-
由于塑料具有比其他材料(如木材、陶瓷、金属等)成本低、易加工、重量轻等显著优势[1],可广泛应用到包装、建筑、电子电器、医疗器械等行业。据国家统计局统计,2017年,我国塑料品的产量为7.5×107 t,同比2016年增长3.4%。但是,我国的塑料品消耗量更为巨大,约为产量的150%,我国已经成为废塑料产量最多的国家。而废塑料回收量仅为1.70×107 t,较2016年的1.88×107 t下降了1.8×106 t,降幅为9.6%[2]。由此可见,大部分塑料没有得到有效回收和利用。这些未回收利用的塑料废弃物在自然界中难以生物降解,对土壤和水资源造成了严重的污染[3]。因此,提高废塑料的回收利用率便成为当前需要解决的关键问题。
废塑料回收利用的方法主要包括填埋法[4]、焚烧法、热解法[5]、气化法等,但这些方法存在土地占用率高、造成水污染和大气污染等缺点。而采用废塑料制备较高价值的离子交换树脂,则是对废弃物进行资源化不错的选择。离子交换树脂主要应用于废水处理、工业催化[6]、吸附和可回收稀有金属等方面,SULKOWSKI等[7]将废旧塑料进行化学改性来获得有效的絮凝剂,降低了水的浊度和溶解杂质浓度,改善了纯净水的质量参数。SULKOWSKI等[8]将固体二氧化硅硫酸用于溶解在有机溶剂中的聚苯乙烯废物的非均相磺化,制备有效的阳离子交换剂,主要用于处理污水中的重金属离子,具有较高的金属去除率。ZHANG等[9]以废旧印刷电路板为原料,通过氯甲基化和季胺化反应制备阴离子交换树脂,主要用于吸附水溶液中重金属Cr(Ⅵ)。此外,燃煤电厂化石燃料燃烧产生的大量CO2造成了气候恶化、全球变暖等问题[10],联合国政府间气候变化专门委员会(IPCC)预测,截至2050年,大气中CO2的体积分数将达到0.55×10−3,因此,对CO2的捕集和资源化也越来越受到关注[11]。把废塑料的循环利用和CO2的捕集结合起来,利用废旧塑料制备离子交换树脂并应用于CO2捕集则显得十分有意义,而此类研究鲜见报道。
本研究以废旧电视机外壳(WTVS)为原料,通过磺化反应和溶胀-渗透方法来制备SM-D001,将其作为CO2吸附材料的载体;采用N2吸附-脱附、傅里叶变换红外光谱(FT-IR)、热重分析(TGA)、扫描电镜(SEM)、压汞法等手段对SM-D001进行表征;考察了不同正庚烷的量、乙醇/水质量比和致孔时间等条件下制备的SM-D001对CO2吸附能力的影响;并以SM-D001为载体,五乙烯六胺(PEHA)为改性剂,制备固态胺吸附剂,考察了PEHA负载量对CO2吸附能力的影响,对其进行吸附动力学研究,为进一步证明固态胺吸附剂对CO2吸附过程是物理吸附和化学吸附共同作用的结论提供参考。
全文HTML
-
废旧电视机外壳(WTVS):青岛海信电器股份有限公司;二氯甲烷(M=84.94 g·mol−1,ω≥99.8%):天津市科密欧化学试剂有限公司;浓硫酸(H2SO4,M=98.08 g·mol−1,95%≤ω≤98%,分析纯):莱阳经济技术开发区精细化工厂;氢氧化钠(NaOH,M=40 g·mol−1,ω≥96.0%,分析纯):天津市北辰方正试剂厂;正庚烷(M=100.20 g·mol−1,97%≤ω≤98%,分析纯):天津市富宇精细化工有限公司;无水乙醇(M=46.07 g·mol−1,ω≥99.7%,分析纯):天津市北联精细化学品开发有限公司;氯化铜(CuCl2·2H2O,M=170.48 g·mol−1,ω≥99.0%):天津市广成化学试剂有限公司;五乙烯六胺(PEHA,M=232.38 g·mol−1):上海麦克林生化科技有限公司;N2(高纯,ω=99.999%)和混合气(15% CO2/85% N2,体积分数):华金工业气体公司。
-
将2 g废旧电视机外壳溶解在20 mL的二氯甲烷中,再向其加入适量浓硫酸,搅拌均匀,在室温下放置一段时间后,滴加NaOH溶液,直至溶液为中性,再多次用无水乙醇洗涤,来除去残留在磺酸钠型树脂中的二氯甲烷,在室温下干燥,得到磺酸钠型树脂颗粒。先将2 g磺酸钠型树脂颗粒倒入装有100 g乙醇/水混合液的500 mL四口烧瓶中,待树脂颗粒在乙醇/水混合液中分散均匀后,再加入正庚烷,在一定温度下反应5 h后,停止加热,待温度冷却至室温,经过多次过滤和洗涤,最终得到SM-D001。
-
将SM-D001置于无水乙醇中浸泡6 h,除去残留在树脂中的有机物。把干燥的SM-D001置于氯化铜溶液(0.25 mol·L−1)浸泡12 h,使Cu2+均匀地分布在SM-D001表面,将SM-D001-Cu加入20 mL的PEHA乙醇溶液中,超声3 h,在室温下,干燥得到SM-D001-Cu-xPEHA,其中x代表PEHA占固态胺吸附剂的质量分数,计算方法见式(1)。
式中:mPEHA为PEHA的质量,g;mSM-D001-Cu为SM-D001-Cu的质量,g。
-
样品研磨后,用KBr压片,采用傅里叶变换红外光谱仪(TENSOR-27型,德国BRUKER公司),在4 000~500 cm−1测定吸附剂官能团的变化。利用热分析仪(NETZSCH STA409PC型,德国Netzsch公司)测试吸附剂的热稳定性,在40 mL·min−1的N2环境下,以10 K·min−1的升温速率,从室温升至700 ℃。采用SEM(JEOL JSM-6700F型,日本电子株式会社)观察吸附剂表面形貌。利用比表面积测定仪(ASAP 2020V4.01型,美国Micromeritics公司),在77 K下测得N2吸附-脱附等温线。根据吸附-脱附等温线中的数据,利用Barrett Joyner-Halenda(BJH)方法确定孔径分布曲线。采用孔径测定仪(Poremaster33型,美国Quantachrome公司),其基本原理是压汞法,在真空条件下,将汞注入样品管中,然后将样品管放入高压站进行分析,最高压力为227.5 MPa。
-
CO2吸脱附实验在如图1所示的固定床反应器(内径10 mm,长200 mm的不锈钢管)中进行。称取1 g吸附剂置于反应器内,在30 mL·min−1的高纯N2氛围中,80 ℃吹扫1 h,除去物理吸附的H2O和CO2。将吸附剂冷却至吸附温度,进样气体切换为15% CO2/85% N2,开始吸附实验。出口CO2浓度由气相色谱仪(PE Clarus 500,美国)检测,当出口CO2浓度等于进口CO2浓度时,CO2吸附完成。将吸附剂升温至80 ℃,进样气体切换为N2,开始脱附实验。当出口检测不到CO2时,CO2脱附完成。CO2吸附量由式(2)计算得出。
式中:qt为t时刻时吸附剂对CO2的吸附量,mmol·g−1;Q为进气流量,mL·min−1;m为吸附剂的质量,g;c0、c分别为进口CO2浓度和出口CO2浓度,mmol·L−1;t为吸附时间,min。
-
吸附动力学是评价吸附剂吸附性能的重要指标,目前,已报道的有机胺负载多孔固体材料吸附CO2的动力学模型主要包括拟一阶动力学模型、拟二阶动力学模型、Avrami吸附模型。由于拟一阶和拟二阶动力学模型对固态胺吸附剂吸附CO2具有一定的局限性,用Avrami吸附模型与固态胺吸附剂吸附CO2,实验数据拟合较好[12-13]。本研究采用Avrami吸附模型研究固态胺吸附剂的吸附动力学。Avrami吸附方程见式(3)。
式中:kA为Avrami动力学模型的吸附速率常数,min−1;qs为CO2的饱和吸附量,mmol·g−1;nA为Avrami吸附模型的反应级数。
1.1. 实验原料
1.2. SM-D001的制备
1.3. 固态胺吸附剂的制备
1.4. 材料表征
1.5. CO2吸脱附实验
1.6. 吸附动力学模型
-
1) FT-IR表征。图2表示WTVS、磺酸钠型树脂和SM-D001的FT-IR谱图。在WTVS的FT-IR谱图中,3 022 cm−1是烯烃上的=C—H伸缩振动吸收峰,2 925 cm−1和2 850 cm−1是饱和烃—CH2反对称伸缩振动和对称伸缩振动吸收峰,1 600、1 492和1 450 cm−1为苯环骨架伸缩振动吸收峰,757 cm−1和700 cm−1是苯环上C—H的面外变形振动[14],说明WTVS主要成分为聚丁二烯聚苯乙烯(HIPS)。磺酸钠型树脂颗粒的FT-IR谱图中存在1 037 cm−1与1 002 cm−1双峰,是苯环C—H面内弯曲振动受S=O对称伸缩振动的影响产生的。1 174 cm−1与1 124 cm−1是S=O反对称伸缩振动产生的[15]。3 458 cm−1 是磺酸基团上—OH的伸缩振动吸收峰。表明硫酸和HIPS成功地发生了亲电取代反应[16],生成了带有磺酸基团的磺酸钠型树脂颗粒(如图3所示)。加入致孔剂后制备的SM-D001与磺酸钠型树脂颗粒的振动吸收峰相比,基本不发生变化,表明用乙醇、水和正庚烷作为致孔剂进行致孔时,致孔剂与磺酸钠型树脂不发生化学反应。
2)热重分析。图4为WTVS改性前后的热重分析图。由此可见,WTVS只出现1个失重峰,其失重区域为315~450 ℃,失重量约为总重量的96.4%,其主要由于WTVS样品在空气中的热分解所致,说明WTVS样品的最高耐热温度为315 ℃。磺酸钠型树脂和SM-D001都出现2个失重峰,第1个失重区域均在50~150 ℃,失重率分别为12%和8%,其主要是样品吸附的空气中的水和CO2经加热后解吸排出的[17]。当温度高于300 ℃时,磺酸钠型树脂和SM-D001快速失重(分别约为72%和71%),主要是磺酸钠型树脂和SM-D001在空气中的热分解[18]所致。TGA-DTG实验结果表明,SM-D001在300 ℃温度以下是热稳定的,和商用D001[19]一样,能够满足整个吸附脱附的温度要求。
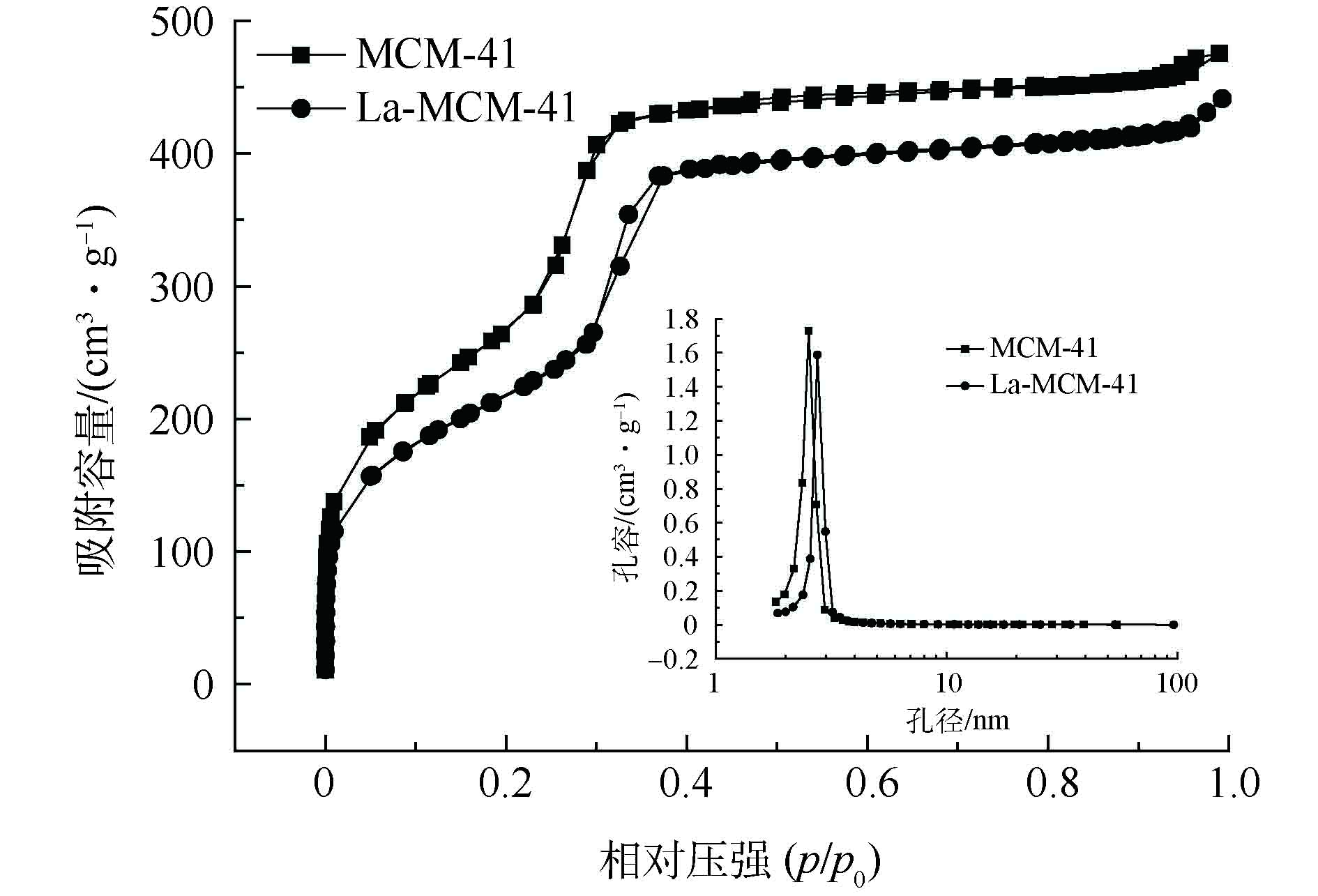
3) N2吸附-脱附等温线。WTVS、磺酸钠型树脂和SM-D001 N2吸附-脱附等温线和BJH孔径分布如图5所示。磺酸钠型树脂和SM-D001 N2吸附-脱附等温线与商用D001[20]一样,均是典型的Ⅳ型等温线。磺酸钠型树脂和SM-D001在相对压力p/p0<0.40时,N2吸附量缓慢上升,磺酸钠型树脂和SM-D001在相对压力为0.65~0.95时,出现明显的H3型滞后环,其主要是由片状粒子堆积而成的狭缝孔,具有不规则的孔形状。在p/p0≈1时,吸附曲线陡然增加,表明磺酸钠型树脂和SM-D001都以大孔分布为主[21]。磺酸钠型树脂和SM-D001在孔径为5~10 nm和≥50 nm处具有较大的对数微分孔体积,且在50~200 nm,对数微分孔体积呈现逐渐增加的趋势,因此,磺酸钠型树脂和SM-D001在大于200 nm范围内也有孔径分布。氮吸附方法测量孔隙半径范围一般为中孔或微孔。压汞法测量孔径分布时,由于压力以及样品性质的限制,液态汞无法进入细小的微孔中,因此,只能测量出样品中大孔和中孔的孔径分布[22]。因此,单一的手段不能满足全范围孔径分布测量,采用压汞法对磺酸钠型树脂和SM-D001进行进一步孔径分布测试。
4)压汞法。图6表示磺酸钠型树脂和SM-D001的进退汞曲线和孔径分布。由此可见,磺酸钠型树脂和SM-D001的进汞曲线随着压力的增大,孔容不断增大,在低压过程中,累积进汞量最多,说明制备的材料是以大孔分布为主;在高压时,此时的再进汞量最少,表明在这个阶段时,与之相应的孔隙分布少。由磺酸钠型树脂和SM-D001的孔径分布曲线可知,磺酸钠型树脂孔径分布主要集中在5.00×104 nm以上,表明磺酸钠型树脂主要是微米级孔结构。SM-D001不同孔径的对数微分孔体积增量主要集中在1 219.67 ~3 106.84 nm和3 618.75~6 466.13 nm 2个孔径段[23],表明SM-D001以大孔分布为主,且孔径比磺酸钠型树脂小,比表面积大。表1和表2分别表示SM-D001各孔径段孔容和比表面积及其分布比例。由此可见,磺酸钠型树脂孔体积为1.08 mL·g−1,比表面积为2 m2·g−1,SM-D001孔体积为3.52 mL·g−1,比表面积为30.70 m2·g−1,由此说明,加入致孔剂能够提高材料的孔隙率和比表面积。2种材料的大孔体积占总体积比例分别为99.07%和97.73%,进一步表明磺酸钠型树脂和SM-D001以大孔分布为主[24-25]。与商用D001(比表面积106.8 m2·g−1)[19]相比,其存在比表面积小、平均孔径较大等缺点。
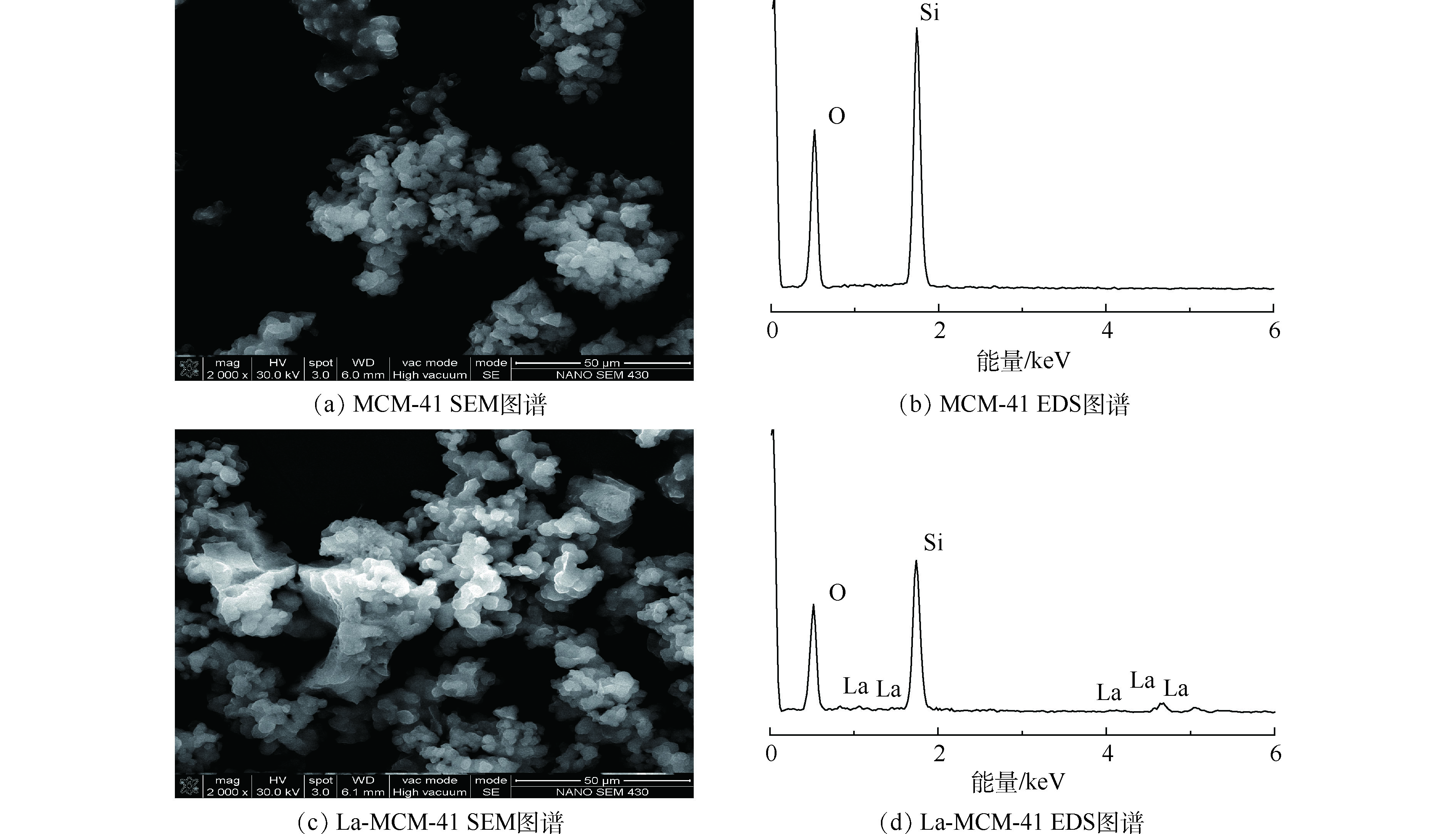
5)扫描电镜分析。由图7(a)可以看出,WTVS表面光滑,没有孔结构。在图7(b)中,硫酸与HIPS反应后,用自来水洗去残留在树脂中的硫酸的过程中,树脂吸水膨胀,同时残留在树脂中的二氯甲烷将树脂内部的部分溶解。在室温下干燥过程中,树脂内部水分蒸发,得到表面粗糙、内部具有孔结构的磺酸钠型树脂。图7(c)表示加入致孔剂为正庚烷、乙醇和水的混合液后的SEM图像。由于磺酸钠型树脂具有很强的亲水性,其颗粒在没有表面活性剂的帮助下可以作为单个固体颗粒分散在水和乙醇中。添加正庚烷,大部分磺酸钠型树脂固体颗粒扩散到正庚烷溶液与水/乙醇的界面上,形成稳定的皮克林乳液。在加热过程中,聚合物链的流动性增强,正庚烷和乙醇会穿透磺酸钠型树脂使其溶胀,同时也会使停留在磺酸钠型树脂颗粒内部的乙醇和正庚烷混合液蒸发,实现液固分离,使树脂内部产生空隙,最后得到SM-D001[26-27]。
-
在致孔温度为70 ℃、正庚烷用量为10 g、乙醇和水的混合液总量为100 g时,考察了不同乙醇/水质量比下制备的SM-D001对CO2吸附能力的影响,图8表示不同乙醇/水质量比下制备的树脂的吸附穿透曲线和吸附能力曲线。由此可见,当乙醇/水质量比为90∶10时,吸附穿透时间最高达到6 min,随着乙醇和水的混合液中水含量的增加,其穿透吸附量和平衡吸附量呈先增加后减小的趋势。这是由于磺酸钠型树脂具有亲水性,随着水含量的增加,磺酸钠型树脂能够吸水膨胀,促使正庚烷随乙醇溶液一起渗透到磺酸钠型树脂内部;同时,在加热过程中,停留在磺酸钠型树脂颗粒内部的乙醇和正庚烷混合液蒸发,实现液固分离,使树脂内部产生空隙,得到具有较多孔隙的SM-D001。但当混合液中水含量继续增加时,此时乙醇含量相对减少,正庚烷与水之间的表面张力增加,只有少量的正庚烷随乙醇一起渗透到树脂内部,大量的正庚烷停留在树脂表面,使制备的SM-D001孔隙较少。由于SM-D001对CO2的吸附主要是物理吸附,其比表面积越大,与CO2接触面积就越大,同时对CO2吸附能力就越强。因此,当乙醇/水质量比为90∶10时,制备的树脂对CO2吸附能力达到最高,其吸附量达到1.72 mmol·g−1。
在致孔温度为70 ℃、正庚烷用量为10 g、致孔剂乙醇/水质量比为90∶10时,考察了不同致孔时间制备的树脂对CO2吸附性能的影响。图9表示不同致孔时间下制备的SM-D001的吸附穿透曲线和吸附能力曲线,由此可见,随着致孔时间的延长,树脂对CO2的吸附穿透时间呈先不变后减小的趋势,穿透吸附量和平衡吸附量呈先增加后减小的趋势。这是由于在致孔反应开始时,磺酸钠型树脂由于表面磺酸基团量较少,其吸水作用较弱,但随着时间的延长,磺酸型树脂缓慢吸水膨胀,使正庚烷能够随乙醇充分地渗透到树脂内部,使SM-D001比表面积增加,CO2吸附能力增强。继续增加致孔时间,其CO2吸附能力呈现下降的趋势,主要是因为随着反应时间的延长,残留在树脂中正庚烷不断地使磺酸型树脂内部溶解,造成树脂内部孔坍塌,比表面积减小,CO2吸附能力不断减小。
在致孔温度为70 ℃,致孔剂乙醇/水质量比为90∶10,反应时间为5 h时,考察了正庚烷的用量对CO2吸附能力的影响。图10表示不同正庚烷的量制备的SM-D001的吸附穿透曲线和吸附能力曲线,由此可见,当正庚烷的量为0 g时,其CO2吸附穿透时间为4 min;当开始加入正庚烷时,其穿透吸附时间增加到6 min,同时穿透吸附量和平衡吸附量呈先增加后减小的趋势。这是由于正庚烷为相分离剂,在致孔过程中,正庚烷随乙醇一起渗透到树脂内部。当树脂内部正庚烷的量较少时,正庚烷对树脂的致孔能力较弱,比表面积和孔体积较小;随着正庚烷量的增加,停留在磺酸钠型树脂颗粒内部乙醇和正庚烷混合液蒸发,实现液固分离,使树脂内部产生空隙,得到SM-D001,其比表面积和孔体积较大;随着正庚烷量的继续增加,大量的正庚烷使磺酸型树脂内部溶解,溶解的树脂与未溶解的树脂发生黏结,导致其比表面积与孔体积减小,因而CO2吸附能力下降。
-
将不同PEHA负载量的固态胺吸附剂在温度为60 ℃和进气流量为40 mL·min−1条件下进行CO2吸附实验。由表3可以看出,当SM-D001-Cu负载PEHA的量增加时,其CO2吸附能力呈现先增加后减少的趋势,这是由于随着PEHA负载量的增加,吸附剂表面和内部孔道胺活性位点增加,但由于模板和内部孔道的限制,当PEHA负载量大于0.3时,部分PEHA会聚集堵塞孔道,导致CO2只能与吸附剂表面负载的PEHA发生反应[20],使CO2吸附能力下降。以SM-D001为载体的固态胺吸附剂对CO2吸附能力最高达到3.61 mmol·g−1,与以商用D001为载体制备的固态胺吸附剂对CO2吸附性能(吸附量4 mmol·g−1)[19]相比,其吸附量较小,主要是因为当商用D001负载有机胺后,吸附剂部分大孔结构被有机胺占据,形成具有空间分割的微孔结构,使得其微孔结构比例增加,大孔结构比例减少。吸附剂中存在的微孔促进了物理吸附的发生[28]。同时孔道内引入的氨基活性组分与CO2发生化学反应,形成氨基甲酸盐,剩余的孔道为CO2扩散提供通道。但由于SM-D001比表面积较小,平均孔径较大,有机胺负载后的吸附剂大孔和微孔比例不发生变化,因此,以SM-D001为载体的固态胺吸附剂对CO2吸附能力相对较弱。为了增加其CO2吸附能力,应进一步进行优化实验,制备比表面积大、平均孔径较小的SM-D001。
-
将不同PEHA负载量的固态胺吸附剂在吸附温度为60 ℃、CO2分压为15 kPa、进气流量为40 mL·min−1条件下,对吸附实验数据进行Avrami吸附模型非线性拟合,拟合结果见图11和表4。由此可知,固态胺吸附剂对CO2的吸附过程分为快速的穿透吸附和相对缓慢的逐渐平衡阶段。在吸附初期,CO2分子的扩散速度较快,原因是CO2分子从气体流动主体扩散到吸附剂的外表面时,其扩散阻力较小。当颗粒外表面吸附达到平衡时,CO2分子通过吸附剂孔扩散到颗粒内部,内扩散过程中由于PEHA占据了一部分颗粒内部孔道,其内部扩散阻力较大,CO2在吸附剂内部扩散速率较慢,因此,内扩散是CO2吸附过程的速率控制步骤,当吸附一段时间后,吸附和脱附达到平衡,CO2吸附量保持不变[29]。用Avrami吸附模型[30]对吸附数据进行拟合,具有较高的拟合度,在不同PEHA负载量下(R2>0.999),说明固态胺吸附剂对CO2的吸附过程能够用Avrami吸附模型较好地描述,同时表明SM-D001-Cu-0.3PEHA对CO2的吸附是物理吸附与化学吸附共同作用的结果。
2.1. 材料表征
2.2. 致孔条件对SM-D001吸附性能的影响
2.3. PEHA负载量对CO2吸附性能的影响
2.4. CO2吸附动力学
-
1)通过将WTVS进行磺化反应,制备磺酸钠型吸水树脂,再加入致孔剂乙醇、水和正庚烷得到SM-D001。该方法操作简单,原料价廉易得,能耗低,同时能够实现保护环境和节约资源的双重效果。
2)在致孔剂正庚烷用量为25 g、乙醇/水质量比为90∶10、反应时间为5 h条件下,采用溶胀-渗透方法制备SM-D001,并将其进行CO2吸附实验,CO2吸附量达到1.87 mmol·g−1,用压汞法测得其比表面积为30.70 m2·g−1,平均孔径为458.52 nm,孔体积为3.52 mL·g−1。
3)以SM-D001为模板,通过配位法制备固体胺吸附剂SM-D001-Cu-0.3PEHA,在吸附温度为60 ℃、进气流量为40 mL·min−1、CO2分压为15 kPa条件下,其CO2吸附量达到3.61 mmol·g−1,符合工业上对燃煤电厂中CO2吸附的要求。
4)通过动力学的研究可知,吸附过程是由物理吸附和化学吸附共同作用的结果,内扩散是整个吸附过程的速率控制步骤,同时吸附过程符合Avrami吸附模型。



 下载:
下载: