餐厨垃圾是指餐馆、饭店、单位食堂等的饮食剩余物,以及后厨的果蔬、肉食、油脂、面点等的加工过程废弃物。近年来,我国的餐厨垃圾产生量每年以超过10%的速度持续增长[1-2]。若不经妥善处理,餐厨垃圾会腐烂变质,进而污染土壤和水体,同时散发恶臭气体、传播疾病,危害人群健康[3]。传统的餐厨垃圾处理方式如填埋、焚烧等处理过程中可能会导致渗滤液中的高浓度有机污染物进入土壤和地下水,再加上填埋产生的甲烷、焚烧产生的二噁英等也可能进入空气,因此,传统的处理方式存在二次污染的可能[4]。利用厌氧发酵等生化方法对餐厨垃圾进行无害化和资源化利用是绿色有效的[5]。将餐厨垃圾固液分离后,固相经一定的处理可制得生物蛋白,液相经过定向生物转化、高效分离加以利用。该过程可获得的产品包括乳酸、乙醇、丁醇、己酸、氢气、沼气等。这些产物为增值化学品,具有较高的利用价值[6]。
乳酸是三大有机酸之一,已广泛应用于酿酒、医药、食品、化妆品、卷烟、制革等领域。此外,乳酸还是一种重要的生物基平台化合物,可作为生产原料制造其他化学品,如聚乳酸、丙烯酸、丙酸、2,3-戊二酮、丙酮酸、丙烯、乳酸酯(绿色环保溶剂)、乳酸盐等[7],具有广阔的应用前景。以乳酸为主要原料聚合生成的聚乳酸,是一种新型的生物降解材料,为重要的塑料替代品。随着各国对抗塑料污染、鼓励开发生物降解塑料等政策的实施,聚乳酸市场需求快速增长,并成为乳酸的第一大应用领域。以乳酸为原料制造聚乳酸,其市场占比约为37.5%。
由于化学法生产乳酸往往产生DL-乳酸的外消旋混合物,因此,目前在工业上应用较多的是发酵法,约占乳酸生产的90%以上[8]。但微生物发酵生产乳酸存在3个主要瓶颈:一是适应复杂底物的优势乳酸工程菌的选育;二是发酵底物和营养物质的高成本;三是下游工艺(分离和纯化步骤)的复杂和高成本。
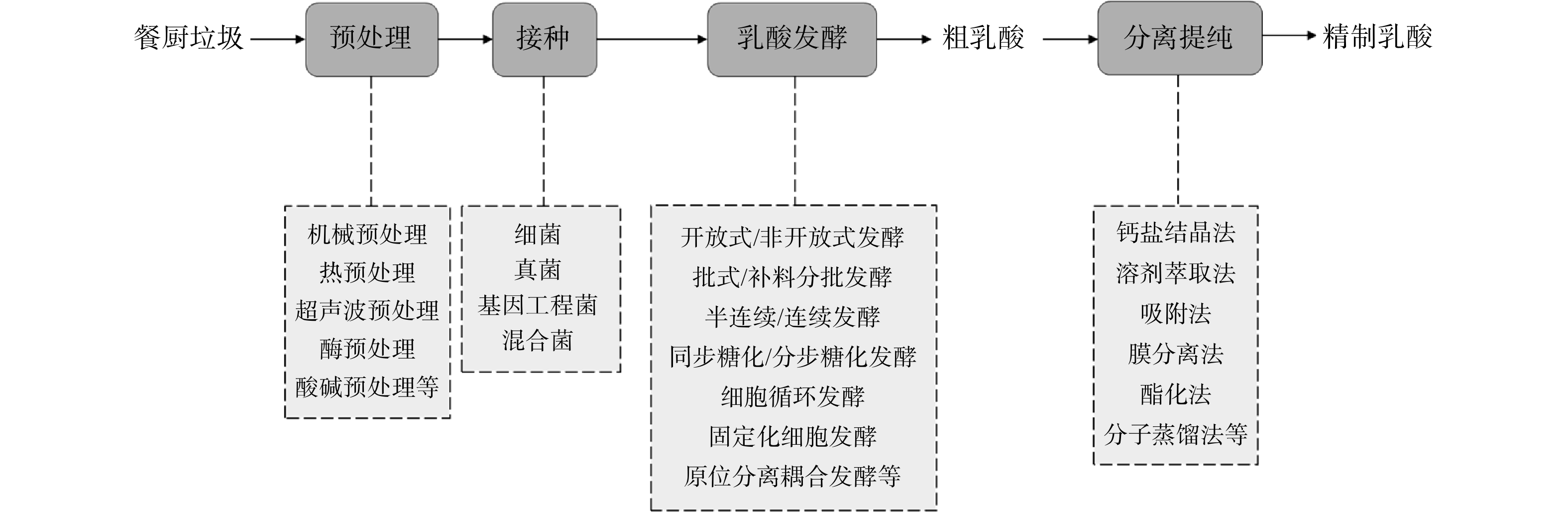
餐厨垃圾有机物含量高,含有丰富的氮、磷及微量元素,是很好的生物发酵培养基。在利用餐厨垃圾进行乳酸发酵前往往需要先经过预处理过程,以便于微生物更好地利用底物。生物发酵产乳酸的流程图如图1所示。
本文从餐厨垃圾乳酸发酵技术的高产菌株选育、与其他废物协同发酵及缓解产物抑制的原位分离耦合发酵3个方面,对国内外相关研究进行综述,并梳理餐厨垃圾乳酸发酵领域的新进展,以期为解决该领域存在的瓶颈问题、促进其产业化提供参考。
1. 乳酸发酵用微生物
1.1 细菌
乳酸菌(lactic acid bacteria,LAB)是最常见且在工业中使用最多的细菌,为革兰氏阳性菌,是无芽孢的球菌或棒状菌。LAB利用单糖或二糖来发酵产乳酸,其适宜的生长温度为5~45 ℃,pH为5.5~6.5。氨基酸、核苷酸、矿物质、维生素、脂肪、碳水化合物等均可作为其营养来源[9]。另外,LAB分布广泛,种类众多,从各种物质中分离和筛选出的野生型LAB是获得可发酵和遗传稳定菌株的最主要来源,已有多种菌株用于乳酸生产中[10]。然而,受自身和发酵环境所限制,野生乳酸菌的发酵能力也有限,因此,分离得到高产量的野生乳酸菌是LAB研究的主要内容。
乳酸发酵可分为同型发酵、异型发酵和双岐发酵3种途径,其反应式分别如式(1)~(3)所示。
同型发酵是通过糖酵解途径(embden meyerhof pathway,EMP)得到乳酸,该过程产物纯度高,糖产乳酸的理论转化率为100%。然而,由于该过程存在微生物的其他生理活动,如细胞生长、蛋白质合成等,故实际转化率会低于理论转化率。一般认为,实际转化率达到80%以上者,即为同型乳酸发酵。异型发酵和双岐发酵途径的理论转化率仅为50%。
从环境中分离得到乳酸菌是相对快捷的方法。WANG等[11]利用自行分离得到能耐高温的TY50乳酸菌,可在温度为45 ℃,pH为5.5~6.0的条件下发酵餐厨垃圾产乳酸。该课题组还利用TD46乳酸菌对餐厨垃圾进行不灭菌发酵,产生的乳酸浓度比未接种乳酸菌的对照组提高了75.1%[12]。KWAN等[13]先用泡盛曲霉或米曲霉对餐厨垃圾进行水解,之后利用干酪乳杆菌(Shirota)对餐厨垃圾发酵产乳酸,乳酸产率可达0.27 g·g−1(以单位干重餐厨垃圾的乳酸产量计)。
芽孢杆菌是革兰氏阴性、兼性厌氧菌,也可用于生产乳酸。PLEISSNER等[14]利用食品废物和烘焙废物的混合物制乳酸,将用真菌酶水解后再进行脱脂的产物作为凝结芽孢杆菌生产乳酸的氮源,效果较好,并降低了成本。SAKAI等[15]分离出一种地衣芽孢杆菌TY7,在50 ℃下对餐厨垃圾进行不灭菌发酵,可产生40 g·L−1的L-乳酸,其光学纯度为97%,生产率为2.5 g·(L·h)−1。该课题组还利用嗜热芽孢杆菌,在55 ℃、pH为5.5的情况下发酵5 d,积累了86 g·L−1的乳酸,光学纯度为97%[16]。高温发酵环境会使该芽孢杆菌在与餐厨垃圾中土著菌群的竞争中处于优势,又由于其对pH相对敏感,故利用该芽孢杆菌发酵的关键就是控制温度的同时也要控制pH,以保证产生高质量的L-乳酸。
1.2 真菌
真菌也可用以生产乳酸。与细菌相比,部分真菌可直接利用淀粉等大分子底物,而不需先进行糖化。其中,米根霉的相关应用较多,它对营养需求相对较低,对环境的适应性很强,可利用底物很广。此外,米根霉丝状结构富含蛋白质,发酵后的残渣可作为粗蛋白原料再利用。液态发酵时,米根霉易沉降,有利于后续菌液分离。然而,菌丝体的形态和氧气供应会影响乳酸的生产率[10]。周群等[17]采用米根霉AS3.819对厨余垃圾进行不灭菌发酵,研究了pH对乳酸生产的影响。该研究发现,发酵液中还原糖浓度呈先上升后下降的趋势,pH为8时乳酸产量最大,达到60 g·L−1,60 h内乳酸的产生速率为1 g·(L·h)−1。L-乳酸为主要的异构体产物,其乳酸光学纯度可达99%。利用真菌来进行餐厨垃圾乳酸发酵的研究相对较少,所能利用的菌种也较为局限,因此,在分离、筛选高效真菌菌株方面还有很多内容可研究。以上内容提到的细菌和真菌产乳酸的研究成果汇总见表1。
1.3 基因工程菌
为满足商业的要求,可利用基因工程技术改造发酵用乳酸菌株,以使其光学纯度更高、营养物质需求更少、乳酸产量和生产率更高,并达到提高其底物特异性、消除质粒及抗生素标记[10]等目的。
雷森林[18]利用基因工程的方法改造并研究了毕赤酵母对餐厨垃圾的发酵作用,分别克隆了凝结芽孢杆菌的乳酸脱氢酶基因、米曲霉的淀粉酶基因、黑曲霉的糖化酶基因,然后将这3种基因同时表达在1株毕赤酵母中。利用此菌株,在不外加商业淀粉酶的情况下,最高乳酸产量是16.8 g·L−1,为理论产值的48%。常被用于改造的菌种中,有乳酸菌、大肠杆菌、酵母菌、米根霉及一些藻类等[19]。将菌种本不具备的相关基因通过基因工程的手段进行表达,可减少所用相关酶的量,从而降低成本。但目前基因工程菌种对工业生产条件和环境的适应能力不强,这也限制了其大规模应用。因此,工程菌的进一步研究方向为改善微生物的代谢能力,提高工程菌的产酸效率,尽量降低副产物,增加工程菌对环境的适应性及对副产物的耐受性,从而进一步降低发酵成本。
1.4 混合菌
单一菌种会受到自身条件及发酵过程中的底物、产物的限制,因此,利用混合菌共发酵来提高底物利用率和乳酸产率也逐渐被关注。KIM等[20]利用以乳酸杆菌为主要菌种的土著混合菌对餐厨垃圾进行乳酸发酵时发现,在50 ℃时发酵效果最好,当水力停留时间为1 d时,乳酸质量浓度可达40 g·L−1。陈佳奇[21]将几种确定的菌种进行混合发酵研究,发现可利用解淀粉芽孢杆菌和德氏乳杆菌对餐厨垃圾进行底物不灭菌混合发酵,当发酵温度为45 ℃、接种间隔为1.5 h、初始发酵pH为6.5时,乳酸产率最高,可达0.351 g·g−1(以每克总固质量所产乳酸质量计)。
餐厨垃圾中含各种各样的土著菌种,如产酶菌、乳酸菌等。利用土著菌群进行发酵亦可取得较好效果。ZHANG等[22]利用餐厨垃圾中的土著菌群进行发酵,并研究了乳酸的纯度和微生物菌群。结果表明:L-乳酸是主要异构体产物,且pH在酸性或碱性条件下光学纯度比中性更高;菌群中乳酸菌和梭菌属占主要部分。WANG等[23]利用分离出的2种野生乳酸菌株TH165和TD175对餐厨垃圾进行发酵,可产生33.80 g·L−1的乳酸,比不接种的对照组高36.9%。黄林丽等[24]分别以调酸发酵和泡菜水发酵菌种作为混合菌种,在灭菌和未灭菌的情况下分别进行餐厨垃圾的发酵。结果表明,未灭菌组的乳酸含量均高于灭菌组,且金黄色葡萄球菌和沙门氏菌没被检出。TASHIRO等[25]使用海洋动物资源堆肥中的微生物群对餐厨垃圾进行分批发酵。通过设置一系列温度梯度进行发酵研究发现,在50 ℃时乳酸产量为34.5 g·L−1。
用产酶菌代替酶制剂,可大大降低原料的预处理成本[26],特别是将糖化酶的生产、酶解糖化和乳酸发酵在同一反应器内进行。该过程可节省反应器体积,降低购酶费用和投资成本,是一个新的发展方向。例如,可利用一些糖化酶生产菌与乳酸生产菌混合培养,直接转化淀粉等碳水化合物生产乳酸,如黑曲霉(糖化酶生产菌)和米根霉(乳酸生产菌)同为好氧菌,有望能在同一反应器中混合培养产乳酸,可增加底物糖的浓度,实现同步糖化发酵,从而增加发酵效率。
虽然混合菌进行发酵效果更好,但需要考虑各菌种的最适生长条件、功能及协同性,需开发具有共生作用和协同作用的酶生产菌和乳酸生产菌。因此,选择合适的菌种搭配、进行不同菌群的发酵过程研究是当前使用混合菌发酵的重点。
2. 餐厨垃圾与其他生物质废物混合发酵
利用单一餐厨垃圾为底物时,虽然其营养丰富,但所含淀粉和葡萄糖等极易降解,碳源消耗速度较快,乳酸迅速生成积累使发酵系统易酸化而发生产物抑制进而影响乳酸产量。因此,将餐厨垃圾与木质纤维素、活性污泥等生物质废物进行混合发酵,有利于发酵底物营养元素的优势互补,并缓解餐厨垃圾易酸化的问题。
2.1 餐厨垃圾与木质纤维类废物的混合发酵
利用木质纤维素类废物发酵产乳酸需外加氮源和生长因子(如酵母膏),而且木质纤维素本身结构复杂,不易被微生物降解,其预处理成本较高。将餐厨垃圾与木质纤维类废物混合发酵,可调节底物碳氮比,使底物营养均衡,也可延缓系统的酸化,从而促进乳酸发酵过程。
ZHENG等[27]分别将苦参药渣(含纤维素28.3%、半纤维素23.6%、木质素14.6%)与餐厨垃圾以不同混合比(以干重计)(0∶1,1∶0.5,1∶1,1∶1.5,1∶2,1∶0)混合后,接入种子培养液,再加入酶制剂,并在35 ℃、140 r·min−1的恒温摇床中进行乳酸发酵。结果表明,混合物料共发酵生成的乳酸浓度均比仅餐厨垃圾或仅苦参药渣发酵的乳酸浓度高;当苦参药渣与餐厨垃圾质量比为1∶1.5时,得到的L-乳酸浓度最高,可达48.4 g·L−1,说明物料之间具有明显协同效应。此混合比条件下的糖酸转化率为0.904 g·g−1,分别是相同质量单独原料分别产乳酸量的6倍(苦参药渣)或1.8倍(餐厨垃圾)以上。WANG等[28]同样研究了两种原料的协同作用,将碱预处理后的苦参残渣与餐厨垃圾以1∶1.5的比例混合,并进行同步糖化发酵,获得乳酸浓度可达67.5 g·L−1。同时还发现,将碱预处理液间隔24 h添加到发酵体系并调节pH,可提高乳酸产量;特别是当碱预处理液回用50%的乳酸产量时,比不回用的对照组增加了34.3%。这是由于碱预处理液的间歇式添加使得发酵体系pH更稳定,可减轻餐厨垃圾酸化;又因为碱预处理液中含有某些抑制性物质,抑制了产乙醇酵母的生长但不影响乳酸菌生长和代谢,使得更多的可发酵糖转化为乳酸。而餐厨垃圾为整个体系提供了丰富的氮源,从而降低了药渣发酵的额外氮源添加量。
汪群慧等[29]将菌糠添加到餐厨垃圾中发酵产乳酸。菌糠是栽培并采收食用菇后的剩余培养基或培养料,含有氨基酸、微量元素及水解效率高的微生物等。当10 g菌糠与50 g餐厨垃圾共发酵时,乳酸产率(以每克底物(干重)产乳酸的质量计)为0.30 g·g−1,比仅用餐厨垃圾时的产率提高了2.33倍。后续还需研究餐厨垃圾与菌糠在不同混合比条件下的共发酵系统中碳氮营养互补、多组分协同降解的机理,探究菌糠中所含高木质纤维素物料、pH调理性物料(石灰粉或石膏粉等)对缓解系统酸化的作用,以及菌糠中多种微量元素(如Zn、Se、Mn和Fe等)、生物活性物质(纤维素酶、半纤维素酶、蛋白酶等)对餐厨垃圾-菌糠共发酵产乳酸的促进作用。菌糠中含有很多细菌和真菌,当它与餐厨垃圾共发酵时,存在与乳酸发酵菌群竞争底物的问题。同时,氧化乳酸的微生物、底物投加方式、发酵系统温度和pH调控方式等必将影响该发酵过程核心功能微生物群落的变化。如何抑制与乳酸菌竞争的菌群,从而保证产乳酸发酵菌群的优势生长,是餐厨垃圾-菌糠混合发酵系统成功构建的关键。
2.2 餐厨垃圾与活性污泥混合发酵
我国餐厨垃圾的碳氮比(C/N)一般为10~30,而活性污泥的C/N较低,约为6~16[30]。由于污泥中微生物种类多,故将二者进行混合发酵并进行组分性质互补研究。XUE等[31]以活性污泥和餐厨垃圾为混合底物,利用混合微生物菌群进行阴极电发酵产乳酸,在阴极上施加了−100 mV电压来刺激发酵过程,得到乳酸生产率为0.657 8 g·(L·h)−1,比没有外部电压的另一组提高了4.73倍。这可能是由于施加电压增加了某些微生物的生命活动或者酶活。
ZHANG等[32]研究了pH和温度对餐厨垃圾和活性污泥混合发酵过程的乳酸浓度和光学纯度变化的影响,并通过响应面(response surface methodology,RSM)分析验证多因素交互作用效果。结果表明,随着温度的升高,乳酸生产的最佳pH会降低;在发酵温度为35 ℃、初始底物(以COD计)质量浓度 25.51 g ·L−1时,最佳pH为9;若发酵温度50 ℃、初始底物(以COD计)质量浓度21.01 g·L−1时,最适pH为7。最高速率均为活性污泥和餐厨垃圾共发酵3 d后获得,且产乳酸菌的最佳生存条件与高速率生产条件相一致。
XU等[33]以餐厨垃圾和活性污泥作为底物,利用重复分批发酵方式进行乳酸发酵,乳酸产率可达(以每克有机物(单位体积底物COD)对应产生乳酸的质量计)0.72 g·g−1,乳酸生产率可达0.53 g·(L·h)−1。与分批反应器相比,重复分批反应器中关键水解酶活性得以增加。废活性污泥含有丰富的微生物群落,虽可以提高乳酸发酵过程中的水解酸化作用,但也会不可避免引入消耗乳酸及产生挥发性脂肪酸(volatile fatty acids,VFA)的菌种。因此,在将餐厨垃圾和废活性污泥混合发酵中,需严格控制温度和pH,以避免产生过多的VFA及L-乳酸产量的下降。LI等[34]在餐厨垃圾发酵产乳酸时添加活性污泥,并开发了碱性发酵技术。结果表明,添加污泥及间歇性碱性发酵不仅明显改善了水解步骤,还抑制了乳酸消耗和D-乳酸的产生,L-乳酸产率以每克有机物(以单位体积底物COD对应产生乳酸的质量计)为0.52 g·g −1,光学纯度为100%。
3. 原位分离耦合乳酸发酵
在乳酸发酵过程中,乳酸不断积累会造成体系的pH下降,导致发酵液酸化并使发酵过程减慢、底物利用率低,进而影响乳酸产率及产量。因此,需要采用合适的方法边发酵边将乳酸分离出来(即原位分离乳酸发酵技术),以降低乳酸的抑制作用,提高发酵效率。表2所示的3种原位分离乳酸发酵技术已受到较多关注和研究。
3.1 原位萃取发酵技术
原位乳酸萃取发酵也称为萃取分离耦合乳酸发酵,即在发酵过程中利用有机溶剂连续萃取发酵产物,以消除乳酸抑制。以液体为萃取剂时,如含有目标产物的原料也是液体,则称为液液萃取。根据萃取剂的不同,又分为溶剂萃取、双水相萃取、超临界流体萃取、液膜萃取等。萃取剂的基本要求即分配系数高,自身消耗少,对发酵体系的毒害影响小。十二烷醇、油醇、叔胺Alamine-336等是常用的萃取剂,萃取剂与细胞直接接触会产生毒害作用。常用的减轻溶剂毒性的方法有:使用膜将溶剂与细胞分开(渗透萃取);细胞固定化;在固定化载体中包埋豆油等植物油。陈敏等[35]使用三辛胺和油醇混合溶剂,利用固定化细胞方法进行了萃取发酵,经过24 h发酵后,乳酸产量比对照组提高了60%,且可有效提取乳酸,同时对乳酸菌毒性也较小。WASEWAR等[36]研究了反应萃取在原位分离中的使用,比较了辛醇、甲基异丁酮(methylisobutylketone,MIBK)和癸醇的添加对从一种叔胺(Alamine 336)中提取乳酸的效果。结果表明,辛醇的提取效果最好,并且进行半连续操作更便捷,且不消耗额外试剂,不产生大量废物。
相较于传统有机相-水相的溶剂萃取,双水相萃取是全新的替代方法。这两相大多数情况下由水与非挥发性成分组成,可避免挥发性有机成分的使用。除此之外,该萃取方法还具备如下优势:含水量高(70%~90%)适宜提取水溶性的蛋白质、酶等生物活性物质,且不易引起蛋白质的变性失活;不存在有机溶剂残留问题;易于放大,各种参数可按比例放大而产物收率并不降低。这是其他分离技术无法比拟的。KWON等[37]探究了聚合阳离子、聚乙基亚胺(polyethylimine,PEI)和不带电聚合物(羟乙基)纤维素(hydroxyethyl cellulose,HEC)组成的双水相体系萃取乳酸发酵的潜力。在无pH控制的情况下,乳酸乳球菌在PEI/ HEC两相发酵体系中添加质量分数为20 g·L−1的葡萄糖进行分批发酵,与PEI或HEC单相发酵体系相比,乳酸产量和细胞生物膜分别提高了3~4倍,乳酸优先分布到富含PEI的底相中。
萃取剂本身的性质、对乳酸的分配系数及可能的毒性,都是制约其大规模应用于发酵生产的影响因素。为了能将萃取法更多应用于原位发酵提取中,需进一步开发高效、绿色、成本更低的萃取剂。
3.2 原位吸附发酵技术
原位乳酸吸附发酵也称吸附分离耦合乳酸发酵,即利用特定固体吸附剂(如树脂、活性炭等)在发酵过程中通过吸附分离将乳酸从发酵液中移除。BONK等[38]在餐厨垃圾发酵产乳酸的同时,采用活性炭进行原位分离:实验前将活性炭洗涤筛分;实验中直接与发酵液接触;吸附后利用丙酮来进行脱附。该项研究结果表明,即使在含有颗粒物的餐厨垃圾发酵液中,也可以用活性炭进行原位分离,并没有发生明显的孔隙堵塞导致效率下降的情况。该研究实现了更高的产率,并提高了乳酸的纯度。然而,在实际应用中,活性炭和丙酮的循环使用次数还有待进一步研究。
由于活性炭易吸附饱和,不但吸附乳酸还会吸附糖等其他物质,因此,从工业化生产的角度来对比,离子交换树脂法以选择性强、交换(吸附)容量大、操作简单、易于自动化控制等优点具有较强的竞争力。ATAEI等[39]使用Amberlite树脂(IRA-400,Cl−)在自动控制pH的条件下从发酵液中原位分离乳酸。在pH=6.1,温度为37 ℃时生产率最大,使用该原位分离系统发酵38 h后,乳酸的最大质量浓度为37.4 g·L−1,其乳酸生产率是不采用原位分离系统的5倍。GARRETT等[40]使用Amberlite™ IRA-67弱碱树脂进行乳酸提取,将乳酸质量浓度控制在20 g·L−1以内;该系统产率是常规分批补料发酵的1.3倍;对该树脂进行表征,其吸附曲线与Langmuir等温线相吻合,且发酵108 d后,树脂仍具有较好的稳定性,可有效原位分离乳酸。ZHANG等[41]利用凝结芽孢杆菌进行乳酸发酵,利用10种离子交换树脂进行原位分离,发现其中的335树脂效果最好。WANG等[42]利用树脂吸附回收乳酸,发酵液循环回发酵罐,使乳酸产量提高了23%,达到183.4 g·L−1。
吸附剂的高吸附容量有利于产物的及时分离,可明显降低产物抑制作用。开发选择性更好、吸附容量更高、成本更低、寿命更长、机械强度和化学稳定性更好的吸附剂是进一步研究的主要内容。
3.3 原位膜分离发酵技术
将膜组件与常规发酵罐集成在一起,当目标产物达到一定浓度时,及时将其从发酵体系中移除,可减少产物抑制,并使发酵过程保持较高的细胞浓度。应用较多的膜类型有:渗析(扩散排阻)、电渗析(离子排阻)、微滤和超滤(分子排阻)、纳滤等。
JEANTET等[43]研究了生物反应器中乳酸的半连续生产与纳滤膜耦合技术,纳滤膜部分是MSP006239 Prolab 系统配备Nanomax 50 Millipore (R76A) 膜,使用该技术实现的最高生产率为7.1 g·(L·h)−1,乳酸质量浓度为55 g·L−1,发酵44 d后超过99%的膜污染是可逆的,并且通过水冲洗即很容易恢复到初始渗透通量。SIKDER等[44]将纳滤分离和细胞循环发酵产乳酸相结合,发现NF3复合聚酰胺膜可保留94%的糖,乳酸的光学纯度为85.6%。WANG等[45]将中空纤维超滤膜和发酵罐耦合,进行了6批次的发酵,乳酸的产率及细胞密度持续上升,且重复分批发酵的反应稳定性较高,底物可被有效利用,是一种提高产率的有效方法。TALEGHANI等[46]制备了一种纳滤膜TFC-NF,并与另外3种商用纳滤膜进行了膜分离发酵效果比较,结果显示自制膜对目标产物的排斥因子更低。
电渗析技术也是常用的膜分离技术。BOONTAWAN等[47]研究了电渗析法提取发酵乳酸中乳酸浓度的影响,发现临界浓度为80 g·L−1,溶液中的乳酸浓度最高可达185 g·L−1。GAO等[48]使用连续电渗析发酵法(electrodialysis fermentation,EDF)来生产乳酸,进料葡萄糖浓度为175 g·L−1,发酵持续了350 h以上,乳酸最大生产率为8.18 g·(L·h)−1,转化率为71%,乳酸产量是常规EDF的19.5倍、间歇EDF的9.7倍。WANG等[49]将双极膜电渗析和发酵过程耦合进行连续发酵,乳酸回收率达到69.5%,净产量约为1.32 mol·L−1。
原位膜分离发酵中,体系内的细胞浓度比常规发酵中更高,底物利用更快,在减少产物抑制的情况下能保持一定的底物量,可明显提高乳酸产率。但是,膜生物反应器的生物膜控制及系统运行的监控是必须要考虑的因素,而且膜组件成本较高,且在运行中需要注意膜污染问题及膜组件的更换和清洗,因此,开发新型膜组件以降低成本、延长寿命、提高效率是原位膜分离发酵技术推广应用的关键。
3.4 乳酸镁结晶分离耦合乳酸发酵
在温度高于34.5 ℃时,乳酸镁的溶解度低于乳酸钙[50]。WANG等[51]用镁碱(MgO)为中和剂,利用乳酸镁在水溶液中具有较低溶解度的优势,实现了乳酸镁结晶在线移除(或原位分离),降低了产物抑制效应,并采用HCl酸化、异戊醇萃取、浓缩萃余液得到MgCl沉淀,再进行热解得到MgO和HCl,实现了重复利用。含乳酸的萃取液用水反萃取,浓缩后得到乳酸产品,萃取液异戊醇回用。该工艺相比于钙盐分离工艺,没有任何固体或液体废物排放,可减少活性炭脱色、阴阳离子交换等工艺,且操作简单,是一种新的原位分离耦合乳酸发酵模式。
4. 餐厨垃圾乳酸发酵的新进展
4.1 开放式发酵体系中各菌群的协同调控
传统的纯物质乳酸发酵一般为原料灭菌后接入乳酸菌,再在无菌条件下进行发酵,称为非开放式发酵模式。而发酵原料不灭菌接入乳酸菌,无需严格的无菌操作即进行乳酸发酵则称为开放式发酵模式。开放式发酵的工艺简单、节能,可降低乳酸生产成本。
本课题组在前期研究中多次验证了餐厨垃圾采用不灭菌的开放式发酵是可行的,且乳酸菌在发酵过程中成为优势菌株,其乳酸产量达到、甚至超过原料灭菌的非开放式发酵[52-53]。SAKAI等[54]也发现,厨余垃圾在不灭菌的开放式乳酸发酵时,间歇调节pH到中性可有效富集产乳酸细菌群。TANAKA等[55]在开放式发酵过程中通过控制pH、抑制土著微生物生长,进而提高了脱脂麦麸产乳酸的光学纯度。邹慧等[56]将嗜淀粉乳杆菌接种到餐厨垃圾中,比较了开放式与非开放式发酵中乳酸的产量及占比。在发酵96 h后,开放式发酵体系中的乳酸产量比非开放式高54%。姜华[57]关注了开放式发酵过程中的乳酸、总糖、可溶性糖及细菌数量变化,认为餐厨垃圾适合作为乳酸发酵的底物,且相比传统非开放式发酵,开放式发酵明显提高了乳酸产量。其原因可能是:土著菌参与了大分子的水解,将其转化成小分子可溶糖,为乳酸菌提供了丰富的底物。张波等[58]通过对温度和pH的调控,提高开放式发酵系统中目标产物-乳酸的光学纯度,使餐厨垃圾在45℃条件下发酵144 h后,获得的乳酸光学纯度达到97%。
在实际乳酸发酵工程应用中常通过延迟添加碱性缓冲剂的办法,使发酵液呈酸性来抑制开放式发酵体系中的杂菌繁殖。如可在发酵开始后8 h左右分批添加碳酸钙,这样可利用发酵初期产生的少量乳酸来抑制杂菌生长。ZHAO等[59]的研究表明,底物不灭菌,仅添加乳酸菌至餐厨垃圾后放置1~3 d,即会生成高浓度的乳酸,从而使pH下降,病原菌类的金黄色葡萄球菌落和大肠杆菌落数比不加乳酸菌的对照组分别减少了99.9%和99.8%。
针对不灭菌的开放式发酵系统,可采用宏基因组、宏转录组学及荧光定量PCR等现代分子生物学手段,定性与定量解析其微生物群落的变化;需确定复杂底物乳酸发酵过程的关键功能菌群,阐明乳酸菌(尤其是L-乳酸菌)、水解酸化菌、真菌等各菌群之间的相互作用和关系(拮抗、协同和共生作用);需通过复杂底物胞外水解酶、乳酸菌胞内分解代谢关键酶等相关酶活的测定,揭示酶活与系统运行状态之间的动态关联,阐明底物向乳酸转化的机理。
4.2 高细胞密度发酵技术
高细胞密度是一个相对概念,一般认为上限值为150~200 g·L−1,下限值为20~30 g·L−1。然而,对于一些极端微生物或自养微生物,若细胞浓度达到1 g·L−1也可算高细胞密度。广义来讲,凡是细胞密度比较高,以至接近其理论值均可称为高细胞密度。采用分批补料发酵、细胞循环发酵和固定化细胞发酵等培养技术,均可提高细胞密度(即菌体生物量),从而提高体积产率。
分批补料发酵是在微生物的分批发酵中,向反应器内补加一定量的底物,从而使培养液中的底物浓度保持在一定范围内,以满足微生物的生长需要。与批式发酵相比,相同量的底物分多次添加,可明显降低底物抑制作用,但添加底物的量、添加次数及添加频率等都是需要考虑的因素。
细胞循环发酵即将上一批次发酵的部分或全部乳酸菌细胞接种于下一批次。与普通的批次发酵或分批补料发酵相比,细胞循环发酵提高了乳酸菌的生物量,降低了设备投资和生产成本,节省了发酵罐清洁和灭菌及种子液培养的时间和成本,也便于下游分离纯化。
固定化细胞发酵是将细胞通过一定的方法约束在一定空间界限内,限制其自由移动,但保留其催化活性。固定化细胞技术可获得更高的细胞浓度,细胞可重复利用,同时在稀释率较高时也不会发生洗脱现象。此外,其单位容积的产率高,发酵液中菌体含量少,有利于产品的分离纯化。陶静等[60]探索了海藻酸钠、琼脂和明胶-戊二醛3种载体,进行固定化乳酸菌发酵实验。结果表明,使用海藻酸钠更为合理,其最佳反应条件为:海藻酸钠浓度2%,接种量15%,凝胶珠直径为2~2.5 mm,氯化钙浓度为5%。目前,固定化细胞技术在乳酸发酵中使用较少,载体也限制了生化反应所需的扩散作用,但其成本低且可操作性好的特点使固定化细胞乳酸发酵引起了业界越来越多的关注。
4.3 生物强化乳酸发酵技术
为提高乳酸产率,添加一些物质来强化生物发酵过程也是一种新思路。之前较多的研究集中在铁材料促进甲烷发酵,而对发酵过程中产生的有机酸的影响关注较少[61]。JIN等[62]探究了纳米零价铁(nanoscale zero-valent iron,NZVI)对餐厨垃圾发酵产VFA的影响,反应48 h后乙酸比例最大,占到了72%,并发现NZVI可提高多种关键酶的活性,改变微生物群落结构。此外,在发酵过程中乳酸产量与NZVI剂量呈正相关。铁材料作为微生物营养元素和高效催化剂,可促进底物水解,还可与有机酸直接反应,达到缓解底物酸化的作用(Fe0 +2H+ ⇌ Fe2+ +H2),并对氧化还原电位也有调整作用。WANG等[61]探究了NZVI、纳米氧化铁(nFO)及磁铁矿(nM)对餐厨垃圾连续发酵产有机酸、醇等的作用。其中,NZVI和nFO对底物溶解和转化的促进作用更明显,也可促进乳酸生产。由于纳米铁材料比普通铁材料具有更大的比表面积和更好的反应性,故在餐厨垃圾发酵产乳酸的过程中,添加纳米铁材料会产生积极效果。
Fe2+ +H2),并对氧化还原电位也有调整作用。WANG等[61]探究了NZVI、纳米氧化铁(nFO)及磁铁矿(nM)对餐厨垃圾连续发酵产有机酸、醇等的作用。其中,NZVI和nFO对底物溶解和转化的促进作用更明显,也可促进乳酸生产。由于纳米铁材料比普通铁材料具有更大的比表面积和更好的反应性,故在餐厨垃圾发酵产乳酸的过程中,添加纳米铁材料会产生积极效果。
添加NZVI可促进乳酸产生,而氧化还原电位的调整可能来自于NZVI的电子供应,因此,向微生物群落提供电子也会影响乳酸发酵。施加一定电压以提供电子是一个新的思路。XUE等[31]使用两级电化学系统,发酵反应室连接负电极,通电为之提供负电压,与未通电相比,乳酸的生产率和光学纯度均得到了提高,说明电流提供的电子可能促进了丙酮酸到乳酸的转化。有研究已证实,微生物电解池(microbial electrolysis cells,MEC)可有效缓解VFA带来的酸抑制作用[63],构建阴极电化学发酵产乳酸系统,在阴极室提高还原力,可使得乳酸产量和产率明显提高。将电化学技术更多地应用到乳酸发酵系统,甚至作为预处理,亦可作为发酵方法研究的重要方向。
4.4 更高附加值产品的延伸制取
为获得更有价值的产品,将乳酸作为中间产物向其他产物转化的研究亦越来越多。ZHU等[64]进行了接种Ruminococcaceae bacterium CPB6菌将乳酸转化为正己酸的研究,在pH为6.0~6.5、温度为(30±1)℃的条件下,初始乳酸质量浓度为20 g·L−1,分批进行实验,中间补充乳酸,最终产生己酸23.41 g·L−1。己酸在食品、医药及化学工业中用途广泛,是食品添加剂、香水、抗癌药己雷琐辛等的主要原料,也可作为表面活性剂生产中的添加剂。
由于发酵液中的葡萄糖和甘露糖会抑制木糖代谢途径,因此,直接发酵葡萄糖半纤维素水解液生产木糖醇的效率较低。RIVAS等[65]采用先接种乳酸菌、转换葡萄糖和甘露糖为乳酸,然后微滤去除乳酸菌,并接种发酵微生物转化木糖为木糖醇,从而使发酵的效率得以提高。
程琪越[66]将玉米秸秆和淀粉作为底物,接种乳酸乳球菌(Lactococcus lactis subsp.lactis ATCC 11454)进行同步糖化发酵,生产乳链菌肽和乳酸。若在食品中加入十万分之几到万分之几乳链菌肽,将可抑制引起食物腐败的许多革兰氏阳性菌(包括葡萄球菌、链球菌、微球菌等)生长和繁殖,并生成一种高效、无毒的天然食品防腐剂。该项研究表明,在淀粉质量浓度为40 g·L−1, 糖化酶添加量为100 U·g−1、CaCO3添加量为2.5%、Tween-80添加量为1 mL的条件下,得到的产物乳链菌肽最高效价为2 516.42 IU·mL−1, 乳酸质量浓度为37 g·L−1,达到理论产率的84%。
有些乳酸菌还可产生一种新型的天然抗菌物质-苯乳酸,与乳酸菌产生的细菌素(如乳链球菌素)相比,苯乳酸是小分子物质,其抑菌谱宽、稳定性高,已成为乳酸菌抑菌能力的有效标志之一。作为一种新型生物防腐剂,苯乳酸在乳品工业中具有广阔应用前景。
此外,生物法乳酸脱水制造丙烯酸的成本约为1.1×104元·t−1。而目前丙烯酸售价已经高达1.4×104~1.8×104元·t−1。南京工业大学开发的利用微生物将乳酸脱水制丙烯酸技术的转化率已达到85%[67]。可以通过减少石化原料的消耗、减少有毒中间产物的产生,使乳酸转化成丙烯酸的成本大大降低。
近年来,用木糖渣发酵联产乳酸和聚氨基葡萄糖、生物发酵联产L-乳酸和L-赖氨酸、粗甘油生物发酵联产1,3-丙二醇和乳酸、用玉米芯发酵联产低聚木糖和乳酸等的可行性研究亦有报道,已实现了高附加值产品的生物炼制过程。此外,餐厨垃圾与其他生物质废物混合发酵联产乳酸和有机肥等的研究也获得较好的经济效益和环境效益,可最大限度地实现废物的减量化和资源化。
5. 结语
1)市场对于乳酸和聚乳酸的需求量日益增加。尽管相关研究取得了一定进展,但目前利用餐厨垃圾发酵产乳酸技术仍有一些限制因素。
2)发酵菌种是乳酸生产的主体。可从环境中分离发酵效果好的野生乳酸细菌,或利用已分离得到的效果较好的乳酸细菌作为发酵菌株。再将其接种至餐厨垃圾中,通过相关实验优化对餐厨垃圾这类复杂底物的适应性等,进而获得适宜参与餐厨垃圾发酵的相关菌种。米根霉等真菌因可直接利用淀粉及所产乳酸的高光学纯度而受到较多关注。利用基因工程技术筛选或改造出可产生高效水解酶、生命活动受pH等环境因素影响小、能适应餐厨垃圾复杂底物,并可高效利用糖类、生产更高质量、更高纯度乳酸的菌株,将是乳酸发酵领域急待突破的关键技术。
3)此外,还需开发新的发酵工艺,如将不同菌种混合进行发酵,改变单一菌种发酵的方式或者利用不同配比的底物等,可有效地改善微生物对底物的利用。尤其是利用细胞循环、固定化细胞等高细胞密度发酵技术,可缩小反应器体积,缩短生产周期,减少设备投资,并在一定程度上减少废水量,降低生产成本,提高综合生产效率。
4)各种原位分离耦合乳酸发酵技术可从发酵系统中选择性地分离乳酸,可有效减少产物抑制作用。其中,原位萃取发酵技术重点在选择对微生物无害,且萃取效果好、成本低的萃取剂。在原位吸附发酵技术中,要选择性质稳定、吸附容量大、可回收利用、寿命长的吸附剂,采用负载金属、改变孔隙结构等方法来加强其吸附能力。开发新的膜材料、优化膜分离工艺、改善膜清洗方式等,都有益于解决原位膜分离发酵技术的膜污染堵塞、膜寿命和成本问题。
5)生物炼制被认为是化学炼制的替代方式。餐厨垃圾作为生物质废物的一种,其乳酸衍生物及发酵联产高附加值产品的潜力很大,需继续深入研究,以期更好地实现餐厨垃圾的无害化与资源化利用。









 下载:
下载: