搅拌均匀后放入塑料碗中,温室25 ℃培养14 d,再加入不同施用量的磷肥,每种处理设3个重复。其中,MPP是以水溶液的形式加入,CMP是以粉末形式加入(再加入与MPP等量的水),经过人工反复搓土使其充分拌匀[14]。加盖并留有透气孔,在温室25 ℃下培养。期间采用称重法对土壤进行加水,保持土壤含水量稳定,加入磷肥90 d后,对土样进行取样,自然风干、磨细,全部过筛,待测。
土柱淋溶实验。首先,在每个土柱底部装上45 cm污染土壤,再在上部分别装上20 cm未添加磷肥(作为对照)和添加不同磷肥及用量的污染土壤(模拟农田耕作施肥),在填装过程中均匀填压土柱四周的土壤。为使土壤达到田间持水量,装土完成后将土柱下部放置于去离子水溶液中,并维持饱和状态7 d,以清除气泡并保持化学平衡,之后垂直放在淋溶架上,待土柱中多余的水滴完后开始进行淋溶。采用合成降水浸出液(Synthetic Precipitation Leaching Procedure, SPLP)[22]来模拟雨水淋溶,按浓硫酸和浓硝酸(均为分析纯)重量比60∶40混合得到储备液,再经过适当稀释得到淋溶液的pH在5.60±0.05(模拟雨水)。实验共分4个周期淋溶,每1周期7 d淋溶1.2 L(安徽铜陵年降雨量为1 400 mm左右,地表径流量约为30%,根据土柱截面积计算出年淋溶量约为4.8 L,设计淋溶总量为4.8 L,近似于1年降水量[23]),采用间歇淋溶模式,每天8:00到20:00淋溶,将淋溶液从土柱上方加入,向下淋溶,待1个周期淋完,进行土样和水样采集,共采集4次。土样每列有12个取样口,共4个方向,分别从2.5和7.5 cm上下左右相邻的4个取土孔取样混合,作为5 cm处土样,另外4个取土孔土样混合作为平行样,同理可得15、25、35、45和55 cm处土样,自然风干、磨细,过筛,待测。水样通过Rhizon土壤溶液取样器取25 mL水溶液(连续取2次做平行样),底部水样通过收集瓶收集(每淋溶1.2 L收集1次),待测。
-
土壤pH采用《土壤pH的测定》[20],水土质量比2.5∶1浸提,pH计(pHS-3C雷磁)测定;有机质质量分数采用《土壤有机质的测定》[20],过量重络酸钾-硫酸溶液氧化,硫酸亚铁标准溶液滴定法测定;有效磷质量分数采用《土壤有效磷的测定》[20],氟化铵-盐酸溶液浸提,钼锑抗比色法(UV9000元析,下同)测定;全磷质量分数采用《土壤全磷测定法》[20],NaOH熔融-钼锑抗比色法测定;重金属铅、镉有效态质量分数采用《有效态铅和镉的测定》[20],称取5.00 g土壤,加入25.00mL DTPA(二乙三胺五乙酸)提取,火焰原子吸收(NovAA300 安捷伦,下同)法测定;重金属铜、锌有效态质量分数采用《土壤有效态锌、锰、铁、铜含量的测定》[20],称取10.00 g土壤,加入20.00 mL DTPA提取,火焰原子吸收法测定;全量重金属铅、镉质量分数采用《土壤质量 铅、镉的测定》[20],四酸消解,火焰原子吸收法测定;全量重金属铜、锌质量分数采用《土壤和沉积物铜、锌、铅、镍、铬的测定》[20],四酸消解,火焰原子吸收法测定;磷肥中磷的质量分数采用《复混肥料中有效磷含量的测定》[24],2%柠檬酸提取,钼锑抗比色法测定;溶液总磷质量浓度采用《水质总磷的测定》[24],过硫酸钾氧化-钼酸铵分光光度法测定。
-
实验数据处理分析采用Excel 2010和SPSS 26.0,处理之间的显著检验是采用LSD法和Duncan检验相结合,差异显著性水平皆为5%显著性水平,图表制作采用Photo shop 2019和Origin 2018。
-
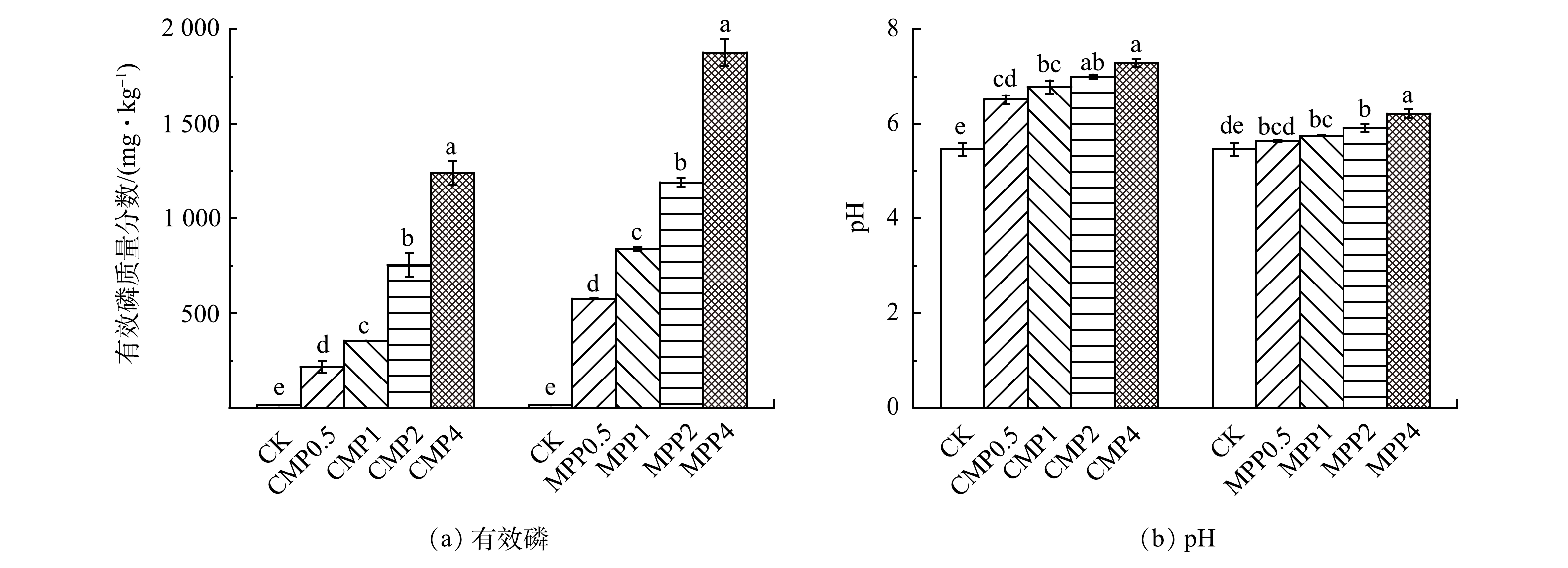
施加不同磷肥及用量培养90 d后,土壤有效磷质量分数、pH均随着磷肥施用量的增加而增加。相同磷肥施加水平下,土壤有效磷质量分数MPP均大于CMP(图2(a))。这可能是由于MPP是水溶性磷肥且是纯P试剂,而CMP是商品性磷肥,水溶性P质量分数较低导致的[25]。这与周佚群等[12]研究的4种磷肥加入污染土壤,其中相同施加水平下MPP使土壤有效磷质量分数增加幅度最大的结果类似。相同磷肥施加水平下,CMP使pH增加幅度比MPP更大(图2(b))。这是因为,CMP含有较多的CaCO3使其具有碱性特征,而MPP是强碱弱酸盐,在土壤中主要以H2PO4−离子形式存在,会与土壤中OH−发生交换解吸,释放吸附在土壤胶体上的OH−从而增加土壤pH[25]。
-
2种磷肥施加土壤90 d后对4种重金属的钝化效果见图3。从图3(a)、图3(b)可以看出,CMP和MPP均能有效降低土壤中Pb、Cd的有效态质量分数,且对Pb的效果好于Cd。与Pb、Cd不同的是,CMP和MPP对Cu、Zn的钝化效果不好,甚至导致了它们的活化(图3(c)、图3(d))。磷肥对Pb、Cd钝化效果较好的原因可能是,磷肥中的磷酸盐与土壤中的硫酸铅、碳酸铅等发生反应生成更加稳定的磷氯铅矿。同时,磷酸盐对土壤中Cd的吸附能促进两者之间发生配位螯合作用,生成难溶性金属矿物,从而显著降低Pb、Cd的生物有效性[26]。而且,磷酸盐对Pb具有较强的亲和性,通常会优先生成磷酸铅沉淀[27],故导致Pb的钝化效果好于Cd。而磷肥导致Cu、Zn活化的原因可能是,磷肥对Cu和Zn的稳定分别仅有25%Cu和5%Zn形成了磷酸盐沉淀,磷酸盐对Cu、Zn的钝化机理主要依靠表面吸附和络合作用[28-29]。而且,有研究指出,土壤中含有复合重金属Pb、Cu、Zn时,会使Pb、Cu、Zn的吸附容量分别下降15.20%、48.30%和75.60%,使重金属之间出现竞争性吸附,从而导致Cu、Zn吸附量减少[29]。同时,磷肥的添加会使土壤pH增加(图2(b)),土壤pH>6会导致Cu的解离[30]。另外,磷肥带入的钾离子(MPP)、钙离子、镁离子(CMP)等阳离子与重金属离子竞争吸附位点,从而抑制土壤对重金属的吸附,甚至置换解离出重金属离子导致活化[31-32]。因此,导致复合污染土壤中2种磷肥对Pb的钝化效果最好,Cd次之,Cu、Zn反而活化的原因可能是,磷肥对Pb、Cd是与磷酸盐结合量超过与钾、钙等阳离子的解离量,而Cu、Zn则是由于磷酸盐与其反应生成的磷酸盐沉淀较少(25%Cu、5%Zn),Pb和Cd的存在使其吸附容量显著降低(48.3%Cu、75.6%Zn)、土壤pH升高、钾钙等阳离子的解离等多种原因导致,使得Cu、Zn解离量超过与磷酸盐的结合量。魏晓欣等[33]采用磷酸盐钝化修复Pb、Cd、Cu、Zn复合污染土壤时,也出现了对有效态Cd、Pb钝化效果明显,而对有效态Cu、Zn钝化效果不明显甚至活化的现象。
磷肥用量成倍递增时,Pb、Cd有效态质量分数降低的相对幅度并不显著,仅不同用量下MPP对Pb的钝化差异较为显著,而其它各处理对Pb或Cd的钝化效果差异甚微。同时,磷肥用量的增加也会导致Cu、Zn活化风险的增加。通过不同磷肥添加量对复合污染土壤Pb、Cd、Cu、Zn的钝化效果对比,发现枸溶性磷肥CMP与重金属的摩尔比为0.5、水溶性磷肥MPP与重金属的摩尔比为1时,能实现对Pb、Cd具有较好的钝化效果,同时也能有效降低Cu、Zn的活化风险。
MPP对Pb、Cd的钝化效果要好于CMP,同时也更易造成Cu、Zn的活化。这主要是因为,MPP有效成分更高(图2(a)),水溶性也更强,使得加入土壤中释放的磷酸根、钾离子(MPP)的量相较于CMP加入土壤中释放的磷酸根、钙离子、镁离子的量更多,故导致更多的磷酸根与Pb、Cd反应,从而降低了Pb、Cd的有效性。然而,更多的钾离子与Cu、Zn竞争吸附位点,抑制了土壤对重金属的吸附,甚至置换出更多Cu、Zn,最终使得MPP对Pb、Cd的钝化效果更好,同时导致了Cu、Zn活化风险更大。有研究指出,向污染土壤中施加MPP会释放大量钾离子,使土壤对Zn的吸附能力降低,同时,钾离子能够与重金属之间竞争土壤吸附位点,从而导致土壤对Zn的吸附能力减小,使土壤中Zn活化[34-35]。
-
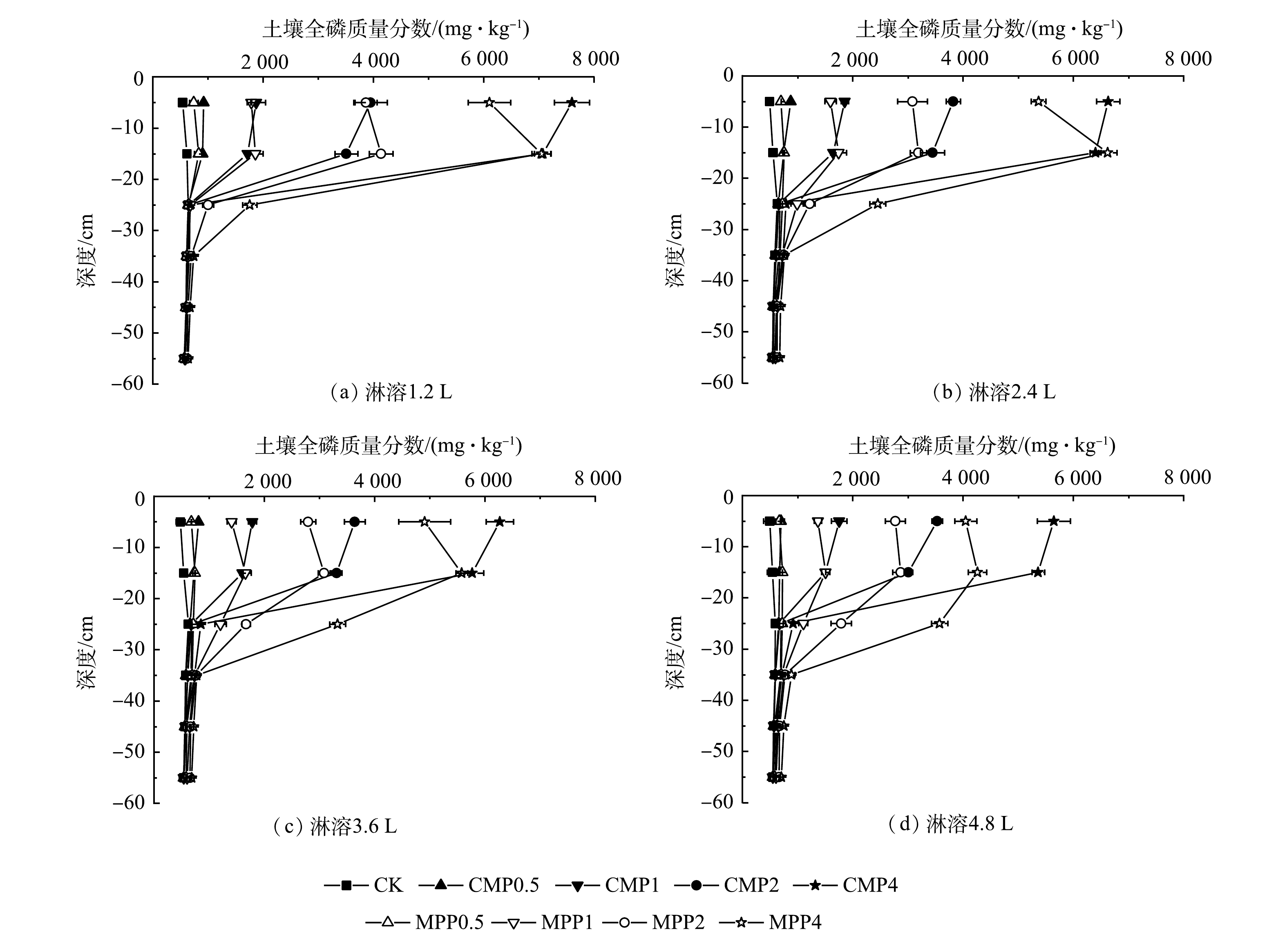
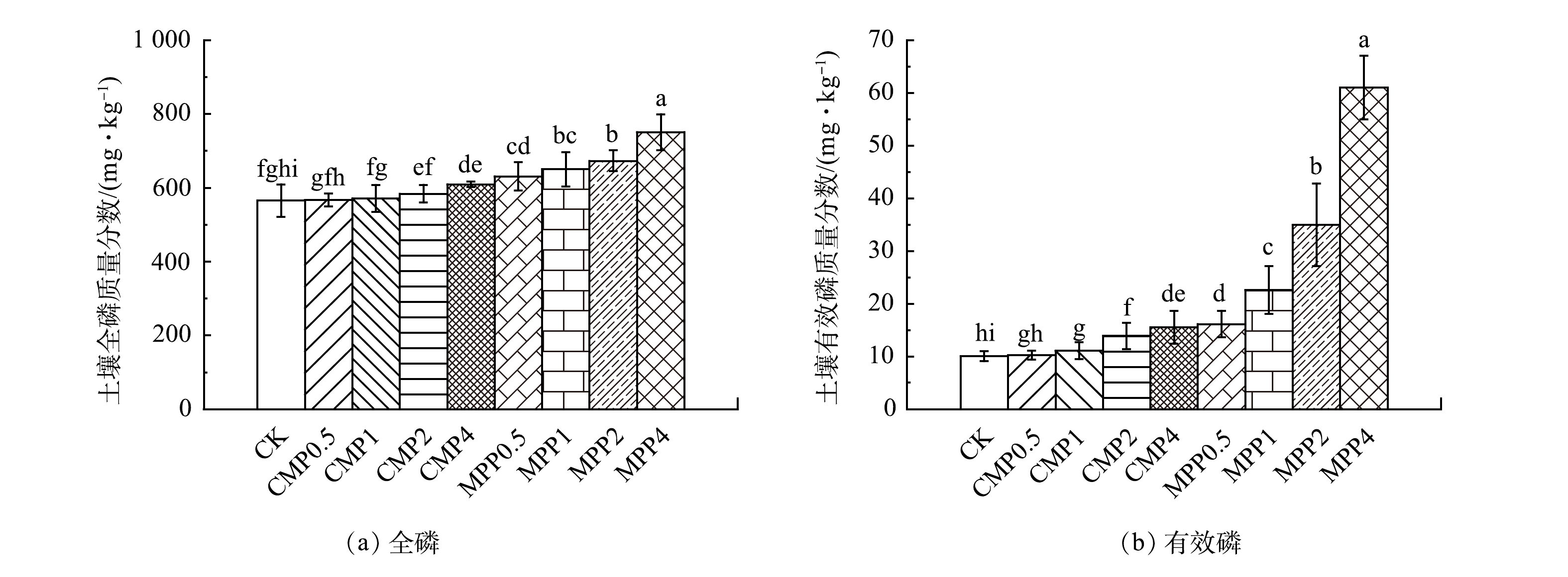
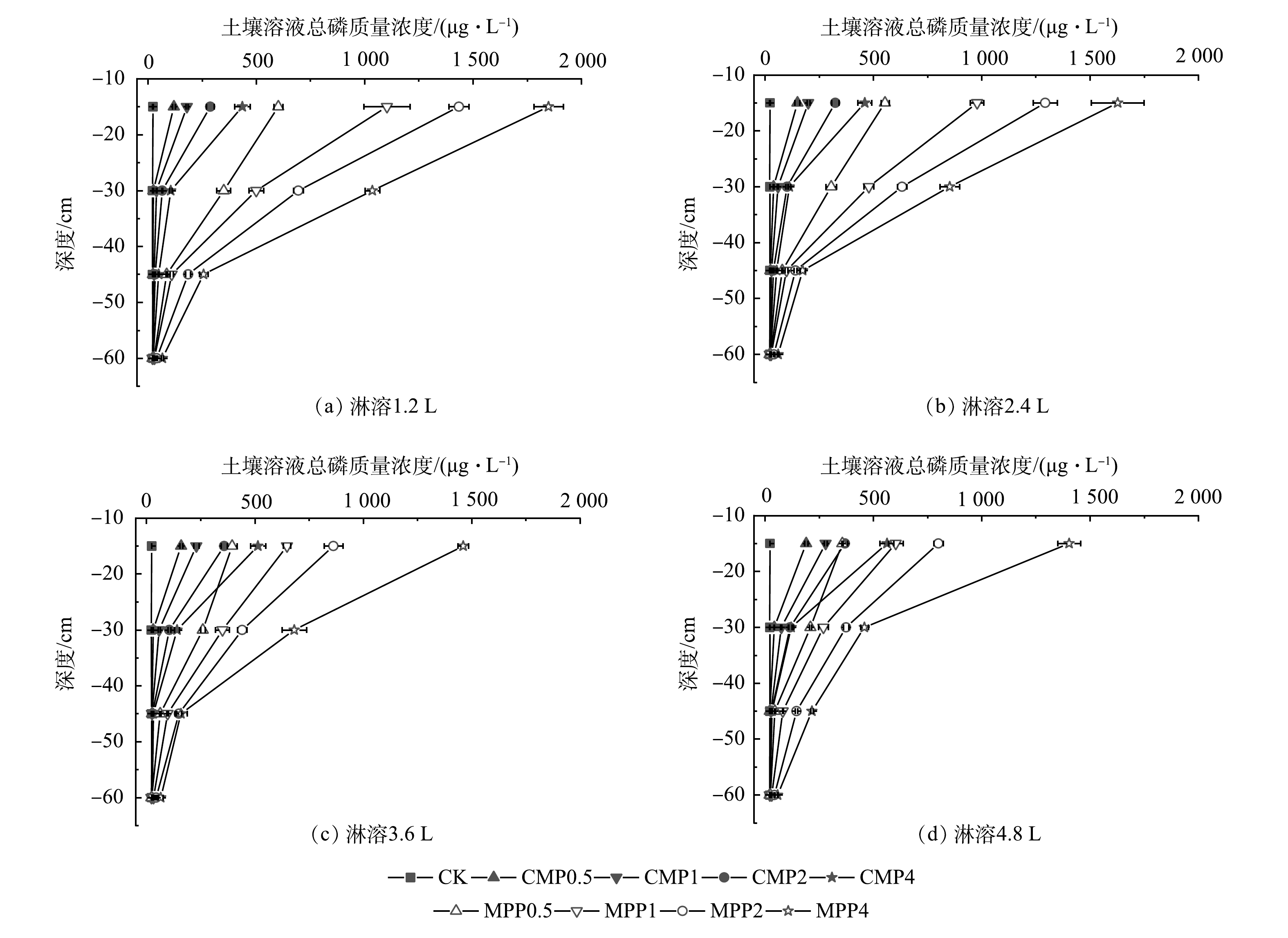
不同磷肥处理在不同淋溶量下对土壤不同深度全磷、有效磷质量分数的影响见图4、图5。从图4、图5可以看出,土柱淋溶过程中,各磷肥处理组表层20 cm土壤全磷和有效磷均呈现往下层迁移的趋势,20~65 cm土层全磷和有效磷质量分数均缓慢升高。其中,20~35 cm土层全磷和有效磷质量分数显著增加。从土壤不同深度全磷和有效磷质量分数的对比中发现,以淋溶4.8 L为例(图4(d),图5(d)),在20~65 cm土层,相同磷肥施加水平下MPP各处理使土壤全磷和有效磷质量分数均远大于CMP。这说明,向土壤表层添加磷肥,会使土壤表层以下全磷和有效磷质量分数随土壤剖面深度的增加而显著下降。这是因为,磷肥受到自身体积、重力和土壤本身的阻力等综合效应,使磷肥向土壤下层迁移效率降低,从而导致土壤中磷的质量分数随着深度的增加而显著降低[36]。MPP相比于CMP会使更多的磷肥随着淋溶液向土壤下层迁移累积。这可能是因为,MPP是水溶性磷肥,通过淋溶液向土壤下层迁移的有效磷和全磷量更多,而CMP属于枸溶性磷肥,淋溶液的加入仅使少量的磷向下迁移,故导致20~65 cm处全磷和有效磷质量分数相较于MPP较低,甚至55 cm深度土壤全磷和有效磷质量分数与对照组无显著差异。这与陈世宝等[18]研究的土壤全磷和有效磷质量分数随土壤剖面深度的增加累积量大幅下降。其中,水溶性磷肥累积量明显大于枸溶性磷肥的规律类似。
图6为淋溶4.8 L深度45 cm处不同磷肥处理组土壤全磷、有效磷质量分数图。从图6中可以看出,随着磷肥施加量的成倍增加,土壤不同深度全磷和有效磷质量分数均出现不同程度的增加,且MPP在各施磷水平下土壤全磷和有效磷质量分数均显著大于CMP。可见,与CMP相比,成倍施用MPP更易导致磷迁移至土壤下层并大量累积。
-
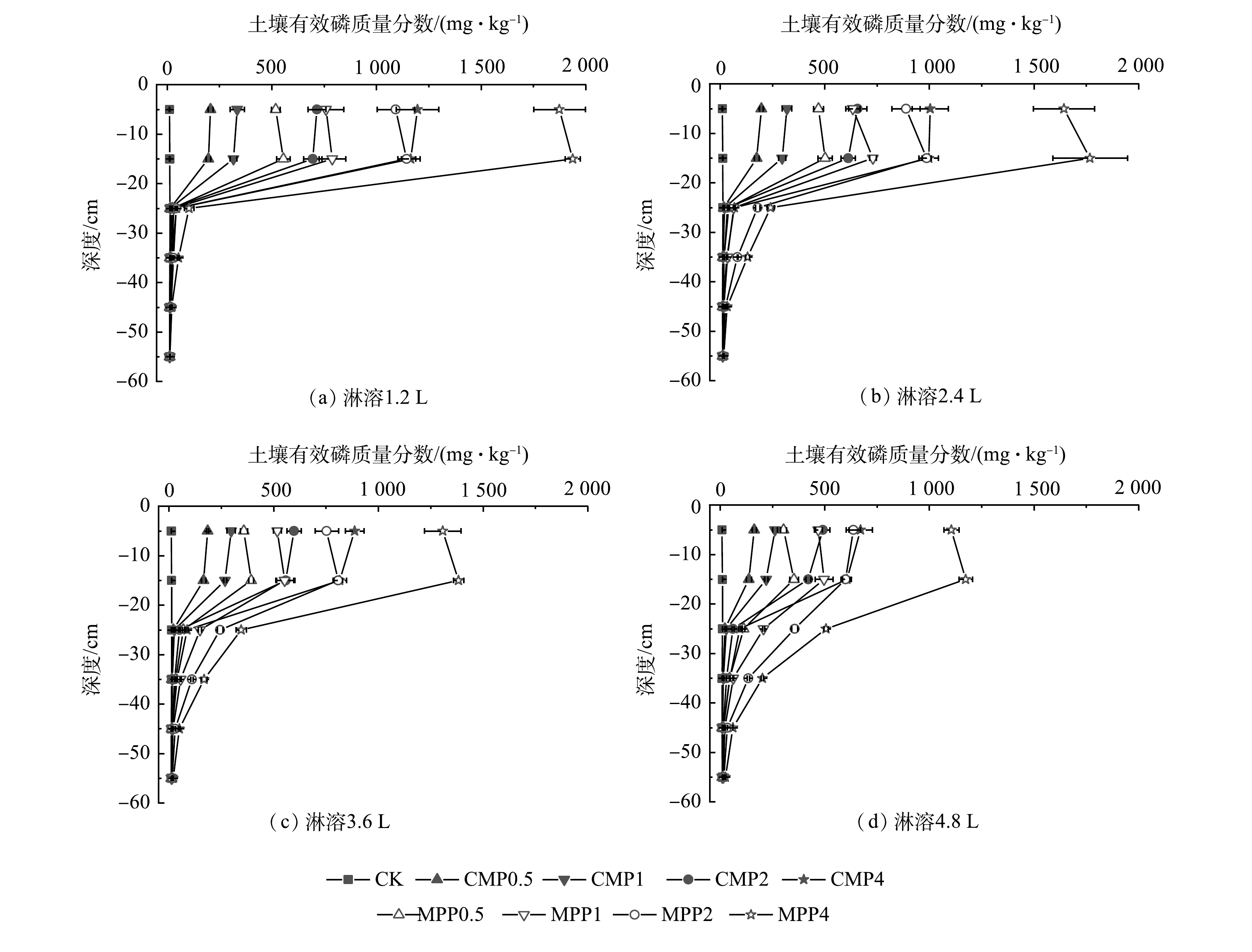
不同磷肥处理在不同淋溶量下对土壤不同深度溶液总磷质量浓度影响结果如图7所示,结果显示,各施磷肥处理组均随着深度的增加,土壤溶液总磷质量浓度显著下降。同时,MPP各处理使土壤总磷质量浓度均显著高于CMP各处理。这说明,施用MPP更容易造成磷淋溶损失,其潜在的地下水污染风险更大。
图8为淋溶4.8 L深度45 cm处不同磷肥处理组土壤溶液总磷质量浓度变化。可见,随着磷肥施加水平的增加,土壤不同深度溶液总磷质量浓度均出现不同程度的增加。这进一步说明,随着磷肥施加水平的增加,施用MPP更易导致磷淋溶损失,地下水二次污染风险更大。李学平等[37]亦发现,磷肥随着施磷水平的增加,会导致酸性土壤不同深度渗滤液总磷质量浓度显著增加,且对地下水产生潜在风险更大。
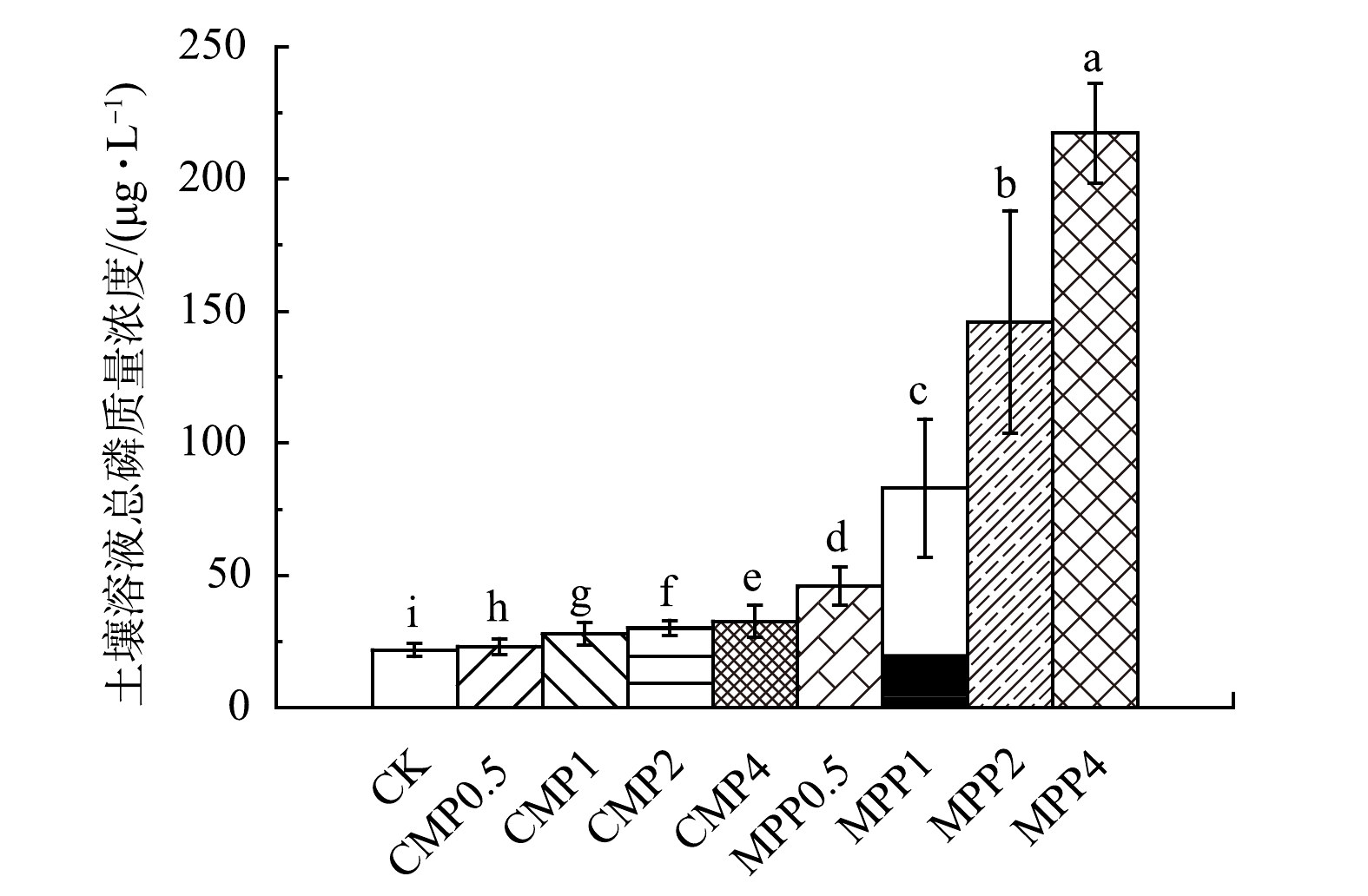
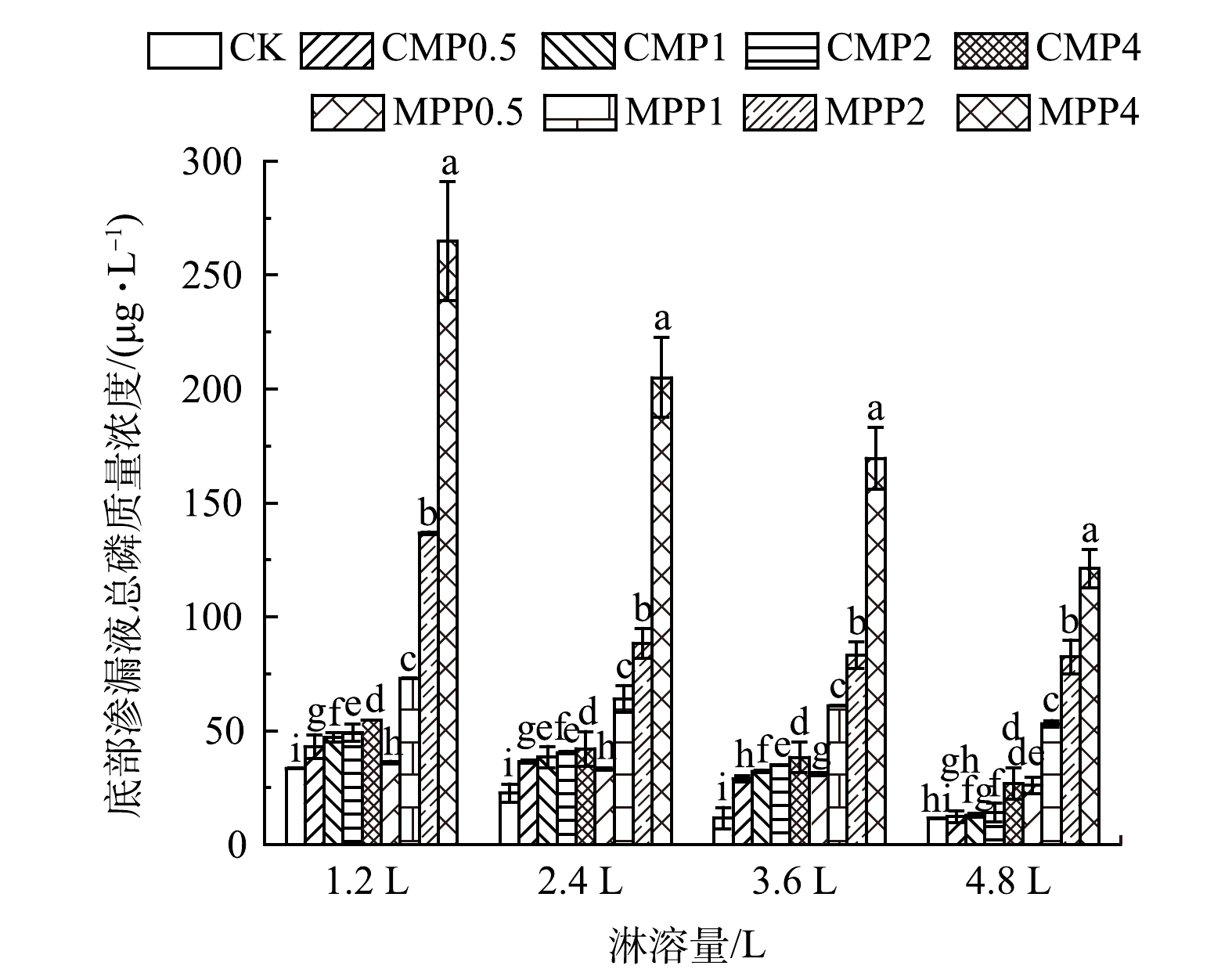
图9为不同磷肥处理土壤底部渗滤液总磷平均质量浓度(每次淋溶1.2 L收集),各磷肥处理均使土壤底部渗滤液总磷平均质量浓度出现不同程度的增加,且随着淋溶量1.2 L到4.8 L的增加而减少。这是因为,淋溶前期土壤磷会形成优势流[38],即淋溶前期淋溶液在土壤中流失会使土壤产生相应的孔隙,磷会随着孔隙较容易向土壤下层流失;同时,在实验前期施加的磷肥可能还未被土壤胶体完全固定,导致大部分向下淋溶。这两方面原因使得土壤底部渗滤液在淋溶前期总磷质量浓度较高。各施磷处理均使土壤底部渗滤液总磷质量浓度增加。其中,MPP1、MPP2、MPP4各处理相比于对照组显著增加,MPP0.5、CMP0.5、CMP1、CMP2、CMP4仅出现少量增加。这说明,水溶性磷肥MPP施加量超过2 g·kg−1容易造成土壤磷肥向65 cm深度的大量流失,CMP施加量低于8 g·kg−1不易造成土壤磷肥向65 cm以下的大量流失。JIN等[39]采用不溶性磷肥磷矿粉和可溶性磷肥MPP钝化土壤重金属Pb,也出现了不溶性磷肥不易造成磷向土壤下层累积,而可溶性磷肥MPP随着用量的增加会显著增加土壤下层磷累积的现象。
综上所述,磷肥用于修复复合重金属污染土壤应综合重金属类型、钝化效果以及潜在的磷迁移流失风险,选择适当的磷肥种类和用量。结合磷肥对重金属的钝化效果(图3)与底部渗滤液总磷质量浓度的研究结果(图9)可知,CMP与重金属的摩尔比为0.5、MPP与重金属的摩尔比为1,对Pb、Cd、Cu、Zn复合污染土壤整体钝化效果较好;MPP施加量超过2 g·kg−1容易造成磷肥向土壤65 cm深度的大量流失;CMP施加量低于8 g·kg−1不易造成磷肥向土壤65 cm以下的大量流失。因此,建议水溶性磷肥(MPP)与重金属摩尔比为1且施磷量控制在2 g·kg−1以下,枸溶性磷肥(CMP)与重金属摩尔比为0.5且施磷量控制在8 g·kg−1以下施用较为合理。
-
1) CMP和MPP施加土壤90 d后,Pb的钝化效果均较好,Cd次之,Cu、Zn存在活化现象,磷肥更适用于Pb、Cd污染土壤修复。
2)水溶性磷肥(MPP)对Pb、Cd的钝化效果优于枸溶性磷肥(CMP),但水溶性磷肥在土壤中释放的有效磷质量分数更多,向土壤下层迁移性也更强,更易导致磷肥迁移流失。而且,磷肥的成倍施用并不能显著提高Pb、Cd钝化效率,反而会增加Cu、Zn活化、磷肥迁移流失的风险。
3)各磷肥处理使土壤全磷、有效磷质量分数和溶液总磷质量浓度都表现出,随着深度(20~65 cm)的增加而不断显著下降和随着施磷水平(1~8 g·kg−1)的增加而不断显著增加的趋势。土壤底部渗滤液总磷平均质量浓度随着淋溶水平(1.2~4.8 L)的增加显著下降。
4)为尽可能实现对Pb、Cd、Cu、Zn复合污染土壤具有较好的钝化效果和减少磷的二次污染,建议水溶性磷肥(MPP)与重金属摩尔比为1且施磷量控制在2 g·kg−1以下,枸溶性磷肥(CMP)与重金属摩尔比为0.5且施磷量控制在8 g·kg−1以下施用较为合适。
磷肥钝化修复重金属污染土壤及其环境风险
Immobilization remediation of heavy metal contaminated soil by phosphate fertilizer and its environmental risk
-
摘要: 磷肥广泛应用于农田重金属污染土壤钝化修复,但不同类型磷肥对多种重金属复合污染土壤的修复效果及其环境风险尚不明确。通过室内土壤培养和土柱淋溶模拟实验,研究枸溶性磷肥-钙镁磷肥(CMP)和水溶性磷肥-磷酸二氢钾(MPP)对重金属Pb、Cd、Cu、Zn复合污染土壤的钝化效果以及磷在土壤剖面中的淋溶损失特征。结果表明,CMP和MPP施用量(以P2O5计)为8 g·kg−1时,Pb、Cd的钝化率分别为35.05%和71.72%、31.76%和40.99%,而Cu、Zn则出现一定程度的活化(最高达29.62%)。MPP对Pb的钝化效果显著优于CMP,但对Cd而言,2者差异不明显,且磷肥用量的成倍增加并不能显著提升钝化效果。土柱中土壤全磷、有效磷和淋溶液总磷的质量分数均随着深度(20~65 cm)递增而显著下降,且在某一深度上随着施磷量(1~8 g·kg−1)的增加而显著升高。MPP在高用量下造成的磷淋溶风险显著大于CMP。采用磷肥钝化修复复合重金属污染土壤应综合重金属类型、钝化效果以及潜在的磷流失风险,选择适当的磷肥种类和用量。本研究结果可为磷肥钝化修复重金属污染土壤提供参考。Abstract: Phosphate fertilizer is widely used in the passivation remediation of heavy metal contaminated soils in farmland. However, the remediation effect and environmental risk of different types of phosphate fertilizer on soil contaminated by multiple heavy metals have not been fully concerned. By indoor soil culture and soil column leaching simulation experiments, the passivation effect of calcium-magnesia phosphate (CMP) (citric soluble) and monopotassium phosphate (MPP) (water soluble) on Pb, Cd, Cu, Zn contaminated soil and the characteristics of phosphorus leaching loss in soil profile were investigated. The results showed that when the application rate of CMP and MPP (measured by P2O5) was 8 g·kg−1, the passivation rates of Pb, Cd were 35.05% and 71.72%, 31.76% and 40.99%, respectively, while Cu and Zn were activated to a certain extent (up to 29.62%). The passivation effect of MPP on Pb was obvious better than that of CMP, but for Cd, the difference was not obvious, and the doubling of the amount of phosphate fertilizer did not obvious improve the passivation effect. The mass fraction of soil total P, available P and total P in leaching solution in the soil column decreased significantly with the increase of depth (20~65 cm), and at a certain depth, with the application of phosphorus (1~8 g·kg−1) increased significantly. The risk of phosphorus leaching caused by MPP at high application rate was obvious higher than that caused by CMP. When using phosphate fertilizer passivation to remediate multiple heavy metal contaminated soil, the types of heavy metals, the passivation effect and the potential risk of phosphorus loss should be synthetically considered, and the appropriate type and amount of phosphate fertilizer should be selected. The results of this study can provide a reference for the remediation of heavy metal contaminated soil by phosphate fertilizer passivation.
-
Key words:
- heavy metal /
- phosphate fertilizer /
- passivation /
- soil column leaching /
- phosphorus migration and loss
-
表 1 土壤基本理化性质
Table 1. Basic physical and chemical properties of soil
pH 有机质/(g·kg−1) 有效磷/(mg·kg−1) 全磷/(g·kg−1) 总Pb/(mg·kg−1) 总Cd/(mg·kg−1) 总Cu/(mg·kg−1) 总Zn/(mg·kg−1) 5.34 32.25 13.49 0.61 533.37 7.99 761.46 707.43 表 2 2种磷肥的性质
Table 2. Properties of two phosphate fertilizers
供试肥料 pH 溶解性 P2O5量 磷酸二氢钾(MPP) 4.64 水溶性 52% 钙镁磷肥(CMP) 8.43 枸溶性 12% -
[1] 环境保护部, 国土资源部. 全国土壤污染状况调查公报[R]. 北京: 环境保护部, 国土资源部, 2014: 8-10. [2] 陈世宝, 王萌, 李杉杉, 等. 中国农田土壤重金属污染防治现状与问题思考[J]. 地学前缘, 2019, 26(6): 35-41. [3] LI C, ZHOU K, QIN W, et al. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques[J]. Soil and Sediment Contamination, 2019, 28(2): 1-15. [4] 武晓微, 翟文珺, 高超, 等. 钝化剂对土壤性质及镉生物有效性的影响研究[J]. 农业环境科学学报, 2021, 40(3): 562-569. doi: 10.11654/jaes.2020-0826 [5] 丁淑芳, 谢正苗, 吴卫红, 等. 含磷物质原位化学钝化重金属污染土壤的研究进展[J]. 安徽农业科学, 2012, 40(35): 17093-17097. doi: 10.3969/j.issn.0517-6611.2012.35.045 [6] VALDEMIR R, JOAN E, MAURICIO R, et al. Effects of land use and seasonality on stream water quality in a small tropical catchment: The headwater of Córrego Água Limpa, São Paulo (Brazil)[J]. Science of the Total Environment, 2018: 622-623. [7] RIBAUDO M O, HEIMLICH R, CLASSEN R, et al. Least-cost management of nonpoint source pollution: source reduction versus interception strategies for controlling nitrogen loss in the Mississippi Basin[J]. Ecological Economics, 2001, 37(2): 183-197. doi: 10.1016/S0921-8009(00)00273-1 [8] 周世伟, 徐明岗. 磷酸盐修复重金属污染土壤的研究进展[J]. 生态学报, 2007, 27(7): 3043-3050. doi: 10.3321/j.issn:1000-0933.2007.07.046 [9] BASTA N T, MCGOWEN S L. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil[J]. Environmental Pollution, 2004, 127(1): 73-82. doi: 10.1016/S0269-7491(03)00250-1 [10] HETTIARACHCHI G M, PIERZYNSKI G M, RANSOM M D. In Situ stabilization of soil lead using phosphorus and manganese oxide[J]. Journal of Environmental Quality, 2001, 30(4): 1214. doi: 10.2134/jeq2001.3041214x [11] CAO X, MA L Q, CHEN M, et al. Impacts of phosphate amendments on lead biogeochemistry at a contaminated site[J]. Environmental Science & Technology, 2002, 36(24): 5296-304. [12] 周佚群, 梁成华, 杜立宇, 等. 不同施磷水平对土壤中重金属镉的钝化效果评价[J]. 水土保持通报, 2014, 34(6): 68-72. [13] 钱海燕, 王兴祥, 黄国勤, 等. 钙镁磷肥和石灰对受Cu Zn污染的菜园土壤的改良作用[J]. 农业环境科学学报, 2007, 26(1): 235-239. doi: 10.3321/j.issn:1672-2043.2007.01.046 [14] 刘洁, 陈杰, 李顺奇, 等. 几种含磷材料对紫色土铅稳定条件优化及磷淋失环境风险评价[J]. 环境工程学报, 2018, 12(8): 2301-2310. doi: 10.12030/j.cjee.201801073 [15] CAO X, WAHBI A, MA L, et al. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid[J]. Journal of Hazardous Materials, 2009, 164(2/3): 555-564. [16] 施尧. 磷基材料钝化修复重金属Pb、Cu、Zn复合污染土壤[D]. 上海:上海交通大学, 2011. [17] 姚臻晖, 涂理达, 周慧平, 等. 稻田镉污染原位钝化修复及磷积累与迁移特征[J]. 中国环境科学, 2021, 41(5): 2374-2379. [18] 陈世宝, 朱永官, 马义兵. 不同磷处理对污染土壤中有效态铅及磷迁移的影响[J]. 环境科学学报, 2006, 26(7): 1140-1144. doi: 10.3321/j.issn:0253-2468.2006.07.016 [19] MIGNNADI S, C0RAMI A, FERRINI V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn[J]. Chemosphere, 2012, 86(4): 354-360. doi: 10.1016/j.chemosphere.2011.09.050 [20] 刘凤枝, 李玉浸. 土壤监测分析技术[M]. 北京:化学工业出版社, 2015(7). [21] 吴龙华, 骆永明. 根际土壤溶液取样器: 介绍一种新型原位土壤溶液采集装置[J]. 土壤, 1999, 31(1): 55-57. [22] TOWNSEND T, DUBEY B, TOLAYMAT T. Interpretation of Synthetic Precipitation Leaching Procedure (SPLP) Results for Assessing Risk to Groundwater from Land-Applied Granular Waste[J]. Environmental Engineering Science, 2006, 23(1): 239-251. doi: 10.1089/ees.2006.23.239 [23] CUI H B, ZHANG S W, Li R Y, et al. Leaching of Cu, Cd, Pb, and phosphorus and their availability in the phosphate-amended contaminated soils under simulated acid rain[J]. Environmental Science & Pollution Research, 2017, 24(26): 21128-21137. [24] 鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 1999. [25] 陈建军, 俞天明, 王碧玲, 等. 用TCLP和形态法评估含磷物质修复铅锌矿污染土壤的效果及其影响因素[J]. 环境科学, 2010, 31(1): 185-191. [26] 付煜恒, 张惠灵, 王宇, 等. 磷酸盐对铅镉复合污染土壤的钝化修复研究[J]. 环境工程, 2017, 35(9): 176-180. [27] APPEL C, MA L Q, RHUE R D, et al. Sequential sorption of lead and cadmium in three tropical soils[J]. Environmental Pollution, 2008, 155(1): 132-140. doi: 10.1016/j.envpol.2007.10.026 [28] 王碧玲. 含磷物质修复铅锌矿污染土壤的机理和技术[D]. 杭州:浙江大学, 2008. [29] CAO X D, WAHBI A, RHUE D R, et al. Mechanisms of lead, copper, and zinc retention by phosphate rock[J]. Environment Pollution, 2004, 131(3): 435-444. doi: 10.1016/j.envpol.2004.03.003 [30] 何振立. 污染及有益元素的土壤化学平衡[M]. 北京:中国环境科学出版社, 1998. [31] 徐明岗, 刘平, 宋正国, 等. 施肥对污染土壤中重金属行为影响的研究进展[J]. 农业环境科学学报, 2006, 25(S1): 328-333. [32] 郭亮, 李忠武, 黄斌, 等. 不同施磷量(KH2PO4)作用对Cu、Zn在红壤中的迁移转化[J]. 环境科学, 2014, 35(9): 3546-3552. [33] 魏晓欣. 含磷物质钝化修复重金属复合污染土壤[D]. 西安:西安科技大学, 2010. [34] SHUMAN L M. Effect of ionic strength and anions on zinc adsorption by two soils[J]. Soil Science Society of America Journal, 1986, 50(6): 1438-1442. doi: 10.2136/sssaj1986.03615995005000060012x [35] KAUSHIK R D, GUPTA V K, SINGH J P. Distribution of zinc, cadmium, and copper forms in soils as influenced by phosphorus application[J]. Arid Land Research and Management, 1993, 7(2): 163-171. doi: 10.1080/15324989309381345 [36] 张磊, 宋航, 陈小琴, 等. 穴施条件下肥料养分在土壤中迁移规律的初步研究[J]. 土壤, 2020, 52(6): 1145-1151. [37] 李学平, 孙燕, 石孝均. 紫色土稻田磷素淋失特征及其对地下水的影响[J]. 环境科学学报, 2008, 28(9): 1832-1838. doi: 10.3321/j.issn:0253-2468.2008.09.018 [38] GAZIS C, FENG X. A stable isotope study of soil water: evidence for mixing and preferential flow paths[J]. Geoderma, 2004, 119(1/2): 97-111. [39] JIN H P, BOLAN N, MEGHARAJ M, et al. Comparative value of phosphate sources on the immobilization of lead, and leaching of lead and phosphorus in lead contaminated soils[J]. Science of the Total Environment, 2011, 409(4): 853-860. doi: 10.1016/j.scitotenv.2010.11.003 -




 下载:
下载: