-
尾矿是世界上最大的工业固体废物之一。钢铁行业的飞速发展产生了大量铁尾矿,截至2013年底,我国累积了5.00×109 t铁尾矿[1]。这些铁尾矿不仅污染了土地和水体,还威胁着人群健康[2]。同时,随着选矿技术的进步和可开发利用矿产资源的减少,铁尾矿的资源化利用已成为缓解铁矿供需矛盾的可行途径。
目前,对铁尾矿大多采取无害化和资源化处理两条路径。一是将重金属从铁尾矿中分离出来。YE等[3]利用生物浸出从铁尾矿中回收Pb(4.12%)和Zn(97.85%);SIRKECI等[4]利用浮选技术从铁尾矿中分离Co(83.1%)、Ni(57.7%)和Cu(59.0%)。另一途径是直接对铁尾矿进行资源化利用。WANG等[5]在铁尾矿中添加高岭土和飞灰制烧结砖;LI等[6]以铁尾矿为原料,采用泡沫凝胶法制备了高孔隙率(89%)、高强度、低导热率(导热系数0.032 W·(m·K)−1)的多孔砖;HU等[7]通过铁尾矿、K2CO3和KOH混合950 ℃烧结制备缓释硅肥,但该方法将重金属固定在了硅肥中。上述方法都会将重金属元素固定在产品中,存在二次暴露的风险,潜在危害性高[8]。生物浸出和浮选也存在微生物生存条件苛刻的难题[3],浮选产生的二次污染处理[4]等会导致工业化管理成本提高。因此,开发出更适合工业化处理铁尾矿重金属污染的技术是十分必要的。
一般来说,金属氯化物的沸点较氧化物的低,因此,氯化焙烧能够在较低焙烧温度下有效分离去除固体废物中的重金属[9]。LI等[10]在添加4% CaCl2、温度1 050 ℃、时间2 h的条件下,通过氯化焙烧回收氰化尾矿中的Au(91.6%)和Ag(54.7%)。YU等[11]以MgCl2·6H2O为氯化剂,在Cl与飞灰质量比为0.15、温度1 000 ℃条件下,对生活垃圾飞灰进行焙烧,结果发现,Cd和Pb挥发率接近100%。氯化焙烧相对于氧化焙烧,能够在较低温度下将重金属挥发[11]。氯化焙烧工艺在固体废物分离回收重金属中具有较好的效果,但目前很少有关于氯化焙烧在回收和安全处理铁尾矿方面的应用研究。铁尾矿作为亟需处理并可资源化的固体废物,在氯化焙烧分离重金属上值得进行深入研究。考虑到固体氯化剂更易控制Cl的添加量从而控制尾气污染,多数学者选择固体氯化剂来进行氯化焙烧。CaCl2的氯含量高、价格低廉[12]、释放HCl的能力比NaCl和KCl强[13],因此,CaCl2是最为常用的氯化剂之一。
本研究以CaCl2为氯化剂对铁尾矿进行氯化焙烧,研究不同焙烧温度和CaCl2添加量对Pb、Cu和Cd挥发率的影响;并利用X射线荧光分析(XRF)、热重分析(TG)、X射线衍射(XRD)和扫描电子显微镜(SEM)探究焙烧前后铁尾矿的基本特性和晶相结构的变化,从而了解在氯化焙烧过程中的物相和元素转变机理。本研究结果可为铁尾矿的无害化处理和资源化利用提供参考。
全文HTML
-
铁尾矿样品来自广东省韶关大宝山,呈红褐色土壤状,含水率较高。经自然通风和电加热鼓风干燥箱105 ℃干燥的铁尾矿采用球磨机(QM-3SP2行星式球磨机,南京南大)磨细过200目。铁尾矿的化学成分如表1所示。铁尾矿中目标金属Pb、Cu和Cd的含量分别为2 230.04、4 568.00和6.44 mg·kg−1。
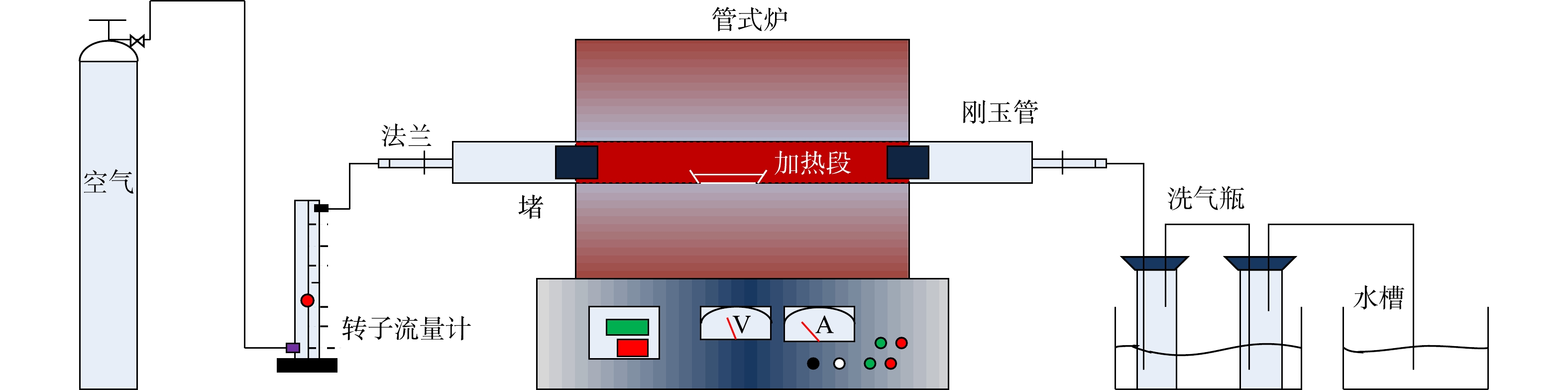
氯化焙烧实验系统如图1所示,主要由供气系统、管式炉(SK2-2-130天津中环,刚玉管直径8 cm,长度120 cm)、冷凝水收集系统,尾气处理系统4个部分组成。
-
将铁尾矿样品和无水氯化钙(CaCl2,AR)以0、5%、10%、20%和35%的CaCl2质量比混合,共10 g。把所需的CaCl2颗粒置于烘干至恒重的4 cm×8 cm刚玉方舟中,用移液枪向刚玉方舟添加10.0 mL超纯水,轻微振荡搅拌,待CaCl2完全溶解后,加入称好的铁尾矿,振荡搅拌混合。将样品在烘箱中于105 ℃干燥至恒重,后选择在600、800、1 000和1 200 ℃焙烧温度下进行氯化焙烧实验。本实验中供试气体为干燥空气,流速约为25 mL·min−1。每个氯化焙烧实验设置了2个平行样。最后,将反应气体连续通过3%的HNO3和5%的NaOH以充分吸收,然后排放到空气中。
为了量化在各种处理条件下铁尾矿中重金属的挥发作用,使用了NOWAK等[14]提出的重金属挥发率计算公式(式(1))。
式中:R是重金属元素的挥发率;C0是高温处理前原铁尾矿样品中重金属元素的浓度,mg·g−1;M0是高温处理前铁尾矿样品的添加量,g;C是高温处理后残渣中重金属元素浓度,mg·g−1;M是高温处理后残渣的总质量,g。
-
微波消解程序根据国家标准《土壤和沉积物金属元素总量的消解微波消解》(HJ 832-2017)[15]执行;使用电感耦合等离子体质谱仪(ICP-MS)(Thermo Scientific-ICAP RQ)检测重金属浓度并计算总量;使用热重分析仪(METZSCH-TG 209 F1德国)分析铁尾矿的热力学行为,载气为空气,升温速度为10 ℃·min−1;使用X射线衍射仪(XRD)(Bruker D8 ADVANCE)来识别原铁尾矿和焙烧渣在10°~90°时的主要结晶化合物;使用扫描电子显微镜-能量色散光谱仪(SEM-EDX)(Zeiss Merlin & Oxford X-MaxN)观察原铁尾矿和焙烧渣的形貌;使用X射线荧光光谱仪(XRF)(SHIMADZU-EDX-7000,日本)确定原铁尾矿样品和焙烧渣的主要化学成分,并通过面积归一化方法确定组分的质量分数。
重金属浸出浓度实验根据工业标准《固体废物毒性浸出方法硫酸硝酸法》(HJ/T 299-2007)[16]制备浸提剂,过滤浸出液后使用火焰原子吸收分光光度计(F-AAS、Hitachi Z2000)检测金属浸出浓度。
1.1. 供试铁尾矿原料与实验装置
1.2. 氯化焙烧实验方法
1.3. 分析方法
-
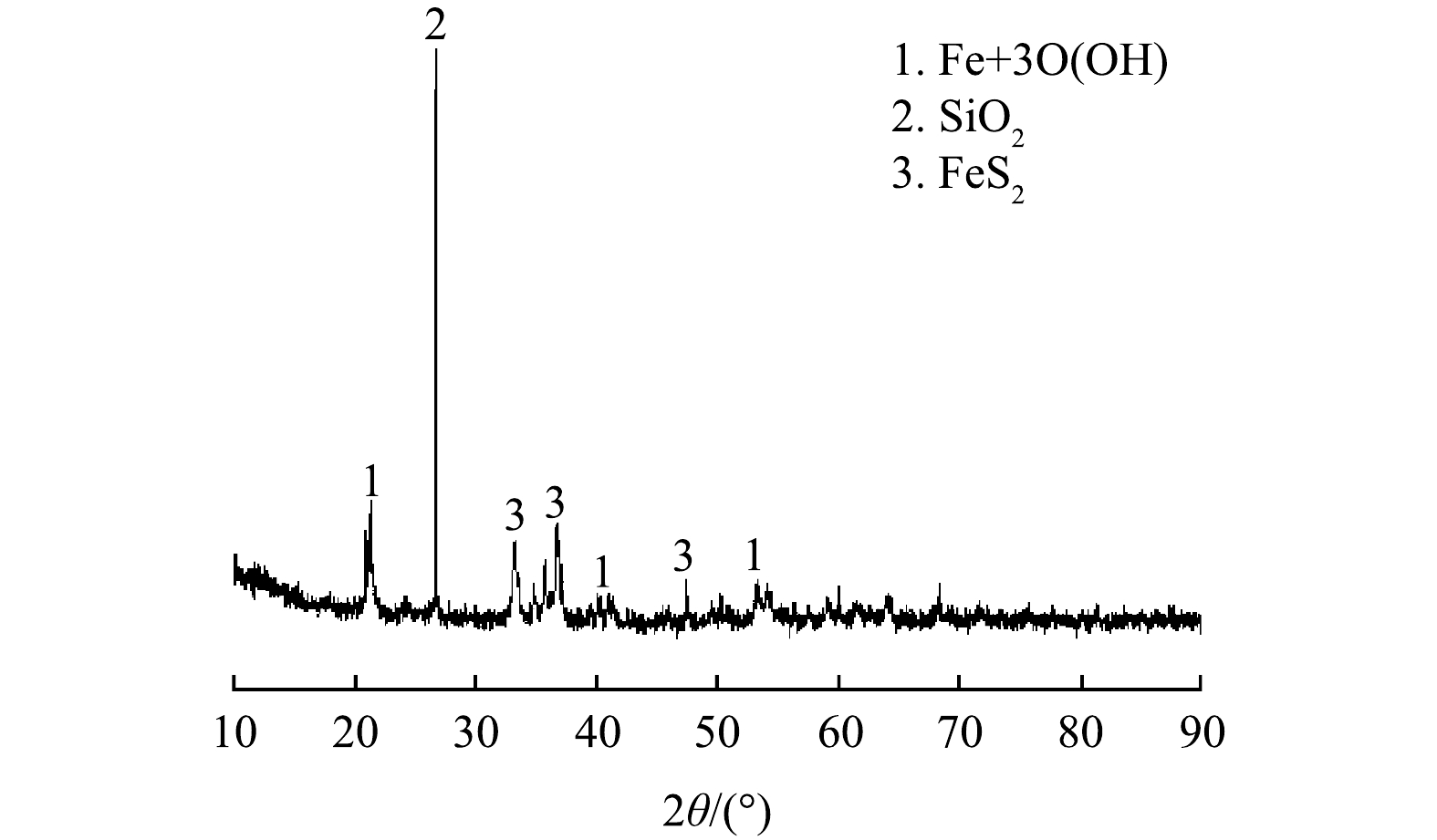
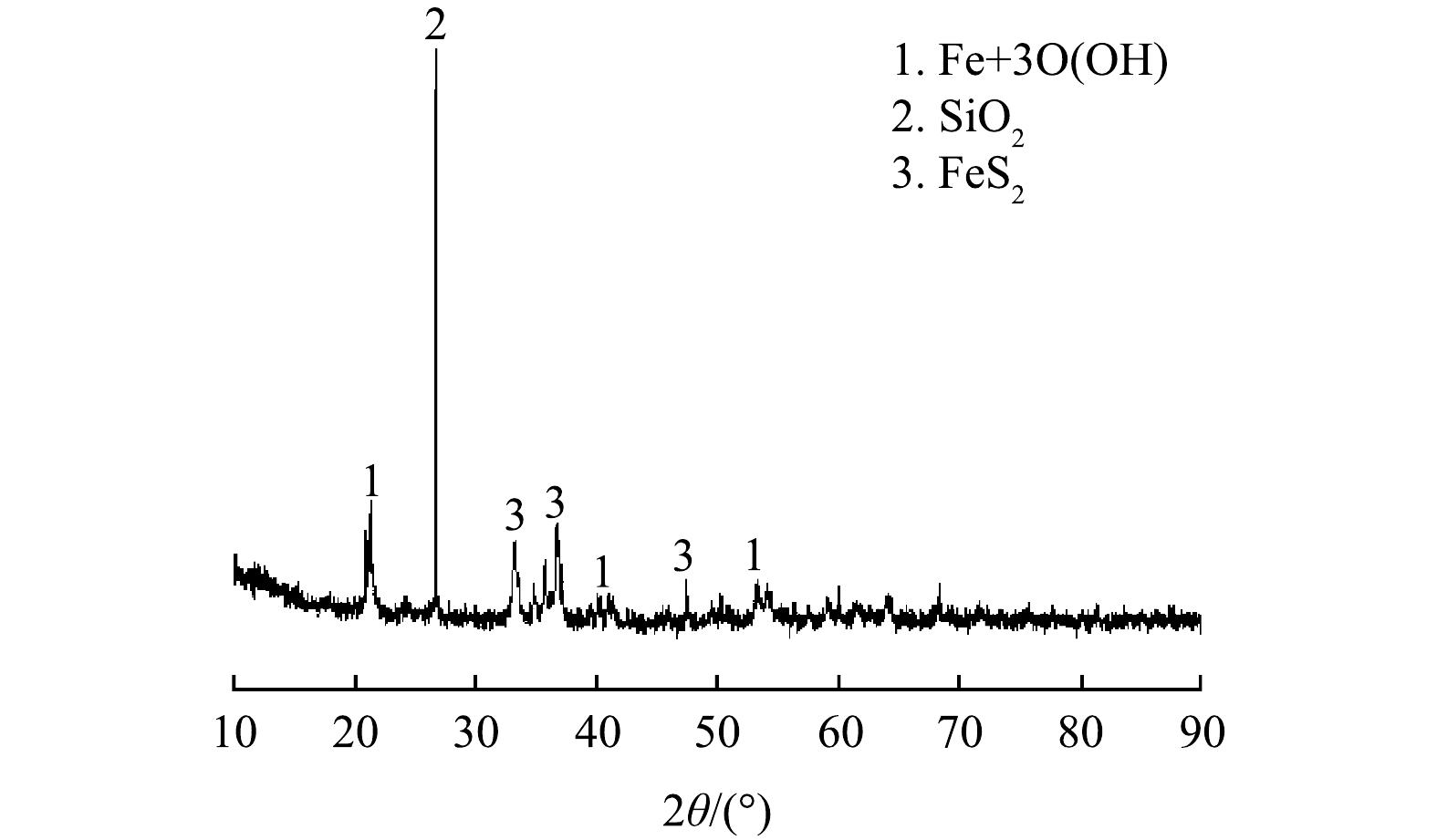
由表1可知,铁尾矿主要由Fe、Si、Al、S,其他元素(K、Mg、Cu、Mn、Zn、Pb)和少量稀有金属组成。Fe2O3含量最高,其他含量较高的化合物有SiO2、Al2O3、SO3。如图2所示,铁尾矿中的Fe主要以针铁矿形式存在,并同时存在SiO2和FeS2相。
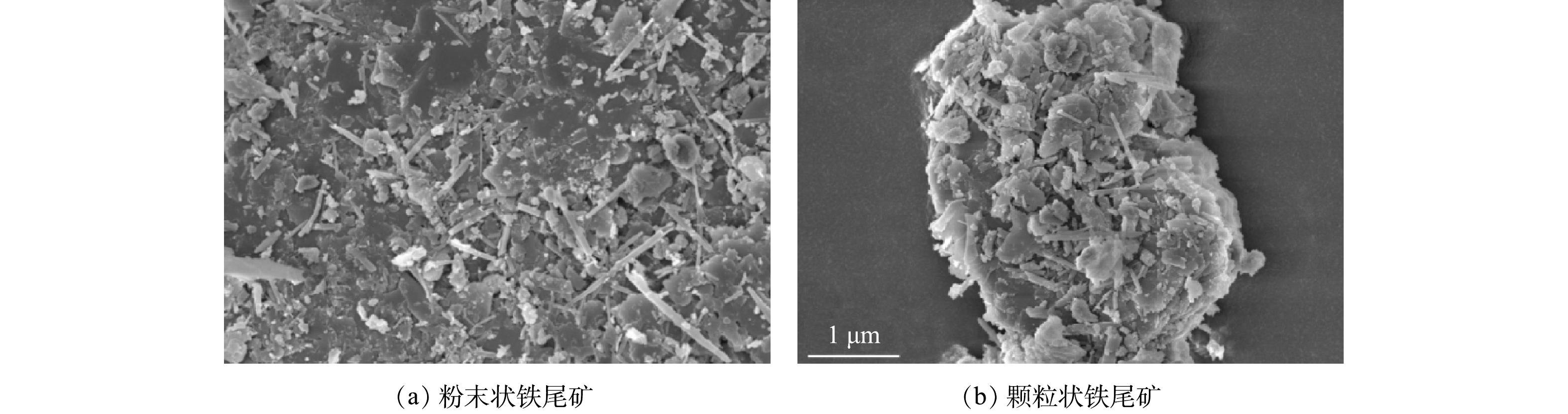
铁尾矿的微观形态如图3所示。由图3(a)可知,粉末状铁尾矿的粒度分布均匀,较多小尺寸片状或长条状的颗粒被吸附在原始颗粒表面上。这种吸附物为矿物相中的铁存在形式[17]。结合表1和图2可知,Fe2O3和FeO(OH)含量占比最高,铁尾矿的表面吸附物可以推测为Fe2O3和FeO(OH)。将图3(a)与图3(b)对比可知,颗粒状铁尾矿呈团聚现象,不利于重金属氯化物的挥发。
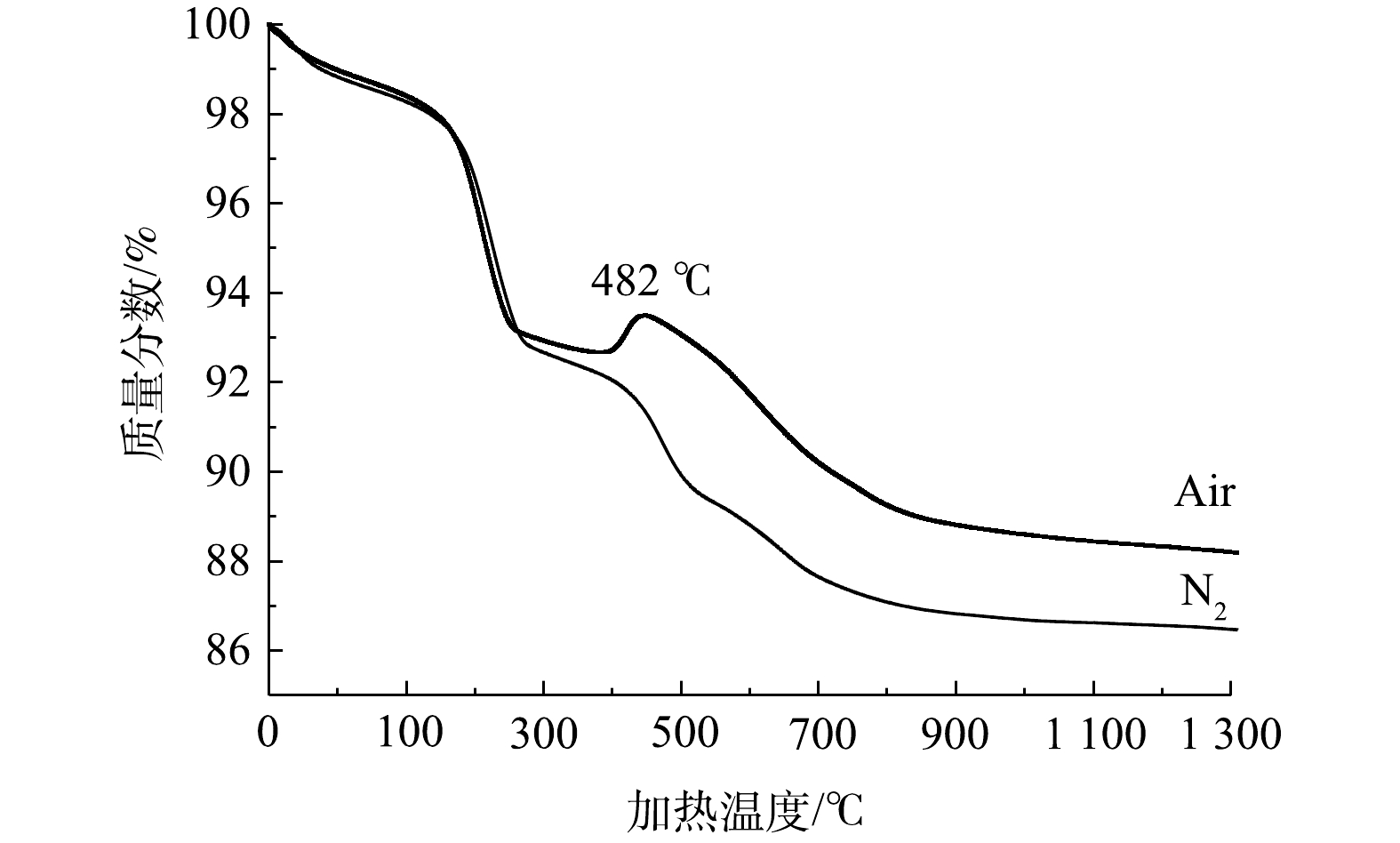
铁尾矿在10 ℃·min−1升温速率下的热重分析(TG)曲线如图4所示。据TG曲线显示,在200~300 ℃内,由于铁尾矿中的针铁矿分解,重量损失显著[18]。对比氮气条件下的情况可知,空气热重曲线在482 ℃左右时铁尾矿重量有显著增加,这可能与热处理过程中氧化铁的转化有关,其中铁尾矿中的某些非晶铁与空气中的氧发生反应,逐渐生成氧化铁和氢氧化铁。当温度达到600 ℃时,重量下降了8%。当温度达到1 000~1 200 ℃时,尾矿的失重率接近12%。
-
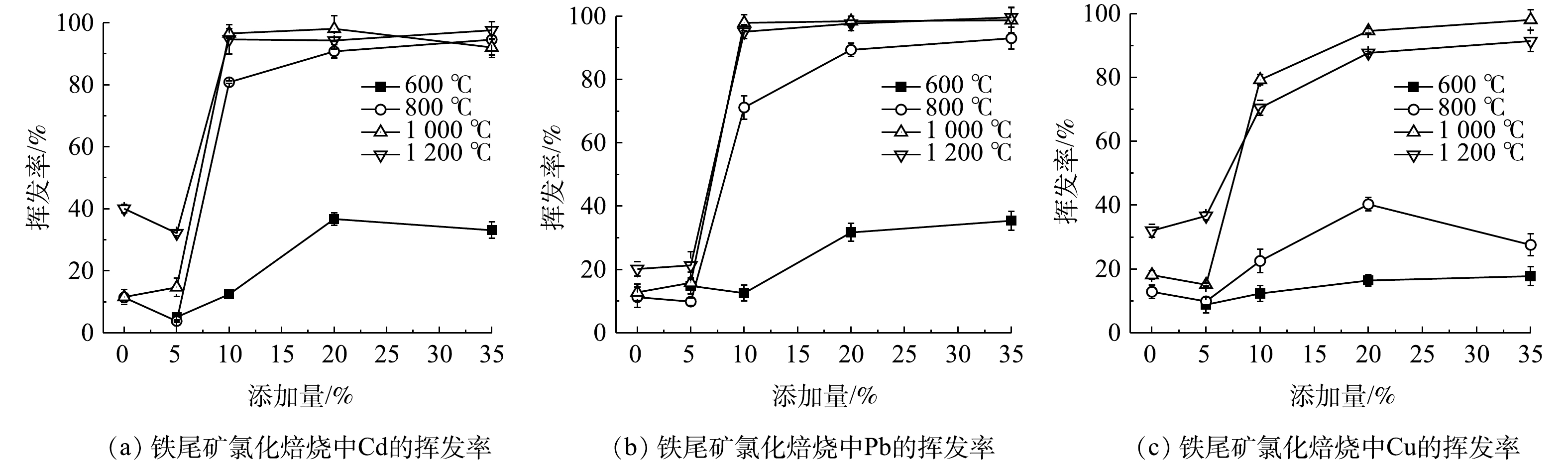
不同焙烧温度和不同CaCl2添加比下铁尾矿中Cd、Pb和Cu的挥发率如图5所示。由图5可知,随着温度自600 ℃升高到1 200 ℃,铁尾矿中Pb和Cd的挥发率相似,总体呈现先急速上升后平稳的趋势,而Cu的挥发率呈现先上升后下降趋势。在600~800 ℃过程中,Cd和Pb挥发率随着温度的上升而迅速上升,Cd的挥发率(10% CaCl2)从12.45%上升到80.84%,Pb的挥发率(10% CaCl2)从12.54%上升到71.15%。在800 ℃以上,Pb、Cd挥发率受温度的影响较小,均稳定在90.00%左右的较高水平。Cu挥发率在所有条件下均比Pb、Cd低。这与PVC氯化PbO、CdO和CuO时Pb、Cd比Cu更易挥发这一结果一致[19]。随着温度自600 ℃上升到1 000 ℃,Cu的挥发率上升,出现先缓后急的趋势,但当温度上升至1 200 ℃时,Cu挥发率反而下降,因此,不是温度越高越利于重金属挥发。纯PbCl2通过空气热重检测可知,在400 ℃即出现急剧挥发[20],但本实验Pb在600~800 ℃温度上挥发率才急剧上升。故可知,本实验600 ℃后才大量产生PbCl2,而制约600 ℃以下PbCl2生成的原因是此时CaCl2的分解效率低[21]。高挥发性元素Cd和Pb在较低温度下就能高效挥发[22],所以生成的PbCl2和CdCl2在900 ℃时基本上完全挥发[23]。Cu的挥发率较低是因为Cu属于中度挥发性重金属,氯化挥发受其氯化物扩散的限制[24],所以,随着温度的上升,铜的氯化物扩散加快,挥发率上升。但温度过高,物料烧结玻璃化也可能阻碍了铜的氯化物挥发,导致过高温度下Cu的低挥发率。添加10% CaCl2,温度为1 000 ℃时,Pb、Cd和Cu的挥发率分别为97.80%、96.57%和79.80%。
当焙烧温度为1 000 ℃时,随着CaCl2添加比的增加,Pb、Cd和Cu的挥发率趋于上升。对于Pb和Cd,在温度高于600 ℃、CaCl2添加比在0~5%之间时,挥发率均低于40%这一较低水平。当1 000 ℃,CaCl2添加比从5%到10%时,Pb和Cd的挥发率迅速上升并超过98%,几乎达到最高值。这一结果与氯化焙烧对固体废物污泥飞灰重金属挥发的结论基本相同[24-25]。在温度不变的情况下(600 ℃以上),CaCl2添加比达到10%之后,继续增加CaCl2添加量并不会对Pb和Cd的挥发率产生明显影响。在1 000 ℃下,当CaCl2比例从10%增加到20%时,Cu的挥发率从79.23%增加到94.54%。这一结果相对于其他固体废物要高,其主要原因是铁尾矿中含有大量的SiO2,可促进CaCl2转化为HCl[13],使反应往更利于生成金属氯化物挥发的方向进行。充足的氯可保证Pb转化为易挥发的PbCl2[26],所以,足够的氯含量是氯化焙烧去除铁尾矿中Pb、Cd的保证。综上所述,全面考虑工业成本后,确定CaCl2添加量10%、温度1 000 ℃为铁尾矿氯化焙烧去除Pb、Cd和Cu的最佳条件。
-
保持CaCl2添加量为10%,不同焙烧温度(600、800、1 000、1 200 ℃)和固定焙烧温度1 000 ℃,不同CaCl2添加量(0、5%、10%、20%)下的焙烧渣XRD谱图见图6。由图6(a)可知,在添加10% CaCl2,不同焙烧温度的条件下,尾矿和焙烧渣中衍射峰的主要成分是SiO2、Fe2O3和FeO(OH)。随着温度的上升,SiO2衍射峰的相对强度降低,且Fe2O3和铁的氢氧化物(Fe1.833(OH)0.5O2.5)的相对强度增加。经过焙烧,尾矿中的FeO(OH)转化为Fe2O3和铁的氢氧化物(Fe1.833(OH)0.5O2.5),与铁尾矿的热重分析一致。
由图6(b)可知,随着CaCl2添加比的增加,SiO2的相对强度逐渐降低,Fe2O3和FeO(OH)的相对强度略有增加,并逐渐形成CaSO4。有研究发现,添加NaCl的垃圾焚烧烧渣中产生了Ca2SiO4[27],本研究中SiO2的降低也可能是与SiO2形成了Ca2SiO4有关;图6中观察不到Ca2SiO4相应晶相可能是其以无定形形式存在或是含量过低[17]。在本研究各种焙烧条件下,焙烧渣XRD谱图并没有显示出重金属Cu、Pb和Cd对应的尖晶石或硅酸盐结构[5],可知Cu、Pb和Cd与铁尾矿得到了有效分离。
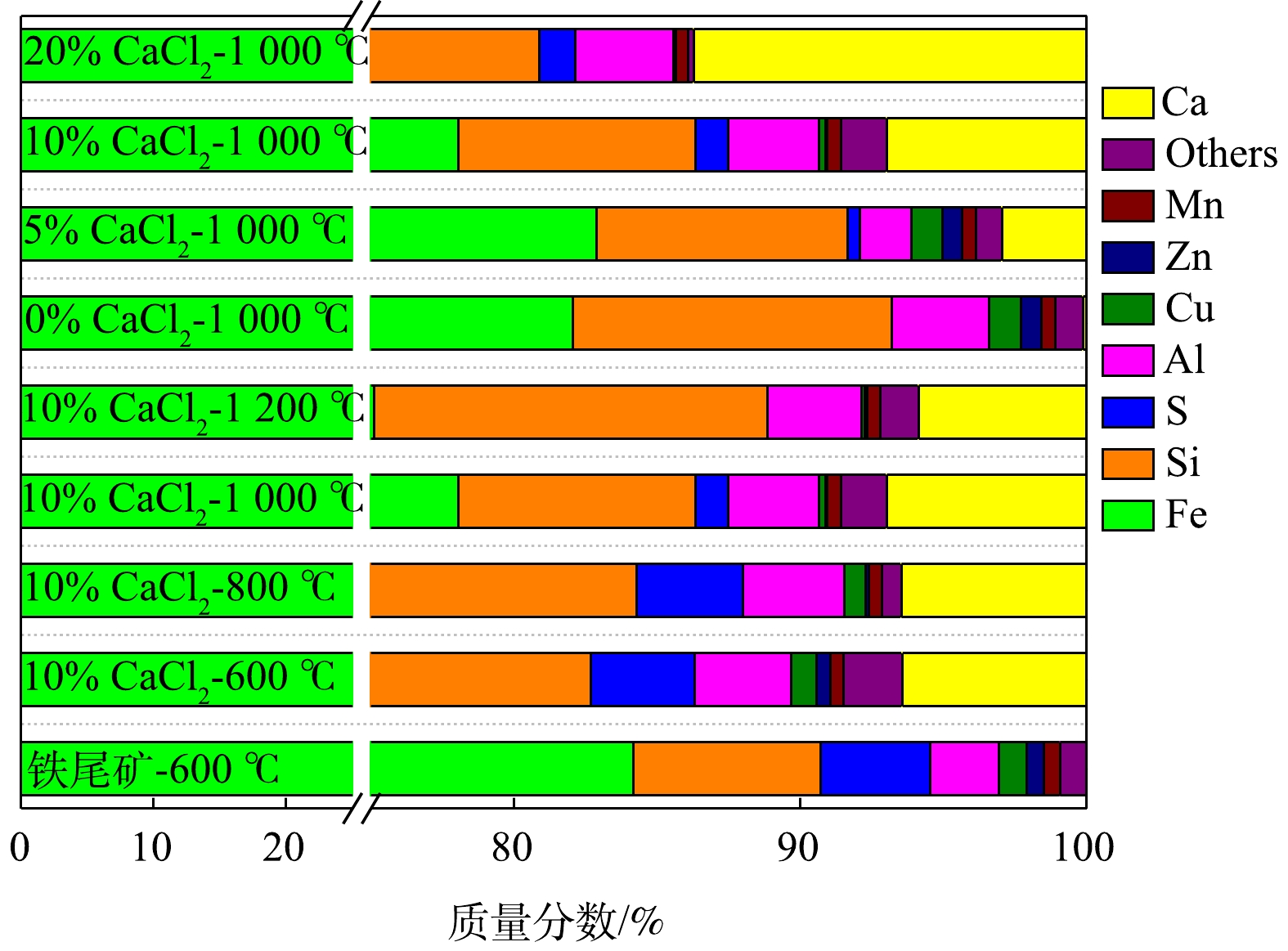
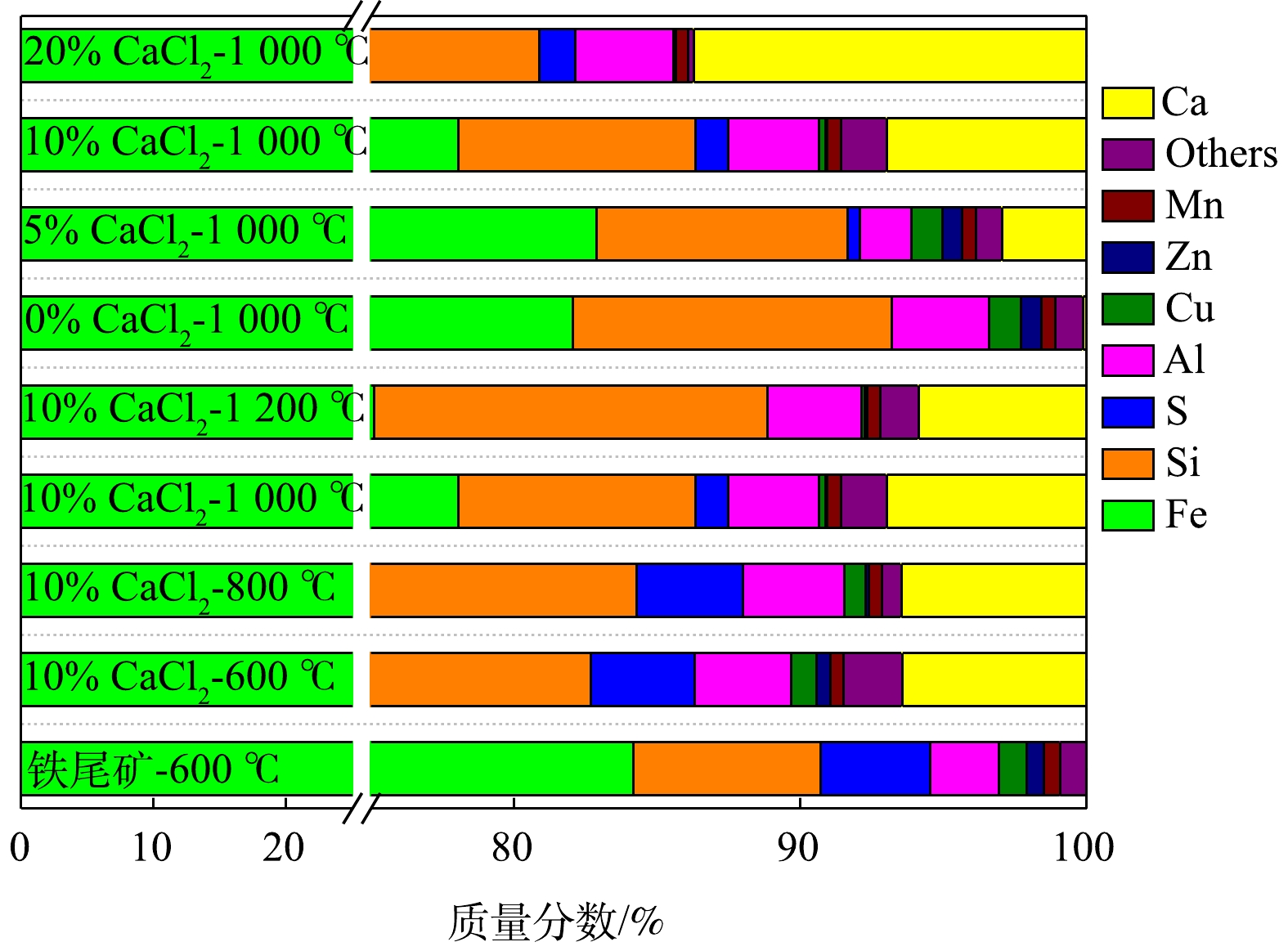
在不同焙烧条件下,铁尾矿混合物的XRF元素分析结果如图7所示。随着焙烧温度的升高和CaCl2添加量的增加,焙烧渣中Cu、Zn含量逐渐降低。当焙烧温度低于1 000 ℃时,S含量变化不明显,但在1 200 ℃时,S含量出现降低。在1 000 ℃焙烧温度下,随着CaCl2添加量的增加,S含量出现升高。这是因为,在空气条件下CaCl2、O2、H2O和SO2反应生成CaSO4[13],且CaCl2的增加导致Ca含量增加,促使S以固体的形式存在于烧渣中。Ca的相对含量受温度影响较小,但受CaCl2添加量的影响较大。Ca含量随所添加CaCl2的增加成比例增加,而铁尾矿或焙烧炉渣中均不存在Cl。因此,在温度1 000 ℃、10% CaCl2添加量的焙烧条件下,Pb、Cd和Cu等微量重金属成分挥发后,焙烧渣的主要成分与铁尾矿相比变化不大,尤其是Fe、Si、Al。
CaCl2与O2反应释放出样品中的Cl2,且当H2O与铁尾矿混合时会与CaCl2反应生成HCl[11, 28]。其反应方程式如式(2)~式(4)所示。
在本研究中,CaCl2达到778 ℃的熔点后,与铁尾矿中的SiO2反应释放出HCl[13]。Cl2和HCl在高温下与铁尾矿中的重金属反应形成金属氯化物,而Cu、Pb和Cd主要分别以CuCl(g)、PbCl2(g)、Pb2O3(g)和CdCl2(g)形式挥发[25]。铁尾矿中的Ca、S和SiO2经过氯化焙烧后以CaSO4和Ca2SiO4形式残留在焙烧炉渣中,大部分Ca以钙盐形式留在焙烧炉渣中;而Cl以金属氯化物、Cl2和HCl的形式挥发。此外,铁尾矿中的Fe和Al很难在氧化条件下氯化[29],而以金属氧化物的形式继续残留在烧渣中。
-
不同焙烧温度和不同CaCl2添加量下,铁尾矿焙烧渣中重金属的浸出毒性结果见表2。由表2可知,与原铁尾矿相比,经过氯化焙烧的烧渣中重金属Zn、Cu、Ni和Pb浸出浓度均大大降低。在干燥空气环境下,不同温度和不同CaCl2添加量下氯化焙烧渣的重金属浸出浓度远低于《危险废物鉴别标准浸出毒性鉴别》(GB 5085.3-2007)[30]标准限值。CaCl2添加比为10%时,600 ℃后依旧有重金属浸出,达到800 ℃时浸出液重金属浓度就全部低于检出限。在1 000 ℃、CaCl2添加比低于5%时,有重金属浸出,而大于5%后,重金属浓度就全部低于检出限。经氯化焙烧后,焙烧渣的重金属浸出毒性满足相关标准,且其中的Fe2O3、SiO2和Al2O3的与初始铁尾矿相比变化不大,含量较多,可以利用其制成建材产品。此外,添加CaCl2可能会增加焙烧渣中Ca的含量,这部分Ca可以降低建材利用时Ca的使用量,例如在制作高压加气混凝土时,可以减少石灰的消耗[1]。综上所述,经过氯化焙烧的铁尾矿焙烧渣可进行资源化利用。
2.1. 铁尾矿的基本特性
2.2. 铁尾矿氯化焙烧影响因素
2.3. 铁尾矿氯化焙烧过程成分变化及机理分析
2.4. 铁尾矿焙烧渣重金属浸出毒性分析
-
1)在铁尾矿氯化焙烧过程中,相比Pb和Cd而言,Cu更难挥发,Cu的挥发需要更高温度和更多CaCl2添加量。铁尾矿的最佳氯化焙烧条件为温度1 000 ℃、10% CaCl2添加量,此时Pb、Cd和Cu的挥发率分别为97.80%、96.57%和79.80%。
2)铁尾矿中的Ca、S和SiO2经过氯化焙烧后,以CaSO4和Ca2SiO4形式残留在焙烧残渣中,而Fe和Al以金属氧化物的形式残留在焙烧残渣中。
3)焙烧残渣中的Pb、Cd、Cu和Ni浸出浓度均远低于国家标准浓度限值,氯化焙烧后的残渣可以进行资源化利用。





 DownLoad:
DownLoad: