-
全球工业化与城市化的高速发展导致重金属严重污染水资源,人类社会正遭受严峻的健康挑战[1-3]。铅作为一种毒性重金属,由于其在生态系统中具有生物累积性和持久性的特点,已被世界环保组织列为优先治理的一种污染物[4]。长期饮用含铅废水会损坏人体器官,增加癌症的发病率。目前,污水中铅的治理技术主要有化学沉淀、膜过滤、电渗析以及生物修复等,但这些技术在实际的污水处理中存在诸多限制因素,且可能会带来二次污染[5-7]。吸附法因其具有成本低、高效、安全、无副产物生成等优点被认为是当前最具应用前景的技术[8]。
生物炭是由生物质原料经高温无氧或微氧热解而成的高度芳香化的多孔富炭固体。因其具有优良的表面性能(大比表面、多孔结构、丰富的官能团等)、来源广泛、对环境友好等优点,受到各研究领域高度关注[9]。近年来,生物炭在治理污水中的有机污染物与重金属方面表现出了极大的潜力,是一种应用范围较为广泛的环境污染吸附剂[10-11]。有研究[9]发现,生物炭的表面理化性质决定其吸附性能,而原料与热解条件(尤其是热解温度)又是影响生物炭表面理化性质的主要因素。因此,选择合适的原料以及适宜的热解温度是生产高效吸附产品的必要条件。
我国食用菌产业规模庞大,2017年食用菌总产量约为3.7×107 t,随之而产生的菌糠总量高达6.0×107 t[12]。大规模的菌糠堆积会对生态环境造成巨大压力,同时也对废弃物处理工作提出了严峻的挑战。菌糠的传统处理方式为室外堆积或就地焚烧,这样不仅浪费资源,而且污染环境[13]。由于菌糠表面富含羧基、羟基、磷酸基团与羰基等官能团,国内外学者已将其作为重金属吸附剂进行了大量研究。HU等[14]研究发现,香菇菌糠吸附Cu2+主要是由于其表面富含羟基、氨基以及羧基等官能团;宋涛[15]利用NaOH改性黑木耳菌糠吸附水中的Pb2+,其最大吸附量达到了49.53 mg·g−1;JIN等[16]根据Langmuir模型计算得出平菇菌糠对Cd2+的最大吸附量为87.2 mg·g−1。然而,关于以菌糠为原料制备生物炭去除水体中的Pb2+研究却鲜有报道,且其吸附性能及吸附机制尚不清楚。因此,本研究以平菇菌糠为生物质原料,在250、450和650 ℃下慢速热解制备生物炭,通过BET、SEM-EDS、XRD、FT-IR等多种现代化技术对生物炭样品进行表征,采用批量吸附实验研究其对水体中Pb2+的吸附特性,利用机制定性、定量分析实验,深入解析其吸附机理,为固废管理以及污水中Pb2+的去除提供参考。
-
平菇菌糠(PS)取自山西农业大学食用菌研究中心,样品经60 ℃烘干48 h后,粉碎筛分至1 mm。将粉碎后的样品装入100 mL耐高温瓷坩埚中,经压实密封后,放入马弗炉。热解前,充入N2 10~20 min,马弗炉的升温速率设置为10 ℃·min−1,然后分别在250、450、650 ℃下热解2 h,热解温度的选择参考文献中的方法[17-18];待自然冷却至室温后,研磨过100目筛,最后将制备样品收集在密封袋中保存,以备进一步实验利用。根据原料名称和热解温度,将平菇菌糠生物炭分别命名为PS250、PS450和PS650。
-
采用马弗炉测定PSBC灰分,灼烧温度为800 ℃;使用pH计(PHS-4C+,上海,雷磁)测定PSBC的pH,在测定前,振荡1 h,固水比设为1∶20;使用元素分析仪(Vario Macrocube,德国,Elementar)测定PSBC 中的C、H、N的含量,根据质量平衡计算O含量,并依次计算O/C与H/C值;使用扫描电镜能谱仪(JEOL,JSM-6490LV,日本,Hitachi)对PSBC吸附前后的表面形态进行表征,放大4 000倍;基于Brunner-Emmet-Teller(BET)与Barrett-Joyner-Halenda(BJH)理论,使用比表面与孔径分析仪(ASAP 2020,美国,Micromeritics)测定PSBC比表面积、孔径及孔径体积;使用Zeta电位分析仪(ZS90,英国,Malvern Panalytical)测定PSBC表面Zeta电位;使用X射线衍射仪 (XRD,D8 Advance,德国,Bruker)在2θ为2°~70°内扫描获得XRD图像,分析PSBC吸附前后表面晶体结构;使用傅里叶红外光谱仪(FTIR,Nicolet,美国,Thermo)对PSBC吸附前后的表面官能团进行定性分析,样品经KBr压片后,在波数500~4 000 cm−1内扫描64次获得光谱。
-
在批量吸附实验中,Pb2+储备液(1 000 mg·L−1)采用Pb(NO3)2与蒸馏水制备,以0.01 mol·L−1 NaNO3作为溶液背景电解质;在后续实验中,所需Pb2+浓度通过稀释储备液获得;稀释后的Pb2+溶液经0.1 mol·L−1的HNO3和NaOH调节pH后,在恒温振荡箱中分批进行吸附实验。
在考察初始pH对吸附的影响时,将Pb2+储备液稀释至300 mg·L−1,然后调节溶液pH至3~7(过高的pH会导致沉淀产生)。准确称取0.05 g 生物炭样品(PS250、PS450与PS650),分别加入100 mL锥形瓶中(含40 mL稀释液),在25 ℃下,以150 r·min−1转速振荡24 h后,过滤取样。滤液中Pb2+浓度使用ICP-OES(Optima 5300 DV,美国,Perkin Elmer)测定,实验重复3次,根据吸附量结果确定最佳pH。
生物炭的平衡吸附量(qe)与去除率(ƞ)分别由式(1)和式(2)计算。
式中:qe为平衡吸附量,mg·g−1;η为去除率;C0与Ce分别为Pb2+溶液的初始浓度与吸附平衡浓度,mg·L−1;V为溶液体积,mL;m为生物炭的质量,g。
在进行吸附动力学实验时,将Pb2+储备液稀释至300 mg·L−1,溶液pH调节为6.0。分别称取0.05 g生物炭加入100 mL锥形瓶中(含40 mL稀释液),在室温下以150 r·min−1转速振荡24 h,并分别在预定时间取样过滤,滤液中Pb2+的浓度由ICP-OES测定,实验重复3次。
利用准一级动力与准二级动力模型拟合生物炭对Pb2+的吸附过程,方程如式(3)和式(4)所示。
式中:qt与qe分别为t时刻和吸附平衡时的Pb2+吸附量,mg·g−1;k1为准一级吸附速率常数,min−1;k2为准二级吸附速率常数,g·(mg·min) −1。
在进行吸附等温线实验时,将Pb2+储备液分别稀释至100、200、300、400、500、600和700 mg·L−1,溶液pH调节为6.0。分别称取0.05 g生物炭加入含40 mL不同浓度稀释液的100 mL锥形瓶中,在室温下以150 r·min−1转速振荡后过滤,使用ICP-OES检测滤液中剩余Pb2+的浓度,实验重复3次。
利用Langmuir与Freundlich模型对吸附等温线进行拟合,方程如式(5)和式(6)所示。
式中:qe与qmax分别为平衡吸附量和最大吸附容量,mg·g−1;Ce为吸附达到平衡时溶液中Pb2+的溶度,mg·L−1;kL为与吸附强度有关的Langmuir常数;kF与n分别为反应吸附容量和吸附强度的Freundlich常数。
定量分析生物炭吸附Pb2+机制主要围绕3种吸附作用[8, 17]:1)生物炭与Pb2+之间的沉淀作用(Qp),酸洗会去除生物炭大部分矿物质,但对表面官能团无影响,脱矿前后生物炭对Pb2+吸附量变化值则为沉淀机制贡献的吸附量(Qp),计算方法如式(7)所示;2)含氧官能团对Pb2+的络合作用(Qf),由于羟基、羧基等含氧官能团的存在,经酸洗后的生物炭,吸附Pb2+前后的pH会降低,具体反应方程如式(8)和式(9)所示,据ZHANG等[19]的研究报道,将pH变化值换算成H+的浓度,即为含氧官能团的络合对Pb2+贡献的吸附量(Qf);3) π电子与Pb2+之间的相互作用(Qπ),酸洗后的生物炭对Pb2+的吸附量(Qa)是由含氧官能团的络合以及π电子的结合共同作用的结果,因此,π电子结合作用对Pb2+贡献的吸附量(Qπ)由式(10)计算。不同吸附机制的Pb2+吸附量对总吸附量(Qt)的贡献率分别由Qp/Qt、Qf/Qt与Qπ/Qt表示。
式中:Qt为总吸附量,mg·g−1;Qp为沉淀机制贡献的吸附量,mg·g−1;Qf为含氧官能团的络合贡献的吸附量,mg·g−1;Qa为π电子结合贡献的吸附量,mg·g−1。
-
准确称取0.05 g生物炭,置于40 mL浓度为300 mg·L−1的Pb2+稀释溶液中,吸附24 h后,过滤获得负载Pb2+的固体样品。选用0.1 mol·L−1 HCl作为解吸剂[18],分别称取0.05 g负载Pb2+的生物炭加入含40 mL HCl的100 mL锥形瓶中,解吸1 h,之后通过离心法进行固液分离,收集滤液待测。同时,使用去离子水对分离出的固体样品进行多次冲洗后,分别加入40 mL含Pb2+稀释液(300 mg·L−1),再次进行吸附、过滤,实验条件与1.3节中的描述一致。使用ICP-OES测定剩余滤液的Pb2+浓度,实验重复3次。
-
实验考察了热解温度对PSBC性质的影响。3种热解温度条件下制备的PSBC主要理化性质见表1。随热解温度的升高,PSBC的产率逐渐降低,灰分含量与pH明显增加。同时,PSBC中的C元素含量增加,O、H与N元素含量降低。O与H含量降低主要是由于PS在热解过程发生了脱水作用,N含量降低可能原因是高温导致挥发性含氮物质(
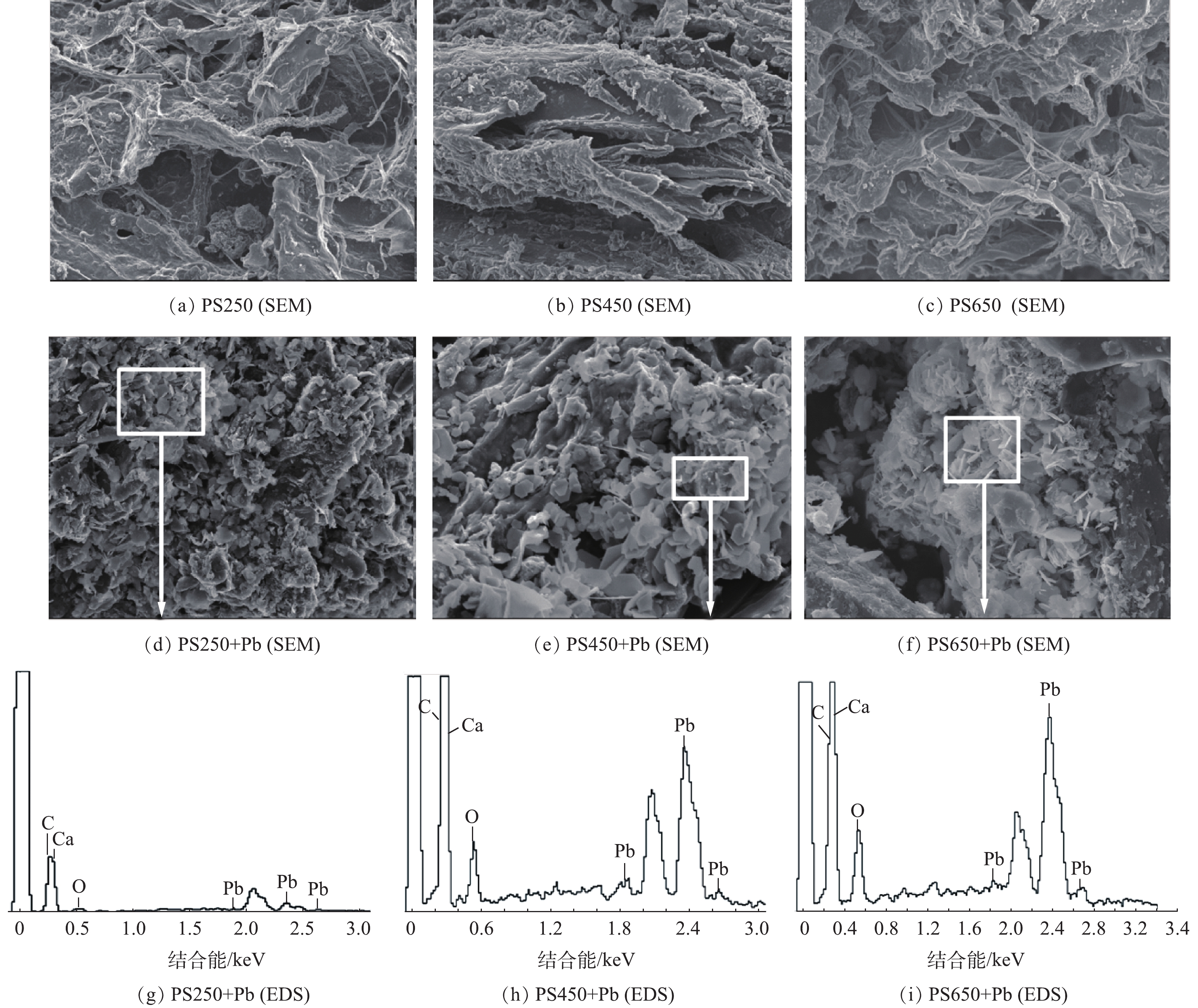
NH+4/NO−3 )损耗[20]。O/C与H/C指标可分别反映生物炭的炭化程度和芳香性[21]。随着热解温度的升高,O/C与H/C均明显降低。这表明高温下制备的PSBC炭化程度高且具有较好的芳香性。这与ZHANG等[19]报道的生物炭在高温下形成更多芳香结构的研究结果一致。如表1所示,PS250、PS450、PS650的Zeta电位分别为−29.7、−27.2与−26.4 mV,表明热解温度对PSBC表面所带负电荷量影响较小。生物炭表面带负电荷,有利于吸附溶液中重金属阳离子。基于BJH理论计算出的不同PSBC的孔径结果表明,3种生物炭的平均孔径均介于2~50 nm之间,属于介孔结构[3]。随着热解温度由250 ℃上升至650 ℃,PSBC的比表面由5.37 m2·g−1大幅度增加为75.63 m2·g−1,孔径体积由0.01 cm3·g−1增加为0.11 cm3·g−1。这说明高温热解(650 ℃)制备的生物炭具有较高的比表面积和良好的孔径结构。这些特点可为其吸附水体中Pb2+提供更多的结合点位[3],从而有利于重金属Pb2+的去除。图1(a)~图1(c)为不同PSBC的扫描电镜图谱。在放大4 000倍的条件下观察发现,随着热解温度的升高,PSBC表面变得越来越粗糙,无规则,且形成了更多的孔隙结构。这说明较高的热解温度有利于多孔结构的形成,同时也验证了随热解温度的升高比表面积增大的结论。图1(d)~图1(i)为吸附后不同PSBC的扫描电镜图谱,可以看出,吸附后的PSBC表面均有白色针状物质的出现。对这些物质进行EDS分析后结果表明,吸附后的PSBC表面附着了一些含Pb2+的化合物,且能谱图中代表Pb2+的吸收峰随着热解温度的升高而变得更加尖锐。
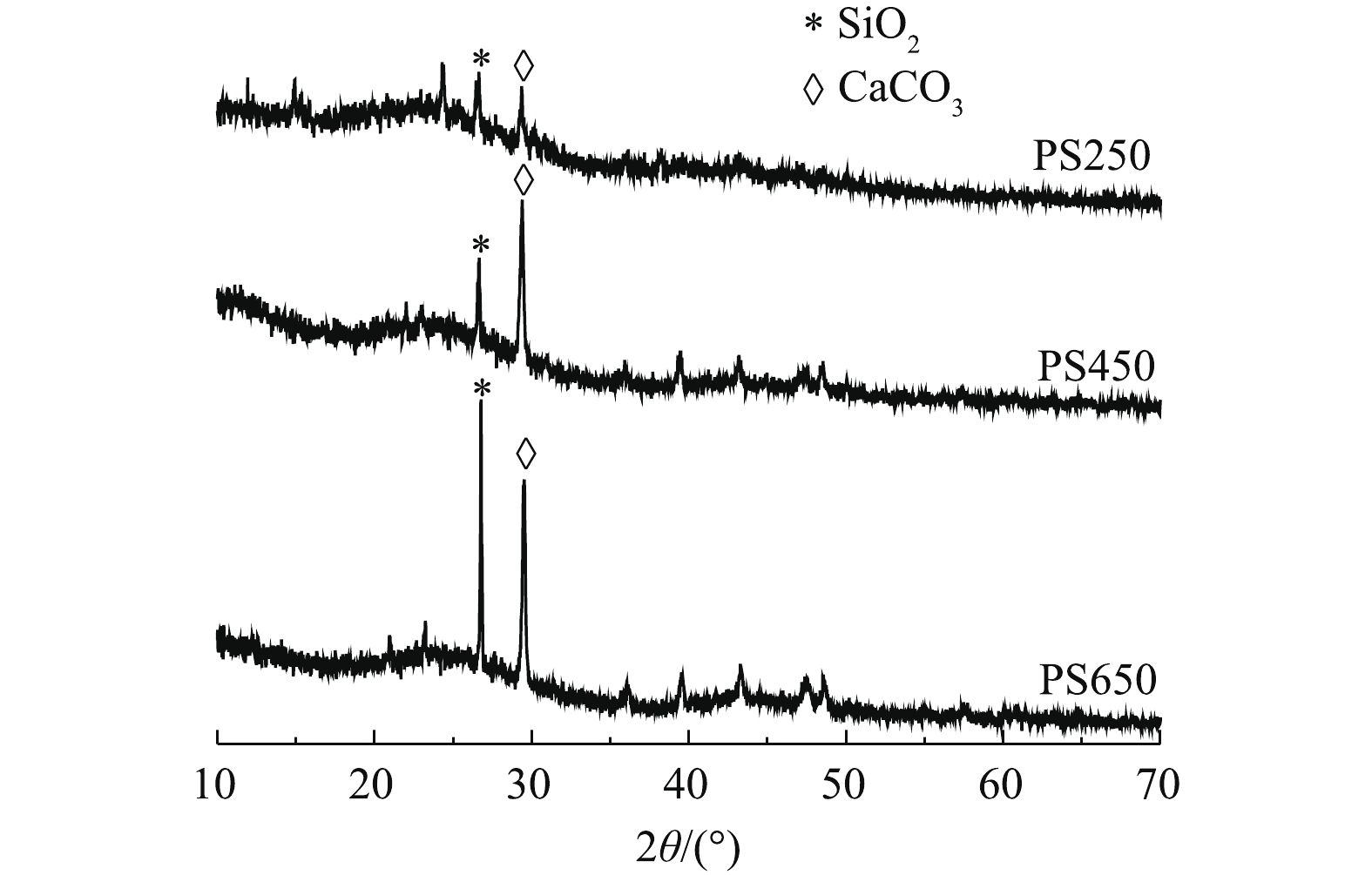
图2为不同PSBC的XRD衍射图谱,可以看出,热解温度对XRD衍射峰强度影响较大。3种生物炭的XRD图谱均出现2种明显的衍射峰,分别代表SiO2(2θ=26.6°)与CaCO3(2θ=29.4°)晶体相,说明PSBC富含石英和方解石。随热解温度的升高,代表SiO2(2θ=26.6°)与CaCO3(2θ=29.4°)衍射峰的峰值强度明显增强,表明高温热解有利于形成更多的SiO2和CaCO3矿物,从而间接解释了生物炭的灰分含量随热解温度的升高而增加的现象。同时,PS650中富含CaCO3,其可释放到溶液中与Pb2+形成沉淀,促进对Pb2+的吸附[19]。
不同PSBC的红外光谱图见图3。从图3中可清楚观察到不同PSBC在3 417、1 638、1 585、1 413、1 384、875与762 cm−1处存在明显吸收峰,表明其富含官能团。随热解温度由250 ℃上升至650 ℃,3 417 cm−1处的—OH伸缩振动峰[22]、1 638 cm−1处的羧基与羰基C=O振动峰[23]、1 585 cm−1处的芳香族C=O吸收峰[18]以及1 384 cm−1处的羧酸C—O环振动峰均减弱[17],这主要是由于高温热解的脱水、脱羟和脱羧作用。这些含氧官能团的损失可能会影响PSBC对Pb2+的吸附能力[23]。875 cm−1处出现的振动带为CaCO3晶体典型吸收峰[7],随热解温度的升高,其峰值强度增强,说明高温热解促使更多的CaCO3的形成,这种现象与XRD分析结果一致。1 413 cm−1与762 cm−1处的振动带分别代表芳香C=C环与芳香C—H振动的吸收峰[24],随热解温度的升高,2条吸收峰均有不同程度增强。说明高温热解有利于PSBC中更多芳香结构的生成,这一现象与表1中芳香性指标(H/C)随着热解温度的变化规律一致。
-
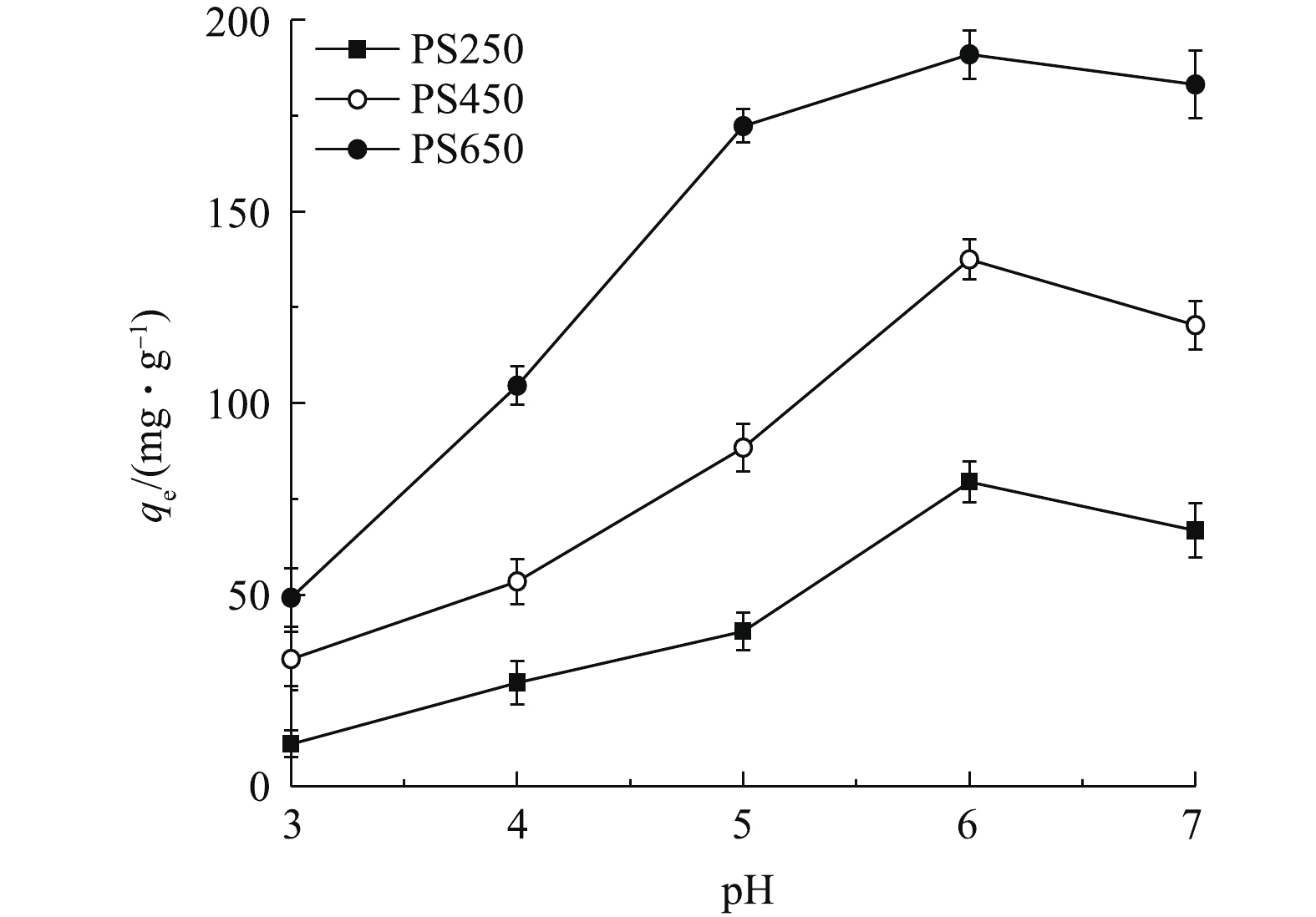
由图4可知,随溶液初始pH由3.0上升至6.0,PS250、PS450与PS650的吸附量由11.02、33.22和49.26 mg·g−1增加为79.47、137.53和191.03 mg·g−1,随后均呈降低趋势。低pH条件下,PSBC对Pb2+的吸附效果极差,这主要是由于溶液中大量的H+会与Pb2+形成激烈竞争吸附,导致PSBC表面大量的结合位点被占据,从而削弱其对Pb2+的吸附能力。随溶液pH升高,H+含量减少,这会使PSBC表面出现更多的结合位点,从而增加其对Pb2+的吸附量[7]。因此,PSBC对Pb2+吸附的最佳pH为6.0,在后续实验中,将Pb2+溶液的pH均调节为最佳。
-
反应时间对Pb2+吸附量的影响如图5所示。可以看出,3种PSBC对Pb2+的吸附是一种典型的动力学过程,在吸附开始后的2 h内,吸附量快速增加,随后增长幅度缓慢,直到24 h吸附达到平衡。在快速吸附阶段,PS250、PS450与PS650对Pb2+的吸附量分别占到总吸附量的74.37%、90.16%与97.52%。传质驱动力与表面结合位点是导致快速吸附过程产生的2个主要原因[7],在吸附初期,由于高浓度Pb2+产生的较大传质驱动力以及PSBC表面较多的结合位点,这2种原因导致溶液中的Pb2+可以快速的附着于PSBC的表面;随着吸附过程的进行,溶液中传质驱动力的减弱以及PSBC表面结合位点的减少使溶液中Pb2+的吸附趋于缓慢[3]。
准一阶与准二阶模型的拟合参数如表2所示。准二阶模型的相关系数均大于准一阶模型(R2>0.99),且由准二阶模型计算出的理论吸附量更接近实际吸附量,表明准二阶动力学模型更适合对Pb2+的吸附过程拟合。同时,也说明PSBC对Pb2+的吸附主要以化学吸附为主[22]。另外,PS650的吸附速率常数k2远大于其他2种生物炭,表明其可以更快地达到吸附平衡。
-
不同PSBC对Pb2+的等温吸附线如图6所示,模型拟合参数见表3。可以看出,在低浓度范围内,不同PSBC对Pb2+的吸附量迅速增加,随着初始浓度的增加,Pb2+吸附量持续缓慢增长,直至趋于稳定。这主要是由于低浓度条件下,PSBC表面可提供充足的吸附结合位点,有利于Pb2+吸附量的快速增加。而随Pb2+浓度的增加,PSBC表面的结合位点的逐渐饱和导致其吸附量逐渐趋于平稳。
由表3可知,Freundlich模型(R2为0.995 2~0.997 6)比Langmuir模型(R2为0.879 9~0.969 5)更适合描述PSBC对Pb2+的吸附过程(表3),说明Pb2+的吸附过程以多分子层吸附为主[24]。此外,不同PSBC的Freundlich常数n为1~10,表明PSBC对Pb2+的吸附属于有利吸附[25]。对于吸附容量常数kF,其值随着热解温度的升高大幅度增加,说明PS650较其他2种生物炭具有更大的吸附容量。同时,由Langmuir模型计算出的PS250、PS450与PS650的最大吸附量分别达到了94.02、156.22与215.30 mg·g−1。结果也表明PS650对溶液中Pb2+吸附能力最强,这主要归根于PS650具有较大的比表面积及良好的介孔结构(表1)。本研究所制备的PS650对Pb2+的吸附量高于大多数吸附材料,甚至高于一些改性生物炭(表4),表明高温制备的PSBC在去除Pb2+方面具有一定的应用潜力。
-
本研究对吸附机制进行了定性分析。由SEM-EDS的分析结果(图1)可知,随着吸附反应的进行,溶液中的Pb2+会以某种化合物的形态附着在不同PSBC的表面,且随着热解温度由250 ℃升高至650 ℃,能谱图中代表Pb2+的吸收峰变得更加尖锐,说明PS650上吸附的Pb2+含量高于PS250与PS450,这与批量吸附实验所得出的结论相吻合。
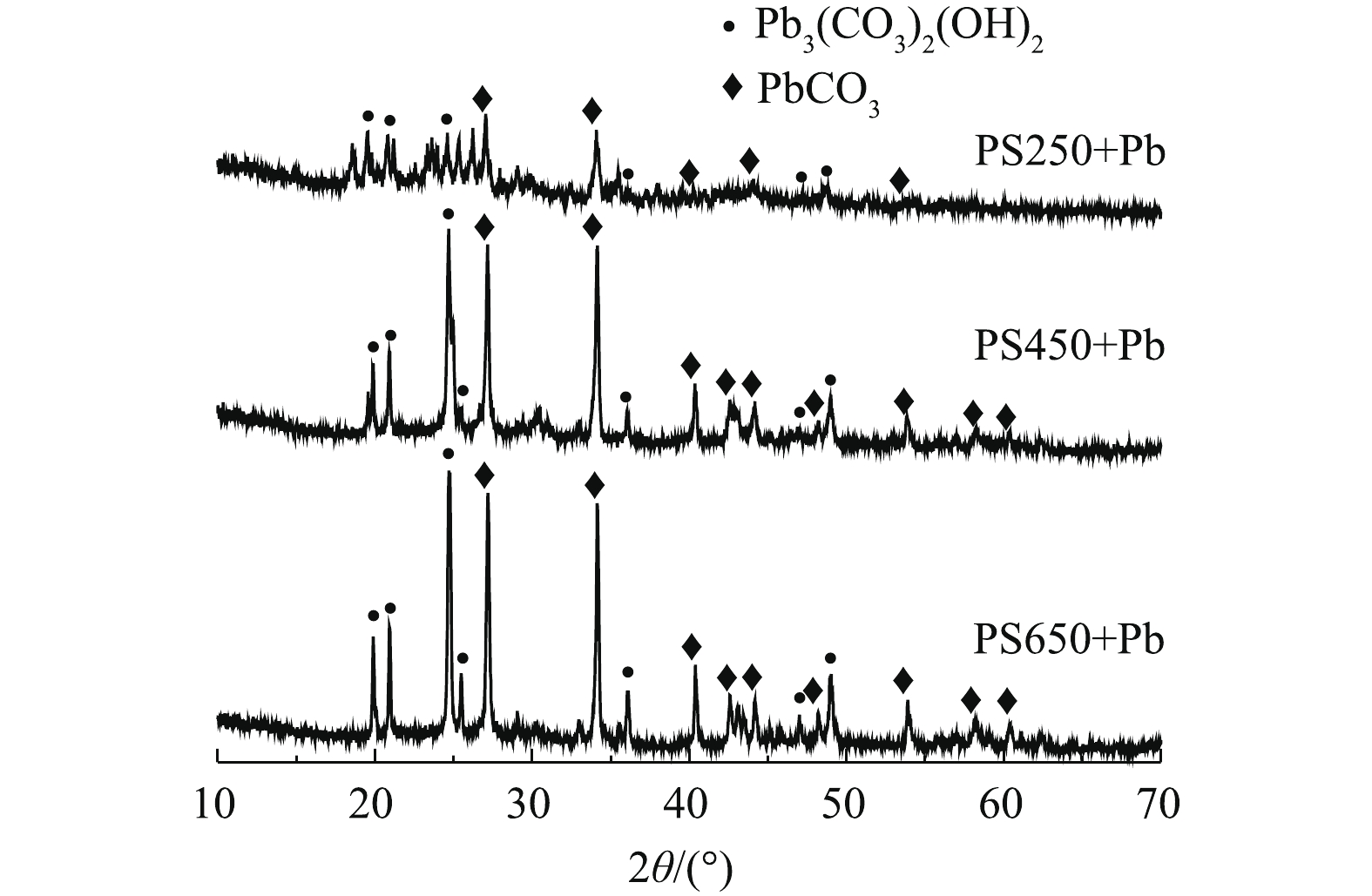
为进一步了解Pb2+在PSBC表面的存在形态,通过XRD对吸附后PSBC表面的晶体结构进行分析(图7)。与未吸附PSBC相比(图2),吸附后的XRD图谱上出现了2种新的尖锐峰,这2种峰分别代表白铅矿(PbCO3)与水镁石(Pb3(CO3)2(OH)2)矿物,且其峰值强度随着热解温度的升高在不断增强。而代表CaCO3晶体的尖锐峰均消失。溶液中的Pb2+主要通过置换CaCO3中的Ca2+,从而在PSBC上形成新的沉淀物质。由于PbCO3的溶解平衡常数(Ksp=7.4×10−14)比CaCO3(Ksp=2.8×10−9)小5个数量级,因此,溶液中的Pb2+很容易将CaCO3上的Ca2+替换[9]。上述分析结果表明,沉淀机制确实参与了PSBC对Pb2+的吸附过程,并且沉淀量随着生物炭热解温度的升高而增大。
对比吸附前后不同PSBC的红外光谱图(图3)发现,吸附后PSBC上的含氧官能团的特征峰均发生了变化,例如3 417 cm−1(—OH)与1 638 cm−1(羧基C=O)处的吸收峰明显减弱,1 585 cm−1(芳香C=O)与1 384 cm−1(C—O)处的吸收峰发生了明显的位移,这些结果表明含氧官能团参与了PSBC对Pb2+的吸附。图谱中875 cm−1处代表CaCO3吸收峰的消失,说明CaCO3与溶液中的Pb2+发生了反应,进一步证实了沉淀机制存在于Pb2+吸附过程,这与吸附后XRD所呈现的结果一致。此外,1 413 cm−1 (C=C)及762 cm−1(芳香C—H)处的吸收峰发生了明显的减弱,表明PSBC上的芳香结构发生了变化。这可能是由于环芳香族π体系提供的π电子与Pb2+形成了络合物,且π-Pb2+间的相互作用会随着生物炭热解温度的升高不断增强[17]。
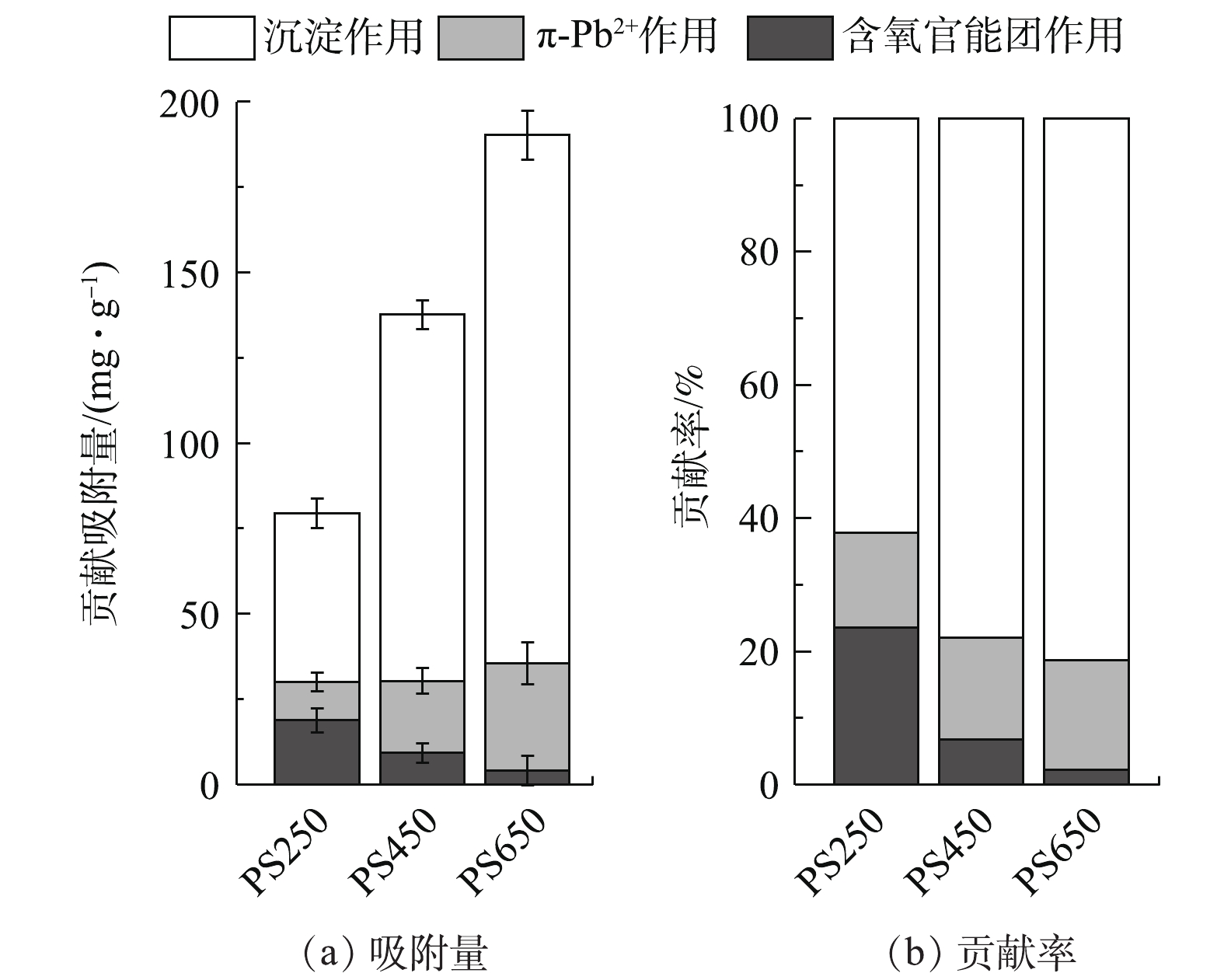
本研究对吸附机制进行了定量分析。沉淀作用(Qp)、含氧官能团的络合(Qf)以及π-Pb2+间的相互作用(Qπ)对整体吸附量(Qt)的贡献如图8所示。可以看出,不同吸附机制对PSBC吸附Pb2+的贡献依次为Qp>Qπ>Qf,说明沉淀作用是PSBC吸附Pb2+的主要机制。随热解温度由250 ℃上升至650 ℃,Qp由49.33 mg·g−1增加为154.77 mg·g−1,Qp/Qt比值由62.14%增加为81.36%,表明Pb2+的矿物沉淀作用随热解温度的升高而增加,这与之前分析结果(图1和图7)一致。对于π-Pb2+间的相互作用,PS250、PS450、PS650的Qπ分别为11.30、21.14、31.15 mg·g−1,Qπ/Qt的比值分别到达了14.23%、15.36%、16.48%,说明π-Pb2+间的相互作用会随着热解温度的升高逐渐增强,这与PSBC的芳构化程度和环芳香族π体系提供的π电子会随热解温度的升高而增大的结论(图3)一致。与Qπ和Qπ/Qt的变化规律相反,随热解温度由250 ℃升高至650 ℃,Qf由18.76 mg·g−1降低为4.11 mg·g−1,Qf/Qt的比值由23.63%下降至2.16%,表明高温热解会降低PSBC表面含氧官能团对Pb2+的络合作用,此现象进一步验证了PSBC上含氧官能团数量会随热解温度的升高而降低,如图3揭示了高温生物炭上的—OH、C=O以及C—O基团的吸收峰值较低温生物炭弱。
-
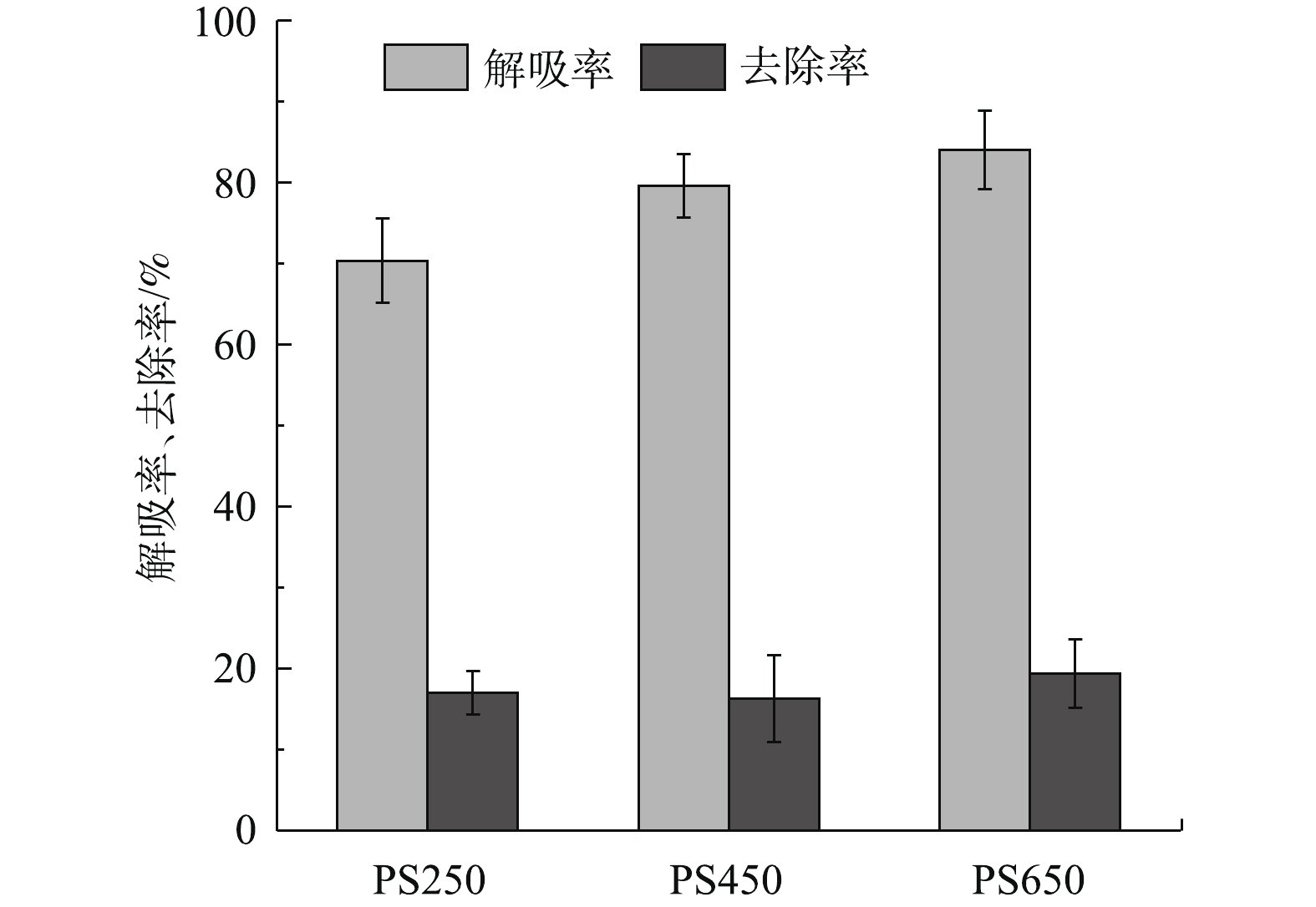
在实际的污水修复过程中,重金属回收以及吸附产品的重复再利用是一项非常重要的工作。使用0.1 mol·L−1的HCl对吸附后的PSBC进行解吸,不仅可以了解其解吸效率,同时也有助于对吸附机制进一步解析。由图9可知,PS250、PS450与PS650的解吸效率分别达到了70.33%、79.57%与83.94%,这主要是由于低pH条件下吸附后的PSBC表面上Pb沉淀矿物(白铅矿与水镁石)的溶解造成的[30]。高的解吸效率不仅说明了PSBC具有高Pb2+回收潜力,而且再次证明了沉淀作用是PSBC吸附的Pb2+的主要机制。同时,解吸结果也表明PSBC表面上形成的沉淀矿物含量随着热解温度的升高而增加,这与机理分析中得到的结论(图7)一致。对于再利用实验,解吸附后,不同PSBC对Pb2+的吸附能力大幅度降低,去除率仅达到了16.28%~19.35%,这说明使用HCl作为解吸剂不利于PSBC的重复再利用。虽然HCl可以通过与PSBC表面附着的PbCO3发生反应导致Pb2+溶解,但过量H+也会造成PSBC表面CaCO3分解,从而大幅度降低PSBC对Pb2+的吸附能力(沉淀作用),导致其可重复利用性能差[31]。因此,寻找到一种具有高的解吸效率且不会影响CaCO3结构的解吸剂是提高PSBC可重复利用性能的关键,这有待于在今后的工作中作进一步的研究。
-
1) PSBC是一种介孔材料,随热解温度由250 ℃上升至650 ℃,其产率、O/C与H/C的比值、孔径大小以及表面含氧官能团均减少,而灰分含量、pH、孔径体积以及比表面积均明显增加。同时,PSBC表面芳构化程度随热解温度的升高而增大。
2) PSBC对Pb2+的吸附依赖于溶液的pH,当pH为6.0时达到最大吸附量。准二阶动力模型与Freundlich模型适用于描述PSBC对Pb2+的吸附过程,说明Pb2+的吸附主要是发生在多分子层上的化学吸附。由Langmuir模型计算出的PS250、PS450与PS650的最大吸附量分别为94.02、156.22与215.30 mg·g−1,PS650所具有的高效除Pb2+能力归因于其拥有的大比表面积与良好的介孔结构,其吸附能力甚至高于一些改性生物炭。因此,PS650可以作为一种廉价、高效的吸附剂应用于污水Pb2+的处理。
3)根据SEM-EDS、XRD、FTIR等定性以及定量分析结果,碳酸盐的沉淀作用为PSBC吸附Pb2+的主要机制,同时还伴随着π-Pb2+间相互作用以及含氧官能团络合作用的贡献。沉淀与π-Pb2+相互作用的贡献比例会随着热解温度的升高不断增加,而含氧官能团的络合作用却在不断减弱。
平菇菌糠生物炭对水体中Pb2+的吸附特性与机制
Adsorption characteristics and mechanisms of Pb2+ in water on biochar derived from spent Pleurotus ostreatus substrate
-
摘要: 为缓解固废处理压力及开发高效廉价Pb2+吸附产品,以平菇菌糠(PS)为原料,在250、450、650 ℃温度下,限氧热解制备生物炭(PSBC),并采用BET、SEM-EDS、XRD、FT-IR对样品的表面理化性质进行了探究。通过批量吸附、机制定性和定量分析实验,研究了PSBC对水体中Pb2+的吸附特性和吸附机理。结果表明:当热解温度由250 ℃升至650 ℃,含氧官能团数量减少,比表面积与芳构化程度急剧增加;PSBC对Pb2+的吸附依赖于溶液pH,当pH为6.0时吸附量最大。吸附过程符合准二级动力学与Freundlich模型,说明以多分子层上的化学吸附为主。相较PS250与PS450,PS650拥有较大比表面积与良好的介孔结构,可为Pb2+的吸附提供更多的结合位点。经Langmuir模型计算,PS650最大吸附量为215.30 mg·g−1,其吸附效果甚至高于一些改性生物炭。机理分析表明,PSBC吸附Pb2+的主要机制为碳酸盐的沉淀作用,同时还伴随着含氧官能团的络合以及π-Pb2+间相互作用的贡献。本研究结果可为菌糠固废管理以及污水中Pb2+的去除提供参考。Abstract: In order to alleviate the pressure of solid waste treatment and develop cheap and efficient Pb2+ adsorbent products, spent Pleurotus ostreatus substrate (PS) was used as raw material to prepare biochar (PSBC) by limited oxygen pyrolysis at temperature of 250, 450 and 650 ℃, and the surface physical and chemical properties of the samples were studied by BET, SEM-EDS, XRD and FT-IR. The characteristics and mechanism of Pb2+ adsorption on PSBC were studied by the experiments of batch adsorption, mechanism qualitative and quantitative analysis. The results showed that the specific surface area and aromatization of PSBC increased sharply and oxygen-containing functional groups decreased when the pyrolysis temperature increased from 250 ℃ to 650 ℃. The adsorption of Pb2+ on PSBC was pH-dependent, and the maximum adsorption amount occurred at pH 6.0. The pseudo second order kinetic model and the Freundlich model were suitable for fitting the adsorption process of Pb2+ onto PSBC, which proved that the process was chemical adsorption occurring on the multi-molecular layer. Compared with PS250 and PS450, PS650 had larger specific surface area, better-developed mesoporous structure and more binding sites for Pb2+ adsorption. The maximum adsorption capacity calculated by Langmuir model was 215.30 mg∙g−1, which was even higher than those of some modified biochars. The results of qualitative and quantitative analysis showed that the precipitation of carbonate always dominated the adsorption process of Pb2+ on PSBC, being accompanied by the complexation of oxygen-containing functional groups and the interaction between π-Pb2+.The results can provide reference for solid waste management of spent mushroom substrate and the removal of Pb2+ from sewage.
-
农村生活污水作为面源污染的主要来源,具有氮磷营养素较高、分散、面广、量少、不易收集、管理水平低等特点[1-2],是导致河流、湖泊富营养化的重要原因[3-4]。因此,为提高农村生活污水处理率、研究开发投资成本低、工艺设备操作简单、低能高效、运行维护方便且适用于农村污水处理设备具有重要意义。
近年来,生物膜法凭借剩余污泥量少、抗冲击负荷能力强、运行管理方便等特点,已在水处理中被大量运用[5-6],生物滤池利用生物膜的过滤、絮凝及生物氧化作用,能够有效地去除水中引起水体富营养化的氮磷元素[7]。多级生物滤池通过设置两级及以上独立的缺氧、好氧段来进行污水处理,具有抗冲击负荷能力强、充分利用原水碳源、较好地有机物降解和脱氮等能力[8-9]。填料作为生物滤池重要的组成部分,其结构、空隙率和亲水性等属性对生物膜的生长、结构和更新均具有显著影响[10-12]。相比于其他填料,聚氨酯填料(海绵)具有寿命长、损耗低(年损耗5%)、不易堵塞、氧利用率高等优点,其独特的多孔结构可使微生物在流动状态下自动聚集于载体内部,形成三维固定化生物膜,避免了流体剪切力对微生物冲刷剥离的影响,在其外表面实现亚硝化反应,在内部实现亚硝酸盐的反硝化,不仅有简单的物理吸附,还有正、负电荷的引力。此外,聚氨酯海绵填料市售价格为860~1 200元·m−3,价格低廉,故其在水处理中受到了重视,但也存在着生物亲和性不足等缺点[13-19]。为此,本研究将改性后的海绵作为填料,构建了4级斜板生物填料滤池,并对该处理系统的硝化液回流比参数进行了考察,采用高通量测序技术分析处理池中生物膜微生物生长分布情况,为该污水处理系统的达标稳定运行提供理论依据。本研究可为实际工程中工艺结构及参数的选取提供参考,有着重要的现实意义。
1. 实验材料与装置
1.1 实验材料
1)填料。本研究使用的聚氨酯发泡海绵材料购买于江苏常州市某公司,堆积比重为26 kg·m−3,孔隙率为96.20%,整张填料的尺寸为1.00 m×2.00 m×0.02 m,将填料裁剪为0.37 m×0.15 m×0.02 m大小的尺寸,采用化学氧化-壳聚糖接枝[20-21]改性方法获得的改性聚氨酯填料以备用。
2)实验用水。根据现场调研及相关资料查阅,按照浙江省农村地区生活污水水质特征,本研究采用稀释加药法配制了实验用进水,即取一定体积的原水(北京科技大学校区内的生活污水)稀释,稀释后降低的浓度差采用乙酸钠、葡萄糖、氯化铵和磷酸氢二钾进行添加,使进水中的COD、氨氮、TP和SS浓度达到模拟农村生活污水的设计浓度值,设计浓度值见表1。出水水质需要达到浙江省《农村生活污水处理设施水污染物排放标准》(DB 33/973-2015)一级标准,其中pH的标准限值为6~9,其他主要监测指标及浓度见表1。
表 1 进/出水水质Table 1. Water quality of inlet / outlet进出水浓度 COD/(mg·L−1) 氨氮/(mg·L−1) TP/(mg·L−1) SS/(mg·L−1) 进水设计浓度 250~350 33~40 3.2~3.8 60~100 出水浓度标准 ≤60 ≤15 ≤2 ≤20 1.2 污水处理装置系统
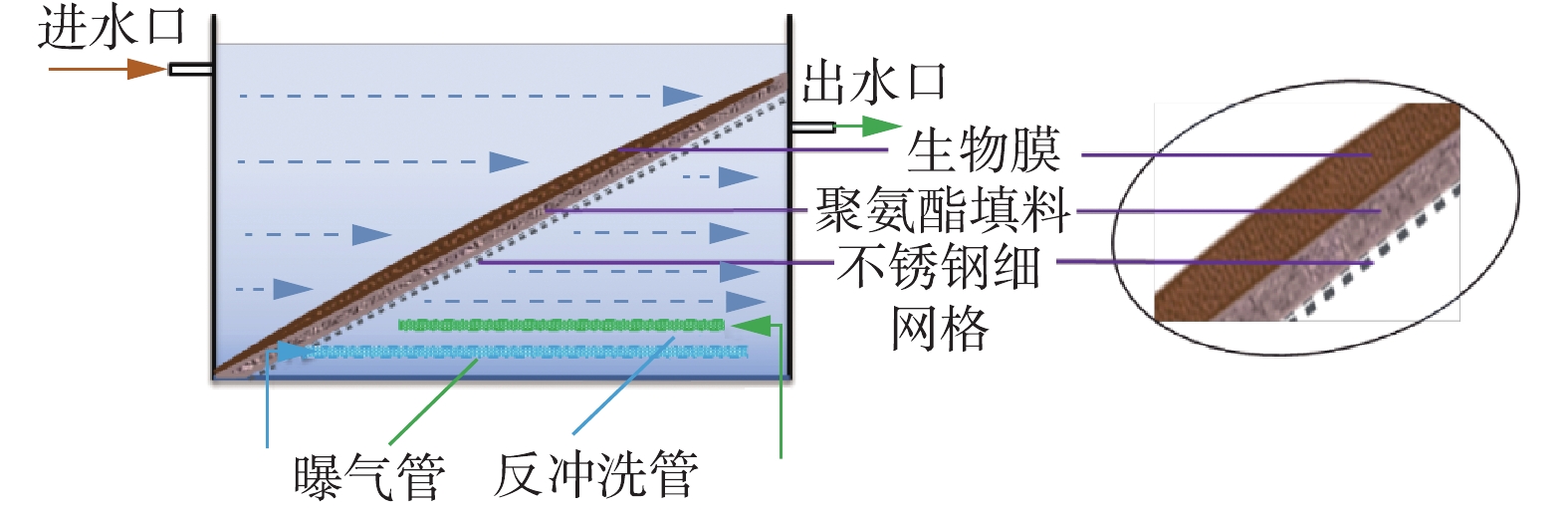
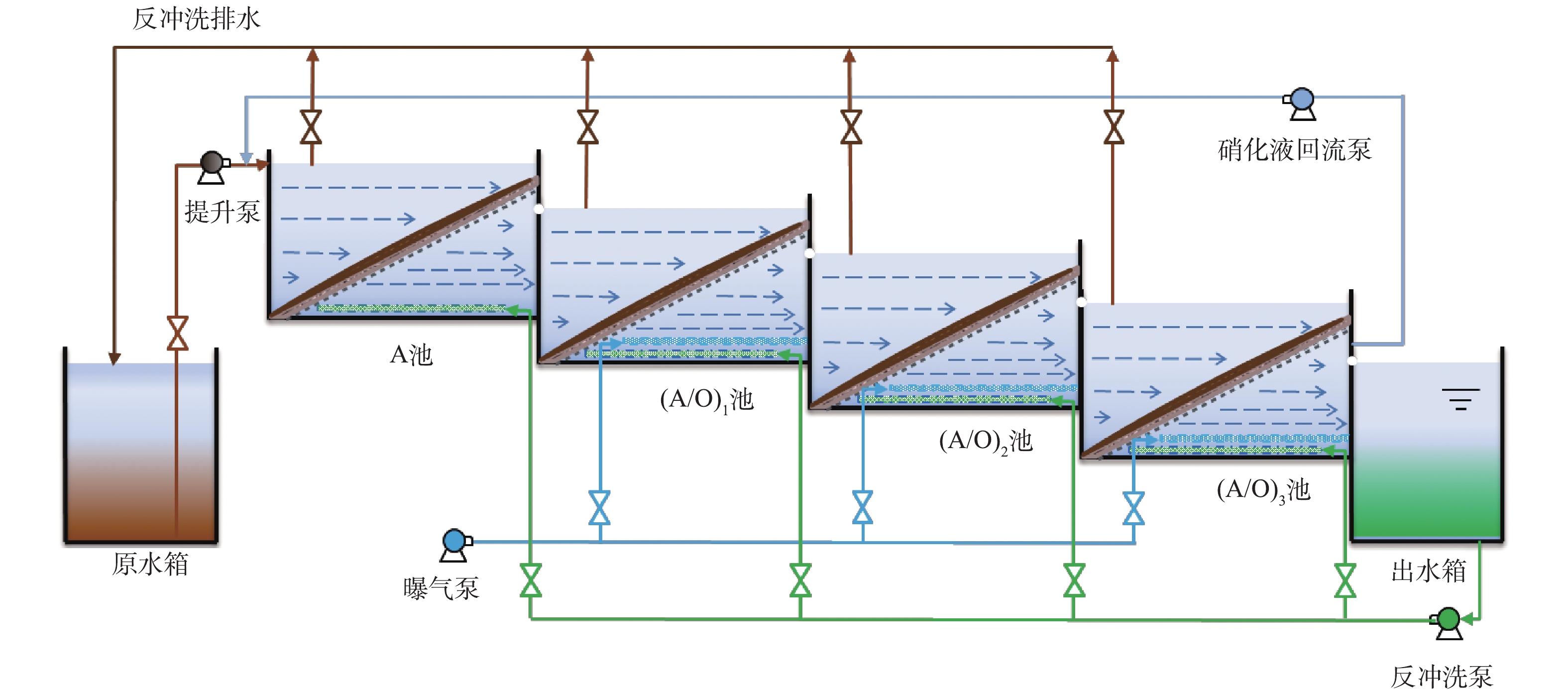
本研究实验装置如图1和图2所示。在笔者前期工作的基础上,确定了该系统由4个斜板生物滤池串联构成,运行时,第1个池体不进行曝气,后3个池体进行曝气,将其分别记为A、(A/O)1、(A/O)2、(A/O)3。
1)单池结构。单个斜板生物滤池的尺寸为0.30 m×0.15 m×0.25 m,有效容积为10.10 L,主要由进出水管、斜板式生物填料、不锈钢支撑网、曝气系统、反冲洗系统、回流液系统等构成。为防止因水流冲击造成填料的变形和移动,生物膜填料平铺在不锈钢支撑网上并以36°角进行固定,同时采用弹性压条将斜板填料与四周池壁缝隙处进行压实,防止在进水过程中造成壁流效应。斜板填料固定好后的尺寸为0.37 m×0.15 m×0.02 m,填充率约为11%,其将水池分割为上下2个相对独立的部分,可在一个处理池中同时创造好氧和缺氧的条件,这样每个A/O池均可视为一个局部的A/O系统,使同步硝化反硝化成为可能,这也是该处理系统的优势之一。
2)运行参数。实验系统采用前段单点进水,单个滤池水力负荷为1.40 m3·(m2·d)−1,进水流量约为(24±1) L·d−1,废水在每一级池体内的水力停留时间约为10 h,系统总体停留时间约为40 h。A/O池斜板填料下方好氧区溶解氧浓度控制在(2.0±0.2) mg·L−1,控制斜板上方缺氧区溶解氧浓度≤0.50 mg·L−1,第一个A池不曝气。为达到脱氮的目标,本研究将A/O池处理后的混合液通过回流泵回流至A池,利用A池中的反硝化菌将回流混合液中亚硝态氮和硝态氮转化成氮气[22],将硝化液回流比分别设为0、50%、100%、200%。
3)填料反冲洗。该污水处理系统进水开始运行初期主要依靠填料的机械截留和过滤作用来去除污水中的悬浮物质,随着水处理的进行,斜板填料截留的悬浮物质增多,逐渐形成一个生物膜,起到截留、吸收、降解有机物及氮、磷等污染物的作用[23]。随着系统运行,生物膜增厚,填料膜两侧压头损失逐渐增大。在实验阶段每12 d对填料进行1次水反冲洗,反冲洗时填料上老化的生物膜随着冲洗水而脱落并随反冲洗水排出系统,达到净化滤层的目的,同时减少了滤料两侧压差。
1.3 微生物群落分析
为了研究4级填料生物滤池污水处理系统的细菌群落,利于系统的操作和优化,将最优条件下的斜板生物膜样品在干冰冷冻条件下送至生工生物工程(上海)股份有限公司,采用基于16S rRNA基因V3~V4区高通量测序[24-25]分析生物膜中细菌的种类及多样性,在97%的分类水平上,通过应用Mothur软件计算各样品的Alpha多样性[26],用RDP Classifier方法与Green Gene数据库进行物种注释分析[27]。
2. 结果与讨论
对于污水处理系统,在不同回流比条件下分别进行了12 d的运行实验,每2 d检测1次进出水COD、氨氮、TN、TP水质。本实验装置系统对于SS的去除主要来自于四个池体内斜板填料的机械截留过滤作用,在初期摸索实验阶段得知,当实验进水SS浓度为60~100 mg·L−1时,出水中SS浓度均在10 mg·L−1以下,随着运行时间的延长,出水SS浓度会进一步降低到5 mg·L−1,满足DB 33/973-2015一级标准对SS的要求,由于该指标基本不受回流比的影响,在本文中不做详细分析。
2.1 对COD的去除效果
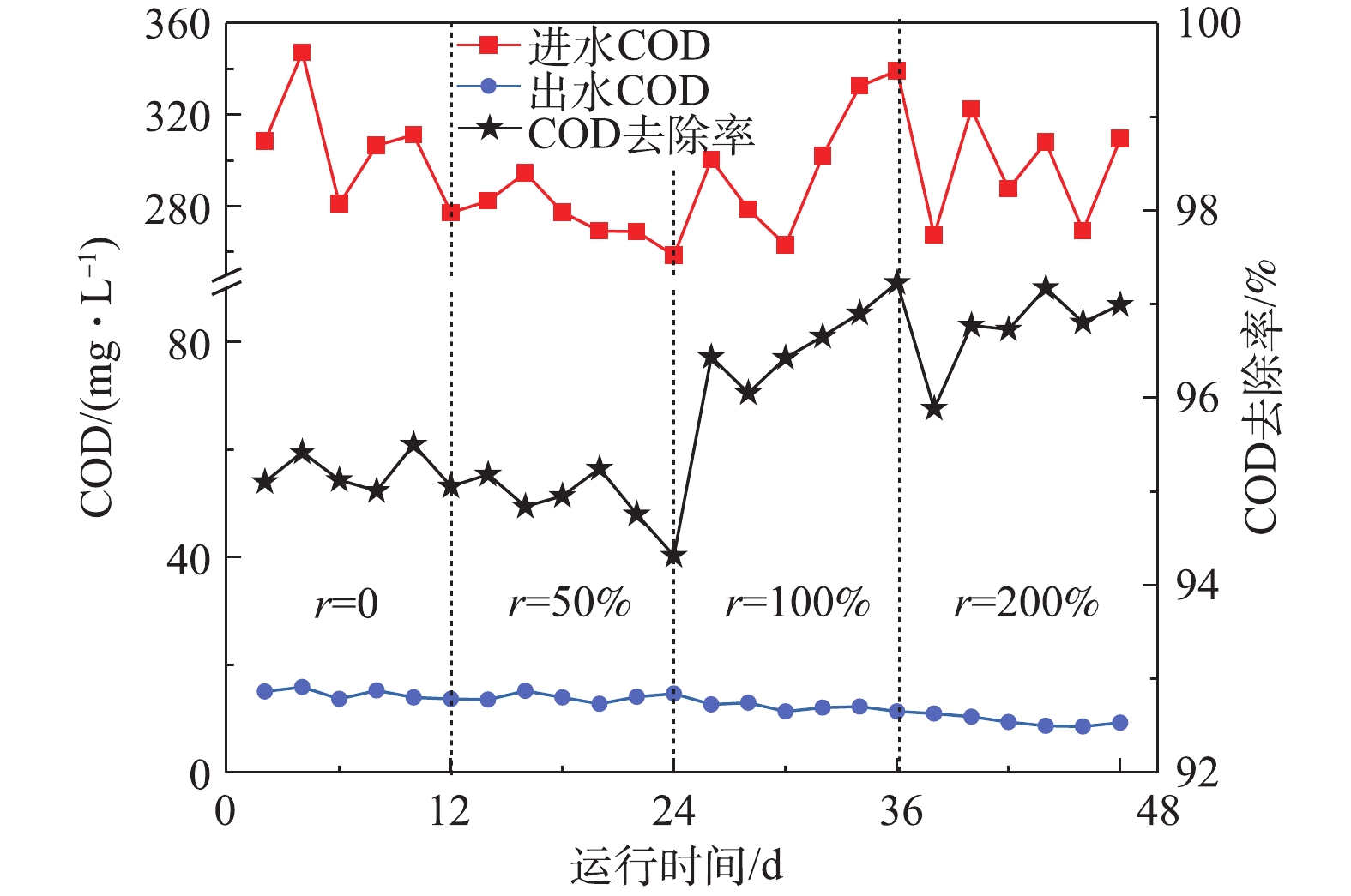
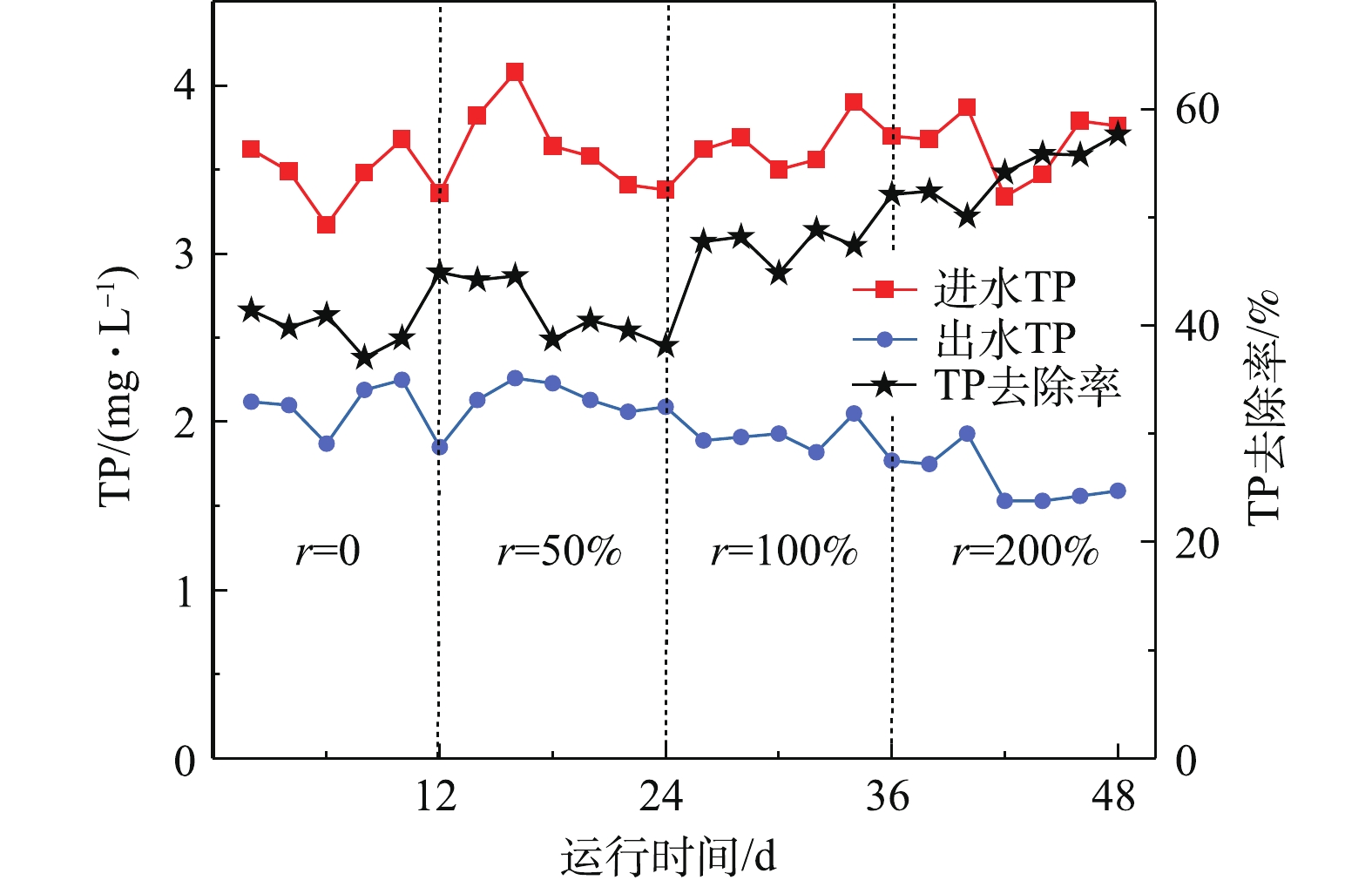
由图3可知,回流比从0逐渐增大到200%时,COD去除率逐渐升高并趋于平稳,平均出水COD由14.62 mg·L−1降低到9.57 mg·L−1,达到DB 33/973-2015一级排放标准,由此可见,改系统对有机物的去除率整体较高。这是由于A/O池作为污水处理系统的重要组成,在聚氨酯填料表面及内部生长的大量异养微生物的降解和吸收作用下,有机物被大量去除[28]。同时可以看到,回流比的增大促进了有机污染物的降解,这是由于回流比的增加使水力剪切作用加大,加快了生物膜的脱落和更新,提高了传质效果,同时反硝化细菌利用回流液中的硝酸盐作为电子受体,以有机污染物作为碳源来进行反硝化作用,促进了COD的去除。剩余的COD没有被微生物利用,其原因可能是难降解的有机物以及微生物通过内源呼吸的产物是微生物较难利用的碳源。
2.2 对氮元素的去除效果
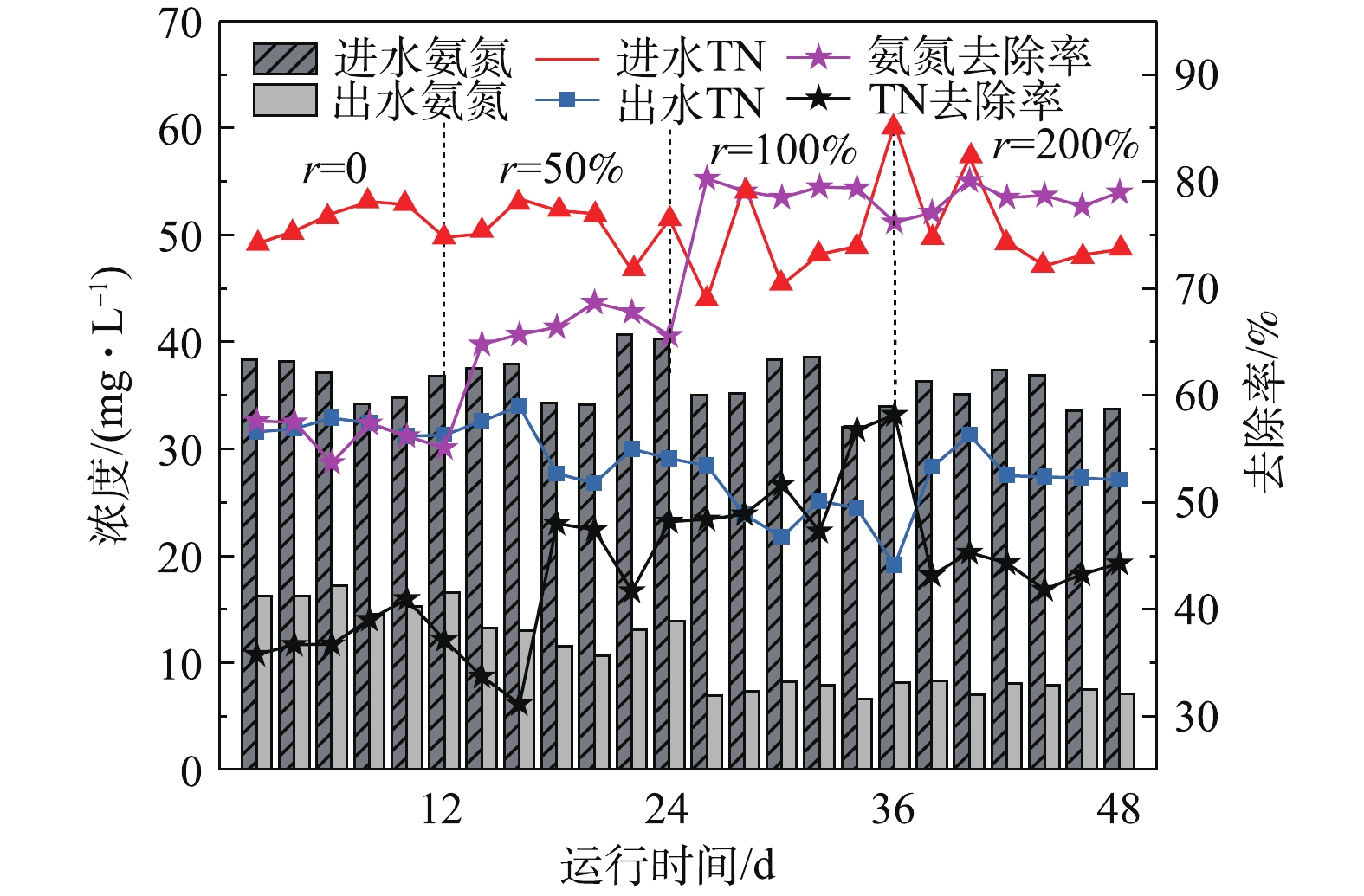
处理系统在不同回流比下对氨氮和TN的去除效果和去除率如图4所示。由图4可知,当回流比由0提高到100%时,氨氮的平均去除率由56.25%提高到78.83%;而当回流比继续增加到200%时,系统对氨氮去除率并没有持续增大,平均去除率为78.50%。污染物去除率的逐步升高是由于回流硝化液稀释了进水中的有机物浓度,使得A/O池内异养菌竞争力下降,硝化自养菌成为优势菌,这有利于硝化反应的进行,进而提高了氨氮的去除率。而当硝化液回流比超过100%时,回流液携带大量的溶解氧进入A池,破坏了缺氧环境,使得反硝化菌活性降低,对有机污染物的消耗降低,A/O段的有机负荷增大,自养硝化细菌与异养菌的竞争力减弱,降低氨氮去除率[29]。此外,回流比的增大导致系统的水力负荷增大,进水流速和水力剪切作用增大,使得一部分硝化菌脱落随出水流失。在这些因素的共同作用下,硝化液回流比从100%增加到200%时,氨氮去除率无明显增高。
TN的去除率随着回流比的增大呈先上升后下降的趋势,当回流比由0增大到100%时,TN的去除率明显上升且达到最高,由37.73%增大到51.89%,出水TN平均浓度由31.88 mg·L−1降低到23.79 mg·L−1。这是由于随着回流比增大,进入A池的硝化液量也相应增大,反硝化菌利用原水中的有机物作为碳源,将A/O池中回流的硝态氮作为电子受体进行反硝化反应来脱氮。当回流比继续增加到200%时,TN的去除率下降,其原因与氨氮去除率下降的原因相同,均是由于回流比的增大,使得更多的溶解氧进入A池,破坏了缺氧环境,从而降低了反硝化菌活性,进而导致反硝化脱氮作用减少。
2.3 对磷元素的去除效果
由图5可知,随着回流比的增加,有机负荷也随之增加,聚磷菌在A池获得充足的碳源,释磷量增多,在A/O池吸磷量也随着增加,磷的去除率升高,TP的平均去除率由40.52%提高到54.36%,出水TP平均浓度由2.06 mg·L−1减少到1.65 mg·L−1,当回流比大于等于100%时,出水TP达到DB33/973-2015一级标准。系统对TP的去除主要依靠聚磷菌(PAOs)在厌氧条件下释放磷,将易降解的有机物和挥发性脂肪酸VFA转化为聚羟基链烷酸(PHAs)的形式,污水进入A/O池后,聚磷菌以PHAs为电子供体在好氧状态下过量吸磷[30-31]。本研究在每一种工况结束后对填料进行水反冲洗,老化的生物膜最终随反冲洗水以富磷污泥的形式流出系统,实现脱磷的效果。
综上所述,该多级A/O填料滤池处理系统在回流比变化较大的情况下,综合对各污染物的去除效果确定回流比为100%是该系统的最优运行条件,出水水质均达到浙江省《农村生活污水处理设施水污染物排放标准》(DB33/973-2015)一级标准。
2.4 细菌群落多样性分析
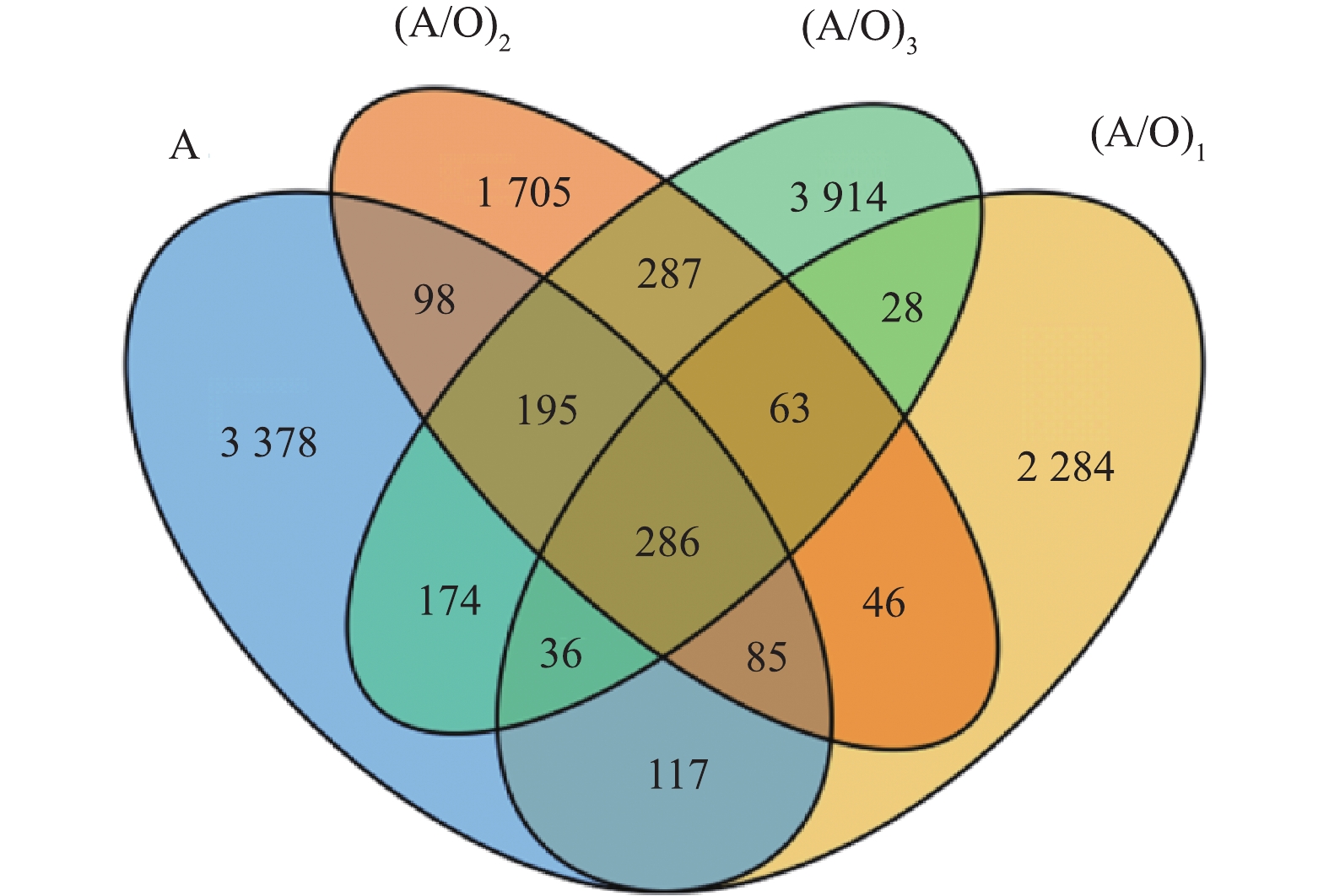
对4个斜板生物膜样品进行通量测序分析,得到有效序列信息后以97%的相似性将其聚类成为OTU[32],Veen图可以直观地展现样品的OTU组成的多样性、相似性及重叠情况。本实验4个生物膜样品属水平的Veen图如图6所示。4个池体的生物膜样品聚类得到的OTU总数目分别为4 369、2 945、2 765、4 983个,(A/O)3池物种种数最多[33]。2个处理池共同拥有的OTU个数越多时,其细菌组成的相似度越大,由图6可知,4个生物池内(A/O)2和(A/O)3池内细菌组成的相似度最大。A、(A/O)1、(A/O)2和(A/O)3生物膜样品特有的OTU数目分别是3 378、2 284、1 705、3 914个,各处理池之间共有OTU数目明显低于其各自特有的OTU数目。这说明各处理池在细菌群落组成上存在较大的差异,在一定程度上表明各处理池在处理系统中起着不同的作用。
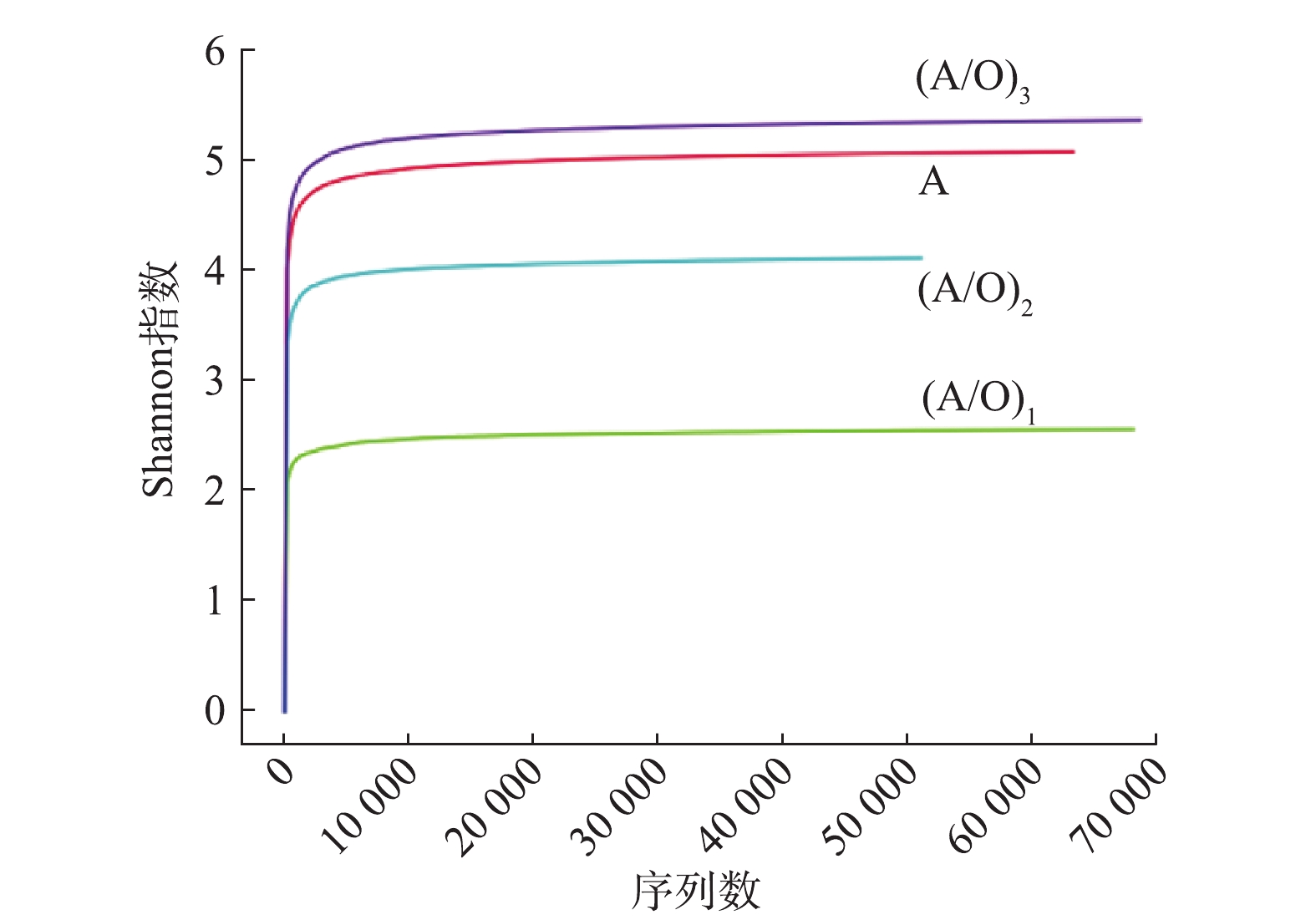
Alpha多样性包括覆盖率、ACE指数、Chao1指数及Simpson指数。Chao1指数和ACE指数可反应群落分布丰富度,其越大群落丰富度越高。Shannon指数越大,群落多样性越高,Simpson指数越大,群落多样性越低[34-35]。由表2中的ACE和Chao1指数可知,4个生物膜样品中细菌群落丰富度由大到小顺序排列依次是(A/O)1>(A/O)3>A>(A/O)2。Shannon指数表明各生物膜样品的细菌群落多样性由大到小是(A/O)3>A>(A/O)2>(A/O)1,Simpson指数也印证了这一点。(A/O)1样品的细菌群落多样性最低,这可能是由于进入(A/O)1池的污水中有机物浓度高、营养物质丰富,从而造成丝状菌大量繁殖,丝状菌与菌胶团细菌竞争营养物质和氧,导致池内溶解氧浓度大幅降低。最终,丝状菌成为优势菌,破坏了菌胶团的结构、减少了菌胶团细菌的多样性。由4个样品的文库覆盖率(coverage)可以看出,所有样本的样本序列的覆盖率为95%~96%,获取了绝大多数样本信息,能够很好地反映各样品的细菌群落组成。利用R语言工具制作Shannon稀疏曲线见图7。由图7可见,当序列数增加到10 000以上时,4个样本的Shannon稀疏曲线均趋向平坦,同样说明4个样本的测序数据量足够大,可以反映4个生物膜样本的绝大多数微生物的物种信息。
表 2 样品多样性及丰度指数统计Table 2. Sample diversity and abundance index statistics样本名称 OTU ACE Chao1 Shannon Simpson 覆盖率/% A 4 369 41 920.70 22 856.80 5.21 0.02 95 (A/O)1 2 945 72 636.75 27 675.05 2.63 0.25 96 (A/O)2 2 765 27 145.44 13 244.00 4.22 0.07 96 (A/O)3 4 983 55 977.55 26 508.83 5.50 0.02 95 2.5 各处理池细菌在门及属分类层面的分布规律
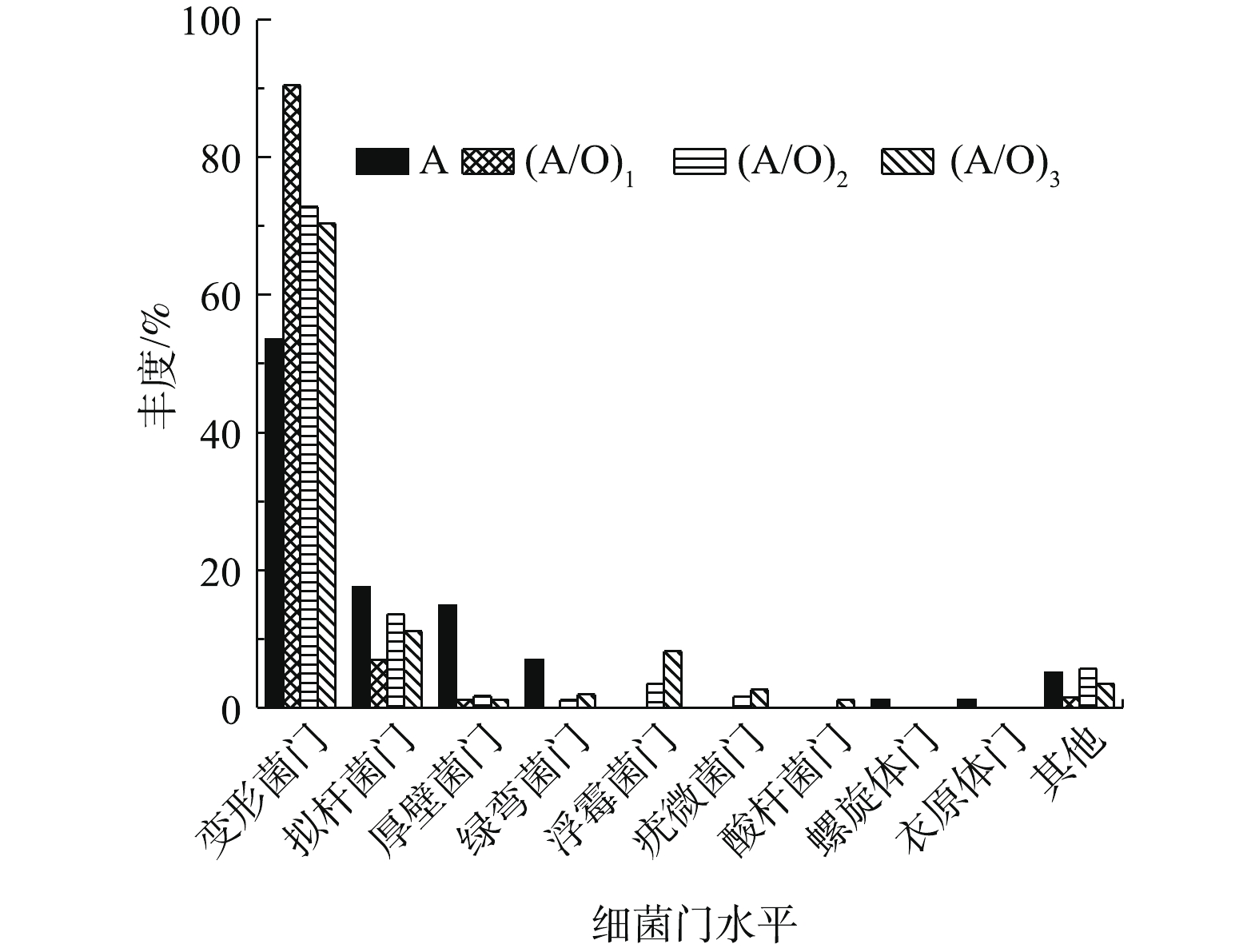
微生物作为生物废水处理工艺的核心,在降解和转化污染物过程中起着关键的作用[36-37]。4个生物膜样品的可操作分类单元(OTU)在门(phylum)水平上的细菌群落分布如图8所示。由图8可知,该处理系统主要存在变形菌门、拟杆菌门、厚壁菌门等门类群。变形菌门参与氨化、反硝化等生化过程,在生物脱氮、除磷及诸多污染物降解过程中起着重要的作用[38],其在各个池中占比最大,在A/O池中更是高达90.50%、72.80%和70.40%。考虑在A/O池内水中NH3-N进行硝化反应生成
NO−3 ,同时微生物进行生物代谢降解有机物供给聚磷菌能量,使其以PHAs为电子供体在好氧状态下过量吸磷,磷进入细胞组织,最后以富磷污泥的形式从系统中排出现除磷的去除;在A池中,拟杆菌门、厚壁菌门、绿弯菌门的占比情况分别为17.50%、14.80%和6.90%,相较于A/O池高。有研究[39-40]表明,该菌类对于污水中的碳水化合物、大分子有机物的降解具有重要的作用。例如,绿弯菌门中的厌氧绳菌科是典型的产氢产乙酸细菌,在厌氧或缺氧条件下较常见,厚壁菌门则可以进行反硝化脱氮反应,具有较强的氨化作用。可见,在A池中细菌主要以有机物为碳源将A/O池回流进来的NO−3 还原为N2释放,实现污水中氮的去除,期间,高分子有机物被分解为小分子,可降低后续生物处理的生物负荷并提高了生化性。此外,在A/O池中也存在有拟杆菌门、厚壁菌门等门类,这是因为每一个A/O池都兼具好氧池和缺氧池的部分功能。除以上几大门类外,还存在浮霉菌门、酸杆菌门等,但其占比相对较小。为进一步了解反应器在运行过程中细菌群落的演化,在属(genus)水平进一步进行细菌群落结构分析并对其功能菌属进行统计,统计结果如表3所示。由表3可见,优势菌属大多为反硝化菌、聚磷菌这类具有脱氮除磷作用的功能微生物,解释了水体N、P良好的去除作用。其中,硝化细菌主要有4个属,反硝化细菌主要有9个属,聚磷菌有3个属。有研究[41]表明,Nitrosomonas是亚硝化单胞菌属,是能够调节硝化过程中氨氮转变为亚硝酸盐氮的限速酶,起到控制硝化反应的作用。Nitrospira是硝化螺旋菌属,在处理系统中具有将亚硝酸盐还原为硝酸盐的能力,A/O池样品中该菌属的丰度值较高;Pseudomonas属于变形菌门,是常见的反硝化细菌,对废水生物脱氮、除磷均有重要意义,同时,Pseudomonas能够显著降低系统中的COD,其在A池中的含量最高,在其他A/O池的样品中的含量也较高,这也是系统对COD具有较高去除率的原因;除磷微生物主要有Acinetobacter[42]、Pseudomonas、Thiopseudomonas,其中以Acinetobacter细菌为主。
表 3 脱氮除磷功能菌及其丰度Table 3. Nitrogen and phosphorus removal bacteria and their abundances% 功能菌 A (A/O)1 (A/O)2 (A/O)3 Nitrospira(硝化) 0 0 0.02 0.44 Nitrobacter(硝化) 0 0 0 0.04 Nitrosomonas(硝化) 0 0 0.01 0.03 Nitrosospira(硝化) 0 0 0 0.01 Rosebaccilus(反硝化) 0.03 0 0.97 0.59 Pseudomonas(反硝化、聚磷) 0.25 0.15 0.04 0.08 Halothiobaccilus(反硝化) 0.3 0.01 0 0.01 Thiopseudomonas(反硝化、聚磷) 0.01 0 0.03 0.22 Cloacibaccilus(反硝化) 0.17 0 0.01 0 Acetanaerobacterium(反硝化) 0.15 0 0 0 Anaerobacterium(反硝化) 0.1 0 0 0 Thiobaccilus(反硝化) 0.01 0 0.01 0.01 Baccilus(反硝化) 0 0 0 0.01 Acinetobacter(聚磷) 0.43 6.91 1.3 0.55 3. 结论
1) 100%是该系统的最优硝化液回流比,在此条件下系统对COD、氨氮、TN、TP的综合去除率最高,包括SS指标均优于浙江省地方标准《农村生活污水处理设施水污染物排放标准》(DB 33/973-2015)一级标准。该系统具有较高的污染物去除率,同时具有流程简单、无辅助药剂添加等优势,具有良好的应用价值。
2)本研究所有样本的覆盖率为95%~96%,获取了绝大多数微生物的物种信息,保证了后续的微生物分析结果的准确性。不同生物膜样品共有的OTU数目远低于其特有的OTU,A/O池、A池在细菌群落组成上存在很大差异,发挥着不同的功能和作用。不同样品细菌群落丰富度由大到小排列依次为(A/O)1>(A/O)3>A>(A/O)2,细菌群落的多样性由大到小排列依次为(A/O)3>A>(A/O)2>(A/O)1。
3)在A/O池中,以变形菌门为主要门类群。在此,NH3-N进行硝化反应,聚磷菌在好氧状态下吸磷,有机物被降解;在A池中,拟杆菌门、厚壁菌门、绿弯菌门的占比较大,
NO−3 在此还原为N2释放;同时高分子有机物分解为小分子,降低后续生物处理的生物负荷。细菌属水平的统计结果为硝化菌、反硝化菌、聚磷菌等具有脱氮除磷作用的微生物,解释了系统稳定高效的的氮磷处理能力。 -
表 1 PSBC的理化性质
Table 1. Physical-chemical properties of PSBC
生物炭 产率/% 灰分/% pH Zeta电位/mV C/% H/% O/% N/% O/C H/C 比表面积/(m2·g−1) 孔径/nm 孔径体积/(cm3·g−1) PS250 50.86 8.71 8.24 −29.7 65.6 4.68 27.19 2.5 0.41 0.07 5.37 7.32 0.01 PS450 38.67 11.74 9.92 −27.2 69 3.19 25.6 2.26 0.37 0.05 22.75 3.81 0.03 PS650 31.37 14.31 10.08 −26.4 71.5 1.36 25.21 1.93 0.35 0.02 75.63 3.24 0.11 表 2 吸附动力学模型拟合参数
Table 2. Constant and correlation coefficients of the adsorption kinetic equation
生物炭 准一阶动力学模型 准二阶动力学模型 qe,实际 qe,理论 k1 R2 qe,理论 k2 R2 PS250 79.38 74.85 0.021 7 0.960 4 81.47 0.000 4 0.994 2 PS450 137.63 130.72 0.060 4 0.911 5 138.26 0.000 7 0.995 4 PS650 190.23 185.89 0.148 5 0.875 3 191.09 0.001 9 0.998 7 表 3 吸附等温线拟合
Table 3. Isotherm constants for fitting equation
生物炭 Langmuir模型 Freundlich模型 qmax kL R2 kF n R2 PS250 94.02 0.047 3 0.969 5 31.89 5.741 3 0.995 2 PS450 156.22 0.414 3 0.944 7 70.59 6.920 5 0.996 6 PS650 215.30 28.920 1 0.879 9 120.44 8.185 2 0.997 6 表 4 不同吸附剂对Pb2+的吸附容量
Table 4. Adsorption capacity of Pb2+ on different adsorbents
-
[1] GUO S Z, DUAN N, NAN Z G, et al. g-C3N4 modified magnetic Fe3O4 adsorbent: Preparation, characterization, and performance of Zn(II), Pb(II) and Cd(II) removal from aqueous solution[J]. Journal of Molecular Liquids, 2018, 258: 225-234. doi: 10.1016/j.molliq.2018.03.029 [2] AHMAD Z, GAO B, MOSA A, et al. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass[J]. Journal of Cleaner Production, 2018, 180: 437-449. doi: 10.1016/j.jclepro.2018.01.133 [3] HE J Y, LI Y L, WANG C M, et al. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers[J]. Applied Surface Science, 2017, 426: 29-39. doi: 10.1016/j.apsusc.2017.07.103 [4] YAN Y B, LI Q, SUN X Y. Recycling flue gas desulphurization (FGD) gypsum for removal of Pb(II) and Cd(II) from wastewater[J]. Journal of Colloid and Interface Science, 2015, 457: 86-95. doi: 10.1016/j.jcis.2015.06.035 [5] 罗小锋, 张玉玲. 儿童血铅水平及其暴露因素调查[J]. 医疗装备, 2018, 31(13): 49-50. doi: 10.3969/j.issn.1002-2376.2018.13.029 [6] ABU-DANSO E, PERANIEMI S, LEIVISKA T, et al. Synthesis of S-ligand tethered cellulose nanofibers for efficient removal of Pb(II) and Cd(II) ions from synthetic and industrial wastewater[J]. Environmental Pollution, 2018, 242: 1988-1997. doi: 10.1016/j.envpol.2018.07.044 [7] 计海洋, 汪玉瑛, 吕豪豪, 等. 不同炭化温度制备的蚕丝被废弃物生物炭对重金属Cd2+的吸附性能[J]. 应用生态学报, 2018, 29(4): 1328-1338. [8] WANG R Z, HUANG D L, LIU Y G, et al. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock[J]. Bioresource Technology, 2018, 261: 265-271. doi: 10.1016/j.biortech.2018.04.032 [9] ZHOU Z, XU Z H, FENG Q J, et al. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar[J]. Journal of Cleaner Production, 2018, 187: 996-1005. doi: 10.1016/j.jclepro.2018.03.268 [10] ZAZYCKI M A, GODINHO M, PERONDI D, et al. New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions[J]. Journal of Cleaner Production, 2018, 171: 57-65. doi: 10.1016/j.jclepro.2017.10.007 [11] CIBATI A, FOEREID B, BISSESSUR A, et al. Assessment of Miscanthus giganteus derived biochar as copper and zinc adsorbent: Study of the effect of pyrolysis temperature, pH and hydrogen peroxide modification[J]. Journal of Cleaner Production, 2017, 162: 1285-1296. doi: 10.1016/j.jclepro.2017.06.114 [12] 刘宁, 张桂芹, 王奉强. 菌糠的资源化研究与开发利用进展[J]. 安徽农业科学, 2019, 47(14): 7-11. doi: 10.3969/j.issn.0517-6611.2019.14.003 [13] LOU Z, SUN Y, BIAN S, et al. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar[J]. Chemosphere, 2017, 169: 23-31. doi: 10.1016/j.chemosphere.2016.11.044 [14] HU X J, GU H D, ZANG T T, et al. Biosorption mechanism of Cu2+ by innovative immobilized spent substrate of fragrant mushroom biomass[J]. Ecological Engineering, 2014, 73: 509-513. doi: 10.1016/j.ecoleng.2014.09.067 [15] 宋涛. 氢氧化钠改性及固定化黑木耳菌糠对水中Pb(II)的吸附特征[D]. 哈尔滨: 东北农业大学, 2018. [16] JIN Y, TENG C Y, YU S M, et al. Batch and fixed-bed biosorption of Cd(II) from aqueous solution using immobilized Pleurotus ostreatus spent substrate[J]. Chemosphere, 2018, 191: 799-808. doi: 10.1016/j.chemosphere.2017.08.154 [17] WANG Z Y, LIU G C, ZHENG H, et al. Investigating the mechanisms of biochar’s removal of lead from solution[J]. Bioresource Technology, 2015, 177: 308-317. doi: 10.1016/j.biortech.2014.11.077 [18] DING W C, DONG X L, IME I M, et al. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere[J]. Chemosphere, 2014, 105: 68-74. doi: 10.1016/j.chemosphere.2013.12.042 [19] ZHANG C, SHAN B Q, TANG W Z, et al. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere[J]. Bioresource Technology, 2017, 238: 352-360. doi: 10.1016/j.biortech.2017.04.051 [20] TANG Y, ALAM M S, KONHAUSER K O, et al. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater[J]. Journal of Cleaner Production, 2019, 209: 927-936. doi: 10.1016/j.jclepro.2018.10.268 [21] CHOI Y K, KAN E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water[J]. Chemosphere, 2019, 218: 741-748. doi: 10.1016/j.chemosphere.2018.11.151 [22] 郜礼阳, 邓金环, 唐国强, 等. 不同温度桉树叶生物炭对Cd2+的吸附特性及机制[J]. 中国环境科学, 2018, 38(3): 1001-1009. doi: 10.3969/j.issn.1000-6923.2018.03.025 [23] GOH C L, SETHUPATHI S, BASHIR M, et al. Adsorptive behaviour of palm oil mill sludge biochar pyrolyzed at low temperature for copper and cadmium removal[J]. Journal of Environmental Management, 2019, 237: 281-288. [24] 邓金环, 郜礼阳, 周皖婉, 等. 不同温度制备香根草生物炭对Cd2+的吸附特性与机制[J]. 农业环境科学学报, 2018, 37(2): 340-349. doi: 10.11654/jaes.2017-1066 [25] WANG C Q, WANG H, CAO Y J. Pb(II) sorption by biochar derived from Cinnamomum camphora and its improvement with ultrasound-assisted alkali activation[J]. Colloid and Surfaces A, 2018, 556: 177-184. doi: 10.1016/j.colsurfa.2018.08.036 [26] 郑凯琪, 王俊超, 刘姝彤, 等. 不同热解温度污泥生物炭对Pb2+、Cd2+的吸附特性[J]. 环境工程学报, 2016, 10(12): 7277-7282. doi: 10.12030/j.cjee.201507083 [27] ASUQUO E, MARTIN A, NZEREM P, et al. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies[J]. Journal of Environmental Chemical Engineering, 2017, 5: 679-698. doi: 10.1016/j.jece.2016.12.043 [28] 杨婷婷, 孟莉蓉, 李晖, 等. 两种生物炭对Pb的吸附特性研究[J]. 农业环境科学学报, 2017, 36(8): 1627-1633. doi: 10.11654/jaes.2017-0276 [29] 于长江, 董心雨, 王苗, 等. 海藻酸钙/生物炭复合材料的制备及其对Pb(II)的吸附性能与机制[J]. 环境科学, 2018, 39(8): 3719-3728. [30] TRAKAL L, VESELSKA V, SAFARIK I, et al. Lead and cadmium sorption mechanisms on magnetically modified biochars[J]. Bioresource Technology, 2016, 203: 318-324. doi: 10.1016/j.biortech.2015.12.056 [31] ZHANGJ Q, HU X L, ZHANG K J, et al. Desorption of calcium-rich crayfish shell biochar for the removal of lead from aqueous solutions[J]. Journal of Colloid and Interface Science, 2019, 554: 417-423. doi: 10.1016/j.jcis.2019.06.096 -






 DownLoad:
DownLoad: