-
冷冻法作为一种清洁、高效且具有广阔应用前景的水处理技术,在废水处理[1-3]和食品工业[4-5]等方面展现出众多优势,例如设备腐蚀低、没有二次污染和无需化学添加剂等[6]。冷冻过程中,水分子在凝固点结晶为冰晶,同时可溶性污染物则在液相中积聚[7]。因此,通过分离冰-液体混合物可以得到洁净水。根据冰晶的生长方式,冷冻法可分为渐进冷冻法和悬浮结晶法[8]。与渐进冷冻法相比,悬浮结晶法具有更大的固液界面,更高的能量传递效率和更快的冰晶生长速度[9]。因此,悬浮结晶法在实验和理论研究方面均受到了更加广泛的关注。
近年来,研究人员从多个方面对冷冻法进行研究,例如通过建立数学模型预测冰晶生长速度,将数字图像处理技术运用于研究冰晶的形状和大小[10-11]。有研究[12-13]表明,由于在悬浮结晶过程中,污染物会进入冰晶中,从而降低冰晶纯度,因此,冰晶纯度是冷冻法的研究核心。但是,从分子水平层面来看,对杂质进入冰晶的机理仍然缺乏相关的报道和研究。
本研究以4种不同官能团有机物污染物(即己烷、正己酸、正己醇和正己醛)为研究对象,利用悬浮结晶法进行污染物分离实验,探讨了不同有机污染物在冷冻浓缩过程中所形成的冰晶中的杂质浓度变化。此外,运用量子化学计算,通过几何优化和约化密度梯度(RDG)分析,从理论上研究了拥有不同官能团的有机物污染物与水分子之间形成的氢键强度,以探究官能团与水分子形成的氢键强度对冰晶纯度的影响。
全文HTML
-
图1为悬浮结晶实验装置示意图。该装置配有低温恒温槽(1.5 kW,DC-4006,郑州北润仪器有限公司),并搭载数显电动搅拌器(JJ-1A,60W Tenke科学仪器公司,中国)。在整个悬浮结晶过程,用温度记录仪(sh-x24,多通道东莞联谊仪器有限公司,中国)监控溶液温度的变化。500 mL广口瓶置于装有无水乙醇的低温恒温槽中,通过搅拌器改变悬浮结晶过程中的转速。用软木塞密封容量瓶,以减少外部环境因素的干扰并防止有机物挥发。在整个悬浮结晶过程中,用温度记录仪监控溶液温度的变化。
-
分别将250 mL浓度为1.6 mmol·L−1的模拟溶液(己烷、正己醇、正己醛和正己酸)(购自阿拉丁,纯度大于99%)放入500 mL广口瓶中。冷冻悬浮结晶实验工艺参数设置如表1所示。该悬浮结晶实验中,冷冻温度均设定为溶液的冰点以下。为进一步研究冰晶杂质浓度差异,本文分别在不同冷冻温度、冷冻时间及转速等实验条件下,研究在不同有机污染物中冰晶中杂质浓度的差异。
在实验过程中,当溶液温度降低至设定温度时,向广口瓶中投入0.1 g晶种,并从此时开始记录冷冻时间。悬浮结晶完成后,将冰晶收集用自制分离装置在离心机中以1 000 r·min−1离心1 min,从而将冰晶与表面残留污染物分离。随后,在室温(25±2) ℃下将其融化,并用TOC分析仪(TOC-VCPH,Shimadzu,Japan)测量原水和冰晶融化后的总有机碳(TOC)。最后,根据式(1)计算冰晶不纯度。
式中:R为冰晶不纯度;CRW为原溶液的TOC,mg·L−1;CI为冰融水的TOC,mg·L−1。
-
本研究模拟了水分子与四种有机物杂质之间形成的氢键。通过DFT和Becke的3参数混合泛函[14]结合Gaussian 09,选取6-31+G**基组,使用Lee-Yang-Parr的B3LYP泛函[15]优化,分别对有机物和水分子进行几何优化,从而得到能量最低的初始构型,再基于该初始构型进行几何优化,得到能量最低的构型,并且引入了吸附能以研究氢键的特性[16]。通常认为,如果在定义为R1-A-H和B-R2的2个物种之间存在分子间力,则可以通过式(2)证明这种相互作用的能量。
式中:E(R1—A—H···B—R2)、E(R1—A—H)、E(B—R2)分别为各个化合物在稳定状态下的单点能,kJ·mol−1;R1—A—H···B—R2体系由于形成结合键而稳定,因此,ΔE值为负。在O—H···O体系中,深入研究氢键强度和H···B间距离的关系。由于氢键体系易以线性呈现,因此,将A—H···B角度作为氢键的几何特征进行研究。通过RDG的分析结果[17]实现对有机物与水分子之间的范德华相互作用和氢键的可视化呈现,从而有利于深入理解有机物与水分子之间的相互作用[18]。
1.1. 悬浮结晶实验装置
1.2. 悬浮结晶实验
1.3. 理论计算
-
如图2所示,在己烷、正己酸、正己醇和正己醛4种具有不同官能团有机物的悬浮结晶分离实验中,转速200 r·min−1,−0.9 ℃冷冻温度,30 min反应时长,发现其去除率分别为67.07%、87.75%、94.71%和95.32%。其中对于非极性有机物己烷,其去除率最低仅有67.07%,而对于3种极性有机物,其去除率呈现一定差异。结果表明,对于具有不同官能团的有机物,在悬浮结晶过程中形成的冰晶杂质浓度有明显差异。
通过冰点测定实验,分别得到4种有机物的冰点温度(己烷−0.21 ℃、正已醇−0.25 ℃、正己醛−0.23 ℃正己酸−0.25 ℃)(图3),发现其冰点温度均在−0.2 ℃左右。由于溶液结冰时需要一定的过冷度,因此,将初始实验温度设定为低于冰点的−0.4 ℃。并分别在过冷度为0.2、0.7、1.7、2.4 ℃的工况下,进行悬浮结晶测试。
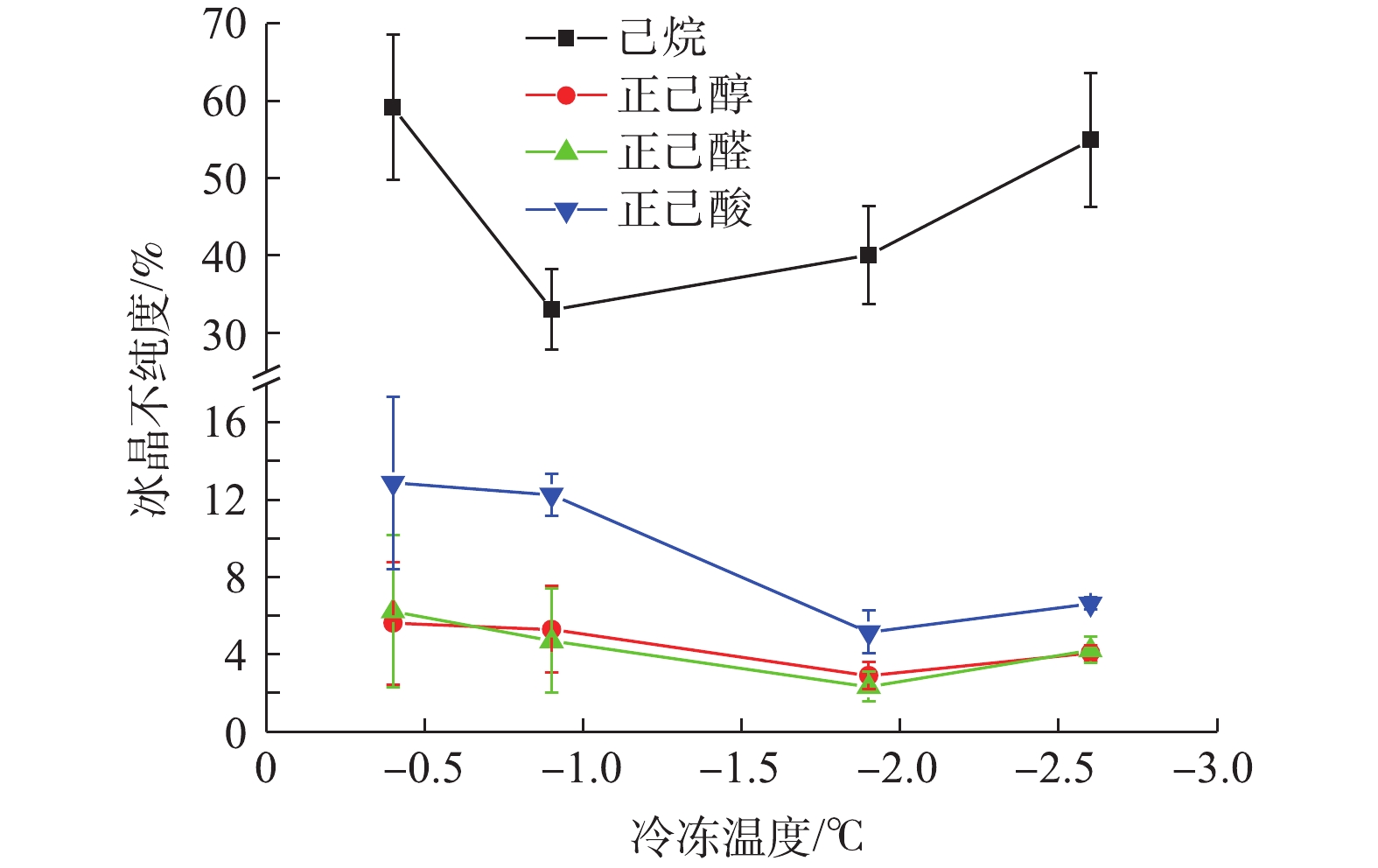
如图4所示,当固定转速为200 r·min−1,冷冻时间为30 min,随着冷冻温度降低,4种有机杂质在冰晶中的浓度呈下降趋势,但当温度低于一定限值时,会发生过冷效应,部分杂质重新进入冰晶中,从而使得冰晶杂质浓度升高。更重要的是,由图4中可明显看出,在己烷溶液中,在各温度下所形成的冰晶杂质浓度均要明显高于其他3种有机溶液。其主要原因在于,己烷是非极性有机物,其在水中的溶解度较低,在低温下易于析出,且悬浮在溶液表面,易掺入溶液上层悬浮的冰晶中,从而使得冰晶杂质浓度明显上升。
如图5所示,当固定的冷冻温度为−0.9 ℃,转速为200 r·min−1,随着冷冻时间的延长,在4种有机物溶液中,悬浮结晶形成的冰晶中有机物杂质浓度均呈降低的趋势。但是,在己烷溶液中,冰晶的杂质浓度要明显高于其他3种有机溶液。
在研究转速对冰晶杂质浓度的过程中可以发现,转速的影响主要体现在结冰速率和有机物掺杂这2个方面。如图6所示,当固定的冷冻温度为−0.9 ℃,冷冻时间为30 min,对于正己醇,正己醛和正己酸这3种极性有机物杂质,当转速处于低速区间时(200 r·min−1内),结冰速率相对较慢,且由于这3种极性有机物溶解度较高,在搅拌的作用下,转速的增加会加速杂质进入冰晶中。而当转速处于高速区间时(大于200 r·min−1),随着转速的增加,结冰速率明显加快,不利于杂质的进入,从而使得冰晶杂质浓度逐渐降低。此时,转速的提高起到加速杂质分离的作用。然而,转速对于己烷的作用恰好相反。经过分析认为,由于己烷溶解度较低,因此,当转速在低速区间时,更利于杂质和冰晶的分离;当转速超过临界值后,会加速己烷析出并和溶液上层悬浮的冰晶混合,从而使得最终冰晶的杂质浓度上升。另外,在己烷溶液中,冰晶的杂质浓度依然明显高于其他3种有机物中形成的冰晶杂质浓度。
通过上述分析可以发现,转速、温度等实验工况对不同有机物杂质进入冰晶的浓度具有影响。并且,己烷溶液的冰晶杂质浓度明显高于正己醇、正己醛和正己酸溶液的冰晶杂质浓度。该结果表明,相比于有官能团的极性有机物(正己醇、正己醛和正己酸),在没有官能团的非极性己烷的溶液中,更容易导致冰晶杂质浓度上升。其原因在于,非极性己烷在水中的溶解度较低,在浓缩效应的作用下,在冷冻过程中更容易析出。析出的正己烷和悬浮结晶形成的冰晶,由于密度较低,会漂浮在溶液上层并逐渐发生混合。因此,己烷会附着在冰晶的表面,并且在离心过程中很难被去除,从而使得冰杂质浓度要明显高于具有较高溶解度的极性正己醇、正己醛和正己酸。但是,在相同冷冻条件下,对于具有不同官能团的正己醇、正己醛和正己酸溶液中仍然呈现出冰晶杂质浓度的差异。通过量子化学计算其氢键强度,可以进一步深入分析杂质浓度差异与氢键强度相关性。
-
模型几何结构优化结果见图7,通过对不同有机物分子和水分子进行结构优化以达到能量最低的稳定状态。如图7(a)可见,在己烷分子中,17C—19H键由原来的0.109 5 nm,经过结构优化后缩短至0.109 nm。氢原子(己烷分子)与氧原子(水分子)距离为0.259 nm。由己烷分子和水分子优化后的总能量与单个己烷和水分子之和的能量差为−1.93 kJ·mol−1,这表明己烷分子和水分子没有缔合形成氢键,仅存在较弱的分子间作用力。
正己醇和正己醛分别可以与水分子形成一个氢键(图7(b)和图7(c))。其中,正己醇的羟基中氧原子和水分子中氢原子形成的氢键键长为0.189 nm。并且形成氢键后,正己醇的17C—20O和20O—21H键长分别由原来的0.143 nm和0.096 nm伸长至0.144 nm和0.097 nm。经过计算可知,正己醇和水分子间的氢键键能为−26.48 kJ·mol−1,20O—24H—22O的键角为172.46°。正己醛中的醛基中氧原子和水分子中氢原子形成的氢键键长为0.192 nm,醛基中的17C=19O的键长由原来的0.121 nm伸长至0.122 nm。经过计算可知,氢键作用能为−24.31 kJ·mol−1,19O—22H—20O键角为160.18°。由此可知,正己醇和正己醛与水分子形成的氢键键能十分接近。该理论计算的结果和上述悬浮结晶实验(图4~图6)中,正己醇和正己醛溶液中相似的冰晶杂质浓度结果相一致。
与正己醇和正己醛不同,正己酸和水分子可以形成2个氢键(图7(d)),其中,羰基氧原子和水分子的氢形成氢键,键长为0.196 nm,羟基氢原子和水分子的氧原子形成氢键,键长为0.179 nm。正己酸和水分子形成氢键后,正己酸的17C=20O和18O—19H的键长由原来的0.121 nm和0.097 nm伸长至0.123 nm和0.099 nm。由计算可得,氢键键能为−44.10 kJ·mol−1。18O—19H—21O和20O—22H—21O的键角分别为156.26°和136.98°。
为了提高4种有机物分子和水分子间作用力的可视化程度,利用RDG分析法对其相互作用力进行分析,其结果如图8所示,有机物和水分子之间的分子间力由彩色等值面表示,蓝色表示氢键,绿色表示范德华相互作用,红色表示强排斥力。
已知正己酸和水分子间的氢键键能(−44.10 kJ·mol−1)明显高于正己醇和正己醛。如图8所示,正己酸和水分子间有明显蓝色等值面。而正己醇和正己醛与水分子间形成的氢键键能较弱(分别为−26.48 kJ·mol−1和−24.31 kJ·mol−1),因而其间等值面的蓝色区域面积明显较小。己烷和水分子间由于没有缔结氢键,因此,在其间的等值面完全呈现对应于范德华相互作用的绿色。
通过计算结果(表2)可知,正己酸和水分子间缔结的氢键键能要远大于正己醇和正己醛。较大的氢键键能使得正己酸与水分子间存在较强的吸附能,从而使得正己酸在悬浮结晶过程中更容易进入冰晶中。因此,其冰晶的杂质浓度明显高于正己醇和正己醛中冰晶的杂质浓度。而正己烷由于是非极性有机物,其和水分子间无法缔结氢键,因此,其溶解性较差,从而在悬浮结晶过程中易于析出,漂浮在溶液表层并于悬浮在表层的冰晶混合,附着于冰晶表面,并难以通过离心分离,从而使得其冰晶的杂质浓度要远高于另外3种极性有机物杂质。本研究在探讨水分子和有机物分子物理吸附体系的理论计算过程中,是在基态即绝对零度下进行的,通过结构优化获得体系最低单点能,进而通过能量差算得分子间作用力。然而在现实情况下,无法实现绝对零度的理想条件,当环境温度升高时,体系焓则随之增加,不利于体系的稳定,而当温度降低时,则反之,有利于体系吉布斯自由能下降。根据吉布斯状态函数可知,熵的增加可导致吉布斯自由能降低,这有利于体系温度降低,而当熵减小时则反之。
2.1. 实验结果
2.2. 理论计算结果
-
1)悬浮冷冻结晶法对废水中己烷、正己酸、正己醇和正己醛的去除率分别可达67.07%、87.75%、94.71%和95.32%,且有机污染物的官能团在冷冻结晶过程中发挥重要作用。
2)非极性己烷溶液中形成的冰晶杂质浓度要明显高于其余3种极性有机溶液。
3)量子化学理论分析对冰晶杂质浓度的变化机理的研究结果表明,极性有机污染物官能团和水分子之间缔结的氢键强度会明显影响冰晶杂质浓度,氢键吸附能越大,其冰晶杂质浓度越高,对应分离效果越差。






 下载:
下载: