持久性有机污染物(persistent organic pollutants, POPs)作为一类可在环境中持久性存在,并可长距离迁移的有机污染物,其对生态以及人体健康的影响受到广泛关注。为有效控制POPs污染带来的生态和健康危害,自2001年起包括中国在内的多个国家和地区签署并加入了《关于持久性有机污染物的斯德哥尔摩公约》,对包括有机氯杀虫剂和多氯联苯等首批传统POPs实行禁止或管控措施[1]。然而随着人类活动需求和化工产业的发展,人工化学品合成和使用的增速导致大量新污染物进入到环境中,给生态环境健康带来新一轮威胁和挑战[2]。相比于传统POPs其污染上升趋势明显,污染种类多样,且部分新污染物特别是传统POPs替代品的生物累积性和毒性并未有明显改观,因此对该类新污染物的风险评估和管控是当前生态环境保护的重中之重[3]。迄今为止,已有包括多溴二苯醚、全氟辛基磺酸盐(PFOS)等16种新型有机污染物被列入《斯德哥尔摩公约》受控名单[4]。中国生态环境部发布的《重点管控新污染物清单(2022年版)》中,也将全氟辛酸及其盐类和相关化合物(PFOA类)、全氟辛基磺酸及其盐类(PFOS类)、全氟己基磺酸及其盐类和相关化合物(PFHxS类)和短链氯化石蜡(SCCPs)等列入首批进行严格环境风险管控的新污染物[5]。
海洋是地球最大的生态系统之一,同时也是多种有机污染物的汇集地[6]。受水体交换作用对入海污染物稀释的影响,海水水体中POPs的浓度水平通常在pg·L-1~ng·L-1之间[7]。尽管其海水浓度很低,该类物质的高亲脂性和生物代谢惰性等使其可以通过水相和食物相暴露等途径在海洋生物体内富集并在食物链中传递,因此污染物的生物富集和沿食物链放大潜力也成为评估其生态环境安全的重要依据之一。此外,随着环境痕量化学分析技术的发展,新型持久性有机污染物对海洋生物体暴露风险也受到了广泛关注。相比于传统POPs,该类污染物的分子结构、理化性质以及与生物的互作关系更为复杂,有关其在海洋环境中的生物富集和沿食物链(网)的生物放大效应的认知仍有待进一步明确。本文针对全球海洋环境中重点关注的POPs包括有机氯农药(OCPs)、多环芳烃(PAHs)、多氯联苯(PCBs)以及多溴联苯醚(PBDEs)、氯化石蜡(CPs)和全/多氟烷基化合物(PFAS),重点分析和综述传统和新型持久性有机污染物在海洋生态系统中的富集和沿食物链放大潜力,以及其关键的影响因素,有助于加深对POPs生物累积性和持久性的认识,对评估其生态效应、建立海洋风险管理标准有十分重要的意义。
1 海洋环境中POPs的生物富集(Bioaccumulation of POPs in the marine environment)
国际社会对POPs生物富集的标准为,当化合物的食物链放大因子(TMF)>1,其具有显著的生物富集效应;当化合物的生物浓缩因子(BCF)或生物富集因子(BAF)>5 000,其可能具有生物富集效应;而当化合物的辛醇-水分配系数(logKow>4)且辛醇-空气分配系数(logKOA>5)时,其具有一定的生物富集潜力[8]。BCF和BAF均定义为平衡状态下生物体内污染物浓度与水体浓度的比值。其中,BCF仅适用于水相暴露,通常在实验室测得。而对于复杂的野外环境,BAF还同时包含了食物相暴露对生物体富集污染物的贡献[8]。相对于淡水生物,POPs在海洋生物中富集的相关参数仍较为缺乏。Berrojalbiz等[9]对PAHs在海洋浮游植物(Rhodomonas salina)和桡足类(Paracartia(Acartia) grani)的生物富集研究发现,浮游生物对PAHs吸收速率和同化效率随PAHs的疏水性增加而增加,其logBCF和logBAF均与PAHs的logKow呈现显著的正相关关系。然而受桡足类对PAHs生物转化的影响,桡足类的logBCF(1.1~2.8)要比浮游植物的logBCF(3.6~6.7)低[9]。对部分OCPs(logKow>5)和PCBs的研究也同样发现,其海洋浮游植物的logBCF(PCBs:6.0~8.0)以及海洋动物的logBAF包括浮游动物(OCPs:4.7~6.1;PCBs:6.4~8.9)、双壳类(OCPs:2.9~6.8)、甲壳类(OCPs:4.0~8.9)、鱼类(OCPs:4.1~7.2;PCBs:6.4~13.8)和哺乳类(PCBs:9.0~10.7)均随着其logKow的增加而增加,且与生物所处营养级水平呈现显著的正相关关系[10-12]。此外,海洋沉积物作为海洋环境中POPs的储存库[13],栖息在海洋沉积物表层及内部的底栖生物对沉积物有机质及食物碎屑等的摄入不仅是其富集POPs的重要来源,同时也在一定程度上影响着POPs的生物放大效应[14]。Sun等[15]研究SCCPs在河口生态系统中的生物富集发现,SCCPs在底栖无脊椎动物(虾、蟹和双壳类)中的浓度高于非底栖物种如中上层鱼类和鱿鱼。Martín等[16]对海洋棘皮动物(Holothuria tubulosa Gmelin)体内全氟羧酸类(PFCAs)的研究也同样表明,其logBAF和生物-沉积物富集因子(logBSAF)分别为1.2~4.4和1.4~2.9,且均随着全氟烷基碳链的增加而显著增加。BSAF为生物体内污染物脂肪归一化浓度与沉积物中污染物总有机碳归一化浓度的比值,常用于表征底栖生物富集污染物的能力[17]。对于多数POPs,其logBSAF与logKow呈一定的正相关关系。如波斯湾北部双壳类中,PCB-153、PCB-138、PCB-118、滴滴涕(DDTs)和PBDEs的logBSAF(2.4~3.1)均远高于六氯环己烷(HCHs) (logBSAF:0.2)[18]。而对于PAHs,部分研究表明,PAHs在我国舟山湾渔场和海南珊瑚礁底栖生物中的logBSAF与logKow呈现一定的负相关关系[19-20]。Thomann和Komlos[21]对底栖食物网中PAHs的分析发现,当logKow<5时,底栖鱼类对沉积物孔隙水中PAHs的生物可利用度增加,导致其logBSAF随着logKow的增加而增加,而当logKow>5时,其在肠道同化效率和logBSAF将随着logKow的增加而显著降低。
此外,海洋生物的脂肪含量、体型、年龄、性别、生殖、摄食生态学和营养级水平等也是影响海洋生物富集POPs的重要因素[22-24]。绝大多数POPs为高度脂溶性化合物,其在生物体内分布与生物体的脂肪含量密切相关,因此常采取脂肪校正浓度来表示其富集能力。海洋生物体型和年龄的变化在一定程度上不仅改变了POPs的吸收代谢动力学,并从时间尺度上,影响其在海洋生物中的富集[23]。脂溶性化合物的吸收方式主要为简单扩散,其扩散速率会随着体型的增加以及比表面积和呼吸速率的降低而降低,进而影响了其在生物体的吸收和消除速率[25]。对于高脂溶性(logKow>5)化合物,其在大体型海洋生物体内通常具有较高的半衰期和富集浓度[26]。此外,受母体传递和饮食限制的影响,部分海洋生物幼体在生长初期体内的POPs浓度会呈现一定的生长稀释现象[27]。母体传递也是导致POPs生物富集性别差异的重要因素之一。Tuerk等[28]对成年海豚体内POPs分析发现,雌性大西洋斑纹豚(Lagenorhynchus acutus)和粗齿海豚(Steno bredanensis)体内PBDEs和PCBs浓度均显著低于雄性。基于对幼体和成年雌性里海海豹(Pusa caspica)鲸脂中POPs富集趋势的分析也同样表明,POPs在哺乳期母体和幼体之间存在选择性转移的现象[29]。
食物相暴露通常被认为是POPs生物富集的主要途径,污染物可通过高营养级生物的捕食作用随着营养级的增加而逐级积累[23]。作为处于海洋食物链中较高营养级的捕食者,海洋哺乳动物脂肪含量高、生命周期较长,体内细胞色素P450酶系对POPs代谢能力弱,其POPs累积在短时间内不会被清除,因此可反映周围海域的污染程度[30]。一项对阿拉斯加到纳维亚半岛的海洋生物群(鱼类和哺乳动物)体内传统POPs的30年时间序列浓度分析表明,PCBs以及OCPs的大部分种类包括HCHs、六氯苯(HCBs)、DDTs和氯丹(CHLs)等均有检出,其最早可追溯至20世纪70年代[6]。在所有的时间序列中,其体内总浓度水平呈现一定的下降趋势,然其年度平均下降率不足5%,表明传统POPs的环境稳定性和持久性对海洋生物仍是一个长期的威胁[6-7]。对于新型持久性有机污染物,其海洋生物富集数据相对较少(表1)[18,31-63]。一项对PFAS的最新研究发现,在2009—2010以及2018—2019年间,PFOS和替代品氯代多氟烷醚磺酸盐(F-53B)和六氟环氧丙烷二聚体羧酸(HPFO-DA)在中国东海江豚(Neophocaena asiaeorientalis sunameri)的生物富集随时间的推移呈现一定的上升趋势,且F-53B富集浓度与PFOS富集浓度相当,其在海洋环境的生态风险应进一步引起重视[64]。
表1 海洋生物体内持久性有机污染物(POPs)浓度
Table 1 Bioaccumulation level of persistent organic pollutants (POPs) in marine organisms

污染物种类Pollutants生物类群Biological group浓度/(ng·g-1)(以单位脂肪质量计)Concentration/(ng·g-1) (Based on lipid mass)时间Sampling time地点Sampling location有机氯农药Organochlorine浮游动物 Zooplankton甲壳类 Crustacean双壳类 Bivalve头足类 Cephalopoda鱼类 Fish哺乳类 Mammal12.0~17.02010—2014中国舟山[31] Zhoushan, China[31]47.0~205.02010—2014中国舟山[31] Zhoushan, China[31]126.2~338.02014中国南海[32] South China Sea[32]2.5~191.02009—2012波斯湾北部[18] North Arabian Gulf[18]11.1~28.92010—2019西班牙北海岸[33] Northern Spanish coast[33]97.0~142.02010—2014中国舟山[31] Zhoushan, China[31]8.8~14.22014中国南海[32] South China Sea[32]51.0~1 204.02010—2014中国舟山[31] Zhoushan, China[31]282.0~12 308.02017—2018南非伊丽莎白港[34] Port Elizabeth, South Africa[34]190.0~9 622.02010—2014中国舟山[31] Zhoushan, China[31]104 739.0~202 926.02003—2011西班牙加那利群岛[35] Canary Islands, Spain[35]62 700.0~57 700.02004—2014中国珠江河口[36] Pearl River Estuary, China[36]多环芳烃Polycyclic aromatic hydrocarbons浮游动物 Zooplankton甲壳类 Crustacean双壳类 Bivalve鱼类 Fish哺乳类 Mammal哺乳类 Mammal8.2~88.02015—2016北极斯瓦尔巴群岛[37] West Spitsbergen Fjords[37]23.9~61.92017中国北部湾[38] Beibu Gulf, China[38]10.4~40.12010—2019西班牙北海岸[33] Northern Spanish coast[33]8.7~86.72016中国南海[39] South China Sea[39]22.4~72.62015—2020中国珠江河口[40] Pearl River Estuary, China[40]4.8~432.02003中国黄海和南海[41] South China Sea and Yellow Sea[41]1 394.0~42 577.02003—2011西班牙加那利群岛[35] Canary Islands, Spain[35]多氯联苯Polychlorinated biphenyls浮游动物 Zooplankton甲壳类 Crustacean双壳类 Bivalve头足类 Cephalopoda鱼类 Fish哺乳类 Mammal41.1~911.32015—2016北极斯瓦尔巴群岛[37] West Spitsbergen Fjords[37]125.6~748.32017地中海[42] Mediterranean Sea[42]117.0~294.02005日本东京湾[43] Tokyo Bay, Japan[43]32.1~118.02012中国珠江河口[40] Pearl River Estuary, China[40]10.3~66.12014中国南海[39] South China Sea[39]13.7~127.02005日本东京湾[43] Tokyo Bay, Japan[43]15.6~73.62010—2019西班牙北海岸[33] Northern Spanish coast[33]8.8~14.22014中国南海[32] South China Sea[32]93.9~1 550.02010—2014美国普吉特海湾[44] Puget Sound, USA[44]0.1~30 982.02011孙德尔本斯红树林(孟加拉国所辖部分)[45]Sundarbans (the part in Bangladesh)[45]35.0~466.02012中国珠江河口[46] Pearl River Estuary, China[46]15.7~117.92014中国南海[32] South China Sea[32]2 300.0~318 000.02010—2013北大西洋[47] North Atlantic Ocean[47]12 935.0~64 397.02006—2013地中海[48] Mediterranean Sea[48]多溴联苯醚Polybrominated diphenyl ethers浮游动物 Zooplankton6.9~333.32010地中海[42] Mediterranean Sea[42]甲壳类 Crustacean92.1~97.32005日本东京湾[43] Tokyo Bay, Japan[43]
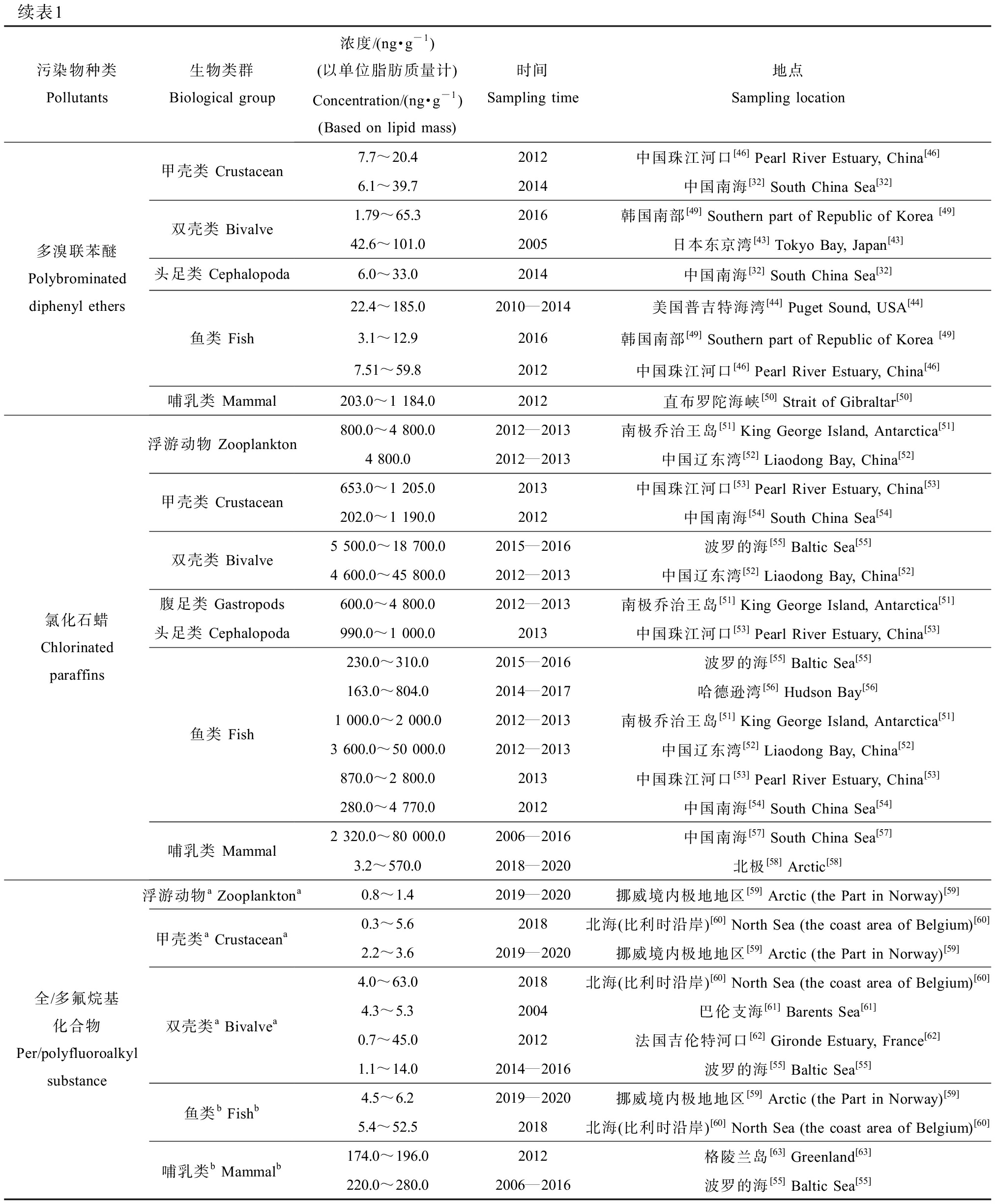
续表1污染物种类Pollutants生物类群Biological group浓度/(ng·g-1)(以单位脂肪质量计)Concentration/(ng·g-1) (Based on lipid mass)时间Sampling time地点Sampling location多溴联苯醚Polybrominated diphenyl ethers甲壳类 Crustacean双壳类 Bivalve头足类 Cephalopoda鱼类 Fish哺乳类 Mammal7.7~20.42012中国珠江河口[46] Pearl River Estuary, China[46]6.1~39.72014中国南海[32] South China Sea[32]1.79~65.32016韩国南部[49] Southern part of Republic of Korea [49]42.6~101.02005日本东京湾[43] Tokyo Bay, Japan[43]6.0~33.02014中国南海[32] South China Sea[32]22.4~185.02010—2014美国普吉特海湾[44] Puget Sound, USA[44]3.1~12.92016韩国南部[49] Southern part of Republic of Korea [49]7.51~59.82012中国珠江河口[46] Pearl River Estuary, China[46]203.0~1 184.02012直布罗陀海峡[50] Strait of Gibraltar[50]氯化石蜡Chlorinated paraffins浮游动物 Zooplankton甲壳类 Crustacean双壳类 Bivalve腹足类 Gastropods头足类 Cephalopoda鱼类 Fish哺乳类 Mammal800.0~4 800.02012—2013南极乔治王岛[51] King George Island, Antarctica[51]4 800.02012—2013中国辽东湾[52] Liaodong Bay, China[52]653.0~1 205.02013中国珠江河口[53] Pearl River Estuary, China[53]202.0~1 190.02012中国南海[54] South China Sea[54]5 500.0~18 700.02015—2016波罗的海[55] Baltic Sea[55]4 600.0~45 800.02012—2013中国辽东湾[52] Liaodong Bay, China[52]600.0~4 800.02012—2013南极乔治王岛[51] King George Island, Antarctica[51]990.0~1 000.02013中国珠江河口[53] Pearl River Estuary, China[53]230.0~310.02015—2016波罗的海[55] Baltic Sea[55]163.0~804.02014—2017哈德逊湾[56] Hudson Bay[56]1 000.0~2 000.02012—2013南极乔治王岛[51] King George Island, Antarctica[51]3 600.0~50 000.02012—2013中国辽东湾[52] Liaodong Bay, China[52]870.0~2 800.02013中国珠江河口[53] Pearl River Estuary, China[53]280.0~4 770.02012中国南海[54] South China Sea[54]2 320.0~80 000.02006—2016中国南海[57] South China Sea[57]3.2~570.02018—2020北极[58] Arctic[58]全/多氟烷基化合物Per/polyfluoroalkyl substance浮游动物a Zooplanktona甲壳类a Crustaceana双壳类a Bivalvea鱼类b Fishb哺乳类b Mammalb0.8~1.42019—2020挪威境内极地地区[59] Arctic (the Part in Norway)[59]0.3~5.62018北海(比利时沿岸)[60] North Sea (the coast area of Belgium)[60]2.2~3.62019—2020挪威境内极地地区[59] Arctic (the Part in Norway)[59]4.0~63.02018北海(比利时沿岸)[60] North Sea (the coast area of Belgium)[60]4.3~5.32004巴伦支海[61] Barents Sea[61]0.7~45.02012法国吉伦特河口[62] Gironde Estuary, France[62]1.1~14.02014—2016波罗的海[55] Baltic Sea[55]4.5~6.22019—2020挪威境内极地地区[59] Arctic (the Part in Norway)[59]5.4~52.52018北海(比利时沿岸)[60] North Sea (the coast area of Belgium)[60]174.0~196.02012格陵兰岛[63] Greenland[63]220.0~280.02006—2016波罗的海[55] Baltic Sea[55]
注:a个体浓度,以单位湿体质量计;b肝脏浓度,以单位肝脏湿质量计。
Note: a Whole-body concentration, based on wet body mass; b Liver concentration, based on wet liver mass.
栖息地环境的污染水平、气候等也极大程度决定了海洋生物POPs富集的分布趋势[22,24]。低、中纬度地区是POPs的主要生产和使用区,其密集的污染源分布以及沿海港口的人为活动等导致了该地区周围海域存在较高的POPs污染和富集总量[24]。由于全球温度差异,低、中纬度地区的温度相对较高,POPs易于从各种介质挥发到大气中进行远距离迁移至寒冷地区,导致了POPs可沿纬度跃迁沉降并累积在全球海洋生态系统中[3,65]。Bustnes等[66]对不同纬度海洋鱼类体内POPs分析研究表明,其总浓度呈现由南至北下降的趋势,而低氯代PCBs同系物的浓度占比随着纬度的增加而增加,呈现一定的纬度分化现象。Vorkamp等[67]对挪威海岸的贻贝(Mytilus edulis)和大西洋鳕鱼(Gadus morhua)体内的CPs分析也同样发现,SCCPs的脂肪浓度也随着纬度的增加以及与污染源的距离增加而普遍下降。
其他环境因素如全球气候变化对海洋理化性质(海洋酸化和温度上升等)的改变在不同程度上也会影响POPs在海洋中的环境行为。海洋酸化可破坏有机物与金属的络合作用,影响POPs的生物降解过程,而温度上升可改变POPs在水和颗粒物之间的相态分配,并进而影响其在海洋中的暴露水平[11,65]。海洋环境中新污染物微塑料作为POPs在海洋环境介质的重要载体之一,其在海洋环境中的广泛分布对POPs生物富集的影响也不容忽视[68]。
目前研究表明,微塑料对POPs在海洋生物体内富集的贡献相对较低,海洋哺乳动物从食物链中富集的POPs总量要远超于微塑料中富集的POPs总量[69]。野外试验也显示,微塑料的摄入可能会增加污染物的生物清除率,这意味着微塑料的载体作用不太可能会增加POPs对海洋生物的暴露风险[70]。
2 POPs在海洋食物链/网的生物放大潜力(Trophic magnification potential of legacy and emerging POPs in the marine food web)
生物放大因子(BMF)和TMF是表征污染物生物放大的重要参数,两者均描述污染物在捕食者和被捕食者之间的传递过程。其中,BMF仅考虑2个营养级之间捕食者和被捕食者的关系,而TMF可通过评估污染物在整个食物网中的传递,提供基于食物网结构整体性的生物放大定量依据。因此TMF被欧洲化学品管理局(ECHA)和化学品注册、评估、授权和限制法规(REACH)纳为评估化学品生物累积性和是否具有生物放大效应的最佳证据权重,以综合性地反映污染物在食物链/网中的生物放大潜力[8]。
2.1 有机氯农药
OCPs主要包括以苯为原料合成的HCHs、HCBs、DDTs和以环戊二烯为原料合成的CHLs、艾氏剂、狄氏剂和异狄氏剂等。虽然全球绝大多数国家和地区已禁止生产该类化合物,但OCPs的高亲脂性和环境稳定性导致这类污染物可以长期累积在海洋生物体内[71]。对于绝大多数的HCHs、HCBs、CHLs和DDTs,其TMF与logKow呈一定的正相关关系[12]。Zhou等[31]对多种OCPs的TMF研究发现,除了o,p’-DDE的TMF<1,其他OCPs包括HCHs、CHLs和DDTs等均具有显著的沿食物链放大潜力;其中,脂溶性较低(logKow=3.89)的HCHs及其同分异构体的TMF均低于DDTs和CHLs。Hop等[72]对巴伦支海边缘冰区OCPs的食物链放大研究也发现,在浮游动物和鱼类脂肪中HCHs、CHLs和p,p’-DDE的浓度没有明显差异,而在鞍背海豹(Phocagroenlandica)和环斑海豹(Phoca hispida)脂肪中HCHs浓度最低,其TMF(1.6~8.1)也均低于其他OCPs。生物转化是影响OCPs沿食物链放大的重要因素之一。在海洋生物中,DDTs可被生物转化为DDDs和DDEs,该转化能力随着营养级的增加而增强[73]。其中,p,p’-DDE是p,p’-DDT主要的生物转化产物,在海洋哺乳动物体内,其含量可达DDTs总浓度的95.7%[34]。因此,高营养级海洋生物对DDTs的生物转化也成为其富集DDEs的主要来源之一[73-74]。
2.2 多环芳烃
PAHs是一类多环芳香烃化合物,按其物化性质可分为低分子量芳烃(2~3环)和高分子量芳烃(4~7环)。PAHs的logKow在3.3到6.8之间,随着环数增加,其脂溶性增加,挥发性和稳定性降低,在沉积物和土壤中的半衰期为0.2~5.0 a。相关研究表明,PAHs在海洋环境中的食物链放大效应并不显著。Wan等[75]对中国渤海湾中18种PAHs分析发现,logKow在3.5~6.5范围内的PAHs在海洋食物链中的脂肪中的浓度随着营养级增加而降低,呈现“营养级稀释”效应。对东京湾和波斯湾食物网的研究也同样发现PAHs不具有生物放大现象[76-77]。
其中,大分子量PAHs对细胞膜的渗透性下降以及生物体内普遍存在的细胞色素P450酶系对PAHs的生物转化是影响其在海洋环境中沿食物链转移的重要因素[76-78]。此外,最新研究发现,不同纬度海洋生物对PAHs的生物转化差异可能会影响其生物放大效应,在低纬度热带海洋生态系统中3和4环PAHs具有显著的营养级放大现象,其BAF和TMF与纬度呈一定的负相关关系[39]。
2.3 多氯联苯和多溴联苯醚
PCBs和PBDEs分别是一类多氯代芳烃类和多溴代联苯类化合物,两者的logKow范围分别在4.0~8.0和5.9~10.0之间,logKOA分别为9.3~11.5和9.3~15.3,并随着氯/溴化程度增加而增加[79]。一项对全球生态系统中PCBs的研究表明,PCBs的生物放大效应具有普适性,在南北极、太平洋、大西洋和印度洋海域中,均发现PCBs同系物(PCB-28、PCB-52、PCB-101、PCB-118、PCB-138、PCB-153和PCB-180)可沿食物链传递并在高营养级海洋生物中长期累积,其TMF与logKow在一定范围内(logKow:5.8~7.0)呈现显著的正相关关系[80-81]。而对于logKow不在该范围的PCBs,生物的呼吸代谢、生物转化以及生物可利用度降低等均会影响其在生物体内的富集[82-83]。García-Alvarez等[35]对西班牙加那利群岛宽吻海豚(Tursiops truncatus)脂肪组织中PCBs含量分析,发现高氯代的PCBs(PCB-180、PCB-153和PCB-138等)是其主要的富集类型,占其总量的86%。该规律与高度城市化地区如墨西哥湾和偏远地区如大西洋中宽吻海豚的体内分布趋势相似[84-85]。而对于氯化程度较低以及部分logKow>7.0的PCBs,其在海洋生物如鱼类和哺乳动物体内更倾向于被生物转化和清除。Buckman等[82]对PCBs生物转化效率的研究发现,其在鱼体内的半衰期主要取决于不同氯原子的取代方式。PCBs氯代方式主要分为5类,第1类在同一苯环上没有邻接的氢原子包括PCB-153、PCB-146、PCB-183、PCB-191、PCB-128和PCB-178等,第2类仅在正位和元位上有邻位氢原子且具有2个及以上的正位氯原子,第3类与第2类相似但仅具有1个或0个正位氯原子,第4类在元位和对位上有邻接的氢原子以及2个或更少的正位氯原子,第5类与第4类相似但具有3个及以上的正位氯原子[86-87]。其中,第1类几乎不被生物转化,第2类和第3类可能会参与由CYP1A介导的生物转化,然该过程尚未得到证实。而第4类(PCB-41、PCB-42、PCB-83、PCB-91和PCB-110等)和第5类(PCB-45、PCB-95、PCB-132、PCB-136、PCB-149和PCB-176等)为主要参与由CYP450酶系介导的生物转化的PCBs同系物[88]。Hoekstra等[89]对弓头鲸(Balaena mysticetus)肝脏和鲸脂中PCBs分析显示,相比于第1、2、5类PCBs,第3类和第4类的生物富集较低,表明该类化合物可能参与了由CYP2B介导的生物转化[90]。在其他海洋鲸类动物中也发现了同样的富集模式,该模式可能进一步影响PCBs在海洋食物链的放大效应。
同样作为一类高亲脂性的有机污染物,PBDEs在海洋环境中具有较为显著的生物放大潜力,且其TMF与logKow呈现一定的正相关关系。研究表明,在不同海域(地中海、南北极、巴西沿海等)哺乳动物如鲸类、北极熊(Ursus maritimus)和海豚脂肪组织中,BDE-47、BDE-99、BDE-100和BDE-153为主要检出物,其中五溴联苯醚BDE-47的检出率和浓度占比最高[48,91-92]。Dorneles等[93]对远离污染源的大西洋西南海域(河口、大陆架和远海)鲸类肝脏中的PBDEs分析也同样发现,四溴、五溴和六溴联苯醚浓度占其总量的80%以上。通过线性回归模型对8种不同溴化程度的PBDEs(BDE-17、BDE-28、BDE-47、BDE-49、BDE-85、BDE-99、BDE-100和BDE-153)在食物链生物放大的分析发现,其TMF值均>1,且低溴化PBDEs(BDE-17、BDE-28和BDE-47)的TMF值随着溴化程度增加而增加,BDE-47的TMF值最高,其次分别为BDE-100、BDE-85和BDE-99[94]。造成该现象的原因主要包括,一方面,高溴化PBDEs的“超疏水性”可能导致其在生物体内的吸收和同化效率下降[95]。另一方面,对PBDEs的污染来源分析表明,虽然PBDEs有209种同系物,但商品化PBDEs的种类有限,主要包括五溴、八溴和十溴联苯醚3个大类。其中,五溴联苯醚是商品化PBDEs的主要成分之一,该类PBDEs的大量使用导致了其在海洋生物体内的高富集量[96]。此外,PBDEs的生物脱溴作用也可影响高溴化PBDEs在海洋高营养级生物体内的累积[96]。对东京湾和渤海沿海食物链中PBDEs同系物比例的研究发现,BDE-209、BDE-99和BDE-153在虾、蟹和鱼类等体内可被生物代谢为BDE-47和BDE-154,并可通过食物链传递至高营养级生物体内[97-98]。
2.4 氯化石蜡
氯化石蜡是石蜡烃的氯化衍生物,根据链长可分为短链氯化石蜡(C10~13-SCCPs)、中长链氯化石蜡(C14~17-MCCPs)、长链氯化石蜡(C18~20-LCCPs)以及碳链>20的长链氯化石蜡(C>20-LCCPs)等。在一定氯含量范围内(55%~70%),其logKow随着链长的增加而增加,C10~13-SCCPs、C14~17-MCCPs、C18~20-LCCPs和C>20-LCCPs的logKow范围分别为4.7~6.9、5.5~8.2、7.3~7.6和7.5~12.8[99]。在海洋环境中,SCCPs和MCCPs是2类主要的检出物质[99]。Zeng等[57]对中国南海中江豚和印度驼背海豚(Sousa chinensis)脂肪组织的SCCPs和MCCPs含量分析发现,脂肪中MCCPs的浓度比SCCPs高,其中,C14-MCCP为主要MCCPs检出物,占总浓度的40%以上。此外,SCCPs和MCCPs较高的logKOA和较长的半衰期使其具有长距离迁移的能力[100-101]。Li等[101]在南极地区采集的生物样品中均发现了SCCPs的存在,其较强的生物富集和放大潜力可能对该海域生物造成一定的生态风险。最新研究发现,在格陵兰岛、冰岛和瑞典等地区的海洋野生动物体内首次检测到高浓度的LCCPs,占总CPs含量的52%,证实了LCCPs同样具有长距离迁移和富集潜力[58]。相比于MCCPs,SCCPs的生物富集和放大效应更为显著[57]。研究表明,SCCPs的碳链长度和氯化程度是影响其沿食物链放大的重要因素之一[102]。CPs的TMF与logKow呈显著的正相关关系[52]。作为一类新型持久性有机污染物,CPs的TMF(1.5~5.7)略低于PBDEs(2.6~7.3)[103]和PCBs(3.4~12.3)[104],然而其长期环境释放的危害不容忽视,未来仍需加强对该类化合物在海洋环境的生物累积和毒性效应等研究。
2.5 全/多氟烷基化合物
PFAS是一类由人工合成的高度氟化脂肪族化合物,主要包括全氟磺酸类(PFSAs)和PFCAs,以及其前体和替代物。PFAS具有较高的肠道吸收效率,因此食物相暴露是其生物富集的主要途径[105]。与其他亲脂类POPs不同,该类化合物对蛋白具有很高的亲和力,其体内浓度与蛋白含量呈正相关,并易于富集在血液、肝脏和肾脏中[106]。对PFASs的吸收代谢动力学研究发现,基于蛋白归一化浓度更能准确地反映其生物富集,其生物放大相关参数也应表征为蛋白-水分配系数(logKPW)和蛋白-空气分配系数(logKPA)[107]。在海洋食物链中,相同氟化链长的PFSAs比PFCAs具有更高的生物富集潜力。对北极环斑海豹取样分析表明,PFOS的检出浓度最高,而PFOA在极地生物中富集量普遍较低[108-110]。通过模型对PFAS在鱼体的吸收、消除速率以及BCF的研究发现,在血液和肝脏中,相比于PFOS和长链的PFCAs,PFOA吸收速率低,生物消除速率较高,进而导致其生物富集和放大潜力相对较低[106]。
与此同时,PFOS前体化合物的生物转化可能成为其在海洋生物中富集的贡献源之一[111]。对北极海洋食物链2种PFOS的前体化合物分析表明,N-乙基全氟辛烷磺酰胺(N-EtPFOSA)和全氟辛烷磺酰胺酸(PFOSA)在白海豚(Lagenorhynchus albirostris)和鼠海豚(Phocoena phocoena)的体内浓度均高于浮游植物和甲壳类,表明哺乳动物具有更高的PFOS转化来源[111]。此外,不同哺乳动物对PFOS前体化合物的生物转化能力不同,对各类哺乳动物肝脏中PFOS与PFOSA的浓度比值分析显示,北极熊的转化效率最高,其次分别为斑海豹(Phoca largha)、环斑海豹等[112]。
对于PFCAs,大量研究表明,仅有长链(C9~C12) PFCAs在海洋食物网中具有一定的沿食物链放大效应[113]。Butt等[109]对加拿大境内的北极地区不同海域中环斑海豹肝脏中的PFAS分析发现,全氟壬酸(PFNA)、全氟癸酸(PFDA)和全氟十一烷酸(PFUnDA)为主要PFCAs检出物。该检出模式与东格陵兰极地地区的北极熊和虎鲸(Orcinus orca)中相似[114]。Rayne和Forest[115]对PFCAs在海洋食物链生物放大的研究表明,部分全氟羧酸(C7~C14 PFCAs)的TMFPW>1,且随着KPW和KPA(KPW:102~104;KPA:104~106)的增加而增加,对于不在此范围的PFCAs,其吸收速率、同化效率以及生物可利用度的降低等可影响其在海洋生物体内的累积。
随着PFOS和PFOA被《斯德哥尔摩公约》列为持久性有机污染物,其生产和使用的禁止和限制促进了一系列替代品的研发和投入使用。其中,全氟烷醚磺酸(PFESAs)和全氟烷醚羧酸(PFECAs)是当下在海洋生态系统中被广泛检出的2类替代品,其潜在的生态环境危害备受关注。在海洋环境中,氯代多氟烷醚磺酸盐(F-53B)和六氟环氧丙烷二聚体羧酸(HPFO-DA)及其同系物六氟环氧丙烷三聚体羧酸(HPFO-TA)、六氟环氧丙烷四聚体羧酸(HFPO-TeA)等是目前报道较多的化合物。作为PFOS的替代品,F-53B的生物富集潜力和毒性均不容忽视。Liu等[116]对中国渤海食物网研究发现,F-53B具有显著的生物富集和沿食物链生物放大效应,其TMF(3.4~4.3)与PFOS(3.8~3.9)相当。与此同时,PFOA的替代品及其同系物包括HFPO-TA、HFPO-TeA、全氟-3,5,7,9,11-五氧杂十二烷酸(PFO5DoDA)和7-氢-全氟-4-甲基-3,6-二氧辛烷磺酸(H-PFMO2OSA)在我国东北的小清河河口食物网中均呈现显著的沿食物链放大效应,其TMF与全氟碳链长度和醚氧基数量呈一定的正相关关系[117]。以上研究表明,相比于传统PFOS和PFOA,新型全氟和多氟烷醚类化合物的生物累积性并未得到有效的改善,并可能成为当前海洋环境健康领域需面对的新问题。
3 总结和展望(Summary and prospect)
海洋生态系统受纳了大量来自大陆、河流等排入的污染物质。POPs作为一类可在环境中持久性存在,并可长距离迁移的有机污染物,其在海洋环境中的长期赋存和生态危害是当前海洋环境保护的核心问题之一。随着工业发展和合成化学品物质的不断涌现,新型持久性有机污染物在海洋环境被广泛检出。然而对其环境持久性和生物累积性的认知仍较为缺乏。本文重点综述POPs在海洋环境中的生物富集、沿食物链放大潜力以及相关的影响因素,针对现有的研究提出以下几点总结和展望:(1)传统POPs在海洋环境中的生物累积和生态危害仍需进行长期的跟踪和评估;(2)新型持久性有机污染物在海洋环境中富集和放大的研究数据较为缺乏,对该类污染物的生物累积性尚未形成明确的认知;(3)POPs在全球不同海域食物链/网的生物累积特征和差异仍需重点关注;(4)全球气候变化以及其他因素对POPs生物累积的综合性影响不容忽视,在该方面的研究仍需加强。
[1] 阮挺, 江桂斌. 发现新型环境有机污染物的基本理论与方法[J]. 中国科学院院刊, 2020, 35(11): 1328-1336
Ruan T, Jiang G B. Basic theory and analytical methodology for identification of novel environmental organic pollutants [J]. Bulletin of Chinese Academy of Sciences, 2020, 35(11): 1328-1336 (in Chinese)
[2] Yang Y, Zhang X R, Jiang J Y, et al. Which micropollutants in water environments deserve more attention globally? [J]. Environmental Science & Technology, 2022, 56(1): 13-29
[3] 陈家苗, 王建设. 新型全氟和多氟烷醚类化合物的环境分布与毒性研究进展[J]. 生态毒理学报, 2020, 15(5): 28-34
Chen J M, Wang J S. Research progress in environmental distribution and toxicity of per-and polyfluoroalkyl ether substances [J]. Asian Journal of Ecotoxicology, 2020, 15(5): 28-34 (in Chinese)
[4] United Nations Environmental Programme. Stockholm Convention [EB/OL]. (2020-09-01) [2022-10-16]. http://pops.int.
[5] 中国生态环境部. 重点管控新污染物清单(2022年版)(征求意见)[EB/OL]. [2022-09-24]. https://www.mee.gov.cn/xxhk2018/xxgk/xxgk06/202209/t20220927_995054.html.
[6] O’Driscoll K, Mayer B, Ilyina T, et al. Modelling the cycling of persistent organic pollutants (POPs) in the North Sea system: Fluxes, loading, seasonality, trends [J]. Journal of Marine Systems, 2013, 111-112: 69-82
[7] van Ael E, Covaci A, Das K, et al. Factors influencing the bioaccumulation of persistent organic pollutants in food webs of the Scheldt Estuary [J]. Environmental Science & Technology, 2013, 47(19): 11221-11231
[8] Gobas F A, de Wolf W, Burkhard L P, et al. Revisiting bioaccumulation criteria for POPs and PBT assessments [J]. Integrated Environmental Assessment and Management, 2009, 5(4): 624-637
[9] Berrojalbiz N, Lacorte S, Calbet A, et al. Accumulation and cycling of polycyclic aromatic hydrocarbons in zooplankton [J]. Environmental Science & Technology, 2009, 43(7): 2295-2301
[10] Gerofke A, Kömp P, McLachlan M S. Bioconcentration of persistent organic pollutants in four species of marine phytoplankton [J]. Environmental Toxicology and Chemistry, 2005, 24(11): 2908-2917
[11] Sobek A, McLachlan M S, Borgå K, et al. A comparison of PCB bioaccumulation factors between an Arctic and a temperate marine food web [J]. The Science of the Total Environment, 2010, 408(13): 2753-2760
[12] Kim S K. Trophic transfer of organochlorine pesticides through food-chain in coastal marine ecosystem [J]. Environmental Engineering Research, 2020, 25(1): 43-51
[13] Zhong H F, Zheng M G, Liang Y, et al. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in sediments from the East China Sea and the Yellow Sea: Occurrence, source apportionment and environmental risk assessment [J]. Chemosphere, 2021, 282: 131042
[14] Sun Y X, Hao Q, Xu X R, et al. Persistent organic pollutants in marine fish from Yongxing Island, South China Sea: Levels, composition profiles and human dietary exposure assessment [J]. Chemosphere, 2014, 98: 84-90
[15] Sun R X, Luo X J, Tang B, et al. Short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary in South China: Residue levels and interspecies differences [J]. The Science of the Total Environment, 2016, 553: 196-203
[16] Martín J, Hidalgo F, García-Corcoles M T, et al. Bioaccumulation of perfluoroalkyl substances in marine echinoderms: Results of laboratory-scale experiments with Holothuria tubulosa Gmelin, 1791 [J]. Chemosphere, 2019, 215: 261-271
[17] 姚文君, 薛文平, 国文, 等. 环渤海近岸海域表层沉积物及底栖生物中PBDEs的赋存特征及富集行为[J]. 生态毒理学报, 2016, 11(2): 413-420
Yao W J, Xue W P, Guo W, et al. Occurrence and bioaccumulation of polybrominated diphenyl ethers(PBDEs) in surficial sediment and benthic organism in the Bohai Sea [J]. Asian Journal of Ecotoxicology, 2016, 11(2): 413-420 (in Chinese)
[18] Ali N, Ali L N, Eqani S A, et al. Organohalogenated contaminants in sediments and bivalves from the northern Arabian Gulf [J]. Ecotoxicology and Environmental Safety, 2015, 122: 432-439
[19] Zhang C C, Li Y L, Wang C L, et al. Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: Bioaccumulation and human health risk assessment [J]. Marine Pollution Bulletin, 2020, 153: 110995
[20] Xiang N, Jiang C X, Yang T H, et al. Occurrence and distribution of polycyclic aromatic hydrocarbons (PAHs) in seawater, sediments and corals from Hainan Island, China [J]. Ecotoxicology and Environmental Safety, 2018, 152: 8-15
[21] Thomann R V, Komlos J. Model of biota-sediment accumulation factor for polycyclic aromatic hydrocarbons [J]. Environmental Toxicology and Chemistry, 1999, 18(5): 1060-1068
[22] Borgå K, Kidd K A, Muir D C, et al. Trophic magnification factors: Considerations of ecology, ecosystems, and study design [J]. Integrated Environmental Assessment and Management, 2012, 8(1): 64-84
[23] Borgå K, Fisk A T, Hoekstra P E, et al. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs [J]. Environmental Toxicology and Chemistry, 2004, 23(10): 2367-2385
[24] Kelly B C, Gobas F A P C. An Arctic terrestrial food-chain bioaccumulation model for persistent organic pollutants [J]. Environmental Science & Technology, 2003, 37(13): 2966-2974
[25] Landrum P F. Toxicokinetics of organic xenobiotics in the amphipod, Pontoporeia hoyi: role of physiological and environmental variables [J]. Aquatic Toxicology, 1988, 12(3): 245-271
[26] O’Connor A T, Robinson D, Dasgupta T P, et al. Bioaccumulation of polychlorinated biphenyls (PCBs) in Atlantic Sea bream (Archosargus rhomboidalis) from Kingston Harbour, Jamaica [J]. Bulletin of Environmental Contamination and Toxicology, 2017, 99(3): 328-332
[27] Bustnes J O, Bårdsen B J, Herzke D, et al. Plasma concentrations of organohalogenated pollutants in predatory bird nestlings: Associations to growth rate and dietary tracers [J]. Environmental Toxicology and Chemistry, 2013, 32(11): 2520-2527
[28] Tuerk K J S, Kucklick J R, Becker P R, et al. Persistent organic pollutants in two dolphin species with focus on toxaphene and polybrominated diphenyl ethers [J]. Environmental Science & Technology, 2005, 39(3): 692-698
[29] Ranjbar Jafarabadi A, Mashjoor S, Mohamadjafari Dehkordi S, et al. Emerging POPs-type cocktail signatures in Pusa caspica in quantitative structure-activity relationship of Caspian Sea [J]. Journal of Hazardous Materials, 2021, 406: 124334
[30] Tanabe S, Iwata H, Tatsukawa R. Global contamination by persistent organochlorines and their ecotoxicological impact on marine mammals [J]. Science of the Total Environment, 1994, 154(2-3): 163-177
[31] Zhou S S, Pan Y Q, Zhang L N, et al. Biomagnification and enantiomeric profiles of organochlorine pesticides in food web components from Zhoushan Fishing Ground, China [J]. Marine Pollution Bulletin, 2018, 131(Pt A): 602-610
[32] Sun Y X, Hu Y X, Zhang Z W, et al. Halogenated organic pollutants in marine biota from the Xuande Atoll, South China Sea: Levels, biomagnification and dietary exposure [J]. Marine Pollution Bulletin, 2017, 118(1-2): 413-419
[33] Carro N, Cobas J, García I, et al. Organochlorine compounds and polycyclic aromatic hydrocarbons in mussels from Ria de Vigo (the Northern Spanish coast). Current levels and long-term trends (2010-2019). Relationship with human pressures [J]. Regional Studies in Marine Science, 2021, 44: 101742
[34] Olisah C, Okoh O O, Okoh A I. Distribution of organochlorine pesticides in fresh fish carcasses from selected estuaries in Eastern Cape Province, South Africa, and the associated health risk assessment [J]. Marine Pollution Bulletin, 2019, 149: 110605
[35] García-Alvarez N, Martín V, Fernández A, et al. Levels and profiles of POPs (organochlorine pesticides, PCBs, and PAHs) in free-ranging common bottlenose dolphins of the Canary Islands, Spain [J]. The Science of the Total Environment, 2014, 493: 22-31
[36] Gui D, Yu R Q, He X, et al. Tissue distribution and fate of persistent organic pollutants in Indo-Pacific humpback dolphins from the Pearl River Estuary, China [J]. Marine Pollution Bulletin, 2014, 86(1-2): 266-273
[37] Pouch A, Zaborska A, Dąbrowska A M, et al. Bioaccumulation of PCBs, HCB and PAHs in the summer plankton from West Spitsbergen Fjords [J]. Marine Pollution Bulletin, 2022, 177: 113488
[38] Han M W, Liu F, Kang Y R, et al. Occurrence, distribution, sources, and bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in multi environmental media in estuaries and the coast of the Beibu Gulf, China: A health risk assessment through seafood consumption [J]. Environmental Science and Pollution Research International, 2022, 29(35): 52493-52506
[39] Han M W, Li H L, Kang Y R, et al. Bioaccumulation and trophic transfer of PAHs in tropical marine food webs from coral reef ecosystems, the South China Sea: Compositional pattern, driving factors, ecological aspects, and risk assessment [J]. Chemosphere, 2022, 308(Pt 1): 136295
[40] Li H Y, Wang X S, Peng S Y, et al. Seasonal variation of temperature affects HMW-PAH accumulation in fishery species by bacterially mediated LMW-PAH degradation [J]. The Science of the Total Environment, 2022, 853: 158617
[41] Moon H B, An Y R, Park K J, et al. Occurrence and accumulation features of polycyclic aromatic hydrocarbons and synthetic musk compounds in finless porpoises (Neophocaena phocaenoides) from Korean coastal waters [J]. Marine Pollution Bulletin, 2011, 62(9): 1963-1968
[42] Castro-Jiménez J, Bănaru D, Chen C T, et al. Persistent organic pollutants burden, trophic magnification and risk in a pelagic food web from coastal NW Mediterranean Sea [J]. Environmental Science & Technology, 2021, 55(14): 9557-9568
[43] Mizukawa K, Takada H, Takeuchi I, et al. Bioconcentration and biomagnification of polybrominated diphenyl ethers (PBDEs) through lower-trophic-level coastal marine food web [J]. Marine Pollution Bulletin, 2009, 58(8): 1217-1224
[44] Conn K E, Liedtke T L, Takesue R K, et al. Legacy and current-use toxic contaminants in Pacific sand lance (Ammodytes personatus) from Puget Sound, Washington, USA [J]. Marine Pollution Bulletin, 2020, 158: 111287
[45] Borrell A, Tornero V, Bhattacharjee D, et al. Organochlorine concentrations in aquatic organisms from different trophic levels of the Sundarbans mangrove ecosystem and their implications for human consumption [J]. Environmental Pollution, 2019, 251: 681-688
[46] Sun Y X, Zhang Z W, Xu X R, et al. Bioaccumulation and biomagnification of halogenated organic pollutants in mangrove biota from the Pearl River Estuary, South China [J]. Marine Pollution Bulletin, 2015, 99(1-2): 150-156
[47] Megson D, Brown T, Jones G R, et al. Polychlorinated biphenyl (PCB) concentrations and profiles in marine mammals from the North Atlantic Ocean [J]. Chemosphere, 2022, 288(Pt 3): 132639
[48] Pinzone M, Budzinski H, Tasciotti A, et al. POPs in free-ranging pilot whales, sperm whales and fin whales from the Mediterranean Sea: Influence of biological and ecological factors [J]. Environmental Research, 2015, 142: 185-196
[49] Choo G, Lee I S, Oh J E. Species and habitat-dependent accumulation and biomagnification of brominated flame retardants and PBDE metabolites [J]. Journal of Hazardous Materials, 2019, 371: 175-182
[50] Barón E, Giménez J, Verborgh P, et al. Bioaccumulation and biomagnification of classical flame retardants, related halogenated natural compounds and alternative flame retardants in three delphinids from Southern European waters [J]. Environmental Pollution, 2015, 203: 107-115
[51] Li H J, Fu J J, Zhang A Q, et al. Occurrence, bioaccumulation and long-range transport of short-chain chlorinated paraffins on the Fildes Peninsula at King George Island, Antarctica [J]. Environment International, 2016, 94: 408-414
[52] Ma X D, Zhang H J, Wang Z, et al. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China [J]. Environmental Science & Technology, 2014, 48(10): 5964-5971
[53] Huang Y M, Chen L G, Jiang G, et al. Bioaccumulation and biomagnification of short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary, South China [J]. The Science of the Total Environment, 2019, 671: 262-269
[54] Zeng L X, Lam J C W, Chen H, et al. Tracking dietary sources of short- and medium-chain chlorinated paraffins in marine mammals through a subtropical marine food web [J]. Environmental Science & Technology, 2017, 51(17): 9543-9552
[55] de Wit C A, Bossi R, Dietz R, et al. Organohalogen compounds of emerging concern in Baltic Sea biota: Levels, biomagnification potential and comparisons with legacy contaminants [J]. Environment International, 2020, 144: 106037
[56] Facciola N, Pedro S, Houde M, et al. Measurable levels of short-chain chlorinated paraffins in western Hudson Bay fishes but limited biomagnification from fish to ringed seals [J]. Environmental Toxicology and Chemistry, 2021, 40(11): 2990-2999
[57] Zeng L X, Lam J C W, Wang Y W, et al. Temporal trends and pattern changes of short- and medium-chain chlorinated paraffins in marine mammals from the South China Sea over the past decade [J]. Environmental Science & Technology, 2015, 49(19): 11348-11355
[58] Yuan B, McLachlan M S, Roos A M, et al. Long-chain chlorinated paraffins have reached the Arctic [J]. Environmental Science & Technology Letters, 2021, 8(9): 753-759
[59] Ali A M, Langberg H A, Hale S E, et al. The fate of poly- and perfluoroalkyl substances in a marine food web influenced by land-based sources in the Norwegian Arctic [J]. Environmental Science Processes & Impacts, 2021, 23(4): 588-604
[60] Cara B, Lies T, Thimo G, et al. Bioaccumulation and trophic transfer of perfluorinated alkyl substances (PFAS) in marine biota from the Belgian North Sea: Distribution and human health risk implications [J]. Environmental Pollution, 2022, 311: 119907
[61] Haukås M, Berger U, Hop H, et al. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web [J]. Environmental Pollution, 2007, 148(1): 360-371
[62] Munoz G, Budzinski H, Babut M, et al. Evidence for the trophic transfer of perfluoroalkylated substances in a temperate macrotidal estuary [J]. Environmental Science & Technology, 2017, 51(15): 8450-8459
[63] Boisvert G, Sonne C, Rigét F F, et al. Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey [J]. Environmental Pollution, 2019, 252(Pt B): 1335-1343
[64] Zhang B, He Y, Yang G, et al. Legacy and emerging poly- and perfluoroalkyl substances in finless porpoises from East China Sea: Temporal trends and tissue-specific accumulation [J]. Environmental Science & Technology, 2022, 56(10): 6113-6122
[65] Jurado E, Jaward F, Lohmann R, et al. Wet deposition of persistent organic pollutants to the global oceans [J]. Environmental Science & Technology, 2005, 39(8): 2426-2435
[66] Bustnes J O, Borgå K, Dempster T, et al. Latitudinal distribution of persistent organic pollutants in pelagic and demersal marine fish on the Norwegian Coast [J]. Environmental Science & Technology, 2012, 46(14): 7836-7843
[67] Vorkamp K, Balmer J, Hung H, et al. A review of chlorinated paraffin contamination in Arctic ecosystems [J]. Emerging Contaminants, 2019, 5: 219-231
[68] Teuten E L, Rowland S J, Galloway T S, et al. Potential for plastics to transport hydrophobic contaminants [J]. Environmental Science & Technology, 2007, 41(22): 7759-7764
[69] Koelmans A A. Modeling the Role of Microplastics in Bioaccumulation of Organic Chemicals to Marine Aquatic Organisms. A Critical Review [M]//Marine Anthropogenic Litter. Cham: Springer International Publishing, 2015: 309-324
[70] Koelmans A A, Bakir A, Burton G A, et al. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies [J]. Environmental Science & Technology, 2016, 50(7): 3315-3326
[71] Nfon E, Cousins I T, Broman D. Biomagnification of organic pollutants in benthic and pelagic marine food chains from the Baltic Sea [J]. The Science of the Total Environment, 2008, 397(1-3): 190-204
[72] Hop H, Borgá K, Gabrielsen G W, et al. Food web magnificaton of persistent organic pollutants in poikilotherms and homeotherms [J]. Environmental Science & Technology, 2002, 36(12): 2589-2597
[73] Borgå K, Gabrielsen G W, Skaare J U. Biomagnification of organochlorines along a Barents Sea food chain [J]. Environmental Pollution, 2001, 113(2): 187-198
[74] Northcott G L, Jones K C. Partitioning, extractability, and formation of nonextractable PAH residues in soil. 2. Effects on compound dissolution behavior [J]. Environmental Science & Technology, 2001, 35(6): 1111-1117
[75] Wan Y, Jin X H, Hu J Y, et al. Trophic dilution of polycyclic aromatic hydrocarbons (PAHs) in a marine food web from Bohai Bay, North China [J]. Environmental Science & Technology, 2007, 41(9): 3109-3114
[76] Takeuchi I, Miyoshi N, Mizukawa K, et al. Biomagnification profiles of polycyclic aromatic hydrocarbons, alkylphenols and polychlorinated biphenyls in Tokyo Bay elucidated by delta 13C and delta 15N isotope ratios as guides to trophic web structure [J]. Marine Pollution Bulletin, 2009, 58(5): 663-671
[77] Akhbarizadeh R, Moore F, Keshavarzi B. Polycyclic aromatic hydrocarbons and potentially toxic elements in seafood from the Persian Gulf: Presence, trophic transfer, and chronic intake risk assessment [J]. Environmental Geochemistry and Health, 2019, 41(6): 2803-2820
[78] Malmquist L M, Selck H, Jørgensen K B, et al. Polycyclic aromatic acids are primary metabolites of alkyl-PAHs-A case study with Nereis diversicolor [J]. Environmental Science & Technology, 2015, 49(9): 5713-5721
[79] 于海瀛. 部分有机化合物空气/颗粒物分配系数与正辛醇/空气分配系数的预测研究[D]. 大连: 大连理工大学, 2008: 75-78
Yu H Y. Prediction for gas-particle partition coefficient and octanol-air partition coefficient of selected organic compounds [D]. Dalian: Dalian University of Technology, 2008: 75-78 (in Chinese)
[80] Prince K D, Taylor S D, Angelini C. A global, cross-system meta-analysis of polychlorinated biphenyl biomagnification [J]. Environmental Science & Technology, 2020, 54(18): 10989-11001
[81] Walters D M, Mills M A, Cade B S, et al. Trophic magnification of PCBs and its relationship to the octanol-water partition coefficient [J]. Environmental Science & Technology, 2011, 45(9): 3917-3924
[82] Buckman A H, Wong C S, Chow E A, et al. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish [J]. Aquatic Toxicology, 2006, 78(2): 176-185
[83] Kelly B C, Ikonomou M G, Blair J D, et al. Food web-specific biomagnification of persistent organic pollutants [J]. Science, 2007, 317(5835): 236-239
[84] Kucklick J, Schwacke L, Wells R, et al. Bottlenose dolphins as indicators of persistent organic pollutants in the western North Atlantic Ocean and northern Gulf of Mexico [J]. Environmental Science & Technology, 2011, 45(10): 4270-4277
[85] García- lvarez N, Boada L D, Fernández A, et al. Assessment of the levels of polycyclic aromatic hydrocarbons and organochlorine contaminants in bottlenose dolphins (Tursiops truncatus) from the Eastern Atlantic Ocean [J]. Marine Environmental Research, 2014, 100: 48-56
lvarez N, Boada L D, Fernández A, et al. Assessment of the levels of polycyclic aromatic hydrocarbons and organochlorine contaminants in bottlenose dolphins (Tursiops truncatus) from the Eastern Atlantic Ocean [J]. Marine Environmental Research, 2014, 100: 48-56
[86] Boon J P, Oostingh I, van der Meer J, et al. A model for the bioaccumulation of chlorobiphenyl congeners in marine mammals [J]. European Journal of Pharmacology, 1994, 270(2-3): 237-251
[87] Boon J P, van der Meer J, Allchin C R, et al. Concentration-dependent changes of PCB patterns in fish-eating mammals: Structural evidence for induction of cytochrome P450 [J]. Archives of Environmental Contamination and Toxicology, 1997, 33(3): 298-311
[88] Kannan N, Reusch T B, Schulz-Bull D E, et al. Chlorobiphenyls: Model compounds for metabolism in food chain organisms and their potential use as ecotoxicological stress indicators by application of the metabolic slope concept [J]. Environmental Science & Technology, 1995, 29(7): 1851-1859
[89] Hoekstra P F, Wong C S, O’Hara T M, et al. Enantiomer-specific accumulation of PCB atropisomers in the bowhead whale (Balaena mysticetus) [J]. Environmental Science & Technology, 2002, 36(7): 1419-1425
[90] Tanabe S, Watanabe S, Kan H, et al. Capacity and mode of PCB metabolism in small cetaceans1 [J]. Marine Mammal Science, 1988, 4(2): 103-124
[91] Lavandier R, Arêas J, Quinete N, et al. PCB and PBDE levels in a highly threatened dolphin species from the Southeastern Brazilian coast [J]. Environmental Pollution, 2016, 208(Pt B): 442-449
[92] Letcher R J, Morris A D, Dyck M, et al. Legacy and new halogenated persistent organic pollutants in polar bears from a contamination hotspot in the Arctic, Hudson Bay Canada [J]. The Science of the Total Environment, 2018, 610-611: 121-136
[93] Dorneles P R, Lailson-Brito J, Dirtu A C, et al. Anthropogenic and naturally-produced organobrominated compounds in marine mammals from Brazil [J]. Environment International, 2010, 36(1): 60-67
[94] Shao M H, Tao P, Wang M, et al. Trophic magnification of polybrominated diphenyl ethers in the marine food web from coastal area of Bohai Bay, North China [J]. Environmental Pollution, 2016, 213: 379-385
[95] Thomann R V. Bioaccumulation model of organic chemical distribution in aquatic food chains [J]. Environmental Science & Technology, 1989, 23(6): 699-707
[96] Stapleton H M, Letcher R J, Baker J E. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) [J]. Environmental Science & Technology, 2004, 38(4): 1054-1061
[97] Mizukawa K, Yamada T, Matsuo H, et al. Biomagnification and debromination of polybrominated diphenyl ethers in a coastal ecosystem in Tokyo Bay [J]. The Science of the Total Environment, 2013, 449: 401-409
[98] Zheng B H, Zhao X R, Ni X J, et al. Bioaccumulation characteristics of polybrominated diphenyl ethers in the marine food web of Bohai Bay [J]. Chemosphere, 2016, 150: 424-430
[99] van Mourik L M, Gaus C, Leonards P E G, et al. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015 [J]. Chemosphere, 2016, 155: 415-428
[100] Scheringer M. Characterization of the environmental distribution behavior of organic chemicals by means of persistence and spatial range [J]. Environmental Science & Technology, 1997, 31(10): 2891-2897
[101] Li C, Xie H B, Chen J W, et al. Predicting gaseous reaction rates of short chain chlorinated paraffins with ·OH: Overcoming the difficulty in experimental determination [J]. Environmental Science & Technology, 2014, 48(23): 13808-13816
[102] Huang H T, Gao L R, Xia D, et al. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China [J]. Scientific Reports, 2017, 7(1): 10749
[103] Ma X D, Zhang H J, Yao Z W, et al. Bioaccumulation and trophic transfer of polybrominated diphenyl ethers (PBDEs) in a marine food web from Liaodong Bay, North China [J]. Marine Pollution Bulletin, 2013, 74(1): 110-115
[104] Wan Y, Hu J Y, Yang M, et al. Characterization of trophic transfer for polychlorinated dibenzo-p-dioxins, dibenzofurans, non- and mono-ortho polychlorinated biphenyls in the marine food web of Bohai Bay, North China [J]. Environmental Science & Technology, 2005, 39(8): 2417-2425
[105] Goecke-Flora C M, Reo N V. Influence of carbon chain length on the hepatic effects of perfluorinated fatty acids. A 19F- and 31P-NMR investigation [J]. Chemical Research in Toxicology, 1996, 9(4): 689-695
[106] Martin J W, Mabury S A, Solomon K R, et al. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss) [J]. Environmental Toxicology and Chemistry, 2003, 22(1): 196-204
[107] Hoekman D. Exploring QSAR fundamentals and applications in chemistry and biology [J]. Journal of the American Chemical Society, 1996, 118(43): 10678
[108] Conder J M, Hoke R A, de Wolf W, et al. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds [J]. Environmental Science & Technology, 2008, 42(4): 995-1003
[109] Butt C M, Mabury S A, Kwan M, et al. Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic [J]. Environmental Toxicology and Chemistry, 2008, 27(3): 542-553
[110] Kannan K. Perfluoroalkyl and polyfluoroalkyl substances: Current and future perspectives [J]. Environmental Chemistry, 2011, 8(4): 333
[111] Tomy G T, Budakowski W, Halldorson T, et al. Fluorinated organic compounds in an eastern Arctic marine food web [J]. Environmental Science & Technology, 2004, 38(24): 6475-6481
[112] Galatius A, Bossi R, Sonne C, et al. PFAS profiles in three North Sea top predators: Metabolic differences among species? [J]. Environmental Science and Pollution Research International, 2013, 20(11): 8013-8020
[113] Miranda D A, Peaslee G F, Zachritz A M, et al. A worldwide evaluation of trophic magnification of per- and polyfluoroalkyl substances in aquatic ecosystems [J]. Integrated Environmental Assessment and Management, 2022, 18(6): 1500-1512
[114] Gebbink W A, Bossi R, Rigét F F, et al. Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals [J]. Chemosphere, 2016, 144: 2384-2391
[115] Rayne S, Forest K. Perfluoroalkyl contaminants in an Arctic marine food web: Trophic magnification and wildlife exposure [J]. Environmental Science & Technology, 2009, 43(11): 4037-4043
[116] Liu Y W, Ruan T, Lin Y F, et al. Chlorinated polyfluoroalkyl ether sulfonic acids in marine organisms from Bohai Sea, China: Occurrence, temporal variations, and trophic transfer behavior [J]. Environmental Science & Technology, 2017, 51(8): 4407-4414
[117] Li Y N, Yao J Z, Zhang J, et al. First report on the bioaccumulation and trophic transfer of perfluoroalkyl ether carboxylic acids in estuarine food web [J]. Environmental Science & Technology, 2022, 56(10): 6046-6055