-
近年来,随着环保意识的增强、执法力度的加大以及污水处理技术的不断成熟,工业废水排放量持续增长的趋势得到有效遏制,但是工业废水的排放量依然十分巨大。据有关资料显示,2017年工业废水的排放量达到1.8×1010 t左右,占废水排放总量的30%左右,其中高盐高浓度有机废水因其成分复杂、盐度高、难以处理而备受关注[1]。高盐废水是指总含盐量大于1%(质量分数)的废水[2],这类废水中往往含有高浓度的有机物。高盐高浓度有机废水广泛存在于制药、染料、化工、制革等多个行业[3],如果直接排放会对自然环境和人类生活环境造成严重的危害。同时,高浓度的盐分也会抑制有机废水生物处理中微生物的生长,因此,对高盐废水的脱盐处理,具有十分重要的意义[4]。如何处理高盐高浓度的有机废水一直是工业废水处理中的历史性难题[5]。目前,市场上处理此类废水的方法主要有热分离法[6-7]、膜分离法[8]、离子交换法[9]、电渗析法[10]、电吸附法[11]等,但是这些方法往往伴有占地面积大、结构复杂、运行成本高的缺点。
冷冻法是利用水分子在结晶过程中会排斥杂质的原理,从而获得较为纯净的冰和浓缩的溶液[12]。纯净的冰溶解后,COD和含盐量大幅度下降,可满足排放要求,而多次浓缩的溶液可以通过焚烧等方式进行处置。此外,冷冻法具有低能耗(冻结比蒸发过程能耗更低,水的蒸发热(40.6 kJ·mo1−1)几乎是融合热的7倍(6.01 kJ·mo1−1)[13]、少污染、腐蚀结垢低、适用范围广等优点[14]。
本研究采用多级冷冻的技术处理高盐高浓度模拟有机废水,探究了结冰率、冷冻温度、初始浓度(COD和盐浓度)、冷冻接触面积与脱盐率和有机物去除率的关系,将该工艺应用于实际的化工废水,并通过多级冷冻的方式,使化工废水的盐浓度与COD满足《污水综合排放标准》(GB 8978-1996)三级标准的要求。研究结合了两元相图,在分子水平上提出了多级冷冻脱盐、去除有机物的机理,探索了冷冻结晶工艺对其他水质指标(氨氮、总氮)的去除效果。
全文HTML
-
氯化钠(≥99.5%,分析纯)、浓硫酸(95%~98%,分析纯)、重铬酸钾(≥99.8%,分析纯)购自于国药集团化学试剂有限公司;硫酸汞(99%,分析纯)、葡萄糖(98%,分析纯)购自于上海阿拉丁生化科技股份有限公司;硫酸银(≥99.7%,分析纯)、酒石酸锑钾(99%,分析纯)购自于西陇化工股份有限公司;实验用水全部为超纯水(电导率=18 MΩ·cm, 25 ℃),其他试剂均为分析纯。
-
美的BCD-468WTPM(E)型可控温冰箱;HACH senION7型电导率仪;HK-UP-10型超纯水机;YM-040S型超声波清洗器;YXQ50A立式压力蒸汽灭菌器;Thermo Evolution 220 紫外可见分光光度计。
-
由于实际工业废水中离子的多样性和复杂性,本研究首先使用氯化钠、葡萄糖配置不同浓度的模拟废水进行研究。实验选用氯化钠模拟废水中的盐分,盐浓度为8 000~20 000 mg·L−1[15];选用葡萄糖模拟废水中的有机物,COD为2 000~8 000 mg·L−1 [16]。本实验所用的蓄水槽均为自制有机玻璃容器,尺寸为15 cm×10 cm×18 cm,冷冻接触面积为150 cm2;尺寸为20 cm×10 cm×15 cm,冷冻接触面积为200 cm2。实验过程中溶液体积设置为2 000 mL。
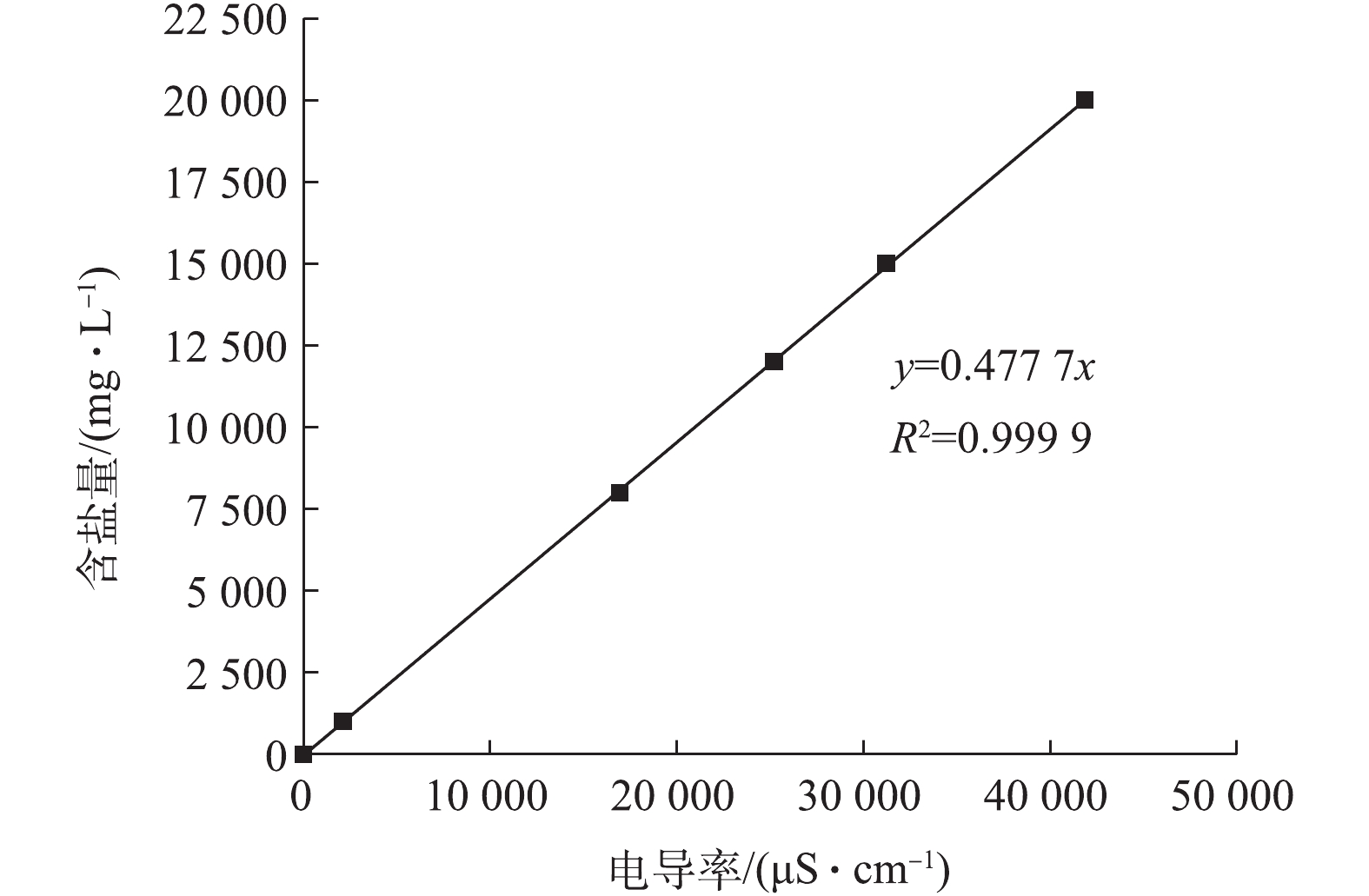
在一定温度下,溶液中的电解质浓度与电导率呈现良好的线性关系[17],即电导率可以用来表示溶液中的电解质浓度。又因为模拟溶液中只添加葡萄糖(不导电)、氯化钠2种物质,因此,在温度相同时,可用电导率来计算溶液中的含盐量。配置氯化钠浓度分别为1 000、8 000、12 000、15 000、20 000 mg·L−1的溶液,分别测定电导率,绘制溶液浓度随电导率的变化曲线,结果如图1所示。
本研究所涉及的其他水质指标的测定方法如下: COD采用重铬酸钾法(HJ 828-2017)测定;氨氮浓度采用纳氏试剂比色法(HJ 535-2009)测定;总磷浓度采用钼酸铵分光光度法(GB 11893-1989)测定;总氮浓度采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012)测定。除水质指标外,本研究涉及的其他指标按照式(1)~式(3)进行计算。
式中:S为结冰率;Vi为冰体积,mL;V0为初始模拟废水体积,mL。
式中:R1为脱盐率;C0为模拟废水的初始盐浓度,mg·L−1;C1为冰融水的盐浓度,mg·L−1。
式中:R2为有机物去除率;D0为模拟废水的初始COD,mg·L−1;D1为冰融水的COD,mg·L−1。
在实验过程中,首先,自制高盐高浓度有机物模拟废水和有机玻璃容器;然后,分别取2 000 mL不同浓度的模拟废水置于可控温冰箱中,分析结冰率、冷冻温度、初始浓度(盐浓度和COD)和冷冻接触面积4个因素对高盐高有机物废水的脱盐和去除有机物的影响;将上层冰置于常温下融化,测定上层冰融水的盐浓度与COD,从而计算得到脱盐率和有机物去除率;最后,对特定浓度的模拟废水和实际化工废水通过多级冷冻的方式进行了脱盐和去除有机物的研究。
1.1. 实验试剂
1.2. 实验仪器
1.3. 实验方法
-
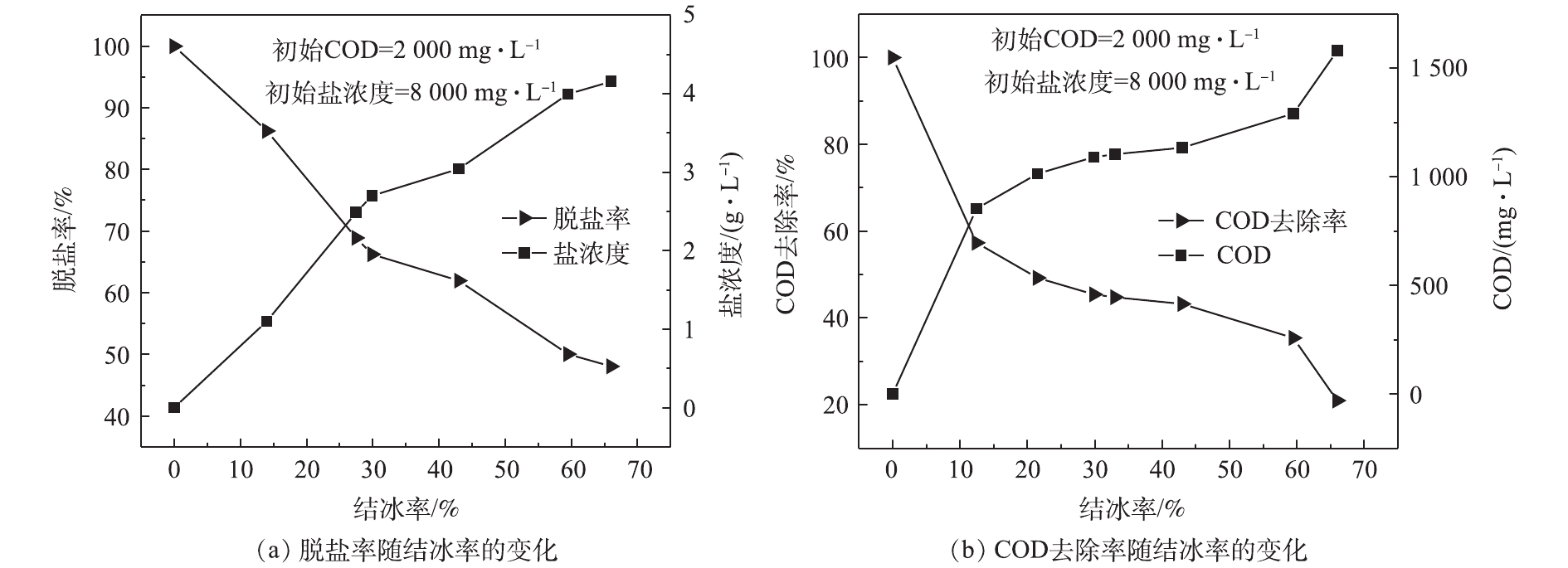
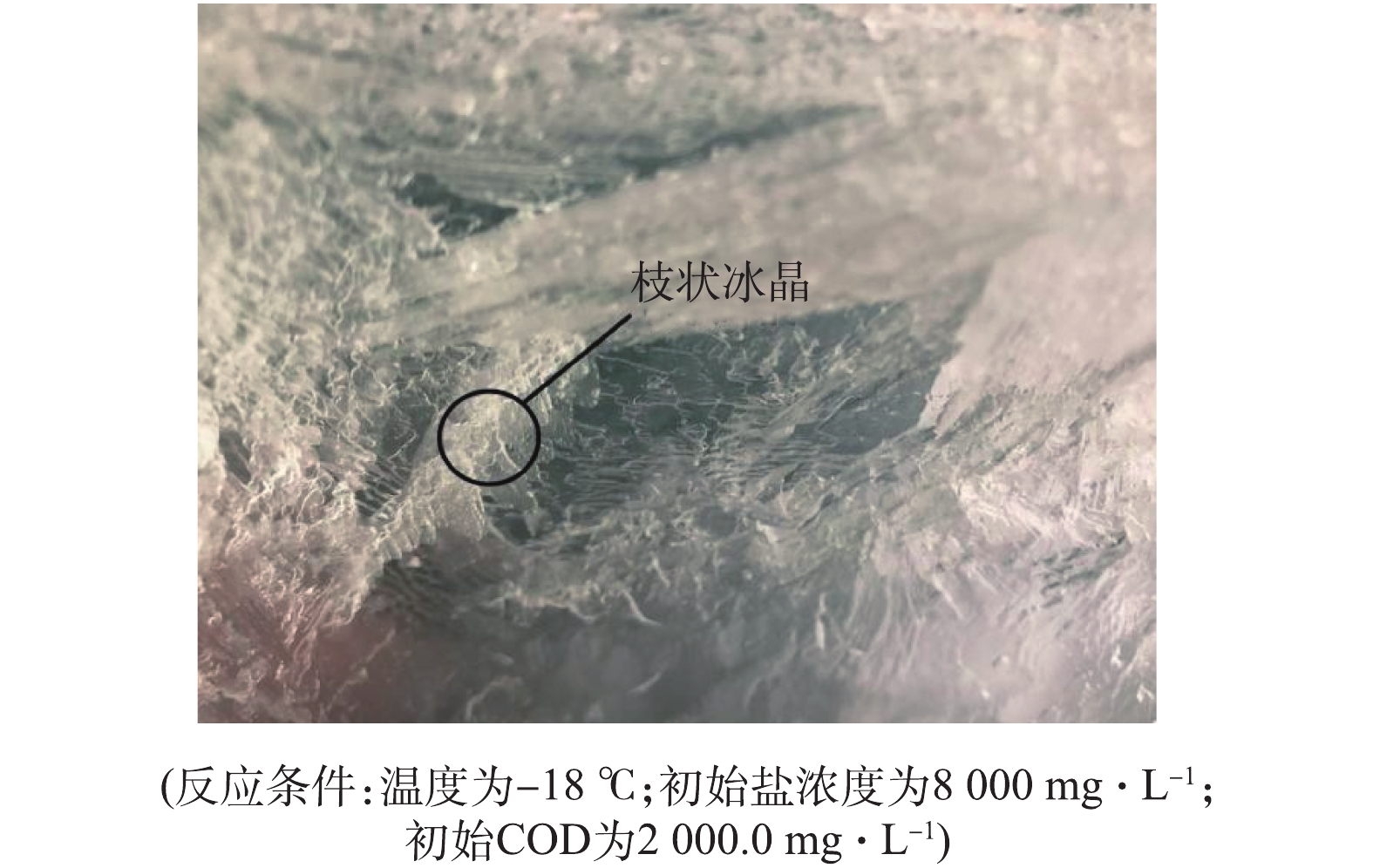
有研究[15]表明,结冰率会影响固液两相中介质的传递,从而影响脱盐率和有机物去除率。为探究结冰率的影响,在冷冻温度为−18 ℃、冷冻接触面积为200 cm2、初始盐浓度为8 000.0 mg·L−1、初始COD为2 000.0 mg·L−1的实验条件下,得到模拟废水上层冰融水的脱盐率和有机物去除率随结冰率的变化,如图2所示。由图2可知,当结冰率从14%增长到66%时,脱盐率可从86.3%降低至48.1%,表明上层冰融水的脱盐率随结冰率的增大而减小;当结冰率从12.5%增长到66%时,有机物的去除率从57.3%降低至21.0%,表明上层冰融水中有机物去除率的变化规律与脱盐率相似,均随结冰率的增大而减小。其原因为:一方面,在高盐高有机物废水结晶过程中,分子从界面向冰体的扩散速度比热从冰体向界面的传递速度更快,促进了树枝状生长[18],从而容易形成较多的枝状冰晶(图3),且呈现柔软的性质[19-20];另一方面,随着结冰率的增加,下层水溶液中的盐浓度和COD越来越大,枝状冰晶末端的缝隙就更容易捕获到溶液中的盐和有机物,从而导致上层冰中的盐浓度和COD越来越高[21]。因此,在后续实验过程中的结冰率均设置为50%。
-
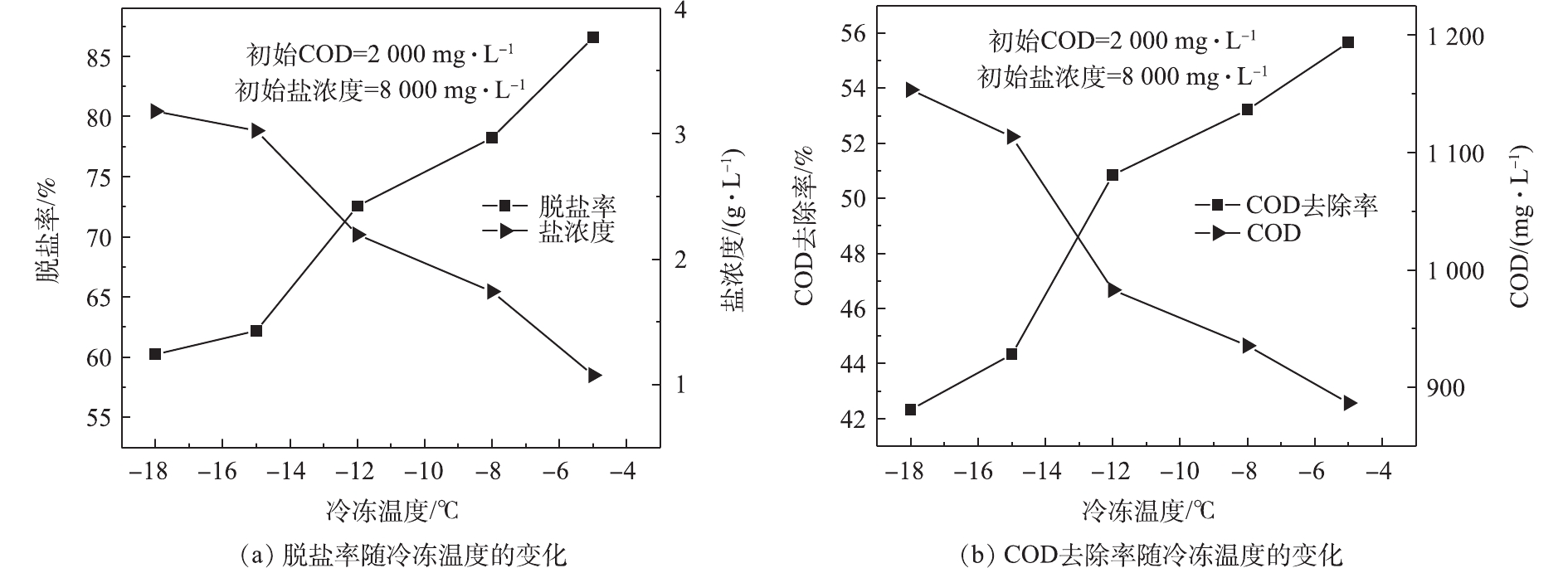
根据NaCl的两元相图和结晶的原理,冷冻温度必须低于冰的成核温度才会使其发生结晶,但是当温度低于共晶温度时,溶液需要大面积散发潜热,此时,大部分冰晶开始呈枝状结构生长,下层溶液开始析出结晶氯化钠,从而影响污染物在冰与水之间的分配,各种溶液均存在着与之相对应的有效冷冻温度和最佳冷冻温度[22]。本研究在模拟废水的初始盐浓度为8 000.0 mg·L−1、COD为2 000.0 mg·L−1、冷冻接触面积为200 cm2、结冰率为50%的条件下,分别设置−5、−8、−12、−15、−18 ℃ 5个温度梯度,探究了冰冻温度对上层冰融水脱盐率和有机物去除率的影响(图4)。如图4所示,当温度从−18 ℃升至−5 ℃时,脱盐率从60.2%升至86.6%,有机物去除率从42.3%升至55.7%,脱盐率和有机物去除率均随冷冻温度的升高而升高。分析高盐高有机物废水的冷冻过程可知:一方面,冷冻温度越低结冰速率越快,此时,液相与固/液两相界面之间水分子的含量差将会增大,从而加速了水分子由液相向固/液两相界面的迁移,一旦这个速率大过盐分和有机物从固/液两相界面向液相迁移的速度,盐分和有机物分子就会被截留在冰晶内,从而导致脱盐率和有机物去除率降低;另一方面,当冷冻温度降低时,脱盐速率将会随之加快。因此,综合脱盐率和脱盐速率2个方面的影响,后续研究的冷冻温度设置为−18 ℃。
-
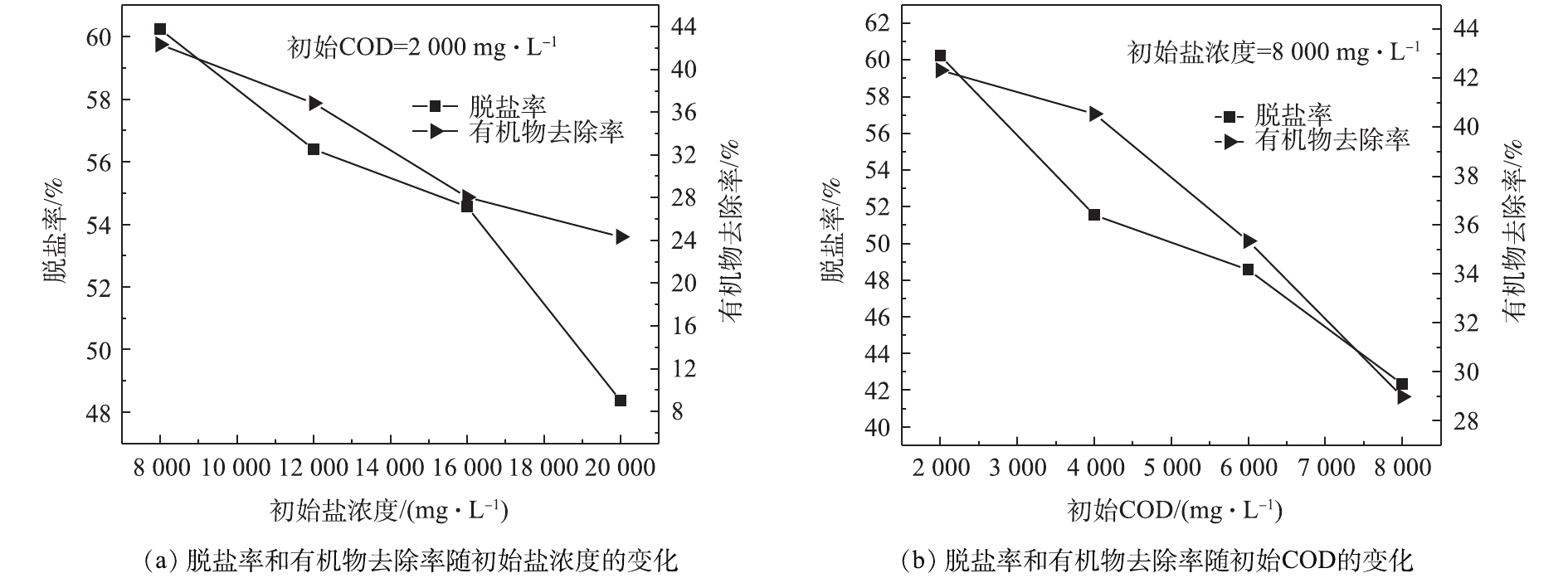
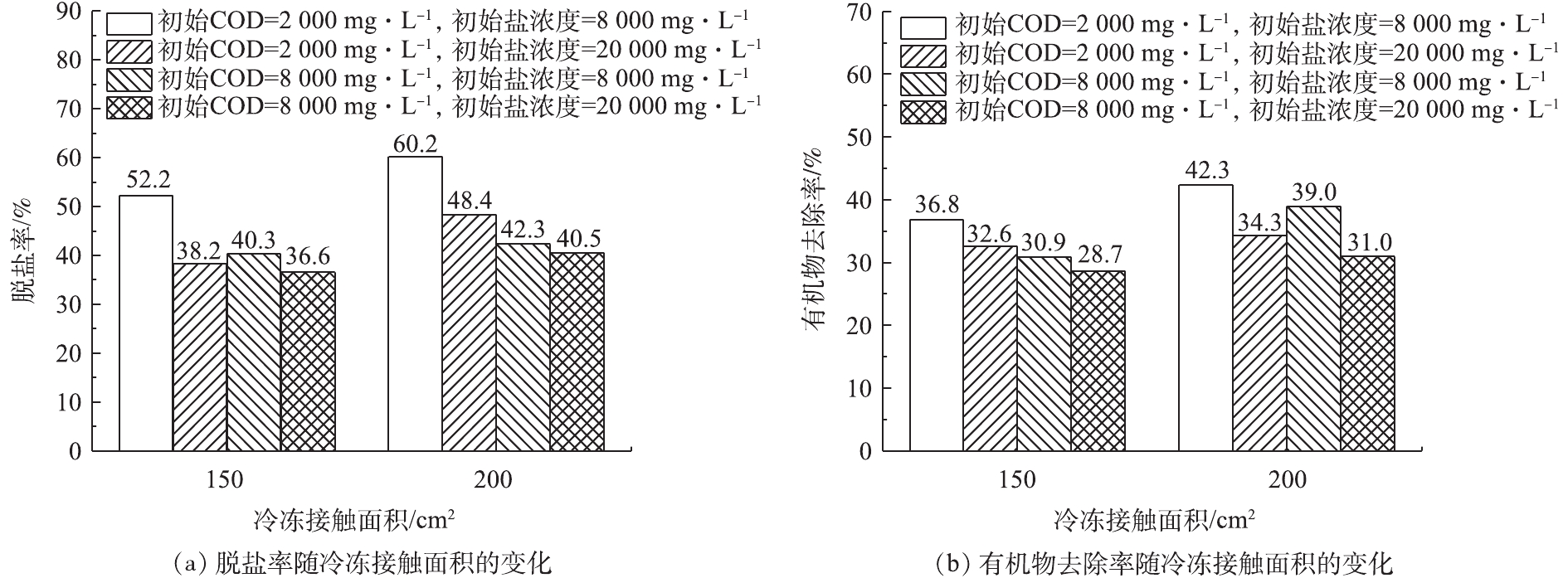
为了探究初始盐浓度和初始COD对高盐高浓度有机废水的脱盐率和有机物去除率影响。本研究在冷冻温度为−18 ℃,结冰率为50%,冷冻接触面积为200 cm2的条件下,设计了不同初始盐浓度(8 000~20 000 mg·L−1)和COD(2 000~8 000 mg·L−1)的浓度梯度,初始盐浓度和初始COD对脱盐率和有机物去除率的影响结果如图5所示。由图5可知,初始盐浓度和初始COD对冷冻结晶工艺脱盐和去除有机物有很大的影响。当固定初始COD为2 000.0 mg·L−1, 初始盐浓度从8 000.0 mg·L−1上升到20 000.0 mg·L−1时,脱盐率从60.2%下降至48.4%,有机物去除率从42.3%下降至24.3%;当固定初始盐浓度为8 000.0 mg·L−1,在初始COD为2 000.0 mg·L−1时,脱盐率和有机物去除率分别可达到60.2%和42.3%,当初始COD升高至8 000.0 mg·L−1时,脱盐率和有机物去除率分别下降至42.3%和29.0%。这是因为:当初始盐浓度和初始COD升高时,溶液的黏度升高,扩散系数降低,这将导致二次结晶和微小冰晶的形成;同时,含盐量和COD越高,形成的冰晶颗粒就会越小,表面积就越大,进而导致冰体中包裹的溶质越多,最终导致脱盐率和有机物去除率越低[23]。
-
为了探究在相同水样体积(2 000 mL)下,冷冻接触面积是否对脱盐率和有机物去除率产生影响,将毛巾包裹自制有机玻璃容器,使水样自上表面结晶。在冷冻温度为−18 ℃,结冰率为50%的条件下,分别设置150 cm2和200 cm2 2个冷冻接触面积,探究不同初始COD和初始盐浓度下,冷冻接触面积对脱盐率和有机物去除率的影响(图6)。如图6所示,在不同初始COD和初始盐浓度下,均有类似的实验结果:接触面积为200 cm2下的脱盐率和有机物去除率均略高于接触面积为150 cm2下脱盐率和有机物去除率, 这说明冷冻接触面积也是影响脱盐率和有机物去除率的主要因素。其原因可能为:随着温度的降低,溶液需要散发大量的潜热,当接触面积较大时,有足够的面积进行散热;但当接触面积较小时,冰晶将会形成枝状结构,并会不断产生新的分支,且分支末端的缝隙很容易捕获溶液中的杂质,从而导致脱盐率和有机物去除率下降。
-
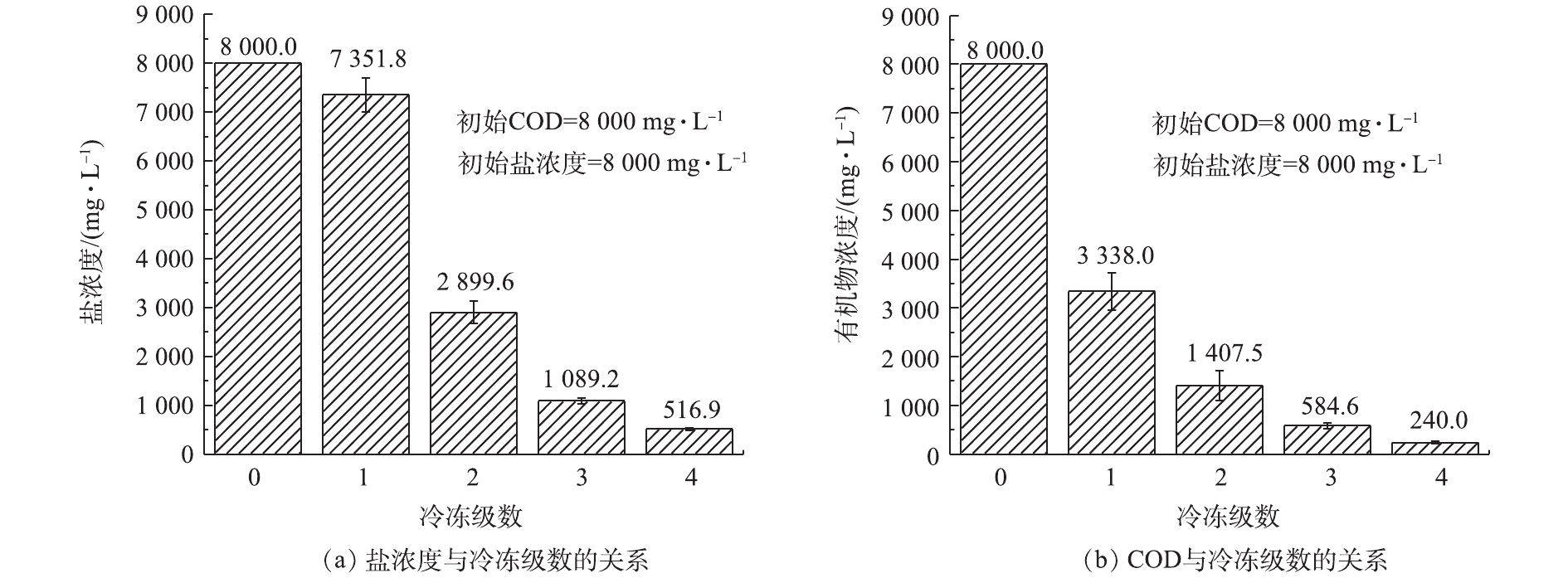
根据《污水综合排放标准》(GB 8978-1996)对化工企业的要求,化工企业排入设置二级污水处理厂的城镇排水系统的污水应执行三级标准,COD的限值为500 mg·L−1。此外,以生化系统为主体的污水处理站的进水盐分极限浓度约为4 000.0 mg·L−1[10],盐分过高将导致微生物死亡,从而导致污水处理站难以运行。本研究在冷冻温度为−18 ℃,结冰率为50%的条件下,采用多级冷冻结晶的工艺,处理初始盐浓度为8 000.0 mg·L−1、初始COD为8 000.0 mg·L−1的高盐高浓度模拟有机废水,结果如图7所示。由图7可知,溶液中的含盐量在二级冷冻便降低为2 899.6 mg·L−1,满足生化工艺废水处理技术中对含盐量的极限要求,在四级冷冻后,溶液中的含盐量下降为516.9 mg·L−1,去除率为93.5%;同时,溶液中的COD在4级冷冻后下降为240.0 mg·L−1,去除率为97.0%,满足化工企业的接管要求。
-
与实际废水相比,模拟废水具有成分复杂,含有多种离子、有机成分和无机成分的特点,为探究多级冷冻工艺对实际废水的除有机物和脱盐效率,本实验在多级冷冻去除模拟高盐高浓度有机废水的基础上,选用江苏连云港某化工企业实际废水为研究对象,在实验室条件下,对其进行多级冷冻处理。该废水的水质指标、执行标准和标准限值如表1所示。
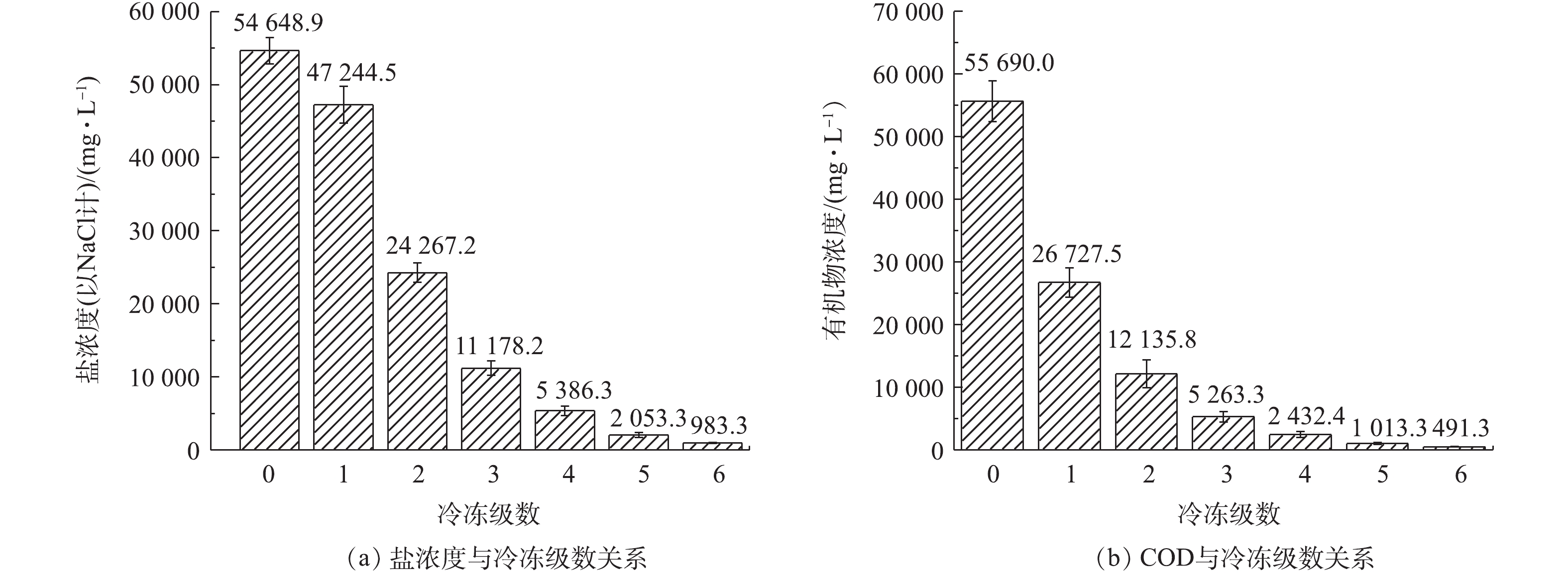
根据《污水综合排放标准》(GB 8978-1996)对化工企业废水的接管要求,采用多级冷冻结晶的工艺对该化工厂废水进行处理,盐分和有机物的去除情况如图8所示。由图8可知,该化工废水的初始COD高达55 690.0 mg·L−1,初始盐浓度高达54 648.9 mg·L−1(以NaCl计),属于高盐高浓度有机废水。根据表1出水标准限值,在5级冷冻后,盐浓度即小于4 000 mg·L−1,达到生化处理有机物的要求,脱盐率为96.2%,在6级冷冻后盐浓度低达983.3 mg·L−1(以NaCl计),此时脱盐率为98.2%。COD在6级冷冻后满足化工企业排放入市政管道的要求,浓度为491.3 mg·L−1,有机物去除率达到99.1%。
-
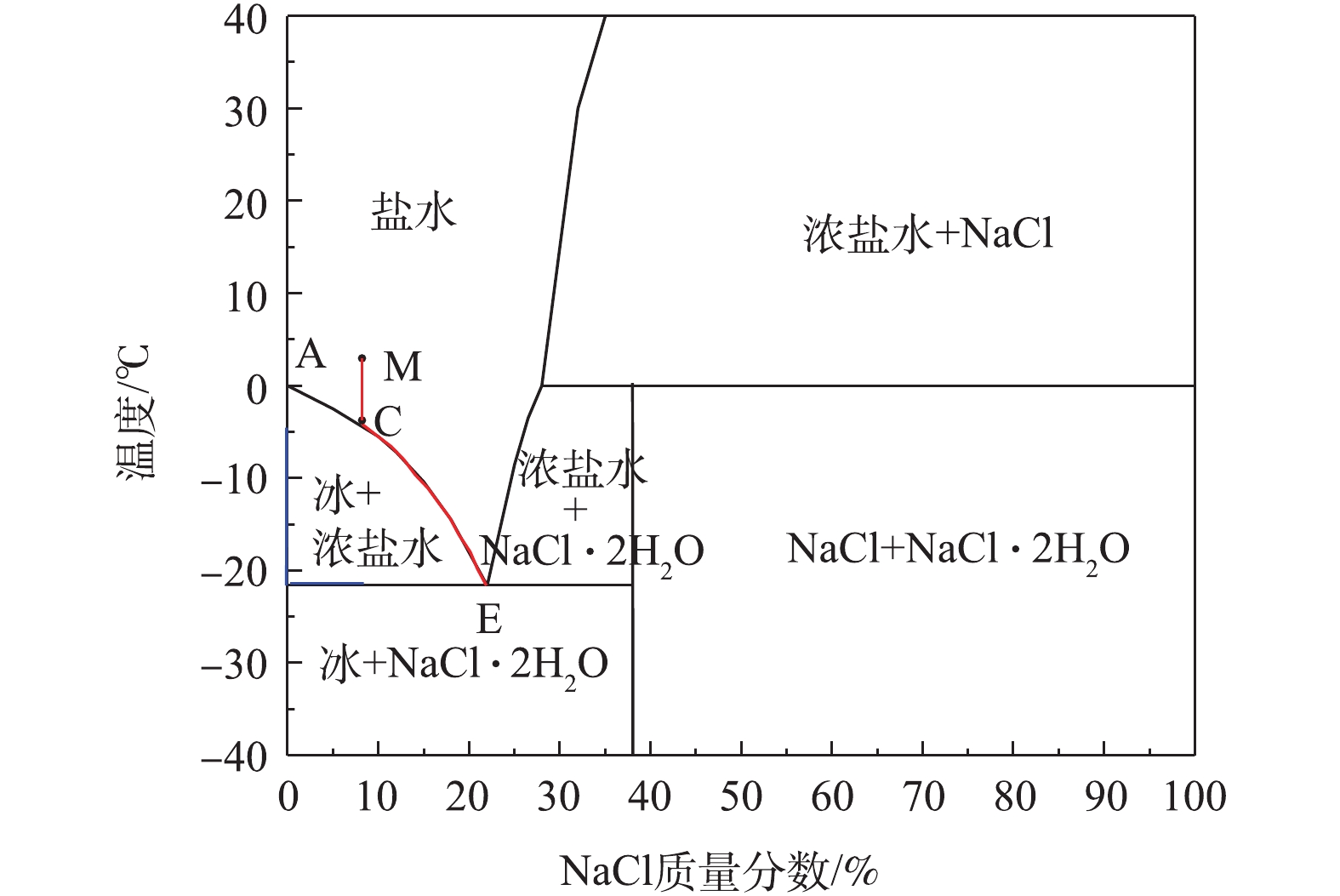
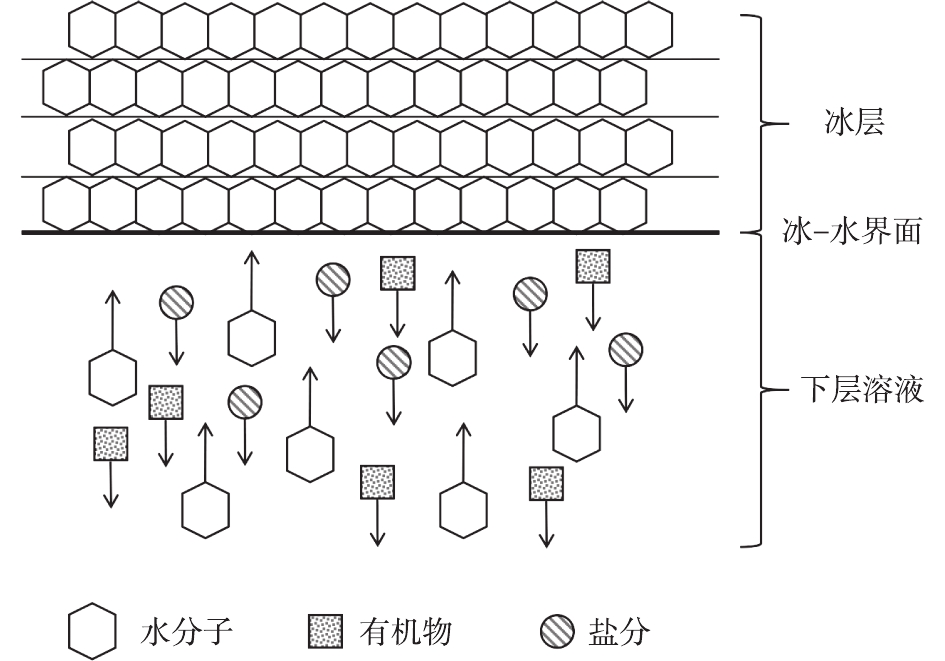
图9为NaCl溶液的二元相图,显示了氯化钠溶液中各物相之间相互联系和转化的规律[15]。其中E点为NaCl溶液的共晶点,共晶温度为−21.2 ℃,共晶浓度为22.4%。把处于M点的盐溶液放置在低温条件下(−18 ℃),当溶液温度到达TC后,溶液开始析出冰,继续下降温度,液相组成沿着AE曲线由C点向E点变化,当温度到达TE后,将从液相中按E点组成中NaCl和水的比例同时析出NaCl晶体和冰晶,此时,系统处于三相(冰、盐水、NaCl)共存点[24-25]。根据以上结果与讨论,提出了冷冻脱盐、去除有机物的机理,如图10所示。结冰是一种从液相变为固相的过程,主要可以分为2个阶段[26-27]:一是晶核形成的阶段,结冰过程开始;二是新相生长阶段,此时晶核长大成为冰晶。在高盐高浓度有机废水结冰过程中,两相界面处的水分子会在氢键的作用下缔结析出,附着在冰层下表面冰冻结成冰。同时,由于结冰过程有排斥盐分和有机物的效应,盐分和有机物会从上层冰体中迁移至下层溶液中, 而水分子在浓度差的推动下,不断朝两相界面处扩散[28],从而导致上层冰体中的盐浓度和有机物浓度不断降低。
-
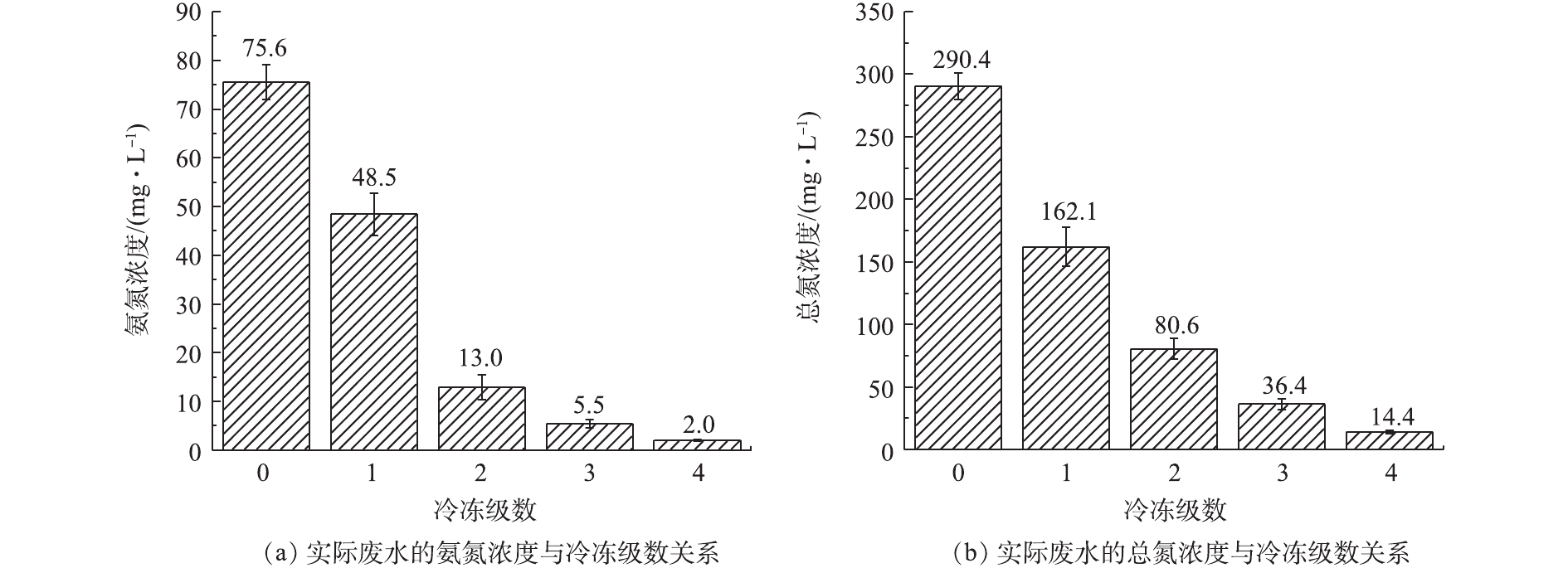
冷冻结晶工艺除对废水中的有机物和盐分有分离效果外,对废水中的其他污染物也有效果。RODRIGUEZ等[29]对含有不同浓度的硝酸盐和磷酸盐废水进行冷冻净化处理,结果表明冷冻净化技术对这2种污染物的去除率均可达到99%以上。郝利娜等[30]通过室内冷冻实验,分析了冷冻法对生活污水中的有机物和氨氮的效果;MARTINEZ等[31]研究了冷冻净水法对高Cr3+含量的制革废水的净化效果。本实验初步探究了冷冻结晶工艺是否对废水中的氨氮和总氮有去除效果,实验结果如图11所示。由图11可知,冷冻结晶对废水中的总氮和氨氮去除效果明显,该废水的初始氨氮浓度为75.6 mg·L−1,在3级冷冻后,氨氮浓度降低为5.5 mg·L−1,在4级冷冻后,降低为2.0 mg·L−1,去除率达到97.3%;初始总氮浓度为290.4 mg·L−1,在4级冷冻后,去除率为95.1%,达到14.4 mg·L−1。可见在4级冷冻结晶后,上层冰融水的氨氮和总氮浓度即可满足《城镇污水处理厂污染物排放标准》一级B的要求。
2.1. 结冰率对上层冰融水脱盐率和有机物去除率的影响
2.2. 冷冻温度对上层冰融水脱盐率和有机物去除率的影响
2.3. 初始盐浓度和初始COD对上层冰融水脱盐率和有机物去除率的影响
2.4. 冷冻接触面积对上层冰融水脱盐率和有机物去除率的影响
2.5. 多级冷冻处理模拟高盐高浓度有机废水
2.6. 多级冷冻处理实际化工废水
2.7. 冷冻脱盐、去除有机物的机理
2.8. 冷冻结晶工艺对废水中其他水质指标去除效果的探索
-
1)冷冻温度、初始盐浓度、初始COD、结冰率和冷冻接触面积均会影响有机物去除率和脱盐率。冷冻温度越低,冷冻速度变快,但脱盐率和有机物去除率下降;随着冷冻时间的增加,结冰率变大,但是脱盐率和有机物去除率随之下降;初始盐浓度或初始COD越高,脱盐和有机物去除效果越差;体积相同的水样,当冷冻接触面积越大时,脱盐率和有机物去除率随之越高。
2)多级冷冻工艺去除模拟高盐高浓度有机废水和实际化工废水中的有机物和盐分都有明显的效果。初始COD为8 000.0 mg·L−1、初始盐浓度为8 000.0 mg·L−1的模拟废水在4级冷冻后脱盐率为93.5%,有机物去除率为97.0%,可满足接管要求。初始COD为55 690.0 mg·L−1, 初始盐浓度为54 648.9 mg·L−1(以NaCl计)的实际化工废水在6级冷冻后,COD降至491.3 mg·L−1,有机物去除率为99.1%,盐浓度降为983.0 mg·L−1(以NaCl计),脱盐率为98.2%,可满足《污水综合排放标准》(GB 8978-1996)对化工企业废水的接管要求。
3)冷冻结晶工艺除对有机物和盐分有去除效果外,对废水中的氨氮和总氮也有去除效果。
4)鉴于冷冻结晶工艺良好的脱盐和有机物去除效果,因此,可以考虑将冷冻结晶技术作为污水处理厂的预处理工艺,去除盐分和降低有机物负荷。



 下载:
下载: