1 有毒藻华、藻毒素与气候变化(Toxic algal blooms, algal toxins and climate changes)
1.1 有毒藻华
有害藻华(harmful algal blooms, HABs)是指海洋中的浮游生物,如浮游微藻、原生动物或细菌等,在一定的环境条件下,短时间内异常增殖和聚集,引起水色变化的灾害性海洋生态现象[1]。HABs会导致海洋生态系统的严重破坏[2]。近20年来,近海HABs不断加剧,成为全球性的海洋环境问题。20世纪90年代以来,对HABs的研究开始强调其毒性和危害性,HABs中的有毒藻华越来越受到关注[3]。本文综述主要指的是海洋有毒微藻暴发性增殖形成的藻华。
甲藻和硅藻是形成有毒微藻藻华的主要藻种。世界各地已报道的4 000余种微藻中,能引发HABs的种类有337种[4]。其中,至少有100种可以产生并分泌藻毒素[5]。我国已有记录的海洋有毒微藻有17种[6-20](表1)。近年来,随着人类活动对近海环境污染的加剧,有毒藻华灾害频繁暴发。根据2016—2020年的中国海洋灾害公报统计,我国近海多次引发HABs的藻种以东海原甲藻(Prorocentrum donghaiense)和米氏凯伦藻(Karenia mikimotoi)等产毒甲藻为主(表2)。在2018年,米氏凯伦藻作为中国近海有毒藻华的主要优势种,引发有毒赤潮7次,累计面积214 km2[8]。有毒藻华不仅影响植食性鱼类和滤食性贝类等海产品品质,对海洋渔业造成经济损失,还可引发人类中毒事件,危害人类健康。
表1 中国近海已有记录产毒藻种统计
Table 1 Statistics of recorded toxigenic algae species in offshore China
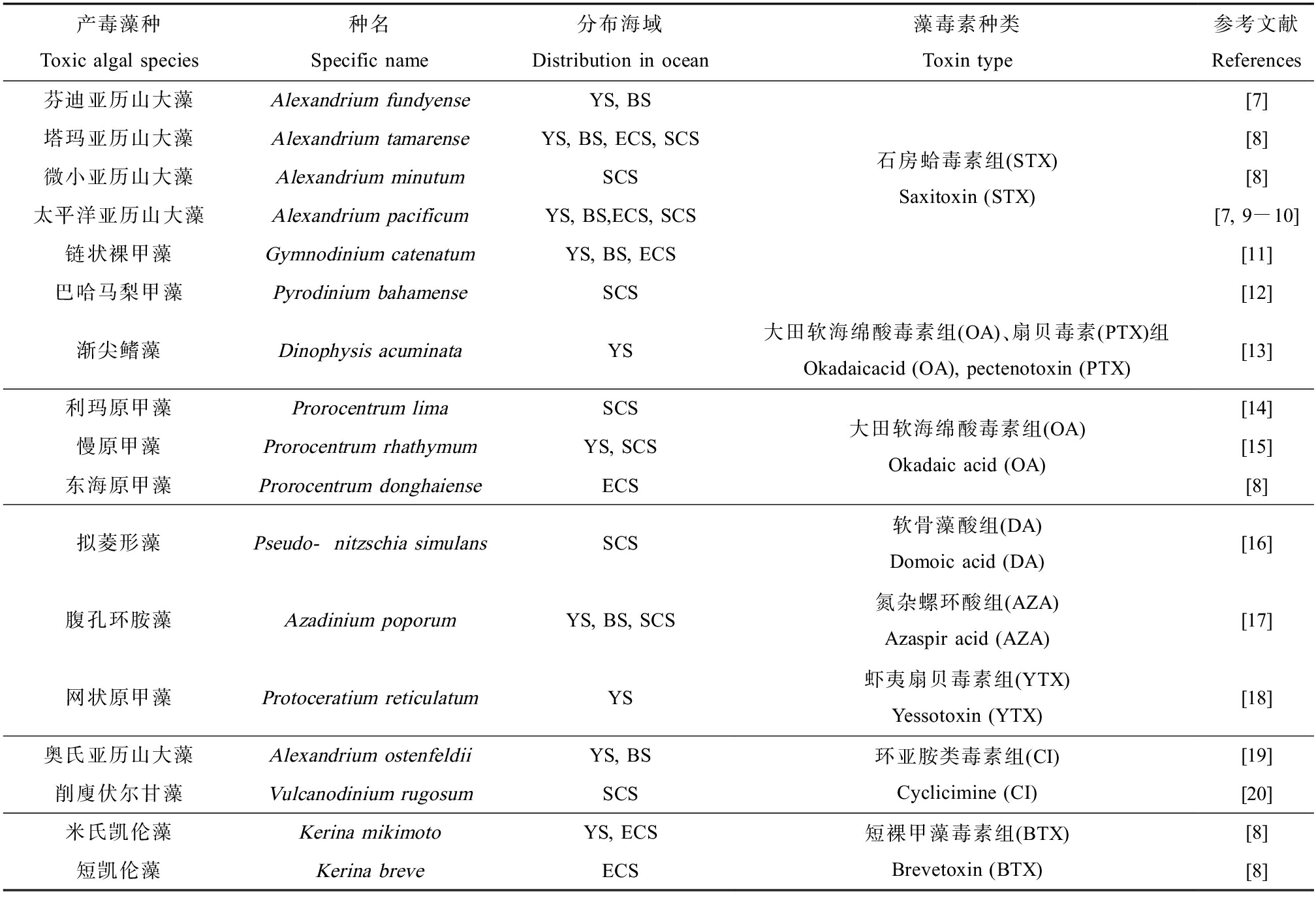
产毒藻种Toxic algal species种名Specific name分布海域Distribution in ocean藻毒素种类Toxin type参考文献References芬迪亚历山大藻Alexandrium fundyenseYS, BS塔玛亚历山大藻Alexandrium tamarenseYS, BS, ECS, SCS微小亚历山大藻Alexandrium minutumSCS太平洋亚历山大藻Alexandrium pacificumYS, BS,ECS, SCS链状裸甲藻Gymnodinium catenatumYS, BS, ECS巴哈马梨甲藻Pyrodinium bahamenseSCS石房蛤毒素组(STX)Saxitoxin (STX)[7][8][8][7, 9-10][11][12]渐尖鳍藻Dinophysis acuminataYS大田软海绵酸毒素组(OA)、扇贝毒素(PTX)组Okadaicacid (OA), pectenotoxin (PTX)[13]利玛原甲藻Prorocentrum limaSCS慢原甲藻Prorocentrum rhathymumYS, SCS东海原甲藻Prorocentrum donghaienseECS大田软海绵酸毒素组(OA)Okadaic acid (OA)[14][15][8]拟菱形藻Pseudo-nitzschia simulansSCS软骨藻酸组(DA)Domoic acid (DA)[16]腹孔环胺藻Azadinium poporumYS, BS, SCS氮杂螺环酸组(AZA)Azaspir acid (AZA)[17]网状原甲藻Protoceratium reticulatumYS虾夷扇贝毒素组(YTX)Yessotoxin (YTX)[18]奥氏亚历山大藻Alexandrium ostenfeldiiYS, BS削廋伏尔甘藻Vulcanodinium rugosumSCS环亚胺类毒素组(CI)Cyclicimine (CI)[19][20]米氏凯伦藻Kerina mikimotoYS, ECS短凯伦藻Kerina breveECS短裸甲藻毒素组(BTX)Brevetoxin (BTX)[8][8]
注:BS代表渤海,YS代表黄海,ECS代表东海,SCS代表南海。
Note:BS means the Bohai Sea, YS means the Yellow Sea, ECS means the East China Sea, and SCS means the South China Sea.
表2 2016—2020年中国近海有害藻华(HABs)优势种、藻华次数和累计受灾面积
Table 2 The main dominant species, event numbers and cumulative areas of harmful algal blooms (HABs) in coastal sea areas during 2016—2020 in China

年份Year有害藻华优势种数Numbers of HABs dominant species主要有害藻华优势生物Main HABs dominant species引发有害藻华次数Number of times累计受灾面积/km2Cumulative affected area/km2202014东海原甲藻 P. donghaiense101 215201916东海原甲藻 P. donghaiense121 251201818米氏凯伦藻 K. mikimotoi7214201734米氏凯伦藻 K. mikimotoi121 247201628东海原甲藻 P. donghaiense152 966
1.2 藻毒素
海洋有毒微藻产生的藻毒素各不相同且结构复杂。海洋藻毒素最初是按照引起人类中毒的症状以及毒素传递媒介被分为:麻痹性贝类毒素(paralytic shellfish poisoning toxins, PSTs)、腹泻性贝类毒素(diarrhetic shellfish toxins, DSTs)、神经性贝类毒素(neurotoxic shellfish toxins, NSTs)、记忆缺失性贝类毒素(amnesic shellfish toxins, ASP)、西加鱼毒素(ciguatera fish toxins, CFTs)和溶血性毒素(haemolytic toxins, HTs)等[21]。后来随着分析化学的发展,越来越多的海洋藻毒素结构被解析出来,2004年,联合国粮农组织、联合国政府间海洋学委会和世界健康组织,根据海洋藻毒素的化学结构,将其划分为八大类,包括氮杂螺环酸组(AZA)、短裸甲藻毒素组(BTX)、环亚胺类毒素组(CI)、大田软海绵酸毒素组(OA)、扇贝毒素组(PTX)、虾夷扇贝毒素组(YTX)、石房蛤毒素组(STX)和软骨藻酸组(DA)等[22]。这种分类方式较早前根据中毒症状对毒素的分类方法能更准确地将各种毒素进行归类比较,因此也逐渐成为目前使用更普遍的分类方式。
STX和DA是水溶性毒素[23]。STX分子式为C10H17N7O4,在高温和酸性条件溶液中稳定,是毒性最强的神经毒素之一,主要来源为膝沟藻属(Gonyaulax)和亚历山大藻属(Alexandrium)[24]。DA是一种非蛋白氨基酸,分子式为C15H21NO6,可导致记忆力丧失,主要来源于拟菱形藻属(Pseudonitzschia)[24],在近海被检出较多[23]。AZA、BTX、CI、OA、PTX和YTX均为脂溶性毒素,且除CI外均为聚醚类化合物。AZA的结构包含6,5,6-三螺环、一个环胺结构和羧基,主要来源为环胺藻属(Azadinium)[25]。OA分子式为C44H68O13,主要来源于鳍藻属(Dinophysis)和原甲藻属[24]。BTX是包含10~11个环状结构的大环聚醚类物质,主要由短裸甲藻(G. brevis)产生。PTX和YTX同为大环内酯聚醚毒素。PTX包含8个小环醚,产毒藻主要为鳍藻属[26]。YTX含有2个磺酰基,已报道的产毒藻有网状原甲藻、多边舌甲藻(Lingulodinium polyedrum)和具刺膝沟藻(G. spinifera)[27]。CI大多由海洋甲藻产生,也可以在贝类等生物中通过代谢作用形成[26]。脂溶性藻毒素在近海海水中及贝类样品中较易被检出,其中OA组和PTX组毒素分布最为广泛,在大西洋、太平洋及地中海沿岸多个国家近海均有检出[23]。
1.3 全球气候变化与CO2浓度升高
全球气候变化包括了温度、降水、营养盐、太阳辐射和温室气体浓度等诸多因素,这些因素对浮游植物的生命活动产生重要的影响,加剧近海海洋有毒微藻暴发性增殖带来的危害。降水的变化可能改变表层海水营养盐条件,其中磷(P)或氮(N)营养盐的限制可以显著影响海洋有毒微藻的细胞毒性[28-30]。温室气体的过度排放导致了温室效应,据模型预测,到21世纪末全球表层海水平均温度将会持续升高1~4 ℃[31]。海水温度升高可在特定海域促进部分产毒甲藻的短期快速增殖[32],使细胞毒素含量增加[33],增加有毒藻华形成的可能性,使低温下毒性更强的藻种引发的有毒藻华在纬度更高的海区出现[34-35],也可能使有毒藻华更快进入衰退期[28]。有研究预测温室效应引起的水温升高还可使上层海水更稳定,导致全球多处混合层变浅[36],这将使海洋有毒微藻收到的光照强度增加,也可促进其生长[37],影响有毒微藻合成毒素的浓度[38]。
CO2是主要的温室气体,在燃烧化石燃料、砍伐森林、工业化和水泥生产等过程中排放。CO2在大气中的浓度已从工业革命前的284 Pa又上升到2013年的400 Pa[31]。联合国政府间气候变化专门委员会(Intergovernmental Panel on Climate Change, IPCC)第5次评估报告预估,到21世纪末(2100年)大气CO2分压可能达到1 013 Pa[31]。海洋是地球表面最大的碳库,吸收了人类排放CO2总量的1/3[32]。随着海洋吸收CO2量的增加,海水pH也随之降低,引起海洋酸化。与19世纪70年代前相比,表层海水H+浓度已增加了32%,pH下降了0.1[34-35]。IPCC预测,到21世纪末表层海水pH将下降0.3~0.4[31]。Jiang等[39]预测,到21世纪末,海洋表层的溶解性无机碳将増加12%。同时,海水pH降低及CO2浓度增加将影响氮的氧化还原反应以及自养生物在调节氮循环中的生物反应,影响营养盐的形态和循环,进而影响海洋有毒藻华的发生[40-41]。环境CO2浓度的升高不仅会影响海洋有毒微藻光合作用效率、固碳和生长等,还可能促使某些微藻所产毒素的毒性增强,加剧有毒藻华暴发的危害,并和其他气候变化因素共同作用,影响海洋有毒微藻的生长和产毒。
2 环境CO2浓度升高对海洋有毒微藻生长及产毒的影响(Effect of elevated CO2 on growth and toxicity of marine toxic microalgae)
浮游植物是海洋生态系统的初级生产者,贡献了地球约50%的初级生产力[42]。包括海洋有毒微藻在内的浮游植物能够通过吸收CO2调节气候,在海洋和全球碳循环中起着举足轻重的作用。同时,环境CO2浓度升高也会引起海水碳酸盐系统的改变,使得海洋生物赖以生存的生境发生变化,反过来影响有毒微藻的生理、生长和新陈代谢过程。
2.1 环境CO2浓度升高对海洋有毒微藻生长的影响
海洋有毒微藻的生长除了受温度、营养盐、光照和盐度的影响外,还往往受到碳限制[43]。为克服碳限制,藻类利用其碳浓缩机制(CO2 concentrating mechanism, CCM),催化![]() 和CO2之间的相互转化。CO2浓度升高有助于缓解藻类的碳限制,改变它们的生长周期,促进初级生产力的提高,但这种影响会因藻种不同而存在明显差异[44],表现为对有毒微藻生长的促进或抑制,具有Ⅱ型核酮糖-1,5-二磷酸羧化酶(Rubisco酶)的甲藻对碳限制更敏感[45],因此高浓度的CO2能显著提高这些有毒甲藻的生长速度。已有研究表明,环境CO2浓度的提高,会增强某些甲藻,如强壮前沟藻(Amphidinium carterae)、塔玛亚历山大藻(A. tamarense)和网状原甲藻(P. reticulatum)等的Rubisco酶和碳酸酐酶(carbonic anhydrase, CA)的活性,进而促进光合作用[44]。然而,也有研究指出,高浓度CO2会降低藻类对溶解无机碳(DIC)的亲和力,下调CCM [46]。例如,假微型海链藻(Thalassiosira pseudonana)和中肋骨条藻(Skeletonema costatum)等在高浓度CO2环境下CA酶活性显著降低
和CO2之间的相互转化。CO2浓度升高有助于缓解藻类的碳限制,改变它们的生长周期,促进初级生产力的提高,但这种影响会因藻种不同而存在明显差异[44],表现为对有毒微藻生长的促进或抑制,具有Ⅱ型核酮糖-1,5-二磷酸羧化酶(Rubisco酶)的甲藻对碳限制更敏感[45],因此高浓度的CO2能显著提高这些有毒甲藻的生长速度。已有研究表明,环境CO2浓度的提高,会增强某些甲藻,如强壮前沟藻(Amphidinium carterae)、塔玛亚历山大藻(A. tamarense)和网状原甲藻(P. reticulatum)等的Rubisco酶和碳酸酐酶(carbonic anhydrase, CA)的活性,进而促进光合作用[44]。然而,也有研究指出,高浓度CO2会降低藻类对溶解无机碳(DIC)的亲和力,下调CCM [46]。例如,假微型海链藻(Thalassiosira pseudonana)和中肋骨条藻(Skeletonema costatum)等在高浓度CO2环境下CA酶活性显著降低![]() 向CO2的转化受到抑制,光合作用效率降低[38]。此外,环境CO2浓度升高也会影响藻类Rubisco酶的活性,影响氧化还原作用和电子传递作用,抑制或促进藻类的光呼吸,从而改变细胞的光合效率及呼吸效率,影响有毒微藻的生长[47-49]。部分有毒微藻在环境CO2升高条件下的生长情况如表3所示[44-45.47, 50-54]。
向CO2的转化受到抑制,光合作用效率降低[38]。此外,环境CO2浓度升高也会影响藻类Rubisco酶的活性,影响氧化还原作用和电子传递作用,抑制或促进藻类的光呼吸,从而改变细胞的光合效率及呼吸效率,影响有毒微藻的生长[47-49]。部分有毒微藻在环境CO2升高条件下的生长情况如表3所示[44-45.47, 50-54]。
表3 部分海洋有毒微藻在环境CO2升高条件下生长情况的变化
Table 3 Growth changes of some toxic microalgae under the condition of elevated CO2

有毒微藻种类Toxin microalgae species藻种分选源地Source of toxic algaeCO2胁迫条件(pCO2/Pa)CO2 stress condition (pCO2/Pa)促进或抑制生长Promote or inhibit growth参考文献References赤潮异弯藻Heterosigma akaahiwo中国长江口Yangtze Estuary, China1 013↑[50]中国秦皇岛Qinhuangdao, China1 013;2 026↑[51]微小亚历山大藻A.minutum中国东海East China Sea810;1 216↑[52]中国南海South China Sea810;1 216↑[45]塔玛亚历山大藻A. tamarense中国东海East China Sea1 013↑[53]北海(德国近岸区域)North Sea (German coastal area)810;1 216↓[44]东海原甲藻P. donghaiense中国近海China offshore810;1 520↓[47]米氏凯伦藻K. mikimotoi中国近海China offshore810;1 520↓[47]中国近海China offshore1 013;2 026↑[54]锥状斯氏藻Scrippsiella trochoidea北海(德国近岸区域)North Sea (German coastal area)810;1 216↓[44]
注:↑表示促进生长,↓表示抑制生长;pCO2为CO2浓度。
Note:↑ means promote toxin algae growth, and ↓ means inhibit toxin algae growth; pCO2 stands for concentration of CO2.
如前所述,海洋吸收大气中过量的CO2使海水pH下降,部分藻类因其生理结构或特性,可能对海水pH降低产生反应[55],如高CO2浓度和低pH会降低碳酸酐酶对溶解性无机碳的亲和力,抑制部分甲藻的生长[56]。另外,Brandenburg等[57]采用荟萃分析方法评估CO2富集对亚历山大藻生长和毒性的影响得出,与其他浮游植物物种相比,由CO2驱动的生长速度增加可能为有毒藻华物种带来额外的竞争优势。高浓度的环境CO2对不同藻类生长影响的差异,也可能改变生物的种间竞争关系,导致浮游植物群落物种组成改变、优势种更替。例如,米氏凯伦藻和盐生杜氏藻(Dunaliella salina)在共培养体系中,2种微藻的最大环境容纳量相对于单培养体系均受到显著(P<0.05)抑制,环境CO2升高使2种微藻的竞争关系表现为向有利于米氏凯伦藻的方向发展[54]。环境CO2的升高对海洋有毒微藻生长的促进作用使其能在更短的时间达到预警密度,或者其峰值密度更高,产生危害的相应增大。
2.2 环境CO2浓度升高对海洋有毒微藻产毒的影响
环境CO2浓度升高往往会影响海洋有毒微藻的新陈代谢,进而影响其代谢产物——藻毒素。多种海洋有毒微藻在环境CO2浓度升高条件下毒素产量增加。例如,Tatters等[55]发现CO2浓度升高刺激链式亚历山大藻(A. catenella)产生更多毒素。在环境CO2水平升高的条件下,从东海分离的塔玛亚历山大藻株的生长和细胞毒性均显著(P<0.05)增加[58]。Sun等[59]的研究表明高浓度CO2刺激多列拟菱形藻(Pseudo-nitzschia multiseries)产生更多的DA。胡顺鑫等[54]发现米氏凯伦藻在高浓度CO2环境中的无细胞过滤液对盐生杜氏藻有明显的抑制作用,据此推测高浓度的CO2使米式凯伦藻分泌的溶血性毒素等化感物质增多。Jin等[60]通过海洋酸化的中尺度宇宙实验(mesocosm experiment),发现海洋球石藻(Emiliania huxleyi)产生的有毒酚类化合物随着CO2浓度升高而明显增多。有研究报道比较了CO2限制和CO2饱和条件下短凯伦藻(K. breve)产毒情况,发现藻细胞在CO2饱和条件下产生的DA是CO2限制条件下的3倍~4倍[61]。也有研究发现,高CO2浓度可延长芬迪亚历山大藻(A. fundyense)的分裂周期,增加藻毒性[62]。当然,环境CO2浓度升高并不是对所有海洋有毒微藻的产毒都具有促进作用,也有一些藻种,如红褐藻(Chrysochromulina polylepis)、多列拟菱形藻等产生的毒素含量随着CO2增加而显著降低(P<0.05) [63]。
CO2浓度升高不仅影响海洋有毒微藻的毒素产量,同样也能影响其所产毒素组分。van de Waal等[64]的研究显示,环境CO2分压高达1 216 Pa时,塔玛亚历山大藻所产的毒素总量基本不变但毒性降低,因其麻痹性贝类毒素(PSP)向毒性更小的无磺基毒素转化。Fu等[65]证明了高CO2分压导致剧毒卡尔藻(Karlodinium veneficum)胞内毒素向毒性更强的衍生物转化。Kremp等[66]发现分离自波罗的海的亚历山大藻PSTs毒素组成随着CO2浓度的增加而改变,其中西加毒素(GTX)含量在不断增加。Pang等[58]的研究结果表明,与400 Pa相比,在环境CO2分压为1 013 Pa条件下我国东海的塔玛亚历山大藻虽然单细胞毒素产量减少了一半,但是由于细胞内毒性较强的GTX 1和GTX 4组分浓度的增加,单个藻细胞的毒性增强了近60%。表4总结了部分海洋有毒微藻在高浓度环境CO2条件下主要致毒组分的变化及毒性增减情况。
表4 部分海洋有毒微藻在高浓度环境CO2条件下主要致毒组分的变化及毒性增减情况
Table 4 Changes of major toxic components and the toxicity of some toxic microalgae under high concentrations of CO2
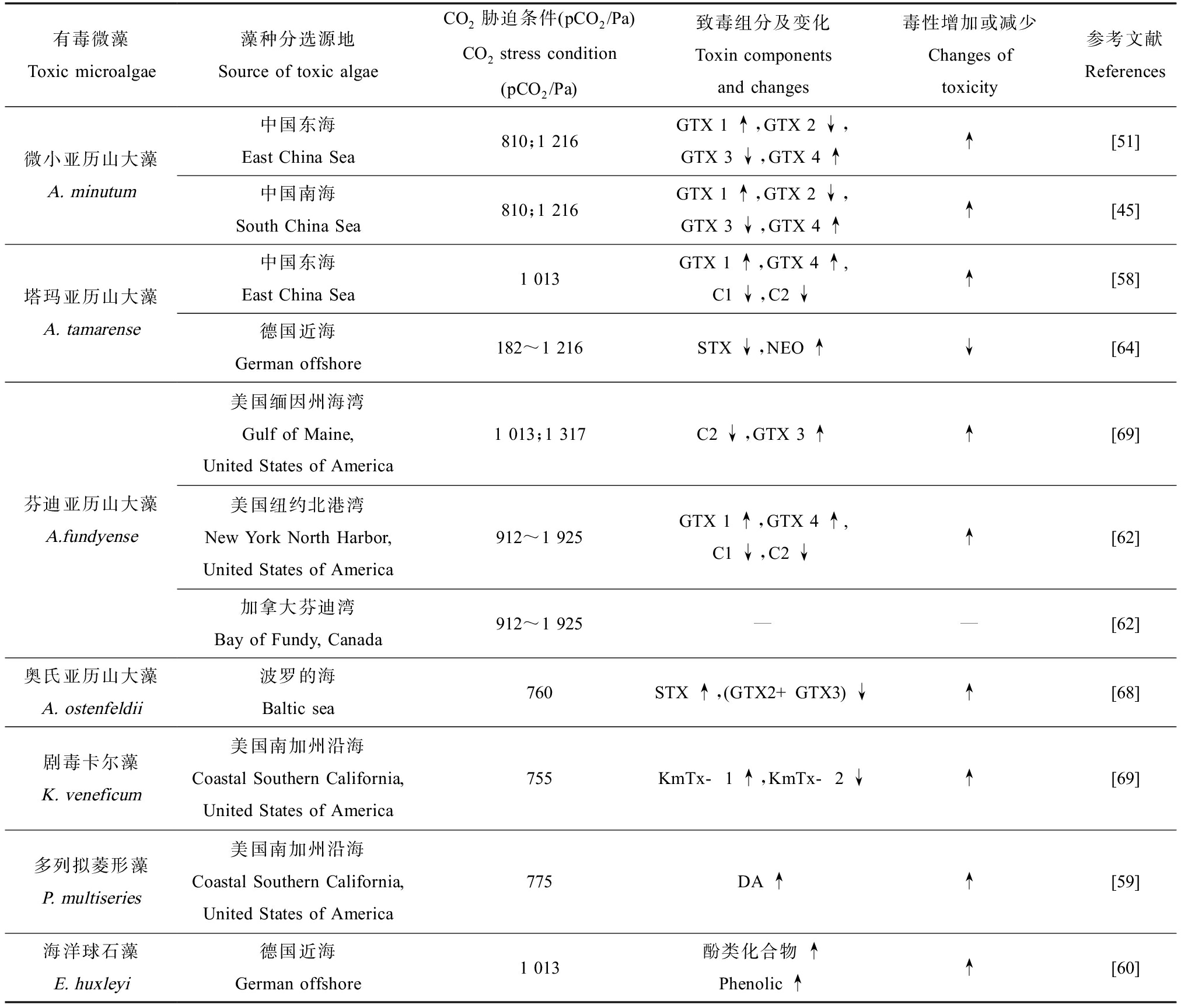
有毒微藻Toxic microalgae藻种分选源地Source of toxic algaeCO2胁迫条件(pCO2/Pa)CO2 stress condition (pCO2/Pa)致毒组分及变化Toxin components and changes毒性增加或减少Changes of toxicity参考文献References微小亚历山大藻A. minutum中国东海East China Sea810;1 216GTX 1 ↑,GTX 2 ↓,GTX 3 ↓,GTX 4 ↑↑[51]中国南海South China Sea810;1 216GTX 1 ↑,GTX 2 ↓,GTX 3 ↓,GTX 4 ↑↑[45]塔玛亚历山大藻A. tamarense中国东海East China Sea1 013GTX 1 ↑,GTX 4 ↑,C1 ↓,C2 ↓↑[58]德国近海German offshore182~1 216STX ↓,NEO ↑↓[64]芬迪亚历山大藻A.fundyense美国缅因州海湾Gulf of Maine, United States of America1 013;1 317C2 ↓,GTX 3 ↑↑[69]美国纽约北港湾New York North Harbor, United States of America912~1 925GTX 1 ↑,GTX 4 ↑,C1 ↓,C2 ↓↑[62]加拿大芬迪湾Bay of Fundy, Canada912~1 925——[62]奥氏亚历山大藻A. ostenfeldii波罗的海Baltic sea760STX ↑,(GTX2+GTX3) ↓↑[68]剧毒卡尔藻K. veneficum美国南加州沿海Coastal Southern California, United States of America755KmTx-1 ↑,KmTx-2 ↓↑[69]多列拟菱形藻P. multiseries美国南加州沿海Coastal Southern California, United States of America775DA ↑↑[59]海洋球石藻E. huxleyi德国近海German offshore1 013酚类化合物 ↑Phenolic ↑↑[60]
注:↑表示毒素含量增加,↓表示毒素含量减少,—表示无明显变化;pCO2为CO2浓度;GTX 1~4为膝沟藻毒素(Gonyautoxins),C1、C2为N-磺酰氨甲酰基类毒素(N-sulfocarbamoyl toxins),STX表示石房蛤毒素(saxitoxin),NEO表示新石房蛤毒素(neosaxitoxin),KmTx-1、KmTx-2表示卡尔藻毒素(karlotoxins),DA表示软骨藻酸(domoic acid)。
Note:↑ means an increase of toxin, ↓ means a decrease of toxin, and — means there is no significant change of toxin; pCO2 stands for concentration of CO2; GTX 1~4 stand for gonyautoxin 1~4, C1, C2 stand for N-sulfocarbamoyl toxins, STX stands for saxitoxin, NEO stands for neosaxitoxin, KmTx-1, KmTx-2 stand for karlotoxins and DA stands for domoic acid.
已有研究结果显示,不同海洋有毒微藻产毒对CO2浓度升高的响应不同,且同一藻种的不同地域株之间也有差异,如Hattenrath-Lehmann Theresa等[62]的研究结果显示,在较高水平的CO2分压下,分离自纽约北港湾的芬迪亚历山大藻总细胞毒性显著提高71%~81% (P<0.05),而分离自芬迪湾的芬迪亚历山大藻则无变化。也有研究发现分离自我国广东省珠江口海域的塔玛亚历山大藻,高浓度的环境CO2(1 114 Pa)可以减轻富营养化氮源对毒性的影响[67]。因此未来气候变化对有海洋有毒微藻产毒的影响可能需要结合不同海域的环境理化因素综合考虑和具体分析。
另外,早期有研究通过直接在培养体系内添加酸(HCl)或碱(NaOH)调节水体的pH,改变水体的CO2溶解饱和度,以分析CO2对海洋有毒微藻生长和产毒的影响[68-69]。但添加酸碱改变pH会影响毒素的手性性质,进而影响毒性,并不能真实反映环境CO2浓度变化对海洋有毒微藻生长和产毒的影响。现在更多的研究采用向培养环境中泵入不同CO2分压的气体或CO2饱和水的方式,使实验结果更具可比性[56,59]。
2.3 其他环境因子与CO2升高的协同作用
随着全球气候变化加剧,海水变暖、海洋酸化对有毒藻华的影响受到广泛关注,也有研究将环境CO2浓度与其他环境因素如营养盐、海水温度结合,探究它们对海洋有毒微藻生长和产毒的协同影响。例如在酸化环境中升高海水中N∶P的比例可促进微小亚历山大藻细胞毒素含量和毒性的增加,但不能促进其生长[70];在N限制和CO2升高的协同作用下,芬迪亚历山大藻的细胞毒性高于任一因素单独作用的结果[71-72];在硅酸盐限制条件下升高CO2水平会使拟菱形藻(P. fraudulenta)的细胞毒性显著增强(P<0.05) [55]。CO2浓度升高与海水温度升高能够协同作用,刺激铜绿微囊藻(Microcystis aeruginosa)的生长,但主要是通过提高铜绿微囊藻的生物量来影响总毒素含量,而单个细胞的产毒量并不随CO2浓度和温度升高而增加[73]。这也提示我们对多个影响因素的综合探究有助于进一步了解毒素变化的机制,并预估这些气候因素的耦合作用对海洋中有毒藻华在实际海区中暴发的条件、毒性和危害有怎样的影响。
目前探究环境CO2分压对有毒微藻生长及产毒的影响多为室内模拟实验,但考虑生物和非生物因素之间复杂的相互作用,已有研究通过设计更接近自然条件的中宇宙野外实验探究CO2分压升高及其他环境因素共同作用对一些浮游植物的影响。如Wohlrab等[74]在瑞典Gullmar海湾进行的中宇宙实验表明,高CO2分压的硅藻群落中软骨藻酸含量显著增加,这可能与群落结构向拟菱形藻的转变和/或拟菱形藻体内软骨藻酸产量的增加有关,且在营养有限的条件下,CO2分压水平升高会协同增加软骨藻酸的水平。目前针对有毒微藻的野外围隔实验研究还很有限,在未来的研究中应该着重开展此类方法的实验工作。
3 研究展望(Research prospect)
综上所述,环境CO2浓度的升高会影响海洋有毒微藻的生长和产毒,使有毒微藻暴发性增殖时的环境风险发生变化。尽管迄今已有大量的研究致力于揭示海洋酸化对海洋生物的影响,但由于海洋有毒微藻地域分布较广,且种类分支较多,因此目前的研究结果尚未获得较为统一的结论。未来研究气候变化对有海洋有毒微藻产毒的影响可结合不同海域的环境理化因素综合考虑,将这些研究结果进行比较和联系。
另外,气候变化对浮游植物尤其是海洋有毒微藻的影响研究,目前多为通过室内模拟某种气候变化条件进行实验研究,难以实现自然海区中,海水温度、海水pH、光照强度和营养盐等多种理化因素的变化,可能与实际情况存在差距,温室气体对气候变化背景下的海洋环境产生的复杂多元交互影响仍待继续探究。目前已有研究人员通过中宇宙野外实验体系加入CO2气体或饱和水的方式来模拟研究环境CO2升高对常见水生生态系统中无毒HAB微藻演替的影响。但由于海洋有毒微藻的产毒特性,对其开展野外实验时藻毒素进入海洋造成环境污染的安全隐患,以及如短裸甲藻毒素能形成气溶胶进而引发气喘、神经伤害等的安全问题,如何在安全保障的前提下开展有毒藻的中宇宙生态系统模拟实验也是亟待在未来进一步解决的技术瓶颈。
在应用的层面,已知多种气候因素对环境条件的改变,使海洋有毒微藻在浮游生物群落中相对于其他非产毒藻变得更具竞争力[57,67,74],增强了部分有毒微藻产生、分泌藻毒素,使得海洋环境中有毒藻华发生的可能性和危害性都将增加。因此,在实际藻华尤其是有毒藻华的生态监控,需考虑变化的环境因素下海洋有毒微藻的毒性变化和暴发性增殖后对海产品质量的影响,调整水体有毒微藻密度的监测指标。例如,在监测海区中有毒甲藻的生物多样性的同时,可结合海区生态环境中的CO2分压或pH、营养盐浓度和海水温度等指标,对环境CO2浓度升高(或表现为水体pH降低)条件下生长或产毒加强的有毒甲藻种类,在水体富营养化程度较高时,可增加关注度和监测频次,下调有毒藻华预警的藻密度;对于随季节温差较大的水体,可根据水体温度或按季节划分适当调整有毒藻华预警的藻密度阈值,以期加强对海洋有毒微藻的危害防控。
[1] Berdalet E, Kudela R, Urban E, et al. GlobalHAB: A new program to promote international research, observations, and modeling of harmful algal blooms in aquatic systems [J]. Oceanography, 2017, 30(1): 70-81
[2] 赵冬至, 赵玲, 张丰收. 我国海域赤潮灾害的类型、分布与变化趋势[J]. 海洋环境科学, 2003, 22(3): 7-11
Zhao D Z, Zhao L, Zhang F S. Type of formation, distribution and temporal trend of red tides occurred in the China Sea [J]. Marine Environmental Science, 2003, 22(3): 7-11 (in Chinese)
[3] 于仁成, 吕颂辉, 齐雨藻, 等. 中国近海有害藻华研究现状与展望[J]. 海洋与湖沼, 2020, 51(4): 768-788
Yu R C, Lv S H, Qi Y Z, et al. Progress and perspectives of harmful algal bloom studies in China [J]. Oceanologia et Limnologia Sinica, 2020, 51(4): 768-788 (in Chinese)
[4] Smayda T. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea [J]. Limnology and Oceanography, 1997, 42(5): 1137-1153
[5] Visciano P, Schirone M, Berti M, et al. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods [J]. Frontiers in Microbiology, 2016, 7: 1051
[6] Lu D, Qi Y, Gu H, et al. Causative species of harmful algal blooms in Chinese coastal waters [J]. Algological Studies International Journal of Phycological Research, 2014, 145: 145-168
[7] Gao Y, Yu R C, Chen J H, et al. Distribution of Alexandrium fundyense and A. pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea [J]. Marine Pollution Bulletin, 2015, 96(1-2): 210-219
[8] 国家海洋局. 中国海洋灾害公报[R]. 北京: 国家海洋局, 2020
[9] Genovesi B, Berrebi P, Nagai S, et al. Geographic structure evidenced in the toxic dinoflagellate Alexandrium pacificum Litaker (A. catenella - group Ⅳ (Whedon &Kofoid) Balech) along Japanese and Chinese coastal waters [J]. Marine Pollution Bulletin, 2015, 98(1-2): 95-105
[10] Gao Y, Yu R C, Murray S A, et al. High specificity of a quantitative PCR assay targeting a saxitoxin gene for monitoring toxic algae associated with paralytic shellfish toxins in the Yellow Sea [J]. Applied and Environmental Microbiology, 2015, 81(20): 6973-6981
[11] Gu H F, Liu T T, Vale P, et al. Morphology, phylogeny and toxin profiles of Gymnodinium inusitatum sp. nov., Gymnodinium catenatum and Gymnodinium microreticulatum (Dinophyceae) from the Yellow Sea, China [J]. Harmful Algae, 2013, 28: 97-107
[12] Usup G, Ahmad A, Matsuoka K, et al. Biology, ecology and bloom dynamics of the toxic marine dinoflagellate Pyrodinium bahamense [J]. Harmful Algae, 2012, 14: 301-312
[13] 罗璇, 于仁成, 周名江. 应用LC-MS联用方法分析青岛近海渐尖鳍藻(Dinophysis acuminata)细胞中的毒素成分[J]. 海洋环境科学, 2014, 33(5): 781-787
Luo X, Yu R C, Zhou M J. Analysis of toxins in cells of Dinophysis acuminata collected from the coastal waters of Qingdao with a LC-MS method [J]. Marine Environmental Science, 2014, 33(5): 781-787 (in Chinese)
[14] Luo Z H, Zhang H, Krock B, et al. Morphology, molecular phylogeny and okadaic acid production of epibenthic Prorocentrum (Dinophyceae) species from the northern South China Sea [J]. Algal Research, 2017, 22: 14-30
[15] 勾玉晓, 刘磊, 李冬梅, 等. 北黄海慢原甲藻形态结构与腹泻性贝类毒素组成[J]. 中国渔业质量与标准, 2018, 8(3): 11-18
Gou Y X, Liu L, Li D M, et al. Morphological and toxicological characterization of DSP producing dinoflagellate,Prorocentrum rhathymum, isolated from the North Yellow Sea, China [J]. Chinese Fishery Quality and Standards, 2018, 8(3): 11-18 (in Chinese)
[16] Li Y, Huang C X, Xu G S, et al. Pseudo-nitzschia simulans sp. nov. (Bacillariophyceae), the first domoic acid producer from Chinese waters [J]. Harmful Algae, 2017, 67: 119-130
[17] Gu H F, Luo Z H, Krock B, et al. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea [J]. Harmful Algae, 2013, 21-22: 64-75
[18] Liu L, Wei N, Gou Y X, et al. Seasonal variability of Protoceratium reticulatum and yessotoxins in Japanese scallop Patinopecten yessoensis in northern Yellow Sea of China [J]. Toxicon, 2017, 139: 31-40
[19] Salgado P, Riobó P, Rodríguez F, et al. Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: Evidence of paralytic toxin, spirolide, and gymnodimine [J]. Toxicon: Official Journal of the International Society on Toxinology, 2015, 103: 85-98
[20] Selwood A I, Wilkins A L, Munday R, et al. Pinnatoxin H: A new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate [J]. Tetrahedron Letters, 2014, 55(40): 5508-5510
[21] 于仁成, 罗璇. 我国近海有毒藻和藻毒素的研究现状与展望[J]. 海洋科学集刊, 2016(1): 155-166
[22] Toyofuku H. Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report) [J]. Marine Pollution Bulletin, 2006, 52(12): 1735-1745
[23] 陈军辉, 吴丹妮, 何秀平, 等. 海洋水环境中藻毒素的检测技术及分布研究进展[J]. 海洋科学进展, 2019, 37(3): 355-373
Chen J H, Wu D N, He X P, et al. The research advances in detection technology and distribution characteristics of algae toxins in marine water environment [J]. Advances in Marine Science, 2019, 37(3): 355-373 (in Chinese)
[24] 柳俊秀, 何培民. 赤潮藻毒素种类与化学结构研究进展[J]. 中国医药生物技术, 2009, 4(2): 144-147
[25] 吕金金, 李宏业, 刘洁生, 等. 氮杂螺环酸贝类毒素的研究进展[J]. 海洋科学, 2018, 42(9): 127-134
Lü J J, Li H Y, Liu J S, et al. Advances in research on azaspiracids [J]. Marine Sciences, 2018, 42(9): 127-134 (in Chinese)
[26] 吴海燕, 郭萌萌, 谭志军, 等. 环亚胺毒素研究进展[J]. 中国渔业质量与标准, 2012, 2(3): 21-32
Wu H Y, Guo M M, Tan Z J, et al. Research progress of cyclic imines toxins [J]. Chinese Fishery Quality and Standards, 2012, 2(3): 21-32 (in Chinese)
[27] 高春蕾, 刘仁沿, 梁玉波, 等. 虾夷扇贝毒素yessotoxins(YTXs), 中国沿海贝类中首次发现的一组贝类生物毒素[J]. 海洋学报, 2010, 32(3): 129-137
Gao C L, Liu R Y, Liang Y B, et al. First report of the presence of yessotoxins (YTXs) in shellfish from China’s coastal areas [J]. Acta Oceanologica Sinica, 2010, 32(3): 129-137 (in Chinese)
[28] 高春蕾, 孙萍, 贾智慧, 等. 温度和营养盐限制对网状原角藻生长与产毒的影响[J]. 生态学报, 2017, 37(12): 4217-4226
Gao C L, Sun P, Jia Z H, et al. Effects of temperature and nutrient limitation on growth and yessotoxin production of Protoceratium reticulatum [J]. Acta Ecologica Sinica, 2017, 37(12): 4217-4226 (in Chinese)
[29] Fu F X, Warner M, Zhang Y H, et al. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria) [J]. Journal of Phycology, 2007, 43(3): 485-496
[30] Howard M D A, Cochlan W P, Ladizinsky N, et al. Nitrogenous preference of toxigenic Pseudo-nitzschia australis (Bacillariophyceae) from field and laboratory experiments [J]. Harmful Algae, 2007, 6(2): 206-217
[31] Stocker T F, Qin D, Plattner G K, et al. IPCC, 2013: Climate Change 2013: the Physical Science Basis. Contribution of Working Group Ⅰ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [R]. Cambridge: Cambridge University Press, 2013: 525-527
[32] Sabine C L, Feely R A. The Oceanic Sink for Carbon Dioxide [M]// Smith K A. Greenhouse Gas Sinks. Oxford: Oxford University Press, 2007: 31-49
[33] Sala-Pérez M, Alpermann T J, Krock B, et al. Growth and bioactive secondary metabolites of Arctic Protoceratium reticulatum (Dinophyceae) [J]. Harmful Algae, 2016, 55: 85-96
[34] Caldeira K, Wickett M E. Oceanography: Anthropogenic carbon and ocean pH [J]. Nature, 2003, 425(6956): 365
[35] Doney S C, Fabry V J, Feely R A, et al. Ocean acidification [J]. Annual Review of Marine Science, 2009, 1(1): 169-192
[36] Shi H, Jin F F, Wills R C J, et al. Global decline in ocean memory over the 21st Century [J]. Science Advances, 2022, 8(18): eabm3468
[37] Riebesell U, Wolf-Gladrow D A, Smetacek V. Carbon dioxide limitation of marine phytoplankton growth rates [J]. Nature, 1993, 361(6409): 249-251
[38] Giovagnetti V, Brunet C, Conversano F, et al. Assessing the role of dust deposition on phytoplankton ecophysiology and succession in a low-nutrient low-chlorophyll ecosystem: A mesocosm experiment in the Mediterranean Sea [J]. Biogeosciences, 2012, 10: 2973-2991
[39] Jiang L Q, Feely R, Brendan R, et al. Climatological distribution of aragonite saturation state in the global oceans [J]. Global Biogeochemical Cycles, 2015, 29(10): 1656-1673
[40] Aluwihare L, Meador T. Chemical Composition of Marine Dissolved Organic Nitrogen [M]. Capone D G, Bronk D, Mulholland M R, et al. Eds. Nitrogen in the Marine Environment. 2nd Edition. Elsevier Inc., 2008: 95-140
[41] Rost B, Zondervan I, Wolf-Gladrow D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: Current knowledge, contradictions and research directions [J]. Marine Ecology Progress Series, 2008, 373: 227-237
[42] Field C B, Behrenfeld M J, Randerson J T, et al. Primary production of the biosphere: Integrating terrestrial and oceanic components [J]. Science, 1998, 281(5374): 237-240
[43] Torstensson A, Chierici M, Wulff A. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula [J]. Polar Biology, 2012, 35(2): 205-214
[44] Eberlein T, Van de Waal D B, Rost B. Differential effects of ocean acidification on carbon acquisition in two bloom-forming dinoflagellate species [J]. Physiologia Plantarum, 2014, 151(4): 468-479
[45] Lian Z R, Li F, He X P, et al. Rising CO2 will increase toxicity of marine dinoflagellate Alexandrium minutum [J]. Journal of Hazardous Materials, 2022, 431: 128627
[46] Clement R, Lignon S, Mansuelle P, et al. Responses of the marine diatom Thalassiosira pseudonana to changes in CO2 concentration: A proteomic approach [J]. Scientific Reports, 2017, 7: 42333
[47] 贺云凤, 逄凯, 李克强, 等. NH4-N氮源下海洋酸化对东海原甲藻和米氏凯伦藻生长的影响[J]. 中国海洋大学学报(自然科学版), 2020, 50(5): 94-103
He Y F, Pang K, Li K Q, et al. Effects of ocean acidification on the growth of Prorocentrum donghaiense and Karenia mikimotoi under NH4-N source [J]. Periodical of Ocean University of China, 2020, 50(5): 94-103 (in Chinese)
[48] Spilling K, Paul A J, Virkkala N, et al. Ocean acidification decreases plankton respiration: Evidence from a mesocosm experiment [J]. Biogeosciences, 2016, 13(16): 1-35
[49] Mercado J M, Figueroa F L, Niell F X, et al. A new method for estimating external, carbonic anhydrase activity in macroalgae [J]. Journal of Phycology, 1997, 33(6): 999-1006
[50] 杨安强. 多重环境因子变化对赤潮异弯藻生长的影响[D]. 上海: 华东师范大学, 2021: 78-79
Yang A Q. Effect of multiple environment factors on the growth of Heterosigma akashiwo [D]. Shanghai: East China Normal University, 2021: 78-79 (in Chinese)
[51] 贾民娟. 不同CO2浓度对微藻生长的影响[D]. 济南: 山东大学, 2019: 24-25, 36-37
[52] 郝爽. 海洋酸化对微小亚历山大藻产毒的影响和调控机制初探[D]. 济南: 山东大学, 2021: 41, 53-55
[53] 徐金涛, 庞敏, 马新, 等. CO2加富对塔玛亚历山大藻叶绿素荧光参数及产毒的影响[J]. 海洋与湖沼, 2016, 47(3): 557-563
Xu J T, Pang M, Ma X, et al. Carbon dioxide enrichment affects chlorophyll fluorescence and toxin production of Alexandrium tamarense [J]. Oceanologia et Limnologia Sinica, 2016, 47(3): 557-563 (in Chinese)
[54] 胡顺鑫, 杨丁, 唐学玺, 等. 海水酸化对米氏凯伦藻和盐生杜氏藻种群增长和种间竞争的影响[J]. 海洋与湖沼, 2017, 48(4): 777-785
Hu S X, Yang D, Tang X X, et al. Ocean acidification on population growth and inter-species competition between Karenia mikimotoi and Dunaliella salina [J]. Oceanologia et Limnologia Sinica, 2017, 48(4): 777-785 (in Chinese)
[55] Tatters A O, Fu F X, Hutchins D A. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta [J]. PLoS One, 2012, 7(2): e32116
[56] Clement R, Lignon S, Mansuelle P, et al. Responses of the marine diatom Thalassiosira pseudonana to changes in CO2 concentration: A proteomic approach [J]. Scientific Reports, 2017, 7: 1-12
[57] Brandenburg K M, Velthuis M, Van de Waal D B. Meta-analysis reveals enhanced growth of marine harmful algae from temperate regions with warming and elevated CO2 levels [J]. Global Change Biology, 2019, 25(8): 2607-2618
[58] Pang M, Xu J T, Qu P, et al. Effect of CO2 on growth and toxicity of Alexandrium tamarense from the East China Sea, a major producer of paralytic shellfish toxins [J]. Harmful Algae, 2017, 68: 240-247
[59] Sun J, Hutchins D, Feng Y Y, et al. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries [J]. Limnology and Oceanography, 2011, 56(3): 829-840
[60] Jin P, Wang T F, Liu N N, et al. Ocean acidification increases the accumulation of toxic phenolic compounds across trophic levels [J]. Nature Communications, 2015, 6: 8714
[61] Hardison D, Sunda W, Tester P, et al. Increased cellular brevetoxins in the red tide dinoflagellate Karenia brevis under CO2 limitation of growth rate: Evolutionary implications and potential effects on bloom toxicity [J]. Limnology and Oceanography, 2014, 59(2): 560-577
[62] Hattenrath-Lehmann Theresa K, Smith Juliette L, Wallace Ryan B, et al. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense [J]. Limnology and Oceanography, 2015, 60(1): 198-214
[63] Schmidt L, Hansen P J. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: Effect of cell concentration, growth phase and pH [J]. Marine Ecology Progress Series, 2001, 216: 67-81
[64] van de Waal D B, Eberlein T, John U, et al. Impact of elevated pCO2 on paralytic shellfish poisoning toxin content and composition in Alexandrium tamarense [J]. Toxicon: Official Journal of the International Society on Toxinology, 2014, 78: 58-67
[65] Fu F X, Place A R, Garcia N S, et al. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum [J]. Aquatic Microbial Ecology, 2010, 59: 55-65
[66] Kremp A, Godhe A, Egardt J, et al. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions [J]. Ecology and Evolution, 2012, 2(6): 1195-1207
[67] 司冉冉, 关万春, 蔡景波, 等. 氮源对塔玛亚历山大藻生长和毒性的影响[J]. 生态学杂志, 2017, 36(10): 2880-2885
[68] 杨晶晶. 富营养化和海洋酸化对典型浮游植物群落生理生态特征的影响[D]. 杭州: 浙江大学, 2017: 56-61
[69] Lundholm N, Hansen P J, Kotaki Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the Genera Pseudo-nitzschia and Nitzschia [J]. Marine Ecology Progress Series, 2004, 273: 1-15
[70] Trimborn S, Lundholm N, Thoms S, et al. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry [J]. Physiologia Plantarum, 2008, 133(1): 92-105
[71] 郝爽, 刘丽君, 陈军辉, 等. 高氮磷比与酸化共同作用对微小亚历山大藻生长和产毒的影响[J]. 海洋科学, 2021, 45(2): 1-10
Hao S, Liu L J, Chen J H, et al. Effects of acidification and high N/P ratios on toxin production in Alexandrium minutum [J]. Marine Sciences, 2021, 45(2): 1-10 (in Chinese)
[72] Eberlein T, Waal D V D, Brandenburg K, et al. Interactive effects of ocean acidification and nitrogen limitation on two bloom-forming dinoflagellate species [J]. Marine Ecology Progress Series, 2016, 543: 127-140
[73] 高欣, 许敏, 薛学洋, 等. CO2浓度升高和温度升高对铜绿微囊藻生长及产毒影响[J]. 环境科学与技术, 2014, 37(9): 1-4
Gao X, Xu M, Xue X Y, et al. Effects of elevated CO2 and temperature on growth and microcystin production of Microcystis aeruginosa [J]. Environmental Science &Technology, 2014, 37(9): 1-4 (in Chinese)
[74] Wohlrab S, John U, Klemm K, et al. Ocean acidification increases domoic acid contents during a spring to summer succession of coastal phytoplankton [J]. Harmful Algae, 2020, 92: 101697