-
硝酸盐和高氯酸盐是地下水中常见的共存污染物。农业氮肥的大量施用是地下水硝酸盐污染的最大来源[1]。在美国,约有22%的农业地下水硝酸盐浓度超过饮用水标准[2];在欧洲,有1/3的地下水硝酸盐超过了饮用水标准,硝酸盐达到了100~150 mg·L−1[3];在中国,有61.2%的地下水井存在着硝酸盐严重超标的问题[4]。当长期饮用硝酸盐超标的地下水时,人体患高铁血红蛋白症和癌症的几率会增加[5]。地下水中高氯酸盐主要来源于火箭助推剂、烟花、染料和油漆等工业产品的生产和加工过程[6-7]。有研究表明,智利阿塔卡马沙漠中地表水中的高氯酸盐质量浓度为744~1 480 μg·L−1[8]。有研究[9]表明,由于将含有高氯酸盐的制造业废水排到地下水中,导致位于美国加利福尼亚州东部的萨克拉门地下水高氯酸盐含量达到了8 mg·L−1。KOSAKA等[10]在2007年对日本Usui河和Tone河调研发现,受污域河水中高氯酸盐质量浓度为0.34~2.38 mg·L−1。据调查,我国部分水体存在高浓度高氯酸盐污染状况,如湖南省衡阳市某地表水高氯酸盐含量高达6.8~54.4 mg·L−1[11]。由于高氯酸根离子在电荷和离子半径上都与碘相似,因此,可能会破坏甲状腺中碘的吸收,从而可能影响甲状腺功能,引起甲状腺疾病[12-14],严重威胁着人类的健康。
目前处理水体硝酸盐和高氯酸盐复合污染的方法主要包括离子交换、膜分离、化学还原和生物法[15-16]。离子交换和膜分离工艺虽然能够去除水中的硝酸盐和高氯酸盐,但只是将污染物浓缩转移,并没有将污染物无害转化,且操作成本较高;对于化学还原法,多采用贵金属催化剂,价格昂贵且催化剂易失活;生物还原工艺能够实现硝酸盐和高氯酸盐的高效去除,易于推广应用,故逐渐成为本领域的研究热点[17-18]。根据所需电子供体的不同,生物还原法分为异养还原和自养还原法,对于异养还原过程,常用的有机碳源包括葡萄糖、甲醇、乙酸盐等[19-20]。采用乙酸盐作为电子供体,还原去除硝酸盐和高氯酸盐的反应分别如式(1)和式(2)所示。
需要指出的是,虽然异养生物还原反应速率快,但其反应过程会产生碱度,造成出水pH的升高。此外,碳源投加不易控制,投加过少则处理不彻底,投加过多则残余水中容易造成二次污染。生物自养过程利用无机碳作为电子供体,避免了有机源的投加,近年来受到研究人员的关注。其中,微生物利用单质硫作为电子供体,还原硝酸盐和高氯酸盐的反应如式(3)和式(4)所示。对于硫自养生物还原过程来说,虽然避免了有机碳源投加带来的隐患,但反应过程中产生副产物硫酸盐,同时消耗水体碱度,造成出水pH降低。
基于上述研究背景,为了克服异养和自养还原各自的缺点,本研究建立了异养和硫自养协同作用的一体式生物反应器,其中有机碳源投加量低于理论值,这可有效避免水中有机物的二次污染;硫自养仅承担部分负荷,从而削弱硫酸盐的产生;同时平衡了2种反应过程碱度产生与消耗,稳定出水pH;考察了在不同HRT和不同碳源投加量条件下,混合营养生物工艺对废水中硝酸盐和高氯酸盐的去除效果,以期为该工艺在实际水处理工程中的应用提供参考。
全文HTML
-
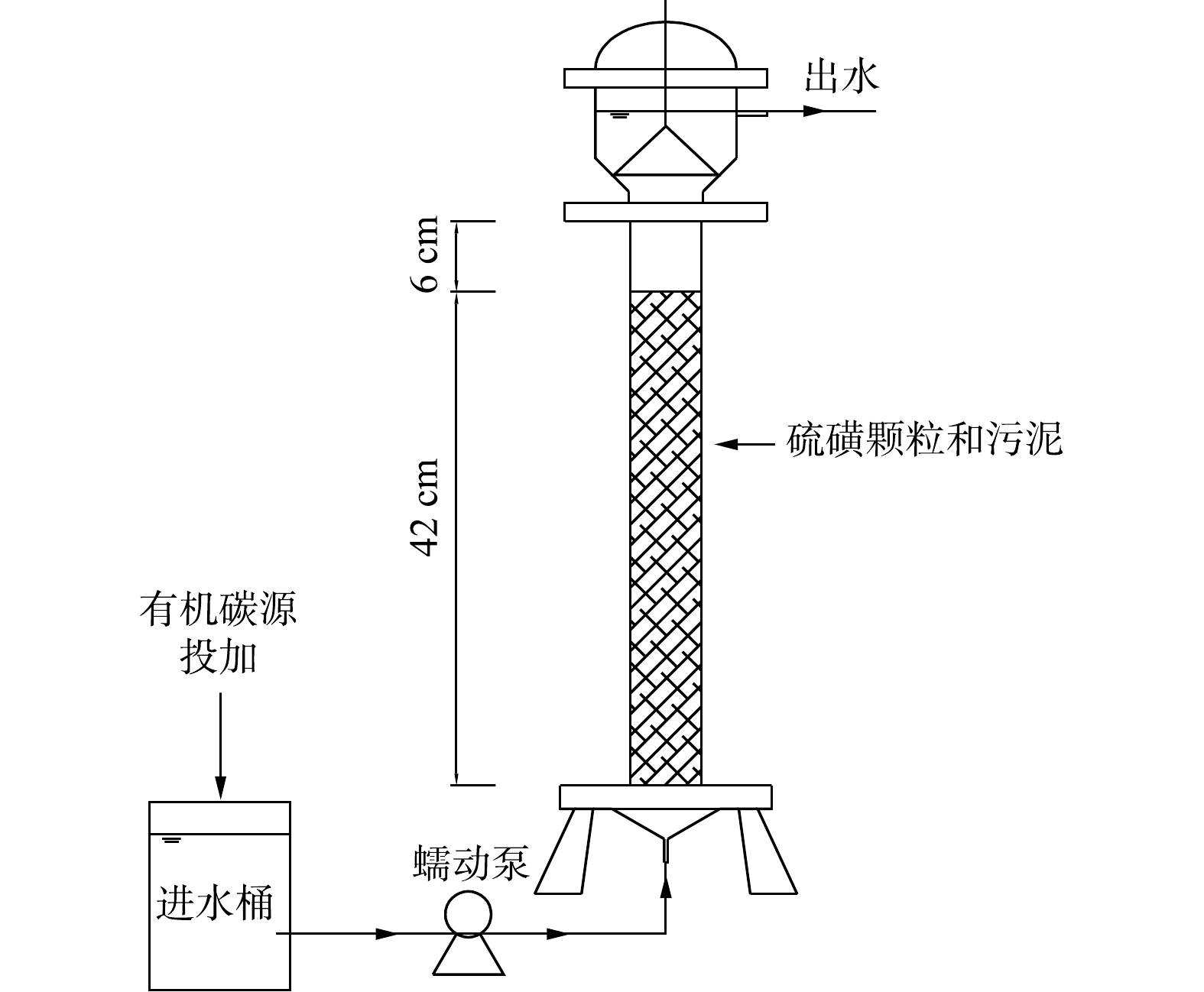
建立了混合营养一体式微生物反应器(图1)。反应器采用有机玻璃柱制成,柱内径为5 cm,柱内填充硫磺颗粒(10目筛子筛分,燕山石化),填充高度42 cm,反应器有效体积310 mL,外部有水浴保温夹层,温度为(30±2) ℃。反应器顶部采用溢流的方式出水,底部通过蠕动泵(YZ1513,保定兰格)均匀的将进水送进反应器。
-
反应器进水采用去离子水和自来水(1∶4)配制而成,加入优级纯高氯酸钠和硝酸钠作为目标污染物。进水组成:
ClO−4 质量浓度(20.12±0.12) mg·L−1,NO−3 -N质量浓度(20.21±0.23) mg·L−1,CH3COO−质量浓度60 mg·L−1(第I~IV阶段添加,第Ⅴ阶段无添加),KH2PO4 0.5 mg·L−1(以磷计),缓冲物质NaHCO3 300 mg·L−1。另外,为了给微生物提供足够的营养成分,进水加入1 mL·L−1的微量元素溶液,溶液成分为0.05 g·L−1 ZnCl2·H2O、0.11 g·L−1 NiCl2·6H2O、0.49 g·L−1 MnCl2·4H2O、0.05 g·L−1 H3BO3、2.00 g·L−1 CoCl2·6H2O、0.11 g·L−1 CuCl2·2H2O。其余进水水质参数为 72.26~138.52 mg·L−1SO2−4 、380.23~497.26 mg·L−1碱度、pH为8.23~8.76。 -
取郑州市五龙口污水处理厂厌氧段污泥,将污泥与硫磺颗粒均匀的填充到反应器中,污泥接种量为6.5 g·L−1。驯化启动阶段进水
ClO−4 质量浓度为40 mg·L−1,NO−3 -N质量浓度为40 mg·L−1,CH3COO−质量浓度为180 mg·L−1,磷源KH2PO4 为0.5 mg·L−1(以磷计),缓冲物质NaHCO3 500 mg·L−1。反应器在HRT为8 h的情况下连续运行14 d后完成驯化。 -
反应器驯化启动完成后,开始正式运行,运行方案如表1所示。实验分为5个阶段,前4个阶段为混合营养工况,调整HRT分别为4、2、1和0.5 h,每个阶段运行10 d。根据反应式(1)和式(2),理论上还原20 mg·L−1的
NO−3 -N和ClO−4 需要164 mg·L−1的CH3COO−。在前4个阶段,进水投加CH3COO−的质量浓度为60 mg·L−1,以营造低碳源投加的混合营养条件。第Ⅴ阶段,保持HRT为0.5 h,取消有机碳源投加,为单独硫自养工艺,研究反应器的处理效能,并与混合营养工艺作对比。 -
所有出水水样均经过0.22 μm滤膜过滤,并在4 ℃条件下保存,采用离子色谱仪(ICS-2100,美国赛默飞)测定水样中的
ClO−4 (最低检测限为0.2 mg·L−1)和SO2−4 (最低检测限为0.4 mg·L−1)。用紫外可见分光光度法测NO−3 ,N-(1-萘基)-乙二胺光度法测NO−2 ,利用TOC分析仪(TOCL-CPN CN200,日本岛津)来测定进出水NPOC,使用pH计(PHS-3C,雷磁,中国)测定进出水pH,采用酸碱滴定法测定进出水的碱度。
1.1. 实验装置
1.2. 进水组成
1.3. 反应器接种和驯化启动
1.4. 实验方案
1.5. 分析方法
-
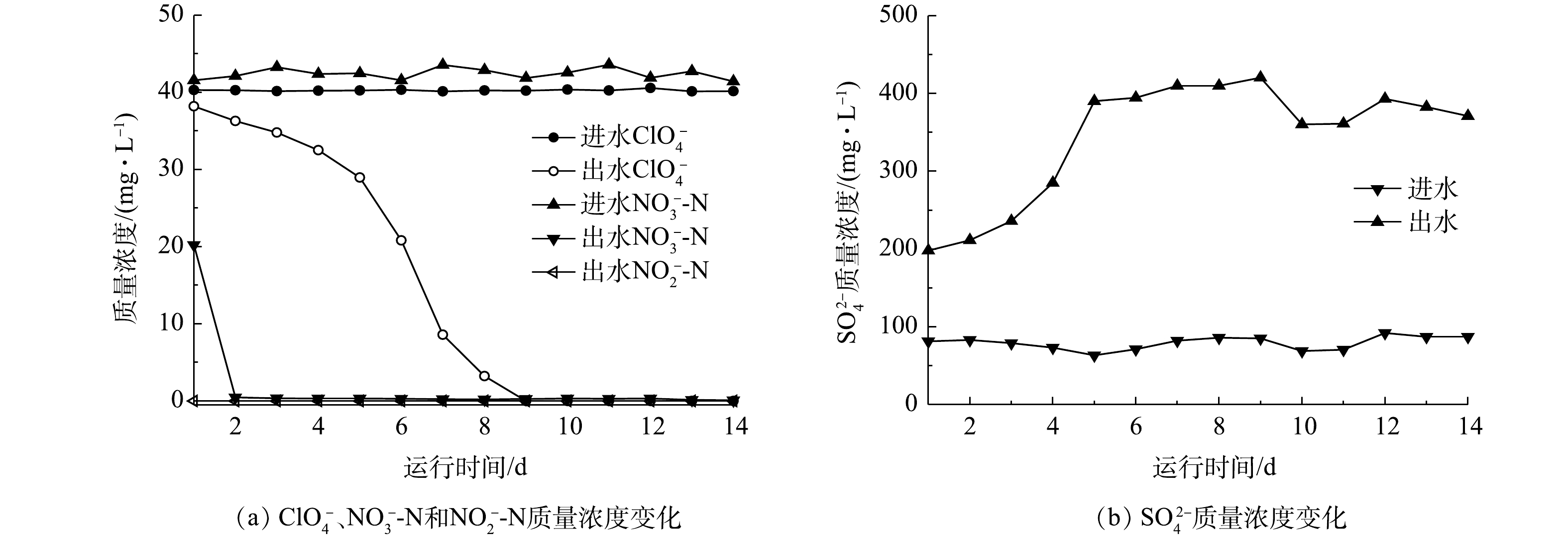
混合营养生物反应器启动期运行效果如图2所示。在HRT为8 h的条件下,反应器只需要2 d的适应期就可以将40 mg·L−1的
NO−3 -N还原,出水NO−3 -N质量浓度为(0.25±0.06) mg·L−1,并且驯化期间没有出现NO−2 的积累,出水浓度始终低于检出限;而对于ClO−4 ,生物反应器则需要8 d的适应期才能够逐渐将40 mg·L−1的ClO−4 还原,出水ClO−4 浓度低于检出限。产生这种现象的原因可能是,接种污泥取自郑州市五龙口污水处理厂的厌氧段污泥,污泥本身就具有反硝化功能,因此,对NO−3 具有较强的适应能力,而对于ClO−4 ,需要一定的适应时间。至第5天,反应器出水SO2−4 质量浓度增加量由116.8 mg·L−1升高至326.7 mg·L−1,这表明硫自养菌正在逐渐驯化成熟,至驯化末期,反应器出水SO2−4 质量浓度增加量稳定在(292.4±6.3) mg·L−1。 -
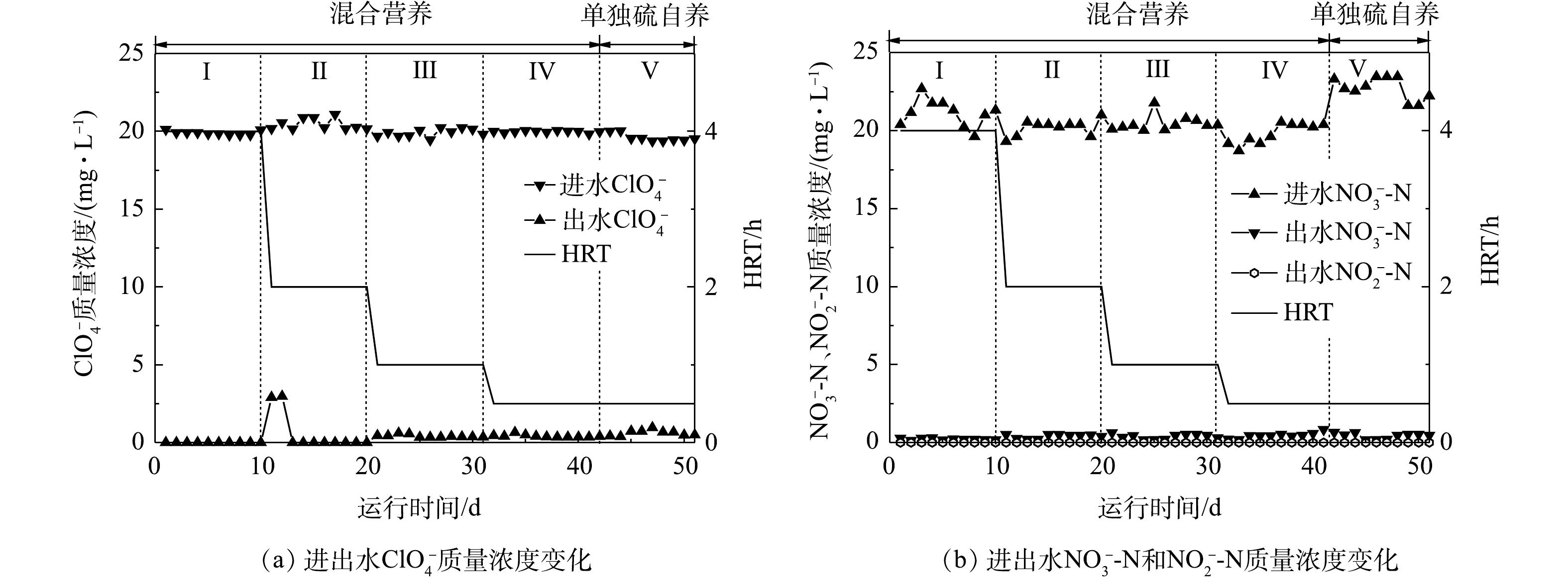
各运行阶段,反应器对
ClO−4 和NO−3 的去除效果如图3所示,在整个运行期间,反应器对ClO−4 和NO−3 均表现出较好的去除效果。对于ClO−4 来说,当HRT从4 h缩短至2 h时,经过3 d的适应期,出水去除率趋于稳定,出水ClO−4 质量浓度低于检出限,当HRT降低到1.0 h和0.5 h时,出水ClO−4 质量浓度分别降低到(0.34±0.06) mg·L−1和(0.35±0.09) mg·L−1。第Ⅴ阶段,单独硫自养工艺下,反应器出水ClO−4 质量浓度升高至(0.58±0.12) mg·L−1,对应去除率为96.2%,相较于混合营养工艺,ClO−4 去除率降低了2%,表明混合营养工艺对ClO−4 的去除效果优于单独硫自养。LV等[21]用甲烷膜生物反应器还原ClO−4 和NO−3 时发现:当进水中只有ClO−4 时,ClO−4 还原速率为1.7 mmol·(m2·d)−1;而当NO−3 存在时,ClO−4 还原速率降为0.64 mmol·(m2·d)−1,当NO−3 被完全去除后,ClO−4 还原速率又恢复到1.6 mmol·(m2·d)−1。DENIZ等[22]用自养-异养组合工艺去除ClO−4 和NO−3 -N时发现:NO−3 -N在被完全还原之后ClO−4 才能被完全还原。在本研究中,混合营养工艺条件下,反应器实现了对2种污染物的高效去除,加入60 mg·L−1 CH3COO−首先促进了NO−3 的还原效率,而后提高了ClO−4 的去除率,使得混合营养生物反应器在0.5 h时对ClO−4 的去除效果优于硫自养生物反应器。反应器运行期间始终保持对
NO−3 较高的去除效率,几乎不受HRT的影响,出水NO−3 -N质量浓度小于0.62 mg·L−1,去除率大于96.9%,实验期间不存在NO−2 -N的积累,出水NO−2 -N度低于检出限。停止添加碳源,反应器对NO−3 -N的去除几乎没有受到影响。围绕2种污染物异养和硫自养过程,本课题组已经开展了部分研究。2017年,本课题组[23]在硫自养固定床生物反应器还原
ClO−4 和NO−3 复合污染过程的研究中发现,2种污染物的还原同时发生,且硝酸盐的还原速率快于高氯酸盐,硫歧化反应伴随着高氯酸盐的还原,共存的NO−3 对硫歧化反应有抑制作用。2018年,本课题组[24]采用乙酸钠为碳源还原水中的高氯酸盐,发现以高氯酸盐为电子受体,长期驯化得到的混合菌群,对水中的硝酸盐依然有着较好的反硝化效果,在碳源有限的条件下,2种污染物的还原同时发生,且硝酸盐的还原率高于高氯酸盐。一般来说,生物过程对硝酸盐的去除优先于高氯酸盐。在本研究中,在混合营养条件下(HRT为4、2、1和0.5 h),反应器稳态运行时对硝酸盐的去除率高于对高氯酸盐的去除率。当停止碳源投加时,对高氯酸盐的去除受到一定程度的影响,去除率降低了2%,而对硝酸盐的去除没有受到影响,与DENIZ等报道的硝酸盐的去除优先于高氯酸盐的结果相一致[22]。需要指出的是,第Ⅳ阶段,HRT仅为0.5 h,本反应器对
NO−3 -N去除负荷达到930.24 g·(m3·d)−1,对ClO−4 的去除负荷达到了943.20 g·(m3·d)−1。WAN等[25]建立了硫自养填充床生物反应器,其对ClO−4 和NO−3 的最大去除负荷分别为705.92 g·(m3·d)−1和721.60 g·(m3·d)−1,去除负荷低于本实验。ZHU等[26]用生物活性炭填充床生物反应器,发现实验期间反应器出水高氯酸盐质量浓度为1.82~2.16 mg·L−1,出水高氯酸盐质量浓度远高于混合营养生物反应器。因此,本研究建立的混合营养生物反应器对ClO−4 和NO−3 具有较好的去除效果。 -
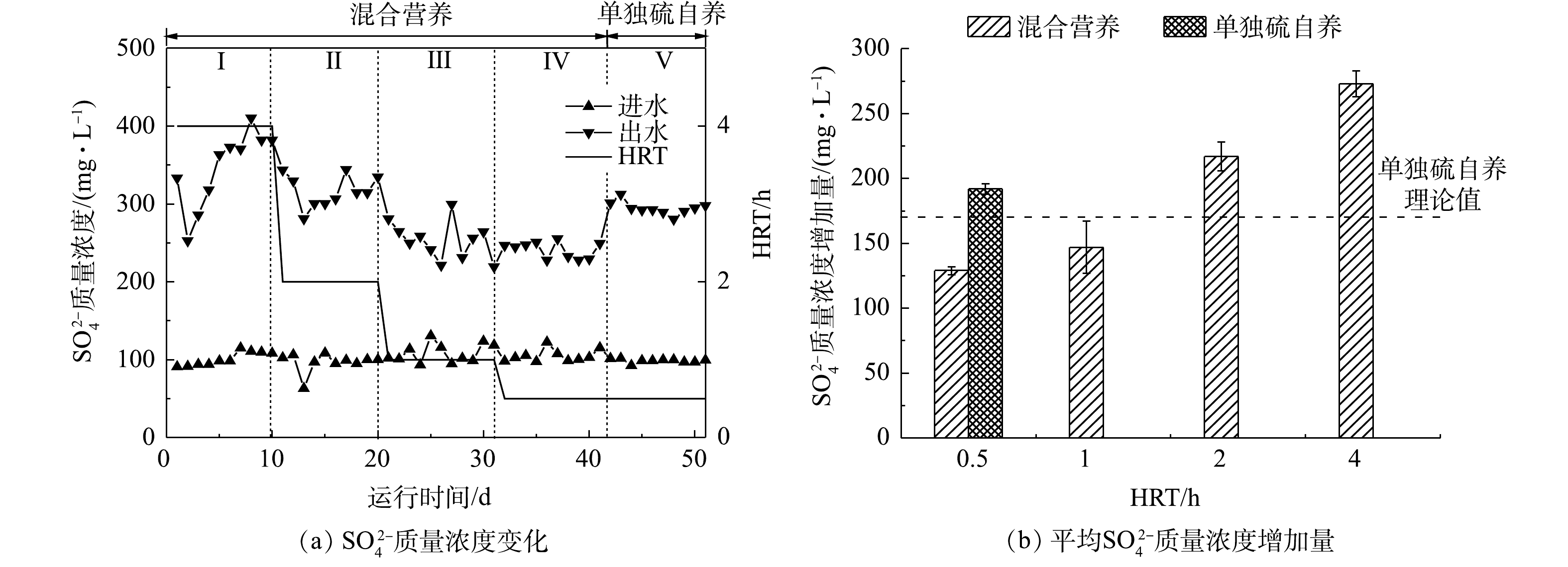
反应器进出水
SO2−4 质量浓度变化如图4所示。在第Ⅰ~Ⅳ阶段,混合营养工艺条件下,出水SO2−4 质量浓度随着HRT的缩短而减少。当HRT为4 h时,出水SO2−4 质量浓度增加量为(273±10) mg·L−1,随着HRT降低到2、1和0.5 h,出水SO2−4 质量浓度增加量分别为(217±11)、(147±20)和(129±3) mg·L−1。第Ⅴ阶段,单独硫自养工艺条件下,出水SO2−4 质量浓度增加量为(192±4) mg·L−1,同有混合营养工艺相比,SO2−4 的产量增加63 mg·L−1。WAN等[27]利用氢自养还原水中高氯酸盐,探究了共存的硝酸盐和硫酸盐对高氯酸盐去除的影响。实验结果表明,还原菌群对3种电子受体的还原速率顺序为
NO−3 >ClO−4 >SO2−4 。此外,有研究[28]表明,无论从热力学或动力学方面分析,硝酸盐还原均优先于硫酸盐。理论上,还原20 mg·L−1的NO−3 -N,需要156 mg·L−1的CH3COO−。在本研究中,进水投加CH3COO−的质量浓度仅为60 mg·L−1,投加碳源不足,硝酸盐将优先于硫酸盐还原,故可推测,硫酸盐直接被有机碳源还原的可能性较低。根据式(3)和式(4),硫自养还原20 mg·L−1的
NO−3 -N和ClO−4 共计产生169 mg·L−1的SO2−4 。因此,当HRT为1 h和0.5 h时,投加60 mg·L−1 CH3COO−能够有效地减少SO2−4 产生,SO2−4 的产量分别减少了22 mg·L−1和40 mg·L−1,HRT越短,SO2−4 的生成受到的抑制越显著。当HRT为4 h和2 h时,虽然投加了60 mg·L−1 CH3COO−,但SO2−4 产生量依然大于硫自养的理论值,由此可推测,在较长的HRT下,发生了硫歧化反应。JU等[29]报道,在硫自养还原
ClO−4 过程中,出水SO2−4 质量浓度远大于理论值,据此推测出有硫歧化反应发生。WAN等[30]发现,在硫自养还原ClO−4 和NO−3 复合污染过程中,硝酸盐的还原速率快于高氯酸盐,硫歧化反应伴随着高氯酸盐的还原,共存的NO−3 对该反应有抑制作用,HRT越长,该反应进行越剧烈,反应如式(5)所示。在混合营养条件下,当HRT为4 h和2 h时,
SO2−4 质量浓度增加量高于硫自养理论值(104±10) mg·L−1 和(48±10) mg·L−1,证实有硫歧化反应发生。因此,为削弱副产物SO2−4 的产生,在保证NO−3 和ClO−4 去除率的同时,工程上应当尽可能地采用较短的HRT。 -
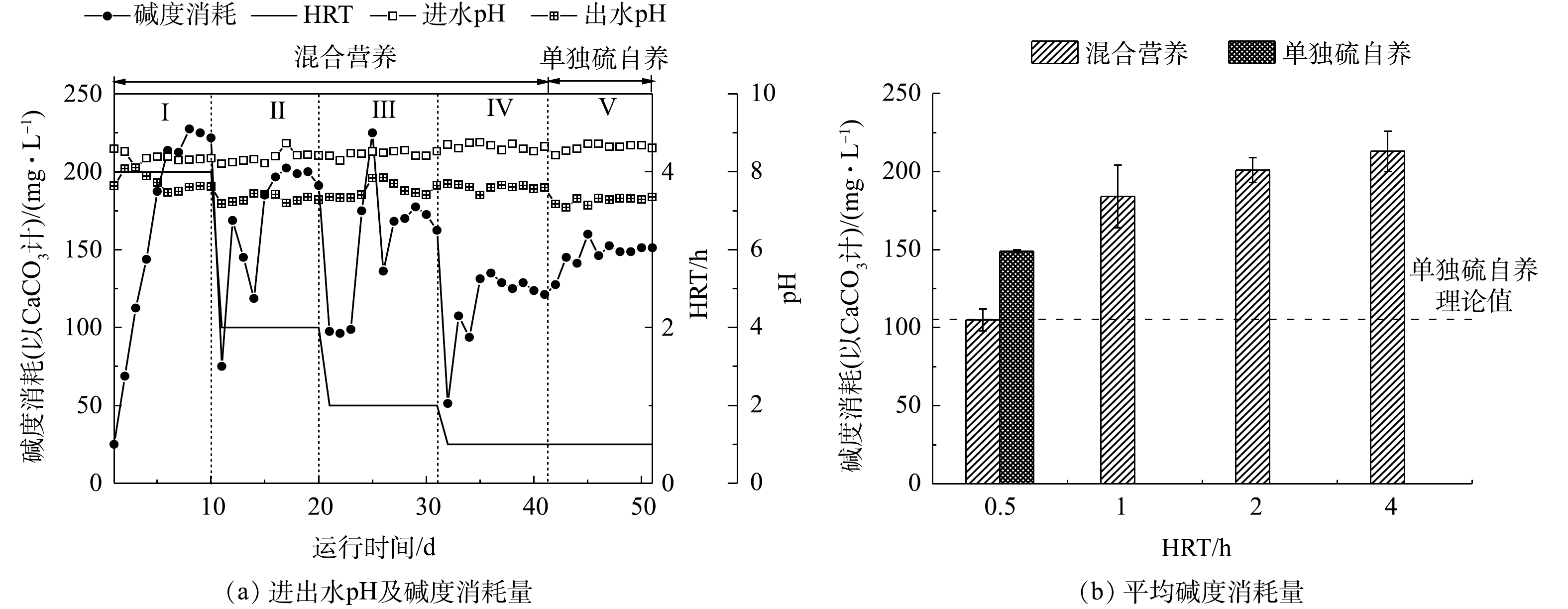
反应器进出水pH和碱度的变化如图5所示。在运行期间,进水pH为8.51~8.72,出水pH稳定在7.18~7.68。实验结果表明,出水碱度消耗量随着HRT的降低呈现降低的趋势。在混合营养工艺下,当HRT为4 h时,碱度消耗量为(213±13) mg·L−1(以CaCO3计);当HRT缩短至0.5 h时,碱度消耗量降为(105±7) mg·L−1,碱度消耗削减了49%。结合式(3)和式(4),理论上硫自养还原20 mg·L−1的
ClO−4 和NO−3 -N的碱度消耗为105 mg·L−1(以CaCO3计)。对比第IV和第Ⅴ阶段,单独硫自养出水pH低于混合营养工艺,碱度消耗量为(150±2) mg·L−1,比混合营养工艺的碱度消耗量增加了45 mg·L−1。这是因为硫自养承担所有负荷,导致碱度消耗增加。在混合营养条件下,异养还原硝酸盐和高氯酸过程产生碱度,硫自养还原硝酸盐和高氯酸盐过程消耗碱度,此外,硫歧化反应也消耗碱度。整个实验运行期间,异养产生的碱度能够抵消一部分自养消耗的碱度,自养-异养在加快去除效率的同时,还能够降低反应物的碱度消耗和硫酸根浓度的产生。在HRT为4 h的条件下,混合营养工艺碱度消耗高于硫自养理论值(108±6) mg·L−1,表明即使在混合营养条件下,较长的HRT也会发生较为剧烈的硫歧化反应,导致碱度消耗较高。 -
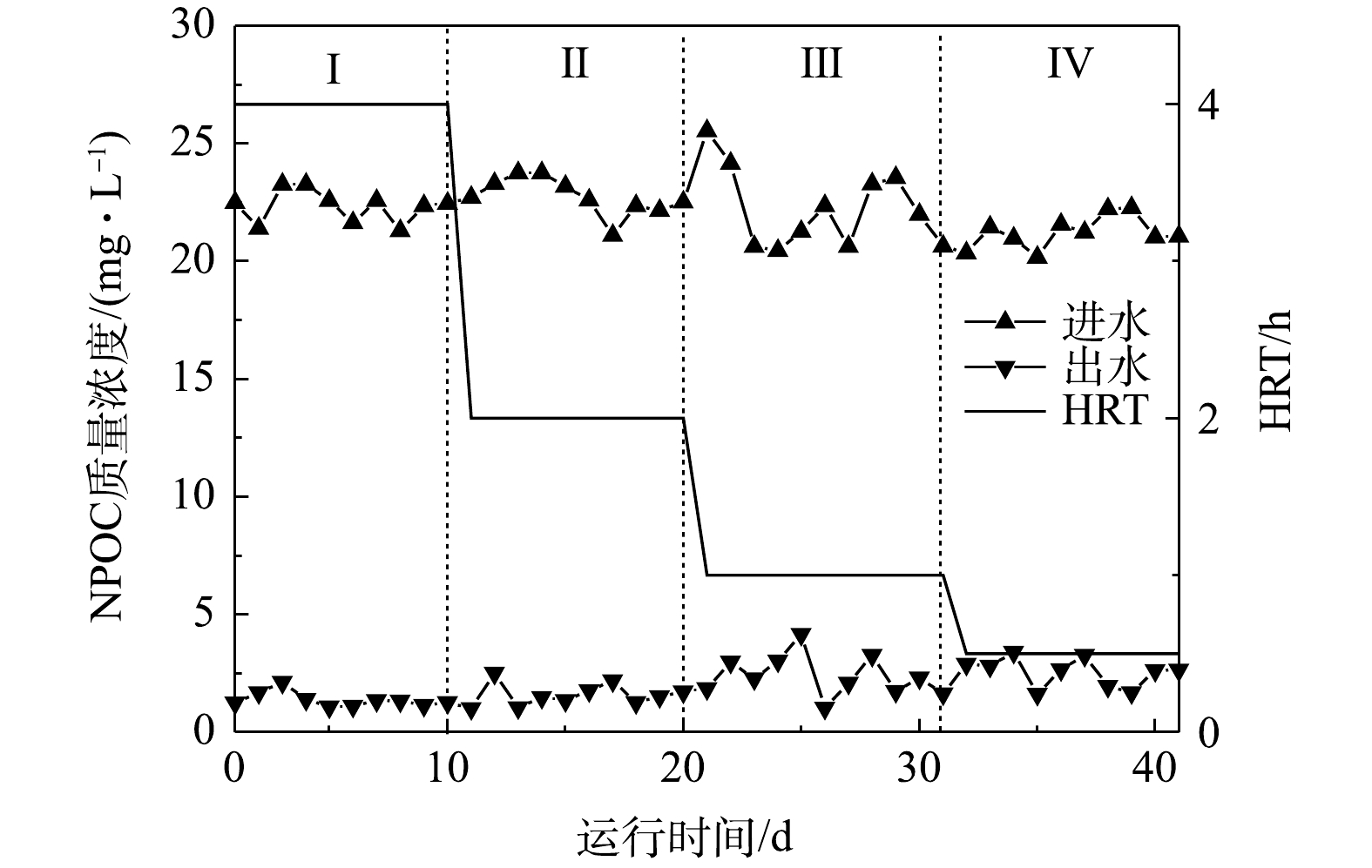
有机碳源是否有残余是影响反应器出水水质的关键所在。本反应器在运行期间,进出水NPOC质量浓度变化如图6所示。在混合营养条件下,反应器出水的NPOC始终维持在较低的水平,出水NPOC小于(2.68±0.13) mg·L−1,且不受HRT的影响。完全去除20 mg·L−1的
NO−3 -N和ClO−4 需要164 mg·L−1的CH3COO−。本研究中,进水仅加入60 mg·L−1CH3COO−,有机碳源的投加远低于理论值,因此,反应器出水中无有机碳源残余。整个实验运行期间,进水COD为(60.56±0.23) mg·L−1,出水COD小于5.68 mg·L−1。
2.1. 反应器的启动
2.2.
反应器对ClO−4 和NO−3 的去除效果
2.3.
反应器进出水SO2−4 质量浓度的变化
2.4. 反应器进出水pH和碱度的变化
2.5. 反应器中进出水NPOC和COD的变化
-
1)混合营养一体式生物反应器能够同步高效的去除
ClO−4 和NO−3 -N。对于20 mg·L−1的进水ClO−4 和NO−3 -N,在HRT分别为4 h和2 h时,出水ClO−4 质量浓度均低于检出限,当HRT为1 h和0.5 h时,出水ClO−4 <0.34 mg·L−1;HRT对NO−3 -N的去除影响较小,出水NO3− -N<0.62 mg·L−1。2)在混合营养条件下,当HRT为0.5 h、温度为(30±2) ℃ 时,反应器中
ClO−4 和NO−3 的去除负荷达到最大,分别为943.20 g·(m3·d)−1和930.24 g·(m3·d)−1。3)混合营养工艺能够有效降低
SO2−4 的产生量和碱度消耗量。当HRT为0.5 h时,与单独硫自养相比,SO2−4 的产生减少了63 mg·L−1,碱度消耗减少了45 mg·L−1。4)由于异养施加不足量碳源,混合营养工艺能有效避免有机碳源残余水中造成二次污染的问题,出水NPOC低于(2.68±0.13) mg·L−1。







 下载:
下载:

















 百度学术
百度学术













































































































