双酚A(bisphenol A, BPA)于1891年被首次合成,作为聚碳酸酯和环氧树脂等高分子材料的合成原料和显色剂,在工业生产中被广泛使用[1-3],是全球年均生产量最大的化学原料之一[4]。至2013年,BPA全球年产量已超过580万t,且仍有增长趋势[5]。塑料或涂层制品中的BPA在长时间的高温、酸碱或紫外辐射下,可通过水解或氨解迁移至食物、环境和皮肤[6-8],进而被人体接触并摄入。目前已在多种环境介质、人体介质中检出BPA,包括空气、室内灰尘、地表水、废水和沉积物等环境样品[9-11]以及包括人类在内多种动物的血液、尿液、唾液、毛发、母乳和羊水等生物样本[12-15],甚至在母体胎盘、脐带血和新生儿尿液中也有检出[7, 16]。进入人体的BPA可与多种受体相互作用,如雌激素受体(ER)、雄激素受体(AR)、雌激素相关受体γ(ERRγ)、甲状腺激素受体(TR)和芳香烃受体(AHR)等[17-19],对人体内分泌系统产生干扰,甚至会对后代的生长发育和生殖功能造成危害[7, 20-23]。流行病学调查显示性欲减退、生殖障碍、多囊卵巢综合征、出生缺陷和包括乳腺癌在内的多种癌症发生等都与BPA的持续暴露有关[5, 8, 20, 24-28]。正因如此,BPA于2008年和2017年先后被美国国家毒理学计划(National Toxicology Program, NTP)和成员国委员会(Member State Committee, MSC)列为环境内分泌干扰物(environmental endocrine-disrupting chemical, EDC)[8]。
随着2008年开始各国陆续发布法律条令限制BPA在塑料制品中的使用[1, 12],BPA替代物的开发成为工业生产亟待解决的问题,这直接刺激了与BPA结构类似的其他双酚类化合物(BPs),如双酚S(bisphenol S, BPS)、双酚F(bisphenol F, BPF)、双酚AF(bisphenol AF, BPAF)、双酚B(bisphenol B, BPB)、双酚E(bisphenol E, BPE)、双酚C(bisphenol C, BPC)、双酚Z(bisphenol Z, BPZ)、双酚FL(bisphenol FL, BPFL)、双酚AP(bisphenol AP, BPAP)、双酚BP(bisphenol BP, BPBP)、双酚M(bisphenol M, BPM)和双酚P(bisphenol P, BPP)等BPA类似物的开发、生产和工业应用。随着BPA类似物的不断投产和使用,越来越多BPA类似物在食物、空气、室内灰尘、水体和沉积物等环境样本和人体尿液、血清等生物样本中被检出,人类尿液排泄数据表明世界人类BPs日均摄入量为每人0.06~0.68 μg·d-1,而另一项研究表明人类血清中各BPs中位数水平为0.07~11.94 ng·mL-1,尽管部分BPs可由人体代谢排出,但BPs对人体的持续暴露仍然增加了体内游离BPs风险[9, 29-35]。且实验表明多种BPA类似物(如BPB、BPF和BPE等)可通过胎盘转移[36],提示BPA类似物的生物安全性有待进一步研究。研究表明,BPA类似物与BPA具有相似的生殖内分泌干扰效应,且部分类似物所展现的生殖毒性比BPA更强[2, 17, 25, 31, 37-39]。
相较于BPA而言,双酚类似物的生殖毒性及相关分子机制的研究较少,这阻碍了对BPA及其类似物于环境安全和人体健康的综合评价。本文将综述近年来国内外针对BPA类似物的生殖毒性、致毒分子机制和人体生殖健康风险的研究进展、存在的问题及展望。
1 BPA类似物的生殖毒性效应(Reproductive toxic effects of BPA analogues)
针对BPA类似物生殖毒性的探索主要来源于小鼠、大鼠和斑马鱼等模式动物,下文则是在动物研究的基础上对BPA类似物对雄性及雌性生殖系统的影响做出的统计。
1.1 BPA类似物对雄性生殖系统的影响
BPA类似物对雄性动物生殖系统的危害主要体现在一些生殖毒性指标的改变,包括雄性的性器官及性附器官质量、性器官组织学变化、生殖细胞发生过程、精子产量及活力、体内激素含量、类固醇激素合成相关基因的表达、卵黄蛋白原表达量(斑马鱼)、氧化应激反应、表观遗传变化和后代数量及健康程度(表1)。值得注意的是环境浓度、低浓度和较高浓度BPs暴露对精子数量及质量均会产生不良影响,以环境相关浓度的BPS(10 ng·kg-1·d-1)[40]暴露小鼠后会破坏睾丸组织结构致使精子数量显著下降,经BPF(25 μg·L-1) [41]、BPAF(25 mg·kg-1·d-1) [42]、BPB(37.5 mg·kg-1·d-1) [43]和BPE(10 mg·kg-1·d-1)[44]暴露的小鼠中观察到同样的现象,其会破坏睾丸及附睾组织,而除了致使精子数降低,BPs还会显著降低精子活力,最终造成小鼠生育能力下降,这可能与BPs暴露导致雄性生殖系统氧化应激水平的增加有关[41-43, 45-46](表1)。除BPC和BPFL,不同剂量BPA类似物暴露雄性动物后均会提高体内雌二醇(E2)水平,显示出同BPA相似的类雌激素效应[41, 46-49]。BPC(0.5 μmol·L-1)暴露青鳉8 h便可引起体内雌激素响应生物标记基因的表达水平显著升高,引起生殖调控系统紊乱[50]。与其他BPs有所不同的是,BPS(10 mg·kg-1·d-1)和BPE(0.05 mg·kg-1·d-1)暴露后引起小鼠体内睾酮(T)水平升高,这可能与不同暴露方式下的BPs在体内不同代谢过程所导致的生物体组织浓度差异有关[44](表1)。除此之外,BPs暴露甚至会引起多代毒性效应,BPS(50 μg·kg-1·d-1)和BPE(50 μg·kg-1·d-1)暴露孕鼠,会对F3代小鼠的生殖系统产生损害,这可能是雄鼠睾丸的表观遗传修饰发生改变所致[51-52]。
表1 双酚A(BPA)类似物对雄性动物生殖系统的危害
Table 1 The effect of bisphenol A (BPA) analogies on male reproductive systems
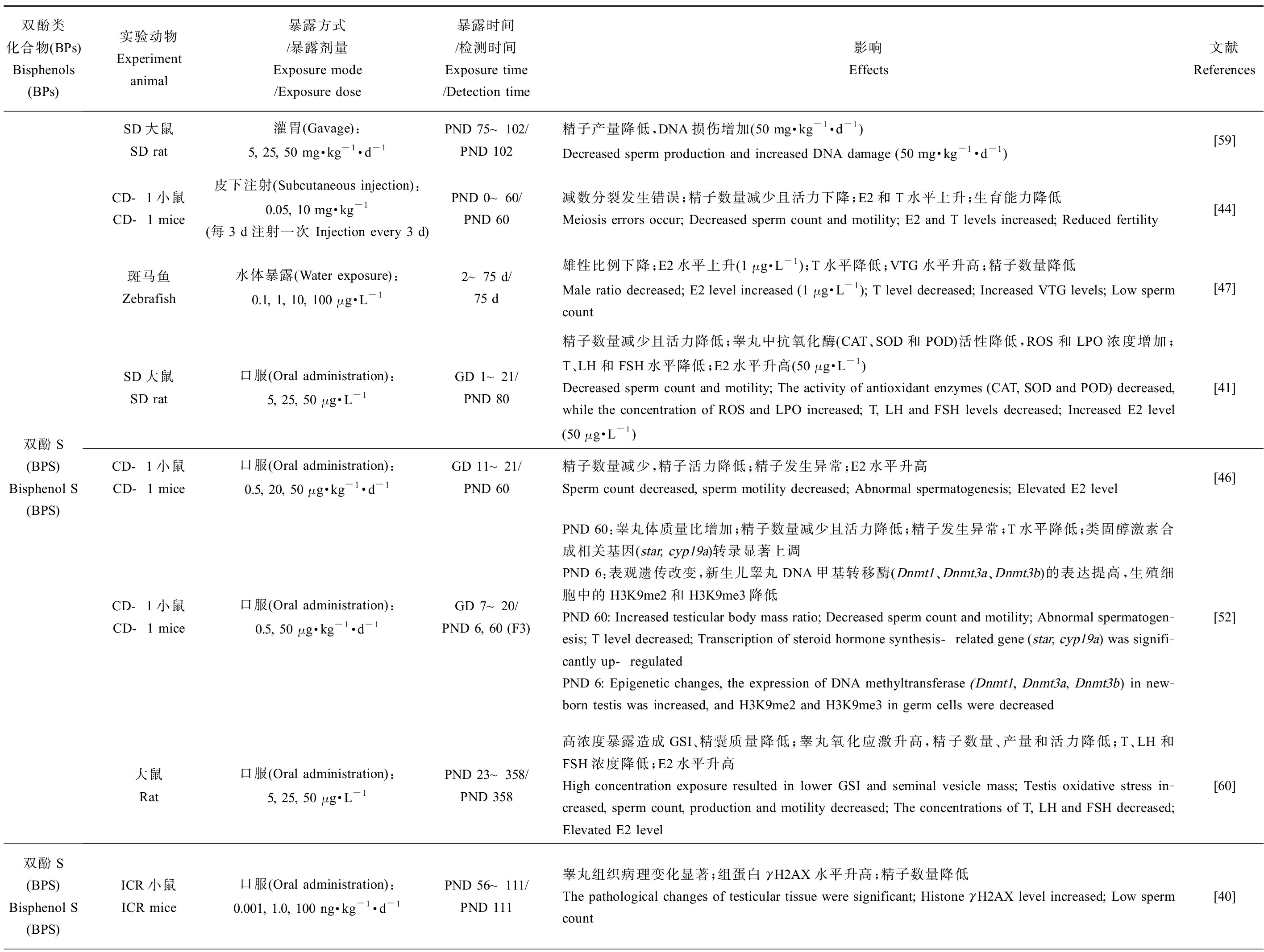
双酚类化合物(BPs)Bisphenols (BPs)实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献References双酚S(BPS) Bisphenol S (BPS)SD大鼠SD rat灌胃(Gavage):5, 25, 50 mg·kg-1·d-1PND 75~102/PND 102精子产量降低,DNA损伤增加(50 mg·kg-1·d-1)Decreased sperm production and increased DNA damage (50 mg·kg-1·d-1)[59]CD-1小鼠CD-1 mice皮下注射(Subcutaneous injection):0.05, 10 mg·kg-1(每3 d注射一次 Injection every 3 d)PND 0~60/PND 60减数分裂发生错误;精子数量减少且活力下降;E2和T水平上升;生育能力降低Meiosis errors occur; Decreased sperm count and motility; E2 and T levels increased; Reduced fertility[44]斑马鱼Zebrafish水体暴露(Water exposure):0.1, 1, 10, 100 μg·L-12~75 d/75 d雄性比例下降;E2水平上升(1 μg·L-1);T水平降低;VTG水平升高;精子数量降低Male ratio decreased; E2 level increased (1 μg·L-1); T level decreased; Increased VTG levels; Low sperm count[47]SD大鼠SD rat口服(Oral administration):5, 25, 50 μg·L-1GD 1~21/PND 80精子数量减少且活力降低;睾丸中抗氧化酶(CAT、SOD和POD)活性降低,ROS和LPO浓度增加;T、LH和FSH水平降低;E2水平升高(50 μg·L-1)Decreased sperm count and motility; The activity of antioxidant enzymes (CAT, SOD and POD) decreased, while the concentration of ROS and LPO increased; T, LH and FSH levels decreased; Increased E2 level (50 μg·L-1)[41]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 20, 50 μg·kg-1·d-1GD 11~21/PND 60精子数量减少,精子活力降低;精子发生异常;E2水平升高 Sperm count decreased, sperm motility decreased; Abnormal spermatogenesis; Elevated E2 level[46]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 50 μg·kg-1·d-1GD 7~20/PND 6, 60 (F3)PND 60:睾丸体质量比增加;精子数量减少且活力降低;精子发生异常;T水平降低;类固醇激素合成相关基因(star, cyp19a)转录显著上调PND 6:表观遗传改变,新生儿睾丸DNA甲基转移酶(Dnmt1、Dnmt3a、Dnmt3b)的表达提高,生殖细胞中的H3K9me2和H3K9me3降低PND 60: Increased testicular body mass ratio; Decreased sperm count and motility; Abnormal spermatogen-esis; T level decreased; Transcription of steroid hormone synthesis-related gene (star, cyp19a) was signifi-cantly up-regulatedPND 6: Epigenetic changes, the expression of DNA methyltransferase (Dnmt1, Dnmt3a, Dnmt3b) in new-born testis was increased, and H3K9me2 and H3K9me3 in germ cells were decreased[52]大鼠Rat口服(Oral administration):5, 25, 50 μg·L-1PND 23~358/PND 358高浓度暴露造成GSI、精囊质量降低;睾丸氧化应激升高,精子数量、产量和活力降低;T、LH和FSH浓度降低;E2水平升高High concentration exposure resulted in lower GSI and seminal vesicle mass; Testis oxidative stress in-creased, sperm count, production and motility decreased; The concentrations of T, LH and FSH decreased; Elevated E2 level[60]双酚S(BPS) Bisphenol S (BPS)ICR小鼠ICR mice口服(Oral administration):0.001, 1.0, 100 ng·kg-1·d-1PND 56~111/PND 111睾丸组织病理变化显著;组蛋白γH2AX水平升高;精子数量降低The pathological changes of testicular tissue were significant; Histone γH2AX level increased; Low sperm count[40]

续表1双酚类化合物(BPs)Bisphenols (BPs)实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献References双酚F(BPF) Bisphenol F (BPF)SD大鼠SD rat灌胃(Gavage):5, 25, 50 mg·kg-1·d-1PND 75~102/PND 102精子产量降低;DNA损伤增加(50 mg·kg-1·d-1)Decreased sperm production; Increased DNA damage (50 mg·kg-1·d-1)[59]SD大鼠SD rat口服(Oral administration):5, 25, 50 μg·L-1GD 1~21/PND 80精子数量、活力降低;睾丸细胞形态改变(25, 50 μg·L-1);睾丸中抗氧化酶(CAT、SOD和POD)活性降低;ROS和LPO浓度增加;T、LH、FSH水平降低;E2水平升高Decreased sperm count and motility; Morphological changes of testicular cells (25, 50 μg·L-1); The activi-ty of antioxidant enzymes (CAT, SOD and POD) in testis decreased; ROS and LPO concentrations in-creased; T, LH, FSH levels decreased; Elevated E2 level[41]大鼠Rat口服(Oral administration):5, 25, 50 μg·L-1PND 23~358/PND 358高浓度暴露时GSI、精囊质量降低;睾丸氧化应激升高;精子数量、活力和日产量降低;T、LH、FSH浓度降低;E2水平升高The GSI and seminal vesicle mass decreased in high concentration exposure; Elevated testicular oxidative stress; Decreased sperm count, motility and daily production; The concentration of T, LH and FSH de-creased; Elevated E2 level[60]双酚AF(BPAF) Bisphenol AF (BPAF)SD大鼠SD rat灌胃(Gavage):50, 200 mg·kg-1·d-1PND 56~69/PND 69血清总胆固醇水平降低;高剂量组T水平降低;LH和FSH水平升高Serum total cholesterol level decreased; The T level of the high-dose group was decreased; Elevated LH and FSH levels[61]昆明小鼠Kunming mice口服(Oral administration):5, 25, 50 mg·kg-1·d-1PND 21~48/PND 48血睾屏障受损;精子数量减少、质量降低(严重损害顶体功能);精子ROS水平升高;DNA损伤(50 mg·kg-1·d-1)Impairment of blood-testis barrier; Decreased sperm count and quality (severely impaired acrosomal function); ROS level of sperm increased; DNA damage (50 mg·kg-1·d-1)[42]斑马鱼Zebrafish水体暴露(Water exposure):5, 25, 125 μg·L-14 hpf~120 dT水平降低;E2水平升高(25, 125 μg·L-1);F0代HPG轴相关基因(cyp19b、star、vtg1等)转录水平改变(125 μg·L-1);F0代交配后卵子受精率下降;F1代畸形率升高,存活率降低T level decreased; E2 levels increased (25, 125 μg·L-1); Transcriptional changes of HPG axis related genes (cyp19b, star, vtg1, etc.) in F0 generation (125 μg·L-1); The ovum fertilization rate decreased after F0 generation mating; The F1 generation has an increased rate of deformity and a decreased survival rate[48]
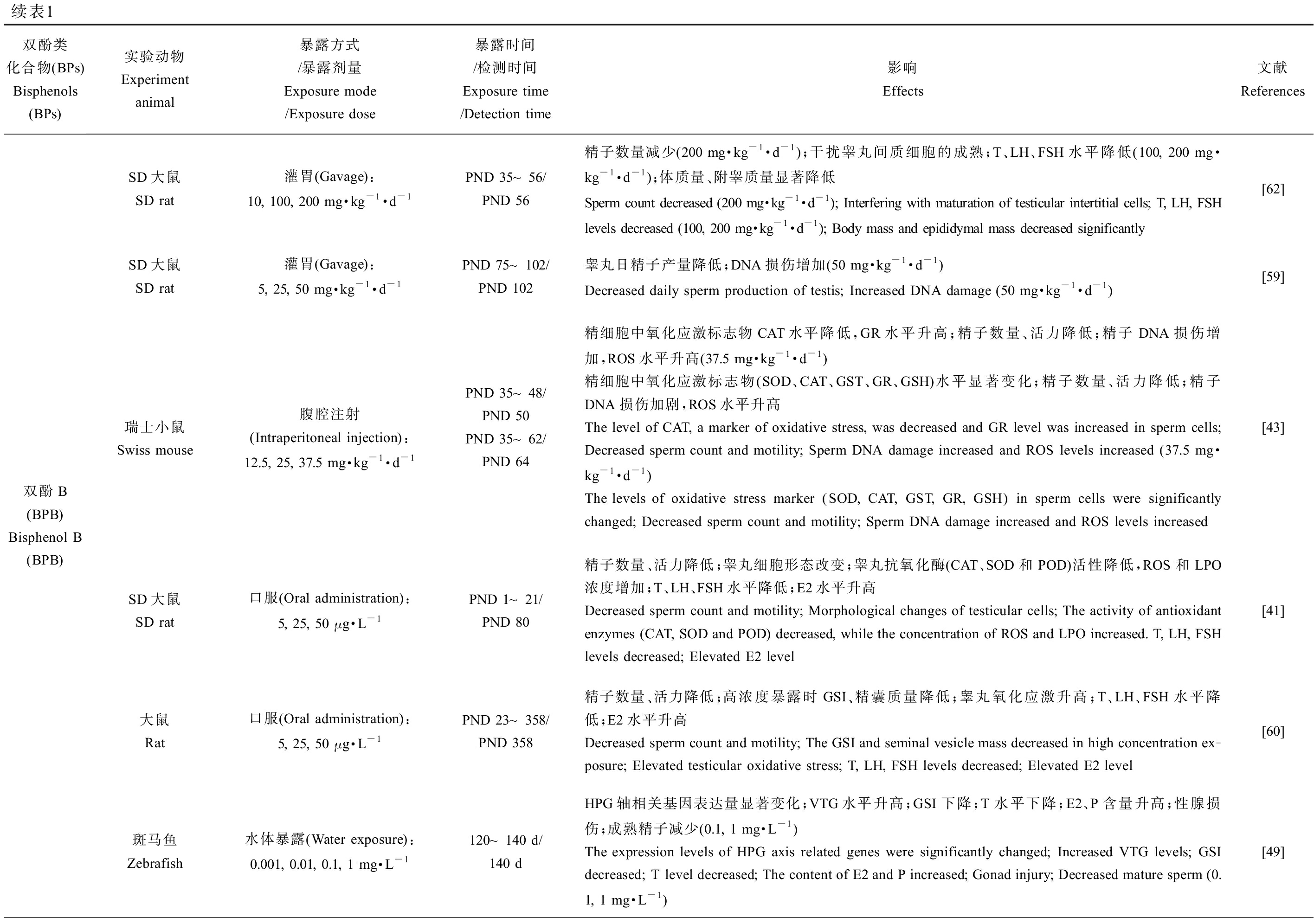
续表1双酚类化合物(BPs)Bisphenols (BPs)实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献References双酚B(BPB) Bisphenol B (BPB)SD大鼠SD rat灌胃(Gavage):10, 100, 200 mg·kg-1·d-1PND 35~56/PND 56精子数量减少(200 mg·kg-1·d-1);干扰睾丸间质细胞的成熟;T、LH、FSH水平降低(100, 200 mg·kg-1·d-1);体质量、附睾质量显著降低Sperm count decreased (200 mg·kg-1·d-1); Interfering with maturation of testicular intertitial cells; T, LH, FSH levels decreased (100, 200 mg·kg-1·d-1); Body mass and epididymal mass decreased significantly[62]SD大鼠SD rat灌胃(Gavage):5, 25, 50 mg·kg-1·d-1PND 75~102/PND 102睾丸日精子产量降低;DNA损伤增加(50 mg·kg-1·d-1)Decreased daily sperm production of testis; Increased DNA damage (50 mg·kg-1·d-1)[59]瑞士小鼠Swiss mouse腹腔注射(Intraperitoneal injection):12.5, 25, 37.5 mg·kg-1·d-1PND 35~48/PND 50PND 35~62/PND 64精细胞中氧化应激标志物CAT水平降低,GR水平升高;精子数量、活力降低;精子DNA损伤增加,ROS水平升高(37.5 mg·kg-1·d-1)精细胞中氧化应激标志物(SOD、CAT、GST、GR、GSH)水平显著变化;精子数量、活力降低;精子DNA损伤加剧,ROS水平升高The level of CAT, a marker of oxidative stress, was decreased and GR level was increased in sperm cells; Decreased sperm count and motility; Sperm DNA damage increased and ROS levels increased (37.5 mg·kg-1·d-1) The levels of oxidative stress marker (SOD, CAT, GST, GR, GSH) in sperm cells were significantly changed; Decreased sperm count and motility; Sperm DNA damage increased and ROS levels increased[43]SD大鼠SD rat口服(Oral administration):5, 25, 50 μg·L-1PND 1~21/PND 80精子数量、活力降低;睾丸细胞形态改变;睾丸抗氧化酶(CAT、SOD和POD)活性降低,ROS和LPO浓度增加;T、LH、FSH水平降低;E2水平升高Decreased sperm count and motility; Morphological changes of testicular cells; The activity of antioxidant enzymes (CAT, SOD and POD) decreased, while the concentration of ROS and LPO increased. T, LH, FSH levels decreased; Elevated E2 level[41]大鼠Rat口服(Oral administration):5, 25, 50 μg·L-1PND 23~358/PND 358精子数量、活力降低;高浓度暴露时GSI、精囊质量降低;睾丸氧化应激升高;T、LH、FSH水平降低;E2水平升高Decreased sperm count and motility; The GSI and seminal vesicle mass decreased in high concentration ex-posure; Elevated testicular oxidative stress; T, LH, FSH levels decreased; Elevated E2 level[60]斑马鱼Zebrafish水体暴露(Water exposure):0.001, 0.01, 0.1, 1 mg·L-1120~140 d/140 dHPG轴相关基因表达量显著变化;VTG水平升高;GSI下降;T水平下降;E2、P含量升高;性腺损伤;成熟精子减少(0.1, 1 mg·L-1) The expression levels of HPG axis related genes were significantly changed; Increased VTG levels; GSI decreased; T level decreased; The content of E2 and P increased; Gonad injury; Decreased mature sperm (0.1, 1 mg·L-1)[49]
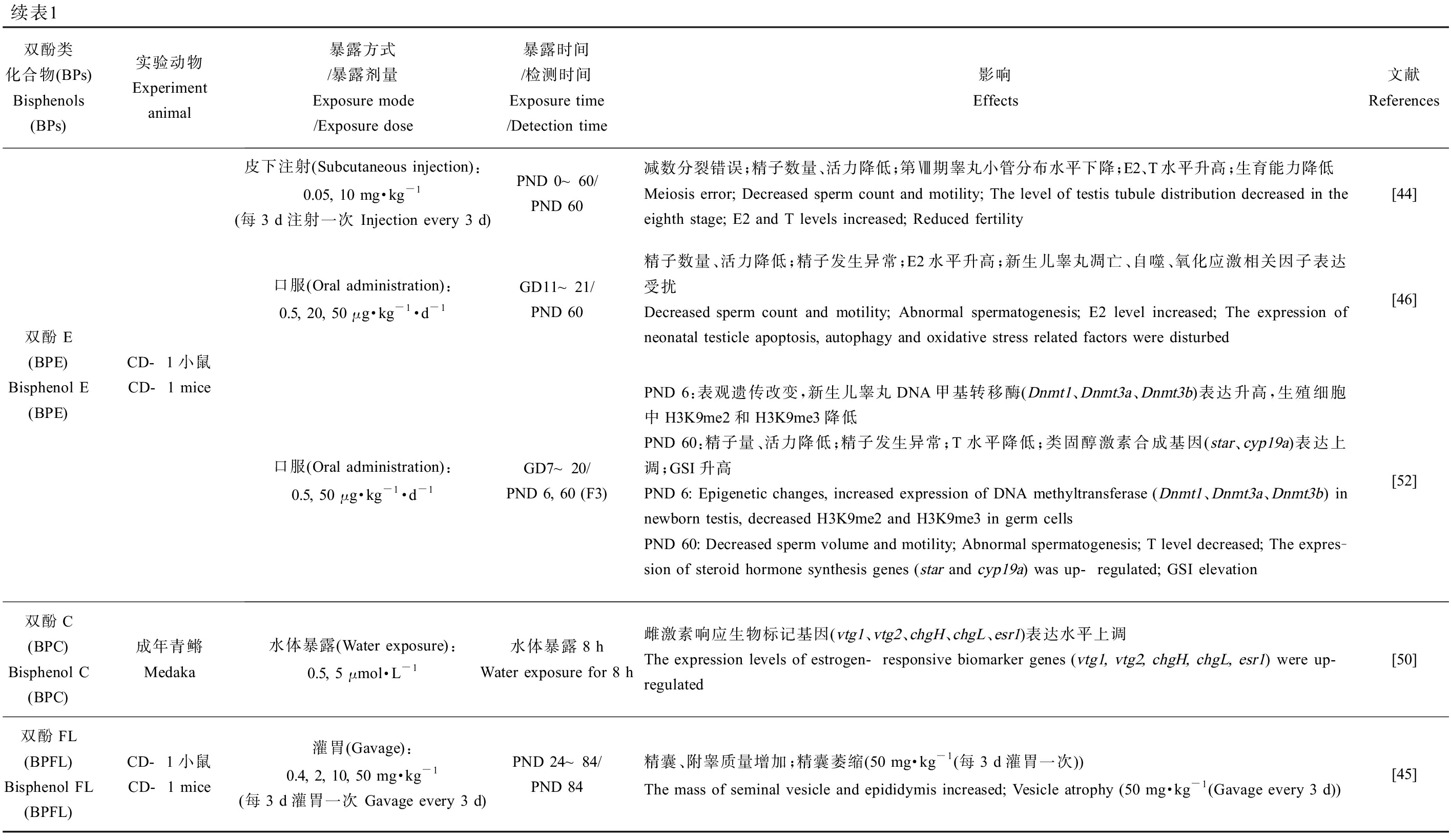
续表1双酚类化合物(BPs)Bisphenols (BPs)实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献References双酚E(BPE) Bisphenol E (BPE)CD-1小鼠CD-1 mice皮下注射(Subcutaneous injection):0.05, 10 mg·kg-1(每3 d注射一次 Injection every 3 d)PND 0~60/PND 60减数分裂错误;精子数量、活力降低;第Ⅷ期睾丸小管分布水平下降;E2、T水平升高;生育能力降低Meiosis error; Decreased sperm count and motility; The level of testis tubule distribution decreased in the eighth stage; E2 and T levels increased; Reduced fertility[44]口服(Oral administration):0.5, 20, 50 μg·kg-1·d-1GD11~21/PND 60精子数量、活力降低;精子发生异常;E2水平升高;新生儿睾丸凋亡、自噬、氧化应激相关因子表达受扰 Decreased sperm count and motility; Abnormal spermatogenesis; E2 level increased; The expression of neonatal testicle apoptosis, autophagy and oxidative stress related factors were disturbed[46]口服(Oral administration):0.5, 50 μg·kg-1·d-1GD7~20/PND 6, 60 (F3)PND 6:表观遗传改变,新生儿睾丸DNA甲基转移酶(Dnmt1、Dnmt3a、Dnmt3b)表达升高,生殖细胞中H3K9me2和H3K9me3降低PND 60:精子量、活力降低;精子发生异常;T水平降低;类固醇激素合成基因(star、cyp19a)表达上调;GSI升高PND 6: Epigenetic changes, increased expression of DNA methyltransferase (Dnmt1、Dnmt3a、Dnmt3b) in newborn testis, decreased H3K9me2 and H3K9me3 in germ cellsPND 60: Decreased sperm volume and motility; Abnormal spermatogenesis; T level decreased; The expres-sion of steroid hormone synthesis genes (star and cyp19a) was up-regulated; GSI elevation[52]双酚C(BPC) Bisphenol C (BPC)成年青鳉Medaka水体暴露(Water exposure):0.5, 5 μmol·L-1水体暴露8 hWater exposure for 8 h雌激素响应生物标记基因(vtg1、vtg2、chgH、chgL、esr1)表达水平上调The expression levels of estrogen-responsive biomarker genes (vtg1, vtg2, chgH, chgL, esr1) were up-regulated[50]双酚FL(BPFL) Bisphenol FL (BPFL)CD-1小鼠CD-1 mice灌胃(Gavage):0.4, 2, 10, 50 mg·kg-1(每3 d灌胃一次 Gavage every 3 d)PND 24~84/PND 84精囊、附睾质量增加;精囊萎缩(50 mg·kg-1(每3 d灌胃一次))The mass of seminal vesicle and epididymis increased; Vesicle atrophy (50 mg·kg-1 (Gavage every 3 d))[45]
注:PND 为出生后天数;GD为怀孕天数;hpf为受精后小时数;F0为亲代;F1/2/3为子一/二/三代;GSI为性腺指数;T为睾酮;E2为雌二醇;P为孕酮;FSH为促卵泡生成素;LH为黄体生成素;ROS为活性氧;LPO为脂质过氧化;SOD为超氧化物歧化酶;CAT为过氧化氢酶;GST为谷胱甘肽-S-转移酶;GR为谷胱甘肽还原酶;GSH为还原型谷胱甘肽;POD为过氧化物酶;HPG为下丘脑-垂体-性腺轴;VTG为卵黄蛋白原。
Note: PND stands for postnatal day; GD stands for gestational day; hpf stands for hour post fertilization; F0 stands for parent generation; F1/2/3 stands for filial generation 1/2/3; GSI stands for gonadosomatic index; T stands for testosterone; E2 stands for estradiol; P stands for progesterone; FSH stands for follicle-stimulating hormone; LH stands for luteinizing hormone; ROS stands for reactive oxygen species; LPO stands for lipid peroxidation; SOD stands for superoxide dismutase; CAT stands for catalase; GST stands for glutathione -S- transferase; GR stands for glutathione reductase; GSH stands for reduced glutathione; POD stands for peroxidase; HPG stands for hypothalamic-pituitary-gonadal; VTG stands for vitellogenin.
1.2 BPA类似物对雌性生殖系统的影响
BPs于雌性生殖健康的研究中,子宫和卵巢质量(相对质量及绝对质量,湿质量及干质量)、性腺体质量比、卵巢中各时期卵泡数量和结构变化、青春期启动时间、发情周期和受精时间、怀孕率、体内激素水平、类固醇激素生成酶相关基因表达、分娩情况及后代存活率、氧化应激水平、表观遗传修饰变化以及后代生殖健康等方面是主要靶点。在环境浓度、低浓度和较高浓度下,不同实验动物中的研究均表明BPA类似物会对雌性动物的生殖系统造成损害,如表2所示。BPS(10 μg·kg-1·d-1) [53-54]、BPF(50 mg·kg-1·d-1) [54]、BPB(5 mg·kg-1·d-1) [54]、BPC(45 mg·kg-1·d-1) [55]、BPZ(30 mg·kg-1·d-1) [56]、BPAP(80 μg·kg-1·d-1) [57]和BPFL(2 mg·kg-1,每3 d给药1次) [45]暴露后均会显著改变实验对象的子宫或卵巢质量,并影响卵巢中各时期卵泡的发育、数量以及卵母细胞质量,表明雌性动物的生殖系统同样是BPs的主要作用靶点。上述BPs会显著影响实验动物体内的E2水平,同时改变睾酮(T)、孕酮(P)、黄体生成素(LH)和促卵泡生成素(FSH)等在雌性动物生殖系统发育及繁殖中起重要作用的激素水平,扰乱激素平衡,严重干扰内分泌,从而损害生殖健康(表2)。在去除卵巢的SD大鼠暴露于BPAF(50 mg·kg-1·d-1)或BPC(45 mg·kg-1·d-1)后,其子宫出现雌激素样变化,表明BPAF和BPC具有强雌激素效应[55]。孕期暴露BPS(50 μg·kg-1·d-1)或BPE(50 μg·kg-1·d-1)致使F3代雌性小鼠出现性早熟、发情周期紊乱、怀孕率降低和幼鼠存活率降低等问题,产生多代毒性效应[58]。
2 BPA类似物生殖毒性的分子机制(Molecular mechanisms of reproductive toxicity of BPA analogues)
研究者已发现BPs对生殖系统的多种危害(表1和表2)[40-50,52-56,58-59,60-68]。对BPs生殖毒性分子机制的研究一直持续探索着。对BPs生殖毒性的分子机制研究最早从BPA开始,其化学结构决定了它与雌激素受体(ER)具有一定的亲和性。而随着研究的深入,发现BPA除了能与E2竞争性结合ER外还会对雄激素受体(AR)、雌激素相关受体γ(ERRγ)、甲状腺激素受体(TR)和芳香烃受体(AHR)等生物体内核受体产生干扰作用。除了核受体,BPA与膜相关雌激素受体——G蛋白偶联受体30(G protein-coupled receptor 30, GPR30)也表现出很强的结合亲和力。BPA通过对以上与生物体生殖相关受体产生干扰,从而扰乱生物体内激素的正常功能并最终对生殖功能造成损害。BPA类似物生殖毒性的分子机制研究大多是基于BPA类似物与BPA结构上的相似性,利用细胞、体外竞争性结合受体和计算机模拟的方法进行研究和探讨。
2.1 雌激素受体途径
BPA可与E2竞争性结合ER(包括ERα、ERβ),尽管其对ER的结合亲和力仅有E2的千分之一,但即使是低剂量的BPA也具有生殖毒性[1]。由于BPA与2种ER亚型的配体结合域结合后引起的ER构象变化的不同使得BPA同E2一样是ERα的激动剂,而成了ERβ的拮抗剂。BPA与ER的作用方式分为基因组作用模式和非基因组作用模式。经典基因组模式为BPA与细胞内的ERα和ERβ完全结合,形成配体-受体复合物,通过与细胞核内的雌激素反应元件(estrogen responsive element, ERE)结合调控靶基因表达;非基因组作用模式中BPA通过与细胞膜上的ERα和ERβ结合使其活化并与膜内其他信号蛋白(如生长因子受体等)相互作用,从而形成多分子复合物介导快速信号传导的发生[69]。非基因组模式下BPA可通过活化ERα介导的核外信号激活ERK/MAPK、PI3K/Akt和Ca2+流(与E2作用模式相同)以及抑制ERβ介导的p38/MAPK信号通路(与E2作用模式相反)发挥作用[70],不少研究表明BPA的非基因组雌激素活性与E2相当[71]。
表2 BPA类似物对雌性动物生殖系统的危害
Table 2 The effect of BPA analogies on female reproductive systems
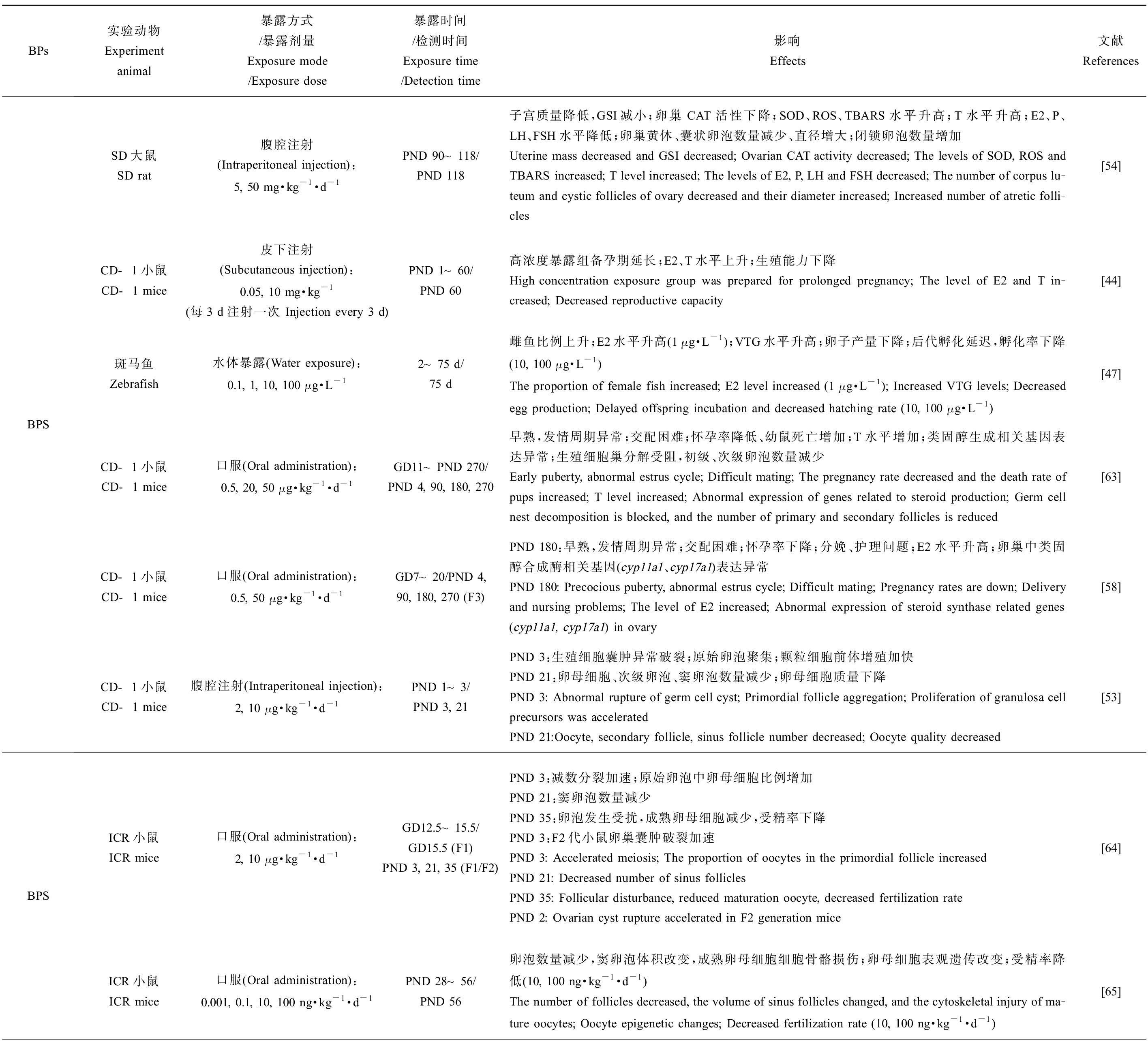
BPs实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献ReferencesBPSSD大鼠SD rat腹腔注射(Intraperitoneal injection):5, 50 mg·kg-1·d-1PND 90~118/PND 118子宫质量降低,GSI减小;卵巢CAT活性下降;SOD、ROS、TBARS水平升高;T水平升高;E2、P、LH、FSH水平降低;卵巢黄体、囊状卵泡数量减少、直径增大;闭锁卵泡数量增加Uterine mass decreased and GSI decreased; Ovarian CAT activity decreased; The levels of SOD, ROS and TBARS increased; T level increased; The levels of E2, P, LH and FSH decreased; The number of corpus lu-teum and cystic follicles of ovary decreased and their diameter increased; Increased number of atretic folli-cles[54]CD-1小鼠CD-1 mice皮下注射(Subcutaneous injection):0.05, 10 mg·kg-1(每3 d注射一次 Injection every 3 d)PND 1~60/PND 60高浓度暴露组备孕期延长;E2、T水平上升;生殖能力下降High concentration exposure group was prepared for prolonged pregnancy; The level of E2 and T in-creased; Decreased reproductive capacity[44]斑马鱼Zebrafish水体暴露(Water exposure):0.1, 1, 10, 100 μg·L-12~75 d/75 d雌鱼比例上升;E2水平升高(1 μg·L-1);VTG水平升高;卵子产量下降;后代孵化延迟,孵化率下降(10, 100 μg·L-1)The proportion of female fish increased; E2 level increased (1 μg·L-1); Increased VTG levels; Decreased egg production; Delayed offspring incubation and decreased hatching rate (10, 100 μg·L-1)[47]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 20, 50 μg·kg-1·d-1GD11~PND 270/PND 4, 90, 180, 270早熟,发情周期异常;交配困难;怀孕率降低、幼鼠死亡增加;T水平增加;类固醇生成相关基因表达异常;生殖细胞巢分解受阻,初级、次级卵泡数量减少Early puberty, abnormal estrus cycle; Difficult mating; The pregnancy rate decreased and the death rate of pups increased; T level increased; Abnormal expression of genes related to steroid production; Germ cell nest decomposition is blocked, and the number of primary and secondary follicles is reduced[63]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 50 μg·kg-1·d-1GD7~20/PND 4, 90, 180, 270 (F3)PND 180:早熟,发情周期异常;交配困难;怀孕率下降;分娩、护理问题;E2水平升高;卵巢中类固醇合成酶相关基因(cyp11a1、cyp17a1)表达异常PND 180: Precocious puberty, abnormal estrus cycle; Difficult mating; Pregnancy rates are down; Delivery and nursing problems; The level of E2 increased; Abnormal expression of steroid synthase related genes (cyp11a1, cyp17a1) in ovary[58]CD-1小鼠CD-1 mice腹腔注射(Intraperitoneal injection):2, 10 μg·kg-1·d-1PND 1~3/PND 3, 21PND 3:生殖细胞囊肿异常破裂;原始卵泡聚集;颗粒细胞前体增殖加快PND 21:卵母细胞、次级卵泡、窦卵泡数量减少;卵母细胞质量下降PND 3: Abnormal rupture of germ cell cyst; Primordial follicle aggregation; Proliferation of granulosa cell precursors was acceleratedPND 21:Oocyte, secondary follicle, sinus follicle number decreased; Oocyte quality decreased[53]BPSICR小鼠ICR mice口服(Oral administration):2, 10 μg·kg-1·d-1GD12.5~15.5/GD15.5 (F1)PND 3, 21, 35 (F1/F2)PND 3:减数分裂加速;原始卵泡中卵母细胞比例增加PND 21:窦卵泡数量减少PND 35:卵泡发生受扰,成熟卵母细胞减少,受精率下降PND 3:F2代小鼠卵巢囊肿破裂加速PND 3: Accelerated meiosis; The proportion of oocytes in the primordial follicle increasedPND 21: Decreased number of sinus folliclesPND 35: Follicular disturbance, reduced maturation oocyte, decreased fertilization ratePND 2: Ovarian cyst rupture accelerated in F2 generation mice[64]ICR小鼠ICR mice口服(Oral administration):0.001, 0.1, 10, 100 ng·kg-1·d-1PND 28~56/PND 56卵泡数量减少,窦卵泡体积改变,成熟卵母细胞细胞骨骼损伤;卵母细胞表观遗传改变;受精率降低(10, 100 ng·kg-1·d-1)The number of follicles decreased, the volume of sinus follicles changed, and the cytoskeletal injury of ma-ture oocytes; Oocyte epigenetic changes; Decreased fertilization rate (10, 100 ng·kg-1·d-1)[65]
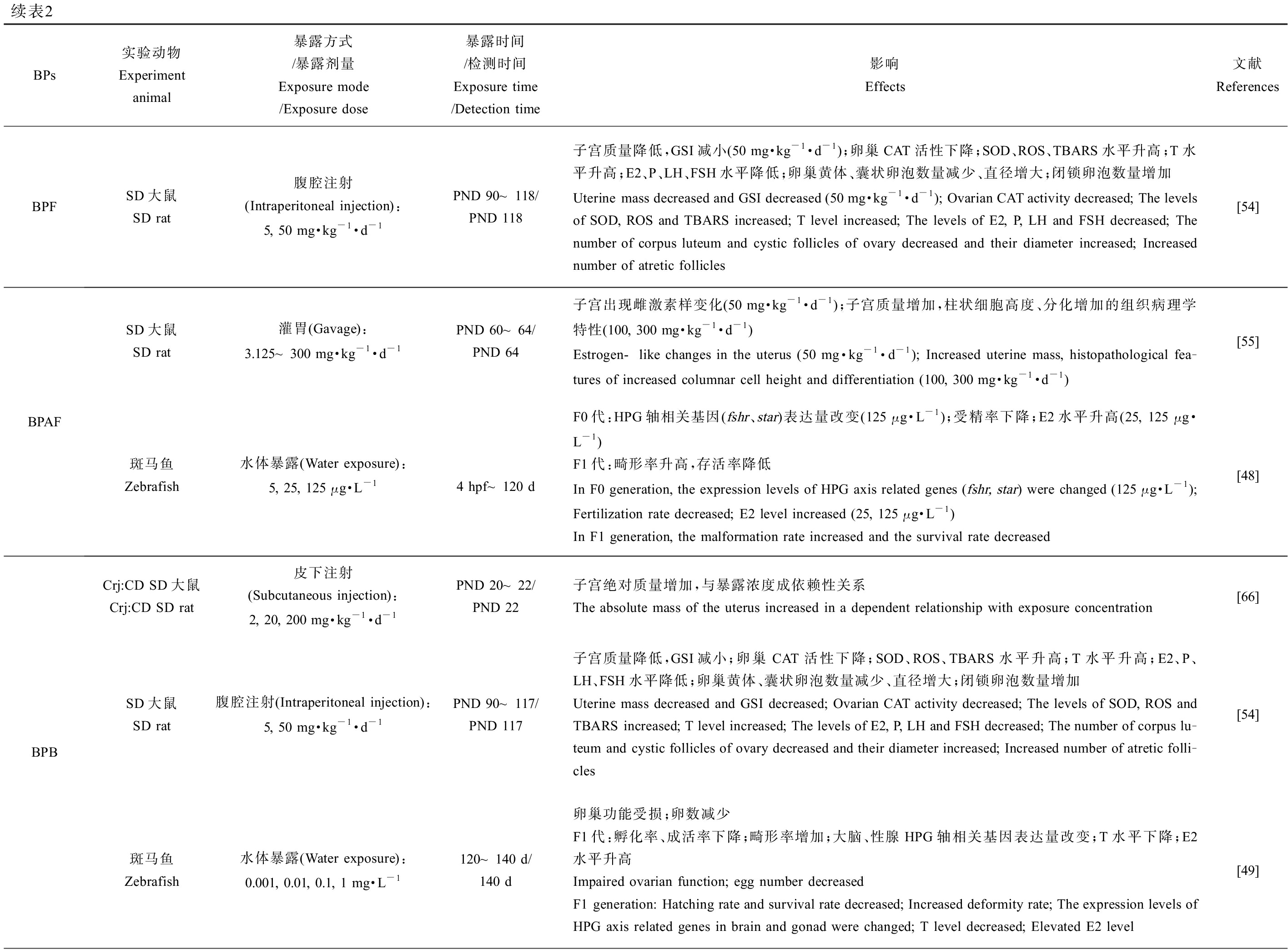
续表2BPs实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献ReferencesBPFSD大鼠SD rat腹腔注射(Intraperitoneal injection):5, 50 mg·kg-1·d-1PND 90~118/PND 118子宫质量降低,GSI减小(50 mg·kg-1·d-1);卵巢CAT活性下降;SOD、ROS、TBARS水平升高;T水平升高;E2、P、LH、FSH水平降低;卵巢黄体、囊状卵泡数量减少、直径增大;闭锁卵泡数量增加Uterine mass decreased and GSI decreased (50 mg·kg-1·d-1); Ovarian CAT activity decreased; The levels of SOD, ROS and TBARS increased; T level increased; The levels of E2, P, LH and FSH decreased; The number of corpus luteum and cystic follicles of ovary decreased and their diameter increased; Increased number of atretic follicles[54]BPAFSD大鼠SD rat灌胃(Gavage):3.125~300 mg·kg-1·d-1PND 60~64/PND 64子宫出现雌激素样变化(50 mg·kg-1·d-1);子宫质量增加,柱状细胞高度、分化增加的组织病理学特性(100, 300 mg·kg-1·d-1)Estrogen-like changes in the uterus (50 mg·kg-1·d-1); Increased uterine mass, histopathological fea-tures of increased columnar cell height and differentiation (100, 300 mg·kg-1·d-1)[55]斑马鱼Zebrafish水体暴露(Water exposure):5, 25, 125 μg·L-14 hpf~120 dF0代:HPG轴相关基因(fshr、star)表达量改变(125 μg·L-1);受精率下降;E2水平升高(25, 125 μg·L-1)F1代:畸形率升高,存活率降低In F0 generation, the expression levels of HPG axis related genes (fshr, star) were changed (125 μg·L-1); Fertilization rate decreased; E2 level increased (25, 125 μg·L-1) In F1 generation, the malformation rate increased and the survival rate decreased[48]BPBCrj:CD SD大鼠Crj:CD SD rat皮下注射(Subcutaneous injection):2, 20, 200 mg·kg-1·d-1PND 20~22/PND 22子宫绝对质量增加,与暴露浓度成依赖性关系The absolute mass of the uterus increased in a dependent relationship with exposure concentration[66]SD大鼠SD rat腹腔注射(Intraperitoneal injection):5, 50 mg·kg-1·d-1PND 90~117/PND 117子宫质量降低,GSI减小;卵巢CAT活性下降;SOD、ROS、TBARS水平升高;T水平升高;E2、P、LH、FSH水平降低;卵巢黄体、囊状卵泡数量减少、直径增大;闭锁卵泡数量增加Uterine mass decreased and GSI decreased; Ovarian CAT activity decreased; The levels of SOD, ROS and TBARS increased; T level increased; The levels of E2, P, LH and FSH decreased; The number of corpus lu-teum and cystic follicles of ovary decreased and their diameter increased; Increased number of atretic folli-cles[54]斑马鱼Zebrafish水体暴露(Water exposure):0.001, 0.01, 0.1, 1 mg·L-1120~140 d/140 d卵巢功能受损;卵数减少F1代:孵化率、成活率下降;畸形率增加;大脑、性腺HPG轴相关基因表达量改变;T水平下降;E2水平升高Impaired ovarian function; egg number decreased F1 generation: Hatching rate and survival rate decreased; Increased deformity rate; The expression levels of HPG axis related genes in brain and gonad were changed; T level decreased; Elevated E2 level[49]
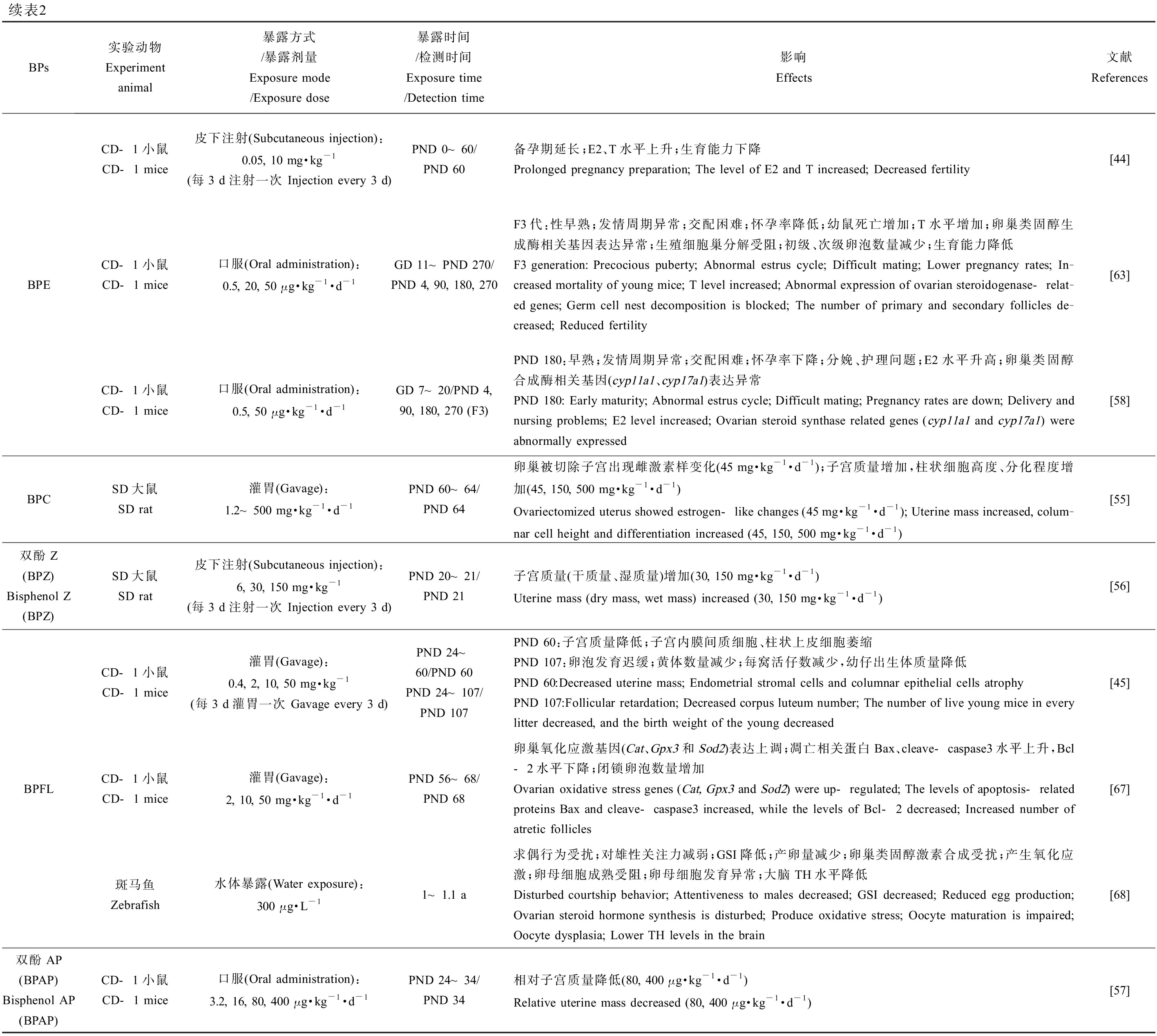
续表2BPs实验动物Experiment animal暴露方式/暴露剂量Exposure mode/Exposure dose暴露时间/检测时间Exposure time/Detection time影响Effects文献ReferencesBPECD-1小鼠CD-1 mice皮下注射(Subcutaneous injection):0.05, 10 mg·kg-1(每3 d注射一次 Injection every 3 d)PND 0~60/PND 60备孕期延长;E2、T水平上升;生育能力下降Prolonged pregnancy preparation; The level of E2 and T increased; Decreased fertility[44]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 20, 50 μg·kg-1·d-1GD 11~PND 270/PND 4, 90, 180, 270F3代:性早熟;发情周期异常;交配困难;怀孕率降低;幼鼠死亡增加;T水平增加;卵巢类固醇生成酶相关基因表达异常;生殖细胞巢分解受阻;初级、次级卵泡数量减少;生育能力降低F3 generation: Precocious puberty; Abnormal estrus cycle; Difficult mating; Lower pregnancy rates; In-creased mortality of young mice; T level increased; Abnormal expression of ovarian steroidogenase-relat-ed genes; Germ cell nest decomposition is blocked; The number of primary and secondary follicles de-creased; Reduced fertility[63]CD-1小鼠CD-1 mice口服(Oral administration):0.5, 50 μg·kg-1·d-1GD 7~20/PND 4, 90, 180, 270 (F3)PND 180:早熟;发情周期异常;交配困难;怀孕率下降;分娩、护理问题;E2水平升高;卵巢类固醇合成酶相关基因(cyp11a1、cyp17a1)表达异常PND 180: Early maturity; Abnormal estrus cycle; Difficult mating; Pregnancy rates are down; Delivery and nursing problems; E2 level increased; Ovarian steroid synthase related genes (cyp11a1 and cyp17a1) were abnormally expressed[58]BPCSD大鼠SD rat灌胃(Gavage):1.2~500 mg·kg-1·d-1PND 60~64/PND 64卵巢被切除子宫出现雌激素样变化(45 mg·kg-1·d-1);子宫质量增加,柱状细胞高度、分化程度增加(45, 150, 500 mg·kg-1·d-1)Ovariectomized uterus showed estrogen-like changes (45 mg·kg-1·d-1); Uterine mass increased, colum-nar cell height and differentiation increased (45, 150, 500 mg·kg-1·d-1)[55]双酚Z(BPZ) Bisphenol Z (BPZ)SD大鼠SD rat皮下注射(Subcutaneous injection):6, 30, 150 mg·kg-1(每3 d注射一次 Injection every 3 d)PND 20~21/PND 21子宫质量(干质量、湿质量)增加(30, 150 mg·kg-1·d-1)Uterine mass (dry mass, wet mass) increased (30, 150 mg·kg-1·d-1)[56]CD-1小鼠CD-1 mice灌胃(Gavage):0.4, 2, 10, 50 mg·kg-1(每3 d灌胃一次 Gavage every 3 d)PND 24~60/PND 60PND 24~107/PND 107PND 60:子宫质量降低;子宫内膜间质细胞、柱状上皮细胞萎缩PND 107:卵泡发育迟缓;黄体数量减少;每窝活仔数减少,幼仔出生体质量降低PND 60:Decreased uterine mass; Endometrial stromal cells and columnar epithelial cells atrophyPND 107:Follicular retardation; Decreased corpus luteum number; The number of live young mice in every litter decreased, and the birth weight of the young decreased[45]BPFLCD-1小鼠CD-1 mice灌胃(Gavage):2, 10, 50 mg·kg-1·d-1PND 56~68/PND 68卵巢氧化应激基因(Cat、Gpx3和Sod2)表达上调;凋亡相关蛋白Bax、cleave-caspase3水平上升,Bcl-2水平下降;闭锁卵泡数量增加Ovarian oxidative stress genes (Cat, Gpx3 and Sod2) were up-regulated; The levels of apoptosis-related proteins Bax and cleave-caspase3 increased, while the levels of Bcl-2 decreased; Increased number of atretic follicles[67]斑马鱼Zebrafish水体暴露(Water exposure):300 μg·L-11~1.1 a求偶行为受扰;对雄性关注力减弱;GSI降低;产卵量减少;卵巢类固醇激素合成受扰;产生氧化应激;卵母细胞成熟受阻;卵母细胞发育异常;大脑TH水平降低 Disturbed courtship behavior; Attentiveness to males decreased; GSI decreased; Reduced egg production; Ovarian steroid hormone synthesis is disturbed; Produce oxidative stress; Oocyte maturation is impaired; Oocyte dysplasia; Lower TH levels in the brain[68]双酚AP(BPAP) Bisphenol AP (BPAP)CD-1小鼠CD-1 mice口服(Oral administration):3.2, 16, 80, 400 μg·kg-1·d-1PND 24~34/PND 34相对子宫质量降低(80, 400 μg·kg-1·d-1)Relative uterine mass decreased (80, 400 μg·kg-1·d-1)[57]
注:PND 为出生后天数;GD为怀孕天数;hpf为受精后小时数;F0为亲代;F1/2/3为子一/二/三代;GSI为性腺指数;T为睾酮;E2为雌二醇;P为孕酮;FSH为促卵泡生成素;LH为黄体生成素;VTG为卵黄蛋白原;ROS为活性氧;LPO为脂质过氧化;SOD为超氧化物歧化酶;CAT为过氧化氢酶;TBARS为硫代巴比妥酸反应物;NKB为神经肽B;TH为甲状腺激素。
Note: PND stands for postnatal day; GD stands for gestational day; hpf stands for hour post fertilization; F0 stands for parent generation; F1/2/3 stands for filial generation 1/2/3; GSI stands for gonadosomatic index; T stands for testosterone; E2 stands for estradiol; P stands for progesterone; FSH stands for follicle-stimulating hormone; LH stands for luteinizing hormone; VTG stands for vitellogenin; ROS stands for reactive oxygen species; LPO stands for lipid peroxidation; SOD stands for superoxide dismutase; CAT stands for catalase; TBARS stands for thiobarbituric acid reactants; NKB stands for neuropeptide B; TH stands for thyroid hormones.
体内和体外实验均发现BPS和BPF具有和BPA类似的雌激素和抗雌激素效应[39],BPS在低剂量下也可高效激活ERα介导的ERK/MAPK通路;通过离体模型推测BPS和BPF的雌激素受体结合活性约为BPA的0.32倍和1.07倍。利用cyp19b-GFP转基因斑马鱼(ER特异性介导E2调控cyp19b基因的表达)的研究也表明BPF同BPA有相当的雌激素活性,而BPS的雌激素活性较弱[72]。在Tox21的比较中,BPAF不仅是BPA类似物中活性最高的ER激动剂,离体研究表明BPAF对ERβ的结合活性比ERα的更高[2, 73]。BPAF可通过ERα诱导的C-X-C趋化因子配体12的表达而促进ERα阳性乳腺癌细胞(T47D和MCF-7)的增殖,但对ERα阴性细胞的增殖无影响[74];同时有研究指出BPAF可激活ERβ通路和Ca2+流超载致使维持卵巢颗粒细胞生理特性的KGN细胞出现凋亡[75]。而利用干扰RNA对ER进行沉默的研究也表明BPAF通过经典基因组通路和ERK1/2依赖的非基因组通路调控雌激素活性通路[76]。动物模型和体外研究均表明BPB有与BPA相似甚至更高的雌激素活性效价,可与E2对ER产生竞争性结合[77];此外,BPB可通过破坏卵母细胞中ERα的定位模式并诱导其功能障碍而影响卵母细胞的减数分裂及其细胞质量,BPB可能通过胞外ERK1/2信号通路和PI3K/AKT信号通路诱导睾丸间质细胞增殖[62, 78]。采用青鳉进行的体内实验以及HELN-ERα和HELN-ERβ荧光素酶报告体系的体外实验均表明,BPC可结合ERα和ERβ并激活下游通路最终表现出强雌激素效应,其作用强度与BPAF和BPB在同一水平(均强于BPA)[50, 73, 79]。同时,BPC、BPAF、BPE与BPA类似,均可通过ER介导的ERK1/2信号通路提高细胞内Ca2+水平,进一步表明其可通过ER途径影响生殖[80]。分子动力学(molecular dynamics, MD)模拟联合体外的竞争性结合实验或细胞实验的研究结果也表明BPE、BPZ、BPBP、BPAP和四甲基双酚A(TMBPA)通过与ERα-LBD结合这一分子起始事件激活下游通路引发雌激素效应[81-83],而BPM、BPP和BPFL这3种BPA类似物在分子对接、MD模拟和体外实验中被证明为ER的拮抗剂,抑制HELN-ERα/ERβ的转录活性[79, 84-85],且Cao等[86]认为hERα中的Thr347是区分BPs拮抗或激动雌激素效应的关键残基。
2.2 膜雌激素受体途径
除了与经典的ER结合,BPA类似物还被证明可通过膜雌激素受体GPR30发挥内分泌干扰效应。GPR30是与ER作用模式不同的一种膜相关雌激素受体,主要通过激活第二信使环腺苷酸(cyclic adenosine monophosphate, cAMP)和表皮生长因子受体(epidermal growth factor receptor, EGFR)等的蛋白激酶途径介导快速非基因效应和转录调节[87]。BPA在低浓度时主要是通过激活GPR30介导的非基因组途径产生毒性效应[88]。环境相关剂量下BPA可通过激活GPR30介导的cGMP-PKG信号通路、cAMP-PKA信号通路、EGFR-MAPK通路介导的ERK1/2快速磷酸化等途径影响精原细胞、精母细胞的增殖和卵母细胞减数分裂[87, 89-90]。通过SKBR-3细胞荧光竞争结合实验,发现BPS、BPF、BPAF、BPB与BPA类似同样可与GPR30直接结合,且BPAF和BPB的结合亲和力比BPA高9倍[91]。除此之外,低剂量的BPF可通过GPR30激活Akt和ERK1/2信号从而诱导细胞增殖,这与E2的作用模式相似[92]。与BPF相同,不同的研究均表明BPAF可通过GPR30单独(不需要ERα参与)激活ERK1/2和Akt磷酸化等信号通路诱导的细胞增殖,这一结果也为BPAF在乳腺癌产生和发展中的作用提供了解释[93-94]。Li等[62]发现BPB可能通过GPR30介导的ERK1/2信号级联放大反应诱导青春期后期大鼠睾丸间质细胞的增殖。BPC同样被报道为GPR30的激动剂,且其诱导细胞内Ca2+水平增加可能同样与GPR30介导的信号通路有关[80, 95]。Liu等[96]通过MD模拟发现,Leu137TM3和Trp272TM6是这几种BPs识别结合并激活GPR30的关键残基,为BPs与GPR30分子识别机制研究提供了理论依据。
2.3 雄激素受体途径
与BPs和ER作用会产生类雌激素效应和抗雌激素效应不同,几乎所有已被报道的在雄激素受体途径发生作用的BPs都会产生抗雄激素作用[1-2]。BPA产生抗雄激素效应可能是因为:(1) BPA与AR的结合干扰了正常雄激素-AR复合物氨基/羧基末端结构域(N/C)之间的分子内的二聚化作用,导致AR稳定性降低而自然降解[97];(2) BPA促进AR泛素化而降解;(3) BPA结合AR-LBD导致受体与其辅助伴侣蛋白——90-kDa热休克蛋白(Hsp90)稳定性增加,而阻止AR与之解离,最终导致AR无法转移至细胞核以介导下游基因的转录;(4) BPA占用AR-LBD位点后干扰了辅助AR核转位的配体依赖的核定位序列,导致配体-受体复合物介导的基因转录不会被刺激,从而产生生殖毒害作用[98]。BPS、BPF、BPAF、BPB、BPE、BPC、BPZ、BPAP、TMBPA和BPP也在不同的体外雄激素受体终点检测中显示为抗雄激素效应,其中BPAF、TMBPA和BPE可能具有更强的雄激素拮抗效应,而BPS的拮抗效应最弱[2, 99]。Conroy-Ben等[81]通过QSAR的方法对比雄激素拮抗剂——羟基氟他胺和BPs与AR结合模型发现BPBP、BPZ、BPB、BPA、BPE、BPF和BPS均通过与AR-LBD的Thr877和Asn705形成氢键而结合(与羟基氟他胺相同),且QSAR模型研究结果与酵母报告基因雄激素试验(YAS)测定的结果一致。目前,BPs与雄激素受体相互结合并发挥生理相关作用的研究有限,且对其产生抗雄激素效应的相关机制了解不足,未来应予以关注。
2.4 雌激素相关受体途径
ERRγ是一种孤儿受体,也是人类48个核受体之一,在发育期哺乳动物大脑以及成年动物大脑和肺等器官中高表达,有研究指出ERRγ在胎儿大脑和胎盘中均有表达,并且ERRγ可能参与前列腺的生长和发育[100]。而不少体外竞争性结合ERRγ-LBD的实验中表明BPA与ERRγ有很强的结合亲和力并且通过保持或锁定ERRγ-LBD的helix12的活性构象而维持ERRγ的高转录活性[73, 101-102]。BPA可与下丘脑[103]、海马区神经元[104]和胎盘[105]中的ERRγ结合而影响下游生殖调控、棘突发生(MAPK信号途径诱导)及BPA在胎盘中的积累。体外实验表明,BPA以时间依赖性的方式诱导ERRγ核易位并增加BPA/ERRγ靶基因的表达,BPA通过促进Ca2+的内流和表皮生长因子(EGF)的分泌,导致EGFR/ERK通路被激活,最终使得BPA通过BPA/ERRγ/EGF/EGFR/ERK信号通路诱导细胞增殖[106]。BPS、BPF、BPAF、BPB、BPC、BPE、BPZ、BPAP、BPP和BPM与ERRγ的体外结合实验同样证明这些BPs具有较高的ERRγ受体亲和力,且BPB和BPE的结合亲和力与BPA相当[73]。Okada等[107]在2008年也得到类似的结果,而且部分BPs的结合活性与人ERRγ荧光素酶报告基因的测定结果吻合。低浓度BPA、BPS和BPF暴露小鼠精母细胞GC-2会显著提高细胞中Errγ的转录,表明ERRγ也是BPS和BPF对生物体生殖产生影响的靶点之一[108]。而对BPAF和BPC进行分子比较对接的结果显示,BPAF和BPC在与ERRγ-LBD结合时也表现为激动构象,发挥关键作用的可能是ERRγ-LBD的helix7上的N346与BPs形成的氢键,且这一计算机预测结果与HG5LN GAL4-ERRγ细胞暴露的活性检测结果相关联[109]。虽然通过体外的配体-受体竞争性结合实验和转染细胞活力及基因表达测定研究可知晓大部分BPs都可与ERRγ进行结合,但是BPs在ERRγ相关通路上对生物体产生生殖毒性的研究仍然有限,也需要更进一步的探究。
2.5 甲状腺激素相关受体途径
甲状腺激素(TH,包括T3和T4)是哺乳动物卵巢正常发育所必需的激素,可改变促性腺激素的释放进而影响卵泡命运,甲状腺激素失衡则会导致卵巢质量下降,精子发生、数量及活力下降,生殖激素合成和分泌紊乱,甚至增加不孕风险[110-111]。在非洲爪蟾[112]、青鳉[113]、啮齿动物[111-112]和猪颗粒细胞[110]中的实验均表明BPA可通过干扰TH与TR的结合影响信号的传导。BPA暴露促进斑马鱼幼鱼体内甲状腺激素运转蛋白(TTR)的表达,且体外荧光竞争实验也表明BPA可与TTR结合,干扰体内正常TH的运输[114-115]。另外也有证据表明BPA可能会通过干扰TH的合成而对生物体产生干扰,BPA暴露斑马鱼后,TH合成相关基因(tg、tshr)的表达量显著上调,而大鼠甲状腺肿瘤细胞FRTL-5暴露于较低浓度BPA后同样使得TH合成相关基因(tg、tshr、nis和tpo)的表达上调[114, 116]。综上所述,BPA可通过对TH的产生、运输和作用环节产生干扰效应,从而间接影响生殖健康。虽然BPA类似物于甲状腺干扰的研究较少,但是已有研究均表明BPA类似物会造成甲状腺干扰。Lu等[117]利用MD模拟结合体内及体外实验发现BPA、BPS等BPs对TRβ有不同程度的抗甲状腺激素活性。而暴露于BPF和BPS的斑马鱼甲状腺发育相关基因(hhex和tg)、甲状腺激素运输相关基因(ttr)和甲状腺激素代谢相关基因(ugt1ab)的转录发生了变化,且幼鱼体内的T3和T4含量显著增加,说明BPS和BPF同样可能干扰TH的合成、分泌及运输[114]。分子对接结果提示BPS与BPF对TR有结合能力,体外荧光竞争结合实验证实其能与TR结合(与BPA类似);此外,3种BPs均能诱导TH依赖的垂体GH3细胞增殖,而在T3存在时抑制T3的诱导,表明BPS和BPF与BPA一样具有干扰TH信号通路的潜力[118]。类似的结果也在BPAF、BPAP、BPC、BPB和BPZ暴露大鼠垂体GH3细胞后产生,表明BPA类似物的TH干扰能力[119]。结合上述研究可知这些BPA类似物的甲状腺干扰效应和机制与BPA类似,需引起研究者的关注并进一步研究。
2.6 芳香烃受体途径
芳香烃受体(AhR)是一种配体激活的转录因子,AhR与配体结合后会被芳香烃受体核转位蛋白(ARNT)带入细胞核与特异性DNA反应元件结合,从而启动包括细胞色素P450家族在内的异源物质代谢相关基因的转录,其在生物体的发育、代谢过程和免疫炎症中起着重要的作用。野生型和AhR-KO小鼠卵泡的BPA暴露实验显示,BPA可抑制2种卵泡生长并降低E2水平,且BPA对AhR-KO卵泡生长的抑制作用要弱于野生型,表明BPA可能通过AhR信号途径部分抑制卵泡生长[120]。BPA暴露妊娠期大鼠可诱导胚胎中芳香烃受体抑制剂(AhR repressor, AhRR)的基因表达上调从而抑制胚胎中AhR的功能[121]。此外,围产期暴露BPA导致的雄性后代的精子发生能力降低及精子畸形率增加,可能与睾丸中AhR信号通路所激活的炎症反应有关[122]。最近的一项研究表明BPA类似物BPF、BPS和BPAF在低浓度(1~100 nmol L-1)下暴露HepG2细胞系均会诱导AhR的RNA和蛋白质水平的增加,从而使受其调节的相关CYP酶的表达出现异常,最终加剧染色体损伤,且分子对接和分子动力学模拟结果也显示上述BPs与AhR的结合亲和力同强效激动剂(PCB 126)的亲和力水平相当[123]。关于BPs与AhR相互作用的相关研究仍停留在计算模拟水平,需要更多的实验证据来支撑这一观点。
L-1)下暴露HepG2细胞系均会诱导AhR的RNA和蛋白质水平的增加,从而使受其调节的相关CYP酶的表达出现异常,最终加剧染色体损伤,且分子对接和分子动力学模拟结果也显示上述BPs与AhR的结合亲和力同强效激动剂(PCB 126)的亲和力水平相当[123]。关于BPs与AhR相互作用的相关研究仍停留在计算模拟水平,需要更多的实验证据来支撑这一观点。
2.7 其他受体途径
体外竞争结合受体测定的研究表明,除了能与上述几种人核受体结合外,BPA、BPS、BPF、BPAF、BPB、BPC、BPE、BPZ、BPAP、BPP和BPM均可与孕烷X受体(PXR)进行结合;除BPS和BPF外,其他测试的BPs可与组成型雄烷受体(CAR)结合,且结合作用极强;而除BPS外,其他BPs均可结合糖皮质激素受体(GR)[73]。Kojima等[99]在测定BPs暴露转染人PXR的COS-7细胞的荧光素酶活性实验中发现BPA、BPAF、BPB、BPZ、BPAP和BPP均是人PXR的激动剂,且BPZ、BPAP和BPP比BPA的激动效应更强。另一项关于HG5LN-PXR的研究显示BPM和BPBP同样是PXR的激动剂[79]。同时,Kojima等[99]还首次通过COS-7细胞的反式激活实验证明了BPA及其类似物BPAF、BPB、BPZ、BPAP和BPP具有CAR逆向激动活性,同时通过基于CHO-K1细胞的反式激活实验证明这6种BPs都是GR的拮抗剂。在一项暴露于BPA、BPS和BPF的重组酵母(稳定表达人GR)系统中GR激活实验的结果也同样表明了BPs的GR拮抗作用[124]。
除了PXR、CAR和GR,BPAF、BPC、BPM和BPZ被证明可与人过氧化物酶体增殖物激活受体(hPPARs)和人视黄X受体(hRXRs)相互作用[125],且与它们相比BPA具有更高的hPPARs和hRXRs结合亲和力。综上所述,BPA及其类似物同样会对PXR、CAR、GR、PPAR和RXR这些同属核受体家族的受体蛋白产生结合作用,即便最终激动或拮抗效应有所不同,但由于核受体作为配体激活的转录因子在特异性调节参与代谢、发育和繁殖的靶基因的表达上起着至关重要的作用,理应对这些污染物的核受体影响做更深层次的机制相关研究。
3 BPA类似物的人体生殖健康风险(Human reproductive health risks of BPA analogues)
BPA类似物主要通过消化道被人体接触吸收,2013年的研究估算人体每日通过膳食摄入BPs的量为幼儿243 ng·kg-1(以体质量计)和成人58.6 ng·kg-1(以体质量计),表明了BPs对人体的较高浓度持续暴露,由于BPA类似物在环境中的检出量日益增多,人类对BPs的接触也呈上升趋势[126]。最近一项基于废水流行病学评估人类暴露于BPA类似物的研究则表明,人类平均每日BPF、BPS、BPP、BPZ、BPB、BPAF和BPAP的暴露水平分别为10.2、5.21、1.15、0.66、0.61、0.58和0.35 μg,说明BPA类似物的人体暴露风险正在升高[32]。相较于多数模式动物研究中的BPs暴露浓度而言,人类暴露剂量水平相对较低,但已有环境相关浓度的研究表明在人类可接触的BPs浓度下,动物研究仍显示出了BPs的生殖毒性,提示BPA类似物的人体生殖健康风险不容忽视[40, 47, 49, 65]。BPS、BPAF和BPB被人体摄入后主要在肝脏和肠道中被代谢为BPs-葡糖苷酸,少量代谢为BPs-硫酸盐、羟基化BPs和其他代谢产物,而BPF主要代谢为BPF-硫酸盐后随尿液排出[31, 126]。一项d4-BPS于人体内的药代动力学研究表明,与BPA相比,BPS吸收快、代谢慢,其在口服后1 h内被迅速吸收,人体内半衰期为6.8 h,且在人体内主要以毒性更强的未偶联形式存在,48 h时BPS在男性中通过尿液排泄率约为92.17%,女性排泄率为70.36%,提示女性可能有更高的BPs暴露风险[127]。BPF和BPAF同样在动物研究中被报道有比BPA更长的体内半衰期和更高的残留量[128]。Karrer等[129]基于生理毒物动力学计算机模型分析发现,在环境相关浓度下BPs在肠道和肝脏中代谢速率的排序为:BPA>BPF>BPS。此外,BPs在体内的代谢产物同样存在健康风险,研究表明BPAF的主要代谢产物BPAF-葡糖苷酸可对PXR和PPARγ产生拮抗作用[130];BPB、BPC和BPF可通过多种途径产生二聚代谢产物,尽管对其毒性研究甚少,但由于BPA的二聚代谢物被证明比BPA具有更强的雌激素效应,故而BPs的代谢产物毒性也应引起关注[9]。虽然大部分BPs可能通过人体代谢降低毒性后排出,但因环境中BPs的广泛存在和日益升高的含量,人体处在持续暴露中,使得体内游离BPs暴露风险增加。
由于BPA类似物的酚类结构与人体性激素结构相似,被认为可干扰人体内雌二醇、孕酮、黄体生成素、促卵泡激素、睾酮和雄烯二酮等性激素水平,从而对人体的生殖系统造成扰乱,影响人类生殖健康[131-133]。BPE、BPF同BPA一样可抑制人类睾丸外植体睾酮的生成,BPS和BPB则会加快睾丸间质细胞INSL3的生成,表明BPA类似物对雄性生殖具干扰效应[134]。BPAF、BPB和BPC等BPs可通过激活CatSper影响人类精子细胞Ca2+信号通路,而破坏人类精子功能[135]。流行病学研究表明BPA类似物的暴露与男性生殖障碍、前列腺癌、女性不孕、乳腺癌和多囊卵巢综合征等生殖相关疾病的发生有关[2, 136-137]。除此之外,BPA类似物与女性孕产风险及胎儿生长缓慢具有相关性。孕妇尿液中BPS含量与女性妊娠年龄和女婴晚产风险的增加相关[138],另有报道孕妇尿液中BPS浓度与早产相关[139]。母亲尿液BPS含量与女婴体质量偏低和下腹围及股骨长度相关[140]。而产前暴露于BPF可能与男婴出生体质量降低有相关性[141]。BPB则被报道与子宫内膜异位有关[128]。最新的一项横断面分析显示适孕女性尿液BPC含量与甲状腺体积呈显著负相关,与促甲状腺激素含量呈显著正相关,而BPC的持续暴露可能会导致甲状腺功能退化进而造成孕妇先兆子痫、胎盘早剥、胎儿宫内发育迟缓、早产以及流产等生殖健康风险[142]。此外,在叶酸补充不足条件下,孕期女性尿液中总双酚类物质(包括BPA、BPS、BPF、BPAF、BPZ、BPB、BPAP和BPP)含量与孕期时间延长有关[143]。综上所述,尽管BPs在人体内可能被代谢排出,但是持续暴露于BPs仍然可能引发生殖健康风险。
综合计算机模拟(QSAR和MD模拟)和体外实验(酵母或细胞报告基因检测)的结果,对各BPA类似物的ER结合亲和力和类雌激素或抗雌激素效应作为指标进行评估得知,相较于BPA而言,除BPS外,其他BPA类似物均表现出与BPA相似或更强的ER结合能力且体外实验同样证实这一结果(表3)[79, 81, 83-85]。在众多BPs中,BPAF所展现出的类雌激素作用最强,而这与BPAF分子结构中酚环间烷基部分的卤化作用相关,其对配体-ERα结合过程中的静电相互作用和氢键的产生有较大影响[144]。相比之下,BPS表现出比BPA更低的ER结合亲和力以及更弱的雌激素效应,这可能与其结构中碳氧双键饱和的连接方式有关,在今后新型BPA类似物的设计中应充分考虑。这提示我们计算机模拟是对新型BPA替代物毒性预测的有效手段,未来应该综合开发基于不同核受体(NRs)的毒性预测模型以期更准确地预测新型化合物毒性。
表3 BPA类似物的内分泌干扰能力(基于竞争性结合人雌激素受体(ER)的实验结果)[79, 81, 83-85]
Table 3 Endocrine activity of BPA analogues (based on the competitive receptor-binding
assays of human estrogen receptor (ER)) [79, 81, 83-85]
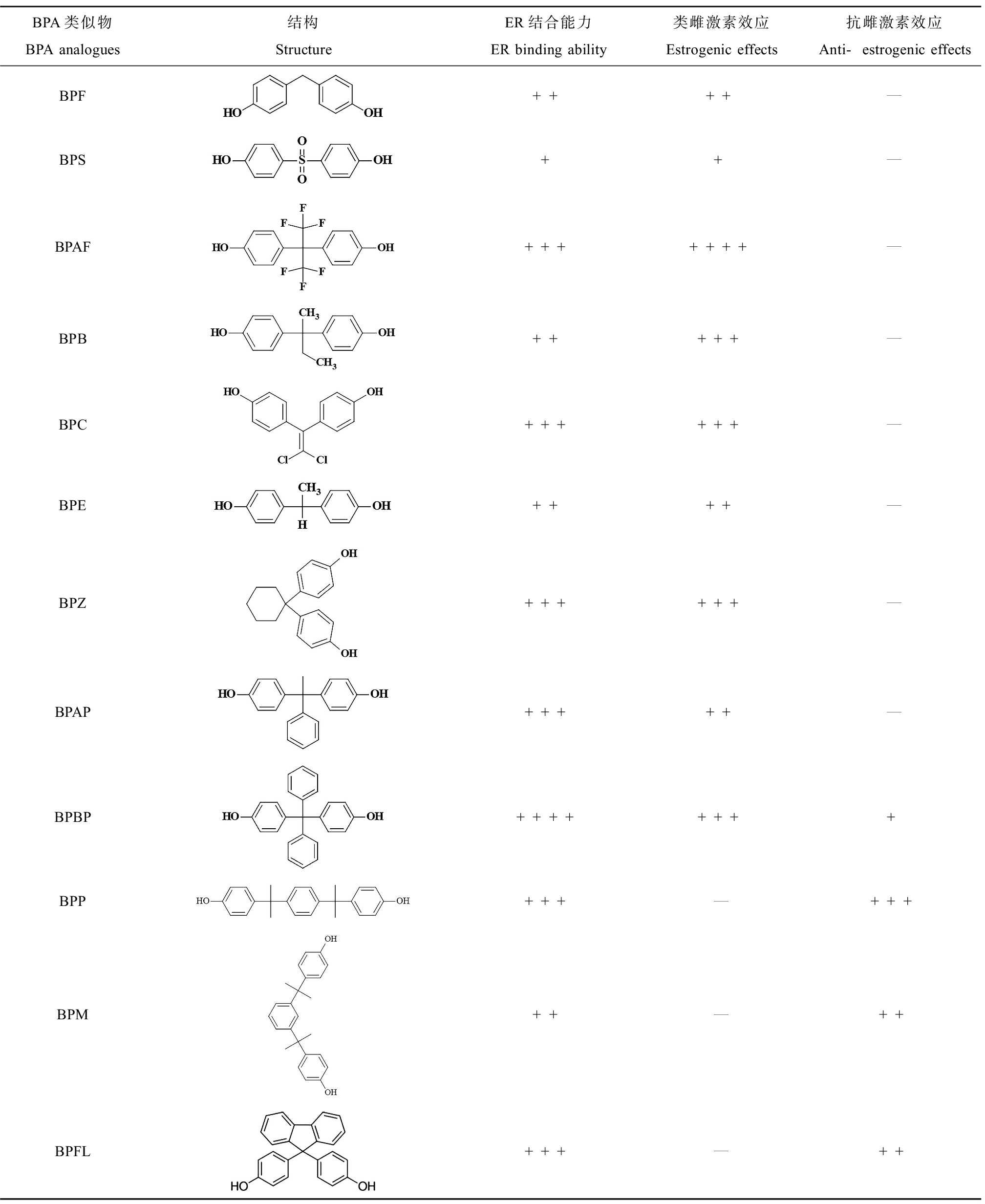
BPA类似物BPA analogues结构StructureER结合能力ER binding ability类雌激素效应Estrogenic effects抗雌激素效应Anti-estrogenic effectsBPF++++—BPS++—BPAF+++++++—BPB+++++—BPC++++++—BPE++++—BPZ++++++—BPAP+++++—BPBP++++++++BPP+++—+++BPM++—++BPFL+++—++
注:+表示计算模拟或体外检测中BPs展现出的与ER结合亲和力强度或类/抗雌激素能力,+越多则能力越强,—表示未见报道。
Note: :+ indicates the intensity of ER binding affinity or estrogenic/anti-estrogenic ability of BPs by computational simulation or in vitro test; the more + represent the stronger ability; — means not been reported.
4 展望(Perspectives)
BPs作为环境内分泌干扰物,尽管BPA在人类直接接触途径上因各国政策的调整而使其风险降低,但是BPA类似物的开发以及应用已然成了新的问题,且目前已经在世界范围内多种人体样本中普遍存在,其能通过胎盘屏障和血睾屏障影响生殖腺而最终可能对子代产生危害,在职业接触人群的分析中也发现这类化学品的长期接触会对人类生殖产生不利影响,严重时可导致不孕不育。通过在哺乳类模式动物和水生鱼类模式动物上的研究也进一步说明了其在雄性和雌性生殖系统上的毒性作用,但目前关于BPA类似物在动物模型研究中的暴露浓度主要是基于BPA对于实验室动物的最低可见有害作用浓度和人体每日耐受摄入量进行设置,普遍高于环境浓度且暴露时间相对较短,真实环境浓度下BPA类似物对生物体长期暴露的研究偏少,不利于评估BPA类似物对人体健康的影响。随着相关研究的增多,环境相关浓度已经受到更多国际同行的重视,所以暴露剂量的降低、暴露时间的延长以及模拟人群的暴露途径是环境毒理学领域的关注重点。另外,目前的研究仍旧以BPA环境检测和人体检出为基础,但随着BPA限用,多种类似物可能取而代之,因此化学品的实际使用情况在确定毒理研究的优先次序中也很重要。此外,生活环境的复杂性使得人体暴露于多种BPs下,在单一BPA类似物的研究基础上也应考察复合暴露可能带来的影响。
BPA类似物的生物利用度、偶联度以及体内半衰期存在差异,相关研究较少且多为体外代谢模型,无法模拟化合物的体内Ⅱ期代谢,故而BPA类似物的动物体内代谢同样是这一领域的新关注点。尽管进入人体的BPs大部分可通过葡萄糖醛酸化和硫酸化而排出体外,但不少研究仍表明一些已投入生产应用的新型BPA类似物(如BPAF、BPB)的生殖毒性相较BPA反而更高,而对这些类似物的生物安全性的研究仍然较少,在其生殖毒性的机制方面同样需要更深的探究。现有研究表明BPA的体内代谢产物具有比BPA更强的雌激素效应,但BPA类似物的相关研究同样较少,了解BPs在体内的代谢过程和代谢产物的毒性能对BPs生殖毒性发生的机制有更全面的解释,在未来的研究中应该更加关注BPA类似物在人体内代谢产物的毒性评估。
伴随着计算机科学在环境领域的广泛应用和蛋白质结构解析方法的日益成熟,开发高通量、稳定、易操作的体外模拟技术对化合物毒性进行预测同样是毒理学领域的发展重点,由于其在化学品毒性预测方面的极大潜力,如今已经得到了广泛应用并受到了领域内的认可。依托于上述计算机模拟技术,BPA及其类似物对ER蛋白结合和效应产生机制已得到了较为充分的研究,但随着对其他核受体和膜受体在EDC引起生殖和其他内分泌干扰研究中的关注,相关的机制研究仍然极为缺乏,也需要更多从业者探究和解释,并且需要更多体内研究以补充体外研究的不足。
[1] Ma Y, Liu H H, Wu J X, et al. The adverse health effects of bisphenol A and related toxicity mechanisms [J]. Environmental Research, 2019, 176: 108575
[2] Pelch K, Wignall J A, Goldstone A E, et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives [J]. Toxicology, 2019, 424: 152235
[3] Huang Y Q, Wong C K C, Zheng J S, et al. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts [J]. Environment International, 2012, 42: 91-99
[4] Ng H W, Shu M, Luo H, et al. Estrogenic activity data extraction and in silico prediction show the endocrine disruption potential of bisphenol A replacement compounds [J]. Chemical Research in Toxicology, 2015, 28(9): 1784-1795
[5] Rosenfeld C S. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues [J]. Frontiers in Neuroendocrinology, 2017, 47: 123-133
[6] Flint S, Markle T, Thompson S, et al. Bisphenol A exposure, effects, and policy: A wildlife perspective [J]. Journal of Environmental Management, 2012, 104: 19-34
[7] Zulkifli S, Rahman A A, Kadir S H S A, et al. Bisphenol A and its effects on the systemic organs of children [J]. European Journal of Pediatrics, 2021, 180(10): 3111-3127
[8] Peng Y, Wang J L, Wu C Q. Determination of endocrine disruption potential of bisphenol A alternatives in food contact materials using in vitro assays: State of the art and future challenges [J]. Journal of Agricultural and Food Chemistry, 2019, 67(46): 12613-12625
[9] Chen D, Kannan K, Tan H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review [J]. Environmental Science & Technology, 2016, 50(11): 5438-5453
[10] Martínez M A, Rovira J, Prasad Sharma R, et al. Comparing dietary and non-dietary source contribution of BPA and DEHP to prenatal exposure: A Catalonia (Spain) case study [J]. Environmental Research, 2018, 166: 25-34
[11] Noszczyńska M, Piotrowska-Seget Z. Bisphenols: Application, occurrence, safety, and biodegradation mediated by bacterial communities in wastewater treatment plants and rivers [J]. Chemosphere, 2018, 201: 214-223
[12] EFSA Panel on Food Contact Materials, Enzymes and Processing Aids. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs [J]. EFSA Journal, 2015, 13(1): 3978
[13] Teeguarden J G. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women [J]. Food and Chemical Toxicology, 2016, 92: 129-142
[14] Martín J, Santos J L, Aparicio I, et al. Analytical method for biomonitoring of endocrine-disrupting compounds (bisphenol A, parabens, perfluoroalkyl compounds and a brominated flame retardant) in human hair by liquid chromatography-tandem mass spectrometry [J]. Analytica Chimica Acta, 2016, 945: 95-101
[15] Rochester J R. Bisphenol A and human health: A review of the literature [J]. Reproductive Toxicology, 2013, 42: 132-155
[16] Lee J, Choi K, Park J, et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs [J]. Science of the Total Environment, 2018, 626: 1494-1501
[17] Qiu W H, Liu S, Chen H, et al. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms [J]. Journal of Hazardous Materials, 2021, 406: 124303
[18] Perera L, Li Y, Coons L A, et al. Binding of bisphenol A, bisphenol AF, and bisphenol S on the androgen receptor: Coregulator recruitment and stimulation of potential interaction sites [J]. Toxicology in Vitro, 2017, 44: 287-302
[19] Vrzal R, Zenata O, Doricakova A, et al. Environmental pollutants parathion, paraquat and bisphenol A show distinct effects towards nuclear receptors-mediated induction of xenobiotics-metabolizing cytochromes P450 in human hepatocytes [J]. Toxicology Letters, 2015, 238(1): 43-53
[20] Nesan D, Kurrasch D M. Gestational exposure to common endocrine disrupting chemicals and their impact on neurodevelopment and behavior [J]. Annual Review of Physiology, 2020, 82: 177-202
[21] den Braver-Sewradj S P, van Spronsen R, Hessel E V S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances [J]. Critical Reviews in Toxicology, 2020, 50(2): 128-147
[22] Pallotti F, Pelloni M, Gianfrilli D, et al. Mechanisms of testicular disruption from exposure to bisphenol A and phtalates [J]. Journal of Clinical Medicine, 2020, 9(2): 471
[23] Casals-Casas C, Desvergne B. Endocrine disruptors: From endocrine to metabolic disruption [J]. Annual Review of Physiology, 2011, 73: 135-162
[24] Chevalier N, Fénichel P. Bisphenol A: Targeting metabolic tissues [J]. Reviews in Endocrine & Metabolic Disorders, 2015, 16(4): 299-309
[25] Žalmanová T, Hošková K, Nevoral J, et al. Bisphenol S instead of bisphenol A: A story of reproductive disruption by regretable substitution—A review [J]. Czech Journal of Animal Science, 2016, 61(10): 433-449
[26] Knower K C, To S Q, Leung Y K, et al. Endocrine disruption of the epigenome: A breast cancer link [J]. Endocrine-Related Cancer, 2014, 21(2): T33-T55
[27] Cristian M, Ionut P R, Aida P, et al. Bisphenol-A and other plastics: Review of endocrine disrupting effects on prostate cancer [J]. Materiale Plastice, 2020, 57(2): 239-252
[28] Konieczna A, Rutkowska A, Rachoń D. Health risk of exposure to bisphenol A (BPA) [J]. Roczniki Panstwowego Zakladu Higieny, 2015, 66(1): 5-11
[29] Yang Y J, Guan J, Yin J, et al. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in South China [J]. Chemosphere, 2014, 112: 481-486
[30] Owczarek K, Kubica P, Kud ak B, et al. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography-tandem mass spectrometry [J]. The Science of the Total Environment, 2018, 628-629: 1362-1368
ak B, et al. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography-tandem mass spectrometry [J]. The Science of the Total Environment, 2018, 628-629: 1362-1368
[31] Usman A. Occurrence, toxicity and endocrine disrupting potential of bisphenol-B and bisphenol-F: A mini-review [J]. Toxicology Letters, 2019, 312: 222-227
[32] Wang H, Liu Z H, Zhang J, et al. Human exposure of bisphenol A and its analogues: Understandings from human urinary excretion data and wastewater-based epidemiology [J]. Environmental Science and Pollution Research International, 2020, 27(3): 3247-3256
[33] Liao C Y, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure [J]. Journal of Agricultural and Food Chemistry, 2013, 61(19): 4655-4662
[34] Liao C Y, Liu F, Moon H B, et al. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions [J]. Environmental Science & Technology, 2012, 46(21): 11558-11565
[35] Zhao X, Qiu W H, Zheng Y, et al. Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China [J]. Ecotoxicology and Environmental Safety, 2019, 180: 43-52
[36] Gély C A, Lacroix M Z, Morin M, et al. Comparison of the materno-fetal transfer of fifteen structurally related bisphenol analogues using an ex vivo human placental perfusion model [J]. Chemosphere, 2021, 276: 130213
[37] Pinto C, Hao R X, Grimaldi M, et al. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo [J]. Toxicology and Applied Pharmacology, 2019, 380: 114709
[38] Beg M A, Sheikh I A. Endocrine disruption: Molecular interactions of environmental bisphenol contaminants with thyroid hormone receptor and thyroxine-binding globulin [J]. Toxicology and Industrial Health, 2020, 36(5): 322-335
[39] Rochester J R, Bolden A L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes [J]. Environmental Health Perspectives, 2015, 123(7): 643-650
![]() á
á![]() á H,
á H,  tiavnická M, Moravec J, et al. Low doses of bisphenol S affect post-translational modifications of sperm proteins in male mice [J]. Reproductive Biology and Endocrinology, 2020, 18(1): 56
tiavnická M, Moravec J, et al. Low doses of bisphenol S affect post-translational modifications of sperm proteins in male mice [J]. Reproductive Biology and Endocrinology, 2020, 18(1): 56
[41] Ullah A, Pirzada M, Jahan S, et al. Prenatal BPA and its analogs BPB, BPF, and BPS exposure and reproductive axis function in the male offspring of Sprague Dawley rats [J]. Human & Experimental Toxicology, 2019, 38(12): 1344-1365
[42] Wu D, Huang C J, Jiao X F, et al. Bisphenol AF compromises blood-testis barrier integrity and sperm quality in mice [J]. Chemosphere, 2019, 237: 124410
[43] Ikhlas S, Ahmad M. Acute and sub-acute bisphenol-B exposures adversely affect sperm count and quality in adolescent male mice [J]. Chemosphere, 2020, 242: 125286
[44] Shi M X, Sekulovski N, MacLean J A 2nd, et al. Effects of bisphenol A analogues on reproductive functions in mice [J]. Reproductive Toxicology, 2017, 73: 280-291
[45] Zhang Z B, Hu Y, Guo J L, et al. Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice [J]. Nature Communications, 2017, 8: 14585
[46] Shi M X, Sekulovski N, MacLean J A 2nd, et al. Prenatal exposure to bisphenol A analogues on male reproductive functions in mice [J]. Toxicological Sciences, 2018, 163(2): 620-631
[47] Naderi M, Wong M Y L, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults [J]. Aquatic Toxicology, 2014, 148: 195-203
[48] Shi J C, Jiao Z H, Zheng S, et al. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring [J]. Chemosphere, 2015, 128: 252-257
[49] Yang Q, Yang X H, Liu J N, et al. Exposure to bisphenol B disrupts steroid hormone homeostasis and gene expression in the hypothalamic-pituitary-gonadal axis of zebrafish [J]. Water, Air, & Soil Pollution, 2017, 228(3): 112
[50] Yamaguchi A, Ishibashi H, Arizono K, et al. In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (Oryzias latipes) and common carp (Cyprinus carpio) [J]. Ecotoxicology and Environmental Safety, 2015, 120: 198-205
[51] Rahman M S, Pang W K, Ryu D Y, et al. Multigenerational impacts of gestational bisphenol A exposure on the sperm function and fertility of male mice [J]. Journal of Hazardous Materials, 2021, 416: 125791
[52] Shi M X, Whorton A E, Sekulovski N, et al. Prenatal exposure to bisphenol A, E, and S induces transgenerational effects on male reproductive functions in mice [J]. Toxicological Sciences, 2019, 172(2): 303-315
[53] Liu W X, Donatella F, Tan S J, et al. Detrimental effect of bisphenol S in mouse germ cell cyst breakdown and primordial follicle assembly [J]. Chemosphere, 2021, 264(Pt 1): 128445
[54] Ijaz S, Ullah A, Shaheen G, et al. Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats [J]. Toxicology Mechanisms and Methods, 2020, 30(1): 60-72
[55] Conley J M, Hannas B R, Furr J R, et al. A demonstration of the uncertainty in predicting the estrogenic activity of individual chemicals and mixtures from an in vitro estrogen receptor transcriptional activation assay (T47D-KBluc) to the in vivo uterotrophic assay using oral exposure [J]. Toxicological Sciences, 2016, 153(2): 382-395
[56] Yamasaki K, Noda S, Imatanaka N, et al. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity [J]. Toxicology Letters, 2004, 146(2): 111-120
[57] Xiao X, Li J Y, Yu T, et al. Bisphenol AP is anti-estrogenic and may cause adverse effects at low doses relevant to human exposure [J]. Environmental Pollution, 2018, 242(Pt B): 1625-1632
[58] Shi M X, Sekulovski N, MacLean J A, et al. Prenatal exposure to bisphenol A analogues on female reproductive functions in mice [J]. Toxicological Sciences, 2019, 168(2): 561-571
[59] Ullah A, Pirzada M, Jahan S, et al. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study [J]. Toxicology and Industrial Health, 2019, 35(4): 294-303
[60] Ullah A, Pirzada M, Jahan S, et al. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action [J]. Food and Chemical Toxicology, 2018, 121: 24-36
[61] Feng Y X, Yin J, Jiao Z H, et al. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats [J]. Toxicology Letters, 2012, 211(2): 201-209
[62] Li Y, Yan H, Yu Y, et al. Bisphenol B stimulates Leydig cell proliferation but inhibits maturation in late pubertal rats [J]. Food and Chemical Toxicology, 2021, 153: 112248
[63] Shi M X, Sekulovski N, MacLean J A, et al. Prenatal exposure to bisphenol A analogues on female reproductive functions in mice [J]. Toxicological Sciences, 2019, 168(2): 561-571
[64] Zhang M Y, Tian Y, Yan Z H, et al. Maternal bisphenol S exposure affects the reproductive capacity of F1 and F2 offspring in mice [J]. Environmental Pollution, 2020, 267: 115382
[65] Nevoral J, Kolinko Y, Moravec J, et al. Long-term exposure to very low doses of bisphenol S affects female reproduction [J]. Reproduction, 2018, 156(1): 47-57
[66] Yamasaki K, Takeyoshi M, Yakabe Y, et al. Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals [J]. Toxicology, 2002, 170(1-2): 21-30
[67] Jia Z Z, Wang H Y, Feng Z Y, et al. Fluorene-9-bisphenol exposure induces cytotoxicity in mouse oocytes and causes ovarian damage [J]. Ecotoxicology and Environmental Safety, 2019, 180: 168-178
[68] Mi P, Zhang Q P, Zhang S H, et al. The effects of fluorene-9-bisphenol on female zebrafish (Danio rerio) reproductive and exploratory behaviors [J]. Chemosphere, 2019, 228: 398-411
[69] Alonso-Magdalena P, Ropero A B, Soriano S, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways [J]. Molecular and Cellular Endocrinology, 2012, 355(2): 201-207
[70] Acconcia F, Pallottini V, Marino M. Molecular mechanisms of action of BPA [J]. Dose-Response, 2015, 13(4): 1559325815610582
[71] Vi as R, Watson C S. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: Effects on cell functions [J]. Environmental Health Perspectives, 2013, 121(3): 352-358
as R, Watson C S. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: Effects on cell functions [J]. Environmental Health Perspectives, 2013, 121(3): 352-358
[72] Le Fol V, Aït-Aïssa S, Sonavane M, et al. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays [J]. Ecotoxicology and Environmental Safety, 2017, 142: 150-156
[73] Liu X H, Sakai H, Nishigori M, et al. Receptor-binding affinities of bisphenol A and its next-generation analogs for human nuclear receptors [J]. Toxicology and Applied Pharmacology, 2019, 377: 114610
[74] Li M, Han X Y, Gao W H, et al. Bisphenol AF stimulates transcription and secretion of C-X-C chemokine ligand 12 to promote proliferation of cultured T47D breast cancer cells [J]. Toxicology, 2015, 338: 30-36
[75] Huang M Q, Li X J, Jia S J, et al. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN [J]. Environmental Pollution, 2021, 270: 116051
[76] Li M, Guo J, Gao W H, et al. Bisphenol AF-induced endogenous transcription is mediated by ERα and ERK1/2 activation in human breast cancer cells [J]. PLoS One, 2014, 9(4): e94725
[77] Serra H, Beausoleil C, Habert R, et al. Evidence for bisphenol B endocrine properties: Scientific and regulatory perspectives [J]. Environmental Health Perspectives, 2019, 127(10): 106001
[78] Zhang S X, Ding Z M, Ahmad M J, et al. Bisphenol B exposure disrupts mouse oocyte meiotic maturation in vitro through affecting spindle assembly and chromosome alignment [J]. Frontiers in Cell and Developmental Biology, 2020, 8: 616771
[79] Grimaldi M, Boulahtouf A, Toporova L, et al. Functional profiling of bisphenols for nuclear receptors [J]. Toxicology, 2019, 420: 39-45
[80] Lei B L, Xu J, Peng W, et al. In vitro profiling of toxicity and endocrine disrupting effects of bisphenol analogues by employing MCF-7 cells and two-hybrid yeast bioassay [J]. Environmental Toxicology, 2017, 32(1): 278-289
[81] Conroy-Ben O, Garcia I, Teske S S. In silico binding of 4,4’-bisphenols predicts in vitro estrogenic and antiandrogenic activity [J]. Environmental Toxicology, 2018, 33(5): 569-578
[82] Cao H M, Wang F B, Liang Y, et al. Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds [J]. Archives of Toxicology, 2017, 91(12): 3897-3912
[83] Pelch K E, Li Y, Perera L, et al. Characterization of estrogenic and androgenic activities for bisphenol A-like chemicals (BPs): In vitro estrogen and androgen receptors transcriptional activation, gene regulation, and binding profiles [J]. Toxicological Sciences, 2019, 172(1): 23-37
[84] Zhang J, Wu W F, Wang Y J, et al. Estrogen receptor-based fluorescence polarization assay for bisphenol analogues and molecular modeling study of their complexation mechanism [J]. Analytica Chimica Acta, 2018, 1032: 107-113
[85] Dvorakova M, Kejlová K, Rucki M, et al. Selected bisphenols and phthalates screened for estrogen and androgen disruption by in silico and in vitro methods [J]. Neuro Endocrinology Letters, 2018, 39(5): 409-416
[86] Cao H M, Wang L, Cao M X, et al. Computational insights on agonist and antagonist mechanisms of estrogen receptor α induced by bisphenol A analogues [J]. Environmental Pollution, 2019, 248: 536-545
[87] 朱本占, 沈忱, 盛治国. 膜受体介导双酚A低剂量内分泌干扰效应的分子机制[J]. 化学进展, 2019, 31(1): 167-179
Zhu B Z, Shen C, Sheng Z G. Mechanism of the endocrine-disruptive effects of low-dose bisphenol A via transmembrane receptor [J]. Progress in Chemistry, 2019, 31(1): 167-179 (in Chinese)
[88] 周炳升, 杨丽华, 刘春生. 持久性有机污染物的内分泌干扰效应[M]. 北京: 科学出版社, 2018: 249-251
[89] Wang C L, Zhang J X, Li Q, et al. Low concentration of BPA induces mice spermatocytes apoptosis via GPR30 [J]. Oncotarget, 2017, 8(30): 49005-49015
[90] Thomas P. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes [J]. The Journal of Steroid Biochemistry and Molecular Biology, 2017, 167: 153-161
[91] Cao L Y, Ren X M, Li C H, et al. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein-coupled estrogen receptor pathway [J]. Environmental Science & Technology, 2017, 51(19): 11423-11430
[92] Lei B L, Huang Y Y, Liu Y, et al. Low-concentration BPF induced cell biological responses by the ERα and GPER1-mediated signaling pathways in MCF-7 breast cancer cells [J]. Ecotoxicology and Environmental Safety, 2018, 165: 144-152
[93] Lei B L, Xu L B, Tang Q Q, et al. Molecular mechanism study of BPAF-induced proliferation of ERα-negative SKBR-3 human breast cancer cells in vitro/in vivo [J]. Science of the Total Environment, 2021, 775: 145814
[94] Lei B L, Sun S, Zhang X L, et al. Bisphenol AF exerts estrogenic activity in MCF-7-cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway [J]. Chemosphere, 2019, 220: 362-370
[95] Périan S, Cerutti C, Forcet C, et al. A cell-based method to detect agonist and antagonist activities of endocrine-disrupting chemicals on GPER [J]. Frontiers in Endocrinology, 2020, 11: 547
[96] Liu X C, Xue Q, Zhang H Z, et al. Structural basis for molecular recognition of G protein-coupled estrogen receptor by selected bisphenols [J]. The Science of the Total Environment, 2021, 793: 148558
[97] Wang H, Ding Z, Shi Q M, et al. Anti-androgenic mechanisms of bisphenol A involve androgen receptor signaling pathway [J]. Toxicology, 2017, 387: 10-16
[98] Huang X, Cang X, Liu J, et al. Molecular mechanism of bisphenol A on androgen receptor antagonism [J]. Toxicology in Vitro, 2019, 61: 104621
[99] Kojima H, Takeuchi S, Sanoh S, et al. Profiling of bisphenol A and eight of its analogues on transcriptional activity via human nuclear receptors [J]. Toxicology, 2019, 413: 48-55
[100] Liu X H, Matsushima A, Shimohigashi M, et al. A characteristic back support structure in the bisphenol A-binding pocket in the human nuclear receptor ERRγ [J]. PLoS One, 2014, 9(6): e101252
[101] Takayanagi S. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity [J]. Toxicology Letters, 2006, 167(2): 95-105
[102] Matsushima A, Kakuta Y, Teramoto T, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma [J]. Journal of Biochemistry, 2007, 142(4): 517-524
[103] Klenke U, Constantin S, Wray S. BPA directly decreases GnRH neuronal activity via noncanonical pathway [J]. Endocrinology, 2016, 157(5): 1980-1990
[104] Tanabe N, Yoshino H, Kimoto T, et al. Nanomolar dose of bisphenol A rapidly modulates spinogenesis in adult hippocampal neurons [J]. Molecular and Cellular Endocrinology, 2012, 351(2): 317-325
[105] Takeda Y, Liu X H, Sumiyoshi M, et al. Placenta expressing the greatest quantity of bisphenol A receptor ERR gamma among the human reproductive tissues: Predominant expression of type-1 ERR gamma isoform [J]. Journal of Biochemistry, 2009, 146(1): 113-122
[106] Yaguchi T. The endocrine disruptor bisphenol A promotes nuclear ERRγ translocation, facilitating cell proliferation of Grade Ⅰ endometrial cancer cells via EGF-dependent and EGF-independent pathways [J]. Molecular and Cellular Biochemistry, 2019, 452(1): 41-50
[107] Okada H, Tokunaga T, Liu X H, et al. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma [J]. Environmental Health Perspectives, 2008, 116(1): 32-38
[108] Sidorkiewicz I, Czerniecki J, Jarząbek K, et al. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line [J]. Toxicology and Applied Pharmacology, 2018, 359: 1-11
[109] Delfosse V, Grimaldi M, Pons J L, et al. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(37): 14930-14935
[110] Song D, Wu G Y, Wei Q W, et al. Bisphenol A attenuates thyroxine-induced apoptosis in ovarian granulosa cells of pigs [J]. Zuchthygiene, 2019, 54(6): 864-872
[111] Vissenberg R, Manders V D, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction [J]. Human Reproduction Update, 2015, 21(3): 378-387
[112] Faheem M, Bhandari R K. Detrimental effects of bisphenol compounds on physiology and reproduction in fish: A literature review [J]. Environmental Toxicology and Pharmacology, 2021, 81: 103497
[113] Naderi M, Wong M Y, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults [J]. Aquatic Toxicology, 2014, 148: 195-203
[114] Lee S, Kim C, Shin H, et al. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish [J]. Chemosphere, 2019, 221: 115-123
[115] Cao J, Guo L H, Wan B, et al. In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A [J]. Journal of Environmental Sciences (China), 2011, 23(2): 315-321
[116] Lee S, Kim C, Youn H, et al. Thyroid hormone disrupting potentials of bisphenol A and its analogues - in vitro comparison study employing rat pituitary (GH3) and thyroid follicular (FRTL-5) cells [J]. Toxicology in Vitro, 2017, 40: 297-304
[117] Lu L P, Zhan T J, Ma M, et al. Thyroid disruption by bisphenol S analogues via thyroid hormone receptor β: in vitro, in vivo, and molecular dynamics simulation study [J]. Environmental Science & Technology, 2018, 52(11): 6617-6625
[118] Zhang Y F, Ren X M, Li Y Y, et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo [J]. Environmental Pollution, 2018, 237: 1072-1079
[119] Lee J Y, Kim S, Choi K, et al. Effects of bisphenol analogs on thyroid endocrine system and possible interaction with 17β-estradiol using GH3 cells [J]. Toxicology in Vitro, 2018, 53: 107-113
[120] Ziv-Gal A, Craig Z R, Wang W, et al. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway [J]. Reproductive Toxicology, 2013, 42: 58-67
[121] Juricek L, Coumoul X. The aryl hydrocarbon receptor and the nervous system [J]. International Journal of Molecular Sciences, 2018, 19(9): 2504
[122] Meng Y, Lin R, Wu F J, et al. Decreased capacity for sperm production induced by perinatal bisphenol A exposure is associated with an increased inflammatory response in the offspring of C57BL/6 male mice [J]. International Journal of Environmental Research and Public Health, 2018, 15(10): 2158
[123] Yu H, Song M Q, Hu K Q, et al. Influence of bisphenol compounds at nanomolar concentrations on chromosome damage induced by metabolically activated carcinogens in HepG2 cells [J]. Environmental Science & Technology, 2021, 55(14): 10001-10011
[124] Roelofs M J, van den Berg M, Bovee T F, et al. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor [J]. Toxicology, 2015, 329: 10-20
[125] Sharma S, Ahmad S, Khan M F, et al. In silico molecular interaction of bisphenol analogues with human nuclear receptors reveals their stronger affinity vs. classical bisphenol A [J]. Toxicology Mechanisms and Methods, 2018, 28(9): 660-669
[126] Gramec Skledar D, Peterlin ![]() L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? [J]. Environmental Toxicology and Pharmacology, 2016, 47: 182-199
L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? [J]. Environmental Toxicology and Pharmacology, 2016, 47: 182-199
[127] Oh J, Choi J W, Ahn Y A, et al. Pharmacokinetics of bisphenol S in humans after single oral administration [J]. Environment International, 2018, 112: 127-133
[128] Usman A, Ahmad M. From BPA to its analogues: Is it a safe journey? [J]. Chemosphere, 2016, 158: 131-142
[129] Karrer C, Roiss T, von Goetz N, et al. Physiologically based pharmacokinetic (PBPK) modeling of the bisphenols BPA, BPS, BPF, and BPAF with new experimental metabolic parameters: Comparing the pharmacokinetic behavior of BPA with its substitutes [J]. Environmental Health Perspectives, 2018, 126(7): 077002
[130] Skledar D G, Carino A, Trontelj J, et al. Endocrine activities and adipogenic effects of bisphenol AF and its main metabolite [J]. Chemosphere, 2019, 215: 870-880
[131] Meeker J D, Calafat A M, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic [J]. Environmental Science & Technology, 2010, 44(4): 1458-1463
[132] Liu X Q, Miao M H, Zhou Z J, et al. Exposure to bisphenol-A and reproductive hormones among male adults [J]. Environmental Toxicology and Pharmacology, 2015, 39(2): 934-941
[133] Pollack A Z, Mumford S L, Krall J R, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach [J]. Environment International, 2018, 120: 137-144
[134] Desdoits-Lethimonier C, Lesné L, Gaudriault P, et al. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis [J]. Human Reproduction, 2017, 32(7): 1465-1473
[135] Rehfeld A, Andersson A M, Skakkebæk N E. Bisphenol A diglycidyl ether (BADGE) and bisphenol analogs, but not bisphenol A (BPA), activate the CatSper Ca2+ channel in human sperm [J]. Frontiers in Endocrinology, 2020, 11: 324
[136] Siracusa J S, Yin L, Measel E, et al. Effects of bisphenol A and its analogs on reproductive health: A mini review [J]. Reproductive Toxicology, 2018, 79: 96-123
[137] Catenza C J, Farooq A, Shubear N S, et al. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues [J]. Chemosphere, 2021, 268: 129273
[138] Wan Y J, Huo W Q, Xu S Q, et al. Relationship between maternal exposure to bisphenol S and pregnancy duration [J]. Environmental Pollution, 2018, 238: 717-724
[139] Aung M T, Ferguson K K, Cantonwine D E, et al. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens [J]. Environmental Research, 2019, 169: 131-138
[140] Ferguson K K, Meeker J D, Cantonwine D E, et al. Environmental phenol associations with ultrasound and delivery measures of fetal growth [J]. Environment International, 2018, 112: 243-250
[141] Liang J, Liu S, Liu T, et al. Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns [J]. Chemosphere, 2020, 252: 126422
[142] Milczarek-Banach J, Rachoń D, Bednarczuk T, et al. Exposure to bisphenol A analogs and the thyroid function and volume in women of reproductive age-cross-sectional study [J]. Frontiers in Endocrinology, 2020, 11: 587252
[143] Philips E M, Kahn L G, Jaddoe V W V, et al. First trimester urinary bisphenol and phthalate concentrations and time to pregnancy: A population-based cohort analysis [J]. The Journal of Clinical Endocrinology and Metabolism, 2018, 103(9): 3540-3547
[144] Zhuang S L, Zhang C L, Liu W P. Atomic insights into distinct hormonal activities of bisphenol A analogues toward PPARγ and ERα receptors [J]. Chemical Research in Toxicology, 2014, 27(10): 1769-1779