镉是一种生物半衰期相对较长的非必需重金属元素,容易在生物体中积累并行使生理功能[1],具有高致毒性[2];如镉与巯基紧密结合,是一种酶抑制剂[3],破坏细胞内部离子平衡并与蛋白质中的金属离子发生置换反应导致其失去活性[4];镉激发细胞的氧化应激造成脂质过氧化、引起蛋白质/DNA损伤[5];镉还可通过干扰DNA合成与修复,破坏细胞的增殖和分化、细胞周期进程和细胞凋亡等[6]。即使在较低浓度下,镉也能通过诱导氧化应激和影响生长、代谢、营养吸收、光合作用和呼吸作用对光合生物产生毒害效应[7-9]。镉对藻类的毒性试验表明,镉胁迫能显著抑制栅藻生长并破坏细胞超微结构[2];镉对藻类光合系统具有毒性效应,破坏叶绿素合成[10-12],竞争性结合放氧复合体(OEC)上Ca2+位点和叶绿素a的Mg2+等[13]。长期以来,镉被认为是没有任何有益功能的有毒重金属。但最近研究表明,植物需要微量Cd2+才能达到最佳生长状态[14];在硅藻中,金属镉、钴和锌可以在功能上互相替换,以保持最佳的生长速度[15]。可见,镉的确切生物效应仍不甚明了,需要深入研究。
蓝藻作为水生生态系统总初级生产力的重要贡献者,是重金属进入食物链的主要途径之一;暴露在环境中的蓝藻细胞对重金属胁迫敏感,集胞藻作为光合作用研究的模式生物,是研究水体重金属潜在生态风险的重要模型[16]。尽管Cd2+对藻细胞光合系统影响的研究已非常深入,但此前的研究主要集中在藻细胞对重金属胁迫的生理响应和生物吸附能力[17-21],Cd2+的毒性机制仍不明确,且Cd2+的急性毒性胁迫影响光合系统中的位点存在一些相互矛盾的结果[10, 22],例如,PSⅠ和PSⅡ对镉的敏感性存在争议;Cd2+的胁迫位点位于电子传递链的确切部位存在分歧。本文选择集胞藻PCC 6803为对象,探究不同浓度Cd2+对细胞抗氧化酶系统和光合活性的毒性效应,以期寻找Cd2+作用于蓝藻细胞的靶标位点,进一步认识重金属对淡水蓝藻的毒性作用及其急性致毒机理、评估重金属的生态风险提供理论依据。
集胞藻(Synechocystis sp.)PCC 6803来自中国科学院水生生物研究所淡水藻种库(FACHB-collection)。以改良BG11培养基(不含EDTA)为培养液,在(30±1) ℃、30~40 μmol·m-2·s-1持续光照条件下,以130 r·min-1转速进行摇床培养,采用紫外分光光度计(UV1701,日本岛津)测定培养物在730 nm的吸光值,通过A730 nm监测其生长。初始接种浓度A730 nm=0.1接入250 mL三角瓶,收集处于对数生长期(A730 nm=0.8~1.0)的藻细胞用于本研究。
3 000 r·min-1转速离心5 min收集对数生长期的藻细胞,无菌水冲洗3次后,置于BG11培养基中,接种量少于培养基的1%,接入含有100 mL BG11的三角瓶。分析纯的试剂CdCl2·2.5H2O购于中国国药有限公司。设置各处理组中Cd2+浓度分别为0.05、0.25、0.50和1.00 mg·L-1,以0 mg·L-1作为对照组。每个浓度设置3个重复,试验周期为24 h。
1.2.1 光合色素含量的测定
光合色素含量的测定如前人[23]描述,取5 mL培养物4 000 r·min-1离心10 min,弃去上清液,加入5 mL预冷的90%的甲醇,混匀后在4 ℃冰箱避光提取24 h,4 000 r·min-1离心10 min后,取上清液,采用紫外分光光度计测定665、652和470 nm波长的吸光值,参照公式计算叶绿素a(Chl a)和类胡萝卜素(Car)的含量:
Chl a (mg·L-1)=16.82A665-9.28A652
Car (mg·L-1)=(1000A470-1.91Chl a)/225
1.2.2 活性氧(ROS)含量测定
集胞藻细胞内的ROS用二氯二氢荧光素双醋酸盐(DCFH-DA)检测。DCFH-DA能进入细胞内,被酯酶分解为DCFH,DCFH与H2O2等小分子过氧化物结合而被氧化成具有荧光性的DCF。在24 h取样,离心,重悬于100 mmol·L-1 PBS (pH 7.2)缓冲液中,再使用PBS缓冲液洗涤细胞2次,200 mL培养物加入96孔板用酶标仪(PerkinElmer VICTOR Nivo,美国)检测DCF荧光强度(激发波长485 nm和发射波长530 nm)和叶绿素荧光(激发波长485 nm和发射波长670 nm)。使用叶绿素荧光法归一化单个细胞ROS含量,使用公式FDCF/FChl计算相对ROS丰度。
1.2.3 超氧化物歧化酶(superoxide dismutase, SOD)活性测定
取适量藻液4 000 r·min-1离心10 min后,将藻泥悬浮于1 mL生理盐水(0.9% NaCl),加入适量锆珠,4 ℃冷冻破碎,4 ℃、1 800 r·min-1离心10 min,取上清用于蛋白含量与酶活测定。SOD的测定使用试剂盒(南京建成),蛋白质测定使用微孔BCA蛋白试剂盒WST-1法(康为世纪),使用蛋白量归一化SOD含量。
1.2.4 快速荧光诱导动力学曲线(OJIP)和PSⅡ最大光化学效率(Fv/Fm)的检测
OJIP曲线和Fv/Fm使用AquaPen-C AP-C 100荧光仪 (Photon Systems Instruments,Brno,捷克)测定,其中Fv=Fm-F0,F0和Fm分别是藻细胞在暗适应状态下的最小和最大荧光产量[24-25]。调节合适的细胞浓度,室温暗适应10 min,蓝藻F0的测定使用弱蓝色测量光(450 nm,<0.1 μmol·m-2·s-1),该光强不足以引起光化学电子传递,Fm采用饱和脉冲光(1 800 μmol·m-2 s-1)照射800 ms测得。以照射时间为横坐标,荧光强度为纵坐标,对快速叶绿素荧光诱导动力学曲线作图,为了更好地观察J点和I点,把横坐标改为对数坐标,结果得到OJIP诱导曲线。
![]() 再氧化曲线的测定
再氧化曲线的测定
叶绿素荧光衰减的测定用双调制荧光仪FL6000(Photon Systems Instruments,Brno,捷克)测定[26-27]。室温暗适应10 min。取3 mL暗适应后的藻液放入样品池,打开测量闪光测定F0,其闪光长度为4 μs。之后打开单次反转饱和闪光还原电子受体![]() 闪光长度为50 μs。单次反转饱和闪光关闭后,荧光强度下降,此时即为
闪光长度为50 μs。单次反转饱和闪光关闭后,荧光强度下降,此时即为![]() 的再氧化过程,仪器每间隔101/6 μs记录一次荧光强度值。参照文献[27-28],对
的再氧化过程,仪器每间隔101/6 μs记录一次荧光强度值。参照文献[27-28],对![]() 再氧化曲线进行三指数衰减拟合,拟合方程如下:
再氧化曲线进行三指数衰减拟合,拟合方程如下:
Ft=A1exp(-t/T1)+A2exp(-t/T2)+A3exp(-t/T3)+A0
式中:Ft代表可变荧光产量,A0~A3指幅度,T1~T3是时间常数,半衰期由时间常数T计算得来,公式t1/2=ln 2T。
1.2.6 S-state检测
取3 mL待测样品放入样品室,打开测量光测定F0,测量光照射时间是1 ms。随后打开光化光闪光照射样品,光化光闪光次数为10次,每次闪光时间间隔为100 ms。以照射时间为横坐标,荧光强度为纵坐标作图即为S-state曲线[29]。根据S-state曲线,无活性PSⅡ反应中心(PSⅡX)比例可通过F0第4次光化光闪光后100 ms处荧光值的差值进行估算,具体计算公式为:ΔF4=F400 ms/F0-1。由于本试验中相对可变荧光强度降低,使用修改后公式[30]来计算PSⅡX的比例:PSⅡx(%)=ΔF4×100(F300 ms/F0-1)。
本试验各组均设置3个平行。统计软件使用SPSS25.0(IBM,USA),处理组和对照组间采用单因素方差(One-way ANOVA)分析检验,若差异显著进行最小显著差异(LSD)多重比较检验组间差异显著性。绘图和数据处理使用R语言中的tidyverse包。
不同浓度Cd2+对叶绿素a含量影响如图1所示。0.05 mg·L-1浓度处理后,叶绿素a与对照组相比呈现上升趋势;以0.25、0.50和1.00 mg·L-1处理集胞藻后,叶绿素a含量与对照组相比显著下降(P<0.01)。

图1 不同浓度镉离子处理24 h对集胞藻光合
色素含量的影响
注:*表示与对照组相比存在显著性差异。
Fig. 1 Effect on the pigment content in Synechocystis sp. after
exposure to different concentrations of cadmium for 24 h
Note: *indicates significant difference compared with the control group.
如图2所示,处理24 h后,Cd2+浓度0.05 mg·L-1处理组与对照组相比ROS含量显著上升(P<0.01),Cd2+浓度为0.25、0.50和1.00 mg·L-1的处理组ROS含量分别是对照组的1.47倍、2.58倍和2.69倍。

图2 不同浓度镉离子处理24 h集胞藻活性氧(ROS)含量和超氧化物歧化酶(SOD)活性的变化
注:*表示与对照组相比存在显著性差异。
Fig. 2 Change of reactive oxygen species (ROS) content and superoxide dismutase (SOD) activity in Synechocystis
sp. after exposure to different concentrations of cadmium for 24 h
Note: *indicates significant difference compared with the control group.
与对照组相比,Cd2+浓度为0.05、0.25和0.50 mg·L-1的处理组藻细胞中SOD活性显著上升(P<0.05);Cd2+浓度为1.00 mg·L-1的处理组藻细胞中SOD活性与对照组差异不显著(P>0.05)。
集胞藻在不同Cd2+环境中,叶绿素荧光诱导曲线和PSⅡ最大光化学效率(Fv/Fm)如图3和图4所示。由图3可知,Cd2+浓度为1.00 mg·L-1,P峰消失。归一化的OJIP曲线可以更好比较各峰之间的变化;Cd2+浓度0.50 mg·L-1,J峰显著上升(P<0.01)。由图4可知,Cd2+浓度为0.50 mg·L-1和1.00 mg·L-1,处理组Fv/Fm相对于对照组显著下降(P<0.01)。

图3 不同浓度镉离子处理24 h集胞藻的OJIP曲线
注:相对可变荧光计算公式为Vt=(Ft-F0)/(Fm-F0)。
Fig. 3 OJIP fluorescence transients of Synechocystis sp. after exposure to different concentrations of cadmium for 24 h
Note: Relative variable fluorescence is calculated by Vt=(Ft-F0)/(Fm-F0).
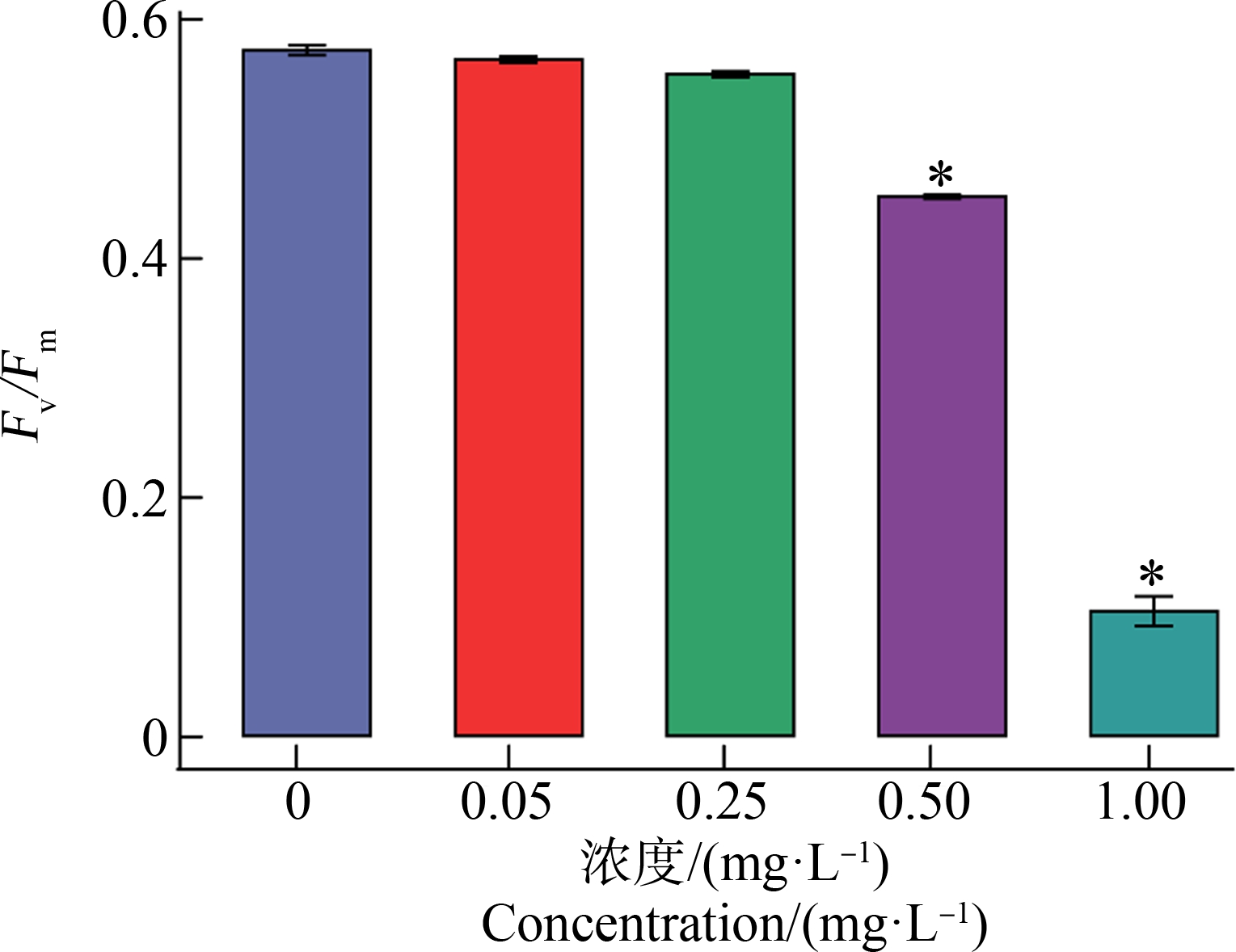
图4 不同浓度镉离子处理24 h集胞藻的
PSⅡ最大光化学效率
(Fv/Fm)变化
注:*表示与对照组相比存在显著性差异。
Fig. 4 Change of PSⅡ maximum photochemical efficiency
(Fv/Fm) in Synechocystis sp. after exposure to different
concentrations of cadmium for 24 h
Note: *indicates significant difference compared with the control group.
如图5所示,不同浓度Cd2+作用下集胞藻![]() 再氧化动力学曲线分析中,Cd2+浓度在0.50 mg·L-1和1.00 mg·L-1时,可变荧光(Fv)振幅显著(P<0.01)减小,衰减期减慢。表1显示
再氧化动力学曲线分析中,Cd2+浓度在0.50 mg·L-1和1.00 mg·L-1时,可变荧光(Fv)振幅显著(P<0.01)减小,衰减期减慢。表1显示![]() 再氧化的参数,Cd2+浓度为0.25、0.50和1.00 mg·L-1,快相、中相半衰期随着浓度显著持续增加(P<0.01)。
再氧化的参数,Cd2+浓度为0.25、0.50和1.00 mg·L-1,快相、中相半衰期随着浓度显著持续增加(P<0.01)。
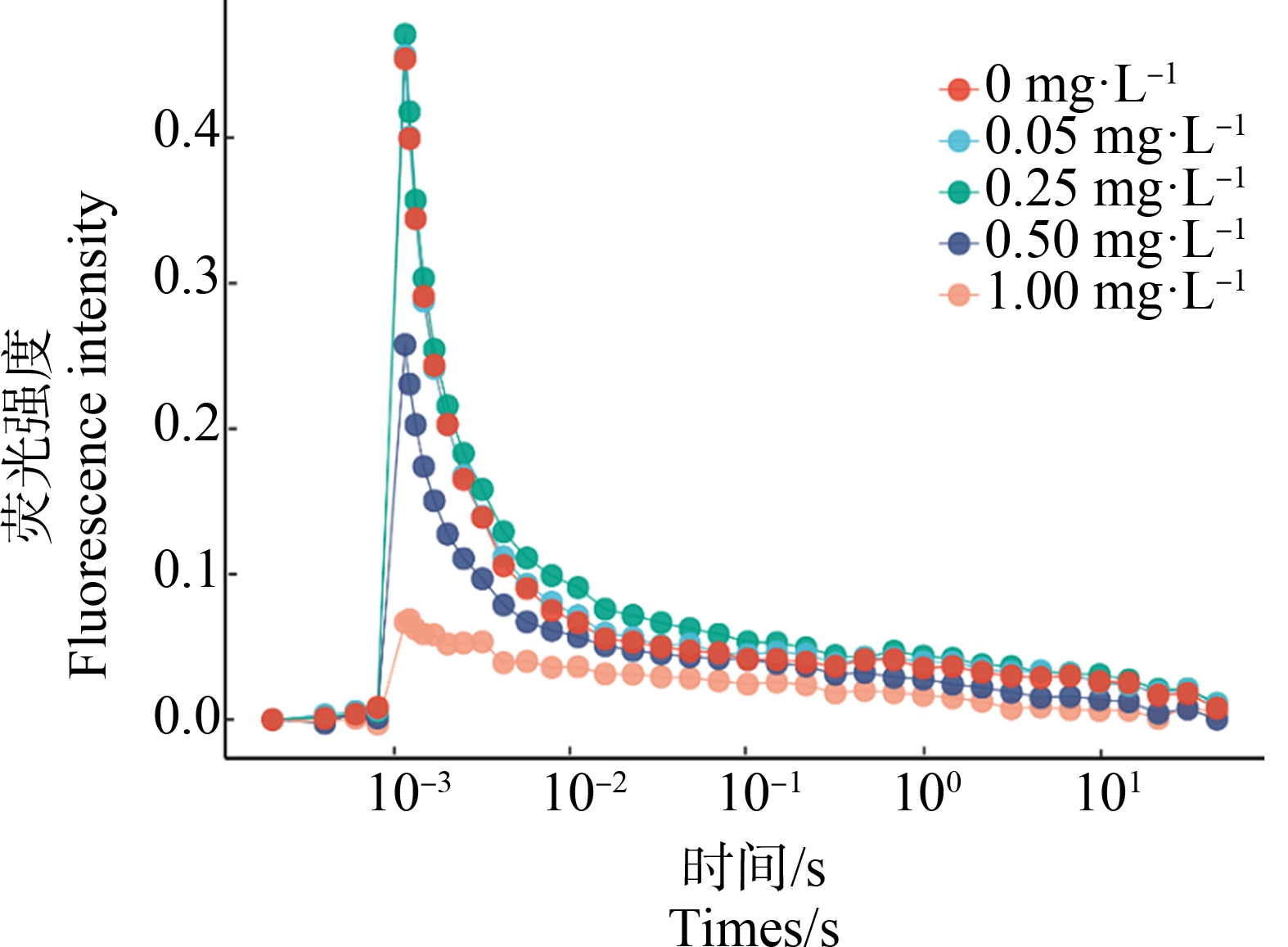
图5 不同浓度镉离子处理24 h集胞藻![]() 再氧化曲线
再氧化曲线
注:荧光强度计算公式为Ft-F0。
Fig. ![]() reoxidation kinetic curves of Synechocystis
reoxidation kinetic curves of Synechocystis
sp. after exposure to different concentrations
of cadmium for 24 h
Note: Fluorescence intensity is calculated by Ft-F0.
表1 不同浓度镉离子处理24 h集胞藻的![]() 再氧化曲线参数
再氧化曲线参数
Table 1 Parameters of ![]() reoxidation kinetics in Synechocystis sp. after exposure
reoxidation kinetics in Synechocystis sp. after exposure
to different concentrations of cadmium for 24 h

浓度/(mg·L-1)Concentration/(mg·L-1)快相Fast phase中相Middle phase慢相Slow phaseA1/%T1/μsA2/%T2/msA3/%T3/s095.403813.904.10.7019.20.0596.503502.904.20.6018.50.2595.904023.107.00.9018.20.5093.004534.805.82.105.11.0061.203 32826.25826.412.562.5
注:A1~A3指幅度,T1~T3是时间常数。
Note: A1~A3 refer to the amplitude, and T1~T3 is the time constant.
不同浓度Cd2+作用下连续单饱和反转闪光激发后S-state曲线如图6(a)所示。PS Ⅱ反应中心的一部分不能将电子从初级受体QA传递给次级受体QB,成为PSⅡX。据此计算细胞内的PSⅡX的比例的结果如图6(b)所示,Cd2+浓度为0.50 mg·L-1和1.00 mg·L-1,集胞藻PSⅡX比例显著上升。

图6 不同浓度镉离子处理24 h集胞藻荧光衰减曲线(a)和无活性PSⅡ反应中心(PSⅡX)比例(b)的变化
注:*表示与对照组相比存在显著性差异,荧光强度计算公式为Ft/F0。
Fig. 6 Fluorescence decay curves (a) and the proportion of inactive PSⅡ reaction center (PSⅡX) (b) of
Synechocystis sp. after exposure to different concentrations of cadmium for 24 h
Note: *indicates significant difference compared with the control group, and fluorescence intensity is calculated by Ft/F0.
尽管地壳中镉元素含量很低,但人类通过采矿、有色金属冶炼和肥料生产使其浓度在局部环境中增加到有毒水平,如化工园区或密集种植区土壤中的Cd2+浓度达到100 mg·kg-1以上。这些Cd2+最终汇集到江河湖海中,威胁水域生态系统的安全与健康。蓝藻作为水域生态系统的重要成员,对镉具有较强的吸附/吸收能力;研究表明蓝藻细胞能在30 min吸附70%以上Cd2+,胞内聚集的Cd2+抑制叶绿素合成并破坏类囊体膜蛋白结构,可直接或间接损伤电子传递系统、酶活和光合系统,进而降低蓝藻细胞的光合能力;可见,蓝藻细胞对Cd2+的胁迫极为敏感[22]。不高于0.50 mg·L-1的低剂量镉浓度能够激发集胞藻细胞的氧化应激和抗氧化酶系统,该结果与曲壳藻[31]暴露在镉胁迫中的表现基本一致。本试验也发现,镉胁迫对细胞光合系统造成损伤,破坏光合电子传递链导致电子外溢,胞内的氧与外溢电子结合产生ROS[32];镉离子含量的增加会导致ROS的产生量持续增加,引起胞内ROS聚集;过量的ROS导致亚细胞结构的破坏,并使其他酶失活,破坏色素和光合系统,导致藻细胞光合作用和其他代谢过程发生紊乱[33-34]。为了应对镉胁迫产生的ROS氧化压力,细胞合成SOD消除ROS的毒性效应;这使得SOD酶活性应激性增强。
Fv/Fm是表征藻细胞PSⅡ光合活性的重要参数,是反映藻类光合作用效率的重要指标。当环境条件中存在胁迫,叶绿素荧光发生相应改变,Fv/Fm也随着改变,这个过程可以反映胁迫对于藻细胞影响状况[24]。本研究为确认Cd2+对藻细胞光合系统的毒性效应,首先考察Fv/Fm的变化,证实了Cd2+对藻细胞光合系统的毒性效应;这个结果与文献[10]报道的结果明显不同。在此基础上进一步考察Cd2+对集胞藻OJIP曲线的影响,确认了二者间具有明显的剂量效应关系。当Cd2+浓度高于0.5 mg·L-1,Fv/Fm显著降低,J峰显著上升,表明最大光量子产量减小,光能转化效率下降;镉胁迫下,集胞藻电子传递链被扰乱,导致PSⅡ活性降低,说明了PSⅡ是Cd2+的作用靶标[26]。当Cd2+为1.00 mg·L-1时,J-I-P荧光强度降低到接近平缓,说明PSⅡ供体侧电子传递受到严重抑制[35],导致![]() 的聚集,进一步表明高浓度Cd2+会损伤光合电子传递链。
的聚集,进一步表明高浓度Cd2+会损伤光合电子传递链。
为了进一步验证Cd2+造成PSⅡ受体侧损伤,进行![]() 再氧化动力学曲线拟合分析。结果显示,Cd2+浓度高于0.25 mg·L-1,快相时长明显增加,镉延缓PSⅡ中QB介导的
再氧化动力学曲线拟合分析。结果显示,Cd2+浓度高于0.25 mg·L-1,快相时长明显增加,镉延缓PSⅡ中QB介导的![]() 再氧化,导致
再氧化,导致![]() 到QB电子转移速率减缓;中相时长明显增加,镉抑制QB结合位点上PQ的结合[36],综上说明镉胁迫可导致PSⅡ受体侧受损,空QB位点对PQ的结合减慢,
到QB电子转移速率减缓;中相时长明显增加,镉抑制QB结合位点上PQ的结合[36],综上说明镉胁迫可导致PSⅡ受体侧受损,空QB位点对PQ的结合减慢,![]() 到QB电子转移受到抑制[30]。PSⅡ反应中心存在异质性,PSⅡX位于类囊体暴露区,不能有效地从电子受体
到QB电子转移受到抑制[30]。PSⅡ反应中心存在异质性,PSⅡX位于类囊体暴露区,不能有效地从电子受体![]() 转移电子到次级电子受体QB,通过单反转光测定S-state,用以确定镉处理下PSⅡ反应中心损伤的比例。S-state分析显示,Cd2+高于0.25 mg·L-1短期处理时PSⅡX比例显著增高,镉将活跃的PSⅡ反应中心转化为不活跃的PSⅡX,进一步解释镉处理下
转移电子到次级电子受体QB,通过单反转光测定S-state,用以确定镉处理下PSⅡ反应中心损伤的比例。S-state分析显示,Cd2+高于0.25 mg·L-1短期处理时PSⅡX比例显著增高,镉将活跃的PSⅡ反应中心转化为不活跃的PSⅡX,进一步解释镉处理下![]() 和QB之间电子传递延迟。由此确认PS Ⅱ反应中心是Cd2+的主要作用靶点。可见,较低浓度的镉也会诱导氧化应激、损伤蛋白结构、影响光合系统。
和QB之间电子传递延迟。由此确认PS Ⅱ反应中心是Cd2+的主要作用靶点。可见,较低浓度的镉也会诱导氧化应激、损伤蛋白结构、影响光合系统。
Cd2+会直接影响藻细胞的色素成分和生长增殖[7, 37-40],本研究发现,0.25、0.50和1.00 mg·L-1 Cd2+处理集胞藻时,其叶绿素含量受到显著抑制。该结果与已有文献的研究结果类似[26, 41]。另一方面,本试验0.05 mg·L-1的Cd2+能促进集胞藻叶绿素的合成。与之前的研究结果“低浓度Cd2+ (0.1 mmol·L-1)能促进集胞藻生长和叶绿素合成”[38],导致蛋白质合成以及细胞中可溶性糖含量增多[23]等类似;这些结果均表明集胞藻能够耐受一定浓度的Cd2+。推测该现象为“毒性兴奋效应”,低毒性能激发藻细胞的代谢能力,促进藻细胞大分子的合成。综合以上分析,集胞藻能耐受不高于0.25 mg·L-1的Cd2+浓度胁迫,高浓度的Cd2+对集胞藻生长具有显著抑制作用。
[1] Wagner G. Accumulation of cadmium in crop plants and its consequences to human health [J]. Advances in Agronomy, 1993, 51: 173-212
[2] Tukaj Z, ![]() A, Skowroński T, et al. Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration [J]. Environmental and Experimental Botany, 2007, 60(3): 291-299
A, Skowroński T, et al. Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration [J]. Environmental and Experimental Botany, 2007, 60(3): 291-299
[3] Xia X, Wu S J, Zhou Z J, et al. Microbial Cd(Ⅱ) and Cr(Ⅵ) resistance mechanisms and application in bioremediation [J]. Journal of Hazardous Materials, 2021, 401: 123685
[4] Ammendola S, Cerasi M, Battistoni A. Deregulation of transition metals homeostasis is a key feature of cadmium toxicity in Salmonella [J]. BioMetals, 2014, 27(4): 703-714
[5] Rani A, Kumar A, Lal A, et al. Cellular mechanisms of cadmium-induced toxicity: A review [J]. International Journal of Environmental Health Research, 2014, 24(4): 378-399
[6] Aimola P, Carmignani M, Volpe A R, et al. Cadmium induces p53-dependent apoptosis in human prostate epithelial cells [J]. PLoS One, 2012, 7(3): e33647
[7] Srivastava A, Biswas S, Yadav S, et al. Acute cadmium toxicity and post-stress recovery: Insights into coordinated and integrated response/recovery strategies of Anabaena sp. PCC 7120 [J]. Journal of Hazardous Materials, 2021, 411: 124822
[8] Blasi B, Peca L, Vass I, et al. Characterization of stress responses of heavy metal and metalloid inducible promoters in synechocystis PCC6803 [J]. Journal of Microbiology and Biotechnology, 2012, 22(2): 166-169
[9] Luo Z X, Wang Z H, Liu A F, et al. New insights into toxic effects of arsenate on four Microcystis species under different phosphorus regimes [J]. Environmental Science and Pollution Research International, 2020, 27(35): 44460-44469
[10] Zhou W B, Juneau P, Qiu B S. Growth and photosynthetic responses of the bloom-forming cyanobacterium Microcystis aeruginosa to elevated levels of cadmium [J]. Chemosphere, 2006, 65(10): 1738-1746
[11] Wang Y, Wang J C, Zhang H H, et al. A intermediate concentration of atmospheric nitrogen dioxide enhances PSⅡ activity and inhibits PSⅠ activity in expanded leaves of tobacco seedlings [J]. Ecotoxicology and Environmental Safety, 2021, 209: 111844
[12] Yu Z, Hao R, Zhang L, et al. Effects of TiO2, SiO2, Ag and CdTe/CdS quantum dots nanoparticles on toxicity of cadmium towards Chlamydomonas reinhardtii [J]. Ecotoxicology and Environmental Safety, 2018, 156: 75-86
[13] Faller P, Kienzler K, Krieger-Liszkay A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of photosystem Ⅱ by competitive binding to the essential Ca2+ site [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2005, 1706(1-2): 158-164
[14] Liu M Q, Yanai J, Jiang R F, et al. Does cadmium play a physiological role in the hyperaccumulator Thlaspi caerulescens? [J]. Chemosphere, 2008, 71(7): 1276-1283
[15] Poole J H, Tyack P L, Stoeger-Horwath A S, et al. Animal behaviour: Elephants are capable of vocal learning [J]. Nature, 2005, 434(7032): 455-456
[16] Glatz A Vass I, Los D A, et al. The Synechocystis model of stress: From molecular chaperones to membranes [J]. Plant Physiology and Biochemistry, 1999, 37(1): 1-12
[17] Igiri B E, Okoduwa S I R, Idoko G O, et al. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review [J]. Journal of Toxicology, 2018, 2018: 2568038
[18] Deng L P. Sorption and desorption of lead (Ⅱ) from wastewater by green algae Cladophora fascicularis [J]. Journal of Hazardous Materials, 2007, 143(1-2): 220-225
[19] Ao D, Lei Z, Dzakpasu M, et al. Role of divalent metals Cu2+ and Zn2+ in Microcystis aeruginosa proliferation and production of toxic microcystins [J]. Toxicon: Official Journal of the International Society on Toxinology, 2019, 170: 51-59
[20] Shivaji S, Dronamaraju S V L. Scenedesnus rotundus isolated from the petroleum effluent employs alternate mechanisms of tolerance to elevated levels of cadmium and zinc [J]. Scientific Reports, 2019, 9: 8485
[21] Newby R Jr, Lee L H, Perez J L, et al. Characterization of zinc stress response in Cyanobacterium synechococcus sp. IU 625 [J]. Aquatic Toxicology, 2017, 186: 159-170
[22] Tóth T, Zsiros O, Kis M, et al. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803 [J]. Plant, Cell & Environment, 2012, 35(12): 2075-2086
[23] Lichtenthaler H K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes [J]. Methods in Enzymology, 1987, 148: 350-382
[24] Pan X L, Zhang D Y, Chen X, et al. Effects of levofloxacin hydrochloride on photosystem Ⅱ activity and heterogeneity of Synechocystis sp. [J]. Chemosphere, 2009, 77(3): 413-418
[25] Maxwell K, Johnson G N. Chlorophyll fluorescence—A practical guide [J]. Journal of Experimental Botany, 2000, 51(345): 659-668
[26] Wang S Z, Zhang D, Pan X. Effects of arsenic on growth and photosystem Ⅱ (PSⅡ) activity of Microcystis aeruginosa [J]. Ecotoxicology and Environmental Safety, 2012, 84: 104-111
[27] Beauchemin R, Gauthier A, Harnois J, et al. Spermine and spermidine inhibition of photosystem Ⅱ: Disassembly of the oxygen evolving complex and consequent perturbation in electron donation from TyrZ to P680+ and the quinone acceptors ![]() to QB [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2007, 1767(7): 905-912
to QB [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2007, 1767(7): 905-912
[28] Wodala B, Deák Z, Vass I, et al. In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves [J]. Plant Physiology, 2008, 146(4): 1920-1927
[29] Kaftan D, Meszaros T, Whitmarsh J, et al. Characterization of photosystem Ⅱ activity and heterogeneity during the cell cycle of the green alga Scenedesmus quadricauda [J]. Plant Physiology, 1999, 120(2): 433-442
[30] Pan X L, Deng C N, Zhang D Y, et al. Toxic effects of amoxicillin on the photosystem Ⅱ of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests [J]. Aquatic Toxicology, 2008, 89(4): 207-213
[31] 杨雨嘉, 支崇远, 李培林, 等. 重金属Cd2+和Cu2+对一种曲壳藻生长情况及其抗氧化酶活性的影响[J]. 生态科学, 2015, 34(6): 75-80
Yang Y J, Zhi C Y, Li P L, et al. Effects of heavy metal Cd2+ and Cu2+ on growth and antioxidant enzymes of Achnanthes kryophila [J]. Ecological Science, 2015, 34(6): 75-80 (in Chinese)
[32] Latifi A, Ruiz M, Zhang C C. Oxidative stress in cyanobacteria [J]. FEMS Microbiology Reviews, 2009, 33(2): 258-278
[33] Rutherford A W, Krieger-Liszkay A. Herbicide-induced oxidative stress in photosystem Ⅱ [J]. Trends in Biochemical Sciences, 2001, 26(11): 648-653
[34] Ezraty B, Gennaris A, Barras F, et al. Oxidative stress, protein damage and repair in bacteria [J]. Nature Reviews Microbiology, 2017, 15(7): 385-396
[35] Govindje E. Sixty-three years since Kautsky: Chlorophyll a fluorescence [J]. Functional Plant Biology, 1995, 22(2): 131-160
[36] Zhang X, Chen H, Wang H, et al. Time-course effects of Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) on Chlorella pyrenoidosa: Growth inhibition and adaptability mechanisms [J]. Journal of Hazardous Materials, 2021, 402: 123784
![]() á E, Sofrová D. Response of intact cyanobacterial cells and their photosynthetic apparatus to Cd2+ ion treatment [J]. Photosynthetica, 2002, 40(1): 103-108
á E, Sofrová D. Response of intact cyanobacterial cells and their photosynthetic apparatus to Cd2+ ion treatment [J]. Photosynthetica, 2002, 40(1): 103-108
[38] 苏甜, 李义刚, 欧瑞康, 等. 镉离子对羊角月牙藻光合作用及其抗氧化酶的毒性影响[J]. 生态科学, 2014, 33(2): 301-306
Su T, Li Y G, Ou R K, et al. Toxic effects of Cd2+ on the photosynthesis and antioxidase activity of Selenastrum capricornutum [J]. Ecological Science, 2014, 33(2): 301-306 (in Chinese)
[39] 邱昌恩, 况琪军, 毕永红, 等. Cd2+对绿球藻生长及生理特性的影响研究[J]. 水生生物学报, 2007, 31(1): 142-145
Qiu C G, Kuang Q J, Bi Y H, et al. The effects of Cd2+ on the growth and physiological characteristics of chlorococcum sp. [J]. Acta Hydrobiologica Sinica, 2007, 31(1): 142-145 (in Chinese)
[40] 聂利华, 杨东娟, 刘亚群, 等. 一株虾池来源的螺旋拟柱孢藻藻株的分离鉴定及重金属离子Cu2+、Cd2+和Pb2+对其生长的影响[J]. 微生物学报, 2019, 59(7): 1307-1317
Nie L H, Yang D J, Liu Y Q, et al. Isolation, identification and effect of heavy metals on the growth of Cylindrospermopsis raciborskii helix from shrimp ponds [J]. Acta Microbiologica Sinica, 2019, 59(7): 1307-1317 (in Chinese)
[41] Xu C X, Sun T, Li S B, et al. Adaptive laboratory evolution of cadmium tolerance in Synechocystis sp. PCC 6803 [J]. Biotechnology for Biofuels, 2018, 11: 205