-
纳米银具有独特的抗菌性能,广泛应用于家用电器[1]、纺织品[2]、食品容器、医疗用品、化妆品等领域[3],AgNPs已经成为全球应用最多的纳米材料之一。AgNPs产品在生产、运输和使用过程中不可避免地直接或间接释放到环境中[4],最终进入污水处理厂[5]。有研究结果[6-7]表明,纳米颗粒进入水体后,粒子本身性质(粒径、形状、溶解性、表面电位和包被材料等)、存在状态(离子态和络合态)和环境因素(pH、溶液离子强度、有机物等)均会显著影响其在水体中的行为。贾俊彩等[8]发现,AgNPs粒径越小,对斑马鱼的毒性越强,易导致斑马鱼胚胎发生多种畸形。BURCHARDT等[9]发现,AgNPs及其溶解释放的Ag+对藻类具有毒性效应。AgNPs的粒径、Ag+溶出强度、表面电位等理化性质均会直接影响其在水体中的反应活性及生物毒性[10-12]。
活性污泥工艺具有流程简洁、操作简易、运行效果稳定等优点,主要利用活性污泥(微生物聚集体)对水中各种污染物进行吸收、转化和降解[13]。有研究结果表明,AgNPs可导致活性污泥中硝化细菌丰度显著减少,降低活性污泥系统的出水水质,从而影响受纳水体的氮循环[14]。也有研究结果[15]表明,进入活性污泥污水处理系统中的AgNPs溶解释放出的Ag+抑制了系统中微生物活性,从而降低活性污泥系统对污染物的去除效率。关于AgNPs影响活性污泥污水处理系统污染物去除效率的原因是其自身还是其释放Ag+所至,仍有争议。LIANG等[16]分别测定了1 mg·L−1 AgNPs和1 mg·L−1 Ag+对SBR中活性污泥硝化性能的影响,认为AgNPs对活性污泥微生物硝化反应的抑制作用来自其本身。WIRTH等[17]发现,AgNPs对Pseudomomas fluorescens细胞膜的毒性大于AgNPs释放出的Ag+毒性。而RADNIECKI等[18]的研究结果表明,AgNPs释放出的Ag+是对活性污泥中Nitrosomonas europaea产生硝化抑制的主要原因。HOQUE等[19]的研究结果表明,污水中AgNPs质量浓度为0.1~0.2 µg·L−1,随着包含AgNPs的材料广泛应用,AgNPs在污水中的浓度有可能继续升高。
本研究采用序批式反应器(sequencing batch reactor,SBR)模拟活性污泥污水处理系统,在进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs以及相同质量浓度的AgNPs可在水中分别溶解释放出的Ag+,在连续运行50 d后,测定SBR出水的各项水质指标,以期为全面评价AgNPs及其释放的Ag+对污水生物处理系统的生态风险提供参考。
全文HTML
-
实验用水为人工模拟中等强度的城市污水,主要组成成分为30 mg·L−1 C6H12O6、400 mg·L−1 CH3COONa、150 mg·L−1 NH4Cl、45 mg·L−1 KH2PO4、20 mg·L−1 MgSO4·7H2O、1 mL·L−1微量元素溶液。微量元素溶液组成为150 mg·L−1 H3BO3、150 mg·L−1 CoCl2·6H2O、30 mg·L−1 CuSO4·5H2O、150 mg·L−1 FeCl2·6H2O、30 mg·L−1 KI、120 mg·L−1 MnCl2·2H2O、60 mg·L−1 Na2Mo7O4·2H2O、120 mg·L−1 ZnSO4·7H2O。使用NaHCO3调节人工污水的pH,使其保持在6.5~7.5。接种污泥取自南京某城市污水处理厂生化池的回流污泥。
-
SBR有效容积为1.6 L,采用空气压缩机曝气,空气流速为2.0 L·min−1。实验期间SBR每日运行2个周期,每周期5 h,包括进水15 min、静置90 min、曝气90 min、静置90 min、排水15 min。接种污泥与人工污水体积比为1∶2,运行14 d后,各反应器污泥混合液悬浮固体(mixed liquor suspended solids,MLSS)质量浓度为(4 430±148) mg·L−1,污泥容积指数(settling velocity index,SVI)达到(83±4) mL·g−1,SBR运行稳定,在进水中分别加入不同质量浓度AgNPs和Ag+,这个时间点记为实验开始,即第1天。将进水中AgNPs质量浓度分别设置为1 mg·L−1和10 mg·L−1。根据孙秀玥[20]超滤法测得AgNPs在纯水中释放出的Ag+为AgNPs质量浓度的30%,人工污水配制时采用纯水(15 MΩ·cm)。当进水中添加的AgNPs质量浓度分别为1 mg·L−1和10 mg·L−1时,添加的Ag+质量浓度分别为0.3 mg·L−1和3.0 mg·L−1,Ag+质量浓度分别对应1 mg·L−1和10 mg·L−1 AgNPs在纯水中的Ag+释放量。SBR分为5组,每组SBR设置3个重复,以进水中不添加AgNPs、也不添加Ag+的SBR组作为对照组(CK),5组实验平行同步进行。Ag+以AgNO3配制,运行期间水温为20~30 ℃,运行周期内换水率为50%。
-
AgNPs购自北京德科岛金科技有限公司,平均粒径10~12 nm,表面包裹物为聚乙烯吡咯烷酮。将AgNPs分别分散于纯水和人工污水,表观质量浓度均为2 mg·L−1,经超声仪(KQ-700DE,昆山市超声仪器公司)在100 W、40 kHz条件下超声5 min,采用紫外-可见光分光光度计(UV-1800,Shimadzu,Japan)光谱扫描模式对其进行扫描。将分别分散于纯水中的1 mg·L−1和10 mg·L−1 AgNPs,在100 W、40 kHz条件下超声30 min后,采用纳米粒度电位仪(Zs90,Malvern Zetasizer Nano,UK)测定AgNPs在纯水和人工污水中的粒径分布及Zeta电位。采用透射电子显微镜(H-7650,HITACHI,Japan)观察分散于纯水和人工污水的AgNPs颗粒的形态。
-
溶解氧(dissolved oxygen,DO)采用便携式溶解氧仪(JPB-607A,上海雷磁)测定;pH采用pH测定仪(PB-10,赛多利斯科学仪器(北京)有限公司)测定;化学需氧量(chemical oxygen demand,COD)采用HACH COD快速测定仪(DR1010,HACH,USA)测定;
NH+4 -N、NO−2 -N、NO−3 -N、PO3−4 采用离子色谱仪(ICS-600,赛默飞世尔(上海)仪器有限公司)测定。 -
采用Microsoft Excel 2016对实验数据进行统计分析,结果用平均值±标准误差(Mean±SE)表示,用SPSS Statistics 25软件中单因素方差分析并检验显著性,P<0.05代表数据之间有显著性差异;采用Origin8.1软件绘图。
1.1. 实验用水与接种污泥
1.2. 实验装置的构建
1.3. AgNPs在污水中的物理性状表征
1.4. 指标及测定方法
1.5. 数据统计与分析
-
1) AgNPs的紫外-可见吸收光谱表征。由于量子尺寸效应,纳米粒子具有独特的非线性光学效应,粒子的尺寸和形状决定着这种非线性光学效应。有研究者发现,AgNPs在390~450 nm处存在由表面等离子体共振引起的特征吸收峰[21-22]。利用紫外-可见分光光度计对AgNPs溶液进行检测,结果见图1。采用纯水和人工污水稀释的AgNPs溶液吸收峰均出现在410 nm处,这与文献报道中AgNPs特征吸收峰的位置相同[21-22]。
2) AgNPs在纯水和人工污水中的水动力学粒径和Zeta电位。采用动态光散射技术测定AgNPs在纯水和人工污水中的粒径分布及Zeta电位,结果见表1。当AgNPs质量浓度为1 mg·L−1时,纯水中AgNPs平均粒径均为(48.88±13.34) nm,人工污水中有43.70%的AgNPs平均粒径为(170.23±36.03) nm;当AgNPs质量浓度为10 mg·L−1时,纯水中AgNPs平均粒径为(52.25±6.67) nm,人工污水中有18.18%的AgNPs平均粒径为(309.30±25.28) nm。由此可见,AgNPs在人工污水中更易发生团聚,团聚体粒径更大。吴其圣等[23]发现,水中离子强度的增加会促进纳米颗粒的聚集。MUKHERJEE等[24]的研究结果也表明,二价阳离子如Ca2+可显著诱导纳米TiO2、AgNPs等纳米颗粒的团聚。实验采用的人工污水中含有丰富的Mg2+和Na+,这些离子可能是造成AgNPs团聚的主要原因。表1表明,在同种分散介质中,AgNPs质量浓度越高,AgNPs颗粒的水动力学粒径越大。这与其他研究的结果类似,即随着纳米材料浓度增加,纳米颗粒团聚体尺寸也随之增加[25-26]。如表1所示,同一分散介质稀释的AgNPs的Zeta电位无统计学上的显著差异,但不同分散介质稀释的AgNPs间的Zeta电位差异显著。
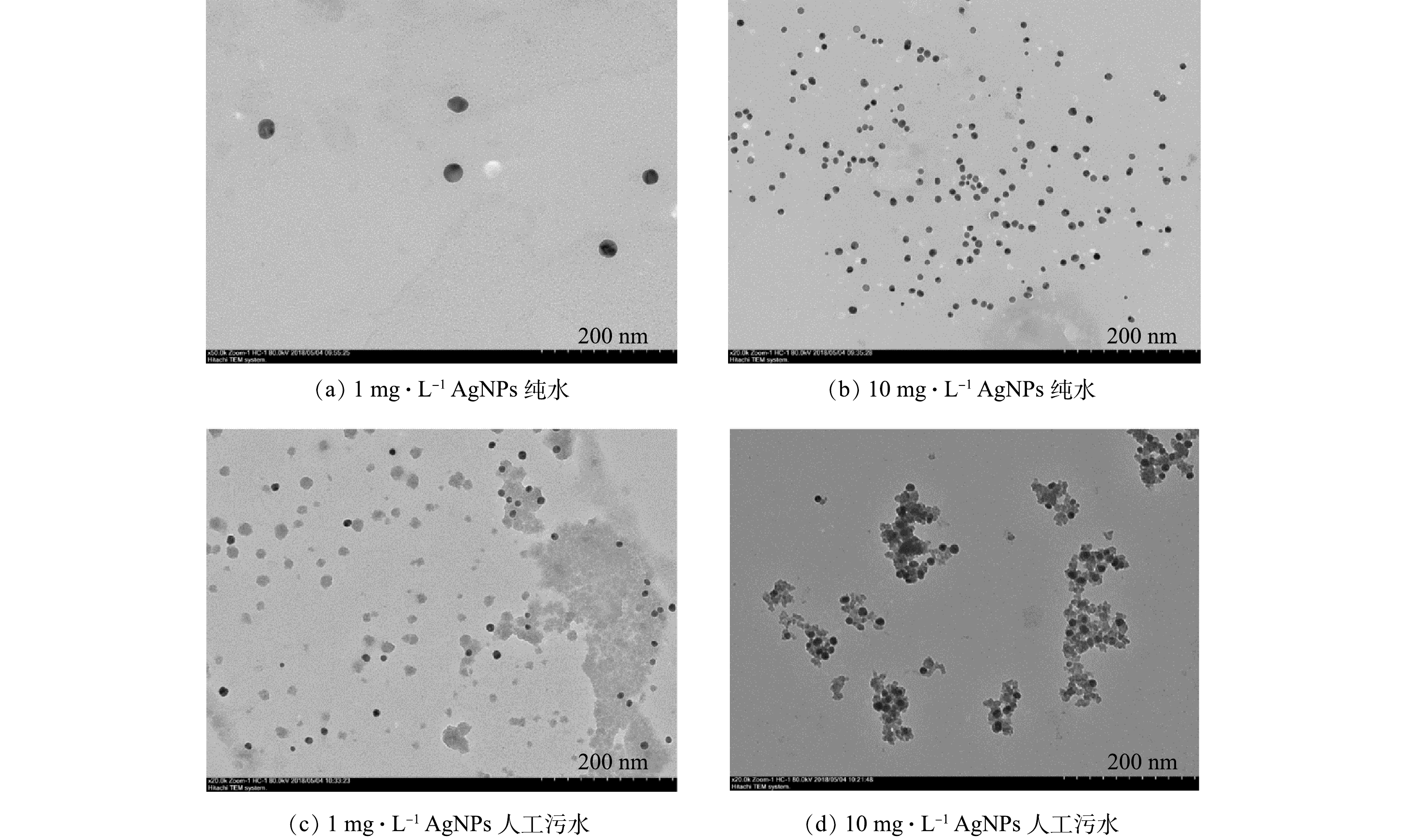
3) AgNPs的透射电镜表征。利用透射电子显微镜观察AgNPs在纯水和人工污水中的形态。如图2(a)和图2(b)所示,1 mg·L−1和10 mg·L−1 AgNPs在纯水中均以分散的颗粒形式存在,未出现较大团聚体。如图2(c)所示,1 mg·L−1 AgNPs在人工污水中大部分仍以分散颗粒形式存在;由图2(d)可见,10 mg·L−1 AgNPs在人工污水中以小块团聚体形式存在,且团聚体粒径部分大于300 nm。有研究结果[27]表明,纳米颗粒尺寸小,比表面积大,其溶液为不稳定体系,加之颗粒间范德华力的作用,颗粒之间容易聚集出现团聚现象。
-
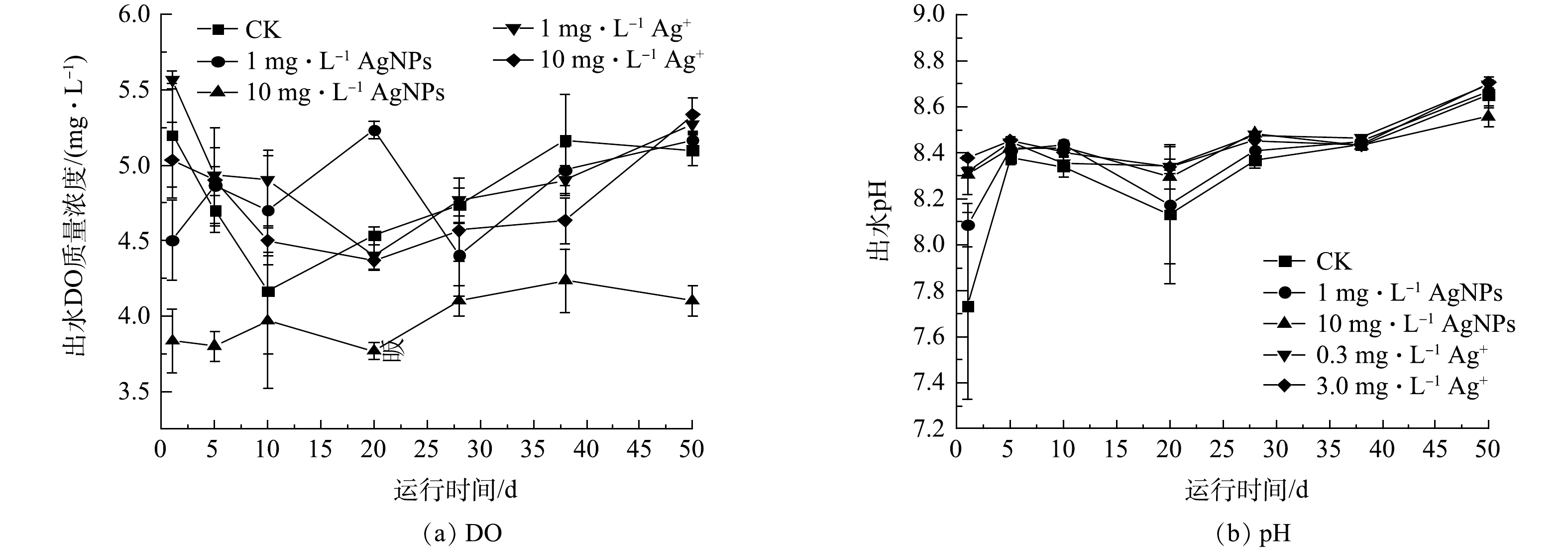
SBR在50 d的连续运行期间,进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对SBR出水DO和pH的影响结果如图3所示。在SBR 50 d的运行期内,10 mg·L−1 AgNPs处理下的SBR出水DO均低于其他处理,但高于3.0 mg·L−1的处理。SBR出水pH在50 d的运行期间均稳定在7.73~8.71,这表明1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对活性污泥系统出水pH没有显著影响(P>0.05)。在整个运行期间,SBR出水DO、pH均符合城镇污水处理厂污染物排放标准(GB 18918-2002)。
-
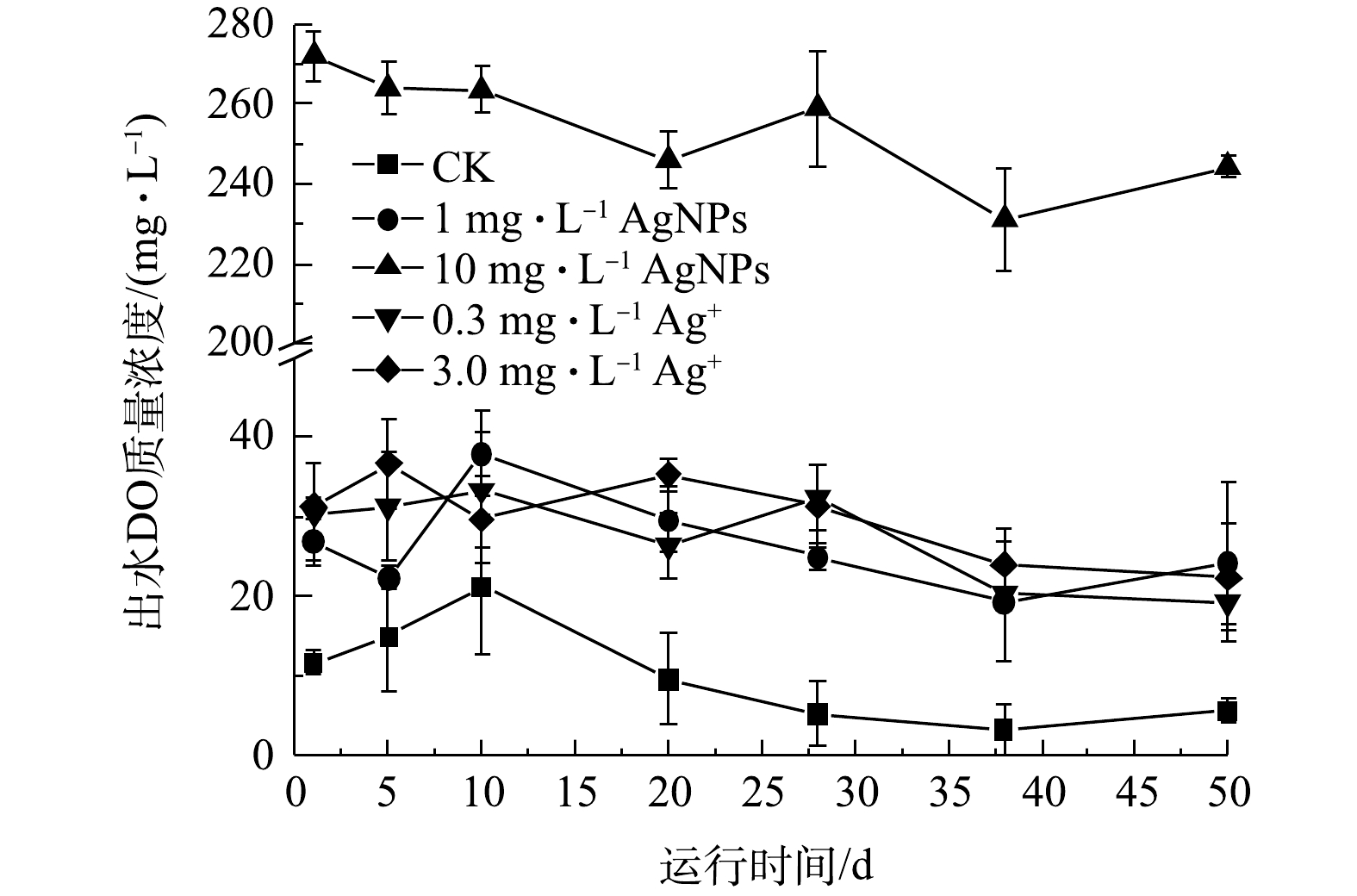
活性污泥系统的进水COD保持在(335.0±1.0) mg·L−1,进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+处理后,SBR出水COD变化如图4所示。在50 d的运行期内,与CK相比,进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对活性污泥去除COD的性能均有显著抑制效应(P<0.05)。进水中分别添加1 mg·L−1 AgNPs、0.3 mg·L−1 Ag+时,SBR出水COD无显著性差异,但进水中添加10 mg·L−1 AgNPs时,出水COD显著高于进水中添加3.0 mg·L−1 Ag+的反应器。这说明进水中添加低质量浓度(1 mg·L−1)AgNPs,对活性污泥去除COD的抑制可能在于其释放的Ag+。JEMEC等[28]采用超滤法和超速离心法分别测量了分散在纯水中AgNPs贮备液(标准质量浓度为40 g·L−1)释放Ag+,分别为AgNPs浓度的68%和46%,并进行斑马鱼、微藻、海洋甲壳类动物等毒性实验,发现AgNPs毒性来源于释放的Ag+而不是AgNPs自身。ALITO等[29]发现在SBR中分别加入0.2 mg·L−1 AgNPs和0.2 mg·L−1 Ag+后,COD去除率由99%分别下降至71%和90%,但3 d后COD去除率均恢复至起初的92%。李墨青[30]的研究结果表明,较高质量浓度AgNPs (10 mg·L−1)对SBR的COD去除率可稳定降低至70%。如图4所示,本实验进水中添加10 mg·L−1 AgNPs时,SBR中出水COD显著高于进水中添加3.0 mg·L−1 Ag+的反应器出水,表明10 mg·L−1 AgNPs对活性污泥去除COD的抑制不仅来自于其溶解释放的Ag+,更主要来自AgNPs自身。
-
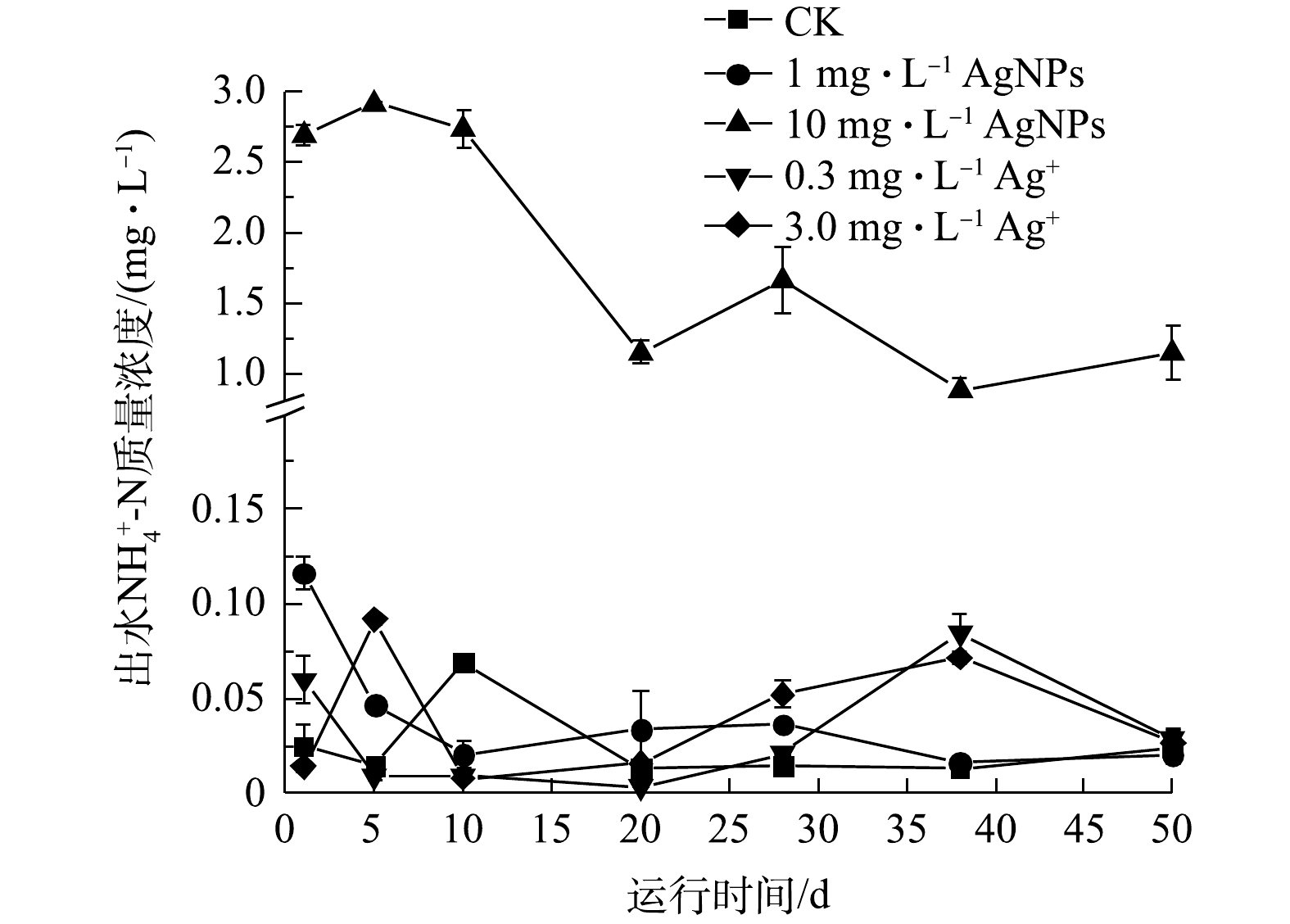
SBR活性污泥系统的进水
NH+4 -N为(42.69±1.85) mg·L−1,进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+,出水NH+4 -N质量浓度变化如图5所示。CK、0.3 mg·L−1和3.0 mg·L−1 Ag+处理下,SBR出水中的NH+4 -N质量浓度在50 d的运行期间内无显著变化,出水NH+4 -N质量浓度为0.01~0.09 mg·L−1。1 mg·L−1 AgNPs处理下的SBR出水NH+4 -N质量浓度逐渐降低,运行20 d后,1 mg·L−1 AgNPs处理的SBR出水NH+4 -N质量浓度与CK相比无显著差别(P>0.05)。上述结果表明,进水中添加1 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+的SBR对NH+4 -N去除率无显著影响(P>0.05)。ALITO等[29]发现,SBR进水中分别添加0.2 mg·L−1 Ag+和0.2 mg·L−1 AgNPs,初始运行时对NH+4 -N去除率有抑制效应,但3 d后便得到恢复。与进水中分别添加1 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+的SBR相比,进水中添加10 mg·L−1 AgNPs明显抑制了SBR对NH+4 -N的去除。在50 d的运行期,进水中添加10 mg·L−1 AgNPs的SBR出水NH+4 -N质量浓度显著高于CK及1 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+处理的SBR,10 mg·L−1 AgNPs对活性污泥去除NH+4 -N的抑制效应主要源于AgNPs自身毒性,这与JEONG等[31]的研究结果一致。 -
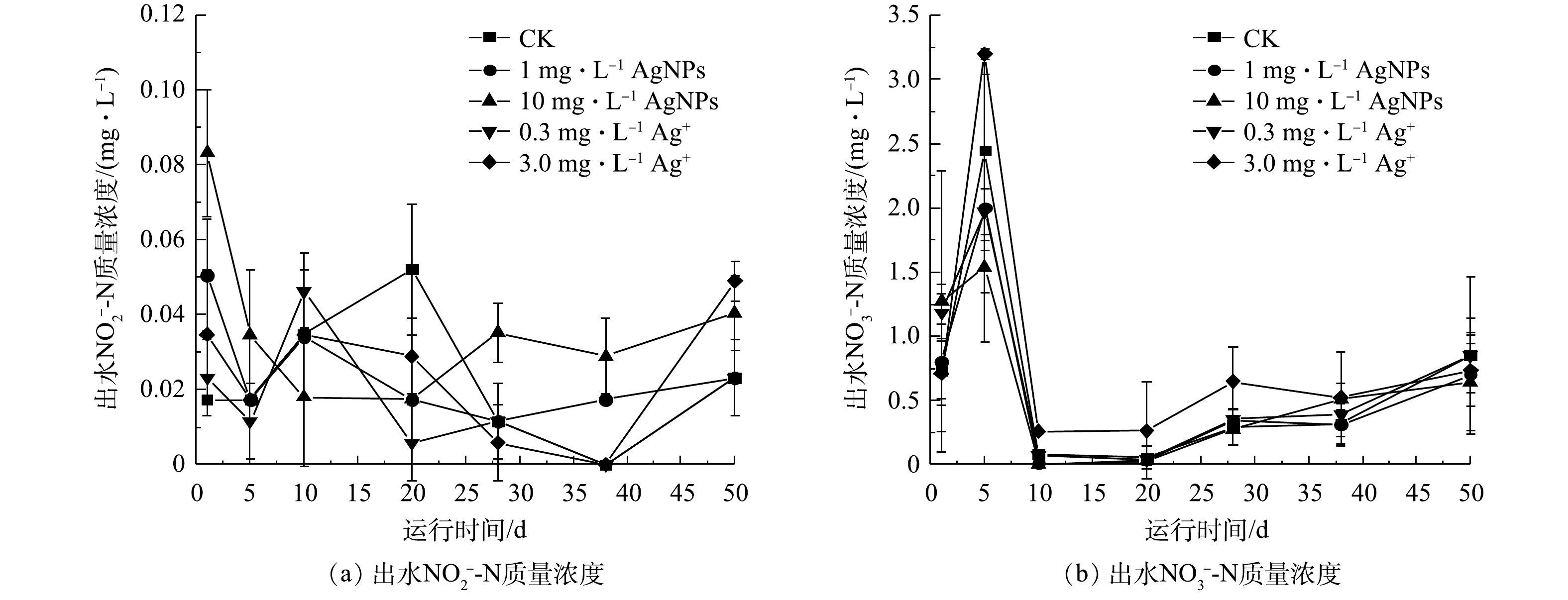
SBR系统进水
NO−2 -N和NO3-N的质量浓度分别为(1.13±0.03) mg·L−1和(10.57±0.13) mg·L−1,在进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+后,SBR出水NO−2 -N、NO3-N质量浓度变化如图6所示。由图6(a)可知,1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对SBR出水NO2-N质量浓度均无显著影响(P>0.05),出水NO2-N质量浓度低于0.1 mg·L−1。由图6(b)可知,在1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+处理下,运行15 d后,各SBR出水NO−3 -N浓度无显著差异(P<0.05),第50天出水NO3-N质量浓度为0.65~0.85 mg·L−1。上述结果表明,1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对活性污泥微生物反硝化过程影响较小,这与YANG等[14]和张汝嘉[32]报道的研究结果类似。 -
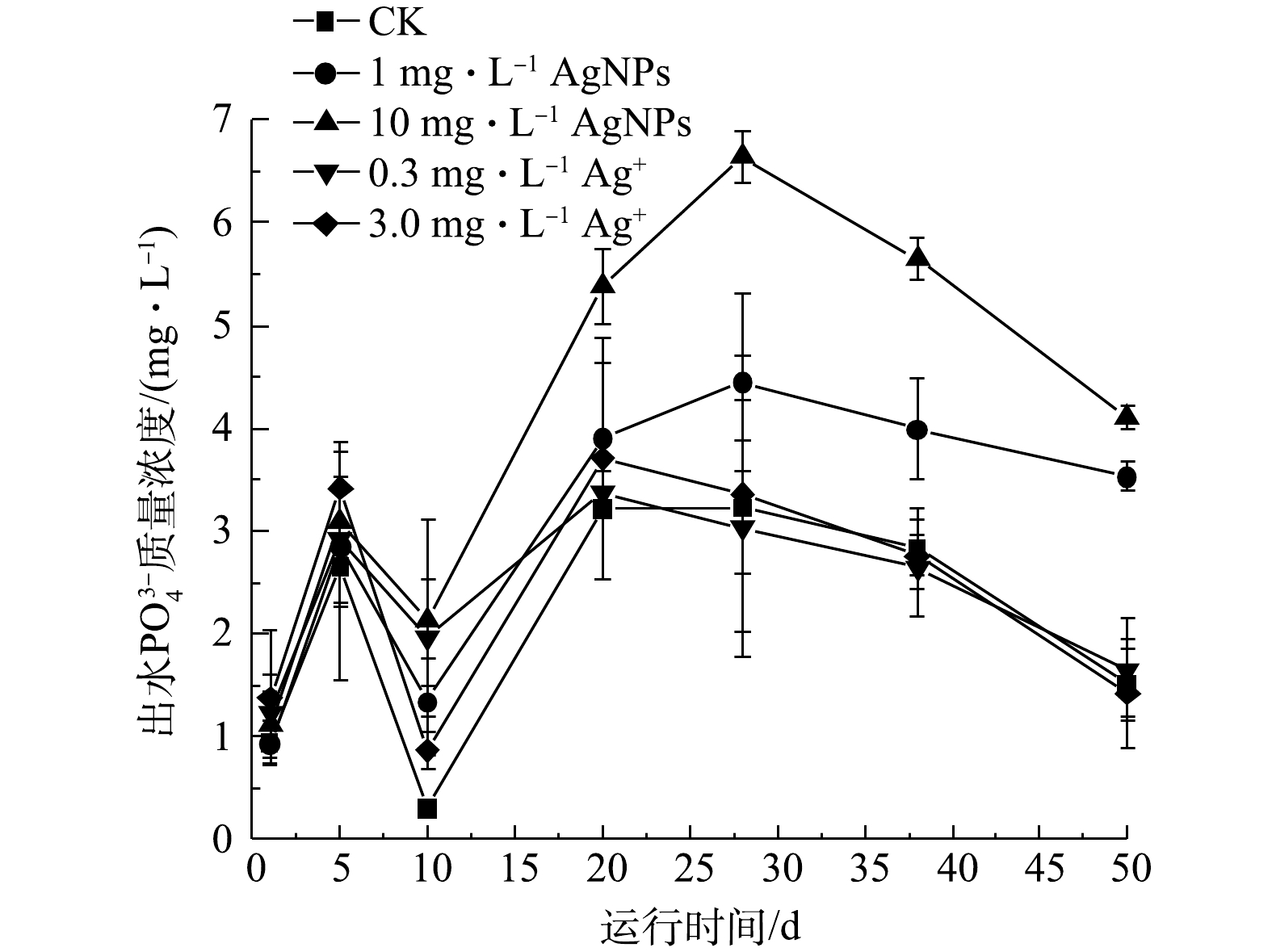
SBR系统进水
PO3−4 为(10.64±0.63) mg·L−1,进水中分别添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+的SBR出水中PO3−4 质量浓度变化如图7所示。运行至第10天后,进水中添加10 mg·L−1 AgNPs的SBR出水中PO3−4 质量浓度显著高于其他处理(P<0.05),运行至第38天后,进水中添加1 mg·L−1 AgNPs的SBR出水中PO3−4 质量浓度显著高于对照(P<0.05),这可能是连续运行中,AgNPs影响聚磷菌好氧吸磷能力,最终导致除磷效果减弱[33]。进水中AgNPs质量浓度越高,出水PO3−4 质量浓度越高,这与苑志华等[33]的研究结果一致。在SBR 50 d的运行期间,进水中分别添加0.3 mg·L−1、3.0 mg·L−1 Ag+的SBR出水中PO34 质量浓度与对照相比均无显著性差异(P<0.05)。1 mg·L−1 AgNPs、10 mg·L−1 AgNPs对活性污泥去除PO3−4 的抑制程度均高于其释放出的Ag+单独作用于活性污泥时的抑制程度,这表明AgNPs对活性污泥去除PO3−4 的抑制作用来源于AgNPs自身。综合上述结果可知,1 mg·L−1 AgNPs对SBR出水中的
NH+4 -N、NO−2 -N和NO3-N质量浓度无明显影响,对COD和PO3−4 的去除有明显抑制效应,但出水COD低于50 mg·L−1,符合城镇污水处理厂污染物排放一级A标准(GB 18918-2002),但系统出水中PO3−4 质量浓度很难达到污染物排放的一级A标准(GB 18918-2002)。AgNPs通过影响活性污泥中微生物群落结构和抑制微生物活性,从而降低活性污泥系统对碳、氮、磷的去除效率。李墨青[30]通过高通量测序发现,AgNPs质量浓度越高,具有脱氮除磷功效的菌群种类逐渐减少。LIANG等[16]的研究结果表明,AgNPs对污水处理系统中硝化细菌活性有抑制作用,最大抑制率为46.5%,从而导致废水中NH+4 -N和NO2-N质量浓度增加。AgNPs通过诱导活性氧产生细胞凋亡机制[34],抑制了活性污泥微生物的脱氮除磷性能[35]。AgNPs在污水或者水体中的存在形式多样,除AgNPs和Ag+外,Ag+经硫化、氯化后与S2-和Cl−结合可能生成Ag2S、AgCl沉淀或胶体AgClx(x−1)-;Ag+也可以与
SO2−4 、CO2−3 结合生成Ag2SO4、Ag2CO3等物质,这些物质会影响AgNPs及Ag+对微生物的毒性。CHOI等[36]发现,AgNPs可形成Ag2S沉淀物,导致AgNPs对硝化生物的毒性降低80%;LEVARD等[37]的研究也证明,硫化物的存在会导致AgNPs释放Ag+的速率降低,对AgNPs的毒性产生较大的影响。因此,今后的研究需关注AgNPs在水中的不同赋存形态对活性污泥微生物去除污染物的影响。JOHNSON等[38]通过检测英国9座污水处理厂的污水中总Ag质量浓度(实际测量中很难区分水中AgNPs、Ag+及其他Ag形态,均是以总Ag质量浓度表示),得出污水中总Ag的平均质量浓度为8.4×10−5 mg·L−1。本课题组在南京农业大学学生宿舍区16处化粪池、污水检查井中取水样,检测结果表明污水中总Ag质量浓度为2.1×10−4~4.30×10−2 mg·L−1,远低于1 mg·L−1[39]。从目前国内外报道的城镇污水中总Ag质量浓度,结合本研究与其他研究者所得的结果可以看出,城镇污水处理厂中的AgNPs并未对活性污泥去除污染物的性能产生显著的抑制效应。
2.1. AgNPs物理性状的表征
2.2. SBR出水DO和pH变化
2.3. AgNPs及Ag+对活性污泥系统去除COD的影响
2.4.
AgNPs及Ag+对活性污泥去除NH+4 -N的影响
2.5.
AgNPs及Ag+对活性污泥去除NO−2 -N和NO3-N效果的影响
2.6.
AgNPs及Ag+对活性污泥去除PO3−4 的影响
-
1)表面被聚乙烯吡咯烷酮包被的AgNPs在污水中仍然易团聚,AgNPs质量浓度越高,其粒径越大,团聚现象越明显。
2)进水中添加10 mg·L−1 AgNPs可显著抑制活性污泥系统对COD、
NH+4 -N及PO3−4 的去除,10 mg·L−1 AgNPs对活性污泥系统的显著抑制效应主要源于AgNPs自身而不是其释放的Ag+;添加1 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对活性污泥系统去除COD有抑制效应,但去除率仍高于60%,可以满足城镇污水处理厂污染物排放一级A标准(GB 18918-2002);添加1 mg·L−1 AgNPs可显著抑制活性污泥系统对PO3−4 的去除;添加1 mg·L−1、10 mg·L−1 AgNPs和0.3 mg·L−1、3.0 mg·L−1 Ag+对SBR出水中NO−2 -N和NO−3 -N去除率没有影响。3)目前污水处理系统中AgNPs质量浓度通常在ng·L−1~μg·L−1级别,远低于1 mg·L−1,低质量浓度AgNPs对活性污泥系统污水处理性能的影响有待进一步研究。














 下载:
下载: