-
随着现代工业的快速发展,铬、镉、汞、铜等重金属的大规模开发和广泛应用,造成了严重的土壤和地下水污染[1]。其中,铬是污染最为严重的重金属之一,在环境中主要以Cr(Ⅲ)和Cr(Ⅵ) 2种形态存在,Cr(Ⅵ)的毒性是Cr(Ⅲ)的500倍[2-3],且在土壤地下水中的迁移扩散能力较强[4]。铬污染已经成为影响人类健康、生态环境及社会可持续发展的严重威胁[5]。用于Cr(Ⅵ)修复的还原剂有零价铁、二价铁和多硫化物等。其中,零价纳米铁是应用最为广泛的修复剂之一[6-7]。然而在实际修复工程中,由于零价纳米铁表面易氧化、颗粒易团聚,在很大程度上降低了其反应活性及其在环境介质中的迁移扩散能力[8-9]。
近年来,通过绿色合成法制备零价纳米铁逐渐成为研究热点[10],该方法是使用具有高还原能力的植物提取液替代硼氢化钠溶液。与传统方法相比,其优点在于:提取液中的多酚类物质既可作为还原剂又能包覆在纳米铁的表面,提高其抗氧化活性[11];具有更低的环境影响和制备成本[12];植物提取液中的有机质可为微生物提供养分,为利用微生物协同修复创造了条件[13]。MACHADO等[14]使用葡萄、红茶、葡萄藤叶提取液合成纳米铁,对布洛芬的去除率高达95%;ESSIEN等[15]将橄榄叶提取液作为还原剂制备绿色纳米铁,利用纳米铁静电吸附和表面络合去除水中的Ni2+,去除率最高可达97%;郭梦羽等[16]使用红背桂叶、菩提叶和龙眼叶的提取液制备纳米铁,其对亚甲基蓝的脱色率分别为99.2%、54.2%和20.8%。但是,由于绿色纳米铁对目标污染物的去除能力受到植物提取液性质的影响,在一些研究中,往往更多地关注修复剂对污染物的去除效果,而忽视了修复剂自身的悬浮稳定性和迁移扩散能力,同时保持修复剂的反应活性和悬浮稳定性是修复技术发挥作用的关键。
本研究选取了富含植物多酚的绿茶、红茶、乌龙茶、桑树叶,以及成都市资源丰富的银杏叶、石榴树叶、枇杷叶、悬铃木、木芙蓉共9种样本植物。通过对不同植物提取液的抗氧化活性测试,初步筛选出3种还原能力较高的绿色纳米铁,并对其进行了较为深入的表征分析,通过考察不同绿色纳米铁对Cr(Ⅵ)的去除效果,比较了绿色纳米铁的反应活性,以期为绿色纳米铁制备技术应用于土壤地下水污染修复提供参考。
全文HTML
-
实验材料:四水合氯化亚铁(FeCl2·4H2O)、硫氰酸钾(KSCN)、二苯碳酰二肼(C13H14N4O)为分析纯,重铬酸钾(K2Cr2O7)为优级纯,均购于科隆化工试剂厂;三吡啶三吖嗪(TPTZ, C18H12N6)为分析纯,购于上海阿拉丁生化科技股份有限公司;银杏叶、石榴树叶、枇杷叶、悬铃木、木芙蓉采摘自成都理工大学校园,桑树叶、乌龙茶、绿茶及红茶在市场购买。
实验仪器:恒温水浴振荡器(KW-400,上虞佳星仪器厂);真空干燥箱(DZF-6 021,上海精宏实验设备有限公司);数显加热磁力搅拌器(HSC-19T,群安实验仪器有限公司);紫外-可见分光光度计(TU-1 901,北京谱析通用仪器有限责任公司);电热恒温鼓风干燥箱(DHG-9 240B,上海申贤恒温设备厂);马尔文激光粒度分析仪(Nano series 3 600,英国马尔文仪器有限公司)。
-
在进行植物叶子提取液的制备时,将9种植物叶子(绿茶、红茶、乌龙茶、银杏叶、石榴叶、枇杷叶、悬铃木、木芙蓉和桑叶)洗净烘干,剪碎后过2 mm筛,各称取2 g,置于烧杯中。加入300 mL超纯水,在温度95 ℃,转速300 r·min−1的条件下磁力搅拌40 min,冷却至室温后过滤,即制得提取液。
利用铁离子抗氧化能力法(FRAP法)测试不同植物叶子提取液的抗氧化活性。FRAP试剂包括:0.3 mol·L−1 醋酸缓冲液(pH=3.6)、10 mmol·L−1 TPTZ溶液、20 mmol·L−1 FeCl3溶液,上述溶液以10∶1∶1的体积比例混合;在比色管中加入0.1 mL不同植物叶子提取液后,加入2.4 mL FRAP试剂,在37 ℃下水浴10 min,于593 nm处测定吸光度;以0.1~1.6 mmol·L−1的FeSO4标准液替代样品绘制标准曲线。
在进行绿色纳米铁的制备时,称取0.01 mol的FeCl2·4H2O,加300 mL超纯水在烧杯中搅拌溶解,配制成0.03 mol·L−1的Fe(Ⅱ)溶液。将新鲜制备的叶子提取液和Fe(Ⅱ)溶液以体积比2∶1的比例混合,采用恒速搅拌器搅拌,控制温度为(25±0.5) ℃,转速为(300±25) r·min−1,1 h后,得到纳米铁悬浊液并过滤,分别使用超纯水和无水乙醇进行清洗,所得的固体置于真空干燥箱中90 ℃干燥至恒重,即得到绿色纳米铁颗粒。
在进行绿色纳米铁的悬浮稳定性测试时,分别取650 mg·L−1绿色纳米铁的悬浊液,盛于50 mL的比色管中静置12 h。在不同的时间观察悬浊液的分层团聚情况,并取一定量的上清液,利用紫外可见分光光度计和硫氰酸盐比色法,在波长为510 nm处测定其中的绿色纳米铁浓度,并计算相对浓度(Ct/C0)。
-
配制不同浓度的纳米铁悬浊液10、20、30、40、70、100 mg·L−1,各取15 mL于50 mL锥形瓶内,分别加入15 mL Cr(Ⅵ)浓度为50 mg·L−1的 K2Cr2O7 溶液,在pH=4、温度25 ℃的条件下反应24 h。取1 mL反应后的悬浊液于10 mL的比色管中稀释10倍,采用二苯碳酰二肼分光光度法,在最大吸收波长540 nm下测定其吸光度,算出反应后的Cr(Ⅵ)浓度,根据式(1)计算Cr(Ⅵ)的去除率。
式中:η为Cr(Ⅵ)的去除率;C0为悬浊液中Cr(Ⅵ)的初始浓度,mg·L−1;C为反应后悬浊液中Cr(Ⅵ)的浓度,mg·L−1。
绿色纳米铁去除Cr(Ⅵ)的动力学使用拟一级模型(pseudo first order model, PFOM)和双室一级模型(two-compartment first order model,2-PFOM)描述。拟一级模型和双室一级模型分别如式(2)和式(3)所示。
式中:Ct为t时刻Cr(Ⅵ)的浓度,mg·L−1;k1为拟一级模型去除速率常数,min−1;kfast和kslow分别为双室一级动力学去除速率常数,min−1;ffast和fslow分别为快、慢室去除的比例,并且ffast+fslow=1。
1.1. 实验材料与仪器
1.2. 绿色纳米铁的制备及表征
1.3. 绿色纳米铁去除Cr(Ⅵ)性能
-
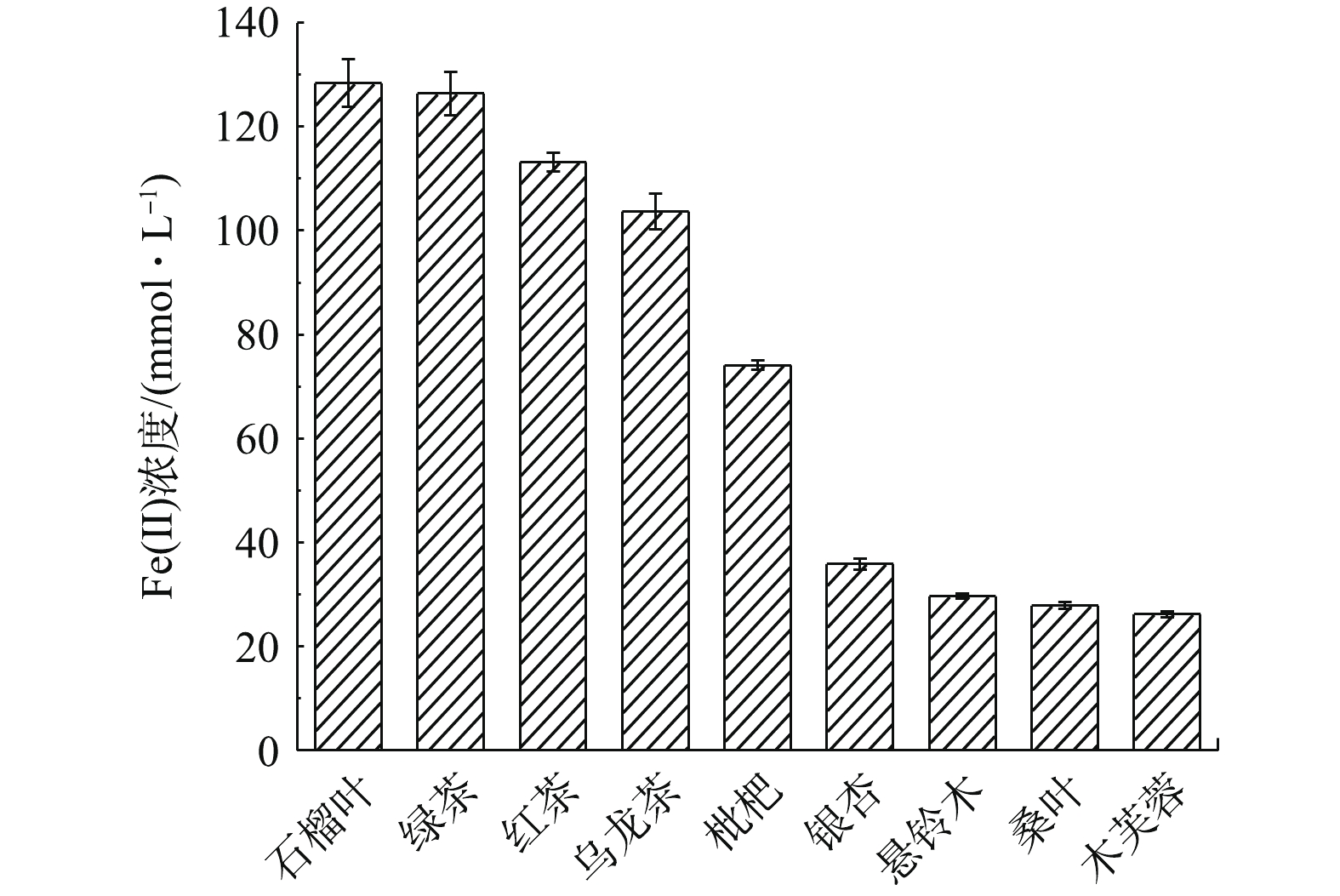
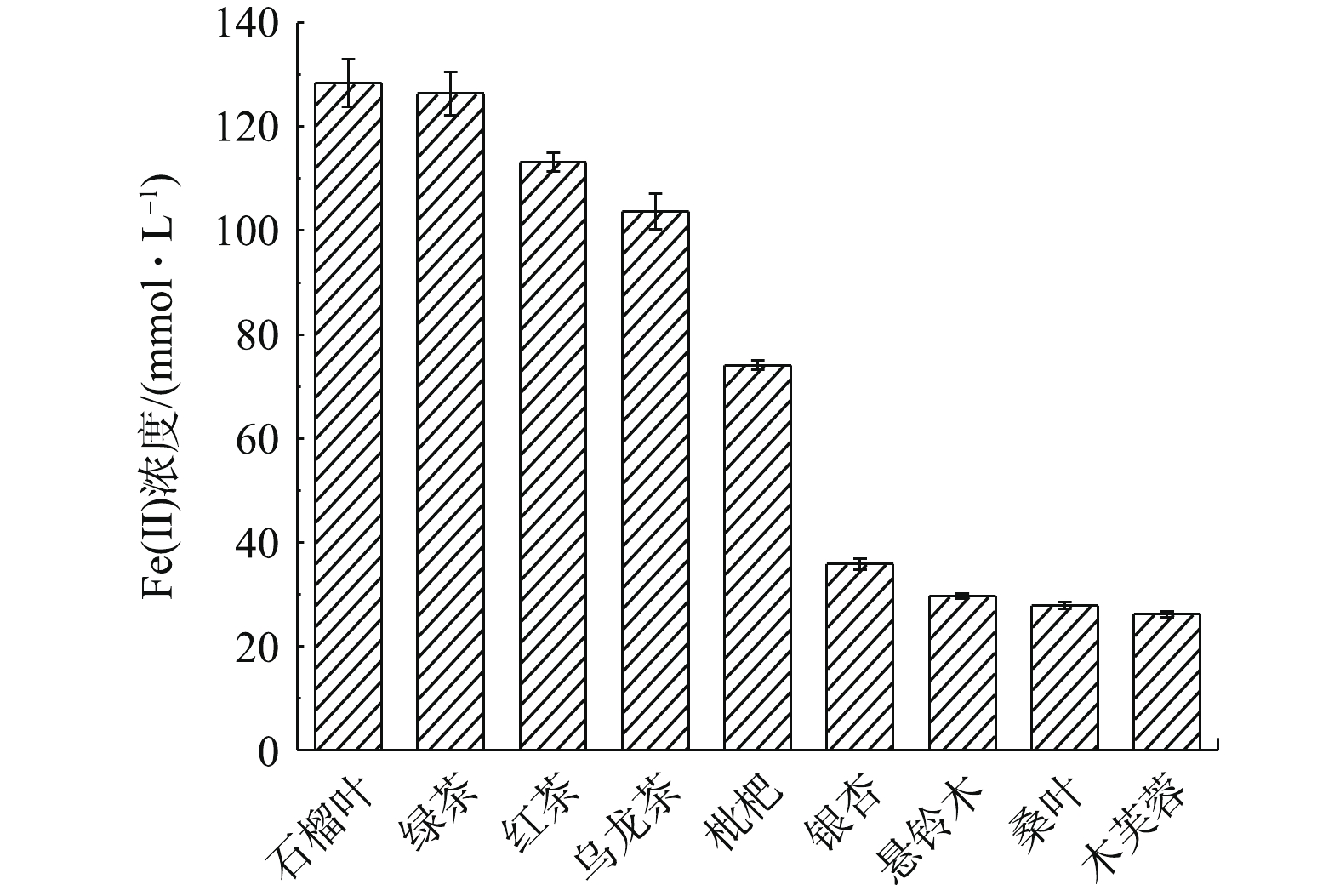
植物多酚是一类存在于植物体内的次生代谢物,具有抗菌、抗病毒和抗氧化的特性[17]。在制备零价纳米铁的过程中,植物多酚的抗氧化活性起到关键作用。利用FRAP法测定9种植物叶子提取液的抗氧化活性,结果如图1所示。
以还原产物Fe(Ⅱ)的浓度作为衡量标准,9种植物叶子提取液的抗氧化能力在25.67~133.48 mmol·L−1以内,其中绿茶、石榴叶提取液的抗氧化能力最强,红茶提取液次之,表明他们可能是作为绿色合成纳米铁最合适的原材料。多酚类物质首先与Fe(Ⅱ)络合,将其还原为Fe0,提取液中包括多酚在内的天然抗氧化有机分子具有多个极性原子和丰富的电子键,能够提供电子至铁原子d轨道的空位上,形成配位键,从而促进了抗氧化有机分子在铁表面的吸附[18],从而将生成的Fe0包裹,故混合液的颜色从黄绿色逐渐加深,最终转变为黑色,以此作为Fe0形成的初步指示,颜色越深则Fe0颗粒的生成率越高。
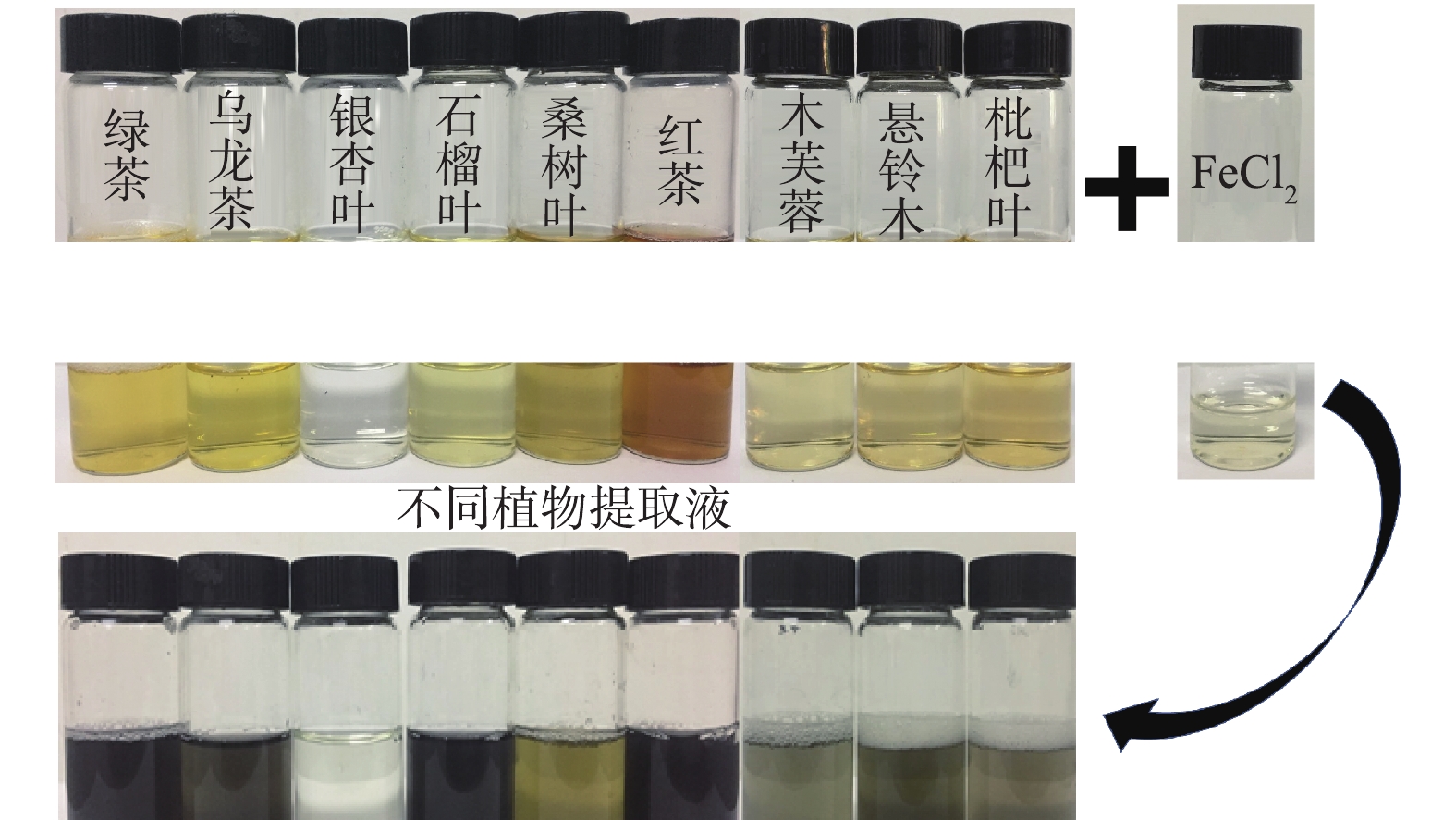
FeCl2溶液与不同植物提取液反应之后的颜色变化如图2所示。反应后颜色明显变黑的是绿茶、石榴叶和红茶提取液,乌龙茶提取液虽然在一定程度上变黑,但仍有明显的提取液原有颜色,这说明Fe0的生成量较少。其余的提取液反应后颜色变化并不明显,表明这些植物提取液对Fe(Ⅱ)的还原效果不佳。以上结果与FRAP法的测试结果一致,因此,在下一步研究中选取绿茶(GT)、石榴叶(PG)和红茶(BT)的提取液用于绿色纳米铁颗粒的合成,生成的纳米铁分别记为GT-nZVI、PG-nZVI及BT-nZVI。
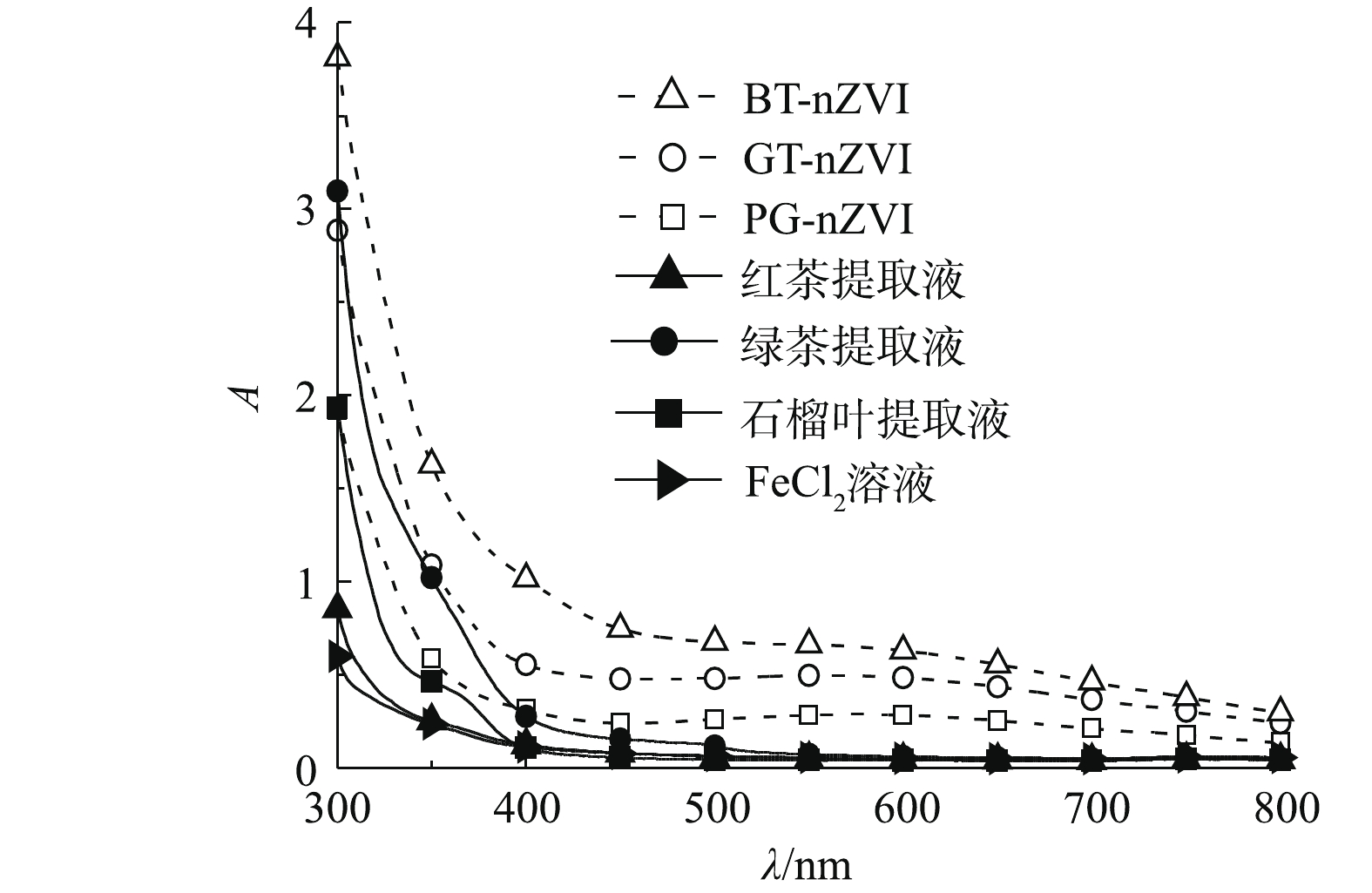
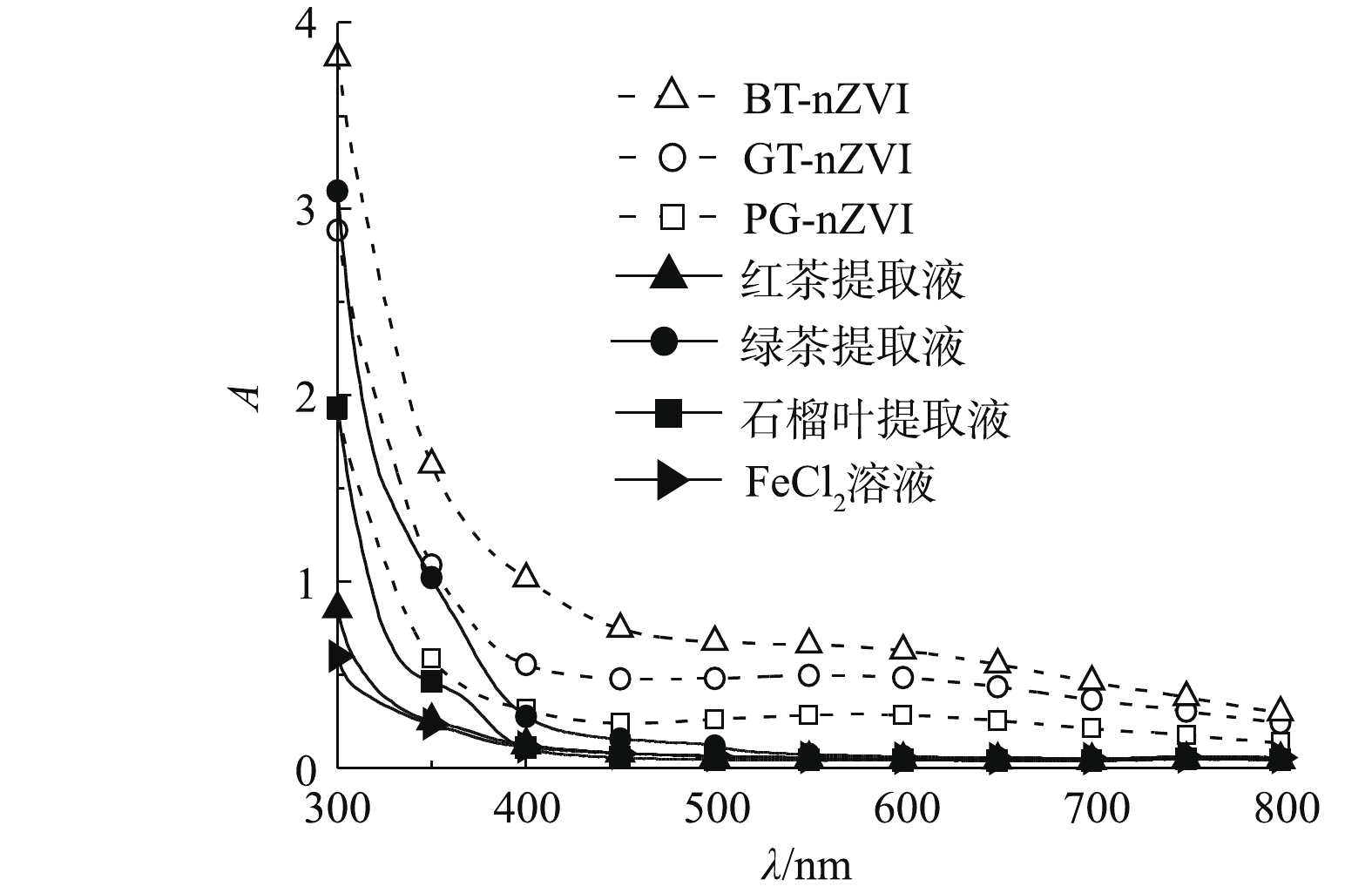
对3种植物提取液、混合反应后的悬浊液及FeCl2溶液进行紫外-可见光谱扫描。全波长扫描结果如图3所示。绿茶(GT)、石榴叶(PG)及红茶(BT)提取液与FeCl2溶液在波长大于500 nm时,吸光度几乎为0,而反应之后的悬浊液在600 nm左右出现较宽的吸收峰,根据相关研究[19-21]表明,这是由于植物提取液与FeCl2溶液反应生成了新的产物,即Fe(Ⅱ)可能被还原为Fe0。
-
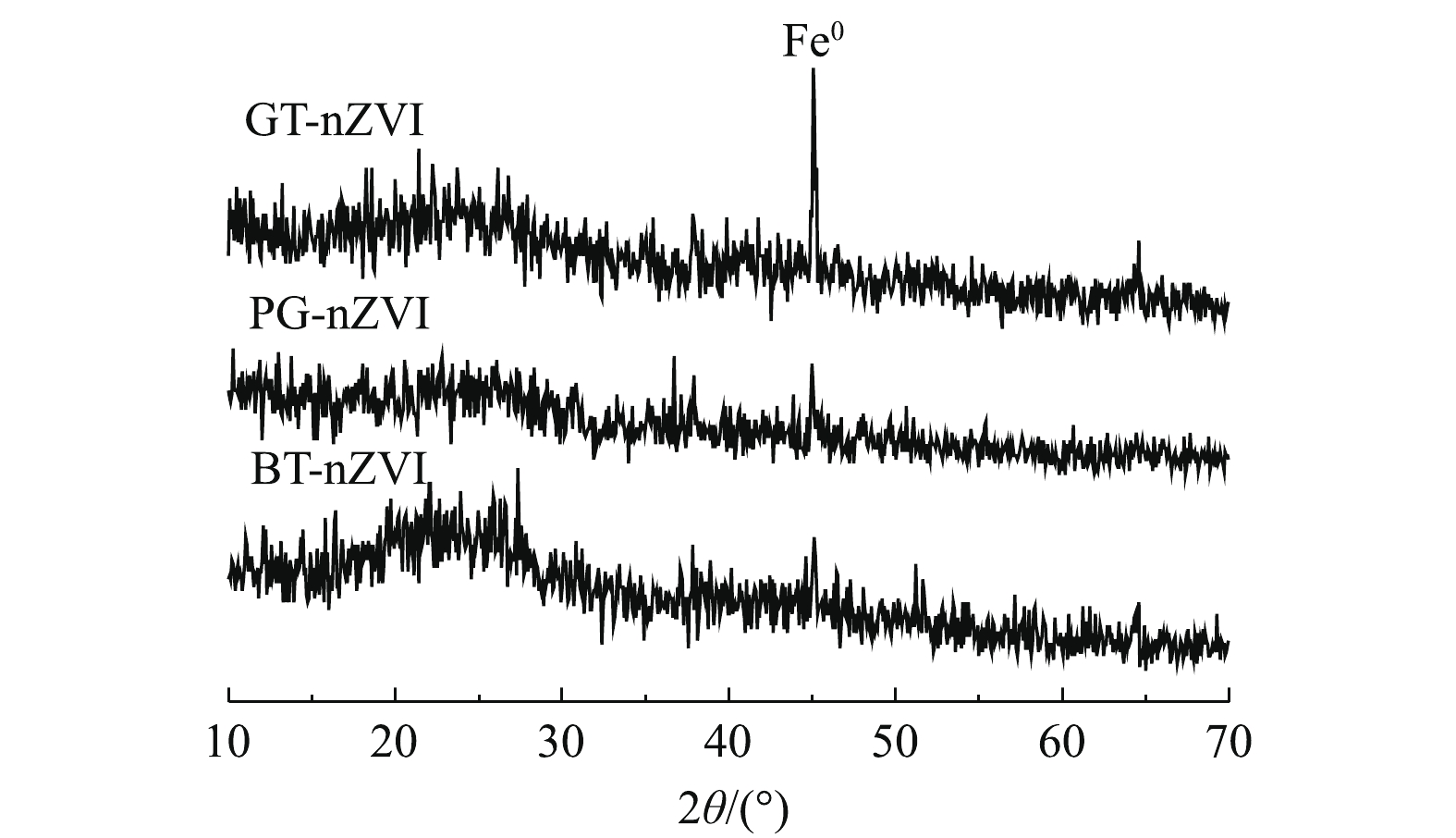
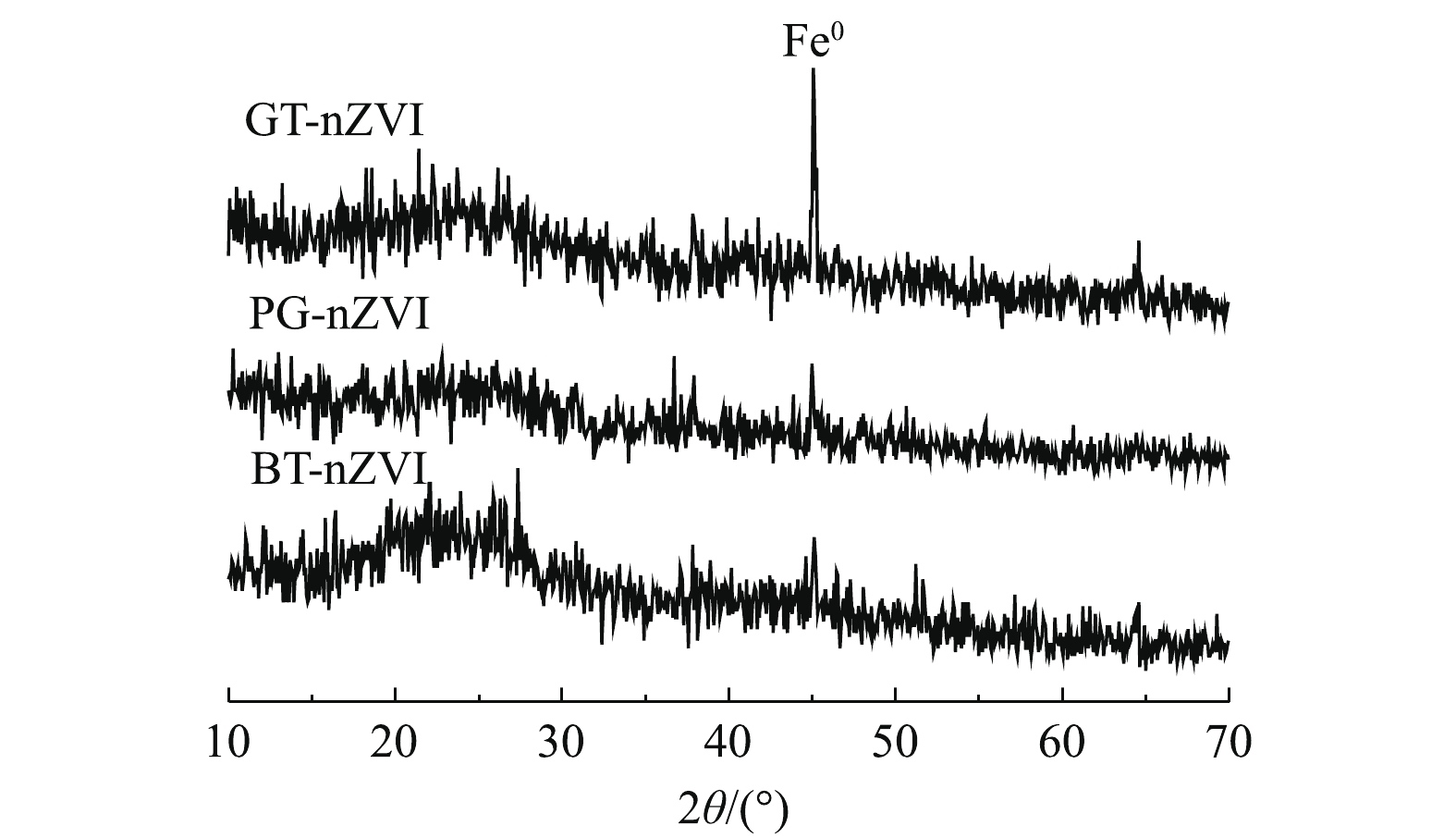
为进一步确认体系中纳米零价铁的生成,将制备的GT-nZVI、PG-nZVI和BT-nZVI进行了XRD测试,结果如图4所示。2θ = 44.9°为Fe0的特征峰[20, 22-23],这表明了纳米Fe0的成功制备。GT-nZVI的衍射峰比另外2种纳米铁更明显,BT-nZVI的衍射峰最微弱,这可能归因于生成的纳米铁颗粒为无定型态或者纳米铁表面包裹的不同有机成分。
-
通过SEM对GT-nZVI、PG-nZVI和BT-nZVI的表面形态进行了观察,由图5(a)可以看出,经过干燥后的GT-nZVI整体为不规则的球状。由图5(b)和图5(c)可知,PG-nZVI、BT-nZVI在干燥后呈现出较明显的团聚,粒径增大,形貌上更接近片状堆叠物,GT-nZVI的粒径最小,分散性能最好。
利用马尔文激光粒度仪分析了GT-nZVI、PG-nZVI及BT-nZVI 3种纳米零价铁颗粒的粒径分布[24],其粒径分别为24~140、43~255、28~221 nm,平均尺寸为88、118、124 nm。一般来说,纳米铁颗粒粒径越小,比表面积则越大,代表其拥有更高的反应活性,因此,基于粒径分析结果可知,GT-nZVI的性能要优于PG-nZVI和BT-nZVI,这与污染物去除批实验结果一致。
-
纳米铁具有较大的比表面积和本身具有的磁性,极易团聚,导致自身比表面积、反应活性以及迁移性降低,易在含水层中沉淀造成介质孔隙堵塞,进而降低含水层的渗透性[25]。而植物提取液制备纳米铁过程中,一些大分子物质包覆在Fe0的表面,能够有效控制纳米Fe0的粒径并减少团聚,从而增强了纳米Fe0悬浊液的悬浮稳定性。
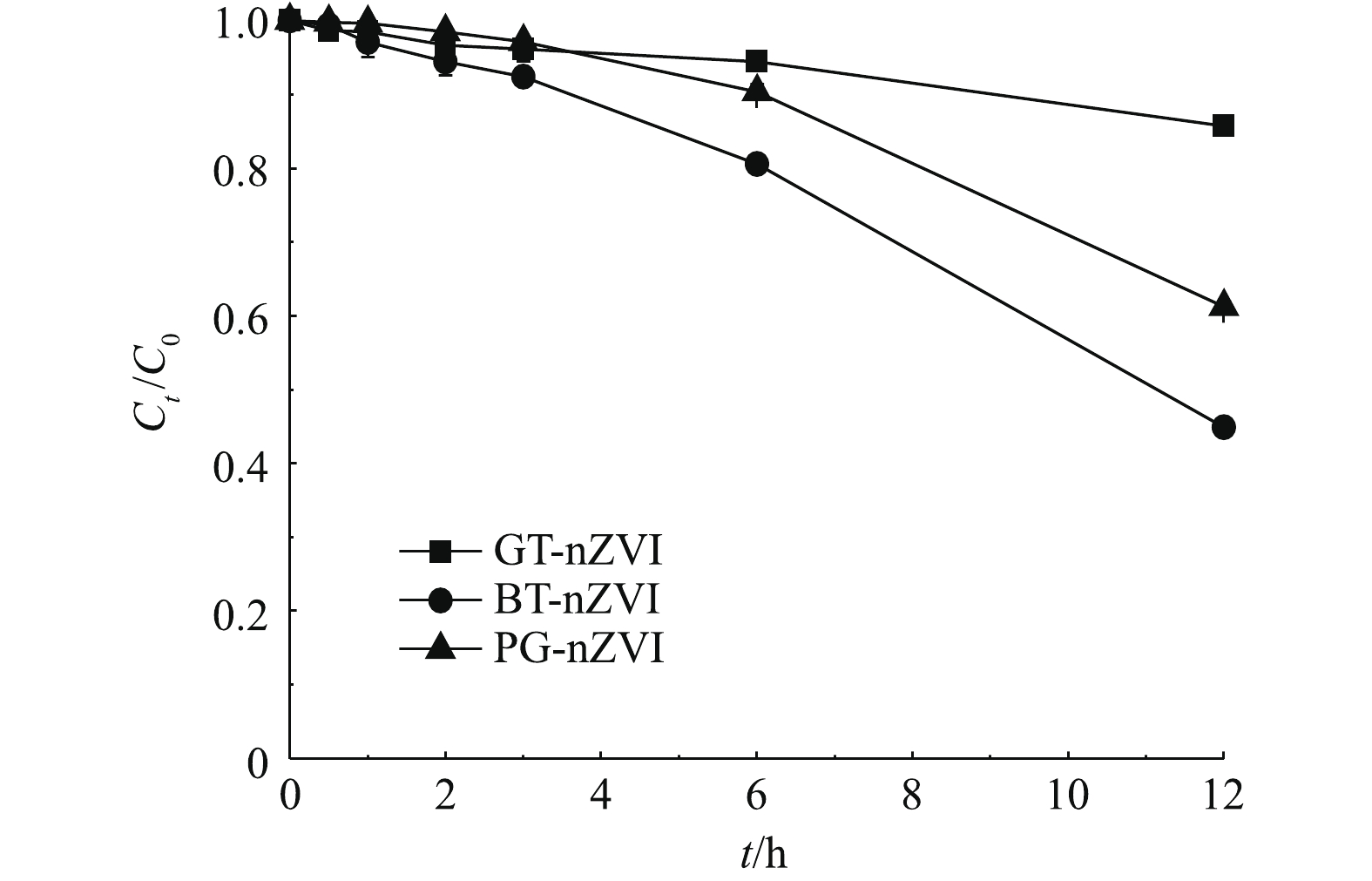
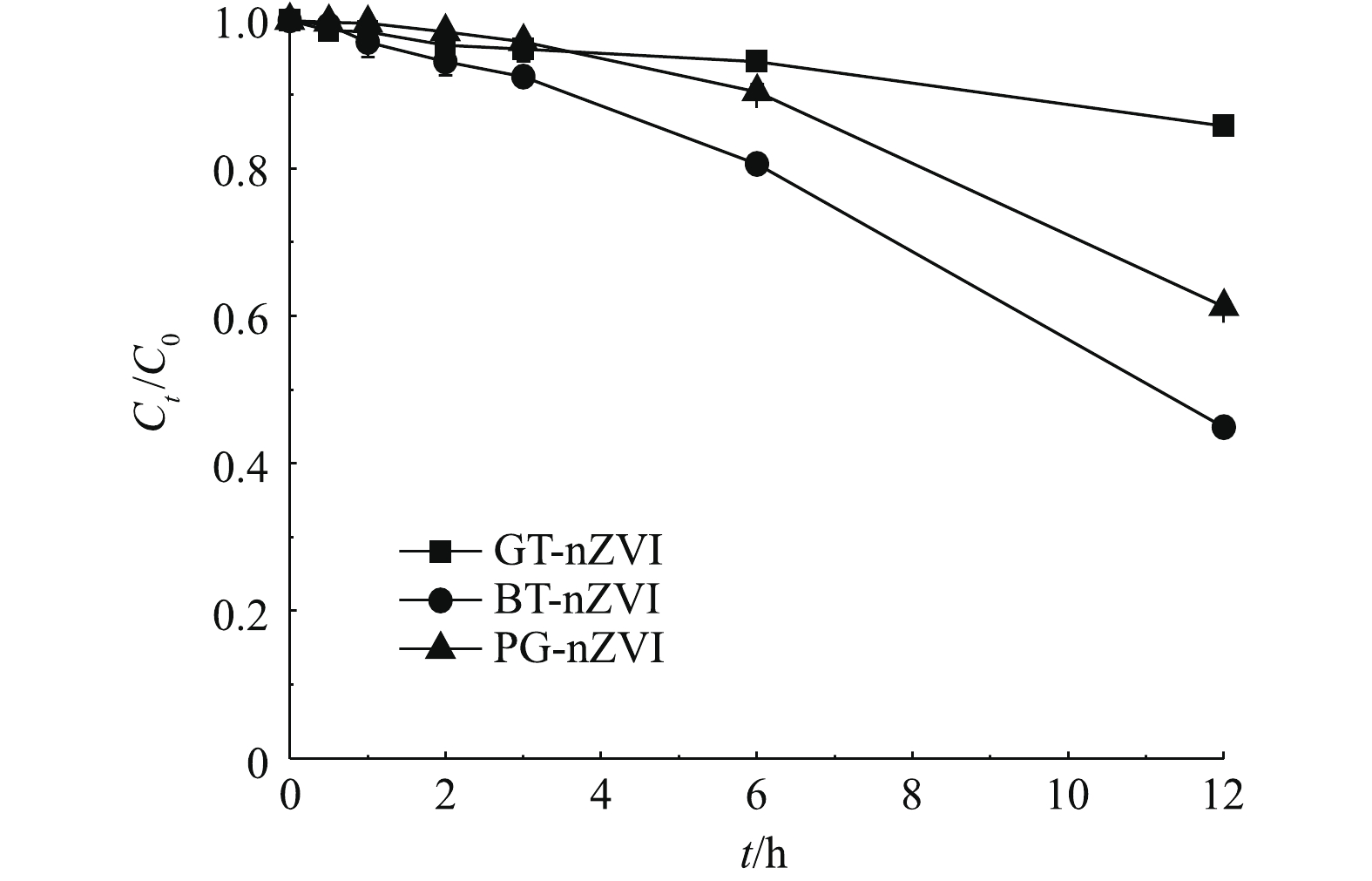
由图6可知,3种悬浊液均含650 mg·L−1的绿色纳米铁,在开始的4 h静置时间内,上清液中的铁含量一直保持在初始浓度的85%以上,在静置6 h后,BT-nZVI的上清液中铁含量约为初始浓度的80%,GT-nZVI与PG-nZVI的上清液中铁含量的比例在90%以上。在静置12 h后,BT-nZVI与PG-nZVI的上清液铁含量分别降至45%与60%,而GT-nZVI则在85%左右。这说明3种绿色纳米铁悬浊液在4 h以内均未出现明显的团聚,而之后的稳定性开始变差,其中,GT-nZVI的悬浮稳定性最好。
-
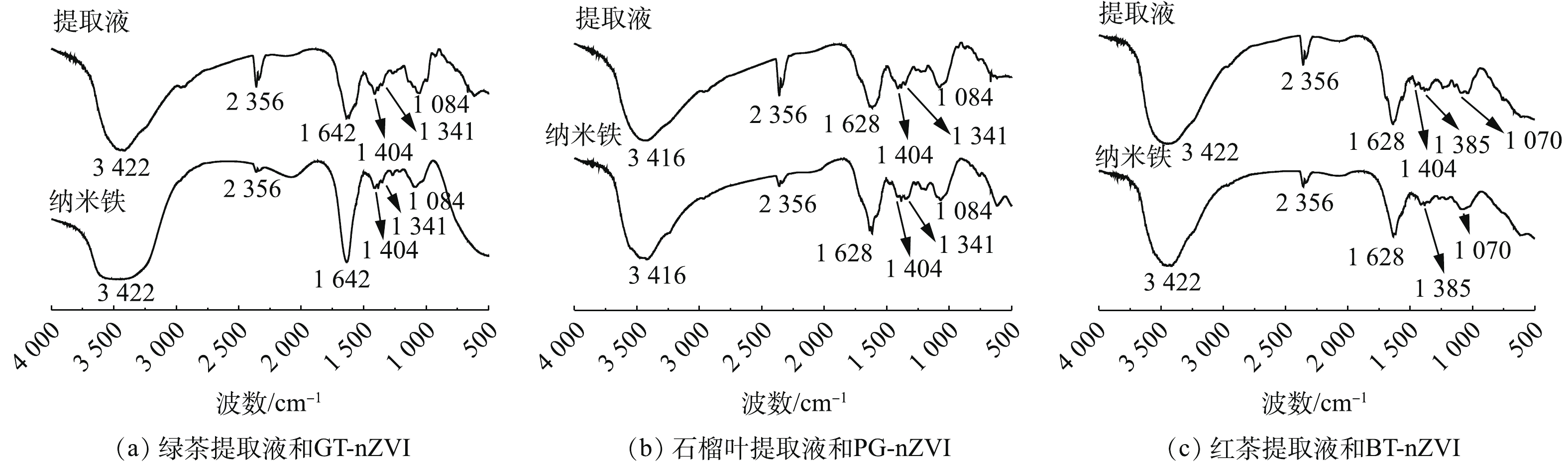
对绿色合成的纳米铁进行了FT-IR测定,以确定不同植物提取液还原FeCl2后潜在的生物分子官能团。图7为3种绿色纳米铁的FT-IR光谱。
红外光谱中3 422、3 416、2 356 cm−1处的峰表示绿色纳米铁和植物提取液存在多酚中的羟基,多酚是还原Fe(Ⅱ)的有效成分,以及纳米铁颗粒表面的覆盖层[22, 26-27]。通过对比提取液与纳米铁的峰强,3 422、3 416、2 356 cm−1处的峰强度明显降低,这表明提取物中的多酚参与了纳米铁的生成。1 642 cm−1和1 628 cm−1附近是C=C的振动吸收峰,当有C=C基团包覆在纳米铁表面时,能够减缓纳米铁的氧化,防止磁性团聚,进而保证了绿色纳米铁在长时间内较高的活性[19, 28],相较于另外2种绿色纳米铁,GT-nZVI在此处的峰强度更高,这说明表面覆盖了更多的C=C基团,故具有更好的稳定性和反应活性。1 404 cm−1附近是C—H键的伸缩振动吸收峰,1 385 cm−1和1 341 cm−1处的吸收峰则对应于芳香胺的C—N键,1 084 cm−1附近吸收峰是—NH的伸缩振动引起的,在约1 070 cm−1处可以看到由C—O键的伸缩振动吸收峰,这意味着酚类、脂肪族胺、氨基酸也可能是合成中的稳定剂[28-31]。
-
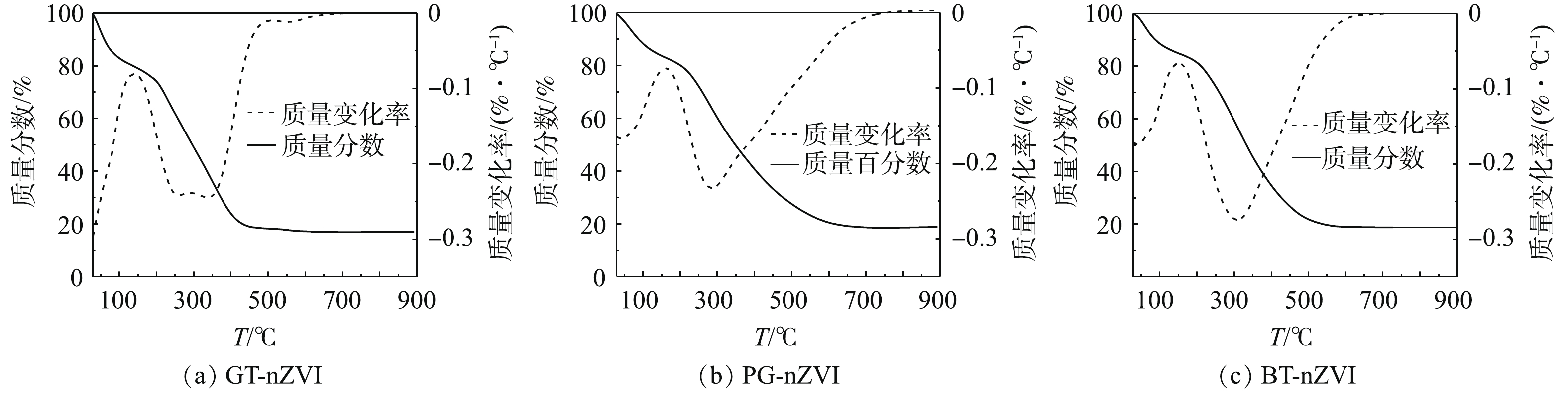
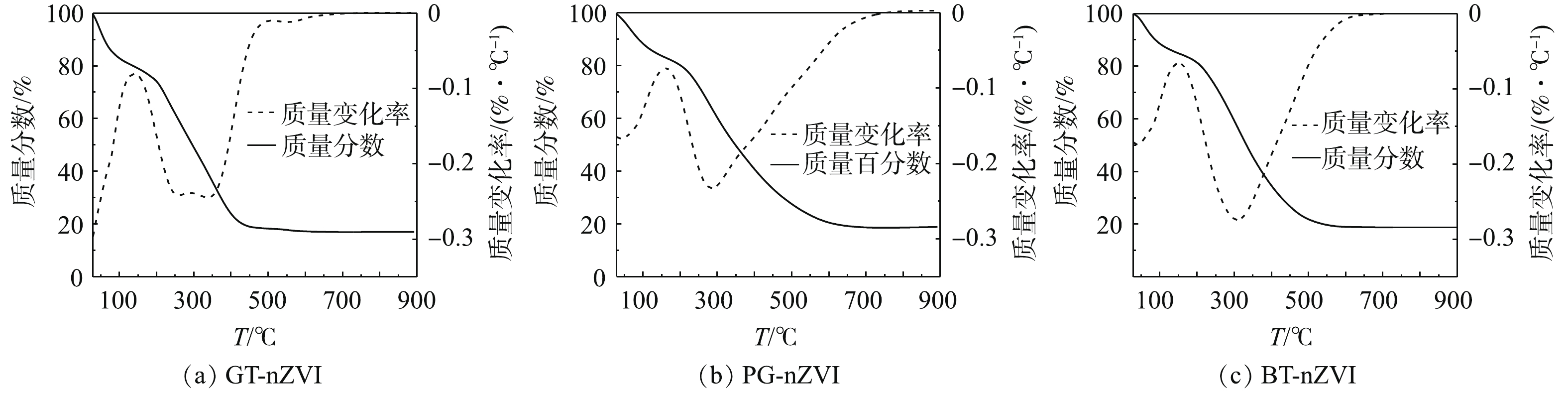
通过热重分析表征了3种绿色纳米铁的热力学效应。图8为GT-nZVI、PG-nZVI和BT-nZVI的热重曲线图,实验在氮气氛围中进行,起始温度29 ℃,终点温度900 ℃,升温速度为10 ℃·min−1。由热重分析曲线(图8)可知,合成的绿色纳米铁随着温度的升高出现了显著的质量损失。当温度从29 ℃升高至100 ℃时,3种绿色纳米铁的质量损失在15.0%左右[32],低于200 ℃的初始质量损失通常归因于残留水分和吸附水分的蒸发。在100~200 ℃的温度范围内,质量变化不明显,有机化合物在高达200 ℃的温度下表现出明显的热稳定性,当温度升到更高时,有机物会发生分解[33]。温度从200 ℃升至600 ℃和700 ℃时,3种绿色纳米铁的质量趋于稳定,包覆在纳米铁表面的有机物分解殆尽,残留物是其中的纳米铁颗粒。GT-nZVI的残留物大约有17.0%,PG-nZVI与BT-nZVI的残留物大约有18.8%。
以上结果表明,绿茶提取液中有更多的有机质包覆在纳米铁的表面,而包覆的厚度与空间位阻呈正相关关系,从而增强了对纳米铁颗粒之间磁力势能、范德华引力的抵抗[34]。结合粒径测试结果可知,3种绿色纳米铁中GT-nZVI的粒径最小,因此,颗粒之间的磁力势能也更弱,促使GT-nZVI的稳定性与分散性最好,与悬浮稳定性测试结果一致。
-
本研究以Cr(Ⅵ)为目标污染物,考察了3种绿色纳米铁的反应活性。在已有研究中,刘勇等[35]以桉树叶提取液绿色合成纳米铁去除Cr(Ⅵ),并与商品化纳米铁的去除效果对比,发现以桉树叶提取液制备的绿色合成纳米铁对铬的去除率可达77.2%,而商品化纳米铁的去除率仅为37.3%。林国庆等[36]使用羊栖菜提取液绿色合成纳米铁去除Cr(Ⅵ),反应90 min后,去除率高达92.76%。赵玲子等[37]利用琼脂改性纳米铁,与水中Cr(Ⅵ)反应180 min后,去除率可达95%以上。
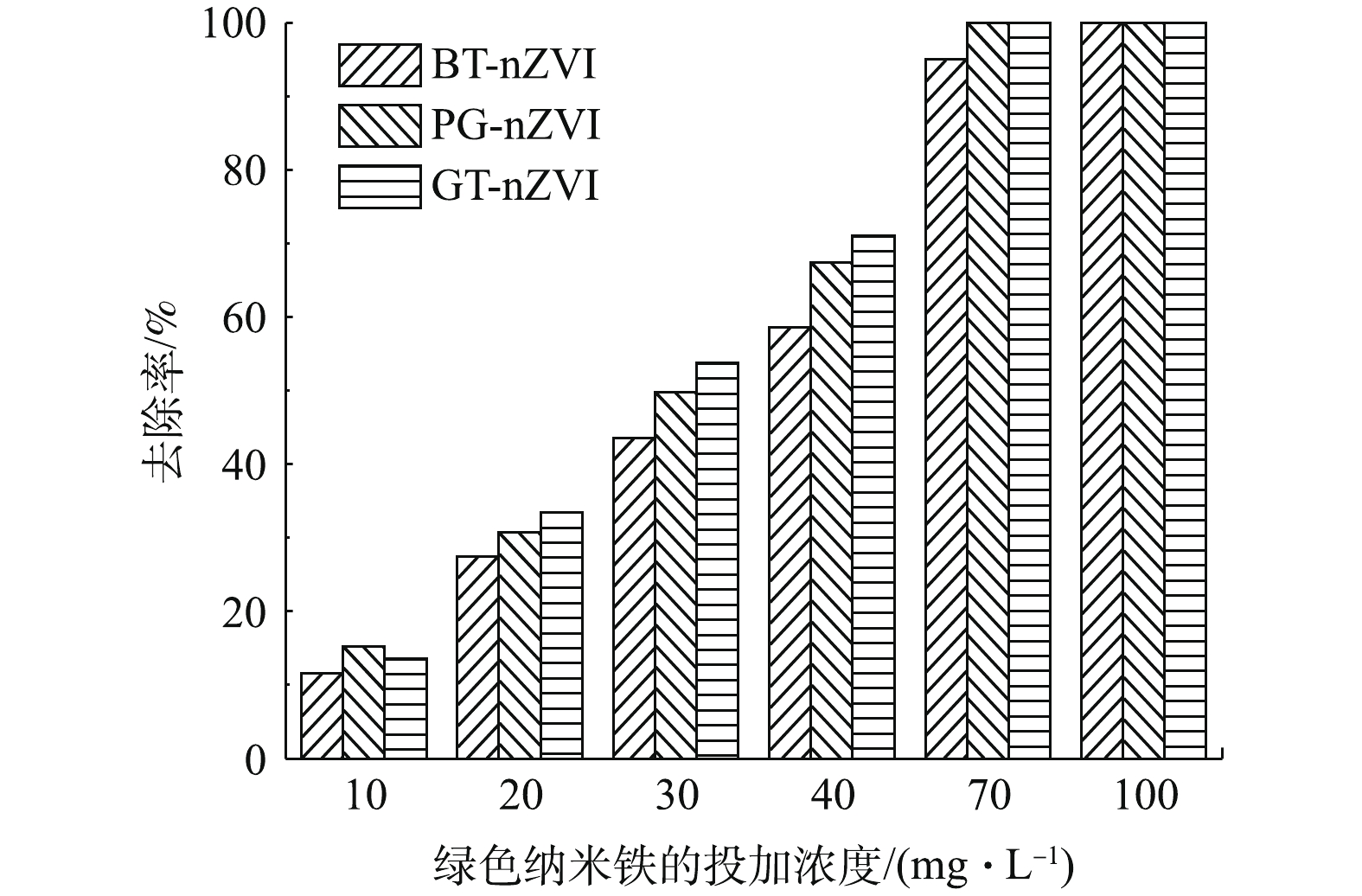
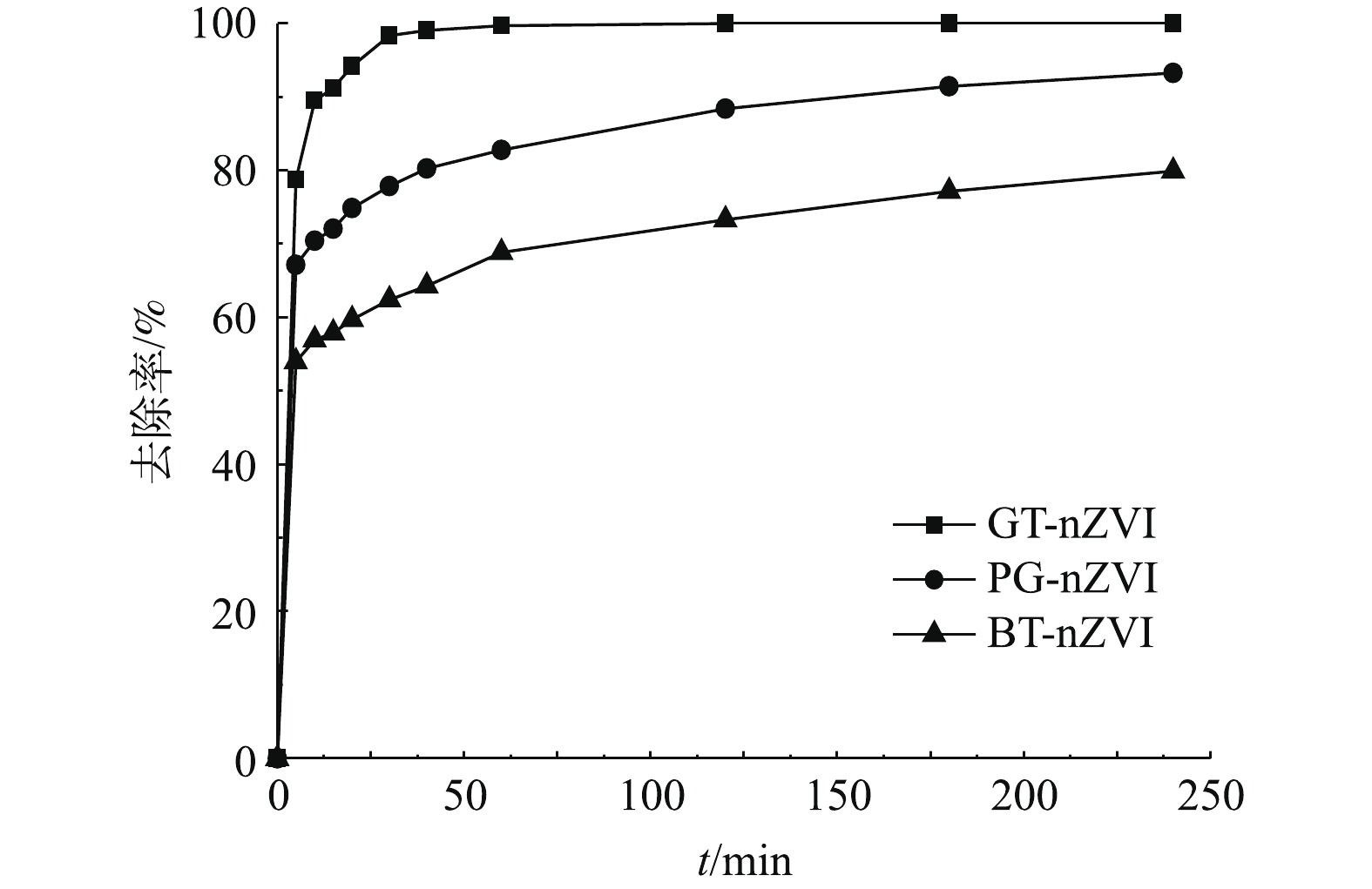
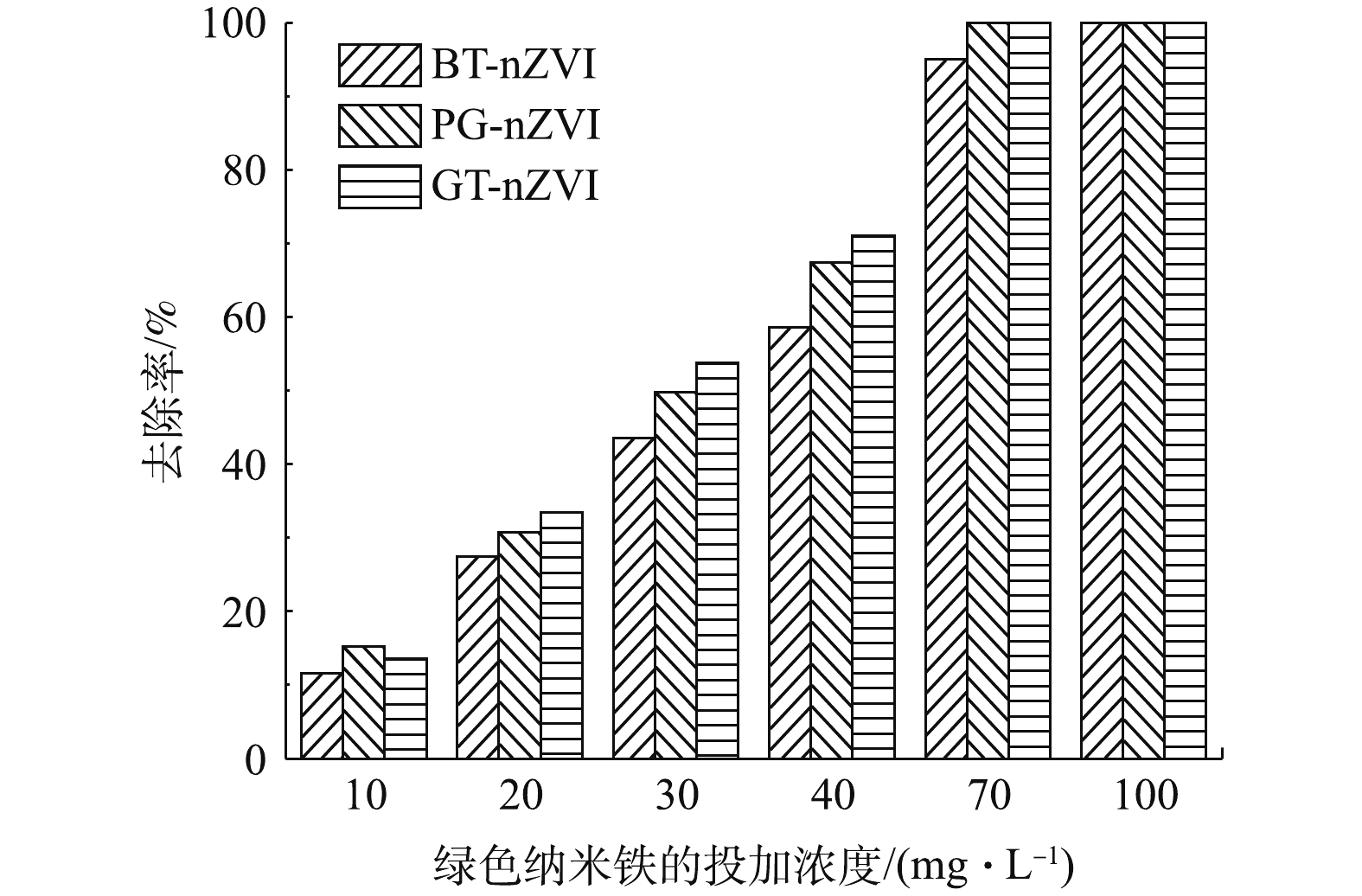
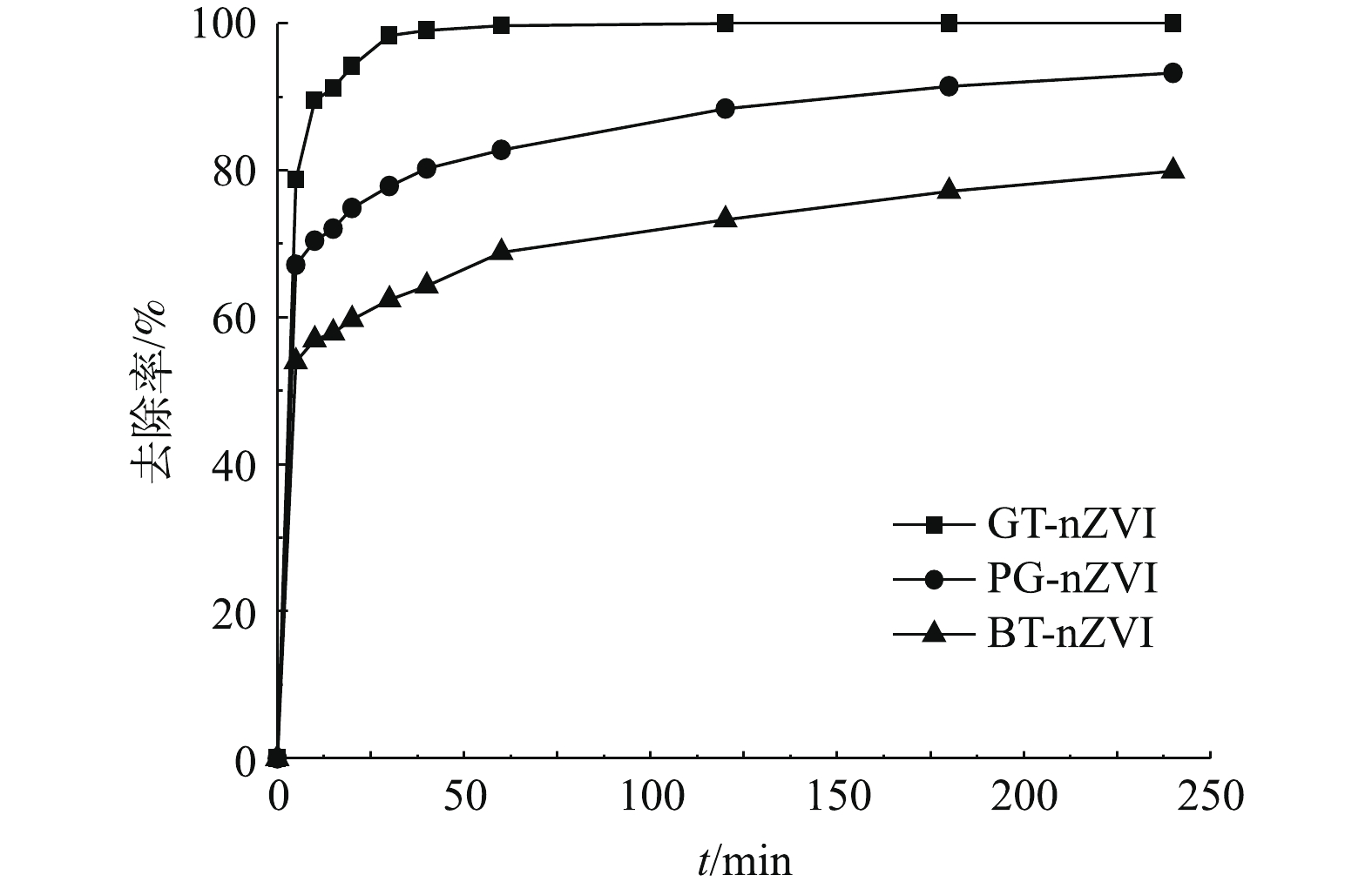
本实验中不同浓度GT-nZVI、PG-nZVI和BT-nZVI在24 h内对Cr(Ⅵ)的去除率如图9所示,由图9可以看出,3种绿色纳米铁对Cr(Ⅵ)都有还原效果。在绿色纳米铁浓度低于70 mg·L−1时,GT-nZVI对Cr(Ⅵ)的去除率最高,PG-nZVI的去除效果与之接近。在初始投加浓度在70 mg·L−1时,GT-nZVI与PG-nZVI对Cr(Ⅵ)的去除率到达100%,BT-nZVI对Cr(Ⅵ)的去除率到达94.9%,随着初始浓度到达100 mg·L−1,3种材料对Cr(Ⅵ)的去除率均达到100%。图10为绿色纳米铁投加浓度为100 mg·L−1时对Cr(Ⅵ)的去除率,反应约4 h达到平衡。其中GT-nZVI在反应时间为60 min时,去除率达到99.7%,可见GT-nZVI与Cr(Ⅵ)的反应速率较快,去除能力强。
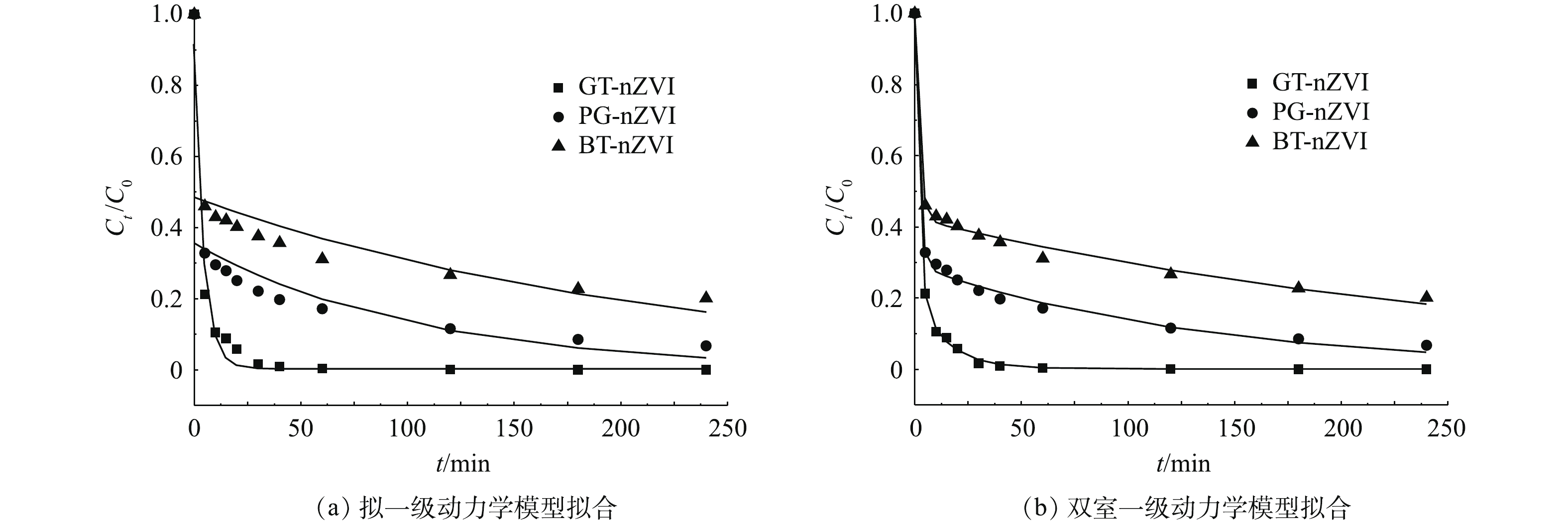
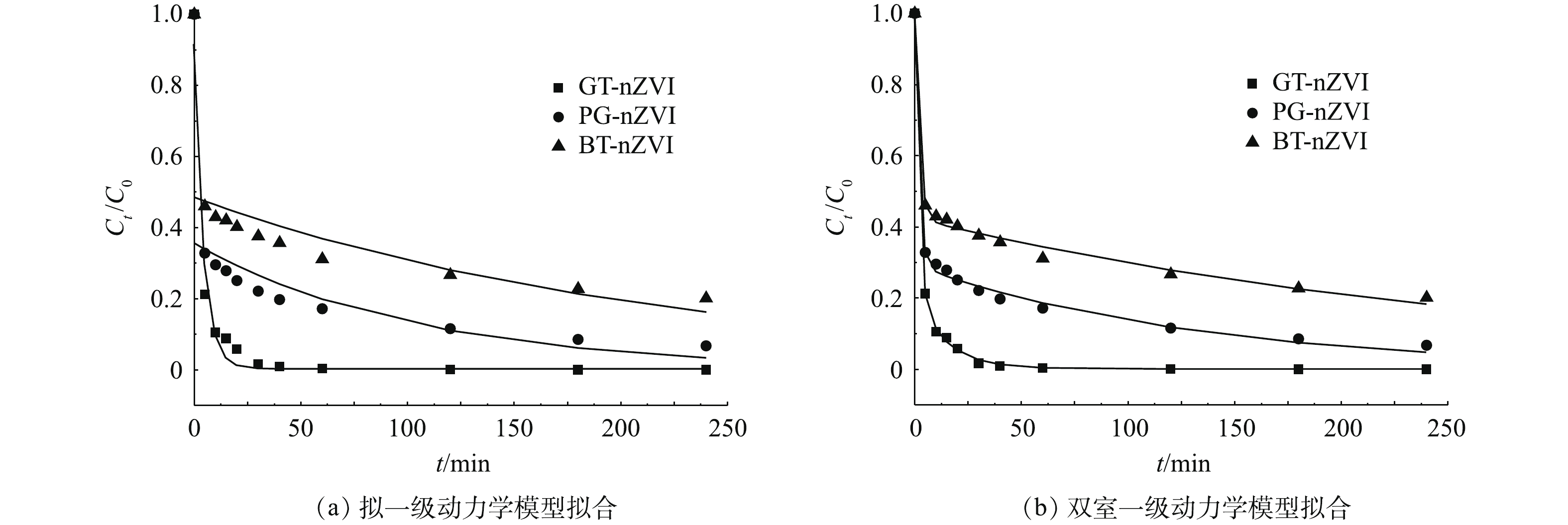
由于绿色纳米铁对Cr(Ⅵ)的去除速率表现出初始快速与随后较缓慢的2个阶段,本实验选用拟一级动力学模型和区分快、慢去除动力学的双室一级模型进行拟合,拟合结果见表1,动力学拟合曲线如图11(a)和图11(b)所示。双室一级模型具有更高的动力学曲线拟合度,其可调可决系数
R2adj 均大于拟一级动力学模型,因此,双室一级模型更适用于描述本实验中绿色纳米铁去除Cr(Ⅵ)的动力学过程。从双室一级模型拟合结果可以看出,绿色纳米铁对Cr(Ⅵ)的去除表现出明显的快室与慢室,其中GT-nZVI对Cr(Ⅵ)的去除反应表现出比其他2种绿色纳米铁更快的速率。具体而言,GT-nZVI的快、慢速去除速率常数(kfast和kslow)均大于PG-nZVI、BT-nZVI的快、慢速去除速率常数。此外,GT-nZVI快速去除约为总去除的0.77,高于PG-nZVI的0.70和BT-nZVI的0.58,表明GT-nZVI的还原反应对其总去除的贡献高于另外2种纳米铁。根据傅里叶红外光谱测试及热重测试结果推测,这是因为GT-nZVI中零价纳米铁表面被包覆了更多的有机质,且含有更多的C=C基团,因此,能够有效防止环境中的自由基对纳米铁的干扰,从而保持纳米铁较高的反应活性。
绿色纳米铁去除Cr(Ⅵ)主要存在还原反应和吸附作用2个方面。初始反应非常迅速,零价铁将重铬酸根还原为Cr(Ⅲ),反应生成的Fe(Ⅱ)可进一步还原Cr(Ⅵ),主要的反应如式(4)和式(5)所示,且零价铁还与混合液中的氢离子、溶解氧反应生成Fe(Ⅱ),继续充当还原剂,混合液中的Cr(Ⅵ)浓度快速降低,如式(6)和式(7)所示。随后的反应由吸附作用占主导,反应速率降低,由还原反应过程中生成Fe(Ⅲ)与Fe(Ⅱ)在混合液中发生水解反应,生成Fe(OH)3和Fe(OH)2,随着这2种沉淀的析出铬离子会被吸附,使得目标污染物进一步被去除[38-39]。
绿色纳米铁颗粒表面有机质含有特定作用的官能团,能够促进对Cr(Ⅵ)的去除,如氨基与羧基能够与Fe(Ⅲ)发生螯合作用,增强对Cr(Ⅵ)的吸附[40];C=C、C—H、C—N等官能团通过吸附作用,增加绿色纳米铁表面Cr(Ⅵ)的浓度,同时还原反应也在进行,因此,绿色纳米铁对Cr(Ⅵ)的去除过程是吸附与还原的共同作用[41-42]。纳米铁的活性与植物提取液的抗氧化性强弱或多酚含量高低呈正相关[21],故结合上述的表征分析结果,与另外2种绿色纳米铁比,GT-nZVI粒径最小、分散稳定性最好,因此,能够更充分地与重金属污染物接触,且具有更快的反应速率和更强的抗氧化性,对地下水污染具有较好的修复效果。
2.1. 零价纳米铁的抗氧化活性
2.2. X射线衍射(XRD)分析
2.3. 绿色合成纳米铁的粒径与形貌
2.4. 纳米铁的悬浮稳定性
2.5. 傅里叶红外光谱(FT-IR)分析
2.6. 热重分析(TGA)
2.7. GT-nZVI、PG-nZVI和BT-nZVI对Cr(Ⅵ)的去除效果
-
1)筛选的3种绿色纳米铁的悬浮稳定性由高到低为GT-nZVI、PG-nZVI、BT-nZVI。其中,GT-nZVI能在12 h内保持上清液中铁含量在初始浓度的80%以上。
2)测试分析结果表明,GT-nZVI呈现椭球状,PG-nZVI和BT-nZVI形貌上更接近片状堆叠物,平均粒径分别为88、118、124 nm,表面被大量有机物包裹,其中GT-nZVI表面包裹的有机质含量最高。酚类化合物在还原Fe(Ⅱ)的反应中起到了主要作用,酚类、氨基酸、脂肪族胺可能在合成中起到了表面保护的作用。
3) 3种绿色纳米铁对Cr(Ⅵ)的去除过程中既有还原反应也有吸附作用,还原反应起主导作用。其中GT-nZVI具有更高的还原性,较小的粒径,对Cr(Ⅵ)的去除速率最快。





 下载:
下载: