-
氮及其化合物会污染地表水体,还会经微生物作用转化为硝酸盐氮积累在土壤中造成地下水污染[1],已成为水体污染治理中的重要污染物。因此,寻求高效、低耗的脱氮技术成为国内外水处理领域亟待解决的重要课题。相较于离子交换、膜分离、化学还原等物理化学手段,生物脱氮具有高效低耗、稳定运行等优点,并已被广泛应用于实际污水处理中[2]。由于反硝化过程中微生物所需碳源种类不同,可将反硝化过程分为异养反硝化和自养反硝化两大类[3]。常见的自养反硝化过程包括氢自养反硝化、硫自养反硝化[2]、铁自养反硝化[4]等。有研究表明,在厌氧条件下甲烷能直接作为碳源及电子供体发生反硝化脱氮[5],这一过程被称为厌氧甲烷氧化的反硝化(denitrifying anaerobic methane oxidation, DAMO)。与其他电子供体相比,甲烷获得途径广泛、无毒且经济便宜[6]。同时,甲烷是温室气体,所产生的温室效应是等质量二氧化碳的26倍,对全球变暖的贡献率约占20%[7]。因此,厌氧甲烷氧化与自养反硝化的耦合反应将人为产生的甲烷用于废水反硝化脱氮处理,可为低物耗废水处理和节能减排提供新思路。
由于DAMO微生物为自养型微生物,生长缓慢,富集培养比较困难,故基于此类微生物的生物膜反应器研究较少。因此本研究拟采用序批实验,利用人工模拟低氮负荷废水,比较不同环境因素对DAMO系统中间产物的积累与脱氮效果的影响,并通过高通量测序技术,探究不同pH作用下的污泥中微生物的生态分布、群落结构组成和演替变化规律,以揭示系统pH对DAMO功能微生物种群分布及变迁情况的影响,以期为甲烷厌氧氧化的自养反硝化系统的pH调控及条件优化提供参考。
-
本研究通过序批实验考察CH4供应、初始硝氮质量浓度和溶液pH对厌氧甲烷氧化型自养反硝化性能的影响。将经过前期培养的50 mL污泥接种至具塞摇瓶(250 mL)内,再加入150 mL模拟硝酸盐废水,调节溶液pH;每日向具塞摇瓶内以0.01 MPa压力注入足量甲烷;为避免光照影响,用双层锡箔纸包住瓶身以保证反应在黑暗环境下进行;将摇瓶置于恒温振荡培养箱中进行反应,调节温度为(30±1) ℃,转速为(150±5) r·min−1;定时检测溶液中
NO−3 -N、NO−2 -N和NH+4 -N的质量浓度。 -
本实验采用的接种种泥取自武汉市沙湖污水处理厂二沉池,污泥的初始MLSS约为8 900 mg·L−1。实验开始前,将取回的活性污泥放置在5 L的密闭容器内进行为期30 d的培养驯化。在污泥驯化期间,向容器中加入模拟硝酸盐废水([NO3−-N]=20 mg·L−1)以促进微生物的增殖,并每天更换新鲜模拟硝酸盐废水。此外,向上述容器中通15 min氮气以形成厌氧氛围,然后再通入足量甲烷气体,以达到对污泥培养驯化的目的。将经驯化后能够发生基于甲烷厌氧氧化的反硝化作用的污泥用于后续实验。模拟硝酸盐废水的具体成分及质量浓度为:
NO−3 -N 20~50 mg·L−1,K2HPO4 25 mg·L−1,KH2PO4 20 mg·L−1,CaCl2 10 mg·L−1,NaHCO3 2 000 mg·L−1。添加微量元素液的体积分数为0.5 mL·L−1,调节pH至7.3~7.5。微量元素液的成分及质量浓度为:ZnSO4·7H2O 0.5 g·L−1,CaCl2 2 g·L−1,MnCl2·4H2O 2.5 g·L−1,Na2MoO4·4H2O 0.5 g·L−1,KI 0.18 g·L−1,CuSO4·5H2O 0.1 g·L−1,CoCl2·6H2O 0.15 g·L−1,FeCl3·6H2O 1.5 g·L−1。 -
水质指标的检测方法依照国家环保总局编制的《水与废水监测分析方法(第4版)》,以及美国公共卫生协会编写的《Standard Methods for the Examination of Water and Wastewater》(第十九版)中的标准方法检测。其中,[
NO−3 -N]采用紫外分光光度法测试,[NO−2 -N]采用N-(1-萘基)-乙二胺分光光度法测试,[NH+4 -N]用纳氏试剂分光光度法测试。采用SPSS 23.0进行方差分析(ANOVA),以确定数据集之间是否存在差异,如果p<0.05,则认为存在显著差异。为探究环境pH对甲烷厌氧氧化耦合自养反硝化微生物的多样性和种群群落结构变化的影响,分别取pH为6、7、8、9时的污泥样品进行高通量测序,测序结果分别标记为P1、P2、P3和P4。本实验中,高通量测序在上海美吉生物医药科技有限公司进行,利用Illumina Miseq平台,以515F(5′-GTGCCAGCMGCCGCGG-3′)与907R(5′-CCGTCAATTCMTTTRAGTTT-3′)作为引物进行PCR扩增与焦磷酸测序。
-
为探究甲烷在自养反硝化体系中的作用,分别向S1、S2系统中通入氮气和甲烷,将pH调至7.5,并检测系统的反硝化速率(见图1),未检测到NO2−-N和
NH+4 -N的积累。由图1可知,通入氮气时(S1),NO−3 -N含量基本保持不变,几乎没有降解;而在充足甲烷的供应下(S2),6 d内NO−3 -N基本完全降解,平均脱氮速率为3.67 mg·(L·d)−1。这说明甲烷供应是反硝化过程中的重要条件,也证明了经前期培养驯化的污泥能发生基于甲烷厌氧氧化的反硝化作用。 -
将摇瓶内溶液pH保持在7.5,供以充足的甲烷气体,初始
NO−3 -N质量浓度分别设为20、30、40和50 mg·L−1,探究不同NO−3 -N质量浓度对反硝化速率的影响,实验结果如图2所示。由图2可知,整个过程几乎没有NH+4 -N的积累,NH+4 -N质量均维持在0.3 mg·L−1以下。当初始硝氮浓度从20 mg·L−1增加至30 mg·L−1时,平均去除速率也从3.33 mg·(L·d)−1增加至4.28 mg·(L·d)−1 (p<0.05)。这表明在一定范围内,随着初始NO−3 -N质量浓度的增加,脱氮速率呈上升趋势。这可能是由于:一方面增加了氮源,有利于微生物的增长繁殖;另一方面,在甲烷厌氧氧化耦合自养反硝化过程中,NO−3 -N经过一系列还原酶的作用被转化成N2,同时还有O2生成,而甲烷在甲烷氧化菌的作用下被O2氧化成甲醇,最终氧化为CO2[8]。NO−3 -N质量分数的增加有可能促进了O2的生成,加快了甲烷被氧化成甲醇的速率,而甲醇又可以为NO−3 -N及NO2−-N的还原提供电子,最终导致脱氮速率的提升。此外,自养反硝化菌的一系列脱氮过程属于酶促反应,底物浓度是影响酶促反应速率的重要因素,在系统中底物浓度较低的情况下,酶促反应的速率会随着底物浓度的增加而提高。然而,当系统中底物浓度增大到一定值时,酶促反应速率便不再提升。当初始NO−3 -N质量浓度增至40和50 mg·L−1时,平均反硝化速率有所降低,分别为4和3.85 mg·(L·d)−1。这说明过高的NO−3 -N质量浓度抑制了自养反硝化菌的活性[9]。由于系统中底物浓度过高会抑制酶促反应,从而导致系统脱氮速率的降低。由图2 (b)可知,
NO−2 -N质量浓度整体都呈现先增加后减小的趋势。这说明在甲烷厌氧氧化反硝化反应中存在NO−2 -N的积累及进一步的还原过程。当初始NO−3 -N质量浓度从20 mg·L−1增至50 mg·L−1时,NO−2 -N积累量峰值从0.27 mg·L−1增至2.69 mg·L−1。由此可见,当NO−3 -N质量浓度增加时,NO−2 -N的积累量也逐渐增加,而中间产物的不断积累也会影响反硝化效果,这也是导致脱氮速率有所下降的原因之一。 -
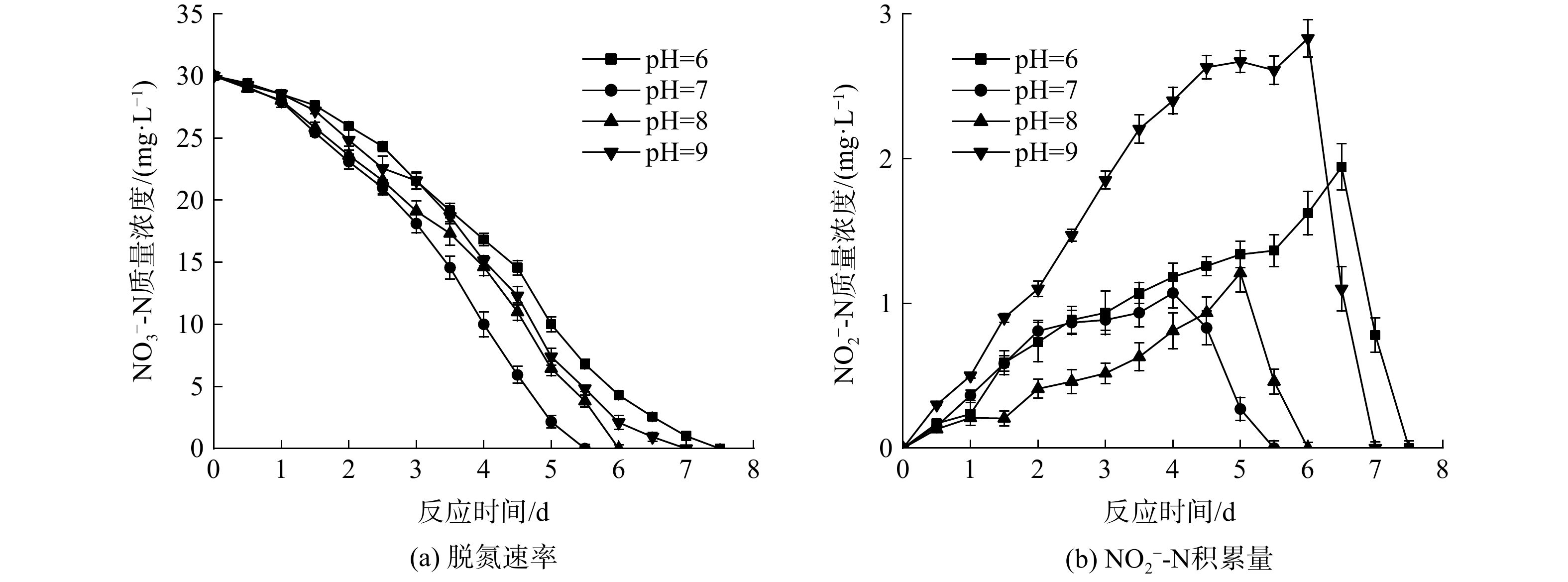
为探究初始pH对反硝化速率的影响,将初始
NO−3 -N质量浓度控制在30 mg·L−1,分别调节系统pH为6、7、8、9,研究在不同pH下系统脱氮速率的变化,结果如图3所示。实验过程中几乎没有NH+4 -N的积累,NH+4 -N质量浓度均在0.3 mg·L−1以下。由图3 (a)可知,4个反应器均能在一定时间内将NH+4 -N降解完全。当初始pH为7时,系统对NH+4 -N的平均去除速率最高,为5.45 mg·(L·d)−1;当pH增大至8时,反应器平均脱氮速率虽有所下降,但仍保持在5 mg·(L·d)−1左右。然而,当pH继续升至9时,NO−3 -N平均去除速率降至4.28 mg·(L·d)−1;当pH为6时,反应器的平均脱氮速率最低,仅为4 mg·(L·d)−1。不同pH条件下的脱氮速率之间均存在显著差异(p<0.05)。由此可见,反应器在中性和微碱性环境中能保持良好的脱氮速率,过高或过低的pH都会影响反应器对NO−3 -N的去除效果。在HE等[10]的研究中,厌氧甲烷氧化反硝化菌在pH为7.0~8.0时保持较高活性,在高pH(pH=9)及低pH(pH=6)时,其活性均会下降,pH=6时其活性最低。这与本实验的结论相似。由于甲烷厌氧氧化的自养反硝化主要靠微生物的反硝化作用来实现,而各种微生物都有其生存适宜的pH范围,故当环境pH偏离适宜pH过多时,微生物的生长和繁殖就会受到影响,甚至死亡。环境中的pH主要通过3个方面限制微生物的反硝化过程。一是影响微生物胞外水解酶的活性并引起细胞膜上蛋白质的变性,从而影响微生物对物质的降解和吸收;二是通过影响膜的通透性并引起膜表面电荷性质的变化,进一步抑制细胞对物质的分解和吸收;三是影响营养物质的降解和吸收。其中,反硝化过程又由一系列的酶促反应组成,环境pH的变化会影响酶的离子化程度,改变蛋白质结构,从而影响酶的活性。当环境pH过高或过低时,酶也会失去活性[11]。因此,当pH为7~8时,系统反硝化速率良好,差别不大,但随着环境的初始pH增大到9或者减小至6时,反应器的平均脱氮效率也会随之下降。
由图3 (b)可知,系统初始pH对甲烷厌氧氧化的自养反硝化过程中亚硝氮的积累也会产生一定程度的影响。当pH为7和8时,系统
NO−2 -N积累量较低;但随着pH升高至9时,NO−2 -N积累量也随之增加;而当pH减小至6时,反应过程中NO−2 -N质量浓度的峰值也较高。这可能是由于:在反应过程中,亚硝酸盐还原酶有迟缓期,而迟缓期的长短由驯化时间的长短和反应条件决定,如pH和营养物质浓度等,因此,不适宜的环境pH(pH<7或pH>8)会延长亚硝酸盐还原酶的迟缓期,从而造成NO−2 -N的积累[12]。另外,虽然系统处于酸性环境中的平均反硝化速率最低,但pH为6时NO−2 -N的积累量却比pH为9时要少。这可能是由于:NO−3 -N首先在硝酸盐还原酶的作用下被还原成NO−2 -N,然后在亚硝酸盐还原酶的作用下进一步被还原、降解。有研究表明,亚硝酸盐还原酶在中性和微碱性环境中活性最高,但在酸性环境中反应器的平均脱氮速率下降幅度比碱性环境中更大。因此,当初始pH为6的时候,由于系统反硝化效率低,并没有过多的NO−3 -N被还原成NO−2 -N。尽管此时亚硝酸盐还原酶活性较低,但由于NO−2 -N含量的减少,也使得反应器中没有过多的NO−2 -N积累。 -
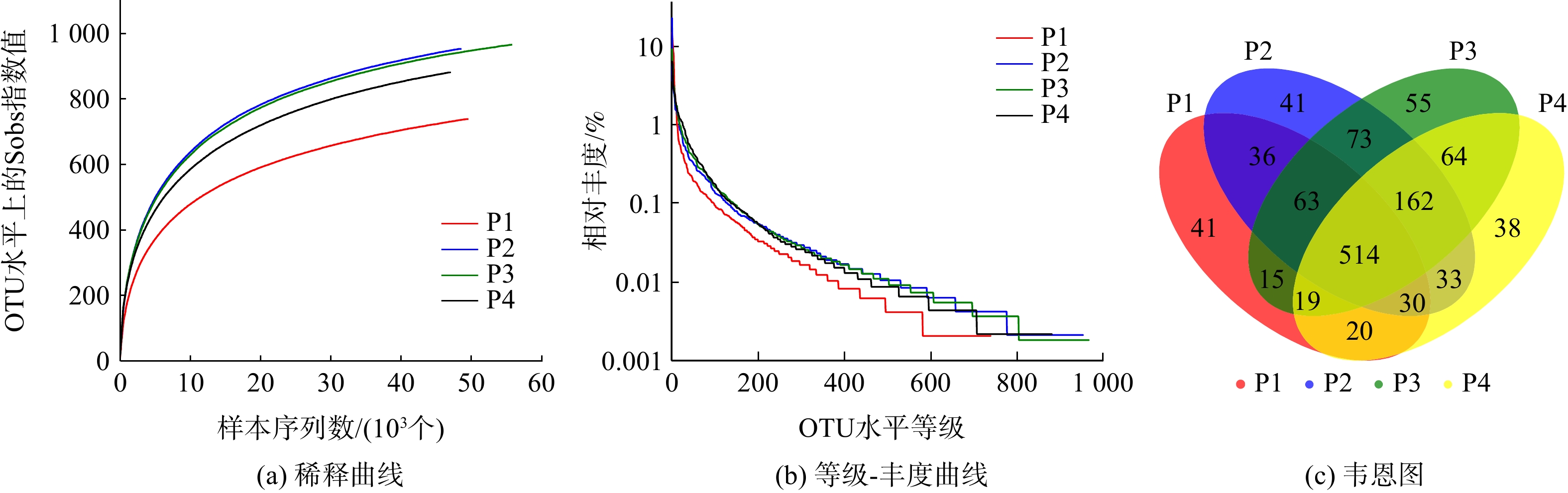
对初始pH分别为6、7、8、9的摇瓶内污泥取样后进行高通量测序,分析其微生物种群多样性和群落结构。反映系统微生物种群丰度和多样性的各种指标可由样品的Alpha多样性分析得出,见表1。由表1可知,当相似度为97%时,4个样品中分别获得49 400、48 248、55 726和46 796条有效序列(Reads)。4个样品的Coverage值均大于0.99,这表明样品测序得到的序列可覆盖大部分的区域,测序深度能较好地代表4个样品中的微生物群落,结果可有效反映样品的真实状况。
Ace和Chao指数可用来估计物种的丰度,数值越大说明微生物的丰度越高,微生物数目也就越多。由表1可知,这2组数据呈现相同的变化趋势,大小顺序均为P2≈P3>P4>P1。这表明在pH=7和pH=8的环境下,微生物丰度最高,微生物的数目也最多。然而,随着环境pH的增大或减小,系统内微生物的丰度均有所下降,并且在酸性环境下丰度最低。Shannon和Simpson指数能够代表测序样本的生物多样性,Shannon越大,Simpson越小,表明微生物的多样性越高,因此4个样品按多样性高低排序为P4>P3>P2>P1。Shannoneven与Simpsoneven指数为反映样品均匀度的指标,Shannoneven越大,Simpsoneven越小,表明微生物的均匀度越好。4个样本按均匀度高低排序为P4>P3>P2>P1。
另外,pH=7和pH=8的反应器微生物丰度最高,多样性及均匀度适中。这主要是由于中性和微碱性环境有利于微生物的生长繁殖,并且对微生物中功能菌的筛选作用强。随着pH的升高,微生物多样性和均匀度也越高,说明碱性环境可提高微生物的多样性和均匀度。
以上结果与图4中的稀释曲线和等级-丰度曲线所显示的结果相同,4个样品的稀释曲线最后都趋于平缓。这表明此次测序的取样合理,继续增加序列数只会产生少量的OTU,且从曲线中得到4个样品的OTU数目变化趋势与前述相同。等级-丰度曲线可用来表示微生物中物种丰度和物种均匀度2个方面的内容。其中,物种的丰度由水平方向曲线的宽度来反映,曲线在横轴上的范围越大,物种的丰富度就越高;而曲线的形状(平缓程度)反映了样本中群落的均匀度,曲线越平缓,物种分布越均匀。本结果表明,当pH=7和pH=8时,物种丰度较大,均匀度较好。
韦恩图可用来统计多个样本中所共有和独有OTU数量,能直观表达不同样品间物种的相似性。由图4 (c)所示,4个样品共获得了1 204个OTU,P1、P2、P3、P4所单独特有的OTU数分别为41、41、55和38,分别占总数的3.4%、3.4%、4.6%和3.2%。而4个样品共有的OTU数目为514,占各样品OTU总数的50%~70%。这说明不同pH下微生物物种的相似性较高,pH的改变并未使系统中微生物的种类发生太大变化。因此,不同条件下微生物仍具备一定的脱氮能力,而环境pH的改变可能引起系统中主要脱氮功能菌丰度的变化,从而导致脱氮效率的不同。
-
为进一步揭示pH对微生物群落的影响,分别在门、纲、属水平上分析了4个样品的微生物群落结构。图5显示了门水平的种群分布,4个样品中丰度最多的8个菌门分别是Proteobacteria(变形菌门)、Bacteroidetes(拟杆菌门)、Chloroflexi(绿弯菌门)、Planctomycetes(浮霉菌门)、Latescibacteria(匿杆菌门)、Firmicutes(厚壁菌门)、Acidobacteria(酸杆菌门)和Zixibacteria(河床菌门)。这些菌门总和在4个样品中占90%以上。其中,Proteobacteria在4个样品中所占比例最高,分别为47.58%、45.79%、38.89%和35.96%;其次是Bacteroidetes和Chloroflexi,Bacteroidetes在4个样品中占比分别28.12%、21.33%、22.76%和26.52%,Chloroflexi占比分别为4.73%、10.79%、15.61%和14.49%。钱祝胜等[13]在中空纤维膜反应器内富集的反硝化厌氧甲烷氧化菌群中,占优势的菌门依次为Chloroflexi(绿弯菌门)、Proteobacteria(变形菌门)、Planctomycetes(浮霉菌门)、Chlorobi(绿菌门)、Acidobacteria(酸杆菌门)、Bacteroidetes(拟杆菌门),与本研究中占优势的菌门相似。Proteobacteria和Bacteroidetes一直被认为是反硝化脱氮过程中最常见的自养反硝化微生物,这2种菌门包含各种类型的反硝化菌及甲烷氧化菌[14]。图5表明,在4个样品中,这2种菌门所占比例的总和差别不大,即各种pH环境下都具备一定的反硝化脱氮能力。在LUO等[15]构建的以甲烷作为电子供体去除地下水中硝酸盐的生物膜反应器中,Planctomycetes作为主要菌门被检出。Chloroflexi作为厌氧污泥中一种常见的复杂菌门[16],包含了好氧嗜热菌、厌氧光养菌、利用卤化物或有机物的厌氧微生物等多种微生物,并被认为能够参与自养反硝化过程。绿弯菌门细菌能够将多糖、蛋白质等大分子有机物分解为乙酸等低分子有机酸,这些产物又能够被产甲烷菌利用进行产甲烷作用[17-18]。由图5可知,当初始pH从6逐渐升高时,系统内Chloroflexi所占比例也有一定的增长,这表明环境pH对Chloroflexi有较强的选择作用,过酸的环境不利于它的增长繁殖。除以上4种菌门外,Firmicutes和Acidobacteria也被证明具有脱氮基因,具备相应的反硝化能力[19]。除以上所述的主要菌门外,其他如Synergistetes、Gracilibacteria、Gemmatimonadetes、Hydrogenedentes和Cyanobacteria等菌门微生物由于在样品中具有较低的丰度(<1%)而被归为“Other”类,但这些菌种也在系统反硝化脱氮的过程中发挥着作用[20-24]。总的来说,虽然初始pH会影响不同菌门的相对丰度,但系统中同时存在的多种与反硝化相关的微生物还是使得系统具有高效的脱氮性能。
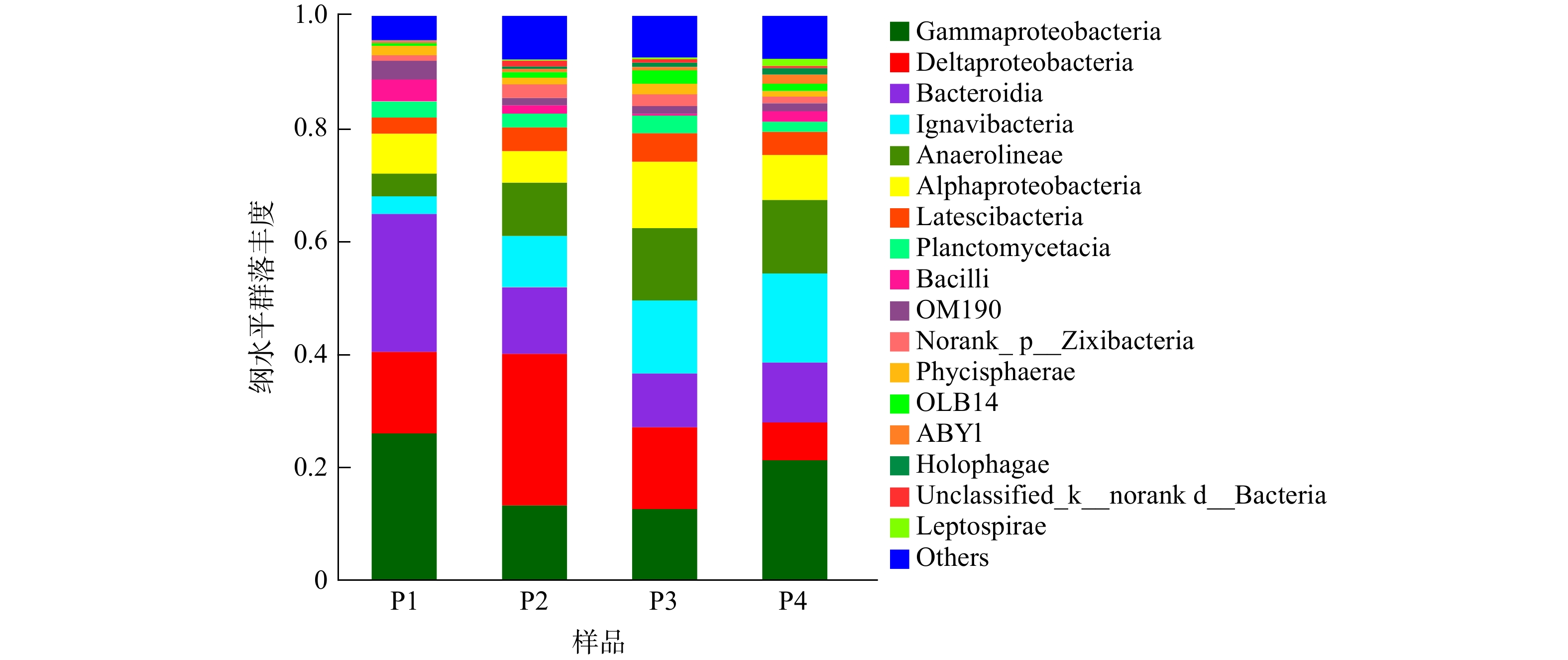
图6显示了在纲水平上4个样品的菌群结构。由图6可知,系统中的主要优势菌纲为Gammaproteobacteria、Deltaproteobacteria、Bacterodia、Ignavibacteria、Anaerolineae和Alphaproteobacteria,这些菌纲总和占各个样品总数比例的70%以上。其中Gammaproteobacteria、Deltaproteobacteria和Alphaproteobacteria同属于变形菌门,在此前多项自养反硝化的研究中被证明具有去除硝酸盐的能力[19]。在pH=7和pH=8的环境下,Gammaproteobacteria在样品中所占比例分别为13.22%和12.58%;随着pH的增大或减小,其含量均有一定的增加,在pH=6时占比为26.02%,pH=9时占比为21.29%。而Deltaproteobacteria菌纲的占比随着pH的变化趋势与此相反,在4个样品中所占比例分别为14.44%、26.91%、14.50%和6.65%。这说明这2种菌纲存在一定的竞争关系,中性和弱碱性环境适合Deltaproteobacteria的生长繁殖,从而导致丰度增加,而Gammaproteobacteria适合在酸性和强碱性环境下生长,因此表现出相反的变化趋势。Alphaproteobacteria在4个样品中所占比例分别为7.05%、5.58%、11.77%和7.96%,其中,在初始pH为8时其丰度最高。以上3种菌纲具有反硝化能力,可利用甲烷氧化生成的甲醇将系统中的NO3−-N转化成N2;同时,某些菌纲中包含同时在甲烷厌氧氧化和反硝化过程中发挥作用的细菌,如Chitinophagaceae(Alphaproteobacteria菌纲)在甲烷厌氧氧化和反硝化过程中扮演重要角色[25]。同属于拟杆菌门的Bacterodia和Ignavibacteria菌纲也有相似的变化趋势,它们在4个样品中所占比例分别为24.43%、11.79%、9.57%、10.62%和3.12%、9.07%、12.91%、15.83%。由此可见,两者也可能存在一定的竞争关系,酸性条件下更利于Bacterodia的增长繁殖。Anaerolineae是一种典型的自养反硝化菌[26],它在4个样品中的相对丰度分别为4.05%、9.48%、12.83%和13.02%。这说明环境pH的增加有利于该菌纲的富集,在酸性环境下其含量最低,也与前文得出的在酸性环境下系统脱氮效果较差的结果相吻合。
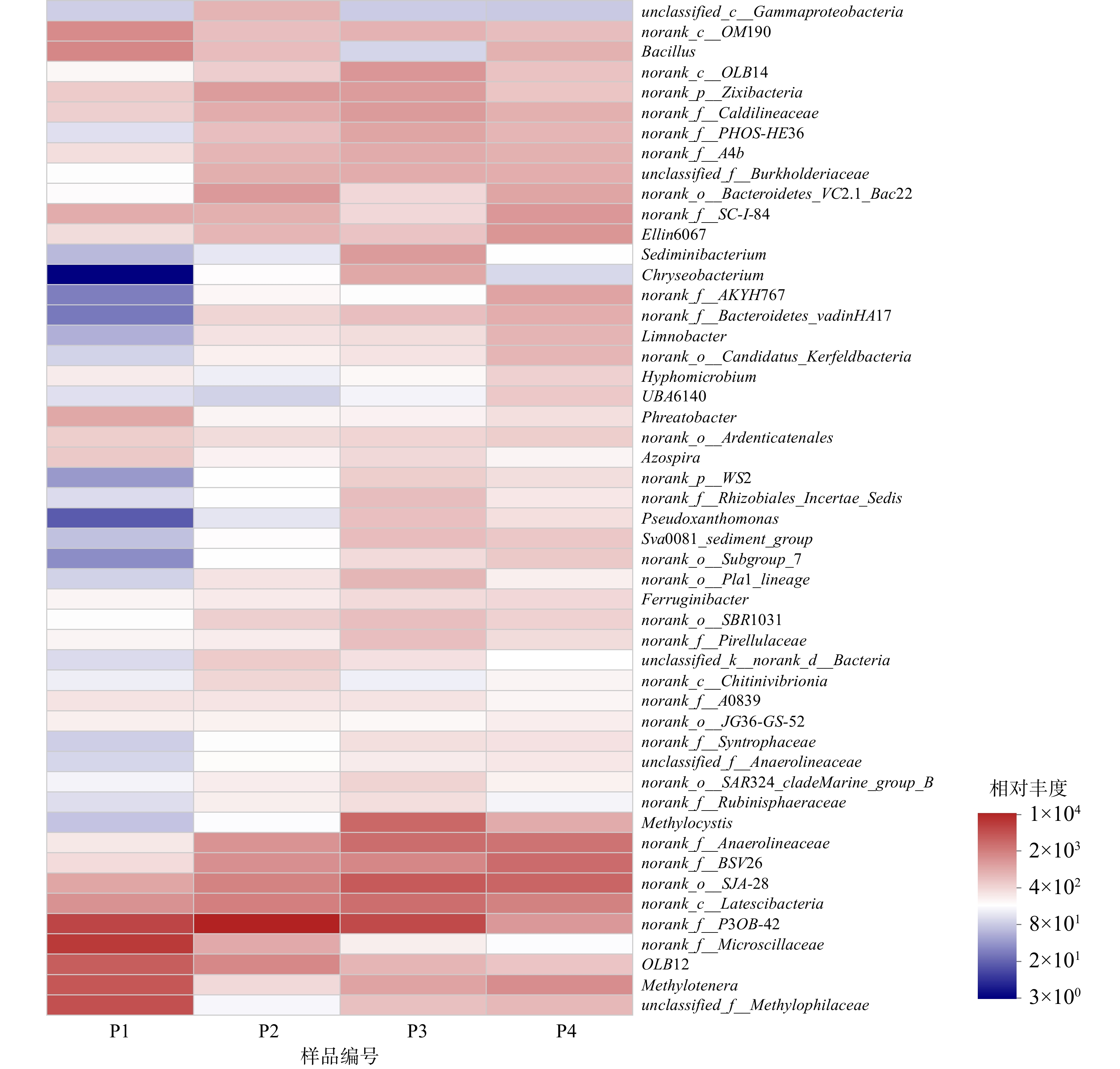
初始pH的变化会引起系统内微生物在门、纲水平上的丰度变化,从而对微生物的群落结构变化产生了一定影响。为进一步了解环境pH对种群结构的影响,对4个样品中的物种进行了属水平上的分析,并挑选相对丰度最高的50个菌属作热图(图7)。
4个样品中的微生物在属水平的分布上也均存在相似性和差异性。具体来说,P1中相对丰度最高的10个菌属及其所占的比例分别为norank_f_Microscillaceae(15.07%)、norank_f__P3OB-42(12.57%)、unclassified_f__Methylophilaceae(10.10%)、Methylotenera(8.85%)、OLB12(7.81%)、Bacillus(3.71%)、norank_c__OM190(3.35%)、norank_c__Latescibacteria(2.94%)、norank_o__SJA-28(2.07%)、Phreatobacter(1.95%);P2中相对丰度最高的10个菌属及其所占的比例分别为norank_f__P3OB-42(24.09%)、norank_c__Latescibacteria(7.18%)、norank_o__SJA-28(6.70%)、OLB12(3.58%)、norank_f_BSV26(3.14%)、norank_f__Anaerolineaceae(2.94%)、norank_o__Bacteroidetes_VC2.1_Bac22 (2.61%)、norank_p__Zixibacteria (2.45%)、norank_f__Caldilineaceae (1.81%)、unclassified_f__Burkholderiaceae (1.77%);P3中相对丰度最高的10个菌属及其所占的比例分别为norank_f__P3OB-42 (9.88%)、norank_o__SJA-28 (7.30%)、Methylocystis (5.79%)、norank_f__Anaerolineaceae (5.12%)、norank_c__Latescibacteria (5.03%)、norank_f__BSV26 (3.19%)、norank_c__OM190(2.39%)、norank_f__Caldilineaceae (2.17%)、Sediminibacterium (2.16%)、norank_p__Zixibacteria (2.12%);P4中相对丰度最高的10个菌属及其所占比例分别为norank_o__SJA-28 (7.08%)、norank_f__BSV26 (6.37%)、norank_f__Anaerolineaceae (5.43%)、norank_c__Latescibacteria (4.13%)、Methylotenera (3.37%)、Ellin6067 (2.87%)、norank_f__SC-I-84 (2.81%)、norank_f__P3OB-42 (2.75%)、norank_f__AKYH767 (2.24%)、norank_o__Bacteroidetes_VC2.1_Bac22 (2.20%)。
由此可见,随着初始pH的变化,4个样品中微生物群落结构发生了较大变化,主要微生物菌属也有较大差异。其中,Methylocystis在初始pH为8的环境下有明显富集。Methylocystis是常见的甲烷氧化菌II型菌株,能以甲烷作为唯一碳源和能量来源,在大多数含有甲烷和氧气的环境中都被发现。同时,LAI等[27]也在以甲烷作为电子供体同步去除硝酸盐和溴酸盐的系统中发现了Methylocystis的存在,并且认为其在甲烷氧化、硝酸盐及溴酸盐的还原过程中发挥了重要作用。在本系统中,Methylocystis作为甲烷氧化菌,氧化甲烷生成甲醇,甲醇为硝氮及亚硝氮的还原提供电子进行反硝化反应脱氮,其对甲烷氧化及硝酸盐的还原均发挥重要作用。Methylotenera和未分类的Methylophilaceae同属于嗜甲基菌科,能利用甲醇作为碳源和能量来源,使其氧化分解[28]。LONG等[29]在关于甲烷作为电子供体还原六价铬和硝酸盐的研究中也发现了这2种微生物的存在。在初始pH为6的环境中,这2种微生物可得到大量富集。这可能是由于:在内部好氧的亚硝酸盐依赖型厌氧甲烷氧化途径中,酸性条件加快了甲烷被甲烷氧化菌氧化成甲醇的过程,从而使得Methylotenera和Methylophilaceae大量富集。Methylotenera和Methylophilaceae也是甲烷氧化菌,能利用O2将甲烷转化为甲醇。同时,生成的甲醇可用于反硝化脱氮。此外,norank_f__Anaerolineaceae属于Anaerolineaceae科,随着初始pH的增大,相对丰度也有所增加。Anaerolineaceae菌科被证明存在于许多以甲烷为电子供体的自养反硝化体系中[30],同时,这种丝状菌能为微生物的附着提供骨架[31],有利于微生物的生长繁殖。另外,还有诸如norank_o__SJA-28、norank_f__P3OB-42 、norank_c__Latescibacteria等菌属也在4个污泥样品中被检测出来。虽然有研究表明这些菌可在有硝酸盐负荷的环境中存在,但暂时还无法明确其能否参与甲烷厌氧氧化的自养反硝化过程,故还需进一步研究来证明。
虽然单一菌属的相对丰度随环境初始pH变化的差异较大,但除污性能是由系统内不同菌群、多种功能微生物间的相互协作实现的。不同pH下,污泥样品的微生物种群结构虽有所差别,但其大多具备甲烷氧化或反硝化能力,且不同初始pH下功能菌在整个系统中所占比例仍然较高,这也为甲烷厌氧氧化型反硝化工艺能在较广pH范围内维持可靠的脱氮效率奠定了微生物基础。
-
1)在CH4供应充足的情况下,系统具备良好的反硝化速率,且没有
NO−2 -N的积累,而空白组几乎没有还原NO−3 -N的能力,表明经前期培养驯化的污泥能够以甲烷作为电子供体进行自养反硝化。2)随着初始
NO−3 -N质量浓度的升高,系统平均脱氮速率呈现先升高后下降的趋势,表明在一定范围内增加NO−3 -N质量浓度可提高系统反硝化速率,平均脱氮速率最高可达到4.28 mg·(L·d)−1;反应器内出现不同程度的NO−2 -N积累,当初始NO−3 -N质量浓度为50 mg·L−1时,NO−2 -N积累量达到峰值,为2.69 mg·L−1。3)系统在不同pH环境下均具备一定的脱氮能力,而反硝化速率有所差异。在中性和弱碱性环境下,系统脱氮效果最优,脱氮速率最高可达5.45 mg·(L·d)−1。此时,
NO−2 -N积累量最少,峰值仅为1.07 mg·L−1。当系统初始pH增大或减小,平均脱氮速率都会有所下降,且NO−2 -N积累量明显增加。4)由微生物菌群分析结果可知,环境pH对微生物种群结构具有选择作用。在pH=7和pH=8时,系统内厌氧污泥微生物的丰度最高,物种多样性和均匀性适中;不同pH下,厌氧污泥内微生物群落的主要菌门为Proteobacteria和Bacteroidetes,在纲水平上,Gammaproteobacteria、Deltaproteobacteria、Bacterodia、Ignavibacteria和Anaerolineae为优势菌纲。在适宜的pH(pH=8)下,常见甲烷氧化菌Methylocystis大量富集。不同pH环境下均存在常见于许多以甲烷为电子供体的自养反硝化体系中的Anaerolineaceae,其具体功能有待进一步探究。
不同环境因素对厌氧甲烷氧化型自养反硝化系统脱氮性能的影响及其微生物群落分析
Effects of different environmental factors on the denitrification performance and microbial community of an autotrophic denitrification system based on anaerobic methane oxidation
-
摘要: 采用序批实验研究了不同环境因素(甲烷供应、初始硝氮浓度和pH值)对厌氧甲烷氧化型反硝化系统脱氮性能的影响,并采用高通量测序对不同pH下反应器内微生物群落结构进行了分析。结果表明:当甲烷供给充足时,系统反硝化效果明显;随着初始硝氮浓度的升高,系统平均脱氮率呈现先升高后下降的趋势,表明适当增大硝氮质量浓度(<30 mg·L−1)可提高反硝化速率;在不同pH下(pH=6~9)系统均表现出较强的脱氮能力,在中性和弱碱性条件下的脱氮速率最高。基于高通量测序结果表明:pH为7和8时微生物丰度最高,多样性及均匀度适中;随着pH的升高,微生物多样性和均匀度也越高,说明碱性环境可以提高微生物的多样性和均匀度;不同pH下,Gammaproteobacteria,Deltaproteobacteria,Bacterodia,Ignavibacteria和Anaerolineae为优势菌纲;pH为8时,常见的甲烷氧化菌Methylocystis大量富集;pH为6时,甲烷氧化菌Methylotenera和Methylophilaceae得到富集;环境pH对微生物种群结构具有选择作用,不同pH环境中优势微生物亦不同。本研究可为厌氧甲烷氧化型自养反硝化系统的条件优化和性能提升提供参考。Abstract: Effects of different environmental factors (methane supply, initial nitrate concentration and pH value) on the denitrification performance of the anaerobic methane oxidized denitrification system were investigated through batch experiments, and the microbial community structure in the reactor at different pH values was analyzed by high-throughput sequencing. Results indicated that effective denitrification could be achieved with the sufficient supply of methane. With the increase of the initial nitrate concentration, the average denitrification rate of the system primarily increased and then decreased, indicating that denitrification rate could be improved by appropriately increasing the initial nitrate concentration (< 30 mg·L−1). The system exhibited good denitrification capability under different ambient pH (pH=6~9), with the highest denitrification rate achieved at neutral and slightly alkaline conditions. High-throughput sequencing found that the microbial abundance was the highest at pH=7 and pH=8, and the diversity and uniformity were moderate. With the increase of pH, the diversity and uniformity of microorganisms increased, indicating that the alkaline environment can improve microorganisms diversity and uniformity. Gammaproteobacteria, Deltaproteobacteria, Bacterodia, Ignavibacteria and Anaerolineae were the dominant classes at different pH values. When pH=8, methanotroph Methylocystis was abundantly enriched, and when pH=6, methanotroph Methylotenera and Methylophilaceae were enriched. Environmental pH had a selective effect on the microbial community structure, and the dominant microorganisms in different pH environments were different.
-
Key words:
- anaerobic methane oxidation /
- denitrification /
- pH value /
- high throughput sequencing
-
氮及其化合物会污染地表水体,还会经微生物作用转化为硝酸盐氮积累在土壤中造成地下水污染[1],已成为水体污染治理中的重要污染物。因此,寻求高效、低耗的脱氮技术成为国内外水处理领域亟待解决的重要课题。相较于离子交换、膜分离、化学还原等物理化学手段,生物脱氮具有高效低耗、稳定运行等优点,并已被广泛应用于实际污水处理中[2]。由于反硝化过程中微生物所需碳源种类不同,可将反硝化过程分为异养反硝化和自养反硝化两大类[3]。常见的自养反硝化过程包括氢自养反硝化、硫自养反硝化[2]、铁自养反硝化[4]等。有研究表明,在厌氧条件下甲烷能直接作为碳源及电子供体发生反硝化脱氮[5],这一过程被称为厌氧甲烷氧化的反硝化(denitrifying anaerobic methane oxidation, DAMO)。与其他电子供体相比,甲烷获得途径广泛、无毒且经济便宜[6]。同时,甲烷是温室气体,所产生的温室效应是等质量二氧化碳的26倍,对全球变暖的贡献率约占20%[7]。因此,厌氧甲烷氧化与自养反硝化的耦合反应将人为产生的甲烷用于废水反硝化脱氮处理,可为低物耗废水处理和节能减排提供新思路。
由于DAMO微生物为自养型微生物,生长缓慢,富集培养比较困难,故基于此类微生物的生物膜反应器研究较少。因此本研究拟采用序批实验,利用人工模拟低氮负荷废水,比较不同环境因素对DAMO系统中间产物的积累与脱氮效果的影响,并通过高通量测序技术,探究不同pH作用下的污泥中微生物的生态分布、群落结构组成和演替变化规律,以揭示系统pH对DAMO功能微生物种群分布及变迁情况的影响,以期为甲烷厌氧氧化的自养反硝化系统的pH调控及条件优化提供参考。
1. 材料与方法
1.1 实验方法
本研究通过序批实验考察CH4供应、初始硝氮质量浓度和溶液pH对厌氧甲烷氧化型自养反硝化性能的影响。将经过前期培养的50 mL污泥接种至具塞摇瓶(250 mL)内,再加入150 mL模拟硝酸盐废水,调节溶液pH;每日向具塞摇瓶内以0.01 MPa压力注入足量甲烷;为避免光照影响,用双层锡箔纸包住瓶身以保证反应在黑暗环境下进行;将摇瓶置于恒温振荡培养箱中进行反应,调节温度为(30±1) ℃,转速为(150±5) r·min−1;定时检测溶液中
NO−3 NO−2 NH+4 1.2 接种污泥与实验用水
本实验采用的接种种泥取自武汉市沙湖污水处理厂二沉池,污泥的初始MLSS约为8 900 mg·L−1。实验开始前,将取回的活性污泥放置在5 L的密闭容器内进行为期30 d的培养驯化。在污泥驯化期间,向容器中加入模拟硝酸盐废水([NO3−-N]=20 mg·L−1)以促进微生物的增殖,并每天更换新鲜模拟硝酸盐废水。此外,向上述容器中通15 min氮气以形成厌氧氛围,然后再通入足量甲烷气体,以达到对污泥培养驯化的目的。将经驯化后能够发生基于甲烷厌氧氧化的反硝化作用的污泥用于后续实验。模拟硝酸盐废水的具体成分及质量浓度为:
NO−3 1.3 分析方法
水质指标的检测方法依照国家环保总局编制的《水与废水监测分析方法(第4版)》,以及美国公共卫生协会编写的《Standard Methods for the Examination of Water and Wastewater》(第十九版)中的标准方法检测。其中,[
NO−3 NO−2 NH+4 为探究环境pH对甲烷厌氧氧化耦合自养反硝化微生物的多样性和种群群落结构变化的影响,分别取pH为6、7、8、9时的污泥样品进行高通量测序,测序结果分别标记为P1、P2、P3和P4。本实验中,高通量测序在上海美吉生物医药科技有限公司进行,利用Illumina Miseq平台,以515F(5′-GTGCCAGCMGCCGCGG-3′)与907R(5′-CCGTCAATTCMTTTRAGTTT-3′)作为引物进行PCR扩增与焦磷酸测序。
2. 结果与讨论
2.1 CH4供应对反硝化过程的影响
为探究甲烷在自养反硝化体系中的作用,分别向S1、S2系统中通入氮气和甲烷,将pH调至7.5,并检测系统的反硝化速率(见图1),未检测到NO2−-N和
NH+4 NO−3 NO−3 2.2 初始
NO−3 将摇瓶内溶液pH保持在7.5,供以充足的甲烷气体,初始
NO−3 NO−3 NH+4 NH+4 NO−3 NO−3 NO−3 NO−3 NO−3 NO−3 由图2 (b)可知,
NO−2 NO−2 NO−3 NO−2 NO−3 NO−2 2.3 初始pH对反硝化过程的影响
为探究初始pH对反硝化速率的影响,将初始
NO−3 NH+4 NH+4 NH+4 NH+4 NO−3 NO−3 由于甲烷厌氧氧化的自养反硝化主要靠微生物的反硝化作用来实现,而各种微生物都有其生存适宜的pH范围,故当环境pH偏离适宜pH过多时,微生物的生长和繁殖就会受到影响,甚至死亡。环境中的pH主要通过3个方面限制微生物的反硝化过程。一是影响微生物胞外水解酶的活性并引起细胞膜上蛋白质的变性,从而影响微生物对物质的降解和吸收;二是通过影响膜的通透性并引起膜表面电荷性质的变化,进一步抑制细胞对物质的分解和吸收;三是影响营养物质的降解和吸收。其中,反硝化过程又由一系列的酶促反应组成,环境pH的变化会影响酶的离子化程度,改变蛋白质结构,从而影响酶的活性。当环境pH过高或过低时,酶也会失去活性[11]。因此,当pH为7~8时,系统反硝化速率良好,差别不大,但随着环境的初始pH增大到9或者减小至6时,反应器的平均脱氮效率也会随之下降。
由图3 (b)可知,系统初始pH对甲烷厌氧氧化的自养反硝化过程中亚硝氮的积累也会产生一定程度的影响。当pH为7和8时,系统
NO−2 NO−2 NO−2 NO−2 NO−2 NO−3 NO−2 NO−3 NO−2 NO−2 NO−2 2.4 微生物群落分析
2.4.1 微生物种群的多样性分析
对初始pH分别为6、7、8、9的摇瓶内污泥取样后进行高通量测序,分析其微生物种群多样性和群落结构。反映系统微生物种群丰度和多样性的各种指标可由样品的Alpha多样性分析得出,见表1。由表1可知,当相似度为97%时,4个样品中分别获得49 400、48 248、55 726和46 796条有效序列(Reads)。4个样品的Coverage值均大于0.99,这表明样品测序得到的序列可覆盖大部分的区域,测序深度能较好地代表4个样品中的微生物群落,结果可有效反映样品的真实状况。
表 1 不同pH下微生物Alpha多样性指数表Table 1. Microbial Alpha diversity index at different pH values样品编号 Reads OTUs Ace Chao Shannon Simpson Shannoneven Simpsoneven Coverage P1 49 400 738 869 881 3.86 0.063 5 0.584 0 0.065 09 0.997 P2 48 248 952 1 077 1 082 4.50 0.060 7 0.656 4 0.044 79 0.996 P3 55 726 965 1 076 1 087 4.84 0.023 1 0.704 3 0.021 35 0.997 P4 46 796 880 1 017 1 016 4.90 0.017 5 0.723 4 0.017 30 0.996 Ace和Chao指数可用来估计物种的丰度,数值越大说明微生物的丰度越高,微生物数目也就越多。由表1可知,这2组数据呈现相同的变化趋势,大小顺序均为P2≈P3>P4>P1。这表明在pH=7和pH=8的环境下,微生物丰度最高,微生物的数目也最多。然而,随着环境pH的增大或减小,系统内微生物的丰度均有所下降,并且在酸性环境下丰度最低。Shannon和Simpson指数能够代表测序样本的生物多样性,Shannon越大,Simpson越小,表明微生物的多样性越高,因此4个样品按多样性高低排序为P4>P3>P2>P1。Shannoneven与Simpsoneven指数为反映样品均匀度的指标,Shannoneven越大,Simpsoneven越小,表明微生物的均匀度越好。4个样本按均匀度高低排序为P4>P3>P2>P1。
另外,pH=7和pH=8的反应器微生物丰度最高,多样性及均匀度适中。这主要是由于中性和微碱性环境有利于微生物的生长繁殖,并且对微生物中功能菌的筛选作用强。随着pH的升高,微生物多样性和均匀度也越高,说明碱性环境可提高微生物的多样性和均匀度。
以上结果与图4中的稀释曲线和等级-丰度曲线所显示的结果相同,4个样品的稀释曲线最后都趋于平缓。这表明此次测序的取样合理,继续增加序列数只会产生少量的OTU,且从曲线中得到4个样品的OTU数目变化趋势与前述相同。等级-丰度曲线可用来表示微生物中物种丰度和物种均匀度2个方面的内容。其中,物种的丰度由水平方向曲线的宽度来反映,曲线在横轴上的范围越大,物种的丰富度就越高;而曲线的形状(平缓程度)反映了样本中群落的均匀度,曲线越平缓,物种分布越均匀。本结果表明,当pH=7和pH=8时,物种丰度较大,均匀度较好。
韦恩图可用来统计多个样本中所共有和独有OTU数量,能直观表达不同样品间物种的相似性。由图4 (c)所示,4个样品共获得了1 204个OTU,P1、P2、P3、P4所单独特有的OTU数分别为41、41、55和38,分别占总数的3.4%、3.4%、4.6%和3.2%。而4个样品共有的OTU数目为514,占各样品OTU总数的50%~70%。这说明不同pH下微生物物种的相似性较高,pH的改变并未使系统中微生物的种类发生太大变化。因此,不同条件下微生物仍具备一定的脱氮能力,而环境pH的改变可能引起系统中主要脱氮功能菌丰度的变化,从而导致脱氮效率的不同。
2.4.2 微生物群落结构分析
为进一步揭示pH对微生物群落的影响,分别在门、纲、属水平上分析了4个样品的微生物群落结构。图5显示了门水平的种群分布,4个样品中丰度最多的8个菌门分别是Proteobacteria(变形菌门)、Bacteroidetes(拟杆菌门)、Chloroflexi(绿弯菌门)、Planctomycetes(浮霉菌门)、Latescibacteria(匿杆菌门)、Firmicutes(厚壁菌门)、Acidobacteria(酸杆菌门)和Zixibacteria(河床菌门)。这些菌门总和在4个样品中占90%以上。其中,Proteobacteria在4个样品中所占比例最高,分别为47.58%、45.79%、38.89%和35.96%;其次是Bacteroidetes和Chloroflexi,Bacteroidetes在4个样品中占比分别28.12%、21.33%、22.76%和26.52%,Chloroflexi占比分别为4.73%、10.79%、15.61%和14.49%。钱祝胜等[13]在中空纤维膜反应器内富集的反硝化厌氧甲烷氧化菌群中,占优势的菌门依次为Chloroflexi(绿弯菌门)、Proteobacteria(变形菌门)、Planctomycetes(浮霉菌门)、Chlorobi(绿菌门)、Acidobacteria(酸杆菌门)、Bacteroidetes(拟杆菌门),与本研究中占优势的菌门相似。Proteobacteria和Bacteroidetes一直被认为是反硝化脱氮过程中最常见的自养反硝化微生物,这2种菌门包含各种类型的反硝化菌及甲烷氧化菌[14]。图5表明,在4个样品中,这2种菌门所占比例的总和差别不大,即各种pH环境下都具备一定的反硝化脱氮能力。在LUO等[15]构建的以甲烷作为电子供体去除地下水中硝酸盐的生物膜反应器中,Planctomycetes作为主要菌门被检出。Chloroflexi作为厌氧污泥中一种常见的复杂菌门[16],包含了好氧嗜热菌、厌氧光养菌、利用卤化物或有机物的厌氧微生物等多种微生物,并被认为能够参与自养反硝化过程。绿弯菌门细菌能够将多糖、蛋白质等大分子有机物分解为乙酸等低分子有机酸,这些产物又能够被产甲烷菌利用进行产甲烷作用[17-18]。由图5可知,当初始pH从6逐渐升高时,系统内Chloroflexi所占比例也有一定的增长,这表明环境pH对Chloroflexi有较强的选择作用,过酸的环境不利于它的增长繁殖。除以上4种菌门外,Firmicutes和Acidobacteria也被证明具有脱氮基因,具备相应的反硝化能力[19]。除以上所述的主要菌门外,其他如Synergistetes、Gracilibacteria、Gemmatimonadetes、Hydrogenedentes和Cyanobacteria等菌门微生物由于在样品中具有较低的丰度(<1%)而被归为“Other”类,但这些菌种也在系统反硝化脱氮的过程中发挥着作用[20-24]。总的来说,虽然初始pH会影响不同菌门的相对丰度,但系统中同时存在的多种与反硝化相关的微生物还是使得系统具有高效的脱氮性能。
图6显示了在纲水平上4个样品的菌群结构。由图6可知,系统中的主要优势菌纲为Gammaproteobacteria、Deltaproteobacteria、Bacterodia、Ignavibacteria、Anaerolineae和Alphaproteobacteria,这些菌纲总和占各个样品总数比例的70%以上。其中Gammaproteobacteria、Deltaproteobacteria和Alphaproteobacteria同属于变形菌门,在此前多项自养反硝化的研究中被证明具有去除硝酸盐的能力[19]。在pH=7和pH=8的环境下,Gammaproteobacteria在样品中所占比例分别为13.22%和12.58%;随着pH的增大或减小,其含量均有一定的增加,在pH=6时占比为26.02%,pH=9时占比为21.29%。而Deltaproteobacteria菌纲的占比随着pH的变化趋势与此相反,在4个样品中所占比例分别为14.44%、26.91%、14.50%和6.65%。这说明这2种菌纲存在一定的竞争关系,中性和弱碱性环境适合Deltaproteobacteria的生长繁殖,从而导致丰度增加,而Gammaproteobacteria适合在酸性和强碱性环境下生长,因此表现出相反的变化趋势。Alphaproteobacteria在4个样品中所占比例分别为7.05%、5.58%、11.77%和7.96%,其中,在初始pH为8时其丰度最高。以上3种菌纲具有反硝化能力,可利用甲烷氧化生成的甲醇将系统中的NO3−-N转化成N2;同时,某些菌纲中包含同时在甲烷厌氧氧化和反硝化过程中发挥作用的细菌,如Chitinophagaceae(Alphaproteobacteria菌纲)在甲烷厌氧氧化和反硝化过程中扮演重要角色[25]。同属于拟杆菌门的Bacterodia和Ignavibacteria菌纲也有相似的变化趋势,它们在4个样品中所占比例分别为24.43%、11.79%、9.57%、10.62%和3.12%、9.07%、12.91%、15.83%。由此可见,两者也可能存在一定的竞争关系,酸性条件下更利于Bacterodia的增长繁殖。Anaerolineae是一种典型的自养反硝化菌[26],它在4个样品中的相对丰度分别为4.05%、9.48%、12.83%和13.02%。这说明环境pH的增加有利于该菌纲的富集,在酸性环境下其含量最低,也与前文得出的在酸性环境下系统脱氮效果较差的结果相吻合。
初始pH的变化会引起系统内微生物在门、纲水平上的丰度变化,从而对微生物的群落结构变化产生了一定影响。为进一步了解环境pH对种群结构的影响,对4个样品中的物种进行了属水平上的分析,并挑选相对丰度最高的50个菌属作热图(图7)。
4个样品中的微生物在属水平的分布上也均存在相似性和差异性。具体来说,P1中相对丰度最高的10个菌属及其所占的比例分别为norank_f_Microscillaceae(15.07%)、norank_f__P3OB-42(12.57%)、unclassified_f__Methylophilaceae(10.10%)、Methylotenera(8.85%)、OLB12(7.81%)、Bacillus(3.71%)、norank_c__OM190(3.35%)、norank_c__Latescibacteria(2.94%)、norank_o__SJA-28(2.07%)、Phreatobacter(1.95%);P2中相对丰度最高的10个菌属及其所占的比例分别为norank_f__P3OB-42(24.09%)、norank_c__Latescibacteria(7.18%)、norank_o__SJA-28(6.70%)、OLB12(3.58%)、norank_f_BSV26(3.14%)、norank_f__Anaerolineaceae(2.94%)、norank_o__Bacteroidetes_VC2.1_Bac22 (2.61%)、norank_p__Zixibacteria (2.45%)、norank_f__Caldilineaceae (1.81%)、unclassified_f__Burkholderiaceae (1.77%);P3中相对丰度最高的10个菌属及其所占的比例分别为norank_f__P3OB-42 (9.88%)、norank_o__SJA-28 (7.30%)、Methylocystis (5.79%)、norank_f__Anaerolineaceae (5.12%)、norank_c__Latescibacteria (5.03%)、norank_f__BSV26 (3.19%)、norank_c__OM190(2.39%)、norank_f__Caldilineaceae (2.17%)、Sediminibacterium (2.16%)、norank_p__Zixibacteria (2.12%);P4中相对丰度最高的10个菌属及其所占比例分别为norank_o__SJA-28 (7.08%)、norank_f__BSV26 (6.37%)、norank_f__Anaerolineaceae (5.43%)、norank_c__Latescibacteria (4.13%)、Methylotenera (3.37%)、Ellin6067 (2.87%)、norank_f__SC-I-84 (2.81%)、norank_f__P3OB-42 (2.75%)、norank_f__AKYH767 (2.24%)、norank_o__Bacteroidetes_VC2.1_Bac22 (2.20%)。
由此可见,随着初始pH的变化,4个样品中微生物群落结构发生了较大变化,主要微生物菌属也有较大差异。其中,Methylocystis在初始pH为8的环境下有明显富集。Methylocystis是常见的甲烷氧化菌II型菌株,能以甲烷作为唯一碳源和能量来源,在大多数含有甲烷和氧气的环境中都被发现。同时,LAI等[27]也在以甲烷作为电子供体同步去除硝酸盐和溴酸盐的系统中发现了Methylocystis的存在,并且认为其在甲烷氧化、硝酸盐及溴酸盐的还原过程中发挥了重要作用。在本系统中,Methylocystis作为甲烷氧化菌,氧化甲烷生成甲醇,甲醇为硝氮及亚硝氮的还原提供电子进行反硝化反应脱氮,其对甲烷氧化及硝酸盐的还原均发挥重要作用。Methylotenera和未分类的Methylophilaceae同属于嗜甲基菌科,能利用甲醇作为碳源和能量来源,使其氧化分解[28]。LONG等[29]在关于甲烷作为电子供体还原六价铬和硝酸盐的研究中也发现了这2种微生物的存在。在初始pH为6的环境中,这2种微生物可得到大量富集。这可能是由于:在内部好氧的亚硝酸盐依赖型厌氧甲烷氧化途径中,酸性条件加快了甲烷被甲烷氧化菌氧化成甲醇的过程,从而使得Methylotenera和Methylophilaceae大量富集。Methylotenera和Methylophilaceae也是甲烷氧化菌,能利用O2将甲烷转化为甲醇。同时,生成的甲醇可用于反硝化脱氮。此外,norank_f__Anaerolineaceae属于Anaerolineaceae科,随着初始pH的增大,相对丰度也有所增加。Anaerolineaceae菌科被证明存在于许多以甲烷为电子供体的自养反硝化体系中[30],同时,这种丝状菌能为微生物的附着提供骨架[31],有利于微生物的生长繁殖。另外,还有诸如norank_o__SJA-28、norank_f__P3OB-42 、norank_c__Latescibacteria等菌属也在4个污泥样品中被检测出来。虽然有研究表明这些菌可在有硝酸盐负荷的环境中存在,但暂时还无法明确其能否参与甲烷厌氧氧化的自养反硝化过程,故还需进一步研究来证明。
虽然单一菌属的相对丰度随环境初始pH变化的差异较大,但除污性能是由系统内不同菌群、多种功能微生物间的相互协作实现的。不同pH下,污泥样品的微生物种群结构虽有所差别,但其大多具备甲烷氧化或反硝化能力,且不同初始pH下功能菌在整个系统中所占比例仍然较高,这也为甲烷厌氧氧化型反硝化工艺能在较广pH范围内维持可靠的脱氮效率奠定了微生物基础。
3. 结论
1)在CH4供应充足的情况下,系统具备良好的反硝化速率,且没有
NO−2 NO−3 2)随着初始
NO−3 NO−3 NO−2 NO−3 NO−2 3)系统在不同pH环境下均具备一定的脱氮能力,而反硝化速率有所差异。在中性和弱碱性环境下,系统脱氮效果最优,脱氮速率最高可达5.45 mg·(L·d)−1。此时,
NO−2 NO−2 4)由微生物菌群分析结果可知,环境pH对微生物种群结构具有选择作用。在pH=7和pH=8时,系统内厌氧污泥微生物的丰度最高,物种多样性和均匀性适中;不同pH下,厌氧污泥内微生物群落的主要菌门为Proteobacteria和Bacteroidetes,在纲水平上,Gammaproteobacteria、Deltaproteobacteria、Bacterodia、Ignavibacteria和Anaerolineae为优势菌纲。在适宜的pH(pH=8)下,常见甲烷氧化菌Methylocystis大量富集。不同pH环境下均存在常见于许多以甲烷为电子供体的自养反硝化体系中的Anaerolineaceae,其具体功能有待进一步探究。
-
表 1 不同pH下微生物Alpha多样性指数表
Table 1. Microbial Alpha diversity index at different pH values
样品编号 Reads OTUs Ace Chao Shannon Simpson Shannoneven Simpsoneven Coverage P1 49 400 738 869 881 3.86 0.063 5 0.584 0 0.065 09 0.997 P2 48 248 952 1 077 1 082 4.50 0.060 7 0.656 4 0.044 79 0.996 P3 55 726 965 1 076 1 087 4.84 0.023 1 0.704 3 0.021 35 0.997 P4 46 796 880 1 017 1 016 4.90 0.017 5 0.723 4 0.017 30 0.996 -
[1] 董萌. 地下水硝酸盐污染的原位修复试验研究[D]. 邯郸: 河北工程大学, 2015. [2] GHAFARI S, HASAN M, AROUA M K. Bio-electrochemical removal of nitrate from water and wastewater:A review[J]. Bioresource Technology, 2008, 99(10): 3965-3974. doi: 10.1016/j.biortech.2007.05.026 [3] FENG S S, LIN X, TONG Y J, et al. Biodesulfurization of sulfide wastewater for elemental sulfur recovery by isolated Halothiobacillus neapolitanus in an internal airlift loop reactor[J]. Bioresource Technology, 2018, 264: 244-252. doi: 10.1016/j.biortech.2018.05.079 [4] ZHOU J, WANG H Y, YANG K, et al. Autotrophic denitrification by nitrate-dependent Fe(II) oxidation in a continuous up-flow biofilter[J]. Bioprocess and Biosystems Engineering, 2016, 39(2): 277-284. doi: 10.1007/s00449-015-1511-7 [5] 董文艺, 赵志军, 李继. 甲烷作为反硝化气体碳源的研究进展[J]. 安全与环境工程, 2011, 18(4): 64-69. doi: 10.3969/j.issn.1671-1556.2011.04.015 [6] 王劲松, 张召基, 王晓君, 等. 反硝化型甲烷厌氧氧化微生物人工富集与应用研究进展[J]. 水处理技术, 2017, 43(12): 8-13. [7] 楼菊青, 王析镭, 杨东叶, 等. 不同接种物对富集反硝化型甲烷厌氧氧化微生物的影响[J]. 环境科学学报, 2016, 36(11): 4087-4095. [8] 薛松, 张梦竹, 李琳, 等. 甲烷厌氧氧化协同硝酸盐还原菌群驯化及其群落特征[J]. 环境科学, 2018, 39(3): 1357-1364. [9] VASILIADOU I A, KARANASIOS K A, PAVLOU S, et al. Experimental and modelling study of drinking water hydrogenotrophic denitrification in packed-bed reactors[J]. Journal of Hazardous Materials, 2009, 165(1/2/3): 812-824. [10] HE Z F, GENG S, SHEN L D, et al. The short- and long-term effects of environmental conditions on anaerobic methane oxidation coupled to nitrite reduction[J]. Water Research, 2015, 68: 554-562. doi: 10.1016/j.watres.2014.09.055 [11] HUANG B, CHI G Y, CHEN X, et al. Removal of highly elevated nitrate from drinking water by pH-heterogenized heterotrophic denitrification facilitated with ferrous sulfide-based autotrophic denitrification[J]. Bioresource Technology, 2011, 102(21): 10154-10157. doi: 10.1016/j.biortech.2011.08.048 [12] 徐亚同. pH值、温度对反硝化的影响[J]. 中国环境科学, 1994, 14(4): 308-313. doi: 10.3321/j.issn:1000-6923.1994.04.010 [13] 钱祝胜, 付亮, 丁静, 等. 中空纤维膜生物反应器富集反硝化厌氧甲烷氧化菌群的研究[J]. 中国科学技术大学学报, 2014, 44(11): 887-892. doi: 10.3969/j.issn.0253-2778.2014.11.002 [14] CHEN D, WANG H Y, YANG K, et al. Performance and microbial communities in a combined bioelectrochemical and sulfur autotrophic denitrification system at low temperature[J]. Chemosphere, 2018, 193: 337-342. doi: 10.1016/j.chemosphere.2017.11.017 [15] LUO J H, CHEN H, YUAN Z G, et al. Methane-supported nitrate removal from groundwater in a membrane biofilm reactor[J]. Water Research, 2018, 132: 71-78. doi: 10.1016/j.watres.2017.12.064 [16] SHU D T, HE Y L, YUE H, et al. Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing[J]. Bioresource Technology, 2015, 186: 163-172. doi: 10.1016/j.biortech.2015.03.072 [17] LU Y Z, CHEN G J, BAI Y N, et al. Chromium isotope fractionation during Cr(VI) reduction in a methane-based hollow-fiber membrane biofilm reactor[J]. Water Research, 2018, 130: 263-270. doi: 10.1016/j.watres.2017.11.045 [18] CAI C, HU S H, CHEN X M, et al. Effect of methane partial pressure on the performance of a membrane biofilm reactor coupling methane-dependent denitrification and anammox[J]. Science of the Total Environment, 2018, 639: 278-285. doi: 10.1016/j.scitotenv.2018.05.164 [19] LIAO R H, LI Y, YU X M, et al. Performance and microbial diversity of an expanded granular sludge bed reactor for high sulfate and nitrate waste brine treatment[J]. Journal of Environmental Sciences, 2014, 26(4): 717-725. doi: 10.1016/S1001-0742(13)60479-9 [20] CAO S B, DU R, ZHANG H Y, et al. Understanding the granulation of partial denitrification sludge for nitrite production[J]. Chemosphere, 2019, 236: 124389. doi: 10.1016/j.chemosphere.2019.124389 [21] ZHANG Z Q, CHEN H Z, MU X Y, et al. Nitrate application decreased microbial biodiversity but stimulated denitrifiers in epiphytic biofilms on Ceratophyllum demersum[J]. Journal of Environmental Management, 2020, 269: 110814. doi: 10.1016/j.jenvman.2020.110814 [22] HAN B, MO L Y, FANG Y T, et al. Rates and microbial communities of denitrification and anammox across coastal tidal flat lands and inland paddy soils in East China[J]. Applied Soil Ecology, 2021, 157: 103768. doi: 10.1016/j.apsoil.2020.103768 [23] XU X J, CHEN C, GUAN X, et al. Performance and microbial community analysis of a microaerophilic sulfate and nitrate co-reduction system[J]. Chemical Engineering Journal, 2017, 330: 63-70. doi: 10.1016/j.cej.2017.07.136 [24] NOBU M K, NARIHIRO T, RINKE C, et al. Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor[J]. The ISME Journal, 2015, 9(8): 1710-1722. doi: 10.1038/ismej.2014.256 [25] WAKI M, YASUDA T, YOKOYAMA H, et al. Nitrogen removal by co-occurring methane oxidation, denitrification, aerobic ammonium oxidation, and anammox[J]. Applied Microbiology and Biotechnology, 2009, 84(5): 977-985. doi: 10.1007/s00253-009-2112-7 [26] XIAO Y, ZHENG Y, WU S, et al. Bacterial community structure of autotrophic denitrification biocathode by 454 pyrosequencing of the 16S rRNA gene[J]. Microbial Ecology, 2015, 69(3): 492-499. doi: 10.1007/s00248-014-0492-4 [27] LAI C Y, LV P L, DONG Q Y, et al. Bromate and nitrate bioreduction coupled with poly-β-hydroxybutyrate production in a methane-based membrane biofilm reactor[J]. Environmental Science & Technology, 2018, 52(12): 7024-7031. [28] ALRASHED W, LEE J, PARK J, et al. Hypoxic methane oxidation coupled to denitrification in a membrane biofilm[J]. Chemical Engineering Journal, 2018, 348: 745-753. doi: 10.1016/j.cej.2018.04.202 [29] LONG M, ZHOU C, XIA S Q, et al. Concomitant Cr(VI) reduction and Cr(III) precipitation with nitrate in a methane/oxygen-based membrane biofilm reactor[J]. Chemical Engineering Journal, 2017, 315: 58-66. doi: 10.1016/j.cej.2017.01.018 [30] 关亮炯. 我国水污染现状及治理对策[J]. 科技情报开发与经济, 2004, 14(6): 80-81. [31] ZHANG M, YANG Q, ZHANG J H, et al. Enhancement of denitrifying phosphorus removal and microbial community of long-term operation in an anaerobic anoxic oxic-biological contact oxidation system[J]. Journal of Bioscience and Bioengineering, 2016, 122(4): 456-466. doi: 10.1016/j.jbiosc.2016.03.019 -

















 下载:
下载: