-
厌氧氨氧化工艺氮去除负荷高、无需外加有机碳源、污泥产量低、运行成本低,但因厌氧氨氧化菌生长速度慢、倍增时间长,且对环境条件变化较为敏感,使得厌氧氨氧化工艺启动耗时较长,这极大地限制了厌氧氨氧化技术的工程应用[1]。Dokhaven污水厂的厌氧氨氧化(anaerobic ammonia oxidation,ANAMMOX)工艺耗时3.5 a成功启动[2]。因此,厌氧氨氧化菌(anaerobic ammonia oxidizing bacteria,AnAOB)的高效富集、ANAMMOX工艺的快速启动及稳定运行引起研究者的广泛关注。
ANAMMOX工艺的快速启动与接种污泥性质、反应器类型密切相关[3]。不同类型的好氧、厌氧污泥均可用于ANAMMOX工艺启动。好氧污泥虽菌群丰富,但其所含厌氧菌属较少,启动期会相应较长;厌氧反硝化污泥中含有的反硝化菌与AnAOB同属厌氧菌,可省去好氧转向厌氧环境时的污泥适应阶段,完成AnAOB的富集,可缩短工艺启动市场,但其沉降性能较差,启动过程中易出现污泥流失[4];而经厌氧消化后的剩余污泥具有良好沉降性能,且来源广泛、易于获取,污泥碳氮比含量低、高氨氮适应性强、厌氧菌含量高且与AnAOB的代谢基质相近,较适合作为ANAMMOX工艺启动的接种污泥。反应器类型直接影响污泥固体停留时间(solid retention time,SRT)、水流上升流速等,进而影响AnAOB富集、工艺启动速度、工艺运行稳定性等;序批式反应器(sequencing batch reactor,SBR)、序批式生物膜反应器(sequencing batch biofilm reactor,SBBR)、膜生物反应器(membrane bioreactor,MBR)、生物滤池均可成功启动ANAMMOX工艺[5-7]。上流式厌氧污泥床(upflow anaerobic sludge blanket,UASB)反应器作为第2代厌氧反应器的杰出代表,具有较好的污泥持留能力和基质传质效果,可为AnAOB的生长提供良好的环境,其在ANAMMOX工艺运行中的优势已逐步显现。WANG等[8]将厌氧氨氧化颗粒污泥接种于UASB反应器(22 L),经过178 d启动及稳定运行,氮容积负荷(nitrogen loading rate,NLR)和氮去除负荷(nitrogen removal rate,NRR)可高达8.25 kg ·(m3·d)−1(以N计)和6.93 kg·(m3·d)−1(以N计)。
因此,本研究以厌氧消化污泥为接种污泥,以UASB反应器为反应装置启动ANAMMMOX工艺,研究了ANAMMOX-UASB反应器启动过程的生物特性,考察了ANAMMOX- UASB启动运行过程中的污泥表观形态、脱氢酶及胞外聚合物的变化,分析了启动过程中胞外聚合物结构、组成的特征,解析了功能菌群动态演替规律,以期探明ANAMMOX-UASB启动过程中的生物特性,为厌氧氨氧化工艺的快速启动与工程应用提供参考。
-
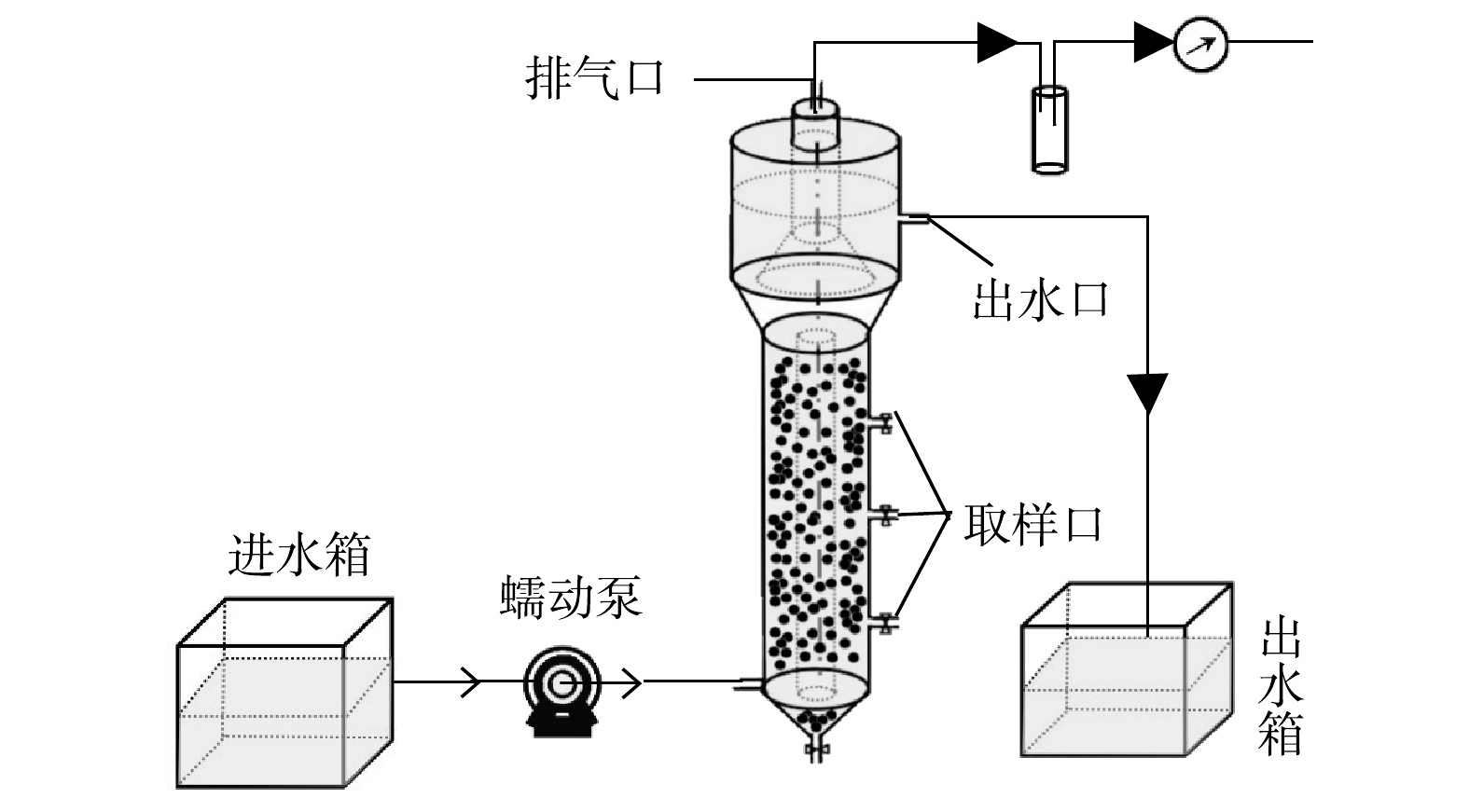
所采用的实验装置如图1所示,UASB反应器呈圆柱结构,由有机玻璃制成,总容积为3.5 L,有效容积为1.4 L,其中反应区内径6 cm,高度50 cm。模拟废水由蠕动泵从UASB反应器底部连续泵入,从下至上依次经反应区、沉淀区后,最后经出水口排出;产生的气体经顶部排气孔排出。反应区部分采用黑布包裹以避免光照对AnAOB产生的不利影响。反应器工作温度为(35±1) ℃。
-
实验中所用接种污泥取自青岛市某污水处理厂厌氧消化罐,污泥形态呈黑褐色絮状。混合液悬浮固体质量浓度(mixed liquid suspended solids,MLSS)约为47.39 g·L−1,混合液挥发性悬浮固体质量浓度(mixed liquor volatile suspended solids,MLVSS)约为22.77 g·L−1,MLVSS/MLSS为0.48。接种污泥量为1.2 L。
-
实验废水采用人工模拟废水,
NH+4 -N和NO−2 -N分别由NH4Cl和NaNO2按需配置,其他主要成分为27 mg·L−1 KH2PO4、500 mg·L−1 NaHCO3、180 mg·L−1 CaCl2·2H2O、300 mg·L−1 MgSO4·7H2O。微量元素Ⅰ、Ⅱ添加量均为1 mL·L−1。微量元素Ⅰ的组成成分及含量为5 mg·L−1 EDTA、5 mg·L−1 FeSO4·7H2O、微量元素Ⅱ为15 mg·L−1 EDTA、0.99 mg·L−1 MnCl2·4H2O、0.25 mg·L−1 CuSO4·5H2O、0.43 mg·L−1 ZnSO4·7H2O、0.014 mg·L−1 H3BO4、0.19 mg·L−1 NiCl2·6H2O、0.22 mg·L−1 Na2MoO4·2H2O。废水pH为7.5~7.8。 -
NH+4 -N采用纳氏试剂分光光度法测定;NO−2 -N采用N-(1-萘基)乙二胺分光光度法测定;NO−3 -N采用紫外分光光度法测定;pH采用玻璃电极法测定;SS和VSS采用重量法[9]测定。脱氢酶(dehydrogenase activity,DHA)的提取与测定采用TTC还原法[10]。胞外聚合物(extracellular polymeric substance,EPS)提取与测定参照WANG等[11]的方法分层提取松散型胞外聚合物(loosely bound EPS,LB-EPS)与紧密型胞外聚合物(tightly bound EPS,TB-EPS)。采用硫酸-蒽酮比色法[12]测定多糖(polysaccharide,PS)含量,采用改进的Folin-酚试剂法[13]测定蛋白质(protein,PN)的含量。三维荧光光谱(three-dimensional excitation-emission matrix fluorescence spectroscopy,3D-EEM)采用荧光光度计(F-4 600, Hitachi, Japan)测定样品中LB-EPS和TB-EPS的三维荧光光谱[14]。激发波长(Ex)和发射波长(Em)分别为200~450 nm和240~550 nm,扫描间隔为5 nm,扫描速度采用1 200 nm·min−1。用超纯水作为空白样品校正水的拉曼散射。实验数据采用Origin绘图分析。
聚合酶链式反应(polymerase chain reaction,PCR)及高通量测序:采用Power Soil DNA Isolation Kit按照操作步骤提取样品中DNA,使用1%(质量分数)的琼脂糖凝胶电泳检测DNA浓度和质量。采用细菌16S rRNA基因V3+V4扩增区域,引物为341F(CCTACGGGNGGCWGCAG)和805R(GACTACHVGGGTATCTAATCC),对DNA进行PCR扩增,扩增体系及扩增条件按彭广生等[15]方法进行。PCR扩增产物使用OMEGA胶回收纯化试剂盒纯化后4 ℃保存。样本交由北京诺禾致源生物信息科技有限公司进行DNA提取和测序。利用Illumina HiSeq高通量测序技术在HiSeq 2500系统进行测序。
-
ANAMMOX-UASB反应器启动过程共持续250 d,整个过程分为菌体水解期(阶段Ⅰ)、活性迟滞期(阶段Ⅱ)、活性提高期(阶段Ⅲ)和稳定运行期(阶段Ⅳ和阶段Ⅴ)[16]。启动过程运行情况见表1。
-
ANAMMOX-UASB反应器接种污泥为絮状黑褐色厌氧消化污泥(图2(a))。随着启动运行,污泥内有机物不断消耗、异养菌逐渐死亡,导致反应器污泥层高度逐渐下降。第53天,絮状污泥颜色逐渐转变为黄褐色,且部分区域污泥呈现团聚体状。自第100天起,反应器内出现小颗粒状污泥,且部分已呈现浅红色(图2(b)),结合此过程反应器脱氮性能可断定厌氧氨氧化反应已明显显现。此时,反应器内的污泥与郑平等[17]报导的厌氧氨氧化颗粒污泥形状一致,说明絮状污泥逐渐颗粒化,厌氧氨氧化污泥初步形成。颗粒污泥呈现红色是由于厌氧氨氧化菌体内含有丰富的细胞色素c,污泥发红程度可以反映厌氧氨氧化菌的富集程度,可以作为肉眼判断厌氧氨氧化活性的依据[18]。随着ANAMMOX-UASB反应器继续运行,絮状污泥颗粒化进一步加强,至第250天时,反应器内以不规则状的红色颗粒污泥和褐色絮状污泥为主(图2(c)),此时NRR维持在2 kg·(m3·d)−1左右,厌氧氨氧化菌已成功富集。
-
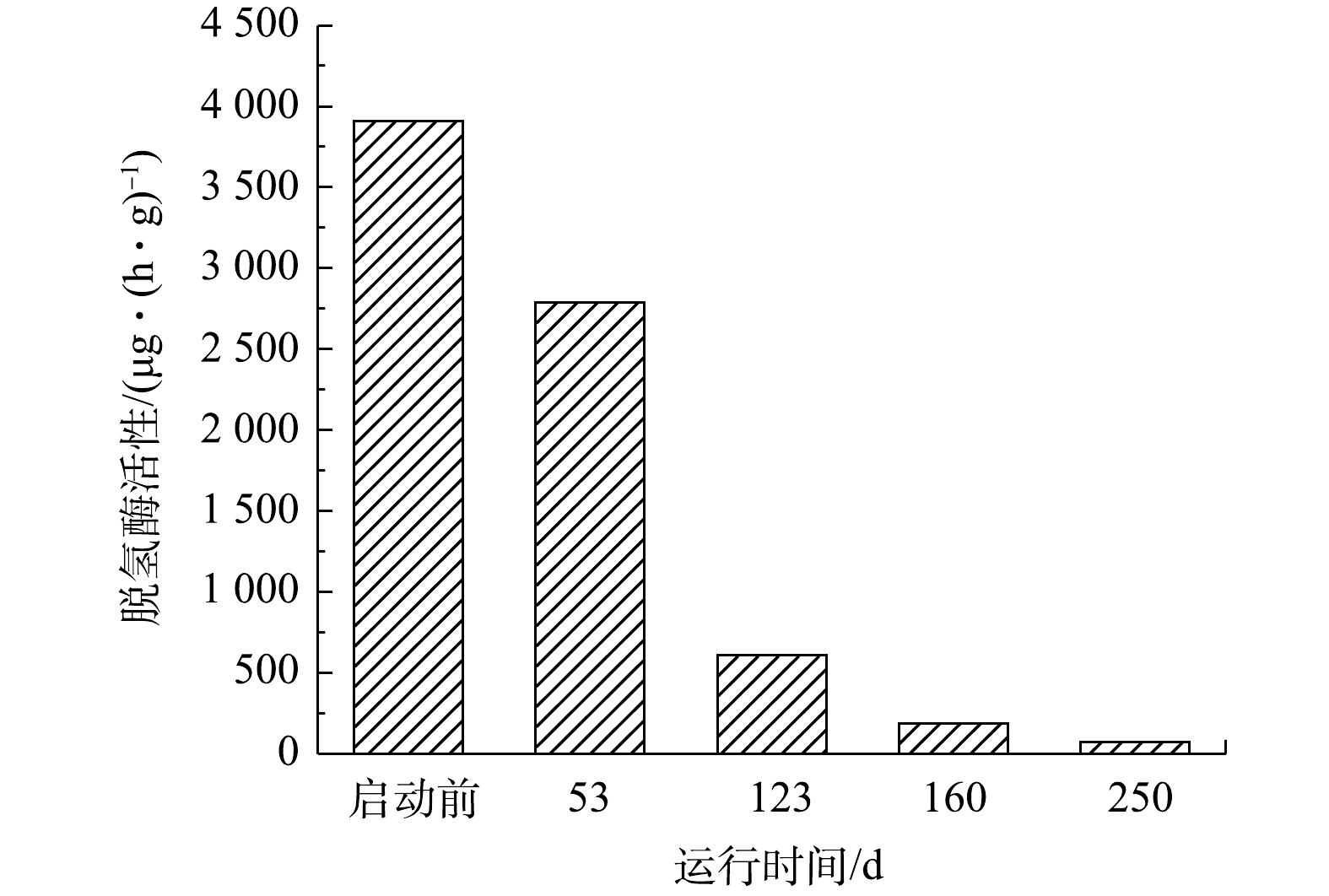
脱氢酶主要参与微生物降解有机物的过程[10],也可以参与某些细胞的合成。一氧化碳脱氢酶是乙酰辅酶A途径的关键酶。尽管厌氧氨氧化菌不以有机物为碳源,但仍有脱氢酶活性。ANAMMOX-UASB反应器启动过程中脱氢酶活性变化,可以反映出厌氧氨氧化工艺启动过程中污泥中异养菌的消长情况,结果见图3。由图3可知,因接种污泥为厌氧消化污泥,其异养微生物丰度较高,脱氢酶活性为3 909.51 μg·(h·g)−1(以TF计)。在第53天,污泥的脱氢酶活性降低至2 788.809 μg·(h·g)−1,这主要是由内源有机物量降低,异养微生物逐步衰亡、自溶等造成。随着启动过程的继续(53~250 d),厌氧氨氧化菌丰度逐步增加,异养菌含量进一步降低,脱氢酶活性大幅度降低,最终降至72.13 μg·(h·g)−1。LIN等[19]利用厌氧氨氧化污泥作为接种污泥启动厌氧氨氧化工艺时,启动前脱氢酶活性为684 μg·(h·g)−1,成功启动后脱氢酶活性降为252 μg·(h·g)−1。KIM等[20]的研究表明,异养菌脱氢酶参与了有机化合物的同化和异化反应,自养菌的脱氢酶主要参与有机化合物的同化,异养菌的脱氢酶活性远高于自养菌脱氢酶。在厌氧氨氧化工艺启动中,因进水基质无有机物,污泥中异养菌大量裂解死亡,导致脱氢酶活性大幅下降;厌氧氨氧化菌虽然不利用有机物进行分解代谢,但仍以CO2作为无机碳源,通过乙酰辅酶A途径合成自身细胞物质,故工艺在成功启动后仍具有一定的脱氢酶活性。
-
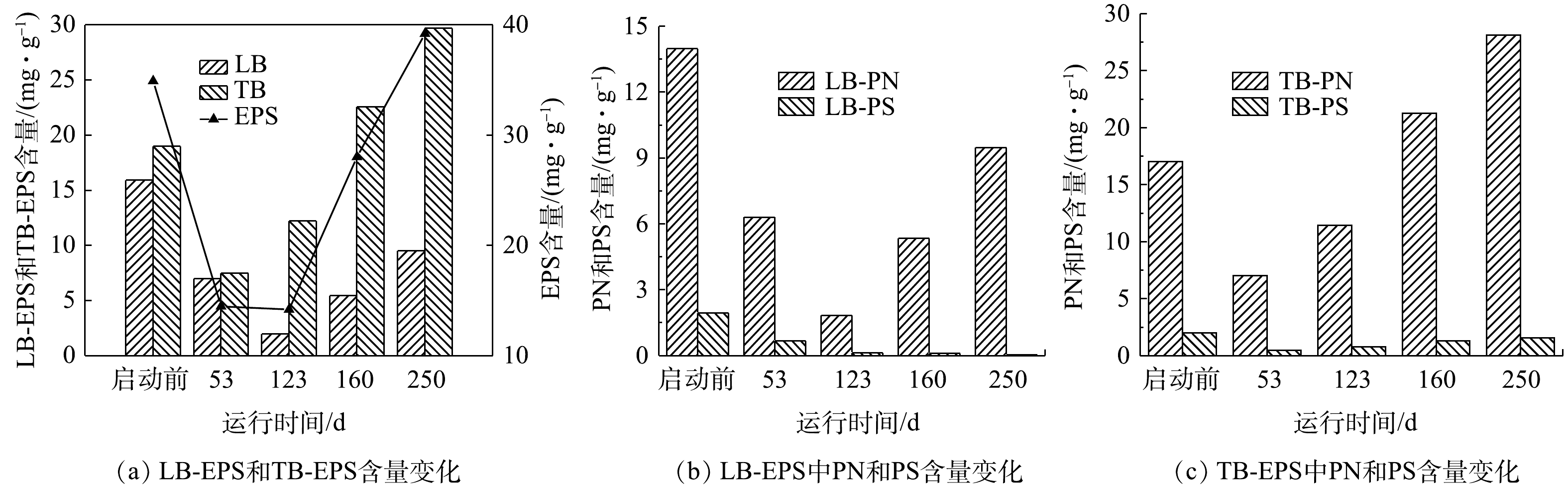
1) EPS含量。EPS是厌氧氨氧化污泥的重要组成部分,厌氧氨氧化细菌会嵌入到由细胞和EPS组成的聚集体中[21]。EPS具有流动性的双层结构,是由松散附着的外层(LB)和紧密黏附的内层(TB)组成,EPS的主要成分是蛋白质(PN)和多糖(PS)[22]。ANAMMOX-UASB反应器启动过程中EPS含量及组成见图4。由图4可知,第0~53天,EPS含量快速降低,由初期的34.91 mg·g−1(以VSS计)(0 d)下降为14.48 mg·g−1(53 d),PN/PS由7.88(0 d)升至11.58(53 d);EPS中TB/LB由1.19变为1.07。第53~123天,EPS含量略降至14.19 mg·g−1,PN/PS由11.58升至14.25;EPS中TB/LB为6.21。第123~160天,EPS含量逐渐上升至28.02 mg·g−1,PN/PS进一步升至18.59;EPS中TB/LB为4.15。第160~250天,EPS含量继续升至39.21 mg·g−1,PN/PS升至23.20;EPS中TB/LB变为3.12。EPS中PN含量变化大,PS含量变化相对较小,且在启动过程中PN/PS持续上升。启动过程中EPS均以TB-EPS为主;LB-EPS中LB-PN/LB平均值93.8%,TB-EPS中TB-PN/TB平均值为93.1%,各分层EPS中均以PN为主,表明EPS中蛋白质在ANAMMOX-UASB启动过程中起关键作用,这与GUO等[23]报道的研究结果一致。大量的PN产生可能来源于细胞死亡后的释放、细胞的分泌以及EPS中含有大量胞外酶所致。PN是疏水性基团,PS是亲水性基团,MA等[24]的研究表明,EPS中PN的含量与污泥的絮凝和沉降能力成正相关,较高的PN含量可以增强微生物聚集体的凝聚性。DONG等[25]的研究表明,PN中带正电荷的氨基可以中和羧基的负电荷,从而加剧污泥的絮凝。CHEN等[21]发现,PN/PS越大,污泥的沉降性能越好。在ANAMMOX-UASB启动过程中,PN/PS由启动前的7.88增至23.20,与絮状污泥逐渐颗粒化及其沉降性能逐渐增强的变化趋势一致。
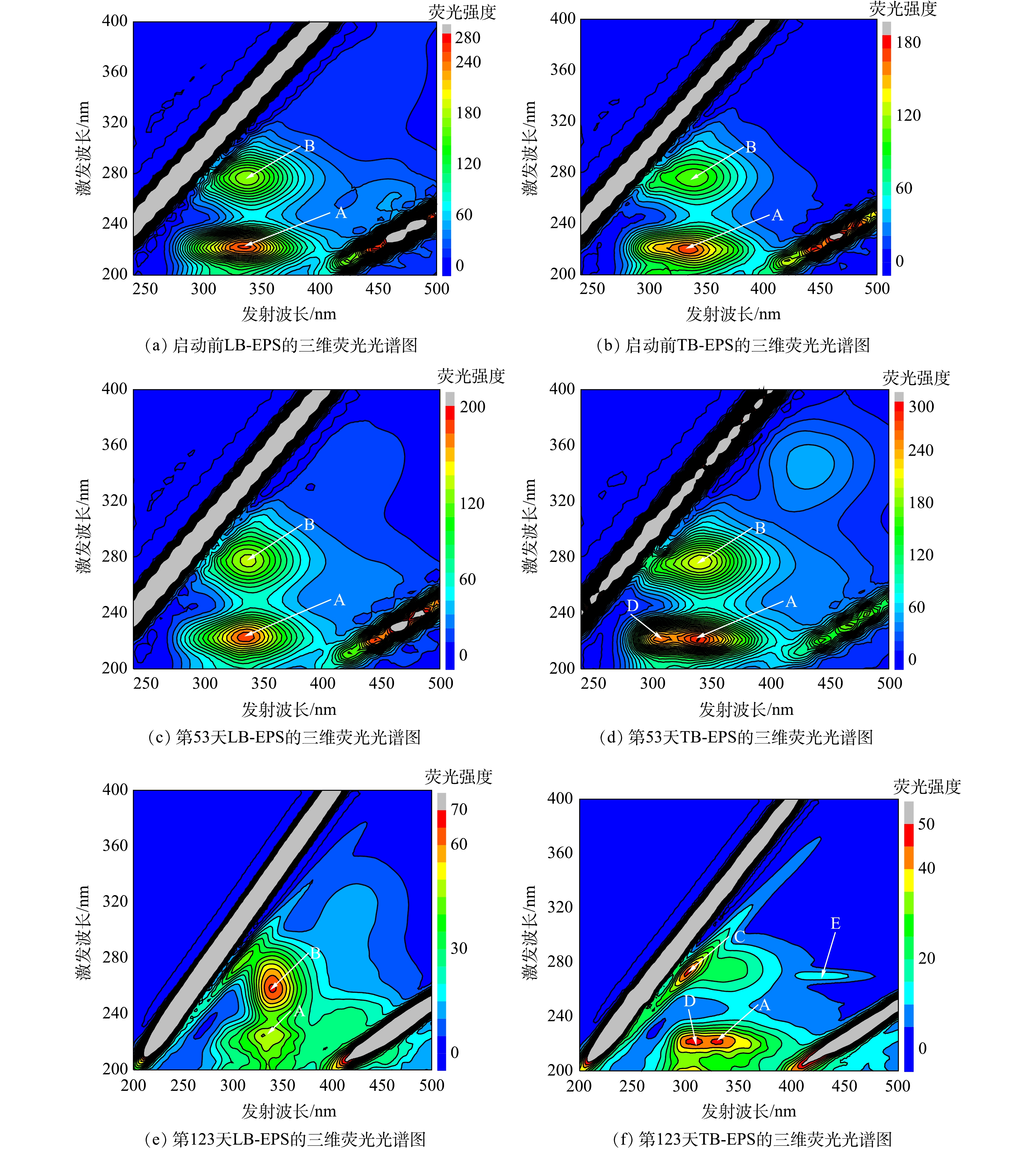
2) EPS组分的荧光特性。污泥EPS中含有大量荧光特性的物质,可利用三维荧光分光光度法分析EPS组成特征。启动过程中第0、53、123天的LB-EPS和TB-EPS三维荧光光谱如图5所示。由图5可知,ANAMMOX-UASB在启动过程中共观察到5个荧光峰(A~E)。其中,荧光峰A(220~225 nm/330~340 nm)为芳香族蛋白类物质;荧光峰B(260~275 nm/335~340 nm)为色氨酸蛋白类物质;荧光峰C(270~275 nm/300~305 nm)与酪氨酸蛋白类物质有关;荧光峰D(220 nm/310~320 nm)与简单芳环蛋白类物质有关,荧光峰E(270 nm/425 nm)为富里酸类物质[26]。
荧光峰A、B在污泥启动过程的LB-EPS和TB-EPS中均存在,说明在启动过程中的EPS均以蛋白质为主;虽然LB-EPS和TB-EPS所处位置不同,但其组分和结构基本相同。在LB-EPS中仅存在荧光峰A和B,无其他荧光峰,且启动过程中LB-EPS的结构与组成并未发生较大变化。在第53天时TB-EPS中,除存在荧光峰A和B,还出现荧光峰D,说明此阶段有简单芳环蛋白类物质产生;第123天时,出现荧光峰C和E,荧光峰B消失,说明部分色氨酸蛋白类物质转化为酪氨酸蛋白类物质[27]。三维荧光结果表明,在ANAMMOX-UASB启动过程中污泥EPS中的TB-EPS的结构和组成均会发生变化。
ANAMMOX-UASB启动过程中LB-EPS和TB-EPS中各峰光谱参数见表2。由表2可知,荧光峰会向更长的波长移动(称为红移),或向更短的波长移动(称为蓝移)。在LB-EPS中,第123天荧光峰A沿Ex方向蓝移5 nm,沿Em方向红移5 nm;荧光峰B沿Ex方向蓝移15 nm,沿Em方向红移5 nm。在TB-EPS中,第53天时荧光峰A沿Em方向红移10 nm,但至第123天时沿Em方向蓝移10 nm,与启动前相同;荧光峰B沿Em方向红移5 nm。上述研究结果表明,波长移动与EPS中各成分的结构变化密切相关,其中波长红移与含羰基取代基、羟基、烷氧基、氨基和羧基含量增加相关联,而波长蓝移则与芳香酯基减少,芳香环数下降,共轭键数的降低和羰基、羟基和氨基的减少直接相关。荧光峰波长及峰强度的变化说明ANAMMOX-UASB启动过程中污泥EPS中的组分、结构均发生变化。
-
1)微生物多样性和丰富度分析。ANAMMOX-UASB启动过程中的微生物多样性和丰富度如表3所示。由表3可知,所有样品的覆盖率均大于99%。这表明样品数据涵盖的物种充足,微生物群落丰富度和多样性结果具有较高的可靠性和真实性[28]。ACE和Chao1指数可以表征物种的丰富度,其值越大,所含物种越丰富。Shannon指数常用来评价生物群落组成复杂程度,其值越大,表明群落复杂程度越高[29]。Simpson指数更倾向于反应群落的均匀性,其值越大,表示优势菌群占总体生物菌群比例越大[30]。由表3可知,接种污泥的Chao1和ACE指数分别为594.16和583.74,第53天的Chao1和ACE指数分别上升至最大值,为695.08和682.31。这表明此时的群落中所含物种最丰富,结合启动中出水指标可得,此时反硝化菌、氨化菌、厌氧氨氧化菌共存。第250天分别下降到351.00和312.34,表明工艺启动成功后,微生物以自养厌氧氨氧化菌为主,群落丰富度有所下降。接种污泥的Shannon指数为5.19,第53天上升至最大值为6.06,表明此时群落复杂度最大;第250天降低至4.43,其原因是反应器成功启动后,厌氧氨氧化菌的丰度不断升高,其他微生物的丰度逐渐降低,导致微生物群落复杂度有所下降。接种污泥的Simpson指数为0.92,第53天上升至最大值0.96,表明此时反应器内优势菌群所占的比例也最大;第250天下降为0.87,较接种污泥略有降低,表明反应器启动成功后,优势菌群所占的比例有所下降。
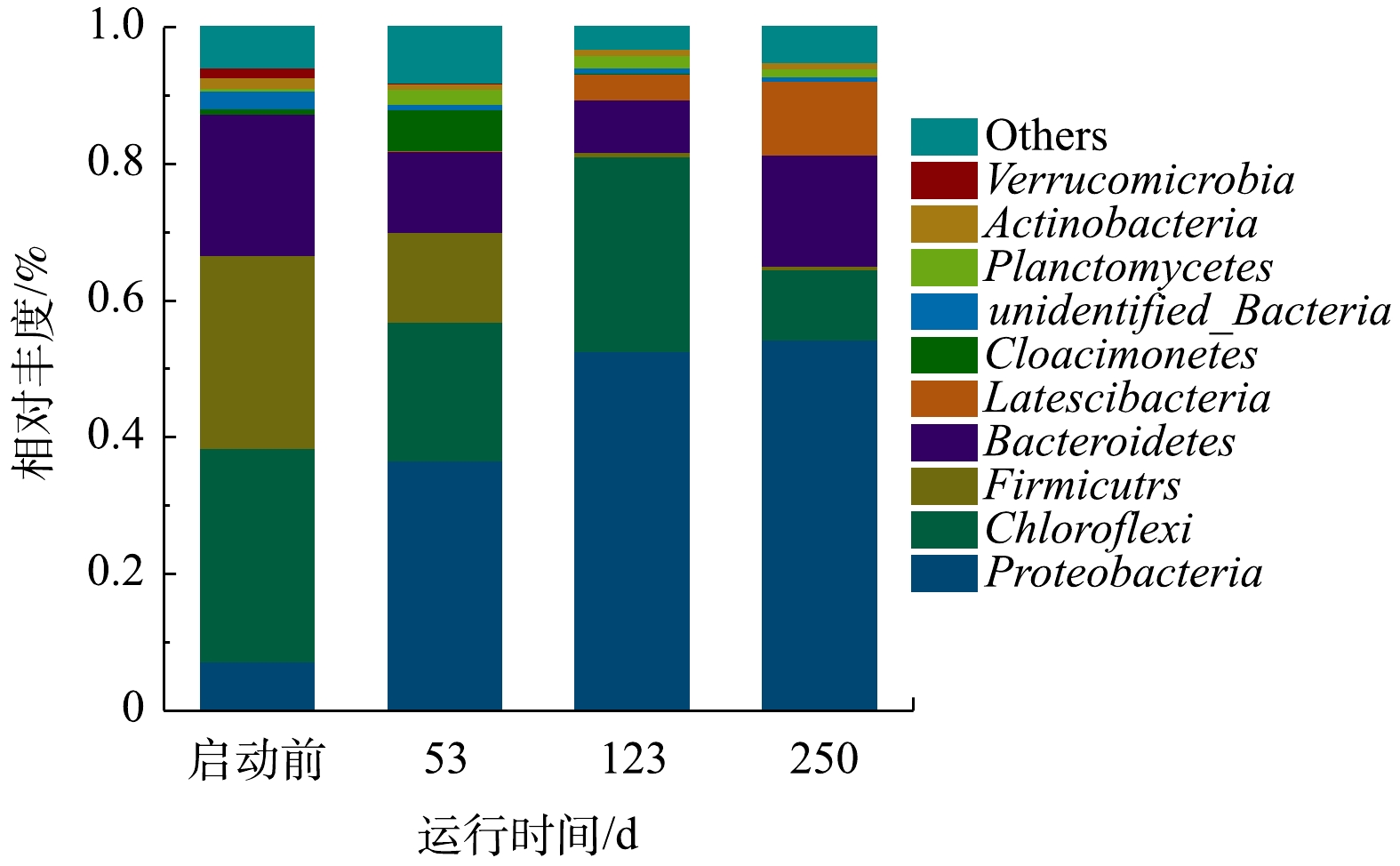
2)微生物门水平物种丰度分析。将微生物在门水平上丰度前10的物种绘制成物种相对丰度柱形累加图(图6)。主要为变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、Latescibacteria菌门、Cloacimonetes菌门、浮霉菌门(Planctomycetes)、放线菌门(Actinobacteria)和疣微菌门(Verrucomicrobia)。其中浮霉菌门和变形菌门为脱氮功能菌。变形菌门在各个样品中占最大比例,在启动过程中丰度逐渐上升,由7.22%上升到54.40%。曹雁等[31]利用上流式厌氧过滤床(upflow blanket filter,UBF)反应器启动厌氧氨氧化工艺,启动成功后变形菌门增加了12%。杨开亮等[32]利用SBR接种活性污泥启动厌氧氨氧化工艺时,变形菌门在启动过程中丰度逐渐下降。上述研究结果不同,可能与反应器类型、接种污泥类型、运行参数及环境条件等不同有关。沈耀良等[33]在厌氧折流板(anaerobic baffled reactor,ABR)反应器中发现各隔室中变形菌门为主要菌种,其在脱氮方面占有重要地位。绿弯菌门是厌氧氨氧化系统中常见的伴生菌门,接种污泥的丰度为31.23%,第53天下降为20.32%,第123天反应器启动成功时丰度上升为28.52%。CHEN等[34]研究发现,绿弯菌门是兼性厌氧菌,在厌氧氨氧化污泥中起到支撑和骨架作用,可作为形成小颗粒污泥的框架粒子,也可作为颗粒污泥的载体,从而有利于厌氧氨氧化颗粒污泥的形成。MIAO等[35]的研究表明,厚壁菌门对反硝化有重要作用,厚壁菌门丰度由28.27%下降为5.55%,说明ANAMMOX-UASB启动过程中异养反硝化菌因环境条件改变大量裂解死亡。浮霉菌门为主要的自养脱氮功能菌群,厌氧氨氧化菌归属此菌门;接种污泥浮霉菌门丰度仅为0.34%,第123天启动成功时其丰度增至1.89%,表明反应器内富集了一定量的厌氧氨氧化菌。其他研究者也发现,厌氧氨氧化工艺启动过程中变形菌门丰度始终高于浮霉菌门丰度。如朱彤等[36]发现,在厌氧氨氧化反应器启动成功后,虽然变形菌门丰度有所下降,但仍高于浮霉菌门。
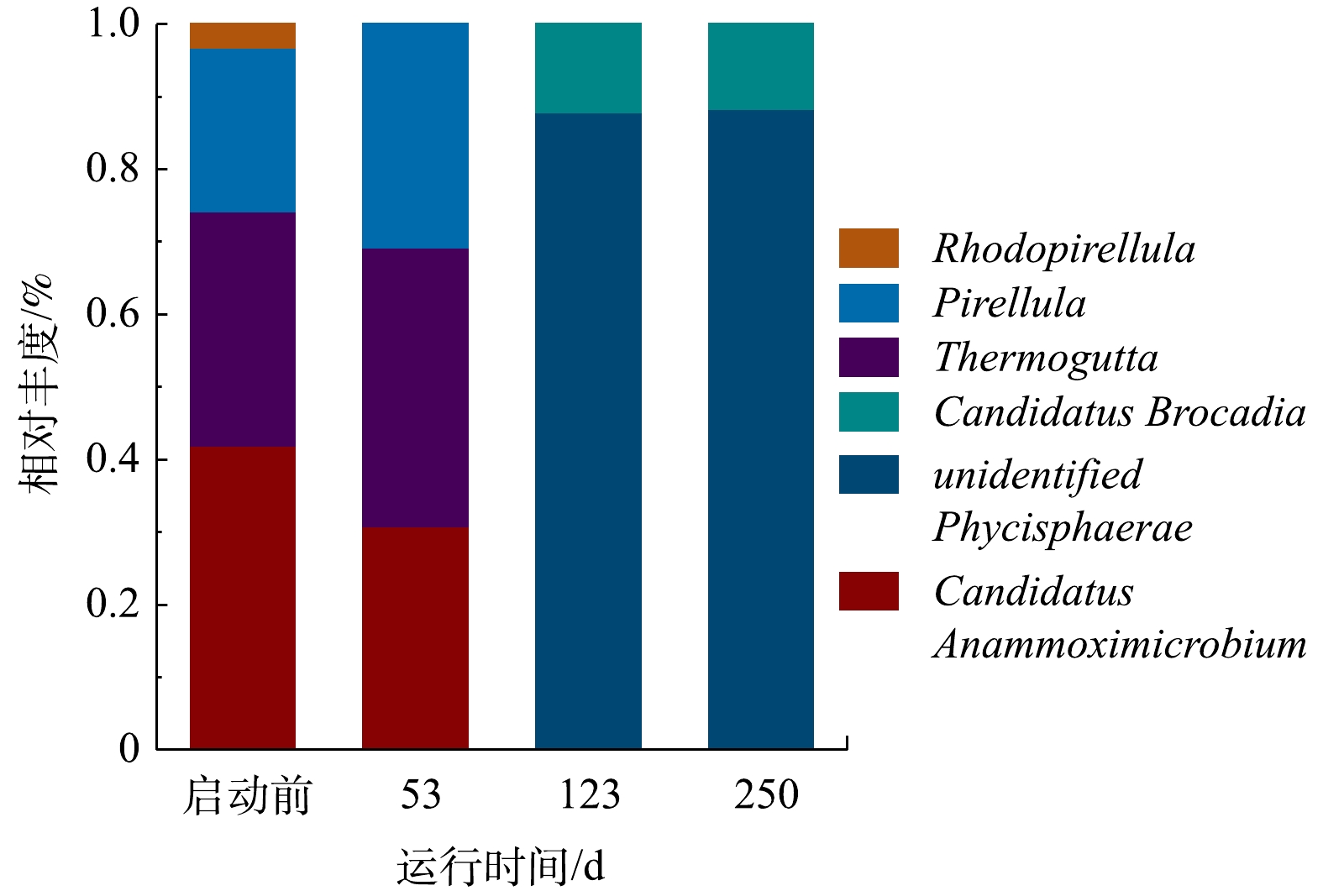
3)微生物属水平物种丰度分析。由于厌氧氨氧化菌属于浮霉菌门,因此,对浮霉菌门进行属水平的分析(图7)。浮霉菌门有unidentified_Phycisphaerae、Candidatus_Anammoximicrobium、Candidatus_Brocadia、Thermogutta、Pirellula和Rhodopirellula。在已知的6种厌氧氨氧化菌属中,启动过程中存在2种厌氧氨氧化菌属,分别为Candidatus Anammoximicrobium和Candidatus Brocadia。接种污泥中Candidatus Anammoximicrobium的丰度为40.94%,第53天下降到30.77%,第123天下降为0。杨瑞丽等[29]进行厌氧氨氧化工艺启动时,观察到Candidatus Anammoximicrobium丰度随NLR增大而逐渐降低,第172天下降到0,其可能的原因是Candidatus Anammoximicrobium生长速率较慢、亚硝酸盐亲和力较低以及对水质变化的抵抗力较弱。第123天,Candidatus Brocadia是反应器内唯一的厌氧氨氧化菌,其丰度为12.15%,第250天,下降到11.63%。曹雁等[31]利用UBF反应器培养厌氧氨氧化细菌时,Candidatus Brocadia的丰度由0.01%增加到1.00%。VAN DER STAR等[37]发现Candidatus Brocadia适合存在于高含氮浓度废水中。本实验中,进水氨氮质量浓度由50 mg·L−1提高到300 mg·L−1,属于高含氮废水,更适合Candidatus Brocadia生长,故Candidatus Brocadia启动过程中可得到有效富集,成为优势菌属。有研究[38]表明,由于生存环境的不同,厌氧氨氧化菌的群落结构存在差异,在稳定的生长环境中,通常只有1个属种的厌氧氨氧化菌占优势,这与本研究结果类似。
-
1) ANAMMOX-UASB启动过程中絮状污泥逐渐颗粒化,最终以不规则状的红色颗粒污泥和褐色絮状污泥为主。微生物的脱氢酶活性由3 909.51 μg·(h·g)−1持续降至72.13 μg·(h·g)−1。EPS主要以TB-EPS为主,占比为75.7%;且LB-EPS和TB-EPS中PN占比稳步增大,分别增至99.6%和94.7%,PN在厌氧氨氧化工艺启动过程中起关键作用。
2)在ANAMMOX-UASB启动过程中,Chao1、ACE、Shannon和Simpson指数均呈现先升后降的趋势,启动过程中污泥的优势菌门为变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、浮霉菌门(Planctomycetes)。浮霉菌门中Candidatus Anammoximicrobium丰度逐渐降低直至消失,而Candidatus Brocadia丰度最终增至12.15%。
ANAMMOX-UASB反应器启动过程中的生物特性
Biological characteristics of ANAMMOX-UASB reactor during startup process
-
摘要: 以絮状厌氧消化污泥为接种污泥,经过250 d运行后成功启动了ANAMMOX-UASB反应器。结果表明:在启动过程中,絮体污泥逐渐颗粒化并以不规则状的红色颗粒污泥和褐色絮状污泥为主;脱氢酶活性由启动前的3 909.51 μg·(h·g)−1最终降至72.13 μg·(h·g)−1;EPS含量在启动过程中先降后升,EPS组成中主要为TB-EPS,占比由54.4%升至75.7%;启动过程中LB-EPS和TB-EPS中均以PN为主,且PN占比逐步增大,分别由初始的88.7%和89.5%增至99.6%和94.7%;启动过程中EPS的结构与组成均发生变化。ANAMMOX-UASB启动过程中微生物Chao1、ACE、Shannon和Simpson指数均先升后降,启动成功后微生物多样性和丰富度均降低。污泥中微生物的优势菌门为变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、浮霉菌门(Planctomycetes)。浮霉菌门中Candidatus Anammoximicrobium丰度逐渐降低直至消失,而Candidatus Brocadia丰度最终增至12.15%。Abstract: The ANAMMOX-UASB reactor inoculated with flocculent anaerobic digestion sludge was successfully started up after 250 days operation. During the full startup process, floc sludge was gradually granulated, and mainly consists of irregular red granular sludge and brown flocculent sludge. The activity of dehydrogenase decreased from 3 909.51 μg·(h·g)−1 to 72.13 μg·(h·g)−1 during the entire startup process. Furthermore, the EPS content declined initially and then increased, and TB-EPS proportion increased from 54.4% to 75.7%, which was the main constituent of EPS. Meanwhile, PN was also the main component in both LB-EPS and TB-EPS, and the proportion of PN increased gradually from 88.7% and 89.5% to 99.6% and 94.7%, respectively. During the startup of ANAMMOX-UASB, both the composition and structure of EPS changed, the indexes of Chao1, ACE, Shannon and Simpson increased firstly and then declined, and the microbial diversity and richness decreased after successful startup. The dominant phyla of microorganisms were Proteobacteria, Chloroflexi, Firmicutes, Bacteroidetes and Planctomycetes. The abundance of Candidatus anammoximicrobium decreased gradually and vanished eventually, while the abundance of Candidatus brocadia increased ultimately to 12.15%.
-
Key words:
- anammox /
- UASB /
- dehydrogenase /
- extracellular polymeric substance /
- microbial community
-
厌氧氨氧化工艺氮去除负荷高、无需外加有机碳源、污泥产量低、运行成本低,但因厌氧氨氧化菌生长速度慢、倍增时间长,且对环境条件变化较为敏感,使得厌氧氨氧化工艺启动耗时较长,这极大地限制了厌氧氨氧化技术的工程应用[1]。Dokhaven污水厂的厌氧氨氧化(anaerobic ammonia oxidation,ANAMMOX)工艺耗时3.5 a成功启动[2]。因此,厌氧氨氧化菌(anaerobic ammonia oxidizing bacteria,AnAOB)的高效富集、ANAMMOX工艺的快速启动及稳定运行引起研究者的广泛关注。
ANAMMOX工艺的快速启动与接种污泥性质、反应器类型密切相关[3]。不同类型的好氧、厌氧污泥均可用于ANAMMOX工艺启动。好氧污泥虽菌群丰富,但其所含厌氧菌属较少,启动期会相应较长;厌氧反硝化污泥中含有的反硝化菌与AnAOB同属厌氧菌,可省去好氧转向厌氧环境时的污泥适应阶段,完成AnAOB的富集,可缩短工艺启动市场,但其沉降性能较差,启动过程中易出现污泥流失[4];而经厌氧消化后的剩余污泥具有良好沉降性能,且来源广泛、易于获取,污泥碳氮比含量低、高氨氮适应性强、厌氧菌含量高且与AnAOB的代谢基质相近,较适合作为ANAMMOX工艺启动的接种污泥。反应器类型直接影响污泥固体停留时间(solid retention time,SRT)、水流上升流速等,进而影响AnAOB富集、工艺启动速度、工艺运行稳定性等;序批式反应器(sequencing batch reactor,SBR)、序批式生物膜反应器(sequencing batch biofilm reactor,SBBR)、膜生物反应器(membrane bioreactor,MBR)、生物滤池均可成功启动ANAMMOX工艺[5-7]。上流式厌氧污泥床(upflow anaerobic sludge blanket,UASB)反应器作为第2代厌氧反应器的杰出代表,具有较好的污泥持留能力和基质传质效果,可为AnAOB的生长提供良好的环境,其在ANAMMOX工艺运行中的优势已逐步显现。WANG等[8]将厌氧氨氧化颗粒污泥接种于UASB反应器(22 L),经过178 d启动及稳定运行,氮容积负荷(nitrogen loading rate,NLR)和氮去除负荷(nitrogen removal rate,NRR)可高达8.25 kg ·(m3·d)−1(以N计)和6.93 kg·(m3·d)−1(以N计)。
因此,本研究以厌氧消化污泥为接种污泥,以UASB反应器为反应装置启动ANAMMMOX工艺,研究了ANAMMOX-UASB反应器启动过程的生物特性,考察了ANAMMOX- UASB启动运行过程中的污泥表观形态、脱氢酶及胞外聚合物的变化,分析了启动过程中胞外聚合物结构、组成的特征,解析了功能菌群动态演替规律,以期探明ANAMMOX-UASB启动过程中的生物特性,为厌氧氨氧化工艺的快速启动与工程应用提供参考。
1. 材料与方法
1.1 实验装置
所采用的实验装置如图1所示,UASB反应器呈圆柱结构,由有机玻璃制成,总容积为3.5 L,有效容积为1.4 L,其中反应区内径6 cm,高度50 cm。模拟废水由蠕动泵从UASB反应器底部连续泵入,从下至上依次经反应区、沉淀区后,最后经出水口排出;产生的气体经顶部排气孔排出。反应区部分采用黑布包裹以避免光照对AnAOB产生的不利影响。反应器工作温度为(35±1) ℃。
1.2 接种污泥
实验中所用接种污泥取自青岛市某污水处理厂厌氧消化罐,污泥形态呈黑褐色絮状。混合液悬浮固体质量浓度(mixed liquid suspended solids,MLSS)约为47.39 g·L−1,混合液挥发性悬浮固体质量浓度(mixed liquor volatile suspended solids,MLVSS)约为22.77 g·L−1,MLVSS/MLSS为0.48。接种污泥量为1.2 L。
1.3 实验废水
实验废水采用人工模拟废水,
NH+4 NO−2 1.4 分析测定方法
NH+4 NO−2 NO−3 三维荧光光谱(three-dimensional excitation-emission matrix fluorescence spectroscopy,3D-EEM)采用荧光光度计(F-4 600, Hitachi, Japan)测定样品中LB-EPS和TB-EPS的三维荧光光谱[14]。激发波长(Ex)和发射波长(Em)分别为200~450 nm和240~550 nm,扫描间隔为5 nm,扫描速度采用1 200 nm·min−1。用超纯水作为空白样品校正水的拉曼散射。实验数据采用Origin绘图分析。
聚合酶链式反应(polymerase chain reaction,PCR)及高通量测序:采用Power Soil DNA Isolation Kit按照操作步骤提取样品中DNA,使用1%(质量分数)的琼脂糖凝胶电泳检测DNA浓度和质量。采用细菌16S rRNA基因V3+V4扩增区域,引物为341F(CCTACGGGNGGCWGCAG)和805R(GACTACHVGGGTATCTAATCC),对DNA进行PCR扩增,扩增体系及扩增条件按彭广生等[15]方法进行。PCR扩增产物使用OMEGA胶回收纯化试剂盒纯化后4 ℃保存。样本交由北京诺禾致源生物信息科技有限公司进行DNA提取和测序。利用Illumina HiSeq高通量测序技术在HiSeq 2500系统进行测序。
2. 结果与讨论
ANAMMOX-UASB反应器启动过程共持续250 d,整个过程分为菌体水解期(阶段Ⅰ)、活性迟滞期(阶段Ⅱ)、活性提高期(阶段Ⅲ)和稳定运行期(阶段Ⅳ和阶段Ⅴ)[16]。启动过程运行情况见表1。
表 1 ANAMMOX-UASB启动过程中各阶段运行情况Table 1. Performance of each stage during ANAMMOX-UASB startup process阶段 运行时间/d HRT/h 进水N质量浓度/(mg·L−1) 出水N质量浓度/(mg·L−1) TN去除率/% NH+4-N NO−2-N NH+4-N NO−2-N NO−3-N Ⅰ 1~13 24 50 50 152.36±85.15 1.22±1.16 4.07±1.76 −57.65±84.85 Ⅱ 14~53 24 50 50 4.68±7.92 7.91±6.31 3.4±1.73 84.19±10.6 Ⅲ 54~123 24 50~300 50~300 0.17±0.40 0.37±0.42 14.71±18.2 96.6±2.75 Ⅳ 124~160 24~12 300 300 1.86±2.62 0.83±0.77 56.21±0.77 90.18±2.49 Ⅴ 161~250 12~6 300 300 8.55±7.24 3.22±2.02 67.54±6.9 86.78±1.72 2.1 污泥表观形态
ANAMMOX-UASB反应器接种污泥为絮状黑褐色厌氧消化污泥(图2(a))。随着启动运行,污泥内有机物不断消耗、异养菌逐渐死亡,导致反应器污泥层高度逐渐下降。第53天,絮状污泥颜色逐渐转变为黄褐色,且部分区域污泥呈现团聚体状。自第100天起,反应器内出现小颗粒状污泥,且部分已呈现浅红色(图2(b)),结合此过程反应器脱氮性能可断定厌氧氨氧化反应已明显显现。此时,反应器内的污泥与郑平等[17]报导的厌氧氨氧化颗粒污泥形状一致,说明絮状污泥逐渐颗粒化,厌氧氨氧化污泥初步形成。颗粒污泥呈现红色是由于厌氧氨氧化菌体内含有丰富的细胞色素c,污泥发红程度可以反映厌氧氨氧化菌的富集程度,可以作为肉眼判断厌氧氨氧化活性的依据[18]。随着ANAMMOX-UASB反应器继续运行,絮状污泥颗粒化进一步加强,至第250天时,反应器内以不规则状的红色颗粒污泥和褐色絮状污泥为主(图2(c)),此时NRR维持在2 kg·(m3·d)−1左右,厌氧氨氧化菌已成功富集。
2.2 脱氢酶(DHA)活性
脱氢酶主要参与微生物降解有机物的过程[10],也可以参与某些细胞的合成。一氧化碳脱氢酶是乙酰辅酶A途径的关键酶。尽管厌氧氨氧化菌不以有机物为碳源,但仍有脱氢酶活性。ANAMMOX-UASB反应器启动过程中脱氢酶活性变化,可以反映出厌氧氨氧化工艺启动过程中污泥中异养菌的消长情况,结果见图3。由图3可知,因接种污泥为厌氧消化污泥,其异养微生物丰度较高,脱氢酶活性为3 909.51 μg·(h·g)−1(以TF计)。在第53天,污泥的脱氢酶活性降低至2 788.809 μg·(h·g)−1,这主要是由内源有机物量降低,异养微生物逐步衰亡、自溶等造成。随着启动过程的继续(53~250 d),厌氧氨氧化菌丰度逐步增加,异养菌含量进一步降低,脱氢酶活性大幅度降低,最终降至72.13 μg·(h·g)−1。LIN等[19]利用厌氧氨氧化污泥作为接种污泥启动厌氧氨氧化工艺时,启动前脱氢酶活性为684 μg·(h·g)−1,成功启动后脱氢酶活性降为252 μg·(h·g)−1。KIM等[20]的研究表明,异养菌脱氢酶参与了有机化合物的同化和异化反应,自养菌的脱氢酶主要参与有机化合物的同化,异养菌的脱氢酶活性远高于自养菌脱氢酶。在厌氧氨氧化工艺启动中,因进水基质无有机物,污泥中异养菌大量裂解死亡,导致脱氢酶活性大幅下降;厌氧氨氧化菌虽然不利用有机物进行分解代谢,但仍以CO2作为无机碳源,通过乙酰辅酶A途径合成自身细胞物质,故工艺在成功启动后仍具有一定的脱氢酶活性。
2.3 胞外聚合物(EPS)
1) EPS含量。EPS是厌氧氨氧化污泥的重要组成部分,厌氧氨氧化细菌会嵌入到由细胞和EPS组成的聚集体中[21]。EPS具有流动性的双层结构,是由松散附着的外层(LB)和紧密黏附的内层(TB)组成,EPS的主要成分是蛋白质(PN)和多糖(PS)[22]。ANAMMOX-UASB反应器启动过程中EPS含量及组成见图4。由图4可知,第0~53天,EPS含量快速降低,由初期的34.91 mg·g−1(以VSS计)(0 d)下降为14.48 mg·g−1(53 d),PN/PS由7.88(0 d)升至11.58(53 d);EPS中TB/LB由1.19变为1.07。第53~123天,EPS含量略降至14.19 mg·g−1,PN/PS由11.58升至14.25;EPS中TB/LB为6.21。第123~160天,EPS含量逐渐上升至28.02 mg·g−1,PN/PS进一步升至18.59;EPS中TB/LB为4.15。第160~250天,EPS含量继续升至39.21 mg·g−1,PN/PS升至23.20;EPS中TB/LB变为3.12。EPS中PN含量变化大,PS含量变化相对较小,且在启动过程中PN/PS持续上升。启动过程中EPS均以TB-EPS为主;LB-EPS中LB-PN/LB平均值93.8%,TB-EPS中TB-PN/TB平均值为93.1%,各分层EPS中均以PN为主,表明EPS中蛋白质在ANAMMOX-UASB启动过程中起关键作用,这与GUO等[23]报道的研究结果一致。大量的PN产生可能来源于细胞死亡后的释放、细胞的分泌以及EPS中含有大量胞外酶所致。PN是疏水性基团,PS是亲水性基团,MA等[24]的研究表明,EPS中PN的含量与污泥的絮凝和沉降能力成正相关,较高的PN含量可以增强微生物聚集体的凝聚性。DONG等[25]的研究表明,PN中带正电荷的氨基可以中和羧基的负电荷,从而加剧污泥的絮凝。CHEN等[21]发现,PN/PS越大,污泥的沉降性能越好。在ANAMMOX-UASB启动过程中,PN/PS由启动前的7.88增至23.20,与絮状污泥逐渐颗粒化及其沉降性能逐渐增强的变化趋势一致。
2) EPS组分的荧光特性。污泥EPS中含有大量荧光特性的物质,可利用三维荧光分光光度法分析EPS组成特征。启动过程中第0、53、123天的LB-EPS和TB-EPS三维荧光光谱如图5所示。由图5可知,ANAMMOX-UASB在启动过程中共观察到5个荧光峰(A~E)。其中,荧光峰A(220~225 nm/330~340 nm)为芳香族蛋白类物质;荧光峰B(260~275 nm/335~340 nm)为色氨酸蛋白类物质;荧光峰C(270~275 nm/300~305 nm)与酪氨酸蛋白类物质有关;荧光峰D(220 nm/310~320 nm)与简单芳环蛋白类物质有关,荧光峰E(270 nm/425 nm)为富里酸类物质[26]。
荧光峰A、B在污泥启动过程的LB-EPS和TB-EPS中均存在,说明在启动过程中的EPS均以蛋白质为主;虽然LB-EPS和TB-EPS所处位置不同,但其组分和结构基本相同。在LB-EPS中仅存在荧光峰A和B,无其他荧光峰,且启动过程中LB-EPS的结构与组成并未发生较大变化。在第53天时TB-EPS中,除存在荧光峰A和B,还出现荧光峰D,说明此阶段有简单芳环蛋白类物质产生;第123天时,出现荧光峰C和E,荧光峰B消失,说明部分色氨酸蛋白类物质转化为酪氨酸蛋白类物质[27]。三维荧光结果表明,在ANAMMOX-UASB启动过程中污泥EPS中的TB-EPS的结构和组成均会发生变化。
ANAMMOX-UASB启动过程中LB-EPS和TB-EPS中各峰光谱参数见表2。由表2可知,荧光峰会向更长的波长移动(称为红移),或向更短的波长移动(称为蓝移)。在LB-EPS中,第123天荧光峰A沿Ex方向蓝移5 nm,沿Em方向红移5 nm;荧光峰B沿Ex方向蓝移15 nm,沿Em方向红移5 nm。在TB-EPS中,第53天时荧光峰A沿Em方向红移10 nm,但至第123天时沿Em方向蓝移10 nm,与启动前相同;荧光峰B沿Em方向红移5 nm。上述研究结果表明,波长移动与EPS中各成分的结构变化密切相关,其中波长红移与含羰基取代基、羟基、烷氧基、氨基和羧基含量增加相关联,而波长蓝移则与芳香酯基减少,芳香环数下降,共轭键数的降低和羰基、羟基和氨基的减少直接相关。荧光峰波长及峰强度的变化说明ANAMMOX-UASB启动过程中污泥EPS中的组分、结构均发生变化。
表 2 ANAMMOX-UASB启动过程中LB-EPS和TB-EPS的荧光光谱参数Table 2. Fluorescence spectral parameters of LB-EPS and TB-EPS during startup process of ANAMMOX-UASB样品 时间/d 峰A 峰B 峰C 峰D 峰E Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 LB 0 225/330 274.5 275/335 175.1 — — — — — — 53 225/330 193.4 275/335 136.2 — — — — — — 123 220/335 51.0 260/340 65.3 — — — — — — TB 0 220/330 176.5 275/335 109.0 — — — — — — 53 220/340 296.4 275/340 210.0 — — 220/310 260.0 — — 123 220/330 47.7 — — 270/300 48.0 220/310 47.8 270/425 15.3 2.4 微生物群落
1)微生物多样性和丰富度分析。ANAMMOX-UASB启动过程中的微生物多样性和丰富度如表3所示。由表3可知,所有样品的覆盖率均大于99%。这表明样品数据涵盖的物种充足,微生物群落丰富度和多样性结果具有较高的可靠性和真实性[28]。ACE和Chao1指数可以表征物种的丰富度,其值越大,所含物种越丰富。Shannon指数常用来评价生物群落组成复杂程度,其值越大,表明群落复杂程度越高[29]。Simpson指数更倾向于反应群落的均匀性,其值越大,表示优势菌群占总体生物菌群比例越大[30]。由表3可知,接种污泥的Chao1和ACE指数分别为594.16和583.74,第53天的Chao1和ACE指数分别上升至最大值,为695.08和682.31。这表明此时的群落中所含物种最丰富,结合启动中出水指标可得,此时反硝化菌、氨化菌、厌氧氨氧化菌共存。第250天分别下降到351.00和312.34,表明工艺启动成功后,微生物以自养厌氧氨氧化菌为主,群落丰富度有所下降。接种污泥的Shannon指数为5.19,第53天上升至最大值为6.06,表明此时群落复杂度最大;第250天降低至4.43,其原因是反应器成功启动后,厌氧氨氧化菌的丰度不断升高,其他微生物的丰度逐渐降低,导致微生物群落复杂度有所下降。接种污泥的Simpson指数为0.92,第53天上升至最大值0.96,表明此时反应器内优势菌群所占的比例也最大;第250天下降为0.87,较接种污泥略有降低,表明反应器启动成功后,优势菌群所占的比例有所下降。
表 3 ANAMMOX-UASB启动过程中微生物多样性和丰富度Table 3. Microbial diversity and abundance during startup process of ANAMMOX-UASB时间/d Chao1 ACE Shannon Simpson 覆盖率/% 0 594.16 583.74 5.19 0.92 99.5 53 695.08 682.31 6.06 0.96 99.5 123 343.23 328.19 4.78 0.90 99.8 250 351.00 312.34 4.43 0.87 99.7 2)微生物门水平物种丰度分析。将微生物在门水平上丰度前10的物种绘制成物种相对丰度柱形累加图(图6)。主要为变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、Latescibacteria菌门、Cloacimonetes菌门、浮霉菌门(Planctomycetes)、放线菌门(Actinobacteria)和疣微菌门(Verrucomicrobia)。其中浮霉菌门和变形菌门为脱氮功能菌。变形菌门在各个样品中占最大比例,在启动过程中丰度逐渐上升,由7.22%上升到54.40%。曹雁等[31]利用上流式厌氧过滤床(upflow blanket filter,UBF)反应器启动厌氧氨氧化工艺,启动成功后变形菌门增加了12%。杨开亮等[32]利用SBR接种活性污泥启动厌氧氨氧化工艺时,变形菌门在启动过程中丰度逐渐下降。上述研究结果不同,可能与反应器类型、接种污泥类型、运行参数及环境条件等不同有关。沈耀良等[33]在厌氧折流板(anaerobic baffled reactor,ABR)反应器中发现各隔室中变形菌门为主要菌种,其在脱氮方面占有重要地位。绿弯菌门是厌氧氨氧化系统中常见的伴生菌门,接种污泥的丰度为31.23%,第53天下降为20.32%,第123天反应器启动成功时丰度上升为28.52%。CHEN等[34]研究发现,绿弯菌门是兼性厌氧菌,在厌氧氨氧化污泥中起到支撑和骨架作用,可作为形成小颗粒污泥的框架粒子,也可作为颗粒污泥的载体,从而有利于厌氧氨氧化颗粒污泥的形成。MIAO等[35]的研究表明,厚壁菌门对反硝化有重要作用,厚壁菌门丰度由28.27%下降为5.55%,说明ANAMMOX-UASB启动过程中异养反硝化菌因环境条件改变大量裂解死亡。浮霉菌门为主要的自养脱氮功能菌群,厌氧氨氧化菌归属此菌门;接种污泥浮霉菌门丰度仅为0.34%,第123天启动成功时其丰度增至1.89%,表明反应器内富集了一定量的厌氧氨氧化菌。其他研究者也发现,厌氧氨氧化工艺启动过程中变形菌门丰度始终高于浮霉菌门丰度。如朱彤等[36]发现,在厌氧氨氧化反应器启动成功后,虽然变形菌门丰度有所下降,但仍高于浮霉菌门。
3)微生物属水平物种丰度分析。由于厌氧氨氧化菌属于浮霉菌门,因此,对浮霉菌门进行属水平的分析(图7)。浮霉菌门有unidentified_Phycisphaerae、Candidatus_Anammoximicrobium、Candidatus_Brocadia、Thermogutta、Pirellula和Rhodopirellula。在已知的6种厌氧氨氧化菌属中,启动过程中存在2种厌氧氨氧化菌属,分别为Candidatus Anammoximicrobium和Candidatus Brocadia。接种污泥中Candidatus Anammoximicrobium的丰度为40.94%,第53天下降到30.77%,第123天下降为0。杨瑞丽等[29]进行厌氧氨氧化工艺启动时,观察到Candidatus Anammoximicrobium丰度随NLR增大而逐渐降低,第172天下降到0,其可能的原因是Candidatus Anammoximicrobium生长速率较慢、亚硝酸盐亲和力较低以及对水质变化的抵抗力较弱。第123天,Candidatus Brocadia是反应器内唯一的厌氧氨氧化菌,其丰度为12.15%,第250天,下降到11.63%。曹雁等[31]利用UBF反应器培养厌氧氨氧化细菌时,Candidatus Brocadia的丰度由0.01%增加到1.00%。VAN DER STAR等[37]发现Candidatus Brocadia适合存在于高含氮浓度废水中。本实验中,进水氨氮质量浓度由50 mg·L−1提高到300 mg·L−1,属于高含氮废水,更适合Candidatus Brocadia生长,故Candidatus Brocadia启动过程中可得到有效富集,成为优势菌属。有研究[38]表明,由于生存环境的不同,厌氧氨氧化菌的群落结构存在差异,在稳定的生长环境中,通常只有1个属种的厌氧氨氧化菌占优势,这与本研究结果类似。
3. 结论
1) ANAMMOX-UASB启动过程中絮状污泥逐渐颗粒化,最终以不规则状的红色颗粒污泥和褐色絮状污泥为主。微生物的脱氢酶活性由3 909.51 μg·(h·g)−1持续降至72.13 μg·(h·g)−1。EPS主要以TB-EPS为主,占比为75.7%;且LB-EPS和TB-EPS中PN占比稳步增大,分别增至99.6%和94.7%,PN在厌氧氨氧化工艺启动过程中起关键作用。
2)在ANAMMOX-UASB启动过程中,Chao1、ACE、Shannon和Simpson指数均呈现先升后降的趋势,启动过程中污泥的优势菌门为变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、浮霉菌门(Planctomycetes)。浮霉菌门中Candidatus Anammoximicrobium丰度逐渐降低直至消失,而Candidatus Brocadia丰度最终增至12.15%。
-
表 1 ANAMMOX-UASB启动过程中各阶段运行情况
Table 1. Performance of each stage during ANAMMOX-UASB startup process
阶段 运行时间/d HRT/h 进水N质量浓度/(mg·L−1) 出水N质量浓度/(mg·L−1) TN去除率/% NH+4-N NO−2-N NH+4-N NO−2-N NO−3-N Ⅰ 1~13 24 50 50 152.36±85.15 1.22±1.16 4.07±1.76 −57.65±84.85 Ⅱ 14~53 24 50 50 4.68±7.92 7.91±6.31 3.4±1.73 84.19±10.6 Ⅲ 54~123 24 50~300 50~300 0.17±0.40 0.37±0.42 14.71±18.2 96.6±2.75 Ⅳ 124~160 24~12 300 300 1.86±2.62 0.83±0.77 56.21±0.77 90.18±2.49 Ⅴ 161~250 12~6 300 300 8.55±7.24 3.22±2.02 67.54±6.9 86.78±1.72 表 2 ANAMMOX-UASB启动过程中LB-EPS和TB-EPS的荧光光谱参数
Table 2. Fluorescence spectral parameters of LB-EPS and TB-EPS during startup process of ANAMMOX-UASB
样品 时间/d 峰A 峰B 峰C 峰D 峰E Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 Ex/Em 强度 LB 0 225/330 274.5 275/335 175.1 — — — — — — 53 225/330 193.4 275/335 136.2 — — — — — — 123 220/335 51.0 260/340 65.3 — — — — — — TB 0 220/330 176.5 275/335 109.0 — — — — — — 53 220/340 296.4 275/340 210.0 — — 220/310 260.0 — — 123 220/330 47.7 — — 270/300 48.0 220/310 47.8 270/425 15.3 表 3 ANAMMOX-UASB启动过程中微生物多样性和丰富度
Table 3. Microbial diversity and abundance during startup process of ANAMMOX-UASB
时间/d Chao1 ACE Shannon Simpson 覆盖率/% 0 594.16 583.74 5.19 0.92 99.5 53 695.08 682.31 6.06 0.96 99.5 123 343.23 328.19 4.78 0.90 99.8 250 351.00 312.34 4.43 0.87 99.7 -
[1] MENG F, SU G, HU Y, et al. Improving nitrogen removal in an ANAMMOX reactor using a permeable reactive biobarrier[J]. Water Research, 2014, 58: 82-91. doi: 10.1016/j.watres.2014.03.049 [2] ABMA W R, SCHULTZ C E, MULDER J W, et al. Full-scale granular sludge ANAMMOX process[J]. Water Science and Technology, 2007, 55(8/9): 27-33. [3] 沈耀, 陈重军, 张海芹, 等. 基于高通量测序的ABR厌氧氨氧化反应器各隔室细菌群落特征分析[J]. 环境科学, 2016, 37(7): 2652-2658. [4] 陈文静, 陈圣东, 梁佳茵, 等. Anammox脱氮工艺启动研究进展[J]. 环境科学与技术, 2019, 42(11): 130-140. [5] 徐师, 张大超, 肖隆文, 等. 厌氧氨氧化反应快速启动方法的研究进展[J]. 环境工程, 2018, 36(6): 18-21. [6] 吕玮, 张立秋, 黄奕亮, 等. 常温低基质下两种厌氧氨氧化反应器启动特性比较[J]. 中国给水排水, 2019, 35(3): 31-37. [7] GAO F, ZHANG H, YANG F, et al. The effects of zero-valent iron (ZVI) and ferroferric oxide (Fe3O4) on anammox activity and granulation in anaerobic continuously stirred tank reactors (CSTR)[J]. Process Biochemistry, 2014, 49(11): 1970-1978. doi: 10.1016/j.procbio.2014.07.019 [8] WANG Y, HU X, JIANG B, et al. Symbiotic relationship analysis of predominant bacteria in a lab-scale anammox UASB bioreactor[J]. Environmental Science and Pollution Research, 2016, 23(8): 7615-7626. doi: 10.1007/s11356-015-6016-z [9] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [10] 朱南文, 闺航, 陈美慈, 等. TTC-脱氢酶测定方法的探讨[J]. 中国沼气, 1996, 14(2): 3-5. [11] WANG Z, GAO M, WANG Z, et al. Effect of salinity on extracellular polymeric substances of activated sludge from an anoxic-aerobic sequencing batch reactor[J]. Chemosphere, 2013, 93(11): 2789-2795. doi: 10.1016/j.chemosphere.2013.09.038 [12] HOU J, MIAO L, WANG C, et al. Effect of CuO nanoparticles on the production and composition of extracellular polymeric substances and physicochemical stability of activated sludge flocs[J]. Bioresource Technology, 2015, 176: 65-70. doi: 10.1016/j.biortech.2014.11.020 [13] FRØLUND B B K. Extraction of extracellular polymers from activated sludge using a cation exchange resin[J]. Water Research, 1996, 8(30): 1749-1758. [14] 杨明明, 刘子涵, 周杨, 等. 厌氧氨氧化颗粒污泥EPS及其对污泥表面特性的影响[J]. 环境科学, 2019, 40(5): 2341-2348. [15] 彭广生, 陆燕青, 曾鸿鹄, 等. 人工湿地β-六六六去除效果及细菌群落特征分析[J]. 水处理技术, 2020, 46(8): 34-38. [16] 朱晓桐, 于冰洁, 林久淑, 等. ANAMMOX-UASB反应器启动特性[J]. 环境科学与技术, 2020, 43(12): 143-150. [17] 郑平, 许冬冬, 康达, 等. 厌氧氨氧化颗粒污泥研究进展[J]. 微生物学通报, 2019, 46(8): 1988-1995. [18] BORAN K, JAN T K. Anammox biochemistry: A tale of heme c proteins[J]. Trends in Biochemical Science, 2016, 41(12): 998-1011. doi: 10.1016/j.tibs.2016.08.015 [19] LIN Q, KANG D, ZHANG M, et al. The performance of anammox reactor during start-up: Enzymes tell the story[J]. Process Safety and Environmental Protection, 2019, 121: 247-253. doi: 10.1016/j.psep.2018.10.029 [20] KIM S, PARK J, CHO Y, et al. Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions[J]. Bioresource Technology, 2013, 144: 8-13. doi: 10.1016/j.biortech.2013.06.068 [21] CHEN H, HU H, CHEN Q, et al. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties[J]. Bioresource Technology, 2016, 211: 594-602. doi: 10.1016/j.biortech.2016.03.139 [22] 杨敏, 胡学伟, 宁平, 等. 废水生物处理中胞外聚合物(EPS)的研究进展[J]. 工业水处理, 2011, 31(7): 7-12. doi: 10.3969/j.issn.1005-829X.2011.07.002 [23] GUO J, WANG S, LIAN J, et al. Rapid start-up of the anammox process: Effects of five different sludge extracellular polymeric substances on the activity of anammox bacteria[J]. Bioresource Technology, 2016, 220: 641-646. doi: 10.1016/j.biortech.2016.08.084 [24] MA B, LI Z, WANG S, et al. Insights into the effect of nickel (Ni(II)) on the performance, microbial enzymatic activity and extracellular polymeric substances of activated sludge[J]. Environmental Pollution, 2019, 251: 81-89. doi: 10.1016/j.envpol.2019.04.094 [25] DONG J, ZHANG Z, YU Z, et al. Evolution and functional analysis of extracellular polymeric substances during the granulation of aerobic sludge used to treat p-chloroaniline wastewater[J]. Chemical Engineering Journal, 2017, 330: 596-604. doi: 10.1016/j.cej.2017.07.174 [26] ZHU L, QI H, LV M, et al. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies[J]. Bioresource Technology, 2012, 124: 455-459. doi: 10.1016/j.biortech.2012.08.059 [27] 王子超. 盐度和重金属对序批式生物反应器性能及微生物群落结构影响的研究[D]. 青岛: 中国海洋大学, 2014. [28] 何承兴, 储昭瑞, 谭炳琰, 等. 厌氧氨氧化SBBR启动过程中菌群演替分析[J]. 水处理技术, 2019, 45(7): 93-96. [29] 杨瑞丽, 王晓君, 吴俊斌, 等. 厌氧氨氧化工艺快速启动策略及其微生物特性[J]. 环境工程学报, 2018, 12(12): 3341-3350. [30] 宋壮壮, 吕爽, 刘哲, 等. 厌氧氨氧化耦合反硝化工艺的启动及微生物群落变化特征[J]. 环境科学, 2019, 40(11): 5057-5065. [31] 曹雁, 王桐屿, 秦玉洁, 等. 厌氧氨氧化反应器脱氮性能及细菌群落多样性分析[J]. 环境科学, 2017, 38(4): 1544-1550. [32] 杨开亮, 廖德祥, 王莹, 等. 厌氧氨氧化快速启动及微生物群落演替研究[J]. 水处理技术, 2020, 46(5): 65-70. [33] 沈耀良, 张海芹, 王翻翻, 等. 不同接种污泥ABR厌氧氨氧化的启动特征[J]. 环境科学, 2015, 36(6): 2216-2221. [34] CHEN C, HUANG X, LEI C, et al. Effect of organic matter strength on anammox for modified greenhouse turtle breeding wastewater treatment[J]. Bioresource Technology, 2013, 148: 172-179. doi: 10.1016/j.biortech.2013.08.132 [35] MIAO Y, LIAO R, ZHANG X, et al. Metagenomic insights into Cr(VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater[J]. Water Research, 2015, 76: 43-52. doi: 10.1016/j.watres.2015.02.042 [36] 朱彤, 贾若坦, 梁启煜, 等. 厌氧氨氧化反应器运行过程微生物群落演替分析[J]. 东北大学学报(自然科学版), 2018, 39(5): 693-698. doi: 10.12068/j.issn.1005-3026.2018.05.018 [37] VAN DER STAR W R L, MICLEA A I, VAN DONGEN U G J M, et al. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells[J]. Biotechnology and Bioengineering, 2008, 101(2): 286-294. doi: 10.1002/bit.21891 [38] VAN DE VOSSENBERG J, RATTRAY J E, GEERTS W, et al. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production[J]. Environmental Microbiology, 2008, 10(11): 3120-3129. doi: 10.1111/j.1462-2920.2008.01643.x -




 下载:
下载: