UV/O3降解水样中COD的动力学建模
Kinetics modeling for degradation of COD in water sample by UV/O3
-
摘要: 建立动力学模型可以对UV/O3降解有机物的过程进行有效的分析和预测,帮助改进水处理工艺以提高有机物的降解效率和降低能源消耗。然而,已有的方法无法满足实际水样中未知有机物降解过程的建模需求。在化学反应机理的基础上,结合对有机物降解生成CO2浓度演化规律的分析,建立了一种可预测未知有机物降解反应的数学模型。通过改变氧化消解的条件,从理论上检验了该模型的灵敏度;同时,实验采用了含有葡萄糖、尿素和邻苯二甲酸氢钾的混合溶液作为测试水样,考察了模型的准确性,实验值与预测值的趋势一致。Abstract: Kinetics modeling can be used to effectively analyze and predict the process of the degradation of organics using the UV/O3 technique.It can also be used to improve the removal rate and reduce power consumption;however,the methods of kinetics modeling that were reported previously cannot be applied when there are unknown organics in the sample solution.Based on the chemical reaction mechanism,a mathematic model that can predict the treatment of a water sample with unknown organic species was established by including the evolution of CO2.The sensitivity of the model was tested under a variety of digestion conditions.Meanwhile,a sample solution mixed with glucose,urea,and potassium acid phthalate was used to validate the accuracy of the model.The experimental and simulated results followed the same trend.
-
Key words:
- advanced oxidation processes /
- kinetics model /
- hydroxyl radical /
- ozone /
- reaction order /
- reaction rate constant
-
盐酸强力霉素(DC)是一种半合成的四环素类抗生素。大量的四环素直接排泄到环境中,对生态系统和人类健康具有潜在风险。目前,已在水生环境中被普遍检测出此类抗生素[1]。由于其复杂的结构,DC不能通过常规的生物处理工艺被有效地去除。因此,通常采用许多物理和化学处理方法予以去除,例如吸附[2]、基于臭氧的高级氧化过程[3]、光催化[4]等。光催化方法由于其低成本,高效率和环境友好性等特点被广泛应用于处理印染废水[5]、抗生素废水[6]等。类石墨氮化碳(g-C3N4)因其合适的带隙,无毒,稳定性好而被认为是潜在的去除有机污染物的可见光光催化剂。但该催化剂存在对可见光的响应效率较低,光生电子和空穴的重组率较高等缺陷[7]。因此,需要寻找有效的方法去改善g-C3N4的光催化性能。其中,构建基于g-C3N4的异质结复合材料是最有效的方法之一,这可以有效地促进光诱导电荷的分离并加速光催化反应进程[8]。宋思扬等[9]通过化学浴沉淀法制备了Co掺杂的FeOOH与石墨相氮化碳复合材料(Co-FeOOH/g-C3N4),以罗丹明B(RhB)为目标污染物,在最佳反应条件下,Co-FeOOH、g-C3N4和Co-FeOOH/g-C3N4对RhB的去除率分别为23.7%、59.6%和91.5%。CeO2作为一种活性稀土金属氧化物,由于具有Ce4+和Ce3+的化合价变化而引起了广泛关注。Ce4+和Ce3+的氧化还原循环将改善光生电子和空穴对的界面电荷转移和分离速率[10]。据报道,CeO2的CB和VB分别为−0.39 eV和2.50 eV,而g-C3N4的CB和VB分别为−1.13 eV和1.57 eV[11],因此,CeO2和g-C3N4因具有良好匹配的能带结构而可以形成高效的异质结构。HUMAYUN等[12]制备了g-C3N4/CeO2,在可见光下考察了其对2,4-二氯苯酚(2,4-DCP)的降解效果,发现羟基自由基(˙OH)是降解2,4-DCP的主要活性物质。此外,基于密度泛函方法的理论算术,铈具有像Pt一样的能垒,而g-C3N4负载的铈可以产生更多的活性位点[13]。目前的研究中,CeO2/g-C3N4仅作为光催化剂降解污染物[14-16],但由于CeO2可以与H2O2产生类芬顿反应[17],因此,构建新型CeO2/g-C3N4非均相光芬顿体系,有望进一步提高对污染物的降解效率。
基于上述原因,本研究通过水热法制备了CeO2/g-C3N4,并在可见光下采用光催化-芬顿法降解盐酸强力霉素(DC)。使用扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线粉末衍射(XRD)、X射线光电子能谱(XPS)、电阻抗能谱(EIS)、电子自旋共振(ESR)和漫反射光谱(UV-Vis)等手段对合成的CeO2/g-C3N4进行了表征;分别考察了初始pH、H2O2浓度、不同的铈掺杂量、催化剂用量和DC浓度对DC降解的影响,优化了反应条件;评价了复合光催化剂的重复使用性和稳定性;探讨了CeO2/g-C3N4光催化-芬顿体系的降解机理。
1. 材料与方法
1.1 实验试剂与仪器
试剂:六水合硝酸铈、糠醇、异丙醇、对苯醌、乙二胺四乙酸二钠、盐酸(均为分析纯,国药集团化学试剂有限公司);30%双氧水(优级纯,国药集团);尿素(分析纯,上海麦克林生化科技有限公司);5,5-二甲基-1-吡咯啉-N-氧化物(DMPO,98%,上海九鼎生物科技有限公司);盐酸强力霉素(纯度96%,上海阿拉丁生化科技有限公司);实验用水为超纯水。
仪器:KSL-1100X控温马弗炉(合肥科晶材料技术有限公司);XPA光化学反应仪(南京胥江机电厂);UV-2600紫外-可见分光光度计(日本岛津公司);Gemini 50扫描电子显微镜(德国卡尔蔡司公司);ESCALAB250Xi型光电子能谱仪(美国赛默飞世尔公司);X-Pert PRO MPD固定靶X射线衍射仪(荷兰帕纳科公司);JES-FA200电子顺磁共振波谱仪(日本捷欧路公司)。
1.2 材料制备
采用水热法[18]用于制备CeO2/g-C3N4复合催化剂。首先称取5.0 g尿素置于坩埚中,以2.5 ℃·min−1的升温速率加热至550 ℃,煅烧2 h,待冷却至室温后,将淡黄色固体取出并研磨得到粉末状的g-C3N4;将0.4 g的g-C3N4溶于50 mL去离子水中,超声波处理30 min;将一定量的Ce(NO3)3·6H2O添加到悬浮液中,搅拌30 min后将其转移到高压釜中,并在160 ℃下加热10 h,再将其冷却到室温,并用去离子水反复洗涤几次,最后在90 ℃下干燥8 h,得到浅黄色材料。对所制备的催化剂命名为X%CeO2/g-C3N4。按照与上述相同的方法,还制备了纯CeO2。
1.3 实验方法
在光催化反应仪(XPA-7)中进行了DC的降解实验。为了使反应溶液温度恒定在(18±2) °C,仪器中的光反应装置与冷却装置连接。垂直可见光源是500 W氙气灯(用滤光片过滤了波长小于420 nm的紫外线)。首先,使用浓度为0.5 mol·L−1的NaOH或H2SO4溶液调节DC溶液的初始pH。之后,将称量的0.025 g CeO2/g-C3N4放入到50 mL DC溶液中,并在黑暗中搅拌30 min达到吸附平衡,转速为500 r·min−1。最后,加入5 mmol·L−1 H2O2并打开灯反应120 min。在此过程中,每20 min取1 mL反应悬浮液样品并使用0.22 μm PES滤头进行过滤。将反应后的溶液进行离心分离,使用纯水洗涤离心3次,进行真空干燥,得到反应后的CeO2/g-C3N4。并再按照上述步骤反复4次,考察其重复性和稳定性。
1.4 分析方法
将获得的样品通过高效液相色谱(HPLC)测量DC浓度。测试DC浓度的最佳流动相是0.01 mol·L−1的乙二酸,甲醇和乙腈的混合溶液(乙二酸∶甲醇∶乙腈=65∶17∶18),检测波长为357 nm,柱温为30 ℃。
2. 结果与讨论
2.1 催化剂表征结果
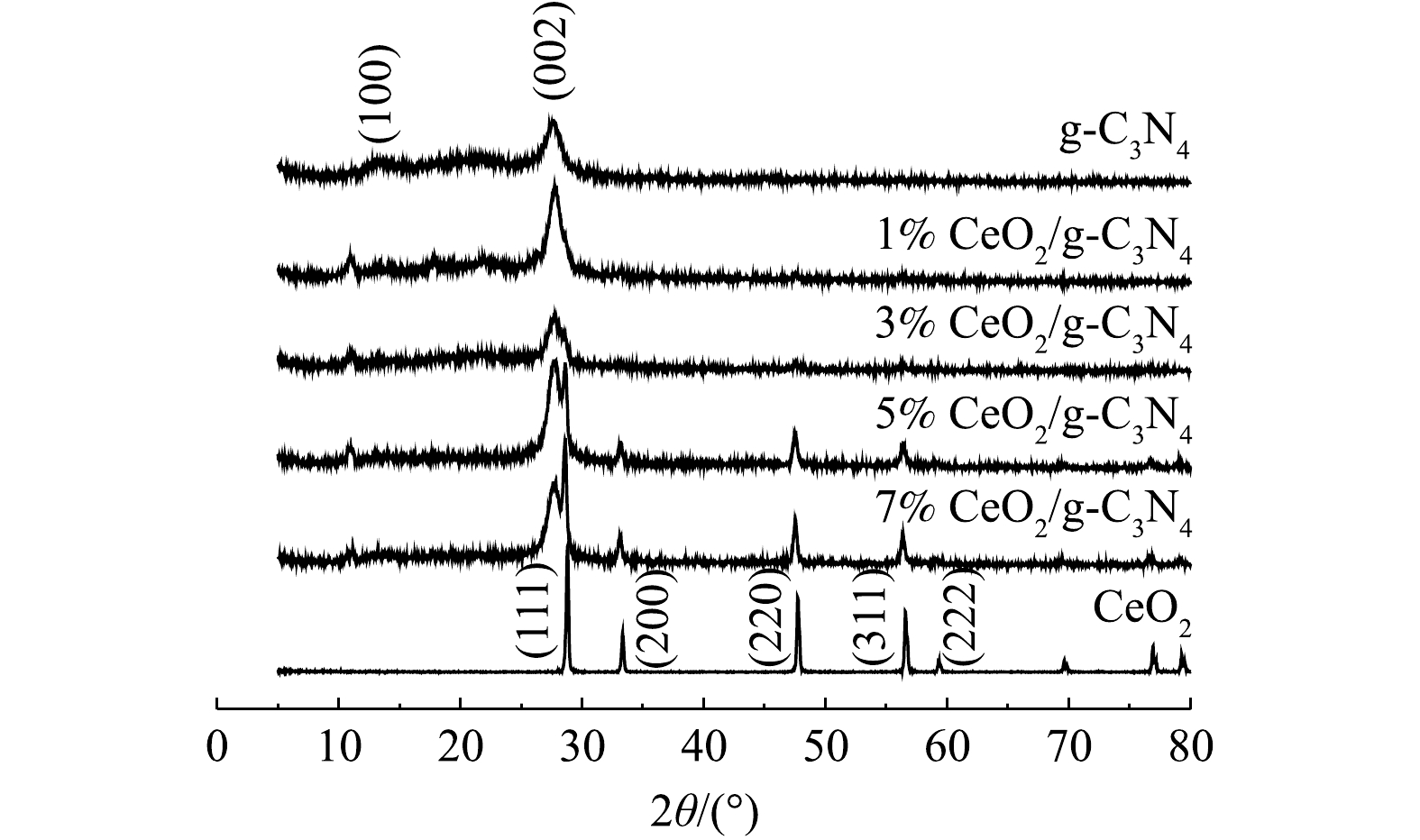
通过粉末XRD分析研究了所制备的纳米复合材料中各相的晶体结构。图1显示了X%CeO2/g-C3N4、CeO2和g-C3N4的XRD谱图。由图1可以看出,g-C3N4的谱图中显示出有2个明显的特征衍射峰,典型的衍射峰位于13.1°和27.6°,可分别归因与g-C3N4(100)和(002)晶面[19]。纯CeO2和X%CeO2/g-C3N4复合材料的XRD光谱显示出立方CeO2的典型XRD图谱(JCPDS 78-0694)[20]。在28.8°、33.3°、47.7°、56.58°和59.34°处CeO2的特征衍射峰对应(111)、(200)、(220)、(311)和(222)平面[21]。从图1中可以看出,在X%CeO2/g-C3N4复合材料中,显示了位于27.6°的特征衍射峰,这表明存在g-C3N4相。
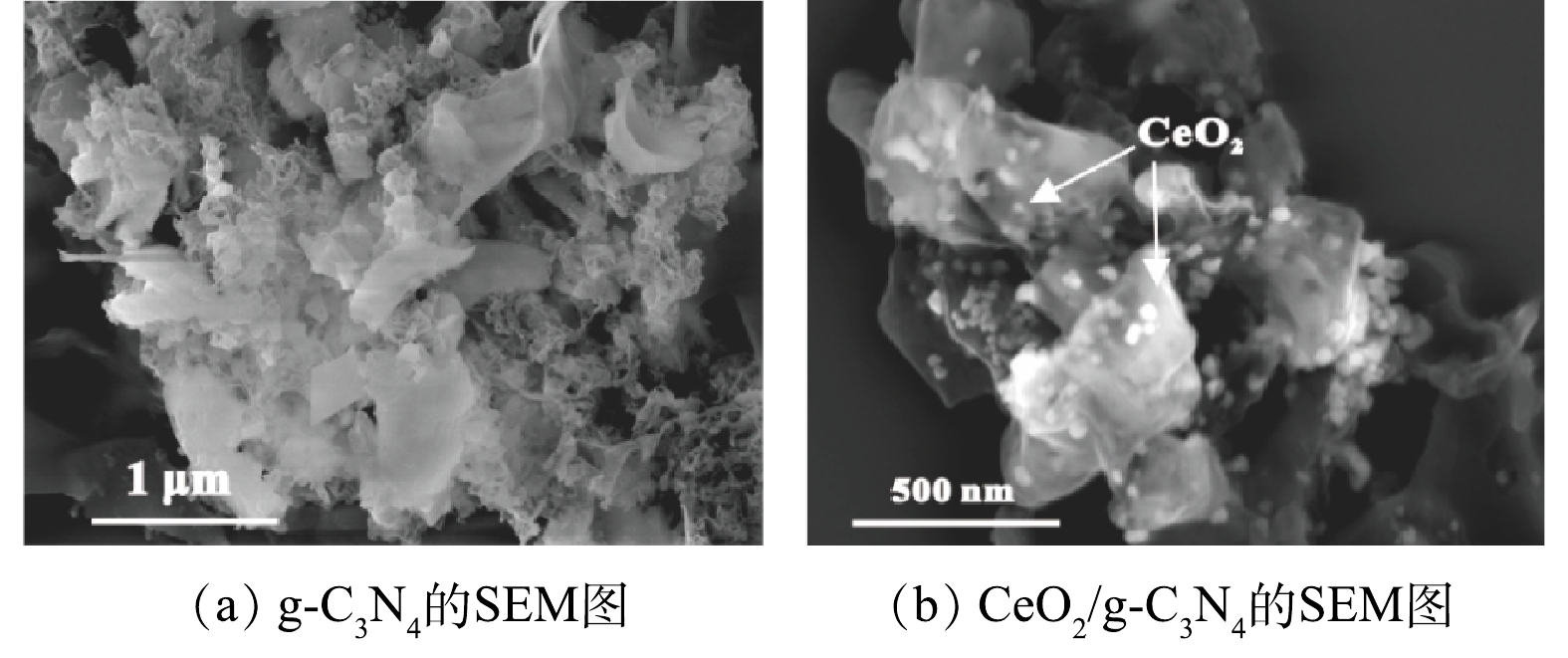
为了表征CeO2/g-C3N4的形态和微观结构,进行了SEM分析。图2(a)和图2(b)显示了g-C3N4和5%CeO2/g-C3N4的SEM图像。所有的催化剂均含有介孔状结构,在图2(b)中,CeO2颗粒均匀分布在g-C3N4的表面,这表明CeO2已经成功掺杂到g-C3N4中并且不会改变其结构。
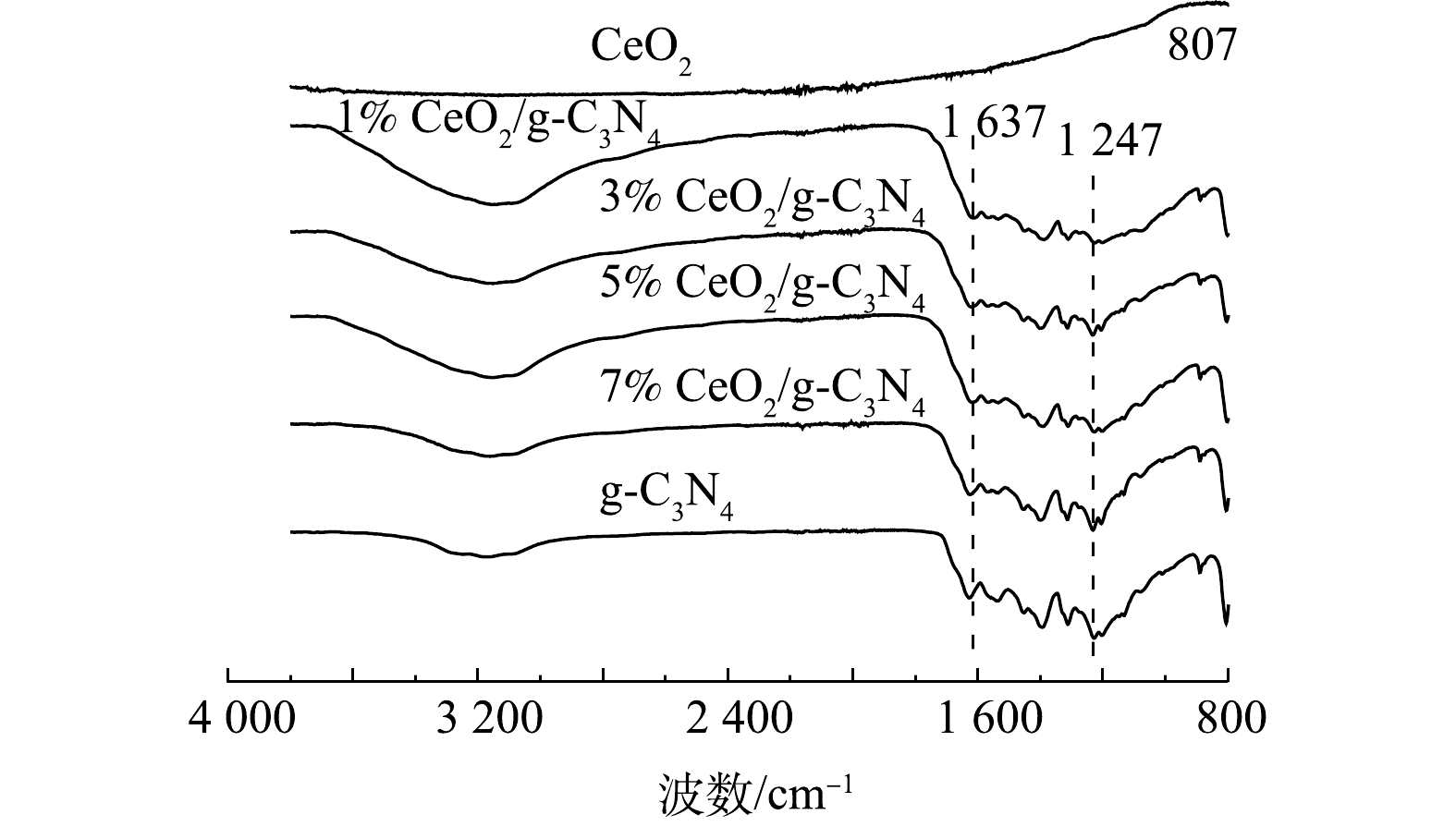
图3显示了g-C3N4、CeO2和X%CeO2/g-C3N4的FTIR光谱。由图3中可以看出,对于X%CeO2/g-C3N4复合材料,显示了g-C3N4的典型分子结构,也观察到g-C3N4的所有特征吸收峰,这证实了复合材料中有g-C3N4的骨架。1 247~1 637 cm−1的强吸收带(在1 247 cm−1和1 637 cm−1处具有特征峰)可以归因于CN杂环的典型拉伸振动[22]。3 000~3 600 cm−1处的峰应该是NH的拉伸振动吸收峰[23]。在含Ce催化剂的制备中,CeO2是通常产生的物质,且在500~700 cm−1处观察到Ce—O拉伸振动[24]。上述表征结果表明,在掺杂Ce后,g-C3N4的主要结构并未发生明显变化。此外,由于掺杂含量低和峰重叠影响,因此,看不到Ce相关基团的振动带[25]。
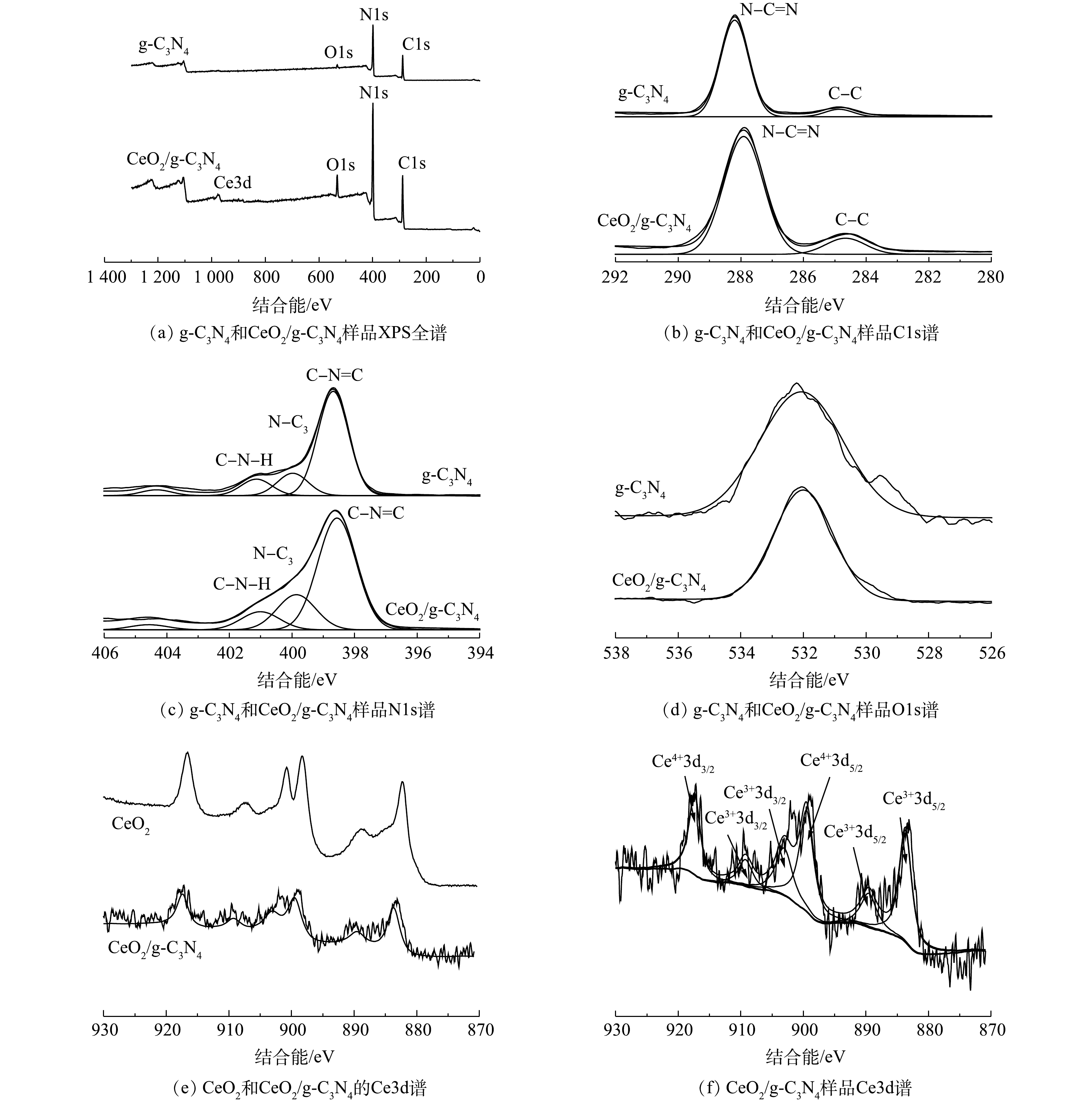
图4(a)显示了g-C3N4和CeO2/g-C3N4的所有元素的全光谱。在g-C3N4的XPS光谱中发现了C、N、O的峰,在CeO2/g-C3N4的XPS光谱中发现了C、N、O和Ce的峰,这表明CeO2已成功引入g-C3N4。可以看出,C和N是主要元素。图4(b)显示了g-C3N4和CeO2/g-C3N4的Cls光谱。g-C3N4的C1s谱可分解为2个不同的高斯-洛伦兹峰,中心峰的结合能为284.88 eV和288.21 eV。284.88 eV(19.82%)处的峰可归因于表面无定形碳的C—C配位,288.21 eV(80.18%)处的峰可归因于C—N或C—(N)3[26]。CeO2/g-C3N4的C1s光谱与g-C3N4的相似,中心峰的结合能为283.6 5eV和287.78 eV。在g-C3N4的N1s光谱中(图4(c)),可以观察到3个峰:398.69 eV(76.54%)处的峰可归因于sp2轨道杂化的芳族氮(C—N=C);399.93 eV(10.46%)处的峰是由于sp3轨道杂化N—(C)3引起的; 401.14 eV处的的峰(9.28%)可归因于C—N—H组[27]。在CeO2/g-C3N4的N 1s光谱中,中心峰的结合能为398.04、399.88和401.17 eV。在532.3 eV处的O1s峰与在催化剂表面上的羟基基团或水分子的存在有关[15, 28](图4(d))。图4(e)显示了纯CeO2和所制备的CeO2/g-C3N4的Ce3d光谱,在纯CeO2的Ce3d光谱观察到6个峰,分别位于882.3、888.9、898.3、900.8、907.3和916.7 eV,而在CeO2/g-C3N4的Ce3d光谱也观察到相对应的6个峰。在CeO2/g-C3N4的Ce3d光谱中(图4(f)),结合能峰位于883.6 eV和889.5 eV,说明存在Ce3+3d5/2,结合能峰位于899.4 eV,则说明存在Ce4+3d5/2,而结合能峰位于903.1 eV和909.2 eV则说明Ce3+3d3/2的存在,结合能峰位于917.3 eV则是由于Ce4+3d3/2的存在,证明Ce以Ce(Ⅲ)和Ce(Ⅳ)态的形式存在[11, 29]。CeO2/g-C3N4峰的位置相较于纯CeO2有所偏移,这可能是由于g-C3N4与CeO2之间存在相互作用[15]。
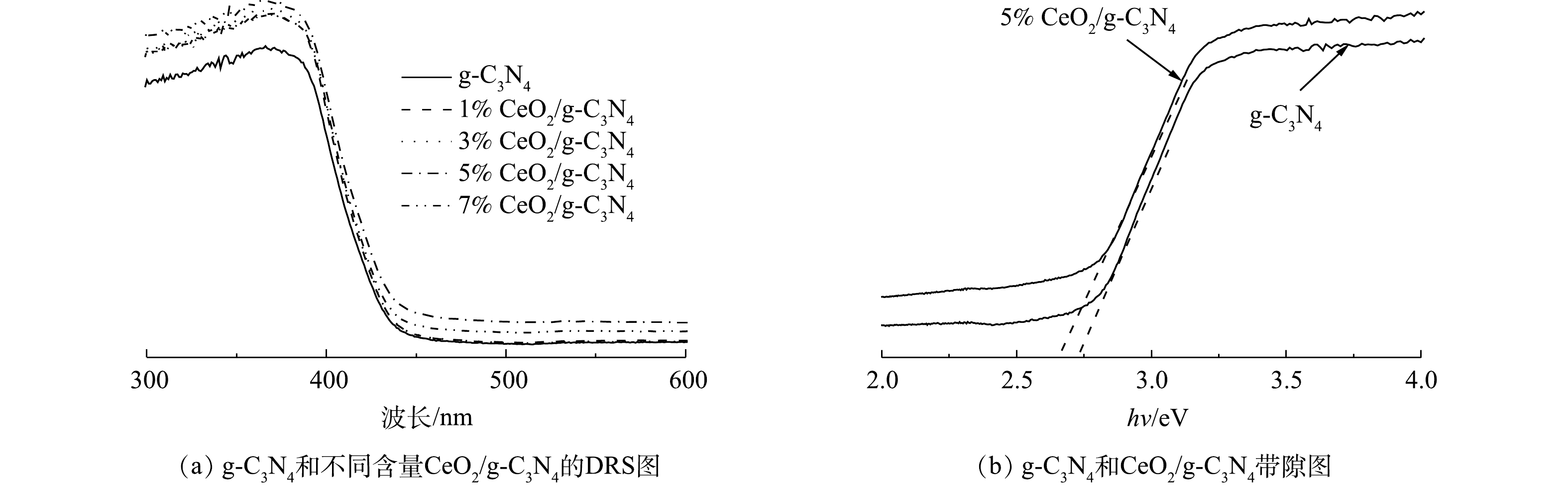
为了研究催化剂的光吸收性能,测量了g-C3N4和不同含量CeO2/g-C3N4的UV-Vis漫反射光谱。从图5(a)中可以看出,随着引入Ce掺杂剂,CeO2/g-C3N4的吸收边缘在大约420~460 nm处出现红移。这可能是因为Ce和g-C3N4之间的共轭和电荷转移。此外,他们的吸收范围更宽更强,从而增强了可见光吸收和光催化性能。掺杂CeO2可以改善催化剂的光吸收性能,不同CeO2含量的催化剂的光吸收性能差别不大,其中5%CeO2/g-C3N4的光吸收性能略好。此外,基于UV-vis DRS数据,通过Kubelk-Munk方法(式(1))计算了CeO2、g-C3N4和CeO2/g-C3N4的带隙值。
αhv=A(hv−Eg)n/2 (1) 式中:α,hν,Eg和A分别代表吸收系数,光能,光带隙能量和常数。
n取决于半导体中的跃迁特性(直接跃迁n = 1;间接跃迁n = 4)。对于g-C3N4,n=1[30]。根据式(1)计算得出,g-C3N4和5%CeO2/g-C3N4的带隙分别为2.73 eV和2.59 eV(图5(b))。带隙变窄有利于光吸收,这意味着激发电子从价带(VB)跃迁至导带(CB)所需的能量更少,通过掺杂Ce可以增强光的吸收。由此可见,Ce掺杂对g-C3N4的作用可以扩展可见光吸收,最终导致光催化活性的提高。
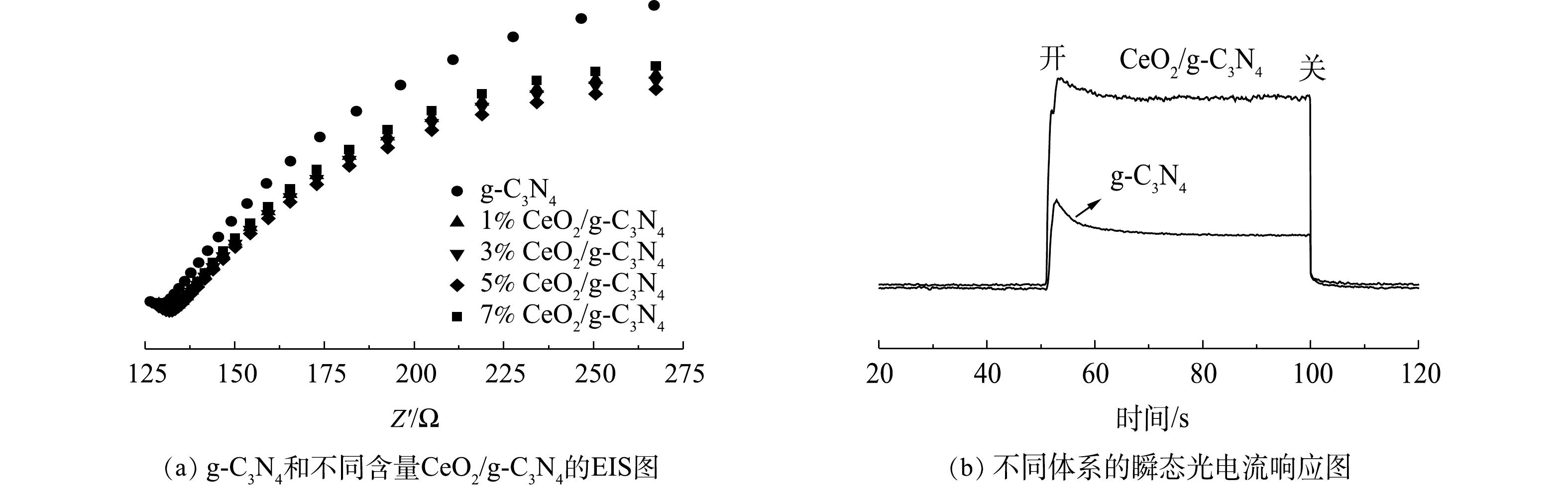
为了更好地了解CeO2/g-C3N4中的光诱导电流分离行为,对其进行了电化学阻抗谱(EIS)的测量,结果如图6所示。EIS电化学阻抗谱上的电弧反映了电极/电解质界面处电荷转移层的电阻。较小的电弧半径表示较低的电阻和较高的电荷转移效率[31]。由图6(a)可以看出,CeO2/g-C3N4复合光催化剂的电弧半径小于g-C3N4,其中5%CeO2/g-C3N4催化剂的电弧半径最小,这表明,在5%CeO2/g-C3N4复合光催化剂界面处的电子-空穴对的转换和分离更有效。为了进一步评估不同催化剂的电荷分离效率,对其进行了瞬态光电流响应的测量。图6(b)显示了纯g-C3N4和CeO2/g-C3N4的光电流响应。当打开和关闭光源时,CeO2/g-C3N4产生的光电流最高,这表明与纯g-C3N4相比,CeO2/g-C3N4复合光催化剂具有更低的电子-空穴对复合率。
2.2 CeO2/g-C3N4催化降解盐酸强力霉素的性能
1)不同体系下DC的降解效果。为了探索CeO2/g-C3N4的光催化活性,在催化剂投加量为500 mg·L−1,初始H2O2浓度为5 mmol·L−1,初始pH为2.0,DC浓度为10 mg·L−1的条件下进行实验。图7显示了DC在不同的反应体系中的降解效果,这些体系分别是非均相芬顿体系,光催化体系和光催化-芬顿体系。为排除催化剂的吸附作用对污染物浓度变化的影响,制备的催化剂在光照实验前均进行了30 min避光搅拌。由图7可见,单独的CeO2/g-C3N4在黑暗条件下对DC的吸附去除率只有5.1%。在含有H2O2/Vis系统中,DC的去除率为11.9%,这表明,在没有催化剂的情况下,H2O2在可见光下对DC的氧化能力有限。在g-C3N4和CeO2/g-C3N4的光催化体系中DC的去除率在120 min内分别为38.1%和46.9%。在单独CeO2的和5%CeO2/g-C3N4的非均相芬顿体系中,在120 min内对DC的去除率为27.3%和31.5%。但是,在5%CeO2/g-C3N4的光芬顿体系中,DC的去除率在120 min内可达到99.1%。上述结果表明,5%CeO2/g-C3N4复合催化剂的去除率高于其他催化剂。
2)初始pH对DC降解效率的影响。如图8所示,当初始pH为2.0、3.0和5.0时,DC的去除率分别为97.3%、81.8%和73.5%。当初始pH进一步提高至中性条件时,DC降解则受到抑制。在初始pH为7.0时,DC的去除率降低至62.2%。该结果可能是由于,在酸性条件下,溶液中存在大量H+离子,促进了Ce与H2O2发生芬顿反应产生·OH。另一方面,当溶液pH偏高时,H2O2容易分解为H2O和O2[32]。
3)不同铈掺杂量对DC去除率的影响。如图9所示,当使用CeO2/g-C3N4作为催化剂时,对DC的去除率大大增加。随着铈掺杂量由1%增加到5%,DC去除率由67%提高至97%;而当铈掺杂量增加到7%时,对DC的去除率降低到58%。有研究表明,g-C3N4中金属离子的存在会引起表面缺陷的形成,一方面,可以通过增加掺杂金属的量来改善催化反应性;另一方面,过量的CeO2物种可能充当载流子的复合中心并覆盖表面上的活性位点,从而降低了光催化效率[12,33]。本实验研究结果表明,当铈掺杂量达到5%时表现出最佳的降解效果。
4) CeO2/g-C3N4投加量对DC去除效果的影响。图10显示了在120 min下,不同质量浓度催化剂(0.25、0.5、0.75和1.0 g·L−1)对DC的去除效果。随着催化剂投加量由0.25 g·L−1增加到0.5 g·L−1,DC的去除率在120 min内由70.1%增加到99.3%。这是因为,对于一定浓度的DC溶液,在一定的催化剂用量范围内,催化剂浓度的增加可以增加活性位点,从而提高DC的降解效率。但是,当催化剂质量浓度从0.5 g·L−1增加到1.0 g·L−1时,一方面过量的催化剂会使不透明性增加,光透过率降低,从而会阻碍光和活性位点在催化剂表面的渗透,导致DC去除率下降[34];另一方面,催化剂投加量过大也有可能导致产生自由基过多,造成自我淬灭[35]。
5) H2O2浓度对DC去除效果的影响。图11显示了加入不同H2O2浓度对CeO2/g-C3N4复合催化剂降解DC的影响。当H2O2浓度由1 mmol·L−1增加到5 mmol·L−1时,DC去除率在100 min内由75%提高到97.3%。该结果表明,随着H2O2浓度的增加,DC去除率有所增加。但是,当H2O2的浓度进一步增加到7 mmol·L−1时,DC的去除率会降低至86%。这是因为当H2O2浓度低于临界值时,催化反应产生的˙OH自由基的数量随H2O2浓度的加大而增加;相反,当H2O2浓度高于临界值时,生成的˙OH可能被过量的H2O2捕获而形成活性较低的˙HO2自由基[36](式(2)~(4))。大量的˙OH被消耗,˙OH无法与DC有效反应,导致去除率降低和H2O2浪费。
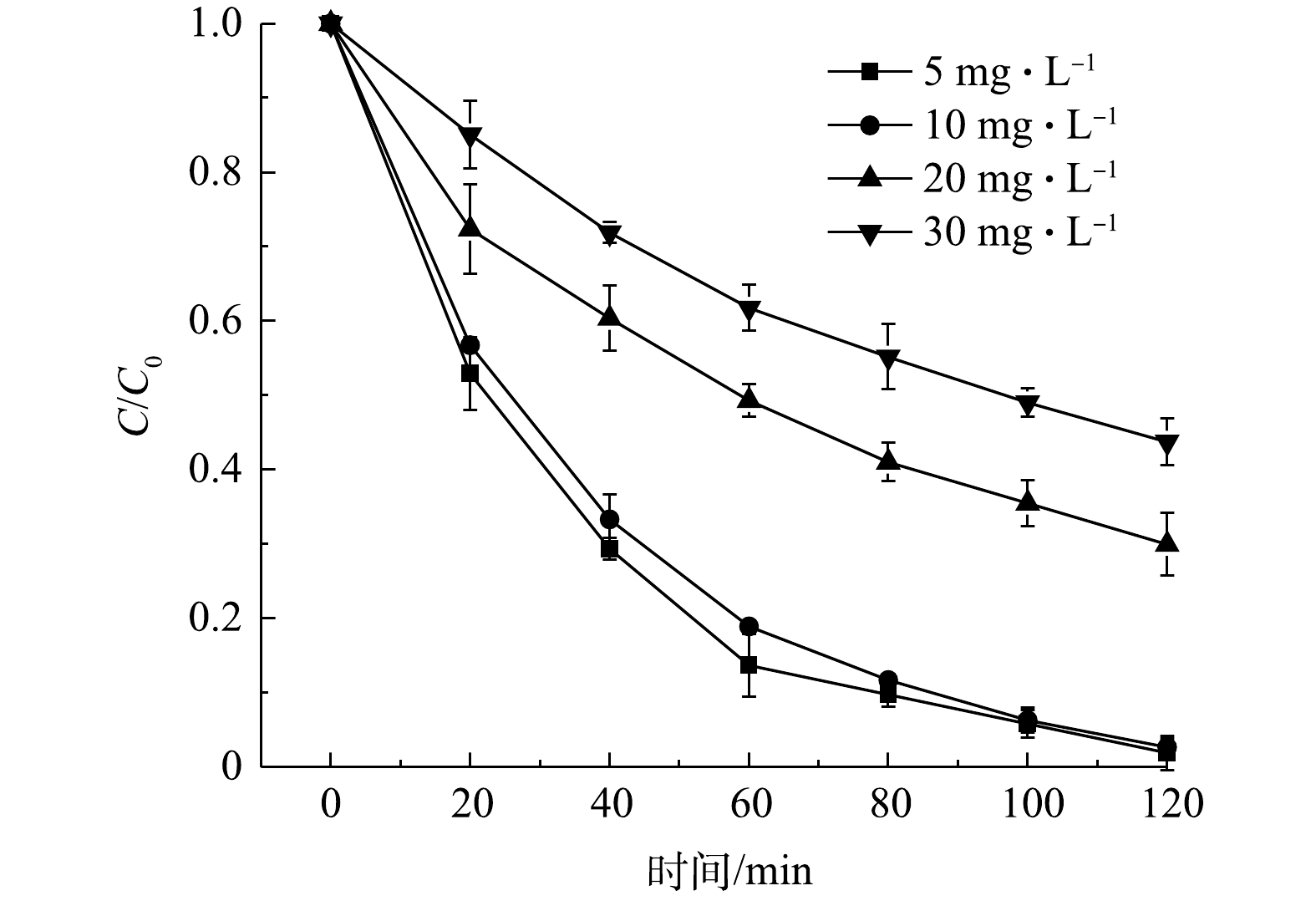
H2O2+⋅OH→⋅HO2+H2O (2) ⋅HO2+⋅OH→H2O+O2 (3) ⋅OH+⋅OH→H2O2 (4) 6) DC初始浓度对其去除效果的影响。图12反映了DC的初始浓度对其降解效率的影响。可以看出,DC的去除率与其初始浓度成反比。当DC的初始浓度为10 mg·L−1时,可以在120 min内完全降解;但是,当DC的初始质量浓度增加到20 mg·L−1和30 mg·L−1时,反应120 min后的DC去除率为70.6%和56.5%。这是因为过量的DC分子将占据催化剂表面部位并阻止其与H2O2接触,从而无法生成足够的羟基自由基来降解DC[37]。
2.3 CeO2/g-C3N4催化剂的重复性与稳定性
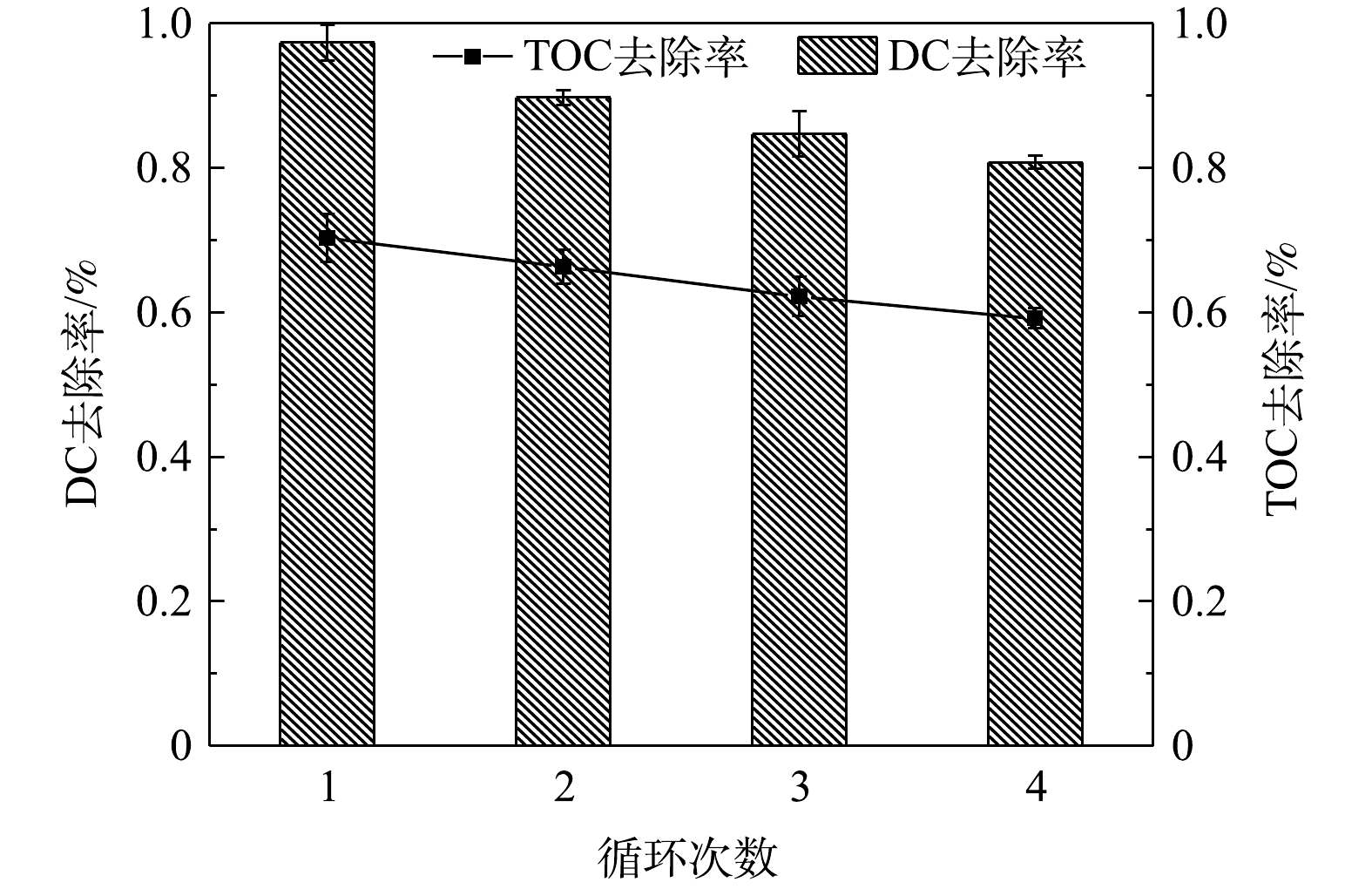
催化剂的重复性与稳定性是技术实际应用中的重要因素。为了评估CeO2/g-C3N4的化学稳定性和重复使用性,在可见光下对光催化剂进行了4次连续的重复实验。在每个反应之后,通过静置分离,然后用纯水反复洗涤并真空干燥,干燥后的样品研磨收集以便用于后续降解实验。实验结果如图13所示。经过4次循环,DC的去除率从97.8%降低到81.5%,仅下降16.3%。TOC的去除率从69.2%降低到58.5%,仅下降为10.7%。此外,在循环过程中光催化剂的量略有减少。上述结果表明,CeO2/g-C3N4具有较高的重复性和稳定性。
2.4 CeO2/g-C3N4催化H2O2降解DC机理
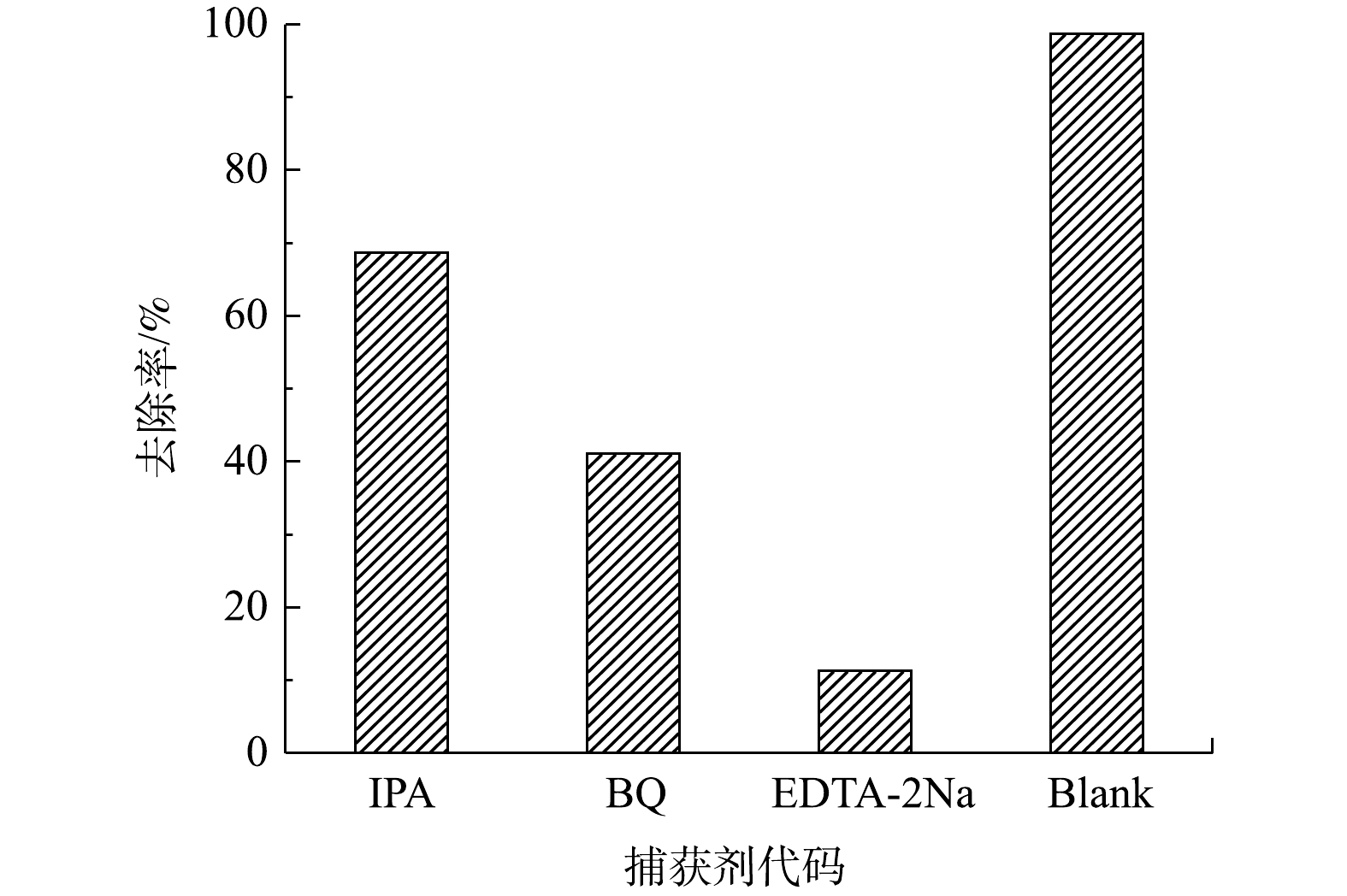
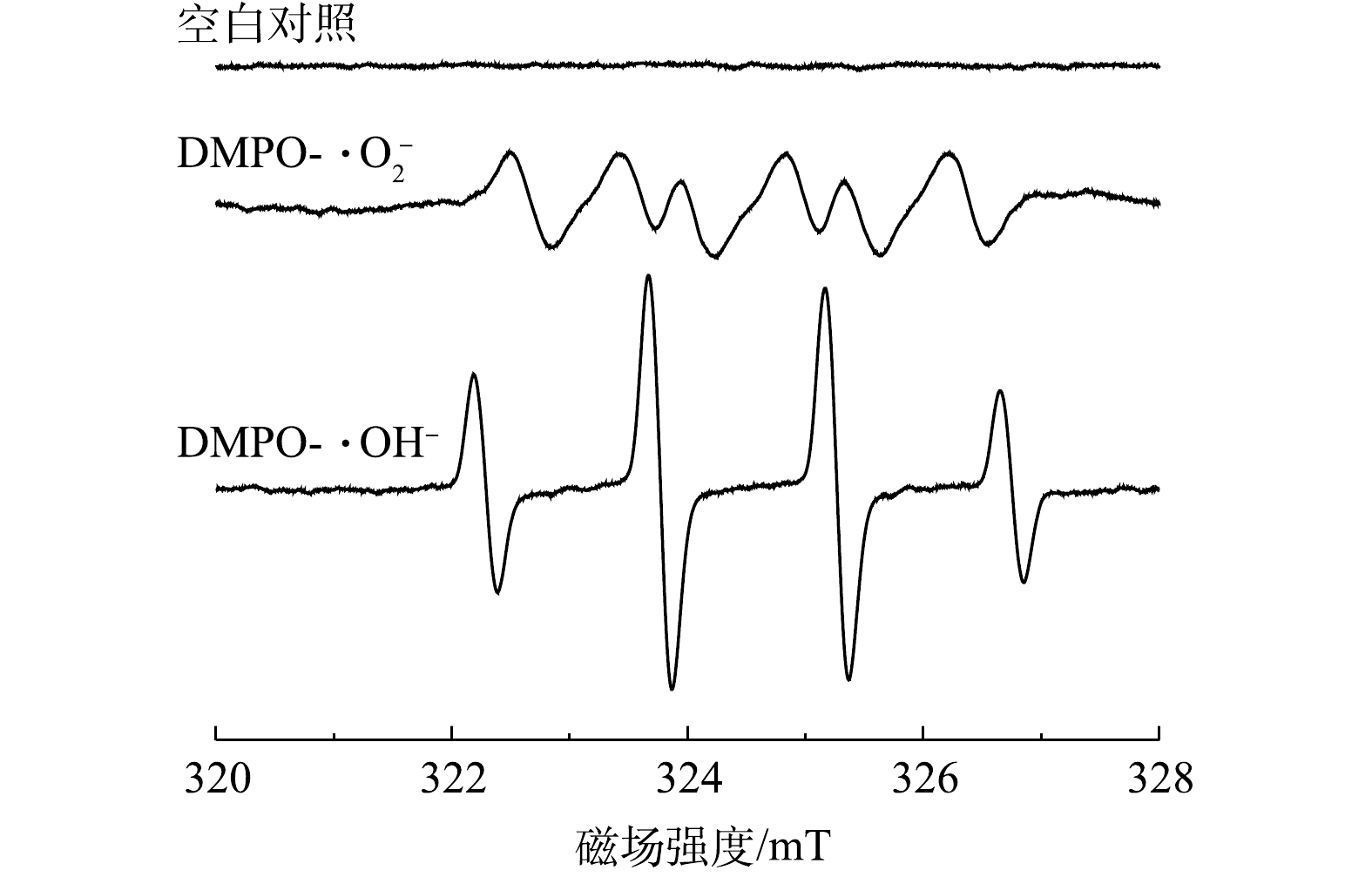
为了确定CeO2/g-C3N4/H2O2体系降解DC反应中主要的自由基种类,进行了自由基淬灭实验。用于实验的淬灭剂为异丙醇(˙OH)、对苯醌(
⋅O−2 ⋅O−2 ⋅O−2 ⋅O−2 综合以上信息可以推测,CeO2/g-C3N4催化H2O2降解DC的催化机理为:首先,g-C3N4在见光照下产生光生电子和空穴h+。g-C3N4和CeO2的CB分别为−1.09 eV和−0.79 eV,VB分别为1.61 eV和2.03 eV[39]。因为g-C3N4和CeO2的CB比E0 (O2/
⋅O−2 ⋅O−2 ⋅O−2 g-C3N4+hv→g-C3N4(h++e−) (5) O2+e−→⋅O−2 (6) Ce4++e−→Ce3+ (7) Ce3++H2O2→Ce4++⋅OH+OH− (8) Ce4++H2O2→Ce3++⋅OOH+H+ (9) h++H2O2→⋅O−2+2h+ (10) ⋅O−2+h++⋅OH+DC→小分子/离子 (11) 3. 结论
1)在pH为2.0、H2O2为5 mmol·L−1、催化剂投加量为0.5 g·L−1的最佳条件下,5%CeO2/g-C3N4可有效去除10 mg·L−1的DC,DC去除率可达到99.1%。
2)在可见光和H2O2同时存在下催化降解DC,CeO2/g-C3N4的光催化活性比纯g-C3N4的光催化活性有明显提高,其中5%CeO2/g-C3N4显示最优的催化活性,反应速率是g-C3N4的2.6倍,分别比单独的光催化体系和非均相芬顿体系的去除率提高了61%和72%。上述结果说明,光催化技术和非均相芬顿技术之间存在协同效应。
3) CeO2/g-C3N4降解DC可能的反应机理为:光催化促进了类芬顿反应中Ce4+和Ce3+的循环,也提高光生电子-空穴分离效率。循环实验结果表明,CeO2/g-C3N4具有很好的重复利用性。
-
[1] RUAN X.,JIN X.,YANG Z.,et al.Photodegradation of tri (chloropropyl) phosphate solution by UV/O3.Water Air Soil Pollution,2014,225(8):1-9 [2] TAKAHASHI N.,HIBINO T.,TORII H.,et al.Evaluation of O3/UV and O3/H2O2 as practical advanced oxidation processes for degradation of 1,4-Dioxane.Ozone Science & Engineering,2013,35(5):331-337 [3] PATTERSON C.L.,CADENA F.,SINHA R.,et al.Field treatment of MTBE-contaminated groundwater using ozone/UV oxidation.Groundwater Monitoring Remediation,2013,33(2):44-52 [4] TOMOVA D.,ILIEV V.,RAKOVSKY S.,et al.Photocatalytic oxidation of 2,4,6-trinitrotoluene in the presence of ozone under irradiation with UV and visible light.Journal of Photochemistry and Photobiology A:Chemistry,2012,231(1):1-8 [5] XU B.,CHEN Z.,QI F.,et al.Comparison of N-nitrosodiethylamine degradation in water by UV irradiation and UV/O3:Efficiency,product and mechanism.Journal of Hazardous Materials,2010,179(1/2/3):976-982 [6] SHANG N.,CHEN Y.,MA H.,et al.Oxidation of methyl methacrylate from semiconductor wastewater by O3 and O3/UV processes.Journal of Hazardous Materials,2007,147(1/2):307-312 [7] GUROL M.D.,AKATA A.Kinetics of ozone photolysis in aqueous solution.AIChE Journal,1996,42(11):3283-3292 [8] GAROMA T.,GUROL M.D.Modeling aqueous ozone/UV process using oxalic acid as probe chemical.Environmental Science & Technology,2005,39(20):7964-7969 [9] STAEHELIN J.,HOIGNE J.Decomposition of ozone in water:Rate of initiation by hydroxide ions and hydrogen peroxide.Environmental Science & Technology,1982,16(10):676-681 [10] BAXENDALE J.H.,WILSON J.A.The photolysis of hydrogen peroxide at high light intensities.Transactions of the Faraday Society,1957,53:344-356 [11] SAUER M.C.,BROWN W.G.,HART E.J.O(3P) atom formation by the photolysis of hydrogen peroxide in alkaline aqueous solutions.Journal of Physical Chemistry,1984,88(7):1398-1400 [12] BHLER R.E.,STAEHELIN J.,HOIGN J.Ozone decomposition in water studied by pulseradiolysis.1.HO2/O2- and HO3/O3- as intermediates.Journal of Physical Chemistry,1984,88(12):2560-2564 [13] SEHESTED K.,HOLCMAN J.,BJERGBAKKE E.,et al. Formation of ozone in the reaction of hydroxyl with O3- and the decay of the ozonide ion radical at pH 10-13.Journal of Physical Chemistry,1984,88(2):269-273 [14] SEHESTED K.,HOLCMAN J.,BJERGBAKKE E.,et al.A pulse radiolytic study of the reaction OH+O3 in aqueousmedium.Journal of Physical Chemistry,1984,88(18):4144-4147 [15] BIELSKI B.H.J.,CABELLI D.E.,ARUDI R.L.,et al.Reactivity of HO2/O2- radicals in aqueous solution.Journal of Physical and Chemical Reference Data,1985,14(4):1041-1100 [16] CHRISTENSEN H.,SEHESTED K.,CORFITZEN H.Reactions of hydroxyl radicals with hydrogenperoxide at ambient and elevatedtemperatures.Journal of Physical Chemistry,1982,86(9):1588-1590 [17] BUXTON G.V.,GREENSTOCK C.L.,HELMAN W.P.,et al.Criticalreview of rate constants for reactions of hydrated electrons,hydrogenatoms and hydroxyl radicals (OH/O-) in aqueoussolution.Journal of Physical and Chemical Reference Data,1988,17(2):513-886 [18] LIDE D.R.CRC Handbook of Chemistry and Physics.Boca Raton,FL:CRC Press,2002 [19] DRAGANIĆ Z.D.,NEGRÓN-MENDOZA A.,SEHESTED K.,et al.Radiolysis of aqueoussolutions of ammonium bicarbonate over a large dose range.Radiation Physics and Chemistry,1991,38(3):317-321 [20] SEHESTED K.,RASMUSSEN O.L.,FRICKE H.Rate constants of OH with HO2,O2-,and H2O2+ from hydrogen peroxide formation in pulse-irradiated oxygenated water.The Journal of Physical Chemistry,1968,72(2):626-631 [21] ERIKSEN T.E.,LIND J., MERENYI G.On the acid-base equilibrium of the carbonate radical.Radiation Physics and Chemistry,1985,26(2):197-199 [22] BADER H.,HOIGNE J.Determination of ozone in water by the indigo method.Water Research,1981,15(4):449-456 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 1832
- HTML全文浏览数: 1428
- PDF下载数: 433
- 施引文献: 0



 下载:
下载: