邻苯二甲酸酯类(phthalate esters, PAEs)能够增加塑料制品的可塑性[1],其在工业生产过程中常作为增塑剂被应用于医疗器械、洗涤护理产品、清洁剂、化妆品和食品包装等各个领域[2],与人们日常生活息息相关。相关产品在生产、使用和处置过程中,可不断释放PAEs到环境中[3],污染土壤[4]、水体[5]和大气[6]。环境中的PAEs能够通过皮肤接触、呼吸和进食等多种途径进入人体[7-8],危害机体健康。血液是人体极其重要的组织,具有携氧、免疫、物质运输、调节体温及信息传递等多种功能。进入人体的部分PAEs会被血液吸收,并在内源性酯酶的作用下被水解代谢为相应的邻苯二甲酸单酯(mPAEs)[9]。此外,环境中的mPAEs也能以各种方式进入生物体血液[10-12]。研究人员已经在男性血液、母血和脐带血中检测到了mPAEs的存在[13-14]。存在于血液中的PAEs及其代谢产物随血液循环被输送到全身各处,损害体内的组织和器官,引发疾病。
目前,关于PAEs及其代谢产物毒性的研究主要集中在生殖发育、神经和遗传等方面[15-21],涉及血液毒性及其作用机制的研究鲜有报道,且研究对象主要针对分子量较大、毒性较强的邻苯二甲酸二(2-乙基己基)酯(DEHP)、邻苯二甲酸二正丁酯(DBP)、邻苯二甲酸丁苄酯(BBP)及其代谢产物邻苯二甲酸单(2-乙基己基)酯(MEHP)、邻苯二甲酸单丁酯(MBP)、邻苯二甲酸单苄酯(MBzP)。张弘等[22]的研究表明,DEHP(500 mg·kg-1)亚慢性染毒90 d会使大鼠白细胞、红细胞数量及血红蛋白含量显著下降。另有研究表明,MEHP也与血液中血红蛋白含量的下降有关[23-24]。Sicińska[25]通过体外实验发现,DBP、BBP(25 μg·mL-1)及其代谢产物MBP、MBzP(250 μg·mL-1)会使红细胞出现溶血现象。此外,DBP、BBP、MBP和MBzP还会增加离体红细胞中活性氧(ROS)含量,改变其抗氧化酶(超氧化物歧化酶(SOD)、过氧化氢酶(CAT))活性,使胞内高铁血红蛋白(MetHb)含量升高[26]。
邻苯二甲酸二甲酯(dimethyl phthalate, DMP)作为一种分子量较低的PAEs已经被列入我国优先污染物黑名单[27],其一级代谢产物邻苯二甲酸单甲酯(monomethyl phthalate, MMP)具有急性毒性、致畸和致癌活性[28-29]。目前,DMP和MMP在人体的各种体液中均已被检测到[30-32]。Babu-Rajendran等[33]采用气相色谱-质谱联用法测定了人体尿液中的PAEs,发现尿液中DMP的浓度范围为nd~141 ng·mL-1。陈甘讷等[34]的研究表明,MMP在孕妇分娩后母血和脐血中的浓度分别为1.89~2.07 μg·L-1和2.41~2.67 μg·L-1。针对研究现状的不足,本实验选取MMP(DMP的代谢产物)为研究对象,以Sprague Dawley(SD)大鼠为受试动物,通过检测血细胞(红细胞、白细胞、淋巴细胞和中性粒细胞)数量、血液生化指标(总胆固醇、尿素氮、总蛋白和碱性磷酸酶)以及氧化应激水平(抗氧化酶SOD、CAT和丙二醛(MDA))以探究MMP(50、250和500 mg·kg-1)对大鼠血液的毒性影响及作用机制,有助于完善PAEs类代谢物对血液毒性效应的研究,为其风险评价和相关疾病预防提供科学依据。
1 材料与方法(Materials and methods)
1.1 主要仪器设备与试剂耗材
主要仪器设备:动物版血液分析仪(HF-3800,济南汉方医疗器械有限公司,中国)、紫外-可见分光光度计(岛津UV-1700,Shimadzu,日本)、离心沉淀机(80-2,上海荣泰生化有限公司,中国)、数显恒温水浴锅(HH-S,常州翔天实验仪器厂,中国)、旋涡混合器(XH-C,金坛市白塔新宝仪器厂,中国)、电热鼓风干燥箱(101-00A,上海路韵仪器设备有限公司,中国)。
主要试剂耗材:邻苯二甲酸单甲酯(MMP;分析纯,国药集团化学试剂有限公司,中国)、乙酸(冰醋酸;分析纯,国药集团化学试剂有限公司,中国)、肝素钠(heparin sodium salt;185 USP units·mg-1,上海麦克林生化科技有限公司,中国)、长寿花金胚玉米油(山东三星玉米产业科技有限公司,中国);总超氧化物歧化酶(total superoxide dismutase, T-SOD)测试盒、过氧化氢酶(catalase, CAT)测试盒、丙二醛(malonicdialdehyde, MDA)测试盒、总胆固醇(total cholesterol, T-CHO)测试盒、尿素氮(BUN)测试盒、总蛋白(total protein, TP)定量试剂盒、酸性磷酸酶(acid phosphatase, ACP)试剂盒(南京建成生物工程研究所,中国)。
1.2 实验动物
本研究选用的SD大鼠购自正规畜牧中心,所有大鼠均健康。在实验过程中,大鼠被饲养在实验室动物房中,温度控制在18~25 ℃,相对湿度控制在(55±5)%,接受正常光照,可自由获取标准动物饲料和经过滤的自来水。按照本地法规进行动物实验。
1.3 实验方法
1.3.1 SD大鼠染毒及采血
设置3个不同剂量的染毒组(低剂量50 mg·kg-1、中剂量250 mg·kg-1、高剂量500 mg·kg-1)和对照组,每组选用3只SD大鼠,按照体质量计算出每只大鼠灌胃所需MMP质量,用玉米油配成1.5 mL染毒液,对大鼠灌胃染毒。对照组灌胃等量的1.5 mL玉米油。
染毒结束后,用固定器将SD大鼠固定,在鼠尾中下部涂抹75%酒精消毒,用5.5号静脉输液针扎入静脉采集所需血液。
1.3.2 血细胞检测
将SD大鼠灌胃染毒3 h后,从尾部采集血液至EDTA-K2抗凝管中,使用HF-3800动物版血液分析仪的全血模式检测血液中红细胞、白细胞、淋巴细胞和中性粒细胞的数量,记录检测结果。
1.3.3 血液生化指标检测
对SD大鼠灌胃染毒24 h(禁食12 h)和连续染毒7 d(每天同一时间灌胃染毒)后,从大鼠尾部取全血于离心管中,以3 000 r·min-1将血样离心15 min,取上层血清用于检测血液中总胆固醇、总蛋白的含量及酸性磷酸酶的活性。从大鼠尾部采取染毒24 h和连续染毒7 d后的全血于肝素抗凝离心管中,在3 000 r·min-1条件下离心15 min,取上层血浆用于检测血液中的尿素氮含量。根据总胆固醇、尿素氮、总蛋白和酸性磷酸酶试剂盒说明书上的操作步骤,使用紫外-可见分光光度计检测。
1.3.4 血液氧化应激水平检测
对SD大鼠灌胃染毒3 h后,从尾部取全血于离心管中。将血样以3 000 r·min-1离心15 min,取上层血清用于检测血液中SOD、CAT活性和MDA含量。根据SOD、CAT和MDA试剂盒说明书上的操作步骤,使用紫外-可见分光光度计检测。
1.4 数据分析
用Origin 2018和SPSS 25.0软件计算平均值和标准差,对各组红细胞、白细胞、中性粒细胞和淋巴细胞等血细胞数量,总胆固醇、尿素氮、总蛋白的含量和酸性磷酸酶活性等血液生化指标,以及SOD、CAT的活性和MDA含量等氧化应激指标之间的差异性进行单因素方差分析(One-Way ANOVA),方差齐性采用LSD检验,并绘制柱状图。
2 结果(Results)
2.1 MMP对SD大鼠血液中血细胞数量的影响
染毒3 h后,MMP对大鼠血液中红细胞、白细胞、淋巴细胞和中性粒细胞的毒性影响如表1所示。统计分析表明,随着MMP剂量的增加,SD大鼠血液中红细胞、白细胞、淋巴细胞和中性粒细胞数量总体上均呈现下降趋势。低、中、高剂量染毒组红细胞数量与对照组相比分别下降1.11%、4.7%和9.13%,但均无显著差异(P>0.05)。淋巴细胞和中性粒细胞数量呈现低剂量兴奋效应[35],高剂量组白细胞、淋巴细胞和中性粒细胞数量与对照组相比分别下降43.57%、30.7%和26.44%,均有显著性差异(P<0.05)。这表明,MMP会导致大鼠血液中的血细胞数量出现不同程度的降低。
表1 不同剂量邻苯二甲酸单甲酯(MMP)染毒3 h后对SD大鼠血液中血细胞数量的影响
Table 1 The number of blood cells of SD rats after monomethyl phthalate (MMP) exposure for 3 h
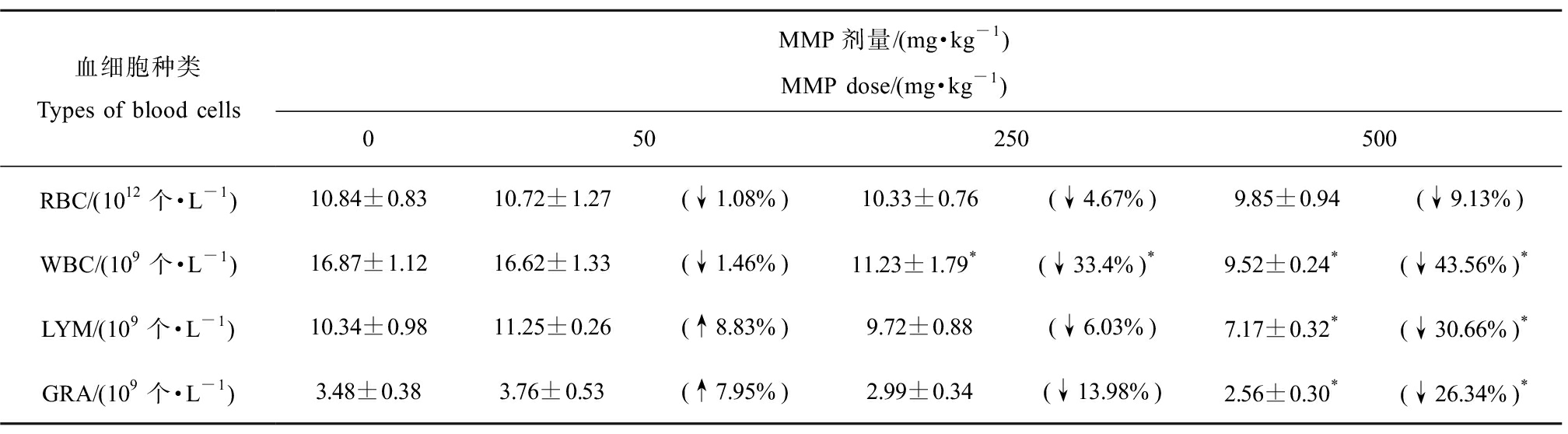
血细胞种类Types of blood cellsMMP剂量/(mg·kg-1)MMP dose/(mg·kg-1)050250500RBC/(1012个·L-1)10.84±0.8310.72±1.27(↓1.08%)10.33±0.76(↓4.67%)9.85±0.94(↓9.13%)WBC/(109个·L-1)16.87±1.1216.62±1.33(↓1.46%)11.23±1.79*(↓33.4%)*9.52±0.24*(↓43.56%)*LYM/(109个·L-1)10.34±0.9811.25±0.26(↑8.83%)9.72±0.88(↓6.03%)7.17±0.32*(↓30.66%)*GRA/(109个·L-1)3.48±0.383.76±0.53(↑7.95%)2.99±0.34(↓13.98%)2.56±0.30*(↓26.34%)*
注:RBC表示红细胞,WBC表示白细胞,LYM表示淋巴细胞,GRA表示中性粒细胞;↑或↓表示与对照组相比上升或下降的百分比;*表示染毒组与对照组有显著性差异(P<0.05)。
Note: RBC stands for red blood cell, WBC stands for white blood cell, LYM stands for lymphocyte, and GRA stands for granulocyte; ↑ or ↓ indicates the percentage increase or decrease compared with the control group; *indicates significant difference from the control (P<0.05).
2.2 MMP对SD大鼠血液生化指标的影响
染毒24 h和连续染毒7 d后,MMP对大鼠血液总胆固醇、尿素氮、总蛋白含量和酸性磷酸酶活性的影响如图1所示。

图1 不同染毒时间下MMP对SD大鼠血液生化指标的影响
注:T-CHO表示总胆固醇,BUN表示尿素氮,TP表示总蛋白,ACP表示酸性磷酸酶;*表示染毒组与对照组有显著性差异(P<0.05)。
Fig. 1 Blood biochemical indexes of SD rats after MMP exposure for different time
Note: T-CHO stands for total cholesterol, BUN stands for blood urea nitrogen, TP stands for total protein, and ACP stands for acid phosphatase;
* indicates significant difference from the control (P<0.05).
随着MMP剂量增大,大鼠血液中总胆固醇含量均呈现出下降趋势,且连续染毒7 d后下降得更快(图1(a))。染毒24 h后,低、中、高剂量组总胆固醇含量分别下降17.47%、31%和36.67%,与对照组相比均差异显著(P<0.05)。连续染毒7 d后,与对照组相比,低、中、高剂量组总胆固醇含量分别下降22.97%、37.4%和43.97%,均有显著差异(P<0.05)。连续染毒7 d后,各剂量组总胆固醇含量均比染毒24 h后低,且500 mg·kg-1剂量下总胆固醇含量为最低。这表明,摄入MMP的时间越久、剂量越大总胆固醇含量降低越快,对血液损害越大。
MMP均会导致血液中尿素氮含量减少,且总体呈现下降趋势(图1(b))。其中,染毒24 h后,低、中、高剂量组尿素氮含量和对照组相比差异均显著(P<0.05),分别下降15.97%、26.43%和34.33%。连续染毒7 d后,尿素氮含量呈现低剂量兴奋效应,中、高剂量MMP使尿素氮含量较对照组分别下降22.13%和28%,差异显著(P<0.05)。这表明,短时间摄入不同剂量MMP均会使大鼠血液尿素氮含量减少;而长时间摄入低剂量MMP时,大鼠血液尿素氮含量会升高;MMP剂量增大后,血液中尿素氮含量会减少。
血液中总蛋白含量均随着MMP剂量增加而减少,呈下降趋势,但连续染毒7 d后下降趋势较慢(图1(c))。其中,高剂量MMP染毒24 h和连续染毒7 d后,总蛋白含量分别减少18.25%和26.35%,与对照组相比差异显著(P<0.05);连续染毒7 d后高剂量组的总蛋白含量最低。这表明,长时间高剂量摄入MMP会使大鼠血液总蛋白含量大幅降低。
MMP能使大鼠血液中酸性磷酸酶活性下降(图1(d))。其中,染毒24 h后,血液中酸性磷酸酶活性随着MMP染毒剂量的增加而下降,低、中、高剂量组较对照组分别显著下降10.63%、19.93%和41.37%(P<0.05)。连续染毒7 d后,低剂量组酸性磷酸酶活性与对照组相比显著上升27.4%(P<0.05),呈现低剂量兴奋效应;中、高剂量MMP分别使酸性磷酸酶活性显著下降21.87%和31.43%(P<0.05)。这表明,无论短时间还是长时间,摄入MMP剂量越大,大鼠血液中酸性磷酸酶活性越低。
2.3 MMP染毒后SD大鼠血液氧化应激水平
不同剂量MMP染毒3 h后,大鼠血液中SOD、CAT相对活性及MDA相对含量如图2所示。
随着MMP剂量增加,SOD活性呈下降趋势(图2(a))。与对照组相比,低、中、高剂量MMP使SOD活性分别下降3.6%、11.7%和17.5%(P>0.05)。这表明,MMP能够抑制SOD活性,但影响较小。
各剂量MMP对大鼠染毒3 h后,血液中CAT活性均低于对照组,且随着MMP剂量的增加呈现下降趋势(图2(b))。低、中、高剂量染毒组CAT活性与对照组相比分别下降19.57%、24.13%和57.68%(P<0.05)。这表明,MMP会使CAT活性下降,损害血液的抗氧化防御体系。
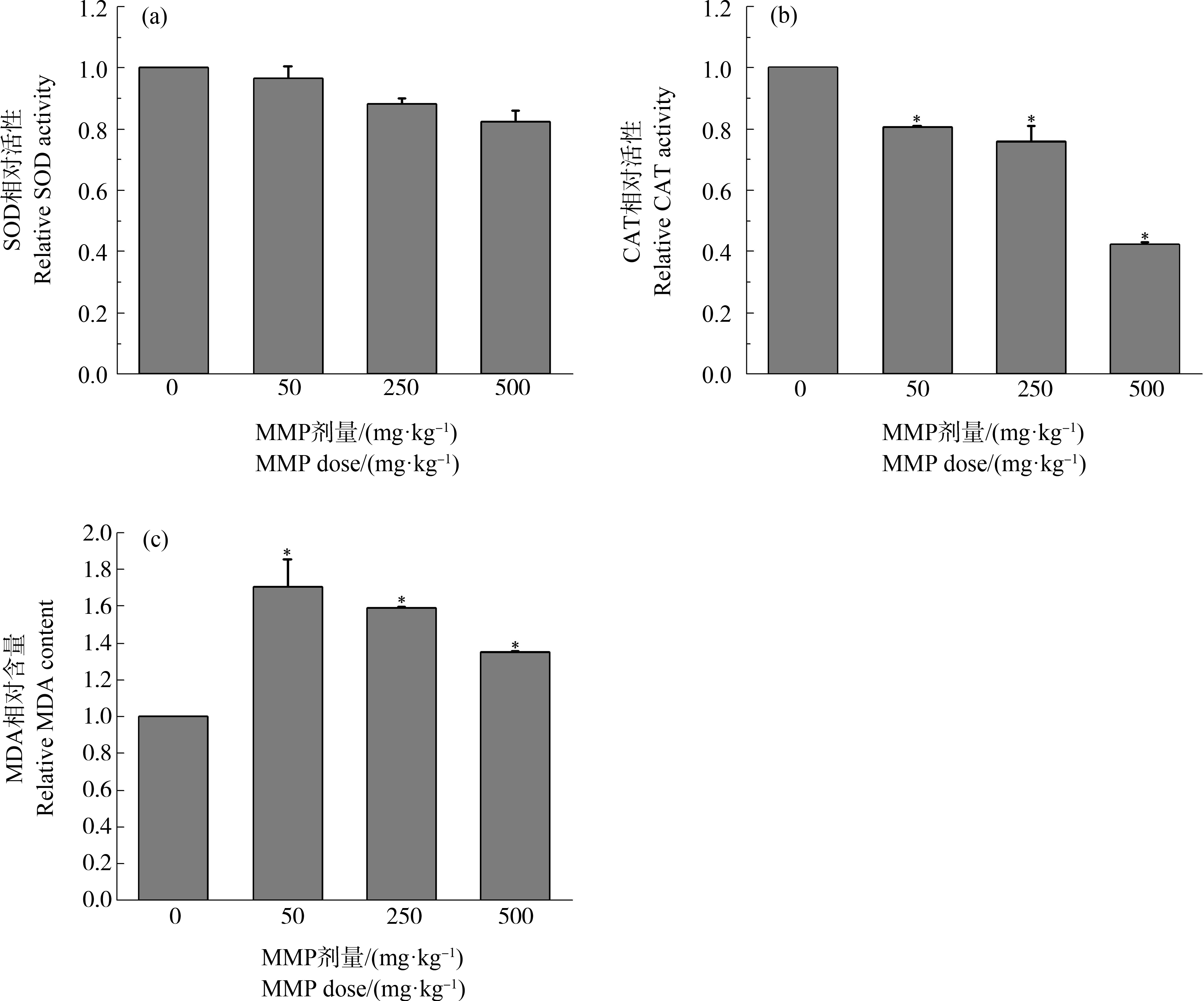
图2 不同剂量MMP染毒3 h后大鼠血液氧化应激水平
注:SOD表示超氧化物歧化酶,CAT表示过氧化氢酶,MDA表示丙二醛;*表示染毒组与对照组有显著性差异(P<0.05)。
Fig. 2 Oxidative stress level in the blood of SD rats after MMP exposure for 3 h
Note: SOD stands for superoxide dismutase, CAT stands for catalase, and MDA stands for malonic dialdehyde;
*indicates significant difference from the control (P<0.05).
不同剂量染毒组MDA含量均高于对照组,且均有显著差异(P<0.05) (图2(c))。与对照组相比,低、中、高剂量组MDA含量分别升高70.43%、59%和34.8%。这表明,MMP进入机体后能够诱导血液发生氧化应激反应,引起血液MDA含量升高。
3 讨论(Discussion)
3.1 MMP对SD大鼠血液功能的影响
血液是机体重要的组织,由血细胞和血浆组成,具有携氧、免疫等功能。血细胞包括红细胞、白细胞和血小板。大量研究证实,红细胞的主要功能是运输氧气和二氧化碳[36-37],同时还具有免疫功能,参与免疫应答调节[38-39]。白细胞是血液中执行免疫功能最主要的细胞[40],其能够通过吞噬细菌等途径来抵御和杀死入侵的病原体[41],对机体防御系统的建立有重要意义。淋巴细胞和中性粒细胞均属于白细胞,分别在机体特异性和非特异性免疫功能方面发挥重要作用[41-43]。
已有研究表明,PAEs会造成血细胞数目下降,影响机体的免疫功能[44],但对其代谢物的影响仍不明确。本研究结果显示,MMP(DMP的代谢产物)会对血液中红细胞、白细胞、淋巴细胞及中性粒细胞造成不同程度的损伤,使其数目下降;且随着MMP剂量的增大,细胞数目下降愈发明显(表1)。这表明,作为PAEs类代谢物MMP也会使血液中的血细胞数量减少,进而造成血液的携氧和免疫等功能下降。
临床上,红细胞、白细胞、淋巴细胞和中性粒细胞也常作为诊断病理变化的重要依据。红细胞数量下降意味着机体可能出现贫血、白血病等疾病;白细胞数目减少代表机体的造血功能出现障碍;淋巴细胞数目偏低会导致机体免疫缺陷;中性粒细胞数量减少表明机体受到化学损伤,出现骨髓造血异常等疾病。由此推测,MMP会影响血液的携氧和免疫等功能,进而可能会引发机体出现贫血、免疫缺陷及骨髓造血功能障碍等疾病。
3.2 MMP对SD大鼠血脂代谢、肾、肝功能的影响
总胆固醇是反映机体内脂肪代谢水平的一项重要指标[45],其含量过多过少都会对机体产生危害。Zhu等[46]研究发现PAEs类代谢物会使胆固醇含量下降。本研究也得到了相似的结果,无论短时间还是长时间摄入MMP,机体血液总胆固醇含量均呈现下降趋势(图1(a))。这表明,MMP会影响血液中脂质的新陈代谢水平。
尿素氮是蛋白质代谢的终末产物,其常作为机体肾功能评价的重要指标[47]。在正常情况下,尿素氮会被肾小球滤过排出体外,若其含量偏低则代表着肾功能失调,含量偏高则意味着过滤功能可能失效,造成器质性肾功能损害。本研究中,机体摄入MMP后,血液中尿素氮含量下降(图1(b))。这表明,MMP会影响机体肾功能,导致其功能失调。
总蛋白含量能够反映肝脏的合成功能以及储备能力[48]。酸性磷酸酶也是描述机体肝功能的一个重要指标[49]。本研究发现,MMP会使血液中总蛋白含量以及酸性磷酸酶活性下降(图1(c)、图1(d))。总蛋白含量下降可能是因为机体摄入MMP后使肝细胞受损,肝脏合成功能出现障碍,最终导致其合成蛋白质减少。由于PAEs类代谢物具有亲脂性容易渗入膜脂质[50],因此推测酸性磷酸酶活性下降可能是因为MMP与溶酶体膜相互作用,抑制了酸性磷酸酶的释放,也可能因为MMP妨碍了溶酶体和质膜的融合,阻止酸性磷酸酶分泌到胞外而导致其活性下降[51]。总蛋白含量和酸性磷酸酶活性下降均反映出MMP对肝功能有一定影响。由于肝脏是胆固醇和尿素氮合成的主要场所,因此推测两者含量的下降也可能是肝功能受损造成的[52-53]。
综上所述,机体摄入MMP后,会导致血脂代谢能力以及肾、肝功能受损。临床上,总胆固醇含量偏低意味着机体可能出现肝硬化、贫血等疾病。尿素氮含量减少表明机体肾功能出现障碍且可能有严重的肝脏疾病。总蛋白含量偏低代表机体可能患有肾炎、肝炎和肝硬化等疾病。酸性磷酸酶活性下降代表机体可能患有贫血、肾炎等疾病。本研究中,4个指标相互印证,且与3.1推测相吻合,因此MMP可能会诱导机体出现贫血、肾炎、肾功能障碍、肝炎和肝硬化等疾病。
3.3 MMP对SD大鼠血液抗氧化能力的影响
氧化应激(oxidative stress, OS)是指机体氧化与抗氧化平衡被破坏,生成过多的氧自由基而无法被清除,从而对机体产生的一种负面作用[54]。由于氧自由基可以直接或间接氧化DNA、脂质和蛋白质,诱发基因突变、脂质过氧化以及蛋白质变性,因此氧化应激通常被认为是污染物对机体产生毒性的作用机制之一[55]。已有研究表明,苯、四氯化碳及重金属铅、镧、钛等污染物能够通过氧化应激引起机体造血、肾、肝功能受损,进而诱发贫血、造血功能障碍、肾炎、肾功能失调以及肝炎、肝硬化等疾病[56-60]。因此,笔者从氧化应激的角度探究MMP对大鼠血液、肾、肝损伤的可能作用机制。
SOD和CAT作为机体抗氧化酶系极其重要的组成部分,其存在可以减轻氧化应激产生的损伤[61]。其中,SOD能够催化超氧阴离子进行歧化反应[62],清除有害的超氧化物自由基;CAT能够催化过氧化氢将其迅速转化为无害或毒性较小的物质[63],防止过氧化氢过多积累对机体造成损伤。本研究发现,机体摄入MMP后会使血液中SOD和CAT活性下降,且随着MMP剂量的增加,SOD和CAT活性呈现出下降趋势(图2(a)、图2(b))。这表明,MMP能够抑制血液中抗氧化酶SOD和CAT的活性,进而损害血液的抗氧化系统。
MDA作为脂质过氧化物的产物,其含量的多少可以反映出机体氧化损伤的程度[64]。本研究发现,各剂量MMP染毒组的MDA含量均高于对照组(图2(c))。这表明,机体摄入MMP后导致血液抗氧化防御体系受损,清除自由基的能力下降,引起氧化应激反应,对血液造成损伤。Holland等[65]研究发现妇女妊娠期尿液中的某些PAEs类代谢物会引起8-异丙醇烷(脂质过氧化的生物标志物)水平升高,使机体发生抗氧化损伤及氧化应激反应。本研究结果与之相符。
综上所述,MMP作为PAEs类代谢物的一种,会使血液的抗氧化能力下降,诱发氧化应激反应,从而对血液中血细胞以及血液生化指标产生影响,损害血液的携氧、免疫等功能;血脂的代谢能力以及机体的肾、肝功能,推测MMP进而可能引发机体发生贫血、造血功能障碍、肾功能失调以及肝硬化等相关疾病。本研究有助于明确邻苯二甲酸酯类代谢物对血液的毒性效应,并为其潜在的风险评估和相关疾病的预防提供一定的理论指导。
[1] 张静, 陈会明. 邻苯二甲酸酯类增塑剂的危害及监管现状[J]. 现代化工, 2011, 31(12): 1-6
Zhang J, Chen H M. Hazards and supervision status of phthalate plasticizer [J]. Modern Chemical Industry, 2011, 31(12): 1-6 (in Chinese)
[2] 吕文涛, 王小逸, 林兴桃, 等. 室内环境中的邻苯二甲酸酯类化合物的研究进展[J]. 环境科技, 2008, 21(S2): 67-70
Lv W T, Wang X Y, Lin X T, et al. General situation on determination of phthalate esters in indoor environment [J]. Environmental Science and Technology, 2008, 21(S2): 67-70 (in Chinese)
[3] Björklund K, Cousins A P, Strömvall A, et al. Phthalates and nonylphenols in urban runoff: Occurrence, distribution and area emission factors [J]. Science of the Total Environment, 2009, 407(16): 4665-4672
[4] 崔学慧, 李炳华, 陈鸿汉, 等. 中国土壤与沉积物中邻苯二甲酸酯污染水平及其吸附研究进展[J]. 生态环境学报, 2010, 19(2): 472-479
Cui X H, Li B H, Chen H H, et al. A review of phthalic acid esters contamination and sorption in soil and sediment, China [J]. Ecology and Environmental Sciences, 2010, 19(2): 472-479 (in Chinese)
[5] 董磊, 汤显强, 林莉, 等. 长江武汉段丰水期水体和沉积物中多环芳烃及邻苯二甲酸酯类有机污染物污染特征及来源分析[J]. 环境科学, 2018, 39(6): 2588-2599
Dong L, Tang X Q, Lin L, et al. Pollution characteristics and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters during high water level periods in the Wuhan section of the Yangtze River, China [J]. Environmental Science, 2018, 39(6): 2588-2599 (in Chinese)
[6] 崔永博, 白莉, 李春辉, 等. 室内空气邻苯二甲酸酯的污染危害及检测方法研究进展[J]. 北方建筑, 2020, 5(5): 35-39
Cui Y B, Bai L, Li C H, et al. Research progress on pollution hazards and detection methods of phthalates in indoor air [J]. Northern Architecture, 2020, 5(5): 35-39 (in Chinese)
[7] Wang X Y, Okoffo E D, Banks A P, et al. Phthalate esters in face masks and associated inhalation exposure risk [J]. Journal of Hazardous Materials, 2022, 423(Pt A): 127001
[8] 陈崇新, 王校, 王哲, 等. 大学生体内邻苯二甲酸酯的暴露分析[J]. 环境与健康杂志, 2016, 33(4): 335-338
Chen C X, Wang X, Wang Z, et al. Analysis of phthalates exposure in college students [J]. Journal of Environment and Health, 2016, 33(4): 335-338 (in Chinese)
[9] Silva M J, Samandar E, Preau J L Jr, et al. Automated solid-phase extraction and quantitative analysis of 14 phthalate metabolites in human serum using isotope dilution-high-performance liquid chromatography-tandem mass spectrometry [J]. Journal of Analytical Toxicology, 2005, 29(8): 819-824
[10] Blair J D, Ikonomou M G, Kelly B C, et al. Ultra-trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI-MS/MS [J]. Environmental Science & Technology, 2009, 43(16): 6262-6268
[11] Liu X T, Peng C F, Shi Y M, et al. Beyond phthalate diesters: Existence of phthalate monoesters in South China house dust and implications for human exposure [J]. Environmental Science & Technology, 2019, 53(20): 11675-11683
[12] Jiang J Q, Mu D, Ding M Y, et al. Simultaneous determination of primary and secondary phthalate monoesters in the Taihu Lake: Exploration of sources [J]. Chemosphere, 2018, 202: 17-24
[13] Mose T, Knudsen L E, Hedegaard M, et al. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system [J]. International Journal of Toxicology, 2007, 26(3): 221-229
[14] 宋小飞, 施召才, 马伟文, 等. 液相色谱-质谱联用法测定血液中邻苯二甲酸酯[J]. 实验技术与管理, 2019, 36(9): 53-56
Song X F, Shi Z C, Ma W W, et al. Determination of phthalate esters in blood by liquid chromatography-mass spectrometry [J]. Experimental Technology and Management, 2019, 36(9): 53-56 (in Chinese)
[15] Ahmad R, Gautam A K, Verma Y, et al. Effects of in utero di-butyl phthalate and butyl benzyl phthalate exposure on offspring development and male reproduction of rat [J]. Environmental Science and Pollution Research, 2014, 21(4): 3156-3165
[16] Banerjee R, Pathmasiri W, Snyder R, et al. Metabolomics of brain and reproductive organs: Characterizing the impact of gestational exposure to butylbenzyl phthalate on dams and resultant offspring [J]. Metabolomics, 2012, 8(6): 1012-1025
[17] Ran D Z, Luo Y, Gan Z J, et al. Neural mechanisms underlying the deficit of learning and memory by exposure to di(2-ethylhexyl) phthalate in rats [J]. Ecotoxicology and Environmental Safety, 2019, 174: 58-65
[18] Song P P, Gao J P, Li X X, et al. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida) [J]. Environment International, 2019, 129: 10-17
[19] Deng T R, Du Y Y, Wang Y X, et al. The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: A prospective single-center study [J]. Ecotoxicology and Environmental Safety, 2020, 188: 109884
[20] Huang P C, Chang W H, Wu M T, et al. Characterization of phthalate exposure in relation to serum thyroid and growth hormones, and estimated daily intake levels in children exposed to phthalate-tainted products: A longitudinal cohort study [J]. Environmental Pollution, 2020, 264: 114648
[21] Sterrett M E, Bloom M S, Jamro E L, et al. Maternal food and beverage consumption behaviors and discrepant phthalate exposure by race [J]. International Journal of Environmental Research and Public Health, 2021, 18(4): 2190
[22] 张弘, 隋自洁, 孙兰, 等. 邻苯二甲酸二(2-乙基己基)酯亚慢性染毒对Wistar大鼠血液学及血生化指标的影响[J]. 中国卫生工程学, 2021, 20(3): 367-370
Zhang H, Sui Z J, Sun L, et al. Subchronic exposure of bis(2-ethylhexyl) phthalate to blood cells and blood cells of Wistar rats and the impact of biochemical indicators [J]. Chinese Journal of Public Health Engineering, 2021, 20(3): 367-370 (in Chinese)
[23] Chu I, Secours V E, Marino I A, et al. Sub-acute and sub-chronic toxicity of mono-2-ethylhexyl phthalate in the rat [J]. Archives of Environmental Contamination and Toxicology, 1981, 10(3): 271-280
[24] Zhu Y D, Zhu B B, Gao H, et al. Repeated measures of prenatal phthalate exposure and maternal hemoglobin concentration trends: The Ma’anshan birth cohort (MABC) study [J]. Environmental Pollution, 2018, 242(Pt B): 1033-1041
[25] Sicińska P. Di-n-butyl phthalate, butylbenzyl phthalate and their metabolites induce haemolysis and eryptosis in human erythrocytes [J]. Chemosphere, 2018, 203: 44-53
[26] Sicińska P, Kik K, Bukowska B. Human erythrocytes exposed to phthalates and their metabolites alter antioxidant enzyme activity and hemoglobin oxidation [J]. International Journal of Molecular Sciences, 2020, 21(12): 4480
[27] Lien Y J, Ku H Y, Su P H, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study [J]. Environmental Health Perspectives, 2015, 123(1): 95-100
[28] Mira M G, Zdenko S, Dinko P, et al. Phthalates in underground waters of the Zagreb area [J]. Croatian Medical Journal, 2002, 43(4): 493-497
[29] 邱东茹, 吴振斌, 贺锋. 内分泌扰乱化学品对动物的影响和作用机制[J]. 环境科学研究, 2000, 13(6): 52-55
Qiu D R, Wu Z B, He F. Effects of endocrine disrupting chemicals on animals and mechanisms of action [J]. Research of Environmental Sciences, 2000, 13(6): 52-55 (in Chinese)
[30] Wang Y X, Liu C, Chen Y J, et al. Predictors and correlations of phthalate metabolite concentrations in urine and seminal plasma among reproductive-aged men [J]. Environmental Research, 2018, 161: 336-344
[31] Olsén L, Lampa E, Birkholz D A, et al. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) [J]. Ecotoxicology and Environmental Safety, 2012, 75(1): 242-248
[32] Högberg J, Hanberg A, Berglund M, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations [J]. Environmental Health Perspectives, 2008, 116(3): 334-339
[33] Babu-Rajendran R, Preethi G, Poopal R K, et al. GC-MS determination of phthalate esters in human urine: A potential biomarker for phthalate bio-monitoring [J]. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 2018, 1079: 15-24
[34] 陈甘讷, 黄伟雯, 李洪庆, 等. 广州孕妇孕期邻苯二甲酸酯暴露水平及其与妊娠结局的关系[J]. 环境与职业医学, 2021, 38(6): 573-579
Chen G, Huang W W, Li H Q, et al. Phthalate exposure during pregnancy and its relationship with birth outcomes in Guangzhou [J]. Journal of Environmental and Occupational Medicine, 2021, 38(6): 573-579 (in Chinese)
[35] 陶功华. 低剂量兴奋效应作用机制的研究进展[J]. 中山大学研究生学刊(自然科学与医学版), 2007, 28(1): 16-21
Tao G H. Research status of the mechanism of hormesis [J]. Journal of the Graduates Sun Yat-Sen University (Natural Sciences Medicine), 2007, 28(1): 16-21 (in Chinese)
[36] Hawkey C M, Bennett P M, Gascoyne S C, et al. Erythrocyte size, number and haemoglobin content in vertebrates [J]. British Journal of Haematology, 1991, 77(3): 392-397
[37] Chi Z X, Liu J, Tan S W, et al. Revealing the toxicity of dimethyl phthalate (DMP) to the oxygen-carrying function of red blood cells (RBCs): The iron release mechanism [J]. Chemosphere, 2021, 263: 128017
[38] 杨汉春. 动物免疫学[M]. 2版. 北京: 中国农业大学出版社, 2003: 76
[39] Siegel I, Liu T L, Gleicher N. The red-cell immune system [J]. The Lancet, 1981, 318(8246): 556-559
[40] 钱国英, 陈永富. 免疫学[M]. 杭州: 浙江大学出版社, 2010: 36-44
[41] 赵雅辉, 王小逸, 林兴桃, 等. 邻苯二甲酸酯类化合物的体内代谢及毒性研究进展[J]. 环境与健康杂志, 2010, 27(2): 184-187
Zhao Y H, Wang X Y, Lin X T, et al. Advances in researches on metabolic mechanism and toxicity of phthalate esters [J]. Journal of Environment and Health, 2010, 27(2): 184-187 (in Chinese)
[42] Rosales C, Uribe-Querol E. Phagocytosis: A fundamental process in immunity [J]. BioMed Research International, 2017, 2017: 9042851
[43] Rainard P, Riollet C, Poutrel B, et al. Phagocytosis and killing of Staphylococcus aureus by bovine neutrophils after priming by tumor necrosis factor-α and the des-arginine derivative of C5a [J]. American Journal of Veterinary Research, 2000, 61(8): 951-959
[44] 赵春风, 刁晓平, 谢嘉, 等. 邻苯二甲酸二乙基己酯(DEHP)对马氏珠母贝(Pinctada martensi)血细胞免疫功能及氧化应激效应的影响[J]. 生态毒理学报, 2014, 9(2): 375-381
Zhao C F, Diao X P, Xie J, et al. Effects of diethylhexyl phthalate on haemocyte immune functions and oxidative stress in Pinctada martensi [J]. Asian Journal of Ecotoxicology, 2014, 9(2): 375-381 (in Chinese)
[45] 刘凤华, 辛萍萍, 温华梅, 等. 饲粮类型对驴驹生长性能和血清生化指标的影响[J]. 饲料研究, 2021, 44(19): 103-106
Liu F H, Xin P P, Wen H M, et al. Effect of feed types on growth performance and serum biochemical indexes of donkey foal [J]. Feed Research, 2021, 44(19): 103-106 (in Chinese)
[46] Zhu Q Q, Hou J, Yin W J, et al. Associations of a mixture of urinary phthalate metabolites with blood lipid traits: A repeated-measures pilot study [J]. Environmental Pollution, 2020, 257: 113509
[47] Bugyei-Twum A, Abadeh A, Thai K, et al. Suppression of NLRP3 inflammasome activation ameliorates chronic kidney disease-induced cardiac fibrosis and diastolic dysfunction [J]. Scientific Reports, 2016, 6: 39551
[48] Akande T, Balogun S T, Gabriel O. The effects of penicillin-streptomycin on liver amino-transferases, alkaline phosphatase and total serum protein in rabbits (Orcytolagus coniculus) [J]. Journal of Applied Pharmaceutical Science, 2012, 2(1): 32-35
[49] 胡冬雪, 马季, 王成强, 等. 拟微绿球藻粉替代鱼粉对大菱鲆幼鱼生长性能、体组成和血清生化指标的影响[J]. 渔业科学进展, 2019, 40(4): 21-30
Hu D X, Ma J, Wang C Q, et al. Effects of replacement of dietary fish meal by Nannochloropsis sp. meal on growth performance, body composition, and serum biochemical indices of juvenile turbot (Scophthalmus maximus L.) [J]. Progress in Fishery Sciences, 2019, 40(4): 21-30 (in Chinese)
[50] Rock G, Tocchi M, Ganz P R, et al. Incorporation of plasticizer into red cells during storage [J]. Transfusion, 1984, 24(6): 493-498
[51] Sonde V, D’Souza A, Tarapore R, et al. Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague-Dawley rats [J]. Toxicology, 2000, 147(1): 23-31
[52] Kumari A. Sweet Biochemistry [M]. Pittsburgh: Academic Press, 2018: 27-31
[53] Dejong C H C, Deutz N E P, Soeters P B. Ammonia and glutamine metabolism during liver insufficiency: The role of kidney and brain in interorgan nitrogen exchange [J]. Scandinavian Journal of Gastroenterology, 1996, 31(suppl.218): 61-77
[54] Betteridge D J. What is oxidative stress? [J]. Metabolism, 2000, 49(2): 3-8
[55] Packer L, Wirtz K W A. Signalling Mechanisms—From Transcription Factors to Oxidative Stress [M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 1995: 165-186
[56] Wang L P, He X Q, Bi Y Y, et al. Stem cell and benzene-induced malignancy and hematotoxicity [J]. Chemical Research in Toxicology, 2012, 25(7): 1303-1315
[57] Unsal V, Cicek M, Sabancilar ⅰ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants [J]. Reviews on Environmental Health, 2021, 36(2): 279-295
[58] Mager E M, Wintz H, Vulpe C D, et al. Toxicogenomics of water chemistry influence on chronic lead exposure to the fathead minnow (Pimephales promelas) [J]. Aquatic Toxicology, 2008, 87(3): 200-209
[59] Zhao H Q, Hong J, Yu X H, et al. Oxidative stress in the kidney injury of mice following exposure to lanthanides trichloride [J]. Chemosphere, 2013, 93(6): 875-884
[60] Li S S, Wang W W, Zhang Q, et al. Co-exposures of TiO2 nanoparticles and cadmium ions at non-lethal doses aggravates liver injury in mice with ConA-induced hepatitis [J]. Environmental Toxicology and Pharmacology, 2021, 86: 103669
[61] Ighodaro O M, et al. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid [J]. Alexandria Journal of Medicine, 2018, 54(4): 287-293
[62] Mittler R. Oxidative stress, antioxidants and stress tolerance [J]. Trends in Plant Science, 2002, 7(9): 405-410
[63] Qin P F, Liu R T. Oxidative stress response of two fluoroquinolones with catalase and erythrocytes: A combined molecular and cellular study [J]. Journal of Hazardous Materials, 2013, 252-253: 321-329
[64] Grotto D, Santa Maria L D, Boeira S, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection [J]. Journal of Pharmaceutical and Biomedical Analysis, 2007, 43(2): 619-624
[65] Holland N, Huen K, Tran V, et al. Urinary phthalate metabolites and biomarkers of oxidative stress in a Mexican-American cohort: Variability in early and late pregnancy [J]. Toxics, 2016, 4(1): 7