-
中国是传统的农业生产大国,每年水稻秸秆产量十分巨大[1]。近年来,水稻秸秆作为一种重要的生物质资源,受到越来越多的关注。目前,中国大部分地区对农作物秸秆常见的处理方式为粉碎还田、就地焚烧、或与畜禽粪便沤肥等。但这些做法不仅使土壤板结、肥力下降、微生态环境遭到破坏,还容易造成生物质能源的浪费和大气环境的污染[1-2]。与常见的处理方式相比,厌氧消化是水稻秸秆资源化利用的有效方法[3],其不仅能产生清洁能源进行资源化利用,而且发酵产生的沼渣沼液含有大量氮、磷、钾等元素,是很好的有机肥料,可用于农业生产,综合效益显著。但是,水稻秸秆中含有大量的木质纤维素成分,其难降解特性显著限制了水稻秸秆的利用效率[4]。
为了更好地利用水稻秸秆,研究者们从各个角度进行了广泛的研究。有的研究[5]使用不同预处理方式来改善水稻秸秆中木质纤维素的水解情况。虽然预处理方式可以提高水稻秸秆的水解效率,但是也出现了很多不可避免的问题,如预处理成本较高、易形成二次污染等,在实际应用中很难推广[6]。ZHANG等[7]的研究表明,在使用瘤胃液预处理水稻秸秆后,进行30 d厌氧消化,累积甲烷产量可以达到285.10 mL·g−1(以VS计);但是,这种通过过滤分离预处理后的产物、再进一步应用于随后的产甲烷过程的方式,规模化运用成本很高。为了解决这个问题,可以采用将微生物接种到厌氧消化系统中来强化木质纤维素的水解过程。SHI等[8]的研究表明,通过接种沼渣,玉米秸秆厌氧消化系统累积甲烷产量可以达到103 L·kg−1(以VS计),然而其挥发性固体(VS)的转化效率仅为23%。同时,在间歇式反应器中,使用瘤胃微生物在25~40 ℃下孵育240 h后,玉米秸秆的转化效率可以达到65%~70%[9]。显然,与其他接种物相比,瘤胃微生物是强化木质纤维素降解中更合理的接种物,这是因为其具有高质量的木质纤维素水解细菌,如Fibrobacter,succinogenes,Ruminococcus等 [10-11]。
通过共接种厌氧污泥能够解决瘤胃中产甲烷菌含量较少的问题;然而,在批次实验中,直接利用水稻秸秆来测试共接种瘤胃内容物和厌氧污泥的厌氧消化系统的产甲烷潜力,得到的沼气中的甲烷含量很低,仅为32%~44%[11]。本研究为中试规模的连续式厌氧消化系统,采用瘤胃微生物群和厌氧污泥共同接种的方式,强化了底物的利用率,并通过逐步提升底物有机负荷的方式分析了水稻秸秆的降解和转化规律,确定了本厌氧消化系统最佳的有机负荷率。
-
本研究中使用的瘤胃内容物来自屠宰场(中国无锡)的牛瘤胃。首先使用2层纱布过滤器过滤瘤胃内容物,然后高速离心分离上清液来获得瘤胃微生物群。从厌氧消化器(江苏清洁环境有限公司,中国苏州)回收厌氧污泥。分离好的瘤胃液和厌氧污泥基于VS含量的1∶1共同接种到反应器中。从稻田(连云港,中国)中收集秸秆。底物和接种物的主要参数如表1所示。
-
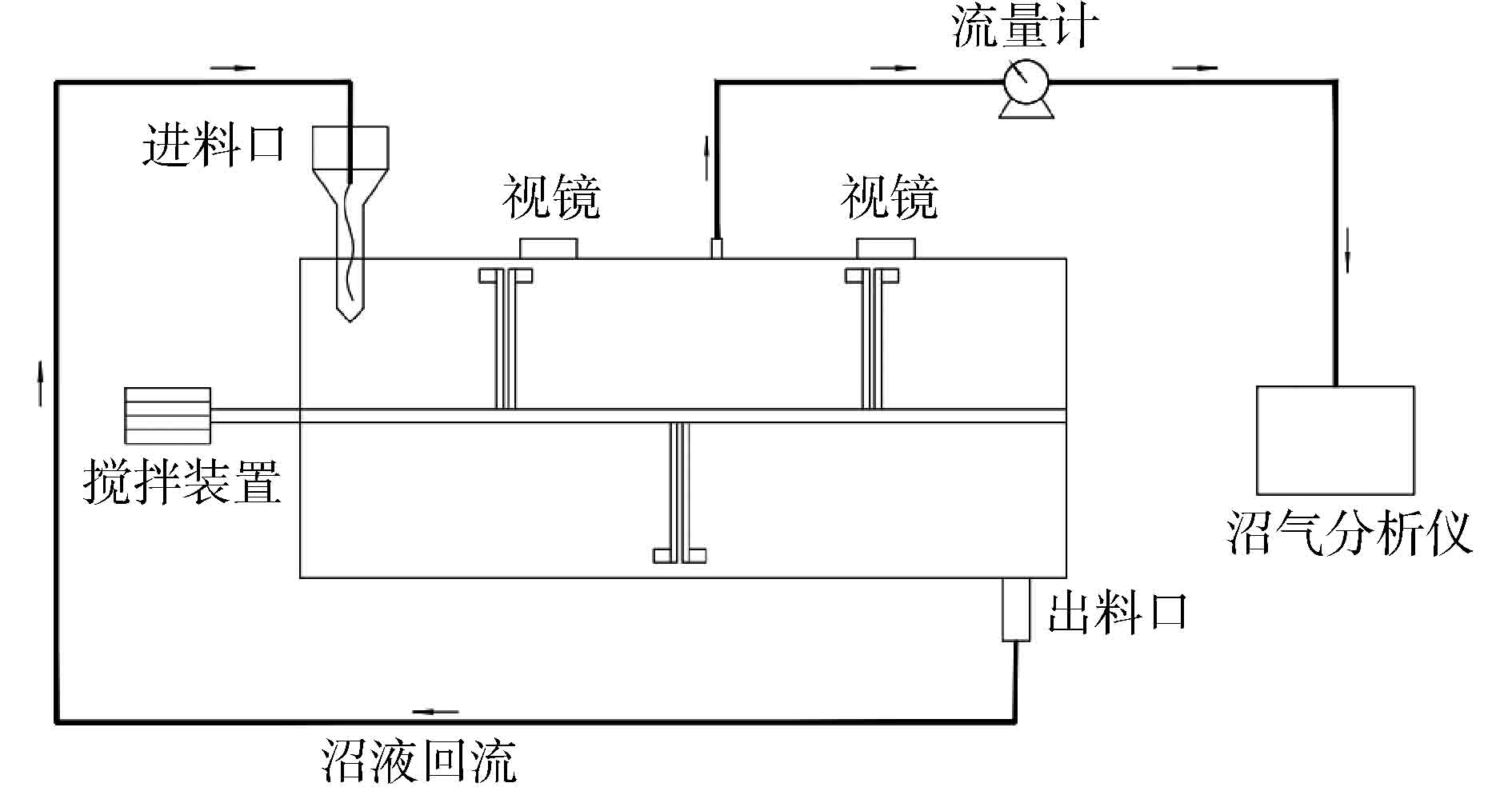
实验在卧式厌氧反应器中进行,反应器容积为200 L,有效体积为190 L,反应装置如图1所示。将湿式气体流量计(LMF-1型,长春汽车滤清器有限公司)连接在反应器出气口以测量沼气产量。便携式红外沼气分析仪(Gasboard-3200L型,武汉立方光电有限公司)用于测定沼气中的甲烷含量。在厌氧消化过程中,从反应器顶部的进料口加入秸秆,通过螺旋提升泵从反应器底部的出料口取泥,经固液分离后,其液体部分回流到反应器中。在本研究中,反应器温度控制在(39 ± 1) ℃。实验过程进行连续搅拌,通过控制柜调节搅拌速率为4 r·min−1。
-
本实验通过逐步提高系统有机负荷的方式,进行了7个阶段的实验,对应参数如表2所示。在反应器运行期间,系统的总固体含量保持在17%~20%,每天固定时间进料,每2 d进料前取1次样,监测相关指标,提供系统有机负荷提升依据。
-
1)总固体(TS):采用105 ℃烘干法[12];挥发性固体(VS):采用600 ℃灼烧法[12]。
2)蛋白质:用凯氏定氮仪(KDN-520型,杭州绿博仪器有限公司)测定凯氏氮含量,乘以6.25得到结果[13]。
3)纤维素、半纤维素和木质素:采用Van Soest洗涤法[14],利用全自动纤维分析仪(ANKOM 2000i型,美国ANKOM科技公司)测定;使用傅里叶变换红外分光光度计(IRTracer-100,日本岛津公司)测定水稻秸秆和沼渣的结构。
4)挥发性脂肪酸(VFA):采用总量比色法[15]测定。
5)木聚糖酶酶活力定义:1 mL酶液1 min 催化水解木聚糖生成1 μg木糖的酶量为1个酶活力单位(U·(mL·min)−1);羧甲基纤维素酶(CMC)活力定义:1 mL酶液1 min 催化水解羧甲基纤维素钠(CMC-Na)生成1 μg葡萄糖的酶量为1个酶活力单位(U·(mL·min)−1),以上酶都采用二硝基水杨酸法(DNS)显色法测定,以含有发酵液上清、但不含底物的反应体系作为比色对照[16]。
-
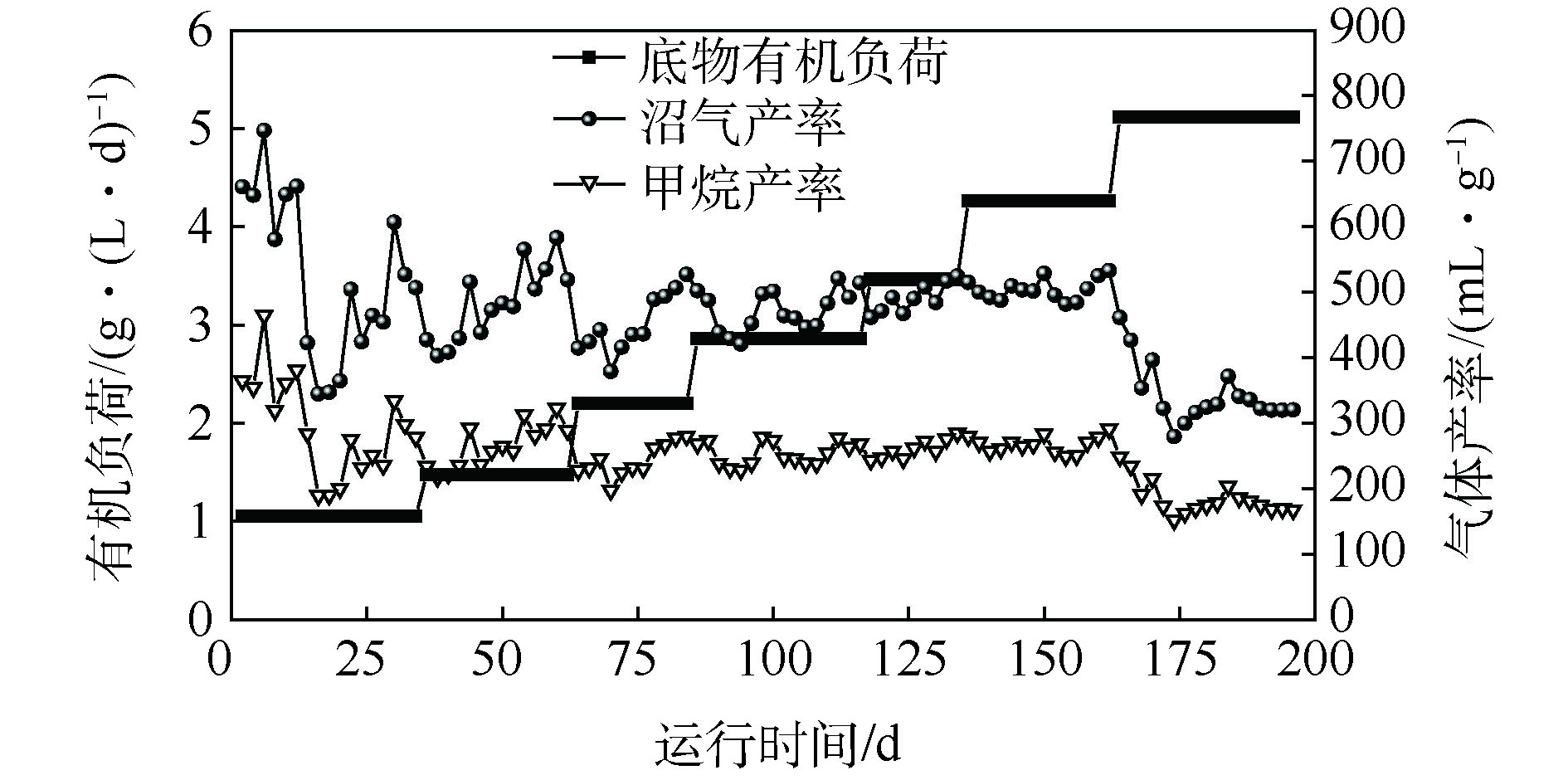
本厌氧消化系统运行期间的沼气产率和甲烷产率如图2所示,总共的运行时间为196 d。从图2可以看出,沼气产率和甲烷产率在反应器启动阶段波动较大,然后逐渐稳定。这表明,随着反应的进行,水稻秸秆厌氧消化系统逐渐稳定。在每个阶段,稳定状态下的沼气产率为533、534、547、542、528、527、329 mL·g−1(以VS计),相应的甲烷产率为284、289、303、299、285、287、171 mL·g−1(以VS计),各个阶段下沼气中的甲烷含量约为55%。随着底物有机负荷(OLR)的增加,稳定的沼气产率和甲烷产率表明水稻秸秆在H7阶段前均可以被系统稳定利用;同时,在底物OLR提升的节点,由于底物对反应体系的冲击作用会出现沼气产率的明显下降,随着反应体系逐渐适应新的有机负荷,沼气产率会逐渐回升并保持相对稳定。此外,本系统的容积沼气产率从H1阶段到H6阶段不断提高,由0.56 L·(L·d)−1增加到2.20 L·(L·d)−1。在H7阶段,沼气生产率和甲烷产量维持在329 mL·g−1和171 mL·g−1的较低水平。结合木质纤维素的降解情况(见下述2.3节),5.12 g·(L·d)−1(以VS计)的OLR超过了本厌氧消化系统的处理能力,其水解能力已经饱和。显然,在H6阶段,本厌氧消化系统获得了最佳的OLR,结果为4.26 g·(L·d)−1。
现有的关于水稻秸秆连续厌氧消化的研究[17]在2.00 g·(L·d)−1(以VS计)的OLR下,仅获得了143 mL·g−1(以VS计)的特定甲烷产量,低于本体系的处理水平;同时,ZHOU等[18]在水稻秸秆OLR达到2.00 g·(L·d)−1时,获得的最高容积沼气产率仅为0.86 L·(L·d)−1,也远小于本体系最佳容积产气率,体现了本厌氧消化系统高效的厌氧消化效率。这表明,经过长时间连续运行和有机负荷提升,成功塑造出了一种利用水稻秸秆的高效厌氧消化系统,对水稻秸秆进行了高效的资源化利用。
-
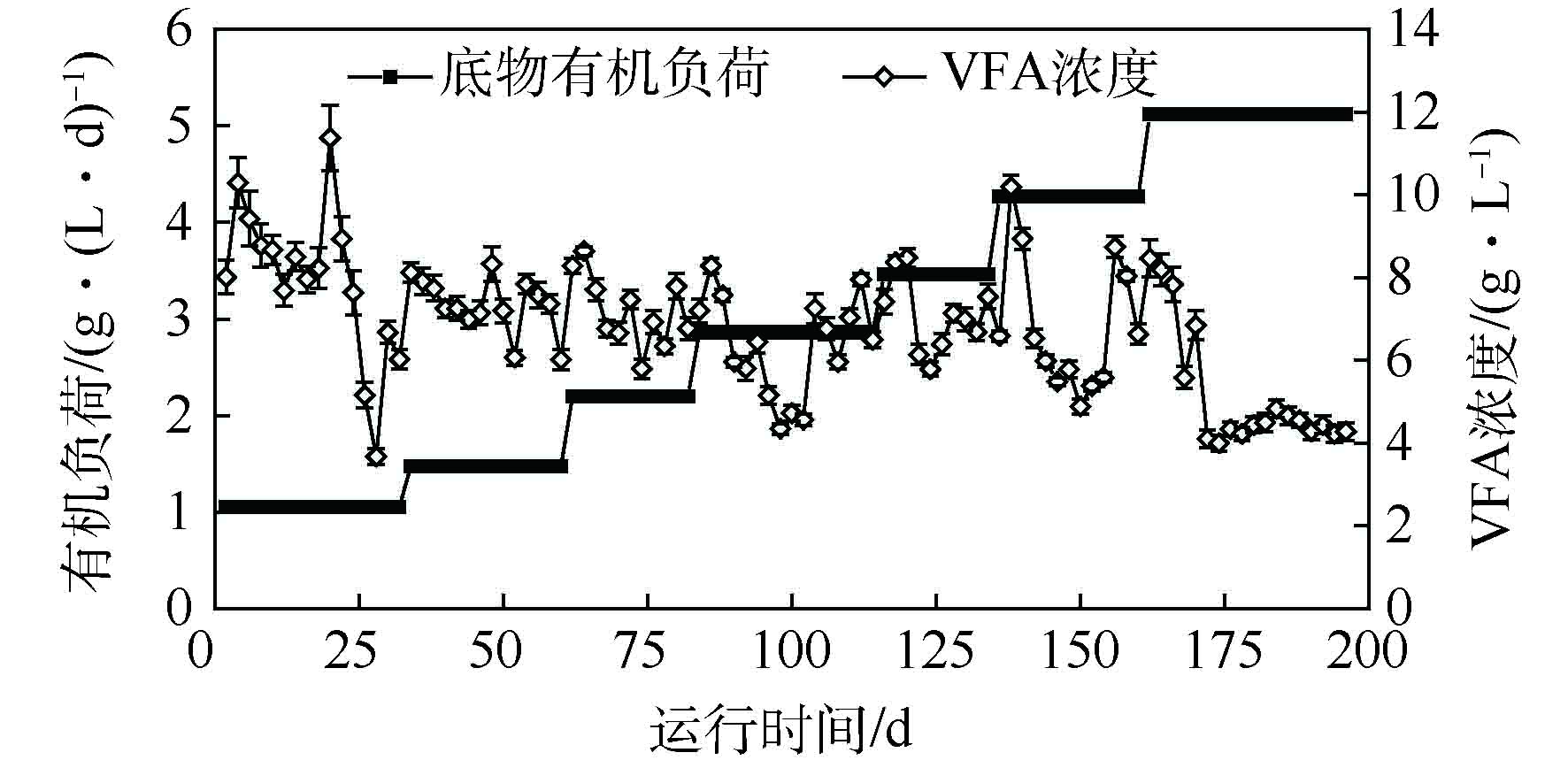
微生物代谢产物是厌氧消化体系的重要运行参数,它能反映消化过程的稳定性以及影响体系的有害物质的积累。在本厌氧消化系统中,主要的微生物代谢产物是挥发性脂肪酸(VFA)。在本体系中,VFA浓度的变化趋势与每日甲烷产率的变化趋势相似。如图3所示,VFA浓度的变化在各阶段开始时呈现相同的趋势,即先上升后下降。本体系中VFA在每个阶段开始的快速积累是由于底物OLR的增加对厌氧消化系统的冲击所造成的,增加的VFA随着本体系产甲烷活性的增强而被逐渐消耗,该结果与产气情况分析的结果(见上述2.1节)一致。这表明,通过逐步提升底物有机负荷,反应体系适应了底物特性,对底物的利用能力持续增强。同时,每个阶段VFA的平均浓度分别为7.93、7.34、6.65、6.22、6.49、6.68、5.21 g·L−1。前6个阶段VFA平均浓度的变化趋势为先下降,然后逐渐稳定。由系统的甲烷产率(见2.1节)可知,本体系内水解过程与产甲烷过程达到动态平衡,系统处于稳定状态,体现了本厌氧消化系统高效的厌氧消化效率。在H7阶段,VFA浓度降低了40%,从6.84 g·L−1下降到4.11 g·L−1,然后持续稳定在较低水平。水解是水稻秸秆厌氧消化的限速步骤,由木质纤维素的降解情况(见2.3节)可知,纤维素和半纤维素降解率的降低可能是H7阶段VFA产量减少的直接原因,这就直接导致了在H7阶段系统低的厌氧消化效率。
-
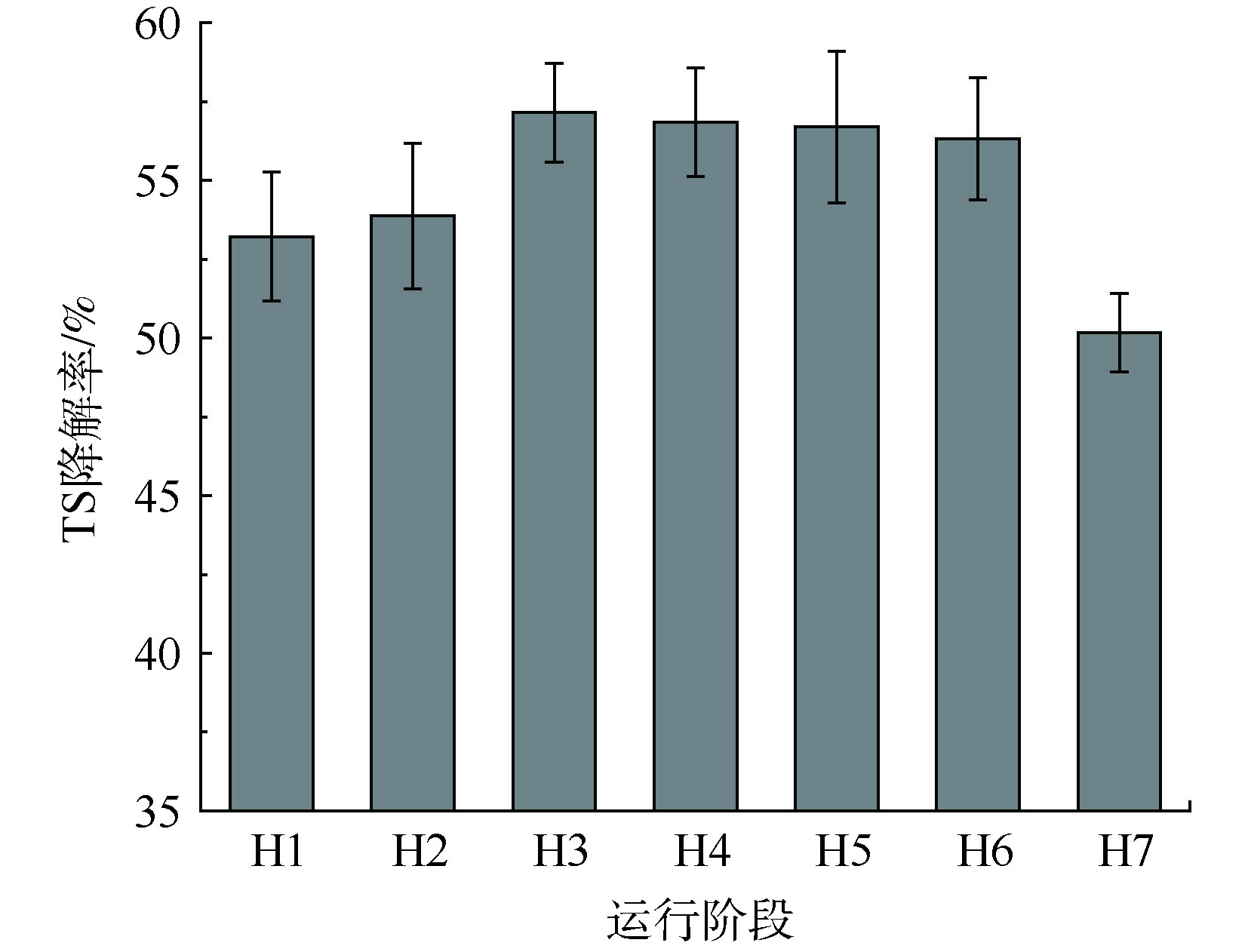
如图4所示,不同底物OLR下水稻秸秆TS的降解率分别为53.22%、53.87%、57.15%、56.84%、56.69%、56.31%和50.18%。显然,底物TS降解率的变化趋势与特定沼气产率的变化趋势一致。从H1阶段到H3阶段,TS降解率的增加主要是由于本厌氧消化系统中微生物对底物的逐渐适应。此后,TS降解率保持相对稳定,同时结合水解酶的分析结果(见2.4节)发现,随着底物OLR的增加,本厌氧消化系统的木质纤维素降解菌群逐渐富集,水解酶的分泌量提高,在较短的物料停留时间下能高效水解底物。然而H7阶段的TS降解率比H6阶段的TS降解率明显降低了6.13%,这揭示了底物水解速率的相对降低是本体系厌氧消化效率降低的主要原因,这与微生物代谢产物的分析结果一致。
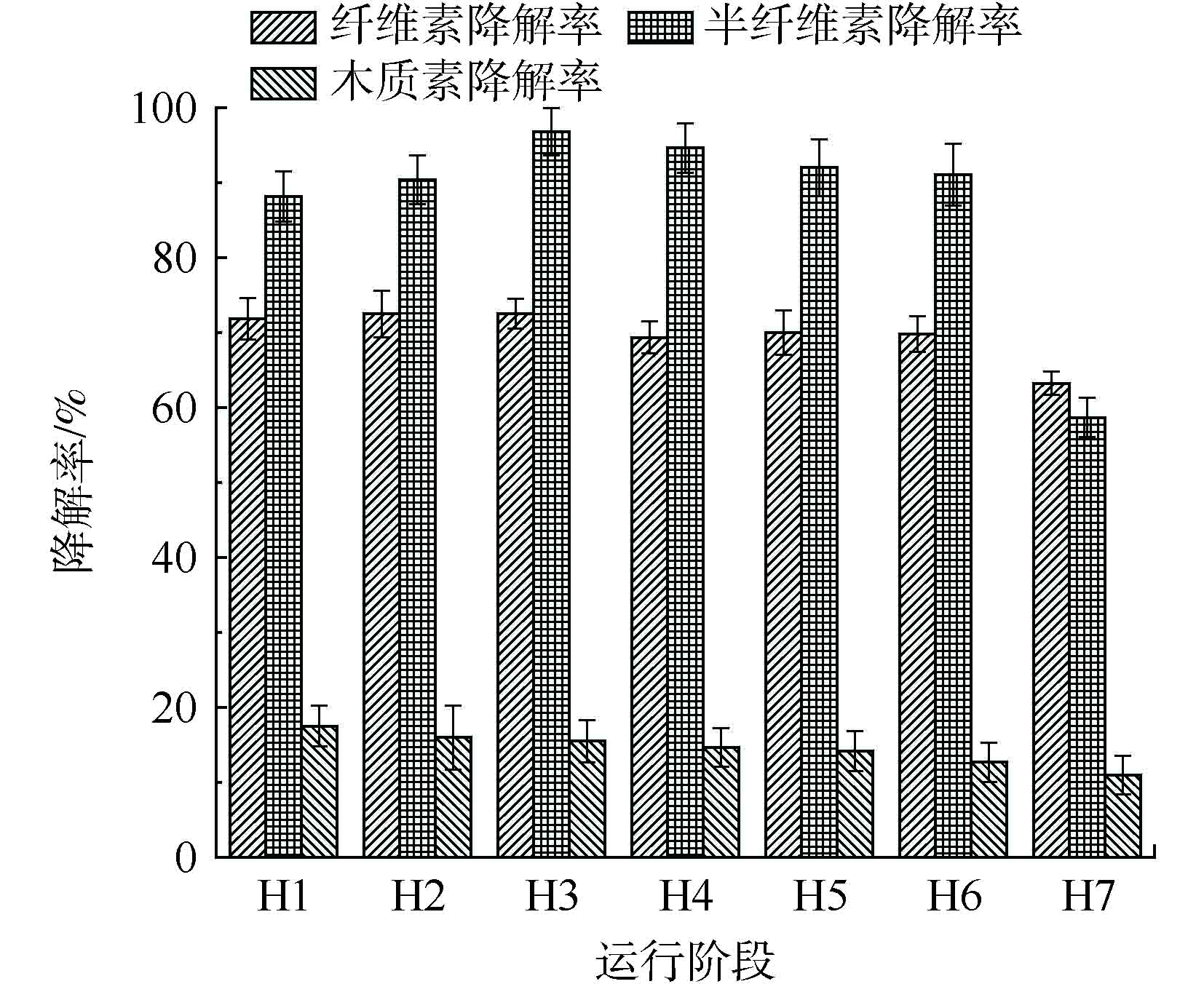
作为底物的主要成分,木质纤维素降解率的变化趋势与底物TS降解率的变化趋势一致。木质纤维素由纤维素、半纤维素和木质素组成,其在不同反应器运行阶段的降解率如图5所示。H1、H2、H3、H4、H5、H6、H7阶段的纤维素降解率分别为71.85%、72.47%、72.53%、69.35%、70.01%、69.79%、63.22%;半纤维素降解率分别为88.14%、90.33%、96.76%、94.63%、92.01%、91.07%、58.66%;木质素降解率分别为17.48%、15.98%、15.49%、14.64%、14.16%、12.66%、10.95%。由此可见,本体系中纤维素降解率和半纤维素降解率几乎贡献了全部的木质纤维素降解率。随着底物OLR的增加,本体系半纤维素降解率逐渐增加,并且在H3阶段之后保持着相对稳定的水平,其最大的降解率达到96.76%。相比之下,木质素具有复杂的酚类聚合物,这种聚合物在厌氧系统中很难降解[19];同时,纤维素和木质素之间存在交联结构,这阻止了纤维素水解酶与纤维素的接触[20]。然而在本系统中,从H1~H6阶段,纤维素降解率保持相对稳定,在69.35%~72.53%范围内。这表明,尽管存在这种交联结构,但本厌氧消化系统仍可在高OLR条件下有效降解纤维素。结合水稻秸秆的组成成分,可清楚地证明,反应器的连续运行成功地形成了高效的木质纤维素降解体系。此外,木质纤维素降解的减少可能是在H7阶段观察到的沼气产率减少的直接原因。
-
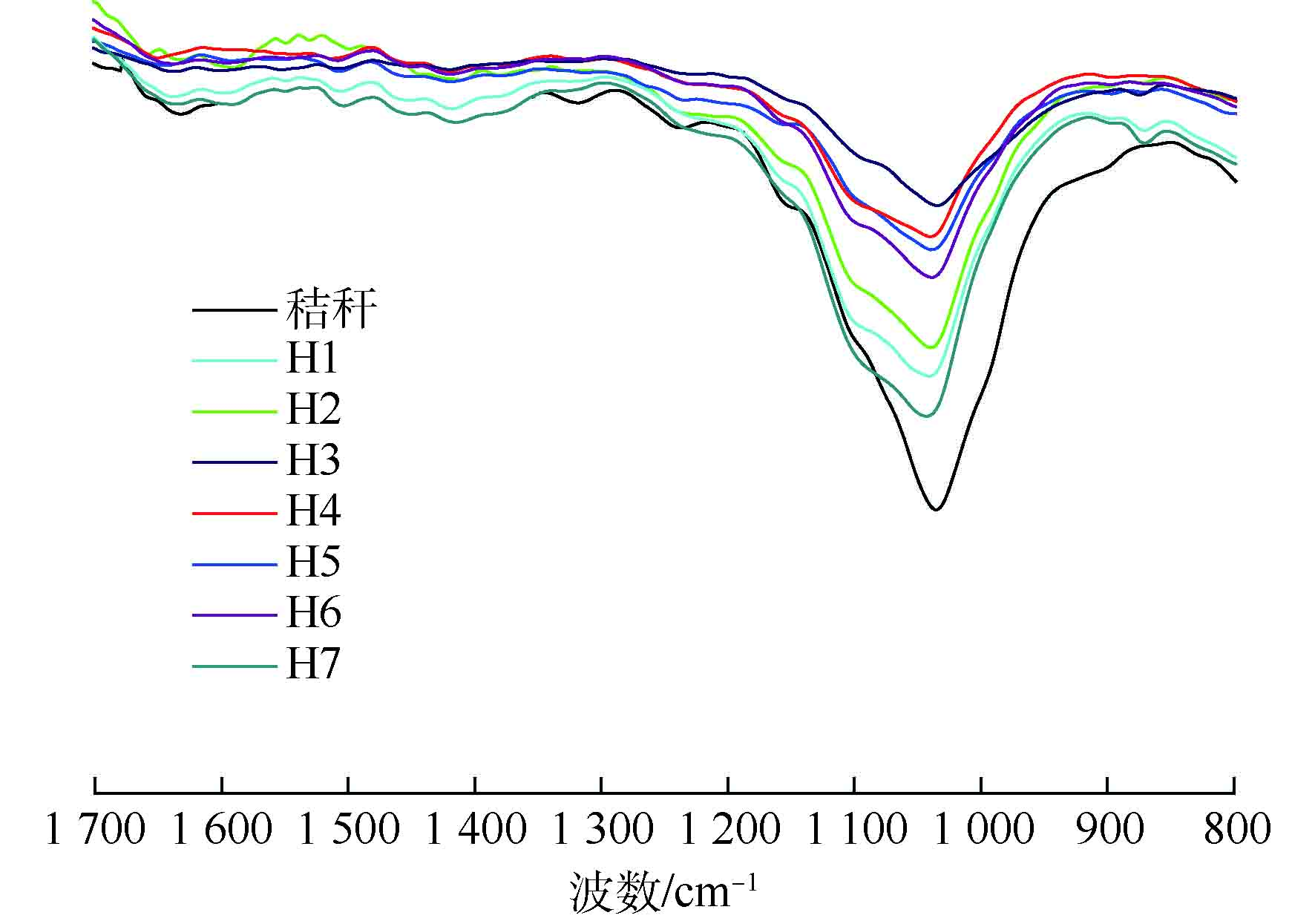
FT-IR技术适用于研究物质的分子结构和化学基团,使用该技术分析了水稻秸秆和不同底物OLR下沼渣的木质纤维素的分子结构,检验水稻秸秆的利用情况,以深入探究其厌氧消化过程中的降解机理。样品的FT-IR光谱图如图6所示。根据FT-IR的图谱,不同位置峰透过率的变化揭示了不同底物OLR下纤维素、半纤维素和木质素化学结构的变化。不同底物OLR下的整体透过率图谱的趋势相似,不同峰的透过率随着本厌氧消化系统厌氧消化效率的提高而增加。对比分析每一阶段和水稻秸秆的FT-IR图谱,1 151 cm−1(纤维素和半纤维素中的C—O—C振动峰),1 105 cm−1(通常与结晶纤维素和半纤维素结构相关),1 035 cm−1(纤维素和半纤维素中的C—O伸展峰)和900 cm−1(纤维素中的C—H变形峰)处的峰强度显著降低[21],这表明,本体系中的纤维素和半纤维素可以被有效利用。同时,微生物对富含木质纤维素环境的适应导致了H1和H2阶段底物利用率降低。相比之下,在1 631 cm−1(木质素中苯环的C=O伸缩峰),1 504 cm−1(苯环骨架),1 452 cm−1(甲氧基的C—H变形峰),1 317 cm−1(紫丁香基和愈创木基缩合木质素)和1 234 cm−1(木质素酚醚键中的C—O—C伸展峰)[22]处观察到的厌氧消化后获得的峰强度的减弱很小。这是由于木质素结构的破坏需要分子氧的参与,而这也是木质素在厌氧环境中难以降解的根本原因[23]。基于FT-IR光谱分析可知,本厌氧消化系统有效降解了水稻秸秆中的纤维素和半纤维素,但对木质素降解的强化效果很弱。上述事实表明,纤维素和半纤维素的有效降解是本厌氧消化系统保持高效厌氧消化效率的关键。
-
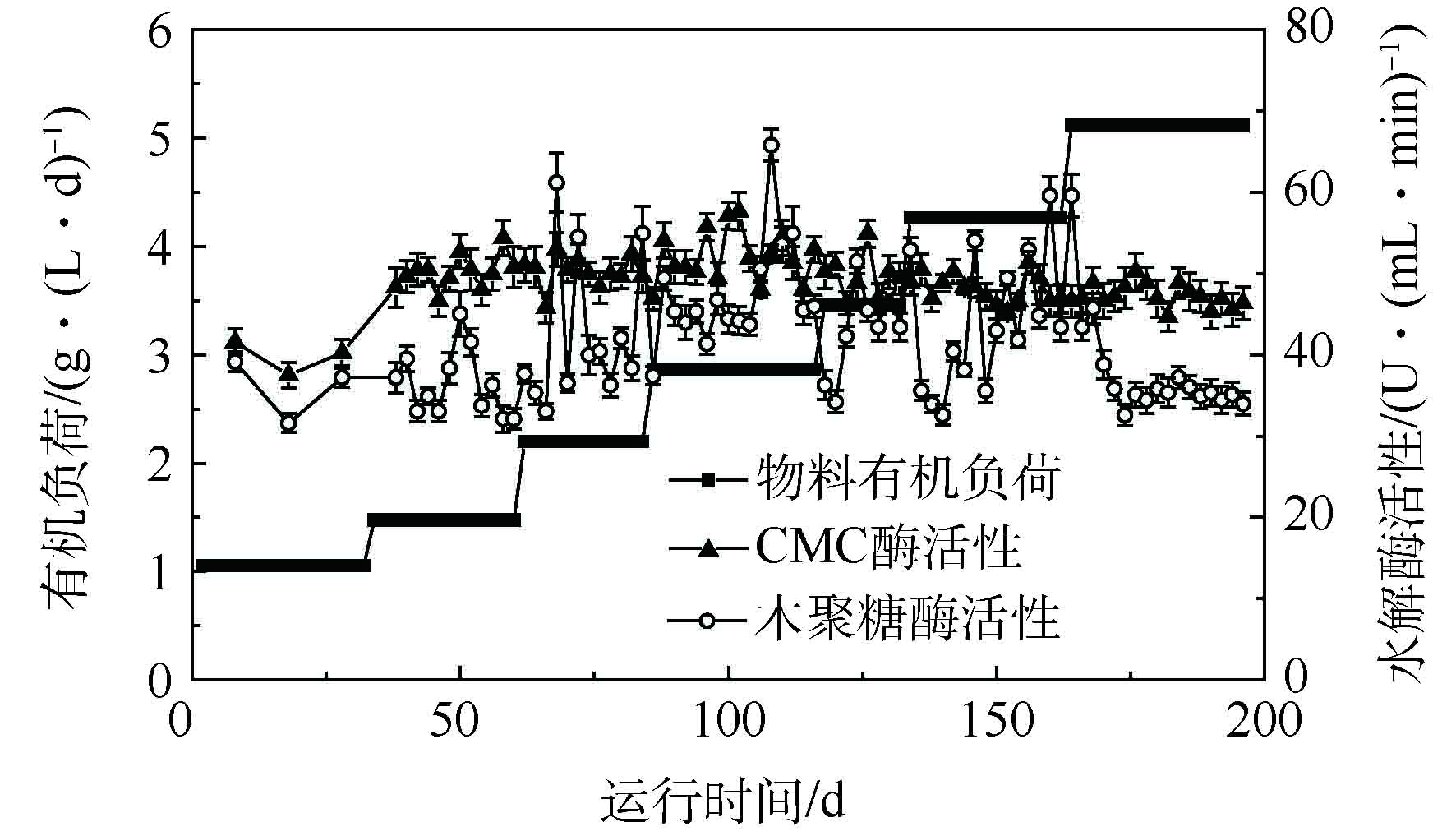
水解阶段是木质纤维素厌氧消化的限速步骤[24]。由于纤维素和半纤维素的有效降解是本厌氧消化系统保持高效厌氧消化效率的关键,同时普遍采用CMC酶来表征的内切葡聚糖酶是纤维素酶系中最重要的成分,而半纤维素的主要成分为木聚糖,所以木聚糖酶是影响半纤维素降解的关键因素。因此,CMC酶和木聚糖酶的活性测定结果可被用于评估本厌氧消化系统的水解潜力。
如图7所示,本体系稳定阶段的木聚糖酶活性从H1阶段的35.9 U·(mL·min)−1逐渐升高至H6阶段的50.2 U·(mL·min)−1,然后到H7阶段,降至34.7 U·(mL·min)−1,这与半纤维素降解率的分析一致;同时,本厌氧消化系统高的木聚糖酶活性可能源于瘤胃微生物中含有的高效水解微生物[25]。此外,本体系中木聚糖酶活性存在波动,这可能是由于提升系统有机负荷时,底物水解产生的VFA未能被产甲烷微生物及时利用,体系内的VFA快速积累,从而抑制了水解细菌系统[26]。CMC酶活性在整个反应体系运行期间保持,相对稳定水平,这证实了上面的结果(见2.3.1节)。由此可知,经过本厌氧消化系统的连续运行塑造了高效的木质纤维素降解体系,可以高效地降解半纤维素和纤维素。值得注意的是,在H7阶段,木聚糖酶活性显著降低,并维持在较低水平,这可能是导致H7阶段底物水解效率和甲烷产量降低(见2.1节)的根本原因。
-
1)本厌氧消化系统在底物OLR达到4.26 g·(L·d)−1(以VS计)时表现出最佳的厌氧消化性能,水稻秸秆沼气产率为528 mL·g−1(以VS计),甲烷产率为287 mL·g−1,容积沼气产率达到2.20 L·(L·d)−1。这表明,本厌氧消化系统可以高效地降解水稻秸秆,也表明本反应体系中的产甲烷微生物活性良好,从而展现出了优异的厌氧消化性能。
2)从初始阶段到最佳底物OLR的阶段,纤维素降解率稳定在(71 ± 2)%,半纤维素降解率稳定在(92 ± 4)%,木质素降解率稳定在(15 ± 3)%。这种稳定性是由于连续的底物刺激和体系内微生物的自我进化成功塑造了高效的木质纤维素降解体系,相比于木质素的难以降解,纤维素和半纤维素的有效利用,贡献了本厌氧消化系统高效的厌氧消化性能。
3)本厌氧消化系统高效的厌氧消化性能表明,通过瘤胃微生物群和厌氧污泥共同接种的方式有效强化了水稻秸秆的利用效率,研究结果可为水稻秸秆厌氧消化的工程化应用提供参考。
共接种瘤胃微生物和厌氧污泥的水稻秸秆中试厌氧消化系统性能评估
Performance evaluation on a pilot-scale anaerobic digestion system of rice straw with co-inoculating ruminal microbiota and anaerobic sludge
-
摘要: 针对富含木质纤维素底物利用效率低的问题,通过在中试厌氧消化系统中共接种瘤胃微生物和厌氧污泥来改善水稻秸秆中木质纤维素的水解,采用逐步提升底物有机负荷(OLR)的方式,评估了接种后水稻秸秆的厌氧消化效率。结果表明,在反应体系底物有机负荷达到4.26 g·(L·d)−1(以VS计)时,系统表现出最佳的厌氧消化性能,此时沼气产率为528 mL·g−1 (以VS计),甲烷产率为287 mL·g−1,容积沼气生产强度达到2.20 L·(L·d)−1。在反应器有机负荷从1.05 g·(L·d)−1提升到4.26 g·(L·d)−1的运行过程中,系统的纤维素降解率稳定在(71 ± 2)%,半纤维素降解率稳定在(92 ± 4)%,木质素降解率稳定在(15 ± 3)%。这种稳定性表明反应器的连续运行成功地形成了高效的木质纤维素降解体系,结果可为实际规模化应用提供参考。
-
关键词:
- 水稻秸秆 /
- 厌氧消化 /
- 有机负荷 (OLR) /
- 木质纤维素 /
- 降解
Abstract: In order to overcome the low bioavailability of lignocellulosic biomass, the hydrolysis of rice straw was enhanced by co-inoculating ruminal microbiota and anaerobic sludge in a pilot-scale anaerobic reactor, and the digestion efficiency was evaluated by gradually increasing organic load rate (OLR). The optimal fermentation performance was obtained at the OLR of 4.26 g·(L·d)−1 (calculated in VS), where the biogas yield, methane yield and volumetric biogas productivity reached 528 mL·g−1 (calculated in VS), 287 mL·g−1 and 2.20 L·(L·d)−1, respectively. With the increase of OLR from 1.05 g·(L·d)−1 to 4.26 g·(L·d)−1, the degradation efficiencies of cellulose, hemicellulose and lignin was maintained at (71 ± 2)%, (92 ± 4)% and (15 ± 3)%, respectively. The operational stability indicated that a highly efficient lignocellulose degradation system was successfully established by the continuous reactor operation, providing a theoretical basis for the practical scale-up application.-
Key words:
- rice straw /
- anaerobic digestion /
- organic loading rate (OLR) /
- lignocellulose /
- degradation
-
受气候和人类活动的影响,许多饮用水水源的水质存在季节性变化,呈现出春季和秋季藻类含量高、冬季浊度低、夏季汛期浊度高的特征。给水生产中常见的沉淀工艺能有效处理浊度较高的原水,但当原水藻类含量较高或低温低浊时,除藻除浊效果较差;另一方面,气浮工艺可有效处理藻含量较高或低温低浊的原水,但当原水浊度较高时,除浊效果较差。因此,给水生产中需同时建设气浮和沉淀2种工艺的构筑物,以应对受季节影响较大的水源水处理。
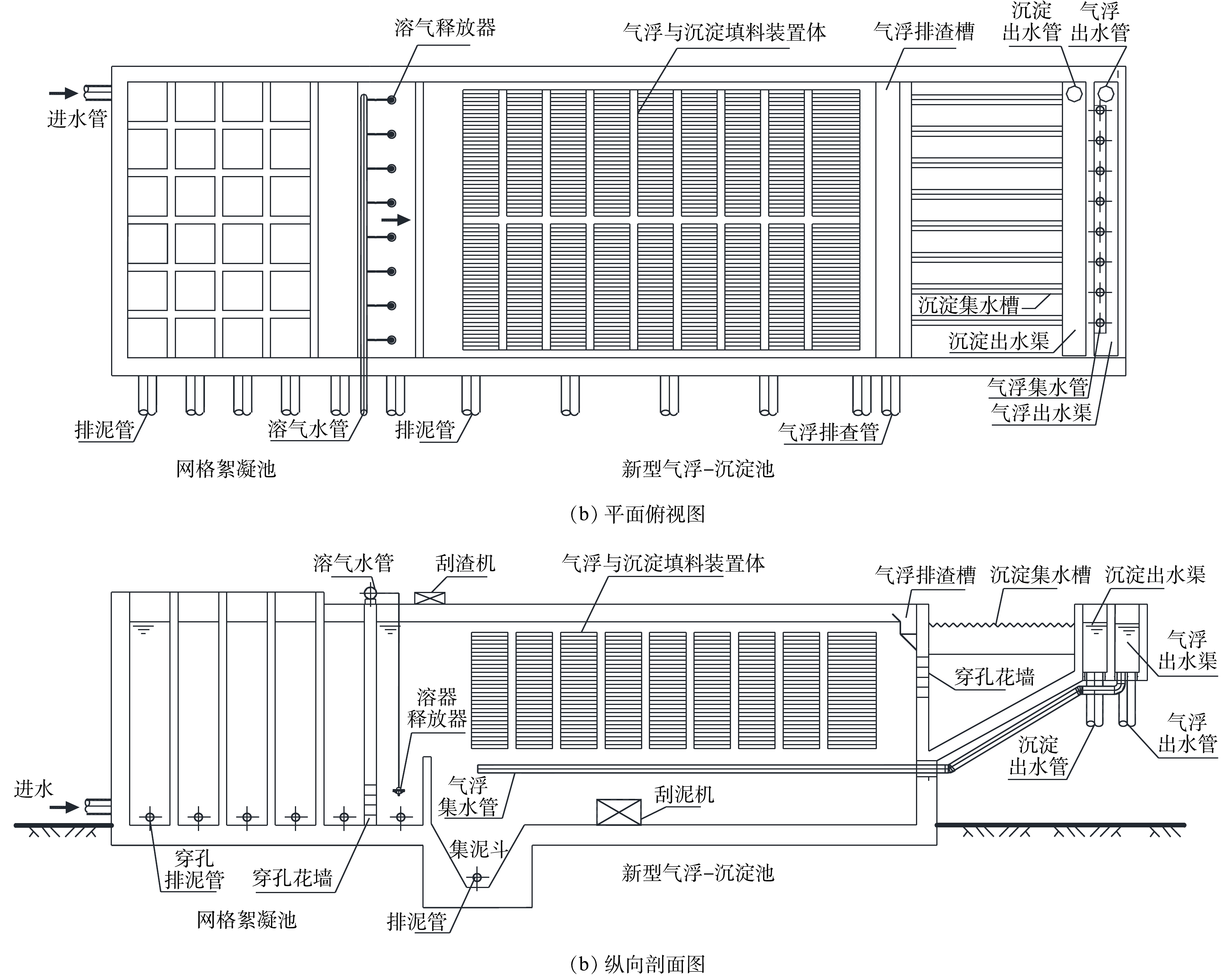
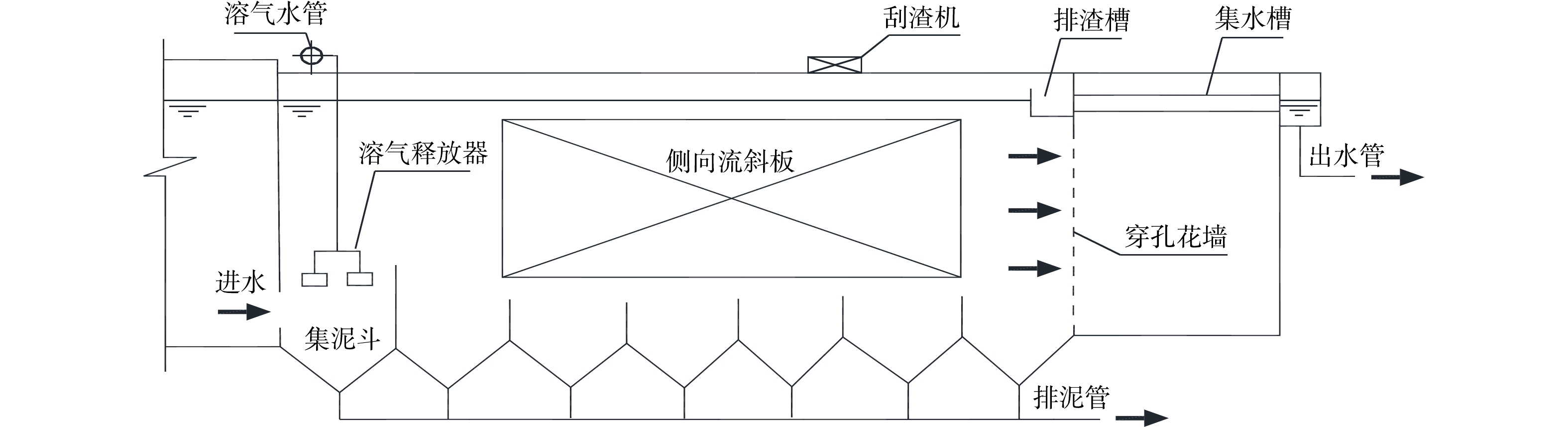
为节省用地和建设成本,孙志民等[1]将气浮工艺和沉淀工艺耦合在一个构筑物内,形成侧向流斜板浮沉池。该工艺已成功应用于自吉林市第一水厂(2×104 m3·d−1)[2]、吉林市第四水厂二期(4.5×104 m3·d−1)[2]、新疆库尔勒市自来水公司水厂(3×104 m3·d−1)[3]、大庆石化总厂水气厂生活水厂(2.04×104 m3·d−1)[4]、三明市下洋水厂二期(5×104 m3·d−1)[5]等给水厂。然而,随着侧向流斜板浮沉池的深入应用,其构造上的一些缺陷暴露出来[6-7]:原本考虑兼顾气浮与沉淀出水要求,共用一个出水集水系统,而出水流态表明,气浮在底部集水、沉淀在顶部集水才能保证出水颗粒物最少、出水水质最优;原本考虑采用多折斜板结构,以便增加斜板面积,提高侧向流斜板的沉淀效率,但实际运行时,斜板折叠处积泥严重,不仅影响斜板沉淀排泥,而且对絮体颗粒上浮形成阻碍,影响浮渣形成;原本考虑底部采用穿孔管排泥,使系统实现均匀排泥,故设置较矮的集泥区,而长期运行中穿孔管的排泥效果较差,池体底部积泥较多;而又因集泥区高度较矮,气浮接触区挡墙高度不足,导致溶气水与絮体颗粒接触时间不足,影响了气浮对絮体颗粒的去除效果。
为解决上述问题,在侧向流斜板浮沉池的基础上,重新构建池体结构、优化布置,形成基于气浮与沉淀切换运行的新型气浮—沉淀工艺。本文剖析新型工艺的原理、运行方式和设计要点,并以珠海三灶水厂(2×104 m3·d−1)的升级改造工程为应用案例,评估该工艺的生产运行状况,以期为给水厂应对水源水质的季节性变化提供参考。
1. 新型气浮-沉淀工艺的优化内容、运行方式及设计要点
1.1 新工艺的优化内容
为减少传统侧向流斜板浮沉池(图1)暴露出的缺陷,改进池体结构、优化布置,形成新型气浮—沉淀工艺(图2)。工艺优化的内容包括:1)分别设置独立的气浮和沉淀出水集水系统,以保证气浮和沉淀出水的最优水力条件,气浮和沉淀不同模式时单独出水、互不干扰,保证出水水质稳定;2)采用单折结构斜板,以减轻运行沉淀工艺时斜板的积泥程度、优化运行气浮工艺时的水力条件;3)设置机械刮泥结构和装置,用以提高排泥效率,避免因池底部积泥引起的水质恶化问题;4)增加接触区挡墙高度,使溶气水与絮体颗粒充分接触,形成状态良好的浮渣,有利于气浮出水的水质提升。
1.2 工艺运行方式
气浮模式的运行条件:1)运行沉淀工艺处理效果较差,从而影响供水水质时;2)对供水水质要求提高时;3)在春秋季节原水藻含量较高(>3×106个·L−1)或浊度较低(<40 NTU)时。沉淀模式的运行条件:1)当运行气浮工艺处理效果较差,从而影响供水水质时;2)考虑节省运行电费、操作简单时;3)在夏季暴雨原水浊度较高(≥40 NTU)时[8]。
1.2.1 气浮模式
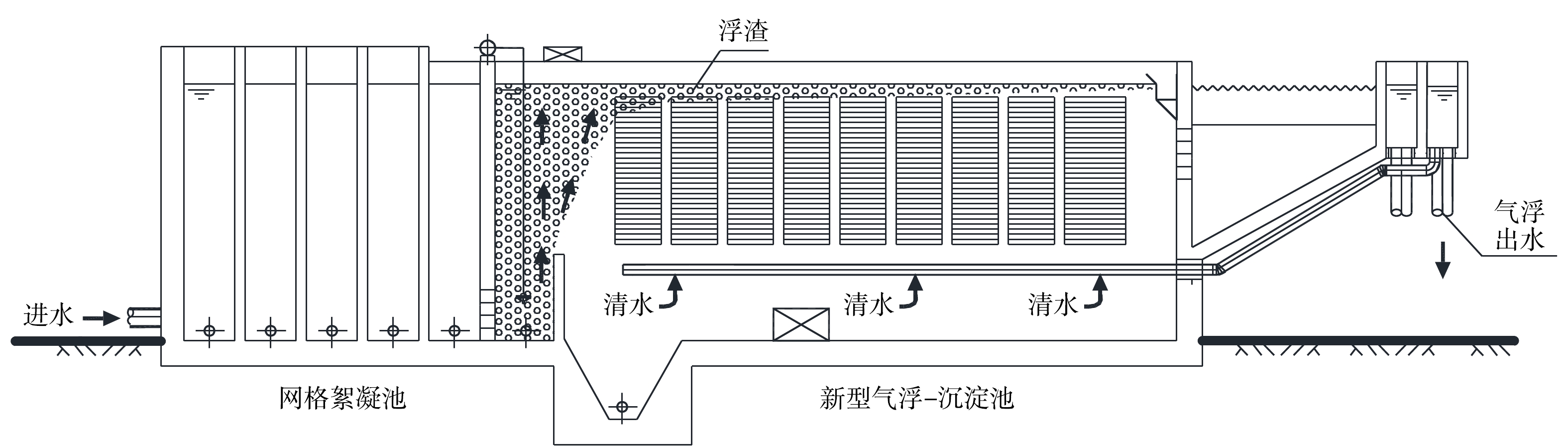
气浮模式工艺(图3)中,絮凝后的水流经配水穿孔花墙后进入接触区,与溶气释放器注入的溶气水充分混合[9];溶气水释放的微小气泡与原水中的絮凝体等颗粒充分黏附,形成微气泡与絮凝体的聚集体颗粒,即带气絮粒;颗粒随水流向上漂浮,一部分上浮至水面形成浮渣,一部分进入侧向流斜板模组内,沿斜板滑动上浮至水面形成浮渣;刮渣机定期将水面浮渣刮至排渣槽排出;杂质颗粒上浮分离后,澄清的水沿斜板缝隙流向池底部,经过气浮穿孔集水管进入气浮出水渠,并通过气浮出水管流入滤池[10]。
1.2.2 沉淀模式
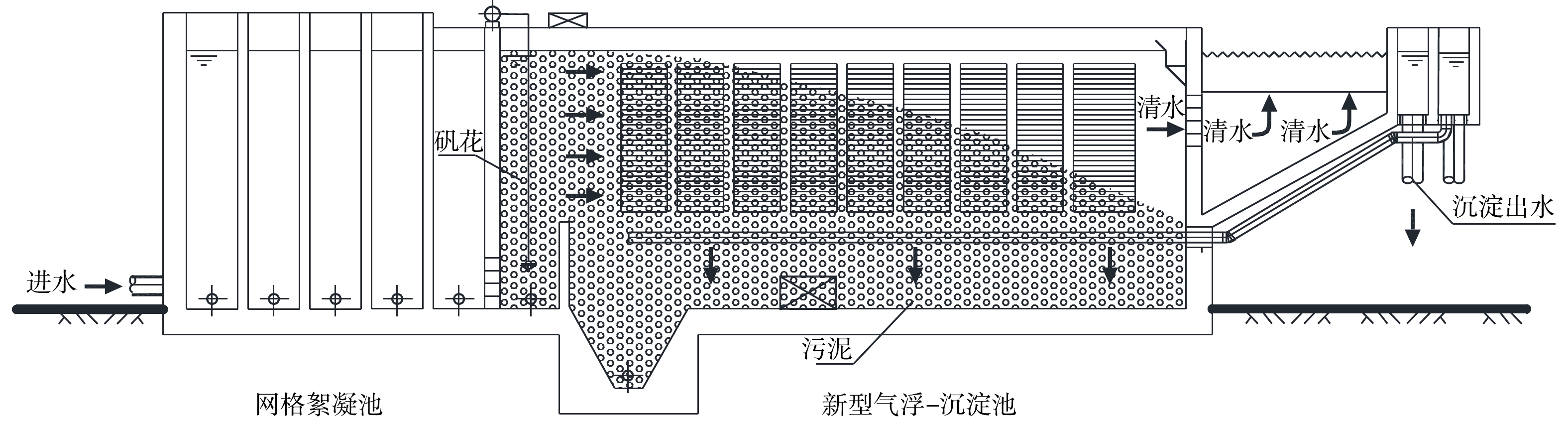
沉淀模式工艺(图4)中絮凝后的水流经配水穿孔花墙后进入接触区,然后进入侧向流斜板模组;进入模组后,絮凝体等杂质颗粒沉降到斜板上,沿斜板下滑至积泥区,由刮泥机刮至集泥斗后,通过管道排出;絮凝体等杂质颗粒沉淀分离后,澄清的水沿斜板缝隙流出侧向流斜板模组,经过上部末端的沉淀出水穿孔花墙,依次进入沉淀集水槽、沉淀出水渠,最后通过沉淀出水管流入滤池。
1.3 工艺设计要点
1)进出水设计要点:①为减少絮凝体破碎,进水配水穿孔花墙过孔流速宜小于0.1 m·s−1;②为保证配水均匀,同时降低沿程水头损失,气浮穿孔集水管内流速不宜超过0.5 m·s−1;③为避免沉淀后絮凝颗粒被带出,沉淀出水穿孔花墙水流速度宜小于0.1 m·s−1;④为应对处理水量的波动,沉淀出水集水槽溢流率不宜超过200 m3·(m·d)−1;
2)气浮模式工艺设计要点:①为使释放器在最佳性能范围内运行,溶气压力宜采用0.3~0.45 MPa;为实现气浮工艺运行的经济性,回流比宜选取8%~10%;②为保证溶气水与絮体颗粒物有足够的接触时间,形成形态较好的带气絮粒,气浮接触区水流上升速度宜采用10~20 mm·s−1,接触区内停留时间不宜少于60 s;③为保障气浮工艺具有较强的抗冲击负荷能力,分离区表面负荷率宜为2.88~5.4 m3·(m2·h)−1;④为提高气浮溶气效率,压力溶气罐宜采用阶梯环为填料,填料层高度宜采用1.0~1.5 m,罐高度宜为2.5~3.5 m[11];⑤为实现出水水质和工程投资之间较好的平衡,气浮分离区的长度宜采用15~20 m;
3)沉淀模式工艺设计要点:①考虑到水库水絮体沉降速度较小,颗粒沉降速度μ宜采用0.8~0.15 mm·s−1,斜板间水流流速v宜采用5~10 mm·s−1;②考虑到应对水质和水量的冲击,斜板需保证一定的富余量,有效系数η宜选用0.7~0.8;③综合考虑不同水质情况下斜板的处理效果,侧向流斜板(气浮与沉淀填料装置)模组,斜板倾斜角宜采用50°~60°、斜板间距宜采用50 mm~80 mm[12];④为实现最优水力条件,应在侧向流斜板(气浮与沉淀填料装置)模组底部设置阻流墙、进出处设置缓冲区;⑤为确保排泥效果,宜采用刮泥机排出沉淀污泥;为确保排渣效果,宜采用刮渣机排除气浮浮渣。
2. 应用实例及成本分析
2.1 工程实例
本研究中,以珠海三灶水厂改造作为工程应用实例,评估新型气浮-沉淀工艺的运行效果及运营成本。该厂原采用重力式单阀滤池直接过滤工艺,但随着原水水质的变化和国家饮用水标准的提高,其出厂水质已不能满足供水水质要求。经过多次论证,改造主体工艺确定为新型气浮-沉淀工艺(见图5)。水厂净水工艺流程见图6,改造后的处理规模为2×104 m3·d−1。
2.2 运行效果
珠海三灶水厂原水为水库水。水质特点为:春秋季节藻类高、浊度低;冬季浊度低;夏季暴雨导致浊度高;春秋与冬季铁锰高。当春秋季节原水藻类高、浊度低,或者浊度低、运行沉淀工艺处理效果较差,影响供水水质时,则切换运行气浮工艺,以满足供水水质要求;当夏季降雨或暴雨、原水浊度较高,气浮工艺运行效果差影响供水水质时,则切换运行沉淀工艺,以满足供水水质要求;另外,采用锰砂滤料滤池,解决原水铁锰含量高问题。改造后的工艺处理效果如下。
2.2.1 除藻效果
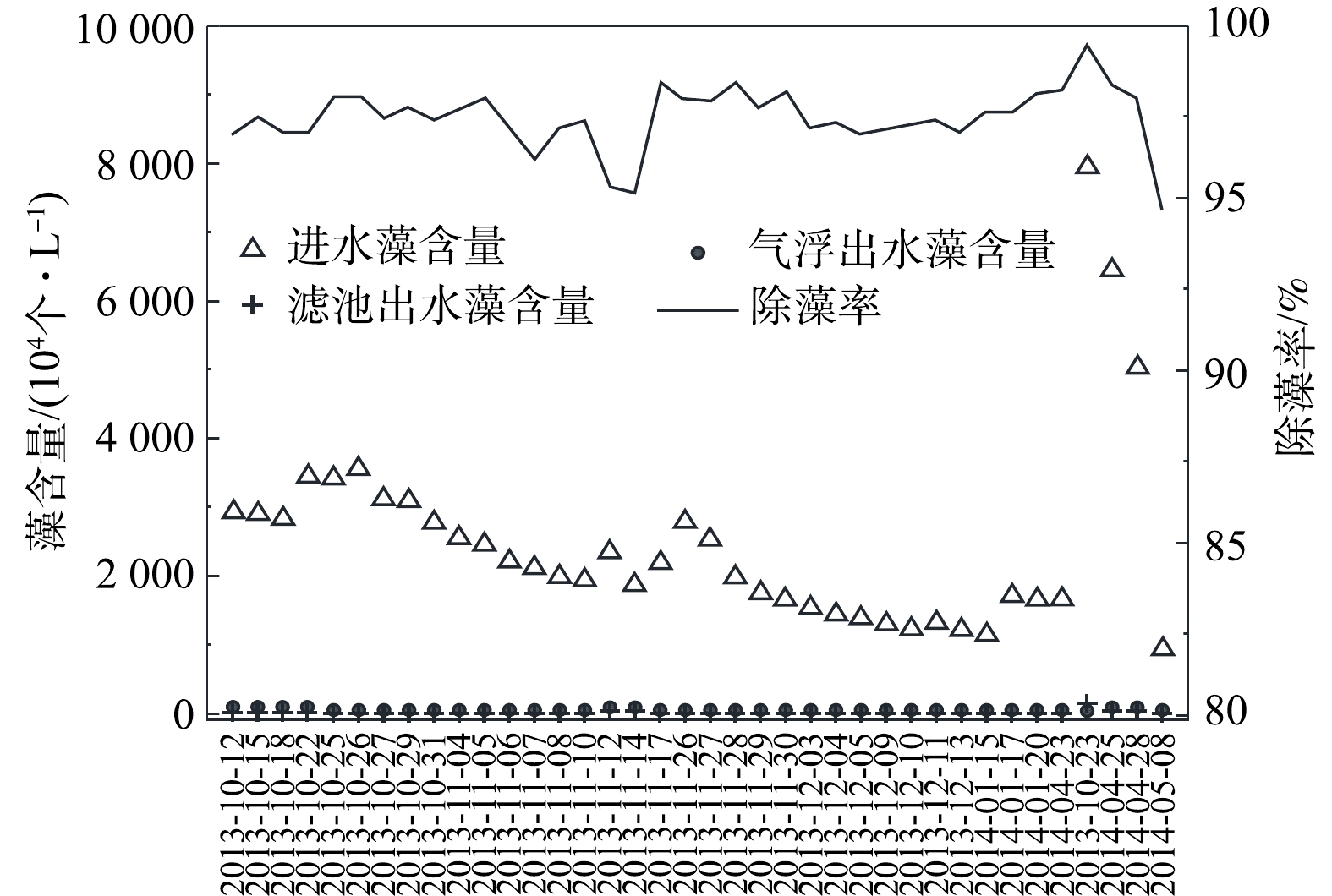
当原水藻含量超过3×106个·L−1时,运行气浮模式,溶气压力0.35~0.4 MPa,回流比8%。由图7数据可知,系统运行较为稳定,进水藻含量为940×104~7 798×104个·L−1,出水藻含量为32×104~112×104个·L−1,除藻率为94.70%~99.14%,锰砂滤料V型滤池滤后水藻含量9.61×104~25.98×104个·L−1。
2.2.2 除浊效果
当原水浊度低于40 NTU时,运行气浮模式除浊,溶气压力0.35~0.4 MPa,回流比8%。由图8可知,系统运行较为稳定,进水浊度5.12~24.50 NTU,出水平均浊度为0.5 NTU,除浊率为90.23%~97.95%,锰砂滤料V型滤池滤后水浊度为0.03~0.14 NTU。
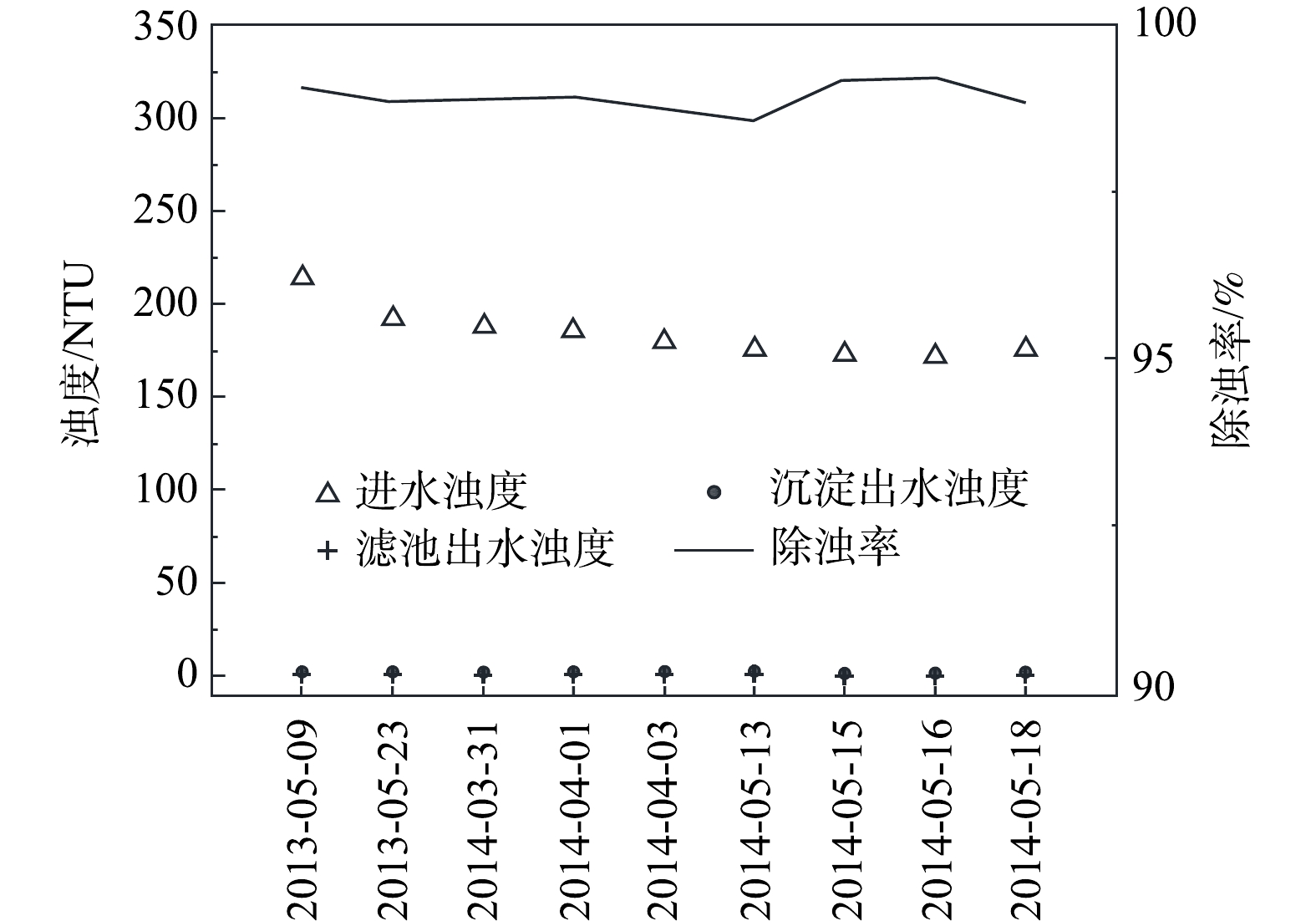
当进水浊度高于40 NTU时,尤其当汛期时原水浊度急剧升高时,则运行沉淀模式。由图9数据可知,系统运行较为稳定,进水浊度174~220 NTU,出水平均浊度为3 NTU,除浊率为98.36%~98.60%,锰砂滤料V型滤池滤后水浊度小于0.5 NTU。
2.2.3 CODMn去除效果
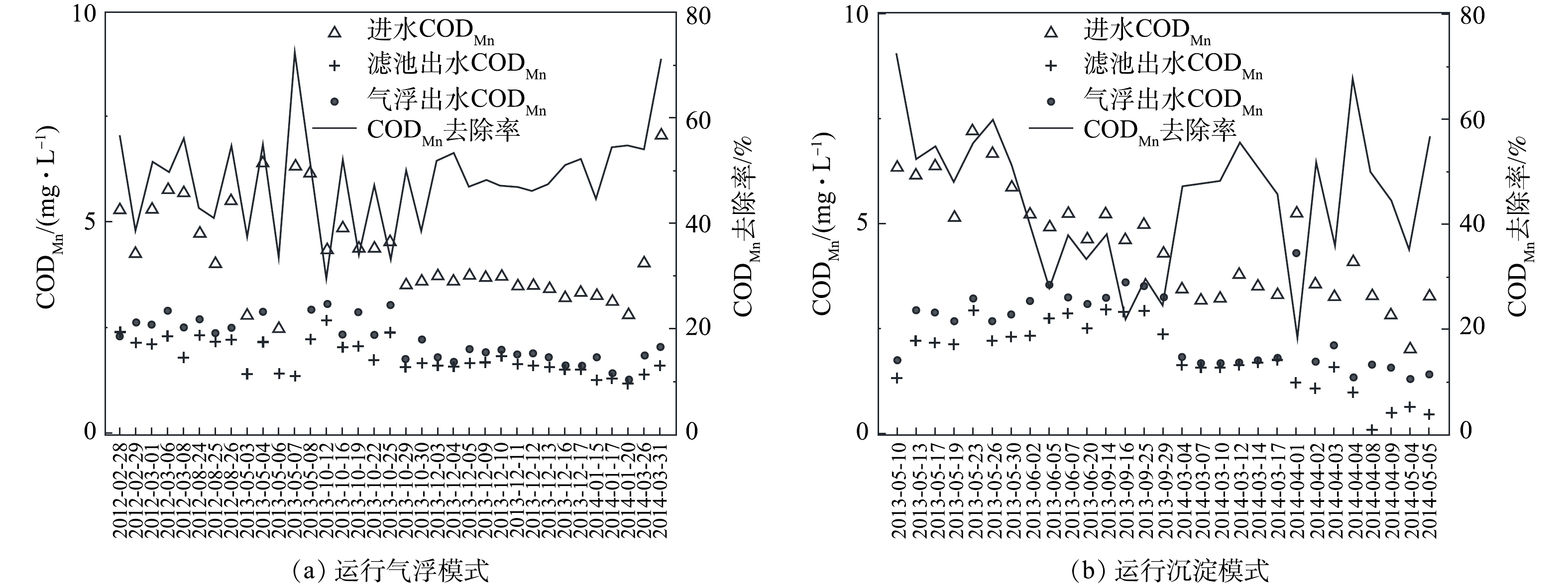
在系统运行气浮和沉淀2种模式时,分别监测了进出水中的高锰酸盐指数。该指标可反映系统对有机物的去除效果。运行气浮模式时(见图10(a)),进水CODMn为2.45~7.04 mg·L−1,出水CODMn为1.26~3.00 mg·L−1,CODMn去除率为29.49%~72.35%。运行沉淀模式时(见图10(b)),进水CODMn为2~7.22 mg·L−1,出水CODMn为1.3~4.3 mg·L−1,CODMn去除率为18.1%~72.35%。运行结果表明,气浮模式对CODMn的去除效果比沉淀工艺模式更加稳定。
2.2.4 氨氮去除效果
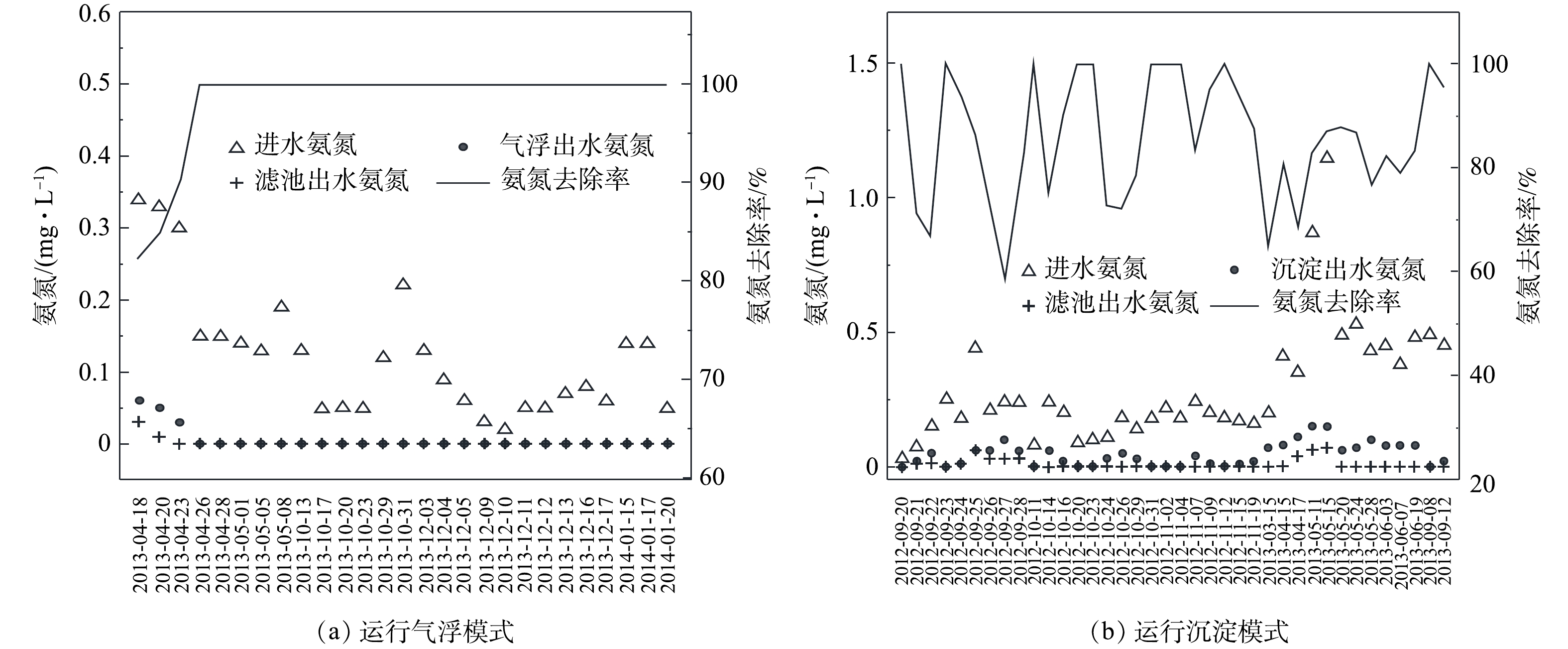
在系统分别运行气浮和沉淀2种模式时,监测了进出水的氨氮含量。该指标可反映系统对引起水体富营养化的有机物去除效果。运行气浮模式时(见图11(a)),进水氨氮含量为0.02~0.34 mg·L−1,出水氨氮含量0~0.06 mg·L−1,氨氮去除率为82.35%~100%;运行沉淀模式时(见图11(b)),进水氨氮含量为0.03~1.15 mg·L−1,出水氨氮含量为0~0.49 mg·L−1,氨氮去除率为44.58%~100%。运行结果表明,气浮模式对氨氮的去除效果比沉淀模式稳定。
2.3 新型气浮-沉淀工艺与同类型工艺的效果及土建费用对比
济南玉清水厂与珠海三灶水厂的源水特征比较类似。两个水厂源水均为水库水,常年低浊,藻类、有机物、嗅味物质呈季节性升高[13-14]。表1为珠海三灶水厂与济南玉清水厂的除藻、除浊效果对比。通过对比类似水源水质的同类型工艺处理效果可知,运行新型气浮-沉淀工艺,藻类去除率不低于95%,浊度去除率不低于92%,与沉淀串联气浮工艺的处理效果相当。土建费用方面,在相同规模(2×104 m3·d−1)下,同时实现气浮与沉淀2种工艺,新型气浮—沉淀池所需的占地面积最少,仅为309 m2;而“絮凝+平流沉淀+气浮”组合池的占地面积为713 m2,是新型气浮—沉淀池的2.31倍[15]。通过对比可知,在保证相同处理效果的前提下,新型气浮—沉淀工艺可减少用地面积,降低工程造价,还可减少构筑物的闲置。
表 1 两种工艺的水厂处理效果对比Table 1. Comparison of algae removal effect of the two treatment processes3. 结语
新型气浮-沉淀工艺经过多年的发展,已在全国多地应用,主要有珠海三灶水厂(2×104 m3·d−1)、中山长坑水库水厂(0.6×104 m3·d−1)、韶关市演山水厂(在建,6×104 m3·d−1)、廉江市九洲江水厂(在建,10×104 m3·d−1)。新型气浮-沉淀工艺相比原侧向流斜板浮沉池工艺,改进了出水集水系统、斜板结构及布置方式、刮泥方式和接触区高度。珠海三灶水厂的生产运行实践表明,新型气浮-沉淀工艺对于地表水源水季节性水质变化适应性较强,在除藻和除浊方面均表现出较高的去除效率,同时占地面积较小,节约工程造价。
-
表 1 底物和接种物的性质
Table 1. Characteristic of the substrate and inoculum
材料 TS/% 1) VS/% 1) VS/TS/% 碳氮比 蛋白质/% 2) 纤维素/% 2) 半纤维素/% 2) 木质素/% 2) 秸秆 91.51±0.34 81.98±0.26 89.59±0.62 33.97±0.42 5.25±0.54 36.93±0.65 24.52±0.33 4.63±0.17 瘤胃内容物 15.15±0.21 12.13±0.12 80.06±1.90 12.48±0.41 NA NA NA NA 厌氧污泥 13.91±0.32 6.91±0.17 49.68±2.37 7.36±0.27 NA NA NA NA 注:TS为总固体;VS为挥发性固体;NA表示未分析;1)占湿物料的比例;2)占干物料的比例。 表 2 半连续式厌氧消化系统各阶段的运行参数
Table 2. Operation parameters of each stage on semi-continuous anaerobic digestion system
运行阶段 进料量/(g·d−1) 有机负荷/(g·(L·d)−1) 停留时间/d H1 250 1.05 760 H2 350 1.47 542 H3 500 2.21 380 H4 650 2.86 292 H5 800 3.46 237 H6 1 000 4.26 190 H7 1 200 5.12 158 -
[1] LI K, LIU R H, SUN C. A review of methane production from agricultural residues in China[J]. Renewable and Sustainable Energy Reviews, 2016, 54: 857-865. doi: 10.1016/j.rser.2015.10.103 [2] 温博婷. 木质纤维素原料的酶解糖化及厌氧发酵转化机理研究[D]. 北京: 中国农业大学, 2015. [3] MUSSOLINE W, ESPOSITO G, GIORDANO A, et al. The anaerobic digestion of rice straw: A review[J]. Critical Reviews in Environmental Science & Technology, 2013, 43(9): 895-915. [4] MONLAU F, SAMBUSITI C, BARAKAT A, et al. Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials[J]. Environmental Science & Technology, 2012, 46(21): 12217-12225. [5] KIM I J, JUNG J Y, LEE H J, et al. Customized optimization of cellulase mixtures for differently pretreated rice straw[J]. Bioprocess and Biosystems Engineering, 2015, 38(5): 929-937. doi: 10.1007/s00449-014-1338-7 [6] LI M, SI S L, HAO B, et al. Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus[J]. Bioresource Technology, 2014, 169: 447-454. doi: 10.1016/j.biortech.2014.07.017 [7] ZHANG H B, ZHANG P Y, YE J, et al. Improvement of methane production from rice straw with rumen fluid pretreatment: A feasibility study[J]. International Biodeterioration & Biodegradation, 2016, 113: 9-16. [8] SHI J, XU F Q, WANG Z J, et al. Effects of microbial and non-microbial factors of liquid anaerobic digestion effluent as inoculum on solid-state anaerobic digestion of corn stover[J]. Bioresource Technology, 2014, 157: 188-196. doi: 10.1016/j.biortech.2014.01.089 [9] HU Z H, Yu H Q. Application of rumen microorganisms for enhanced anaerobic fermentation of corn stover[J]. Process Biochemistry, 2005, 40(7): 2371-2377. doi: 10.1016/j.procbio.2004.09.021 [10] DAI X, TIAN Y, LI J T, et al. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen[J]. Applied and Environmental Microbiology, 2015, 81(4): 1375-1386. doi: 10.1128/AEM.03682-14 [11] DENG Y Y, HUANG Z X, ZHAO M X, et al. Effects of co-inoculating rice straw with ruminal microbiota and anaerobic sludge: Digestion performance and spatial distribution of microbial communities[J]. Applied Microbiology and Biotechnology, 2017, 101(14): 5937-5948. doi: 10.1007/s00253-017-8332-3 [12] APHA, AWWA, WEF. Standard methods for the examination of water & wastewater: 21st Edition[S]. Washington D C: American Public Health Association, 2005. [13] HALL N G, SCHONFELDT H C. Total nitrogen vs amino-acid profile as indicator of protein content of beef[J]. Food Chemistry, 2013, 140(3): 608-612. doi: 10.1016/j.foodchem.2012.08.046 [14] EL ACHKAR J H, LENDORMI T, HOBAIKA Z, et al. Anaerobic digestion of grape pomace: Biochemical characterization of the fractions and methane production in batch and continuous digesters[J]. Waste Management, 2016, 50: 275-282. doi: 10.1016/j.wasman.2016.02.028 [15] 汪婷. 沼气发酵过程中产甲烷菌分子多样性研究及产甲烷菌的分离[D]. 南京: 南京农业大学, 2005. [16] SAMBUSITI C, ROLLINI M, FICARA E, et al. Enzymatic and metabolic activities of four anaerobic sludges and their impact on methane production from ensiled sorghum forage[J]. Bioresource Technology, 2014, 155: 122-128. doi: 10.1016/j.biortech.2013.12.055 [17] ZEALAND A M, ROSKILLY A P, GRAHAM D W. Effect of feeding frequency and organic loading rate on biomethane production in the anaerobic digestion of rice straw[J]. Applied Energy, 2017, 207: 156-165. doi: 10.1016/j.apenergy.2017.05.170 [18] ZHOU J, YANG J, YU Q, et al. Different organic loading rates on the biogas production during the anaerobic digestion of rice straw: A pilot study[J]. Bioresource Technology, 2017, 244: 865-871. [19] CHAPLEUR O, MADIGOU C, CIVADE R, et al. Increasing concentrations of phenol progressively affect anaerobic digestion of cellulose and associated microbial communities[J]. Biodegradation, 2016, 27(1): 15-27. doi: 10.1007/s10532-015-9751-4 [20] TAHERZADEH M J, KARIMI K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review[J]. International Journal of Molecular Sciences, 2008, 9(9): 1621-1651. doi: 10.3390/ijms9091621 [21] YANG S G, LI J H, ZHENG Z, et al. Lignocellulosic structural changes of Spartina alterniflora after anaerobic mono- and co-digestion[J]. International Biodeterioration & Biodegradation, 2009, 63(5): 569-575. [22] AMNUAYCHEEWA P, HENGAROONPRASAN R, RATTANAPORN K, et al. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids[J]. Industrial Crops and Products, 2016, 87: 247-254. doi: 10.1016/j.indcrop.2016.04.069 [23] KOMILIS D P, HAM R K. The effect of lignin and sugars to the aerobic decomposition of solid wastes[J]. Waste Management, 2003, 23(5): 419-423. doi: 10.1016/S0956-053X(03)00062-X [24] ZHAO X B, ZHANG L H, LIU D H. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose[J]. Biofuels, Bioproducts and Biorefining, 2012, 6(4): 465-482. doi: 10.1002/bbb.v6.4 [25] XU F, SHI J, LV W, et al. Comparison of different liquid anaerobic digestion effluents as inocula and nitrogen sources for solid-state batch anaerobic digestion of corn stover[J]. Waste Management, 2013, 33(1): 26-32. doi: 10.1016/j.wasman.2012.08.006 [26] ROMSAIYUD A, SONGKASIRI W, NOPHARATANA A, et al. Combination effect of pH and acetate on enzymatic cellulose hydrolysis[J]. Journal of Environmental Sciences, 2009, 21(7): 965-970. doi: 10.1016/S1001-0742(08)62369-4 -




 下载:
下载: