-
难降解有机污染废水具有生物毒性,甚至致癌等严重问题[1]。因此,迫切需要开发经济有效的有机污染物废水处理技术[2]。高级氧化法作为一种能高效氧化降解有机污染物技术方法,被广泛应用于难降解有机污染物废水处理,其中的光催化法和类Fenton试剂法因其处理成本低、降解效率高和无二次污染等优点,具有很好的应用前景。然而,光催化法需要更加高效的催化剂,以提高废水中有机物的去除率。
水滑石(LDHs)是一种具有特殊层状结构的双金属氢氧化物,其化学式为[M2+1-xM3+x(OH)2]x+(An-)x/n·mH2O,因其具有主体层板金属阳离子可调变、层间阴离子可交换及粒径尺寸可调控等特点,故可成为优良催化材料的前驱体[3-4]。同时,LDHs具有易制备、合成成本低廉、比表面积较大、不产生二次污染等特点,属于环境友好型催化剂,近年来受到国内外各界学者的关注[5]。在可见光催化过程中,污染物的降解效率受限的主要原因有LDHs的电子传递效率低以及较弱的载流子迁移速率和较快的电子-空穴对复合速率,从而导致光催化效率和光催化活性偏低[9]。有研究[8]表明,Cu2+、Co2+及Fe2+等过渡金属具有良好的供电子能力,往往作为金属阳离子用于制备LDHs,以提高电子传递效率。目前,针对二元水滑石制备的报道较多,如MgAl-LDHs[6]、ZnAl-LDHs[7]等。另外,石墨烯是一种具有特殊结构和性质的单层石墨片,其具备高载流子迁移率和高比表面积等优点[10]。同时,石墨烯巨大的比表面积可以有效吸附有机污染分子,且增加光催化反应的活性位点,从而提高污染物降解效率[11]。YANG等[12]成功制备了CoZnAl-LDH/RGO/g-C3N4复合催化剂,可将CO2光催化还原为CO。此外,在可见光或UV氧化体系下,通过添加H2O2 促进Cu2O、CuO等催化效率,提高体系中超氧自由基(·
O2− )和羟基自由基(·OH) 生成效率,实现废水中难降解有机物的快速去除[13]。但在光催化体系下,通过将LDHs、石墨烯、H2O2三者有机组合以实现光催化去除有机物的研究尚鲜有报道[14]。基于此,本研究采用单滴共沉淀方法制备CuCoAl-LDHs/GO复合材料,以RhB和苯酚作为目标污染物,通过添加H2O2协同CuCoAl-LDHs/GO光催化体系降解目标污染物,并进一步分析不同H2O2和催化剂添加量下对目标污染物的降解效率影响。
-
三水合硝酸铜(Cu(NO3)2·3H2O)、六水合硝酸钴(Co(NO3)2·6H2O)、九水合硝酸铝(Al(NO3)3·9H2O)、片状氢氧化钠(NaOH)、无水碳酸钠(Na2CO3)和过氧化氢(H2O2)均购自上海麦克林生化科技有限公司,石墨粉、浓硫酸(H2SO4)、盐酸(HCl)和无水乙醇(C2H5OH)则购自国药化学试剂有限公司。实验用水均为自制超纯水。
-
CuCoAl-LDHs的制备:采用单滴共沉淀法制备三元CuCoAl-LDHs:称取Co(NO3)2·6H2O (12 mmol)、Cu(NO3)2·3H2O (4 mmol)和Al(NO3)3·9H2O (8 mmol) 溶解于200 mL的超纯水中,移入1 L三口烧瓶中,磁力搅拌至混合均匀;用蠕动泵以1 mL·min−1的滴速将2 mol·L−1 NaOH和1 mol·L−1 Na2CO3混合溶液在磁力搅拌下缓缓滴入上述溶液中,至溶液pH达到10,随后65 ℃油浴24 h;将悬浊液取出后抽滤并用超纯水洗涤至上层清液pH至中性后,冷冻干燥得到三元CuCoAl-LDHs产物。
CuCoAl-LDHs/GO的制备:按照改进的Hummers法进行氧化石墨烯(GO)的制备[15],称取Co(NO3)2·6H2O (12 mmol)、Cu(NO3)2·3H2O (4 mmol)和Al(NO3)3·9H2O (8 mmol)加入事先溶解好的5 mg·mL−1的GO分散液40 mL中,超声分散1 h后,用超纯水稀释至200 mL,移入1 L三口烧瓶中,磁力搅拌30 min至混合均匀;用蠕动泵以1 mL·min−1的滴速将2 mol·L−1 NaOH和1 mol·L−1 Na2CO3混合溶液在磁力搅拌下缓缓滴入上述溶液中,至溶液pH达到10,随后65 ℃油浴24 h;将悬浊液取出后抽滤并用超纯水洗涤至上层清液pH至中性后,冷冻干燥得到三元CuCoAl-LDHs/GO复合产物。
-
采用德国Bruker公司生产的X射线粉末衍射仪(XRD)对催化剂进行物相分析。其中,加速电压60 kV,电流80 mA,Cu靶Kα为射线源,λ=0.154 06 nm,扫描范围为5°~80°,扫描速率10°·min−1;催化剂的微区形貌和表面微区成分的定性和半定量通过美国FEI公司所生产的场发射环境扫描电子显微镜(SEM)和英国Oxford的型号为AZtec X-Max 80型X射线能量色散谱仪分析(EDS),工作电压80~200 kV;使用美国赛默飞公司生产的型号为ESCALAB250Xi的X射线光电子能谱仪(XPS)对样品的表面成分、电子结构和能带结构进行分析,测试波长为400~400 cm−1;采用安捷伦科技有限公司的型号为Cary 100的紫外-可见分光光度计测定催化剂的紫外可见漫反射谱图,波长扫描范围为200~800 nm。
-
考察H2O2强化催化剂光催化性能,采用添加H2O2的光催化法降解染料RhB和有机污染物苯酚。在不同体系下对催化剂的投加量和投加H2O2的浓度分别进行了分析,其中光催化降解反应采用北京中教金源光催化专用反应器250 mL,标准磨口,石英上盖,法兰接口,采用10 mL注射器扎针取样。采用300W氙灯模拟太阳光源,镜头采用UVIRCUT400紫外截止滤光片,出射光谱为400~780 nm,光照时间为1 h,取样间隔10 min。采用赛默飞型号为UltiMate300的液相色谱对苯酚浓度进行分析测定,本实验使用的RhB和苯酚浓度均为50 mg·L−1。
RhB在554 nm处有最大吸收波长,利用这一特性采用紫外分光光度法实时检测染料吸光度的变化,再根据朗伯-比尔定律(A=εbc,A为吸光度,c为浓度),吸光度的变化可以反映污染物的残留量,污染物的去除率η按照式(1)计算[16]。
式中:Ci为污染物的初始浓度,mg·L−1;Ci为t时污染物的浓度, mg·L−1。
-
CuCoAl-LDHs和CuCoAl-LDHs/GO材料的XRD表征结果如图1所示。两者的XRD峰形相似,均表现出LDHs结构的特征衍射峰。在2θ=11.6°、23.56°、34.66°、39.16°、46.56°、60.36°、61.7°处均存在强衍射峰,对应水滑石结构的(003)、(006)、(012)、(015)、(018)、(110)和(113)特征衍射峰[17];CuCoAl-LDHs/GO在11.6°左右的峰形比CuCoAl-LDHs有明显增强,对应的是氧化石墨烯(002)特征衍射峰。此外,没有观察到与Co(OH)2、Cu(OH)2或Al(OH)3有关的衍射峰,表明所制备的CuCoAl-LDHs和CuCoAl-LDHs/GO具有较纯的LDHs相[18-19]。
-
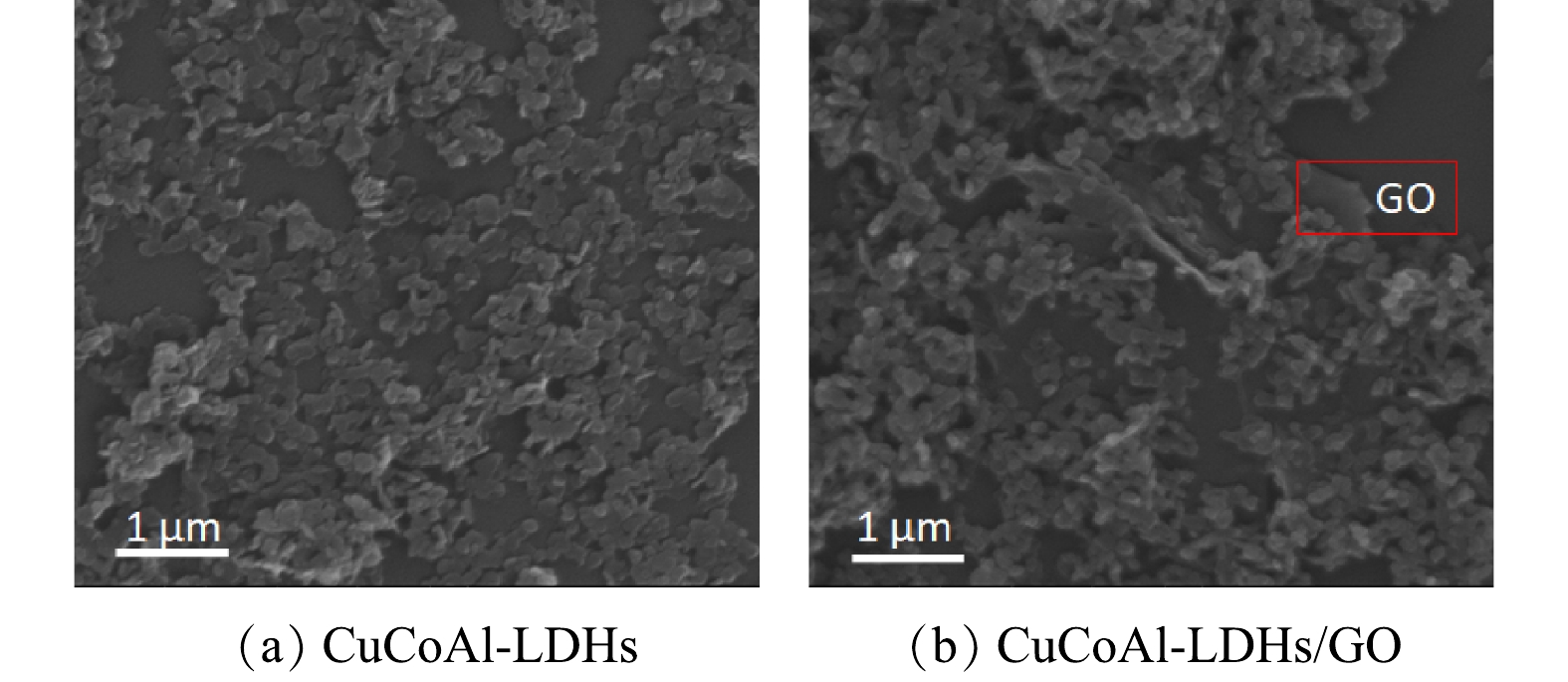
图2为催化剂的SEM电镜和EDS能谱表征图。由图2(a)可知,制备的CuCoAl-LDHs材料具有典型的六边形层状结构,这与CHENG等[20]所报道的水滑石结构相一致,LDHs纳米片均匀的分散在氧化石墨烯表面(图2(b))。
-
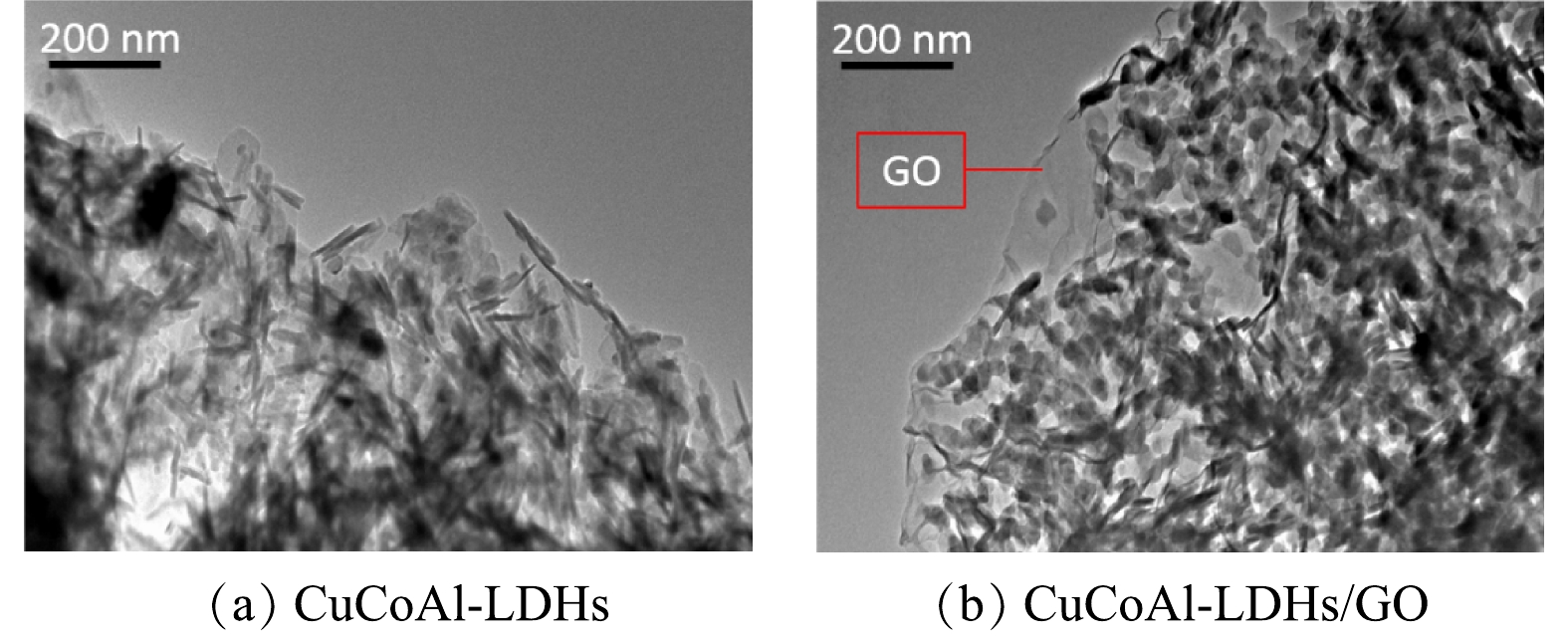
图3为催化剂的TEM电镜表征图。由图3(a)可知,合成的CuCoAl-LDHs材料为薄片状,其大小约为50 nm,厚度约为10 nm。在图3(b)中可清晰地观察到CuCoAl-LDHs材料的存在,且均匀分布在清晰的透明氧化石墨烯薄膜表面,进一步证明所合成出的CuCoAl-LDHs/GO具备石墨烯负载LDHs材料特征。
-
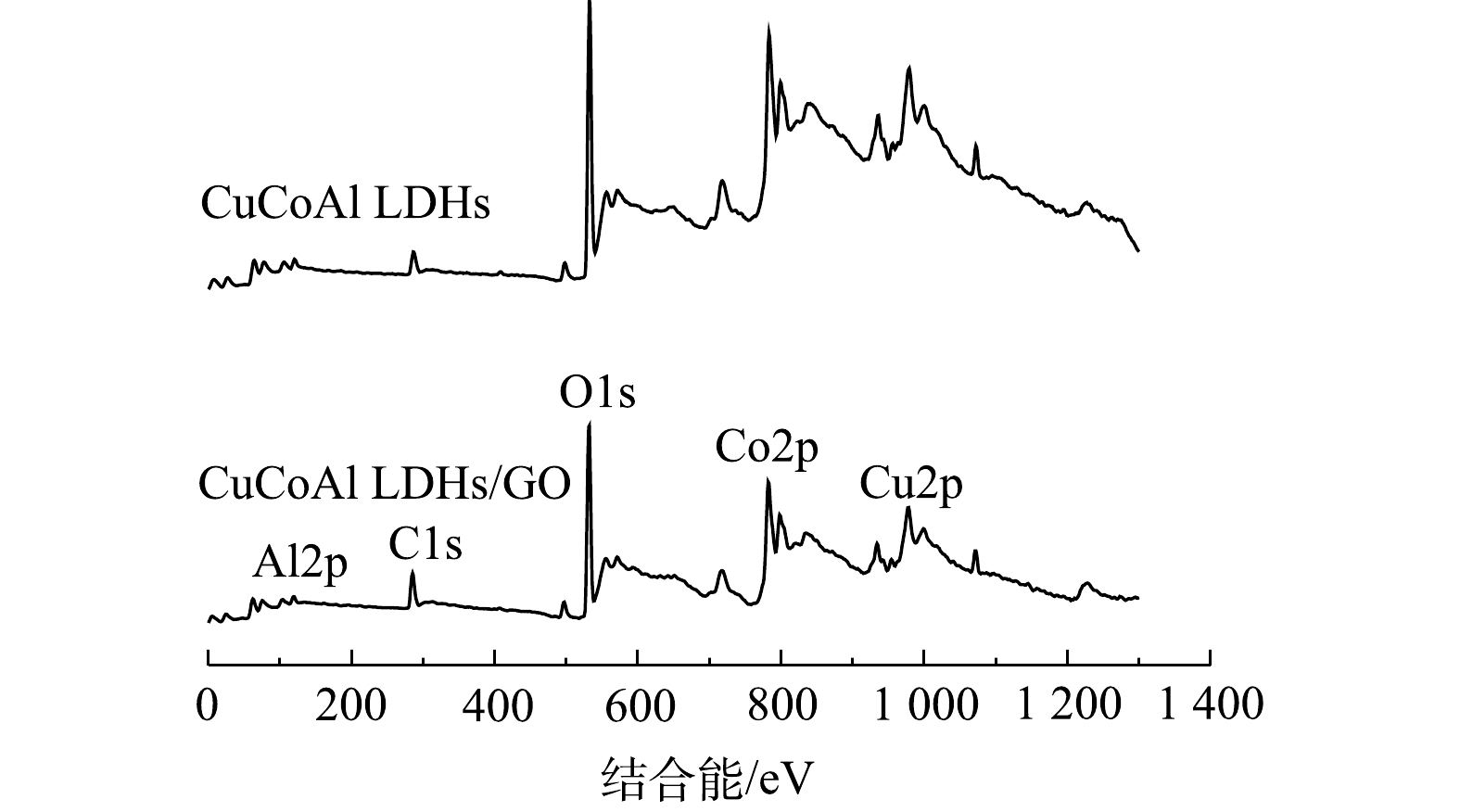
CuCoAl-LDHs和CuCoAl-LDHs/GO的XPS全谱扫描图如图4所示。对比2种材料XPS可知,均存在Co、Cu、Al、C、O元素的峰,表明两者具有相同的Co、Cu、Al组成的三元LDHs结构,而CuCoAl-LDHs/GO中C元素含量明显增强,证明三元结构的LDHs成功引入氧化石墨烯中,这与WANG等[21]报道的结果一致。图5(a)为CuCoAl-LDHs/GO在Co2p处的XPS谱图,在Co2p3/2轨道上,结合能为779.8 eV和780.8 eV处的2个峰对应于Co2+和Co3+,其比例为1.65∶1[22]。由图5(b)可见,在Cu2p3/2轨道上,结合能为934.4 eV和932.5 eV处的2个峰对应于Cu2+和Cu+,其比例为2.06∶1 [23]。图5(c)为C1s处的XPS谱图,其中结合能为284.4 eV的强峰对应于氧化石墨烯上的C—C单键,而在288.4 eV左右的小峰归因于
CO32− 中的C—O键。图5(d)为O1s核水平上的XPS谱图,其中结合能为529.9 eV和531.2 eV处的2个峰对应于晶格氧(O2−)和O—OH,其中O lattice/O-OH的比例为1.2∶1[24],进一步表明催化剂中存在Co3+、Co2+、Cu2+、Cu+和Al3+等金属离子,可参与氧化还原反应。 -
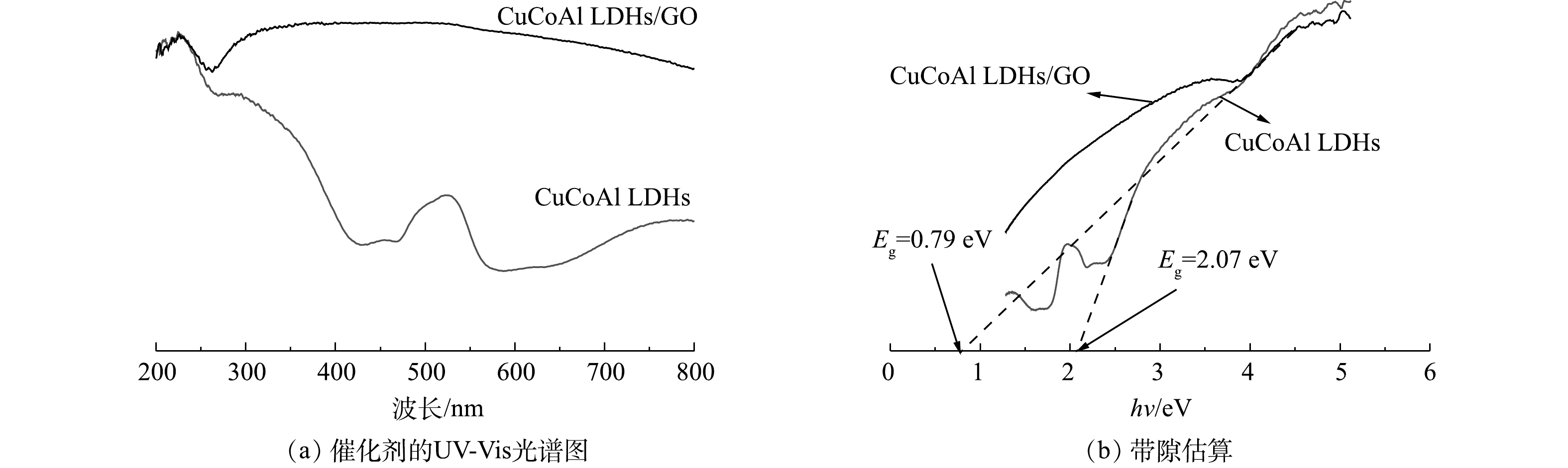
利用UV-Vis DRS研究CuCoAl-LDHs/GO的光响应特性。如图6(a)所示,LDHs具有2个较为明显的吸收峰:在紫外范围内(200~400 nm)出现的波段可归因于配体到金属的电荷转移(O2−→Mn+);位于可见光范围内(490~560 nm)出现的吸收波段则是Co2+在LDHs内层中的跃迁所引起的[25-26]。负载了GO的LDHs符合氧化石墨烯的吸收谱图特征,并且CuCoAl-LDHs/GO复合材料在可见光波段的吸光度存在明显提升。此外,根据Kubelka-Munk函数计算,CuCoAl-LDHs和CuCoAl-LDHs/GO的带隙如图6(b)所示,其带隙分别为2.07 eV和0.79 eV,GO基对光的吸收强度越高,其光催化活性越高[27]。
-
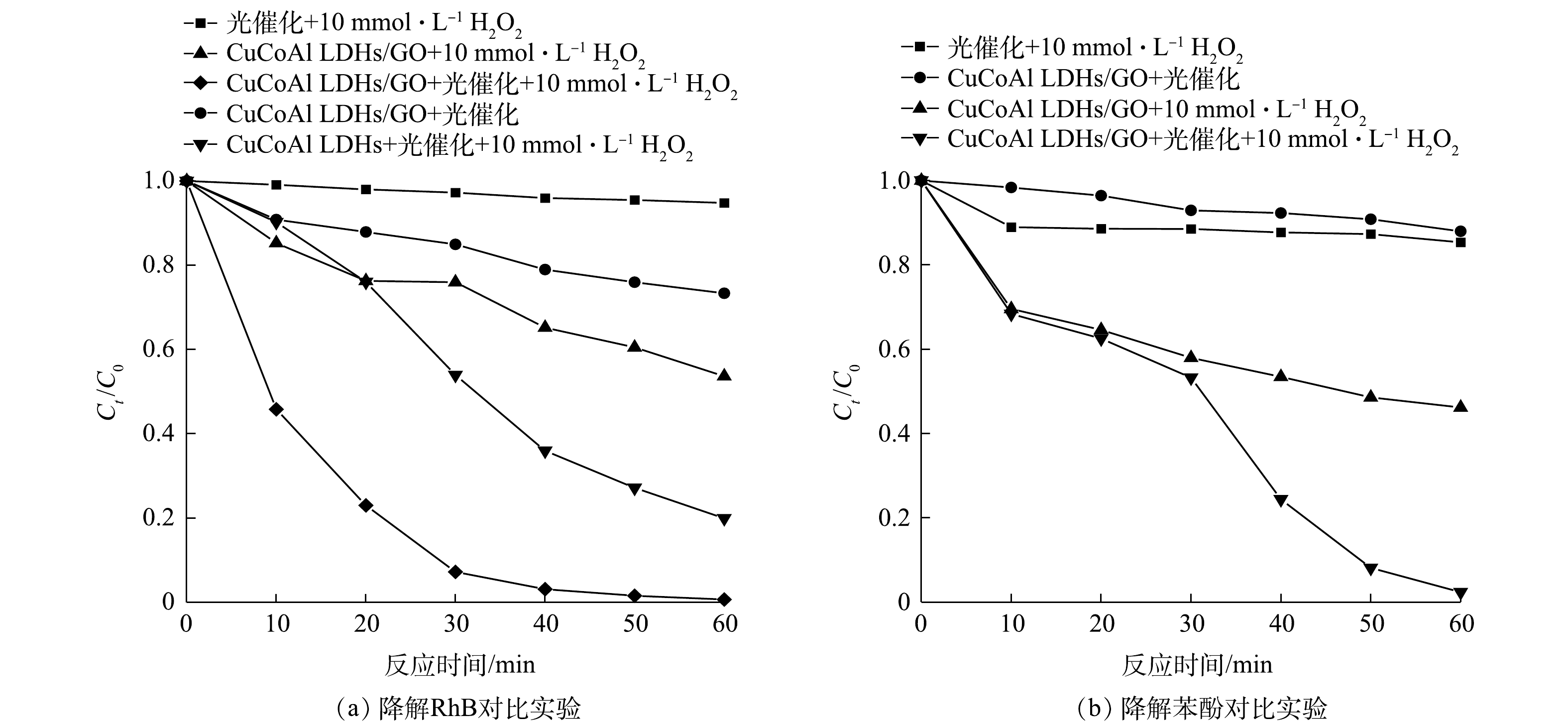
为了探究H2O2的加入是否对CuCoAl-LDHs/GO材料的催化性能起到增效作用,分别对RhB和苯酚进行了不同体系的催化降解。由图7(a)可知,在不添加催化剂且H2O2为10 mmol·L−1和氙灯照射强度为300 W的条件下,对RhB的降解效果不明显。在仅使用可见光和只添加H2O2不使用可见光照射的条件下,在60 min时CuCoAl-LDHs/GO对RhB的光催化降解率分别为26.7%和46.4%。同样,在CuCoAl-LDHs和H2O2共同存在时,光催化降解RhB效率为80.1%;在CuCoAl-LDHs/GO和H2O2共同存在条件下,RhB的光催化降解率达到99.3%,降解率提升了19.2%。由图7(b)可知,仅H2O2存在时,对苯酚存在一定去除效果;在不添加催化剂且仅存在H2O2和可见光照射的条件下,苯酚的降解率为14.6%,这是由于H2O2自身具备一定的氧化能力。在无可见光照射下,H2O2与CuCoAl-LDHs/GO共同作用对苯酚的降解率为53.9%。在可见光照射下,仅CuCoAl-LDHs/GO对苯酚的光催化降解效果同样效率低;但当H2O2和CuCoAl-LDHs/GO复合光催化体系下,CuCoAl-LDHs/GO对苯酚降解率达到了97.6%,可以证明H2O2强化了CuCoAl-LDHs/GO光催化性能。导致以上结果的可能原因为,在可见光和催化剂共同作用下,负载氧化石墨烯可提高CuCoAl-LDHs的光吸收性能,加快电子传递速率,减少光生电子和空穴的复合[28],加速H2O2的分解,从而提高自由基的生成效率,促进污染物的降解。
-
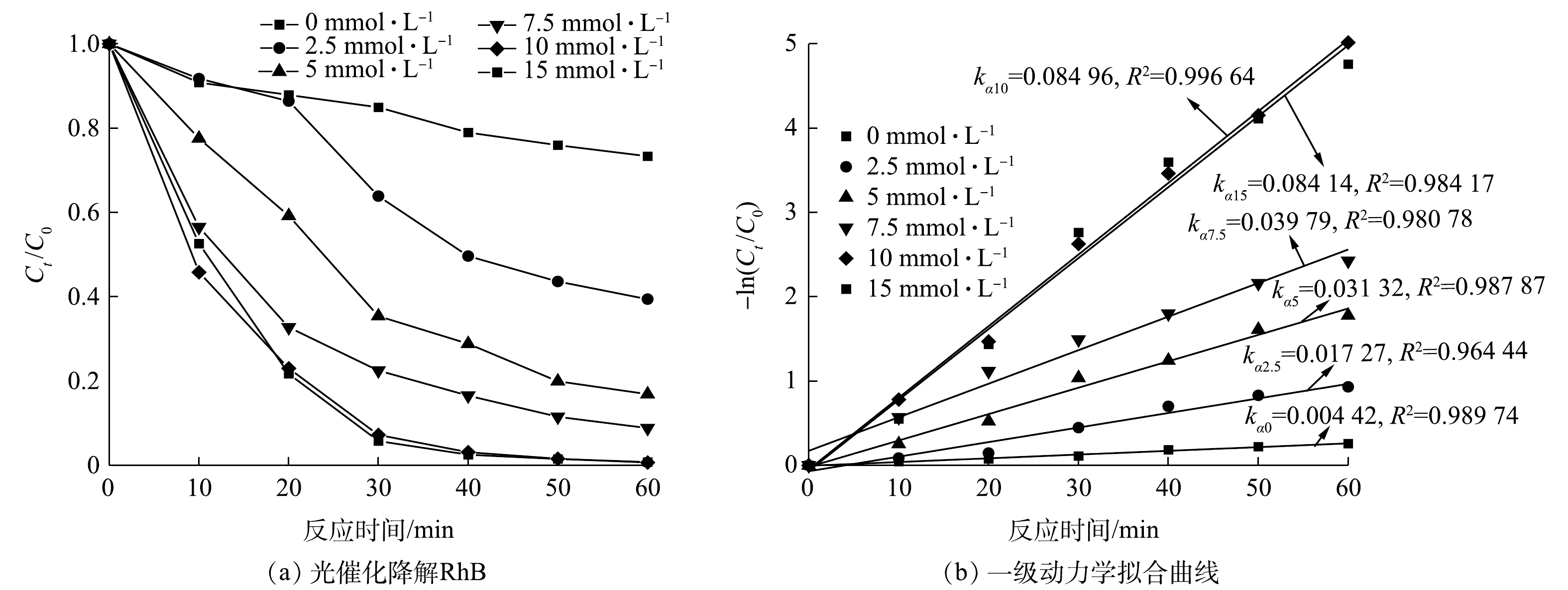
为进一步证明H2O2的加入可提升CuCoAl-LDHs/GO的光催化效率,探究了H2O2浓度对催化剂降解RhB性能的影响。由图8(a)可知,在催化剂的投加量为1 g·L−1且保持可见光照射下,当H2O2浓度分别为0、2.5、5、7.5、10和15 mmol·L−1时,CuCoAl-LDHs/GO对RhB的降解率依次为26.7%、60.6%、83.1%、91.2%、99.3%和99.2%。可见,在H2O2浓度为10 mmol·L−1时降解效果最好,此时RhB几乎完全降解。如图8(b)所示,RhB光催化降解符合一级动力学。可以看出,降解速率常数kα随着H2O2浓度的升高而增大,在H2O2浓度为10 mmol·L−1时,降解速率常数kα达到最大值,继续增大H2O2浓度则kα不再增大。这进一步表明H2O2的增加会强化体系中CuCoAl-LDHs/GO光催化性能,但达到一定浓度后,受到催化剂影响,污染物降解率提升有限。
-
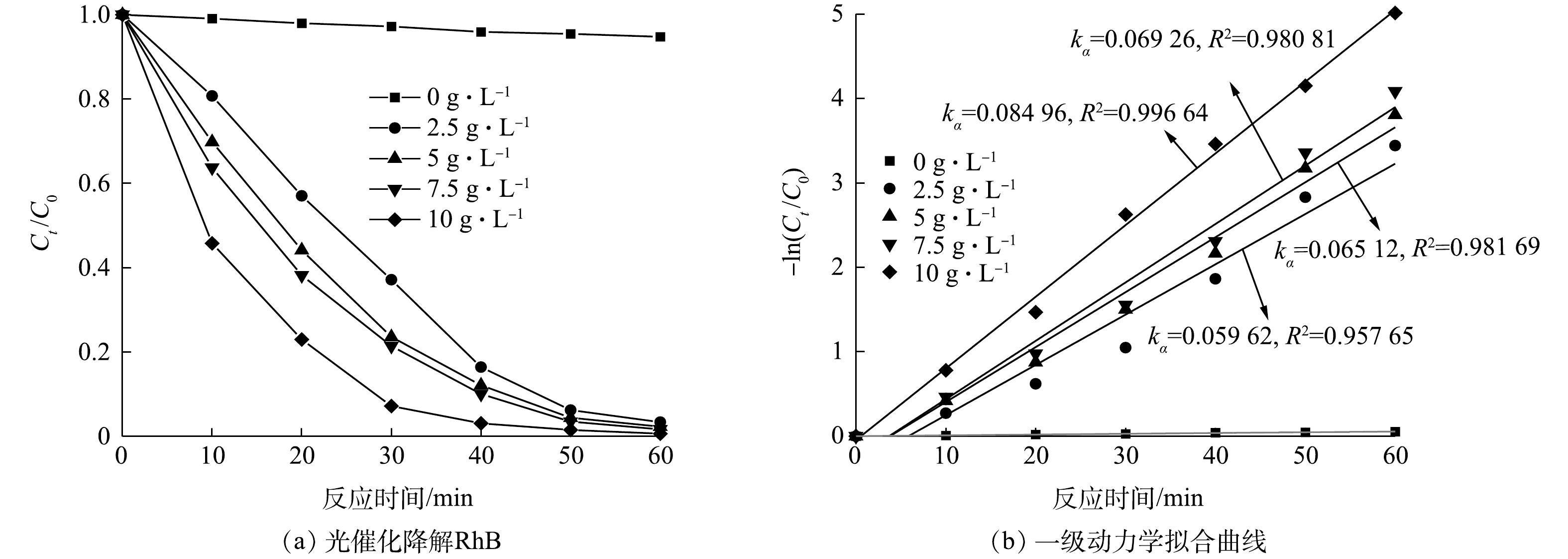
图9(a)反映了CuCoAl-LDHs/GO在保持300 W氙灯光源照射,添加的10 mmol·L−1 H2O2时催化剂投加量对RhB降解效果的影响。在可见光照射下,未投加催化剂时,H2O2对RhB的降解率较低,仅为5%。在H2O2为10 mmol L−1、催化剂投加量为1 g·L−1、反应60 min时,RhB降解率达到最高,继续增大催化剂投加量后RhB降解率变化不明显。结合图9(b)可知,催化剂投加量对RhB的降解速率影响较大,降解速率常数与投加量呈正比例关系,降解速率随着催化剂的投加量的增大而增大。这是由于催化剂投加量的增加,增加活性位点数量,提高了体系的单位时间效率。
-
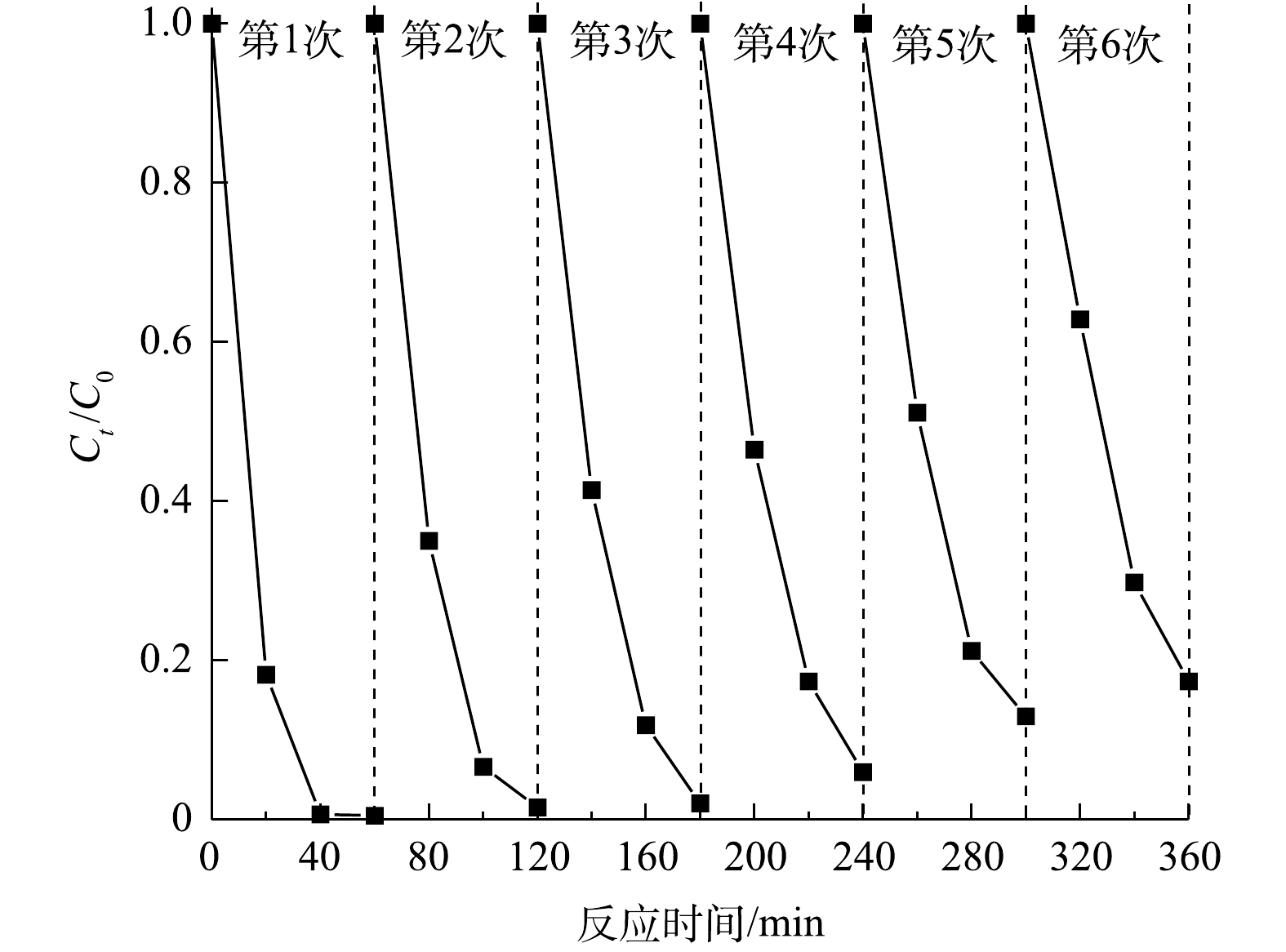
除了良好的催化性能之外,催化剂可循环利用性是实际应用的关键因素。通过每次活化反应结束后的催化剂回收、水洗、干燥后再次进行光催化降解RhB实验,以验证其稳定性和可重复使用性(图10)。由图10可知,在H2O2存在条件下,经过6次循环后,CuCoAl-LDHs/GO仍具备良好的光催化降解性能,降解率达到82.65%,这表明制备的CuCoAl-LDHs/GO复合催化剂具备良好的稳定性和可循环利用性。
-
1)通过单滴共沉淀法成功制备了CuCoAl-LDHs/GO复合材料,该催化剂较普通的三元CuCoAl-LDHs水滑石具有更窄的禁带宽度和更高的催化活性。
2)通过H2O2协同CuCoAl-LDHs/GO对RhB和苯酚的光催化降解,H2O2复合光催化体系较其他体系对催化剂催化性能的提升更大,对RhB和苯酚的降解率分别达到了99.3%和97.6%。因此,光催化体系中,H2O2与CuCoAl-LDHs/GO具备协同增效作用。
3)所制备的CuCoAl-LDHs/GO复合材料在循环6次后降解率仍可以达到82.65%以上,说明其具备良好的稳定性和可循环利用性。
H2O2协同强化CuCoAl-LDHs/GO复合材料光催化效能
Photocatalytic performance of CuCoAl-LDHs/GO composites synergistically enhanced by H2O2
-
摘要: 采用单滴共沉淀法制备了氧化石墨烯负载到铜钴铝水滑石(CuCoAl-LDHs/GO)复合材料,并以罗丹明(RhB)和苯酚为目标降解物,开展了H2O2协同CuCoAl-LDHs/GO强化光催化降解的实验。结果表明:通过XRD、SEM、XPS及UV-Vis表征,发现复合材料中存在石墨烯和金属离子(Co3+、Co2+、Cu2+、Cu+、Al3+),且具备较高的光催化活性;H2O2存在的条件下,1 g·L−1 CuCoAl-LDHs/GO对RhB和苯酚的光催化降解率分别为99.3%和97.6%;H2O2和CuCoAl-LDHs/GO投加量增加有助于RhB的降解,循环6次后降解率仍达82.65%,表明H2O2可有效促进CuCoAl-LDHs/GO光催化降解性能,并具备多次循环利用的能力。以上研究结果可为实际水环境中微污染的治理修复提供参考。
-
关键词:
- CuCoAl-LDHs/GO /
- 可见光 /
- H2O2 /
- 效能 /
- 强化作用
Abstract: The CuCuAl-LDHs/GO composite supported by GO was prepared by single drop co-precipitation method. The photocatalytic performance of CuCoAl-LDHs/GO reinforced by H2O2 was studied using rhodamine (RHB) and phenol as the target degradants. The results showed that through XRD, SEM, XPS and UV-Vis characterization, the presence of graphene and metal ions (CO3+, CO2+, Cu2+, Cu+, Al3+) in the composites was conducive to their high photocatalytic activity. The photocatalytic degradation rates of RhB and phenol by 1 g·L−1 CuCoAl-LDHS /GO were 99.3% and 97.6% in the presence of H2O2, respectively. The dosage of H2O2 and CuCoAl-LDHs/GO were positively proportional to the degradation rate of RhB; In the presence of H2O2, CuCoAl-LDHs/GO catalyst has good stability and photocatalytic degradation performance, the degradation rate of RhB was still up to 82.65% after six cycles, which can provide a new technical support for the treatment and remediation of micropollutants in the actual water environment.-
Key words:
- CuCoAl-LDHs/GO /
- visible light /
- H2O2 /
- efficiency /
- reinforcement
-
-
[1] LUO L, WANG Y, ZHU M, et al. Co-Cu-Al layered double oxides as heterogeneous catalyst for enhanced degradation of organic pollutants in wastewater by activating peroxymonosulfate: performance and synergistic effect[J]. Industrial & Engineering Chemistry Research, 2019, 58: 8699-8711. [2] 张冠华, 陈语芙, 孟跃, 等. AuCu/ZnAl-LDO复合光催化剂的制备及其光催化性能[J]. 无机化学学报, 2020, 36(5): 109-118. [3] ZIARATI A, BADIEI A, GRILLO R, et al. 3D Yolk@Shell TiO2-x/LDH architecture: tailored structure for visible light CO2 conversion[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 5903-5910. [4] MENG Y, CHEN Y F, ZHOU X B, et al. Experimental and theoretical investigations into the activity and mechanism of the water–gas shift reaction catalyzed by Au nanoparticles supported on Zn-Al/Cr/Fe layered double hydroxides[J]. International Journal of Hydrogen Energy, 2020, 45(1): 464-476. doi: 10.1016/j.ijhydene.2019.10.172 [5] 苏荣军, 魏澜, 赵仁波, 等. 可见光催化剂ZnNiAl-LDOs的表征及降解PNP的研究[J]. 南昌大学学报:理科版, 2020(2): 148-154. [6] QIN, Z. , YANG, et al. Synthesis and characterization of polyoxyethylene sulfate intercalated Mg−Al−nitrate layered double hydroxide[J]. Langmuir, 2003, 19(14): 5570-5574. doi: 10.1021/la034526j [7] FENG Y, LI D, WANG Y, et al. Synthesis and characterization of a UV absorbent-intercalated Zn-Al layered double hydroxide[J]. Polymer Degradation and Stability, 2006, 91(4): 789-794. doi: 10.1016/j.polymdegradstab.2005.06.006 [8] ANIPSITAKIS G P, DIONYSIOU D D. RADICAL, et al. Generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705. [9] LIANG J, WEI Y, YAO Y, et al. Constructing high-efficiency photocatalyst for degrading ciprofloxacin: Three-dimensional visible light driven graphene based NiAlFe LDH[J]. Journal of Colloid and Interface Science, 2019, 540: 237-246. doi: 10.1016/j.jcis.2019.01.011 [10] CHOI W, LAHIRI I, SEELABOYINA R, et al. Synthesis of graphene and its applications: A Review[J]. Critical Reviews in Solid State & Materials Sciences, 2010, 35(1): 52-71. [11] NICHELA, D A, BERKOVIC, et al. Nitrobenzene degradation in fenton-like systems using Cu(II) as catalyst. comparison between Cu(II)- and Fe(III)-based systems[J]. Chemical Engineering Journal, 2013, 228: 1148-1157. doi: 10.1016/j.cej.2013.05.002 [12] 徐君君, 张熙茹, 杜义平, 等. UV/Cu2O/H2O2耦合强化降解左旋氧氟沙星[J]. 环境化学, 2021: 1-10. [13] 苏翠伟, 李媛媛, 佟冶, 等. H2O2协同提高单斜晶相BiVO4可见光催化性能的研究[J]. 化工新型材料, 2020, 48(S1): 64-68. [14] 吕来, 胡春. 多相芬顿催化水处理技术与原理[J]. 化学进展, 2017, 29(9): 981-999. doi: 10.7536/PC170552 [15] LI Y, CHEN J, LIANG H, et al. Highly compressible macroporous graphene monoliths via an improved hydrothermal process[J]. Advanced Materials, 2014, 26(28): 4789-4793. doi: 10.1002/adma.201400657 [16] 张启彦. TiO2-GO/LDHs复合材料光催化降解VOCs的研究[D]. 济南: 山东大学, 2020. [17] PEREZ-RAMIREZ J, MUL G, KAPTEIJN F, et al. Insitu investigation of thethermal decomposition of Co–Al hydrotalcite in different atmospheres[J]. Journal of Materials Chemistry, 2001, 11(3): 821-830. doi: 10.1039/b009320n [18] CAI P, HONG Z, CHONG W, et al. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg-Al-CO3 layered double hydroxides[J]. Journal of hazardous materials, 2012, 213-214(30): 100-108. [19] DAS D P, DAS J, PARIDA K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTLC) towards removal of fluoride from aqueous solution[J]. J Colloid Interface Sci, 2003, 261(2): 213-220. doi: 10.1016/S0021-9797(03)00082-1 [20] CHENG X, HUANG X, WANG X, et al. Influence of calcination on the adsorptive removal of phosphate by Zn-Al layered double hydroxides from excess sludge liquor.[J]. Journal of Hazardous Materials, 2010, 177(1-3): 516-523. doi: 10.1016/j.jhazmat.2009.12.063 [21] WANG H, JING M, WU Y, et al. Effective degradation of phenol via Fenton reaction over CuNiFe layered double hydroxides[J]. Journal of Hazardous Materials, 2018(353): 53-61. [22] DUAN, X G, SU C, et al. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals[J]. Applied Catalysis, B. Environmental: An International Journal Devoted to Catalytic Science and Its Applications, 2018, 220: 626-634. [23] LU S, WANG G, CHEN S, et al. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants.[J]. Journal of Hazardous Materials, 2018, 353: 401. doi: 10.1016/j.jhazmat.2018.04.021 [24] REN Y, LIN L, MA J, et al. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Applied Catalysis B Environmental An International Journal Devoted to Catalytic Science & Its Applications, 2015, 165: 572-578. [25] JO W K, TONDA S. Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with notable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants[J]. Journal of Hazardous Materials, 2019, 368(APR. 15): 778-787. [26] RUDOLF C, DRAGOI B, UNGUREANU A, et al. NiAl and CoAl materials derived from takovite-like LDHs and related structures as efficient chemoselective hydrogenation catalysts[J]. Catalysis Science & Technology, 2014, 4(1): 179-189. [27] KUMAR S, ISAACS M A, TROFIMOVAITE R, et al. P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction[J]. Applied Catalysis B Environmental, 2017, 35(1): 394. [28] KUMAR S, KUMAR A, et al. Enhanced photocatalytic activity of rGO-CeO2 nanocomposites driven by sunlight[J]. Materials Science and Engineering B, 2017, 223(9): 98-108. -





 下载:
下载: