-
铝盐类混凝剂在饮用水净化过程中的残余问题一直是混凝工艺面临的技术难题,受到广泛关注。过量摄入的铝会在人体中积累残留,从而形成一些潜在的健康问题,如引发阿尔茨海默症、导致体细胞及生殖细胞发生突变等[1-3]。
在水质净化过程中,经铝盐混凝处理后水中铝的含量会明显升高[4-5]。有研究[6]发现,溶解态铝是饮用水中总残余铝的主要组成部分。混凝剂容易以溶解态的单体或小聚合体的形式与溶解性有机物(DOM)中的羧基、酚基类活性基团络合形成有机络合态的铝,不易通过沉降、过滤等后续工艺去除而残留在饮用水中,该机理被认为是溶解性残余铝的主要成因[7-9]。铁盐也是一种常用混凝剂[10],其价格便宜,矾花易沉降,对DOM也有较好的去除效果[11]。但是铁盐过量使用可能造成出水的感官污染,而且极易腐蚀设备[12]。铁-铝盐混凝剂混合投加是在保证水质的同时,减少铝盐类混凝剂投加量和残余铝的常用工艺。
混凝过程非常复杂,不同种类的混凝剂对DOM中不同组分的去除能力存在差异,影响其分子量分布及特性[10]。此外,在铁-铝盐混合投加时,Fe3+离子同样可以与DOM中的羧基、酚基络合形成有机态的铁,Fe3+、Al3之间会形成竞争作用相互影响[13-15]。由于缺乏有效的表征手段,对混凝环境条件下DOM特性的变化及其与Al3+、Fe3+之间相互作用机理认识不清,影响了工程应用。
最近的研究发现,可以用紫外-可见光谱法定量表征天然环境条件下DOM的水化学特性变化规律,结合非理想竞争吸附模型(NICA),可以从酸碱滴定过程中紫外-可见差分光谱参数(如D(lnA400))的变化获得DOM中羧基、酚基类官能团数量、化学反应平衡常数等定量信息[16-18]。
本研究通过中试实验,在考察使用聚合氯化铝(PACl)和三氯化铁(FeCl3)不同混凝剂投加方案下的水质净化效果及在铝残余量的基础上,利用紫外-可见光谱法,揭示DOM的去除特性及残余铝形成与调控机制,以期为实际工程生产中残余铝的控制提供参考。
全文HTML
-
中试系统由混合池、混凝反应池、斜管沉淀池和石英砂滤池组成,设计流量为1 m3·h−1。原水通过增压泵被提升至混合池,与通过计量泵投加的混凝剂混合,混合池转速为300 r·min−1,停留时间为5 min。混合后原水进入混凝反应池中,池内设三级搅拌桨,转速分别为250、150、50 r·min−1,每级停留时间6.5 min。混凝出水进入斜管沉淀池,停留时间为40 min,然后进入石英砂滤池,滤池设计流速为10 m·h−1。
实验原水为中国北方某大型水厂水源。实验期间原水水质:浊度为1.314~1.865 NTU,pH为 8.10~8.38,DOC为 2.46~2.82 mg·L−1,UV254为 0.026 1~0.030 7 cm−1,溶解性残余铝为0 μg·L−1,溶解性残余铁为0 μg·L−1。
实验重点考察原水和滤池出水水质。浊度和pH分别采用浊度计(Hach turbidimeter 2100P)和pH计(Mettler Toledo S210 Seven Compact)测量。水样经过孔径为0.45 μm的微滤膜过滤后,溶解性有机碳(DOC)由碳分析仪(Shimadzu TOC-Vcsh)来测定;溶解性铝、铁浓度用电感耦合等离子体质谱仪(Element X Series,Thermo Scientific)测定;紫外-可见分光光谱采用紫外-可见分光光度计(Hitachi U-3900)测定。
-
除非特别说明,所用化学品均为试剂级。所有溶液均使用Milli-Q水(18.2 MU,Millipore Corp,MA,USA)制备。混凝剂聚合氯化铝(PACl,Al2O3含量约为10%)、三氯化铁(FeCl3,总Fe含量约为40%)均为商用产品,由当地一家工厂(中国北京万水净水剂有限公司)提供。
-
100 mL水样经孔径为0.45 μm的膜过滤去除水样中颗粒物后,利用阳离子交换树脂去除水样中的Ca2+、Mg2+等背景金属阳离子。利用HClO4将水样酸化至pH 3,室温保存30 min。向样品中逐步滴加0.1 mol·L−1的NaOH,使水样pH缓慢升高,在pH为 3~11内,以0.5个pH为间隔(误差为 −0.05~0.05),取少量样品进行紫外-可见光光谱扫描,测量波长为200~600 nm。
-
DOM的紫外-可见光谱滴定数据分析采用文献中的方法[17-18]。利用式(1)和式(2)分别计算线性差分光谱和差分对数转换光谱参数。
式中:D(Aλ)为线性差分光谱;D(lnAλ)为差分光谱对数转换光谱参数;Aλ, i为选定实验条件(i)在波长λ处测量的DOM吸光度;Aλ, ref为参考光谱在波长λ处测量的DOM吸光度(通常为pH=3时)。
光谱参数DlnA400通过式(2)计算后与修正的NICA模型结合,利用式(3)拟合定量DOM水解(脱质子)引起的光谱参数的变化规律。
式中:D(lnALAS)和D(lnAHAS)分别对应在波长λ处羧基类和酚基类官能团水解(脱质子)引起的吸光度最大变化,这2类官能团分别为低亲和质子活性位点(LAS)以及高亲和质子活性位点(HAS);pKHAS和pKLAS为水解平衡常数;mLAS和mHAS为平衡常数的分布宽度指数[16-19]。值得指出的是,D(lnALAS)和D(lnAHAS)的数值代表单位水体中1 mg的DOC所含活性官能团的数量[16-19]。
1.1. 中试实验
1.2. 试剂与药品
1.3. 光谱滴定方法
1.4. 数据处理
-
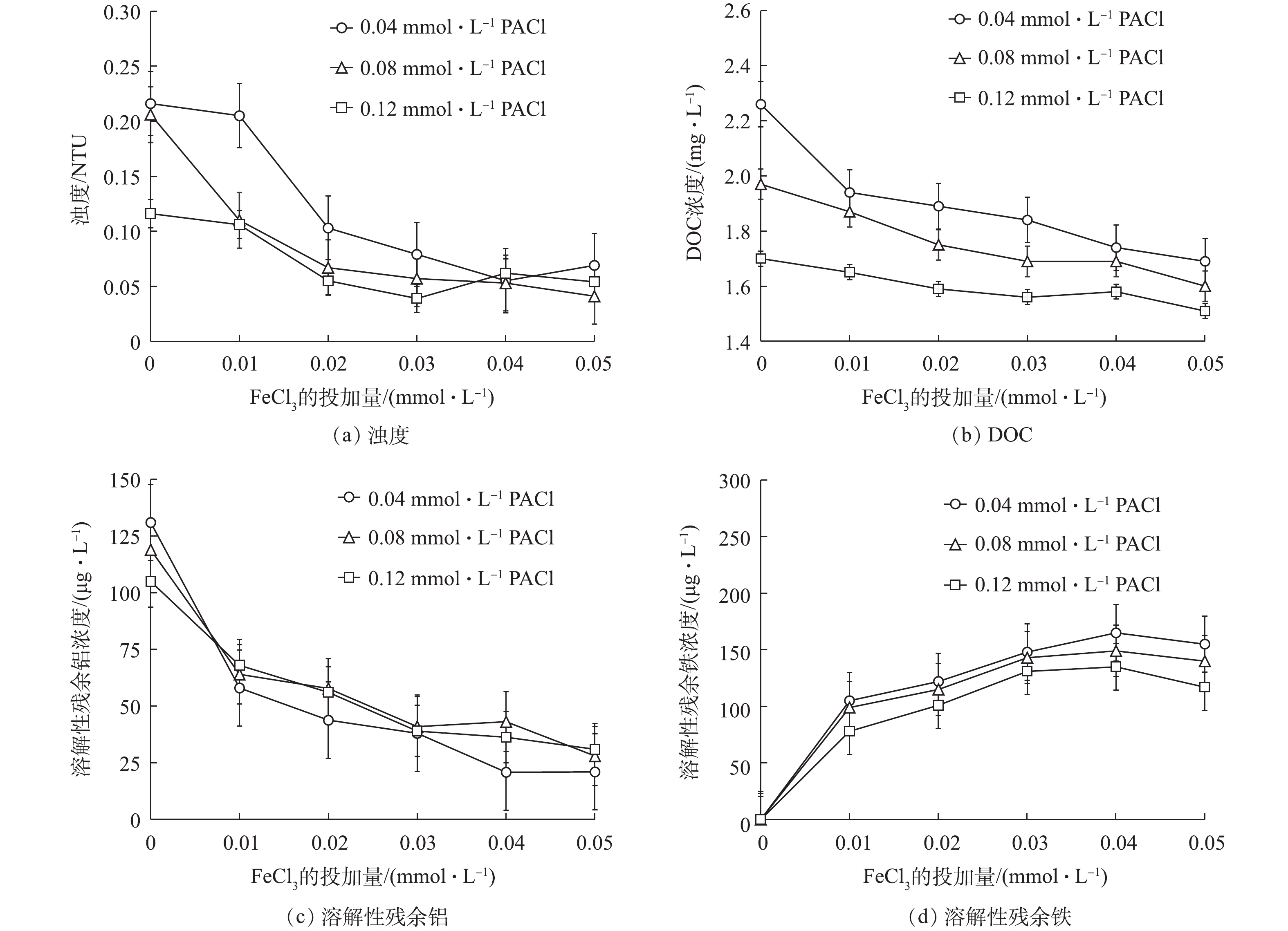
本研究探讨了单独使用混凝剂PACl在低、中、高3种剂量(0.04、0.08、0.12 mmol·L−1,以Al3+计)时,以及同时投加0.01、0.02、0.03、0.04、0.05 mmol·L−1 (以Fe3+计,下同) FeCl3进行铁-铝盐混合混凝时的水质变化。滤池出水的浊度、DOC、溶解性残余铝和铁浓度变化情况如图1所示。
当单独使用PACl时,DOC和浊度的去除效果随着铝盐投加量增加逐渐增加,在低、中、高3种剂量下,DOC去除率逐渐升高,分别为15.0%、25.4%、36.6% (原水DOC为2.66 mg·L−1),浊度分别为0.216、0.206、0.115 NTU (原水浊度为1.580 NTU);溶解性残余铝浓度轻微降低,分别为131、119、105 μg·L−1。
同时投加PACl和FeCl3时,DOC的去除效果会进一步提升,浊度去除效果有轻微改善,溶解性残余铝的浓度会随FeCl3投加量增加显著降低。由图1(a)和图1(b)可知,当铁-铝盐混合投加使用时,0.08 mmol·L−1 PACl + 0.03 mmol·L−1 FeCl3、0.04 mmol·L−1 PACl + 0.05 mmol·L−1 FeCl3这2种投加方案下的浊度和DOC去除效果均达到或超过单独使用高投加量的PACl时的水平。由图1(c)和图1(d)可知,在将混凝剂混合投加后,溶解性残余铝浓度显著降低,由PACl单独混凝的滤池出水中残余铝为105 μg·L−1分别降低到41 μg·L−1和21 μg·L−1;在不使用混凝剂FeCl3时,滤池出水中溶解性铁含量很低(<5 μg·L−1),当投加混凝剂FeCl3后溶解性铁浓度显著升高。
-
为了探究DOM的特性与溶解性残余铝的内在联系,对水样进行紫外-可见光谱酸碱滴定。利用式(2)计算光谱参数D(lnA400),结合修正后的NICA模型拟合定量DOM水解(脱质子)引起的D(lnA400)光谱参数的变化规律,部分结果如图2所示。
不同混凝剂投加方案下的滤池出水对应的NICA模型拟合参数和DOM所含官能团计算结果如表1所示。通常使用CDOC·D(lnALAS)、CDOC·D(lnAHAS)的值分别计算单位水体中DOM含有的羧基类、酚基类官能团总量[16-18]。
由表1可知,在单独使用铝盐混凝的过程中,随着PACl投加量的增加,活性官能团总量相较于原水不断减少。在高PACl投加量下,羧基类官能团总量累计减少约13.3%,酚基类官能团总量累计减少9.4%。
同时投加FeCl3会使DOM中活性官能团总量进一步减少,其中羧基类官能团总量变化规律并不明显,酚基类官能团总量下降显著。在FeCl3的投加量为0.05 mmol·L−1时,低、中、高PACl投加量下酚基类官能团总量较未投加FeCl3时分别减少约31.4%、35.3%、38.4%。
将不同混凝剂投加条件下滤池出水中溶解性残余铝浓度与DOM中活性官能团总量作比较,结果如图3所示。滤池出水中的溶解性残余铝浓度与DOM中活性官能团总量相关(
R2总 =0.73),其中与酚基类官能团总量关系尤为密切(R2酚基 =0.85)。结合表1结果,可以推测,正是由于FeCl3的加入改变了DOM特性及活性官能团总量,尤其是大幅减少了酚基类活性官能团总量,从而使溶解性残余铝浓度降低。 -
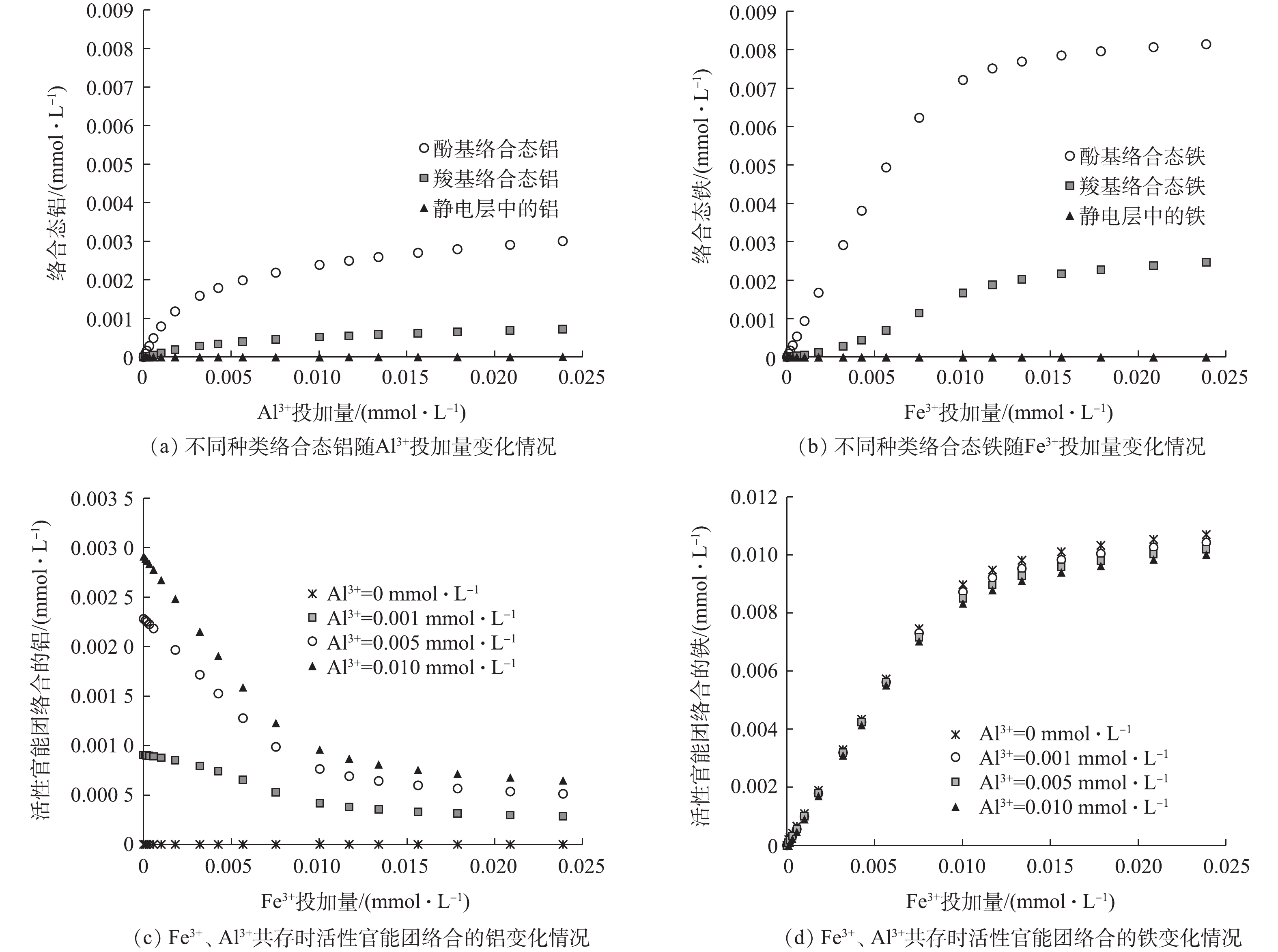
为了进一步验证上述推测,探究Al3+与Fe3+和DOM络合时的竞争机制,分别用检测的铝、铁浓度和活性官能团总量之比表示不同混凝剂使用条件下产水中单位数量活性官能团络合的铝、铁的浓度,结果如图4所示。可以看出,单位数量活性官能团络合的铁浓度随着FeCl3的加入大幅上升,而单位数量活性官能团络合的铝浓度均随着FeCl3的加入显著下降,FeCl3强烈抑制了Al3+与DOM的络合,FeCl3剂量越高,抑制作用越明显。
由于目前尚没有方法能直接测量混凝条件下有机络合态铝、铁的浓度,使用水化学模型软件Visual Minteq 3.1(https://vminteq.lwr.kth.se/download/),基于NICA-Donnan模型[20]预测Fe3+、Al3+离子与腐殖酸(humic, HA)的络合分配机制。不同Al3+、Fe3+投加量下活性官能团络合的铝、铁浓度的模拟计算结果如图5所示。由于实际水源中DOM与HA的特性存在显著差异,而且混凝过程中Fe3+、Al3+离子易水解产生沉淀,模型计算时参数设置与实际混凝环境存在较大差异,仅提供了较低浓度条件下的计算数据作为参考。该结果也表明,DOM与Al3+、Fe3+的络合物中,酚基络合态的铝、铁是主要组成部分,其浓度远高于羧基络合态和静电层中的铝和铁,占总络合态铝、铁的比率均超过了77%;DOM对Fe3+的络合能力要显著强于Al3+,相同Fe3+、Al3+总浓度下Fe3+络合量是Al3+的2倍以上;在Fe3+、Al3+共存时,Fe3+强烈抑制了Al3+与DOM的络合过程,Fe3+投加量越大,抑制作用越明显,而Al3+对Fe3+与DOM络合过程影响较轻微。模型计算结果与实验结果基本一致。
2.1. 不同混凝剂投加量下的水处理效果
2.2. FeCl3对DOM特性的影响
2.3. Fe3+与Al3+竞争机制
-
1)铁-铝盐混凝剂混合投加工艺可以显著降低滤池出水的溶解性残余铝含量,同时能在减少混凝剂总使用量的情况下保证水处理效能。
2)溶解性残余铝与DOM的特性及活性官能团含量密切相关,尤其是酚基类官能团总量。在铁-铝盐混合投加时,FeCl3能显著提高酚基类官能团的去除效率,降低了滤池出水中溶解性残余铝的浓度。
3) Fe3+与DOM的络合能力显著强于Al3+。投加铁盐后,通过竞争络合位点,明显抑制了络合态有机铝的形成,而Al3+对Fe3与+DOM之间的络合过程影响轻微。




 下载:
下载: