-
水体中氮磷营养盐是藻类生长的物质基础,对藻类生长构成上行效应,但过多的营养盐会造成水体的富营养化,引起藻类的增殖暴发甚至形成水华现象。因此,对富营养化水体氮磷营养盐的削减是治理富营养化水体的重要途径。使用镧改性黏土制剂被认为是去除水体磷营养盐的有效手段,近年来在国内外富营养化水体治理工程中得到广泛应用,取得了显著成效[1-3]。镧制剂除磷作用机理为:金属镧可与水体中的可溶性磷酸根结合生成磷镧镨矿沉淀,从而去除水体中的生物活性磷,进而实现对富营养化水体的生态修复[4-5]。在实际的工程应用中,镧改性黏土制剂也存在诸多应用缺陷,镧改性黏土只能与可溶性的磷酸盐反应,难以去除富营养化水体中大量以藻类生物质磷形式存在的颗粒态磷,因此,镧改性黏土用于富营养化水体生态修复通常耗时较长,见效较慢[6]。在藻类暴发的水华时期通常不建议使用该方法。此外,镧改性黏土的使用方式通常是制备成黏土悬液后喷洒于水面,由于黏土自身沉降较为缓慢,因此,常常造成水体浑浊现象并持续一定时间,而黏土颗粒悬浮于水体中对浮游动物、底栖动物、鱼类等水生生物的摄食和呼吸作用造成一定负面影响[7-8]。因此,如何以黏土作为基质材料,对其进行改性使其在具有除磷功能的同时,兼具絮凝除藻降浊的能力是目前亟待解决的问题。
壳聚糖是天然甲壳素部分脱乙酰基后得到高分子化合物,具有良好的生物降解性、生物相容性和无毒性,将壳聚糖溶于酸性溶液,壳聚糖分子具有阳离子型聚电介质的性质,在水生态工程中被成功的用于絮凝除藻,改善富营养化水体的生态环境质量[9-11]。但由于壳聚糖只能溶于pH小于5的酸性溶液中而不溶于中性或高碱性溶液,使得壳聚糖的使用受到较大限制[12]。本研究采用壳聚糖作为黏土改性剂,预先对壳聚糖通过季铵化改性处理,制备出溶于中性水环境的季铵化壳聚糖;再使用季铵化壳聚糖溶液联合镧制剂对膨润土进行复合改性处理,制备出壳聚糖-镧复合改性膨润土;通过絮凝实验验证了其絮凝除藻性能,并通过磷吸附实验分析了其除磷特性,探究了利用复合改性膨润土开展富营养化水体修复实验的效果,以期为富营养化水体的生态修复提供高效的同步除藻固磷的新材料。
全文HTML
-
实验用水样采集自富营养化的天然水体,藻密度为14.93×106 细胞·L−1,水样优势藻主要为绿藻门藻类。实验用壳聚糖和膨润土市购,硝酸镧、氯化钠、磷酸氢二钾等试剂均为分析纯。
-
1)复合改性膨润土的制备,参照文献中的方法[13]进行。取0.5 g壳聚糖溶于50 mL 1%的冰醋酸溶液中,搅拌混匀溶解。缓慢滴加25 mL 20%的二甲基二烯丙基氯化铵溶液,充分混合均匀。立即将锥形瓶置于水浴振荡器中加热反应,反应温度为55 ℃,时间20 min。反应结束后冷却至室温。加入等体积丙酮,生成大量的白色絮状物。离心后将沉淀使用无水乙醇洗涤,最后将产物在干燥箱中65 ℃干燥 24 h得到季铵化壳聚糖。使用时取定量季铵化壳聚糖溶于去离子水中得到1 g·L−1的季铵化壳聚糖溶液。
季铵化壳聚糖-镧改性膨润土(CLMB)的制备:称量过200目筛,粒径小于74 μm的膨润土5 g,加入500 mL 0.1 mol·L−1的LaCl3溶液中搅拌混合均匀,常温下搅拌24 h,离心倒出上清液,然后用去离子水清洗黏土并离心。此过程至少重复3次,以去除多余的未交换的La,加入1 L 1 g·L−1的季铵化壳聚糖溶液中混合均匀,制备得到CLMB黏土悬液,CLMB等电点为10.5,La含量为5.02%。
2) CLMB絮凝除藻性能测试。主要考察CLMB投加量、水样pH、离子浓度对CLMB絮凝除藻的影响,具体实验方法如下。CLMB投加量对CLMB絮凝除藻的影响:分别取 1 L富营养化水体水样置于7个烧杯中,CLMB投加量分别为5、20、30、50、70、90、110 mg·L−1,投加CLMB后以300 r·min−1快速搅拌5 min,再以150 r·min−1搅拌3 min,静置30 min后于液面下2 cm处取上清液进行叶绿素a和浊度测试。水样pH对CLMB絮凝除藻的影响:分别取 1 L富营养化水体水样置于6个烧杯中,调节水样pH分别为4、5、6、7、8、9,CLMB投加量均为50 mg·L−1,后续操作同上。离子浓度对CLMB絮凝除藻的影响:分别取1 L富营养化水样置于6个烧杯中,水样中分别添加0、0.01、0.02、0.04、0.08、0.16 mol·L−1 NaCl,CLMB投加量均为50 mg·L−1,后续操作同上。
3) CLMB对磷等温吸附实验。配制一组100 mL浓度分别为 0.5、1、5、10、15、20、40、60和80 mg·L−1的KH2PO4溶液于150 mL锥形瓶中,调节pH为7,分别加入0.1 g CLMB,在摇床中以25 ℃,150 r·min−1的速度振荡,反应3 h后,取出并以8 000 r·min−1离心5 min,取上清液经0.22 μm孔径微孔滤膜过滤,滤液用钼酸铵分光光度法测定磷含量。由此得到吸附等温线数据,并用Langmuir方程和Freundlich方程对实验结果进行拟合。
4) CLMB对磷吸附动力学实验。配制100 mL质量浓度为10 mg·L−1的KH2PO4溶液于150 mL锥形瓶中,调节pH为7,加入0.1 g CLMB,在摇床中以25 ℃,150 r·min−1的速度振荡,每隔一定时间取样测定平衡液中磷浓度,并计算CLMB对磷的吸附量,用准一级动力学模型和准二级动力学模型对CLMB吸附磷的动力学曲线进行拟合。
5)富营养化水体修复实验。实验用水体为重庆市主城区的2个相邻的混凝土构筑的池塘,平均水深2 m,大小均为约80 m2,底泥呈厌氧灰黑色,总磷平均含量为1.26 g·kg−1,总氮平均含量3.59 g·kg−1,有机质平均含量为7.2%,水质达到重度富营养化水平。在整个实验期间,池塘之间的进出口被关闭,没有连接外部水源。取1个池塘作为对照,不再作任何处理维持原状,另1个池塘全池投加8 kg CLMB。实验期间处于初冬时期,选择冬季适宜性水生植物菹草作为修复水体用水草品种,在投加CLMB后每个水池投撒菹草石芽0.5 kg。实验开始后定期检测水质,连续监测近90 d。
1.1. 实验材料
1.2. 实验方法
-
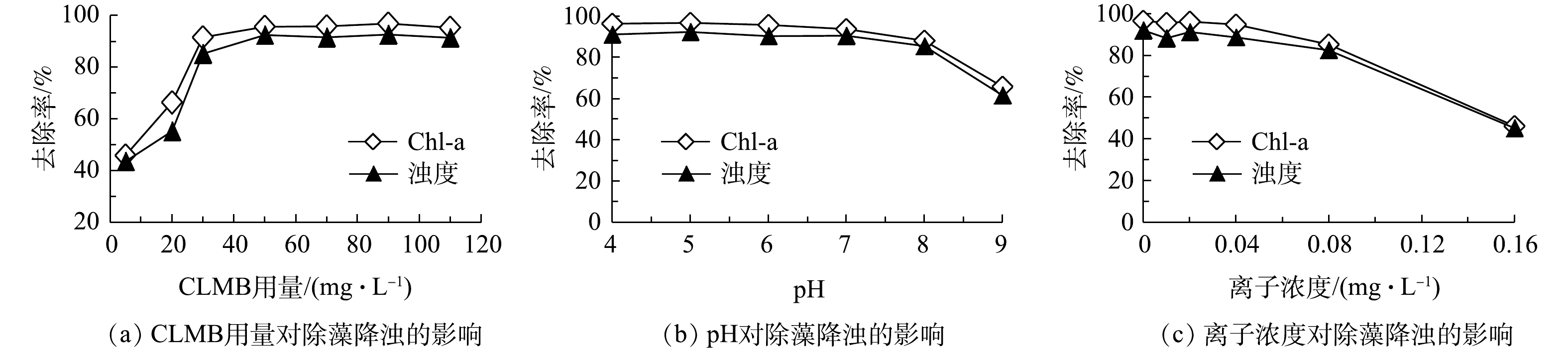
分析CLMB投药量、水体pH、离子浓度等因素对CLMB絮凝除藻效果的影响,结果如图1所示。由图1(a)可知,CLMB除藻效果随着投加量的增大而显著上升,在CLMB投加量为50 mg·L−1时,叶绿素a(Chl-a)去除率达到95.63%,浊度去除率为92.55%。继续增大CLMB投加量,除藻降浊效果并没有明显变化。
天然水体 pH通常在4~9,当CLMB投药量为50 mg·L−1时,水样pH对CLMB除磷效果的影响结果如图1(b)所示。当pH为在4~7时,CLMB均有很好的除藻效果,Chl-a去除率达到95%以上;当水样pH为8时,除藻效果有所下降,但除藻率仍然达到了88.14%;当水样pH为9时,CLMB除藻效率急剧下降,Chl-a去除率为65.82%,浊度去除率为61.58%。pH对CLMB除藻效果的影响与壳聚糖表面电荷有关。在pH大于8时,壳聚糖表面电荷为负电荷,而藻细胞通常带有负电荷,由于同种电荷排斥作用,因此,难于形成藻细胞絮凝体[14]。可见,将CLMB用于富营养化水体絮凝除藻,需要注意水体中pH所处范围,CLMB更适用于pH为4~8的水体,在碱度较大的水体中,例如藻华爆发时期,水环境pH可达到9~10,直接应用CLMB时药效受到较大影响,应搭配一定的水体pH调控措施开展。
离子浓度对CLMB除藻效果的影响如图1(c)所示。由图1(c)可知,在离子浓度较低时,其对CLMB除藻效果无显著影响;随着离子浓度增大到0.08 mol·L−1及以上,CLMB除藻性能逐渐下降;当离子浓度增大到0.16 mol·L−1时,Chl-a去除率仅能达到46.25%。离子浓度的影响在于可屏蔽壳聚糖分子的表面正电荷排斥作用,从而使壳聚糖的网捕作用减弱,使得除藻效率下降[15]。由此可见,CLMB更适合在低离子浓度的淡水水体中应用,而不宜使用于离子浓度较大的水体。
-
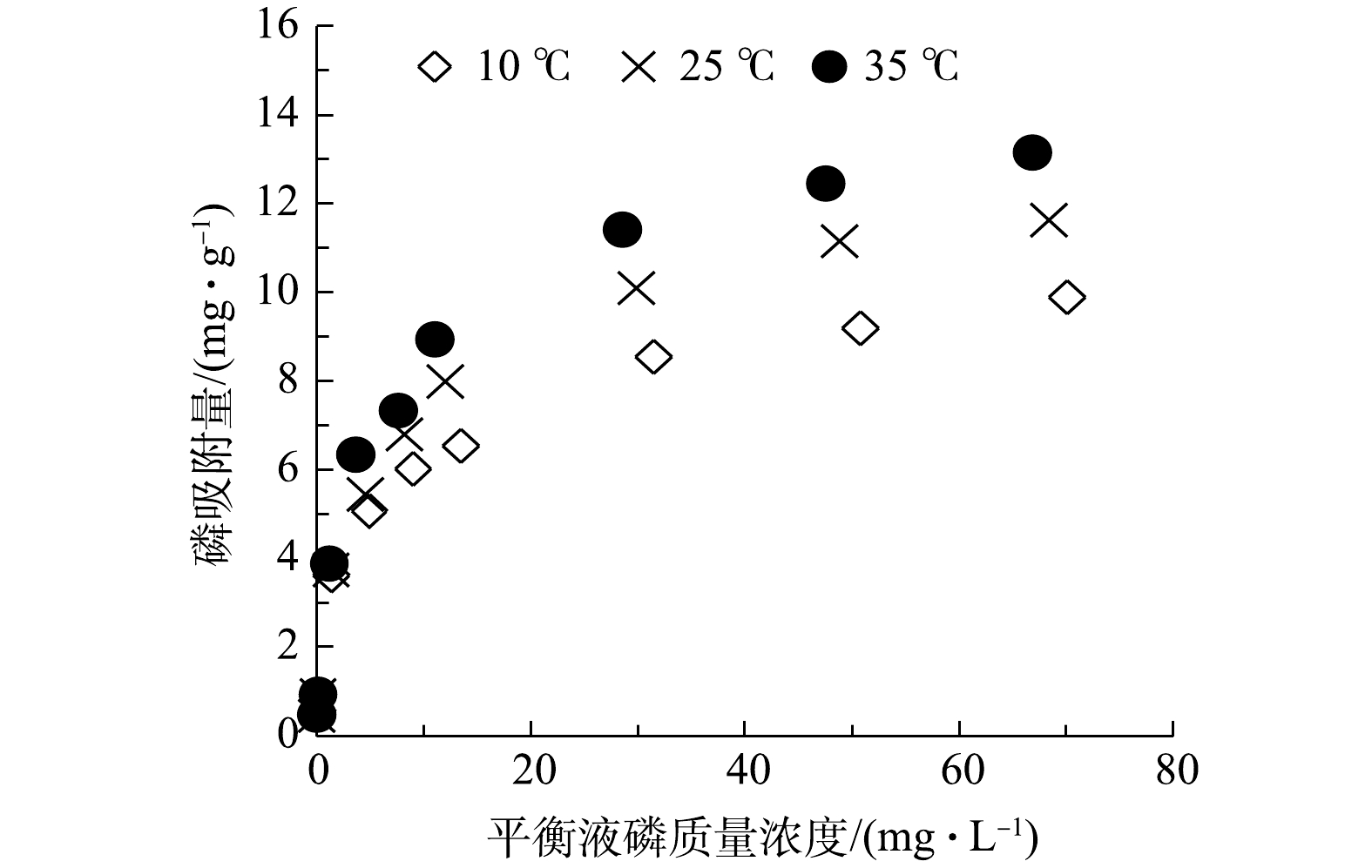
选取10、25、35 ℃ 3个温度条件,分析水温对CLMB吸附磷效果的影响,实验结果见图2。由图2可知,CLMB对磷的等温吸附线更符合 Langmuir 方程(表1),磷的吸附量随着水样中磷浓度的增加而迅速增大,当水样中磷浓度较高时,吸附量增长缓慢,且溶液中残余磷浓度处于较高水平。同时,从等温吸附曲线可以看出,CLMB对磷的饱和吸附容量随着水温的升高而增大,表明CLMB对磷的吸附过程是吸热反应,水温升高有助于CLMB对磷的吸附,35 ℃时CLMB对磷的饱和吸附量达到13.46 mg·g−1。有研究[16]表明,应用广泛的phoslock锁磷剂在10~35 ℃时对磷的饱和吸附量为9.54~10.54 mg·g−1。由此可见,CLMB对磷的饱和吸附量高于phoslock,这可能与2种磷吸附剂中的La含量有关,CLMB制剂的La含量为5.02%,phoslock中La含量不低于4.5%。此外,壳聚糖包裹于黏土颗粒表面,壳聚糖自身具有较好的吸附功能,对磷也存在一定吸附作用,增强了CLMB对磷的吸附性能。考虑到富营养化水体磷浓度水平和水温条件,以及CLMB对磷具有良好的吸附性能,CLMB可适用于富营养化水体中磷的去除。
-
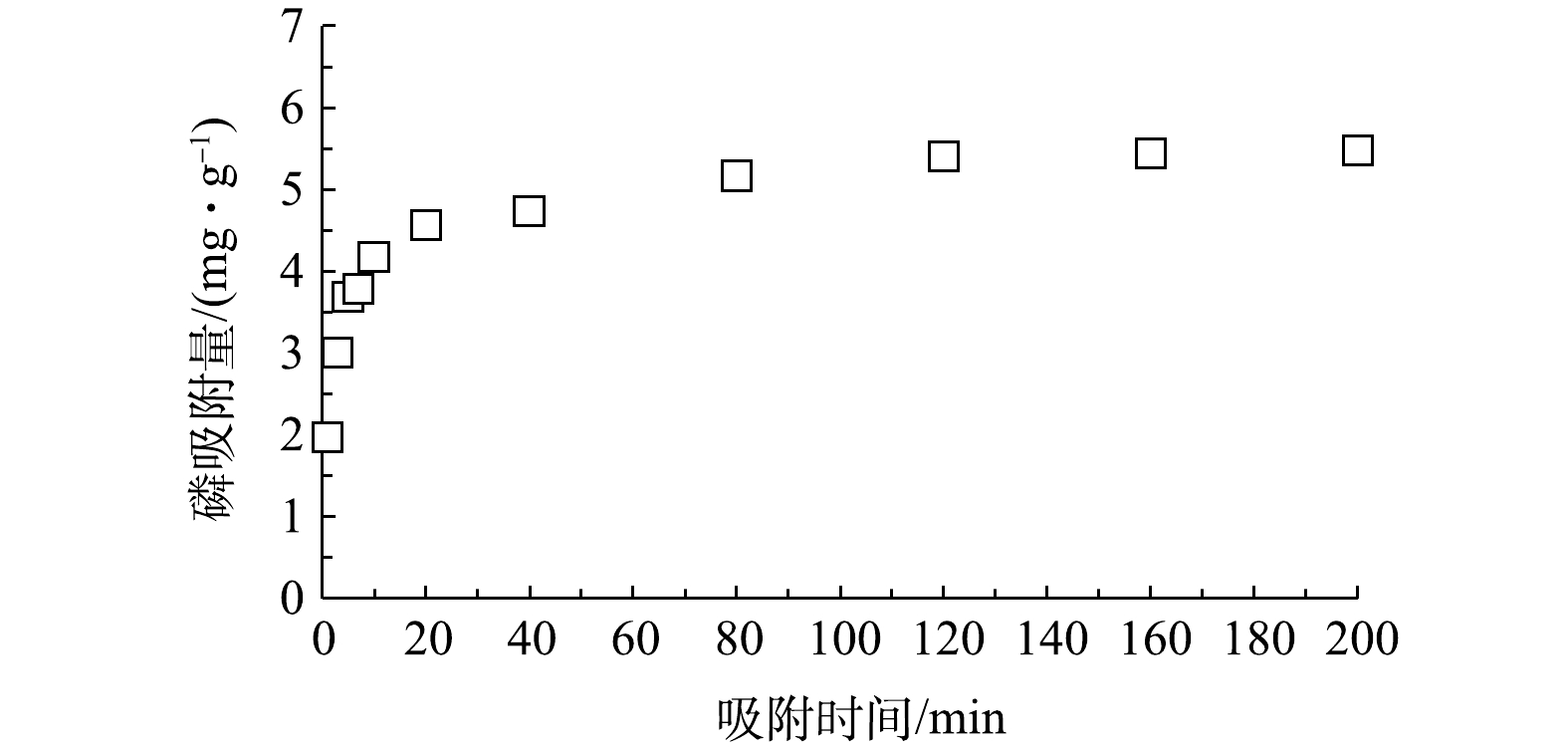
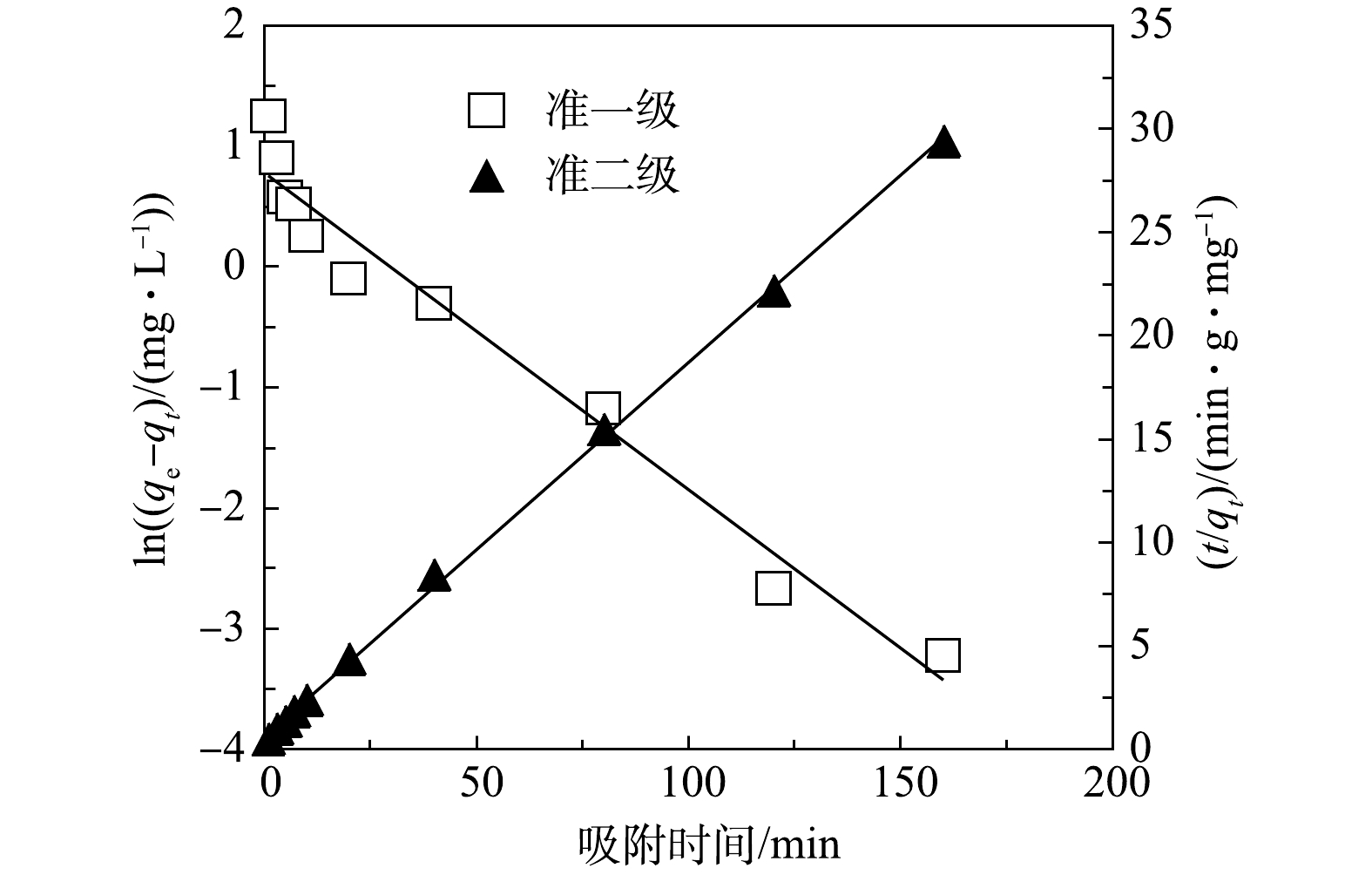
CLMB吸附磷的动力学实验结果如图3所示;分别用准一级动力学模型和准二级动力学模型进行拟合,结果见图4。由图3可知,在CLMB对磷的吸附前20 min内,吸附速率很快,吸附量随时间迅速增大,在20 min内能够达到平衡吸附量的90%以上;后期随吸附时间的延长,吸附量缓慢增长;吸附时间为120 min 时基本达到平衡状态。由图4显示的拟合结果可知,CLMB对磷的吸附更符合准二级动力学模型(R2 = 0.999 3)。采用准二级动力学模型计算出的平衡吸附量为5.519 mg·g−1,与实测平衡吸附量(5.48 mg·g−1)接近。可见,CLMB对磷的吸附过程更适合采用准二级动力学模型进行描述,CLMB对磷的吸附属于化学吸附[17]。
-
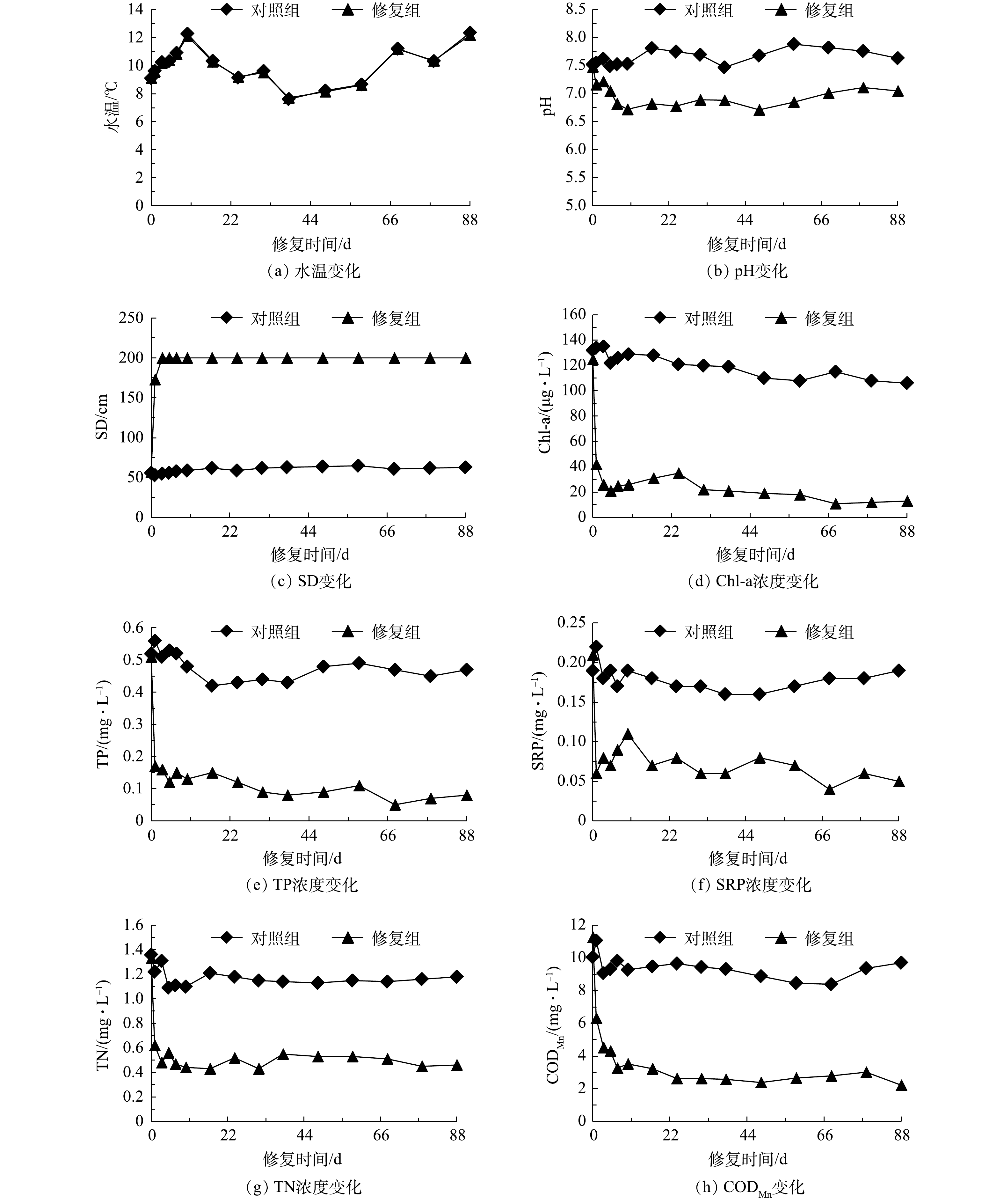
修复前后的水体理化指标变化见图5。整个实验阶段处于冬季,水温较低(7.62~12.36 ℃)。对照组水体初始pH为7.52,实验期间pH波动较小。修复组水体pH在实验开始后pH有所降低,最低pH为6.71。这可能与藻密度的降低有关,通过CLMB的除藻作用,藻类对水体中二氧化碳的消耗降低,使得水体pH偏微酸性。对照组水体透明度为53~65 cm,均值为60 cm,水质显得浑浊。修复组的水体透明度在实验开始后1 d内即得到很大改善,水体透明度显著提升,之后达到最大水深2 m处,并维持至实验结束。对照组中Chl-a质量浓度为106~135 μg·L−1,平均值为121 μg·L−1,而修复组水体在修复措施实施后Chl-a质量浓度迅速得到降低。由于富营养化水体中Chl-a主要来自于藻类,Chl-a质量浓度的降低表明CLMB达到了絮凝除藻的目的,降低了水体中藻类生物量。相比对照组,氮磷营养盐含量在整个实验阶段的相对稳定,修复组中水体氮磷营养盐得到大幅度削减,TP和TN浓度相比实验前的降幅分别达到84.31%和65.41%,SRP浓度降低了76.2%。由于富营养化水体中的氮磷营养盐以颗粒态为主,尤其是以藻类生物质形式的氮磷存在[18],在采用CLMB对富营养水体进行治理后,藻类大量沉降,水体中营养盐随之显著下降,之后趋于稳定且未出现氮磷营养盐的回升。藻类死亡及沉积物中的营养盐未出现向水体二次释放,这表明修复组的水环境条件可促使营养盐能够稳定的存在于沉积物中,尤其是CLMB所具有的较大磷饱和吸附性能和磷形态稳定性能够持续发挥固磷作用,防止磷素向水体的二次释放。修复组中营养盐的有效控制进一步抑制了藻类的再次暴发,使得水体CODMn显著低于对照组,至实验结束时为2.22 mg·L−1,降幅达到80.28%。修复组水质的长效稳定除了与CLMB持续发挥作用有关外,可能还与水体中水生植被的恢复有关,实验后期菹草群落生长情况良好,沉水植物能够在吸收水体中的氮磷营养盐,从而抑制藻类生长。
根据水体SD、TP、TN、Chl-a和CODMn计算了水体综合营养状态指数值,结果表明,对照组和修复组初始综合营养状态指数值均达到70以上,达到重度富营养化水平。在整个实验期间,对照组水体富营养化程度有所降低,部分时段降低至中度富营养化水平,但仍旧逼近重度富营养水平;而修复组富营养化程度迅速改善,指数值下降至50以下,即达到中营养水平,消除了水体富营养化。
-
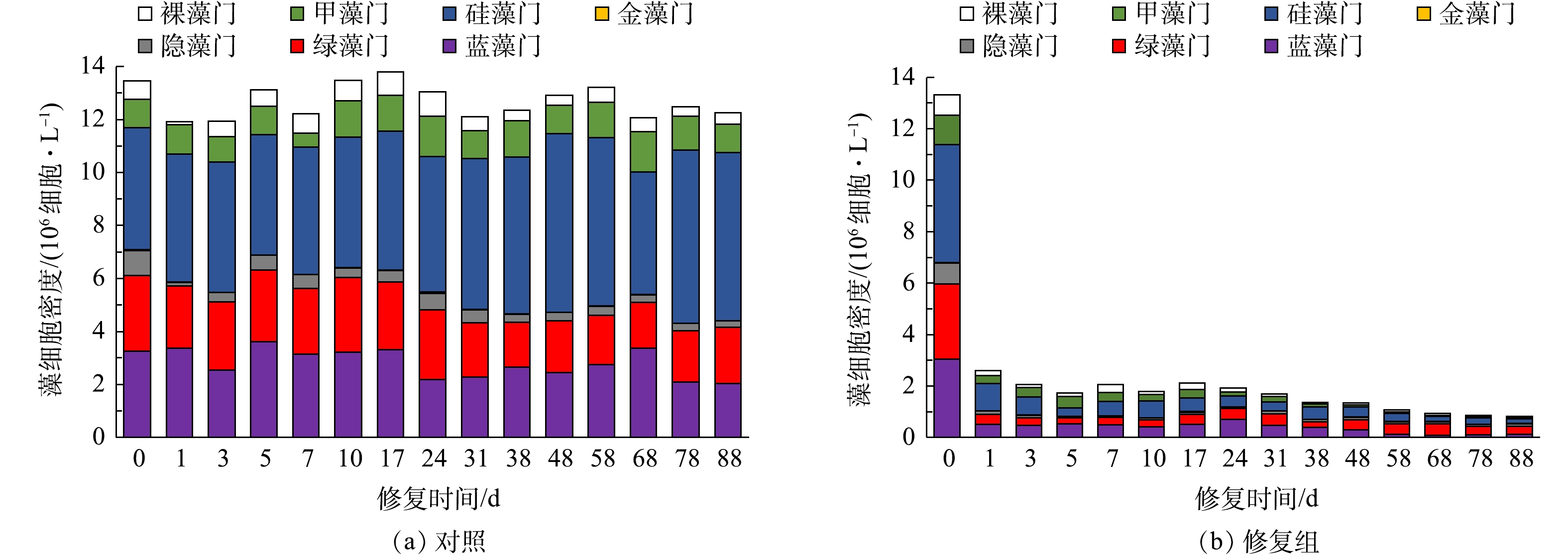
修复前后水体中藻类群落结构变化如图6所示。实验期间对照组藻密度波动较小,为11.91~13.79×106 细胞·L−1,均值为12.68×106 细胞·L−1,藻类优势种主要以硅藻门、蓝藻门、绿藻门等藻类为主,实验期间藻类种群结构有小幅度变动,硅藻门藻密度有进一步上升,优势度有所扩大。修复组在修复措施实施后,藻细胞密度迅速下降,由初始13.33×106 细胞·L−1降至第1天的2.59×106 细胞·L−1。由此可见,投加CLMB药剂能够快速絮凝沉降水体中的藻细胞,降低藻密度,这对于水质的迅速改善起着关键作用。实验末期藻细胞密度总体较为稳定,变化幅度较小,藻类优势种仍旧以硅藻门、蓝藻门、绿藻门藻类为主,但绿藻门优势度有所增大,而硅藻门和蓝藻门优势度有所下降。
实验开始后的30 d,对照组中未见沉水植物的生长,而修复组水体中的菹草生长茂密(图7)。可见,对于富营养化水体,水质浑浊透明度低,藻密度高,竞争力强,不利于沉水植物的恢复;而修复组通过改性膨润土的除藻固磷作用,迅速改善了水质,尤其是提高了水体透明度,为沉水植物的生长创造了良好的条件。而沉水植被的恢复对于水生态系统的平衡稳定具有重要意义,沉水植物能够大量吸收利用水体和沉积物中的氮磷营养盐,通过化感作用抑制藻类生长,减缓水体扰动促进水体中的悬浮物沉降,富营养化的藻型水体向草型水体的转变反映出水生态系统的恢复[19-21]。由于本实验处于冬季,因此,选择菹草作为恢复沉水植被的品种,植物品种选择显得单一,在实际的富营养化水体生态修复工程中,应根据当地气候、湖库水深、基底结构等因素可选择多种品种,以利于恢复多样性更高的沉水植被,促进整个水生态系统的平衡稳定。这值得进一步深入研究探讨。
2.1. CLMB絮凝除藻影响因素分析
2.2. CLMB对磷的等温吸附
2.3. CLMB吸附磷的动力学
2.4. 富营养化水体修复前后理化指标变化特征
2.5. 富营养化水体修复前后藻类及沉水植物变化特征
-
1)当CLMB最佳投药量为50 mg·L−1时,CLMB的除藻率达到95.63%,浊度去除率为92.55%。水体pH和离子浓度对CLMB同步除藻效果具有显著影响,CLMB适用于pH为4~8,离子浓度低于0.08 mol·L−1的淡水。
2) CLMB对磷的等温吸附过程更适合采用Langmuir方程描述,水温升高有助于CLMB对磷的吸附,35 ℃时CLMB对磷的饱和吸附量达到13.46 mg·g−1。CLMB对磷的吸附前20 min内,吸附速率很快,吸附量随时间迅速增大,120 min内即可达到平衡吸附量,吸附过程适合采用准二级动力学模型描述。
3) CLMB用于修复富营养化水体,能够在较短时间内快速削减藻密度,提高水体透明度,降低氮磷营养盐含量,消除水体富营养化现象,改善后的水环境为沉水植物菹草的萌芽生长提供有利条件,水生植被的恢复也会进一步稳固水生态系统的平衡稳定。





 下载:
下载: