Fe2+活化过硫酸氢钾复合盐降解吲哚美辛
Degradation of indomethacin in aqueous by peroxymonosulfate activated by ferrous ion
-
摘要: 通过亚铁离子活化过硫酸氢钾复合盐产生氧化性的硫酸根自由基,以吲哚美辛(IM)为目标污染物,研究了不同亚铁离子浓度和过硫酸氢钾浓度,以及加入Cl-离子和腐殖酸对吲哚美辛降解情况的影响。结果表明,[IM]:[PMS]:[Fe2+]=1:2:2条件下,IM的去除率接近100%;低浓度的Cl-抑制吲哚美辛的降解,高浓度则为促进作用;而腐殖酸都在不同程度上抑制了吲哚美辛的降解。经淬灭实验表明,亚铁离子活化过硫酸氢钾降解吲哚美辛中起主要作用的自由基是SO4-·。该方法能在短时间内高效降解吲哚美辛,为实际废水中吲哚美辛去除提供参考。Abstract: The degradation of indomethacin (IM) by the ferrous-ion-activated sodium peroxymonosulfate (PMS) was investigated.This process is based on the generation of sulfate radicals,which are powerful oxidizing species in aqueous phase.The effects of varying concentrations of Fe2+ and PMS and the addition of Cl- and humic acid (HA) on the degradation efficiencies of IM were studied.The results showed that the removal of IM was close to 100% at the molar ratio of [IM]:[PMS]:[Fe2+]=1:2:2.A low concentration of Cl- had a negative effect and a high concentration of Cl- had a positive effect on the degradation of IM,whereas HA had a negative effect.The results of quenching experiments revealed that SO4-· was the major active radical that contributed to the degradation of IM by ferrous-ion-activated peroxymonosulfate.This method could efficiently degrade IM in a short time,which could help in the treatment of actual wastewater contaminated by IM.
-
Key words:
- indomemthacin /
- ferrous ions /
- peroxymonosulfate /
- SO4-· /
- Cl-
-
镉(Cadmium, Cd)是珠三角地区农田土壤重金属污染的主要元素之一,给农产品及环境安全带来较大威胁[1-2]。以种植超累积植物的方式修复土壤重金属污染可有效保持农田土壤的生态结构和功能,但在实际应用中往往受到土壤理化性质、重金属赋存形态等因素的影响,导致超累积植物生长缓慢、生物量小、对土壤重金属的修复效率低等问题[3-4]。通过将超累积植物与根际促生菌联用,借助根际促生菌与植物间的相互作用,促进植物生长,提高土壤矿质元素的生物有效性[5-7],进而提高植物修复效率的研究已成为近年来的热点 [8-12]。
龙葵是一种能生长在受镉污染严重的矿区,为生长速度快,且地上部分生物量大的镉超累积植物,已被广泛应用于土壤的植物修复研究中。阴沟肠杆菌(Enterobacter cloacae)是本课题组前期从根系土壤筛选并鉴定的具有产铁载体功能,可促进植物生长的根际促生菌。因此,从理论上讲,将龙葵与阴沟肠杆菌联用可大幅提高龙葵对镉污染农田土壤的修复效率。然而,受根际土壤环境多方面的影响,这种联用技术在实际应用中尚存在菌株定殖效果差、生态位竞争力不足的局限性[13-14]。通过液体接种到植物根际土壤菌株的存活能力往往较低,从而影响植物修复效率。此外,修复过程中需要定期接种菌液,亦会给农业生产带来不便[15]。
根际促生菌的封装技术是提高其在植物根际土壤中存活能力,并增强植物修复效率的有效方法[16]。海藻酸盐与钙盐发生交联反应形成的化合物对细菌无毒害作用,具有良好的生物相容性,且能被土壤环境自然降解,因而适用于微生物载体材料[17]。淀粉和乳清能提高细菌在干燥和低温环境下的活性[17],是固体菌剂中提高根际促生菌复苏能力和保存时间的有效添加成分。此外,植物对钙、锌、锰和铁4种金属元素的吸收会影响其自身生长,并参与植物富集和转运镉的生理过程。
本研究选用海藻酸钠、氯化钙、黑麦粉、甜乳清粉为载体材料,阴沟肠杆菌为菌株[14],制备包裹型根际促生菌剂Z16,通过盆栽实验,研究对比菌液和菌剂对龙葵提取土壤重金属镉的影响,并分析该菌剂对龙葵吸收钙、锰、锌和铁这4种金属元素的影响,进一步证实菌剂的作用效果,以期为强化超累积植物-根际促生菌联用技术对镉污染农田的植物修复效率提供参考。
1. 材料和方法
1.1 材料
龙葵种子购自中国科学院沈阳应用生态研究所。阴沟肠杆菌(Enterobacter cloacae, EC)是由课题组前期通过磺酸盐(chrome azurol sulphonate,CAS)筛选培养基从镉污染土壤种植的龙葵根系土中筛选所得的一株具有产铁载体能力的根际促生菌[14],保藏于实验室−80 ℃冰箱。
盆栽土壤采自广东省韶关市大宝山附近农田土壤。样品去除异物后放置温室风干、磨碎和混匀,过 4 mm 筛网。土壤阳离子交换量(cation exchange capacity,CEC)的测定按照《土壤 阳离子交换量的测定 三氯化六氨合钴浸提-分光光度法(HJ 889-2017)》标准执行。土壤理化性质:pH 6.33;总有机碳(total organic carbon,TOC) 29.51 g·kg−1;有效磷 22.40 mg·kg−1;CEC 98.81 cmol+·kg−1;总Cd含量 0.91 mg·kg−1;二乙烯三胺五乙酸提取态Cd 0.43 mg·kg−1;Tessier法[18]提取碳酸盐结合态Cd 0.28 mg·kg−1。
LB 培养基配方:胰蛋白胨(Tryptone)10 g·L−1;酵母提取物(Yeast extract)5 g·L−1;氯化钠(NaCl) 10 g·L−1;pH 7.0~7.5。
菌剂材料:海藻酸钠(Sodium alginate) 质量分数为2.5%,氯化钙(CaCl2)0.1 mol·L−1,黑全麦粉质量分数为7.5%,甜乳清粉质量分数为2%,菌液EC 108 CFU·mL−1。
1.2 实验方法
1) 包裹型菌剂的制备与SEM观察。将EC菌从斜面培养基接种到LB液体培养基中,在28 ℃、150 r·min−1摇床条件下培养14~18 h,以转速4 000 r·min−1离心10 min后收集菌体,用无菌水等体积重悬备用(菌液浓度为108 CFU·mL−1)。称取2.5 g的海藻酸钠和7.5 g的黑麦粉放置于100 mL无菌水溶剂搅拌混合,在高温灭菌、冷却后加入2 g的甜乳清粉。将黏稠的溶液与EC菌液按1∶1的比例搅拌混合。利用滴管吸取适量混合溶液,缓慢滴入0.1 mol·L−1的氯化钙溶液。海藻酸钠和氯化钙发生交联反应形成胶粒状固体小球,过滤后在−50 ℃条件下进行真空冷冻干燥2 d,得到干燥的包裹型菌剂待用。将干燥的包裹型菌剂放置于铜台上,利用导电胶固定,干燥喷镀后进行扫描电镜(scanning electron microscope, SEM)观察其外部。使用灭菌刀片侧切颗粒菌剂后,放置于铜台上,利用导电胶固定,干燥喷镀后观察其内部。称取适量菌剂于无菌蒸馏水中,在28 ℃、150 r·min−1 摇床条件下孵育 48 h。孵育期间,每间隔6 h取出,吸取少量菌液并稀释梯度倍数,涂布平板。最后放置在28 ℃培养箱培养14~18 h,取出计数。
2) 盆栽实验。盆栽实验在温室内进行,温度控制在(30±2)℃。采用直径为18 cm的塑料花盆。每个花盆装有1.5 kg土壤。调节盆中持水量约80%。盆栽设计:共设菌剂Z16组、加菌EC组和对照CK组3个处理,每个处理3个平行。菌液通过灌根的方式加入6 mL。每隔30 d补充1次,共补充2次。菌剂通过与根周土壤混合的方式加入6 g,间隔30 d补充1次,共补充2次。龙葵生长60 d后,分别收集龙葵地上部茎叶、地下部根系和根系土壤,测量龙葵株高,并称量地上部茎叶和地下部根系鲜重。随后,将茎叶和根放入105 ℃烘箱内杀青30 min,然后在60 ℃条件下恒温烘干,称量地上部茎叶和地下部根系干重。将烘干的地上部茎叶和地下部根系粉碎,称取0.1 g茎叶和根,分别加8 mL硝酸进行微波消解,经0.45 μm过滤定容。根系土壤放于温室自然风干,采用Tessier法提取碳酸盐结合态Cd。用石墨原子吸收分光光度计测定消解茎叶、根及提取液中镉浓度,使用火焰原子吸收分光光度计测定消解茎叶和根部的钙、锌、锰和铁元素的浓度,并根据仪器检测浓度与最终定容体积计算含量。
3) 数据统计与分析。每个处理组设置3个平行,使用Excel对数据进行计算整理,通过IBM SPSS Statistics软件Duncan 新复极差法,在0.05水平上分析样品间差异的显著性(p<0.05),使用Origin软件作图。
Cd富集总量、Cd富集系数和Cd转运系数依次按式(1)~(3)计算。
A地上部=C地上部×M地上部,A根部=C根部×M根部 (1) BCF地上部=C地上部C土,BCF根部=C根部C土 (2) TF=C地上部C根部 (3) 式中:A为龙葵Cd富集总量,µg;BCF为Cd富集系数;TF为Cd转运系数;C地上部为地上部Cd富集质量分数,mg·kg−1;C根部为根部Cd富集质量分数,mg·kg−1;M地上部为龙葵地上部干重,g;M根部为龙葵根部干重,g;C土为土壤总Cd质量分数,mg·kg−1。
2. 结果与分析
2.1 包裹型菌剂的外观形状和活性
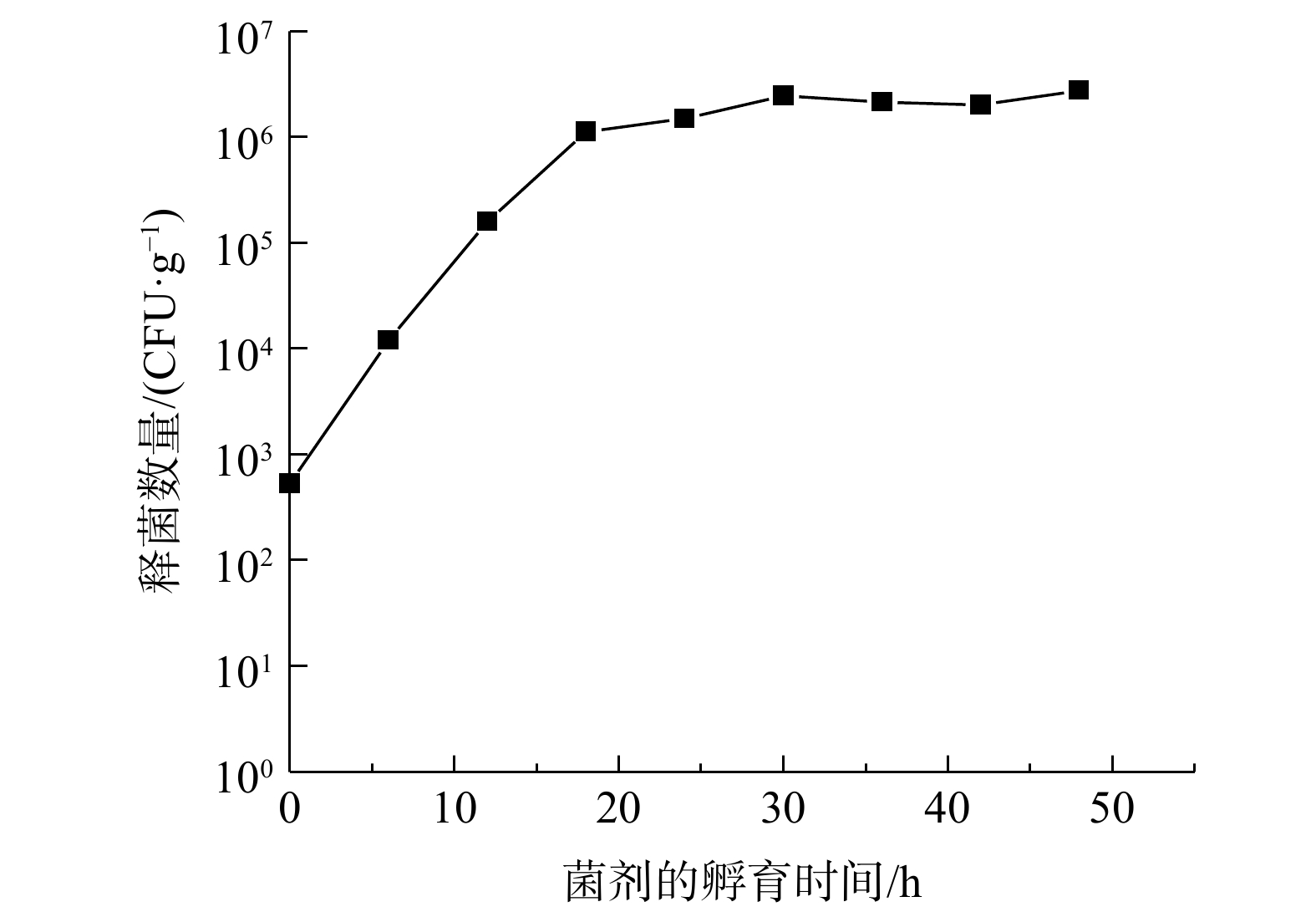
海藻酸钠与氯化钙交联形成稳定性较好的胶囊,从而为菌株提供保护屏障。适当的黑麦和甜乳清粉为菌株的生存提供营养。如图1(a)和(b)所示,包裹型菌剂Z16呈现胶囊状小球颗粒,粒径为2~4 mm。图1(c)和(e)扫描电镜观测结果表明,菌剂的外表面粗糙,带有褶皱,菌株可黏附并密集分布。图1(d)和(f)表明,菌剂内部结构形成不同大小空间的腔室,这种特殊的内部结构为大部分菌株的生存提供了物理空间。内部菌株分泌胞外聚合物(extracellular polymeric substances, EPS)填充腔体内部,成熟的生物膜已形成。菌剂Z16的活性如图2所示。包裹型菌剂在孵育24 h后,释菌数量达到106 CFU·g−1,短时间内形成较高的菌液浓度,具备良好的活性。
2.2 盆栽实验结果
温室种植盆栽在经过生长60 d的种植期后收获。不同处理组龙葵的株高和鲜重如图3所示。其中,加菌的EC和Z16组龙葵株高显著高于不加菌的CK组(p<0.05),分别提高了29.90%和33.44%;而接种菌液EC和菌剂Z16的2个处理组的龙葵株高无显著差异(p>0.05)。接种菌剂Z16和接种EC菌对龙葵地上部和根部鲜重的影响不同,且都显著高于对照组CK(p<0.05)。与对照组CK相比,接种菌液EC和菌剂Z16的龙葵地上部鲜重显著增加(p<0.05),分别提高了54.73%和85.39%。与接种菌液EC相比,接种菌剂Z16可进一步提高龙葵地上部鲜重的19.82%。另外,接种菌液EC和菌剂Z16的龙葵根部鲜重均较CK组显著增加(p<0.05),分别提高了92.31%和76.92%,而菌液EC和菌剂Z16组的根部的鲜重上并没有显著差异(p>0.05)。
不同处理下龙葵对土壤Cd 的富集能力如表1所示。与接种菌液EC组相比,接种菌剂Z16可进一步提高龙葵对Cd的富集质量分数及总量(p<0.05)。菌剂Z16地上部及根部的Cd富集质量分数分别提高了20.98%和168.97%;菌剂Z16地上部和根部Cd富集质量分数分别提高了28.09%和192.02%。
表 1 龙葵的Cd富集能力Table 1. Cd bioconcentration capacity of Solanum nigrum L.处理组 Cd富集质量分数/(mg·kg-1) Cd富集总量/µg 地上部 根部 地上部 根部 CK 4.68±0.02a 3.38±0.29a 2.57±0.17a 0.09±0.01a EC 4.48±0.13a 3.31±0.05a 4.09±0.11b 0.19±0.02b Z16 5.42±0.14b 8.91±0.02b 5.24±0.32c 0.55±0.07c 注:不同字母代表不同处理组差异显著(p<0.05)。 表2显示,与对照组CK相比,接种菌液EC的富集系数并未产生显著变化(p>0.05),而菌剂Z16则显著提高了龙葵地上部和根部的Cd富集系数(p<0.05),分别为15.92%和163.43%。与对照组CK相比,接种菌液EC的龙葵Cd转运系数并未产生显著变化(p>0.05),而接种菌剂Z16则显著降低了龙葵Cd转运系数(p<0.05),降低了56.23%,且其对镉的富集主要集中在龙葵根部。另外,与对照组CK相比,接种菌液EC的龙葵根际土壤难溶性碳酸盐结合态Cd未产生显著变化(p>0.05),而菌剂Z16可显著降低龙葵根际土壤中碳酸盐结合态Cd的浓度(p<0.05),降低了13.89%。
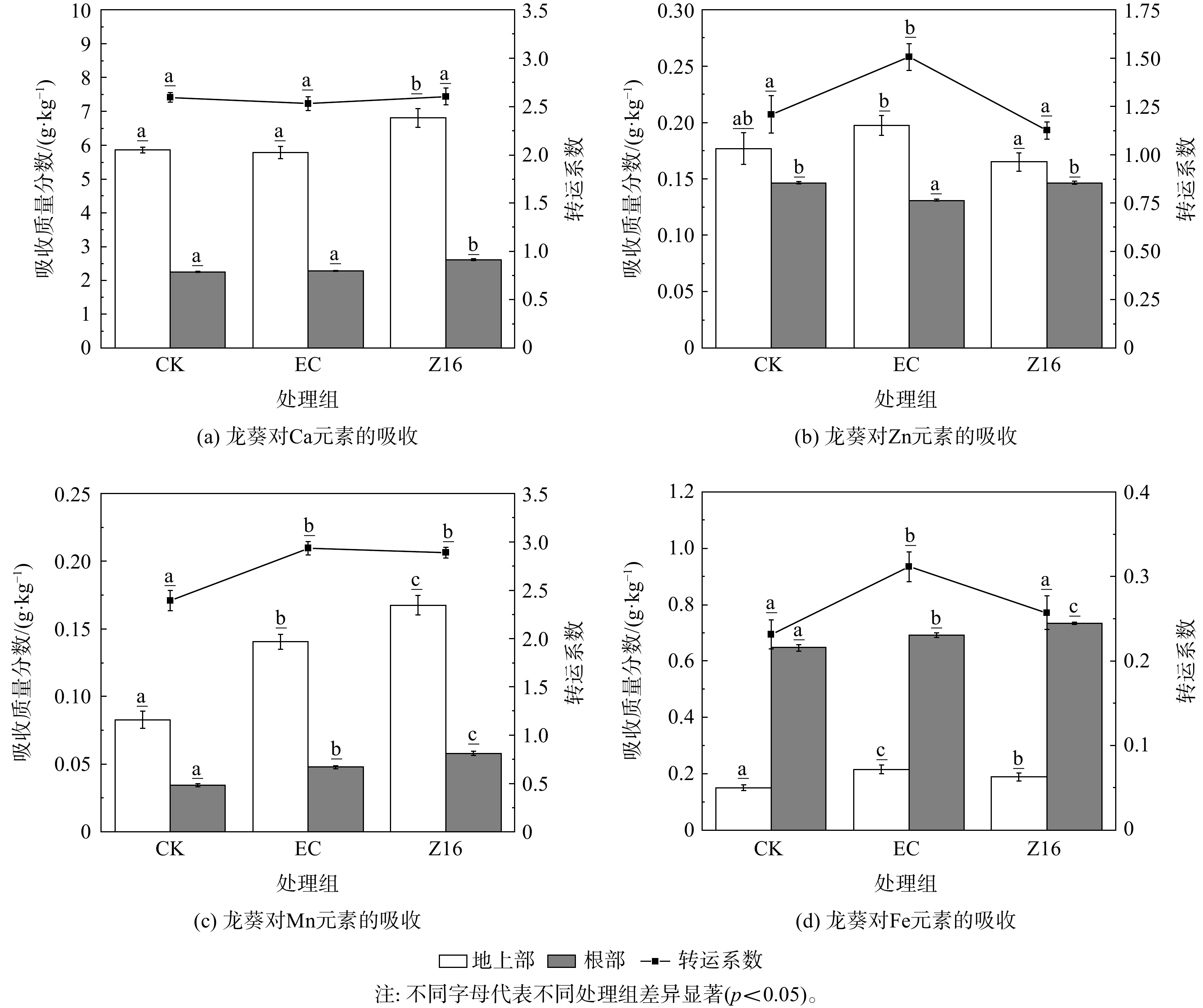
表 2 龙葵的Cd富集和转运系数Table 2. Cd bioconcentration and translocation factor of Solanum nigrum L.处理组 碳酸盐结合态Cd质量分数/(mg·kg−1) 富集系数BCF 转运系数TF 根际土 地上部 根部 CK 0.252±0.006b 5.16±0.02a 3.73±0.32a 1.39±0.12b EC 0.247±0.011b 4.95±0.15a 3.66±0.05a 1.35±0.04b Z16 0.217±0.003a 5.98±0.16b 9.84±0.02b 0.61±0.02a 注:不同字母代表不同处理组差异显著(p<0.05)。 植物生长所必须的金属元素钙、锌、锰和铁在龙葵地上和根部的浓度分布及转运系数如图4所示。不同处理组龙葵地上部和根部对Ca、Zn、Mn和Fe 4种元素的吸收和转运有较大差异。与对照CK相比,接种菌液EC并未提高龙葵地上部对Ca、Zn的吸收量(p>0.05),但可显著提高地上部分对Fe和Mn的吸收量(p<0.05)。与接种菌液EC相比,接种菌剂Z16可明显提高龙葵地上部和根部对Ca和Mn元素的吸收量(p<0.05)。其中,地上部分别提高了17.80%和19.29%;根部分别提高了14.35%和20.83%,但降低了地上部对Zn元素的吸收量。虽然与对照CK处理相比,接种菌剂Z16使龙葵地上部分和根部对Fe元素的吸收量分别提高了26%和13.45%,但接种菌剂Z16对龙葵地上部Fe元素的吸收量较接种菌液EC却降低了12.5%,而根部对Fe元素的吸收量较接种菌液EC略有增加(p<0.05),约增加了6.1%。另外,在龙葵对Ca、Zn、Mn和Fe的转运系数方面,与对照CK相比,接种菌液EC提高了龙葵Zn、Mn和Fe的转运系数(p<0.05),分别提高了24.67%、22.57和35.07%而对龙葵Ca的转运系数影响不大(p>0.05)。与对照CK相比,菌剂Z16将龙葵Mn的转运系数(p<0.05)提高了20.64%;但对Ca、Zn和Fe的转运系数则影响不大(p>0.05)。与接种菌液EC相比,接种菌剂Z16使龙葵Zn和Fe的转运系数有所降低(p<0.05),分别降低了25.23%和17.63%。
3. 讨论
包裹型菌剂Z16的外表面粗糙,多有褶皱,内部结构形成了不同大小的多腔室。这种特殊结构可为内部生存的菌株提供避免外部压力的物理屏障,可有效提高菌株的存活能力[19]。菌剂添加的黑麦与甜乳清粉可为菌株生长提供营养,延长保存菌剂的使用时间,还可缓解真空冷冻干燥对菌株细胞蛋白和膜的损伤,从而提高菌剂复苏过程的细胞活性[20-21]。海藻酸钠与氯化钙交联形成的胶囊颗粒具有缓释特性,使得菌株在根际土壤环境中能保持长时间的效用[22]。另外,菌剂内部的生物膜含有大量具有高持水能力的胞外聚合物(EPS),能改变土壤结构和孔径分布,在增加土壤保水量、减少土壤水分蒸发及增加植物蒸腾作用中发挥重要作用[23]。因此,在土壤中,使用菌剂较施用菌剂发挥的作用更长效。
盆栽结果表明,经菌剂Z16处理后的根际土壤难溶性碳酸盐结合态Cd的质量分数低于对照CK组和菌液EC组。这表明菌剂Z16对根际土壤难溶性碳酸盐结合态镉具有更强活化能力,促进了龙葵对镉的富集。根际促生菌通过分泌植物生长激素,可促进植物生长[14],使得接种菌液EC和菌剂Z16均能显著提高龙葵的株高和鲜重。相较接种菌液EC,菌剂Z16可进一步提高龙葵鲜重。其原因有两方面:一方面由于菌剂内部的多腔室结构为菌株生存提供生态位,提高了菌株在根际土壤中的竞争和存活能力;另一方面,经过土壤微生物的作用,菌剂丰富的有机成分可增加土壤肥力,为龙葵生长提供额外养分[24]。与对照CK组相比,接种菌剂EC和菌剂Z16均显著提高了龙葵对Cd的富集总量,菌液EC组对龙葵地上部和根部富集质量分数和富集系数影响较小,而菌剂Z16则显著提高了龙葵地上部和根部对土壤Cd的富集质量分数和地上部的Cd富集系数。根际促生菌分泌的螯合物质是影响植物根系对重金属富集的重要原因[25]。菌剂的缓释特性让菌株在较长时间内分泌更多螯合物质,从而提高了根际促生菌的存活能力,并促进龙葵对土壤Cd的富集。有研究者报道,可将生物炭作为微生物吸附载体制备固体菌剂[26],然而,生物炭对土壤重金属具有极强的物理化学吸附特性,阻碍了超累积植物对土壤中重金属的提取过程。
Mn和Fe是龙葵生长所需的微量金属元素,参与植物光合作用和细胞中的酶反应[27]。与对照组CK相比,接种菌液EC和菌剂Z16提高了龙葵对Mn和Fe金属元素的吸收。这是由于菌株分泌低分子有机酸物质活化了土壤难溶性Mn和Fe金属元素,而金属元素生物有效性的提高则促进了龙葵根系对土壤金属元素的吸收[28]。铁载体是对环境中铁(III)具有高度亲和力的低分子量化合物,其产出受到生物体周边环境的铁浓度调节[29]。在铁生物利用性较低的土壤环境中,菌剂处理提高了菌株产铁载体的数量,进而增强了龙葵对Fe元素吸收。另外,不同类型的铁载体将引起根际微生物间的铁资源竞争。在菌剂载体的保护下,菌株会分泌出更多抑制型铁载体,从而可提高其与其他根际微生物间的铁资源竞争力。这有利于构建对植物有益的根际土壤核心微生物组网络,以优化根际微生物群落结构,进而发挥更长效作用[30-32]。与对照组CK相比,菌剂Z16提高了龙葵地上部和根部对Ca元素的吸收,而菌液EC却无此作用。这可能与菌剂外表层的钙盐有一定关系,外源添加钙养分可能导致龙葵对Ca元素吸收能力的提高[33]。另外,有研究表明,外源添加低浓度的钙能提高龙葵对Cd的富集,这可能也是造成菌剂处理后的龙葵Cd富集量显著高于菌液处理的原因[34]。金属元素在植物细胞的膜转运过程中存在竞争关系[35],使得菌剂Z16在提高龙葵地上部对Mn和Cd的吸收的同时,降低了其对Fe和Zn的吸收。与接种菌液组的差异表明,尽管在菌剂作用下,龙葵的植物根部可吸收更多Zn和Fe,但转运系数的降低影响了龙葵地上部对这2种金属元素的吸收。
超累积植物富集重金属Cd的过程可分为3步:根系从土壤高效地吸收重金属;通过木质部将重金属转运到地上部;最后在枝叶细胞积累[36]。这一过程中的膜转运是影响土壤重金属元素进入植物细胞的关键因素。金属元素从土壤转运到植物的器官和组织需要不同类型的转运体,其中包括ZIP家族、Nramp家族及ATPases等[37-39];部分金属转运体参与多种金属元素的转运[40],如PEDAS等[41]发现HvIRT1参与Fe、Zn、Mn和Cd元素的转运,WU等[42]发现 HvNramp5参与Mn和Cd元素的金属转运。因此,龙葵地上部和根部对Cd富集与其对其他金属的吸收具有关联性。比如Cd通过其他金属的转运体进入龙葵体内,菌剂处理可能导致了龙葵体内与Mn、Zn、Fe等金属元素相关的转运体表达量增加,在这些微量金属元素被吸收的同时,Cd元素也通过这些转运体被龙葵富集。
4. 结论
1)利用海藻酸盐与钙盐交联形成的载体材料,与根际促生菌结合,研制出包裹型根际促生菌剂Z16。其内部的多腔室结构为菌株提供了生存空间,缓慢释放菌株于24 h孵育后。释菌浓度可达到106 CFU·g−1。与菌液EC相比,菌剂Z16通过增强龙葵对Ca、Mn和Fe 3种金属元素的吸收能力,能进一步促进龙葵生长。
2)与菌液EC相比,菌剂Z16通过持续释放菌株,可发挥更持久的活化镉及促进超累积植物提取镉的作用。根际土壤难溶性碳酸盐结合态镉的质量分数降低了12.15%;地上部和根部的Cd富集系数分别提高了20.98%和168.97%;地上部和根部的Cd富集总量分别提高了28.09%和192.02%。因此,微生物载体与根际促生菌发挥的协同作用可提高该菌株参与的根际促生菌-超累积植物联合修复效率。
-
[1] TERNES T.A.,MEISENHEIMER M.,MCDOWELL D.,et al.Removal of pharmaceuticals during drinking water treatment.Environmental Science & Technology,2002,36(17):3855-3863 [2] EDWARDS M.,TOPP E.,METCALFE C.D.,et al.Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field.Science of the Total Environment,2009,407(14):4220-4230 [3] YU J.T.,BOUWER E.J.,COELHAN M.Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent.Agricultural Water Management,2006,86(1/2):72-80 [4] LIN A.Y.C.,YU T.H.,LIN Chengfang.Pharmaceutical contamination in residential,industrial,and agricultural waste streams:Risk to aqueous environments in Taiwan.Chemosphere,2008,74(1):131-141 [5] RATOLA N.,CINCINELLI A.,ALVES A.,et al.Occurrence of organic microcontaminants in the wastewater treatment process.A mini review.Journal of Hazardous Materials,2012,239-240:1-18 [6] LIANG Chenju,BRUELL C.J.,MARLEY M.C.,et al.Persulfate oxidation for in situ remediation of TCE.I.Activated by ferrous ion with and without a persulfate-thiosulfate redox couple.Chemosphere,2004,55(9):1213-1223 [7] LIANG Chenju,BRUELL C.J.,MARLEY M.C.,et al.Persulfate oxidation for in situ remediation of TCE.II.Activated by chelated ferrous ion.Chemosphere,2004,55(9):1225-1233 [8] HORI H.,YAMAMOTO A.,HAYAKAWA E.,et al.Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant.Environmental Science & Technology,2005,39(7):2383-2388 [9] ANIPSITAKIS G.P.,DIONYSIOU D.D.Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt.Environmental Science & Technology,2003,37(20):4790-4797 [10] ANIPSITAKIS G.P.,DIONYSIOU D.D.Radical generation by the interaction of transition metals with common oxidants.Environmental Science & Technology,2004,38(13):3705-3712 [11] NETA P.,MADHAVAN V.,ZEMEL H.,et al.Rate constants and mechanism of reaction of SO4- with aromatic compounds.Journal of the American Chemical Society,1977,99(1):163-164 [12] RASTOGI A.,AL-ABED S.R.,DIONYSIOU D.D.Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems.Applied Catalysis B:Environmental,2009,85(3/4):171-179 [13] YUAN Ruixia,RAMJAUN S.N.,WANG Zhaohui,et al,Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process:Implications for formation of chlorinated aromatic compounds.Journal of Hazardous Materials,2011,196:173-179 [14] OUYANG Bin,FANG Haojie,ZHU Chengzhu,et al.Reactions between the SO4- radical and some common anions in atmospheric aqueous droplets.Journal of Environmental Sciences,2005,17(5):786-788 [15] YU Xiaoying,BAO Zhenchuan,BARKER J.R.Free radical reactions involving Cl·,Cl2-·,and SO4-· in the 248 nm photolysis of aqueous solutions containing S2O82- and Cl-.The Journal of Physical Chemistry A,2004,108(2):295-308 [16] AHMED M.M.,BARBATI S.,DOUMENQ P.,et al.Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination.Chemical Engineering Journal,2012,197:440-447 [17] WANG G.S.,KANG S.F.,YANG H.J.,et al.Removal of dissolved natural organic matter from source water with alum coagulation.Environmental Technology,2002,23(12):1415-1423 [18] HAYON E.,TREININ A.,WILF J.Electronic spectra,photochemistry,and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems.SO2-,SO3-,SO4-,and SO5- radicals.Journal of the American Chemical Society,1972,94(1):47-57 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 2093
- HTML全文浏览数: 1665
- PDF下载数: 486
- 施引文献: 0






 下载:
下载: