-
丹参首载于《神农本草经》,是中国的传统中药,其有效成分主要包括两大类,分别是水溶性丹参酚酸类和脂溶性丹参酮类[1-4]. 2020年版《中华人民共和国药典》[5]规定了丹参中的主要脂溶性和水溶性活性成分含量测定的方法,采用高效液相色谱法,分两个方法进行分析. 本文利用全谱二维液相,通过优化中心切割条件及柱头聚焦条件,成功建立了丹参提取液中脂溶性活性成分和水溶性活性成分的一针进样分析方法[6-8]. 该方法不仅分离度满足药典要求,同时还有分析时间短、线性范围宽、定量准确度高的优点.
-
岛津全谱二维液相系统. 其中输液泵为两个LC-40B XR和两个LC-40B X3,配备在线脱气机DGU-20A5,自动进样器SIL-40C X3,柱温箱CTO-40C,系统控制器SCL-40,检测器SPD-M40;色谱工作站及LabSolutions Ver. 5.97.
-
液相色谱条件:第一维色谱柱为Shim pack GIST 100 mm×2.1 mm I.D.,2 μm(P/N: 227-30001-04, 岛津(上海)实验器材有限公司);第二维色谱柱为Shim-pack GIST-HP C18-AQ 100 mm×2.1 mm I.D.,3 μm(P/N: 227-30765-03 岛津(上海)实验器材有限公司);第一维流动相A相为0.02%磷酸水溶液、B相为乙腈,第二维A相为0.1%磷酸水溶液,B相为乙腈;柱温20°C;进样体积4μL. 第一维和第二维各自走梯度,见表1,使用的切换阀为FCV-36AH,阀切换时间程序见表2. 丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ使用270nm波长检测,丹酚酸B使用286 nm波长检测.
-
准确称取丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ和丹酚酸B对照品粉末,置于容量瓶中,加甲醇分别定容到1、1 、1 、2 mg·mL−1,为储备溶液. 取适量的储备溶液用甲醇稀释至指定浓度,上机分析.
准确称取丹参样品粉末(过三号筛)约0.15 g,置具塞锥形瓶中,精密加入90%甲醇50 mL,密塞,称定重量,超声处理(功率140 W,频率42 kHz)30 min,放冷,再称定重量,用90%甲醇补足减失的重量,摇匀,滤过,取续滤液,即得.
加标样品制备:准确称取丹参样品粉末(过三号筛)约0.15 g,置具塞锥形瓶中,加入丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ和丹酚酸B对照品溶液适量,精密加入90%甲醇50 mL,密塞,称定重量,超声处理(功率140 W,频率42 kHz)30 min,放冷,再称定重量,用90%甲醇补足重量,摇匀,滤过,取续滤液,即得.
-
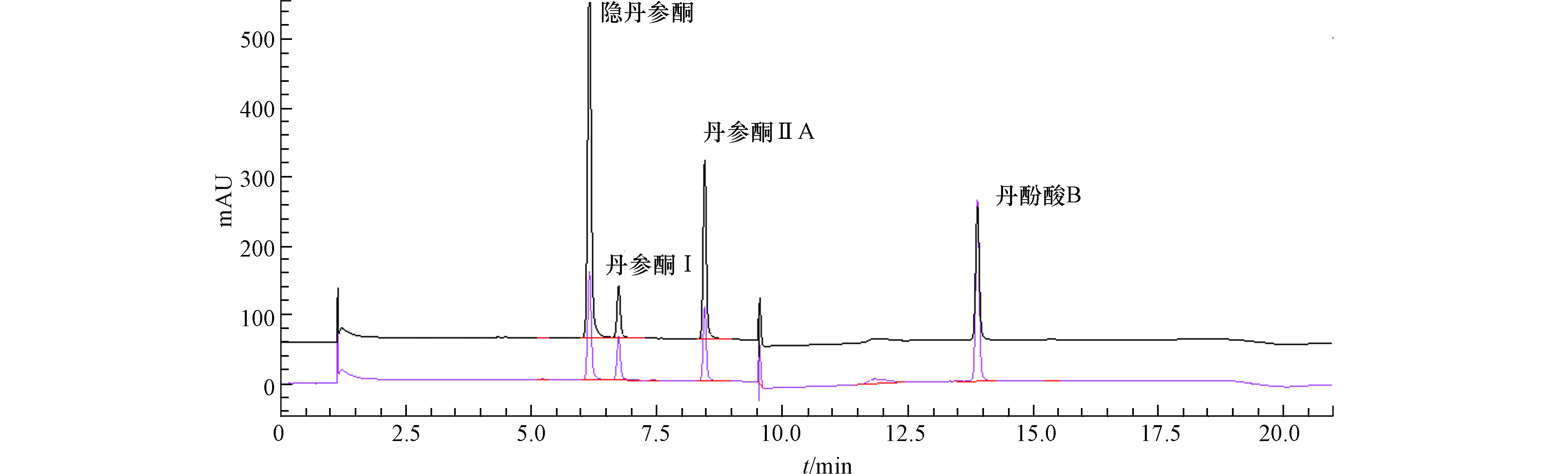
本应用首先分别针对丹参酮类物质和丹酚酸B进行一维液相色谱条件优化和确认,主要优化色谱柱和梯度条件. 丹参酮类物质液相色谱方法优化时,通过微调梯度条件,丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ的分离度良好,相对保留时间见表3,满足药典中相对保留时间规定值的±5%范围之内的要求.
丹酚酸B液相色谱方法优化,主要对比了1.9 μm和3.0 μm的色谱柱,由于柱温较低,1.9 μm的色谱柱虽然有更好的柱效,但是背压更高,因此选择3.0 μm的色谱柱用于丹酚酸B的分离. 丹酚酸B的分离采用梯度洗脱,通过实验验证,丹酚酸B在色谱柱上保留较强,满足柱头聚焦的要求.
-
在全谱二维系统的分析过程中,丹酚酸B从第一维经定量环导入到第二维,并经历了较长时间的柱头稀释聚焦,因此有必要对柱头聚焦条件进行优化以达最佳的聚焦效果. 聚焦体积定义为二维液相中利用有机相将目标物从定量环中携带出来的体积. 以10%为稀释比例,当聚焦体积随聚焦时间延长而增加,考察峰面积随聚焦体积的变化规律,当聚焦体积≥800 μL时,峰面积达到稳定.
-
以800 μL为聚焦体积,在不同稀释比例的条件下,考察丹酚酸B的峰宽、峰高等基本参数. 随着聚焦比例增加,丹酚酸B的峰宽显著变宽,峰面积也随之减小,说明聚焦比例过时丹酚酸B在色谱柱上有一定程度的洗脱,而导致聚焦效果变差. 聚焦比例较小,虽然聚焦效果良好,但是会大幅延长聚焦时间. 因此20%和25%的聚焦比例均比较理想. 通过对比丹酚酸B(峰2)对照品溶液中主峰和附近两个杂质峰的分离度,在聚焦比例为20%条件下,丹酚酸B和两个杂质峰的分离度为分别为3.523和5.791,而25%聚焦比例下,分离度分别为3.121和5.511. 考虑实际样品更为复杂,需要更好的分离效果,因此确定聚焦比例为20%.
-
本文仅考察0.4 、0.5 、0.6 mL·min−1的聚焦流速,实验数据显示,3种聚焦流速对分离效果没有显著影响,选择0.5 mL·min−1流速为最终聚焦流速.
通过优化条件,21 min完成丹参中高级性活性成分和中低极性活性成分的同时分析(图1).
-
对比了100%甲醇、90%甲醇水溶液和80%甲醇水溶液的提取结果,通过对比4种目标物的峰面积,在90%甲醇水溶液中均有良好的提取效率.
-
按照1.3和1.4所述准备对照品溶液和供试品溶液,其中对照品溶液中丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ的浓度为20 μg·mL−1,丹酚酸B的浓度为100 μg·mL−1. 两份溶液均连续进样6次,计算4种成分保留时间和峰面积的重复性,并统计相对标准偏差(RSD%). 结果表明,4种成分的保留时间的RSD在0.018%—0.085%之间,峰面积的RSD在0.10%—0.38%之间,说明本方法稳定性良好.
按1.4节方法配制系列对照品混合标准溶液,以各对照品的色谱峰峰面积(y)对进样质量浓度(x,μg·mL−1)进行回归计算,4种对照品的线性关系,线性回归方程及相关系数(r)如表4所示,4种对照品在线性范围内线性相关性良好,相关系数r均在0.999以上.
向丹参样品中按照低、中、高三个浓度水平分别加入适量的对照品溶液,按照1.4节方法制备加标样品,每个浓度水平平行3次实验,并计算回收率. 实验结果显示(表4),4种成分的加标回收在89.94%—117.40%之间,RSD在0.31%—0.99%.
-
按照上述方法,对丹参样品进行检测,平行6次实验,所得溶液上机分析,结果如表5所示. 结果显示,4种成分的含量在0.43—57.06 mg·g−1之间,RSD在2.64%—4.21%之间.
-
本文利用岛津全谱二维液相系统,建立了一针进样分析丹参样品中脂溶性和水溶性活性成分的液相色谱方法. 本方法仅用21min,不仅可以实现丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ和丹酚酸B的同时分离,同时分离度良好,满足药典要求. 方法学实验中,4种成分的线性相关性、重复性和加标回收均良好,同时还用该方法计算了丹参样品中4种成分的含量. 本方法具有分析时间短、线性范围宽、定量准确度高的优点,可用于丹参中活性物质的含量测定,供相关人员参考.
全谱二维液相色谱检测丹参中的活性成分
Determination of active components in Salvia miltiorrhiza by Polarity Partition two-dimensional liquid chromatography
-
摘要: 本文利用岛津全谱二维液相色谱系统,建立了一针进样分析丹参中脂溶性和水溶性活性成分的液相色谱方法。相对于2020年版《中华人民共和国药典》需要分两个方法进行分析,本方法仅用21 min,可以实现丹参酮ⅡA、隐丹参酮、丹参酮Ⅰ和丹酚酸B的同时分离,分离度良好,满足药典要求。方法学验证结果表明,4种成分在线性范围内相关性良好,线性相关系数均大于0.999;连续6次重复进样分析中,4种成分的保留时间的RSD在0.018%—0.085%之间,峰面积的RSD在0.10%—0.38%之间,说明本方法稳定性良好。加标回收实验,4种成分的加标回收在89.63%—118.15%之间,RSD在0.31%—4.99%之间。使用本方法检测某丹参样品含量,平行6次分析结果的RSD在2.64%—4.21%之间。
-
关键词:
- 全谱二维液相色谱系统 /
- 丹参 /
- 天然产物
Abstract: In this paper, polarity partition two-dimensional liquid chromatography system was used to establish a liquid chromatography method for the analysis of four active components, i.e. tanshinone IIA, cryptotanshinone, tanshinone I and salvianolic acid B, in Salvia miltiorrhiza with different polarity. Compared with the 2020 edition of the Pharmacopoeia of the people's Republic of China, the analysis needs to be divided into two methods. Our method only takes 21 minutes, which can not only realize the simultaneous separation of the four active components, but also has good separation and meets the requirements of the Pharmacopoeia. The results of methodological validation showed that the four components had good correlation in the linear range, and the linear correlation coefficient was greater than 0.999; In six consecutive repeated injection analysis, the RSD of the retention time of the four components is between 0.018%—0.085%, and the RSD of the peak area is between 0.10%—0.38%, indicating that the method is stable. In the standard addition recovery experiment, the standard addition recovery of the four components is between 89.63% —118.15%, and the RSD are between 0.31% —4.99%. The RSD of six parallel analysis results are between 2.64% —4.31%. -
表 1 一维液相色谱梯度洗脱时间程序
Table 1. Time program of fist dimension LC
时间/min 流速/(mL·min−1) 泵A浓度/% 泵B浓度/% 时间/min 流速/(mL·min−1) 泵A浓度/% 泵B浓度/% 一维液相色谱 0.00 0.4 45 55 二维液相色谱 0.00 0.4 80 20 3.00 0.4 45 55 1.10 0.4 80 20 10.00 0.4 10 90 1.11 0.5 80 20 10.10 0.4 45 55 8.11 0.5 80 20 8.12 0.4 80 20 18.12 0.4 50 50 18.20 0.4 80 20 表 2 阀切换时间程序
Table 2. Time program of the valve switching
时间/min 单元 处理命令 值 1.1 柱温箱 Oven Valve 2 1 9.5 柱温箱 Oven Valve 2 0 21 控制器 Stop 表 3 丹参酮类化合物保留时间(min)
Table 3. Retention time of the active chemicals
# 待测成分 保留时间 相对保留时间 药典规定相对保留时间 药典规定参考范围 1 隐丹参酮 6.155 0.728 0.75 0.7125—0.7875 2 丹参酮Ⅰ 6.741 0.797 0.79 0.7505—0.8295 3 丹参酮ⅡA 8.460 1.00 1.00 1 表 4 丹参中4种成分、三个加标浓度的加标回收率(n=3)
Table 4. Recoveries of four components and three spiked concentrations in Salvia miltiorrhiza
成分 样品含量/(mg·g−1) 加标浓度1 加标浓度2 加标浓度3 加标量/(mg·g−1) 回收率/% RSD/% 加标量/(mg·g−1) 回收率/% RSD/% 加标量/(mg·g−1) 回收率/% RSD/% 隐丹参酮 0.43 0.33 97.30 0.42 0.67 97.20 0.64 1.00 98.92 0.31 丹参酮Ⅰ 0.50 0.33 114.00 3.24 0.67 117.40 3.95 1.67 101.02 4.99 丹参酮ⅡA 1.53 0.83 100.32 0.49 1.67 102.28 0.91 3.33 89.94 0.57 丹酚酸B 57.06 11.67 108.80 3.08 16.67 116.43 4.00 33.33 98.87 4.39 表 5 丹参样品检测结果(n=6)
Table 5. Test results of Salvia miltiorrhiza samples
成分 含量/( mg·g−1) RSD/% 隐丹参酮 0.43 4.21 丹参酮Ⅰ 0.50 3.68 丹参酮ⅡA 1.53 3.79 丹酚酸B 57.06 2.64 -
[1] 单晓晓, 洪帮振, 刘洁, 等. 丹参化学成分, 药理作用, 临床应用的研究进展及质量标志物的预测分析 [J]. 中国中药杂志, 2021, 46(21): 5496-5511. [2] 郑琦, 樊慧婷, 张英, 等. 丹参化学成分分析及其抗肿瘤药理作用的研究进展 [J]. 中华中医药学刊, 2020, 38(4): 112-116. [3] 万新焕, 王瑜亮, 周长征, 等. 丹参化学成分及其药理作用研究进展 [J]. 中草药, 2020,51(3): 788-798. [4] 国家药典委员会. 《中华人民共和国药典》一部[S]. 2020年版. [5] FENG J Q, ZHONG Q S,KUANG J M , et al. Simultaneous analysis of the metabolome and lipidome using polarity partition two-dimensional liquid chromatography–mass spectrometry [J]. Anal Chem,2021 , 93(45): 15192-15199. [6] 郑燕, 江媛, 冯展, 等. 基于UPLC-Q-TOF-MS及分子对接技术的丹参抗流感活性探究 [J]. 中草药, 2021, 52(15): 4487-4495. [7] 荣娟娟, 祁俊, 韩晓珂, 等. 高效液相色谱法同时测定五虫化瘀通络胶囊中3种活性成分 [J]. 中国医药导报, 2021, 18(27): 106-109. [8] 王文晓, 杨诺, 高欢, 等. HPLC法同时测定冠心丹参片中10种成分 [J]. 中成药, 2021, 43(5): 1141-1144. -




 下载:
下载:

