-
挥发性有机物(VOCs)是一种有害气体污染物,是雾霾和臭氧污染的前驱物,会对环境和人群健康造成危害。因此,VOCs的控制技术已成为近年来的研究热点[1-2]。近年来,光催化氧化技术因其成本低、无二次污染等优点,得到了广泛的关注。催化剂是光催化技术的核心技术,由于TiO2具有成本低、安全、活性高、稳定性好等优点,目前已成为应用最广泛一种光催化剂[3-4]。然而,它也存在着一些缺点,如易失活、可见光利用率低等。因此,对光催化剂的改性研究成为了热点,目前主要的改性方法主要包括金属离子掺杂、非金属离子掺杂、贵金属表面沉积、表面光敏化和半导体复合材料等[5]。
有研究表明,复合半导体改性方法能有效提高TiO2的光催化活性,特别是ZnO和MnO2[6-8]。SIWINSKA等[9]采用溶胶-凝胶法研究了一系列不同摩尔比的二氧化钛-氧化锌体系。结果表明,与原来的TiO2相比,二氧化钛-氧化锌对3种有机染料具有更高的光催化活性。MA等[10]采用阳极氧化和电沉积方法合成了二氧化锰/二氧化钛纳米管阵列(MnO2/TiO2-NTAS)光电阴极,具有良好的稳定性和可重复利用性,在废水处理中具有很大的应用潜力。
Zn-MnO2干电池具有成本低、容量大等优点,是目前应用最广泛的消费型电池之一。全球锌锰电池的年消耗量约为6×107 t,且仍在增长[11]。废旧锌锰电池含有大量的锰和锌以及其他重金属元素,大量废弃的锌锰电池处理不当会造成土壤、水和大气污染[12-13]。因此,开发出一种经济可行的回收方法非常重要,将废旧电池回收制备功能材料已经逐渐成为研究热点[14-19]。GALLEGOS等[20]使用生物湿法冶金工艺从废电池中回收锌和锰,以制备去除VOCs的催化剂。ZHANG等[21]采用还原酸浸法从废旧锌锰电池中成功合成二氧化锰,并用其负载Cu作为一氧化碳氧化催化剂。将废旧锌锰电池制备成催化剂来处理环境问题是一种具有前景的技术。但截至目前,现有的回收方法过于复杂,成本过高,大部分都只针对废旧电池中的特定金属进行回收,并且回收过程中还存在二次污染问题,因而不利于在实际工程中推广应用。
废旧锌锰电池中含有大量的锰和锌,利用废旧锌锰电池来提高TiO2的光催化性能是一种较为可行的方法。因此,本研究以废旧锌锰电池和二氧化钛为原料,采用球磨法制备复合改性光催化剂,以实现废旧电池中的相关物质全部回收利用。
-
废旧锌锰电池均来源于生活中收集的5号废旧南孚电池,TiO2为重庆新华化工有限公司生产的TiO2-C801,无水乙醇(CH3CH2OH)为分析纯。
-
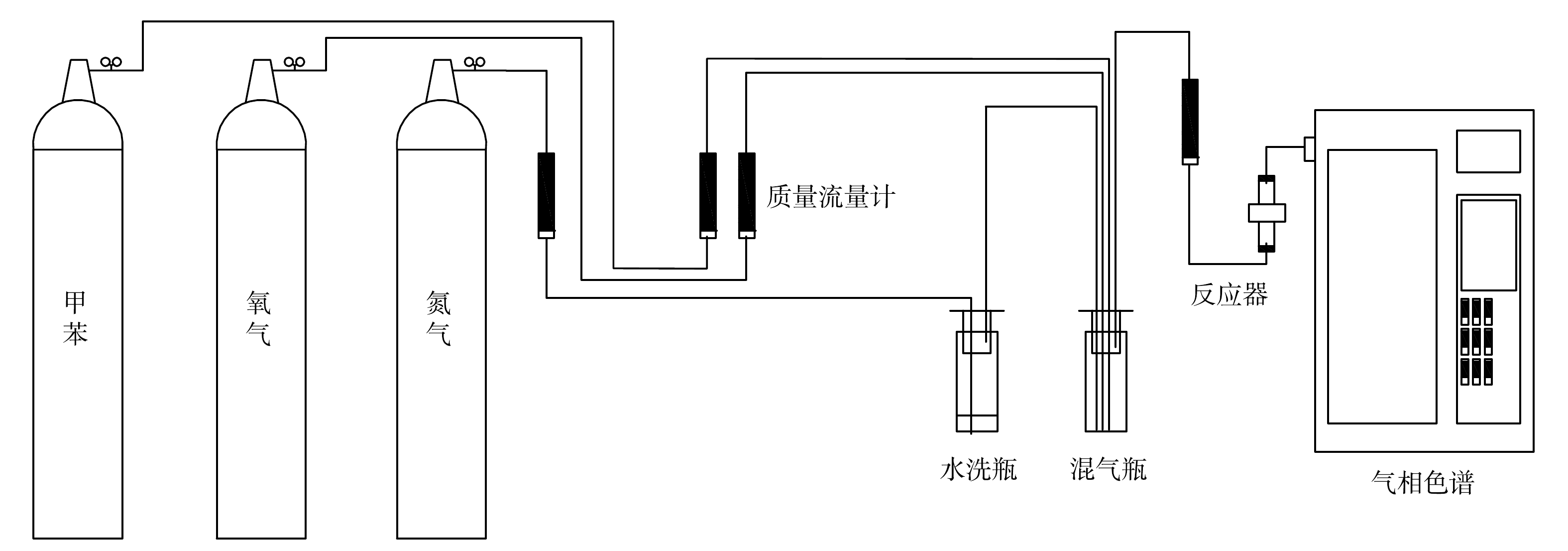
如图1所示,反应器由一个内径为8 mm的石英管制成,催化剂固定在反应器的中心,以4个254 nm的环形紫外灯环绕在反应器外部作为光源。将甲苯混合气体通入反应器,与催化剂接触发生光催化反应。采用气相色谱测定甲苯污染物浓度。
-
1)废旧锌锰电池芯粉的制备。将电池金属外壳机械移除,并收集内部残留物。内部残留物用去离子水反复洗涤,然后于105 ℃干燥并研磨成粉末。制备的废电池芯粉命名为WBP。
2)废旧锌锰电池复合光催化剂的制备。将TiO2和WBP按不同质量比倒入玛瑙研钵中。将一定量的无水乙醇溶液倒入玛瑙砂钵中,用球磨机以500 r·min−1的速度球磨6 h。将混合后的糊状催化剂放入烘箱中,在80 ℃下至完全干燥。然后,将干燥后的催化剂研磨成均匀的粉末,在500 ℃煅烧4 h,制备出废旧锌锰电池芯粉-TiO2催化剂,命名为WBC。根据TiO2与WBP的质量比分别为1∶1、2∶1、3∶1和4∶1,将光催化剂分别命名为WBC1、WBC2、WBC3和WBC4。
-
1)催化剂表征。采用XRF表征测定样品各元素的含量;采用TG热重法测定催化剂在升温加热过程中的质量变化;采用比表面积分析仪测定样品的比表面积和孔径分布;采用扫描电子显微镜(SEM)观察样品的形貌;采用X射线衍射(XRD)分析样品的元素和晶体结构;采用X射线光电子能谱(XPS)测定样品的表面元素和价态;采用EIS和PL表征催化剂的光电性能。
2)光催化降解甲苯实验。首先,将1.5 mL样品置于固定床连续流动反应器中;然后,将初始浓度为62.5 mg·m−3的甲苯以30 mL·min−1的流速引入气体混合室,氮气通过洗气瓶后进入混气瓶,为反应提供水蒸气,氧气直接通入混气瓶,反应气体在进入光催化反应器之前在混气瓶中充分混合;最后,混合均匀的反应气体进入光催化反应器,进行光催化反应。
研究空速(0.2、0.25、0.33、0.5、1.0 s−1)、光强(5 W、10 W、15 W、20 W)、相对湿度(10%、20%、30%、40%、50%、60%)和氧含量(0、10%、15%、21%、30%)对光催化净化效率的影响。以甲苯在光催化反应前后的质量浓度计算其去除效率,以表示催化剂的光催化性能。污染物的空速变化由总气体流量控制,紫外线灯组由4个254 nm圆形紫外线灯组成,光照强度由打开的紫外光灯的数量控制,强度分布用功率表示。
-
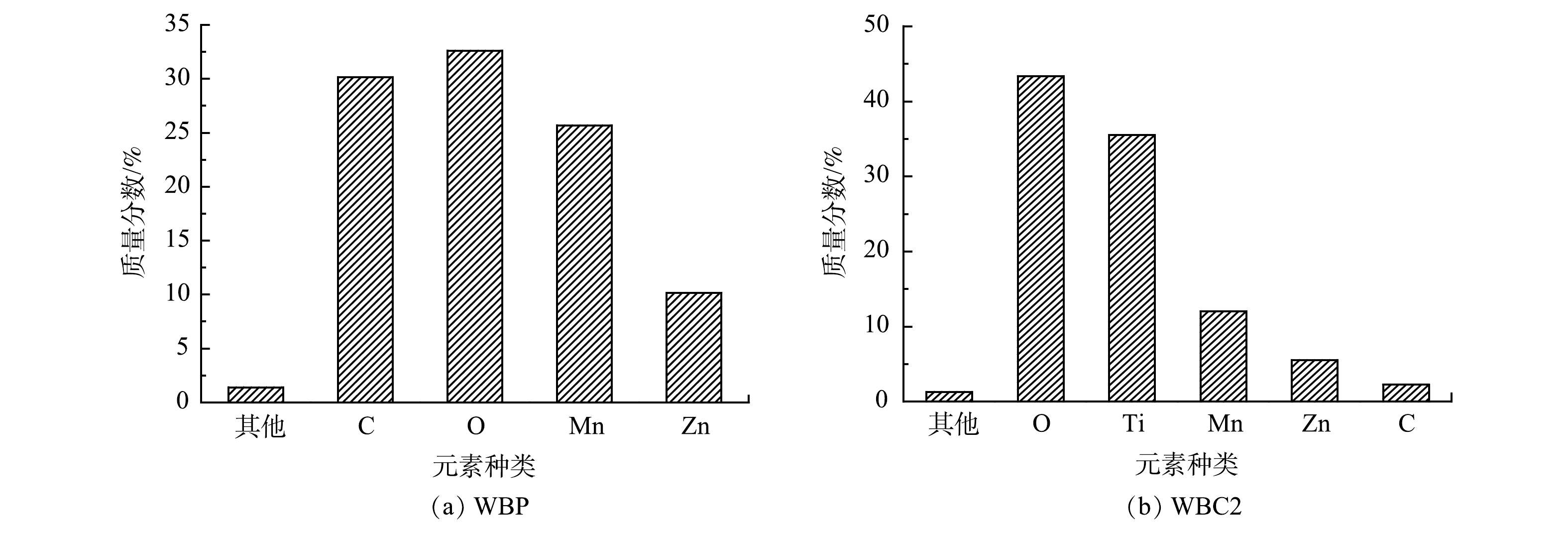
采用XRF对WBP进行了元素分析,结果如图2(a)所示。WBP主要含有Mn、Zn、C和O 4种元素,质量分数分别为25.69%、10.14%、30.16%和32.62%,其他微量元素含量不到1%。复合改性催化剂WBC2的元素质量分数如图2(b)所示,与复合前相比,催化剂中碳的质量分数大幅降低。其原因可能是,在煅烧过程中,碳转化为CO2或其他气态物质而挥发。根据WBC2的元素组成分析可知,催化剂主要以锰、锌和钛的氧化物的形式存在。
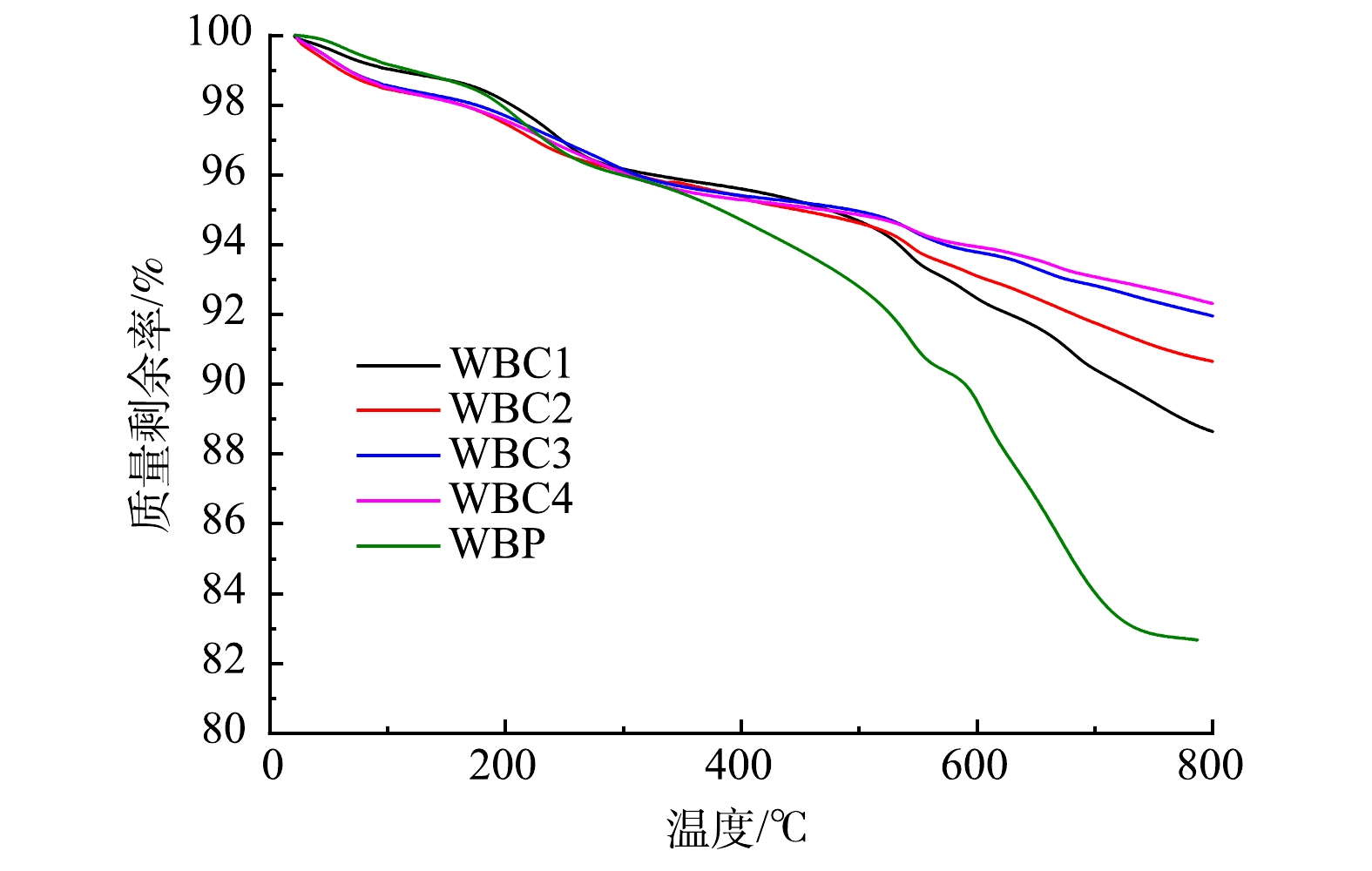
在空气气氛中,以10 ℃·min−1的升温速率,对样品在25~800 ℃进行了热重分析。球磨后催化剂的TG曲线如图3所示。随着温度的升高,催化剂的质量下降。当温度达到500 ℃时,WBC2的质量下降了5%,这可能是由于催化剂中的碳在加热过程中生成为CO2导致的。温度继续上升到800 ℃时,催化剂的质量继续下降。在XRF结果中也可以看出,催化剂在500 ℃时碳的剩余量已经很低了,所以温度升高到500 ℃以上时,碳质量的减少可能是因氧的析出。
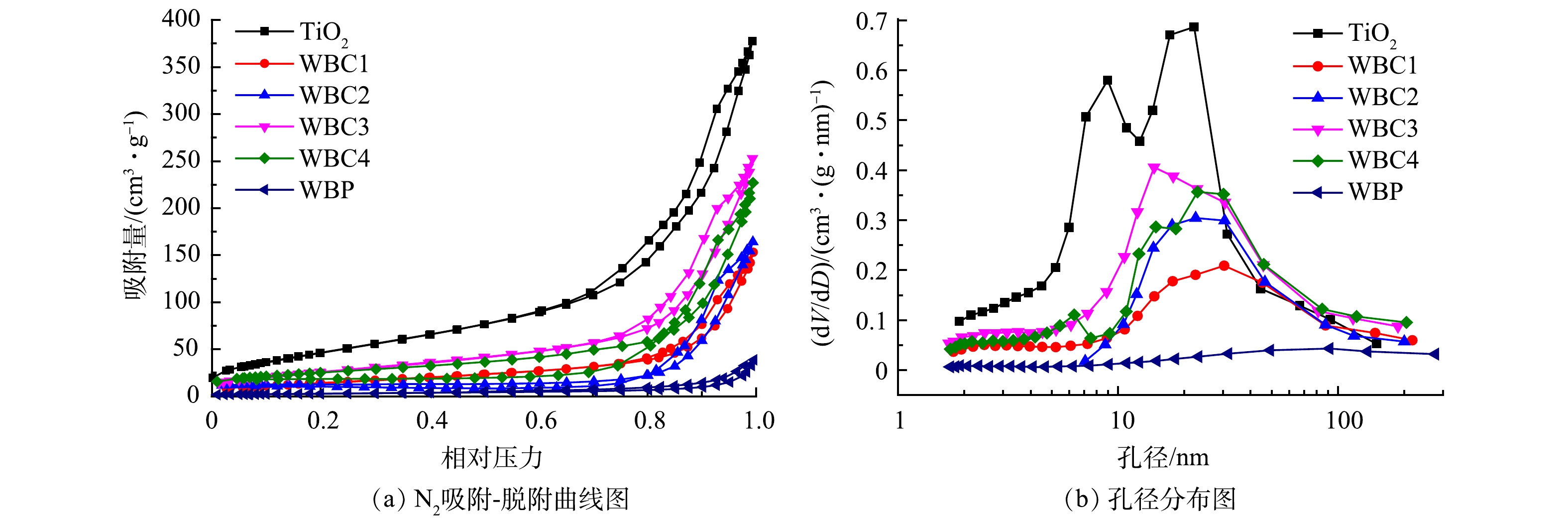
图4(a)显示了TiO2、WBC1、WBC2、WBC3、WBC4和WBP的N2吸附-脱附等温线;图4(b)展示了样品的孔径分布。其中,图4(a)中的样品等温线显示为典型的第Ⅳ类等温线,这是介孔结构的典型特征[22]。如图4(b)所示,与其他改性后催化剂相比较,TiO2的孔径分布范围更大、更平均,孔结构更发达。表1列出了不同样品的比表面积(SBET)、孔体积(Vt)和孔径。TiO2的比表面积、孔容和孔径分别为179.33 m2·g−1、0.583 cm3·g−1和13.022 nm。从表1的数据中可以看出,随着WBP的加入,催化剂的比表面积和总孔体积减小,孔径增大。比表面积和孔体积的降低会导致催化剂吸附性能的降低[23]。因此,WBP的加入会降低光催化剂的吸附性能。
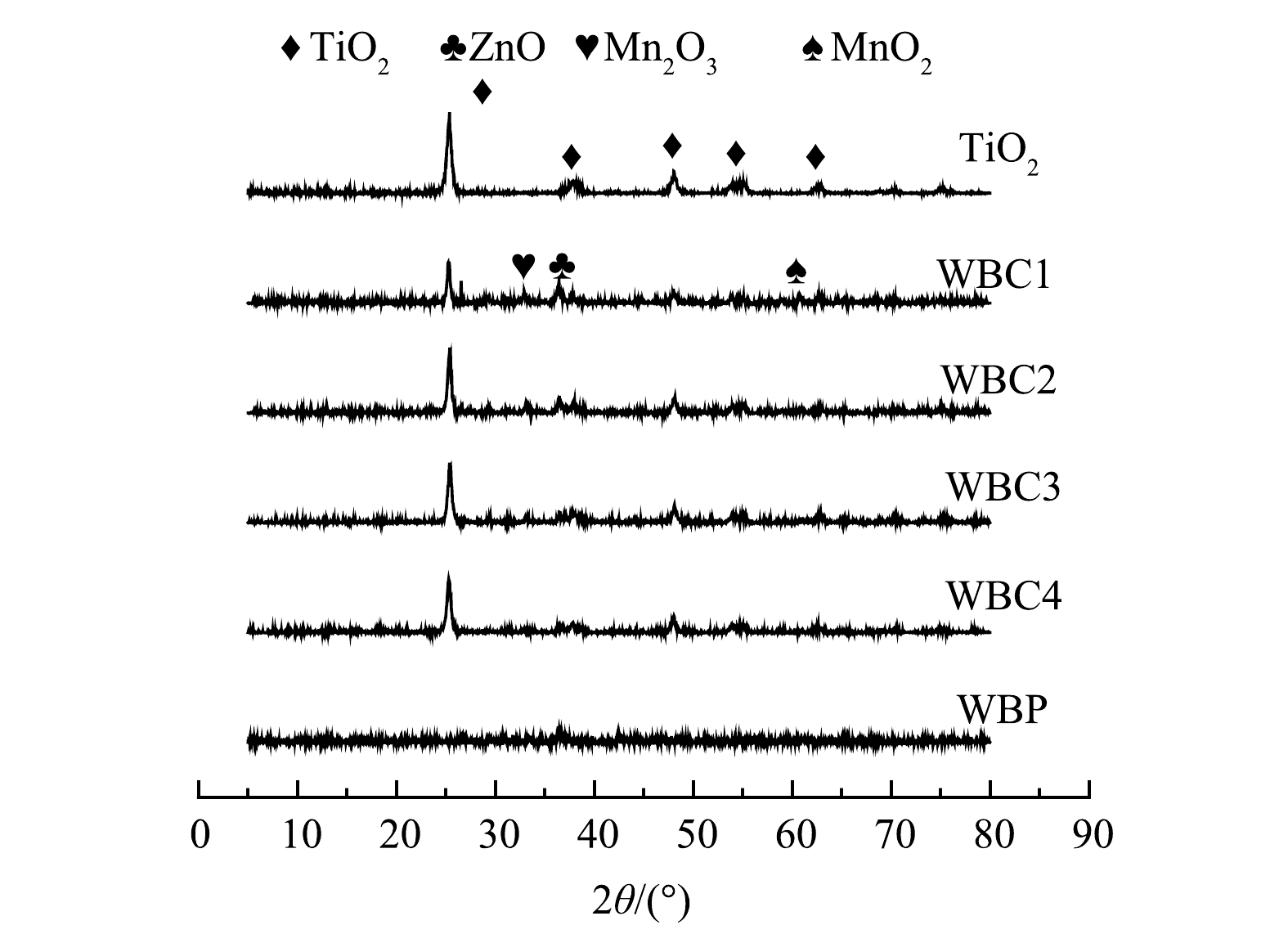
图5为样品TiO2、WBC1、WBC2、WBC3、WBC4和WBP的XRD衍射图。如图5所示,所有样品均呈现典型的(101)、(102)、(204)、(215)锐钛矿衍射峰(JCPDS No.21-1272)[24]。锐钛矿型TiO2经电池芯粉改性后,其特征峰保持完整,这说明锐钛矿型TiO2的晶体结构和晶型没有因电池芯粉的加入和球磨工艺而改变。随着WBP的加入,氧化锌对应的衍射峰出现在2θ=36.1°处[9],同时也出现了相应的二氧化锰衍射峰。随着WBP含量的降低,MnO2和ZnO的XRD衍射峰强度变弱,MnO2和ZnO的负载量降低,进而可能影响催化剂的改性效果。
从图6(a)~图6(d)中可以看出,TiO2和WBP混合均匀。在4种改性样品中,WBC2(图4(b))、WBC3(图4(c))和WBC4(图4(d))样品具有较好的分散性,WBC1(图4(a))样品中WBP的加入量较大,其表面颗粒团聚现象严重,WBC1样品的颗粒增大。随着WBP添加量的增加,催化剂表面发生团聚,使催化剂粒径变大,不利于WBP与TiO2形成较好的接触,进而影响不同物质之间的电子传递,并且使对光生电子空穴对复合的抑制作用降低。此外催化剂表面团聚会造成比表面积下降,进而影响催化剂的吸附性能。这与BET结果相符。
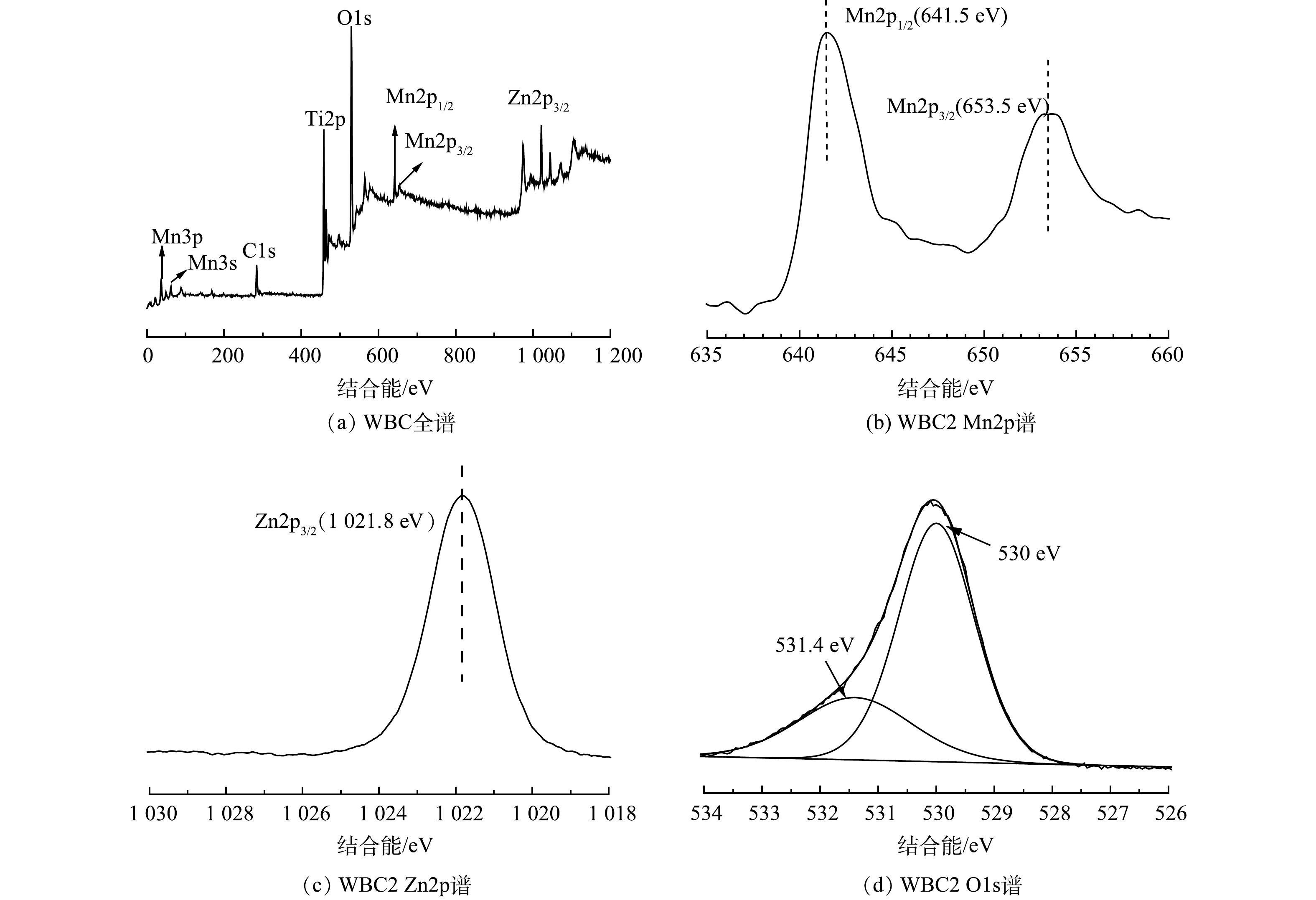
图7(a)显示了WBC2的全尺寸XPS光谱。复合材料中,O 1s、C 1s、Ti 2p、Mn(2p3/2、2p1/2、3s和3p)和Zn 2p的峰值明显,这表明存在O、C、Ti、Mn、Zn等元素。为了研究Mn的化学价态,对Mn 2p进行了高分辨XPS分析。如图7(b)所示,在641.5eV和653.5eV的位置处出现了属于Mn 2p3/2和Mn 2p1/2结合能的Mn 2p峰,根据文献[10,25]推测,这对应于Mn4+的特征峰[9,24]。这表明,催化剂中的Mn以Mn4+氧化物的形式存在,说明MnO2已成功沉积在TiO2表面。图7(c)显示了Zn 2p状态下的电子能级XPS谱。1021.8 eV对应于ZnO晶格中Zn2+的2p3/2。同时,与O1s区相对应的高分辨率XPS光谱如图7(d)所示,O1s峰可以模拟特征结合能为530eV和531.4eV的2个峰,这意味着形成了2种不同类型的O1s态。这些峰可以归因于晶格氧(Ti-O)和羟基-OH。
-
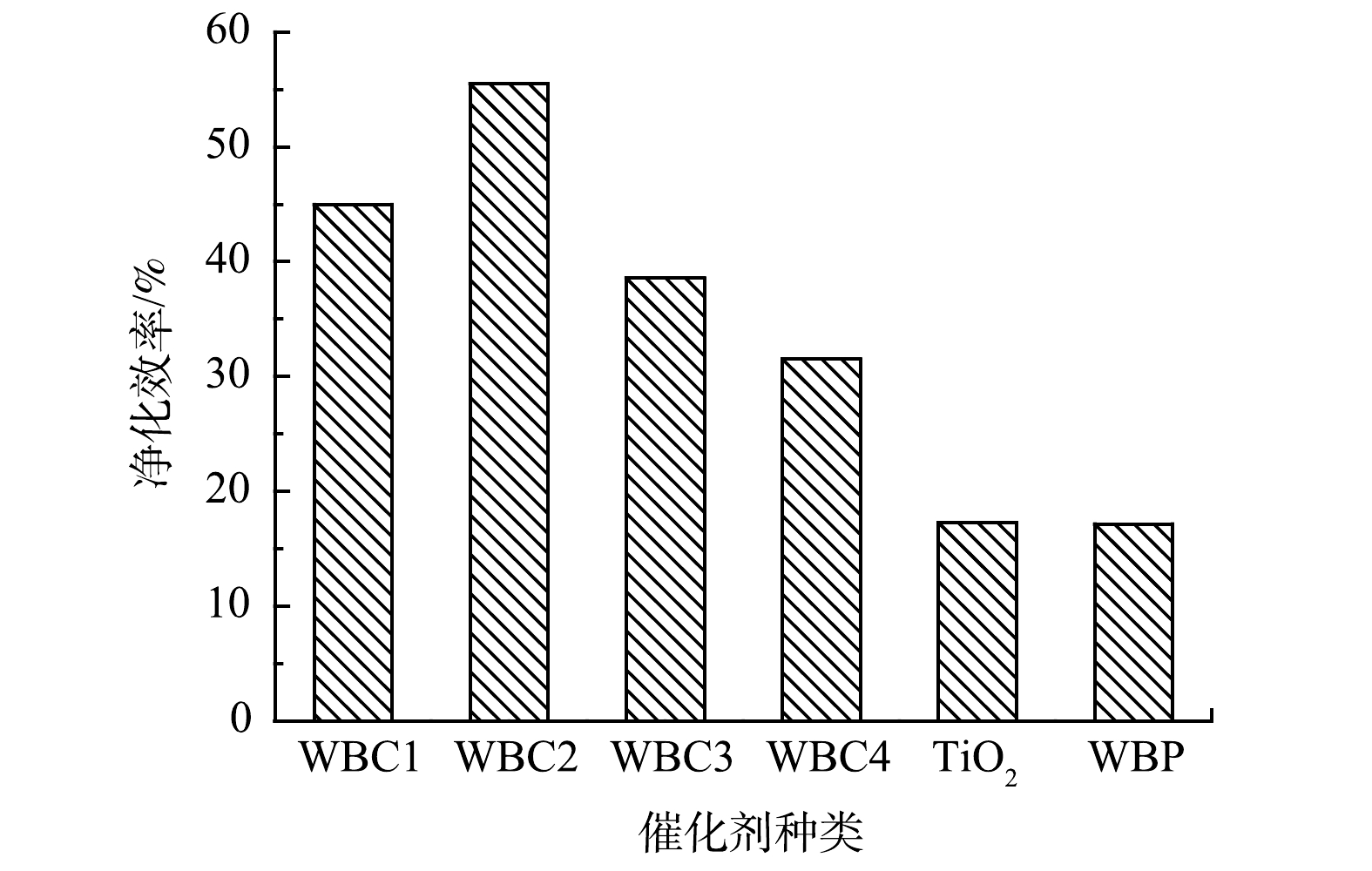
在空速为0.33 s−和甲苯初始质量浓度为62.5 mg·m−3时,在20 W紫外光照射下,分别进行了TiO2、WBC1、WBC2、WBC3、WBC4和WBP对甲苯的光催化净化实验。实验中使用的催化剂均经过穿透吸附试验后达到甲苯吸附饱和状态,排除了吸附性能变化对净化效率的影响。如图8所示,废电池芯粉改性光催化剂的净化效率得到很大提高,其中,WBC2对甲苯的净化效率最高。纯TiO2和WBP的净化效率较低,仅为10%左右。采用球磨法将2种物质混合后,4种混合比例的催化剂对甲苯的净化效率均大大提高,其中改性效果最好的WBC2对甲苯的净化效率提高了近45%。
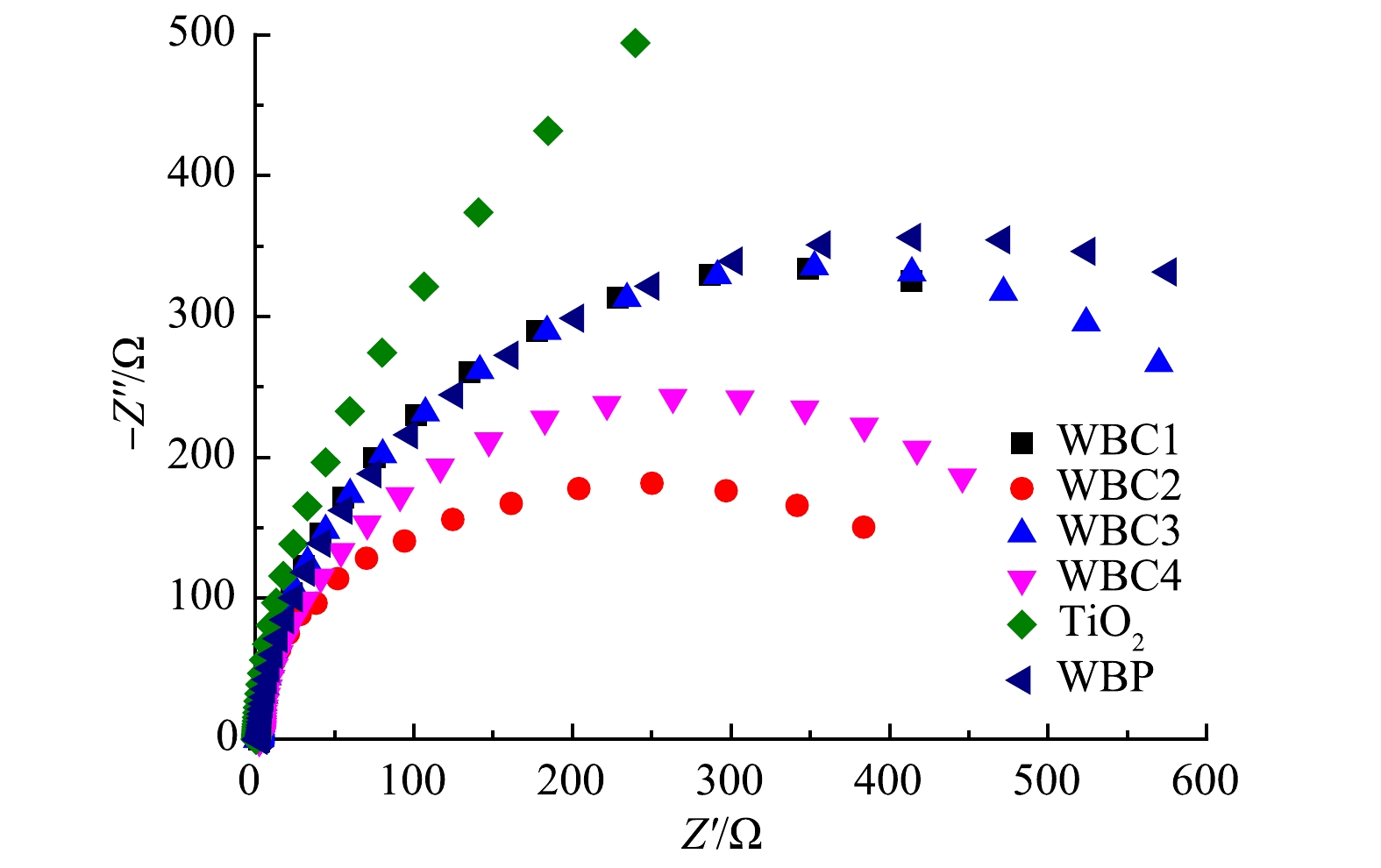
为了更进一步揭示催化剂性能提升的机理,使用EIS-Nyquist图来描述催化剂表面电荷载体的转移特性。阻抗弧半径越小,则物质间的电子转移越快。从图9可以看出,激发光照射下TiO2的Nynquist半径最大,这表明TiO2具有较大的电极阻抗、缓慢的表面电荷转移速率和较低的分离效率[26]。在所有样品中,WBC2的弧半径最小,电荷转移速率最快,电子-空穴对分离效率最好。
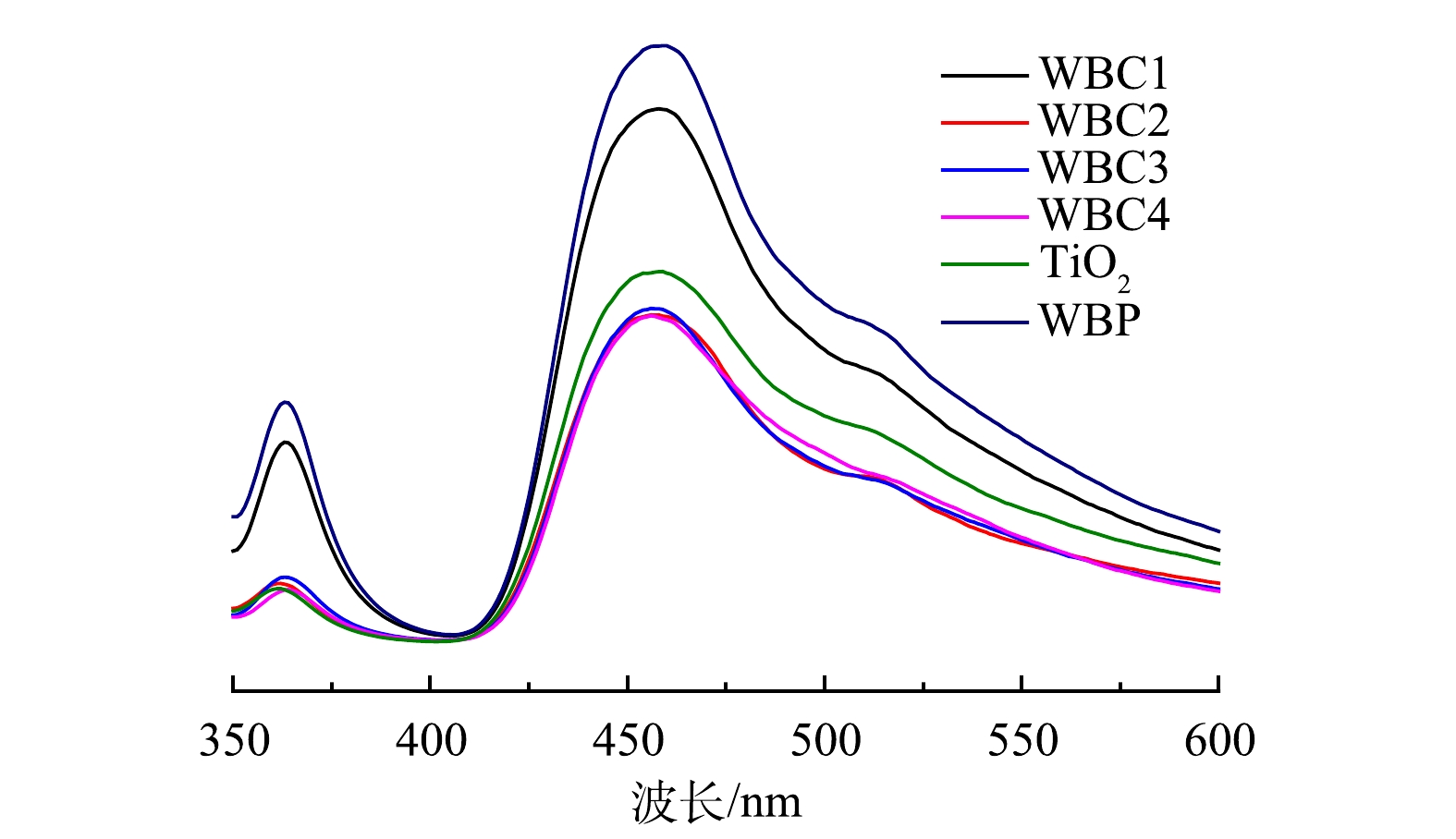
利用室温PL光谱可揭示催化剂电子-空穴对的分离能力。由图10可以看出,在456~458 nm处,6种催化剂均表现出较强的荧光发射峰。当TiO2与WBP复合时,催化剂的发光强度受到强烈抑制。这说明WBP与TiO2之间存在良好的电荷转移,新型复合催化剂的电子-空穴对复合速率降低,光催化活性提高[27]。随着WBP复合量的增加,光生电子-空穴对的分离效率提高,其中WBC2的分离效率最高。EIS和PL光谱的结果与光催化净化实验获得的结果一致。WBC2净化效率最高的原因可能是,随着WBP复合量的增加,可以提供更多的电子-空穴对吸附位点,从而更有效地抑制电子-空穴对的复合,提高催化活性。当WBP的复合量过大时,它会在催化剂表面聚集,如SEM结果所示。这不利于WBP与TiO2的接触,进而影响二者之间的电子传递,导致催化活性降低,所以WBC1对甲苯的净化效率低于WBC2。
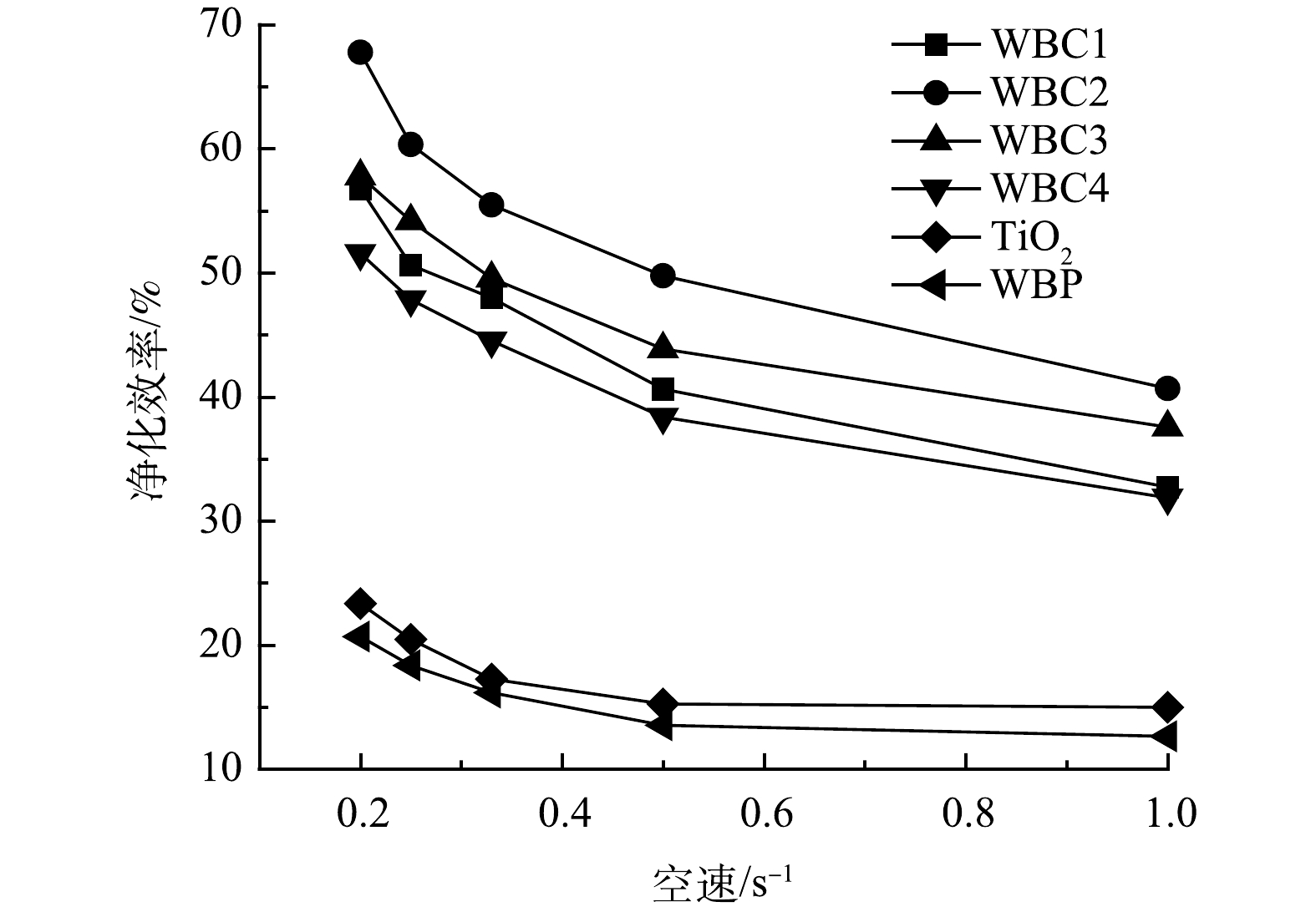
如图11所示,随着空速的降低,污染物的净化效率相应提高。一方面,污染物与催化剂的接触更加充分,污染物分子可以吸收更多的紫外光能量,提高光反应的量子效应;另一方面,也增加了污染物与反应体系中产生的·OH等强氧化活性物质的碰撞概率,提高了污染物的净化效率[28]。当空速从1 s−1降低到0.2 s−1时,TiO2对甲苯的净化效率提高了约10%,而WBC2对甲苯的净化效率提高了近27%。可以看出,经废旧锌锰电池改性后的催化剂可以产生更多的活性组分,降低空速,延长污染物与催化剂的接触时间,催化剂的净化能力将有更大的提升空间。然而,空速的降低也会导致催化剂用量的增加,同时也会增加净化设备的前期投资成本。因此,综合考虑效率和成本因素,确定最优空速具有重要的现实意义。
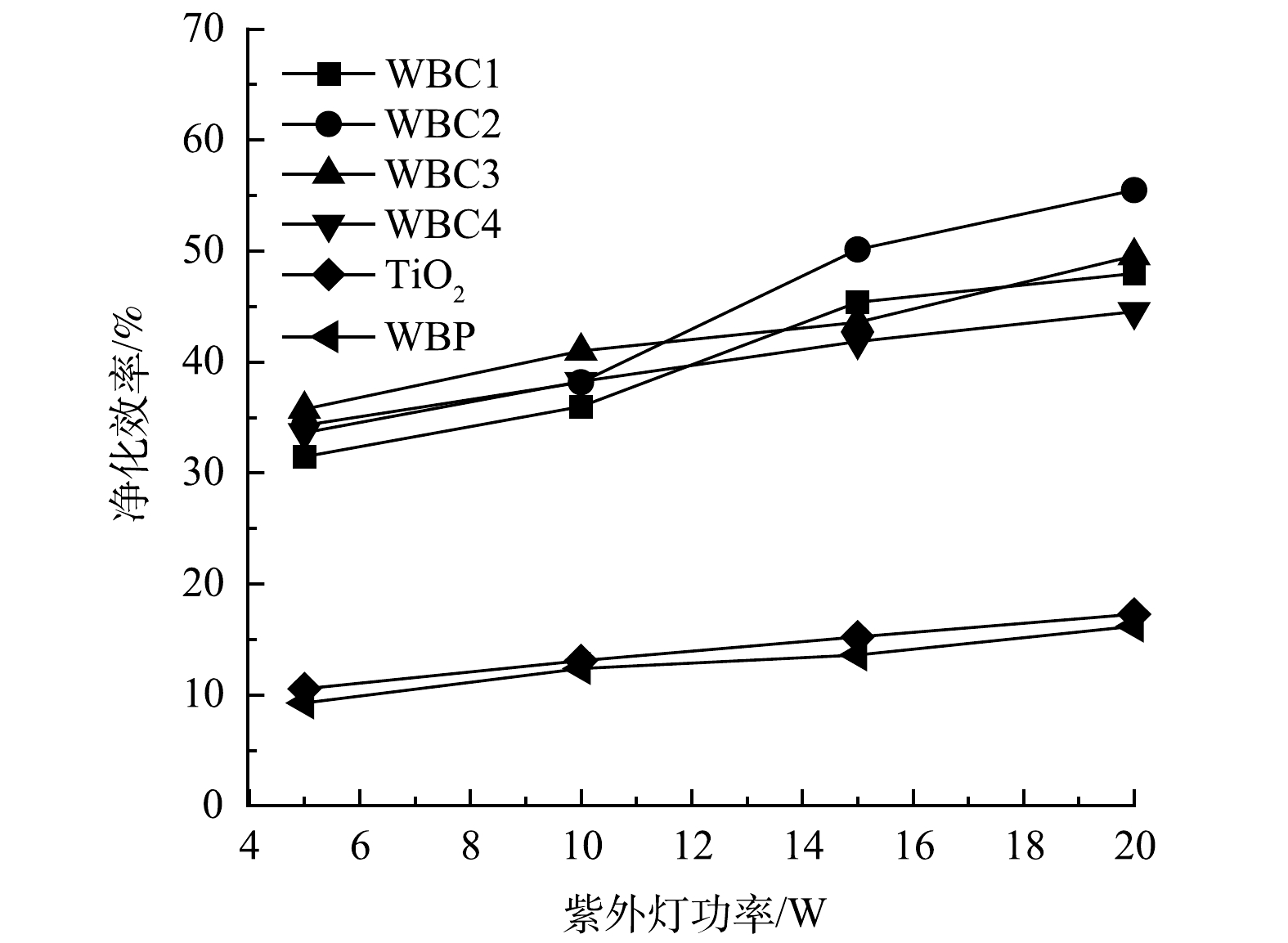
如图12所示,随着紫外光照射强度的增加,光催化净化效率亦相应提高。光照强度越高,单位体积内入射光子越多,催化剂表面产生的活性物质越多,反应越快,则净化效率越高。但是,光的强度并不是越高越好。当光子利用率达到最大值时,光强度的提高并不能转化为更高的净化效率,从而造成能量的浪费。实验结果表明,随着光照强度的增加,WBC2对甲苯的净化效率提高最大。这这说明WBC2催化剂的光子利用率上限较高,具有较高的光子利用率。然而,当紫外灯的功率从15 W上升到20 W时,净化效率的提高幅度会减小,并且随着光强的增加可能达到催化剂光子利用率的上限。因此,在实际应用中需要确定合适的光照强度。
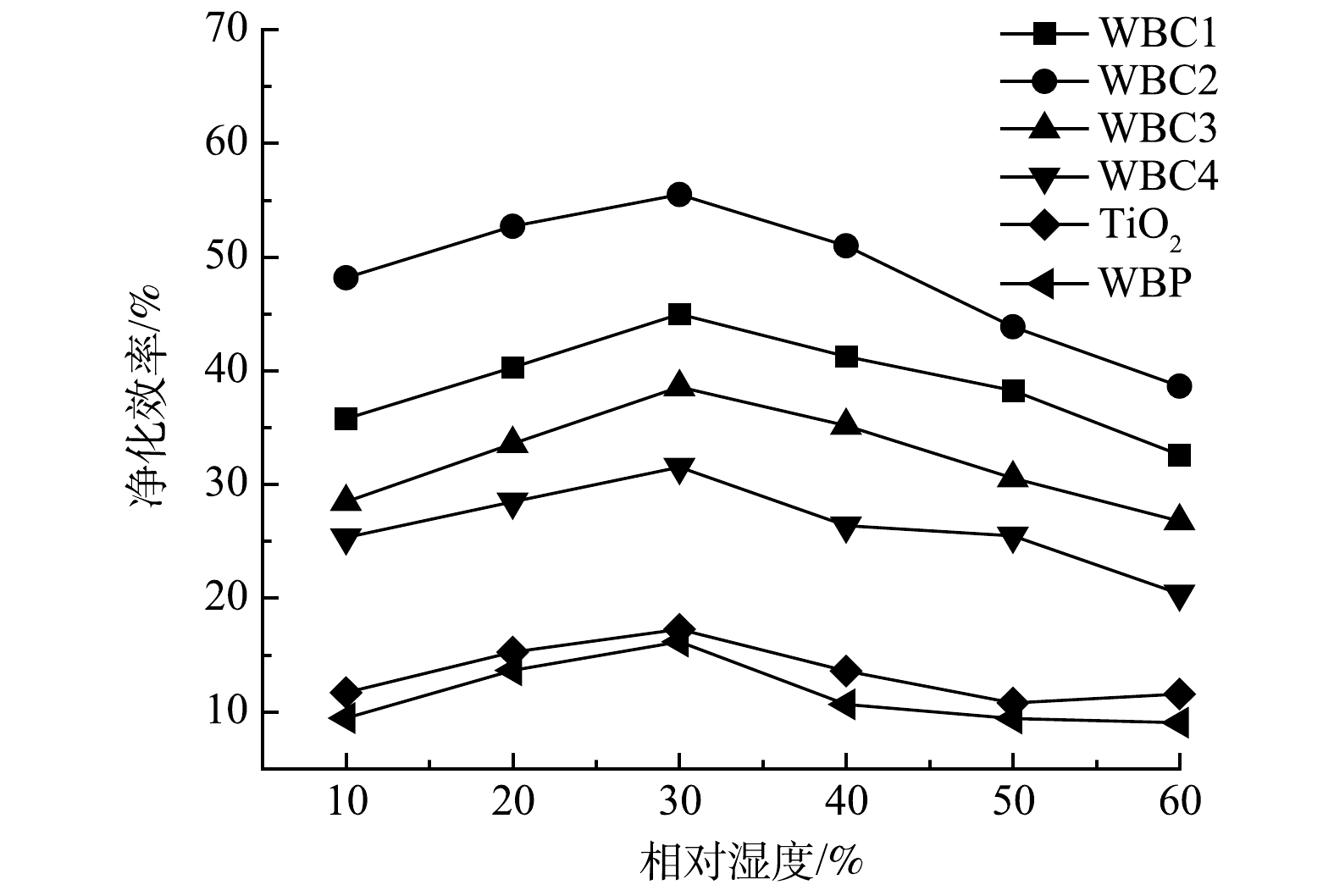
如图13所示,随着相对湿度的增加,净化效率先升高后降低,相对湿度在30%左右时净化效果最好。其原因可能是:随着反应体系中相对湿度的增加,水蒸气在催化剂表面水解形成大量羟基自由基,这会导致反应体系中羟基自由基浓度增加,从而提高污染物净化效率。但是,光催化净化甲苯是先吸附后氧化的过程,当水蒸气含量过高时,水蒸气会被催化剂吸附在表面,占据催化剂表面的活性吸附位点,从而降低催化剂对污染物的吸附,进而降低对甲苯的净化效率[28-29]。当反应系统的相对湿度过高时,污染物的净化效率呈下降趋势。从实验结果可以看出,当相对湿度过高时,WBC2对甲苯的净化效率下降幅度最大。这说明,WBC2对空气湿度较为敏感,不适合高湿度环境。尽管WBC2暴露在高湿度下净化效率会大幅度下降,但其对甲苯的净化效率仍高于其它催化剂,这说明本研究采用的光催化剂改性方法是有效的。
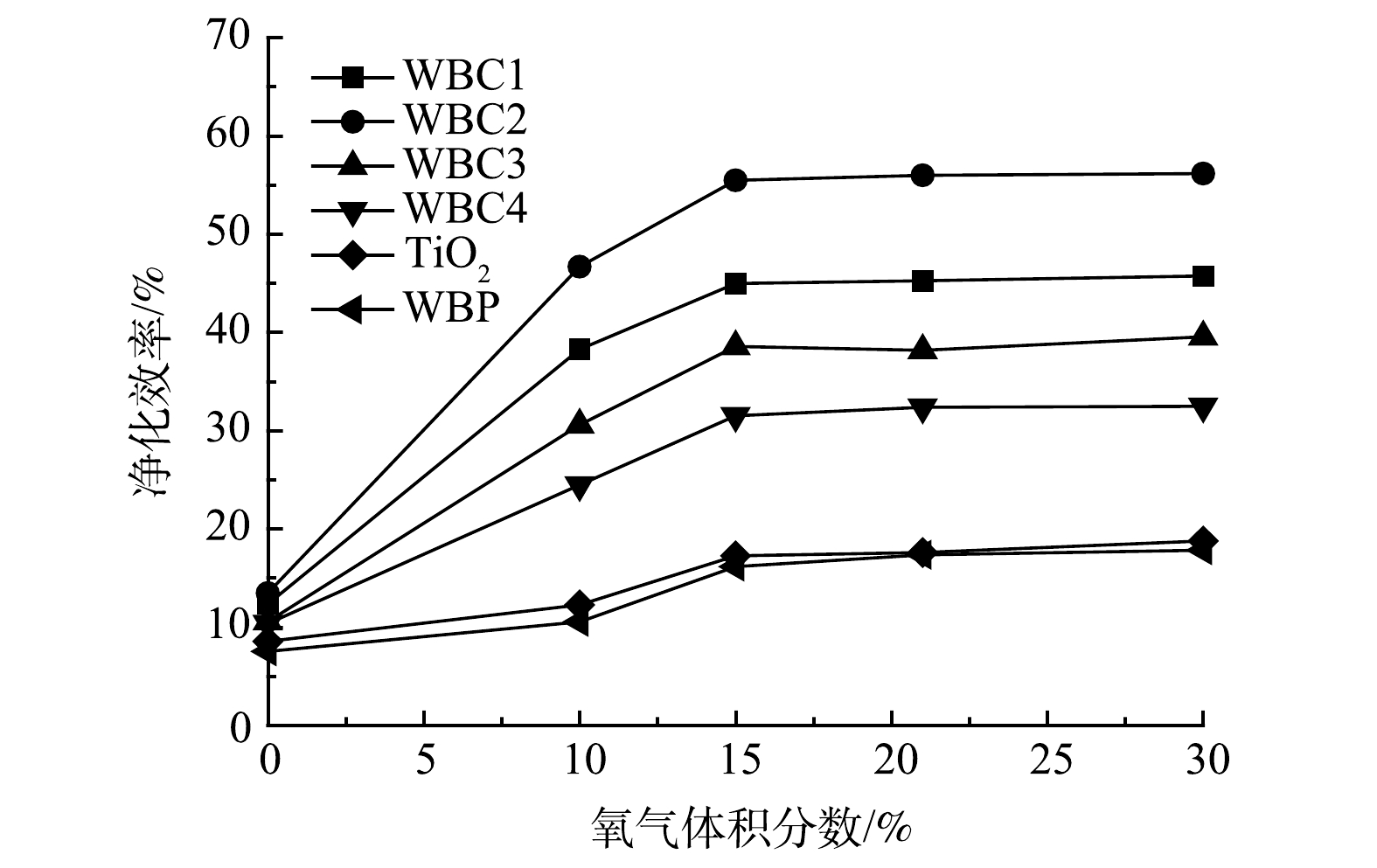
氧气体积分数对催化剂光催化净化效率的影响如图14所示。在缺氧条件下,光催化净化效率很低。随着氧气体积分数的增加,净化效率得到了很大的提高,但当氧气体积分数增加到15%~21%时,净化效率达到稳定状态,且不随氧气体积分数的增加而增加或降低。这可能是因为,吸附在催化剂表面的氧可以捕获催化剂在紫外光照射下产生的表面电子。一方面,它能促进活性基团的形成,如超氧离子(O2−)、过氧化氢(H2O2)和羟基自由基(·OH),从而参与光催化氧化;另一方面,它可以通过捕获电子在一定程度上抑制电子-空穴对的复合,从而提高催化效率[30]。然而,氧气量的不断增加可以提供足够的活性基团来源,但催化剂的转化能力和利用效率有限,不能带来持续的改善。当氧含量为21%时,WBC2对甲苯的净化效率达到峰值。与其它催化剂相比,净化效率的峰值出现在更高氧气浓度处,说明WBC2可以利用更多的氧气,将更多的氧气转化为活性基团。此实验结果还表明,在大气环境下,即可为光催化反应系统可以提供足够的氧气。
综合以上结果,无论反应条件如何变化,废电池复合改性催化剂的净化效率都高于TiO2,而WBC2在所有催化剂中一直处于最优地位。在任何条件下,该催化剂都能保持较高的催化活性,这说明WBC系列催化剂具有较强的环境适应性,能在相对恶劣的反应环境中保证较高的催化活性,当反应条件得到改善时,催化剂的净化能力将有更大的提升空间。
-
1)以TiO2和废旧锌锰电池芯粉为原料,用球磨法制备了复合光催化剂。TiO2与WBP的机械物理结合形成了一种新的复合结构,促进了光生电子-空穴对的分离,极大地提高了光催化活性。
2) WBC2对甲苯的净化效果最好,即二氧化钛与WBP的最佳配比(质量比)为2∶1。
3)空速越大催化剂对甲苯的净化效率越低;净化效率随光照强度的增加呈现先增加后保持不变的规律;催化剂在相对湿度为30%的条件下具有最佳的催化活性;氧气体积分数为15%时为净化效率达到最大。
利用废旧锌锰电池制备光催化剂净化甲苯
Preparation of photocatalyst for toluene purification from waste zinc manganese battery
-
摘要: 针对废旧锌锰电池回收利用难,以及光催化剂TiO2活性低的问题,以废旧锌锰电池和商业二氧化钛为原料,通过球磨法制备了新型复合光催化剂。在紫外光灯照射下,进行了废旧锌锰电池复合改性TiO2对甲苯的光催化氧化实验,并重点探究空速、光照强度、相对湿度和氧气体积分数等关键实验条件对甲苯净化效率的影响。结果表明,改性后的催化剂对甲苯的净化能力大幅提高;当TiO2与废电池芯粉的质量比为2∶1时,催化剂的催化效果最好,甲苯的净化效率提高了近45%;空速越大,催化剂对甲苯的净化效率越低;净化效率随光照强度的增加呈现先增加后保持不变的规律;催化剂在相对湿度为30%的条件下具有最佳的催化活性,氧气体积分数为15%时为净化效率达到最大。本研究结果可为废旧锌锰电池的回收利用提供新的思路。Abstract: In view of the difficult recycling of waste zinc manganese batteries and the low activity of photocatalyst TiO2, a new composite photocatalyst was prepared by ball milling with waste zinc manganese batteries and commercial titanium dioxide as raw materials. Under the irradiation of ultraviolet lamp, the photocatalytic oxidation of toluene by composite modified TiO2 of waste zinc manganese battery was studied, and the effects of key experimental conditions such as space velocity, light intensity, relative humidity and oxygen volume fraction on the purification efficiency of toluene were mainly explored. The results showed that the purification ability of the modified catalyst for toluene was greatly improved. When the mass ratio of TiO2 to waste battery core powder was 2∶1, the catalytic effect of the catalyst was the best, and the purification efficiency of toluene was increased by nearly 45%. It was found that the higher the space velocity, the lower the purification efficiency of the catalyst for toluene. The purification efficiency increased first and then remained unchanged with the increase of light intensity. The catalyst had the best catalytic activity when the relative humidity was 30%, and the purification efficiency reaches the maximum when the oxygen volume fraction was 15%. This study provided a new idea for the recycling of waste zinc manganese batteries.
-
Key words:
- solid waste recycling /
- waste zinc manganese battery /
- TiO2 /
- photocatalysis /
- toluene
-
挥发性有机物(VOCs)是一种有害气体污染物,是雾霾和臭氧污染的前驱物,会对环境和人群健康造成危害。因此,VOCs的控制技术已成为近年来的研究热点[1-2]。近年来,光催化氧化技术因其成本低、无二次污染等优点,得到了广泛的关注。催化剂是光催化技术的核心技术,由于TiO2具有成本低、安全、活性高、稳定性好等优点,目前已成为应用最广泛一种光催化剂[3-4]。然而,它也存在着一些缺点,如易失活、可见光利用率低等。因此,对光催化剂的改性研究成为了热点,目前主要的改性方法主要包括金属离子掺杂、非金属离子掺杂、贵金属表面沉积、表面光敏化和半导体复合材料等[5]。
有研究表明,复合半导体改性方法能有效提高TiO2的光催化活性,特别是ZnO和MnO2[6-8]。SIWINSKA等[9]采用溶胶-凝胶法研究了一系列不同摩尔比的二氧化钛-氧化锌体系。结果表明,与原来的TiO2相比,二氧化钛-氧化锌对3种有机染料具有更高的光催化活性。MA等[10]采用阳极氧化和电沉积方法合成了二氧化锰/二氧化钛纳米管阵列(MnO2/TiO2-NTAS)光电阴极,具有良好的稳定性和可重复利用性,在废水处理中具有很大的应用潜力。
Zn-MnO2干电池具有成本低、容量大等优点,是目前应用最广泛的消费型电池之一。全球锌锰电池的年消耗量约为6×107 t,且仍在增长[11]。废旧锌锰电池含有大量的锰和锌以及其他重金属元素,大量废弃的锌锰电池处理不当会造成土壤、水和大气污染[12-13]。因此,开发出一种经济可行的回收方法非常重要,将废旧电池回收制备功能材料已经逐渐成为研究热点[14-19]。GALLEGOS等[20]使用生物湿法冶金工艺从废电池中回收锌和锰,以制备去除VOCs的催化剂。ZHANG等[21]采用还原酸浸法从废旧锌锰电池中成功合成二氧化锰,并用其负载Cu作为一氧化碳氧化催化剂。将废旧锌锰电池制备成催化剂来处理环境问题是一种具有前景的技术。但截至目前,现有的回收方法过于复杂,成本过高,大部分都只针对废旧电池中的特定金属进行回收,并且回收过程中还存在二次污染问题,因而不利于在实际工程中推广应用。
废旧锌锰电池中含有大量的锰和锌,利用废旧锌锰电池来提高TiO2的光催化性能是一种较为可行的方法。因此,本研究以废旧锌锰电池和二氧化钛为原料,采用球磨法制备复合改性光催化剂,以实现废旧电池中的相关物质全部回收利用。
1. 材料与方法
1.1 实验原料
废旧锌锰电池均来源于生活中收集的5号废旧南孚电池,TiO2为重庆新华化工有限公司生产的TiO2-C801,无水乙醇(CH3CH2OH)为分析纯。
1.2 实验装置
如图1所示,反应器由一个内径为8 mm的石英管制成,催化剂固定在反应器的中心,以4个254 nm的环形紫外灯环绕在反应器外部作为光源。将甲苯混合气体通入反应器,与催化剂接触发生光催化反应。采用气相色谱测定甲苯污染物浓度。
1.3 实验方法
1)废旧锌锰电池芯粉的制备。将电池金属外壳机械移除,并收集内部残留物。内部残留物用去离子水反复洗涤,然后于105 ℃干燥并研磨成粉末。制备的废电池芯粉命名为WBP。
2)废旧锌锰电池复合光催化剂的制备。将TiO2和WBP按不同质量比倒入玛瑙研钵中。将一定量的无水乙醇溶液倒入玛瑙砂钵中,用球磨机以500 r·min−1的速度球磨6 h。将混合后的糊状催化剂放入烘箱中,在80 ℃下至完全干燥。然后,将干燥后的催化剂研磨成均匀的粉末,在500 ℃煅烧4 h,制备出废旧锌锰电池芯粉-TiO2催化剂,命名为WBC。根据TiO2与WBP的质量比分别为1∶1、2∶1、3∶1和4∶1,将光催化剂分别命名为WBC1、WBC2、WBC3和WBC4。
1.4 分析方法
1)催化剂表征。采用XRF表征测定样品各元素的含量;采用TG热重法测定催化剂在升温加热过程中的质量变化;采用比表面积分析仪测定样品的比表面积和孔径分布;采用扫描电子显微镜(SEM)观察样品的形貌;采用X射线衍射(XRD)分析样品的元素和晶体结构;采用X射线光电子能谱(XPS)测定样品的表面元素和价态;采用EIS和PL表征催化剂的光电性能。
2)光催化降解甲苯实验。首先,将1.5 mL样品置于固定床连续流动反应器中;然后,将初始浓度为62.5 mg·m−3的甲苯以30 mL·min−1的流速引入气体混合室,氮气通过洗气瓶后进入混气瓶,为反应提供水蒸气,氧气直接通入混气瓶,反应气体在进入光催化反应器之前在混气瓶中充分混合;最后,混合均匀的反应气体进入光催化反应器,进行光催化反应。
研究空速(0.2、0.25、0.33、0.5、1.0 s−1)、光强(5 W、10 W、15 W、20 W)、相对湿度(10%、20%、30%、40%、50%、60%)和氧含量(0、10%、15%、21%、30%)对光催化净化效率的影响。以甲苯在光催化反应前后的质量浓度计算其去除效率,以表示催化剂的光催化性能。污染物的空速变化由总气体流量控制,紫外线灯组由4个254 nm圆形紫外线灯组成,光照强度由打开的紫外光灯的数量控制,强度分布用功率表示。
2. 结果与讨论
2.1 催化剂表征
采用XRF对WBP进行了元素分析,结果如图2(a)所示。WBP主要含有Mn、Zn、C和O 4种元素,质量分数分别为25.69%、10.14%、30.16%和32.62%,其他微量元素含量不到1%。复合改性催化剂WBC2的元素质量分数如图2(b)所示,与复合前相比,催化剂中碳的质量分数大幅降低。其原因可能是,在煅烧过程中,碳转化为CO2或其他气态物质而挥发。根据WBC2的元素组成分析可知,催化剂主要以锰、锌和钛的氧化物的形式存在。
在空气气氛中,以10 ℃·min−1的升温速率,对样品在25~800 ℃进行了热重分析。球磨后催化剂的TG曲线如图3所示。随着温度的升高,催化剂的质量下降。当温度达到500 ℃时,WBC2的质量下降了5%,这可能是由于催化剂中的碳在加热过程中生成为CO2导致的。温度继续上升到800 ℃时,催化剂的质量继续下降。在XRF结果中也可以看出,催化剂在500 ℃时碳的剩余量已经很低了,所以温度升高到500 ℃以上时,碳质量的减少可能是因氧的析出。
图4(a)显示了TiO2、WBC1、WBC2、WBC3、WBC4和WBP的N2吸附-脱附等温线;图4(b)展示了样品的孔径分布。其中,图4(a)中的样品等温线显示为典型的第Ⅳ类等温线,这是介孔结构的典型特征[22]。如图4(b)所示,与其他改性后催化剂相比较,TiO2的孔径分布范围更大、更平均,孔结构更发达。表1列出了不同样品的比表面积(SBET)、孔体积(Vt)和孔径。TiO2的比表面积、孔容和孔径分别为179.33 m2·g−1、0.583 cm3·g−1和13.022 nm。从表1的数据中可以看出,随着WBP的加入,催化剂的比表面积和总孔体积减小,孔径增大。比表面积和孔体积的降低会导致催化剂吸附性能的降低[23]。因此,WBP的加入会降低光催化剂的吸附性能。
表 1 样品的比表面积、孔容和孔径Table 1. Specific surface area, pore volume, and pore size of the samples样品名称 比表面积/(m2·g−1) 孔体积/(cm3·g−1) 平均孔径/nm TiO2 179.33 0.58 13.02 WBP 10.08 0.06 23.66 WBC1 54.64 0.24 17.32 WBC2 68.9 0.28 16.09 WBC3 89.61 0.35 15.91 WBC4 98.31 0.39 15.67 图5为样品TiO2、WBC1、WBC2、WBC3、WBC4和WBP的XRD衍射图。如图5所示,所有样品均呈现典型的(101)、(102)、(204)、(215)锐钛矿衍射峰(JCPDS No.21-1272)[24]。锐钛矿型TiO2经电池芯粉改性后,其特征峰保持完整,这说明锐钛矿型TiO2的晶体结构和晶型没有因电池芯粉的加入和球磨工艺而改变。随着WBP的加入,氧化锌对应的衍射峰出现在2θ=36.1°处[9],同时也出现了相应的二氧化锰衍射峰。随着WBP含量的降低,MnO2和ZnO的XRD衍射峰强度变弱,MnO2和ZnO的负载量降低,进而可能影响催化剂的改性效果。
从图6(a)~图6(d)中可以看出,TiO2和WBP混合均匀。在4种改性样品中,WBC2(图4(b))、WBC3(图4(c))和WBC4(图4(d))样品具有较好的分散性,WBC1(图4(a))样品中WBP的加入量较大,其表面颗粒团聚现象严重,WBC1样品的颗粒增大。随着WBP添加量的增加,催化剂表面发生团聚,使催化剂粒径变大,不利于WBP与TiO2形成较好的接触,进而影响不同物质之间的电子传递,并且使对光生电子空穴对复合的抑制作用降低。此外催化剂表面团聚会造成比表面积下降,进而影响催化剂的吸附性能。这与BET结果相符。
图7(a)显示了WBC2的全尺寸XPS光谱。复合材料中,O 1s、C 1s、Ti 2p、Mn(2p3/2、2p1/2、3s和3p)和Zn 2p的峰值明显,这表明存在O、C、Ti、Mn、Zn等元素。为了研究Mn的化学价态,对Mn 2p进行了高分辨XPS分析。如图7(b)所示,在641.5eV和653.5eV的位置处出现了属于Mn 2p3/2和Mn 2p1/2结合能的Mn 2p峰,根据文献[10,25]推测,这对应于Mn4+的特征峰[9,24]。这表明,催化剂中的Mn以Mn4+氧化物的形式存在,说明MnO2已成功沉积在TiO2表面。图7(c)显示了Zn 2p状态下的电子能级XPS谱。1021.8 eV对应于ZnO晶格中Zn2+的2p3/2。同时,与O1s区相对应的高分辨率XPS光谱如图7(d)所示,O1s峰可以模拟特征结合能为530eV和531.4eV的2个峰,这意味着形成了2种不同类型的O1s态。这些峰可以归因于晶格氧(Ti-O)和羟基-OH。
2.2 改性催化剂净化甲苯的性能
在空速为0.33 s−和甲苯初始质量浓度为62.5 mg·m−3时,在20 W紫外光照射下,分别进行了TiO2、WBC1、WBC2、WBC3、WBC4和WBP对甲苯的光催化净化实验。实验中使用的催化剂均经过穿透吸附试验后达到甲苯吸附饱和状态,排除了吸附性能变化对净化效率的影响。如图8所示,废电池芯粉改性光催化剂的净化效率得到很大提高,其中,WBC2对甲苯的净化效率最高。纯TiO2和WBP的净化效率较低,仅为10%左右。采用球磨法将2种物质混合后,4种混合比例的催化剂对甲苯的净化效率均大大提高,其中改性效果最好的WBC2对甲苯的净化效率提高了近45%。
为了更进一步揭示催化剂性能提升的机理,使用EIS-Nyquist图来描述催化剂表面电荷载体的转移特性。阻抗弧半径越小,则物质间的电子转移越快。从图9可以看出,激发光照射下TiO2的Nynquist半径最大,这表明TiO2具有较大的电极阻抗、缓慢的表面电荷转移速率和较低的分离效率[26]。在所有样品中,WBC2的弧半径最小,电荷转移速率最快,电子-空穴对分离效率最好。
利用室温PL光谱可揭示催化剂电子-空穴对的分离能力。由图10可以看出,在456~458 nm处,6种催化剂均表现出较强的荧光发射峰。当TiO2与WBP复合时,催化剂的发光强度受到强烈抑制。这说明WBP与TiO2之间存在良好的电荷转移,新型复合催化剂的电子-空穴对复合速率降低,光催化活性提高[27]。随着WBP复合量的增加,光生电子-空穴对的分离效率提高,其中WBC2的分离效率最高。EIS和PL光谱的结果与光催化净化实验获得的结果一致。WBC2净化效率最高的原因可能是,随着WBP复合量的增加,可以提供更多的电子-空穴对吸附位点,从而更有效地抑制电子-空穴对的复合,提高催化活性。当WBP的复合量过大时,它会在催化剂表面聚集,如SEM结果所示。这不利于WBP与TiO2的接触,进而影响二者之间的电子传递,导致催化活性降低,所以WBC1对甲苯的净化效率低于WBC2。
如图11所示,随着空速的降低,污染物的净化效率相应提高。一方面,污染物与催化剂的接触更加充分,污染物分子可以吸收更多的紫外光能量,提高光反应的量子效应;另一方面,也增加了污染物与反应体系中产生的·OH等强氧化活性物质的碰撞概率,提高了污染物的净化效率[28]。当空速从1 s−1降低到0.2 s−1时,TiO2对甲苯的净化效率提高了约10%,而WBC2对甲苯的净化效率提高了近27%。可以看出,经废旧锌锰电池改性后的催化剂可以产生更多的活性组分,降低空速,延长污染物与催化剂的接触时间,催化剂的净化能力将有更大的提升空间。然而,空速的降低也会导致催化剂用量的增加,同时也会增加净化设备的前期投资成本。因此,综合考虑效率和成本因素,确定最优空速具有重要的现实意义。
如图12所示,随着紫外光照射强度的增加,光催化净化效率亦相应提高。光照强度越高,单位体积内入射光子越多,催化剂表面产生的活性物质越多,反应越快,则净化效率越高。但是,光的强度并不是越高越好。当光子利用率达到最大值时,光强度的提高并不能转化为更高的净化效率,从而造成能量的浪费。实验结果表明,随着光照强度的增加,WBC2对甲苯的净化效率提高最大。这这说明WBC2催化剂的光子利用率上限较高,具有较高的光子利用率。然而,当紫外灯的功率从15 W上升到20 W时,净化效率的提高幅度会减小,并且随着光强的增加可能达到催化剂光子利用率的上限。因此,在实际应用中需要确定合适的光照强度。
如图13所示,随着相对湿度的增加,净化效率先升高后降低,相对湿度在30%左右时净化效果最好。其原因可能是:随着反应体系中相对湿度的增加,水蒸气在催化剂表面水解形成大量羟基自由基,这会导致反应体系中羟基自由基浓度增加,从而提高污染物净化效率。但是,光催化净化甲苯是先吸附后氧化的过程,当水蒸气含量过高时,水蒸气会被催化剂吸附在表面,占据催化剂表面的活性吸附位点,从而降低催化剂对污染物的吸附,进而降低对甲苯的净化效率[28-29]。当反应系统的相对湿度过高时,污染物的净化效率呈下降趋势。从实验结果可以看出,当相对湿度过高时,WBC2对甲苯的净化效率下降幅度最大。这说明,WBC2对空气湿度较为敏感,不适合高湿度环境。尽管WBC2暴露在高湿度下净化效率会大幅度下降,但其对甲苯的净化效率仍高于其它催化剂,这说明本研究采用的光催化剂改性方法是有效的。
氧气体积分数对催化剂光催化净化效率的影响如图14所示。在缺氧条件下,光催化净化效率很低。随着氧气体积分数的增加,净化效率得到了很大的提高,但当氧气体积分数增加到15%~21%时,净化效率达到稳定状态,且不随氧气体积分数的增加而增加或降低。这可能是因为,吸附在催化剂表面的氧可以捕获催化剂在紫外光照射下产生的表面电子。一方面,它能促进活性基团的形成,如超氧离子(O2−)、过氧化氢(H2O2)和羟基自由基(·OH),从而参与光催化氧化;另一方面,它可以通过捕获电子在一定程度上抑制电子-空穴对的复合,从而提高催化效率[30]。然而,氧气量的不断增加可以提供足够的活性基团来源,但催化剂的转化能力和利用效率有限,不能带来持续的改善。当氧含量为21%时,WBC2对甲苯的净化效率达到峰值。与其它催化剂相比,净化效率的峰值出现在更高氧气浓度处,说明WBC2可以利用更多的氧气,将更多的氧气转化为活性基团。此实验结果还表明,在大气环境下,即可为光催化反应系统可以提供足够的氧气。
综合以上结果,无论反应条件如何变化,废电池复合改性催化剂的净化效率都高于TiO2,而WBC2在所有催化剂中一直处于最优地位。在任何条件下,该催化剂都能保持较高的催化活性,这说明WBC系列催化剂具有较强的环境适应性,能在相对恶劣的反应环境中保证较高的催化活性,当反应条件得到改善时,催化剂的净化能力将有更大的提升空间。
3. 结论
1)以TiO2和废旧锌锰电池芯粉为原料,用球磨法制备了复合光催化剂。TiO2与WBP的机械物理结合形成了一种新的复合结构,促进了光生电子-空穴对的分离,极大地提高了光催化活性。
2) WBC2对甲苯的净化效果最好,即二氧化钛与WBP的最佳配比(质量比)为2∶1。
3)空速越大催化剂对甲苯的净化效率越低;净化效率随光照强度的增加呈现先增加后保持不变的规律;催化剂在相对湿度为30%的条件下具有最佳的催化活性;氧气体积分数为15%时为净化效率达到最大。
-
表 1 样品的比表面积、孔容和孔径
Table 1. Specific surface area, pore volume, and pore size of the samples
样品名称 比表面积/(m2·g−1) 孔体积/(cm3·g−1) 平均孔径/nm TiO2 179.33 0.58 13.02 WBP 10.08 0.06 23.66 WBC1 54.64 0.24 17.32 WBC2 68.9 0.28 16.09 WBC3 89.61 0.35 15.91 WBC4 98.31 0.39 15.67 -
[1] KAMAL M S, RAZZAK S A, HOSSAIN M M, et al. Catalytic oxidation of volatile organic compounds (VOCs): A review[J]. Atmospheric Environment, 2016, 140: 117-134. doi: 10.1016/j.atmosenv.2016.05.031 [2] 羌宁, 史天哲, 缪海超. 挥发性有机物污染控制方案的运行费用效能比较[J]. 环境科学, 2020, 41(2): 638-646. [3] 崔蕾. TiO2基微纳米复合光催化材料的合成与催化性能研究[D]. 长春: 吉林大学, 2020. [4] 张健伟, 苑鹏, 王建桥, 等. Ce掺杂的CNTs-TiO2光催化剂制备及其NO氧化性能[J]. 环境工程学报, 2020, 14(7): 1852-1861. doi: 10.12030/j.cjee.201909119 [5] PHAM T D, LEE B K, et al. Selective removal of polar VOCs by novel photocatalytic activity of metals co-doped TiO2/PU under visible light[J]. Chemical Engineering Journal, 2017, 307: 63-73. doi: 10.1016/j.cej.2016.08.068 [6] QIN R, MENG F M, MUHAMMAD W K, et al. Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts[J]. Materials Letters, 2019, 240: 84-87. doi: 10.1016/j.matlet.2018.12.139 [7] WEI P, QIN D D, CHEN J Y, et al. Photocatalytic ozonation mechanism of gaseous n-hexane on MOx-TiO2-foam nickel composite (M=Cu, Mn, Ag): Unveiling the role of ˙OH and ˙O2−[J]. Environmental Science: Nano, 2019, 6: 959-969. [8] ZHANG Y, WU M, KWOK Y H, et al. In-situ synthesis of heterojunction TiO2/MnO2 nanostructure with excellent performance in vacuum ultraviolet photocatalytic oxidation of toluene[J]. Applied Catalysis B: Environmental, 2019, 259: 118034. doi: 10.1016/j.apcatb.2019.118034 [9] SIWINSKA K, KUBIAKA A, PIASECKI A, et al. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity[J]. Materials, 2018, 11(5): 841. [10] MA Q, WANG H, ZHANG H, et al. Fabrication of MnO2/TiO2 nano-tube arrays photoelectrode and its enhanced visible light photoelectrocatalytic performance and mechanism[J]. Separation and Purification Technology, 2017, 189: 193-203. doi: 10.1016/j.seppur.2017.08.007 [11] BISWAS R K, KARMAKAR A K, KUMAR S L, et al. Recovery of manganese and zinc from spent Zn-C cell powder: Experimental design of leaching by sulfuric acid solution containing glucose[J]. Waste Management, 2016, 51: 174-181. [12] 李明诗, 郭首义, 李浩东, 等. 废旧碱性锌锰电池综合回收钾、锌、锰[J]. 矿产保护与利用, 2020, 40(5): 134-137. [13] 罗龙海, 闫艳梅, 袁建伟, 等. 废旧锌锰电池中锰和锌在硫酸/草酸溶液中的浸出行为[J]. 湿法冶金, 2019, 38(6): 469-472. [14] XIN B, JIANG W, ASLAM H, et al. Bioleaching of zinc and manganese from spent Zn-Mn batteries and mechanism exploration[J]. Bioresource Technology, 2012, 106: 147-153. doi: 10.1016/j.biortech.2011.12.013 [15] QU J, FENG Y, ZHANG Q, et al. A new insight of recycling of spent Zn-Mn alkaline batteries: Synthesis of ZnxMn1-xO nanoparticles and solar light driven photocatalytic degradation of bisphenol A using them[J]. Journal of Alloys and Compounds, 2015, 622: 703-707. [16] 高培, 沈伯雄, 赵忠. 回收废旧电池制备功能材料研究进展[J]. 化工进展, 2020, 39(10): 4185-4190. [17] 白婷婷, 康静文, 肖坤儒, 等. 废旧锌锰电池回收制备菱形三氧化二锰及其对染料吸附性能研究[J]. 应用化工, 2018, 47(4): 741-745. doi: 10.3969/j.issn.1671-3206.2018.04.027 [18] 田炳阳. 废旧锌锰电池生物浸提液制备锰锌软磁材料的质量控制及关键技术[D]. 北京: 北京理工大学, 2018. [19] 牛志睿, 李彤, 苏沉, 等. 废旧锌锰电池生物淋滤-水热法制备纳米锰锌铁氧体[J]. 环境科学学报, 2017, 37(9): 3356-3363. [20] GALLEGOS M V, PELUSO M A, FINOCCHIO E, et al. Removal of VOCs by catalytic process. A study of MnZnO composites synthesized from waste alkaline and Zn/C batteries[J]. Chemical Engineering Journal, 2016, 313: 1099-1111. [21] ZHANG X, LI H, YANG Y, et al. Facile synthesis of new efficient Cu/MnO2, catalysts from used battery for CO oxidation[J]. Journal of Environmental Chemical Engineering, 2017, 5: 5179-5186. doi: 10.1016/j.jece.2017.09.059 [22] LIU H, MA Y, CHEN J, et al. Highly efficient visible-light-driven photocatalytic degradation of VOCs by CO2-assisted synthesized mesoporous carbon confined mixed-phase TiO2 nanocomposites derived from MOFs[J]. Applied Catalysis B: Environmental, 2019, 250: 337-346. doi: 10.1016/j.apcatb.2019.03.054 [23] AEAUJO, EVANDO S, DACOSTA B P, et al. TiO2/ZnO hierarchical heteronanostructures: Synthesis, characterization and application as photocatalysts[J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 2820-2829. doi: 10.1016/j.jece.2016.05.021 [24] QIAN X, REN M, YUE D, et al. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of VOCs[J]. Applied Catalysis B: Environmental, 2017, 212: 1-6. doi: 10.1016/j.apcatb.2017.04.059 [25] NEVAREZ M M, KOBYLANSKI M, PAWE M, et al. Self-organized TiO2–MnO2 nanotube arrays for efficient photocatalytic degradation of toluene[J]. Molecules, 2017, 22(4): 564-577. doi: 10.3390/molecules22040564 [26] ZHANG J H, HUA Y, QIN J X, et al. TiO2-UiO-66-NH2 nanocomposites as efficient photocatalysts for the oxidation of VOCs[J]. Chemical Engineering Journal, 2020, 385: 123814. doi: 10.1016/j.cej.2019.123814 [27] RAO Z P, SHI G S, WANG Z, et al. Photocatalytic degradation of gaseous VOCs over Tm3+-TiO2: Revealing the activity enhancement mechanism and different reaction paths[J]. Chemical Engineering Journal, 2020, 395: 125078. [28] MAMAGHANI A H, HAGHIGHAT F, LEE C S, et al. Photocatalytic oxidation of MEK over hierarchical TiO2 catalysts: Effect of photocatalyst features and operating conditions[J]. Applied Catalysis B:Environmental, 2019, 251: 1-16. doi: 10.1016/j.apcatb.2019.03.057 [29] HENDERSON M A. A surface science perspective on TiO2 photocatalysis[J]. Surface Science Reports, 2011, 66: 185-297. doi: 10.1016/j.surfrep.2011.01.001 [30] LIN Y T, WENG C H, HSU H J, et al. Effect of oxygen, moisture, and temperature on the photo oxidation of ethylene on N-doped TiO2 catalyst[J]. Separation and Purification Technology, 2014, 134: 117-125. doi: 10.1016/j.seppur.2014.07.039 -




 下载:
下载: