微塑料(micro-plastics, MPs)一般指的是粒径<5 mm的塑料颗粒、纤维、碎片和薄膜等[1],而对于纳米塑料(nano-plastics, NPs)的定义主要分为2种,一种为粒径<1 μm的塑料类型[2-3],另一种为<100 nm的塑料类型[4-5]。微(纳米)塑料(micro(nano)plastics, MNPs)主要分为初级MNPs(直接来自塑料颗粒等的使用,例如塑料生产、化妆品等)和次级MNPs(较大塑料垃圾的破碎) [6]。MNPs来自陆地、河流、海岸和大气,可以通过水循环和大气循环进入大气圈、水圈、土壤圈甚至生物圈,可以从近岸海洋输送到大洋深处、南北两极,在全球广泛分布[7-8]。海洋MNPs具有浓度高、种类多、分布广和危害集中等特点,尤其是在人类活动频繁的渔业水域,其污染问题日益凸显[8-9]。
《中华人民共和国渔业法实施细则》定义海洋渔业水域为中华人民共和国管辖水域中鱼、虾、蟹、贝类的产卵场、索饵场、越冬场、洄游通道和鱼、虾、蟹、贝、藻类及其他水生动植物的养殖场所;各渔业海域具有特有的和有价值的商业渔业物种且一般设置禁渔区和禁渔期[10]。海洋渔业水域中的MNPs主要来自陆域输入和海上人类活动。水域陆源输入是海洋渔业水域MNPs的一个主要来源,海上来源包括渔业养殖用具、捕捞和运输过程等[11]。一般情况下,MNPs可以根据其形态分为颗粒、纤维、碎片、薄膜和泡沫[12]。渔业水域普遍使用塑料养殖用具,如渔网、渔绳、渔线、泡沫浮板、管道和网箱等,这些渔具经磨损和老化,逐渐分解为聚氯乙烯(polyvinyl chloride, PVC)、聚苯乙烯(polystyrene, PS)、聚丙烯(polypropylene, PP)、聚乙烯(polyethene, PE)和聚对苯二甲酸乙二醇酯(polyethylene glycol terephthalate, PET)等类型的MNPs[13-17]。研究发现,不同渔业水域MPs种类存在差异:比如中国四大传统渔场之一的北部湾水域,其表层沉积物(0~5 cm)中PP和PE纤维数占MPs总丰度的61.6%,并且MPs纤维的丰度与不同区域的渔业产量之间存在显著相关性,推测来源于渔业活动中渔具的磨损[18]。我国象山港养殖水域的MPs主要成分为PS,约占总丰度的38.6%[11];而舟山渔场中最常见的MPs是PP和PET[19]。此外,在渔业活动中常用到PVC泡沫制品工具,且PVC管和薄膜可用于搭建人工鱼棚[13]。研究发现PVC在水环境中比其他塑料更容易碎裂并分解为MPs[20-21]。
MNPs之所以引起环境关注,是因为(微米/纳米级)粒径容易被各种生物所摄食且能在食物链中传递[22]。越来越多的研究报道了渔业水域的典型水生生物摄取MPs的情况;例如,Kazmiruk等[23]研究发现在加拿大兰伯特海峡和贝恩斯海峡,太平洋牡蛎(Crassostrea gigas)养殖区受到了MPs的高度污染,影响了牡蛎的健康和品质;Setälä[24]通过荧光标记实验发现小型浮游生物体内MPs会向大型浮游动物转移,说明MNPs可能会通过食物链在各营养级中传递;Zhang等[25]通过调查中国东海舟山渔场周边海域11种野生鱼类和8种野生甲壳类物种体内的MPs污染特征,发现MPs能够通过食物链传递并在高营养级的鱼类体内累积。
海洋MNPs污染是全球环境污染热点问题之一,开展其生态风险评估与风险管控,对于减少污染造成的环境和经济损失等具有重要意义[26-27]。物种敏感性分布(species sensitivity distribution, SSD)评估是水环境MNPs生态风险评估中一种重要的方法,SSD是群落水平上的剂量-效应生态风险评估(ecological risk assessments, ERA)模型,基于不同水域各生态系统中的不同物种对同一环境污染物的敏感性影响存在差异,其横坐标为污染物浓度,纵坐标为物种潜在影响比例(potential affected fractions, PAF),根据物种敏感性差异构建累积概率分布模型,得出定量污染物浓度对应的PAF,即可用于定量某渔业水域污染风险水平[28]。20世纪80年代美国环境保护局(United States Environmental Protection Agency, US EPA)首次将SSD用于建立水质基准,随后作为物种对应激源暴露敏感性的概率模型被广泛用于国内外水生生态系统污染物生态风险评估领域中[28-31]。本研究利用半数有效浓度(median effect concentration, EC50)和SSD曲线通过具体的数值来描述和分析MNPs污染物对海洋生物环境的毒性影响,EC50是指能引起50%受试生物某种效应变化的浓度,是毒性研究中的常用急性数据,此方法一定程度上有利于解决MNPs生态风险评估手段匮乏和评估结果不明确等难题[32-34]。
作为食物链底层重要的初级生产者,微藻代表着物质和能量进入海洋食物链的切入点,也是维持海洋生态系统结构和功能的重要组成部分[35]。微藻具有生长周期短、操作简单、易于观察和对毒性物质响应敏感等特性,是检测多种污染物毒性作用的模式物种[36];且微藻中许多种类本身作为重要饵料藻类具有很强的经济价值和利用价值,不仅可作为医疗和工业加工的原材料和用于生物柴油的制备,而且是鱼虾、贝类、海参、轮虫和卤虫等水产经济动物在不同阶段的营养饵料。因此,若饵料藻类受到MNPs污染,可能对水生食物网产生重大影响[37-38]。基于此,我们认为饵料藻类适合作为渔业水域中MNPs生态风险评估的理想生物。本研究通过文献检索并重新分析渔业水域中MNPs对重要饵料藻类的毒理学研究数据,绘制饵料藻类SSD曲线。通过SSD曲线获得了不同环境浓度的MNPs对饵料藻类的PAF,并对全球13处渔业水域的MNPs的生态风险进行评估,为将来更为全面地开展渔业水域中MNPs的生态风险评估提供参考。
1 材料与方法(Materials and methods)
1.1 EC50的计算
本文首先通过Web of Science数据库和中国知网数据库收集近年来国内外发表的文献,针对MNPs污染暴露对渔业水域中常见的饵料藻类的毒性效应,首先获得共4门12种饵料藻类的研究数据,即在3个及以上MNPs暴露浓度下,对饵料藻类某种毒性效应指标的抑制率或诱导率数据;然后统一将所有MNPs浓度单位换算为μg·L-1,利用GraphPad Prism 8.0®软件计算出海洋饵料藻类受MNPs污染各毒性效应的EC50值,EC50计算方法参考于童等[39]的报道。
1.2 SSD曲线拟合
SSD曲线拟合法主要利用文献中收集到的生物毒理数据来分析污染物对生物的危害程度,进而评估其生态风险。SSD曲线拟合的形式主要包括对数正态累积密度模型(Log-Normal)、逻辑斯蒂累积密度模型(Log-Logistic)、韦布尔累积密度模型(Reweibull)和波尔Ⅲ模型(BurrⅢ)等[40-41]。由于BurrⅢ模型对物种敏感性的拟合特性较好,且函数更灵活[42],因此本研究采用该模型进行后续分析。基于国内外已报道的常见渔业水域中的饵料藻类受MNPs污染暴露的毒理学研究数据(表1),将计算出的结果,上传至Rurrlioz 2.0软件(由澳大利亚联邦科学和工业研究组织提供),选择合适的参数后拟合生成饵料藻类对MNPs的SSD曲线(图1),具体公式及操作方法参考陈锦灿等[43]的研究;在确定的SSD曲线下利用Rurrlioz直接计算得到5%危害浓度值(the hazardous concentration for 5% of the species, HC5)及不同浓度暴露下的PAF值。

图1 饵料藻类对微(纳米)塑料的物种敏感性分布(SSD)曲线
注:实际物种敏感性分布用蓝色实线表示,黑色虚线则分别表示上、下置信区间(95%)。
Fig. 1 Species sensitivity distribution (SSD) curve for bait algae exposed to micro(nano)plastics
Note: Solid blue curve represents concrete SSD; black dotted lines represent the 95% confidence intervals.
表1 微(纳米)塑料对饵料藻类毒性效应数据(EC50)
Table 1 Toxicity effect data of micro(nano)plastics to bait algae (EC50)
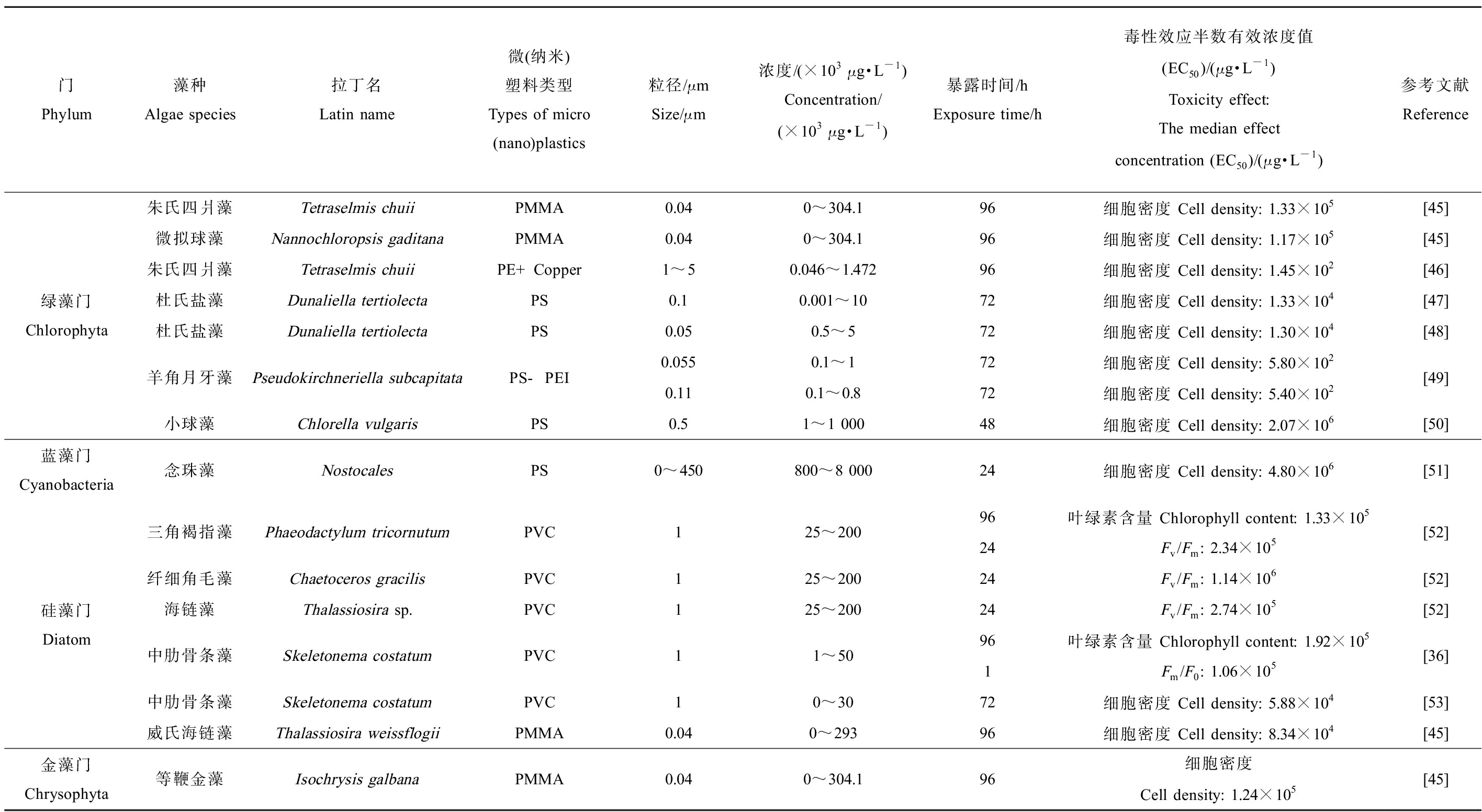
门Phylum藻种Algae species拉丁名Latin name微(纳米)塑料类型Types of micro(nano)plastics粒径/μmSize/μm浓度/(×103 μg·L-1)Concentration/(×103 μg·L-1)暴露时间/hExposure time/h毒性效应半数有效浓度值(EC50)/(μg·L-1)Toxicity effect:The median effect concentration (EC50)/(μg·L-1)参考文献Reference绿藻门Chlorophyta朱氏四爿藻Tetraselmis chuiiPMMA微拟球藻Nannochloropsis gaditanaPMMA朱氏四爿藻Tetraselmis chuiiPE+Copper杜氏盐藻Dunaliella tertiolectaPS杜氏盐藻Dunaliella tertiolectaPS羊角月牙藻Pseudokirchneriella subcapitataPS-PEI小球藻Chlorella vulgarisPS0.040~304.196细胞密度 Cell density: 1.33×105 0.040~304.196细胞密度 Cell density: 1.17×1051~50.046~1.47296细胞密度 Cell density: 1.45×102 0.10.001~1072细胞密度 Cell density: 1.33×1040.050.5~572细胞密度 Cell density: 1.30×1040.0550.1~172细胞密度 Cell density: 5.80×102 0.110.1~0.872细胞密度 Cell density: 5.40×102 0.51~1 00048细胞密度 Cell density: 2.07×106 [45][45][46][47][48][49][50]蓝藻门Cyanobacteria念珠藻NostocalesPS0~450800~8 00024细胞密度 Cell density: 4.80×106 [51]硅藻门Diatom三角褐指藻Phaeodactylum tricornutumPVC125~200纤细角毛藻Chaetoceros gracilisPVC125~200海链藻Thalassiosira sp.PVC125~200中肋骨条藻Skeletonema costatumPVC11~50中肋骨条藻Skeletonema costatumPVC10~30威氏海链藻Thalassiosira weissflogiiPMMA0.040~29396叶绿素含量 Chlorophyll content: 1.33×10524Fv/Fm: 2.34×105 24Fv/Fm: 1.14×10624Fv/Fm: 2.74×105 96叶绿素含量 Chlorophyll content: 1.92×105 1Fm/F0: 1.06×105 72细胞密度 Cell density: 5.88×10496细胞密度 Cell density: 8.34×104 [52][52][52][36][53][45]金藻门Chrysophyta等鞭金藻Isochrysis galbanaPMMA0.040~304.196细胞密度Cell density: 1.24×105 [45]
注:PMMA为聚甲基丙烯酸甲酯;PE为聚乙烯;PS聚苯乙烯;PVC为聚氯乙烯;PS-PEI为聚苯乙烯-聚醚酰亚胺,是一种由羧酸化聚苯乙烯纳米颗粒合成的塑料;Fv/Fm是光系统Ⅱ(PSⅡ)最大光化学量子产量,表示最大光化学效率;Fm/F0表示实际光化学效率,2个指标反映了微藻在光系统中的光能转换。
Note: PMMA stands for polymethyl methacrylate; PE stands for polyethylene; PS stands for polystyrene; PVC stands for polyvinyl chloride; PS-PEI stands for polystyrene-polyetherimide, a kind of plastic synthesized from carboxylated polystyrene nanoparticles; Fv/Fm stands for the maximal photochemical efficiency of PSⅡ which means maximal PSⅡ efficiency; Fm/F0 stands for the actual photochemical efficiency of PSⅡ; Fv/Fm and Fm/F0 reflect the light energy conversion of microalgae PSⅡ.
1.3 渔业水域环境MNPs浓度及PAF的计算
为分析MNPs对饵料藻类的毒性影响与渔业水域环境MNPs浓度之间的关系,我们收集了国内外渔业水域环境中MNPs污染调查数据;由于不同文献中使用的MNPs浓度单位不一致,为了利用SSD曲线开展生态风险评估,首先需要将整理得到的水域MNPs丰度(K) (个·L-1)的单位统一换算为μg·L-1;本研究假设MNPs形状为同等粒径的圆球状(因纤维与碎片体积无法计算);根据Enders等[44]整理的MPs聚合物密度(ρ),取相应的渔业水域常见MPs聚合物密度,通过以下公式得到单个MNPs质量(m):
(1)
m=ρV
(2)
式中:V为单个MPs球体体积,r为球体半径,ρ为MPs密度,m为单个MNPs质量(μg)。最后以m乘以K得到MNPs的水体浓度,单位为μg·L-1。利用Rurrlioz软件将相应水域换算后MPs浓度代入SSD曲线即得到对应PAF值,BurrⅢ型分布计算PAF的公式具体参考陈锦灿等[43]的文献。
2 结果(Results)
2.1 MNPs暴露对饵料藻类毒性效应数据(EC50值)
目前国内外MNPs暴露对饵料藻类毒性效应研究中,常见的塑料种类有PS、PVC、PP、PE和聚甲基丙烯酸甲酯(polymethyl methacrylate, PMMA),粒径集中在0.04~450 μm,所选取的饵料藻类主要为绿藻门、硅藻门、蓝藻门和金藻门中的种类,研究的毒性效应指标集中为藻细胞密度、叶绿素含量、光能转换效率、细胞内化率和抗氧化酶活性等(表1)。
2.2 SSD曲线拟合结果
利用BurrⅢ型分布模型对4门共12种饵料藻类的SSD曲线拟合的结果如图1所示,当MNPs浓度>1 000 μg·L-1,饵料藻类受MNPs损害的程度迅速增强,随着浓度不断升高,最终损害程度在MNPs浓度为1×107 μg·L-1时趋于平稳。
HC5指的是某物种5%的个体受到显著影响时,生存环境中MNPs相对应的浓度,也表示95%的物种个体未受到有害影响时污染物的浓度。HC5的数值越小,表明MNPs对所研究物种的生态风险越大。由SSD曲线得到MNPs对饵料藻类的HC5为240 μg·L-1。
不同浓度MNPs暴露得出的对不同饵料藻类的PAF值,反映其对不同饵料藻类的损害程度。在获得SSD曲线的基础上,通过Rurrlioz 2.0软件可以计算得到不同浓度的MNPs对应的PAF预测值。当MNPs浓度≤1×102 μg·L-1时,有≤4%的饵料藻类会受到MNPs的损害;随着浓度的增大,可能受到影响的物种比例也逐渐增大,当浓度为1×104 μg·L-1时,可能受到危害的物种百分比为20%;而当浓度为1×105 μg·L-1时,受到影响的物种百分比达到47%(表2)。
表2 不同浓度微(纳米)塑料对饵料藻类的
潜在影响比例(PAF)预测值
Table 2 Predicted potential affected fractions (PAF)
values of the bait algae under exposure of
various concentrations of micro(nano)plastics
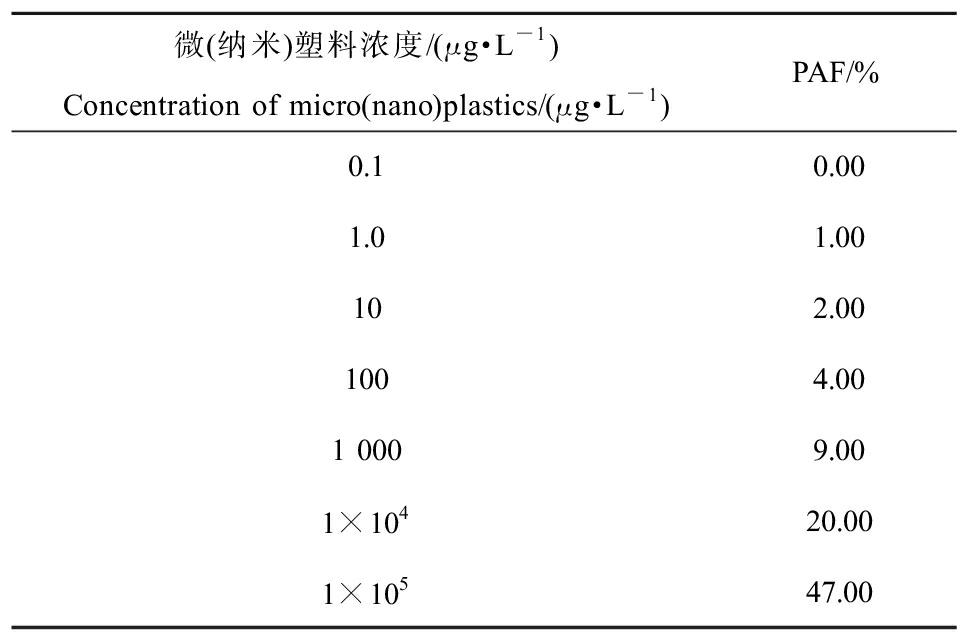
微(纳米)塑料浓度/(μg·L-1)Concentration of micro(nano)plastics/(μg·L-1)PAF/%0.10.001.01.00102.001004.001 0009.001×10420.001×10547.00
2.3 渔业水域环境中MNPs的污染现状和生态风险评估
根据Jambeck等[54]的调查,每年大约有480~1 270万t塑料垃圾进入海洋,如果持续这种状态到2025年输入海洋的塑料垃圾累积数量预计将增加一个数量级;由于MNPs的粒径小,且空间分布非常不均匀,几乎存在于所有生态系统。海洋渔业水域通常集中于沿海范围,更容易受到陆源和海上来源MNPs的双重污染。不同海洋渔业水域MNPs丰度和粒径特征具有很大差异性,主要与各水域离岸距离、人类活动、渔业养殖捕捞和运输等因素有关,主要的MNPs类型为PP、PE、PS和PET[11,20,55]。以国内外共13处代表性渔业水域水体中主要种类和主要粒径的MNPs为评估对象,计算获得丰度数据,将结果统一换算成μg·L-1后代入SSD曲线计算得到对应的PAF值,数据按照PAF值由低到高排序。如表3所示,发现墨西哥湾渔业水域中MNPs的PAF值相对较大,最大值超过了50%。
表3 部分代表性渔业水域微(纳米)塑料污染概况
Table 3 Status of micro(nano)plastics pollution in some representative fishery waters

水域Water area渔业水域主要类型Major type of fishery waters 主要微(纳米)塑料Major micro(nano)plastics主要粒径/μmMajor size/μm丰度/(个·L-1)Abundance/(particles·L-1)单位换算后浓度/(μg·L-1)Concentration after unit conversion /(μg·L-1)PAF/%参考文献Reference中国台湾东部黑潮海域Kuroshio in the eastocean of Taiwan, China鱼类洄游通道Migration channel of fishPP, PE, PS, PET300~1 0000×10-5~9×10-50×10-2~6.63×10-20[56]中国杭州湾Hangzhou Bay, China鱼类养殖场Fish farmPP, PE<1 000(1.4±1.2)×10-4<7.13×10-20[57]北海南部The Southern North Sea鱼类、虾等索饵场Feeding ground of fish and shrimp, etc.PUR, PE, PP11~1000.023~9.71.36×10-5~6.400~1[58]黑海东南海岸The coast of the Southeastern Black Sea鱼类索饵场和洄游通道Feeding ground andmigration channel of fishPE, PET118~1 0003.08×10-3~1.60×10-22.36×10-3~11.80~2[59]中国象山湾 Xiangshan Bay, China鱼类、虾蟹养殖场Aquaculture ground of fish, shrimp, crab, etc.PE, PP, PS250~2 000(4.6±0.5)×10-3~(20.1±0.2)×10-33.20×10-2~89.250~3[14]中国胶州湾Jiaozhou Bay, China鱼类、虾等养殖场Aquaculture ground of fish, shrimp, etc.PE, PET<2 0001.75×10-2~0.13<7.60×102<8[60]中国桑沟湾Sanggou Bay, China鱼类、贝类、藻类等养殖场Aquaculture ground of fish, shellfish, alga, etc.PE<50020.06±4.73<1.29×103<9[61]韩国近海Coastal waters of ROK鱼类、贝类、藻类等养殖场Aquaculture ground of fish, shellfish, alga, etc.PP, PE197±168、752±7110.8719.51×10-3~1.40×103 0~10[62]中国东海马鞍列岛海域人工鱼礁Artificial reefs around the Ma’an Archipelago, East China Sea鱼类、贝类、蟹等养殖场Aquaculture ground of fish, shellfish, crab, etc.PP, PE<1 000(0.16±0.10)~(0.47±0.20)<2.40×103<12[63]印度孟买海岸Mumbai coast, India鱼类、虾等养殖场Aquaculture ground of fish, shrimp, etc.PP, PE, PS, PA, PET, PMMA<250372±143<2.97×103<13[64]中国茅尾海Maowei Sea, China鱼类、贝类养殖场Aquaculture ground of fish, shellfish, etc.PES, PP, PE<1 0000.82~6.90<3.54×103<14[65]
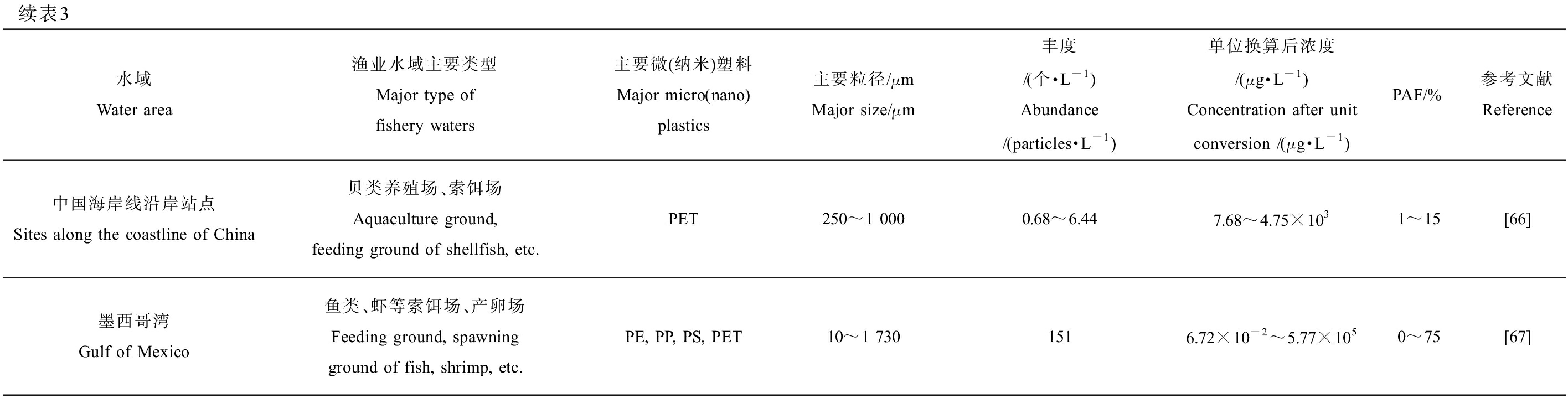
续表3水域Water area渔业水域主要类型Major type of fishery waters 主要微(纳米)塑料Major micro(nano)plastics主要粒径/μmMajor size/μm丰度/(个·L-1)Abundance/(particles·L-1)单位换算后浓度/(μg·L-1)Concentration after unit conversion /(μg·L-1)PAF/%参考文献Reference中国海岸线沿岸站点Sites along the coastline of China贝类养殖场、索饵场Aquaculture ground, feeding ground of shellfish, etc.PET250~1 0000.68~6.447.68~4.75×1031~15[66]墨西哥湾Gulf of Mexico鱼类、虾等索饵场、产卵场Feeding ground, spawning ground of fish, shrimp, etc.PE, PP, PS, PET10~1 7301516.72×10-2~5.77×1050~75[67]
注:PP为聚丙烯;PE为聚乙烯;PS为聚苯乙烯;PET为聚对苯二甲酸乙二醇酯;PUR为聚氨酯;PA为聚酰胺;PES为聚醚砜。
Note: PP stands for polypropylene; PE stands for polyethylene; PS stands for polystyrene; PET stands for polyethylene glycol terephthalate; PUR stands for polyurethane; PA stands for polyamide; PES stands for polyester.
3 讨论(Discussion)
3.1 数据的选取
绘制SSD曲线前的数据准备需要筛选合适的物种、暴露时间、毒性效应数据等以及统一单位,一般遵循适当性、可靠性和精确性3个原则[40, 68-69]。当可用的数据集越丰富,误差的风险就越小,影响预测的不确定性就越低[70]。本研究绘制SSD曲线选取的物种符合US EPA建议的受试生物至少包括3门8科的要求[71]。
尽管欧盟水框架指导共同实施战略(2000/602/EC)和我国《化学物质环境与健康危害评估技术导则(试行)》建议运用慢性毒理学数据进行环境质量评价更为可靠,然而国内外MNPs对微藻毒理学实验研究中十分缺乏无可见效应浓度(no observed effect concentration, NOEC)、最低可见效应浓度(lowest observable effect concentration, LOEC)和10%效应浓度(effective concentration at 10% inhibition, EC10)等慢性毒理学数据[69, 72-73]。通过EC50或半致死浓度(lethal concentration 50%, LC50)等急性数据外推慢性数据NOEC还存在一定争议[74-75];现有选择LC50作为评价指标的毒理学研究中,其研究对象通常为鱼类、甲壳类、软体动物和哺乳动物等[76-77];但由于MNPs暴露造成生物死亡的剂量通常极高,获得LC50相应的MNPs暴露浓度往往远高于实际水环境中的浓度,造成实验结果无法很好地反映真实情况[8]。而EC50的毒性终点可以选择生物响应MNPs最为敏感的生物学指标,相对应的MNPs暴露剂量更接近于环境浓度[49, 66],因此采用EC50结果较LC50更为合理。
3.2 基于PAF评估和预测当前及未来渔业水域MNPs的生态风险
PAF可反映不同污染物对不同营养水平物种的损害程度,目前已有多个研究使用SSD曲线和PAF评估海洋生物受污染物如重金属和MNPs等毒性影响的生态风险[78-80]。本研究总结并预测了全球13处渔业水域中不同环境浓度的MNPs对饵料藻类的潜在影响比例,其中7处不同类型的渔业水域总体PAF预测值为5%~20%(中国胶州湾<中国桑沟湾<韩国近海<中国东海马鞍列岛海域人工鱼礁<印度孟买海岸<中国茅尾海<中国海岸线沿岸站点),有5处水域PAF值<5%(中国台湾东部黑潮海域<中国杭州湾<北海南部<黑海东南海岸<中国象山湾),相应的生态风险较小;另外1处渔业水域(墨西哥湾)MPs浓度对应PAF预测值范围中最大值超过50%。
PAF预测值最高的墨西哥湾污染水域中MNPs的来源广泛,包括近岸旅游活动、工业运输、商业捕捞和海水养殖等人类活动以及河流输入;此外,半封闭式海湾是墨西哥湾污染水域的突出特征,由于海水交换能力较弱,造成MNPs较难扩散而导致累积[57, 67]。2021年10月联合国环境规划署(United Nations Environment Programme, UNEP)发布有关海洋垃圾和塑料污染的综合评估报告称,到2040年流入海洋的塑料垃圾污染量将增加至2016年的3倍(每年2 300~3 700万t)[81]。而塑料垃圾实际测得质量仅仅是模型估算值的1%,剩余99%的海洋塑料垃圾不知去向[82]。因此,在可预见的未来,渔业水域中MNPs的丰度还会不断增加。本研究基于实验室毒理学研究结果绘制的SSD曲线,计算得到的PAF预测值虽与现实环境存在偏差,但对于评估将来水环境中MNPs的生态风险仍具有一定的参考价值。同时,未来渔业水域中MNPs对水生生物的生态风险还需要更全面和持久的监测与评估。
3.3 选取饵料藻类开展渔业水域MNPs生态风险评估的意义
微藻是水生生态系统中最重要的初级生产者,是众多水生生物的能量来源,其数量及结构受到污染影响,最终都可能影响水生生态系统的结构和功能,因此微藻是生态系统维持稳定的第一道防线[35,83]。MNPs的含量以及毒性效应可能通过食物链传递到更高营养级[25],如Gambardella等[47]研究发现PS纳米颗粒可能会通过食物链传递(从藻类到浮游动物再到鱼类),最终影响鱼类的新陈代谢和个体行为,该结果与Cedervall等[84]的研究结果一致。并且微藻对MNPs毒性效应响应较为敏感,主要的毒性效应与机制表现为吸附和遮蔽效应、破坏细胞结构、抑制生长和诱导氧化应激等[43, 85-86]。因此,本研究选取饵料藻类开展渔业水域MNPs毒性效应风险研究,可为渔业水域的生态风险评估提供合理的参考依据。
3.4 当前研究的局限性
(1) 目前MNPs对饵料藻类的实验室暴露浓度单位不统一,且大都采用“名义浓度”(如μg·L-1和mg·L-1)来表征,考虑到塑料是一类难溶颗粒物而不是溶质,故采用名义浓度不是特别恰当。
(2) MNPs的实验室暴露浓度大都采用μg·L-1或mg·L-1来表征,而现实环境中的监测浓度大都采用个·L-1、个·km-2和个·m-3等来表征,因此在利用SSD曲线进行生态风险评估时,需将两者的单位换算成统一格式。由于塑料是难溶颗粒物且形状和尺寸各异,因此在实验室浓度和环境浓度进行互相换算时,不可避免地存在误差和不确定性。
(3) 本研究在进行浓度单位换算时,将所有MNPs都视为规则的微珠,而忽略了纤维和碎片等不规则形状的MNPs,造成结果产生偏差。这一方面是由于当前的实验室暴露实验往往只选取微珠形状的MNPs开展实验,缺少其他形状MNPs的毒理学数据[87];另一方面,野外监测结果仅报道了纤维和碎片等MNPs的长度,而没有提供体积数据,导致无法计算其质量,阻碍了单位间的换算。
(4) 实验室暴露实验采用的MNPs浓度过于追求效应的产生,设置的浓度远高于环境浓度[88],导致MNPs慢性毒理学数据极为匮乏,使生态风险评估结果的实际应用价值受到影响。
(5) 大多数毒性研究只注重MNPs单独暴露对饵料藻类产生的毒性效应;而已知MNPs对重金属、疏水性有机化学品和其他持久性有机污染物具有很强的吸附能力,且MNPs与某些其他污染物混合对藻类的综合毒性具有协同作用[89-92],MNPs单独暴露忽视了MNPs与其他污染物的相互作用。
(6) 针对污染物对微藻产生的毒性效应机制不明确,缺乏统一的敏感生物学效应指标[43],导致毒性效应评估结果之间缺乏可比性。
(7) 利用SSD曲线进行风险评估,本研究存在所选取的饵料藻类种类不全面的问题,缺乏淡水藻的毒性数据,影响了评估结果的全面性[72-73,93]。
3.5 展望与建议
本研究以饵料藻类作为对象,选取了4门9科共12种渔业水域常见饵料藻类受MNPs暴露实验的EC50数据,绘制了SSD曲线,计算得到PAF值,开展渔业水域MNPs风险评估。进一步总结了全球不同渔业水域MNPs污染现状,通过预测PAF值进行生态风险评估,区分了不同渔业水域中MNPs生态风险的水平。因此,本研究为MNPs的生态风险评估提供了新思路和新视角。此外,本研究揭示了当前研究的局限性,并对将来MNPs的毒理学研究和生态风险评估提出了以下建议和展望:
(1) 统一现场监测和实验室暴露实验所使用的浓度单位,建议采用数量单位(如个·L-1)而非浓度单位,使不同研究之间更具可比性。
(2) 开发可快速测定纤维、碎片和颗粒等不规则MNPs体积的三维测量软件,实现这些塑料数量和质量之间的换算。
(3) 由于渔业水域中纤维状MNPs的占比最高,其次为碎片,而不同形状的MNPs具有不同的毒性效应[87,94-95]。因此建议实验室暴露实验应尝试以纤维、碎片和泡沫等形状的MNPs作为材料,研究不同形状的MNPs对饵料藻类的毒性效应和机制。
(4) 统一确立饵料藻类响应MNPs的毒性效应指标,并参考PAF值在SSD曲线上所对应的MNPs浓度来设计实验室内暴露浓度,使其更接近于环境浓度,并通过长期暴露,获得NOEC、LOEC和EC10等数据,完善慢性毒理学数据库。
(5) 开展完善MNPs和其他污染物的单独及联合暴露实验研究,探讨MNPs与其他污染物对饵料藻类的联合毒性效应和机制。
(6) 进一步补充MNPs对海水和淡水渔业水域不同种类饵料藻类的毒理学研究数据,如金藻门如巴夫藻(Pavlova viridis)、蓝藻门如螺旋藻(Spirulina sp.)和隐藻门如卵形隐藻(Cryptomons ovata)等;构建更为完善的SSD曲线,获得更为准确的生态风险评估结论。
(7) 加强对MNPs的环境行为及其在食物链中传递过程的研究。认识到MNPs在环境中的迁移和分布等环境行为以及食物链传递过程是评估其生态风险的重要环节,在将来的生态风险评估模型构建中也需要将它们作为其中一个重要影响因素。
[1] Thompson R C, Olsen Y, Mitchell R P, et al. Lost at sea: Where is all the plastic? [J]. Science, 2004, 304(5672): 838
[2] da Costa J P, Santos P S M, Duarte A C, et al. (Nano)plastics in the environment—Sources, fates and effects [J]. The Science of the Total Environment, 2016, 566-567: 15-26
[3] Ter Halle A, Ladirat L, Gendre X, et al. Understanding the fragmentation pattern of marine plastic debris [J]. Environmental Science & Technology, 2016, 50(11): 5668-5675
[4] Ter Halle A, Jeanneau L, Martignac M, et al. Nanoplastic in the North Atlantic subtropical gyre [J]. Environmental Science & Technology, 2017, 51(23): 13689-13697
[5] Cole M, Lindeque P, Fileman E, et al. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus [J]. Environmental Science & Technology, 2015, 49(2): 1130-1137
[6] Jemec A, Horvat P, Kunej U, et al. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna [J]. Environmental Pollution, 2016, 219: 201-209
[7] Enyoh C E, Verla A W, Verla E N, et al. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks [J]. Environmental Monitoring and Assessment, 2019, 191(11): 668
[8] 李道季. 海洋微塑料研究焦点及存在的科学认知误区[J]. 科技导报, 2020, 38(14): 46-53
Li D J. Focus and scientific cognition misconceptions on marine microplastics studies [J]. Science & Technology Review, 2020, 38(14): 46-53 (in Chinese)
[9] Mathalon A, Hill P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia [J]. Marine Pollution Bulletin, 2014, 81(1): 69-79
[10] Alexander P, Andrei P, Mikhail M, et al. Eco-legal and economic aspects of developing Malomorsky fishing area of Lake Baikal [J]. Aquaculture and Fisheries, 2021(5): 530-534
[11] 夏斌, 杜雨珊, 赵信国, 等. 微塑料在海洋渔业水域中的污染现状及其生物效应研究进展[J]. 渔业科学进展, 2019, 40(3): 178-190
Xia B, Du Y S, Zhao X G, et al. Research progress on microplastics pollution in marine fishery water and their biological effects [J]. Progress in Fishery Sciences, 2019, 40(3): 178-190 (in Chinese)
[12] Hidalgo-Ruz V, Gutow L, Thompson R C, et al. Microplastics in the marine environment: A review of the methods used for identification and quantification [J]. Environmental Science & Technology, 2012, 46(6): 3060-3075
[13] Andrady A. Microplastics in the marine environment [J]. Marine Pollution Bulletin, 2011, 62(8): 1596-1605
[14] Chen M L, Jin M, Tao P R, et al. Assessment of microplastics derived from mariculture in Xiangshan Bay, China [J]. Environmental Pollution, 2018, 242(Pt B): 1146-1156
[15] Ma J L, Niu X J, Zhang D Q, et al. High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou, China [J]. The Science of the Total Environment, 2020, 744: 140679
[16] Vázquez-Rowe I, Ita-Nagy D, Kahhat R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety [J]. Current Opinion in Green and Sustainable Chemistry, 2021, 29: 100464
[17] Wang Q, Zhu X P, Hou C W, et al. Microplastic uptake in commercial fishes from the Bohai Sea, China [J]. Chemosphere, 2021, 263: 127962
[18] Xue B M, Zhang L L, Li R L, et al. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment [J]. Environmental Science & Technology, 2020, 54(4): 2210-2217
[19] Zhang F, Xu J Y, Zhu L X, et al. Seasonal distributions of microplastics and estimation of the microplastic load ingested by wild caught fish in the East China Sea [J]. Journal of Hazardous Materials, 2021, 419: 126456
[20] Rahman M M, Nur N, Mahmud-Al-Hasan M, et al. Effects of light and artificial fish shelter (PVC pipe) on some phenotypic traits of stinging catfish (Heteropneustes fossilis Bloch, 1794) [J]. Aquaculture Research, 2020, 51(1): 124-134
[21] Wu P F, Cai Z W, Jin H B, et al. Adsorption mechanisms of five bisphenol analogues on PVC microplastics [J]. Science of the Total Environment, 2019, 650: 671-678
[22] Law K L, Thompson R C. Oceans. microplastics in the seas [J]. Science, 2014, 345(6193): 144-145
[23] Kazmiruk T N, Kazmiruk V D, Bendell L I. Abundance and distribution of microplastics within surface sediments of a key shellfish growing region of Canada [J]. PLoS One, 2018, 13(5): e0196005
[24] Setälä O. Ingestion and transfer of microplastics in the planktonic food web [J]. Environmental Pollution, 2014, 185: 77-83
[25] Zhang F, Wang X, Xu J, et al. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea [J]. Marine Pollution Bulletin, 2019, 146: 173-182
[26] Mu J L, Zhang S F, Qu L, et al. Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea [J]. Marine Pollution Bulletin, 2019, 143: 58-65
[27] 胡习邦, 曾东, 王俊能, 等. 应用物种敏感性分布评估苯胺的水生生态风险[J]. 生态环境学报, 2016, 25(3): 471-476
Hu X B, Zeng D, Wang J N, et al. Assessing aquatic ecological risk of aniline by species sensitivity distributions [J]. Ecology and Environmental Sciences, 2016, 25(3): 471-476 (in Chinese)
[28] He W, Kong X Z, Qin N, et al. Combining species sensitivity distribution (SSD) model and thermodynamic index (exergy) for system-level ecological risk assessment of contaminates in aquatic ecosystems [J]. Environment International, 2019, 133(Pt B): 105275
[29] Kim D, Kim L, Kim D, et al. Species sensitivity distributions for ethylparaben to derive protective concentrations for soil ecosystems [J]. Environmental Geochemistry and Health, 2022, 44(8): 2435-2449
[30] Flores N Y, Collas F P L, Mehler K, et al. Assessing habitat suitability for native and alien freshwater mussels in the river Waal (the Netherlands), using hydroacoustics and species sensitivity distributions [J]. Environmental Modeling & Assessment, 2022, 27(1): 187-204
[31] Razak M R, Aris A Z, Zakaria N A C, et al. Accumulation and risk assessment of heavy metals employing species sensitivity distributions in Linggi River, Negeri Sembilan, Malaysia [J]. Ecotoxicology and Environmental Safety, 2021, 211: 111905
[32] 《环境科学大辞典》编辑委员会. 环境科学大辞典[M]. 北京: 中国环境科学出版社, 1991: 10
[33] Raimondo S, Vivian D N, Delos C, et al. Protectiveness of species sensitivity distribution hazard concentrations for acute toxicity used in endangered species risk assessment [J]. Environmental Toxicology and Chemistry, 2008, 27(12): 2599-2607
[34] Lithner D, Larsson A, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition [J]. The Science of the Total Environment, 2011, 409(18): 3309-3324
[35] Granum E, Raven J A, Leegood R C. How do marine diatoms fix 10 billion tonnes of inorganic carbon per year? [J]. Canadian Journal of Botany, 2005, 83(7): 898-908
[36] Zhang C, Chen X H, Wang J T, et al. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae [J]. Environmental Pollution, 2017, 220: 1282-1288
[37] 赵东会, 赵丽达, 尤宏, 等. 耐高温球等鞭金藻(Isochrysis galbana)藻种的选育与饵料特性初步分析[J]. 海洋与湖沼, 2021, 52(1): 206-212
Zhao D H, Zhao L D, You H, et al. Breeding and feeding characteristics of high-temperature-resistant strains of Isochrysis galbana [J]. Oceanologia et Limnologia Sinica, 2021, 52(1): 206-212 (in Chinese)
[38] Chen Y X, Ling Y, Li X Y, et al. Size-dependent cellular internalization and effects of polystyrene microplastics in microalgae P. helgolandica var. tsingtaoensis and S. quadricauda [J]. Journal of Hazardous Materials, 2020, 399: 123092
[39] 于童, 白喜云, 解晓晶, 等. 应用GraphPad Prism 5.0 软件计算药物的EC50[J]. 药学进展, 2012(4): 180-183
软件计算药物的EC50[J]. 药学进展, 2012(4): 180-183
Yu T, Bai X Y, Xie X J, et al. Calculation of in vitro EC50 of a drug by GraphPad prism 5.0 software [J]. Progress in Pharmaceutical Sciences, 2012(4): 180-183 (in Chinese)
[40] van Straalen N M. Threshold models for species sensitivity distributions applied to aquatic risk assessment for zinc [J]. Environmental Toxicology and Pharmacology, 2002, 11(3-4): 167-172
[41] 陈波宇, 郑斯瑞, 牛希成, 等. 物种敏感度分布及其在生态毒理学中的应用[J]. 生态毒理学报, 2010, 5(4): 491-497
Chen B Y, Zheng S R, Niu X C, et al. Species sensitivity distribution and its application in ecotoxicology [J]. Asian Journal of Ecotoxicology, 2010, 5(4): 491-497 (in Chinese)
[42] Besseling E, Redondo-Hasselerharm P, Foekema E M, et al. Quantifying ecological risks of aquatic micro- and nanoplastic [J]. Critical Reviews in Environmental Science and Technology, 2019, 49(1): 32-80
[43] 陈锦灿, 方超, 郑榕辉, 等. 应用物种敏感性分布评估微(纳米)塑料对水生生物的生态风险[J]. 生态毒理学报, 2020, 15(1): 242-255
Chen J C, Fang C, Zheng R H, et al. Assessing ecological risks of micro(nano)plastics to aquatic organisms using species sensitivity distributions [J]. Asian Journal of Ecotoxicology, 2020, 15(1): 242-255 (in Chinese)
[44] Enders K, Lenz R, Stedmon C A, et al. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution [J]. Marine Pollution Bulletin, 2015, 100(1): 70-81
[45] Ven ncio C, Ferreira I, Martins M A, et al. The effects of nanoplastics on marine plankton: A case study with polymethylmethacrylate [J]. Ecotoxicology and Environmental Safety, 2019, 184: 109632
ncio C, Ferreira I, Martins M A, et al. The effects of nanoplastics on marine plankton: A case study with polymethylmethacrylate [J]. Ecotoxicology and Environmental Safety, 2019, 184: 109632
[46] Davarpanah E, Guilhermino L. Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii [J]. Estuarine, Coastal and Shelf Science, 2015, 167: 269-275
[47] Gambardella C, Morgana S, Bramini M, et al. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels [J]. Marine Environmental Research, 2018, 141: 313-321
[48] Bergami E, Pugnalini S, Vannuccini M L, et al. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana [J]. Aquatic Toxicology, 2017, 189: 159-169
[49] Casado M P, Macken A, Byrne H J. Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery [J]. Environment International, 2013, 51: 97-105
[50] Tunali M, Uzoefuna E N, Tunali M M, et al. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris [J]. Science of the Total Environment, 2020, 743: 140479
[51] 冯炘, 秦于杰, 解玉红. 微塑料对念珠藻的影响——以聚苯乙烯为例[J]. 价值工程, 2020, 39(12): 263-266
Feng X, Qin Y J, Xie Y H. Effects of microplastics on freshwater organisms: A case study of polystyrene [J]. Value Engineering, 2020, 39(12): 263-266 (in Chinese)
[52] Wang S, Wang Y, Liang Y, et al. The interactions between microplastic polyvinyl chloride and marine diatoms: Physiological, morphological, and growth effects [J]. Ecotoxicology and Environmental Safety, 2020, 203: 111000
[53] Zhu X L, Zhao W H, Chen X H, et al. Growth inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure [J]. Marine Environmental Research, 2020, 158: 105005
[54] Jambeck J R, Geyer R, Wilcox C, et al. Marine pollution. Plastic waste inputs from land into the ocean [J]. Science, 2015, 347(6223): 768-771
[55] 中华人民共和国生态环境部. 2020年中国海洋生态环境状况公报[R]. 北京: 中华人民共和国生态环境部, 2021: 34-36
[56] Shiu R F, Gong G C, Fang M D, et al. Marine microplastics in the surface waters of “pristine” Kuroshio [J]. Marine Pollution Bulletin, 2021, 172: 112808
[57] Wang T, Hu M H, Song L L, et al. Coastal zone use influences the spatial distribution of microplastics in Hangzhou Bay, China [J]. Environmental Pollution, 2020, 266(Pt 2): 115137
[58] Roscher L, Fehres A, Reisel L, et al. Microplastic pollution in the Weser Estuary and the German North Sea [J]. Environmental Pollution, 2021, 288: 117681
[59] Erya ar A R, Gedik K,
ar A R, Gedik K,  ahin A, et al. Characteristics and temporal trends of microplastics in the coastal area in the Southern Black Sea over the past decade [J]. Marine Pollution Bulletin, 2021, 173(Pt A): 112993
ahin A, et al. Characteristics and temporal trends of microplastics in the coastal area in the Southern Black Sea over the past decade [J]. Marine Pollution Bulletin, 2021, 173(Pt A): 112993
[60] Liu Y D, Li Z Z, Jalón-Rojas I, et al. Assessing the potential risk and relationship between microplastics and phthalates in surface seawater of a heavily human-impacted metropolitan bay in Northern China [J]. Ecotoxicology and Environmental Safety, 2020, 204: 111067
[61] Xia B, Sui Q, Sun X M, et al. Microplastic pollution in surface seawater of Sanggou Bay, China: Occurrence, source and inventory [J]. Marine Pollution Bulletin, 2021, 162: 111899
[62] Song Y K, Hong S H, Eo S, et al. Horizontal and vertical distribution of microplastics in Korean coastal waters [J]. Environmental Science & Technology, 2018, 52(21): 12188-12197
[63] Zhang D D, Gui Y Z, Zhou H H, et al. Microplastic pollution in water, sediment, and fish from artificial reefs around the Ma’an Archipelago, Shengsi, China [J]. Science of the Total Environment, 2020, 703: 134768
[64] Gurjar U R, Xavier K A M, Shukla S P, et al. Microplastic pollution in coastal ecosystem off Mumbai coast, India [J]. Chemosphere, 2022, 288: 132484
[65] Zhu J M, Zhang Q, Li Y P, et al. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China [J]. The Science of the Total Environment, 2019, 658: 62-68
[66] Qu X Y, Su L, Li H X, et al. Assessing the relationship between the abundance and properties of microplastics in water and in mussels [J]. The Science of the Total Environment, 2018, 621: 679-686
[67] Shruti V C, Pérez-Guevara F, Kutralam-Muniasamy G, et al. The current state of microplastic pollution in the world’s largest gulf and its future directions [J]. Environmental Pollution, 2021, 291: 118142
[68] Caldwell D J, Mastrocco F, Hutchinson T H, et al. Derivation of an aquatic predicted no-effect concentration for the synthetic hormone, 17 alpha-ethinyl estradiol [J]. Environmental Science & Technology, 2008, 42(19): 7046-7054
[69] 雷炳莉, 黄圣彪, 王子健. 生态风险评价理论和方法[J]. 化学进展, 2009, 21(S1): 350-358
Lei B L, Huang S B, Wang Z J. Theories and methods of ecological risk assessment [J]. Progress in Chemistry, 2009, 21(S1): 350-358 (in Chinese)
[70] Belanger S, Barron M, Craig P, et al. Future needs and recommendations in the development of species sensitivity distributions: Estimating toxicity thresholds for aquatic ecological communities and assessing impacts of chemical exposures [J]. Integrated Environmental Assessment and Management, 2017, 13(4): 664-674
[71] United States Environmental Protection Agency (US EPA). Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses [S]. Washington DC: Office of Research and Development Environmental Research Laboratories, 2010
[72] Connon R E, Geist J, Werner I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment [J]. Sensors, 2012, 12(9): 12741-12771
[73] Wheeler J R, Grist E P, Leung K M, et al. Species sensitivity distributions: Data and model choice [J]. Marine Pollution Bulletin, 2002, 45(1-12): 192-202
[74] Selck H, Riemann B, Christoffersen K, et al. Comparing sensitivity of ecotoxicological effect endpoints between laboratory and field [J]. Ecotoxicology and Environmental Safety, 2002, 52(2): 97-112
[75] Forbes T L, Forbes V E. A critique of the use of distribution-based extrapolation models in ecotoxicology [J]. Functional Ecology, 1993, 7(3): 249
[76] 张潇峮, 王伟, 罗鸣钟, 等. 三苯基锡和聚苯乙烯微塑料联合暴露对胭脂鱼幼鱼的急性毒性效应[J]. 淡水渔业, 2020, 50(4): 33-38
Zhang X Q, Wang W, Luo M Z, et al. Single and combined effects of (polystyrene) microplastics and triphenyltin on juveniles Myxocryprinus asiaticus [J]. Freshwater Fisheries, 2020, 50(4): 33-38 (in Chinese)
[77] Qu H, Ma R X, Barrett H, et al. How microplastics affect chiral illicit drug methamphetamine in aquatic food chain? From green alga (Chlorella pyrenoidosa) to freshwater snail (Cipangopaludian cathayensis) [J]. Environment International, 2020, 136: 105480
[78] Lewis M, Thursby G. Aquatic plants: Test species sensitivity and minimum data requirement evaluations for chemical risk assessments and aquatic life criteria development for the USA [J]. Environmental Pollution, 2018, 238: 270-280
[79] Heijerick D G, Carey S. The toxicity of molybdate to freshwater and marine organisms. Ⅲ. Generating additional chronic toxicity data for the refinement of safe environmental exposure concentrations in the US and Europe [J]. The Science of the Total Environment, 2017, 609: 420-428
[80] 杜建国, 赵佳懿, 陈彬, 等. 应用物种敏感性分布评估重金属对海洋生物的生态风险[J]. 生态毒理学报, 2013, 8(4): 561-570
Du J G, Zhao J Y, Chen B, et al. Assessing ecological risks of heavy metals to marine organisms by species sensitivity distributions [J]. Asian Journal of Ecotoxicology, 2013, 8(4): 561-570 (in Chinese)
[81] McGlade J, Fahim I S, Green D, et al. From pollution to solution: A global assessment of marine litter and plastic pollution [R]. Nairobi: United Nations Environment Programme, 2021
[82] Cózar A, Echevarría F, González-Gordillo J I, et al. Plastic debris in the open ocean [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(28): 10239-10244
[83] Nolte T M, Hartmann N B, Kleijn J M, et al. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption [J]. Aquatic Toxicology, 2017, 183: 11-20
[84] Cedervall T, Hansson L A, Lard M, et al. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish [J]. PLoS One, 2012, 7(2): e32254
[85] 洪喻, 郝立翀, 陈足音. 新兴污染物对微藻的毒性作用与机制研究进展[J]. 生态毒理学报, 2019, 14(5): 22-45
Hong Y, Hao L C, Chen Z Y. Research progress on the toxic effects of emerging pollutants on microalgae and the mechanisms [J]. Asian Journal of Ecotoxicology, 2019, 14(5): 22-45 (in Chinese)
[86] 王素春, 刘光洲, 张欢, 等. 微塑料对微藻的毒性效应研究进展[J]. 海洋环境科学, 2019, 38(2): 192-197
Wang S C, Liu G Z, Zhang H, et al. Toxicity research progress of microplastics on microalgae [J]. Marine Environmental Science, 2019, 38(2): 192-197 (in Chinese)
[87] Frydkjær C K, Iversen N, Roslev P. Ingestion and egestion of microplastics by the cladoceran Daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene [J]. Bulletin of Environmental Contamination and Toxicology, 2017, 99(6): 655-661
[88] Koelmans A A, Besseling E, Foekema E, et al. Risks of plastic debris: Unravelling fact, opinion, perception, and belief [J]. Environmental Science & Technology, 2017, 51(20): 11513-11519
[89] Massos A, Turner A. Cadmium, lead and bromine in beached microplastics [J]. Environmental Pollution, 2017, 227: 139-145
[90] Liu G Z, Zhu Z L, Yang Y X, et al. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater [J]. Environmental Pollution, 2019, 246: 26-33
[91] Prata J C, Lavorante B R B O, Montenegro B S M, et al. Influence of microplastics on the toxicity of the pharmaceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii [J]. Aquatic Toxicology, 2018, 197: 143-152
[92] Yang W F, Gao X X, Wu Y X, et al. The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa [J]. Ecotoxicology and Environmental Safety, 2020, 195: 110484
[93] Huang W, Song B, Liang J, et al. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health [J]. Journal of Hazardous Materials, 2021, 405: 124187
[94] Lusher A L, Burke A, O’Connor I, et al. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling [J]. Marine Pollution Bulletin, 2014, 88(1-2): 325-333
[95] Desforges J P, Galbraith M, Dangerfield N, et al. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean [J]. Marine Pollution Bulletin, 2014, 79(1-2): 94-99