-
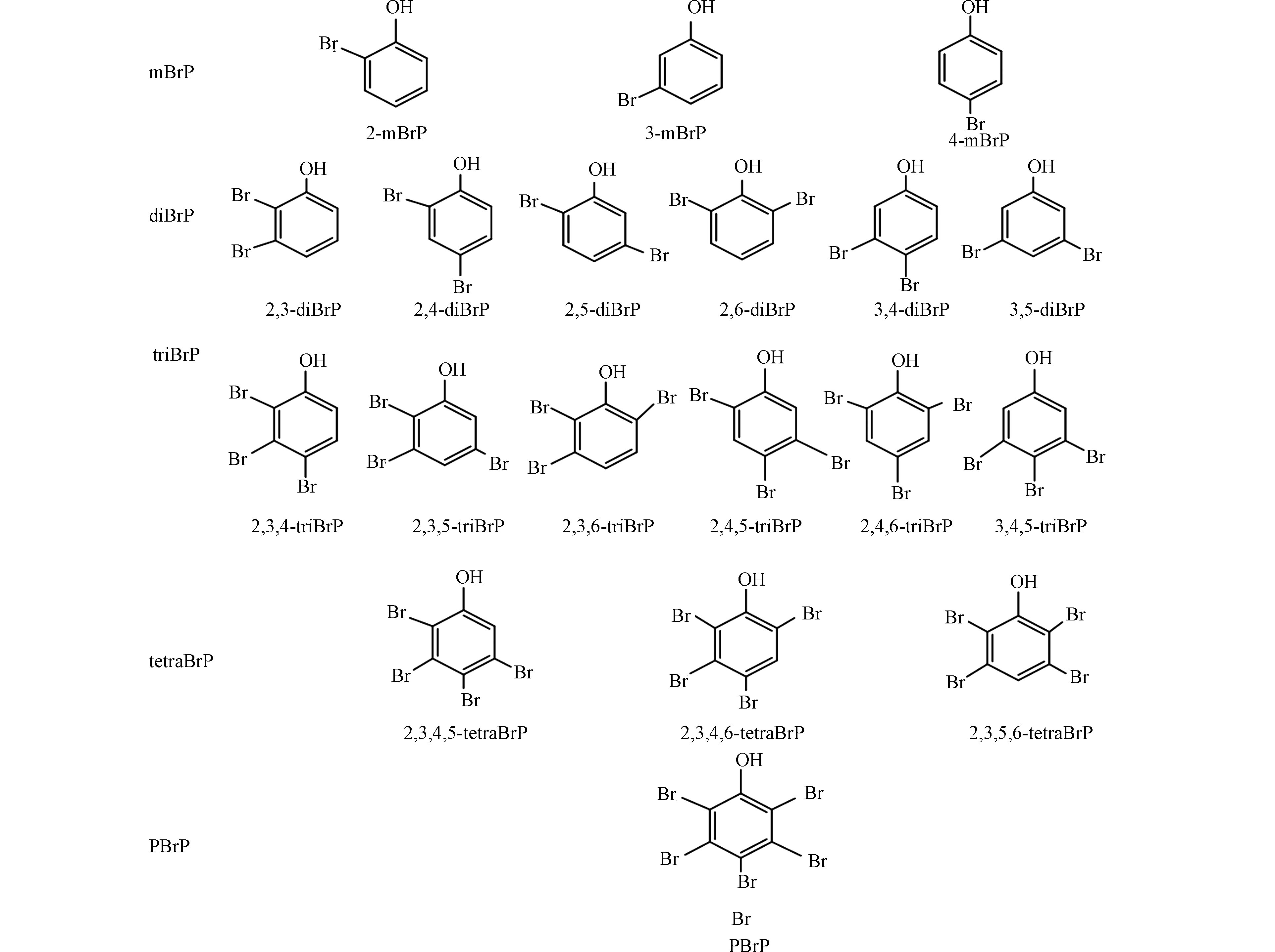
溴酚类化合物(bromophenols,BrPs)不仅具有人为来源,被用作阻燃剂、木材防腐剂等,也具有海洋藻类合成等天然来源,是重要的海洋风味物质[1-2]. 根据苯环上溴原子的取代数目和位置不同,BrPs有19种化合物(图1),在大气、水、土壤、油松树皮及海洋生物等环境样本中均有检出[3-6],甚至在血液和脐带血等人体样本中也有检出[7],电子厂工人血清中检出的BrPs浓度为360 pg·g−1 ww (湿重) [8]. 2,4,6-三溴酚 (2,4,6-bromophenol,2,4,6-triBrP)和五溴酚(pentabromophenol,pBrP)不仅能破坏生物体内甲状腺激素的平衡,也具有显著的抗雌激素效应[9-10]. 因此,BrPs逐渐引起学者们的广泛关注.
海洋中的螺类、贝类和鱼类等动物经摄食藻类可以累积BrPs,经转化等途径也可以将一些人为污染物(如多溴代二苯并二噁英及多溴代二苯并呋喃等)转化为BrPs[11]. 海产品在居民(特别是沿海居民)的膳食结构中占有重要地位,随着人们对健康生活的需求,海产品在膳食中所占的比重呈现显著增加趋势,因此关注海产品质量安全极为必要. 已有研究发现,中国香港市售不同种类海产品中BrPs的含量和分布存在差异[12],但我国其他城市市售海产品中BrPs的赋存情况,特别是海产品中常食用的部位(如贝肉、鱼肉)中BrPs的赋存尚不清晰. 因此,本研究选取9种居民喜食且消费量大的海产品,开展江苏省连云港市海产品中19种BrPs的组织分布及种间差异的研究,为BrPs的生态健康风险和食品安全提供数据支撑.
-
于2021年7月在江苏省连云港市某海鲜市场采集了人们广泛食用、销售量较大的一些海产品,包括双壳类软体动物(牡蛎、紫贻贝和扇贝)、螺类(脉红螺、扁玉螺和花螺)、和鱼类(海鲈鱼、小黄花鱼和金鲳鱼)共9种捕捞的野生海产品. 每种海产品均采集个体大小相近的新鲜样品,在冷藏条件下运回实验室. 贝类和螺类用去离子水清洗后分离去壳,用解剖刀和镊子将每个双壳贝类个体的鳃、外套膜和肉(包含闭壳肌)部分分离,用吸水纸吸干组织表面水分. 由于贝类除鳃和外套膜以外的其他组织以大量贝肉和少量内脏为主,无法将内脏清晰分离,且人们食用这几种贝类通常是净化处理后将贝肉(包括闭壳肌)和内脏团一起食用,因此将内脏合并到贝肉中进行分析和讨论. 螺类样品则分离为螺肉和内脏部分. 为保证足够的样品量并避免个体差异的影响,每种贝与螺的每种组织都由20—35只个体的样品混合而成. 鱼类样品则被分离为鳃、肉和内脏(所有内脏混合在一起)部分,每种组织的样品均由3—4条鱼体的组织混合制备. 每种生物组织的混合样品均进行了准确的质量称量,精确到0.01 g. 随后将所有样品冷冻干燥后用小型粉碎机将其研磨为粉末状固体,密封在棕色玻璃瓶中,置于-20 ℃冰箱中. 海产品个体的干重、湿重等信息详见表1.
-
19种BrPs标准物质,包括2-一溴酚(2-monobromophenol, 2-mBrP)、3-一溴酚(3-bromophenol, 3-mBrP)、4-一溴酚(4-bromophenol, 4-mBrP)、2,3-二溴酚(2,3-dibromophenol, 2,3-diBrP)、2,4-二溴酚(2,4-dibromophenol, 2,4-diBrP)、2,5-二溴酚(2,5-dibromophenol, 2,5-diBrP)、2,6-二溴酚(2,6-dibromophenol, 2,6-diBrP)、3,4-二溴酚(3,4-dibromophenol, 3,4-diBrP)、3,5-二溴酚(3,5-dibromophenol, 3,5-diBrP)、2,3,4-三溴酚(2,3,4-tribromophenol, 2,3,4-triBrP)、2,3,5-三溴酚(2,3,5-tribromophenol, 2,3,5- triBrP)、2,3,6-三溴酚(2,3,6-tribromophenol, 2,3,6-triBrP)、2,4,5-三溴酚(2,4,5-tribromophenol, 2,4,5-triBrP)、2,4,6-triBrP、3,4,5-三溴酚(3,4,5-tribromophenol, 3,4,5-triBrP)、2,3,4,5-四溴酚(2,3,4,5-tetrabromophenol, 2,3,4,5-tetraBrP)、2,3,4,6-四溴酚(2,3,4,6-tetrabromophenol, 2,3,4,6-tetraBrP)、2,4,5,6-四溴酚(2,4,5,6-tetrabromophenol, 2,4,5,6-tetraBrP)、pBrP,均购自加拿大Wellington Laboratories. 同位素内标物质13C6-4-mBrP、13C6-2,4-diBrP、13C6-2,4,6-triBrP、13C6-2,3,4,6-tetraBrP 和13C6-pBrP购自美国Cambridge Isotope Laboratories. 以上标准物质纯度均大于95%. 所有标准溶液均保存于棕色毛细管瓶,并放于4℃冰箱中保存. 色谱级甲醇、乙腈、二氯甲烷等有机溶剂均购自J.T. Baker公司. Poly-Sery WAX(500 mg/6 mL, CNW Technologies GmbH) 和醋酸铵购自中国上海安谱实验科技股份有限公司,盐酸购自国药集团化学试剂有限公司.
-
前处理方法参照已有文献[13],并依据样品性质进行了微调. 准确称量2.00 g样品置于50 mL离心管中,加入20 mL乙腈:二氯甲烷溶液(1:1, 体积比)和20 μL同位素混合内标(13C6-4-mBrP、13C6-2,4-diBrP、13C6-2,4,6-triBrP,浓度分别为(500 ng·mL−1). 分别经超声(53 kHz,20 min)和振荡 (275 次·min−1,20 min)顺序萃取,以3500 r·min−1速度离心10 min取其上清液. 重复萃取3次,合并的萃取液氮吹至近干,用甲醇复溶后过0.22 μm聚四氟乙烯(PTFE)滤膜,随后用盐酸调节样品pH值至2.0±0.01. 随后用Poly-Sery WAX固相萃取柱(500 mg/6 mL)进行浓缩和净化,萃取柱先用6 mL甲醇和6 mL超纯水预处理,上样后用3 mL超纯水洗去杂质. 再用15 mL甲醇进行洗脱,收集洗脱液置于棕色小瓶中,经氮吹定容至500 μL.
-
本研究采用高效液相色谱-三重四极杆串联质谱仪(HPLC-MS/MS)(Ultimate 3000, Thermo Fisher Science, U.S.;Triple-Quad 5500, AB SCIEX, U.S.)检测19种BrPs.
液相色谱条件:色谱分离柱为 Inertsil ODS-4(150 mm×3.0 mm×2 μm, GL Science, Japan),进样量为 5 μL,柱温控制在40 ℃,流动相为含1 mmol·L−1 醋酸铵的水(A)和含0.1%乙酸的乙腈(B)的混合溶剂,流速为0.3 mL·min−1. 流动相梯度为:0 min,45%B/55%A;15 min,70%B/30%A;20—23 min,80%B/20%A;27 min,60%B/40%A;27.5—30 min,45%B/55%A. 质谱条件:在负离子多反应监测模式下对BrPs进行检测,离子源为电喷雾离子源,温度为500℃,离子化电压为-4500 V,气帘气和喷雾气的流速分别设定为38 psi和50 psi. 不同种BrPs检测的母离子、定量离子、碰撞能和去簇电压见表2.
-
基于海产品不同组织混合样品的质量与对应组织中BrPs浓度,计算得到每个海产品中不同种类BrPs及∑4BrPs的个体平均含量,公式如下:
其中,m1、m2、m3、…分别代表不同组织混合样品的质量(g),C1、C2、C3、…分别代表对应的不同组织混合样品中BrPs含量(ng·g−1 dw).
-
每10个样品分析一组9点标准曲线(0.1、 0.2、0.5、1.0、2.0、5.0、10.0、20.0、50.0 ng·mL−1,R2>0.995). 用2.00 g色谱纯硅藻土作为空白样品进行相同的全流程提取分析,在程序空白和仪器空白实验中均未检出BrPs. 每个样品均加入10.0 ng 同位素内标物质(20 μL, 500 ng·mL−1),以校正和消除样品前处理及仪器波动的影响. 除2,3,4,5-tetraBrPs回收率为58.8%外,海产品中其他18种BrPs回收率范围为77.1%—105%. BrPs的浓度值以干重计量,方法检出限(3倍噪声)范围为0.0248—32.1 ng·g−1 dw. 为了便于与文献数据进行对比,文献中BrPs含量如果以湿重或脂重计,则通过文献中给出的含水率(若文献中无含水率数据则按照贝、螺、鱼的含水率(80%)计算)或脂肪含量推算为以干重计的BrPs含量.
-
在采集的螺、贝和海鱼样品中,共检出了4种BrPs,分别为4-mBrP、2,4-diBrP、2,6-diBrP和2,4,6-triBrP,在所有海产品各部位样品中的检出率分别为87.5%、54.2%、50.0%和100%,其他15种BrPs在海产品中均未检出. 有研究表明[12, 14],4-mBrP、2,6-diBrP、2,4-diBrP和2,4,6-triBrP是藻类、鱼类、软体动物、甲壳类动物等海洋生物中广泛检出的BrPs,其中以2,4,6-triBrP占比最高,与本研究结果一致. 在多个海产品相关的研究中还检出了2-mBrP、2,3,4-triBrP、2,3,5-triBrP、2,3,6-triBrP、2,4,5-triBrP、2,4,6-triBrP、3,4,5-triBrP、2,3,4,5-tetraBrP、2,3,4,6-tetraBrP、2,3,5,6-tetraBrP、pBrP等其他多种BrPs[15-17],但这些BrPs在本研究中均未检出,这可能与不同种BrPs的来源、环境暴露浓度、累积特性以及海产品种类有关.
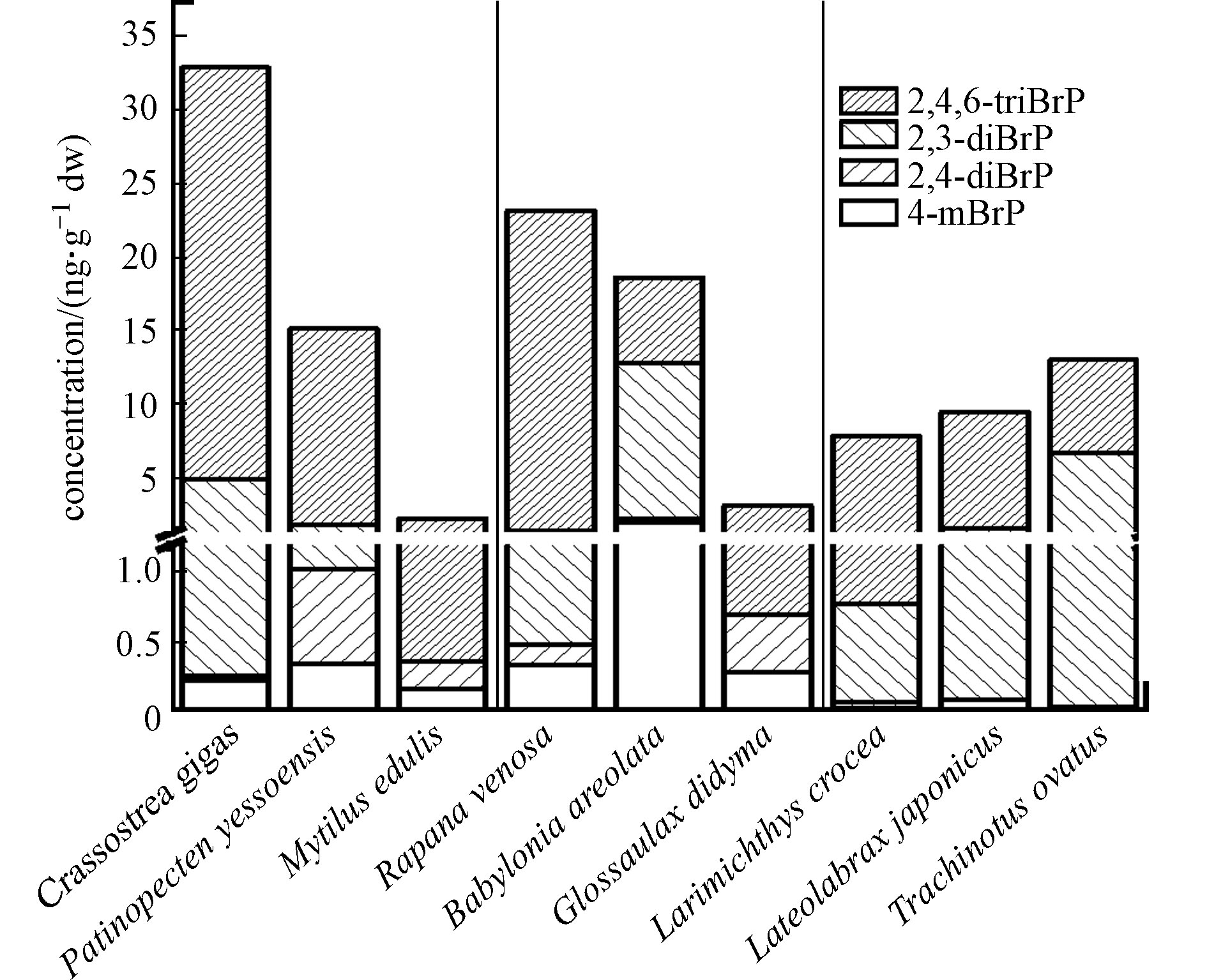
经海产品个体中BrPs含量推算,牡蛎、扇贝、紫贻贝、脉红螺、花螺、扁玉螺、小黄花鱼、海鲈鱼、金鲳鱼中∑4BrPs个体平均含量分别为33.0、15.2、2.25、23.2、18.7、3.11、7.85、9.52、13.0 ng·g−1 dw(图2). 比较不同海产品的含量发现,3种贝类中∑4BrPs、2,4-diBrP、2,4,6-triBrP个体平均含量((16.8±12.6)、(0.304±0.272)、(14.4±10.7) ng·g−1 dw)高于3种螺类((15.0±8.60)、(0.284±0.112)、(10.0±8.43) ng·g−1 dw)和鱼类((10.1±2.17)、(0.0136±0.0193)、(7.10±0.654) ng·g−1 dw),4-mBrP和2,6-diBrP则在螺类((0.863±0.785) ng·g−1 dw和(3.82±4.76) ng·g−1 dw)中高于贝类和鱼类. BrPs在物种间的差异,与生物的习性和生活环境有关,在韩国东南沿海、中国北江等区域的研究显示,BrPs在海洋沉积物中的浓度高于海水[5, 18],因此,贝类样品,特别是牡蛎和扇贝中某些BrPs单体的含量远高于螺类和鱼类,可能与其长期在沉积物中的底栖生活习性相关.
在3种贝类中,牡蛎的∑4BrPs、2,6-diBrP、2,4,6-triBrP个体平均含量最高(33.0、4.70、28.0 ng·g−1 dw),接下来是扇贝(15.2、0.774、13.4 ng·g−1 dw),二者均远高于紫贻贝(2.24、<MDL、1.88 ng·g−1 dw). 扇贝的4-mBrP和2,4-diBrP含量(0.343、0.677 ng·g−1 dw)高于牡蛎(0.221、0.0352 ng·g−1 dw)和紫贻贝(0.161、0.200 ng·g−1 dw). 本研究牡蛎中的溴酚单体4-mBrP、2,4,6-triBrP的个体平均含量高于中国香港地区牡蛎中对应BrPs的含量(<MDL、平均值(8.98±6.82) ng·g−1 dw,范围1.37–18.1 ng·g−1 dw)[12]和美国俄勒冈州州立大学海洋研究站获取的两种牡蛎样品中对应BrPs含量(<MDL、10.0、6.00 ng·g−1 dw)[19];2,6-diBrP处在中等水平,高于中国香港地区牡蛎(平均值(0.858±0.616) ng·g−1 dw,范围0.345—2.18 ng·g−1 dw)[12]低于美国俄勒冈州州立大学海洋研究站的两种牡蛎(含量5.00 ng·g−1 dw和6.00 ng·g−1 dw)[19];但溴酚单体2,4-diBrP的含量远低于中国香港牡蛎(平均值(30.6±18.5) ng·g−1 dw,范围9.8—52.9 ng·g−1 dw)[12]和在美国俄勒冈州的牡蛎(6.00 ng·g−1 dw和6.00 ng·g−1 dw)[19]. 本研究3种贝类中2,4,6-triBrP的个体含量的平均值((14.4±10.7) ng·g−1 dw)远高于在欧洲意大利、丹麦、法国、爱尔兰、西班牙等国家市场上采集的软体动物/甲壳类动物中2,4,6-triBrP的含量(范围0.316—3.73 ng·g−1 dw,数据经文献中脂肪含量换算)[20].
在3种螺类中,∑4BrPs和2,4,6-triBrP的含量按照脉红螺(23.2 ng·g−1 dw和21.8 ng·g−1 dw)、花螺(18.7 ng·g−1 dw和5.85 ng·g−1 dw)、扁玉螺(3.11 ng·g−1 dw和2.41 ng·g−1 dw)依次递减. 花螺中4-mBrP和2,6-diBrP含量(1.97 ng·g−1 dw和10.5 ng·g−1 dw)高于脉红螺(0.337 ng·g−1 dw和0.935 ng·g−1 dw)、扁玉螺(0.278 ng·g−1 dw和<MDL). 与另外3种BrPs不同,2,4-diBrP在扁玉螺中最高为0.419 ng·g−1 dw,接下来依次为花螺(0.288 ng·g−1 dw)和脉红螺(0.144 ng·g−1 dw).
对于3种鱼类,在金鲳鱼中∑4BrPs、2,6-diBrP含量(13.0、6.70 ng·g−1 dw)最高,接下来依次为海鲈鱼(9.52、1.51 ng·g−1 dw)和小黄花鱼(7.85、0.713 ng·g−1 dw). 海鲈鱼中2,4,6-triBrP和4-mBrP含量(7.91 ng·g−1 dw和0.0854 ng·g−1 dw)高于小黄花鱼(7.07 ng·g−1 dw和0.0244 ng·g−1 dw)和金鲳鱼(6.31 ng·g−1 dw和0.0332 ng·g−1 dw). 金鲳鱼和小黄花鱼均为咸水鱼,只能在海洋中生活,而海鲈鱼在海水与淡水中均能生活,因此海鲈鱼还有可能受到淡水生活环境的影响,而2,4,6-triBrP作为人为生产的溴代阻燃剂,往往对流经城市和人类生活区的淡水水体影响更大,这也可能是海鲈鱼体内2,4,6-triBrP含量高的原因.
-
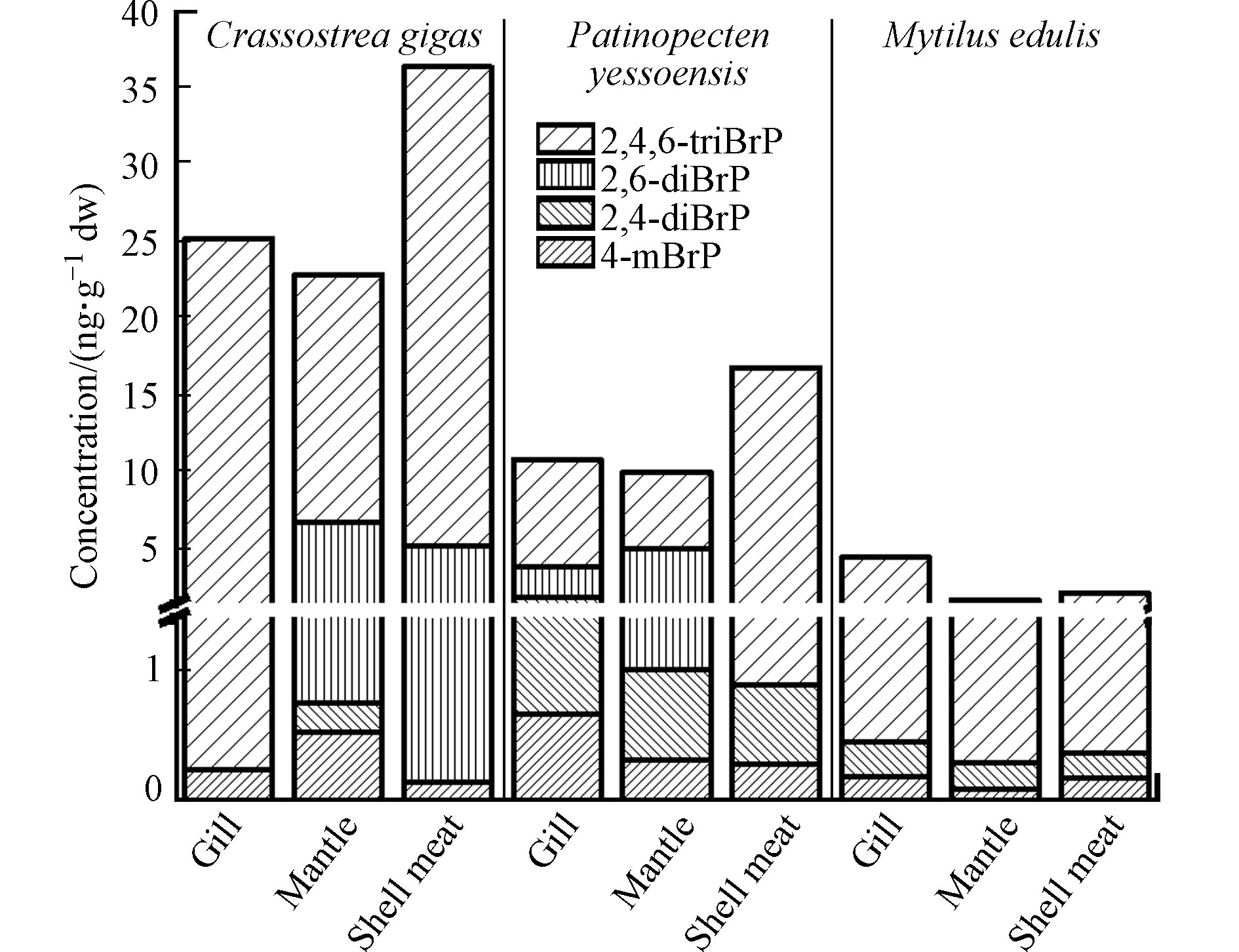
对于紫贻贝,除2,6-diBrP未检出外,检出的BrPs(4-mBrP、2,4-diBrP、2,4,6-triBrP)及∑4BrPs(0.197、0.262、4.00、4.46 ng·g−1 dw)均主要累积在鳃内(图3),高于贝肉(0.187、0.188、1.81、2.19 ng·g−1 dw)和外套膜(0.102、0.204、1.42、1.73 ng·g−1 dw)中对应BrPs的含量. 扇贝鳃中4-mBrP(0.663 ng·g−1 dw)和2,4-diBrP(1.20 ng·g−1 dw)也高于外套膜(0.326 ng·g−1 dw和0.681 ng·g−1 dw)和贝肉(0.295 ng·g−1 dw和0.594 ng·g−1 dw). 这说明鳃也是贝类BrPs暴露及分布的重要组织. 牡蛎和扇贝的贝肉中2,4,6-triBrP(31.0 ng·g−1 dw和15.9 ng·g−1 dw)、∑4BrPs的含量(36.3 ng·g−1 dw 和16.8 ng·g−1 dw)高于鳃和外套膜. 另外,牡蛎与扇贝中2,6-diBrP在外套膜中(6.04 ng·g−1 dw与4.00 ng·g−1 dw)高于鳃和贝肉中含量. 对3种贝类中BrPs的组织分布进行比较,发现紫贻贝各组织中的∑4BrPs均远低于牡蛎和扇贝,BrPs在3种贝类中的组织分布差异与3种贝类不同的生活方式和生长特性有关,牡蛎、扇贝多生活在潮间带、潮下带、低潮带底泥中,而紫贻贝多生活于浅海区附着在岩礁上,从BrPs浓度相对较低的海水中滤食,使其体内BrPs含量相较牡蛎和扇贝要低. 而紫贻贝的外套膜占个体干重的32.0%,远高于牡蛎(15.7%)和扇贝(13.3%). 紫贻贝、牡蛎、扇贝外套膜中∑4BrPs含量分别占对应贝类个体BrPs含量的24.6%、10.9%、8.79%. 由此可见,不同种贝类间相同生物组织在其体重中的占比也影响了BrPs的浓度分布. 整体上,贝类肉中∑4BrPs((18.4±14.0) ng·g−1 dw)高于鳃中((13.5±8.68) ng·g−1 dw)和外套膜((11.5±8.71) ng·g−1 dw).
-
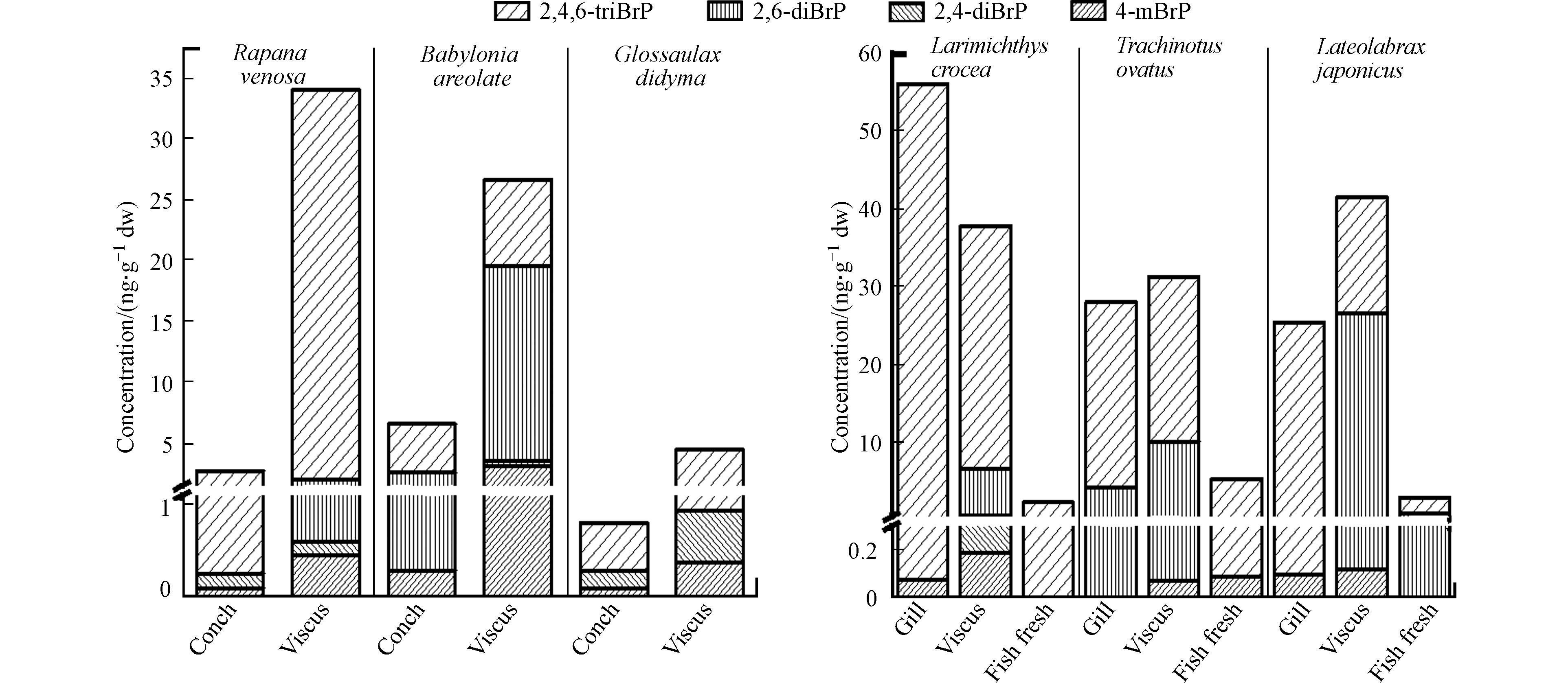
3种螺中各种BrPs及∑4BrPs在内脏中的含量均远高于螺肉中的含量(图4). 3种螺内脏中∑4BrPs平均含量((21.7±12.6) ng·g−1 dw)远高于螺肉((3.37±2.41) ng·g−1 dw). 对于螺肉样品,花螺螺肉中∑4BrPs最高(6.59 ng·g−1 dw)(图4),分别是脉红螺(2.71 ng·g−1 dw)和扁玉螺 (0.806 ng·g−1 dw)的2.43倍和8.18倍. 花螺的螺肉和内脏中4-mBrP、2,6-diBrP、2,4,6-triBrP的含量均高于脉红螺、扁玉螺的对应部位. 2,4-diBrP的最高浓度出现在扁玉螺的螺肉和内脏团中(图4). 3种螺螺肉中∑4BrPs的平均含量((3.37±2.41) ng·g−1 dw,范围0.806—6.59 ng·g−1 dw)远低于3种贝肉的平均含量((18.4±14.0) ng·g−1 dw,范围2.19—36.3 ng·g−1 dw),但花螺和脉红螺螺肉中∑4BrPs的含量则高于紫贻贝贝肉.
-
如图4所示,小黄花鱼、海鲈鱼和金鲳鱼肉中的∑4BrPs含量(2.21、5.29、2.87 ng·g−1 dw)均远低于内脏(37.8、31.3、41.5 ng·g−1 dw)和鳃中的含量(56.1、28.0、25.4 ng·g−1 dw). 检出率最高的2,4,6-triBrP在鱼体中的最高浓度均出现在3种鱼的鱼鳃(56.0、28.0、25.3 ng·g−1 dw)中. 这说明鳃也是鱼类2,4,6-triBrP暴露与分布的重要组织. 4-mBrP和2,6-diBrP在内脏团中的含量均高于鱼肉和鱼鳃中(图4). 其中金鲳鱼内脏中的2,6-diBrP(26.4 ng·g−1 dw)占∑4BrPs(41.5 ng·g−1 dw)的63.6%,超过了检出率最高的2,4,6-triBrP(15.0 ng·g−1 dw,占比36.1%),这一比例也远高于小黄花鱼和海鲈鱼中2,6-diBrP所占比例(15.9%和31.9%)以及文献中报道的鱼类肠胃中2,6-diBrP所占的比例(0—16.9%)[1, 12]. 2,4-diBrP除在小黄花鱼的内脏团中检出(0.344 ng·g−1 dw)外,在小黄花鱼其他组织、海鲈鱼和金鲳鱼的所有组织中均未发现. 3种鱼中检测到的BrPs的∑4BrPs平均含量在内脏中含量最高为(36.9±4.22) ng·g−1 dw,高于鳃((36.5±13.9) ng·g−1 dw)和鱼肉((3.46±1.32) ng·g−1 dw),与其他研究中发现的内脏含量高于肉中含量的现象是一致的.
本研究的海鲈鱼鱼肉中以2,4,6-triBrP为主要BrPs单体,与文献中同种鱼的检测结果一致,但2,4,6-triBrP的含量(5.20 ng·g−1 dw)高于澳大利亚市场上采集的野生与养殖尖吻鲈鱼鱼肉中的含量(平均值(0.0857±0.0481) ng·g−1 dw,范围<MDL—0.800 ng·g−1 dw,经含水率80%计算)[21],低于在瑞典斯德哥尔摩群岛的Nämdö岛周围采集的鲈鱼鱼肉的含量(平均值(6.59±3.15) ng·g−1 dw,范围:(1.45—10.0) ng·g−1 dw,经含水率80%换算)[22];4-mBrP含量((0.0900) ng·g−1 dw)低于澳大利亚尖吻养殖鲈鱼鱼肉中的含量((0.250) ng·g−1 dw,以鱼肉含水率80%计干重)[21]. 本研究海鲈鱼鱼肉中2,4-diBrP和2,6-diBrP均未检出,但在澳大利亚尖吻野生鲈鱼中均有检出.
与其他种类的鱼相比,本研究3种鱼鱼肉中2,4,6-triBrP含量平均值((3.17±1.44) ng·g−1 dw)低于在中国香港市场上采集的不同季节的河豚与褐斑石斑鱼的鱼肉(平均值(15.6±9.91) ng·g−1 dw,范围(2.43–39.2) ng·g−1 dw)[12]和从美国阿拉斯加、密歇根湖、威斯康星州等地采集的鲑鱼、鲱鱼等鱼肉中2,4,6-triBrP的含量((18.5—166) ng·g−1 dw)[19]. 本研究3种鱼内脏中2,4,6-triBrP含量平均值((22.5±6.72) ng·g−1 dw)也均低于在中国香港的河豚与褐斑石斑鱼(平均值(49.1±50.2) ng·g−1 dw,范围(2.18—155) ng·g−1 dw)[12]和澳大利亚新南威尔士州采集的多种底栖食肉性与杂食性鱼类(平均值(203±264) ng·g−1 dw,范围<MDL—850 ng·g−1 dw,按照含水率80%计算)[23] 内脏中2,4,6-triBrP的含量. 本研究3种鱼类的鱼肉及内脏中BrPs含量低于其他研究1—2个数量级,整体上处在较低水平.
-
在贝类、螺类和鱼类的鳃、外套膜、内脏、肉等不同组织中共检出4种BrPs,4-mBrP、2,4-diBrP、2,6-diBrP、2,4,6-triBrP,其中2,4,6-triBrP含量水平和检出率均相对较高,在9种海产品所有组织中的浓度范围为0.512—56.0(平均值:(14.1±13.8) ng·g−1 dw),∑4BrPs的范围为0.806—56.1(平均值:(18.2±15.5) ng·g−1 dw). 3种贝类中∑4BrPs个体平均含量高于3种螺类和鱼类. 3种贝类鳃中∑4BrPs高于外套膜和贝肉. 3种螺的内脏和鳃中∑4BrPs含量平均值远高于螺肉中的含量. 3种鱼内脏中的∑4BrPs含量平均值远高于鳃和鱼肉. 研究证实了BrPs在不同海产品内脏中的高累积,另外鳃也是海产品BrPs暴露及分布的重要组织.
不同种类海产品中溴酚的含量及组织分布
Concentrations and tissue distributions of bromophenols in nine different seafood
-
摘要: 溴酚类化合物(bromophenols,BrPs)可以在藻类等海洋生物中自然生成,是海洋风味物质,在海洋生物中广泛检出.同时,一些BrPs也是广为应用的溴代阻燃剂或溴代阻燃剂的合成前体化合物,不仅具有生物毒性且能与多溴二苯醚、四溴双酚A、甚至多溴代二苯并二噁英及多溴代二苯并呋喃等强毒性有机污染物发生相互转化.本研究利用HPLC-MS/MS分析了我国江苏省连云港市市场上销量较高的贝类、螺类和鱼类(各3种)中,19种BrPs的含量及组织分布.结果可见,样品中共检出4种BrPs,分别是4-一溴酚(4-monobromophenol,4-mBrP)、2,4-二溴酚(2,4-dibromophenol,2,4-diBrP)、2,6-二溴酚(2,6-dibromophenol,2,6-diBrP)和2,4,6-三溴酚(2,4,6-tribromophenol,2,4,6-triBrP),其中2,4,6-triBrP含量水平和检出率最高,在9种海产品不同组织中的浓度范围为0.512—56.0 ng·g−1 dw (干重)(平均值(14.1±13.8) ng·g−1 dw). 生物样本中,∑4BrPs的范围为0.806—56.1 ng·g−1 dw(平均值(18.2±15.5) ng·g−1 dw).海产品中BrPs的含量具有组织分布差异性和物种差异性,其中,3种贝类个体中∑4BrPs平均含量((16.8±12.6) ng·g−1 dw)高于3种螺类((15.0±8.60) ng·g−1 dw)和鱼类((10.1±2.17) ng·g−1 dw).不同种类海产品中∑4BrPs的平均含量显示,贝类肉中含量((18.4±14.0) ng·g−1 dw)高于鳃((13.5±8.68) ng·g−1 dw)和外套膜((11.5±8.71) ng·g−1 dw),螺的内脏含量((21.7±12.6) ng·g−1 dw)远高于螺肉((3.37±2.41) ng·g−1 dw),鱼内脏和鳃中∑4BrPs平均含量((36.9±4.22)和(36.5±13.9)ng·g−1 dw)远高于鱼肉((3.46±1.32) ng·g−1 dw).Abstract: As the seafood flavor components, bromophenols (BrPs) are naturally produced and widely found in marine organisms (e.g. algae). Some of the BrP congeners are also the widely used as flame retardants or the precursors of some flame retardants. BrPs not only have relatively high biological toxicity but also transformed reciprocally with some highly toxic organic pollutant (e.g. polybrominated diphenyl ethers, tetrabromobisphenol A, polybrominated dibenzo-p-dioxins and polybrominated dibenzofurans). The concentrations and tissue distributions of 19 BrPs in 9 seafood (i.e. the shellfish, snail, and fish samples) with the high sales in Chinese market were explored by HPLC-MS/MS in this study. Concentrations and tissue distributions of BrPs in different seafood were specially focused. Four BrPs, including 4-monobromophenol (4-mBrP), 2,4-dibromophenol (2,4-diBrP), 2,6-dibromophenol (2,6-diBrP), and 2,4,6-tribromophenol (2,4,6-triBrP), were found in different tissues of 9 seafood and ∑4BrPs ranged from 0.806 to 56.1 ng·g−1 dw with mean concentration of (18.2±15.5) ng·g−1 dw. The concentration and detection frequency of 2,4,6-triBrP was highest with the concentration ranging from 0.512 to 56.0 ng·g−1 dw (Mean: (14.1±13.8) ng·g−1 dw). Species dependent accumulation and tissues dependent distribution of BrPs were found in seafood. The mean concentration in whole shellfish ((16.8±12.6) ng·g−1 dw) was higher than that in snails ((15.0±8.60) ng·g−1 dw) and fish ((10.1±2.17) ng·g−1 dw). ∑4BrPs in the shell meat ((18.4±14.0) ng·g−1 dw) was higher than that in gill ((13.5±8.68) ng·g−1 dw) and mantle ((11.5±8.71) ng·g−1 dw). For three snails, ∑4BrPs in viscus ((21.7±12.6) ng·g−1 dw) was higher than that in conch ((3.37±2.41) ng·g−1 dw). For three fish, ∑4BrPs in viscus ((36.9±4.22) ng·g−1 dw) and gill ((36.5±13.9) ng·g−1 dw) was higher than that in flesh ((3.46±1.32) ng·g−1 dw).
-
Key words:
- bromophenols /
- tissue distribution /
- seafood.
-
溴酚类化合物(bromophenols,BrPs)不仅具有人为来源,被用作阻燃剂、木材防腐剂等,也具有海洋藻类合成等天然来源,是重要的海洋风味物质[1-2]. 根据苯环上溴原子的取代数目和位置不同,BrPs有19种化合物(图1),在大气、水、土壤、油松树皮及海洋生物等环境样本中均有检出[3-6],甚至在血液和脐带血等人体样本中也有检出[7],电子厂工人血清中检出的BrPs浓度为360 pg·g−1 ww (湿重) [8]. 2,4,6-三溴酚 (2,4,6-bromophenol,2,4,6-triBrP)和五溴酚(pentabromophenol,pBrP)不仅能破坏生物体内甲状腺激素的平衡,也具有显著的抗雌激素效应[9-10]. 因此,BrPs逐渐引起学者们的广泛关注.
海洋中的螺类、贝类和鱼类等动物经摄食藻类可以累积BrPs,经转化等途径也可以将一些人为污染物(如多溴代二苯并二噁英及多溴代二苯并呋喃等)转化为BrPs[11]. 海产品在居民(特别是沿海居民)的膳食结构中占有重要地位,随着人们对健康生活的需求,海产品在膳食中所占的比重呈现显著增加趋势,因此关注海产品质量安全极为必要. 已有研究发现,中国香港市售不同种类海产品中BrPs的含量和分布存在差异[12],但我国其他城市市售海产品中BrPs的赋存情况,特别是海产品中常食用的部位(如贝肉、鱼肉)中BrPs的赋存尚不清晰. 因此,本研究选取9种居民喜食且消费量大的海产品,开展江苏省连云港市海产品中19种BrPs的组织分布及种间差异的研究,为BrPs的生态健康风险和食品安全提供数据支撑.
1. 材料与方法 (Material and methods)
1.1 样品采集和制备
于2021年7月在江苏省连云港市某海鲜市场采集了人们广泛食用、销售量较大的一些海产品,包括双壳类软体动物(牡蛎、紫贻贝和扇贝)、螺类(脉红螺、扁玉螺和花螺)、和鱼类(海鲈鱼、小黄花鱼和金鲳鱼)共9种捕捞的野生海产品. 每种海产品均采集个体大小相近的新鲜样品,在冷藏条件下运回实验室. 贝类和螺类用去离子水清洗后分离去壳,用解剖刀和镊子将每个双壳贝类个体的鳃、外套膜和肉(包含闭壳肌)部分分离,用吸水纸吸干组织表面水分. 由于贝类除鳃和外套膜以外的其他组织以大量贝肉和少量内脏为主,无法将内脏清晰分离,且人们食用这几种贝类通常是净化处理后将贝肉(包括闭壳肌)和内脏团一起食用,因此将内脏合并到贝肉中进行分析和讨论. 螺类样品则分离为螺肉和内脏部分. 为保证足够的样品量并避免个体差异的影响,每种贝与螺的每种组织都由20—35只个体的样品混合而成. 鱼类样品则被分离为鳃、肉和内脏(所有内脏混合在一起)部分,每种组织的样品均由3—4条鱼体的组织混合制备. 每种生物组织的混合样品均进行了准确的质量称量,精确到0.01 g. 随后将所有样品冷冻干燥后用小型粉碎机将其研磨为粉末状固体,密封在棕色玻璃瓶中,置于-20 ℃冰箱中. 海产品个体的干重、湿重等信息详见表1.
表 1 9种海产品的样本数量、组织重量以及含水率.Table 1. Numbers, weights, and the moisture content for the 9 tested seafood samples.种类Species 拉丁名Latin name 数量Quantities 部位Tissues 湿重/gWet weight 干重/gDry weight 含水率/%Moisture content 牡蛎 Crassostrea gigas n=20 鳃 17.0 3.09 81.8 外套膜 20.6 4.49 78.2 肉 91.0 21.0 76.9 扇贝 Patinopecten yessoensis n=30 鳃 23.9 5.96 75.1 外套膜 25.3 6.72 73.4 肉 157 37.7 75.9 紫贻贝 Mytilus edulis n=35 鳃 17.4 3.42 80.3 外套膜 43.8 12.0 72.6 肉 105 22.1 79.0 脉红螺 Rapana venosa n=20 内脏 46.5 14.5 68.9 肉 113 27.4 75.7 花螺 Babylonia areolata n=35 内脏 55.7 18.2 67.2 肉 113 27.4 75.7 扁玉螺 Glossaulax didyma n=35 内脏 99.5 32.4 67.4 肉 200 55.0 72.5 小黄花鱼 Larimichthys crocea n=4 鳃 18.5 6.42 65.3 内脏 67.1 29.2 56.6 鱼肉 553 210 61.9 金鲳鱼 Trachinotus ovatus n=3 鳃 17.8 6.08 65.8 内脏 57.3 35.6 37.8 鱼肉 487 210 57.0 海鲈鱼 Lateolabrax japonicus n=3 鳃 38.4 13.1 66.0 内脏 88.8 58.7 33.9 鱼肉 621 180 70.9 注:湿重、干重、含水率均基于n个个体的混合样品计量. Note:The wet weight, dry weight, and the moisture content were based on the mixed samples of individualities. 1.2 试剂材料
19种BrPs标准物质,包括2-一溴酚(2-monobromophenol, 2-mBrP)、3-一溴酚(3-bromophenol, 3-mBrP)、4-一溴酚(4-bromophenol, 4-mBrP)、2,3-二溴酚(2,3-dibromophenol, 2,3-diBrP)、2,4-二溴酚(2,4-dibromophenol, 2,4-diBrP)、2,5-二溴酚(2,5-dibromophenol, 2,5-diBrP)、2,6-二溴酚(2,6-dibromophenol, 2,6-diBrP)、3,4-二溴酚(3,4-dibromophenol, 3,4-diBrP)、3,5-二溴酚(3,5-dibromophenol, 3,5-diBrP)、2,3,4-三溴酚(2,3,4-tribromophenol, 2,3,4-triBrP)、2,3,5-三溴酚(2,3,5-tribromophenol, 2,3,5- triBrP)、2,3,6-三溴酚(2,3,6-tribromophenol, 2,3,6-triBrP)、2,4,5-三溴酚(2,4,5-tribromophenol, 2,4,5-triBrP)、2,4,6-triBrP、3,4,5-三溴酚(3,4,5-tribromophenol, 3,4,5-triBrP)、2,3,4,5-四溴酚(2,3,4,5-tetrabromophenol, 2,3,4,5-tetraBrP)、2,3,4,6-四溴酚(2,3,4,6-tetrabromophenol, 2,3,4,6-tetraBrP)、2,4,5,6-四溴酚(2,4,5,6-tetrabromophenol, 2,4,5,6-tetraBrP)、pBrP,均购自加拿大Wellington Laboratories. 同位素内标物质13C6-4-mBrP、13C6-2,4-diBrP、13C6-2,4,6-triBrP、13C6-2,3,4,6-tetraBrP 和13C6-pBrP购自美国Cambridge Isotope Laboratories. 以上标准物质纯度均大于95%. 所有标准溶液均保存于棕色毛细管瓶,并放于4℃冰箱中保存. 色谱级甲醇、乙腈、二氯甲烷等有机溶剂均购自J.T. Baker公司. Poly-Sery WAX(500 mg/6 mL, CNW Technologies GmbH) 和醋酸铵购自中国上海安谱实验科技股份有限公司,盐酸购自国药集团化学试剂有限公司.
1.3 样品前处理
前处理方法参照已有文献[13],并依据样品性质进行了微调. 准确称量2.00 g样品置于50 mL离心管中,加入20 mL乙腈:二氯甲烷溶液(1:1, 体积比)和20 μL同位素混合内标(13C6-4-mBrP、13C6-2,4-diBrP、13C6-2,4,6-triBrP,浓度分别为(500 ng·mL−1). 分别经超声(53 kHz,20 min)和振荡 (275 次·min−1,20 min)顺序萃取,以3500 r·min−1速度离心10 min取其上清液. 重复萃取3次,合并的萃取液氮吹至近干,用甲醇复溶后过0.22 μm聚四氟乙烯(PTFE)滤膜,随后用盐酸调节样品pH值至2.0±0.01. 随后用Poly-Sery WAX固相萃取柱(500 mg/6 mL)进行浓缩和净化,萃取柱先用6 mL甲醇和6 mL超纯水预处理,上样后用3 mL超纯水洗去杂质. 再用15 mL甲醇进行洗脱,收集洗脱液置于棕色小瓶中,经氮吹定容至500 μL.
1.4 色谱与质谱条件
本研究采用高效液相色谱-三重四极杆串联质谱仪(HPLC-MS/MS)(Ultimate 3000, Thermo Fisher Science, U.S.;Triple-Quad 5500, AB SCIEX, U.S.)检测19种BrPs.
液相色谱条件:色谱分离柱为 Inertsil ODS-4(150 mm×3.0 mm×2 μm, GL Science, Japan),进样量为 5 μL,柱温控制在40 ℃,流动相为含1 mmol·L−1 醋酸铵的水(A)和含0.1%乙酸的乙腈(B)的混合溶剂,流速为0.3 mL·min−1. 流动相梯度为:0 min,45%B/55%A;15 min,70%B/30%A;20—23 min,80%B/20%A;27 min,60%B/40%A;27.5—30 min,45%B/55%A. 质谱条件:在负离子多反应监测模式下对BrPs进行检测,离子源为电喷雾离子源,温度为500℃,离子化电压为-4500 V,气帘气和喷雾气的流速分别设定为38 psi和50 psi. 不同种BrPs检测的母离子、定量离子、碰撞能和去簇电压见表2.
表 2 不同BrPs的母离子、定量离子、碰撞能和去簇电压Table 2. The precursor and quantitative ion, collision energy, and declustering potential for different BrP congeners.化合物Compounds 母离子(m/z)Precursor ion 定量离子(m/z)Quantitative ion 碰撞能/eVCollision energy 去簇电压/eVDeclustering potential mBrPs 170.8 78.8 −22 −85 172.8 80.8 −22 −85 diBrPs 250.8 78.8 −30 −110 80.8 −30 −110 triBrPs 328.8 78.8 −70 −120 80.8 −70 −120 tetraBrPs 408.6 78.8 −85 −130 80.8 −85 −130 pBrP 488.6 78.8 −82 −130 80.8 −82 −130 13C6−4-mBrP 176.8 78.8 −22 −85 178.8 80.8 −22 −85 13C6−2,4-diBrP 256.8 78.8 −30 −110 80.8 −30 −110 13C6−2,4,6-triBrP 334.9 78.8 −70 −120 80.8 −70 −120 13C6−2,3,4,6-tetraBrP 414.6 78.8 −85 −130 80.8 −85 −130 13C6−PBrP 494.6 78.8 −82 −130 80.8 −82 −130 1.5 海产品个体中BrPs平均含量的计算方法
基于海产品不同组织混合样品的质量与对应组织中BrPs浓度,计算得到每个海产品中不同种类BrPs及∑4BrPs的个体平均含量,公式如下:
海产品个体平均含量=m1×C1+m2×C2+m3×C3+…m1+m2+m3… 其中,m1、m2、m3、…分别代表不同组织混合样品的质量(g),C1、C2、C3、…分别代表对应的不同组织混合样品中BrPs含量(ng·g−1 dw).
1.6 质量控制和质量保证
每10个样品分析一组9点标准曲线(0.1、 0.2、0.5、1.0、2.0、5.0、10.0、20.0、50.0 ng·mL−1,R2>0.995). 用2.00 g色谱纯硅藻土作为空白样品进行相同的全流程提取分析,在程序空白和仪器空白实验中均未检出BrPs. 每个样品均加入10.0 ng 同位素内标物质(20 μL, 500 ng·mL−1),以校正和消除样品前处理及仪器波动的影响. 除2,3,4,5-tetraBrPs回收率为58.8%外,海产品中其他18种BrPs回收率范围为77.1%—105%. BrPs的浓度值以干重计量,方法检出限(3倍噪声)范围为0.0248—32.1 ng·g−1 dw. 为了便于与文献数据进行对比,文献中BrPs含量如果以湿重或脂重计,则通过文献中给出的含水率(若文献中无含水率数据则按照贝、螺、鱼的含水率(80%)计算)或脂肪含量推算为以干重计的BrPs含量.
2. 结果与讨论(Results and discussion)
2.1 不同海产品中的BrPs
在采集的螺、贝和海鱼样品中,共检出了4种BrPs,分别为4-mBrP、2,4-diBrP、2,6-diBrP和2,4,6-triBrP,在所有海产品各部位样品中的检出率分别为87.5%、54.2%、50.0%和100%,其他15种BrPs在海产品中均未检出. 有研究表明[12, 14],4-mBrP、2,6-diBrP、2,4-diBrP和2,4,6-triBrP是藻类、鱼类、软体动物、甲壳类动物等海洋生物中广泛检出的BrPs,其中以2,4,6-triBrP占比最高,与本研究结果一致. 在多个海产品相关的研究中还检出了2-mBrP、2,3,4-triBrP、2,3,5-triBrP、2,3,6-triBrP、2,4,5-triBrP、2,4,6-triBrP、3,4,5-triBrP、2,3,4,5-tetraBrP、2,3,4,6-tetraBrP、2,3,5,6-tetraBrP、pBrP等其他多种BrPs[15-17],但这些BrPs在本研究中均未检出,这可能与不同种BrPs的来源、环境暴露浓度、累积特性以及海产品种类有关.
经海产品个体中BrPs含量推算,牡蛎、扇贝、紫贻贝、脉红螺、花螺、扁玉螺、小黄花鱼、海鲈鱼、金鲳鱼中∑4BrPs个体平均含量分别为33.0、15.2、2.25、23.2、18.7、3.11、7.85、9.52、13.0 ng·g−1 dw(图2). 比较不同海产品的含量发现,3种贝类中∑4BrPs、2,4-diBrP、2,4,6-triBrP个体平均含量((16.8±12.6)、(0.304±0.272)、(14.4±10.7) ng·g−1 dw)高于3种螺类((15.0±8.60)、(0.284±0.112)、(10.0±8.43) ng·g−1 dw)和鱼类((10.1±2.17)、(0.0136±0.0193)、(7.10±0.654) ng·g−1 dw),4-mBrP和2,6-diBrP则在螺类((0.863±0.785) ng·g−1 dw和(3.82±4.76) ng·g−1 dw)中高于贝类和鱼类. BrPs在物种间的差异,与生物的习性和生活环境有关,在韩国东南沿海、中国北江等区域的研究显示,BrPs在海洋沉积物中的浓度高于海水[5, 18],因此,贝类样品,特别是牡蛎和扇贝中某些BrPs单体的含量远高于螺类和鱼类,可能与其长期在沉积物中的底栖生活习性相关.
在3种贝类中,牡蛎的∑4BrPs、2,6-diBrP、2,4,6-triBrP个体平均含量最高(33.0、4.70、28.0 ng·g−1 dw),接下来是扇贝(15.2、0.774、13.4 ng·g−1 dw),二者均远高于紫贻贝(2.24、<MDL、1.88 ng·g−1 dw). 扇贝的4-mBrP和2,4-diBrP含量(0.343、0.677 ng·g−1 dw)高于牡蛎(0.221、0.0352 ng·g−1 dw)和紫贻贝(0.161、0.200 ng·g−1 dw). 本研究牡蛎中的溴酚单体4-mBrP、2,4,6-triBrP的个体平均含量高于中国香港地区牡蛎中对应BrPs的含量(<MDL、平均值(8.98±6.82) ng·g−1 dw,范围1.37–18.1 ng·g−1 dw)[12]和美国俄勒冈州州立大学海洋研究站获取的两种牡蛎样品中对应BrPs含量(<MDL、10.0、6.00 ng·g−1 dw)[19];2,6-diBrP处在中等水平,高于中国香港地区牡蛎(平均值(0.858±0.616) ng·g−1 dw,范围0.345—2.18 ng·g−1 dw)[12]低于美国俄勒冈州州立大学海洋研究站的两种牡蛎(含量5.00 ng·g−1 dw和6.00 ng·g−1 dw)[19];但溴酚单体2,4-diBrP的含量远低于中国香港牡蛎(平均值(30.6±18.5) ng·g−1 dw,范围9.8—52.9 ng·g−1 dw)[12]和在美国俄勒冈州的牡蛎(6.00 ng·g−1 dw和6.00 ng·g−1 dw)[19]. 本研究3种贝类中2,4,6-triBrP的个体含量的平均值((14.4±10.7) ng·g−1 dw)远高于在欧洲意大利、丹麦、法国、爱尔兰、西班牙等国家市场上采集的软体动物/甲壳类动物中2,4,6-triBrP的含量(范围0.316—3.73 ng·g−1 dw,数据经文献中脂肪含量换算)[20].
在3种螺类中,∑4BrPs和2,4,6-triBrP的含量按照脉红螺(23.2 ng·g−1 dw和21.8 ng·g−1 dw)、花螺(18.7 ng·g−1 dw和5.85 ng·g−1 dw)、扁玉螺(3.11 ng·g−1 dw和2.41 ng·g−1 dw)依次递减. 花螺中4-mBrP和2,6-diBrP含量(1.97 ng·g−1 dw和10.5 ng·g−1 dw)高于脉红螺(0.337 ng·g−1 dw和0.935 ng·g−1 dw)、扁玉螺(0.278 ng·g−1 dw和<MDL). 与另外3种BrPs不同,2,4-diBrP在扁玉螺中最高为0.419 ng·g−1 dw,接下来依次为花螺(0.288 ng·g−1 dw)和脉红螺(0.144 ng·g−1 dw).
对于3种鱼类,在金鲳鱼中∑4BrPs、2,6-diBrP含量(13.0、6.70 ng·g−1 dw)最高,接下来依次为海鲈鱼(9.52、1.51 ng·g−1 dw)和小黄花鱼(7.85、0.713 ng·g−1 dw). 海鲈鱼中2,4,6-triBrP和4-mBrP含量(7.91 ng·g−1 dw和0.0854 ng·g−1 dw)高于小黄花鱼(7.07 ng·g−1 dw和0.0244 ng·g−1 dw)和金鲳鱼(6.31 ng·g−1 dw和0.0332 ng·g−1 dw). 金鲳鱼和小黄花鱼均为咸水鱼,只能在海洋中生活,而海鲈鱼在海水与淡水中均能生活,因此海鲈鱼还有可能受到淡水生活环境的影响,而2,4,6-triBrP作为人为生产的溴代阻燃剂,往往对流经城市和人类生活区的淡水水体影响更大,这也可能是海鲈鱼体内2,4,6-triBrP含量高的原因.
2.2 三种贝类中BrPs的组织分布
对于紫贻贝,除2,6-diBrP未检出外,检出的BrPs(4-mBrP、2,4-diBrP、2,4,6-triBrP)及∑4BrPs(0.197、0.262、4.00、4.46 ng·g−1 dw)均主要累积在鳃内(图3),高于贝肉(0.187、0.188、1.81、2.19 ng·g−1 dw)和外套膜(0.102、0.204、1.42、1.73 ng·g−1 dw)中对应BrPs的含量. 扇贝鳃中4-mBrP(0.663 ng·g−1 dw)和2,4-diBrP(1.20 ng·g−1 dw)也高于外套膜(0.326 ng·g−1 dw和0.681 ng·g−1 dw)和贝肉(0.295 ng·g−1 dw和0.594 ng·g−1 dw). 这说明鳃也是贝类BrPs暴露及分布的重要组织. 牡蛎和扇贝的贝肉中2,4,6-triBrP(31.0 ng·g−1 dw和15.9 ng·g−1 dw)、∑4BrPs的含量(36.3 ng·g−1 dw 和16.8 ng·g−1 dw)高于鳃和外套膜. 另外,牡蛎与扇贝中2,6-diBrP在外套膜中(6.04 ng·g−1 dw与4.00 ng·g−1 dw)高于鳃和贝肉中含量. 对3种贝类中BrPs的组织分布进行比较,发现紫贻贝各组织中的∑4BrPs均远低于牡蛎和扇贝,BrPs在3种贝类中的组织分布差异与3种贝类不同的生活方式和生长特性有关,牡蛎、扇贝多生活在潮间带、潮下带、低潮带底泥中,而紫贻贝多生活于浅海区附着在岩礁上,从BrPs浓度相对较低的海水中滤食,使其体内BrPs含量相较牡蛎和扇贝要低. 而紫贻贝的外套膜占个体干重的32.0%,远高于牡蛎(15.7%)和扇贝(13.3%). 紫贻贝、牡蛎、扇贝外套膜中∑4BrPs含量分别占对应贝类个体BrPs含量的24.6%、10.9%、8.79%. 由此可见,不同种贝类间相同生物组织在其体重中的占比也影响了BrPs的浓度分布. 整体上,贝类肉中∑4BrPs((18.4±14.0) ng·g−1 dw)高于鳃中((13.5±8.68) ng·g−1 dw)和外套膜((11.5±8.71) ng·g−1 dw).
2.3 三种螺类中BrPs的组织分布
3种螺中各种BrPs及∑4BrPs在内脏中的含量均远高于螺肉中的含量(图4). 3种螺内脏中∑4BrPs平均含量((21.7±12.6) ng·g−1 dw)远高于螺肉((3.37±2.41) ng·g−1 dw). 对于螺肉样品,花螺螺肉中∑4BrPs最高(6.59 ng·g−1 dw)(图4),分别是脉红螺(2.71 ng·g−1 dw)和扁玉螺 (0.806 ng·g−1 dw)的2.43倍和8.18倍. 花螺的螺肉和内脏中4-mBrP、2,6-diBrP、2,4,6-triBrP的含量均高于脉红螺、扁玉螺的对应部位. 2,4-diBrP的最高浓度出现在扁玉螺的螺肉和内脏团中(图4). 3种螺螺肉中∑4BrPs的平均含量((3.37±2.41) ng·g−1 dw,范围0.806—6.59 ng·g−1 dw)远低于3种贝肉的平均含量((18.4±14.0) ng·g−1 dw,范围2.19—36.3 ng·g−1 dw),但花螺和脉红螺螺肉中∑4BrPs的含量则高于紫贻贝贝肉.
2.4 三种鱼体中BrPs的组织分布
如图4所示,小黄花鱼、海鲈鱼和金鲳鱼肉中的∑4BrPs含量(2.21、5.29、2.87 ng·g−1 dw)均远低于内脏(37.8、31.3、41.5 ng·g−1 dw)和鳃中的含量(56.1、28.0、25.4 ng·g−1 dw). 检出率最高的2,4,6-triBrP在鱼体中的最高浓度均出现在3种鱼的鱼鳃(56.0、28.0、25.3 ng·g−1 dw)中. 这说明鳃也是鱼类2,4,6-triBrP暴露与分布的重要组织. 4-mBrP和2,6-diBrP在内脏团中的含量均高于鱼肉和鱼鳃中(图4). 其中金鲳鱼内脏中的2,6-diBrP(26.4 ng·g−1 dw)占∑4BrPs(41.5 ng·g−1 dw)的63.6%,超过了检出率最高的2,4,6-triBrP(15.0 ng·g−1 dw,占比36.1%),这一比例也远高于小黄花鱼和海鲈鱼中2,6-diBrP所占比例(15.9%和31.9%)以及文献中报道的鱼类肠胃中2,6-diBrP所占的比例(0—16.9%)[1, 12]. 2,4-diBrP除在小黄花鱼的内脏团中检出(0.344 ng·g−1 dw)外,在小黄花鱼其他组织、海鲈鱼和金鲳鱼的所有组织中均未发现. 3种鱼中检测到的BrPs的∑4BrPs平均含量在内脏中含量最高为(36.9±4.22) ng·g−1 dw,高于鳃((36.5±13.9) ng·g−1 dw)和鱼肉((3.46±1.32) ng·g−1 dw),与其他研究中发现的内脏含量高于肉中含量的现象是一致的.
本研究的海鲈鱼鱼肉中以2,4,6-triBrP为主要BrPs单体,与文献中同种鱼的检测结果一致,但2,4,6-triBrP的含量(5.20 ng·g−1 dw)高于澳大利亚市场上采集的野生与养殖尖吻鲈鱼鱼肉中的含量(平均值(0.0857±0.0481) ng·g−1 dw,范围<MDL—0.800 ng·g−1 dw,经含水率80%计算)[21],低于在瑞典斯德哥尔摩群岛的Nämdö岛周围采集的鲈鱼鱼肉的含量(平均值(6.59±3.15) ng·g−1 dw,范围:(1.45—10.0) ng·g−1 dw,经含水率80%换算)[22];4-mBrP含量((0.0900) ng·g−1 dw)低于澳大利亚尖吻养殖鲈鱼鱼肉中的含量((0.250) ng·g−1 dw,以鱼肉含水率80%计干重)[21]. 本研究海鲈鱼鱼肉中2,4-diBrP和2,6-diBrP均未检出,但在澳大利亚尖吻野生鲈鱼中均有检出.
与其他种类的鱼相比,本研究3种鱼鱼肉中2,4,6-triBrP含量平均值((3.17±1.44) ng·g−1 dw)低于在中国香港市场上采集的不同季节的河豚与褐斑石斑鱼的鱼肉(平均值(15.6±9.91) ng·g−1 dw,范围(2.43–39.2) ng·g−1 dw)[12]和从美国阿拉斯加、密歇根湖、威斯康星州等地采集的鲑鱼、鲱鱼等鱼肉中2,4,6-triBrP的含量((18.5—166) ng·g−1 dw)[19]. 本研究3种鱼内脏中2,4,6-triBrP含量平均值((22.5±6.72) ng·g−1 dw)也均低于在中国香港的河豚与褐斑石斑鱼(平均值(49.1±50.2) ng·g−1 dw,范围(2.18—155) ng·g−1 dw)[12]和澳大利亚新南威尔士州采集的多种底栖食肉性与杂食性鱼类(平均值(203±264) ng·g−1 dw,范围<MDL—850 ng·g−1 dw,按照含水率80%计算)[23] 内脏中2,4,6-triBrP的含量. 本研究3种鱼类的鱼肉及内脏中BrPs含量低于其他研究1—2个数量级,整体上处在较低水平.
3. 结论(Conclusion)
在贝类、螺类和鱼类的鳃、外套膜、内脏、肉等不同组织中共检出4种BrPs,4-mBrP、2,4-diBrP、2,6-diBrP、2,4,6-triBrP,其中2,4,6-triBrP含量水平和检出率均相对较高,在9种海产品所有组织中的浓度范围为0.512—56.0(平均值:(14.1±13.8) ng·g−1 dw),∑4BrPs的范围为0.806—56.1(平均值:(18.2±15.5) ng·g−1 dw). 3种贝类中∑4BrPs个体平均含量高于3种螺类和鱼类. 3种贝类鳃中∑4BrPs高于外套膜和贝肉. 3种螺的内脏和鳃中∑4BrPs含量平均值远高于螺肉中的含量. 3种鱼内脏中的∑4BrPs含量平均值远高于鳃和鱼肉. 研究证实了BrPs在不同海产品内脏中的高累积,另外鳃也是海产品BrPs暴露及分布的重要组织.
-
表 1 9种海产品的样本数量、组织重量以及含水率.
Table 1. Numbers, weights, and the moisture content for the 9 tested seafood samples.
种类Species 拉丁名Latin name 数量Quantities 部位Tissues 湿重/gWet weight 干重/gDry weight 含水率/%Moisture content 牡蛎 Crassostrea gigas n=20 鳃 17.0 3.09 81.8 外套膜 20.6 4.49 78.2 肉 91.0 21.0 76.9 扇贝 Patinopecten yessoensis n=30 鳃 23.9 5.96 75.1 外套膜 25.3 6.72 73.4 肉 157 37.7 75.9 紫贻贝 Mytilus edulis n=35 鳃 17.4 3.42 80.3 外套膜 43.8 12.0 72.6 肉 105 22.1 79.0 脉红螺 Rapana venosa n=20 内脏 46.5 14.5 68.9 肉 113 27.4 75.7 花螺 Babylonia areolata n=35 内脏 55.7 18.2 67.2 肉 113 27.4 75.7 扁玉螺 Glossaulax didyma n=35 内脏 99.5 32.4 67.4 肉 200 55.0 72.5 小黄花鱼 Larimichthys crocea n=4 鳃 18.5 6.42 65.3 内脏 67.1 29.2 56.6 鱼肉 553 210 61.9 金鲳鱼 Trachinotus ovatus n=3 鳃 17.8 6.08 65.8 内脏 57.3 35.6 37.8 鱼肉 487 210 57.0 海鲈鱼 Lateolabrax japonicus n=3 鳃 38.4 13.1 66.0 内脏 88.8 58.7 33.9 鱼肉 621 180 70.9 注:湿重、干重、含水率均基于n个个体的混合样品计量. Note:The wet weight, dry weight, and the moisture content were based on the mixed samples of individualities. 表 2 不同BrPs的母离子、定量离子、碰撞能和去簇电压
Table 2. The precursor and quantitative ion, collision energy, and declustering potential for different BrP congeners.
化合物Compounds 母离子(m/z)Precursor ion 定量离子(m/z)Quantitative ion 碰撞能/eVCollision energy 去簇电压/eVDeclustering potential mBrPs 170.8 78.8 −22 −85 172.8 80.8 −22 −85 diBrPs 250.8 78.8 −30 −110 80.8 −30 −110 triBrPs 328.8 78.8 −70 −120 80.8 −70 −120 tetraBrPs 408.6 78.8 −85 −130 80.8 −85 −130 pBrP 488.6 78.8 −82 −130 80.8 −82 −130 13C6−4-mBrP 176.8 78.8 −22 −85 178.8 80.8 −22 −85 13C6−2,4-diBrP 256.8 78.8 −30 −110 80.8 −30 −110 13C6−2,4,6-triBrP 334.9 78.8 −70 −120 80.8 −70 −120 13C6−2,3,4,6-tetraBrP 414.6 78.8 −85 −130 80.8 −85 −130 13C6−PBrP 494.6 78.8 −82 −130 80.8 −82 −130 -
[1] WHITFIELD F, HELIDONIOTIS F, DREW M. The role of diet and environment in the natural flavours of seafoods [J]. Flavour Science, 1996, 197: 3-12. [2] LIN K, SONG L, ZHOU S, et al. Formation of brominated phenolic contaminants from natural manganese oxides-catalyzed oxidation of phenol in the presence of Br [J]. Chemosphere, 2016, 155: 266-273. doi: 10.1016/j.chemosphere.2016.04.064 [3] HAN W, WANG S, HUANG H, et al. Simultaneous determination of brominated phenols in soils [J]. Journal of Environmental Sciences, 2013, 25(11): 2306-2312. doi: 10.1016/S1001-0742(12)60298-8 [4] REINEKE N, BISELLI S, FRANKE S, et al. Brominated indoles and phenols in marine sediment and water extracts from the north and baltic seas-concentrations and effects [J]. Archives of Environmental Contamination and Toxicology, 2006, 51(2): 186-196. doi: 10.1007/s00244-005-0135-3 [5] XIONG J, LI G, AN T, et al. Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China [J]. Environmental Pollution, 2016, 219: 596-603. doi: 10.1016/j.envpol.2016.06.021 [6] 王万洁, 侯兴旺, 栗笑迎, 等. 我国不同城市油松树皮中溴酚的赋存特征 [J]. 环境化学, 2021, 40(11): 3344-3350. doi: 10.7524/j.issn.0254-6108.2021040802 WANG W, HOU X, LI X, et al. Occurrence of bromophenols in pine bark from different cities in China [J]. Environmental Chemistry, 2021, 40(11): 3344-3350(in Chinese). doi: 10.7524/j.issn.0254-6108.2021040802
[7] CHEN Z J, LIU H Y, HO K L, et al. Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) in paired maternal and neonatal samples from South China: Placental transfer and potential risks [J]. Environmental Research, 2016, 148: 72-78. doi: 10.1016/j.envres.2016.03.021 [8] EGUCHI A, NOMIYAMA K, DEVANATHAN G, et al. Different profiles of anthropogenic and naturally produced organohalogen compounds in serum from residents living near a coastal area and e-waste recycling workers in India [J]. Environment International, 2012, 47: 8-16. doi: 10.1016/j.envint.2012.05.003 [9] YANG M T, ZHANG X R. Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete platynereis dumerilii [J]. Environmental Science & Technology, 2013, 47(19): 10868-10876. [10] LIU J Q, ZHANG X R. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: Halophenolic DBPs are generally more toxic than haloaliphatic ones [J]. Water Research, 2014, 65: 64-72. doi: 10.1016/j.watres.2014.07.024 [11] SCHULER D, JAGER J. Formation of chlorinated and brominated dioxins and other organohalogen compounds at the pilot incineration plant VERONA [J]. Chemosphere, 2004, 54(1): 49-59. doi: 10.1016/S0045-6535(03)00756-2 [12] CHUNG H Y, MA W C J, KIM T S. Seasonal distribution of bromophenols in selected Hong Kong seafood [J]. Journal of Agricultural and Food Chemistry, 2003, 51(23): 6752-6760. doi: 10.1021/jf034632r [13] CHI X Y, LIU J Y, YU M, et al. Analysis of bromophenols in various aqueous samples using solid phase extraction followed by HPLC-MS/MS [J]. Talanta, 2017, 164: 57-63. doi: 10.1016/j.talanta.2016.11.010 [14] ESTRELLA L F, FERREIRA V B, GALLISTL C, et al. Occurrence of halogenated natural products in highly consumed fish from polluted and unpolluted tropical bays in SE Brazil [J]. Environmental Pollution, 2018, 242: 684-691. doi: 10.1016/j.envpol.2018.07.030 [15] CHUNG H Y, MA W C J, ANG P O, et al. Seasonal variations of bromophenols in brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegata) collected in Hong Kong [J]. Journal of Agricultural and Food Chemistry, 2003, 51(9): 2619-2624. doi: 10.1021/jf026082n [16] NOMIYAMA K, EGUCHI A, MIZUKAWA H, et al. Anthropogenic and naturally occurring polybrominated phenolic compounds in the blood of cetaceans stranded along Japanese coastal waters [J]. Environmental Pollution, 2011, 159(12): 3364-3373. doi: 10.1016/j.envpol.2011.08.035 [17] WANG H S, DU J, HO K L, et al. Exposure of Hong Kong residents to PBDEs and their structural analogues through market fish consumption [J]. Journal of Hazardous Materials, 2011, 192(1): 374-380. [18] SIM W J, LEE S H, LEE I S, et al. Distribution and formation of chlorophenols and bromophenols in marine and riverine environments [J]. Chemosphere, 2009, 77(4): 552-558. doi: 10.1016/j.chemosphere.2009.07.006 [19] BOYLE J L, LINDSAY R C, STUIBER D A. Bromophenol distribution in salmon and selected seafoods of fresh‐ and saltwater origin [J]. Journal of Food Science, 1992, 57(4): 918-922. doi: 10.1111/j.1365-2621.1992.tb14323.x [20] AZNAR-ALEMANY O, TRABALON L, JACOBS S, et al. Occurrence of halogenated flame retardants in commercial seafood species available in European markets [J]. Food and Chemical Toxicology, 2017, 104: 35-47. doi: 10.1016/j.fct.2016.12.034 [21] FULLER S C, FRANK D C, FITZHENRY M J, et al. Improved approach for analyzing bromophenols in seafood using stable isotope dilution analysis in combination with SPME [J]. Journal of Agricultural and Food Chemistry, 2008, 56(18): 8248-8254. doi: 10.1021/jf801126b [22] DAHLGREN E, LINDQVIST D, DAHLGREN H, et al. Trophic transfer of naturally produced brominated aromatic compounds in a Baltic Sea food chain [J]. Chemosphere, 2016, 144: 1597-1604. doi: 10.1016/j.chemosphere.2015.10.024 [23] WHITFIELD F B, HELIDONIOTIS F, SVORONOS D, et al. The source of bromophenols in some species of Australian ocean fish [J]. Water Science and Technology, 1995, 31(11): 113-120. doi: 10.2166/wst.1995.0416 -




 下载:
下载: