近年来,塑料被广泛应用于工业、农业生产、医疗卫生和商品包装中,且需求量巨大。当前,塑料产量从1950年代的150万t增加至2018年的3.6亿t,2018年中国的塑料产量占世界塑料产量的30%[1]。然而,塑料废弃物处置不当所引发的环境污染问题,引起了世界范围内的广泛忧虑[2]。塑料产品在风化和侵蚀后会破碎成较小的碎片,美国国家海洋和大气管理局(NOAA)将长度<5 mm的塑料碎片定义为微塑料(microplastics, MPs)[3]。如今MPs已成为非常受关注的新污染物,因其难降解、在环境中可长距离运输等特性,对生态系统产生的危害引起了全球关注。塑料薄膜残留物的分解、地表径流、污水灌溉和大气沉降等原因,导致了微塑料MPs容易在土壤中累积,据调查,工业和农业区域土壤中的MPs可高达300~67 500 mg·kg-1[4]。但在风力、地下渗透和雨水冲刷的作用下,微塑料MPs通过地表径流和河流汇入海洋,使其成为微塑料MPs的最终聚集地。据报道,长江口表层水中MPs的平均丰度为900 n·m-3[5-6],珠江三角洲每年估计有390亿个(约66 t)MPs颗粒从河流汇聚入海[7],MPs最终汇聚入海,在全球近海、大洋、深海和极地海洋表面大约有5.25万亿个MPs颗粒漂浮,总质量可达2.7×105 t[8]。
MPs易被水生生物摄入,科学家们在贻贝、鱼类等水生物体内均发现了MPs的存在[3,9-12]。有报道称,当MPs进入机体后会导致斑马鱼(zebrafish)和珊瑚鱼(Acanthochromis polyacanthus)的行为异常、抑制鱼体的生长[13-14];使欧州鲈鱼(Dicentrarchus labrax)[15]、罗非鱼(Oreochromis niloticus)的免疫防御功能降低、扰乱机体的脂质代谢[16]。此外,MPs还会在水生生物的肠道内累积,使机体肠道菌群紊乱[17]。有研究发现MPs可通过影响鱼类肠道菌群组成,进而扰乱机体的营养吸收和能量代谢[18];此外,MPs还会导致斑马鱼肠道菌群紊乱从而引起肠道炎症,并诱导机体产生氧化应激[19]。
目前国内外学者已开展了大量有关微塑料对鱼类的毒性效应研究,但微塑料的种类选择多为聚苯乙烯(PS)[20-23],对聚乙烯的毒性效应研究鲜见报道。尼罗罗非鱼是杂食性鱼,具有生长、繁殖快、抗逆性强等特性,是海南省重要的经济养殖鱼类,也是重要的科研用淡水养殖鱼类[24-25]。因此,本文采用尼罗罗非鱼作为实验对象,研究高密度聚乙烯(HDPE)对其生长性能、生理生化功能以及肠道菌群结构的影响,研究结果可为微聚乙烯塑料对水生生物的毒性效应及其生态风险评估提供科学依据。
1 材料与方法(Materials and methods)
1.1 实验材料
尼罗罗非鱼仔鱼购自海南省海口市海南昌盛鱼鳖种苗场,驯养1周,实验用淡水经200目筛绢过滤,提前曝气24 h后备用。
高密度聚乙烯粉末,粒径61~83 μm(95.5%),密度约为1 000 n·mg-1,购自上海冠步科技有限公司。
1.2 实验方法
1.2.1 尼罗罗非鱼暴露实验
暴露实验在玻璃缸(60 cm×30 cm×35 cm)中进行,实验水体为30 L,每组设置3个重复,随机选择180尾(每缸15尾)大小相近健康幼鱼((23.9±0.46) g)分别放入HDPE浓度为0、0.01、0.1和5 mg·L-1的淡水中,HPDE暴露浓度的设计依据海南海域实测环境浓度和前期预实验。实验期间连续曝气,每日投喂罗非鱼饲料(约占体质量的3%)2次,日换水量100%,暴露28 d后进行取样,取样前禁食24 h,收集鱼脑、肠和肝脏,肝脏称量湿质量,其他分别放入1.5 mL的无菌离心管中,然后将样品置于液氮中速冻,样品储存在-80 ℃超低温冰箱中待后续分析。
1.2.2 生长指标的测定
参考[25-26]的方法,进行生长性能指标的测定:
成活率(survival rate, %)=成活数量/总数量×100%
增重率(weight gain rate, %)=(Wt-W0)/W0×100%
肝体指数(hepatosomatic index, HI, %)=WI/Wt×100%
肥满度(condition factor, %)=Wt/Lt3×100%
式中:W0、WI、Wt和Lt分别为尼罗罗非鱼的初始体质量(g·尾-1),尼罗罗非鱼肝脏质量(g·尾-1),实验终末尼罗罗非鱼体质量(g·尾-1)和终末鱼体长(cm)。
1.2.3 CAT、T-AOC、AKP、ACP、AChE酶活性和MDA含量的测定
每组肝脏和鱼脑称重约0.1 g,用0.86%预冷的生理盐水溶液制成10%(V/m)的匀浆。将匀浆液在4 ℃下2 500 r·min-1离心10 min。立即提取上清液,接着用酶标仪(Bio-RAD,美国)分析其生化参数。丙二醛(MDA)、过氧化氢酶(CAT)、总抗氧化能力(T-AOC)、碱性磷酸酶(AKP)、酸性磷酸酶(ACP)和乙酰胆碱酯酶(AChE)等试剂盒,均购自南京建成生物工程研究所。
1.2.4 16S rDNA高通量测序检测MPs对肠道微生物的影响
使用杭州福来纳米技术有限公司提供的商业磁珠DNA分离试剂盒,从每个肠道中提取基因组DNA(gDNA)。所有提取的gDNA均通过紫外光谱和电泳进行定量,以供进一步分析。此外,采用以下方案通过实时qPCR扩增每个样品中的部分gDNA:50 ℃ 2 min,95 ℃ 10 min,95 ℃ 15 s,56 ℃ 30 s和72 ℃持续1 min,重复40个循环,然后72 ℃持续10 min。用靶向细菌16S rDNA基因V3和V4区的特异性引物(正向引物:5’-ACTCCTACGGGAGGCAGCAG-3’;反向引物:5’-GGACTACHVGGGTWTCTAAT-3’)扩增gDNA样品。此外,在Illumina MiSeq平台上使用双索引扩增和测序,然后进行QIIME(vision 1.9.0)生物信息学分析,检测肠道菌群的组成。为了评估肠道菌群的α多样性,使用QIIME计算丰富度指数(ACE、Chao1指数)和多样性指数(Shannon、Simpson指数)。同时,通过KEGG系统分析罗非鱼肠道微生物基因功能。
1.3 数据统计与分析
所有数据用平均值±标准误(Mean±SE)的方法表示,酶活数据使用SPSS(社会科学统计软件包)25.0软件(美国SPSS Inc.)进行单因素方差分析(One-Way ANOVA)和Duncan检验对数据进行差异性分析,显著水平为P<0.05。肠道微生物数据采用Student’s T检验进行差异性分析,显著水平为P<0.05。
2 结果(Results)
2.1 生长和存活指标
与对照组相比,HDPE暴露28 d后,尼罗罗非鱼的增重率、成活率和肝体比均无明显变化(P>0.05)(图1(a)、(b)和(d))。而0.1 mg·L-1和5 mg·L-1 HDPE暴露组中尼罗罗非鱼的肥满度显著低于对照组(P<0.05)(图1(c))。

图1 高密度聚乙烯微塑料(HDPE)暴露28 d对尼罗罗非鱼的增重率(a)、成活率(b)、肥满度(c)和肝体比(d)的影响
注:不同小写字母表示不同处理组间存在显著差异(P<0.05)。
Fig. 1 Effect of high-density polyethylene (HDPE) on weight gain rate (a), survival rate (b),
condition factor (c) and hepatosomatic index (d) of O. niloticus after exposure for 28 d
Note: Different lowercase letters represent significant difference between each group (P<0.05).
2.2 MDA含量与抗氧化酶活性
经HDPE暴露28 d后,0.01 mg·L-1和5 mg·L-1 HDPE暴露组尼罗罗非鱼肝脏MDA含量显著高于对照组(P<0.05) (图2(a));T-AOC、SOD和CAT活性与对照组相比无显著差异(P>0.05)(图2(b)、(d)和(e))。尼罗罗非鱼肝脏GST活性仅在5 mg·L-1暴露组中显著升高(P<0.05)(图2(c))。
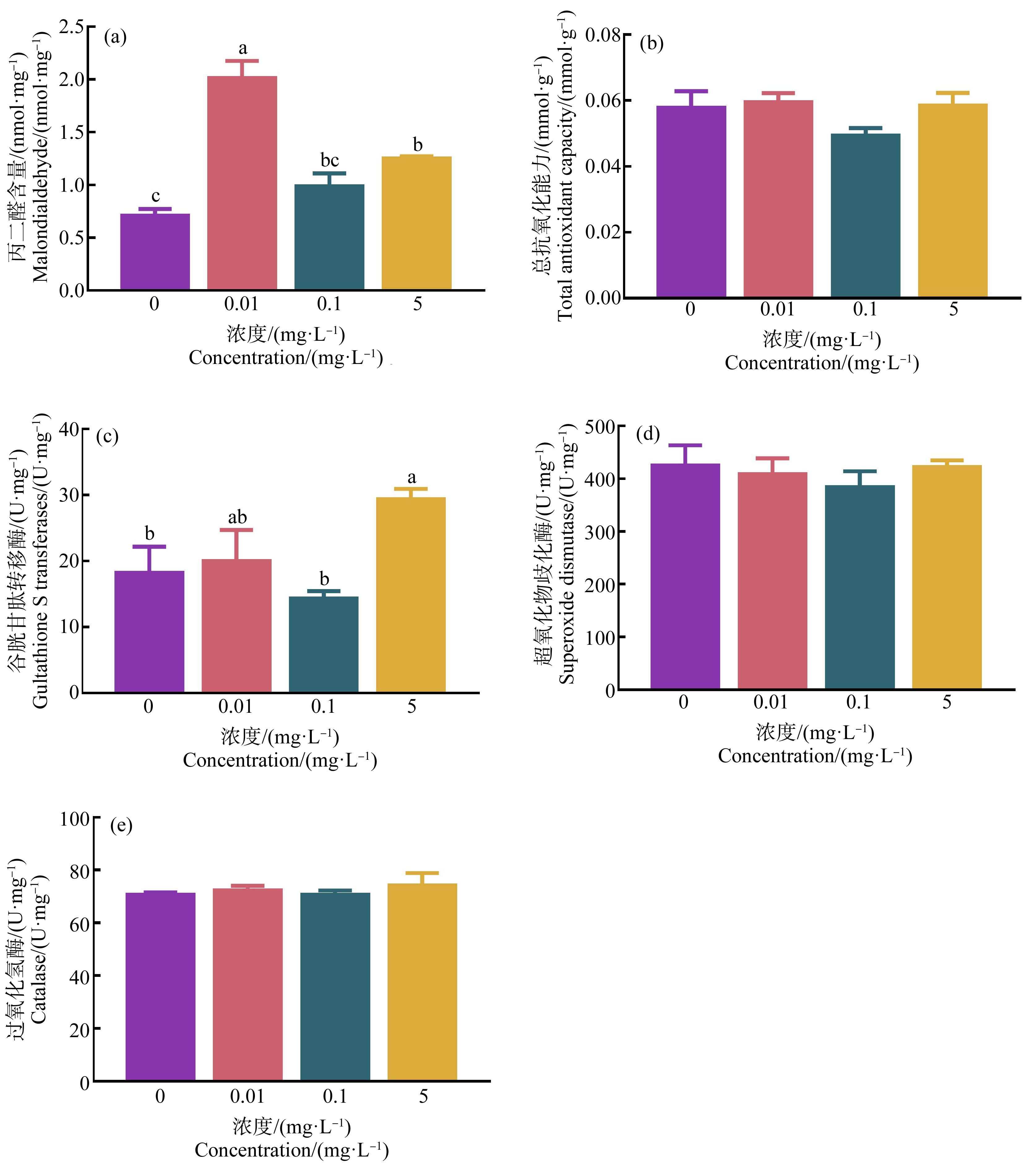
图2 HDPE暴露28 d对尼罗罗非鱼肝脏组织丙二醛(MDA) (a)、总抗氧化能力(T-AOC) (b)、
谷胱甘肽转移酶(GST) (c)、超氧化物歧化酶(SOD) (d)和过氧化氢酶(CAT) (e)的影响
注:不同小写字母表示不同处理组间存在显著差异(P<0.05)。
Fig. 2 Effect of HDPE on malondialdehyde (MDA) (a), total-antioxidant capacity (T-AOC) (b), gultathione S-transferases (GST) (c),
superoxide dismutase (SOD) (d) and catalase (CAT) (e) in liver of O. niloticus after exposure for 28 d
Note: Different lowercase letters represent significant difference between each group (P<0.05).
2.3 神经毒性和免疫功能
经HDPE暴露28 d后,尼罗罗非鱼脑组织中AChE活性以及肝脏组织中AKP活性与对照组相比无显著性差异(P>0.05)(图3(a)和(c)),0.1 mg·L-1暴露组尼罗罗非鱼肝脏组织中ACP活性显著高于对照组(P<0.05)(图3(b))。
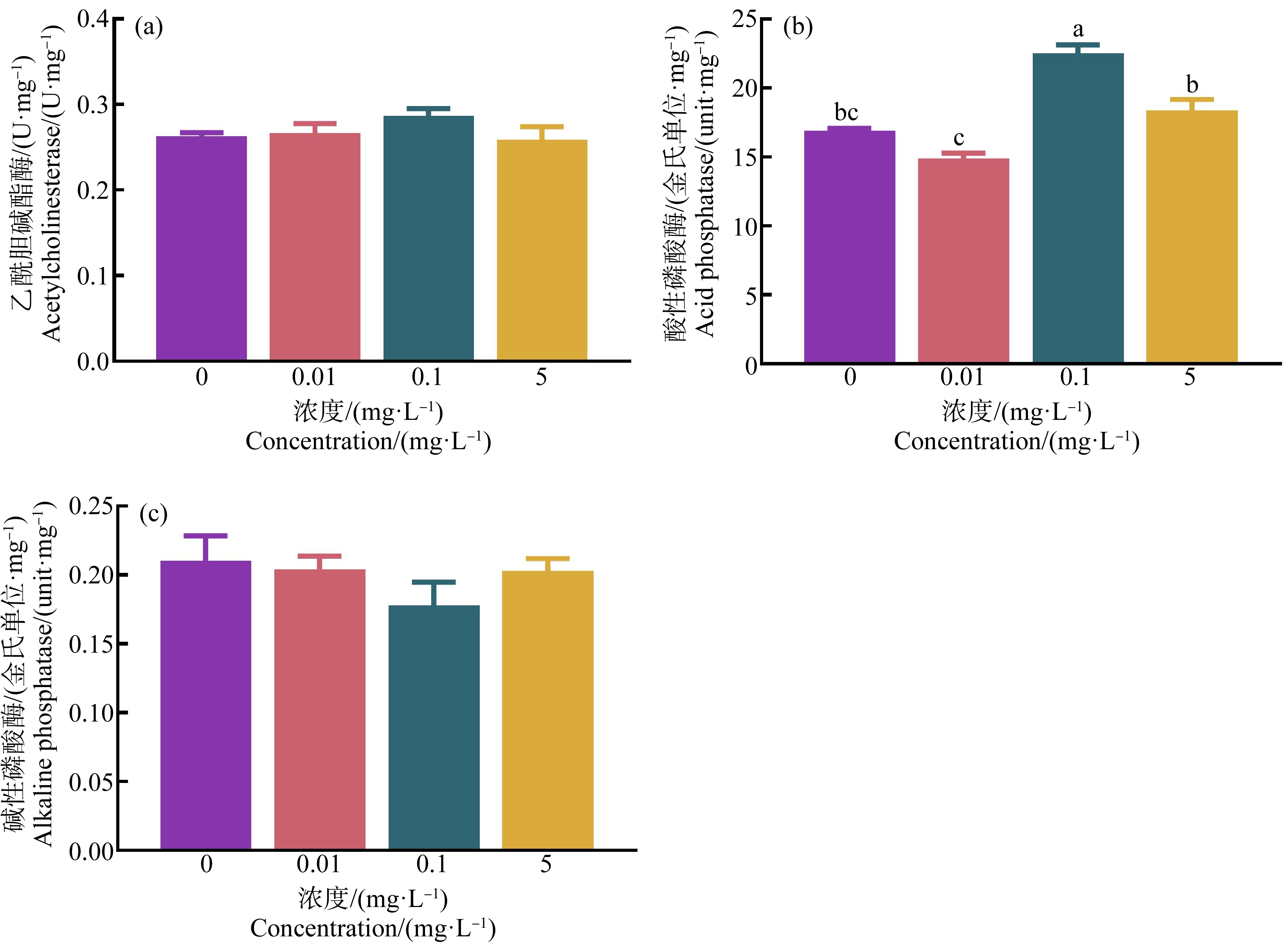
图3 HDPE暴露28 d对尼罗罗非鱼脑组织乙酰胆碱酯酶(AChE) (a)、
肝脏组织酸性磷酸酶(ACP) (b)和碱性磷酸酶(AKP) (c)的影响
注:不同小写字母表示不同处理组间存在显著差异(P<0.05)。
Fig. 3 Effect of HDPE on acetylcholinesterase (AChE) in brain (a), acid phosphatase (ACP) in liver (b) and
alkaline phosphatase (AKP) in liver (c) of O. niloticus after exposure for 28 d
Note: Different lowercase letters represent significant difference between each group (P<0.05).
2.4 肠道菌群丰度与结构功能
OTU(Operational Taxonomic Units)是人为把相似度>97%的分类单元(品系、属、种、分组等)序列进行聚类而设置的统一标志。Veen图用来统计样本之间OTU组成的相似情况,通常情况下,分析时选用相似水平为97%的OTU。如图4显示,0.01、0.1和5 mg·L-1 HDPE暴露组与对照组之间共有的OTU数目分别为34、30和8。

图4 HDPE暴露28 d尼罗罗非鱼肠道微生物Venn图
Fig. 4 Veen diagram of intestinal microbiota after O. niloticus exposed to HDPE for 28 d
Alpha多样性分析显示,Chao1、ACE、Shannon和Simpson指数与对照组相比无显著性差异(P>0.05)(表1)。
表1 HDPE暴露28 d尼罗罗非鱼肠道菌群的Alpha多样性指数
Table 1 Alpha diversity of intestinal microbiota after O. niloticus exposed to HDPE for 28 d
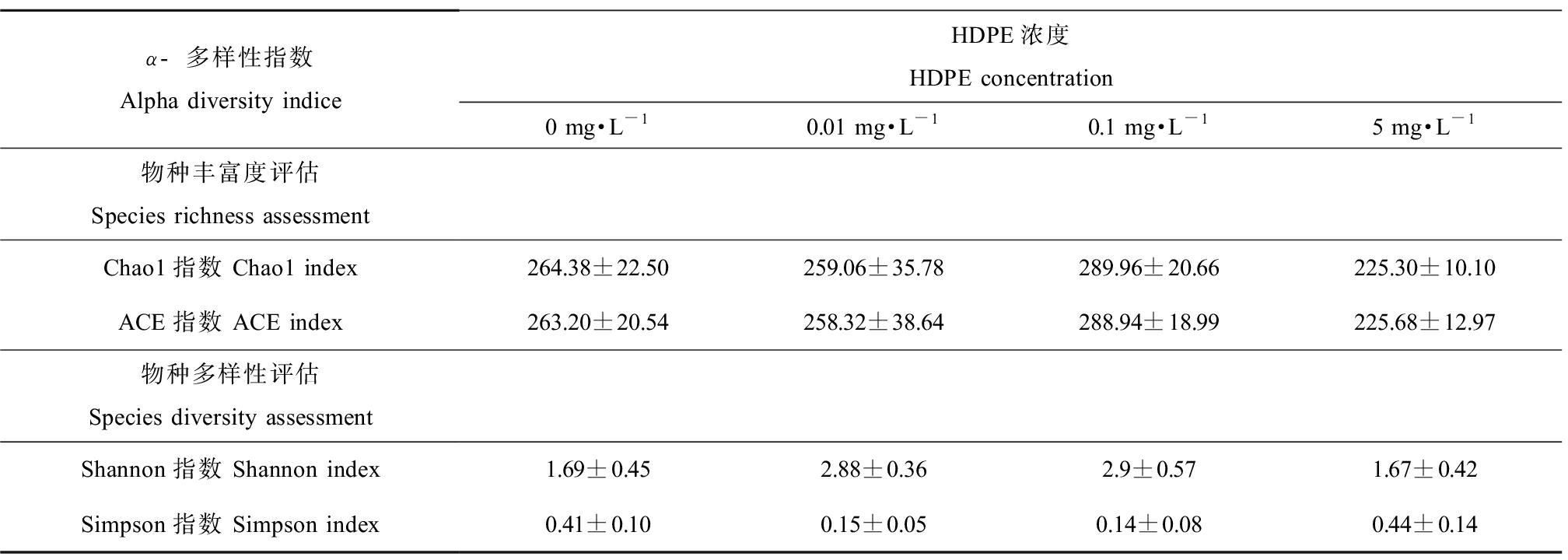
α-多样性指数Alpha diversity indiceHDPE浓度HDPE concentration0 mg·L-10.01 mg·L-10.1 mg·L-15 mg·L-1物种丰富度评估Species richness assessmentChao1指数 Chao1 index264.38±22.50259.06±35.78289.96±20.66225.30±10.10ACE指数 ACE index263.20±20.54258.32±38.64288.94±18.99225.68±12.97物种多样性评估Species diversity assessmentShannon指数 Shannon index1.69±0.452.88±0.362.9±0.571.67±0.42Simpson指数 Simpson index0.41±0.100.15±0.050.14±0.080.44±0.14
注:表中同一列数据右上角相同字母表示差异不显著(P> 0.05),不同表示差异显著(P<0.05)。
Note: The same superscript letters in the same rank represents no significant difference (P>0.05), while the different letters represent significant difference (P<0.05).
在门水平上,放线菌门(Actinobacteria)、变形菌门(Proteobacteria)、梭杆菌门(Fusobacteria)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、绿弯菌门(Chloroflexi)、蓝细菌门(Cyanobacteria)和衣原体门(Chlamydiae)是尼罗罗非鱼肠道内的优势物种,其他物种丰度占比均<1%。其中,5 mg·L-1 HDPE暴露组的尼罗罗非鱼肠道中,衣原体门显著高于对照组(P<0.05)(图5(a)和(b))。

图5 HDPE暴露28 d后尼罗罗非鱼肠道微生物门水平物种组成(a)及组间差异(b)
注:*表示P<0.05。
Fig. 5 Composition of intestine microbiota at the phylum level of O. niloticus (a) and differences
between groups (b) after exposure to HDPE for 28 d
Note: *represents P<0.05.
在属水平上,戈登氏菌属(Gordonia)、分支杆菌属(Mycobacterium)、鲸杆菌属(Cetobacterium)、链球菌属(Streptococcus)、红球菌属(Rhodococcus)和邻单胞菌属(Plesiomonas)为尼罗罗非鱼肠道内的优势属。由Student’s T检验,0.01 mg·L-1 HDPE暴露组未定义到属的芽孢杆菌目(norank_f_norank_o_Bacillales)显著高于对照组(P<0.05);0.1 mg·L-1 HDPE暴露组肠杆菌属(Enterobacter)显著高于对照组(P<0.05);5 mg·L-1暴露组未定义到属的副衣原体科(norank_f Parachlamydiaceae)、生丝微菌属(Hyphomicrobium)、假红游动菌属(Pseudorhodoplanes)、norank_f_JG30-KF-CM45、诺卡菌属(Nocardioides)、井杆菌属(Phreatobacter)、微单胞菌属(Micromonospora)、未定义到属的衣藻科(norank_f_Holosporaceae)和链霉菌属(Streptomyces)均显著高于对照组(P<0.05)(图6(a)和(b))。
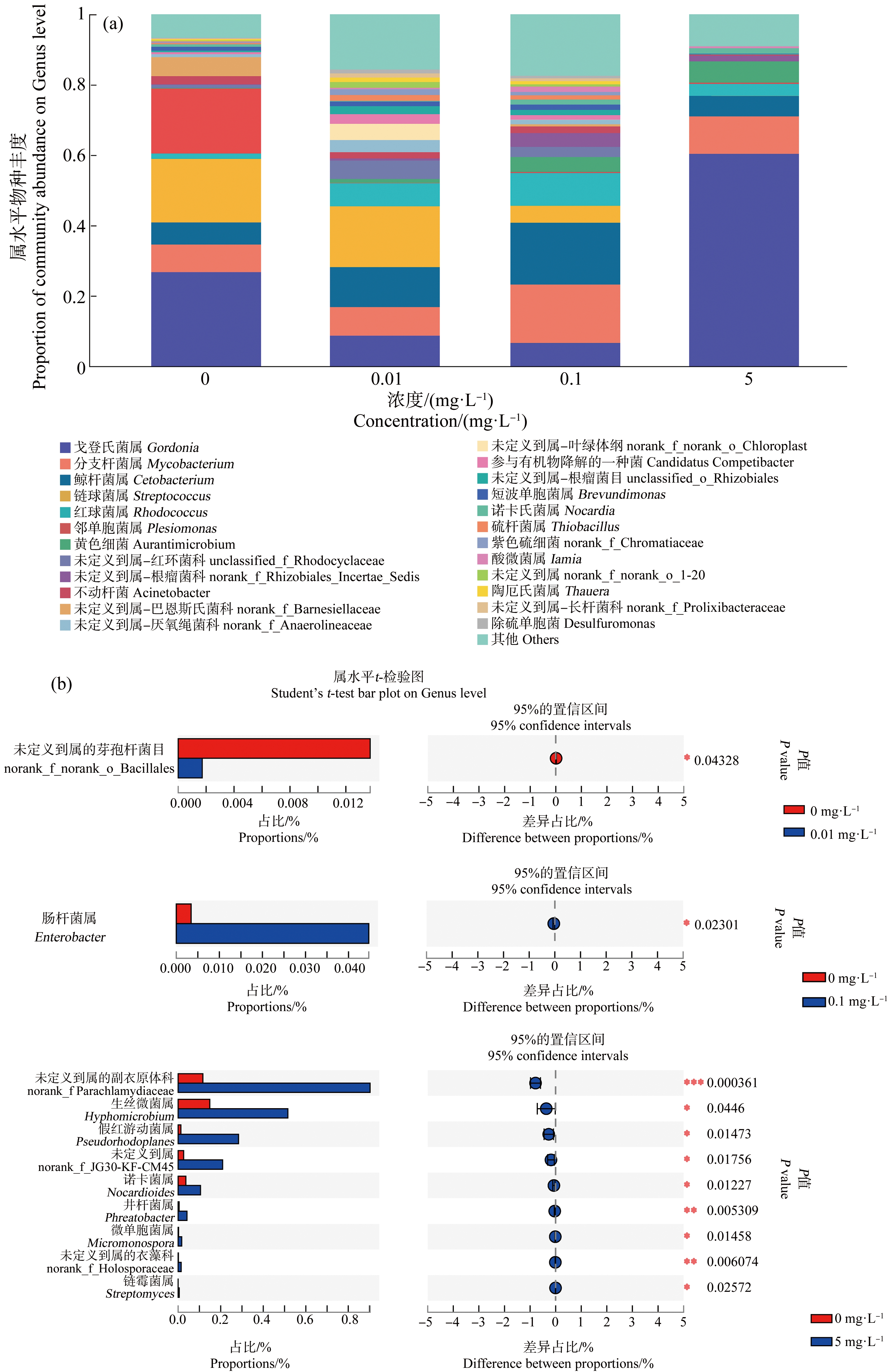
图6 HDPE暴露28 d后尼罗罗非鱼肠道微生物属水平物种组成(a)及组间差异(b)
注:*表示P<0.05,**表示P<0.01。
Fig. 6 Composition of intestine microbiota at the Genus level of O. niloticus (a) and differences
between groups (b) after exposure to HDPE for 28 d
Note: *represents P<0.05, **represents P<0.01.
根据KEGG基因功能预测分析可知,0.1 mg·L-1 HDPE暴露组尼罗罗非鱼的肠道菌群中双组分系统(two-component system)、嘧啶代谢(pyrimidine metabolism)、氨酰基-1RNA生物合成(aminoacyl-1RNA biosynthesis)和同源重组(homologous recombination)通路与对照组相比显著上调(P<0.05),5 mg·L-1 HDPE暴露组中脂肪酸降解(fatty acid degradation)和色氨酸代谢(tryptophan metabolism)通路与对照组相比显著上调(P<0.05)(图7)。

图7 HDPE暴露28 d后尼罗罗非鱼肠道菌群KEGG功能预测分析
注:*表示P<0.05。
Fig. 7 KEGG prediction analysis of intestinal microbiota after O. niloticus exposed to HDPE for 28 d
Note: *represents P<0.05.
3 讨论(Discussion)
本研究中,高密度聚乙烯微塑料未导致尼罗罗非鱼死亡,且与对照组相比,尼罗罗非鱼的增重、成活率和肝体比均无显著变化,这和前人的研究结果相似[22,27-29]。也有报道称,MPs可以降低金鲷鱼的生长[14],这可能与MPs的粒径大小有关,有研究表明,MPs粒径越小对机体的危害越大[15,30]。
当机体受到环境胁迫时,体内活性氧平衡被打破,产生过多的过氧化产物[31]。MDA作为脂质过氧化的终产物,其含量水平可作为机体过氧化程度的指标[32]。SOD、CAT、GST和T-AOC等构成机体抗氧化酶系统。在本研究中,HDPE暴露28 d后,尼罗罗非鱼肝脏中MDA含量和GST酶活性显著升高,但SOD、CAT和T-AOC酶活性无显著变化。这与金鱼暴露于聚氯乙烯微塑料4 d后,肝脏中的MDA含量和GST活性显著升高的结果一致[33]。当鲷鱼暴露于低密度聚乙烯微塑料21 d后,肝脏中GST酶活性也显著升高[34]。以上结果表明,在聚乙烯的MPs胁迫下,会引起尼罗罗非鱼的氧化应激,激活机体的抗氧化防御系统。
AChE是一种与神经冲动传递有关的必需酶,当机体暴露于环境污染物时,其活性易被抑制[35]。本研究结果显示,75 μm HDPE暴露28 d后,对罗非鱼没有神经毒性效应。这与以往的研究不同,据报道,当0.1 μm聚苯乙烯暴露14 d后,红罗非鱼AChE活性被抑制[22];在饲料中添加10~600 μm的聚乙烯后,斑马鱼会因为神经损伤产生癫痫的症状[13];暴露于100~400 μm聚乙烯和聚丙烯混合物,对楔形蛤(Donax trunculus)产生神经毒性[35]。推测这可能和MPs粒径大小和模式生物有关。研究表明,粒径小的MPs可以通过血液循环转移到机体的不同组织[27,29],当MPs通过血脑屏障进入脑组织中时就会对机体产生神经抑制,而粒径大的MPs无法通过血液屏障,因此主要存在于胃肠道中[36-37]。此外,相较于底栖动物,尼罗罗非鱼的生物累积效应低,且具有较强的解毒能力,所以推测HDPE对尼罗罗非鱼的神经毒性影响较小。
鱼类在抵抗外界胁迫的过程中,主要依靠其非特异性免疫防御系统进行防御[38]。非特异性防御机制是通过免疫相关酶来抵抗异物的侵入,从而提高自身免疫力,其中磷酸酶是非常重要的非特异性免疫酶[39],也是动物体内的解毒体系[40],可通过直接溶解或消化外源物质达到防御目的[41]。根据磷酸酶催化作用最适pH的特性,可分为酸性磷酸酶(acid phosphatase, ACP)和碱性磷酸酶(alkaline phosphatase, AKP)[42]。在本研究中,尼罗罗非鱼在HDPE暴露28 d后对AKP活性并未造成显著影响,而0.1 mg·L-1 HDPE暴露组的ACP活性显著升高。有研究表明当黑海参(Holothuria atra)暴露于MPs后,AKP活性没有显著变化[43],而ACP活性升高,表明MPs会影响机体内的免疫防御反应。
肠道微生物可以调节宿主的生长代谢、免疫防御,屏蔽病原微生物入侵,对宿主健康起着非常重要的作用[18]。研究表明,MPs可以在肠道内累积,对肠道微生物群结构和宿主健康存在威胁[29]。本研究发现,HDPE暴露28 d后,尼罗罗非鱼肠道微生物的Alpha多样性与对照组相比无显著差异,此外,罗非鱼肠道菌群Veen图显示随HDPE暴露浓度的升高肠道菌群OTU数逐渐减少。以上结果表明,HDPE胁迫后可能会引起尼罗罗非鱼肠道菌群紊乱,且高浓度HDPE暴露组效应最明显。
据报道,变形菌门、梭杆菌门、厚壁菌门、拟杆菌门和蓝细菌门是罗非鱼肠道中的优势菌群[44-45],其中变形菌门和拟杆菌门丰度下降会降低机体的免疫防御功能[46]。在本研究中,尼罗罗非鱼肠道中变形菌门、拟杆菌门的丰度随着HDPE暴露浓度增加而减少,表明HDPE对尼罗罗非鱼肠道菌群的免疫功能造成潜在影响,且与暴露浓度有关。蓝细菌门能产生自由基分解MPs[47],在本研究中,其丰度减少表明高浓度HDPE暴露可以降低尼罗罗非鱼肠道降解MPs的能力。此外,5 mg·L-1 HDPE暴露组尼罗罗非鱼肠道菌群中衣原体门丰度显著高于对照组(P<0.05)。衣原体作为一种致病菌,可以感染的宿主非常多[48],许多衣原体对动物和人类都有致病性,可作为疾病传播的媒介,构成人畜共患病[49]。对于水产动物而言,衣原体可以引起鱼类产生上皮样囊肿,上皮样囊肿是一种新型的水产养殖疾病,其特征是在鱼类的鳃或皮肤上形成细胞质包涵体,引发鱼类的鳃粘膜粘连坏死或组织增生进而导致鱼体死亡,且衣原体在鱼类中感染率可达4%~100%[50],严重危害养殖动物健康[51-52]。当尼罗罗非鱼暴露于高浓度MPs后,衣原体门丰度显著升高,表明聚乙烯MPs对尼罗罗非鱼的肠道健康造成危害。未定义到属的芽孢杆菌目和肠杆菌属属于肠道益生菌,具有免疫增强作用[53-54],小单胞菌属、链霉菌属和放线菌属可以产生抗菌和抗肿瘤特性物质[55]。当肠道菌群受到MPs胁迫时,其丰度增加,可能与机体的免疫防御反应有关。其中,在高浓度HDPE暴露组中,未定义到属的副衣原体科含量增加最为明显,表明高浓度MPs可以危害尼罗罗非鱼肠道健康,进而对尼罗罗非鱼的机体健康产生潜在风险。
KEGG通路功能预测分析结果显示,机体受到MPs胁迫后0.1 mg·L-1 HDPE暴露组双组分系统、嘧啶代谢、氨酰基-tRNA生物合成和同源重组通路与对照组相比,显著上调(P<0.05)。其中,双组分系统通路属于信号转导功能,可以使细菌接收和响应细胞内外的各种物理、化学和生物刺激[56],其通路上调说明MPs刺激肠道内的信号转导增强;嘧啶代谢和癌细胞增殖有关,在癌细胞中嘧啶代谢酶会过度表达;氨酰基-tRNA生物合成和同源重组属于翻译功能,氨酰基tRNA合成酶(AARS)是翻译机制的重要组成部分,可以催化氨基酸特异性附着在同源tRNA上[57],为蛋白质生物合成提供原料,也可为细胞增殖提供所需的酶[58]。因此,推测聚乙烯微塑料会诱导尼罗罗非鱼肠道内肿瘤细胞或癌细胞增殖。以上4条通路均满足了癌细胞快速增殖的条件,但具体作用机制还需要进一步探究。
在5 mg·L-1 HDPE暴露组中,尼罗罗非鱼肠道中脂肪酸降解和色氨酸代谢通路显著高于对照组。鱼类主要依赖脂代谢为机体提供能量[59],当鱼类暴露于MPs后机体脂代谢增加,表明机体通过调动储备能量来维持生存,进而导致机体储备能量降低。此外,肠道中色氨酸代谢产物可参与肠道通透性、炎症调节和宿主免疫反应[60],还可介导宿主的T细胞和B细胞免疫[61]。色氨酸代谢通路上调表明尼罗罗非鱼暴露于聚乙烯微塑料后,其肠道免疫防御系统被激活,这可能是MPs引起机体储备能量降低,进而导致机体免疫能力下降所致。
综上所述,HDPE暴露尼罗罗非鱼后对其生长、免疫和神经系统没有显著影响,但可以造成氧化损伤,使其肠道菌群发生紊乱,其中,肠道菌群中衣原体门丰度的增加会危害肠道和机体健康。在本研究中已经检测到KEGG功能通路的变化,但其具体影响机制还有待进一步研究。
[1] Plastics Europe. The plastics fact-2019 [EB/OL]. [2022-01-14]. https://www.plasticseurope.org/en/resources/market-data.
[2] 杨光蓉, 陈历睿, 林敦梅. 土壤微塑料污染现状、来源、环境命运及生态效应[J]. 中国环境科学, 2021, 41(1): 353-365
Yang G R, Chen L R, Lin D M. Status, sources, environmental fate and ecological consequences of microplastic pollution in soil [J]. China Environmental Science, 2021, 41(1): 353-365 (in Chinese)
[3] National Oceanic and Atmospheric Administration (NOAA). Feature-story/researchers-probe-orca-poop-microplastics-part-1 [EB/OL]. [2022-01-14]. https://www.fisheries.noaa.gov/
[4] Zhang J J, Chen Y H, Wang X X, et al. A review of microplastics in the soil environment [J]. Chinese Journal of Eco-Agriculture, 2021, 29(6): 937-952
[5] Zhang W W, Zhang S F, Wang J Y, et al. Microplastic pollution in the surface waters of the Bohai Sea, China [J]. Environmental Pollution, 2017, 231: 541-548
[6] Zhao S Y, Wang T, Zhu L X, et al. Analysis of suspended microplastics in the Changjiang Estuary: Implications for riverine plastic load to the ocean [J]. Water Research, 2019, 161: 560-569
[7] Mai L, You S N, He H, et al. Riverine microplastic pollution in the Pearl River Delta, China: Are modeled estimates accurate? [J]. Environmental Science & Technology, 2019, 53(20): 11810-11817
[8] 刘香, 茹小尚, 张立斌. 海洋微塑料污染的生物效应研究进展[J]. 海洋科学, 2021, 45(3): 122-133
Liu X, Ru X S, Zhang L B. Research progress on the biological effects of marine microplastic pollution [J]. Marine Sciences, 2021, 45(3): 122-133 (in Chinese)
[9] Avio C G, Gorbi S, Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea [J]. Marine Environmental Research, 2015, 111: 18-26
[10] Kolandhasamy P, Su L, Li J N, et al. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion [J]. Science of the Total Environment, 2018, 610-611: 635-640
[11] Bellas J, Martínez-Armental J, Martínez-Cámara A, et al. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts [J]. Marine Pollution Bulletin, 2016, 109(1): 55-60
[12] Karlsson T M, Vethaak A D, Almroth B C, et al. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation [J]. Marine Pollution Bulletin, 2017, 122(1-2): 403-408
[13] Mak C W, Ching-Fong Yeung K, Chan K M. Acute toxic effects of polyethylene microplastic on adult zebrafish [J]. Ecotoxicology and Environmental Safety, 2019, 182: 109442
[14] Critchell K, Hoogenboom M O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus) [J]. PLoS One, 2018, 13(3): e0193308
[15] Brandts I, Teles M, Tvarijonaviciute A, et al. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax [J]. Genomics, 2018, 110(6): 435-441
[16] Hamed M, Soliman H A M, Osman A G M, et al. Assessment the effect of exposure to microplastics in Nile tilapia (Oreochromis niloticus) early juvenile: Ⅰ. Blood biomarkers [J]. Chemosphere, 2019, 228: 345-350
[17] Jin Y X, Xia J Z, Pan Z H, et al. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish [J]. Environmental Pollution, 2018, 235: 322-329
[18] Evariste L, Barret M, Mottier A, et al. Gut microbiota of aquatic organisms: A key endpoint for ecotoxicological studies [J]. Environmental Pollution, 2019, 248: 989-999
[19] Lu L, Luo T, Zhao Y, et al. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health [J]. Science of the Total Environment, 2019, 667: 94-100
[20] Song J A, Choi C Y, Park H S. Exposure of bay scallop Argopecten irradians to micro-polystyrene: Bioaccumulation and toxicity [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2020, 236: 108801
[21] Chen Q, Lv W W, Jiao Y, et al. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus [J]. Aquatic Toxicology, 2020, 224: 105497
[22] Ding J N, Zhang S S, Razanajatovo R M, et al. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus) [J]. Environmental Pollution, 2018, 238: 1-9
[23] Elizalde-Velázquez A, Carcano A M, Crago J, et al. Translocation, trophic transfer, accumulation and depuration of polystyrene microplastics in Daphnia magna and Pimephales promelas [J]. Environmental Pollution, 2020, 259: 113937
[24] 吴凡, 田娟, 喻丽娟, 等. 饲料糖脂比对养成中期吉富罗非鱼生长性能、体成分和血清生化指标的影响[J]. 动物营养学报, 2020, 32(12): 5805-5815
Wu F, Tian J, Yu L J, et al. Effects of dietary carbohydrate to lipid ratio on growth performance, body composition and serum biochemical indices of genetic improvement of farmedtilapia in growth mid-stage [J]. Chinese Journal of Animal Nutrition, 2020, 32(12): 5805-5815 (in Chinese)
[25] 陈培赟, 任潇潇, 毕保良. 饲料中添加黄藤素对吉富罗非鱼生长性能、抗氧化能力和非特异性免疫的影响[J]. 上海海洋大学学报, 2021, 30(5): 812-820
Chen P Y, Ren X X, Bi B L. Effects of dietary palmatine on growth performance, antioxidant capacity and non-specific immunity of GIFT strain of Nile tilapia (Oreochromis niloticus) [J]. Journal of Shanghai Ocean University, 2021, 30(5): 812-820 (in Chinese)
[26] 龚福来, 林雪, 王红权. 不同维生素C源对吉富罗非鱼生长性能、抗氧化能力和免疫力的影响[J]. 动物营养学报, 2021, 33(4): 2378-2389
Gong F L, Lin X, Wang H Q. Effects of different vitamin C sources on growth performance, antioxidant capacity and immunity of genetically improved farmed tilapia [J]. Chinese Journal of Animal Nutrition, 2021, 33(4): 2378-2389 (in Chinese)
[27] Pitt J A, Kozal J S, Jayasundara N, et al. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio) [J]. Aquatic Toxicology, 2018, 194: 185-194
[28] Wen B, Zhang N, Jin S R, et al. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid [J]. Aquatic Toxicology, 2018, 195: 67-76
[29] Zhu M, Chernick M, Rittschof D, et al. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes) [J]. Aquatic Toxicology, 2020, 220: 105396
[30] Lei L L, Wu S Y, Lu S B, et al. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans [J]. Science of the Total Environment, 2018, 619-620: 1-8
[31] 王小娇, 胡国成, 张丽娟, 等. 重金属镉胁迫对尼罗罗非鱼抗氧化酶系统的影响[J]. 海洋环境科学, 2016, 35(5): 647-651, 657
Wang X J, Hu G C, Zhang L J, et al. Influence of cadmium on antioxidant defense system in juvenile of Orechromis niloticus [J]. Marine Environmental Science, 2016, 35(5): 647-651, 657 (in Chinese)
[32] 罗其勇. 重金属暴露引起鱼体氧化应激反应的研究进展[J]. 安徽农业科学, 2018, 46(25): 32-35
Luo Q Y. Research progress on oxidative stress reaction of fish body caused by heavy metals [J]. Journal of Anhui Agricultural Sciences, 2018, 46(25): 32-35 (in Chinese)
[33] Romano N, Renukdas N, Fischer H, et al. Differential modulation of oxidative stress, antioxidant defense, histomorphology, ion-regulation and growth marker gene expression in goldfish (Carassius auratus) following exposure to different dose of virgin microplastics [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2020, 238: 108862
[34] Rios-Fuster B, Arechavala-Lopez P, García-Marcos K, et al. Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata [J]. Aquatic Toxicology, 2021, 231: 105737
[35] Tlili S, Jemai D, Brinis S, et al. Microplastics mixture exposure at environmentally relevant conditions induce oxidative stress and neurotoxicity in the wedge clam Donax trunculus [J]. Chemosphere, 2020, 258: 127344
[36] Chen Q Q, Lackmann C, Wang W Y, et al. Microplastics lead to hyperactive swimming behaviour in adult zebrafish [J]. Aquatic Toxicology, 2020, 224: 105521
[37] Dantas N C F M, Duarte O S, Ferreira W C, et al. Plastic intake does not depend on fish eating habits: Identification of microplastics in the stomach contents of fish on an urban beach in Brazil [J]. Marine Pollution Bulletin, 2020, 153: 110959
[38] Limbu S M, Zhou L, Sun S X, et al. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk [J]. Environment International, 2018, 115: 205-219
[39] 韩春艳, 郑清梅, 陈桂丹, 等. 氨氮胁迫对奥尼罗非鱼非特异性免疫的影响[J]. 南方水产科学, 2014, 10(3): 47-52
Han C Y, Zheng Q M, Chen G D, et al. Effect of ammonia-N stress on non-specific immunity of tilapia (Oreochromis niloticus × O. areus) [J]. South China Fisheries Science, 2014, 10(3): 47-52 (in Chinese)
[40] 毕建飞, 张海朋, 曲磊, 等. 维氏气单胞菌3种免疫原对鲫鱼ACP、AKP、POD和SOD活力的影响[J]. 黑龙江畜牧兽医, 2017(9): 12-15
Bi J F, Zhang H P, Qu L, et al. The effects of three types of Aeromonas veronii immunogen on activities of ACP, AKP, POD and SOD in blood of Carassius [J]. Heilongjiang Animal Science and Veterinary Medicine, 2017(9): 12-15 (in Chinese)
[41] 谢丽玲, 谢俊, 赵斌, 等. 重金属污染下龟壳攀鲈组织中ACP和AKP的活力比较[J]. 生态毒理学报, 2016, 11(3): 323-330
Xie L L, Xie J, Zhao B, et al. Activity comparison of ACP and AKP in the tissues of Anabas testudineus from heavy metal-polluted areas [J]. Asian Journal of Ecotoxicology, 2016, 11(3): 323-330 (in Chinese)
[42] 耿艳芳, 赵晓祥. 颜料红23对锦鲤的毒性及对部分组织中磷酸酶活性的影响[J]. 安全与环境学报, 2017, 17(1): 386-391
Geng Y F, Zhao X X. Effect of sublethal pigment red 23 on the acute toxicity of Cyprinus carpio as well as the accumulated phosphatase activities in part of their organs [J]. Journal of Safety and Environment, 2017, 17(1): 386-391 (in Chinese)
[43] 陈孟玲, 高菲, 王新元, 等. 微塑料对黑海参(Holothuria atra)免疫和消化生理的影响[J]. 海洋科学, 2021, 45(4): 126-135
Chen M L, Gao F, Wang X Y, et al. Effects of microplastics on immunity and digestion of sea cucumber, Holothuria atra [J]. Marine Sciences, 2021, 45(4): 126-135 (in Chinese)
[44] 张婧怡, 肖俊, 梁军能, 等. 不同养殖环境下罗非鱼肠道微生物的比较分析[J]. 广西科学院学报, 2020, 36(2): 164-170
Zhang J Y, Xiao J, Liang J N, et al. Comparative analysis of intestinal microorganisms of tilapia under different culture environment [J]. Journal of Guangxi Academy of Sciences, 2020, 36(2): 164-170 (in Chinese)
[45] 佟延南, 李芳远, 李忠琴, 等. 不同养殖阶段罗非鱼肠道微生物多样性的动态分析[J]. 南方农业学报, 2018, 49(7): 1415-1422
Tong Y N, Li F Y, Li Z Q, et al. Dynamic analysis of intestinal microbial diversity in tilapia at different culture stages [J]. Journal of Southern Agriculture, 2018, 49(7): 1415-1422 (in Chinese)
[46] Zhang Y T, Chen H X, He S Q, et al. Subchronic toxicity of dietary sulfamethazine and nanoplastics in marine medaka (Oryzias melastigma): Insights from the gut microbiota and intestinal oxidative status [J]. Ecotoxicology and Environmental Safety, 2021, 226: 112820
[47] Ali S S, Elsamahy T, Koutra E, et al. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal [J]. Science of the Total Environment, 2021, 771: 144719
[48] Burnard D, Polkinghorne A. Chlamydial infections in wildlife-conservation threats and/or reservoirs of ‘spill-over’ infections? [J]. Veterinary Microbiology, 2016, 196: 78-84
[49] Taylor-Brown A, Polkinghorne A. New and emerging chlamydial infections of creatures great and small [J]. New Microbes and New Infections, 2017, 18: 28-33
[50] Sood N, Pradhan P K, Verma D K, et al. Epitheliocystis in rohu Labeo rohita (Hamilton, 1822) is caused by novel Chlamydiales [J]. Aquaculture, 2019, 505: 539-543
[51] Li L L, Amara R, Souissi S, et al. Impacts of microplastics exposure on mussel (Mytilus edulis) gut microbiota [J]. Science of the Total Environment, 2020, 745: 141018
[52] Collingro A, Köstlbacher S, Horn M. Chlamydiae in the environment [J]. Trends in Microbiology, 2020, 28(11): 877-888
[53] Anokyewaa M A, Amoah K, Li Y, et al. Prevalence of virulence genes and antibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China [J]. Aquaculture Reports, 2021, 21: 100784
[54] Xia Y, Lu M X, Chen G, et al. Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus [J]. Fish & Shellfish Immunology, 2018, 76: 368-379
[55] Nafie M S, Awad N M, Tag H M, et al. Micromonospora species from rarely-exploited Egyptian habitats: Chemical profile, antimicrobial, and antitumor activities through antioxidant property [J]. Applied Microbiology and Biotechnology, 2021, 105(6): 2427-2439
[56] Lazar J T, Tabor J J. Bacterial two-component systems as sensors for synthetic biology applications [J]. Current Opinion in Systems Biology, 2021, 28: 100398
[57] Parrot C, Moulinier L, Bernard F, et al. Peculiarities of aminoacyl-tRNA synthetases from trypanosomatids [J]. The Journal of Biological Chemistry, 2021, 297(2): 100913
[58] Zou Y L, Yang Y Y, Fu X X, et al. The regulatory roles of aminoacyl-tRNA synthetase in cardiovascular disease [J]. Molecular Therapy - Nucleic Acids, 2021, 25: 372-387
[59] Mattsson K, Ekvall M T, Hansson L A, et al. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles [J]. Environmental Science & Technology, 2015, 49(1): 553-561
[60] Modoux M, Rolhion N, Mani S, et al. Tryptophan metabolism as a pharmacological target [J]. Trends in Pharmacological Sciences, 2021, 42(1): 60-73
[61] Dagenais-Lussier X, Loucif H, Beji C, et al. Latest developments in tryptophan metabolism: Understanding its role in B cell immunity [J]. Cytokine & Growth Factor Reviews, 2021, 59: 111-117