阿特拉津(C8H14ClN5)的高效性和化学稳定性使其成为世界上最常用的除草剂之一,全球年消费量可达70 000~90 000 t,中国2013年消费量达23 000 t[1-2]。美国环境保护局(US EPA)2007年将其列为潜在地下水污染的优先化学物质,联合国世界卫生组织2017年将其列为Ⅲ类致癌物,欧盟已于2003年10月禁止使用阿特拉津[3],但目前为止,美国和中国等地仍在使用[2]。农业生产中使用大量的除草剂以确保作物质量及产量,但其残留物会产生严重的环境问题[4-5]。作为一种广泛使用的除草剂,阿特拉津及其代谢物在环境中可持续存在数十年[6],会导致地表水和地下水的污染,明显的迁移特性导致在海水和冰中[7]也曾检测到它们的踪迹。此外,由于阿特拉津能与自然环境因素相互作用,且其残留效应难以消除,因此它会对生态系统产生源源不断的威胁[8-9]。阿特拉津会对水环境中鱼类、甲壳类动物、蠕虫和啮齿动物等生物造成毒害作用[10];它的存在会对内分泌、中枢神经和免疫系统产生威胁[11];此外,阿特拉津还可触发人体内卵巢癌细胞芳香化酶活性[12],并导致精液质量变差、睾丸癌发病率增加[13]。
目前关于阿特拉津的研究多集中在生物毒性,部分集中在污染特征[14-15],自梁霞[16]以长江三角洲研究对象,最终推荐阿特拉津短期、长期水质基准值为2.60 μg·L-1和0.0071 μg·L-1后,关于我国其他流域阿特拉津水质基准的研究鲜有报道,且尚未有相应的国家水质基准。而黄河作为我国北方地区的重要水源,大约有15%的农业灌溉面积和12%的中国人口的供水依赖于黄河[17],在我国阿特拉津污染呈现北高南低的趋势[18],对其展开研究势在必行。此外,我国地表水环境质量标准中虽制定了阿特拉津的限值,但在制定时主要依据的是美国、欧洲等发达国家及国际组织的相关水质标准和水生生物毒性数据[19],并非基于我国水生生物相关毒理学研究得出,无法保证能因地制宜地保护我国水生生物。考虑到阿特拉津自身的危害,黄河流域水安全的重要性,亟待填补目前黄河流域阿特拉津水质基准研究的空白,制定出符合中国黄河流域生物区系特征的阿特拉津水生生物水质基准。
为使推导的水生生物水质基准符合黄河流域的特征,基于文献资料整理了黄河流域的水生生物清单,结合阿特拉津对黄河流域水生生物的急性和慢性毒性效应,分别采用《淡水水生生物水质基准制定技术指南》(HJ 831—2017)中推荐使用的物种敏感度分布法(SSD法),以及美国环境保护局(US EPA)推荐使用的毒性百分数排序法(SSR法)进行水生生物水质基准推导,以保证推导结果的准确性,并对国内外相关水环境标准/基准进行讨论分析,以期为黄河流域阿特拉津水质基准制定和水生态环境管理提供科学依据。
1 材料与方法(Materials and methods)
1.1 数据筛查
水生生物初筛:基于现有文献资料,最初以“黄河、水生生物”为主题词进行查找,之后又以“黄河、水生植物、鱼类、浮游生物、植物、底栖动物和两栖动物等”为主题词进行详细检索,数据收集截止时间为2019年12月,最终参考《黄河鱼类志》以及55篇文献进行黄河流域水生生物清单梳理。主要统计的水生生物类型有鱼类、底栖动物、浮游动物、浮游植物、水生植物和两栖动物。在黄河流域水生生物清单的基础上进行毒理数据初筛,使推导基准结果更符合黄河流域生物特征。
毒性数据筛选:阿特拉津的淡水水生生物毒性数据来自US EPA的ECOTOX数据库(http://cfpub.Epa.Gov/ ecotox/)和公开发表的中英文相关文献,中文文献源于中国知网数据库,以“阿特拉津、莠去津、毒性、生物毒性、急性毒性和半数致死浓度等”为主题词进行检索,英文文献源于ScienceDirect数据库,以“atrazine toxicity、reproductive toxicity等”为主题词进行检索,数据收集截止时间为2020年12月。依据US EPA基准技术指南及我国《淡水水生生物水质基准制定技术指南》(HJ 831—2017)的要求进行数据筛选,对筛选后的毒性数据使用Shapiro-Wilk检验方法进行正态分布检验,P>0.05则认为(对数转换)数据符合正态分布。
1.2 水质基准推导方法
目前应用较多的水生生物水质基准推导方法主要有SSD法、SSR法等。SSD法因原理易懂、计算简单和应用方便受到研究者关注[20],建立物种敏感度分布曲线的一般步骤包括:(1)毒性数据获取;(2)数据处理;(3)曲线拟合;(4)5%危害浓度(HC5)的计算[21]。本研究中SSD法的推导过程参考已有文献[22]进行。SSR法将污染物的急性和慢性毒性效应分开考虑,推导的一般步骤是:(1)毒性数据获取;(2)得到属内急性平均值并从低到高排序;(3)得出最终急性值(FAV)、最终慢性值(FCV)、最终植物值(FPV)、最终残留值(FRV);(4)计算得到基准最大浓度(CMC)和基准连续浓度(CCC)[23]。该法简单易行,主要依据公式计算,可操作性和可视性强[24],本研究中SSR法的推导过程参考已有文献[23]进行。
2 结果与讨论(Results and discussion)
基于文献整理了黄河流域水生生物清单,发现黄河流域现有鱼类205种,占水生生物总类别的40.04%,优势物种有餐条、鲫鱼、鲶鱼、黄颡鱼和鲤鱼;现有底栖动物49种,占9.57%,优势物种有摇蚊幼虫、钩虾、椭圆萝卜螺和寡毛类;现有浮游动物89种,占17.38%,优势物种有变形虫、沙壳虫等;现有浮游植物145种,占28.32%,优势物种有微小平裂藻、角甲藻等;现有水生植物17种,占3.32%;现有两栖类动物7种,占1.37%。以上水生生物统计结果将为后期毒性数据初步筛选提供依据,有助于充分利用黄河流域现有水生生物进行毒性数据初筛,并结合受试物种要求进一步筛选,解决黄河流域本土毒性数据缺乏的问题。
2.1 阿特拉津对水生生物的毒性数据
本研究从ECOTOX数据库共导出10 431条阿特拉津毒性数据,另外补充12条文献的中阿特拉津毒性数据。首先,根据4 d≥急性毒性暴露时间≥1 d以及慢性毒性暴露时间≥21 d的条件,将10 431条数据筛选至2 962条;之后,经过筛除重复项剩余数据2 648条;其次,根据淡水生物以及流水式、静态式实验进行筛选,剩余数据量797条;接着,根据黄河流域水生生物清单进行物种筛选,剩余数据121条;最后,由于藻类数据量过多,因此仅保留4 d的急性毒性试验数据,大型溞类等浮游动物尽量选择2 d的急性毒性数据,经筛选后得到39条有效数据,加上补充的12条数据,共得到16个物种51条有效数据(表1),其中47条急性数据涵盖了5门11科15种,慢性数据4条。所获得水生生物毒性数据均来自于水相中阿特拉津的实验结果,急性毒性终点包括半致死浓度(LC50)、半抑制浓度(EC50)和半数抑制效应浓度(IC50),慢性毒性终点包括无可见效应浓度(NOEC)、最低可见效应浓度(LOEC)。
毒性数据满足指南中物种要求,涵盖了水生植物/初级生产者、无脊椎动物/初级消费者和脊椎动物/次级消费者3个营养级。急性毒性数据涵盖了推导水质基准的主要生物类群(硬骨鲤科鱼、硬骨非鲤科鱼、底栖动物、浮游动物和浮游植物),包括了至少3门8科的生物分类单元,而且已通过对数正态分布的Shapiro-Wilk检验(P>0.05),因此急性毒性数据推导的水质基准较为可信。本次选用SSD、SSR这2种方法进行水质基准推导,用到的种平均毒性值(SMAV)和属平均毒性值(GMAV)如表1所示。
表1 阿特拉津对黄河流域本土水生生物的毒性数据
Table 1 Toxicity data of atrazine to native aquatic organisms in the Yellow River Basin

种Species属Genus毒性终点Toxic end pointρ/(mg·L-1)SMAV/(mg·L-1)GMAV/(mg·L-1)参考文献Reference鱼类Fish草鱼Ctenopharyngodon idellus草鱼属Ctenopharyngodon虹鳟Oncorhynchus mykiss鲑属Salmo鲤鱼Cyprinus carpio鲤属Cyprinus泥鳅Misgurnus anguillicadatus泥鳅属Misgurnus4 d-LC5037.0004 d-LC5012.9002 d-LC5020.0004 d-LC504.5004 d-LC500.8704 d-LC5024.0002 d-LC5023.8004 d-LC5014.7004 d-LC505.3004 d-LC502.1424 d-LC5018.8001 d-LC5031.6002 d-LC5026.8204 d-LC5018.98037.00037.0009.0499.0496.3466.34625.24325.243[25][26][26]-[27][28]-[29][29][30][16][31][31][31]两栖动物Amphibians黑斑侧褶蛙Pelophylax nigromaculatus侧褶蛙属Pelophylax4 d-LC5049.25049.25049.250[32]底栖动物Benthic animals日本沼虾Macrobrachium nipponense沼虾属Macrobrachium4 d-LC5022.98022.98022.980[16]浮游动物Zooplankton大型溞Daphnia magna隆线溞Daphnia carinata溞属Misgurnus1 d-LC5035.5002 d-LC50133.0002 d-EC5078.0002 d-LC5026.3002 d-LC5050.4072 d-LC5016.8232 d-LC506.9001 d-EC5039.0002 d-EC5039.0002 d-EC50115.0002 d-EC5031.0002 d-LC509.4002 d-LC5060.6002 d-EC5024.42034.54538.46936.454[33][34][34][35][36][37][38][39][39][29][29][40][41][42]浮游植物Phytoplankton鱼腥藻Anabeana sp.巴豆叶脆杆藻Fragilaria crotonensis钝脆杆藻Fragilaria capucina美丽星杆藻Asterionella formosa谷皮菱形藻Nitzchia palea衣藻Chlamydomonas小球藻Chlorella vulgaris鱼腥藻属Anabeana脆杆藻属Fragilaria星杆藻属Asterionella菱形藻属Nitzschia衣藻属Chlamydomonas小球藻属Chlorella4 d-EC503.0004 d-EC501.2024 d-EC500.6354 d-EC500.2614 d-EC502.1604 d-EC503.9884 d-EC5014.9434 d-IC500.4294 d-EC500.1764 d-EC500.0514 d-EC500.0504 d-IC500.0564 d-EC500.4104 d-EC500.0944 d-EC500.0884 d-EC500.4134 d-EC500.0043.0003.0001.2020.8740.6350.8740.7510.7512.9462.9460.0710.0710.0890.089-[43][44][45][46][47][44][48]-[49][50][51][52][53][45][54][46]
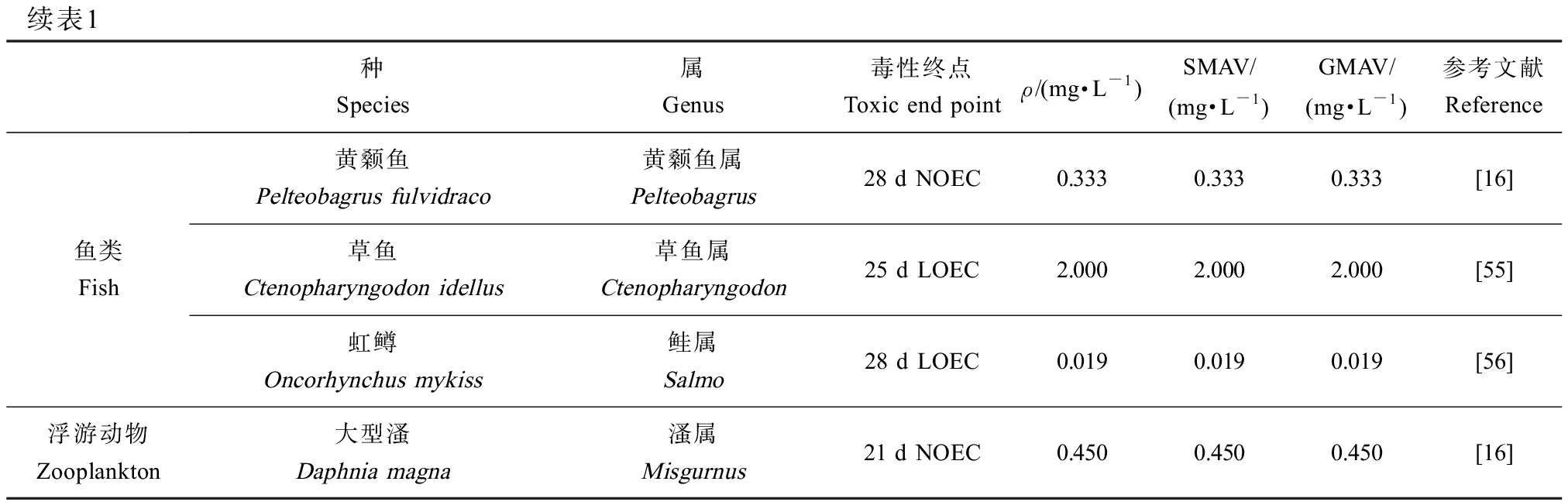
续表1种Species属Genus毒性终点Toxic end pointρ/(mg·L-1)SMAV/(mg·L-1)GMAV/(mg·L-1)参考文献Reference鱼类Fish黄颡鱼Pelteobagrus fulvidraco黄颡鱼属Pelteobagrus28 d NOEC0.3330.3330.333[16]草鱼Ctenopharyngodon idellus草鱼属Ctenopharyngodon25 d LOEC2.0002.0002.000[55]虹鳟Oncorhynchus mykiss鲑属Salmo28 d LOEC0.0190.0190.019[56]浮游动物Zooplankton大型溞Daphnia magna溞属Misgurnus21 d NOEC0.4500.4500.450[16]
注:EC50表示半抑制浓度,LC50表示半致死浓度,IC50表示半数抑制效应浓度,NOEC表示无可见效应浓度,LOEC表示最低可见效应浓度,ρ表示阿特拉津的浓度,GMAV表示属平均毒性值,SMAV表示种平均毒性值,-表示该数据来源于US EPA网站ECOTOX数据库,对应的参考编号(reference number)分别是12999、315、61707、61707。
Note: EC50 is the half inhibitory concentration, LC50 is the half lethal concentration, IC50 is the half inhibitory effect concentration, NOEC is the no observed effect concentration, LOEC is the lowest observed effect concentration, ρ is the concentration of atrazine, GMAV is the genus mean acute value, and SMAV is the species mean acute value ; - indicate that the data comes from the ECOTOX database of US EPA, and the corresponding reference numbers are 12999, 315, 61707 and 61707 respectively.
2.2 黄河流域阿特拉津的水生生物水质基准值
2.2.1 基于SSR法的水质基准推导
利用SPSS 25.0对GMAV取对数后的急性数据进行正态分布检验,经检验发现其分布符合正态分布(均值为0.61,标准差为0.967,在0.05水平下,P值为0.124)。分别采用Origin 2017的Slogistic模型、Stirling模型和ExpDec3模型进行曲线拟合(图1),拟合总结果如表2所示。
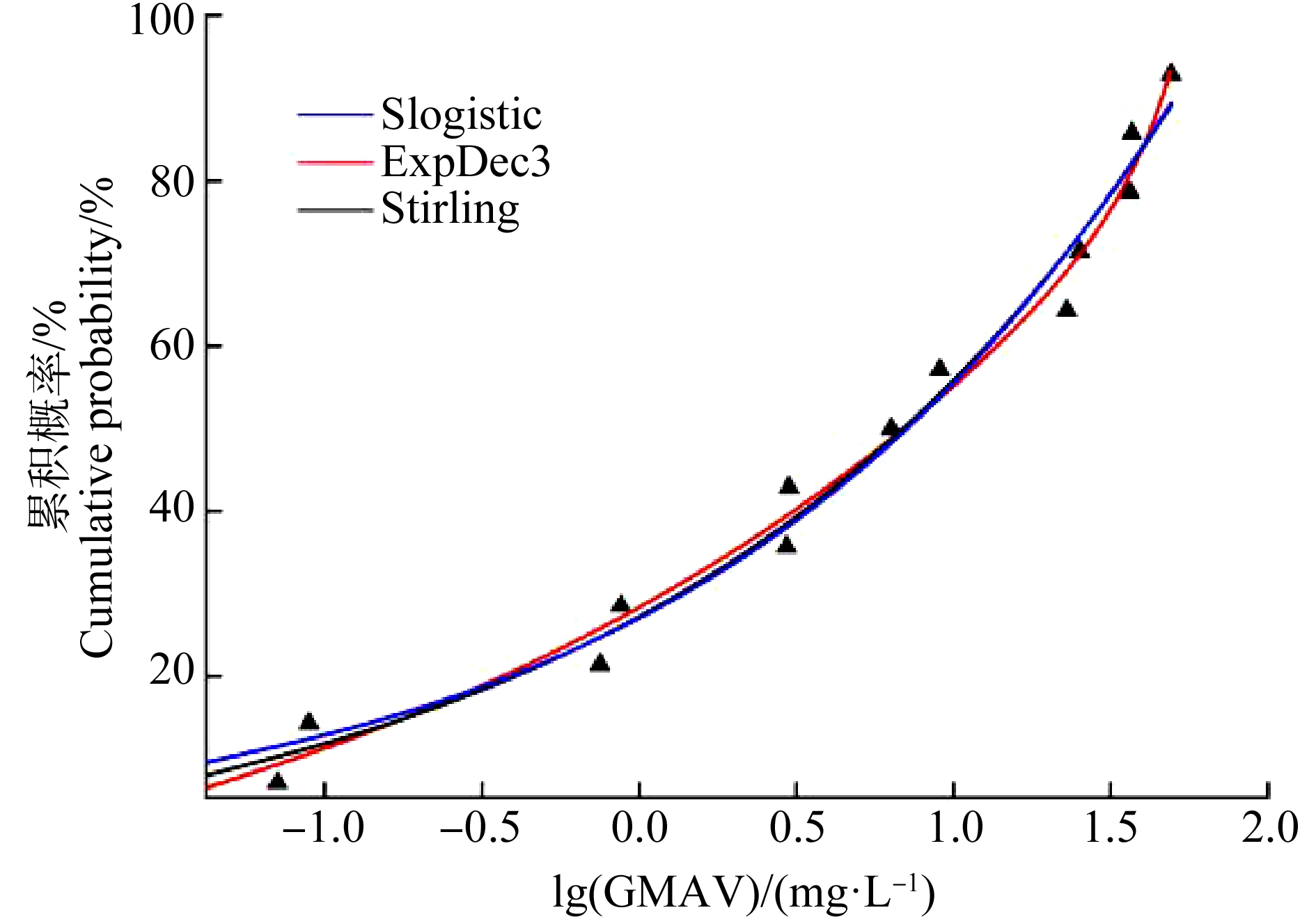
图1 不同模型拟合的阿特拉津急性物种敏感度分布曲线
Fig. 1 Distribution curves of acute species sensitivity of
atrazine fitted by different models
表2 不同模型拟合阿特拉津的急性物种敏感度分布曲线结果
Table 2 Fitting results of acute species sensitivity distribution curves of atrazine in different models
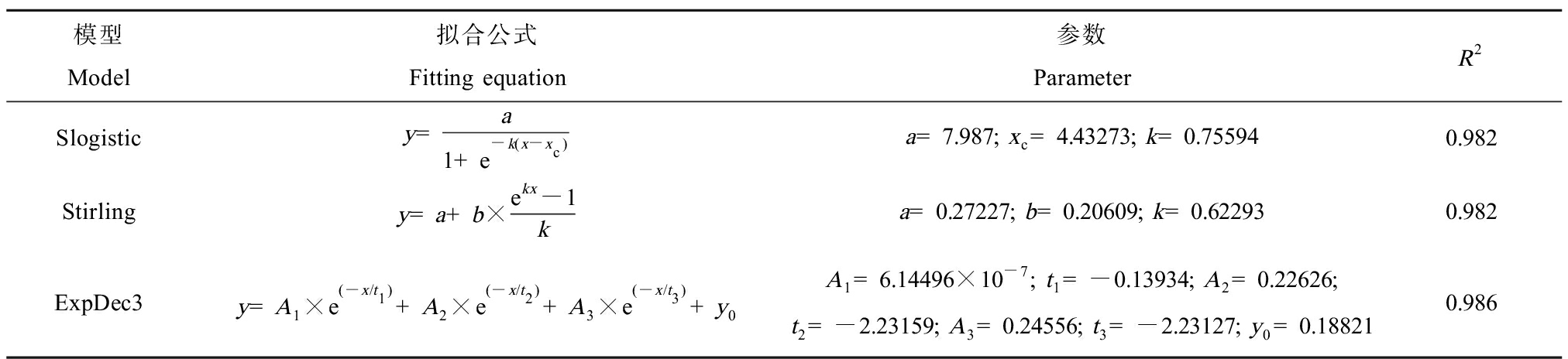
模型Model拟合公式Fitting equation参数ParameterR2Slogisticy=a1+e-k(x-xc)a=7.987; xc=4.43273; k=0.755940.982Stirlingy=a+b×ekx-1ka=0.27227; b=0.20609; k=0.622930.982ExpDec3y=A1×e(-x/t1)+A2×e(-x/t2)+A3×e(-x/t3)+y0A1=6.14496×10-7; t1=-0.13934; A2=0.22626; t2=-2.23159; A3=0.24556; t3=-2.23127; y0=0.188210.986
由表2可知,ExpDec3模型的R2最接近于1,拟合度最好。因此采用ExpDec3模型计算累积概率为0.05时的浓度,得到HC5为29.85 μg·L-1。HC5除以评估因子可确定最终的淡水水生生物水质基准,推导的有效毒性数据有51条且涵盖了足够营养级,因此评估因子取2,得到短期水质基准值为14.90 μg·L-1。只有部分慢性毒性数据满足推导水质基准的要求,不足以建立模型,所以根据HC5与急慢性比的比值计算长期水质基准值,根据草鱼、虹鳟和大型溞3个物种急慢性毒性值计算急慢性比,将其几何平均值87.780作为最终值(表3),得到长期水质基准值为0.34 μg·L-1。综上,基于SSD法得到我国黄河流域水生生物阿特拉津的短期水质基准值和长期水质基准值分别为14.90 μg·L-1和0.34 μg·L-1。
表3 急慢性比率表
Table 3 Ratio of acute to chronic
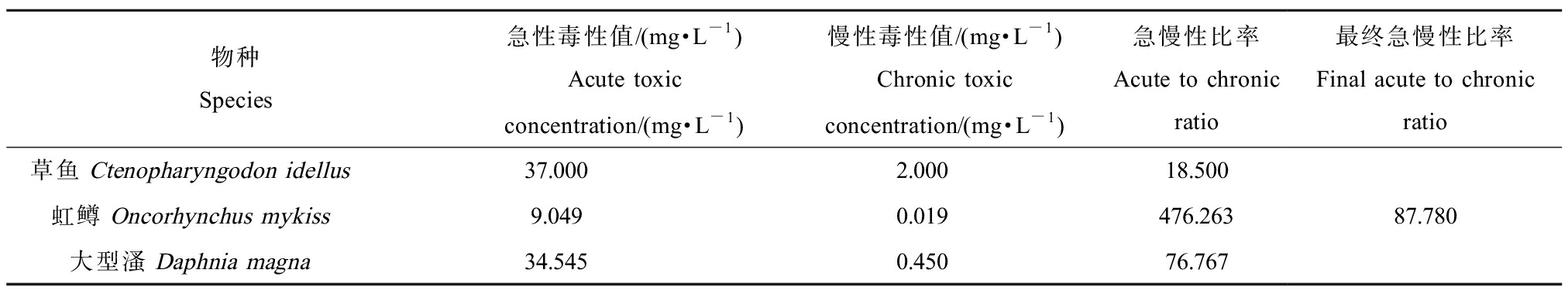
物种Species急性毒性值/(mg·L-1)Acute toxic concentration/(mg·L-1)慢性毒性值/(mg·L-1)Chronic toxic concentration/(mg·L-1)急慢性比率Acute to chronic ratio最终急慢性比率Final acute to chronic ratio草鱼 Ctenopharyngodon idellus37.0002.00018.500虹鳟 Oncorhynchus mykiss9.0490.019476.263大型溞 Daphnia magna34.5450.45076.76787.780
2.2.2 基于SSR法的水质基准推导
参照《淡水水生生物水质基准制定技术指南》(HJ 831—2017),对最敏感的4个属,即P值最小的4个属的GMAV进行拟合。对GMAV进行排序后,得到对阿特拉津毒性最敏感的4个属分别为:衣藻属(P=0.07)、小球藻属(P=0.14)、星杆藻属(P=0.21)和脆杆藻属(P=0.29),四者的GMAV分别为71.00、89.00、751.00和874.00 μg·L-1。由公式计算出FAV为28.50 μg·L-1,CMC为14.20 μg·L-1。由于衣藻世代时间短、分布广泛,对水环境中阿特拉津的存在很敏感,因此可作为水环境阿特拉津污染的指示生物。
符合基准推导要求的阿特拉津慢性毒数据较少(4个),数据量未满足推导FCV要求,因此利用急慢性比(ACR),根据公式FCV=FAV/ACR计算FCV,通过计算得到阿特拉津的FCV为2.85 μg·L-1。CCC为FPV、FRV和FCV中最小者,但本次推导中慢性毒理数据及植物毒理数据较少,不足以对FPV、FRV进行推导,因此CCC直接取FCV,即CCC为2.85 μg·L-1。综上,基于SSR法得到我国黄河流域水生生物阿特拉津的CMC和CCC分别为14.20 μg·L-1和2.85 μg·L-1。
2.2.3 水质基准推导结果对比分析
SSD法和SSR法推导出的阿特拉津短期水质基准值分别是14.90 μg·L-1和14.20 μg·L-1,长期水质基准值分别是0.34 μg·L-1和2.85 μg·L-1。SSD法较好地拟合了不同种属物种对于水体中阿特拉津污染物的敏感程度,且较为全面地利用了所收集数据,得出的基准值可以保护绝大多数水生生物[23];SSR法推导的最终基准值很大程度上依赖于敏感物种的数据[57]。整体上推导出的水质基准较相近,特别是短期水质基准值,这是因为急性数据比较充足,得到的短期水质基准值确定性较高,但慢性数据不足会使得基于SSD法的长期水质基准值确定性降低。而且已有研究表明,当污染物物种敏感度分布连续且呈正态分布时,推荐采用SSR法[58]。综上所述,最终推荐使用的水质基准值为SSR法的结果:短期水质基准值14.20 μg·L-1和长期水质基准值2.85 μg·L-1。与已推导出的长江三角洲[16]短期水质基准值2.60 μg·L-1,长期水质基准值0.0071 μg·L-1相比要大很多,推测原因主要有:(1)最终基准值选用的方法不同,长江三角洲流域、黄河流域分别采用了SSD法、SSR法的推导结果,SSD法的结果相对较严格;(2)所用毒性数据基于的生物物种不同,黄河流域所用毒理数据多集中在藻类,而长江三角洲流域多集中在底栖、鱼类等动物(>70%),曲线拟合时毒性数据点分布较集中;(3)不同流域内生态系统的复杂程度、生物区系分布不同,长江流域鱼类、浮游生物、底栖动物物种数均是黄河流域的数倍,甚至数十倍,故对农药敏感的物种较黄河流域多,水生生物保护需求更大;(4)长江三角洲流域较黄河流域水产养殖业更发达,农药使用量大,故基准制定更为严格。
2.2.4 阿特拉津相关标准对比分析
国内外在相关水质标准/基准中对阿特拉津的安全阈值进行了限定,其主要规定限值如表4所示。《地表水环境质量标准》阿特拉津的浓度阈值(3 μg·L-1)与毒性百分数法推导出的长期水质基准值(2.85 μg·L-1)十分接近。2001年修订的美国国家一级饮用水法规中限值为3 μg·L-1,2002年我国发布的地表水标准制定时主要参考当时已有的指导值,后续地下水、饮用水标准的制定又参考了地表水标准。加拿大Guidelines for Canadian Drinking Water Quality Summary Table提出的标准限值(5 μg·L-1)与我国地表水标准的限值差距不大。美国National Recommended Water Quality Criteria—Aquatic Life Criteria Table中虽将阿特拉津列入,但未指定其水生生物水质基准指导值。加拿大Water Quality Guidelines for the Protection of Aquatic Life中不但将阿特拉津列入,而且限定了淡水水生生物的长期水质基准值1.8 μg·L-1,这与毒性百分数法推导出的长期基准值2.85 μg·L-1处于同一数量级。
表4 相关水质标准/基准中阿特拉津的限值
Table 4 Limits of atrazine in related standards
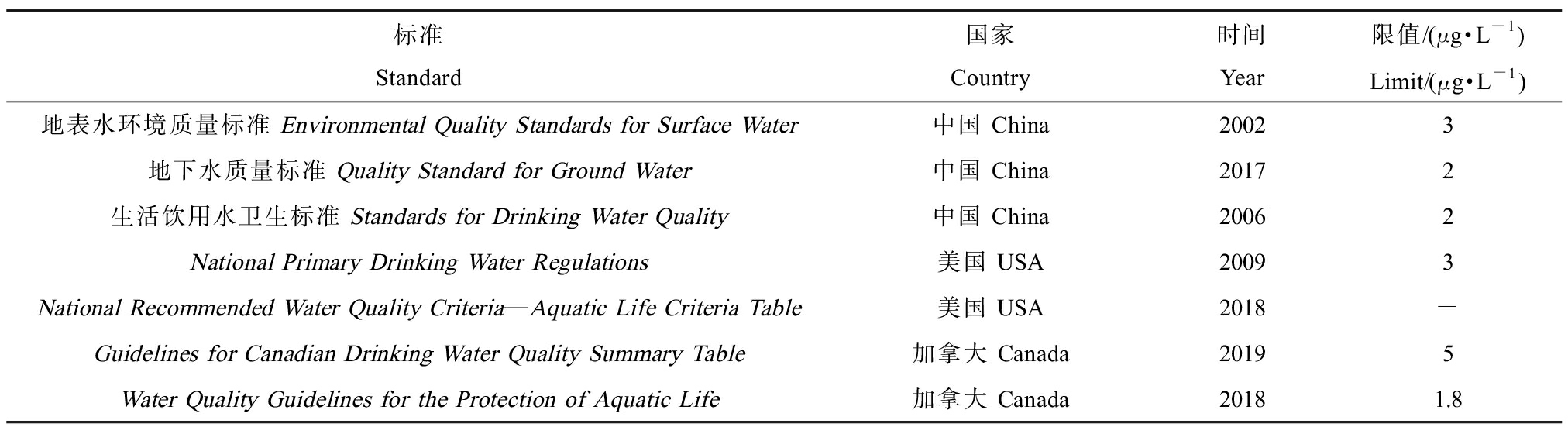
标准Standard国家Country时间Year限值/(μg·L-1)Limit/(μg·L-1)地表水环境质量标准 Environmental Quality Standards for Surface Water中国 China20023地下水质量标准 Quality Standard for Ground Water中国 China20172生活饮用水卫生标准 Standards for Drinking Water Quality中国 China20062National Primary Drinking Water Regulations美国 USA20093National Recommended Water Quality Criteria—Aquatic Life Criteria Table美国 USA2018-Guidelines for Canadian Drinking Water Quality Summary Table加拿大 Canada20195Water Quality Guidelines for the Protection of Aquatic Life加拿大 Canada20181.8
注:-表示无此项数据;National Recommended Water Quality Criteria—Aquatic Life Criteria Table中仅列有该项指标,无水生生物基准指导值;Guidelines for Canadian Drinking Water Quality Summary Table中5 μg·L-1为阿特拉津及其代谢产物的总和;Water Quality Guidelines for the Protection of Aquatic Life中1.8 μg·L-1为长期水质基准值,未给出短期水质基准值。
Note: - indicates that no data available; only this indicator is listed in the National Recommended Water Quality Criteria—Aquatic Life Criteria Table, and there is no guideline value for aquatic organisms; in Guidelines for Canadian Drinking Water Quality Summary Table, 5 μg·L-1 is the sum of atrazine and its metabolites; in Water Quality Guidelines for the Protection of Aquatic Life, 1.8 μg·L-1 is the long-term water quality benchmark value, and the short-term water quality benchmark value is not given.
综上所述,本研究结果表明:以黄河流域水生生物为保护对象,使用SSD法和SSR法推导了阿特拉津的水质基准,二者推导结果较接近。由于慢性数据不足会使得基于SSD法的长期水质基准值确定性降低,而且当污染物物种敏感度分布连续且呈正态分布时推荐使用SSR法,故采用SSR法推导得出的短期水质基准值14.20 μg·L-1和长期水质基准值2.85 μg·L-1作为黄河流域阿特拉津的水生生物水质基准推荐值。
[1] Lin Z, Zhen Z, Liang Y Q, et al. Changes in atrazine speciation and the degradation pathway in red soil during the vermiremediation process [J]. Journal of Hazardous Materials, 2019, 364: 710-719
[2] Yue L, Ge C J, Feng D, et al. Adsorption-desorption behavior of atrazine on agricultural soils in China [J]. Journal of Environmental Sciences (China), 2017, 57: 180-189
[3] Sass J B, Colangelo A. European Union bans atrazine, while the United States negotiates continued use [J]. International Journal of Occupational and Environmental Health, 2006, 12(3): 260-267
[4] Tortella G R, Rubilar O, Cea M, et al. Sorption parameters of carbendazim and iprodione in the presence of copper nanoparticles in two different soils [J]. Journal of Soil Science and Plant Nutrition, 2019, 19(3): 469-476
[5] Mudhoo A, Garg V K S. Sorption, transport and transformation of atrazine in soils, minerals and composts: A review [J]. Pedosphere, 2011, 21(1): 11-25
[6] 李晓宇, 任仲宇, 李芳春, 等. 两种吸附模型对阿特拉津在壤质砂土中的模拟效果分析[J]. 农业环境科学学报, 2020, 39(1): 191-200
Li X Y, Ren Z Y, Li F C, et al. Analysis of simulated migration of atrazine in a type of loamy sand based on two adsorption models [J]. Journal of Agro-Environment Science, 2020, 39(1): 191-200 (in Chinese)
[7] Wu B, Arnold W A, Ma L M. Photolysis of atrazine: Role of triplet dissolved organic matter and limitations of sensitizers and quenchers [J]. Water Research, 2021, 190: 116659
[8] Aggelopoulos C A, Tataraki D, Rassias G. Degradation of atrazine in soil by dielectric barrier discharge plasma—Potential singlet oxygen mediation [J]. Chemical Engineering Journal, 2018, 347: 682-694
[9] Niu B L, Cai J Z, Song W J, et al. Intermediate accumulation and toxicity reduction during the selective photoelectrochemical process of atrazine in complex water bodies [J]. Water Research, 2021, 205: 117663
[10] Zhou R, Liu R, Li W X, et al. The use of different sublethal endpoints to monitor atrazine toxicity in nematode Caenorhabditis elegans [J]. Chemosphere, 2021, 274: 129845
[11] Castro G, Rodríguez I, Ramil M, et al. Evaluation of nitrate effects in the aqueous photodegradability of selected phenolic pollutants [J]. Chemosphere, 2017, 185: 127-136
[12] Albanito L, Lappano R, Madeo A, et al. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells [J]. Environmental Health Perspectives, 2008, 116(12): 1648-1655
[13] Ohlson C G. Testicular cancer and occupational exposures with a focus on xenoestrogens in polyvinyl chloride plastics [J]. Chemosphere, 2000, 40(9-11): 1277-1282
[14] Xie H J, Wang X P, Chen J W, et al. Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China [J]. The Science of the Total Environment, 2019, 656: 946-951
[15] Xue Y, Zhang Z M, Zhang R R, et al. Aquaculture-derived distribution, partitioning, migration, and transformation of atrazine and its metabolites in seawater, sediment, and organisms from a typical semi-closed mariculture bay [J]. Environmental Pollution, 2021, 271: 116362
[16] 梁霞. 长江三角洲流域溴氰菊酯和莠去津水生生物基准研究[D]. 南京: 南京师范大学, 2015: 53-68
Liang X. The aquatic organism criteria deltamethrin and atrazine in the Yangtze River Delta region [D]. Nanjing: Nanjing Normal University, 2015: 53-68 (in Chinese)
[17] Chen Y P, Fu B J, Zhao Y, et al. Sustainable development in the Yellow River Basin: Issues and strategies [J]. Journal of Cleaner Production, 2020, 263: 121223
[18] 徐雄, 李春梅, 孙静, 等. 我国重点流域地表水中29种农药污染及其生态风险评价[J]. 生态毒理学报, 2016, 11(2): 347-354
Xu X, Li C M, Sun J, et al. Residue characteristics and ecological risk assessment of twenty-nine pesticidesin surface water of major river-basin in China [J]. Asian Journal of Ecotoxicology, 2016, 11(2): 347-354 (in Chinese)
[19] 王婷, 张红, 史雅娟, 等. 我国硒淡水水生生物水质基准值推导[J]. 环境科学学报, 2020, 40(4): 1278-1285
Wang T, Zhang H, Shi Y J, et al. Derivation of freshwater quality criteria of selenium for protection of aquatic organisms in China [J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1278-1285 (in Chinese)
[20] 曾勇, 孙霄, 赖雨薇, 等. 基于物种敏感性分布的多环芳烃水生态系统风险评价方法与应用[J]. 生态毒理学报, 2020, 15(5): 235-243
Zeng Y, Sun X, Lai Y W, et al. Aquatic ecosystem risk assessment of polycyclic aromatic hydrocarbons based on species sensitivity distribution [J]. Asian Journal of Ecotoxicology, 2020, 15(5): 235-243 (in Chinese)
[21] 汪贞, 杨先海, 范德玲, 等. 应用物种敏感性分布评估三氯卡班对我国淡水环境的生态风险[J]. 生态与农村环境学报, 2017, 33(10): 921-927
Wang Z, Yang X H, Fan D L, et al. Ecological risk assessment of triclocarban in fresh water of China by species sensitivity distribution [J]. Journal of Ecology and Rural Environment, 2017, 33(10): 921-927 (in Chinese)
[22] 郑磊, 杨文龙, 董亮, 等. 扑草净水环境质量基准及风险评估[J]. 中国环境科学, 2021, 41(8): 3825-3831
Zheng L, Yang W L, Dong L, et al. Derivation of water quality criteria and ecological risk assessment for prometryn [J]. China Environmental Science, 2021, 41(8): 3825-3831 (in Chinese)
[23] 陈曲, 郭继香, 孙乾耀, 等. 甲萘威的淡水水生生物水质基准研究[J]. 环境科学研究, 2016, 29(1): 84-91
Chen Q, Guo J X, Sun Q Y, et al. Aquatic life ambient freshwater quality criteria for carbaryl in China [J]. Research of Environmental Sciences, 2016, 29(1): 84-91 (in Chinese)
[24] 郭文景, 张志勇, 符志友, 等. 锑的淡水水质基准及其对我国水质标准的启示[J]. 中国环境科学, 2020, 40(4): 1628-1636
Guo W J, Zhang Z Y, Fu Z Y, et al. Derivation of aquatic life water quality criteria for antimonyin freshwater and its implication for water quality standard in China [J]. China Environmental Science, 2020, 40(4): 1628-1636 (in Chinese)
[25] Botelho R G, Santos J B D, Fernandes K M, et al. Effects of atrazine and picloram on grass carp: Acute toxicity and histological assessment [J]. Toxicological & Environmental Chemistry, 2012, 94(1): 121-127
[26] Bathe R, Sachsse K, Ullmann L, et al. The evaluation of fish toxicity in the laboratory, proceedings of the european society of toxicology [J]. European Societies, 1975, 16(1): 113-124
[27] Birge W J, Black J A, Westerman A G, et al. Fish and amphibian embryos—A model system for evaluating teratogenicity [J]. Fundamental and Applied Toxicology, 1983, 3(4): 237-242
[28] Mayer F, Ellersieck M. Manual of acute toxicity: Interpretation and data base for 410 chemicals and 66 species of freshwater animals [R]. Washington DC: United States Department of the Interior, Fish and Wildlife Service (USA), 1986
[29] Elderberry T, Jonathan A, Thurman N, et al. Pesticide Ecotoxicity Database (Formerly: Environmental Effects Database (EEDB)) [R]. Washington DC: Office of Pesticide Programs, Environmental Fate and Effects Division, 2000
[30] 韩英, 赵荣伟, 郝其睿, 等. 阿特拉津和毒死蜱对鲤胚胎发育的影响[J]. 东北农业大学学报, 2015, 46(7): 76-82, 89
Han Y, Zhao R W, Hao Q R, et al. Effect of atrazine and chlorpyrifos on embryonic of common carp (Cyprinus carpio L.) [J]. Journal of Northeast Agricultural University, 2015, 46(7): 76-82, 89 (in Chinese)
[31] 王坡, 王芳, 张瑞华, 等. 阿特拉津对泥鳅性腺及性别分化相关基因的影响[J]. 河南师范大学学报(自然科学版), 2017, 45(3): 109-117
Wang P, Wang F, Zhang R H, et al. Effects of atrazine on sex differentiation and expression pattern of related genes in loach [J]. Journal of Henan Normal University (Natural Science Edition), 2017, 45(3): 109-117 (in Chinese)
[32] 曹慧. 阿特拉津对黑斑侧褶蛙免疫毒效应及机理研究[D]. 杭州: 杭州师范大学, 2012: 9-13
Cao H. Immunotoxicity and mechanisms induced by atrazine on the frog (Pelophylax nigromaculata) [D]. Hangzhou: Hangzhou Normal University, 2012: 9-13 (in Chinese)
[33] Palma P, Palma V L, Fernandes R M, et al. Acute toxicity of atrazine, endosulfan sulphate and chlorpyrifos to Vibrio fischeri, Thamnocephalus platyurus and Daphnia magna, relative to their concentrations in surface waters from the Alentejo region of Portugal [J]. Bulletin of Environmental Contamination and Toxicology, 2008, 81(5): 485-489
[34] Rosa R, Materatski P, Moreira-Santos M, et al. A scaled-up system to evaluate zooplankton spatial avoidance and the population immediate decline concentration [J]. Environmental Toxicology and Chemistry, 2012, 31(6): 1301-1305
[35] Choi H J, Kim D, Lee T J. Photochemical degradation of atrazine in UV and UV/H2O2 process: Pathways and toxic effects of products [J]. Journal of Environmental Science and Health, Part B, 2013, 48(11): 927-934
[36] Moreira R A, da Silva Mansano A, da Silva L C, et al. A comparative study of the acute toxicity of the herbicide atrazine to cladocerans Daphnia magna, Ceriodaphnia silvestrii and Macrothrix flabelligera [J]. Acta Limnologica Brasiliensia, 2014, 26(1): 1-8
[37] Sengupta N. The HR96 activator, atrazine, reduces sensitivity of D. magna to triclosan and DHA [J]. Chemosphere, 2015, 128: 299-306
[38] Macek K J, Buxton K S, Sauter S, et al. Chronic toxicity of atrazine to selected aquatic invertebrates and fishes [R]. Washington DC: United States Environmental Protection Agency, 1976
[39] Marchini S, Passerini L, Cesareo D, et al. Herbicidal triazines: Acute toxicity on Daphnia, fish, and plants and analysis of its relationships with structural factors [J]. Ecotoxicology and Environmental Safety, 1988, 16(2): 148-157
[40] Johnson I C, Keller A E, Zam S G. Method for conducting acute toxicity tests with the early life stages of freshwater mussels [J]. ASTM Special Technical Publication, 1993, 1(1): 381-396
[41] He H Z, Yu J, Chen G K, et al. Acute toxicity of butachlor and atrazine to freshwater green alga Scenedesmus obliquus and cladoceran Daphnia carinata [J]. Ecotoxicology and Environmental Safety, 2012, 80: 91-96
[42] Phyu Y L, Warne M S J, Lim R P. Toxicity of atrazine and molinate to the cladoceran Daphnia carinata and the effect of river water and bottom sediment on their bioavailability [J]. Archives of Environmental Contamination and Toxicology, 2004, 46(3): 308-315
[43] Larras F, Keck F, Montuelle B, et al. Linking diatom sensitivity to herbicides to phylogeny: A step forward for biomonitoring? [J]. Environmental Science & Technology, 2014, 48(3): 1921-1930
[44] Larras F, Montuelle B, Bouchez A. Assessment of toxicity thresholds in aquatic environments: Does benthic growth of diatoms affect their exposure and sensitivity to herbicides? [J]. The Science of the Total Environment, 2013, 463-464: 469-477
[45] Seguin F, Leboulanger C, Rimet F, et al. Effects of atrazine and nicosulfuron on phytoplankton in systems of increasing complexity [J]. Archives of Environmental Contamination and Toxicology, 2001, 40(2): 198-208
[46] Bérard A, Dorigo U, Mercier I, et al. Comparison of the ecotoxicological impact of the triazines Irgarol 1051 and atrazine on microalgal cultures and natural microalgal communities in Lake Geneva [J]. Chemosphere, 2003, 53(8): 935-944
[47] Larras F, Bouchez A, Rimet F, et al. Using bioassays and species sensitivity distributions to assess herbicide toxicity towards benthic diatoms [J]. PLoS One, 2012, 7(8): e44458
[48] Chamsi O, Pinelli E, Faucon B, et al. Effects of herbicide mixtures on freshwater microalgae with the potential effect of a safener [J]. Annales de Limnologie - International Journal of Limnology, 2019, 55: 3
[49] Schäfer H, Wenzel A, Fritsche U, et al. Long-term effects of selected xenobiotica on freshwater green algae: Development of a flow-through test system [J]. Science of the Total Environment, 1993, 134: 735-740
[50] Schäfer H, Hettler H, Fritsche U, et al. Biotests using unicellular algae and ciliates for predicting long-term effects of toxicants [J]. Ecotoxicology and Environmental Safety, 1994, 27(1): 64-81
[51] Fernández-Naveira A, Rioboo C, Cid A, et al. Atrazine induced changes in elemental and biochemical composition and nitrate reductase activity in Chlamydomonas reinhardtii [J]. European Journal of Phycology, 2016, 51(3): 338-345
[52] Shitanda I, Takada K, Sakai Y, et al. Compact amperometric algal biosensors for the evaluation of water toxicity [J]. Analytica Chimica Acta, 2005, 530(2): 191-197
[53] Fairchild J F, Ruessler D S, Carlson A R. Comparative sensitivity of five species of macrophytes and six species of algae to atrazine, metribuzin, alachlor, and metolachlor [J]. Environmental Toxicology and Chemistry, 1998, 17(9): 1830-1834
[54] Ma J Y, Xu L, Wang S, et al. Toxicity of 40 herbicides to the green alga Chlorella vulgaris [J]. Ecotoxicology and Environmental Safety, 2002, 51(2): 128-132
[55] Khan A, Shah N, Muhammad M, et al. Quantitative determination of lethal concentration LC50 of atrazine on biochemical parameters; total protein and serum albumin of freshwater fish grass carp (Ctenopharyngodon idella) [J]. Polish Journal of Environmental Studies, 2016, 25(4): 1555-1561
[56] Brüggemann R. Applying Hasse diagram technique for the evaluation of toxicological fish tests [J]. Chemosphere, 1995, 30(9): 1767-1780
[57] 陈丽红, 张瑜, 丁婷婷, 等. 红霉素水生生物基准推导和对中国部分水体生态风险初步评估[J]. 生态环境学报, 2020, 29(8): 1610-1616
Chen L H, Zhang Y, Ding T T, et al. Development of aquatic life criteria for erythromycin and preliminary assessment for the ecological risk of some water bodies in China [J]. Ecology and Environmental Sciences, 2020, 29(8): 1610-1616 (in Chinese)
[58] 陈莉, 蔡文倩, 韩雪萌, 等. 镉对渤海本地种的急性毒性效应及其海水水质基准推导[J]. 中国海洋大学学报(自然科学版), 2021, 51(9): 93-102
Chen L, Cai W Q, Han X M, et al. Acute effect of cadmium on native species and seawater quality criteria derivation in the Bohai Sea [J]. Periodical of Ocean University of China, 2021, 51(9): 93-102 (in Chinese)