全文HTML
1 实验部分
1.1 纳米铁炭材料的制备
1.2 微电解体系实验
1.3 铁碳材料还原硝酸盐途径
1.4 纳米铁炭体系动力学模型的建立
1.5 分析方法
2 结果与讨论
2.1 微电解体系验证
Fig. 1 Change of reduction of nitrate in different materials
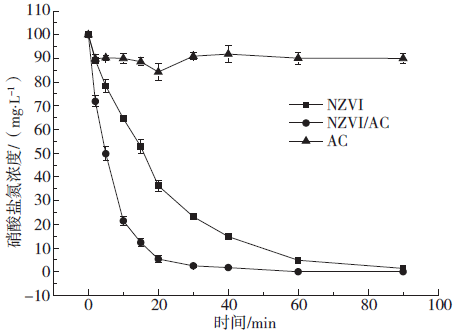
2.2 负载量对微电解体系的影响
Fig. 2 Change of reduction of nitrate by different loads
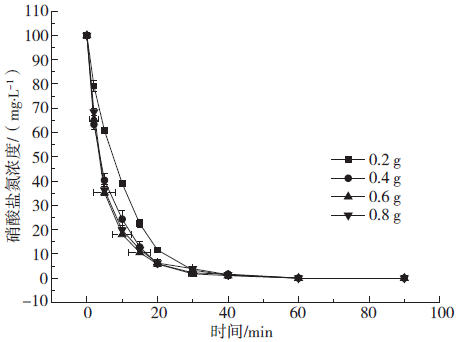
2.3 离子强度对微电解体系的影响
Fig. 3 Effects of different NaCl concentrations on reducing nitrate
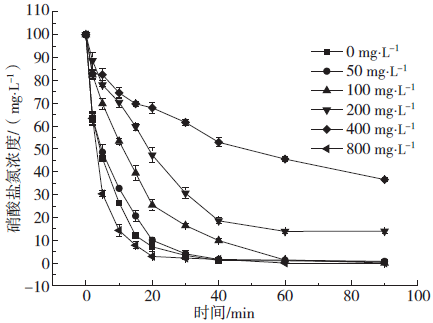
2.4 pH对微电解体系的影响
Fig. 4 Effects of different initial pH reduction nitrates
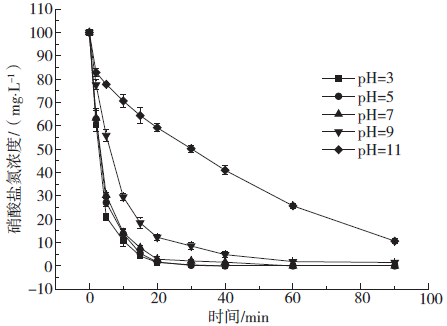
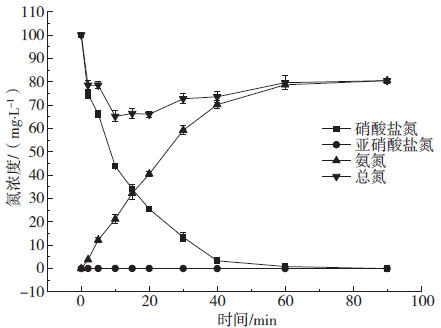
2.5 氮平衡分析
Fig. 5 Change of NZVI / AC reduction of nitrate tri-state nitrogen and total nitrogen
Fig. 5 Change of NZVI / AC reduction of nitrate tri-state nitrogen and total nitrogen
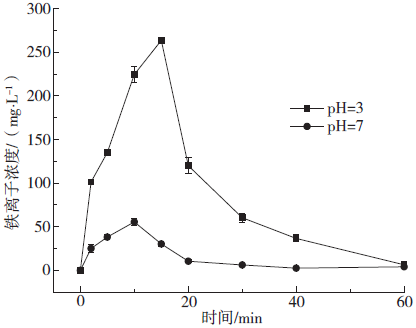
2.6 溶液中铁离子的变化
Fig. 6 Change of Fe2 + in different initial pH solution
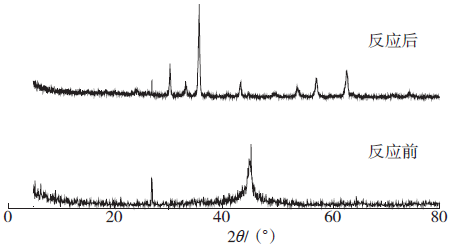
2.7 材料反应前后XRD分析
Fig. 7 NZVI/AC reaction before and after XRD pattern
2.8 动力学模型的建立
2.8.1 硝态氮反应动力学
S + H+→S-H+
| (1) |
S-OH + NO3-→S-NO3- + OH-
| (2) |
S-H+ + S-NO3-→NH4+ + 3H2O + 4Fe2+ + S
| (3) |
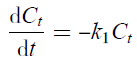 
| (4) |
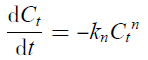 
| (5) |
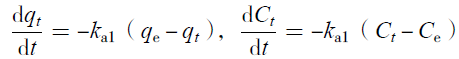 
| (6) |
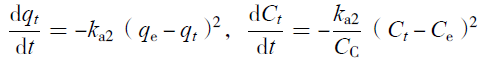 
| (7) |
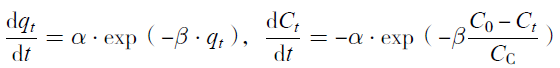 
| (8) |
qt = kt0.5
| (9) |
qe = (C0 - Ce)/CC
| (10) |
qt = (C0 - Ct)/CC
| (11) |
Fig. 8 Fitting result at different NZVI / AC dose
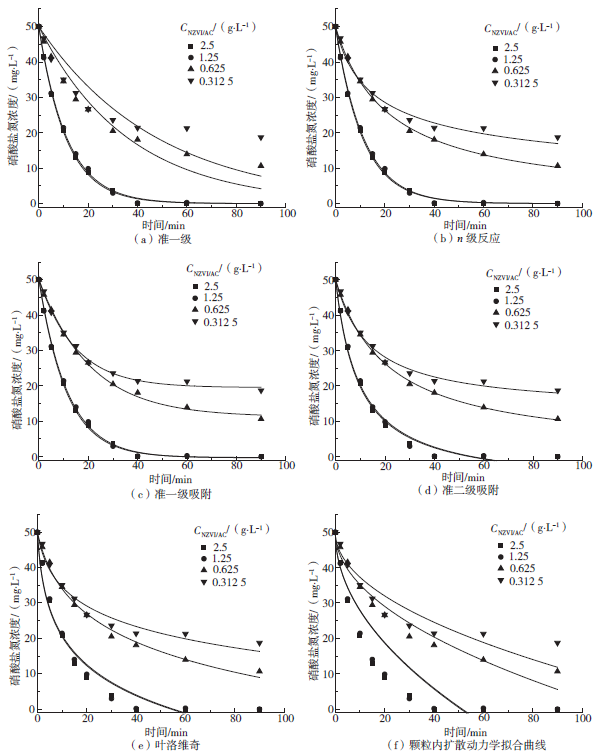
纳米铁炭投加量/(g·L-1) | 准一级反应 | n级反应 | |||
k1/min-1 | R2 | n | kn | R2 | |
2.5 | 9.039×10-2 | 0.998 | 1.019 | 8.495×10-2 | 0.998 |
1.25 | 8.725×10-2 | 0.997 | 1 | 8.725×10-2 | 0.998 |
0.625 | 2.752×10-2 | 0.939 | 2.059 | 7.263×10-4 | 0.999 |
0.312 5 | 2.071×10-2 | 0.735 | 3.454 | 4.061×10-6 | 0.983 |
Table 2 Kinetics fitting parameters of quasi-first-order adsorption and quasi-secondary adsorption
纳米铁炭投加量/(g·L-1) | 准一级吸附 | 准二级吸附 | ||||
Ka1/min-1 | qe/(mg·g -1) | R2 | Ka2/(g·(mg·min-1)) | qe/(mg·g-1) | R2 | |
2.5 | 8.944×10-2 | 20.091 | 0.998 | 4.6×10-3 | 23.405 | 0.991 |
1.25 | 8.597×10-2 | 40.257 | 0.998 | 2.19×10-3 | 47.001 | 0.989 |
0.625 | 4.798×10-2 | 61.988 | 0.997 | 5.961×10-4 | 78.439 | 0.999 |
0.312 5 | 6.758×10-2 | 97.491 | 0.996 | 6.154×10-4 | 118.408 | 0.991 |
Table 3 Kinetics fitting parameters of levovic and particle diffusion
纳米铁炭投加量/(g·L−1) | 叶洛维奇方程 | 颗粒内扩散 | |||
α/(mg·(g·min)−1) | β/(g·mg −1) | R2 | k/(mol·(g·min1/2)−1) | R2 | |
2.5 | 11.625 | 0.196 | 0.956 | 2.803 1 | 0.786 |
1.25 | 11.043 | 0.097 | 0.957 | 5.577 | 0.798 |
0.625 | 3.102 | 0.047 | 0.991 | 7.493 4 | 0.959 |
0.312 5 | 4.091 | 0.035 | 0.968 | 12.873 | 0.883 |
2.8.2 铵的生成动力学
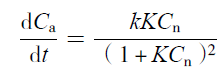 
| (12) |
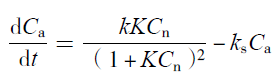 
| (13) |
Fig. 9 Kinetic fitting curve of ammonium generation
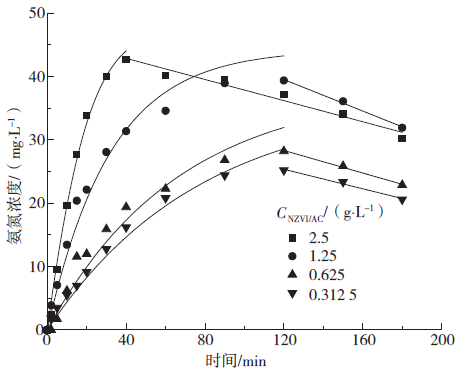
Fig. 10 Change of parameters with dosage
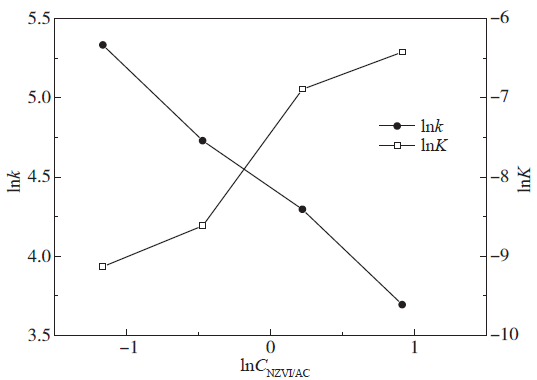
Table 4 Kinetic parameters of ammonium generation
纳米铁碳投加量/(g·L-1) | Langmuir-Hinshelwood | R2 | |
k/(L·(mg·min)-1) | K/(L·mg-1) | ||
2.5 | 40.239 | 1.621×10-3 | 0.994 |
1.25 | 73.418 | 1.015×10-3 | 0.996 |
0.625 | 113.105 | 1.812×10-4 | 0.984 |
0.312 5 | 206.865 | 1.084×10-4 | 0.998 |
2.8.3 模型验证
Fig. 11 Effect of different ionic strength on model parameters
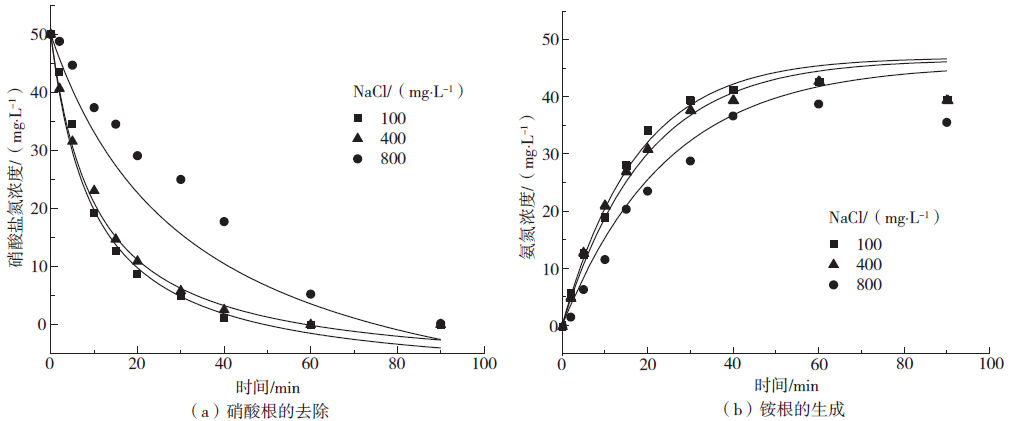
Table 5 Effects of different ionic strengths on model parameters
NaCl浓度/(mg·L-1) | 准二级吸附 | Langmuir-Hinshelwood | ||||
ka2/(g·(mg·min)-1) | qe/(mg·g-1) | R2 | k/(L·(mg·min)-1) | qe/(mg·g-1) | R2 | |
100 | 5.296 3×10-3 | 48.607 864 | 0.980 | 4.324 55×10-4 | 286.253 6 | 0.998 |
400 | 2.27×10-3 | 48.970 352 | 0.984 | 8.395 8×10-4 | 178.585 8 | 0.990 |
800 | 2.21×10-4 | 46.189 728 | 0.908 | 2.138 1×10-4 | 57.545 6 | 0.912 |
Fig. 12 Effect of different initial pH on model parameters
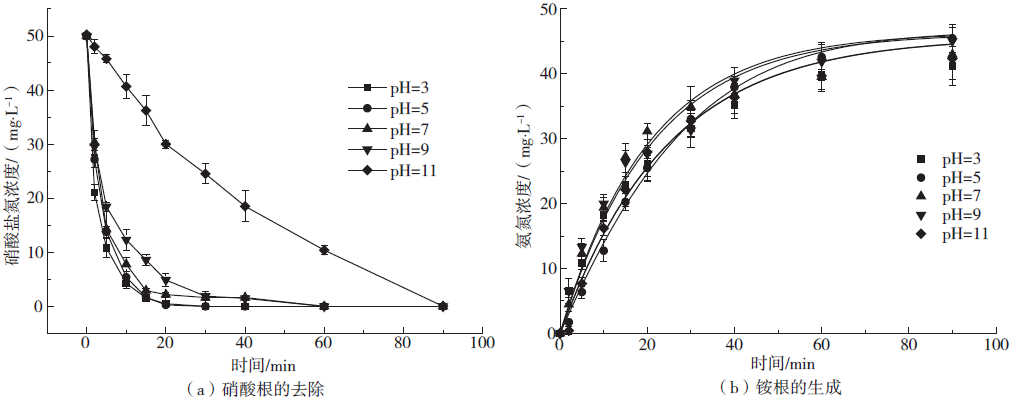
Table 6 Effects of different pH on model parameters
pH | 准二级吸附 | Langmuir-Hinshelwood | ||||
Ka2/(g·(mg·min)-1) | qe/(mg·g-1) | R2 | k/(L·(mg·min)-1) | K/(L·mg-1)
| R2 | |
3 | 3.08×10-2 | 48.607 864 | 0.996 | 286.253 6 | 1.324 5×10-4 | 0.998 |
5 | 2.01×10-2 | 49.711 448 | 0.980 | 208.650 5 | 6.1×10-3 | 0.998 |
7 | 1.75×10-2 | 48.790 352 | 0.981 | 149.375 | 8.395 8×10-4 | 0.990 |
9 | 1.42×10-2 | 46.189 728 | 0.984 | 81.718 7 | 9.874 3×10-4 | 0.992 |
11 | 3.21×10-2 | 49.663 352 | 0.968 | 17.585 8 | 2.138 1×10-4 | 0.992 |
Fig. 13 Effects of different initial nitrate on model
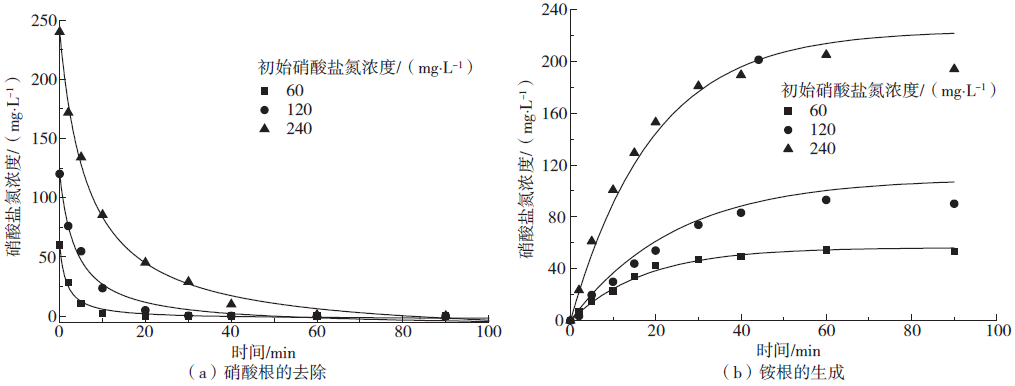
Table 7 Effects of different initial nitrate concentrations on model
NO3-浓度/(mg·L-1) | 准二级吸附 | Langmuir-Hinshelwood | ||||
ka2/(g·(mg·min)-1) | qe/(mg·g-1
)
| R2 | k/(L·(mg·min)-1) | K/(L·mg-1) | R2 | |
60 | 5.03×10-2 | 12.545 | 0.987 | 86.253 | 6.324 5×10-4 | 0.998 |
120 | 9.98×10-3 | 25.866 | 0.986 | 58.355 | 5.395 8×10-4 | 0.991 |
240 | 2.9×10-3 | 51.881 | 0.996 | 37.545 | 5.138 1×10-4 | 0.979 |





 百度学术
百度学术


 下载:
下载:
