-
硫化氢(H2S)特性为剧毒、高腐蚀性,易损害工业管路及设备,威胁相关从业人员生命安全。此外,硫化氢的嗅觉阈值极低(仅为0.012 mg·m−3),是污水处理厂、垃圾填埋场等相关单元不良气味的重要来源[1]。因此,硫化氢的去除在清洁能源制备、烟气达标排放及生活环境优化中十分重要。对于大规模高浓度硫化氢而言,Claus法为首选处理工艺。而对于较低浓度的含硫化氢气体来说,常见脱除方法可分为干法和湿法两大类 [2]。干法多为采用固体吸附剂或催化剂进行硫化氢的吸附/催化脱除;湿法工艺则以碱性或氧化性溶液为吸收剂,利用硫化氢的酸性或还原性来提升其脱硫性能。其中,醇胺溶液吸收法是用于硫化氢、二氧化碳等酸性气体脱除最常见的传统工艺之一[3]。铁基脱硫等氧化脱硫工艺则是利用溶液的强氧化能力,将硫化氢氧化为硫磺,以达到脱硫及硫资源回收的目的[4]。此外,新型非水溶剂(如离子液体、低共熔溶剂)在气态污染物脱除方面的应用也是当前研究热点之一[5]。
纳米流体是指将纳米尺度(小于100 nm)的微粒加入基础流体中获得的稳定、均匀悬浮液[6]。与普通流体相比,不同纳米流体体系可具备独特的热、光、应力-应变及磁特性[7]。因此,纳米流体的使用越来越受到关注。与传统基液相比,纳米流体在增强传热、传质方面具有显著优势。纳米颗粒的布朗运动可增强流体扰动、增大气液传质系数、提升传质通量。向液体中加入适当比例的纳米颗粒,可有效提升其传质特性[8-9]。在气体分离领域,迄今已有较多关于纳米流体在二氧化碳吸收方面的研究。纳米颗粒的加入可强化二氧化碳在吸收剂中的传质,进而显著提升了吸收剂的吸收、再生性能[10-11]。当前利用纳米流体吸收硫化氢气体的研究报道较少,SiC[12]、CuO/Cu[13]、氧化石墨烯[14-16]及其他纳米颗粒[17-19]的加入可有效增强水溶液、醇胺等液体的硫化氢吸收性能,但相关研究使用的基液及纳米颗粒种类仍较少。本团队前期对非水溶液(离子液体、低共熔溶剂)基纳米流体体系的脱硫性能进行了研究[20-21],发现合适的纳米流体构建对气液吸收性能具备显著的增强作用。但纳米流体体系在水溶液气液吸收中的作用研究仍较少,考虑到当前常见的液态脱硫剂仍以碱性或氧化性水溶液为主,对以此类水溶液为基液而形成的纳米流体体系的脱硫性能研究具有重要意义。
为初步探讨纳米流体体系在硫化氢动态吸收脱除中的作用,本研究分别以较为常见的碱性或氧化性水溶液为基液,加入4种常见易得的纳米颗粒,调控原料配比以形成不同种类的纳米流体体系。其中,碱性溶液选择N-甲基二乙醇胺溶液,氧化性水溶液则选取氯化铁溶液。通过系统考察不同体系纳米流体对硫化氢的吸收能力,确定其组成与脱硫性能的内在关系,分析纳米流体体系对水溶液脱硫性能的影响,从而探讨水溶液基纳米流体体系在脱硫方面的作用规律,以期为纳米流体体系在气体脱硫方面的应用提供参考。
-
N-甲基二乙醇胺(MDEA)购自中国阿拉丁试剂(上海)有限公司;无水三氯化铁购自上海展云化工有限公司;纳米铜(Cu)和纳米二氧化硅(SiO2)购自上海麦克林生化科技有限公司;碳纳米管(CNTs)和纳米氧化铝(Al2O3)购自中国阿拉丁试剂(上海)有限公司;所用试剂均为分析纯。
Tecnai G2F20场发射高分辨率透射电子显微镜(TEM,美国FEI公司); ZEISS MERLIN扫描电子显微镜(SEM,英国Oxford仪器公司);TH990FIII智能烟气分析仪(武汉市天虹仪表有限公司)。
-
1) 纳米流体脱硫剂的制备。将一定质量分数(0.01%~0.05%)的纳米颗粒(纳米氧化铝、纳米二氧化硅、碳纳米管、纳米铜)加入质量分数为10%的醇胺水溶液(MDEA)中,磁力搅拌30 min,超声(40 kHz,100 W)间歇震荡40 min,得到稳定均匀的悬浮体系。得到的MDEA纳米流体体系分别记为MDEA@Al2O3-x%,MDEA@SiO2-x%,MDEA@CNTs-x%和MDEA@Cu-x%。其中,x%为纳米颗粒的质量浓度。同样,以0.1 mol·L−1的FeCl3水溶液为基液,加入不同的纳米颗粒,制备FeCl3纳米流体体系,分别记为 FeCl3@Al2O3-x%,FeCl3@SiO2-x%,FeCl3@CNTs-x%和FeCl3@Cu-x%。其中,x%为纳米颗粒的质量分数。
对于氯化铁溶液而言,吸收过程中硫化氢被三价铁离子氧化脱除,其脱硫机理如式(1)[22]所示。
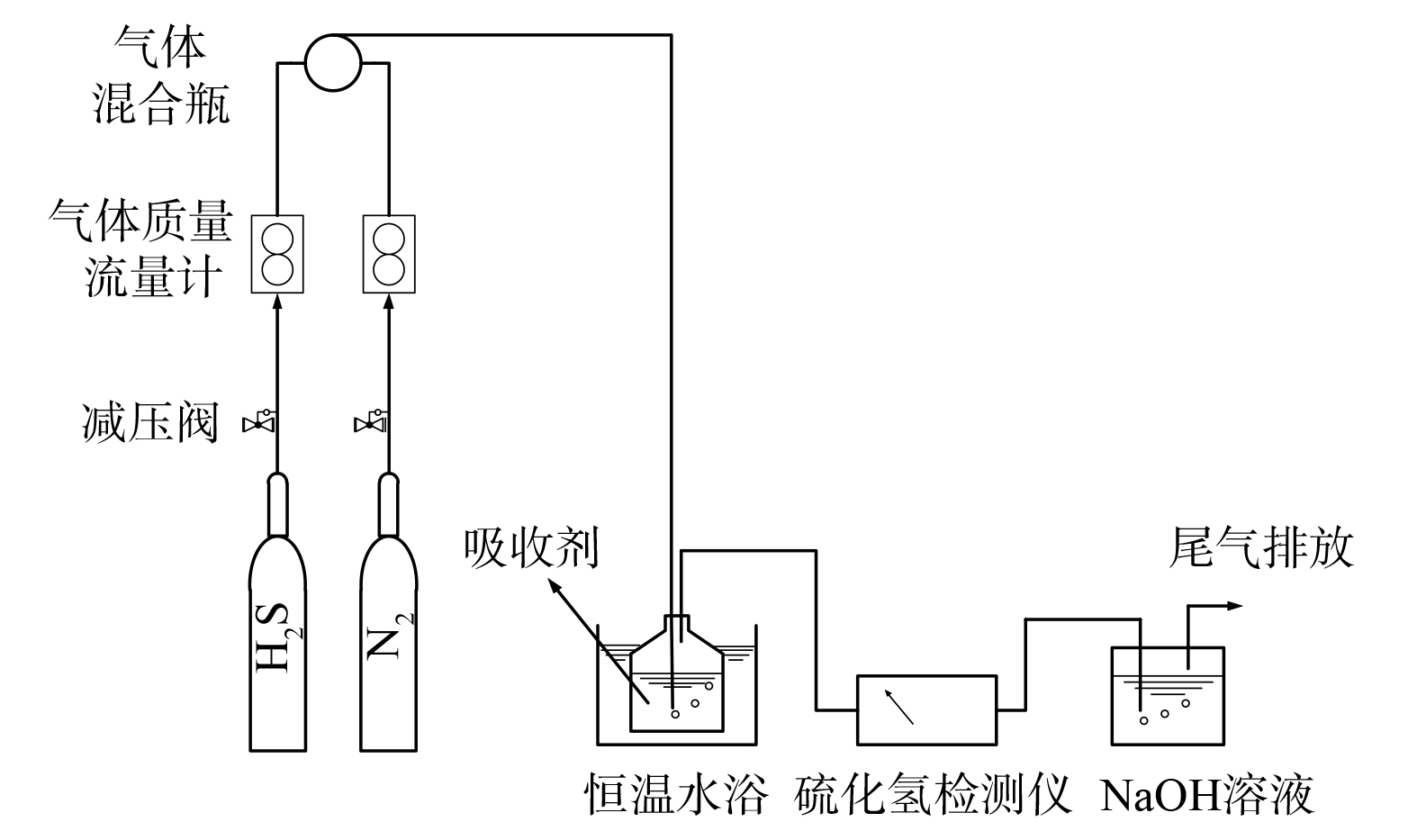
2) 硫化氢动态吸收实验。采用动态硫化氢气体吸收实验装置,流程见图1。所用硫化氢气体质量浓度为1 000 mg·m−3。以氮气为稀释气体,将含硫化氢气体以200 mL·min−1的流速通入装有纳米流体脱硫剂的鼓泡吸收反应器中。气体流量通过质量流量计控制,吸收温度使用恒温水浴控制。采用TH990FIII智能烟气分析仪测定出口气体中的硫化氢质量浓度。尾气通过NaOH溶液除去残余硫化氢气体防止环境污染。吸收液的再生方式为一定温度下鼓入500 mL·min−1空气1 h吹脱。H2S去除效率按式(2)计算。
式中:η为脱硫效率,C0为吸收器入口的H2S质量浓度,Ct为时间t时吸收器出口的H2S质量浓度。
-
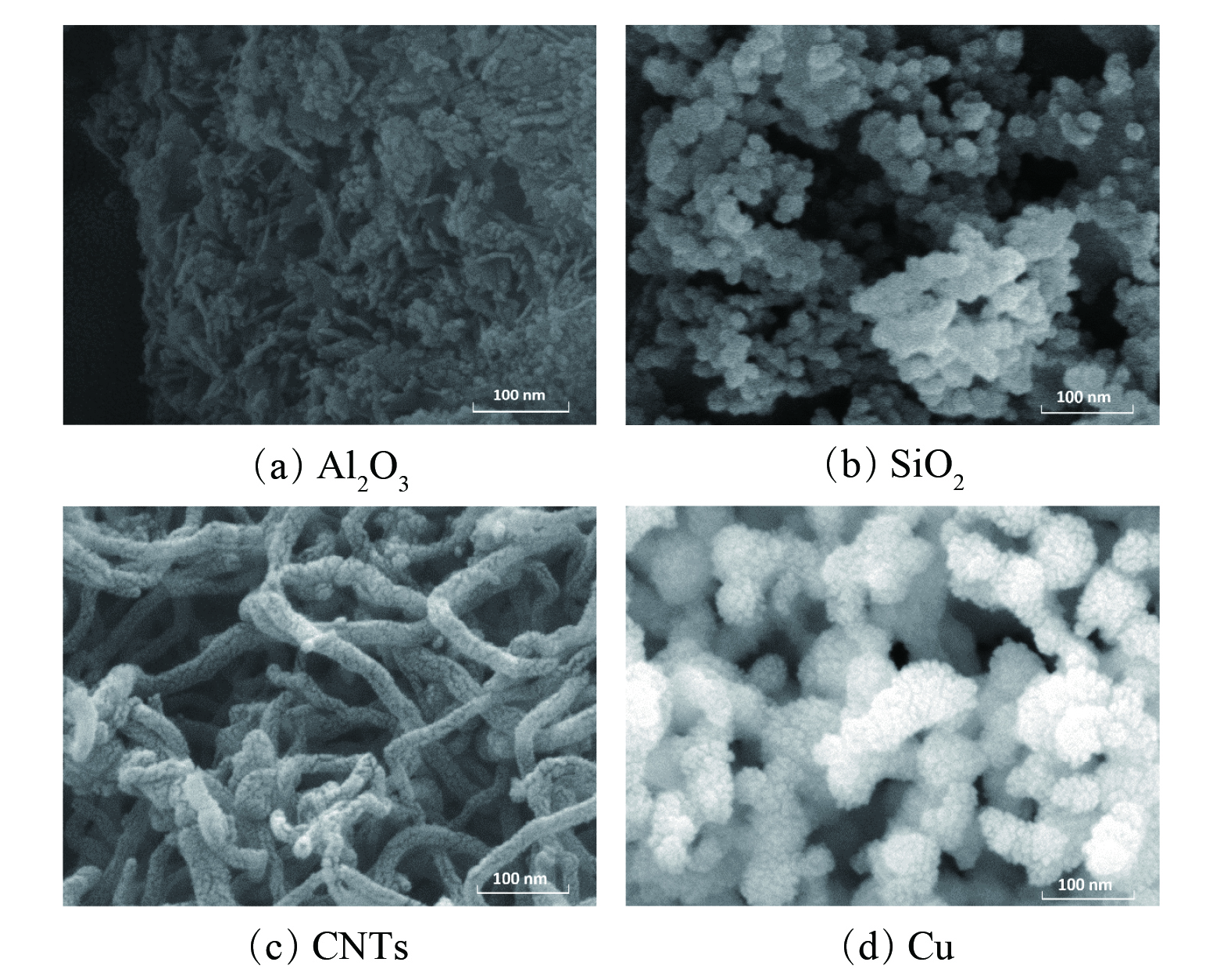
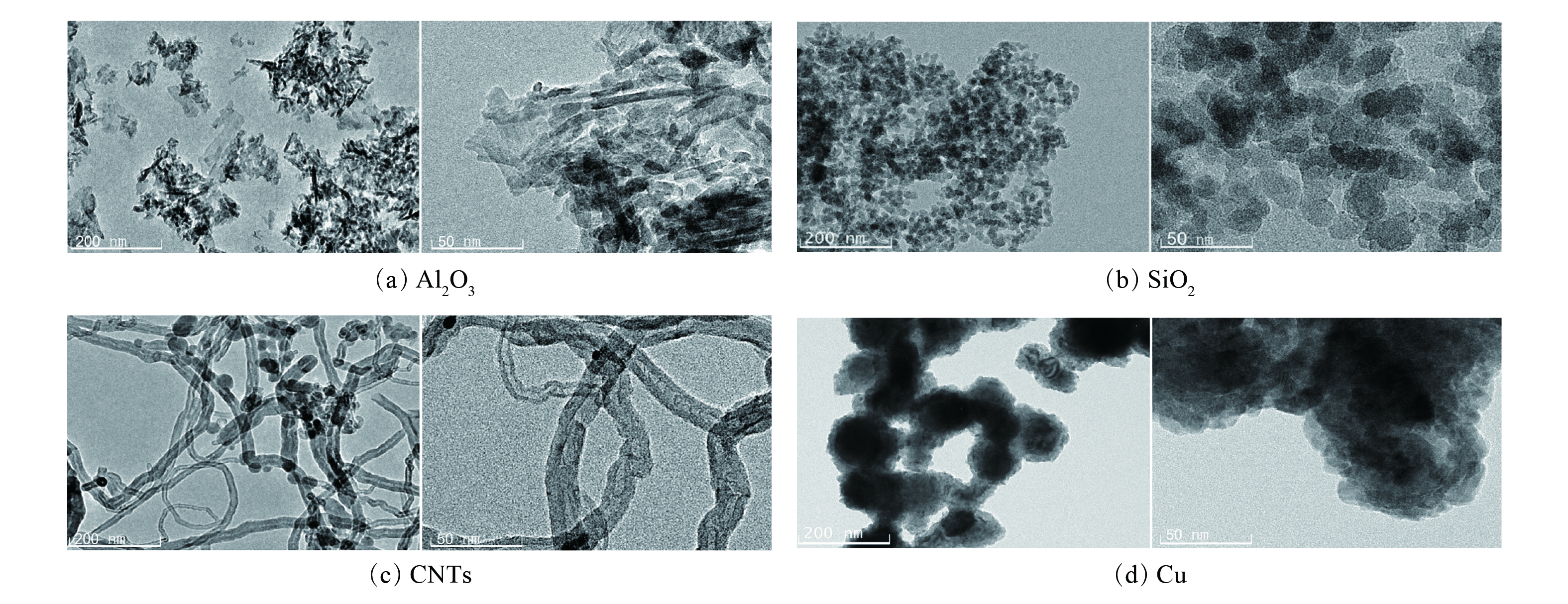
对不同纳米颗粒分别进行SEM和TEM表征,其结果分别如图2和图3所示。纳米SiO2和纳米铜均具有较为均匀规则的球形结构,其粒径均小于100 nm。Al2O3纳米颗粒具有较均匀的棒状结构,碳纳米管则具有管状结构,其直径均小于100 nm。符合纳米流体体系对于纳米颗粒的尺寸(0~100 nm)要求。此外,TEM结果表明,4种纳米颗粒均具备较高的分散度,有利于在溶液中分散均匀。
-
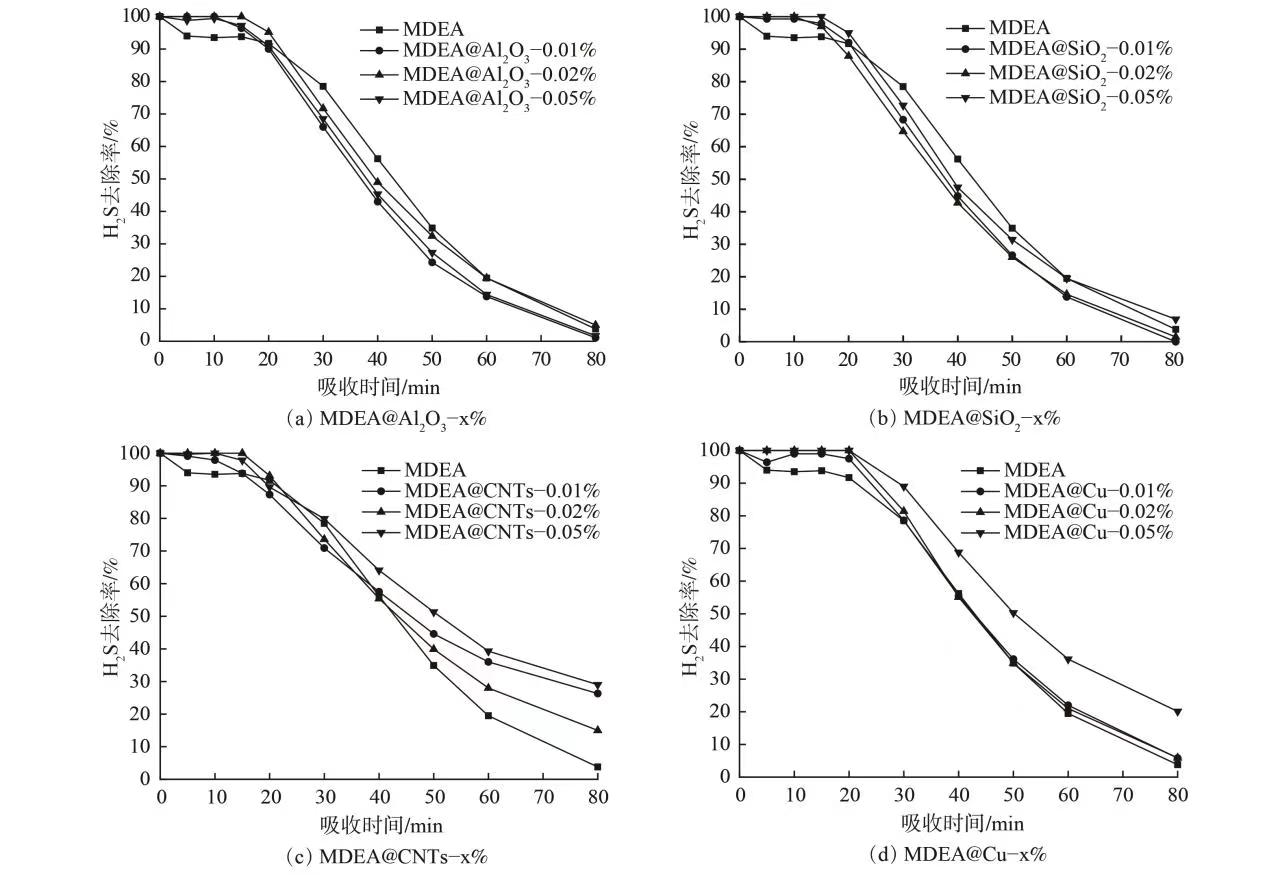
当吸收温度为30 ℃时,添加不同纳米颗粒MDEA溶液的纳米流体体系与未添加纳米颗粒MDEA水溶液的脱硫性能对比如图4所示。添加Al2O3和SiO2纳米颗粒后,形成纳米流体体系的脱硫性能与醇胺水溶液相比未出现显著增强,说明相应2种纳米颗粒的加入对气液吸收过程无积极影响。而添加CNTs和Cu纳米颗粒后,MDEA水溶液对H2S气体的动态去除作用均得到不同程度增强。对于这2种体系而言,80 min内当纳米颗粒质量分数为0.05% 时,形成的纳米流体体系对脱硫作用的提升效果最为显著。对更高质量分数的纳米流体体系性能进行考察时发现,在制备过程中,纳米颗粒质量分数继续升高时,液体中出现明显的颗粒聚结沉淀现象,纳米流体体系的稳定性明显下降。因此,本研究的纳米颗粒质量分数最高选择为0.05%。
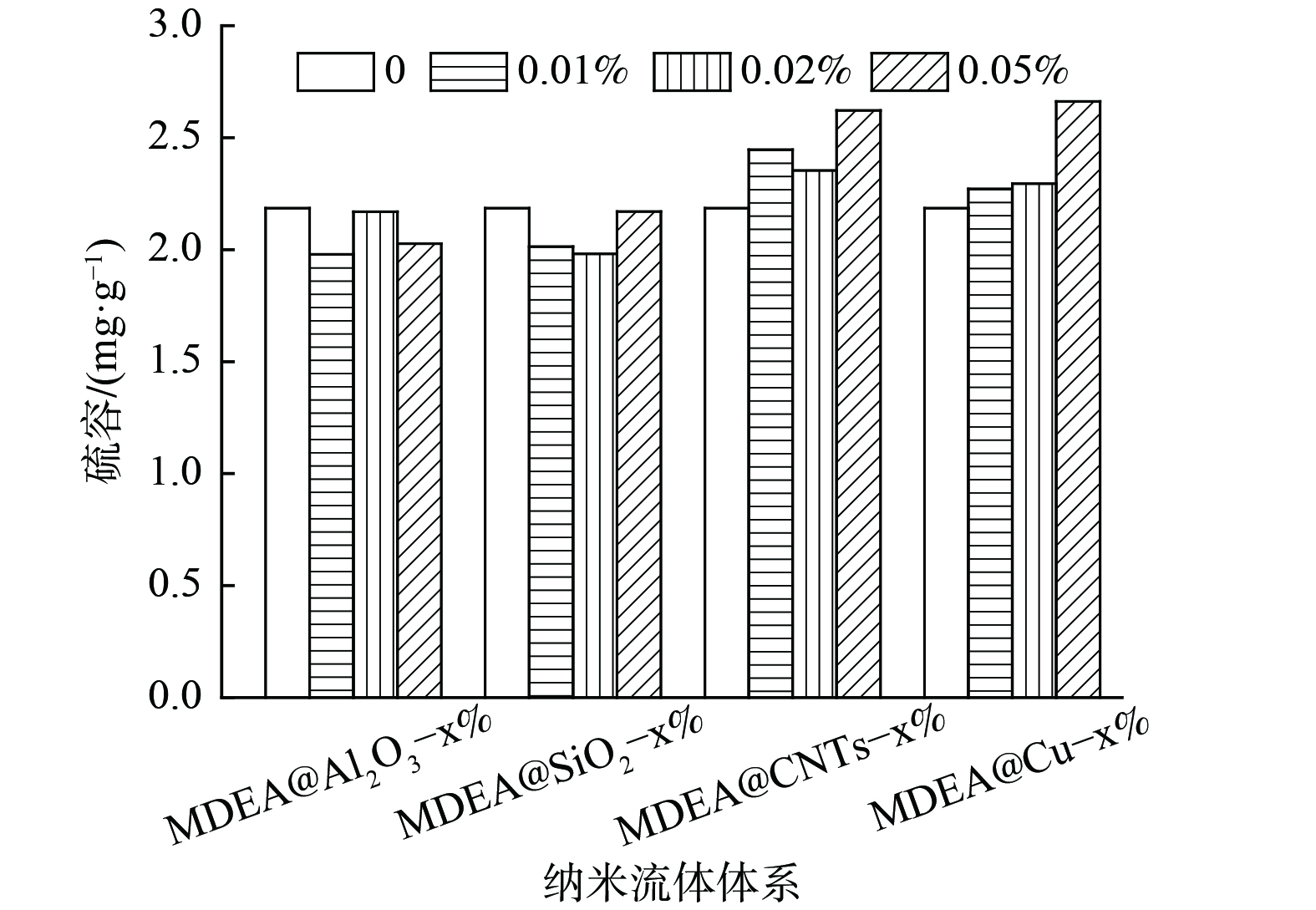
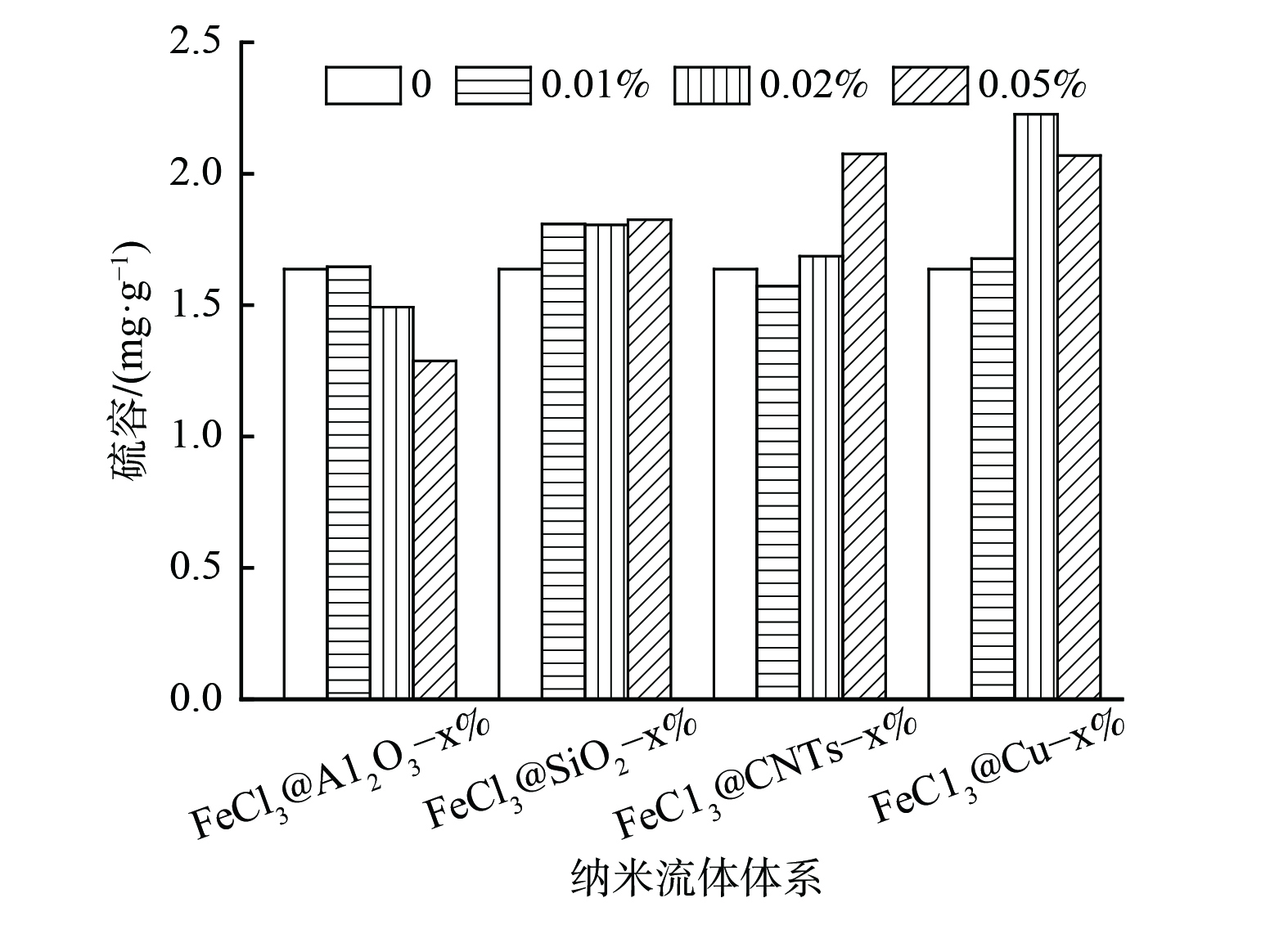
本研究中不同体系的脱硫性能差异较大,部分体系的脱除率一直处于较低水平。同时,饱和硫容的测定时间较长,对测量仪器的压力较大,因此,穿透硫容及饱和硫容不能很好地反映各体系的脱硫性能。考虑到80 min内各体系的脱硫率均低于40%,将80 min内单位质量脱硫剂脱除的硫化氢质量定为硫容,以此对各体系的脱硫性能进行比较。对80 min内不同MDEA水溶液纳米流体体系的硫容进行计算,结果如图5所示。对于纳米氧化铝和纳米二氧化硅来说,纳米颗粒的加入对水溶液的脱硫性能无显著增强作用,甚至略有下降。而对碳纳米管和纳米铜颗粒体系来说,不同质量分数纳米颗粒的加入均对水溶液的硫容有明显提升作用。其中,MDEA@Cu-0.05%纳米流体的硫容最高,MDEA@CNTs-0.05%纳米流体的硫容次之。
-
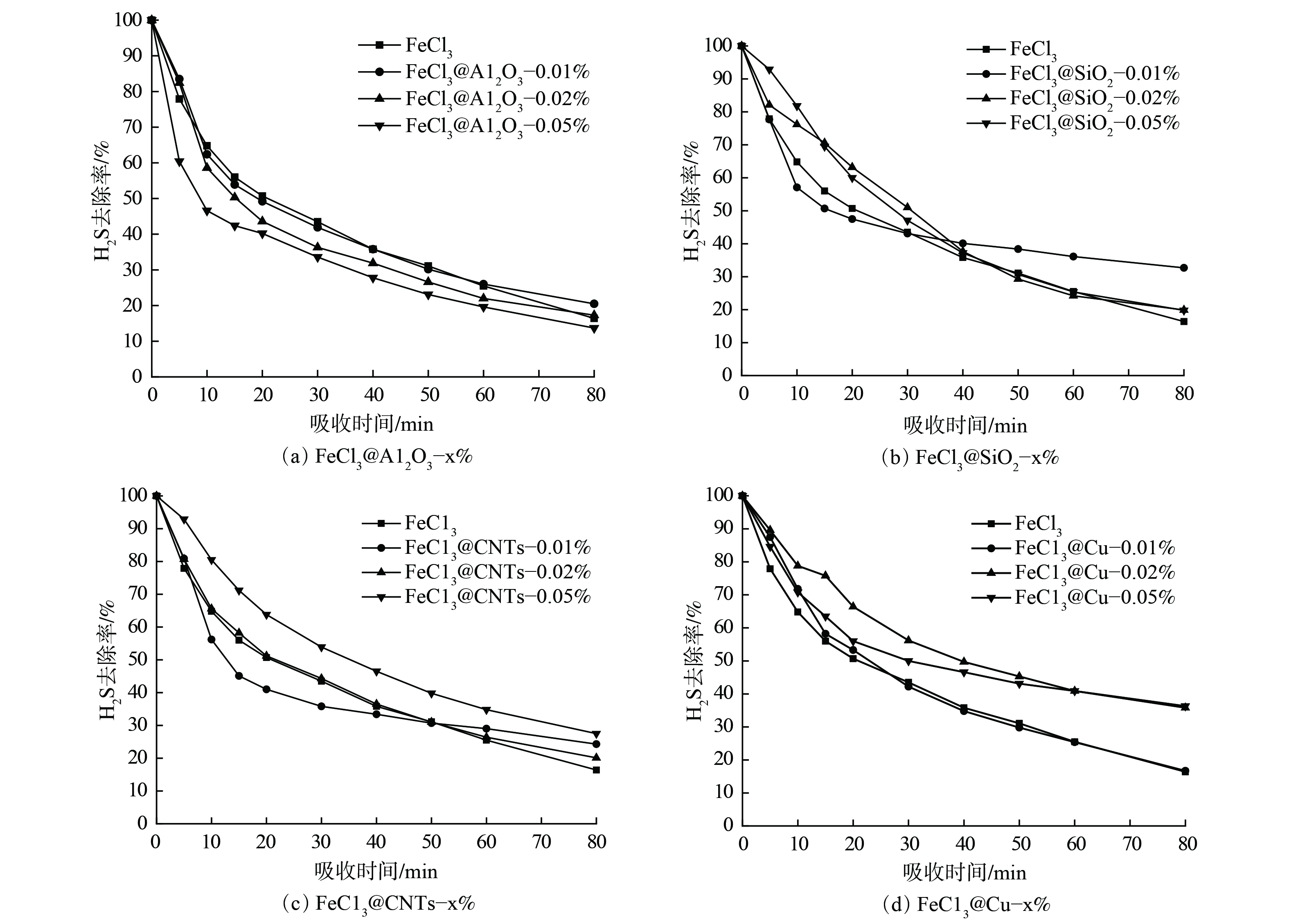
在探讨碱性溶液纳米流体脱硫性能的同时,以氯化铁水溶液为研究对象,探讨以氧化性溶液为基液的纳米流体体系脱硫性能变化。在吸收温度为30 ℃时,将添加不同纳米颗粒FeCl3溶液纳米流体体系与未添加纳米颗粒水溶液的脱硫性能进行对比(见图6)。与未添加纳米颗粒的FeCl3水溶液相比,添加Al2O3纳米颗粒后其脱硫性能未出现明显提升。添加SiO2纳米颗粒后,水溶液动态脱硫性能稍有提升,但提升程度并不明显。2种具备较为显著增强效果的纳米流体体系仍为碳纳米管和纳米铜体系。在碳纳米管纳米流体体系中,只有FeCl3@CNTs-0.05%体系表现出良好的脱硫增强性能。而在纳米铜体系中,FeCl3@Cu-0.02%和FeCl3@Cu-0.05%纳米流体均表现出良好的提升性能。以上结果与MDEA水溶液基纳米流体体系的性能变化规律基本相似。图7为80 min内不同FeCl3水溶液纳米流体体系的硫容计算结果。Al2O3纳米颗粒的加入对FeCl3水溶液脱硫性能并无显著影响,SiO2纳米颗粒的加入则使原溶液的脱硫性能稍有增强。碳纳米管和纳米铜颗粒的引入对FeCl3水溶液的硫化氢脱除能力有较明显的增强作用,其中FeCl3@Cu-0.02%纳米流体体系的增强效果最为明显。
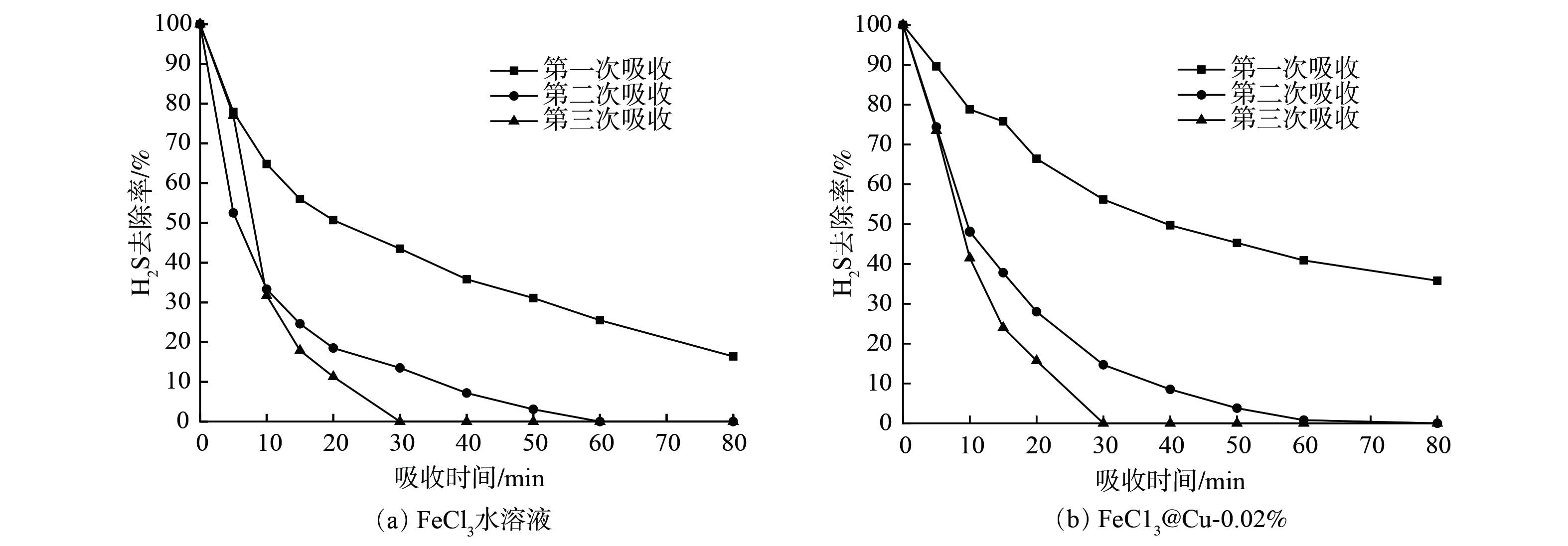
考虑到纳米颗粒易发生团聚现象,可能会对溶液的再生性能造成影响。本研究对添加纳米铜颗粒前后FeCl3水溶液的再生性能进行了考察。再生方法为常温下向吸收硫化氢后的吸收剂中鼓入500 mL·min−1空气,鼓泡时间为1 h。再生效果如图8所示。纳米颗粒的加入未对FeCl3水溶液的再生性能产生显著影响。此外,FeCl3水溶液再生后的脱硫性能出现显著下降,这应该是由于单纯的FeCl3不稳定,易在吸收-再生过程失去活性所致。为提高铁基水溶液的脱硫及再生性能,可考虑向铁基溶液中加入配体,形成络合铁脱硫体系。
-
不同种类、不同比例纳米颗粒的加入对气液吸收性能的影响并不一致,其影响机理可能十分复杂,目前仍无确切机理报道。目前,较为普遍的观点是纳米颗粒对水溶液的影响受多种因素影响。纳米颗粒加入所产生的不利影响包括:导致基础液体的粘度增加、气液接触面积的减少、纳米颗粒的团聚等[14]。而对于纳米流体在传质方面的促进作用,当前较为常见的几种纳米流体强化气液传质机制则包括:掠过效应(传输作用)、抑制气泡聚并机理、边界层混合机理等。掠过效应即纳米颗粒可自由出入传质边界层,在边界层吸附气体后在液相主体中释放,从而加快气液传质速率;抑制气泡聚并机理即固体颗粒附着在气泡表面,阻止气泡聚并,增大了气液传质面积;边界层混合机理则说明纳米颗粒的运动改变了传质边界层的流体力学行为,进而增强气液传质。一般认为以上几种机制相互关联、共同作用。本课题组在前期研究中发现,引入纳米铜颗粒对碱性低共熔溶剂的硫化氢吸收性能具备较为显著的增强作用。对氧化性离子液体溶液的研究则表明,4种纳米颗粒的加入均对离子液体溶液的脱硫性能具有积极影响。表征结果证明,在吸收过程中纳米颗粒无显著物理及化学变化;而动力学实验则证明,纳米颗粒的加入对气液传质具备显著的促进作用[20-21]。同样,在其他研究报道中,不同纳米颗粒对不同基液气液传质的影响作用并不统一,也尚未得出清晰的纳米颗粒、基液性质及传质作用间的构效关系。
在本研究中,对于MDEA溶液而言,碳纳米管和纳米铜颗粒基本不参与气液反应,其对气液吸收过程的强化作用主要为物理作用,纳米颗粒的存在增强了液体湍动,促进了气液传质,从而提高了硫化氢的捕集效率。在氯化铁水溶液中,碳纳米管为惰性颗粒,其强化机制应与在MDEA溶液中类似。而纳米铜颗粒可被三价铁离子部分氧化为铜离子,可快速与硫化氢结合,形成CuS沉淀,进而被铁离子氧化,在一定程度上提高了硫化氢的脱除效率[23]。以上机理仅为初步推断,在后续工作中,应根据基液和纳米颗粒的种类、性质及气液作用进行更为详细的研究工作,进而更为深入地梳理纳米流体气液传质作用的影响规律。
-
1)在基于MDEA水溶液的纳米流体体系中,添加Al2O3和SiO2纳米颗粒的MDEA水溶液纳米流体体系均未表现出明显的提升效果。碳纳米管和纳米铜颗粒的添加对H2S气体的去除均有不同程度的增强作用。MDEA@Cu-0.05%纳米流体显示出较好的提升性能,且硫容最高,MDEA@CNTs-0.05%纳米流体的硫容次之。
2)在基于FeCl3水溶液的纳米流体体系中,与MDEA水溶液纳米流体体系相似,添加Al2O3和SiO2纳米颗粒的FeCl3水溶液纳米流体体系同样均未表现出明显的提升效果。对于CNTs-纳米颗粒来说,只有质量分数为0.05%的CNTs-纳米颗粒表现出较好的增强性能。添加Cu-纳米颗粒的纳米流体体系的硫容最高,质量分数为0.02%的Cu纳米颗粒增强效果最好。这表明构建纳米流体体系是强化气体吸收剂性能的可能途径之一。
以醇胺或氯化铁为基液构建纳米流体体系对硫化氢气体湿法脱除的影响
The effect of nanofluid systems based on alcohol amine or ferric choloride on removal of hydrogen sulfide by wet method
-
摘要: 分别单独以醇胺(MDEA)和氯化铁水溶液为基液,引入4种不同的纳米颗粒(纳米氧化铝、二氧化硅、碳纳米管及纳米铜颗粒),构建不同质量分数的纳米流体体系,并测定不同体系对硫化氢气体的动态脱除性能,以考察纳米流体体系的构建对碱性(MDEA)及氧化性(氯化铁)水溶液脱硫性能的影响。分别对4种纳米颗粒进行了扫描电镜和透射电镜分析,发现其尺寸均在纳米级别。气液吸收研究结果表明,在2种不同基液的纳米流体体系中,添加Al2O3和SiO2纳米颗粒形成的纳米流体体系对原水溶液的脱硫性能均未产生明显影响。而碳纳米管和纳米铜颗粒的添加对H2S气体的去除则有不同程度增强。对于碳纳米管型纳米流体而言,对MDEA和氯化铁水溶液的脱硫强化性能在纳米颗粒质量分数为0.05%时最高。而对纳米铜颗粒而言,在2种水溶液中最佳质量分数分别为0.05%和0.02%。本研究表明纳米铜颗粒的引入对碱性及氧化性水溶液脱硫性能的增强作用最为明显,可为新型纳米流体型脱硫剂的开发提供参考。Abstract: Four kinds of nanoparticles (nano alumina, silica, carbon nanotube and nano copper particles) were introduced into the aqueous solution of alcohol amine (MDEA) and ferric chloride to form nanofluid systems with different concentrations. The dynamic removal performances for H2S of different nanofluid systems were measured, and the effect of nanofluid system on desulfurization performance of alkaline (MDEA) and oxidizing (ferric chloride) aqueous solutions was investigated. The nanoparticles were analyzed by scanning electron microscope and transmission electron microscope, and were found to be in a size of nanometer scale. The experimental results showed that the addition of Al2O3 and SiO2 nanoparticles had no obvious effect on the desulfurization performance of the original aqueous solutions. However, the introduction of carbon nanotubes and copper nanoparticles could enhanced the removal of H2S to different degrees. For carbon nanotube based nanofluid, when the nanoparticle concentration was 0.05%, the enhancement effect on the desulfurization performance of aqueous solution of MDEA and ferric chloride was the highest. As for copper nanoparticles, the optimum concentrations in aqueous solutions of MDEA and ferric chloride was 0.05% and 0.02%, respectively. In general, the addition of copper nanoparticles had the most significant enhancement effect on the desulfurization performance of alkaline and oxidizing aqueous solutions, which can provide a reference for the development of new nano-flow type desulfurizers.
-
Key words:
- Nanofluid /
- H2S /
- MDEA /
- ferric chloride /
- desulfurization /
- enhancement
-
硫化氢(H2S)特性为剧毒、高腐蚀性,易损害工业管路及设备,威胁相关从业人员生命安全。此外,硫化氢的嗅觉阈值极低(仅为0.012 mg·m−3),是污水处理厂、垃圾填埋场等相关单元不良气味的重要来源[1]。因此,硫化氢的去除在清洁能源制备、烟气达标排放及生活环境优化中十分重要。对于大规模高浓度硫化氢而言,Claus法为首选处理工艺。而对于较低浓度的含硫化氢气体来说,常见脱除方法可分为干法和湿法两大类 [2]。干法多为采用固体吸附剂或催化剂进行硫化氢的吸附/催化脱除;湿法工艺则以碱性或氧化性溶液为吸收剂,利用硫化氢的酸性或还原性来提升其脱硫性能。其中,醇胺溶液吸收法是用于硫化氢、二氧化碳等酸性气体脱除最常见的传统工艺之一[3]。铁基脱硫等氧化脱硫工艺则是利用溶液的强氧化能力,将硫化氢氧化为硫磺,以达到脱硫及硫资源回收的目的[4]。此外,新型非水溶剂(如离子液体、低共熔溶剂)在气态污染物脱除方面的应用也是当前研究热点之一[5]。
纳米流体是指将纳米尺度(小于100 nm)的微粒加入基础流体中获得的稳定、均匀悬浮液[6]。与普通流体相比,不同纳米流体体系可具备独特的热、光、应力-应变及磁特性[7]。因此,纳米流体的使用越来越受到关注。与传统基液相比,纳米流体在增强传热、传质方面具有显著优势。纳米颗粒的布朗运动可增强流体扰动、增大气液传质系数、提升传质通量。向液体中加入适当比例的纳米颗粒,可有效提升其传质特性[8-9]。在气体分离领域,迄今已有较多关于纳米流体在二氧化碳吸收方面的研究。纳米颗粒的加入可强化二氧化碳在吸收剂中的传质,进而显著提升了吸收剂的吸收、再生性能[10-11]。当前利用纳米流体吸收硫化氢气体的研究报道较少,SiC[12]、CuO/Cu[13]、氧化石墨烯[14-16]及其他纳米颗粒[17-19]的加入可有效增强水溶液、醇胺等液体的硫化氢吸收性能,但相关研究使用的基液及纳米颗粒种类仍较少。本团队前期对非水溶液(离子液体、低共熔溶剂)基纳米流体体系的脱硫性能进行了研究[20-21],发现合适的纳米流体构建对气液吸收性能具备显著的增强作用。但纳米流体体系在水溶液气液吸收中的作用研究仍较少,考虑到当前常见的液态脱硫剂仍以碱性或氧化性水溶液为主,对以此类水溶液为基液而形成的纳米流体体系的脱硫性能研究具有重要意义。
为初步探讨纳米流体体系在硫化氢动态吸收脱除中的作用,本研究分别以较为常见的碱性或氧化性水溶液为基液,加入4种常见易得的纳米颗粒,调控原料配比以形成不同种类的纳米流体体系。其中,碱性溶液选择N-甲基二乙醇胺溶液,氧化性水溶液则选取氯化铁溶液。通过系统考察不同体系纳米流体对硫化氢的吸收能力,确定其组成与脱硫性能的内在关系,分析纳米流体体系对水溶液脱硫性能的影响,从而探讨水溶液基纳米流体体系在脱硫方面的作用规律,以期为纳米流体体系在气体脱硫方面的应用提供参考。
1. 材料与方法
1.1 试剂与仪器
N-甲基二乙醇胺(MDEA)购自中国阿拉丁试剂(上海)有限公司;无水三氯化铁购自上海展云化工有限公司;纳米铜(Cu)和纳米二氧化硅(SiO2)购自上海麦克林生化科技有限公司;碳纳米管(CNTs)和纳米氧化铝(Al2O3)购自中国阿拉丁试剂(上海)有限公司;所用试剂均为分析纯。
Tecnai G2F20场发射高分辨率透射电子显微镜(TEM,美国FEI公司); ZEISS MERLIN扫描电子显微镜(SEM,英国Oxford仪器公司);TH990FIII智能烟气分析仪(武汉市天虹仪表有限公司)。
1.2 实验过程
1) 纳米流体脱硫剂的制备。将一定质量分数(0.01%~0.05%)的纳米颗粒(纳米氧化铝、纳米二氧化硅、碳纳米管、纳米铜)加入质量分数为10%的醇胺水溶液(MDEA)中,磁力搅拌30 min,超声(40 kHz,100 W)间歇震荡40 min,得到稳定均匀的悬浮体系。得到的MDEA纳米流体体系分别记为MDEA@Al2O3-x%,MDEA@SiO2-x%,MDEA@CNTs-x%和MDEA@Cu-x%。其中,x%为纳米颗粒的质量浓度。同样,以0.1 mol·L−1的FeCl3水溶液为基液,加入不同的纳米颗粒,制备FeCl3纳米流体体系,分别记为 FeCl3@Al2O3-x%,FeCl3@SiO2-x%,FeCl3@CNTs-x%和FeCl3@Cu-x%。其中,x%为纳米颗粒的质量分数。
对于氯化铁溶液而言,吸收过程中硫化氢被三价铁离子氧化脱除,其脱硫机理如式(1)[22]所示。
H2S+2FeCl3=S↓+2FeCl2+2HCl (1) 2) 硫化氢动态吸收实验。采用动态硫化氢气体吸收实验装置,流程见图1。所用硫化氢气体质量浓度为1 000 mg·m−3。以氮气为稀释气体,将含硫化氢气体以200 mL·min−1的流速通入装有纳米流体脱硫剂的鼓泡吸收反应器中。气体流量通过质量流量计控制,吸收温度使用恒温水浴控制。采用TH990FIII智能烟气分析仪测定出口气体中的硫化氢质量浓度。尾气通过NaOH溶液除去残余硫化氢气体防止环境污染。吸收液的再生方式为一定温度下鼓入500 mL·min−1空气1 h吹脱。H2S去除效率按式(2)计算。
η=(C0−Ct)/C0 (2) 式中:η为脱硫效率,C0为吸收器入口的H2S质量浓度,Ct为时间t时吸收器出口的H2S质量浓度。
2. 结果与讨论
2.1 纳米颗粒表征结果
对不同纳米颗粒分别进行SEM和TEM表征,其结果分别如图2和图3所示。纳米SiO2和纳米铜均具有较为均匀规则的球形结构,其粒径均小于100 nm。Al2O3纳米颗粒具有较均匀的棒状结构,碳纳米管则具有管状结构,其直径均小于100 nm。符合纳米流体体系对于纳米颗粒的尺寸(0~100 nm)要求。此外,TEM结果表明,4种纳米颗粒均具备较高的分散度,有利于在溶液中分散均匀。
2.2 基于MDEA水溶液纳米流体体系的脱硫性能
当吸收温度为30 ℃时,添加不同纳米颗粒MDEA溶液的纳米流体体系与未添加纳米颗粒MDEA水溶液的脱硫性能对比如图4所示。添加Al2O3和SiO2纳米颗粒后,形成纳米流体体系的脱硫性能与醇胺水溶液相比未出现显著增强,说明相应2种纳米颗粒的加入对气液吸收过程无积极影响。而添加CNTs和Cu纳米颗粒后,MDEA水溶液对H2S气体的动态去除作用均得到不同程度增强。对于这2种体系而言,80 min内当纳米颗粒质量分数为0.05% 时,形成的纳米流体体系对脱硫作用的提升效果最为显著。对更高质量分数的纳米流体体系性能进行考察时发现,在制备过程中,纳米颗粒质量分数继续升高时,液体中出现明显的颗粒聚结沉淀现象,纳米流体体系的稳定性明显下降。因此,本研究的纳米颗粒质量分数最高选择为0.05%。
本研究中不同体系的脱硫性能差异较大,部分体系的脱除率一直处于较低水平。同时,饱和硫容的测定时间较长,对测量仪器的压力较大,因此,穿透硫容及饱和硫容不能很好地反映各体系的脱硫性能。考虑到80 min内各体系的脱硫率均低于40%,将80 min内单位质量脱硫剂脱除的硫化氢质量定为硫容,以此对各体系的脱硫性能进行比较。对80 min内不同MDEA水溶液纳米流体体系的硫容进行计算,结果如图5所示。对于纳米氧化铝和纳米二氧化硅来说,纳米颗粒的加入对水溶液的脱硫性能无显著增强作用,甚至略有下降。而对碳纳米管和纳米铜颗粒体系来说,不同质量分数纳米颗粒的加入均对水溶液的硫容有明显提升作用。其中,MDEA@Cu-0.05%纳米流体的硫容最高,MDEA@CNTs-0.05%纳米流体的硫容次之。
2.3 基于FeCl3水溶液的纳米流体体系的脱硫性能
在探讨碱性溶液纳米流体脱硫性能的同时,以氯化铁水溶液为研究对象,探讨以氧化性溶液为基液的纳米流体体系脱硫性能变化。在吸收温度为30 ℃时,将添加不同纳米颗粒FeCl3溶液纳米流体体系与未添加纳米颗粒水溶液的脱硫性能进行对比(见图6)。与未添加纳米颗粒的FeCl3水溶液相比,添加Al2O3纳米颗粒后其脱硫性能未出现明显提升。添加SiO2纳米颗粒后,水溶液动态脱硫性能稍有提升,但提升程度并不明显。2种具备较为显著增强效果的纳米流体体系仍为碳纳米管和纳米铜体系。在碳纳米管纳米流体体系中,只有FeCl3@CNTs-0.05%体系表现出良好的脱硫增强性能。而在纳米铜体系中,FeCl3@Cu-0.02%和FeCl3@Cu-0.05%纳米流体均表现出良好的提升性能。以上结果与MDEA水溶液基纳米流体体系的性能变化规律基本相似。图7为80 min内不同FeCl3水溶液纳米流体体系的硫容计算结果。Al2O3纳米颗粒的加入对FeCl3水溶液脱硫性能并无显著影响,SiO2纳米颗粒的加入则使原溶液的脱硫性能稍有增强。碳纳米管和纳米铜颗粒的引入对FeCl3水溶液的硫化氢脱除能力有较明显的增强作用,其中FeCl3@Cu-0.02%纳米流体体系的增强效果最为明显。
考虑到纳米颗粒易发生团聚现象,可能会对溶液的再生性能造成影响。本研究对添加纳米铜颗粒前后FeCl3水溶液的再生性能进行了考察。再生方法为常温下向吸收硫化氢后的吸收剂中鼓入500 mL·min−1空气,鼓泡时间为1 h。再生效果如图8所示。纳米颗粒的加入未对FeCl3水溶液的再生性能产生显著影响。此外,FeCl3水溶液再生后的脱硫性能出现显著下降,这应该是由于单纯的FeCl3不稳定,易在吸收-再生过程失去活性所致。为提高铁基水溶液的脱硫及再生性能,可考虑向铁基溶液中加入配体,形成络合铁脱硫体系。
2.4 纳米流体系促进脱硫性能的机理
不同种类、不同比例纳米颗粒的加入对气液吸收性能的影响并不一致,其影响机理可能十分复杂,目前仍无确切机理报道。目前,较为普遍的观点是纳米颗粒对水溶液的影响受多种因素影响。纳米颗粒加入所产生的不利影响包括:导致基础液体的粘度增加、气液接触面积的减少、纳米颗粒的团聚等[14]。而对于纳米流体在传质方面的促进作用,当前较为常见的几种纳米流体强化气液传质机制则包括:掠过效应(传输作用)、抑制气泡聚并机理、边界层混合机理等。掠过效应即纳米颗粒可自由出入传质边界层,在边界层吸附气体后在液相主体中释放,从而加快气液传质速率;抑制气泡聚并机理即固体颗粒附着在气泡表面,阻止气泡聚并,增大了气液传质面积;边界层混合机理则说明纳米颗粒的运动改变了传质边界层的流体力学行为,进而增强气液传质。一般认为以上几种机制相互关联、共同作用。本课题组在前期研究中发现,引入纳米铜颗粒对碱性低共熔溶剂的硫化氢吸收性能具备较为显著的增强作用。对氧化性离子液体溶液的研究则表明,4种纳米颗粒的加入均对离子液体溶液的脱硫性能具有积极影响。表征结果证明,在吸收过程中纳米颗粒无显著物理及化学变化;而动力学实验则证明,纳米颗粒的加入对气液传质具备显著的促进作用[20-21]。同样,在其他研究报道中,不同纳米颗粒对不同基液气液传质的影响作用并不统一,也尚未得出清晰的纳米颗粒、基液性质及传质作用间的构效关系。
在本研究中,对于MDEA溶液而言,碳纳米管和纳米铜颗粒基本不参与气液反应,其对气液吸收过程的强化作用主要为物理作用,纳米颗粒的存在增强了液体湍动,促进了气液传质,从而提高了硫化氢的捕集效率。在氯化铁水溶液中,碳纳米管为惰性颗粒,其强化机制应与在MDEA溶液中类似。而纳米铜颗粒可被三价铁离子部分氧化为铜离子,可快速与硫化氢结合,形成CuS沉淀,进而被铁离子氧化,在一定程度上提高了硫化氢的脱除效率[23]。以上机理仅为初步推断,在后续工作中,应根据基液和纳米颗粒的种类、性质及气液作用进行更为详细的研究工作,进而更为深入地梳理纳米流体气液传质作用的影响规律。
3. 结论
1)在基于MDEA水溶液的纳米流体体系中,添加Al2O3和SiO2纳米颗粒的MDEA水溶液纳米流体体系均未表现出明显的提升效果。碳纳米管和纳米铜颗粒的添加对H2S气体的去除均有不同程度的增强作用。MDEA@Cu-0.05%纳米流体显示出较好的提升性能,且硫容最高,MDEA@CNTs-0.05%纳米流体的硫容次之。
2)在基于FeCl3水溶液的纳米流体体系中,与MDEA水溶液纳米流体体系相似,添加Al2O3和SiO2纳米颗粒的FeCl3水溶液纳米流体体系同样均未表现出明显的提升效果。对于CNTs-纳米颗粒来说,只有质量分数为0.05%的CNTs-纳米颗粒表现出较好的增强性能。添加Cu-纳米颗粒的纳米流体体系的硫容最高,质量分数为0.02%的Cu纳米颗粒增强效果最好。这表明构建纳米流体体系是强化气体吸收剂性能的可能途径之一。
-
-
[1] WIHEEB A D, SHAMSUDIN I K, AHMAD M A, et al. Present technologies for hydrogen sulfide removal from gaseous mixtures[J]. Reviews in Chemical Engineering, 2013, 29: 449-470. [2] WANG R. Investigation on a new liquid redox method for H2S removal and sulfur recovery with heteropoly compound[J]. Separation and Purification Technology, 2003, 31: 111-121. doi: 10.1016/S1383-5866(02)00153-3 [3] LIU X, LI J, WANG R. Desulfurization and regeneration performance of heteropoly compound/ionic liquid solutions at high temperature[J]. Chemical Engineering Journal, 2017, 316: 171-178. doi: 10.1016/j.cej.2017.01.093 [4] WANG Y, WANG Z, LIU Y. Oxidation absorption of gaseous H2S using Fenton-like advanced oxidation systems[J]. Energy & Fuels, 2018, 32: 11289-11295. [5] LIU X, WANG B, WANG D, et al. Study on the desulfurization performance of metal-based low transition temperature mixtures: Removal of hydrogen sulfide and sulfur recovery[J]. Fuel Processing Technology, 2019, 193: 372-377. doi: 10.1016/j.fuproc.2019.05.029 [6] KUMAR D D, ARASU A V. A comprehensive review of preparation, characterization, properties and stability of hybrid nanofluids[J]. Renewable & Sustainable Energy Reviews, 2018, 81: 1669-1689. [7] 冯一民, 郭蒙, 李丹, 等. 双子表面活性剂修饰金纳米流体的制备及稳定性[J]. 高等学校化学学报, 2017, 38(10): 1829-1833. doi: 10.7503/cjcu20170150 [8] KOMATI S, SURESH A K. Anomalous enhancement of interphase transport rates by nanoparticles: Effect of magnetic iron oxide on gas−liquid mass transfer[J]. Industrial & Engineering Chemistry Research, 2010, 49: 390-405. [9] ZHANG Z, CAI J, CHEN F, et al. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status[J]. Renewable Energy, 2018, 118: 527-535. doi: 10.1016/j.renene.2017.11.031 [10] IRANI V, MALEKI A, TAVASOLI A. CO2 absorption enhancement in graphene-oxide/MDEA nanofluid[J]. Journal of Environmental Chemical Engineering, 2019, 7: 102782. doi: 10.1016/j.jece.2018.11.027 [11] REZAKAZEMI M, DARABI M, SOROUSH E, et al. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor[J]. Separation and Purification Technology, 2019, 210: 920-926. doi: 10.1016/j.seppur.2018.09.005 [12] MA M, ZOU C. Enhancement by SiC nanoparticles of the removal of hydrogen sulfide from natural gas by a traditional desulfurizer[J]. Energy & Fuels, 2017, 31: 8054-8060. [13] MA M, ZOU C. Effect of nanoparticles on the mass transfer process of removal of hydrogen sulfide in biogas by MDEA[J]. International Journal of Heat and Mass Transfer, 2018, 127: 385-392. doi: 10.1016/j.ijheatmasstransfer.2018.06.091 [14] FARAJ S H E, ESFAHANY M N, JAFARIASL M, et al. Hydrogen sulfide bubble absorption enhancement in water-based nanofluids[J]. Industrial & Engineering Chemistry Research, 2014, 53: 16851-16858. [15] ESMAEILIFARAJ S H, ESFAHANY M N. Absorption of hydrogen sulfide and carbon dioxide in water based nanofluids[J]. Industrial & Engineering Chemistry Research, 2016, 55: 4682-4690. [16] ESMAEILIFARAJ S H, ESFAHANY M N, DARVANJOOGHI M H K. Application of water based nanofluids in bioscrubber for improvement of biogas sweetening in a pilot scale[J]. Chemical Engineering and Processing, 2019, 143: 107603. doi: 10.1016/j.cep.2019.107603 [17] IRANI V, TAVASOLI A, VAHIDI M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption[J]. Journal of Colloid and Interface Science, 2018, 527: 57-67. doi: 10.1016/j.jcis.2018.05.018 [18] MALEKI A, IRANI V, TAVASOLI A. H2S solubility enhancement using ethylene diamine functionalized carbon nanotubes and the aqueous solution of N-methyldiethanolamine[J]. Journal of Natural Gas Science and Engineering, 2019, 71: 103004. doi: 10.1016/j.jngse.2019.103004 [19] TAHERI M, MOHEBBI A, HASHEMIPOUR H, et al. Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2–H2S–CH4 gas mixture using amine-based nanofluids in a wetted wall column[J]. Journal of Natural Gas Science and Engineering, 2016, 28: 410-417. doi: 10.1016/j.jngse.2015.12.014 [20] LIU X, WANG B, LV X, et al. Enhanced removal of hydrogen sulfide using novel nanofluid system composed of deep eutectic solvent and Cu nanoparticles[J]. Journal of Hazardous Materials, 2021, 405: 124271. doi: 10.1016/j.jhazmat.2020.124271 [21] LIU X, WANG B, DONG X, et al. Enhancement effect of nanofluids on the desulfurization and regeneration performance of ionic liquid-based system[J]. Journal of Hazardous Materials, 2021, 419: 126394. doi: 10.1016/j.jhazmat.2021.126394 [22] 吕雪, 牟玥, 缪逸文, 等. 三种硫化氢吸收剂吸收效率对比及碘酸钾体系吸收条件优化研究[J]. 化工学报, 2020, 71(10): 4696-4703. [23] 张俊丰, 童志权. Fe/Cu体系湿式催化氧化一步高效脱除 H2S 新方法研究[J]. 环境科学学报, 2005, 25(4): 497-501. doi: 10.3321/j.issn:0253-2468.2005.04.012 -




 下载:
下载: