-
厌氧氨氧化是指在厌氧条件下,厌氧氨氧化菌利用
NO−2 -N将NH+4 -N氧化,最终生成N2和少量NO−3 -N的过程[1-2]。与传统硝化反硝化工艺相比,厌氧氨氧化工艺不消耗有机物,污泥产率低,尤其适合于处理高氨氮、低C/N的废水[3-4],因此,其已受到了国内外广泛关注。2006年,荷兰鹿特丹污水处理厂以厌氧氨氧化工艺进行污泥压滤液的脱氮处理,反应器总氮负荷高达9.5 kg·(m3·d)−1,远高于传统脱氮工艺的去除效率[5]。目前,全球范围内已有超过100座以厌氧氨氧化工艺运行的污水处理装置[6]。尽管厌氧氨氧化工艺具有高效脱氮、运行成本低等优势,然而,由于厌氧氨氧化菌的细胞产率低[7],如何在较短时间内获得足够的厌氧氨氧化菌成为厌氧氨氧化工艺启动和运行的关键所在。微生物的增长是同化和衰减共同作用的结果,而表征微生物衰减的参数为衰减系数。因此,研究衰减系数对探讨厌氧氨氧化微生物的增殖和培养具有重要意义。目前,有关厌氧氨氧化菌衰减系数的研究大多为厌氧条件,而缺氧条件下的较少,探究不同环境下厌氧氨氧化菌的衰减系数,可为厌氧氨氧化工艺的广泛应用提供理论依据和技术指导。
本研究以实验室稳定运行6年的SBR中的厌氧氨氧化菌(Candidatus Brocadia)为研究对象,在温度为35 ℃且无电子供体的条件下,分别进行缺氧(
NO−2 -N、NO−3 -N)和厌氧环境的衰减培养,通过测定厌氧氨氧化污泥的基质利用速率,从而确定了不同环境下厌氧氨氧化菌的衰减系数。
全文HTML
-
衰减培养的污泥取自以SBR方式稳定运行的厌氧氨氧化反应器[8-9]。该污泥的总氮负荷为1.59 kg·(m3·d)−1、总氮去除率为90.64%、Δ
NO−2 -N/ΔNH+4 -N为1.33、ΔNO−3 -N/ΔNH+4 -N为0.26,其参数比LOTTI等[10]报道的理论值偏大。该污泥颗粒化程度较好,呈红棕色。实验用水采用无氧去离子水配制。缺氧环境下衰减系数测定的培养液中分别投加30 mg·L−1 NaNO2和25 mg·L−1 NaNO3,厌氧环境下衰减系数测定的培养液中无NaNO2和NaNO3。基质利用速率测定过程中同时投加25 mg·L−1 NH4Cl和33 mg·L−1 NaNO2。两者均投加500 mg·L−1 KHCO3、180 mg·L−1 CaCl2·2H2O、100 mg·L−1 MgSO4·7H2O、50 mg·L−1 KH2PO4、微量元素Ⅰ和Ⅱ[11-13]均为1 mL·L−1。
-
1) 衰减培养。从反应器中各取150 mL颗粒污泥混合液,采用细胞破碎仪将污泥破碎,使污泥呈现悬浮状态,然后经无氧去离子水淘洗后,置于3个500 mL的培养瓶中,缺氧环境分别投加30 mg·L−1 NaNO2和25 mg·L−1 NaNO3,厌氧环境无NaNO2和NaNO3,3个培养瓶中均投加微量元素后用无氧去离子水定容至400 mL,用橡胶塞塞紧后,向瓶内通入高纯氮气,以去除水中溶解氧,密封后,置于35 ℃恒温水浴中进行衰减培养。
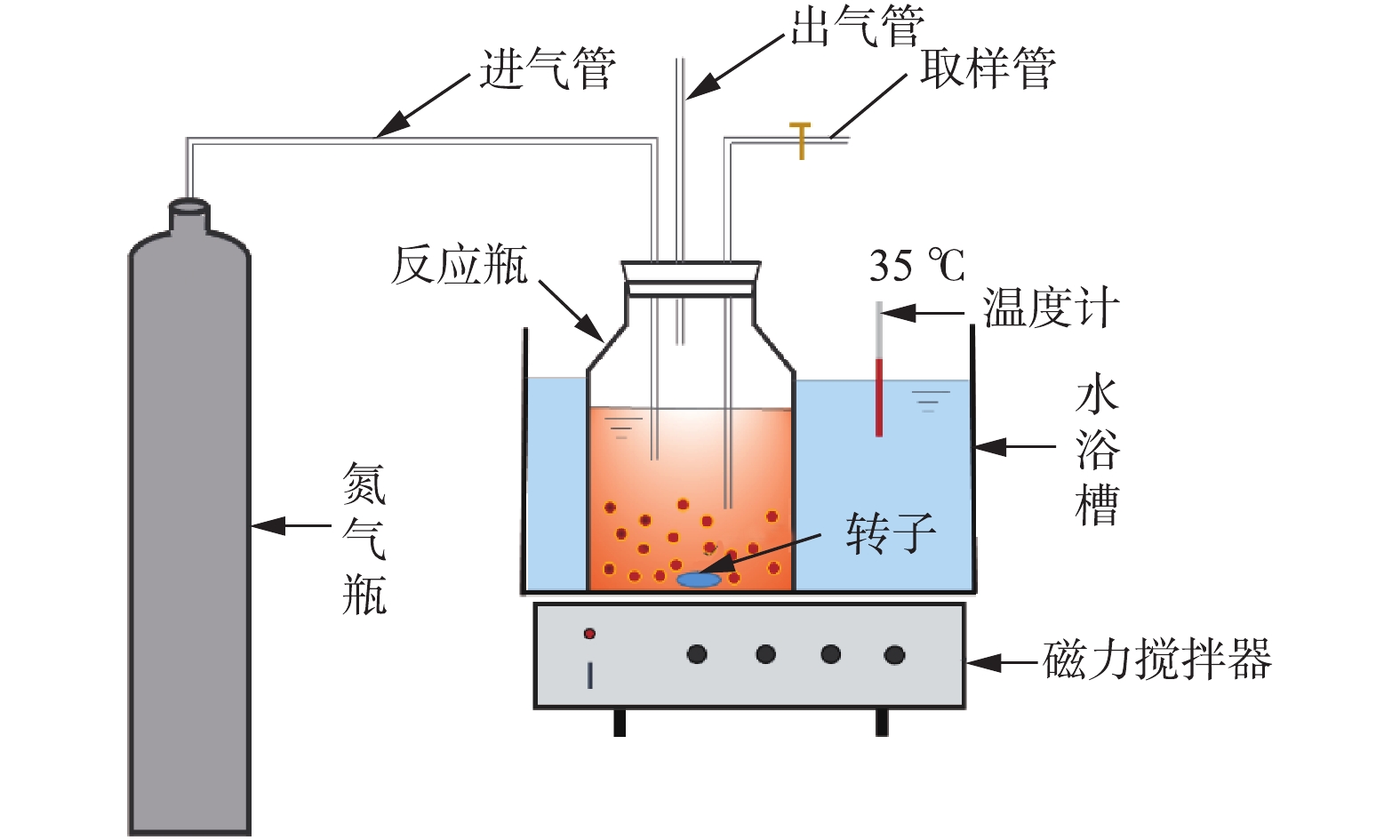
2) 基质利用速率测定。厌氧氨氧化污泥的基质利用速率测定装置见图1,具体操作步骤:从培养瓶中取一定体积的厌氧氨氧化污泥,用无氧去离子水淘洗后,置于250 mL广口瓶中,同时加入25 mg·L−1 NH4Cl、33 mg·L−1 NaNO2和微量元素,然后用无氧去离子水定容至200 mL,采用高纯氮气进行曝气,以去除水中溶解氧。通过恒温水浴控制温度在35 ℃,KHCO3作为缓冲液,将pH控制为7.3~7.8,每隔15 min取样,分析测定样品中
NH+4 -N和NO−2 -N的浓度,由t-c曲线确定厌氧氨氧化污泥的基质利用速率。 -
衰减系数的定义:在无外加基质的条件下,单位质量的微生物在单位时间内减少的细胞量,计算方法如式(1)所示。
式中:
b 为衰减系数;边界条件为t=0时,X=X0。对式(1)进行积分得到式(2)。基质利用速率的定义:在基质充足的条件下,单位体积(反应器)微生物在单位时间内利用基质的量,计算方法如式(3)所示。
当t=0时,对应的污泥浓度X=X0,对应的基质利用速率R=R0,将其代入式(3),得到式(4)。
当t=t时,对应的污泥浓度X=Xt,对应的基质利用速率R=Rt,将其代入式(3),得到式(5)。
将式(5)与式(4)合并,并将式(2)代入,得到式(6)。
式中:X0为初始时刻的污泥浓度,g·L−1;Xt为t时刻的污泥浓度,g·L−1;R0为初始时刻的基质利用速率,mg·(L·d)−1;Rt为t时刻的基质利用速率,mg·(L·d)−1;qmax为单位生物量的最大基质利用速率,d−1;
b 为微生物的衰减系数,d−1。依据式(6),通过测定厌氧氨氧化污泥基质利用速率随时间的变化,即可确定厌氧氨氧化菌的衰减系数。
-
1)常规水质指标分析。实验中各项常规水质指标均按文献中的方法[14]进行测定:
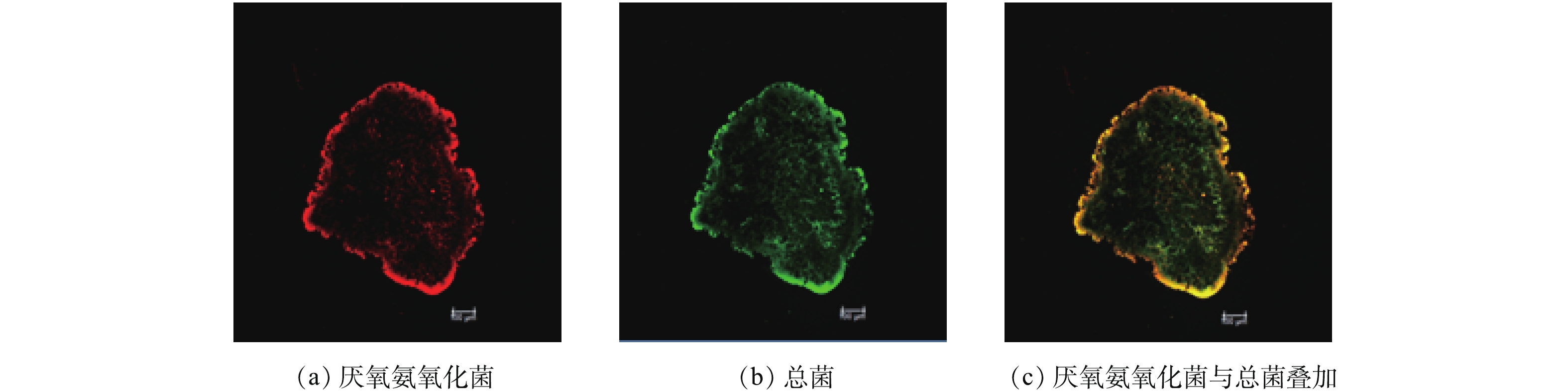
NH+4 -N采用纳氏试剂分光光度法;NO−2 -N采用N-(1-萘基)-乙二胺分光光度法;NO−3 -N采用紫外分光光度法;MLSS和MLVSS均采用重量法;pH采用雷磁PHS-3C型pH计进行测定。2)荧光原位杂交(FISH)。厌氧氨氧化污泥中的微生物种群采用荧光原位杂交法(FISH)进行分析。颗粒污泥经冷冻切片机(Leica CM 1950)进行切片,固定在正电荷黏附玻片上,杂交后,用激光共聚焦显微镜(TCS SP8,莱卡)进行观察和图像采集。实验所用探针如表1所示,总细菌采用Eub338mix(Eub338,Eub338Ⅱ和Eub338Ⅲ等体积混合),厌氧氨氧化菌采用Amx368。厌氧氨氧化菌的定量分析是将污泥样品用细胞破碎仪破碎,杂交后,在激光共聚焦显微镜下观察,并随机采集50幅图像,经Image-Pro Plus软件进行分析后,统计目标微生物占总生物数量的比例。
1.1. 实验材料
1.2. 实验装置
1.3. 基质利用速率确定衰减系数的方法
1.4. 测定项目与方法
-
采用荧光原位杂交对实验所用污泥中的微生物菌群进行分析,结果表明,厌氧氨氧化菌为优势菌(图2),采用Image-Pro Plus软件进行图像分析,确定污泥中厌氧氨氧化菌的含量占总细菌含量的86.03%,因此,采用该污泥进行厌氧氨氧化菌衰减系数的实验测定是可行的。
-
图3为实验所用污泥
NH+4 -N、NO−2 -N利用速率及NO−3 -N产生速率的测定结果。如图3所示,NH+4 -N、NO−2 -N和NO−3 -N的浓度随时间变化的线性关系均较好。依据图3的数据,可得厌氧氨氧化污泥的NH+4 -N利用速率为155.66 mg·(L·d)−1。反应结束时,测得污泥的MLVSS为1.028 g·L−1,对应的以MLVSS计量的NH+4 -N利用速率为151.49 mg·(g·d)−1。然后,以此作为缺氧(NO−2 -N、NO−3 -N)及厌氧环境下衰减培养的厌氧氨氧化污泥的初始基质利用速率(R0)。 -
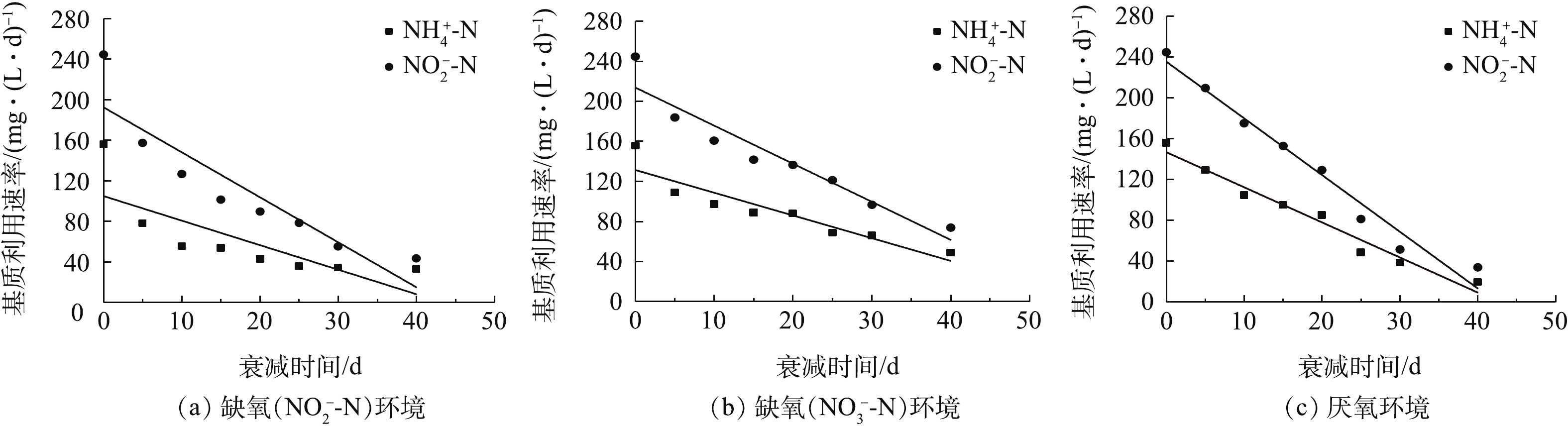
将厌氧氨氧化污泥分别置于缺氧(
NO−2 -N、NO−3 -N)及厌氧环境进行衰减培养,每隔5 d对其进行基质利用速率测定,相应的厌氧氨氧化污泥基质利用速率(Rt)随时间的变化趋势见图4。图4(a)为缺氧(
NO−2 -N)环境下Rt随时间的变化。由图4(a)可见,经过40 d的衰减培养后,Rt的NH+4 -N利用速率为32.69 mg·(L·d)−1,仅为初始值的21%。由FISH检测结果及反应化学计量比可知,系统中可能存在少量反硝化菌,而反硝化菌会利用NO−2 -N产生NH+4 -N,为厌氧氨氧化菌提供了基质,导致厌氧氨氧化污泥基质利用速率在衰减培养初期下降较快,随后呈线性下降的趋势。图4(b)为缺氧(
NO−3 -N)环境下Rt随时间的变化。由图4(b)可见,相比于NO−2 -N衰减环境,NO−3 -N环境下厌氧氨氧化污泥基质利用速率较大,即相应的衰减较小。经过40 d的衰减培养,Rt的NH+4 -N利用速率为48.82 mg·(L·d)−1,为初始值的31.36%。系统中存在的反硝化菌也会利用NO−3 -N产生NH+4 -N,导致衰减培养初期厌氧氨氧化污泥的基质利用速率下降较快,之后呈线性下降的趋势。图4(c)为厌氧环境下Rt随时间的变化。由图4(c)可见,经过40 d的衰减培养,Rt的
NH+4 -N利用速率为19.58 mg·(L·d)−1,基质利用速率大幅减小,仅为初始值的12.58%。在实验过程中,部分污泥颜色变黑,这是因为厌氧氨氧化菌因富含血红素而呈现红色,而汪彩华等[15]的研究结果表明,在无基质的环境下,血红素含量会逐渐减少;另外,死亡菌体水解后会产生硫化物,且硫化物含量随着时间的延长会增多[16],而大部分金属硫化物是黑色沉淀物,最终导致厌氧氨氧化污泥外观颜色呈现黑色。但是,厌氧氨氧化污泥的基质利用速率随时间变化的线性关系良好,这说明厌氧环境下产生的硫化物并未对基质利用速率的测定产生影响。以上实验结果表明,经过40 d的衰减培养后,
NO−3 -N环境下的厌氧氨氧化污泥剩余基质利用速率最大(即相应的衰减最小),NO−2 -N环境次之,厌氧环境最小(即相应的衰减最大)。 -
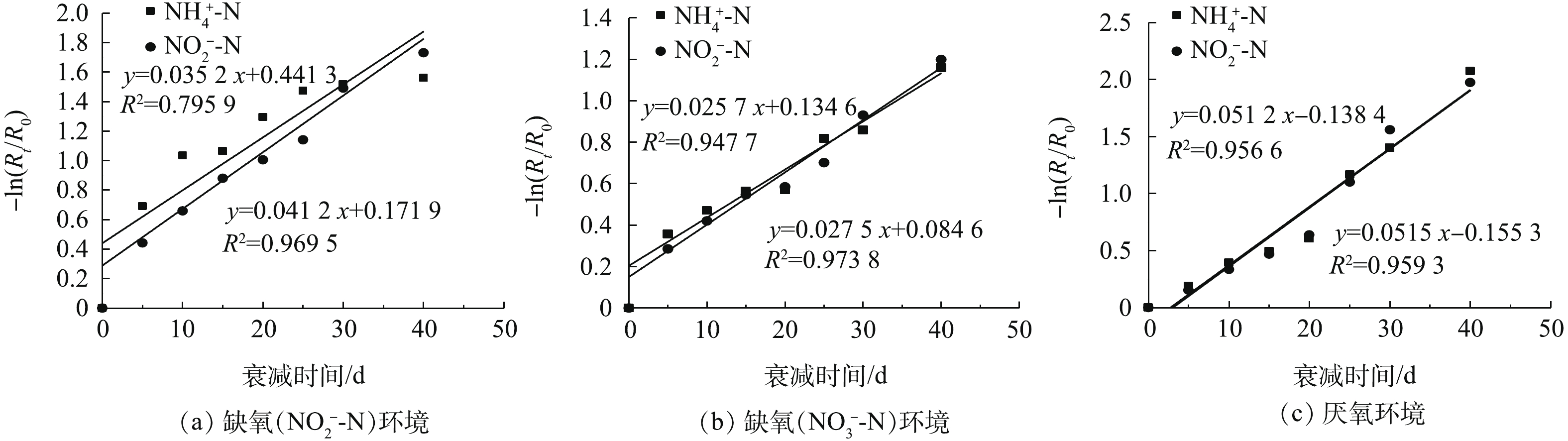
依据测定的厌氧氨氧化污泥的R0与缺氧(
NO−2 -N、NO−3 -N)及厌氧环境下的Rt,采用式(6),可得出不同环境下−ln(Rt/R0)随时间的变化,结果见图5。由此可见,投加NO−2 -N和NO−3 -N的缺氧环境下,厌氧氨氧化菌的衰减系数分别为0.035 2 d−1和0.025 7 d−1,厌氧环境下,厌氧氨氧化菌的衰减系数为0.051 2 d−1。由上述实验结果可知,厌氧及缺氧(
NO−2 -N、NO−3 -N)环境下,厌氧氨氧化菌的衰减系数依次减小。这是因为在厌氧环境中,35 ℃的温度下,厌氧氨氧化菌新陈代谢仍比较旺盛,在没有外源基质的情况下,导致大量细菌进行衰减。而在缺氧环境中,厌氧氨氧化菌以NO−2 -N作为基质,与投加NO−3 -N的环境相比,NO−2 -N的存在会促进厌氧氨氧化菌的衰减。由于厌氧氨氧化菌的增殖速率较小,并且启动一个具有高效脱氮效能的厌氧氨氧化反应器所需时间较长。因此,在进行污泥保存时,维持
NO−3 -N的缺氧环境有利于厌氧氨氧化菌活性和数量的保存。 -
厌氧氨氧化菌的种类、纯度及所处环境不同均会影响衰减系数。表2为文献报道的不同条件下厌氧氨氧化菌的衰减系数。SCAGLIONE等[17]研究了35 ℃下厌氧氨氧化菌的衰减系数,其中含有3种不同种属的厌氧氨氧化菌(Candidatus Brocadia anammoxidans,Candidatus Kuenenia stuttgartiensis以及1种尚未鉴定的浮霉菌),总的衰减系数为0.004 8 d−1,小于本研究的实验结果,这可能是由于实验所用污泥中厌氧氨氧化菌的种类及纯度不同所致。李祥等[18]采用一阶指数衰减模型对厌氧氨氧化菌在(30±2) ℃条件下的衰减系数进行了测定,结果为0.063 0 d−1,略大于本研究的实验结果,这可能是一阶指数衰减模型依据的是衰减培养过程中MLVSS的变化,MLVSS表征的是总菌,而厌氧氨氧化菌不能进行纯培养,污泥中会含有一定数量的其他细菌,从而导致测定结果出现误差。本研究依据厌氧氨氧化污泥基质利用速率随时间的变化确定衰减系数,可有效避免非纯培养体系造成的误差。
目前,硝化菌和异养菌的衰减系数也被众多学者研究。HENZE等[23]的研究结果见表3。异养菌在10 ℃和20 ℃下的衰减系数分别为0.20 d−1和0.40 d−1,而硝化菌在10 ℃和20 ℃下的衰减系数分别为0.05 d−1和0.15 d−1。SALEM等[24]也研究了在20 ℃好氧条件下的AOB和NOB的衰减系数,分别为0.02 d−1和0.08 d−1。一般情况下,温度越高,衰减系数越大,因此,在相同温度下,异养菌、硝化菌和厌氧氨氧化菌的衰减系数依次减小。
2.1. 厌氧氨氧化污泥FISH分析
2.2. 厌氧氨氧化污泥初始基质利用速率
2.3. 不同衰减环境下厌氧氨氧化污泥基质利用速率随时间的变化
2.4. 不同环境下厌氧氨氧化菌的衰减系数
2.5. 衰减系数对比
-
1)依据基质利用速率测定混合菌群中厌氧氨氧化菌的衰减系数,可有效避免由于其他细菌的衰减而引起的实验误差。
2)采用基质利用速率测定方法,确定了在缺氧(
NO−2 -N、NO−3 -N)及厌氧环境下厌氧氨氧化菌的衰减系数分别为0.035 2、0.025 7和0.051 2 d−1,这表明厌氧氨氧化菌的衰减系数与衰减环境密切相关。相比于其他自养菌,厌氧氨氧化菌的衰减系数较小。3)由于厌氧氨氧化菌的增殖速率很小,在进行污泥保存时,厌氧条件下厌氧氨氧化菌衰减较快,维持
NO−3 -N的缺氧环境有利于厌氧氨氧化菌活性和数量的保存。














 DownLoad:
DownLoad:


 百度学术
百度学术












